User login
Opioid clinic physicians report lack of competency in managing patients with HCV
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
FROM THE INTERNATIONAL JOURNAL OF DRUG POLICY
Naltrexone/ketamine combo may reduce depressive symptoms in MDD/AUD patients
Combined naltrexone and ketamine reduced depressive symptoms in a small group of patients with major depressive disorder (MDD) and alcohol use disorder (AUD), according to Gihyun Yoon, MD, of the department of psychiatry at Yale University, New Haven, Conn., and associates.
A total of five patients with major depressive disorder and comorbid alcohol use disorder were included in the 8-week, open-label pilot study. The patients were followed for an additional 4 weeks. The primary outcome was the clinical response, defined as a 50% or higher improvement from baseline in the Montgomery-Åsberg Depression Rating Scale.
After the first ketamine dose, three of the five study participants met the primary outcome, and all five met the outcome after receiving all four doses. Depressive symptoms improved 57%-92% overall. In addition, four of the five patients reported improvement in alcohol craving and consumption; no adverse events were reported.
“Larger randomized clinical trials are needed to better understand whether opiate receptor stimulation contributes to the antidepressant effects of ketamine. If so, then preclinical research will be needed to help us to understand this role for opiates and its implications for future rapid-acting antidepressant treatments,” concluded Dr. Yoon and associates.
Two study authors reported conflicts of interest with numerous companies. All study authors are listed inventors on a patent application by Yale University.
SOURCE: Yoon G et al. JAMA Psychiatry. 2019 Jan 9. doi: 10.1001/jamapsychiatry.2018.3990.
Combined naltrexone and ketamine reduced depressive symptoms in a small group of patients with major depressive disorder (MDD) and alcohol use disorder (AUD), according to Gihyun Yoon, MD, of the department of psychiatry at Yale University, New Haven, Conn., and associates.
A total of five patients with major depressive disorder and comorbid alcohol use disorder were included in the 8-week, open-label pilot study. The patients were followed for an additional 4 weeks. The primary outcome was the clinical response, defined as a 50% or higher improvement from baseline in the Montgomery-Åsberg Depression Rating Scale.
After the first ketamine dose, three of the five study participants met the primary outcome, and all five met the outcome after receiving all four doses. Depressive symptoms improved 57%-92% overall. In addition, four of the five patients reported improvement in alcohol craving and consumption; no adverse events were reported.
“Larger randomized clinical trials are needed to better understand whether opiate receptor stimulation contributes to the antidepressant effects of ketamine. If so, then preclinical research will be needed to help us to understand this role for opiates and its implications for future rapid-acting antidepressant treatments,” concluded Dr. Yoon and associates.
Two study authors reported conflicts of interest with numerous companies. All study authors are listed inventors on a patent application by Yale University.
SOURCE: Yoon G et al. JAMA Psychiatry. 2019 Jan 9. doi: 10.1001/jamapsychiatry.2018.3990.
Combined naltrexone and ketamine reduced depressive symptoms in a small group of patients with major depressive disorder (MDD) and alcohol use disorder (AUD), according to Gihyun Yoon, MD, of the department of psychiatry at Yale University, New Haven, Conn., and associates.
A total of five patients with major depressive disorder and comorbid alcohol use disorder were included in the 8-week, open-label pilot study. The patients were followed for an additional 4 weeks. The primary outcome was the clinical response, defined as a 50% or higher improvement from baseline in the Montgomery-Åsberg Depression Rating Scale.
After the first ketamine dose, three of the five study participants met the primary outcome, and all five met the outcome after receiving all four doses. Depressive symptoms improved 57%-92% overall. In addition, four of the five patients reported improvement in alcohol craving and consumption; no adverse events were reported.
“Larger randomized clinical trials are needed to better understand whether opiate receptor stimulation contributes to the antidepressant effects of ketamine. If so, then preclinical research will be needed to help us to understand this role for opiates and its implications for future rapid-acting antidepressant treatments,” concluded Dr. Yoon and associates.
Two study authors reported conflicts of interest with numerous companies. All study authors are listed inventors on a patent application by Yale University.
SOURCE: Yoon G et al. JAMA Psychiatry. 2019 Jan 9. doi: 10.1001/jamapsychiatry.2018.3990.
