User login
The Journal of Hospital Medicine in 2019 and Beyond
With this issue, I officially assume the role of Editor-in-Chief of the Journal of Hospital Medicine. I am honored and humbled to serve as the third editor for this journal and thankful to my predecessors, Drs. Mark V. Williams and Andrew D. Auerbach, for establishing it as the premier forum for publication of research in hospital medicine.
The journal has always taken a broad view of its mission. Our focus on improving value and quality of healthcare for children and adults will continue. We are also well-positioned to expand our scope and publish the highest quality research and commentary on the evolving healthcare system, including adoption of new technology, population health management, and regionalization in healthcare, and our role within it. There is also increasing recognition that these trends have implications for patient experience and outcomes, healthcare professional well-being, and the learning environment. We welcome qualitative and quantitative research that provides insight into understanding and addressing these new challenges. We also seek your Perspectives in Hospital Medicine to highlight innovations or controversies in healthcare delivery or policy.
The journal landscape has evolved. We consume medical information in many different formats with a rapidly diminishing reliance on paper and ink. Rather than perusing a journal at the end of a busy workday, we now capitalize on small increments of time in between meetings or other activities. The journal has taken a leading role in engaging readers through social media (@JHospMedicine) with Twitter-based features such as journal clubs (#JHMChat) to discuss recently published research as well as visual abstracts to efficiently share scientific advances.1 We will extend these efforts to include “tweetorials,” video abstracts, and a redesigned web presence, allowing us to transcend the constraints of traditional written articles. Our goals are to increase the visibility of authors and accessibility of their research, allow readers to engage with the journal in formats that best meet their needs, and enhance knowledge retention and knowledge translation to improve healthcare systems and patient outcomes.
The Journal of Hospital Medicine also strives to remain relevant to clinical practice through columns that seek to improve diagnostic reasoning (Clinical Care Conundrums), value and innovation in healthcare (Choosing Wisely: Things We Do For No Reason, Choosing Wisely: Next Steps in Improving Healthcare Value), and, through our long-form reviews, core medical knowledge. While in-depth reviews provide an important synthesis of a topic, our work environment and schedules are not always conducive to reading in this manner; busy clinicians may benefit from focused updates. We will introduce new shorter format reviews addressing clinical content, including practice guidelines, and research methodology.
Finally, we are invested in developing a leadership pipeline for academic medicine. Our Editorial Fellowship will provide educational experiences, professional development, and academic and networking opportunities for a cadre of young physicians.2 A new column will highlight leadership and professional development lessons from renowned leaders from a broad range of disciplines. We also value diversity and inclusion. Disparities in academic medical leadership, though well-recognized, persist. For example, women now comprise more than half of all incoming medical students3 and 41% of faculty, yet only 24% of full professors, 18% of department chairs, and 17% of deans.4 This journal will play an important role in creating a diverse pipeline of academic leaders. We will lead by example and, in the coming year, develop approaches to create equity in all facets of journal leadership and authorship.
I am grateful to Dr. Auerbach for his visionary stewardship of the journal. As I take the helm, the journal will continue to evolve with the changing landscape of healthcare. I am fortunate to work with an exceptionally talented team, and I look forward to serving the journal and the field together to accomplish these goals.
Disclosures
The author has no financial conflicts of interest to disclose
1. Wray CM, Auerbach AD, Arora VM. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med 2018;13:764-769. PubMed
2. Wray CM, Olson A, Shah SS, Auerbach AD. Announcing the Journal of Hospital Medicine editorial fellowship. J Hosp Med 2019;14: 8.PubMed
3. American Association of Medical Colleges. Applicants and matriculants data. 2018. https://www.aamc.org/data/facts/applicantmatriculant/. Accessed December 15, 2018.
4. American Association of Medical Colleges. U.S. medical school faculty, 2017. https://www.aamc.org/data/facultyroster/reports/486050/usmsf17.html. Accessed December 15, 2018.
With this issue, I officially assume the role of Editor-in-Chief of the Journal of Hospital Medicine. I am honored and humbled to serve as the third editor for this journal and thankful to my predecessors, Drs. Mark V. Williams and Andrew D. Auerbach, for establishing it as the premier forum for publication of research in hospital medicine.
The journal has always taken a broad view of its mission. Our focus on improving value and quality of healthcare for children and adults will continue. We are also well-positioned to expand our scope and publish the highest quality research and commentary on the evolving healthcare system, including adoption of new technology, population health management, and regionalization in healthcare, and our role within it. There is also increasing recognition that these trends have implications for patient experience and outcomes, healthcare professional well-being, and the learning environment. We welcome qualitative and quantitative research that provides insight into understanding and addressing these new challenges. We also seek your Perspectives in Hospital Medicine to highlight innovations or controversies in healthcare delivery or policy.
The journal landscape has evolved. We consume medical information in many different formats with a rapidly diminishing reliance on paper and ink. Rather than perusing a journal at the end of a busy workday, we now capitalize on small increments of time in between meetings or other activities. The journal has taken a leading role in engaging readers through social media (@JHospMedicine) with Twitter-based features such as journal clubs (#JHMChat) to discuss recently published research as well as visual abstracts to efficiently share scientific advances.1 We will extend these efforts to include “tweetorials,” video abstracts, and a redesigned web presence, allowing us to transcend the constraints of traditional written articles. Our goals are to increase the visibility of authors and accessibility of their research, allow readers to engage with the journal in formats that best meet their needs, and enhance knowledge retention and knowledge translation to improve healthcare systems and patient outcomes.
The Journal of Hospital Medicine also strives to remain relevant to clinical practice through columns that seek to improve diagnostic reasoning (Clinical Care Conundrums), value and innovation in healthcare (Choosing Wisely: Things We Do For No Reason, Choosing Wisely: Next Steps in Improving Healthcare Value), and, through our long-form reviews, core medical knowledge. While in-depth reviews provide an important synthesis of a topic, our work environment and schedules are not always conducive to reading in this manner; busy clinicians may benefit from focused updates. We will introduce new shorter format reviews addressing clinical content, including practice guidelines, and research methodology.
Finally, we are invested in developing a leadership pipeline for academic medicine. Our Editorial Fellowship will provide educational experiences, professional development, and academic and networking opportunities for a cadre of young physicians.2 A new column will highlight leadership and professional development lessons from renowned leaders from a broad range of disciplines. We also value diversity and inclusion. Disparities in academic medical leadership, though well-recognized, persist. For example, women now comprise more than half of all incoming medical students3 and 41% of faculty, yet only 24% of full professors, 18% of department chairs, and 17% of deans.4 This journal will play an important role in creating a diverse pipeline of academic leaders. We will lead by example and, in the coming year, develop approaches to create equity in all facets of journal leadership and authorship.
I am grateful to Dr. Auerbach for his visionary stewardship of the journal. As I take the helm, the journal will continue to evolve with the changing landscape of healthcare. I am fortunate to work with an exceptionally talented team, and I look forward to serving the journal and the field together to accomplish these goals.
Disclosures
The author has no financial conflicts of interest to disclose
With this issue, I officially assume the role of Editor-in-Chief of the Journal of Hospital Medicine. I am honored and humbled to serve as the third editor for this journal and thankful to my predecessors, Drs. Mark V. Williams and Andrew D. Auerbach, for establishing it as the premier forum for publication of research in hospital medicine.
The journal has always taken a broad view of its mission. Our focus on improving value and quality of healthcare for children and adults will continue. We are also well-positioned to expand our scope and publish the highest quality research and commentary on the evolving healthcare system, including adoption of new technology, population health management, and regionalization in healthcare, and our role within it. There is also increasing recognition that these trends have implications for patient experience and outcomes, healthcare professional well-being, and the learning environment. We welcome qualitative and quantitative research that provides insight into understanding and addressing these new challenges. We also seek your Perspectives in Hospital Medicine to highlight innovations or controversies in healthcare delivery or policy.
The journal landscape has evolved. We consume medical information in many different formats with a rapidly diminishing reliance on paper and ink. Rather than perusing a journal at the end of a busy workday, we now capitalize on small increments of time in between meetings or other activities. The journal has taken a leading role in engaging readers through social media (@JHospMedicine) with Twitter-based features such as journal clubs (#JHMChat) to discuss recently published research as well as visual abstracts to efficiently share scientific advances.1 We will extend these efforts to include “tweetorials,” video abstracts, and a redesigned web presence, allowing us to transcend the constraints of traditional written articles. Our goals are to increase the visibility of authors and accessibility of their research, allow readers to engage with the journal in formats that best meet their needs, and enhance knowledge retention and knowledge translation to improve healthcare systems and patient outcomes.
The Journal of Hospital Medicine also strives to remain relevant to clinical practice through columns that seek to improve diagnostic reasoning (Clinical Care Conundrums), value and innovation in healthcare (Choosing Wisely: Things We Do For No Reason, Choosing Wisely: Next Steps in Improving Healthcare Value), and, through our long-form reviews, core medical knowledge. While in-depth reviews provide an important synthesis of a topic, our work environment and schedules are not always conducive to reading in this manner; busy clinicians may benefit from focused updates. We will introduce new shorter format reviews addressing clinical content, including practice guidelines, and research methodology.
Finally, we are invested in developing a leadership pipeline for academic medicine. Our Editorial Fellowship will provide educational experiences, professional development, and academic and networking opportunities for a cadre of young physicians.2 A new column will highlight leadership and professional development lessons from renowned leaders from a broad range of disciplines. We also value diversity and inclusion. Disparities in academic medical leadership, though well-recognized, persist. For example, women now comprise more than half of all incoming medical students3 and 41% of faculty, yet only 24% of full professors, 18% of department chairs, and 17% of deans.4 This journal will play an important role in creating a diverse pipeline of academic leaders. We will lead by example and, in the coming year, develop approaches to create equity in all facets of journal leadership and authorship.
I am grateful to Dr. Auerbach for his visionary stewardship of the journal. As I take the helm, the journal will continue to evolve with the changing landscape of healthcare. I am fortunate to work with an exceptionally talented team, and I look forward to serving the journal and the field together to accomplish these goals.
Disclosures
The author has no financial conflicts of interest to disclose
1. Wray CM, Auerbach AD, Arora VM. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med 2018;13:764-769. PubMed
2. Wray CM, Olson A, Shah SS, Auerbach AD. Announcing the Journal of Hospital Medicine editorial fellowship. J Hosp Med 2019;14: 8.PubMed
3. American Association of Medical Colleges. Applicants and matriculants data. 2018. https://www.aamc.org/data/facts/applicantmatriculant/. Accessed December 15, 2018.
4. American Association of Medical Colleges. U.S. medical school faculty, 2017. https://www.aamc.org/data/facultyroster/reports/486050/usmsf17.html. Accessed December 15, 2018.
1. Wray CM, Auerbach AD, Arora VM. The adoption of an online journal club to improve research dissemination and social media engagement among hospitalists. J Hosp Med 2018;13:764-769. PubMed
2. Wray CM, Olson A, Shah SS, Auerbach AD. Announcing the Journal of Hospital Medicine editorial fellowship. J Hosp Med 2019;14: 8.PubMed
3. American Association of Medical Colleges. Applicants and matriculants data. 2018. https://www.aamc.org/data/facts/applicantmatriculant/. Accessed December 15, 2018.
4. American Association of Medical Colleges. U.S. medical school faculty, 2017. https://www.aamc.org/data/facultyroster/reports/486050/usmsf17.html. Accessed December 15, 2018.
© 2019 Society of Hospital Medicine
Announcing the Journal of Hospital Medicine Editorial Fellowship
The peer review and editorial processes are integral activities in academic medicine that provide ethical, independent, and unbiased critical assessment of submitted manuscripts to academic journals. Recognizing that few trainees or junior faculty are formally exposed to these processes,1 the Journal of Hospital Medicine aims to fill this opportunity gap through the launch of a one-year Editorial Fellowship.
The Fellowship is open to chief residents, hospital medicine fellows, and junior faculty (eg, Assistant Professor or Clinical Instructor). Starting in July of each year, a group of four to six applicants are paired with editorial mentors who are current JHM Deputy or Associate Editors. Structured as a distance-learning program, this program aims to allow Fellows the ability to continue in their full time professional roles while also allowing the opportunity to engage with national leaders in hospital medicine. Regular communication and interactions take place through both synchronous and asynchronous means. Fellows’ responsibilities during the 12-month experience include: completion of six guided peer reviews, preparation of one or two editorials, participation in monthly editorial meetings, and quarterly educational videoconferences. Interested Fellows may also have an opportunity to co-lead the journal’s online journal club, #JHMChat.2 Fellows are expected to attend the editorial staff meeting at the annual Society of Hospital Medicine Conference.
With this program, JHM aims to accomplish several tasks. First, we hope to offer a unique educational experience that allows for further growth, development, inspiration, and experience in academic medicine—specifically around the manuscript review and editorial processes. Second, recognizing that a journal’s quality is frequently a product of its reviewers, JHM hopes to build a cadre of well-trained and experienced reviewers and, hopefully, future members of the JHM editorial leadership team. Third, the program hopes to act as a networking experience, allowing editorial Fellows to learn from, collaborate with, and become academic leaders in the field. Finally, we hope to provide an opportunity for Fellows to be academically productive in their composition of editorial content—an output that will help catalyze their professional development.
We believe that in working closely with the JHM editorial staff, this program will help develop the next generation of leaders in academic hospital medicine. We strongly encourage applications from physicians who have been historically under-represented in leadership in academic medicine. Further details and the application can be found in the appendix and on the JHM website (www.journalofhospitalmedicine.com). It will be announced annually through the @JHospMedicine twitter handle.
Disclosures
The authors have nothing to disclose.
1. Lovejoy TI, Revenson TA, France CR. Reviewing manuscripts for peer-review journals: a primer for novice and seasoned reviewers. Ann Behav Med Publ Soc Behav Med. 2011;42(1):1-13. doi:10.1007/s12160-011-9269-x PubMed
2. Wray CM, Arora VM, Auerbach AD. The Adoption of an Online Journal Club to Improve Research Dissemination and Social Media Engagement Among Hospitalists. J Hosp Med. 2018;13(11). doi:10.12788/jhm.2987 PubMed
The peer review and editorial processes are integral activities in academic medicine that provide ethical, independent, and unbiased critical assessment of submitted manuscripts to academic journals. Recognizing that few trainees or junior faculty are formally exposed to these processes,1 the Journal of Hospital Medicine aims to fill this opportunity gap through the launch of a one-year Editorial Fellowship.
The Fellowship is open to chief residents, hospital medicine fellows, and junior faculty (eg, Assistant Professor or Clinical Instructor). Starting in July of each year, a group of four to six applicants are paired with editorial mentors who are current JHM Deputy or Associate Editors. Structured as a distance-learning program, this program aims to allow Fellows the ability to continue in their full time professional roles while also allowing the opportunity to engage with national leaders in hospital medicine. Regular communication and interactions take place through both synchronous and asynchronous means. Fellows’ responsibilities during the 12-month experience include: completion of six guided peer reviews, preparation of one or two editorials, participation in monthly editorial meetings, and quarterly educational videoconferences. Interested Fellows may also have an opportunity to co-lead the journal’s online journal club, #JHMChat.2 Fellows are expected to attend the editorial staff meeting at the annual Society of Hospital Medicine Conference.
With this program, JHM aims to accomplish several tasks. First, we hope to offer a unique educational experience that allows for further growth, development, inspiration, and experience in academic medicine—specifically around the manuscript review and editorial processes. Second, recognizing that a journal’s quality is frequently a product of its reviewers, JHM hopes to build a cadre of well-trained and experienced reviewers and, hopefully, future members of the JHM editorial leadership team. Third, the program hopes to act as a networking experience, allowing editorial Fellows to learn from, collaborate with, and become academic leaders in the field. Finally, we hope to provide an opportunity for Fellows to be academically productive in their composition of editorial content—an output that will help catalyze their professional development.
We believe that in working closely with the JHM editorial staff, this program will help develop the next generation of leaders in academic hospital medicine. We strongly encourage applications from physicians who have been historically under-represented in leadership in academic medicine. Further details and the application can be found in the appendix and on the JHM website (www.journalofhospitalmedicine.com). It will be announced annually through the @JHospMedicine twitter handle.
Disclosures
The authors have nothing to disclose.
The peer review and editorial processes are integral activities in academic medicine that provide ethical, independent, and unbiased critical assessment of submitted manuscripts to academic journals. Recognizing that few trainees or junior faculty are formally exposed to these processes,1 the Journal of Hospital Medicine aims to fill this opportunity gap through the launch of a one-year Editorial Fellowship.
The Fellowship is open to chief residents, hospital medicine fellows, and junior faculty (eg, Assistant Professor or Clinical Instructor). Starting in July of each year, a group of four to six applicants are paired with editorial mentors who are current JHM Deputy or Associate Editors. Structured as a distance-learning program, this program aims to allow Fellows the ability to continue in their full time professional roles while also allowing the opportunity to engage with national leaders in hospital medicine. Regular communication and interactions take place through both synchronous and asynchronous means. Fellows’ responsibilities during the 12-month experience include: completion of six guided peer reviews, preparation of one or two editorials, participation in monthly editorial meetings, and quarterly educational videoconferences. Interested Fellows may also have an opportunity to co-lead the journal’s online journal club, #JHMChat.2 Fellows are expected to attend the editorial staff meeting at the annual Society of Hospital Medicine Conference.
With this program, JHM aims to accomplish several tasks. First, we hope to offer a unique educational experience that allows for further growth, development, inspiration, and experience in academic medicine—specifically around the manuscript review and editorial processes. Second, recognizing that a journal’s quality is frequently a product of its reviewers, JHM hopes to build a cadre of well-trained and experienced reviewers and, hopefully, future members of the JHM editorial leadership team. Third, the program hopes to act as a networking experience, allowing editorial Fellows to learn from, collaborate with, and become academic leaders in the field. Finally, we hope to provide an opportunity for Fellows to be academically productive in their composition of editorial content—an output that will help catalyze their professional development.
We believe that in working closely with the JHM editorial staff, this program will help develop the next generation of leaders in academic hospital medicine. We strongly encourage applications from physicians who have been historically under-represented in leadership in academic medicine. Further details and the application can be found in the appendix and on the JHM website (www.journalofhospitalmedicine.com). It will be announced annually through the @JHospMedicine twitter handle.
Disclosures
The authors have nothing to disclose.
1. Lovejoy TI, Revenson TA, France CR. Reviewing manuscripts for peer-review journals: a primer for novice and seasoned reviewers. Ann Behav Med Publ Soc Behav Med. 2011;42(1):1-13. doi:10.1007/s12160-011-9269-x PubMed
2. Wray CM, Arora VM, Auerbach AD. The Adoption of an Online Journal Club to Improve Research Dissemination and Social Media Engagement Among Hospitalists. J Hosp Med. 2018;13(11). doi:10.12788/jhm.2987 PubMed
1. Lovejoy TI, Revenson TA, France CR. Reviewing manuscripts for peer-review journals: a primer for novice and seasoned reviewers. Ann Behav Med Publ Soc Behav Med. 2011;42(1):1-13. doi:10.1007/s12160-011-9269-x PubMed
2. Wray CM, Arora VM, Auerbach AD. The Adoption of an Online Journal Club to Improve Research Dissemination and Social Media Engagement Among Hospitalists. J Hosp Med. 2018;13(11). doi:10.12788/jhm.2987 PubMed
© 2019 Society of Hospital Medicine
Association between Hospitalist Productivity Payments and High-Value Care Culture
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
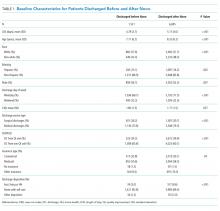
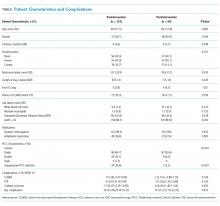
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
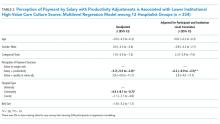
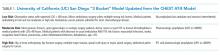
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
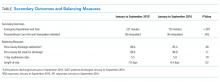
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
The Centers of Medicare and Medicaid Services (CMS) has introduced new payment models that tie quality and value incentives to 90% of fee-for-service payments and provide 50% of Medicare payments through alternative payment models.1 The push toward value comes after productivity-based physician reimbursement (ie, fee for service) has been associated with poor quality care, including delayed diagnoses, complications, readmissions, increased length of stay, and high costs of care.2-5 The method of physician payment is widely believed to affect clinical behavior by incentivizing doing more, coding for more, and billing for more.6-7 Although payment systems may be used to achieve policy objectives,8 little is known about the association of different payment systems with the culture of delivering value-based care among frontline clinicians.
Culture is defined as a system of shared assumptions, values, beliefs, and norms within an environment and has a powerful role in shaping clinician practice patterns.9-12 The culture within medicine currently contributes to the overuse of resources,11,13 and a culture for improvement is correlated with clinical outcomes. A systematic review found a consistent association between positive organization culture and improved outcomes including mortality.14 Across health systems, institutions with high scores on patient safety culture surveys have shown improvements in clinical behaviors and patient outcomes.15-18
In this study, we aim to describe high-value care culture among internal medicine hospitalists across diverse hospitals and evaluate the relationship between physician reimbursement and high-value care culture.
METHODS
Study Design
This study is an observational, cross-sectional survey-based study of hospitalists from 12 hospitals in California between January and June 2016.
Study Population
A total of 12 hospitals with hospitalist programs in California were chosen to represent three types of hospitals (ie, four university, four community, and four safety net). Safety-net hospitals, which traditionally serve low-income and medically and socially vulnerable patients were defined as those in the top quartile (ie, greater than 0.5) of their Disproportionate Share Index (DSH), which measures Medicaid patient load.19-20
To select hospitals with varying value-based care performance, we stratified using CMS value-based purchasing (VBP) scores from fiscal year 2015; these scores have been used to adjust reimbursement for just over 3,000 hospitals in the VBP program of CMS.22,23 CMS calculates the VBP total performance score as a composite of four domains: (1) clinical processes of care (20% of total performance); (2) patient satisfaction (30%); (3) patient outcomes, including mortality and complications (30%); and (4) cost defined by Medicare payment per beneficiary (20%).21 Established quality measures are based on data reported by participating hospitals and chart abstraction during 2011-2014.22 Although other clinical measures of care intensity have been used as proxies of value-based care,23,24 we used the measure of value that has been publically reported by the CMS VBP given its wide use and effects on reimbursements for 80% of hospitals in the CMS VBP program in 2015.25 We obtained institution-level data from the CMS VBP Program and Hospital Compare files. Each of the three types of hospitals represented institutions with low, middle, and high VBP performance (split in tertiles) as reported by the CMS VBP program. To increase the number of participants in tertiles with fewer hospitalists, a fourth hospital was selected for each hospital type.
We excluded individual hospitalists who primarily identified as working in subspecialty divisions and those who spent less than eight weeks during the last year providing direct patient care on inpatient internal medicine services at the studied institution.
Measurement
Hospitalists were asked to complete the High-Value Care Culture Survey (HVCCSTM), which measures the culture of value-based decision making among frontline clinicians.26 Similar to other validated surveys for the assessment of patient safety culture,27,28 the HVCCS can be used to identify target areas for improvement. The survey includes four domains: (1) leadership and health system messaging, (2) data transparency and access, (3) comfort with cost conversations, and (4) blame-free environment. This tool was developed by using a two-phase national modified Delphi process. It was evaluated at two academic centers to complete factor analysis and assess internal consistency, reliability, and validity among internal medicine hospitalists and residents. Validation included estimating product-moment correlation of overall HVCCS scores and domain scores with the CMS institutional VBP scores. HVCCS scores are standardized to a 0-100 point scale for each of the four domains and are then averaged to obtain an overall score.26
In the survey, value was defined as the quality of care provided to patients in relation to the costs required to deliver that care, and high-value care was defined as care that tried to maximize quality while minimizing costs. Quality was defined as the degree to which health services increased the likelihood of desired health outcomes that are safe, effective, patient centered, timely, equitable, and consistent with current professional knowledge. Cost was defined as the negative financial, physical, and emotional effects on patients and the health system.26
Data Analysis
We described the overall institutional mean high-value care culture and domain scores measured by the HVCCS, hospitalist demographics and training experiences, and hospital characteristics. We also described individual survey items. Descriptive statistics were stratified and compared on the basis of hospital type (ie, safety net, community, or university). We assessed the relationship between the clinician perception of reimbursement structure within their divisions and individually reported high-value care culture scores using bivariate and multilevel linear regression. We hypothesized that compared with hospitalists who were paid with salaries or wages, those who reported reimbursement with productivity adjustments may report lower HVCCS scores and those who reported reimbursement with quality or value adjustments may report higher HVCCS scores. We adjusted for physician- and hospital-level characteristics, including age, gender, and training track, and considered hospital type and size as random effects.