FROM JAMA PSYCHIATRY
CRP predicts anti-TNF response in ankylosing spondylitis
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point:
Major finding: Compared with normal baseline CRP, a CRP level more than three times above the upper limit of normal correlated significantly with all four outcomes at week 12.
Study details: A post hoc analysis of data from 867 patients with ankylosing spondylitis who received etanercept during one of four clinical trials (NCT00421915, NCT00418548, NCT00247962, and NCT00356356).
Disclosures: Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
Source: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Biodegradable polymer shows no long-term benefit in heart stents
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
The absence of incremental benefit from a stent with a biodegradable polymer compared with the second generation everolimus-eluting stent with a durable polymer in this 10-year follow-up is consistent with prior reports from medium-term follow-up. At best, drug-eluting coronary stents with a biodegradable polymer are noninferior to the current generation of those with a durable polymer. Late clinical benefit from a biodegradable polymer over a second-generation drug-eluting stent with a durable polymer remains elusive. The findings begs the public health question of whether paying a higher price for a biodegradable coronary stent with a durable-polymer is a good investment.
These 10-year results also highlight that, despite using drug-eluting stent technology that remains more or less standard of care today, the treated patients showed a staggering rate of major adverse cardiac events that exceeded 3% each year. This finding suggests an urgent need to address this residual risk through further improvements in medical therapy and stent technology.
Right now, accumulated evidence supports the notion that thinner struts are less thrombogenic than thicker struts, and that not all durable polymers are equal, with some associated with less inflammation and thrombogenicity. The next frontier for stent design seems to be ultrathin struts that are less than 70 mcm in diameter. We need to now see results from a study that compares an ultrathin-strut stent with a durable polymer against one with a biodegradable polymer.
Sripal Bangalore, MD , is an interventional cardiologist and professor of medicine at New York University. He has been a consultant or adviser to Abbott Vascular, Amgen, Biotronik, and Pfizer, and he has received research funding from Abbott Vascular. He made these comments as designated discussant for ISAR-TEST-4, and in an editorial published online concurrently with his talk (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038378 ).
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
CHICAGO – The idea behind putting a biodegradable polymer on a drug-eluting coronary stent is that, once the antirestenosis drug elutes and the polymer that held it degrades, the bare-metal stent left behind would trigger fewer long-term episodes of in-stent thrombosis than would stents that retain their polymer coating. But 10-year follow-up from a large trial that matched two second-generation drug-eluting stents, one with a biodegradable polymer and the other with a durable polymer, showed no statistically significant difference between the two for any clinical outcome, including the incidence of in-stent thrombosis, Sebastian Kufner, MD, said at the American Heart Association scientific sessions.
The potential advantage of a biodegradable polymer “is expected to occur over time,” and hence following patients for 10 or more years should start to show a clear advantage, at least for the endpoint of stent thrombosis, but that didn’t happen. After a median follow-up of 10.6 years, the cumulative rate of definite or probable stent thrombosis was 1.8% among 1,299 patients who received a sirolimus-eluting stent with a biodegradable polymer (Yukon Choice) and 2.5% among 652 patients who received a second-generation everolimus-eluting stent with a durable polymer (Xience), a difference that was not statistically significant, reported Dr. Kufner, a cardiologist at the The German Heart Centre in Munich.
These two stents also produced comparable 10-year outcomes that showed no statistically significant differences for the outcomes of all-cause death, MI, need for target-lesion revascularization, or the combined incidence of all three of these outcomes. In contrast, both of these second-generation stents showed statistically significant improvements in the combined cardiac endpoint, as well as in all-cause death, and definite stent thrombosis compared with the 652 patients who received the first-generation sirolimus-eluting stent Cypher. Concurrently with Dr. Kufner’s report, the results appeared in an article online (Circulation. 2018 Nov 11. doi: 10.1161/CIRCULATIONAHA.118.038065).
Another notable finding from the 10-year follow-up was the poor prognosis these patients faced after their interventions, including the patients who received second-generation drug-eluting stents. The 10-year rate of all cause death was 30% among patients who received Xience stents, 32% among those treated with Yukon Choice stents, and 37% among patients treated with Cypher stents.
“I’m daunted by this 10-year mortality rate even with the best current drug-eluting stents,” said Roxana Mehran, MD, professor of medicine at Icahn School of Medicine at Mount Sinai in New York and a cochair of the session. “I’m depressed about this as an interventionalist. We need to do better, although it might not just be about the revascularization.” The high mortality in these patients after 10 years may also reflect a lack of optimal medical treatment in some, she suggested.
The ISAR-TEST-4 (Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents) trial randomized 2,603 patients during the period of September 2007–August 2008 at either of two centers in Munich. The study’s primary endpoint was the combined rate of cardiac death, MI, and target-lesion revascularization by 12 months after treatment. The 12-month results showed a similar 14% rate of this combined endpoint in the subgroup of patients treated with the biodegradable-polymer stent and in those treated with stents that used durable polymers, which proved the noninferiority of the stent with a biodegradable polymer (Eur Heart J. 2009 Oct 1;30[20]:2441-9). This initial analysis combined the patients who received Cypher and Xience stents into one comparator group: patients who received stents with durable polymers.
The new, long-term follow-up analysis included 2,153 patients (83%) followed for at least 10 years after their intervention.
ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
SOURCE: Kufner S et al. AHA scientific sessions, Abstract 18630.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The rate of adverse cardiac events was 48% with a biodegradable-polymer stent and 46% with a durable polymer.
Study details: Long-term follow-up of 2,153 patients in the ISAR-TEST-4 trial.
Disclosures: ISAR-TEST-4 received no commercial funding. Dr. Kufner had no disclosures. Dr. Mehran has an ownership interest in Claret Medical and Elixir Medical, has been a consultant or adviser to Abbott Laboratories, Boston Scientific, Bristol-Myers Squibb, Janssen, Roivant Sciences, and Siemens Medical Solutions, and has received research funding from several companies.
Source: Kufner S et al. AHA 2018, Abstract 18630.
MD Anderson–led alliance seeks to advance leukemia drug development
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
The primarily for leukemia.
The collaboration, led by Hagop Kantarjian, MD, chair of leukemia at MD Anderson, will use Ascentage’s proprietary Protein-Protein Interaction drug discovery technology platform to develop the company’s apoptosis-targeted and tyrosine kinase inhibitor drug candidates.
The drug candidates will be studied as single-agent therapies and in combinations with other approved or investigational therapeutics. The candidates, chosen for their potential to treat acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphoblastic leukemia (ALL), myeloproliferative neoplasms, and myelofibrosis, include:
- HQP1351, a third-generation BCR-ABL inhibitor that has been shown to be safe and “highly active” in treating patients with chronic- or accelerated-phase CML, with or without the T3151 mutation. Preliminary results of the phase 1 study were presented at the 2018 annual meeting of the American Society of Hematology (Abstract 791).
- APG-1252, a highly potent Bcl-2 family inhibitor, has high binding affinities to Bcl-2, Bcl-xL and Bcl-w. It has achieved tumor regression in small cell lung cancer, colon, breast, and ALL xenografts. A phase 1, dose-escalating study is currently being conducted (NCT03387332).
- APG-2575, a selective Bcl-2 inhibitor, is being studied in a phase 1, multicenter, single-agent trial in patients with B-cell hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, non-Hodgkin lymphomas, and AML (NCT03537482).
- APG-1387, an inhibitor of apoptosis protein, is being studied in solid tumors and hematologic malignancies (NCT03386526). Investigators asserted that combining it with an anti–programmed death 1 antibody would be “a very attractive approach” for cancer therapy. In advanced solid tumors it has been well tolerated with manageable adverse events, according to a study presented at the 2018 annual meeting of the American Society of Clinical Oncology (Abstract 2593).
- APG-115 is an MDM2-p53 inhibitor that, when combined with radiotherapy, has been shown to enhance the antitumor effect in gastric adenocarcinoma, according to a paper published in the Journal of Experimental & Clinical Cancer Research.
“We will be investigating this pipeline of candidate therapies, and we are interested in the novel mechanism of their actions,” Dr. Kantarjian said in a statement.
Sickle cell infusion gains FDA breakthrough designation
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
Masterclass: First-episode psychosis with Dr. Henry A. Nasrallah
from the Psychopharmacology Update meeting in Cincinnati. Dr. Nasrallah is editor in chief of Current Psychiatry and is the Sydney W. Souers Endowed Chair and professor and chairman of the department of neurology and psychiatry and behavioral neuroscience at Saint Louis University.
If you would like to respond to any of Dr. Nasrallah’s comments in this Masterclass, email us at podcasts@mdedge.com.