This study was approved by the Institutional Review Board at all 12 sites. All analyses were conducted using STATA® 13.0 (College Station, Texas).
RESULTS
Hospitalist Characteristics
A total of 255 (68.9%, 255/370) hospitalists across all sites completed the survey. Of these respondents, 135 were female (50.6%). On average, hospitalists were 39 years of age (SD 6.8), trained in categorical tracks (221; 86.7%), and had previously trained for 14.3 months at a safety-net hospital (SD 14.2). In total, 166 hospitalists (65.1%) reported being paid with salary or wages, 77 (30.2%) with salary plus productivity adjustments, and 12 (4.7%) with salary plus quality or value adjustments. Moreover, 123 (48.6%) hospitalists agreed that funding for their group depended on the volume of services they delivered. Community-based hospitalists reported higher rates of reimbursement with salary plus productivity (47; 32.0%) compared with their counterparts from university-based (24; 28.2%) and safety-net based programs (6; 26.1%). Among the three different hospital types, significant differences exist in hospitalist mean age (P < .001), gender (P = .01), and the number of months training in a safety-net hospital (P = .02; Table 1).
Hospital Characteristics
Of the 12 study sites, four from each type of hospital (ie, safety-net based, community based, and university based) and four representing each value-based purchasing performance tertile (ie, high, middle, and low) were included. Eleven (91.7%) sites were located in urban areas with an average DSH index of 0.40 (SD 0.23), case mix index of 1.97 (SD 0.28), and bed size of 435.5 (SD 146.0; Table 1).
In multilevel regression modeling across all 12 sites, hospitalists from community-based hospitalist programs reported lower mean HVCCS scores (β = −4.4, 95% CI −8.1 to −0.7; Table 2) than those from other hospital types.
High-Value Care Culture Survey Scores
The mean HVCCS score was 50.2 (SD 13.6), and mean domain scores across all sites were 65.4 (SD 15.6) for leadership and health system messaging, 32.4 (SD 22.8) for data transparency and access, 52.1 (SD 19.7) for comfort with cost conversations, and 50.7 (SD 21.4) for blame-free environment (Table 1). For the majority (two-thirds) of individual HVCCS items, more than 30% of hospitalists across all sites agreed or strongly agreed that components of a low-value care culture exist within their institutions. For example, over 80% of hospitalists reported low transparency and limited access to data (see Appendix I for complete survey responses).
Hospitalists reported different HVCCS domains as strengths or weaknesses within their institutions in accordance with hospital type. Compared with university-based and safety-net-based hospitalists, community-based hospitalists reported lower scores in having a blame-free environment (466, SD 21.8). Nearly 50% reported that the clinicians’ fear of legal repercussions affects their frequency of ordering unneeded tests or procedures, and 30% reported that individual clinicians are blamed for complications. Nearly 40% reported that clinicians are uncomfortable discussing the costs of tests or treatments with patients and reported that clinicians do not feel that physicians should discuss costs with patients. Notably, community-based hospitalists uniquely differed in how they reported components of leadership and health system messaging. Over 60% reported a work climate or role modeling supportive of delivering quality care at lower costs. Only 48%, however, reported success seen from implemented efforts, and 45% reported weighing costs in clinical decision making (Table 1, Appendix I).
University-based hospitalists had significantly higher scores in leadership and health system messaging (67.4, SD 16.9) than community-based and safety-net-based hospitalists. They reported that their institutions consider their suggestions to improve quality care at low cost (75%), openly discuss ways to deliver this care (64%), and are actively implementing projects (73%). However, only 54% reported seeing success from implemented high-value care efforts (Table 1, Appendix I).
Safety-net hospitalists reported lower scores in leadership and health system messaging (56.8, SD 10.5) than university-based and community-based hospitalists. Few hospitalists reported a work climate (26%) or role modeling (30%) that is supportive of delivering quality care at low costs, openly discusses ways to deliver this care (35%), encourages frontline clinicians to pursue improvement projects (57%), or actively implements projects (26%). They also reported higher scores in the blame-free environment domain (59.8, SD 22.3; Table 1; Appendix 1).
Productivity Adjustments and High-Value Care Culture
In multilevel regression modeling, hospitalists who reported reimbursement with salary plus productivity adjustments had a lower mean HVCCS score (β = −6.2, 95% CI −9.9 to –2.5) than those who reported payment with salary or wages alone. Further multilevel regression modeling for each HVCCS domain revealed that hospitalists who reported reimbursement with salary plus productivity adjustments had lower scores in the leadership and health system messaging domain (β = −4.9, 95% CI −9.3 to −0.6) and data transparency and access domain (β = −10.7, 95% CI −16.7 to −4.6). No statistically significant difference was found between hospitalists who reported reimbursement with quality or value adjustments.
DISCUSSION
Understanding the drivers that are associated with a high-value care culture is necessary as payment models for hospitals transition from volume-based to value-based care. In this study, we found a meaningful association (β = −6.2) between clinician reimbursement schemes and measures of high-value care culture. A six-point change in the HVCCS score would correspond with a hospital moving from the top quartile to the median, which represents a significant change in performance. The relationship between clinician reimbursement schemes and high-value care culture may be a bidirectional relationship. Fee for service, the predominant payment scheme, places pressure on clinicians to maximize volume, focus on billing, and provide reactive care.7,29 Conversely, payment schemes that avoid these incentives (ie, salary, wages, and adjustments for quality or value), especially if incentives are felt by frontline clinicians, may better align with goals for long-term health outcomes for patient populations and reduce excess visits and services.2-6,8,30-34 At the same time, hospitals with a strong high-value care culture may be more likely to introduce shared savings programs and alternative payment models than those without. Through these decisions, the leadership can play an important role in creating an environment for change.34 Similar to the study sites, hospitals in California have a higher percentage of risk-based payments than hospitals in other states (>22%)35 and may also provide incentives to promote a high-value care culture or affect local physician compensation models.
Hospitals have options in how they choose to pay their clinicians, and these decisions may have downstream effects, such as building or eroding high-value care culture among clinicians or staff. A dose-response relationship between physician compensation models and value culture is plausible (salary with productivity < salary only < salary with value incentive). However, we did not find a statistically significant difference for salary with value incentive. This result may be attributed to the relatively small sample size in this study.
Hospitals can also improve their internal processes, organizational structure, and align their institutional payment contracts with those that emphasize value over fee-for-service-based incentives to increase value in care delivery.36 The operation of hospitals is challenging when competing payment incentives are used at the same time,7 and leadership will likely achieve more success in improving a high-value care culture and value performance when all efforts, including clinician and institutional payment, are aligned.37-38
Enduring large systems redesign will require directing attention to local organizational culture. For the majority of individual HVCCS items, 30% or more hospitalists across all sites agreed or strongly agreed that components of low-value care culture exist within their institutions. This response demonstrates a lack of focus on culture to address high-value care improvement among the study sites. Division and program leaders can begin measuring culture within their groups to develop new interventions that target culture change and improve value.34 No single panacea exists for the value improvement of hospitalist programs in California across all hospital types and sites.
Unique trends, however, emerge among each hospital type that could direct future improvements. In addition to all sites requiring increased transparency and access to data, community-based hospitalists identified the need for improvement in the creation of a blame-free environment, comfort with cost conversations, and aspects of leadership and health system messaging. While a high proportion of these hospitalists reported a work culture and role modeling that support the delivery of quality care at low costs, opportunities to create open discussion and frontline involvement in improvement efforts, weigh costs into clinical decision making, and cost conversations with patients exist. We hypothesize that these opportunities exist because community-based hospitals create infrastructure and technology to drive improvement that is often unseen by frontline providers. University-based hospitalists performed higher on three of the four domains compared with their counterparts but may have opportunities to promote a blame-free environment. A great proportion of these hospitalists reported the occurrence of open discussion and active projects within their institutions but also identified opportunities for the improvement of project implementation. Safety-net hospitalists reported the need to improve leadership and health system messaging across most domain items. Further study is required to evaluate reasons for safety-net hospitalists’ responses. We hypothesize that these responses may be related to having limited institutional resources to provide data and coordinated care and different institutional payment models. Each of these sites could identify trends in specific questions identified by the HVCCS for improvement in the high-value care culture.25
Our study evaluated 12 hospitalist programs in California that represent hospitals of different sizes and institutional VBP performance. A large multisite study that evaluates HVCCS across other specialties and disciplines in medicine, all regions of the country, and ambulatory care settings may be conducted in the future. Community-based hospitalist programs also reported low mean HVCCS scores, and further studies could better understand this relationship.
The limitations of the study include its small subgroup sample size and the lack of a gold standard for the measurement of high-value care. As expected, hospitalist groups among safety-net hospitals in California are small, and we may have been underpowered to determine some correlations presented by safety-net sites when stratifying by hospital type. Other correlations also may have been limited by sample size, including differences in HVCCS scores based on reimbursement and hospital type and the correlation between a blame-free environment and reimbursement type. Additionally, the field lacks a gold standard for the measurement of high-value care to help stratify institutional value performance for site selection. The VBP measure presents policy implications and is currently the best available measure with recent value data for over 3,000 hospitals nationally and representing various types of hospitals. This study is also cross-sectional and may benefit from the further evaluation of organizational culture over time and across other settings.
CONCLUSION
The HVCCS can identify clear targets for improvement and has been evaluated among internal medicine hospitalists. Hospitalists who are paid partly based on productivity reported low measures of high-value care culture at their institutions. As the nation moves toward increasingly value-based payment models, hospitals can strive to improve their understanding of their individual culture for value and begin addressing gaps.
Acknowledgments
The authors wish to thank Michael Lazarus, MD from the University of California Los Angeles; Robert Wachter, MD, James Harrison, PhD; Victoria Valencia, MPH from Dell Medical School at the University of Texas at Austin; Mithu Molla, MD from University of California Davis; Gregory Seymann, MD from the University of California San Diego; Bindu Swaroop, MD and Alpesh Amin, MD from University of California Irvine; Jessica Murphy, DO and Danny Sam, MD from Kaiser Permanente Santa Clara; Thomas Baudendistel, MD and Rajeeva Ranga, MD from Kaiser Permanente Oakland; Yile Ding, MD from California Pacific Medical Center; Soma Wali, MD from Los Angeles County/ OliveView UCLA Medical Center; Anshu Abhat, MD, MPH from the LA BioMed Institute at Los Angeles County/ Harbor-UCLA Medical Center; Steve Tringali, MD from Community Regional Medical Center Fresno; and Dan Dworsky, MD from Scripps Green Hospital for their site leadership and participation with the study.
Disclosures
Dr. Gupta is the Director of the Teaching Value in Healthcare Learning Network at Costs of Care. Dr. Moriates receives royalties from McGraw Hill for the textbook “Understanding Value-based Healthcare” outside of the submitted work and is the Director of Implementation at Costs of Care.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13. Colla CH. Swimming against the current—what might work to reduce low-value care? N Engl J Med. 2014;371(14):1280-1283. doi: 10.1056/NEJMp1404503. PubMed
14.
15.
16. Singer S, Lin S, Falwell A, Gaba D, Baker L. Relationship of safety climate and safety performance in hospitals. Health Serv Res. 2009;44(2 Pt 1):399-421. doi: 10.1111/j.1475-6773.2008.00918.x. PubMed
17.
18. Berry JC, Davis JT, Bartman T, et al. Improved safety culture and teamwork climate are associated with decreases in patient harm and hospital mortality across a hospital system. J Patient Saf. 2016. doi: 10.1097/PTS.0000000000000251. PubMed
19. Chatterjee P, Joynt KE, Orav EJ, Jha AK. Patient experience in safety-net hospitals: implications for improving care and value-based purchasing. Arch Intern Med. 2012;172(16):1204-1210. doi: 10.1001/archinternmed.2012.3158. PubMed
20. Centers for Medicare and Medicaid Services, Disproportionate Share Hospital (DSH). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/dsh.html. Accessed May 1, 2018.
21.
22. Center for Medicare and Medicaid Services, Medicare Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 1, 2018.
23. Sexton JB, Helmreich RL, Neilands TB, et al. The Safety Attitudes Questionnaire: psychometric properties, benchmarking data, and emerging research. BMC Health Serv Res. 2006;6:44. doi: 10.1186/1472-6963-6-44. PubMed
24. Singla AK, Kitch BT, Weissman JS, Campbell EG. Assessing patient safety culture. J Patient Saf. 2006;2(3):105-115. doi: 10.1097/01.jps.0000235388.39149.5a.
25. Centers for Medicare and Medicaid Services, HHS, Medicare Program. Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(26):490-547.
26. Gupta R, Moriates C, Clarke R, et al. Development of a high-value care culture survey: a modified Delphi process and psychometric evaluation. BMJ Qual Saf. 2016:1-9. http://dx.doi.org/10.1136/bmjqs-2016-005612 PubMed
27. Centers for Medicare and Medicaid Services. Medicare program; Hospital inpatient value-based purchasing program. Final rule. Fed Regist. 2011;76(88):26490-26547.
28. Arora A, True A, Dartmouth Atlas of Health Care. What Kind of Physician Will You Be? Variation in Health Care and Its Importance for Residency Training. Dartmouth Institute for Health Policy and Clinical Practice; 2012.
29. Berenson RA, Rich EC. US approaches to physician payment: the deconstruction of primary care. J Gen Intern Med. 2010;25(6):613-618. doi: 10.1007/s11606-010-1295-z. PubMed
30. Rosenthal MB, Dudley RA. Pay-for-performance: will the latest payment trend improve care? JAMA: the Journal of the American Medical Association. 1997;297(7):740-744. doi: 10.1001/jama.297.7.740 PubMed
31. Smith M, Saunders SM, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: the Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; May 10, 2013. PubMed
32. Powers BW, Milstein A, Jain SH. Delivery models for high-risk older patients: Back to the Future? JAMA. 2016;315(1):23-24. doi: 10.1001/jama.2015.17029. PubMed
33. Sinsky CA, Sinsk TA. Lessons from CareMore: A stepping stone to stronger primary care of frail elderly patients. Am J Manag Care. 2015;3(2):2-3.
34. Gupta R, Moriates C. Swimming upstream: creating a culture of high value care. Acad Med. 2016:1-4. doi: 10.1097/ACM.0000000000001485 PubMed
35. Berkeley Forum. California’s delivery system integration and payment system. http://berkeleyhealthcareforum.berkeley.edu/wp-content/uploads/Appendix-II.-California%E2%80%99s-Delivery-System-Integration-and-Payment-System-Methodology.pdf. Accessed July 15, 2018; April 2013.
36. Miller HD. From volume to value: better ways to pay for health care. Health Aff. 2009;28(5):1418-1428. doi: 10.1377/hlthaff.28.5.1418. PubMed
37. Kahn CN, III. Payment reform alone will not transform health care delivery. Health Aff. 2009;28(2):w216-w218. doi: 10.1377/hlthaff.28.2.w216. PubMed
38.
© 2019 Society of Hospital Medicine
Improving Patient Flow: Analysis of an Initiative to Improve Early Discharge
Patient flow throughout the hospital has been shown to be adversely affected by discharge delays.1 When hospitals are operating at peak capacity, these delays impact throughput, length of stay (LOS), and cost of care and block patients from the emergency department (ED), postanesthesia recovery unit (PACU), or home awaiting inpatient beds.2-5 As patients wait in locations not ideal for inpatient care, they may suffer from adverse events and poor satisfaction.3,6 Several studies have analyzed discharge timing as it relates to ED boarding of admitted patients and demonstrated that early discharges (EDCs) can impact boarding times.7-9 A number of recent improvement efforts directed at moving discharges earlier in the day have been published.10-15 However, these improvements are often targeted at specific units or teams within a larger hospital setting and only one is in the pediatric setting.
Lucile Packard Children’s Hospital Stanford (LPCHS) is a 311-bed quaternary care academic women and children’s hospital in Northern California. As our organization expanded, the demand for hospital beds often exceeded capacity. The challenge of overall demand was regularly compounded by a mismatch in bed availability timing – bed demand is early in the day and bed availability is later. This mismatch results in delays for admitted patients waiting in the ED and PACU. Organization leaders identified increasing early discharges (EDCs) as one initiative to contribute to improved patient flow.
Our organization aimed to increase the number of discharges before 11
METHODS
Setting
We focused our EDC interventions on the 87 acute care beds at LPCHS. All patients discharged from these beds were included in the study. We excluded patients discharged from intensive care, maternity, and nursery. Acute care includes five units, one focused on hematology/oncology (Unit A), one focused on cardiology (Unit B), and the others with a surgical and medical pediatric patient mix (Units C, D, and E). Although physician teams have primary units, due to unit size, patients on teams other than cardiology and hematology/oncology are often spread across multiple units wherever there is a bed (including Units A and B). Most of the frontline care physicians are residents supervised by attendings; however, a minority of patients are cared for by nurse practitioners (NPs) or physician assistants (PAs).
Improvement Team
In early 2015, we formed a multidisciplinary group inclusive of a case manager, frontline nurses, nurse management, pediatric residents, and hospitalist physicians with support from performance improvement. We periodically included physician leaders from other specialties to help initiate changes within their own clinical areas. Our group used Lean A3 thinking16 to gather information about the current state, formulate the problem statement, analyze the problem, and consider interventions implemented in three Plan–Do-Check-Act (PDCA) cycles. The A3 is a structured tool to analyze problems before jumping to solutions and communicate with stakeholders. We interviewed leaders, nurses, residents, case managers, etc. and observed work processes around discharge. We met weekly to follow data, assess results of interventions, and problem solve.
Barriers and Interventions
The first barrier we identified and addressed was poor identification and shared team mental model of potential EDC patients and lack of preparation when an EDC was identified. In intervention one starting May 2015, charge nurses on Units C, D, and E were each asked to identify one EDC for the following day. The identified patient was discussed at the previously existing afternoon daily unit huddle17 attended by nurse management, case management, and hospitalist leaders. Following the huddle, the resident, NP, or PA responsible for the patient was paged regarding the EDC plan and tasked with medication reconciliation and discharge paperwork. Others were asked to address their specific area of patient care for discharge (eg, case manager–supplies, nursing–education). The patient was identified on the unit white board with a yellow magnet (use of a visual control18), so that all would be aware of the EDC. An e-mail was sent to case management, nurse leaders, and patient placement coordinators regarding the planned EDCs. Finally, the EDCs were discussed during regularly scheduled huddles throughout the evening and into the next day.17
Despite this first intervention, we noted that progress toward increased EDCs was slow. Thus, we spent approximately seven days (spread over one month) further observing the work processes.19 Over five days, we asked each unit’s charge nurse every hour which patients were waiting to be discharged and the primary reason for waiting. From this information, we created a pareto chart demonstrating that rounds were the highest contributor to waiting (Appendix A). Thus, our second intervention was a daily physician morning huddle that the four nonsurgical physician teams (excluding cardiology, hematology/oncology) implemented one team at a time between November 2015 and February 2016. At the huddle, previously identified EDCs (located on any of the five units) were confirmed and preparatory work was completed (inclusive of the discharge order) before rounds. Further, the attending and resident physicians were to see the patient before or at the start of rounds.
Our working group still observed slow EDC improvement and sought feedback from all providers. EDC was described as “extra” work, apart from routine practices and culture. In addition, our interventions had not addressed most discharges on Units A and B. Consequently, our third intervention in February 2016 aimed to recognize and incentivize teams, units, and individuals for EDC successes. Units and/or physician teams that met 25% of EDCs the previous week were acknowledged through hospital-wide screensavers and certificates of appreciation signed by the Chief Nursing Officer. Units and/or physician teams that met 25% of EDC the previous month were acknowledged with a trophy. Residents received coffee cards for each EDC (though not without controversy among the improvement group as we acknowledged that all providers contributed to EDCs). Finally, weekly, we shared an EDC dashboard displaying unit, team, and organization performance at the hospital-wide leader huddle. We also e-mailed the dashboard regularly to division chiefs, medical directors, and nursing leaders.
Measures
Our primary outcome was percentage of EDCs (based on the time the patient left the room) across acute care. Secondary outcome measures were median wait times for an inpatient bed from the ED (time bed requested to the time patient left the ED) and the average PACU wait time (time the patient is ready to leave the PACU to time the patient left the PACU) per admitted patient. We also assessed balancing measures, including discharge satisfaction, seven-day readmission rates, and LOS. We obtained the mean discharge satisfaction score from the organization’s Press Ganey survey results across acute care (the three discharge questions’ mean – “degree … you felt ready to have your child discharged,” “speed of discharge process …,” and “instructions… to care for your child…”). We obtained seven-day readmission rates from acute care discharges using the hospital’s regularly reported data. We assessed patient characteristics, including sex, age, case mix index (CMI; >2 vs <2), insurance type (nongovernment vs government), day of discharge (weekend vs weekday), and LOS from those patients categorized as inpatients. Complete patient characteristics were not available for observation (InterQual® criteria) status patients.
Analysis
We used descriptive statistics to describe the inpatient population characteristics by analyzing differences when EDC did and did not occur using chi-square and the Mann–Whitney U tests. Patients with missing data were removed from analyses that incorporated patient factors.
To assess our primary outcome, we used an interrupted time series analysis assessing the percentage of EDC in the total population before any intervention (May 2015) and after the last intervention (March 2016). We used the Durbin–Watson statistic to assess autocorrelation of errors in our regression models. As we had only patient characteristics for the inpatient population, we repeated the analysis including only inpatients and accounting for patient factors significantly associated with EDC.
As units and physician teams had differential exposure to the interventions, we performed a subanalysis (using interrupted time series) creating groups based on the combination of interventions to which a patient’s discharge was exposed (based on unit and physician team at discharge). Patient discharges from group 1 (medical patients on Units C, D, and E) were exposed to all three interventions, group 2 patient discharges (medical patients on Units A and B) were exposed to interventions 2 and 3, group 3 (cardiology, hematology/oncology, surgical patients on Units A and B) were exposed to intervention 3, and group 4 (surgical, cardiology, hematology/oncology patients on Units C, D, and E) were exposed to interventions 1 and 3 (Figure 1). Interrupted time series models were fit using the R Statistical Software Package.20
Because of seasonal variation in admissions, we compared secondary outcomes and balancing measures over similar time frames in the calendar year (January to September 2015 vs January to September 2016) using the Mann–Whitney U test and the unpaired t-test, respectively.
The project’s primary purpose was to implement a practice to improve the quality of care, and therefore, the Stanford Institutional Review Board determined it to be nonresearch.
RESULTS
There were 16,175 discharges on acute care from January 2014 through December 2016. Across all acute care units, EDCs increased from an average of 8.8% before the start of interventions (May 2015) to 15.8% after all interventions (February 2016). From the estimated trend in the preintervention period, there was a jump of 3.9% to the start of the postintervention trend (P = .02; Figure 2). Furthermore, there was an increase of 0.48% (95% CI 0.15-0.82%; P < .01) per month in the trend of the slope between the pre- and postintervention. The autocorrelation function and the Durbin–Watson test did not show evidence of autocorrelation (P = .85). Lack of evidence for autocorrelation in this and each of our subsequent fitted models led to excluding an autocorrelation parameter from our models.
From 16,175 discharges, 1,764 (11%) were assigned to observation status. Among inpatients (14,411), patients with missing values (CMI, insurance status) were also excluded (n = 66, 0.5%). Among the remaining 14,345 inpatients, 54% were males, 50% were government-insured, and 1,645 (11.5%) were discharged early. The average age was 8.5 years, the average LOS was seven days, and the median CMI was 2.2. Children who were younger, had shorter LOS, CMI <2, and nongovernment insurance were more likely to be discharged early (P < .01 for all). For each of these variables, F-tests were performed to determine whether there was a statistically significant reduction in variation by adding the variable to our initial model. None of the variables alone or in combination led to a statistically significant reduction in variation. Including these factors in the interrupted time series did not change the significance of the results (jump at postintervention start 3.6%, 95% CI 0.7%-7.2%; P = .02, slope increased by 0.59% per month, 95% CI 0.29-0.89%; P < .01).
In the subgroup analysis, we did not account for patient factors as they did not change the results in the analysis of total population. Though each group had a greater percentage of EDCs in the postintervention period, the changes in slopes and jumps were primarily nonsignificant (Figure 3). Only the change in slope in group 4 was significant (1.1%, 95% CI 0.3-1.9%; P = .01).