Amazon
Apple Podcasts
Google Podcasts
Spotify
from the Psychopharmacology Update meeting in Cincinnati. Dr. Nasrallah is editor in chief of Current Psychiatry and is the Sydney W. Souers Endowed Chair and professor and chairman of the department of neurology and psychiatry and behavioral neuroscience at Saint Louis University.
If you would like to respond to any of Dr. Nasrallah’s comments in this Masterclass, email us at podcasts@mdedge.com.
Amazon
Apple Podcasts
Google Podcasts
Spotify
from the Psychopharmacology Update meeting in Cincinnati. Dr. Nasrallah is editor in chief of Current Psychiatry and is the Sydney W. Souers Endowed Chair and professor and chairman of the department of neurology and psychiatry and behavioral neuroscience at Saint Louis University.
If you would like to respond to any of Dr. Nasrallah’s comments in this Masterclass, email us at podcasts@mdedge.com.
Amazon
Apple Podcasts
Google Podcasts
Spotify
AAP guidance: How to ask about military service
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Necrobiosis Lipoidica With Superimposed Pyoderma Vegetans
Case Report
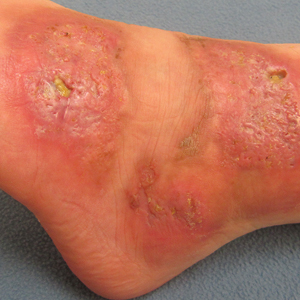
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
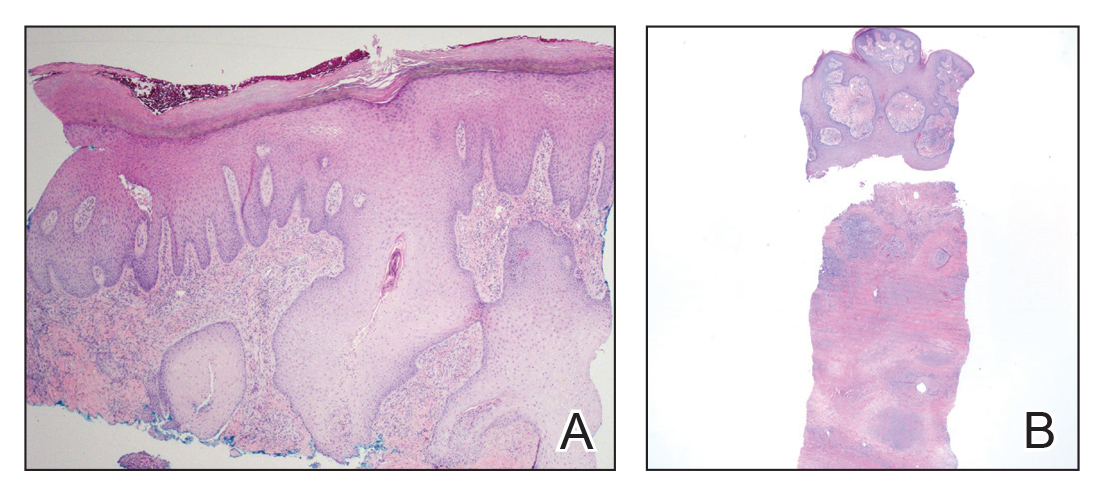
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.
The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.
The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.
The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Practice Points
- Necrobiosis lipoidica (NL), a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial-wall thickening, is most often seen in association with insulin-dependent diabetes mellitus (DM).
- Asymptomatic, well-circumscribed, violaceous papules and nodules coalesce into plaques on the lower extremities, face, or trunk in NL.
- Treatment mainstay is topical and intralesional corticosteroids at active borders of lesions. Other treatments used with some success include tumor necrosis factor 11α inhibitors, topical tretinoin, topical tacrolimus, and skin grafting. Control and management of DM can be helpful.
Daclizumab beta may be superior to interferon beta on MS disability progression
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
FROM MULTIPLE SCLEROSIS JOURNAL
Key clinical point: Daclizumab beta reduces the risk of 24-week sustained worsening on the MSFC by 20%, compared with interferon beta-1a.
Major finding: Daclizumab appears superior to interferon beta-1a regardless of patients’ demographic or disease characteristics.
Study details: A post hoc analysis of the DECIDE study, which included 1,841 patients with relapsing-remitting MS.
Disclosures: Biogen and AbbVie Biotherapeutics supported the DECIDE study.
Source: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.