Between January to September 2015 and 2016, ED wait times decreased by 88 minutes (P <.01) and PACU wait times decreased by 20 minutes per patient admitted (P < .01; Table). There was no statistically significant change in seven-day readmissions (P = .19) or in families feeling ready to discharge (P = .11) or in general discharge satisfaction (P = .48) as measured by Press Ganey survey. Among all discharges (inpatient and observation), the average LOS significantly decreased by 0.6 days (P = .02).
DISCUSSION
The percentage of patients who left the hospital prior to 11
It is difficult to compare our EDC improvements to those of previous studies, as we are unaware of published data on pediatric EDC efforts across an entire hospital. In addition, studies have reported discharges prior to different times in the day (noon, 1
As providers of all types were aware of the constant push for beds due to canceled surgeries, delayed admissions and intensive care transfers, and the inability to accept admission, it is difficult to compare the subgroups directly. Furthermore, although physician teams and units are distinct, individuals (nurses, case managers, trainees) may rotate through different units and teams and we cannot account for individual influences on EDCs depending on exposure to interventions over time. Although all groups improved, the improvement in slope in group 4 (exposed to interventions 1 and 3) was the only significant change. As group 4 contained a large number of surgical patients who often have more predictable hospital stays, perhaps this group was more responsive to the interventions.
Our EDC improvements were associated with a decrease in ED and PACU bed wait times. Importantly, we did not address potential confounding factors impacting these times such as total hospital admission volumes, ED and PACU patient complexity, and distribution of ED and PACU admission requests throughout the day. Modeling has suggested that EDCs could also improve ED flow,7 but studies implementing EDC have not necessarily assessed this outcome.10-15 One study retrospectively evaluated ED boarding times in the context of an EDC improvement effort and found a decrease in boarding times.21 This decrease is important as ED boarders may be at a higher risk for adverse events, a longer LOS, and more readmissions.3,7 Less is known about prolonged PACU wait times; however, studies have reported delays in receiving patients from the operating room (OR), which could presumably impact timeliness of other scheduled procedures and patient satisfaction.22-24 It is worth noting that OR holds as a result of PACU backups happened more frequently at our institution before our EDC work.
Our limitations include that individual providers in the various groups were not completely blind to the interventions and groups often comprised distinct patient populations. Second, LPCHS has a high CMI and LOS relative to most other children’s hospitals, complicating comparison with patient populations at other children’s hospitals. In addition, our work was done at this single institution. However, since a higher CMI was associated with a lower probability of EDC, hospitals with a lower CMI may have a greater opportunity for EDC improvements. Third, hospital systems are more impacted by low EDCs when operating at high occupancy (as we were at LPCHS); thus, improvements in ED and PACU wait times for inpatient beds might not be noted for hospitals operating with a >10% inventory of beds.25 Importantly, our hospital had multiple daily management structures in place, which we harnessed for our interventions, and better patient flow was a key hospital initiative garnering improvement of resources. Hospitals without these resources may have more difficulty implementing similar interventions. Finally, other work to improve patient flow was concurrently implemented, including matching numbers of scheduled OR admissions with anticipated capacity, which probably also contributed to the decrease in ED and PACU wait times.
CONCLUSIONS
We found that a multimodal intervention was associated with more EDCs and improved ED and PACU bed wait times. We observed no impact on discharge satisfaction or readmissions. Our EDC improvement efforts may guide institutions operating at high capacity and aiming to improve EDCs to improve patient flow.
Acknowledgments
The authors would like to acknowledge all those engaged in the early discharge work at LPCHS. They would like to particularly acknowledge Ava Rezvani for her engagement and work in helping to implement the interventions.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose. The authors have no financial relationships relevant to this article to disclose.
Funding
This project was accomplished without specific funding. Funding for incentives was provided by the Lucile Packard Children’s Hospital Stanford.
1. Optimizing Patient Flow: Moving Patients Smoothly Through Acute Care Settings. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. (Available on www.IHI.org)
2. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. doi: 10.1002/jhm.490. PubMed
3. Bekmezian A, Chung PJ. Boarding admitted children in the emergency department impacts inpatient outcomes. Pediatr Emerg Care. 2012;28(3):236-242. doi: 10.1097/PEC.0b013e3182494b94. PubMed
4. Hillier DF, Parry GJ, Shannon MW, Stack AM. The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767-776. doi: 10.1016/j.annemergmed.2008.11.024. PubMed
5. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871. doi: 10.1016/j.jamcollsurg.2007.01.052. PubMed
6. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
7. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
8. Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency department-boarded patients awaiting beds. Ann Emerg Med. 2009;54(3):381-385. doi: 10.1016/j.annemergmed.2009.02.001. PubMed
9. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
10. Beck MJ, Gosik K. Redesigning an inpatient pediatric service using lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220-227. doi: 10.1002/jhm.2300. PubMed
11. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
12. Chaiyachati KH, Sofair AN, Schwartz JI, Chia D. Discharge rounds: implementation of a targeted intervention for improving patient throughput on an inpatient medical teaching service. South Med J. 2016;109(5):313-317. doi: 10.14423/SMJ.0000000000000458. PubMed
13. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag. 2007;26(2):142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
14. Wertheimer B, Ramon EA, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
15. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. doi: 10.1097/QMH.0000000000000049. PubMed
16. Shook J. Managing to Learn: Using the A3 Management Process. Cambridge, MA: Lean Enterprise Institute; 2008.
17. Donnelly, LF. Daily management systems in medicine. Radiographics. 2014;34(2):549-555. doi: 10.1148/rg.342130035.
18. Ching JM, Long CH, Williams BL, Blackmore C. Using lean to improve medication administration safety: in search of the “perfect dose.” Jt Comm J Qual Patient Saf. 2013;39(5):195-204. doi: 10.1016/S1553-7250(13)39026-6. PubMed
19. Kim CS, Spahlinger DA, Kin JM, Billi JE. Lean health care: what can hospitals learn from a world-class automaker. J Hosp Med. 2006;1(3):191-199. doi: 10.1002/jhm.68. PubMed
20. R Version 3.5.1. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
21. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
22. Bruce M. A study in time: performance improvement to reduce excess holding time in PACU. J Perianesth Nurs. 2000;15(4):237-244. doi: 10.1053/jpan.2000.9462. PubMed
23. Dolkart O, Amar E, Weisman D, Flaisho R, Weinbroum AA. Patient dissatisfaction following prolonged stay in the post-anesthesia care unit due to unavailable ward bed in a tertiary hospital. Harefuah. 2013;152(8):446-450. PubMed
24. Lalani SB, Ali F, Kanji Z. Prolonged-stay patients in the PACU: a review of the literature. J Perianesth Nurs. 2013;28(3):151-155. doi: 10.1016/j.jopan.2012.06.009. PubMed
25. Fieldston ES, Hall M, Sills MR, et al. Children’s hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125(5):974-981. doi: 10.1542/peds.2009-1627. PubMed
Patient flow throughout the hospital has been shown to be adversely affected by discharge delays.1 When hospitals are operating at peak capacity, these delays impact throughput, length of stay (LOS), and cost of care and block patients from the emergency department (ED), postanesthesia recovery unit (PACU), or home awaiting inpatient beds.2-5 As patients wait in locations not ideal for inpatient care, they may suffer from adverse events and poor satisfaction.3,6 Several studies have analyzed discharge timing as it relates to ED boarding of admitted patients and demonstrated that early discharges (EDCs) can impact boarding times.7-9 A number of recent improvement efforts directed at moving discharges earlier in the day have been published.10-15 However, these improvements are often targeted at specific units or teams within a larger hospital setting and only one is in the pediatric setting.
Lucile Packard Children’s Hospital Stanford (LPCHS) is a 311-bed quaternary care academic women and children’s hospital in Northern California. As our organization expanded, the demand for hospital beds often exceeded capacity. The challenge of overall demand was regularly compounded by a mismatch in bed availability timing – bed demand is early in the day and bed availability is later. This mismatch results in delays for admitted patients waiting in the ED and PACU. Organization leaders identified increasing early discharges (EDCs) as one initiative to contribute to improved patient flow.
Our organization aimed to increase the number of discharges before 11
METHODS
Setting
We focused our EDC interventions on the 87 acute care beds at LPCHS. All patients discharged from these beds were included in the study. We excluded patients discharged from intensive care, maternity, and nursery. Acute care includes five units, one focused on hematology/oncology (Unit A), one focused on cardiology (Unit B), and the others with a surgical and medical pediatric patient mix (Units C, D, and E). Although physician teams have primary units, due to unit size, patients on teams other than cardiology and hematology/oncology are often spread across multiple units wherever there is a bed (including Units A and B). Most of the frontline care physicians are residents supervised by attendings; however, a minority of patients are cared for by nurse practitioners (NPs) or physician assistants (PAs).
Improvement Team
In early 2015, we formed a multidisciplinary group inclusive of a case manager, frontline nurses, nurse management, pediatric residents, and hospitalist physicians with support from performance improvement. We periodically included physician leaders from other specialties to help initiate changes within their own clinical areas. Our group used Lean A3 thinking16 to gather information about the current state, formulate the problem statement, analyze the problem, and consider interventions implemented in three Plan–Do-Check-Act (PDCA) cycles. The A3 is a structured tool to analyze problems before jumping to solutions and communicate with stakeholders. We interviewed leaders, nurses, residents, case managers, etc. and observed work processes around discharge. We met weekly to follow data, assess results of interventions, and problem solve.
Barriers and Interventions
The first barrier we identified and addressed was poor identification and shared team mental model of potential EDC patients and lack of preparation when an EDC was identified. In intervention one starting May 2015, charge nurses on Units C, D, and E were each asked to identify one EDC for the following day. The identified patient was discussed at the previously existing afternoon daily unit huddle17 attended by nurse management, case management, and hospitalist leaders. Following the huddle, the resident, NP, or PA responsible for the patient was paged regarding the EDC plan and tasked with medication reconciliation and discharge paperwork. Others were asked to address their specific area of patient care for discharge (eg, case manager–supplies, nursing–education). The patient was identified on the unit white board with a yellow magnet (use of a visual control18), so that all would be aware of the EDC. An e-mail was sent to case management, nurse leaders, and patient placement coordinators regarding the planned EDCs. Finally, the EDCs were discussed during regularly scheduled huddles throughout the evening and into the next day.17
Despite this first intervention, we noted that progress toward increased EDCs was slow. Thus, we spent approximately seven days (spread over one month) further observing the work processes.19 Over five days, we asked each unit’s charge nurse every hour which patients were waiting to be discharged and the primary reason for waiting. From this information, we created a pareto chart demonstrating that rounds were the highest contributor to waiting (Appendix A). Thus, our second intervention was a daily physician morning huddle that the four nonsurgical physician teams (excluding cardiology, hematology/oncology) implemented one team at a time between November 2015 and February 2016. At the huddle, previously identified EDCs (located on any of the five units) were confirmed and preparatory work was completed (inclusive of the discharge order) before rounds. Further, the attending and resident physicians were to see the patient before or at the start of rounds.
Our working group still observed slow EDC improvement and sought feedback from all providers. EDC was described as “extra” work, apart from routine practices and culture. In addition, our interventions had not addressed most discharges on Units A and B. Consequently, our third intervention in February 2016 aimed to recognize and incentivize teams, units, and individuals for EDC successes. Units and/or physician teams that met 25% of EDCs the previous week were acknowledged through hospital-wide screensavers and certificates of appreciation signed by the Chief Nursing Officer. Units and/or physician teams that met 25% of EDC the previous month were acknowledged with a trophy. Residents received coffee cards for each EDC (though not without controversy among the improvement group as we acknowledged that all providers contributed to EDCs). Finally, weekly, we shared an EDC dashboard displaying unit, team, and organization performance at the hospital-wide leader huddle. We also e-mailed the dashboard regularly to division chiefs, medical directors, and nursing leaders.
Measures
Our primary outcome was percentage of EDCs (based on the time the patient left the room) across acute care. Secondary outcome measures were median wait times for an inpatient bed from the ED (time bed requested to the time patient left the ED) and the average PACU wait time (time the patient is ready to leave the PACU to time the patient left the PACU) per admitted patient. We also assessed balancing measures, including discharge satisfaction, seven-day readmission rates, and LOS. We obtained the mean discharge satisfaction score from the organization’s Press Ganey survey results across acute care (the three discharge questions’ mean – “degree … you felt ready to have your child discharged,” “speed of discharge process …,” and “instructions… to care for your child…”). We obtained seven-day readmission rates from acute care discharges using the hospital’s regularly reported data. We assessed patient characteristics, including sex, age, case mix index (CMI; >2 vs <2), insurance type (nongovernment vs government), day of discharge (weekend vs weekday), and LOS from those patients categorized as inpatients. Complete patient characteristics were not available for observation (InterQual® criteria) status patients.
Analysis
We used descriptive statistics to describe the inpatient population characteristics by analyzing differences when EDC did and did not occur using chi-square and the Mann–Whitney U tests. Patients with missing data were removed from analyses that incorporated patient factors.
To assess our primary outcome, we used an interrupted time series analysis assessing the percentage of EDC in the total population before any intervention (May 2015) and after the last intervention (March 2016). We used the Durbin–Watson statistic to assess autocorrelation of errors in our regression models. As we had only patient characteristics for the inpatient population, we repeated the analysis including only inpatients and accounting for patient factors significantly associated with EDC.
As units and physician teams had differential exposure to the interventions, we performed a subanalysis (using interrupted time series) creating groups based on the combination of interventions to which a patient’s discharge was exposed (based on unit and physician team at discharge). Patient discharges from group 1 (medical patients on Units C, D, and E) were exposed to all three interventions, group 2 patient discharges (medical patients on Units A and B) were exposed to interventions 2 and 3, group 3 (cardiology, hematology/oncology, surgical patients on Units A and B) were exposed to intervention 3, and group 4 (surgical, cardiology, hematology/oncology patients on Units C, D, and E) were exposed to interventions 1 and 3 (Figure 1). Interrupted time series models were fit using the R Statistical Software Package.20
Because of seasonal variation in admissions, we compared secondary outcomes and balancing measures over similar time frames in the calendar year (January to September 2015 vs January to September 2016) using the Mann–Whitney U test and the unpaired t-test, respectively.
The project’s primary purpose was to implement a practice to improve the quality of care, and therefore, the Stanford Institutional Review Board determined it to be nonresearch.
RESULTS
There were 16,175 discharges on acute care from January 2014 through December 2016. Across all acute care units, EDCs increased from an average of 8.8% before the start of interventions (May 2015) to 15.8% after all interventions (February 2016). From the estimated trend in the preintervention period, there was a jump of 3.9% to the start of the postintervention trend (P = .02; Figure 2). Furthermore, there was an increase of 0.48% (95% CI 0.15-0.82%; P < .01) per month in the trend of the slope between the pre- and postintervention. The autocorrelation function and the Durbin–Watson test did not show evidence of autocorrelation (P = .85). Lack of evidence for autocorrelation in this and each of our subsequent fitted models led to excluding an autocorrelation parameter from our models.
From 16,175 discharges, 1,764 (11%) were assigned to observation status. Among inpatients (14,411), patients with missing values (CMI, insurance status) were also excluded (n = 66, 0.5%). Among the remaining 14,345 inpatients, 54% were males, 50% were government-insured, and 1,645 (11.5%) were discharged early. The average age was 8.5 years, the average LOS was seven days, and the median CMI was 2.2. Children who were younger, had shorter LOS, CMI <2, and nongovernment insurance were more likely to be discharged early (P < .01 for all). For each of these variables, F-tests were performed to determine whether there was a statistically significant reduction in variation by adding the variable to our initial model. None of the variables alone or in combination led to a statistically significant reduction in variation. Including these factors in the interrupted time series did not change the significance of the results (jump at postintervention start 3.6%, 95% CI 0.7%-7.2%; P = .02, slope increased by 0.59% per month, 95% CI 0.29-0.89%; P < .01).
In the subgroup analysis, we did not account for patient factors as they did not change the results in the analysis of total population. Though each group had a greater percentage of EDCs in the postintervention period, the changes in slopes and jumps were primarily nonsignificant (Figure 3). Only the change in slope in group 4 was significant (1.1%, 95% CI 0.3-1.9%; P = .01).
Between January to September 2015 and 2016, ED wait times decreased by 88 minutes (P <.01) and PACU wait times decreased by 20 minutes per patient admitted (P < .01; Table). There was no statistically significant change in seven-day readmissions (P = .19) or in families feeling ready to discharge (P = .11) or in general discharge satisfaction (P = .48) as measured by Press Ganey survey. Among all discharges (inpatient and observation), the average LOS significantly decreased by 0.6 days (P = .02).
DISCUSSION
The percentage of patients who left the hospital prior to 11
It is difficult to compare our EDC improvements to those of previous studies, as we are unaware of published data on pediatric EDC efforts across an entire hospital. In addition, studies have reported discharges prior to different times in the day (noon, 1
As providers of all types were aware of the constant push for beds due to canceled surgeries, delayed admissions and intensive care transfers, and the inability to accept admission, it is difficult to compare the subgroups directly. Furthermore, although physician teams and units are distinct, individuals (nurses, case managers, trainees) may rotate through different units and teams and we cannot account for individual influences on EDCs depending on exposure to interventions over time. Although all groups improved, the improvement in slope in group 4 (exposed to interventions 1 and 3) was the only significant change. As group 4 contained a large number of surgical patients who often have more predictable hospital stays, perhaps this group was more responsive to the interventions.
Our EDC improvements were associated with a decrease in ED and PACU bed wait times. Importantly, we did not address potential confounding factors impacting these times such as total hospital admission volumes, ED and PACU patient complexity, and distribution of ED and PACU admission requests throughout the day. Modeling has suggested that EDCs could also improve ED flow,7 but studies implementing EDC have not necessarily assessed this outcome.10-15 One study retrospectively evaluated ED boarding times in the context of an EDC improvement effort and found a decrease in boarding times.21 This decrease is important as ED boarders may be at a higher risk for adverse events, a longer LOS, and more readmissions.3,7 Less is known about prolonged PACU wait times; however, studies have reported delays in receiving patients from the operating room (OR), which could presumably impact timeliness of other scheduled procedures and patient satisfaction.22-24 It is worth noting that OR holds as a result of PACU backups happened more frequently at our institution before our EDC work.
Our limitations include that individual providers in the various groups were not completely blind to the interventions and groups often comprised distinct patient populations. Second, LPCHS has a high CMI and LOS relative to most other children’s hospitals, complicating comparison with patient populations at other children’s hospitals. In addition, our work was done at this single institution. However, since a higher CMI was associated with a lower probability of EDC, hospitals with a lower CMI may have a greater opportunity for EDC improvements. Third, hospital systems are more impacted by low EDCs when operating at high occupancy (as we were at LPCHS); thus, improvements in ED and PACU wait times for inpatient beds might not be noted for hospitals operating with a >10% inventory of beds.25 Importantly, our hospital had multiple daily management structures in place, which we harnessed for our interventions, and better patient flow was a key hospital initiative garnering improvement of resources. Hospitals without these resources may have more difficulty implementing similar interventions. Finally, other work to improve patient flow was concurrently implemented, including matching numbers of scheduled OR admissions with anticipated capacity, which probably also contributed to the decrease in ED and PACU wait times.
CONCLUSIONS
We found that a multimodal intervention was associated with more EDCs and improved ED and PACU bed wait times. We observed no impact on discharge satisfaction or readmissions. Our EDC improvement efforts may guide institutions operating at high capacity and aiming to improve EDCs to improve patient flow.
Acknowledgments
The authors would like to acknowledge all those engaged in the early discharge work at LPCHS. They would like to particularly acknowledge Ava Rezvani for her engagement and work in helping to implement the interventions.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose. The authors have no financial relationships relevant to this article to disclose.
Funding
This project was accomplished without specific funding. Funding for incentives was provided by the Lucile Packard Children’s Hospital Stanford.
Patient flow throughout the hospital has been shown to be adversely affected by discharge delays.1 When hospitals are operating at peak capacity, these delays impact throughput, length of stay (LOS), and cost of care and block patients from the emergency department (ED), postanesthesia recovery unit (PACU), or home awaiting inpatient beds.2-5 As patients wait in locations not ideal for inpatient care, they may suffer from adverse events and poor satisfaction.3,6 Several studies have analyzed discharge timing as it relates to ED boarding of admitted patients and demonstrated that early discharges (EDCs) can impact boarding times.7-9 A number of recent improvement efforts directed at moving discharges earlier in the day have been published.10-15 However, these improvements are often targeted at specific units or teams within a larger hospital setting and only one is in the pediatric setting.
Lucile Packard Children’s Hospital Stanford (LPCHS) is a 311-bed quaternary care academic women and children’s hospital in Northern California. As our organization expanded, the demand for hospital beds often exceeded capacity. The challenge of overall demand was regularly compounded by a mismatch in bed availability timing – bed demand is early in the day and bed availability is later. This mismatch results in delays for admitted patients waiting in the ED and PACU. Organization leaders identified increasing early discharges (EDCs) as one initiative to contribute to improved patient flow.
Our organization aimed to increase the number of discharges before 11
METHODS
Setting
We focused our EDC interventions on the 87 acute care beds at LPCHS. All patients discharged from these beds were included in the study. We excluded patients discharged from intensive care, maternity, and nursery. Acute care includes five units, one focused on hematology/oncology (Unit A), one focused on cardiology (Unit B), and the others with a surgical and medical pediatric patient mix (Units C, D, and E). Although physician teams have primary units, due to unit size, patients on teams other than cardiology and hematology/oncology are often spread across multiple units wherever there is a bed (including Units A and B). Most of the frontline care physicians are residents supervised by attendings; however, a minority of patients are cared for by nurse practitioners (NPs) or physician assistants (PAs).
Improvement Team
In early 2015, we formed a multidisciplinary group inclusive of a case manager, frontline nurses, nurse management, pediatric residents, and hospitalist physicians with support from performance improvement. We periodically included physician leaders from other specialties to help initiate changes within their own clinical areas. Our group used Lean A3 thinking16 to gather information about the current state, formulate the problem statement, analyze the problem, and consider interventions implemented in three Plan–Do-Check-Act (PDCA) cycles. The A3 is a structured tool to analyze problems before jumping to solutions and communicate with stakeholders. We interviewed leaders, nurses, residents, case managers, etc. and observed work processes around discharge. We met weekly to follow data, assess results of interventions, and problem solve.
Barriers and Interventions
The first barrier we identified and addressed was poor identification and shared team mental model of potential EDC patients and lack of preparation when an EDC was identified. In intervention one starting May 2015, charge nurses on Units C, D, and E were each asked to identify one EDC for the following day. The identified patient was discussed at the previously existing afternoon daily unit huddle17 attended by nurse management, case management, and hospitalist leaders. Following the huddle, the resident, NP, or PA responsible for the patient was paged regarding the EDC plan and tasked with medication reconciliation and discharge paperwork. Others were asked to address their specific area of patient care for discharge (eg, case manager–supplies, nursing–education). The patient was identified on the unit white board with a yellow magnet (use of a visual control18), so that all would be aware of the EDC. An e-mail was sent to case management, nurse leaders, and patient placement coordinators regarding the planned EDCs. Finally, the EDCs were discussed during regularly scheduled huddles throughout the evening and into the next day.17
Despite this first intervention, we noted that progress toward increased EDCs was slow. Thus, we spent approximately seven days (spread over one month) further observing the work processes.19 Over five days, we asked each unit’s charge nurse every hour which patients were waiting to be discharged and the primary reason for waiting. From this information, we created a pareto chart demonstrating that rounds were the highest contributor to waiting (Appendix A). Thus, our second intervention was a daily physician morning huddle that the four nonsurgical physician teams (excluding cardiology, hematology/oncology) implemented one team at a time between November 2015 and February 2016. At the huddle, previously identified EDCs (located on any of the five units) were confirmed and preparatory work was completed (inclusive of the discharge order) before rounds. Further, the attending and resident physicians were to see the patient before or at the start of rounds.
Our working group still observed slow EDC improvement and sought feedback from all providers. EDC was described as “extra” work, apart from routine practices and culture. In addition, our interventions had not addressed most discharges on Units A and B. Consequently, our third intervention in February 2016 aimed to recognize and incentivize teams, units, and individuals for EDC successes. Units and/or physician teams that met 25% of EDCs the previous week were acknowledged through hospital-wide screensavers and certificates of appreciation signed by the Chief Nursing Officer. Units and/or physician teams that met 25% of EDC the previous month were acknowledged with a trophy. Residents received coffee cards for each EDC (though not without controversy among the improvement group as we acknowledged that all providers contributed to EDCs). Finally, weekly, we shared an EDC dashboard displaying unit, team, and organization performance at the hospital-wide leader huddle. We also e-mailed the dashboard regularly to division chiefs, medical directors, and nursing leaders.
Measures
Our primary outcome was percentage of EDCs (based on the time the patient left the room) across acute care. Secondary outcome measures were median wait times for an inpatient bed from the ED (time bed requested to the time patient left the ED) and the average PACU wait time (time the patient is ready to leave the PACU to time the patient left the PACU) per admitted patient. We also assessed balancing measures, including discharge satisfaction, seven-day readmission rates, and LOS. We obtained the mean discharge satisfaction score from the organization’s Press Ganey survey results across acute care (the three discharge questions’ mean – “degree … you felt ready to have your child discharged,” “speed of discharge process …,” and “instructions… to care for your child…”). We obtained seven-day readmission rates from acute care discharges using the hospital’s regularly reported data. We assessed patient characteristics, including sex, age, case mix index (CMI; >2 vs <2), insurance type (nongovernment vs government), day of discharge (weekend vs weekday), and LOS from those patients categorized as inpatients. Complete patient characteristics were not available for observation (InterQual® criteria) status patients.
Analysis
We used descriptive statistics to describe the inpatient population characteristics by analyzing differences when EDC did and did not occur using chi-square and the Mann–Whitney U tests. Patients with missing data were removed from analyses that incorporated patient factors.
To assess our primary outcome, we used an interrupted time series analysis assessing the percentage of EDC in the total population before any intervention (May 2015) and after the last intervention (March 2016). We used the Durbin–Watson statistic to assess autocorrelation of errors in our regression models. As we had only patient characteristics for the inpatient population, we repeated the analysis including only inpatients and accounting for patient factors significantly associated with EDC.
As units and physician teams had differential exposure to the interventions, we performed a subanalysis (using interrupted time series) creating groups based on the combination of interventions to which a patient’s discharge was exposed (based on unit and physician team at discharge). Patient discharges from group 1 (medical patients on Units C, D, and E) were exposed to all three interventions, group 2 patient discharges (medical patients on Units A and B) were exposed to interventions 2 and 3, group 3 (cardiology, hematology/oncology, surgical patients on Units A and B) were exposed to intervention 3, and group 4 (surgical, cardiology, hematology/oncology patients on Units C, D, and E) were exposed to interventions 1 and 3 (Figure 1). Interrupted time series models were fit using the R Statistical Software Package.20
Because of seasonal variation in admissions, we compared secondary outcomes and balancing measures over similar time frames in the calendar year (January to September 2015 vs January to September 2016) using the Mann–Whitney U test and the unpaired t-test, respectively.
The project’s primary purpose was to implement a practice to improve the quality of care, and therefore, the Stanford Institutional Review Board determined it to be nonresearch.
RESULTS
There were 16,175 discharges on acute care from January 2014 through December 2016. Across all acute care units, EDCs increased from an average of 8.8% before the start of interventions (May 2015) to 15.8% after all interventions (February 2016). From the estimated trend in the preintervention period, there was a jump of 3.9% to the start of the postintervention trend (P = .02; Figure 2). Furthermore, there was an increase of 0.48% (95% CI 0.15-0.82%; P < .01) per month in the trend of the slope between the pre- and postintervention. The autocorrelation function and the Durbin–Watson test did not show evidence of autocorrelation (P = .85). Lack of evidence for autocorrelation in this and each of our subsequent fitted models led to excluding an autocorrelation parameter from our models.
From 16,175 discharges, 1,764 (11%) were assigned to observation status. Among inpatients (14,411), patients with missing values (CMI, insurance status) were also excluded (n = 66, 0.5%). Among the remaining 14,345 inpatients, 54% were males, 50% were government-insured, and 1,645 (11.5%) were discharged early. The average age was 8.5 years, the average LOS was seven days, and the median CMI was 2.2. Children who were younger, had shorter LOS, CMI <2, and nongovernment insurance were more likely to be discharged early (P < .01 for all). For each of these variables, F-tests were performed to determine whether there was a statistically significant reduction in variation by adding the variable to our initial model. None of the variables alone or in combination led to a statistically significant reduction in variation. Including these factors in the interrupted time series did not change the significance of the results (jump at postintervention start 3.6%, 95% CI 0.7%-7.2%; P = .02, slope increased by 0.59% per month, 95% CI 0.29-0.89%; P < .01).
In the subgroup analysis, we did not account for patient factors as they did not change the results in the analysis of total population. Though each group had a greater percentage of EDCs in the postintervention period, the changes in slopes and jumps were primarily nonsignificant (Figure 3). Only the change in slope in group 4 was significant (1.1%, 95% CI 0.3-1.9%; P = .01).
Between January to September 2015 and 2016, ED wait times decreased by 88 minutes (P <.01) and PACU wait times decreased by 20 minutes per patient admitted (P < .01; Table). There was no statistically significant change in seven-day readmissions (P = .19) or in families feeling ready to discharge (P = .11) or in general discharge satisfaction (P = .48) as measured by Press Ganey survey. Among all discharges (inpatient and observation), the average LOS significantly decreased by 0.6 days (P = .02).
DISCUSSION
The percentage of patients who left the hospital prior to 11
It is difficult to compare our EDC improvements to those of previous studies, as we are unaware of published data on pediatric EDC efforts across an entire hospital. In addition, studies have reported discharges prior to different times in the day (noon, 1
As providers of all types were aware of the constant push for beds due to canceled surgeries, delayed admissions and intensive care transfers, and the inability to accept admission, it is difficult to compare the subgroups directly. Furthermore, although physician teams and units are distinct, individuals (nurses, case managers, trainees) may rotate through different units and teams and we cannot account for individual influences on EDCs depending on exposure to interventions over time. Although all groups improved, the improvement in slope in group 4 (exposed to interventions 1 and 3) was the only significant change. As group 4 contained a large number of surgical patients who often have more predictable hospital stays, perhaps this group was more responsive to the interventions.
Our EDC improvements were associated with a decrease in ED and PACU bed wait times. Importantly, we did not address potential confounding factors impacting these times such as total hospital admission volumes, ED and PACU patient complexity, and distribution of ED and PACU admission requests throughout the day. Modeling has suggested that EDCs could also improve ED flow,7 but studies implementing EDC have not necessarily assessed this outcome.10-15 One study retrospectively evaluated ED boarding times in the context of an EDC improvement effort and found a decrease in boarding times.21 This decrease is important as ED boarders may be at a higher risk for adverse events, a longer LOS, and more readmissions.3,7 Less is known about prolonged PACU wait times; however, studies have reported delays in receiving patients from the operating room (OR), which could presumably impact timeliness of other scheduled procedures and patient satisfaction.22-24 It is worth noting that OR holds as a result of PACU backups happened more frequently at our institution before our EDC work.
Our limitations include that individual providers in the various groups were not completely blind to the interventions and groups often comprised distinct patient populations. Second, LPCHS has a high CMI and LOS relative to most other children’s hospitals, complicating comparison with patient populations at other children’s hospitals. In addition, our work was done at this single institution. However, since a higher CMI was associated with a lower probability of EDC, hospitals with a lower CMI may have a greater opportunity for EDC improvements. Third, hospital systems are more impacted by low EDCs when operating at high occupancy (as we were at LPCHS); thus, improvements in ED and PACU wait times for inpatient beds might not be noted for hospitals operating with a >10% inventory of beds.25 Importantly, our hospital had multiple daily management structures in place, which we harnessed for our interventions, and better patient flow was a key hospital initiative garnering improvement of resources. Hospitals without these resources may have more difficulty implementing similar interventions. Finally, other work to improve patient flow was concurrently implemented, including matching numbers of scheduled OR admissions with anticipated capacity, which probably also contributed to the decrease in ED and PACU wait times.
CONCLUSIONS
We found that a multimodal intervention was associated with more EDCs and improved ED and PACU bed wait times. We observed no impact on discharge satisfaction or readmissions. Our EDC improvement efforts may guide institutions operating at high capacity and aiming to improve EDCs to improve patient flow.
Acknowledgments
The authors would like to acknowledge all those engaged in the early discharge work at LPCHS. They would like to particularly acknowledge Ava Rezvani for her engagement and work in helping to implement the interventions.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose. The authors have no financial relationships relevant to this article to disclose.
Funding
This project was accomplished without specific funding. Funding for incentives was provided by the Lucile Packard Children’s Hospital Stanford.
1. Optimizing Patient Flow: Moving Patients Smoothly Through Acute Care Settings. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. (Available on www.IHI.org)
2. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. doi: 10.1002/jhm.490. PubMed
3. Bekmezian A, Chung PJ. Boarding admitted children in the emergency department impacts inpatient outcomes. Pediatr Emerg Care. 2012;28(3):236-242. doi: 10.1097/PEC.0b013e3182494b94. PubMed
4. Hillier DF, Parry GJ, Shannon MW, Stack AM. The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767-776. doi: 10.1016/j.annemergmed.2008.11.024. PubMed
5. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871. doi: 10.1016/j.jamcollsurg.2007.01.052. PubMed
6. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
7. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
8. Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency department-boarded patients awaiting beds. Ann Emerg Med. 2009;54(3):381-385. doi: 10.1016/j.annemergmed.2009.02.001. PubMed
9. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
10. Beck MJ, Gosik K. Redesigning an inpatient pediatric service using lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220-227. doi: 10.1002/jhm.2300. PubMed
11. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
12. Chaiyachati KH, Sofair AN, Schwartz JI, Chia D. Discharge rounds: implementation of a targeted intervention for improving patient throughput on an inpatient medical teaching service. South Med J. 2016;109(5):313-317. doi: 10.14423/SMJ.0000000000000458. PubMed
13. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag. 2007;26(2):142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
14. Wertheimer B, Ramon EA, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
15. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. doi: 10.1097/QMH.0000000000000049. PubMed
16. Shook J. Managing to Learn: Using the A3 Management Process. Cambridge, MA: Lean Enterprise Institute; 2008.
17. Donnelly, LF. Daily management systems in medicine. Radiographics. 2014;34(2):549-555. doi: 10.1148/rg.342130035.
18. Ching JM, Long CH, Williams BL, Blackmore C. Using lean to improve medication administration safety: in search of the “perfect dose.” Jt Comm J Qual Patient Saf. 2013;39(5):195-204. doi: 10.1016/S1553-7250(13)39026-6. PubMed
19. Kim CS, Spahlinger DA, Kin JM, Billi JE. Lean health care: what can hospitals learn from a world-class automaker. J Hosp Med. 2006;1(3):191-199. doi: 10.1002/jhm.68. PubMed
20. R Version 3.5.1. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
21. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
22. Bruce M. A study in time: performance improvement to reduce excess holding time in PACU. J Perianesth Nurs. 2000;15(4):237-244. doi: 10.1053/jpan.2000.9462. PubMed
23. Dolkart O, Amar E, Weisman D, Flaisho R, Weinbroum AA. Patient dissatisfaction following prolonged stay in the post-anesthesia care unit due to unavailable ward bed in a tertiary hospital. Harefuah. 2013;152(8):446-450. PubMed
24. Lalani SB, Ali F, Kanji Z. Prolonged-stay patients in the PACU: a review of the literature. J Perianesth Nurs. 2013;28(3):151-155. doi: 10.1016/j.jopan.2012.06.009. PubMed
25. Fieldston ES, Hall M, Sills MR, et al. Children’s hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125(5):974-981. doi: 10.1542/peds.2009-1627. PubMed
1. Optimizing Patient Flow: Moving Patients Smoothly Through Acute Care Settings. IHI Innovation Series white paper. Boston: Institute for Healthcare Improvement; 2003. (Available on www.IHI.org)
2. Srivastava R, Stone BL, Patel R, et al. Delays in discharge in a tertiary care pediatric hospital. J Hosp Med. 2009;4(8):481-485. doi: 10.1002/jhm.490. PubMed
3. Bekmezian A, Chung PJ. Boarding admitted children in the emergency department impacts inpatient outcomes. Pediatr Emerg Care. 2012;28(3):236-242. doi: 10.1097/PEC.0b013e3182494b94. PubMed
4. Hillier DF, Parry GJ, Shannon MW, Stack AM. The effect of hospital bed occupancy on throughput in the pediatric emergency department. Ann Emerg Med. 2009;53(6):767-776. doi: 10.1016/j.annemergmed.2008.11.024. PubMed
5. McGowan JE, Truwit JD, Cipriano P, et al. Operating room efficiency and hospital capacity: factors affecting operating use during maximum hospital census. J Am Coll Surg. 2007;204(5):865-871. doi: 10.1016/j.jamcollsurg.2007.01.052. PubMed
6. Khanna S, Sier D, Boyle J, Zeitz K. Discharge timeliness and its impact on hospital crowding and emergency department flow performance. Emerg Med Australas. 2016;28(2):164-170. doi: 10.1111/1742-6723.12543. PubMed
7. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi: 10.1016/j.jemermed.2010.06.028. PubMed
8. Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency department-boarded patients awaiting beds. Ann Emerg Med. 2009;54(3):381-385. doi: 10.1016/j.annemergmed.2009.02.001. PubMed
9. Khanna S, Boyle J, Good N, Lind J. Impact of admission and discharge peak times on hospital overcrowding. Stud Health Technol Inform. 2011;168:82-88. doi: 10.3233/978-1-60750-791-8-82. PubMed
10. Beck MJ, Gosik K. Redesigning an inpatient pediatric service using lean to improve throughput efficiency. J Hosp Med. 2015;10(4):220-227. doi: 10.1002/jhm.2300. PubMed
11. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
12. Chaiyachati KH, Sofair AN, Schwartz JI, Chia D. Discharge rounds: implementation of a targeted intervention for improving patient throughput on an inpatient medical teaching service. South Med J. 2016;109(5):313-317. doi: 10.14423/SMJ.0000000000000458. PubMed
13. Kravet SJ, Levine RB, Rubin HR, Wright SM. Discharging patients earlier in the day: a concept worth evaluating. Health Care Manag. 2007;26(2):142-146. doi: 10.1097/01.HCM.0000268617.33491.60. PubMed
14. Wertheimer B, Ramon EA, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi: 10.1002/jhm.2412. PubMed
15. Durvasula R, Kayihan A, Del Bene S, et al. A multidisciplinary care pathway significantly increases the number of early morning discharges in a large academic medical center. Qual Manag Health Care. 2015;24(1):45-51. doi: 10.1097/QMH.0000000000000049. PubMed
16. Shook J. Managing to Learn: Using the A3 Management Process. Cambridge, MA: Lean Enterprise Institute; 2008.
17. Donnelly, LF. Daily management systems in medicine. Radiographics. 2014;34(2):549-555. doi: 10.1148/rg.342130035.
18. Ching JM, Long CH, Williams BL, Blackmore C. Using lean to improve medication administration safety: in search of the “perfect dose.” Jt Comm J Qual Patient Saf. 2013;39(5):195-204. doi: 10.1016/S1553-7250(13)39026-6. PubMed
19. Kim CS, Spahlinger DA, Kin JM, Billi JE. Lean health care: what can hospitals learn from a world-class automaker. J Hosp Med. 2006;1(3):191-199. doi: 10.1002/jhm.68. PubMed
20. R Version 3.5.1. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
21. Beck MJ, Okerblom D, Kumar A, Bandyopadhyay S, Scalzi LV. Lean intervention improves patient discharge times, improves emergency department throughput and reduces congestion. Hosp Pract. 2016;44(5):252-259. doi: 10.1080/21548331.2016.1254559. PubMed
22. Bruce M. A study in time: performance improvement to reduce excess holding time in PACU. J Perianesth Nurs. 2000;15(4):237-244. doi: 10.1053/jpan.2000.9462. PubMed
23. Dolkart O, Amar E, Weisman D, Flaisho R, Weinbroum AA. Patient dissatisfaction following prolonged stay in the post-anesthesia care unit due to unavailable ward bed in a tertiary hospital. Harefuah. 2013;152(8):446-450. PubMed
24. Lalani SB, Ali F, Kanji Z. Prolonged-stay patients in the PACU: a review of the literature. J Perianesth Nurs. 2013;28(3):151-155. doi: 10.1016/j.jopan.2012.06.009. PubMed
25. Fieldston ES, Hall M, Sills MR, et al. Children’s hospitals do not acutely respond to high occupancy. Pediatrics. 2010;125(5):974-981. doi: 10.1542/peds.2009-1627. PubMed
© 2019 Society of Hospital Medicine
The Association of Discharge Before Noon and Length of Stay in Hospitalized Pediatric Patients
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
Many hospitals and emergency departments (EDs) face challenges posed by overcrowding and hospital throughput. Slow ED throughput has been associated with worse patient outcomes.1 One strategy increasingly employed to improve hospital throughput is to increase the rate of inpatient discharges earlier in the day, which is often defined as discharges before noon (DCBNs). The hypothesis behind DCBN is that earlier hospital discharges will allow for earlier ED admissions and thus mitigate ED overcrowding while optimizing inpatient hospital flow. Previous quality improvement efforts to increase the percentage of DCBNs have been successfully implemented. For example, Wertheimer et al. implemented a process for earlier discharges and reported a 27-percentage point (11% to 38%) increase in DCBN on general medicine units.2 In a recent survey among leaders in hospital medicine programs, a majority reported early discharge as an important institutional goal.3
Studies of the effectiveness of DCBN initiatives on improving throughput and shortening length of stay (LOS) in adult patients have had mixed results. Computer modeling has supported the idea that earlier inpatient discharges would shorten ED patient boarding time.4
A question of interest for hospitals is if DCBN is a good indicator of shorter LOS, or is DCBN an arbitrary indicator, as morning discharges might just be the result of a delayed discharge of a patient ready for discharge the prior afternoon/evening. Our study objectives were: (1) to determine whether DCBN is associated with a shorter LOS in a pediatric population at an academic medical center, and (2) to examine separately this association in medical and surgical patients given the different provider workflow and patient clinical characteristics in those groups.
PATIENTS AND METHODS
Patients and Settings
We included patients 21 years or younger with an inpatient admission to any of the following pediatric medical or surgical services: cardiac surgery, cardiology, endocrinology, gastroenterology, general services, hematology/oncology, nephrology, orthopedics, otolaryngology, plastic surgery, pulmonology, and urology. Patients whose stay did not extend beyond one midnight were excluded because discharge time of day for these short stays was strongly related to the time of admission. We also excluded patients whose stay extended beyond two standard deviations of the average LOS for the discharge service under the assumption that these patients represented atypical circumstances. Finally, we excluded patients who died or left against medical advice. A consortium diagram of all exclusion criteria can be found in Supplemental Figure 1. Discharge data were extracted from the Carolina Database Warehouse, a data repository of the University of North Carolina Health System. The University of North Carolina Institutional Review Board reviewed and approved this study (IRB 17-0500).
Measures
The outcome of interest was LOS, defined as discharge date and time minus admission date and time, and thus a continuous measure of time in the hospital rather than a number of midnights. Rajkomar et al. used the same definition of LOS.6 The independent variable of interest was whether the discharge occurred before noon. Because discharges between midnight and 8:00
All model covariates were collected at the patient level (Table 1)
Statistical Analysis
Student t tests and χ2 statistics were used to compare baseline characteristics of hospitalizations of patients DCBN and after noon. We used ordinary least squares (OLS) regression models to assess the association between DCBN and LOS. Because DCBN may be correlated with patient characteristics, we used propensity score weighted models. Propensity scores were estimated using a logistic regression predicting DCBN using the variables given in Table 1 (excluding the outcome variable LOS). To estimate the average treatment effect on the entire sample for each model, we weighted each observation by the inverse-probability of treatment as per recent propensity score methods detailed by Garrido et al.9 In the inverse-probability weighted models, we clustered on attending physician to adjust for the autocorrelation caused by unobservable similarities of discharges by the same attending. We tested for multicollinearity using the variance inflation factor (VIF). To test our secondary hypothesis that there was a difference in the relationship between DCBN and LOS based on service type (medical versus surgical), we tested if the service type moderated any of the coefficients using a joint Wald test on the 10 coefficients interacted with the service type.
For our sensitivity analysis, we reran all surgical and medical discharges models changing the LOS outlier exclusion criteria to greater than three and then four standard deviations. Statistical modeling and analysis were completed using Stata version 14 (StataCorp, College Station, Texas).
RESULTS
Our study sample comprised 8,226 pediatric hospitalizations with a LOS mean of 5.10 and a median of 3.91 days respectively (range, 1.25-32.83 days). There were 1,531 (18.6%) DCBNs. Compared to those discharged after noon, patients with DCBN had a higher probability of being surgical patients, having commercial insurance, discharge home with self-care, discharge on the weekend, and discharge from a nonquality improvement unit (Table 1). Patients with DCBN were also more likely to be white, non-Hispanic, and male.
Our propensity score weighted ordinary least score (OLS) LOS regression results are presented in Table 2. In the bivariate analysis, DCBN was associated with an average 0.40 day, or roughly 10 hours, shorter LOS (P < .001). In the multivariate model of all discharges, we found that DCBN was associated with a mean of 0.27 day (P = .010) shorter LOS when compared to discharge in the afternoon when controlling for age, race, ethnicity, weekend discharge, discharge from quality improvement unit, discharge service type, CMI, insurance type, and discharge disposition.
There was no evidence of multicollinearity (mean VIF of 1.14). The Wald test returned an F statistic of 27.50 (P < .001) indicating there was a structural difference in the relationship between LOS and DCBN dependent on discharge service type; thus, we ran separate surgical and medical discharge models to interpret model coefficients for both service types. When we analyzed surgical and medical discharges in separate models, the effect of DCBN on LOS in the medical discharges model was significantly associated with a 0.30 day (P = .017) shorter LOS (Table 2). The association was not significant in the surgical discharges model.
To further test the analysis, we increased the LOS outlier exclusion criteria to three and four standard deviations.
DISCUSSION
The differential effect of DCBN on LOS in surgical and medical discharges suggests that the relationship between DCBN and LOS may be related to provider team workflow. For example, surgical teams may tend to round one time per day early in the morning before spending the entire day in the operating room, and thus completing more early morning discharge orders compared to medical teams. However, if a patient on a surgical service is not ready for discharge first thing in the morning, the patient may be more likely to wait until the following morning for a discharge order. On medical services, physician schedules may allow for more flexibility for rounding and responding with a discharge order when a patient becomes ready; however, medical services may round later in the day compared to surgeons and for a longer period of time, delaying discharges beyond noon that could have been made earlier. Another possibility, given UNC pediatric services are loosely regionalized with surgical patients concentrated more in one unit, is that unit-level differences in how staff processed discharges could have contributed to the difference observed between medical and surgical patients, particularly as there was a unit-level quality improvement effort for decreasing discharge time on one of two medical floors. However, we analyzed for differences based on the discharging unit and found no association. The influence of outliers on the association between DCBN and LOS increases also suggests that this group of children who have extremely long hospital stays might need further exploration.
Our study has some similar and some contrasting results with prior studies in adult patients. Our findings support the modeling literature that suggests DCBN may improve discharge efficiency by shortening patient LOS for some discharges.4 These findings contrast with Rajkomar et al., who reported that DCBN was associated with a longer LOS in adult patients.6 The contrasting findings could be due to differences in pediatric versus adult patients.
Our study has several limitations. While we controlled for observable characteristics using covariates and propensity score weighted analyses, there are likely unobservable characteristics that confound our analysis. We did not measure other factors that may affect discharge time of day such as high occupancy, staffing levels, patient transportation availability, and patient and family preferences. Given these limitations, we caution against interpreting a causal relationship between independent variables and the outcome. Finally, this analysis was conducted at a single tertiary care, academic medical center. The majority of pediatric admissions at this institution are either transferred from other hospitals or scheduled admissions for medical or surgical care. A smaller proportion of discharges are acute, unplanned admissions through our emergency department in children with or without underlying medical complexity. These factors plus the exclusion of observation, extended recovery, and all the less than two-day stays in this study contribute to a relatively higher average LOS. These factors potentially limit generalizability to other care settings. Additionally, the majority of the care teams involve care by resident physicians, and they are often the primary caregivers and write the majority of orders in patient charts such as discharge orders. While we were not able to control for within resident physician similarities between patients, we did control for autocorrelation at the attending level.
CONCLUSION
The results of our study suggest that DCBN is associated with a decreased LOS for medical but not surgical pediatric patients. DCBN may not be an appropriate measure for all services. Further research should be done to identify other feasible but more valid indicators for shorter LOS.
Disclosures
The authors have no financial relationships relative to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Funding
There were no external sources of funding for this work.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
1. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16(1):1-10. doi:10.1111/j.1553-2712.2008.00295.x. PubMed
2. Wertheimer B, Jacobs RE, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
3. Patel H, Fang MC, Mourad M, et al. Hospitalist and internal medicine leaders’ perspectives of early discharge challenges at academic medical centers. J Hosp Med. 2017;13(6):388-391. doi: 10.12788/jhm.2885. PubMed
4. Powell ES, Khare RK, Venkatesh AK, Van Roo BD, Adams JG, Reinhardt G. The relationship between inpatient discharge timing and emergency department boarding. J Emerg Med. 2012;42(2):186-196. doi:10.1016/j.jemermed.2010.06.028. PubMed
5. Wertheimer B, Jacobs RE, Iturrate E, Bailey M, Hochman K. Discharge before noon: effect on throughput and sustainability. J Hosp Med. 2015;10(10):664-669. doi:10.1002/jhm.2412. PubMed
6. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. doi:10.1002/jhm.2529. PubMed
7. Shine D. Discharge before noon: an urban legend. Am J Med. 2015;128(5):445-446. doi:10.1016/j.amjmed.2014.12.011. PubMed
8. Sauer B, Brookhart MA, Roy JA, VanderWeele TJ. Covariate selection. In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. PubMed
9. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49(5):1701-1720. doi:10.1111/1475-6773.12182. PubMed
10. Maguire P. Do discharge-before-noon Intiatives work? 2016. https://www.todayshospitalist.com/do-discharge-before-noon-initiatives-work/. Accessed April, 2018.
© 2019 Society of Hospital Medicine
Screening for Humoral Immunodeficiency in Patients with Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) is the most common infection in hospitalized patients and the eighth most common cause of death in the United States.1 Mortality from CAP is estimated to be 5.1% in the outpatient population,13.6% in hospitalized patients, and 35.1% in patients admitted to the intensive care unit.2,3 CAP accounts for more than 50,000 deaths annually in the United States.2 There are multiple risk factors for CAP, including tobacco use, malnutrition, chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis, and mechanical bronchial obstruction. Underlying immunodeficiency, specifically humoral immunodeficiency, is also a risk factor for CAP.
Primary immunodeficiency (PIDD) is estimated to affect one in 1,800 individuals in the United States.4 The National Institutes of Health (NIH) estimates that only one out of three individuals with PIDD are appropriately diagnosed. Based on probability calculations on known PIDD patients versus incidence of disease, the NIH estimates that more than 500,000 individuals with PIDD remain undiagnosed in the United States.4 Further, there exists an average diagnostic delay of at least five years. This delay increases both morbidity and mortality and leads to increased healthcare utilization.5,6
The most common form of primary immunodeficiency is due to humoral immunodeficiency, including selective IgA deficiency, specific antibody deficiency, and common variable immunodeficiency. Specific antibody deficiency is defined as a lack of response to polysaccharide antigens in the setting of low to normal Ig levels and an intact response to peptide antigens.7 Selective IgA deficiency is defined as the isolated deficiency of serum IgA in the setting of normal serum levels of IgG and IgM in an individual older than four years in whom other causes of hypogammaglobinemia have been excluded.8 Common variable immunodeficiency (CVID) is defined as a decreased serum concentration of IgG in combination with low levels of IgA and/or IgM with a poor or absent response to immunization in the absence of other defined immunodeficiency state.9 In addition to experiencing recurrent infections—namely bronchitis, sinusitis, otitis, and pneumonia—patients with CVID are also at increased risk of autoimmunity and malignancy. In adults, secondary immunodeficiency is more common than primary immunodeficiency. Secondary immunodeficiency occurs commonly with disease states like HIV infection, diabetes, cirrhosis, malnutrition, and autoimmune conditions.10 Additional causes of secondary immune defects due to humoral immunodeficiency include immune-modulating drugs—such as rituximab and ibrutinib—and hematologic malignancies, including chronic lymphocytic leukemia and multiple myeloma. Recurrent infections remain the leading cause of morbidity and mortality in patients with both primary and secondary immunodeficiency.11,12
Evaluation of the humoral immune system begins with measurement of serum immunoglobulin (Ig) levels. Although abnormal Ig levels are not diagnostic of immunodeficiency, abnormal results may prompt additional evaluation. Screening strategies may assist in making an earlier diagnoses, potentially decreasing morbidity and mortality in patients with immunodeficiency.13-15 To date, there have been no studies evaluating the utility of screening Ig levels to evaluate for underlying humoral immunodeficiency in patients hospitalized for CAP.
METHODS
Study Design
This was a prospective cohort study conducted at Rochester General Hospital, a 528-bed tertiary care medical center, from February 2017 to April 2017. We enrolled 100 consecutive patients admitted to the inpatient internal medicine service with a physician diagnosis of CAP. Written consent was obtained from each patient. The study was approved by the institutional review board at Rochester General Hospital.
Case Definition
The following criteria were used to diagnose CAP: (1) Respiratory symptoms of productive cough or pleuritic chest pain, (2) Fever >38°C before or at the time of admission, and (3) chest imaging with infiltrate. Exclusion criteria included a diagnosis of hospital-acquired pneumonia, prior diagnosis of primary immunodeficiency, immunosuppression due to an underlying condition, such as HIV or malignancy, therapy with immunosuppressive medications including chemotherapy, Ig replacement within the past six months, or treatment with >10 mg prednisone for greater than 14 days before hospital admission.
Patients underwent an additional evaluation by a clinical immunologist if they met one of the following criteria: any hypergammaglobinemia (elevated IgG, IgM, or IgA), IgG hypogammaglobinemia <550 mg/dL, undetectable IgM or IgA, or if IgG, IgM, and IgA were all below the lower limit of normal.
CURB-65 was used for estimation of the severity of illness with CAP. The components of the score include age ≥65, confusion, BUN >19 mg/dl, respiratory rate ≥30 breaths per minute and systolic blood pressure <90 mm Hg or diastolic blood pressure ≤60 mm Hg. Each component is scored zero if absent or one if present. Predicted mortality ranges from 0.6% for a score of zero to 27.8% for a score of 5.
Data Collection
Patient health information including age, race, gender, medical history, admission notes, results of chest imaging studies, and relevant laboratory studies including serum levels of IgG, IgM, IgA, IgE on admission was obtained from the electronic medical health record. An additional evaluation by the immunologist occurred within three months of hospital discharge and included repeat Ig levels, pre- and postvaccination titers of polysaccharide and peptide antigens, serum protein electrophoresis, and B & T cell panels.
Description of Normal Levels
The normal levels of immunoglobulins were defined based on standard reference ranges at the laboratory at Rochester General Hospital; IgG (700-1,600 mg/dl), IgM (50-300 mg/dl), IgA (70-400 mg/dl), and IgE (0-378 IU/ml). Although there is no established classification regarding the degree of IgG hypogammaglobinemia,16 clinical immunologists commonly classify the severity of IgG hypogammaglobinemia as follows: mild (550-699 mg/dL), moderate (400-549 mg/dL), and severe (<400 mg/dL) IgG hypogammaglobinemia.
Statistical Analysis
Statistical analysis was performed using STATA software (StataCorp LLC, College Station, Texas). We conducted a Wilcoxon rank-sum test to compare the median difference in length of stay between groups with a low versus normal range of immunoglobulins. A Kruskal–Wallis test was performed to check for the median difference in IgG levels across degrees of illness severity (CURB-65 score categories). We conducted a simple linear regression analysis using the logarithmic data of the length of stay and IgG level variables. A chi-square test was used to determine the association between comorbidities and Ig levels.
RESULTS
Baseline Characteristics
There were 100 patients with CAP enrolled in this study with a median age of 65.04 ± 18.8, and 53% were female. Forty-seven patients reported a previous history of pneumonia and 18 reported a history of recurrent sinusitis or otitis media. Of the 100 enrolled patients, 46 had received pneumococcal polysaccharide vaccine (PPV23), 26 had received the 13-valent pneumococcal conjugate vaccine (PCV13), and 22 had received both (Table 1). The mean white blood cell count on admission was 12.9 ± 7 × 103/uL with 75 ± 12.5% neutrophils. Total protein (6.5 ± 0.8) and albumin (3.7 ± 0.5) were within the normal range for the study population.
Immunoglobulin Analyses
The prevalence of hypogammaglobinemia in the study was 38% (95% CI: 28.47% to 48.25%). The median values of Ig levels for the entire study population and in patients with hypogammaglobinemia are summarized in Table 2.
- IgG hypogammaglobinemia (<700 mg/dl) was found in 27/100 patients, with a median level of 598 mg/dL, IQ range: 459-654. The median age in this group was 76.5 years, and 13 were female. Of these 27 patients, 10 had low IgM, four had low IgA, and four had an elevated IgE. In this group, 11 patients had received PPSV23, nine had received PCV13, and six had received both PPV23 and PCV13 before the index hospital admission.
- IgG hypergammaglobinemia (>1,600 mg/dl) was found in 9/100 patients, with a median level of 1,381 mg/dL, IQ range: 1,237-1,627. The median age was 61 years, and six were female. Of these nine patients, three had low IgM, one had low IgA, and four had elevated IgE.
- IgM hypogammaglobinemia (<50 mg/dl) was found in 23/100 patients with a median level of 38 mg/dL, IQ range: 25-43. In this group, the median age was 69 years, and 10 were female. Of these 23 patients, 10 had low IgG, and three had an elevated IgG.
- IgM hypergammaglobinemia (>300 mg/dl) was noted in two patients, with a median level of 491 mg/dL, IQ range: 418-564. Both patients were female, and one had elevated IgG.
- IgA hypogammaglobinemia (<70 mg/dl) was discovered in six patients, with a median level of 36 mg/dL, IQ range: 18-50. In this group, four patients had low IgG, four had low IgM, one had elevated IgE, and one had elevated IgG.
- IgA hypergammaglobinemia (>400 mg/dl) was noted in five patients, with a median level of 561 mg/dL, IQ range: 442-565: Two patients were female. Of these five patients, one had high IgG, and one had low IgG.
Length of Stay and Severity of Pneumonia
The median length of stay in the hospital for the entire study population was three days (IQ range: 2-5.5 days). Among patients with IgG hypogammaglobinemia, the median length of stay was two days longer as compared with patients who had IgG levels in the normal range (5 days, IQ range [3-10] vs 3days, IQ range [2-5], P = .0085).
The median CURB-65 score for the entire study population was two (IQ range: 1-3). The median CURB-65 score did not differ between patients with low and normal ranges of IgG levels (Median: 2, IQ range [1-3] vs Median: 1, IQ range [0-3], P = .2922). The CURB-65 score was not correlated with IgG levels (ρ = −0.0776, P = .4428). Length of stay, however, was positively correlated with CURB-65 score (ρ = .4673, P = .000)
A simple linear regression analysis using the logarithmic transformation of both length of stay and IgG level revealed a linear relationship between serum IgG levels and hospital length of stay (P = .0335, [R2 = .0453]).
Comorbidities and New Diagnoses
No significant association was found between smoking status, obesity, COPD, asthma, diabetes mellitus, and hypogammaglobinemia.
Fourteen patients with abnormal Ig levels as defined by (1) the presence of hypergammaglobinemia (elevated IgG, IgM, or IgA), (2) IgG levels <550, (3) undetectable IgA or IgM, and (4) either IgG or both IgM and IgA below the lower limit of normal underwent further evaluation. Of these 14 patients, one was diagnosed with multiple myeloma, one with selective IgA deficiency, and three with specific antibody deficiency (Table 3).
DISCUSSION
Previous research has evaluated the humoral immune system during an episode of CAP.17-20 Studies on Ig levels in patients with CAP have shown hypogammaglobinemia to be associated with ICU admission and increased ICU mortality.17,20 Additionally, patients with CAP have been shown to have lower IgG2 levels than healthy controls. The goal of our study was to evaluate patients with CAP for humoral immunodeficiency.
In our study, the prevalence of low Ig levels in CAP was 38%, with IgG hypogammaglobinemia being the most common class of hypogammaglobinemia. This rate is slightly higher than that found in a previous work by de la Torri et al.,21 who reported a prevalence of 28.9% in the inpatient population. The lower prevalence in the de la Torri et al. study was likely secondary to the exclusion of patients who did not have recorded Ig levels.21 Additionally, de la Torri et al. noted an inverse relationship between serum IgG levels and CURB-65. These results were not replicated in our analysis. This is likely due to the relatively low number of patients in each category of CURB-65 score in our study focusing only on inpatients. However, low IgG levels were associated with increased length of stay (5 days, IQ range [3-10] vs 3 days, IQ range [2-5]).
Sepsis can cause hypogammaglobinemia.22,23 The mechanism behind this phenomenon remain unclear, but several theories have been proposed. Sepsis results in endothelial dysfunction, vascular leakage, lymphopenia, and quantitative and qualitative defects in T and B cells.23 This potentially leads to impaired production and increased catabolism of immunoglobulins. Immunoglobulins play an essential role in recovery from sepsis, and there may be increased consumption during acute illness.24-28 Regardless of the mechanism, hypogammaglobinemia with SIRS, sepsis, and septic shock has been shown to be a risk factor for increased mortality in these patients.22,23 There is currently no consensus on the optimal time to screen for humoral immunodeficiency or evaluate the immune system after infection, such as CAP. Some would argue that Ig levels are lower during an active illness and, therefore, this may not be an appropriate time to evaluate Ig levels. However, we believe that inpatient hospitalization for CAP provides a window of opportunity to selectively screen these patients at higher risk for PIDD for underlying immune defects. A hospital-based approach as demonstrated in this study may be more productive than relying on an outpatient evaluation, which often may not occur due to patient recall and/or fragmentation of care, thus leading to the well-recognized delay in diagnosis of immunodeficiency.5,6In our study, one patient was diagnosed with multiple myeloma, three were diagnosed with specific antibody deficiency, and one was diagnosed with selective IgA deficiency. The patient with multiple myeloma was a 79-year old male who presented with his first ever episode of CAP, along with modest anemia and a creatinine of 1.6. His only other infectious history included an episode of sinusitis and one episode of pharyngitis. Additional evaluation included serum and urine electrophoresis, followed by bone marrow biopsy. This patient’s multiple myeloma diagnoses may have been missed if Ig levels had not been evaluated. Three patients were diagnosed with specific antibody deficiency. All these patients were above 50 years of age; two out of the three patients in this group had experienced a previous episode of pneumonia, and one had a history of recurrent sinusitis. Lastly, one patient was diagnosed with selective IgA deficiency as defined by undetectable IgA in the setting of normal IgG and IgM. This 56-year-old patient had a history of multiple episodes of sinusitis and three previous episodes of pneumonia, one requiring inpatient hospitalization. Earlier diagnosis of patients with specific antibody deficiency and selective IgA deficiency can guide management, which focuses on appropriate vaccination, the use of prophylactic antibiotics, and the possible role of Ig replacement in patients with specific antibody deficiency.
Of the 100 patients who underwent screening for immunodeficiency in the setting of CAP, five were found to have clinically significant humoral immunodeficiency, resulting in a number needed to screen of 20 to detect a clinically meaningful immunodeficiency in the setting of CAP. The number needed to screen by colonoscopy to detect one large bowel neoplasm in patients >50 years of age is 23.29 The number needed to screen to diagnose one occult cancer after an unprovoked DVT is 91.30 Based on this information, we feel that future, larger studies are required to evaluate the utility and cost-effectiveness of routine Ig screening for CAP requiring inpatient hospital admission.
We acknowledge limitations to this study. First, this study only evaluated adults in the inpatient floor setting, and therefore the results cannot be applied to the pediatric population or patients in the outpatient or ICU setting. Second, rather than completing a follow-up evaluation in all patients with abnormal immunoglobulins, we selected patients for additional evaluation based on criteria predefined by an immunologist. Although our rationale was to minimize additional diagnostic testing in individuals with mild hypogammaglobinemia, we acknowledge that this could have led to missing subtler humoral defects, such as a patient with near-normal Ig levels but a suboptimal response to vaccination. Third, due to the design of the study, we did not have a healthy matched control group. Despite these limitations, we believe our results are clinically meaningful and warrant future, larger scale investigation.
In conclusion, there is a high prevalence of hypogammaglobinemia in patients admitted with the diagnosis of CAP. IgG hypogammaglobinemia is the most commonly decreased class of Ig, and hospital length of stay is significantly longer in patients with low levels of IgG during admission for CAP. Additional immune evaluation of patients with CAP and abnormal Ig levels may also result in the identification of underlying antibody deficiency or immunoproliferative disorders.
Disclosures
The authors have nothing to disclose
1. File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. doi: 10.3810/pgm.2010.03.2130. PubMed
2. Solomon CG, Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543-551. doi: 10.1056/NEJMcp1214869.
3. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141. doi: 10.1001/jama.1996.03530260048030. PubMed
4. Dantas EO, Aranda CS, Nobre FA, et al. The medical awareness concerning primary immunodeficiency diseases (PID) in the city of Sao Paulo, Brazil. J Allergy Clin Immunol. 2012;129(2):AB86. doi: 10.1016/j.jaci.2011.12.648.
5. Kobrynski L, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. J Clin Immunol. 2014;34(8):954-961. doi: 10.1007/s10875-014-0102-8. PubMed
6. Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J Clin Pathol. 2005;58(5):546-547. doi: 10.1136/jcp.2004.016204. PubMed
7. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 SUPPL.). doi: 10.1016/j.jaci.2012.07.002. PubMed
8. Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10-16. doi: 10.1007/s10875-009-9357-x. PubMed
9. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190-197. doi: 10.1006/clim.1999.4799. PubMed
10. Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S195-S203. doi: 10.1016/j.jaci.2009.08.040. PubMed
11. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107-113. doi: 10.3324/haematol.2014.107714. PubMed
12. Strati P, Chaffee K, Achenbach S, et al. Disease progression and complications are the main cause of death in patients with chronic lymphocytic leukemia (CLL) independent of age and comorbidities at diagnosis. Blood. 2015;126(23):5265.
13. Holding S, Jolles S. Current screening approaches for antibody deficiency. Curr Opin Allergy Clin Immunol. 2015;15(6):547-555. doi: 10.1097/ACI.0000000000000222. PubMed
14. Azar AE, Ballas ZK. Evaluation of the adult with suspected immunodeficiency. Am J Med. 2007;120(9):764-768. doi: 10.1016/j.amjmed.2006.12.013. PubMed
15. Stoop JW, Zegers BJM, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969;4(1):101-112. PubMed
16. Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;9(3):281-283. doi: 10.1016/S1081-1206(10)60665-5. PubMed
17. Justel M, Socias L, Almansa R, et al. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol. 2013;58(3):564-567. doi: 10.1016/j.jcv.2013.09.006. PubMed
18. Gordon CL, Holmes NE, Grayson ML, et al. Comparison of immunoglobulin G subclass concentrations in severe community-acquired pneumonia and severe pandemic 2009 influenza A (H1N1) infection. Clin Vaccine Immunol. 2012;19(3):446-448. doi: 10.1128/CVI.05518-11. PubMed
19. Gordon CL, Johnson PD, Permezel M, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50(5):672-678. doi: 10.1086/650462. PubMed
20. Feldman C, Mahomed AG, Mahida P, et al. IgG subclasses in previously healthy adult patients with acute community-acquired pneumonia. S Afr Med J. 1996;86(5 Suppl):600-602. PubMed
21. de la Torre MC, Torán P, Serra-Prat M, et al. Serum levels of immunoglobulins and severity of community-acquired pneumonia. BMJ Open Respir Res. 2016;3(1):e000152. doi:1 0.1136/bmjresp-2016-000152. PubMed
22. Prucha M, Zazula R, Herold I, Dostal M, Hyanek T, Bellingan G. Presence of hypogammaglobulinemia in patients with severe sepsis, septic shock, and SIRS is associated with increased mortality. J Infect. 2014;68(3):297-299. doi: 10.1016/j.jinf.2013.11.003. PubMed
23. Shankar-Hari M, Culshaw N, Post B, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Intensive Care Med. 2015;41(8):1393-1401. doi: 10.1007/s00134-015-3845-7. PubMed
24. Drewry A, Samra N, Skrupky L, Fuller B, Compton S, Hotchkiss R. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383-391. doi: 10.1097/SHK.0000000000000234. PubMed
25. Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16(3). doi: 10.1186/cc11404. PubMed
26. Nordenfelt P, Waldemarson S, Linder A, et al. Antibody orientation at bacterial surfaces is related to invasive infection. J Exp Med. 2012;209(13):2367-2381. doi: 10.1084/jem.20120325. PubMed
27. Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol. 2009;70(6):553-564. doi: 10.1111/j.1365-3083.2009.02338.x. PubMed
28. Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689-691. doi: 10.1056/NEJMcibr1007320. PubMed
29. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863-1872. doi: 10.1056/NEJMoa054967. PubMed
30. Van Es N, Le Gal G, Otten HM, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism. Ann Intern Med. 2017;167(6):410-417. doi: 10.7326/M17-0868. PubMed
Community-acquired pneumonia (CAP) is the most common infection in hospitalized patients and the eighth most common cause of death in the United States.1 Mortality from CAP is estimated to be 5.1% in the outpatient population,13.6% in hospitalized patients, and 35.1% in patients admitted to the intensive care unit.2,3 CAP accounts for more than 50,000 deaths annually in the United States.2 There are multiple risk factors for CAP, including tobacco use, malnutrition, chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis, and mechanical bronchial obstruction. Underlying immunodeficiency, specifically humoral immunodeficiency, is also a risk factor for CAP.
Primary immunodeficiency (PIDD) is estimated to affect one in 1,800 individuals in the United States.4 The National Institutes of Health (NIH) estimates that only one out of three individuals with PIDD are appropriately diagnosed. Based on probability calculations on known PIDD patients versus incidence of disease, the NIH estimates that more than 500,000 individuals with PIDD remain undiagnosed in the United States.4 Further, there exists an average diagnostic delay of at least five years. This delay increases both morbidity and mortality and leads to increased healthcare utilization.5,6
The most common form of primary immunodeficiency is due to humoral immunodeficiency, including selective IgA deficiency, specific antibody deficiency, and common variable immunodeficiency. Specific antibody deficiency is defined as a lack of response to polysaccharide antigens in the setting of low to normal Ig levels and an intact response to peptide antigens.7 Selective IgA deficiency is defined as the isolated deficiency of serum IgA in the setting of normal serum levels of IgG and IgM in an individual older than four years in whom other causes of hypogammaglobinemia have been excluded.8 Common variable immunodeficiency (CVID) is defined as a decreased serum concentration of IgG in combination with low levels of IgA and/or IgM with a poor or absent response to immunization in the absence of other defined immunodeficiency state.9 In addition to experiencing recurrent infections—namely bronchitis, sinusitis, otitis, and pneumonia—patients with CVID are also at increased risk of autoimmunity and malignancy. In adults, secondary immunodeficiency is more common than primary immunodeficiency. Secondary immunodeficiency occurs commonly with disease states like HIV infection, diabetes, cirrhosis, malnutrition, and autoimmune conditions.10 Additional causes of secondary immune defects due to humoral immunodeficiency include immune-modulating drugs—such as rituximab and ibrutinib—and hematologic malignancies, including chronic lymphocytic leukemia and multiple myeloma. Recurrent infections remain the leading cause of morbidity and mortality in patients with both primary and secondary immunodeficiency.11,12
Evaluation of the humoral immune system begins with measurement of serum immunoglobulin (Ig) levels. Although abnormal Ig levels are not diagnostic of immunodeficiency, abnormal results may prompt additional evaluation. Screening strategies may assist in making an earlier diagnoses, potentially decreasing morbidity and mortality in patients with immunodeficiency.13-15 To date, there have been no studies evaluating the utility of screening Ig levels to evaluate for underlying humoral immunodeficiency in patients hospitalized for CAP.
METHODS
Study Design
This was a prospective cohort study conducted at Rochester General Hospital, a 528-bed tertiary care medical center, from February 2017 to April 2017. We enrolled 100 consecutive patients admitted to the inpatient internal medicine service with a physician diagnosis of CAP. Written consent was obtained from each patient. The study was approved by the institutional review board at Rochester General Hospital.
Case Definition
The following criteria were used to diagnose CAP: (1) Respiratory symptoms of productive cough or pleuritic chest pain, (2) Fever >38°C before or at the time of admission, and (3) chest imaging with infiltrate. Exclusion criteria included a diagnosis of hospital-acquired pneumonia, prior diagnosis of primary immunodeficiency, immunosuppression due to an underlying condition, such as HIV or malignancy, therapy with immunosuppressive medications including chemotherapy, Ig replacement within the past six months, or treatment with >10 mg prednisone for greater than 14 days before hospital admission.
Patients underwent an additional evaluation by a clinical immunologist if they met one of the following criteria: any hypergammaglobinemia (elevated IgG, IgM, or IgA), IgG hypogammaglobinemia <550 mg/dL, undetectable IgM or IgA, or if IgG, IgM, and IgA were all below the lower limit of normal.
CURB-65 was used for estimation of the severity of illness with CAP. The components of the score include age ≥65, confusion, BUN >19 mg/dl, respiratory rate ≥30 breaths per minute and systolic blood pressure <90 mm Hg or diastolic blood pressure ≤60 mm Hg. Each component is scored zero if absent or one if present. Predicted mortality ranges from 0.6% for a score of zero to 27.8% for a score of 5.
Data Collection
Patient health information including age, race, gender, medical history, admission notes, results of chest imaging studies, and relevant laboratory studies including serum levels of IgG, IgM, IgA, IgE on admission was obtained from the electronic medical health record. An additional evaluation by the immunologist occurred within three months of hospital discharge and included repeat Ig levels, pre- and postvaccination titers of polysaccharide and peptide antigens, serum protein electrophoresis, and B & T cell panels.
Description of Normal Levels
The normal levels of immunoglobulins were defined based on standard reference ranges at the laboratory at Rochester General Hospital; IgG (700-1,600 mg/dl), IgM (50-300 mg/dl), IgA (70-400 mg/dl), and IgE (0-378 IU/ml). Although there is no established classification regarding the degree of IgG hypogammaglobinemia,16 clinical immunologists commonly classify the severity of IgG hypogammaglobinemia as follows: mild (550-699 mg/dL), moderate (400-549 mg/dL), and severe (<400 mg/dL) IgG hypogammaglobinemia.
Statistical Analysis
Statistical analysis was performed using STATA software (StataCorp LLC, College Station, Texas). We conducted a Wilcoxon rank-sum test to compare the median difference in length of stay between groups with a low versus normal range of immunoglobulins. A Kruskal–Wallis test was performed to check for the median difference in IgG levels across degrees of illness severity (CURB-65 score categories). We conducted a simple linear regression analysis using the logarithmic data of the length of stay and IgG level variables. A chi-square test was used to determine the association between comorbidities and Ig levels.
RESULTS
Baseline Characteristics
There were 100 patients with CAP enrolled in this study with a median age of 65.04 ± 18.8, and 53% were female. Forty-seven patients reported a previous history of pneumonia and 18 reported a history of recurrent sinusitis or otitis media. Of the 100 enrolled patients, 46 had received pneumococcal polysaccharide vaccine (PPV23), 26 had received the 13-valent pneumococcal conjugate vaccine (PCV13), and 22 had received both (Table 1). The mean white blood cell count on admission was 12.9 ± 7 × 103/uL with 75 ± 12.5% neutrophils. Total protein (6.5 ± 0.8) and albumin (3.7 ± 0.5) were within the normal range for the study population.
Immunoglobulin Analyses
The prevalence of hypogammaglobinemia in the study was 38% (95% CI: 28.47% to 48.25%). The median values of Ig levels for the entire study population and in patients with hypogammaglobinemia are summarized in Table 2.
- IgG hypogammaglobinemia (<700 mg/dl) was found in 27/100 patients, with a median level of 598 mg/dL, IQ range: 459-654. The median age in this group was 76.5 years, and 13 were female. Of these 27 patients, 10 had low IgM, four had low IgA, and four had an elevated IgE. In this group, 11 patients had received PPSV23, nine had received PCV13, and six had received both PPV23 and PCV13 before the index hospital admission.
- IgG hypergammaglobinemia (>1,600 mg/dl) was found in 9/100 patients, with a median level of 1,381 mg/dL, IQ range: 1,237-1,627. The median age was 61 years, and six were female. Of these nine patients, three had low IgM, one had low IgA, and four had elevated IgE.
- IgM hypogammaglobinemia (<50 mg/dl) was found in 23/100 patients with a median level of 38 mg/dL, IQ range: 25-43. In this group, the median age was 69 years, and 10 were female. Of these 23 patients, 10 had low IgG, and three had an elevated IgG.
- IgM hypergammaglobinemia (>300 mg/dl) was noted in two patients, with a median level of 491 mg/dL, IQ range: 418-564. Both patients were female, and one had elevated IgG.
- IgA hypogammaglobinemia (<70 mg/dl) was discovered in six patients, with a median level of 36 mg/dL, IQ range: 18-50. In this group, four patients had low IgG, four had low IgM, one had elevated IgE, and one had elevated IgG.
- IgA hypergammaglobinemia (>400 mg/dl) was noted in five patients, with a median level of 561 mg/dL, IQ range: 442-565: Two patients were female. Of these five patients, one had high IgG, and one had low IgG.
Length of Stay and Severity of Pneumonia
The median length of stay in the hospital for the entire study population was three days (IQ range: 2-5.5 days). Among patients with IgG hypogammaglobinemia, the median length of stay was two days longer as compared with patients who had IgG levels in the normal range (5 days, IQ range [3-10] vs 3days, IQ range [2-5], P = .0085).
The median CURB-65 score for the entire study population was two (IQ range: 1-3). The median CURB-65 score did not differ between patients with low and normal ranges of IgG levels (Median: 2, IQ range [1-3] vs Median: 1, IQ range [0-3], P = .2922). The CURB-65 score was not correlated with IgG levels (ρ = −0.0776, P = .4428). Length of stay, however, was positively correlated with CURB-65 score (ρ = .4673, P = .000)
A simple linear regression analysis using the logarithmic transformation of both length of stay and IgG level revealed a linear relationship between serum IgG levels and hospital length of stay (P = .0335, [R2 = .0453]).
Comorbidities and New Diagnoses
No significant association was found between smoking status, obesity, COPD, asthma, diabetes mellitus, and hypogammaglobinemia.
Fourteen patients with abnormal Ig levels as defined by (1) the presence of hypergammaglobinemia (elevated IgG, IgM, or IgA), (2) IgG levels <550, (3) undetectable IgA or IgM, and (4) either IgG or both IgM and IgA below the lower limit of normal underwent further evaluation. Of these 14 patients, one was diagnosed with multiple myeloma, one with selective IgA deficiency, and three with specific antibody deficiency (Table 3).
DISCUSSION
Previous research has evaluated the humoral immune system during an episode of CAP.17-20 Studies on Ig levels in patients with CAP have shown hypogammaglobinemia to be associated with ICU admission and increased ICU mortality.17,20 Additionally, patients with CAP have been shown to have lower IgG2 levels than healthy controls. The goal of our study was to evaluate patients with CAP for humoral immunodeficiency.
In our study, the prevalence of low Ig levels in CAP was 38%, with IgG hypogammaglobinemia being the most common class of hypogammaglobinemia. This rate is slightly higher than that found in a previous work by de la Torri et al.,21 who reported a prevalence of 28.9% in the inpatient population. The lower prevalence in the de la Torri et al. study was likely secondary to the exclusion of patients who did not have recorded Ig levels.21 Additionally, de la Torri et al. noted an inverse relationship between serum IgG levels and CURB-65. These results were not replicated in our analysis. This is likely due to the relatively low number of patients in each category of CURB-65 score in our study focusing only on inpatients. However, low IgG levels were associated with increased length of stay (5 days, IQ range [3-10] vs 3 days, IQ range [2-5]).
Sepsis can cause hypogammaglobinemia.22,23 The mechanism behind this phenomenon remain unclear, but several theories have been proposed. Sepsis results in endothelial dysfunction, vascular leakage, lymphopenia, and quantitative and qualitative defects in T and B cells.23 This potentially leads to impaired production and increased catabolism of immunoglobulins. Immunoglobulins play an essential role in recovery from sepsis, and there may be increased consumption during acute illness.24-28 Regardless of the mechanism, hypogammaglobinemia with SIRS, sepsis, and septic shock has been shown to be a risk factor for increased mortality in these patients.22,23 There is currently no consensus on the optimal time to screen for humoral immunodeficiency or evaluate the immune system after infection, such as CAP. Some would argue that Ig levels are lower during an active illness and, therefore, this may not be an appropriate time to evaluate Ig levels. However, we believe that inpatient hospitalization for CAP provides a window of opportunity to selectively screen these patients at higher risk for PIDD for underlying immune defects. A hospital-based approach as demonstrated in this study may be more productive than relying on an outpatient evaluation, which often may not occur due to patient recall and/or fragmentation of care, thus leading to the well-recognized delay in diagnosis of immunodeficiency.5,6In our study, one patient was diagnosed with multiple myeloma, three were diagnosed with specific antibody deficiency, and one was diagnosed with selective IgA deficiency. The patient with multiple myeloma was a 79-year old male who presented with his first ever episode of CAP, along with modest anemia and a creatinine of 1.6. His only other infectious history included an episode of sinusitis and one episode of pharyngitis. Additional evaluation included serum and urine electrophoresis, followed by bone marrow biopsy. This patient’s multiple myeloma diagnoses may have been missed if Ig levels had not been evaluated. Three patients were diagnosed with specific antibody deficiency. All these patients were above 50 years of age; two out of the three patients in this group had experienced a previous episode of pneumonia, and one had a history of recurrent sinusitis. Lastly, one patient was diagnosed with selective IgA deficiency as defined by undetectable IgA in the setting of normal IgG and IgM. This 56-year-old patient had a history of multiple episodes of sinusitis and three previous episodes of pneumonia, one requiring inpatient hospitalization. Earlier diagnosis of patients with specific antibody deficiency and selective IgA deficiency can guide management, which focuses on appropriate vaccination, the use of prophylactic antibiotics, and the possible role of Ig replacement in patients with specific antibody deficiency.
Of the 100 patients who underwent screening for immunodeficiency in the setting of CAP, five were found to have clinically significant humoral immunodeficiency, resulting in a number needed to screen of 20 to detect a clinically meaningful immunodeficiency in the setting of CAP. The number needed to screen by colonoscopy to detect one large bowel neoplasm in patients >50 years of age is 23.29 The number needed to screen to diagnose one occult cancer after an unprovoked DVT is 91.30 Based on this information, we feel that future, larger studies are required to evaluate the utility and cost-effectiveness of routine Ig screening for CAP requiring inpatient hospital admission.
We acknowledge limitations to this study. First, this study only evaluated adults in the inpatient floor setting, and therefore the results cannot be applied to the pediatric population or patients in the outpatient or ICU setting. Second, rather than completing a follow-up evaluation in all patients with abnormal immunoglobulins, we selected patients for additional evaluation based on criteria predefined by an immunologist. Although our rationale was to minimize additional diagnostic testing in individuals with mild hypogammaglobinemia, we acknowledge that this could have led to missing subtler humoral defects, such as a patient with near-normal Ig levels but a suboptimal response to vaccination. Third, due to the design of the study, we did not have a healthy matched control group. Despite these limitations, we believe our results are clinically meaningful and warrant future, larger scale investigation.
In conclusion, there is a high prevalence of hypogammaglobinemia in patients admitted with the diagnosis of CAP. IgG hypogammaglobinemia is the most commonly decreased class of Ig, and hospital length of stay is significantly longer in patients with low levels of IgG during admission for CAP. Additional immune evaluation of patients with CAP and abnormal Ig levels may also result in the identification of underlying antibody deficiency or immunoproliferative disorders.
Disclosures
The authors have nothing to disclose
Community-acquired pneumonia (CAP) is the most common infection in hospitalized patients and the eighth most common cause of death in the United States.1 Mortality from CAP is estimated to be 5.1% in the outpatient population,13.6% in hospitalized patients, and 35.1% in patients admitted to the intensive care unit.2,3 CAP accounts for more than 50,000 deaths annually in the United States.2 There are multiple risk factors for CAP, including tobacco use, malnutrition, chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis, and mechanical bronchial obstruction. Underlying immunodeficiency, specifically humoral immunodeficiency, is also a risk factor for CAP.
Primary immunodeficiency (PIDD) is estimated to affect one in 1,800 individuals in the United States.4 The National Institutes of Health (NIH) estimates that only one out of three individuals with PIDD are appropriately diagnosed. Based on probability calculations on known PIDD patients versus incidence of disease, the NIH estimates that more than 500,000 individuals with PIDD remain undiagnosed in the United States.4 Further, there exists an average diagnostic delay of at least five years. This delay increases both morbidity and mortality and leads to increased healthcare utilization.5,6
The most common form of primary immunodeficiency is due to humoral immunodeficiency, including selective IgA deficiency, specific antibody deficiency, and common variable immunodeficiency. Specific antibody deficiency is defined as a lack of response to polysaccharide antigens in the setting of low to normal Ig levels and an intact response to peptide antigens.7 Selective IgA deficiency is defined as the isolated deficiency of serum IgA in the setting of normal serum levels of IgG and IgM in an individual older than four years in whom other causes of hypogammaglobinemia have been excluded.8 Common variable immunodeficiency (CVID) is defined as a decreased serum concentration of IgG in combination with low levels of IgA and/or IgM with a poor or absent response to immunization in the absence of other defined immunodeficiency state.9 In addition to experiencing recurrent infections—namely bronchitis, sinusitis, otitis, and pneumonia—patients with CVID are also at increased risk of autoimmunity and malignancy. In adults, secondary immunodeficiency is more common than primary immunodeficiency. Secondary immunodeficiency occurs commonly with disease states like HIV infection, diabetes, cirrhosis, malnutrition, and autoimmune conditions.10 Additional causes of secondary immune defects due to humoral immunodeficiency include immune-modulating drugs—such as rituximab and ibrutinib—and hematologic malignancies, including chronic lymphocytic leukemia and multiple myeloma. Recurrent infections remain the leading cause of morbidity and mortality in patients with both primary and secondary immunodeficiency.11,12
Evaluation of the humoral immune system begins with measurement of serum immunoglobulin (Ig) levels. Although abnormal Ig levels are not diagnostic of immunodeficiency, abnormal results may prompt additional evaluation. Screening strategies may assist in making an earlier diagnoses, potentially decreasing morbidity and mortality in patients with immunodeficiency.13-15 To date, there have been no studies evaluating the utility of screening Ig levels to evaluate for underlying humoral immunodeficiency in patients hospitalized for CAP.
METHODS
Study Design
This was a prospective cohort study conducted at Rochester General Hospital, a 528-bed tertiary care medical center, from February 2017 to April 2017. We enrolled 100 consecutive patients admitted to the inpatient internal medicine service with a physician diagnosis of CAP. Written consent was obtained from each patient. The study was approved by the institutional review board at Rochester General Hospital.
Case Definition
The following criteria were used to diagnose CAP: (1) Respiratory symptoms of productive cough or pleuritic chest pain, (2) Fever >38°C before or at the time of admission, and (3) chest imaging with infiltrate. Exclusion criteria included a diagnosis of hospital-acquired pneumonia, prior diagnosis of primary immunodeficiency, immunosuppression due to an underlying condition, such as HIV or malignancy, therapy with immunosuppressive medications including chemotherapy, Ig replacement within the past six months, or treatment with >10 mg prednisone for greater than 14 days before hospital admission.
Patients underwent an additional evaluation by a clinical immunologist if they met one of the following criteria: any hypergammaglobinemia (elevated IgG, IgM, or IgA), IgG hypogammaglobinemia <550 mg/dL, undetectable IgM or IgA, or if IgG, IgM, and IgA were all below the lower limit of normal.
CURB-65 was used for estimation of the severity of illness with CAP. The components of the score include age ≥65, confusion, BUN >19 mg/dl, respiratory rate ≥30 breaths per minute and systolic blood pressure <90 mm Hg or diastolic blood pressure ≤60 mm Hg. Each component is scored zero if absent or one if present. Predicted mortality ranges from 0.6% for a score of zero to 27.8% for a score of 5.
Data Collection
Patient health information including age, race, gender, medical history, admission notes, results of chest imaging studies, and relevant laboratory studies including serum levels of IgG, IgM, IgA, IgE on admission was obtained from the electronic medical health record. An additional evaluation by the immunologist occurred within three months of hospital discharge and included repeat Ig levels, pre- and postvaccination titers of polysaccharide and peptide antigens, serum protein electrophoresis, and B & T cell panels.
Description of Normal Levels
The normal levels of immunoglobulins were defined based on standard reference ranges at the laboratory at Rochester General Hospital; IgG (700-1,600 mg/dl), IgM (50-300 mg/dl), IgA (70-400 mg/dl), and IgE (0-378 IU/ml). Although there is no established classification regarding the degree of IgG hypogammaglobinemia,16 clinical immunologists commonly classify the severity of IgG hypogammaglobinemia as follows: mild (550-699 mg/dL), moderate (400-549 mg/dL), and severe (<400 mg/dL) IgG hypogammaglobinemia.
Statistical Analysis
Statistical analysis was performed using STATA software (StataCorp LLC, College Station, Texas). We conducted a Wilcoxon rank-sum test to compare the median difference in length of stay between groups with a low versus normal range of immunoglobulins. A Kruskal–Wallis test was performed to check for the median difference in IgG levels across degrees of illness severity (CURB-65 score categories). We conducted a simple linear regression analysis using the logarithmic data of the length of stay and IgG level variables. A chi-square test was used to determine the association between comorbidities and Ig levels.
RESULTS
Baseline Characteristics
There were 100 patients with CAP enrolled in this study with a median age of 65.04 ± 18.8, and 53% were female. Forty-seven patients reported a previous history of pneumonia and 18 reported a history of recurrent sinusitis or otitis media. Of the 100 enrolled patients, 46 had received pneumococcal polysaccharide vaccine (PPV23), 26 had received the 13-valent pneumococcal conjugate vaccine (PCV13), and 22 had received both (Table 1). The mean white blood cell count on admission was 12.9 ± 7 × 103/uL with 75 ± 12.5% neutrophils. Total protein (6.5 ± 0.8) and albumin (3.7 ± 0.5) were within the normal range for the study population.
Immunoglobulin Analyses
The prevalence of hypogammaglobinemia in the study was 38% (95% CI: 28.47% to 48.25%). The median values of Ig levels for the entire study population and in patients with hypogammaglobinemia are summarized in Table 2.
- IgG hypogammaglobinemia (<700 mg/dl) was found in 27/100 patients, with a median level of 598 mg/dL, IQ range: 459-654. The median age in this group was 76.5 years, and 13 were female. Of these 27 patients, 10 had low IgM, four had low IgA, and four had an elevated IgE. In this group, 11 patients had received PPSV23, nine had received PCV13, and six had received both PPV23 and PCV13 before the index hospital admission.
- IgG hypergammaglobinemia (>1,600 mg/dl) was found in 9/100 patients, with a median level of 1,381 mg/dL, IQ range: 1,237-1,627. The median age was 61 years, and six were female. Of these nine patients, three had low IgM, one had low IgA, and four had elevated IgE.
- IgM hypogammaglobinemia (<50 mg/dl) was found in 23/100 patients with a median level of 38 mg/dL, IQ range: 25-43. In this group, the median age was 69 years, and 10 were female. Of these 23 patients, 10 had low IgG, and three had an elevated IgG.
- IgM hypergammaglobinemia (>300 mg/dl) was noted in two patients, with a median level of 491 mg/dL, IQ range: 418-564. Both patients were female, and one had elevated IgG.
- IgA hypogammaglobinemia (<70 mg/dl) was discovered in six patients, with a median level of 36 mg/dL, IQ range: 18-50. In this group, four patients had low IgG, four had low IgM, one had elevated IgE, and one had elevated IgG.
- IgA hypergammaglobinemia (>400 mg/dl) was noted in five patients, with a median level of 561 mg/dL, IQ range: 442-565: Two patients were female. Of these five patients, one had high IgG, and one had low IgG.
Length of Stay and Severity of Pneumonia
The median length of stay in the hospital for the entire study population was three days (IQ range: 2-5.5 days). Among patients with IgG hypogammaglobinemia, the median length of stay was two days longer as compared with patients who had IgG levels in the normal range (5 days, IQ range [3-10] vs 3days, IQ range [2-5], P = .0085).
The median CURB-65 score for the entire study population was two (IQ range: 1-3). The median CURB-65 score did not differ between patients with low and normal ranges of IgG levels (Median: 2, IQ range [1-3] vs Median: 1, IQ range [0-3], P = .2922). The CURB-65 score was not correlated with IgG levels (ρ = −0.0776, P = .4428). Length of stay, however, was positively correlated with CURB-65 score (ρ = .4673, P = .000)
A simple linear regression analysis using the logarithmic transformation of both length of stay and IgG level revealed a linear relationship between serum IgG levels and hospital length of stay (P = .0335, [R2 = .0453]).
Comorbidities and New Diagnoses
No significant association was found between smoking status, obesity, COPD, asthma, diabetes mellitus, and hypogammaglobinemia.
Fourteen patients with abnormal Ig levels as defined by (1) the presence of hypergammaglobinemia (elevated IgG, IgM, or IgA), (2) IgG levels <550, (3) undetectable IgA or IgM, and (4) either IgG or both IgM and IgA below the lower limit of normal underwent further evaluation. Of these 14 patients, one was diagnosed with multiple myeloma, one with selective IgA deficiency, and three with specific antibody deficiency (Table 3).
DISCUSSION
Previous research has evaluated the humoral immune system during an episode of CAP.17-20 Studies on Ig levels in patients with CAP have shown hypogammaglobinemia to be associated with ICU admission and increased ICU mortality.17,20 Additionally, patients with CAP have been shown to have lower IgG2 levels than healthy controls. The goal of our study was to evaluate patients with CAP for humoral immunodeficiency.
In our study, the prevalence of low Ig levels in CAP was 38%, with IgG hypogammaglobinemia being the most common class of hypogammaglobinemia. This rate is slightly higher than that found in a previous work by de la Torri et al.,21 who reported a prevalence of 28.9% in the inpatient population. The lower prevalence in the de la Torri et al. study was likely secondary to the exclusion of patients who did not have recorded Ig levels.21 Additionally, de la Torri et al. noted an inverse relationship between serum IgG levels and CURB-65. These results were not replicated in our analysis. This is likely due to the relatively low number of patients in each category of CURB-65 score in our study focusing only on inpatients. However, low IgG levels were associated with increased length of stay (5 days, IQ range [3-10] vs 3 days, IQ range [2-5]).
Sepsis can cause hypogammaglobinemia.22,23 The mechanism behind this phenomenon remain unclear, but several theories have been proposed. Sepsis results in endothelial dysfunction, vascular leakage, lymphopenia, and quantitative and qualitative defects in T and B cells.23 This potentially leads to impaired production and increased catabolism of immunoglobulins. Immunoglobulins play an essential role in recovery from sepsis, and there may be increased consumption during acute illness.24-28 Regardless of the mechanism, hypogammaglobinemia with SIRS, sepsis, and septic shock has been shown to be a risk factor for increased mortality in these patients.22,23 There is currently no consensus on the optimal time to screen for humoral immunodeficiency or evaluate the immune system after infection, such as CAP. Some would argue that Ig levels are lower during an active illness and, therefore, this may not be an appropriate time to evaluate Ig levels. However, we believe that inpatient hospitalization for CAP provides a window of opportunity to selectively screen these patients at higher risk for PIDD for underlying immune defects. A hospital-based approach as demonstrated in this study may be more productive than relying on an outpatient evaluation, which often may not occur due to patient recall and/or fragmentation of care, thus leading to the well-recognized delay in diagnosis of immunodeficiency.5,6In our study, one patient was diagnosed with multiple myeloma, three were diagnosed with specific antibody deficiency, and one was diagnosed with selective IgA deficiency. The patient with multiple myeloma was a 79-year old male who presented with his first ever episode of CAP, along with modest anemia and a creatinine of 1.6. His only other infectious history included an episode of sinusitis and one episode of pharyngitis. Additional evaluation included serum and urine electrophoresis, followed by bone marrow biopsy. This patient’s multiple myeloma diagnoses may have been missed if Ig levels had not been evaluated. Three patients were diagnosed with specific antibody deficiency. All these patients were above 50 years of age; two out of the three patients in this group had experienced a previous episode of pneumonia, and one had a history of recurrent sinusitis. Lastly, one patient was diagnosed with selective IgA deficiency as defined by undetectable IgA in the setting of normal IgG and IgM. This 56-year-old patient had a history of multiple episodes of sinusitis and three previous episodes of pneumonia, one requiring inpatient hospitalization. Earlier diagnosis of patients with specific antibody deficiency and selective IgA deficiency can guide management, which focuses on appropriate vaccination, the use of prophylactic antibiotics, and the possible role of Ig replacement in patients with specific antibody deficiency.
Of the 100 patients who underwent screening for immunodeficiency in the setting of CAP, five were found to have clinically significant humoral immunodeficiency, resulting in a number needed to screen of 20 to detect a clinically meaningful immunodeficiency in the setting of CAP. The number needed to screen by colonoscopy to detect one large bowel neoplasm in patients >50 years of age is 23.29 The number needed to screen to diagnose one occult cancer after an unprovoked DVT is 91.30 Based on this information, we feel that future, larger studies are required to evaluate the utility and cost-effectiveness of routine Ig screening for CAP requiring inpatient hospital admission.
We acknowledge limitations to this study. First, this study only evaluated adults in the inpatient floor setting, and therefore the results cannot be applied to the pediatric population or patients in the outpatient or ICU setting. Second, rather than completing a follow-up evaluation in all patients with abnormal immunoglobulins, we selected patients for additional evaluation based on criteria predefined by an immunologist. Although our rationale was to minimize additional diagnostic testing in individuals with mild hypogammaglobinemia, we acknowledge that this could have led to missing subtler humoral defects, such as a patient with near-normal Ig levels but a suboptimal response to vaccination. Third, due to the design of the study, we did not have a healthy matched control group. Despite these limitations, we believe our results are clinically meaningful and warrant future, larger scale investigation.
In conclusion, there is a high prevalence of hypogammaglobinemia in patients admitted with the diagnosis of CAP. IgG hypogammaglobinemia is the most commonly decreased class of Ig, and hospital length of stay is significantly longer in patients with low levels of IgG during admission for CAP. Additional immune evaluation of patients with CAP and abnormal Ig levels may also result in the identification of underlying antibody deficiency or immunoproliferative disorders.
Disclosures
The authors have nothing to disclose
1. File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. doi: 10.3810/pgm.2010.03.2130. PubMed
2. Solomon CG, Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543-551. doi: 10.1056/NEJMcp1214869.
3. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141. doi: 10.1001/jama.1996.03530260048030. PubMed
4. Dantas EO, Aranda CS, Nobre FA, et al. The medical awareness concerning primary immunodeficiency diseases (PID) in the city of Sao Paulo, Brazil. J Allergy Clin Immunol. 2012;129(2):AB86. doi: 10.1016/j.jaci.2011.12.648.
5. Kobrynski L, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. J Clin Immunol. 2014;34(8):954-961. doi: 10.1007/s10875-014-0102-8. PubMed
6. Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J Clin Pathol. 2005;58(5):546-547. doi: 10.1136/jcp.2004.016204. PubMed
7. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 SUPPL.). doi: 10.1016/j.jaci.2012.07.002. PubMed
8. Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10-16. doi: 10.1007/s10875-009-9357-x. PubMed
9. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190-197. doi: 10.1006/clim.1999.4799. PubMed
10. Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S195-S203. doi: 10.1016/j.jaci.2009.08.040. PubMed
11. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107-113. doi: 10.3324/haematol.2014.107714. PubMed
12. Strati P, Chaffee K, Achenbach S, et al. Disease progression and complications are the main cause of death in patients with chronic lymphocytic leukemia (CLL) independent of age and comorbidities at diagnosis. Blood. 2015;126(23):5265.
13. Holding S, Jolles S. Current screening approaches for antibody deficiency. Curr Opin Allergy Clin Immunol. 2015;15(6):547-555. doi: 10.1097/ACI.0000000000000222. PubMed
14. Azar AE, Ballas ZK. Evaluation of the adult with suspected immunodeficiency. Am J Med. 2007;120(9):764-768. doi: 10.1016/j.amjmed.2006.12.013. PubMed
15. Stoop JW, Zegers BJM, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969;4(1):101-112. PubMed
16. Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;9(3):281-283. doi: 10.1016/S1081-1206(10)60665-5. PubMed
17. Justel M, Socias L, Almansa R, et al. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol. 2013;58(3):564-567. doi: 10.1016/j.jcv.2013.09.006. PubMed
18. Gordon CL, Holmes NE, Grayson ML, et al. Comparison of immunoglobulin G subclass concentrations in severe community-acquired pneumonia and severe pandemic 2009 influenza A (H1N1) infection. Clin Vaccine Immunol. 2012;19(3):446-448. doi: 10.1128/CVI.05518-11. PubMed
19. Gordon CL, Johnson PD, Permezel M, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50(5):672-678. doi: 10.1086/650462. PubMed
20. Feldman C, Mahomed AG, Mahida P, et al. IgG subclasses in previously healthy adult patients with acute community-acquired pneumonia. S Afr Med J. 1996;86(5 Suppl):600-602. PubMed
21. de la Torre MC, Torán P, Serra-Prat M, et al. Serum levels of immunoglobulins and severity of community-acquired pneumonia. BMJ Open Respir Res. 2016;3(1):e000152. doi:1 0.1136/bmjresp-2016-000152. PubMed
22. Prucha M, Zazula R, Herold I, Dostal M, Hyanek T, Bellingan G. Presence of hypogammaglobulinemia in patients with severe sepsis, septic shock, and SIRS is associated with increased mortality. J Infect. 2014;68(3):297-299. doi: 10.1016/j.jinf.2013.11.003. PubMed
23. Shankar-Hari M, Culshaw N, Post B, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Intensive Care Med. 2015;41(8):1393-1401. doi: 10.1007/s00134-015-3845-7. PubMed
24. Drewry A, Samra N, Skrupky L, Fuller B, Compton S, Hotchkiss R. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383-391. doi: 10.1097/SHK.0000000000000234. PubMed
25. Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16(3). doi: 10.1186/cc11404. PubMed
26. Nordenfelt P, Waldemarson S, Linder A, et al. Antibody orientation at bacterial surfaces is related to invasive infection. J Exp Med. 2012;209(13):2367-2381. doi: 10.1084/jem.20120325. PubMed
27. Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol. 2009;70(6):553-564. doi: 10.1111/j.1365-3083.2009.02338.x. PubMed
28. Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689-691. doi: 10.1056/NEJMcibr1007320. PubMed
29. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863-1872. doi: 10.1056/NEJMoa054967. PubMed
30. Van Es N, Le Gal G, Otten HM, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism. Ann Intern Med. 2017;167(6):410-417. doi: 10.7326/M17-0868. PubMed
1. File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. doi: 10.3810/pgm.2010.03.2130. PubMed
2. Solomon CG, Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med. 2014;370(6):543-551. doi: 10.1056/NEJMcp1214869.
3. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141. doi: 10.1001/jama.1996.03530260048030. PubMed
4. Dantas EO, Aranda CS, Nobre FA, et al. The medical awareness concerning primary immunodeficiency diseases (PID) in the city of Sao Paulo, Brazil. J Allergy Clin Immunol. 2012;129(2):AB86. doi: 10.1016/j.jaci.2011.12.648.
5. Kobrynski L, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. J Clin Immunol. 2014;34(8):954-961. doi: 10.1007/s10875-014-0102-8. PubMed
6. Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J Clin Pathol. 2005;58(5):546-547. doi: 10.1136/jcp.2004.016204. PubMed
7. Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130(3 SUPPL.). doi: 10.1016/j.jaci.2012.07.002. PubMed
8. Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30(1):10-16. doi: 10.1007/s10875-009-9357-x. PubMed
9. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190-197. doi: 10.1006/clim.1999.4799. PubMed
10. Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S195-S203. doi: 10.1016/j.jaci.2009.08.040. PubMed
11. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107-113. doi: 10.3324/haematol.2014.107714. PubMed
12. Strati P, Chaffee K, Achenbach S, et al. Disease progression and complications are the main cause of death in patients with chronic lymphocytic leukemia (CLL) independent of age and comorbidities at diagnosis. Blood. 2015;126(23):5265.
13. Holding S, Jolles S. Current screening approaches for antibody deficiency. Curr Opin Allergy Clin Immunol. 2015;15(6):547-555. doi: 10.1097/ACI.0000000000000222. PubMed
14. Azar AE, Ballas ZK. Evaluation of the adult with suspected immunodeficiency. Am J Med. 2007;120(9):764-768. doi: 10.1016/j.amjmed.2006.12.013. PubMed
15. Stoop JW, Zegers BJM, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969;4(1):101-112. PubMed
16. Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;9(3):281-283. doi: 10.1016/S1081-1206(10)60665-5. PubMed
17. Justel M, Socias L, Almansa R, et al. IgM levels in plasma predict outcome in severe pandemic influenza. J Clin Virol. 2013;58(3):564-567. doi: 10.1016/j.jcv.2013.09.006. PubMed
18. Gordon CL, Holmes NE, Grayson ML, et al. Comparison of immunoglobulin G subclass concentrations in severe community-acquired pneumonia and severe pandemic 2009 influenza A (H1N1) infection. Clin Vaccine Immunol. 2012;19(3):446-448. doi: 10.1128/CVI.05518-11. PubMed
19. Gordon CL, Johnson PD, Permezel M, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50(5):672-678. doi: 10.1086/650462. PubMed
20. Feldman C, Mahomed AG, Mahida P, et al. IgG subclasses in previously healthy adult patients with acute community-acquired pneumonia. S Afr Med J. 1996;86(5 Suppl):600-602. PubMed
21. de la Torre MC, Torán P, Serra-Prat M, et al. Serum levels of immunoglobulins and severity of community-acquired pneumonia. BMJ Open Respir Res. 2016;3(1):e000152. doi:1 0.1136/bmjresp-2016-000152. PubMed
22. Prucha M, Zazula R, Herold I, Dostal M, Hyanek T, Bellingan G. Presence of hypogammaglobulinemia in patients with severe sepsis, septic shock, and SIRS is associated with increased mortality. J Infect. 2014;68(3):297-299. doi: 10.1016/j.jinf.2013.11.003. PubMed
23. Shankar-Hari M, Culshaw N, Post B, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Intensive Care Med. 2015;41(8):1393-1401. doi: 10.1007/s00134-015-3845-7. PubMed
24. Drewry A, Samra N, Skrupky L, Fuller B, Compton S, Hotchkiss R. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383-391. doi: 10.1097/SHK.0000000000000234. PubMed
25. Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16(3). doi: 10.1186/cc11404. PubMed
26. Nordenfelt P, Waldemarson S, Linder A, et al. Antibody orientation at bacterial surfaces is related to invasive infection. J Exp Med. 2012;209(13):2367-2381. doi: 10.1084/jem.20120325. PubMed
27. Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol. 2009;70(6):553-564. doi: 10.1111/j.1365-3083.2009.02338.x. PubMed
28. Lee WL, Slutsky AS. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689-691. doi: 10.1056/NEJMcibr1007320. PubMed
29. Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355(18):1863-1872. doi: 10.1056/NEJMoa054967. PubMed
30. Van Es N, Le Gal G, Otten HM, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism. Ann Intern Med. 2017;167(6):410-417. doi: 10.7326/M17-0868. PubMed
© 2018 Society of Hospital Medicine
Less Lumens-Less Risk: A Pilot Intervention to Increase the Use of Single-Lumen Peripherally Inserted Central Catheters
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Intermittent Pneumatic Compression for Medical Ward Patients?
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailingTWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
© 2019 Society of Hospital Medicine
The Basement Flight
A 14-year-old girl with a history of asthma presented to the Emergency Department (ED) with three months of persistent, nonproductive cough, and progressive shortness of breath. She reported fatigue, chest tightness, orthopnea, and dyspnea with exertion. She denied fever, rhinorrhea, congestion, hemoptysis, or paroxysmal nocturnal dyspnea.
Her age and past medical history of asthma are incongruent with her new symptoms, as asthma is typified by intermittent exacerbations, not progressive symptoms. Thus, another process, in addition to asthma, is most likely present; it is also important to question the accuracy of previous diagnoses in light of new information. Her symptoms may signify an underlying cardiopulmonary process, such as infiltrative diseases (eg, lymphoma or sarcoidosis), atypical infections, genetic conditions (eg, variant cystic fibrosis), autoimmune conditions, or cardiomyopathy. A detailed symptom history, family history, and careful physical examination will help expand and then refine the differential diagnosis. At this stage, typical infections are less likely.
She had presented two months prior with nonproductive cough and dyspnea. At that presentation, her temperature was 36.3°C, heart rate 110 beats per minute, blood pressure 119/63 mm Hg, respiratory rate 43 breaths per minute, and oxygen saturation 86% while breathing ambient air. A chest CT with contrast demonstrated diffuse patchy multifocal ground-glass opacities in the bilateral lungs as well as a mixture of atelectasis and lobular emphysema in the dependent lobes bilaterally (Figure 1). Her main pulmonary artery was dilated at 3.6 cm (mean of 2.42 cm with SD 0.22). She was diagnosed with atypical pneumonia. She was administered azithromycin, weaned off oxygen, and discharged after a seven-day hospitalization.
Two months prior, she had marked tachypnea, tachycardia, and hypoxemia, and imaging revealed diffuse ground-glass opacities. The differential diagnosis for this constellation of symptoms is extensive and includes many conditions that have an inflammatory component, such as atypical pneumonia caused by Mycoplasma or Chlamydia pneumoniae or a common respiratory virus such as rhinovirus or human metapneumovirus. However, two findings make an acute pneumonia unlikely to be the sole cause of her symptoms: underlying emphysema and an enlarged pulmonary artery. Emphysema is an uncommon finding in children and can be related to congenital or acquired causes; congenital lobar emphysema most often presents earlier in life and is focal, not diffuse. Alpha-1-anti-trypin deficiency and mutations in connective tissue genes such as those encoding for elastin and fibrillin can lead to pulmonary disease. While not diagnostic of pulmonary hypertension, her dilated pulmonary artery, coupled with her history, makes pulmonary hypertension a strong possibility. While her pulmonary hypertension is most likely secondary to chronic lung disease based on the emphysematous changes on CT, it could still be related to a cardiac etiology.
The patient had a history of seasonal allergies and well-controlled asthma. She was hospitalized at age six for an asthma exacerbation associated with a respiratory infection. She was discharged with an albuterol inhaler, but seldom used it. Her parents denied any regular coughing during the day or night. She was morbidly obese. Her tonsils and adenoids were removed to treat obstructive sleep apnea (OSA) at age seven, and a subsequent polysomnography was normal. Her medications included intranasal fluticasone propionate and oral iron supplementation. She had no known allergies or recent travels. She had never smoked. She had two pet cats and a dog. Her mother had a history of obesity, OSA, and eczema. Her father had diabetes and eczema.
The patient’s history prior to the recent few months sheds little light on the cause of her current symptoms. While it is possible that her current symptoms are related to the worsening of a process that had been present for many years which mimicked asthma, this seems implausible given the long period of time between her last asthma exacerbation and her present symptoms. Similarly, while tonsillar and adenoidal hypertrophy can be associated with infiltrative diseases (such as lymphoma), this is less common than the usual (and normal) disproportionate increase in size of the adenoids compared to other airway structures during growth in children.
She was admitted to the hospital. On initial examination, her temperature was 37.4°C, heart rate 125 beats per minute, blood pressure 143/69 mm Hg, respiratory rate 48 breaths per minute, and oxygen saturation 86% breathing ambient air. Her BMI was 58 kg/m2. Her exam demonstrated increased work of breathing with accessory muscle use, and decreased breath sounds at the bases. There were no wheezes or crackles. Cardiovascular, abdominal, and skin exams were normal except for tachycardia. At rest, later in the hospitalization, her oxygen saturation was 97% breathing ambient air and heart rate 110 bpm. After two minutes of walking, her oxygen saturation was 77% and heart rate 132 bpm. Two minutes after resting, her oxygen saturation increased to 91%.
Her white blood cell count was 11.9 x 10 9 /L (67% neutrophils, 24.2% lymphocytes, 6% monocytes, and 2% eosinophils), hemoglobin 11.2 g/dL, and platelet count 278,000/mm 3 . Her complete metabolic panel was normal. The C-reactive protein (CRP) was 24 mg/L (normal range, < 4.9) and erythrocyte sedimentation rate (ESR) 103 mm/hour (normal range, 0-32). A venous blood gas (VBG) showed a pH of 7.42 and pCO2 39. An EKG demonstrated sinus tachycardia.
The combination of the patient’s tachypnea, hypoxemia, respiratory distress, and obesity is striking. Her lack of adventitious lung sounds is surprising given her CT findings, but the sensitivity of chest auscultation may be limited in obese patients. Her laboratory findings help narrow the diagnostic frame: she has mild anemia and leukocytosis along with significant inflammation. The normal CO2 concentration on VBG is concerning given the degree of her tachypnea and reflects significant alveolar hypoventilation.
This marked inflammation with diffuse lung findings again raises the possibility of an inflammatory or, less likely, infectious disorder. Sjogren’s syndrome, systemic lupus erythematosus (SLE), and juvenile dermatomyositis can present in young women with interstitial lung disease. She does have exposure to pets and hypersensitivity pneumonitis can worsen rapidly with continued exposure. Another possibility is that she has an underlying immunodeficiency such as common variable immunodeficiency, although a history of recurrent infections such as pneumonia, bacteremia, or sinusitis is lacking.
An echocardiogram should be performed. In addition, laboratory evaluation for the aforementioned autoimmune causes of interstitial lung disease, immunoglobulin levels, pulmonary function testing (if available as an inpatient), and potentially a bronchoscopy with bronchoalveolar lavage (BAL), and biopsy should be pursued. The BAL and biopsy would be helpful in evaluating for infection and interstitial lung disease in an expeditious manner.
A chest CT without contrast was done and compared to the scan from two months prior. New diffuse, ill-defined centrilobular ground-glass opacities were evident throughout the lung fields; dilation of the main pulmonary artery was unchanged, and previously seen ground-glass opacities had resolved. There were patchy areas of air-trapping and mosaic attenuation in the lower lobes (Figure 2).
Transthoracic echocardiogram demonstrated a right ventricular systolic pressure of 58 mm Hg with flattened intraventricular septum during systole. Left and right ventricular systolic function were normal. The left ventricular diastolic function was normal. Pulmonary function testing demonstrated a FEV1/FVC ratio of 100 (112% predicted), FVC 1.07 L (35 % predicted) and FEV1 1.07 L (39% predicted), and total lung capacity was 2.7L (56% predicted) (Figure 3). Single-breath carbon monoxide uptake in the lung was not interpretable based on 2017 European Respiratory Society (ERS)/American Thoracic Society (ATS) technical standards.
This information is helpful in classifying whether this patient’s primary condition is cardiac or pulmonary in nature. Her normal left ventricular systolic and diastolic function make a cardiac etiology for her pulmonary hypertension less likely. Further, the combination of pulmonary hypertension, a restrictive pattern on pulmonary function testing, and findings consistent with interstitial lung disease on cross-sectional imaging all suggest a primary pulmonary etiology rather than a cardiac, infectious, or thromboembolic condition. While chronic thromboembolic hypertension can result in nonspecific mosaic attenuation, it typically would not cause centrilobular ground-glass opacities nor restrictive lung disease. Thus, it seems most likely that this patient has a progressive pulmonary process resulting in hypoxia, pulmonary hypertension, centrilobular opacities, and lower-lobe mosaic attenuation. Considerations for this process can be broadly categorized as one of the childhood interstitial lung disease (chILD). While this differential diagnosis is broad, strong consideration should be given to hypersensitivity pneumonitis, chronic aspiration, sarcoidosis, and Sjogren’s syndrome. An intriguing possibility is that the patient’s “response to azithromycin” two months prior was due to the avoidance of an inhaled antigen while she was in the hospital; a detailed environmental history should be explored. The normal polysomnography after tonsilloadenoidectomy makes it unlikely that OSA is a major contributor to her current presentation. However, since the surgery was seven years ago, and her BMI is presently 58 kg/m2 she remains at risk for OSA and obesity-hypoventilation syndrome. Polysomnography should be done after her acute symptoms improve.
She was started on 5 mm Hg of continuous positive airway pressure (CPAP) at night after a sleep study on room air demonstrated severe OSA with a respiratory disturbance index of 13 events per hour. Antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibody (ANCA), anti-Jo-1 antibody, anti-RNP antibody, anti-Smith antibody, anti-Ro/SSA and anti-La/SSB antibody were negative as was the histoplasmin antibody. Serum angiotensin-converting enzyme (ACE) level was normal. Mycoplasma IgM and IgG were negative. IgE was 529 kU/L (normal range, <114).
This evaluation reduces the likelihood the patient has Sjogren’s syndrome, SLE, dermatomyositis, or ANCA-associated pulmonary disease. While many patients with dermatomyositis may have negative serologic evaluations, other findings usually present such as rash and myositis are lacking. The negative ANCA evaluation makes granulomatosis with polyangiitis and microscopic polyangiitis very unlikely given the high sensitivity of the ANCA assay for these conditions. ANCA assays are less sensitive for eosinophilic granulomatosis with polyangiitis (EGPA), but the lack of eosinophilia significantly decreases the likelihood of EGPA. ACE levels have relatively poor operating characteristics in the evaluation of sarcoidosis; however, sarcoidosis seems unlikely in this case, especially as patients with sarcoidosis tend to have low or normal IgE levels. Patients with asthma can have elevated IgE levels. However, very elevated IgE levels are more common in other conditions, including allergic bronchopulmonary aspergillosis (ABPA) and the Hyper-IgE syndrome. The latter manifests with recurrent infections and eczema, and is inherited in an autosomal dominant manner. However, both the Hyper-IgE syndrome and ABPA have much higher IgE levels than seen in this case. Allergen-specific IgE testing (including for antibodies to Aspergillus) should be sent. It seems that an interstitial lung disease is present; the waxing and waning pattern and clinical presentation, along with the lack of other systemic findings, make hypersensitivity pneumonitis most likely.
The family lived in an apartment building. Her symptoms started when the family’s neighbor recently moved his outdoor pigeon coop into his basement. The patient often smelled the pigeons and noted feathers coming through the holes in the wall.
One of the key diagnostic features of hypersensitivity pneumonitis (HP) is the history of exposure to a potential offending antigen—in this case likely bird feathers—along with worsening upon reexposure to that antigen. HP is primarily a clinical diagnosis, and testing for serum precipitants has limited value, given the high false negative rate and the frequent lack of clinical symptoms accompanying positive testing. Bronchoalveolar lavage fluid may reveal lymphocytosis and reduced CD4:CD8 ratio. Crackles are commonly heard on examination, but in this case were likely not auscultated due to her obese habitus. The most important treatment is withdrawal of the offending antigen. Limited data suggest that corticosteroid therapy may be helpful in certain HP cases, including subacute, chronic and severe cases as well as patients with hypoxemia, significant imaging findings, and those with significant abnormalities on pulmonary function testing (PFT).
A hypersensitivity pneumonitis precipitins panel was sent with positive antibodies to M. faeni, T. Vulgaris, A. Fumigatus 1 and 6, A. Flavus, and pigeon serum. Her symptoms gradually improved within five days of oral prednisone (60 mg). She was discharged home without dyspnea and normal oxygen saturation while breathing ambient air. A repeat echocardiogram after nighttime CPAP for 1 week demonstrated a right ventricular systolic pressure of 17 mm Hg consistent with improved pulmonary hypertension.
Three weeks later, she returned to clinic for follow up. She had re-experienced dyspnea, cough, and wheezing, which improved when she was outdoors. She was afebrile, tachypneic, tachycardic, and her oxygen saturation was 92% on ambient air.
Her steroid-responsive interstitial lung disease and rapid improvement upon avoidance of the offending antigen is consistent with HP. The positive serum precipitins assay lends further credence to the diagnosis of HP, although serologic analysis with such antibody assays is limited by false positives and false negatives; further, individuals exposed to pigeons often have antibodies present without evidence of HP. History taking at this visit should ask specifically about further pigeon exposure: were the pigeons removed from the home completely, were heating-cooling filters changed, carpets cleaned, and bedding laundered? An in-home evaluation may be helpful before conducting further diagnostic testing.
She was admitted for oxygen therapy and a bronchoscopy, which showed mucosal friability and cobblestoning, suggesting inflammation. BAL revealed a normal CD4:CD8 ratio of 3; BAL cultures were sterile. Her shortness of breath significantly improved following a prolonged course of systemic steroids and removal from the triggering environment. PFTs improved with a FEV1/FVC ratio of 94 (105% predicted), FVC of 2.00 L (66% predicted), FEV1 of 1.88L (69% predicted) (Figure 3B). Her presenting symptoms of persistent cough and progressive dyspnea on exertion, characteristic CT, sterile BAL cultures, positive serum precipitants against pigeon serum, and resolution of her symptoms with withdrawal of the offending antigen were diagnostic of hypersensitivity pneumonitis due to pigeon exposure, also known as bird fancier’s disease.
COMMENTARY
The patient’s original presentation of dyspnea, tachypnea, and hypoxia is commonly associated with pediatric pneumonia and asthma exacerbations.1 However, an alternative diagnosis was suggested by the lack of wheezing, absence of fever, and recurrent presentations with progressive symptoms.
Hypersensitivity pneumonitis (HP) represents an exaggerated T-cell meditated immune response to inhalation of an offending antigen that results in a restrictive ventilatory defect and interstitial infiltrates.2 Bird pneumonitis (also known as bird fancier’s disease) is a frequent cause of HP, accounting for approximately 65-70% of cases.3 HP, however, only manifests in a small number of subjects exposed to culprit antigens, suggesting an underlying genetic susceptibility.4 Prevalence estimates vary depending on bird species, county, climate, and other possible factors.
There are no standard criteria for the diagnosis of HP, though a combination of findings is suggestive. A recent prospective multicenter study created a scoring system for HP based on factors associated with the disease to aid in accurate diagnosis. The most relevant criteria included antigen exposure, recurrent symptoms noted within 4-8 hours after antigen exposure, weight loss, presence of specific IgG antibodies to avian antigens, and inspiratory crackles on exam. Using this rule, the probability that our patient has HP based on clinical characteristics was 93% with an area under the receiver operating curve of 0.93 (96% confidence interval: 0.90-0.95)5. Chest imaging (high resolution CT) often consists of a mosaic pattern of air trapping, as seen in this patient in combination with ground-glass opacities6. Bronchoalveolar lavage (BAL) is sensitive in detecting lung inflammation in a patient with suspected HP. On BAL, a lymphocytic alveolitis can be seen, but absence of this finding does not exclude HP.5,7,8 Pulmonary function tests (PFTs) may be normal in acute HP. When abnormal, PFTs may reveal a restrictive pattern and reduction in carbon monoxide diffusing capacity.7 However, BAL and PFT results are neither specific nor diagnostic of HP; it is important to consider results in the context of the clinical picture.
The respiratory response to inhalation of the avian antigen has traditionally been classified as acute, subacute, or chronic.9 The acute response occurs within hours of exposure to the offending agent and usually resolves within 24 hours after antigen withdrawal. The subacute presentation involves cough and dyspnea over several days to weeks, and can progress to chronic and permanent lung damage if unrecognized and untreated. In chronic presentations, lung abnormalities may persist despite antigen avoidance and pharmacologic interventions.4,10 The patient’s symptoms occurred over a six-month period which coincided with pigeon exposure and resolved during each hospitalization with steroid treatment and removal from the offending agent. Her presentation was consistent with a subacute time course of HP.
The dilated pulmonary artery, elevated right systolic ventricular pressure, and normal right ventricular function in our patient suggested pulmonary hypertension of chronic duration. Her risk factors for pulmonary hypertension included asthma, sleep apnea, possible obesity-hypoventilation syndrome, and HP-associated interstitial lung disease.11
The most important intervention in HP is avoidance of the causative antigen. Medical therapy without removal of antigen is inadequate. Systemic corticosteroids can help ameliorate acute symptoms though dosing and duration remains unclear. For chronic patients unresponsive to steroid therapy, lung transplantation can be considered.4
The key to diagnosis of HP in this patient—and to minimizing repeat testing upon the patient’s recrudescence of symptoms—was the clinician’s consideration that the major impetus for the patient’s improvement in the hospital was removal from the offending antigen in her home environment. As in this case, taking time to delve deeply into a patient’s environment—even by descending the basement stairs—may lead to the diagnosis.
LEARNING POINTS
- Consider hypersensitivity pneumonitis (HP) in patients with recurrent respiratory distress, offending exposure, and resolution of symptoms with removal of culprit antigen.
- The most important treatment of HP is removal of offending antigen; systemic and/or inhaled corticosteroids are indicated until the full resolution of respiratory symptoms.
- Prognosis is dependent on early diagnosis and removal of offending exposures.
- Failure to treat HP might result in end-stage lung disease from pulmonary fibrosis secondary to long-term inflammation.
Disclosures
Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME). The authors declare no conflicts of interests.
1. Ebell MH. Clinical diagnosis of pneumonia in children. Am Fam Physician. 2010;82(2):192-193. PubMed
2. Cormier Y, Lacasse Y. Hypersensitivity pneumonitis and organic dust toxic syndrome. In: Malo J-L, Chan-Yeung M, Bernstein DI, eds. Asthma in the Workplace. Vol 32. Boca Raton, FL: Fourth Informa Healthcare; 2013:392-405.
3. Chan AL, Juarez MM, Leslie KO, Ismail HA, Albertson TE. Bird fancier’s lung: a state-of-the-art review. Clin Rev Allergy Immunol. 2012;43(1-2):69-83. doi: 10.1007/s12016-011-8282-y. PubMed
4. Camarena A, Juárez A, Mejía M, et al. Major histocompatibility complex and tumor necrosis factor-α polymorphisms in pigeon breeder’s disease. Am J Respir Crit Care Med. 2001;163(7):1528-1533. https:/doi.org/10.1164/ajrccm.163.7.2004023. PubMed
5. Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168(8):952-958. doi: 10.1164/rccm.200301-137OC. PubMed
6. Glazer CS, Rose CS, Lynch DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging. 2002;17(4):261-272. PubMed
7. Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314-324. doi: 10.1164/rccm.201203-0513CI. PubMed
8. Calillad DM, Vergnon, JM, Madroszyk A, et al. Bronchoalveolar lavage in hypersensitivity pneumonitis: a series of 139 patients. Inflamm Allergy Drug Targets. 2012;11(1):15-19. doi: 10.2174/187152812798889330. PubMed
9. Richerson HB, Bernstein IL, Fink JN, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol. 1989;84(5 Pt 2):839-844. doi: 10.1016/0091-6749(89)90349-7. PubMed
10. Zacharisen MC, Schlueter DP, Kurup VP, Fink JN. The long-term outcome in acute, subacute, and chronic forms of pigeon breeder’s disease hypersensitivity pneumonitis. Ann Allergy Asthma Immunol. 2002;88(2):175-182. doi: 10.1016/S1081-1206(10)61993-X. PubMed
11. Raymond TE, Khabbaza JE, Yadav R, Tonelli AR. Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc. 2014;11(10):1623-1632. doi: 10.1513/AnnalsATS.201406-253PP. PubMed
A 14-year-old girl with a history of asthma presented to the Emergency Department (ED) with three months of persistent, nonproductive cough, and progressive shortness of breath. She reported fatigue, chest tightness, orthopnea, and dyspnea with exertion. She denied fever, rhinorrhea, congestion, hemoptysis, or paroxysmal nocturnal dyspnea.
Her age and past medical history of asthma are incongruent with her new symptoms, as asthma is typified by intermittent exacerbations, not progressive symptoms. Thus, another process, in addition to asthma, is most likely present; it is also important to question the accuracy of previous diagnoses in light of new information. Her symptoms may signify an underlying cardiopulmonary process, such as infiltrative diseases (eg, lymphoma or sarcoidosis), atypical infections, genetic conditions (eg, variant cystic fibrosis), autoimmune conditions, or cardiomyopathy. A detailed symptom history, family history, and careful physical examination will help expand and then refine the differential diagnosis. At this stage, typical infections are less likely.
She had presented two months prior with nonproductive cough and dyspnea. At that presentation, her temperature was 36.3°C, heart rate 110 beats per minute, blood pressure 119/63 mm Hg, respiratory rate 43 breaths per minute, and oxygen saturation 86% while breathing ambient air. A chest CT with contrast demonstrated diffuse patchy multifocal ground-glass opacities in the bilateral lungs as well as a mixture of atelectasis and lobular emphysema in the dependent lobes bilaterally (Figure 1). Her main pulmonary artery was dilated at 3.6 cm (mean of 2.42 cm with SD 0.22). She was diagnosed with atypical pneumonia. She was administered azithromycin, weaned off oxygen, and discharged after a seven-day hospitalization.
Two months prior, she had marked tachypnea, tachycardia, and hypoxemia, and imaging revealed diffuse ground-glass opacities. The differential diagnosis for this constellation of symptoms is extensive and includes many conditions that have an inflammatory component, such as atypical pneumonia caused by Mycoplasma or Chlamydia pneumoniae or a common respiratory virus such as rhinovirus or human metapneumovirus. However, two findings make an acute pneumonia unlikely to be the sole cause of her symptoms: underlying emphysema and an enlarged pulmonary artery. Emphysema is an uncommon finding in children and can be related to congenital or acquired causes; congenital lobar emphysema most often presents earlier in life and is focal, not diffuse. Alpha-1-anti-trypin deficiency and mutations in connective tissue genes such as those encoding for elastin and fibrillin can lead to pulmonary disease. While not diagnostic of pulmonary hypertension, her dilated pulmonary artery, coupled with her history, makes pulmonary hypertension a strong possibility. While her pulmonary hypertension is most likely secondary to chronic lung disease based on the emphysematous changes on CT, it could still be related to a cardiac etiology.
The patient had a history of seasonal allergies and well-controlled asthma. She was hospitalized at age six for an asthma exacerbation associated with a respiratory infection. She was discharged with an albuterol inhaler, but seldom used it. Her parents denied any regular coughing during the day or night. She was morbidly obese. Her tonsils and adenoids were removed to treat obstructive sleep apnea (OSA) at age seven, and a subsequent polysomnography was normal. Her medications included intranasal fluticasone propionate and oral iron supplementation. She had no known allergies or recent travels. She had never smoked. She had two pet cats and a dog. Her mother had a history of obesity, OSA, and eczema. Her father had diabetes and eczema.
The patient’s history prior to the recent few months sheds little light on the cause of her current symptoms. While it is possible that her current symptoms are related to the worsening of a process that had been present for many years which mimicked asthma, this seems implausible given the long period of time between her last asthma exacerbation and her present symptoms. Similarly, while tonsillar and adenoidal hypertrophy can be associated with infiltrative diseases (such as lymphoma), this is less common than the usual (and normal) disproportionate increase in size of the adenoids compared to other airway structures during growth in children.
She was admitted to the hospital. On initial examination, her temperature was 37.4°C, heart rate 125 beats per minute, blood pressure 143/69 mm Hg, respiratory rate 48 breaths per minute, and oxygen saturation 86% breathing ambient air. Her BMI was 58 kg/m2. Her exam demonstrated increased work of breathing with accessory muscle use, and decreased breath sounds at the bases. There were no wheezes or crackles. Cardiovascular, abdominal, and skin exams were normal except for tachycardia. At rest, later in the hospitalization, her oxygen saturation was 97% breathing ambient air and heart rate 110 bpm. After two minutes of walking, her oxygen saturation was 77% and heart rate 132 bpm. Two minutes after resting, her oxygen saturation increased to 91%.
Her white blood cell count was 11.9 x 10 9 /L (67% neutrophils, 24.2% lymphocytes, 6% monocytes, and 2% eosinophils), hemoglobin 11.2 g/dL, and platelet count 278,000/mm 3 . Her complete metabolic panel was normal. The C-reactive protein (CRP) was 24 mg/L (normal range, < 4.9) and erythrocyte sedimentation rate (ESR) 103 mm/hour (normal range, 0-32). A venous blood gas (VBG) showed a pH of 7.42 and pCO2 39. An EKG demonstrated sinus tachycardia.
The combination of the patient’s tachypnea, hypoxemia, respiratory distress, and obesity is striking. Her lack of adventitious lung sounds is surprising given her CT findings, but the sensitivity of chest auscultation may be limited in obese patients. Her laboratory findings help narrow the diagnostic frame: she has mild anemia and leukocytosis along with significant inflammation. The normal CO2 concentration on VBG is concerning given the degree of her tachypnea and reflects significant alveolar hypoventilation.
This marked inflammation with diffuse lung findings again raises the possibility of an inflammatory or, less likely, infectious disorder. Sjogren’s syndrome, systemic lupus erythematosus (SLE), and juvenile dermatomyositis can present in young women with interstitial lung disease. She does have exposure to pets and hypersensitivity pneumonitis can worsen rapidly with continued exposure. Another possibility is that she has an underlying immunodeficiency such as common variable immunodeficiency, although a history of recurrent infections such as pneumonia, bacteremia, or sinusitis is lacking.
An echocardiogram should be performed. In addition, laboratory evaluation for the aforementioned autoimmune causes of interstitial lung disease, immunoglobulin levels, pulmonary function testing (if available as an inpatient), and potentially a bronchoscopy with bronchoalveolar lavage (BAL), and biopsy should be pursued. The BAL and biopsy would be helpful in evaluating for infection and interstitial lung disease in an expeditious manner.
A chest CT without contrast was done and compared to the scan from two months prior. New diffuse, ill-defined centrilobular ground-glass opacities were evident throughout the lung fields; dilation of the main pulmonary artery was unchanged, and previously seen ground-glass opacities had resolved. There were patchy areas of air-trapping and mosaic attenuation in the lower lobes (Figure 2).
Transthoracic echocardiogram demonstrated a right ventricular systolic pressure of 58 mm Hg with flattened intraventricular septum during systole. Left and right ventricular systolic function were normal. The left ventricular diastolic function was normal. Pulmonary function testing demonstrated a FEV1/FVC ratio of 100 (112% predicted), FVC 1.07 L (35 % predicted) and FEV1 1.07 L (39% predicted), and total lung capacity was 2.7L (56% predicted) (Figure 3). Single-breath carbon monoxide uptake in the lung was not interpretable based on 2017 European Respiratory Society (ERS)/American Thoracic Society (ATS) technical standards.
This information is helpful in classifying whether this patient’s primary condition is cardiac or pulmonary in nature. Her normal left ventricular systolic and diastolic function make a cardiac etiology for her pulmonary hypertension less likely. Further, the combination of pulmonary hypertension, a restrictive pattern on pulmonary function testing, and findings consistent with interstitial lung disease on cross-sectional imaging all suggest a primary pulmonary etiology rather than a cardiac, infectious, or thromboembolic condition. While chronic thromboembolic hypertension can result in nonspecific mosaic attenuation, it typically would not cause centrilobular ground-glass opacities nor restrictive lung disease. Thus, it seems most likely that this patient has a progressive pulmonary process resulting in hypoxia, pulmonary hypertension, centrilobular opacities, and lower-lobe mosaic attenuation. Considerations for this process can be broadly categorized as one of the childhood interstitial lung disease (chILD). While this differential diagnosis is broad, strong consideration should be given to hypersensitivity pneumonitis, chronic aspiration, sarcoidosis, and Sjogren’s syndrome. An intriguing possibility is that the patient’s “response to azithromycin” two months prior was due to the avoidance of an inhaled antigen while she was in the hospital; a detailed environmental history should be explored. The normal polysomnography after tonsilloadenoidectomy makes it unlikely that OSA is a major contributor to her current presentation. However, since the surgery was seven years ago, and her BMI is presently 58 kg/m2 she remains at risk for OSA and obesity-hypoventilation syndrome. Polysomnography should be done after her acute symptoms improve.
She was started on 5 mm Hg of continuous positive airway pressure (CPAP) at night after a sleep study on room air demonstrated severe OSA with a respiratory disturbance index of 13 events per hour. Antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibody (ANCA), anti-Jo-1 antibody, anti-RNP antibody, anti-Smith antibody, anti-Ro/SSA and anti-La/SSB antibody were negative as was the histoplasmin antibody. Serum angiotensin-converting enzyme (ACE) level was normal. Mycoplasma IgM and IgG were negative. IgE was 529 kU/L (normal range, <114).
This evaluation reduces the likelihood the patient has Sjogren’s syndrome, SLE, dermatomyositis, or ANCA-associated pulmonary disease. While many patients with dermatomyositis may have negative serologic evaluations, other findings usually present such as rash and myositis are lacking. The negative ANCA evaluation makes granulomatosis with polyangiitis and microscopic polyangiitis very unlikely given the high sensitivity of the ANCA assay for these conditions. ANCA assays are less sensitive for eosinophilic granulomatosis with polyangiitis (EGPA), but the lack of eosinophilia significantly decreases the likelihood of EGPA. ACE levels have relatively poor operating characteristics in the evaluation of sarcoidosis; however, sarcoidosis seems unlikely in this case, especially as patients with sarcoidosis tend to have low or normal IgE levels. Patients with asthma can have elevated IgE levels. However, very elevated IgE levels are more common in other conditions, including allergic bronchopulmonary aspergillosis (ABPA) and the Hyper-IgE syndrome. The latter manifests with recurrent infections and eczema, and is inherited in an autosomal dominant manner. However, both the Hyper-IgE syndrome and ABPA have much higher IgE levels than seen in this case. Allergen-specific IgE testing (including for antibodies to Aspergillus) should be sent. It seems that an interstitial lung disease is present; the waxing and waning pattern and clinical presentation, along with the lack of other systemic findings, make hypersensitivity pneumonitis most likely.
The family lived in an apartment building. Her symptoms started when the family’s neighbor recently moved his outdoor pigeon coop into his basement. The patient often smelled the pigeons and noted feathers coming through the holes in the wall.
One of the key diagnostic features of hypersensitivity pneumonitis (HP) is the history of exposure to a potential offending antigen—in this case likely bird feathers—along with worsening upon reexposure to that antigen. HP is primarily a clinical diagnosis, and testing for serum precipitants has limited value, given the high false negative rate and the frequent lack of clinical symptoms accompanying positive testing. Bronchoalveolar lavage fluid may reveal lymphocytosis and reduced CD4:CD8 ratio. Crackles are commonly heard on examination, but in this case were likely not auscultated due to her obese habitus. The most important treatment is withdrawal of the offending antigen. Limited data suggest that corticosteroid therapy may be helpful in certain HP cases, including subacute, chronic and severe cases as well as patients with hypoxemia, significant imaging findings, and those with significant abnormalities on pulmonary function testing (PFT).
A hypersensitivity pneumonitis precipitins panel was sent with positive antibodies to M. faeni, T. Vulgaris, A. Fumigatus 1 and 6, A. Flavus, and pigeon serum. Her symptoms gradually improved within five days of oral prednisone (60 mg). She was discharged home without dyspnea and normal oxygen saturation while breathing ambient air. A repeat echocardiogram after nighttime CPAP for 1 week demonstrated a right ventricular systolic pressure of 17 mm Hg consistent with improved pulmonary hypertension.
Three weeks later, she returned to clinic for follow up. She had re-experienced dyspnea, cough, and wheezing, which improved when she was outdoors. She was afebrile, tachypneic, tachycardic, and her oxygen saturation was 92% on ambient air.
Her steroid-responsive interstitial lung disease and rapid improvement upon avoidance of the offending antigen is consistent with HP. The positive serum precipitins assay lends further credence to the diagnosis of HP, although serologic analysis with such antibody assays is limited by false positives and false negatives; further, individuals exposed to pigeons often have antibodies present without evidence of HP. History taking at this visit should ask specifically about further pigeon exposure: were the pigeons removed from the home completely, were heating-cooling filters changed, carpets cleaned, and bedding laundered? An in-home evaluation may be helpful before conducting further diagnostic testing.
She was admitted for oxygen therapy and a bronchoscopy, which showed mucosal friability and cobblestoning, suggesting inflammation. BAL revealed a normal CD4:CD8 ratio of 3; BAL cultures were sterile. Her shortness of breath significantly improved following a prolonged course of systemic steroids and removal from the triggering environment. PFTs improved with a FEV1/FVC ratio of 94 (105% predicted), FVC of 2.00 L (66% predicted), FEV1 of 1.88L (69% predicted) (Figure 3B). Her presenting symptoms of persistent cough and progressive dyspnea on exertion, characteristic CT, sterile BAL cultures, positive serum precipitants against pigeon serum, and resolution of her symptoms with withdrawal of the offending antigen were diagnostic of hypersensitivity pneumonitis due to pigeon exposure, also known as bird fancier’s disease.
COMMENTARY
The patient’s original presentation of dyspnea, tachypnea, and hypoxia is commonly associated with pediatric pneumonia and asthma exacerbations.1 However, an alternative diagnosis was suggested by the lack of wheezing, absence of fever, and recurrent presentations with progressive symptoms.
Hypersensitivity pneumonitis (HP) represents an exaggerated T-cell meditated immune response to inhalation of an offending antigen that results in a restrictive ventilatory defect and interstitial infiltrates.2 Bird pneumonitis (also known as bird fancier’s disease) is a frequent cause of HP, accounting for approximately 65-70% of cases.3 HP, however, only manifests in a small number of subjects exposed to culprit antigens, suggesting an underlying genetic susceptibility.4 Prevalence estimates vary depending on bird species, county, climate, and other possible factors.
There are no standard criteria for the diagnosis of HP, though a combination of findings is suggestive. A recent prospective multicenter study created a scoring system for HP based on factors associated with the disease to aid in accurate diagnosis. The most relevant criteria included antigen exposure, recurrent symptoms noted within 4-8 hours after antigen exposure, weight loss, presence of specific IgG antibodies to avian antigens, and inspiratory crackles on exam. Using this rule, the probability that our patient has HP based on clinical characteristics was 93% with an area under the receiver operating curve of 0.93 (96% confidence interval: 0.90-0.95)5. Chest imaging (high resolution CT) often consists of a mosaic pattern of air trapping, as seen in this patient in combination with ground-glass opacities6. Bronchoalveolar lavage (BAL) is sensitive in detecting lung inflammation in a patient with suspected HP. On BAL, a lymphocytic alveolitis can be seen, but absence of this finding does not exclude HP.5,7,8 Pulmonary function tests (PFTs) may be normal in acute HP. When abnormal, PFTs may reveal a restrictive pattern and reduction in carbon monoxide diffusing capacity.7 However, BAL and PFT results are neither specific nor diagnostic of HP; it is important to consider results in the context of the clinical picture.
The respiratory response to inhalation of the avian antigen has traditionally been classified as acute, subacute, or chronic.9 The acute response occurs within hours of exposure to the offending agent and usually resolves within 24 hours after antigen withdrawal. The subacute presentation involves cough and dyspnea over several days to weeks, and can progress to chronic and permanent lung damage if unrecognized and untreated. In chronic presentations, lung abnormalities may persist despite antigen avoidance and pharmacologic interventions.4,10 The patient’s symptoms occurred over a six-month period which coincided with pigeon exposure and resolved during each hospitalization with steroid treatment and removal from the offending agent. Her presentation was consistent with a subacute time course of HP.
The dilated pulmonary artery, elevated right systolic ventricular pressure, and normal right ventricular function in our patient suggested pulmonary hypertension of chronic duration. Her risk factors for pulmonary hypertension included asthma, sleep apnea, possible obesity-hypoventilation syndrome, and HP-associated interstitial lung disease.11
The most important intervention in HP is avoidance of the causative antigen. Medical therapy without removal of antigen is inadequate. Systemic corticosteroids can help ameliorate acute symptoms though dosing and duration remains unclear. For chronic patients unresponsive to steroid therapy, lung transplantation can be considered.4
The key to diagnosis of HP in this patient—and to minimizing repeat testing upon the patient’s recrudescence of symptoms—was the clinician’s consideration that the major impetus for the patient’s improvement in the hospital was removal from the offending antigen in her home environment. As in this case, taking time to delve deeply into a patient’s environment—even by descending the basement stairs—may lead to the diagnosis.
LEARNING POINTS
- Consider hypersensitivity pneumonitis (HP) in patients with recurrent respiratory distress, offending exposure, and resolution of symptoms with removal of culprit antigen.
- The most important treatment of HP is removal of offending antigen; systemic and/or inhaled corticosteroids are indicated until the full resolution of respiratory symptoms.
- Prognosis is dependent on early diagnosis and removal of offending exposures.
- Failure to treat HP might result in end-stage lung disease from pulmonary fibrosis secondary to long-term inflammation.
Disclosures
Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME). The authors declare no conflicts of interests.
A 14-year-old girl with a history of asthma presented to the Emergency Department (ED) with three months of persistent, nonproductive cough, and progressive shortness of breath. She reported fatigue, chest tightness, orthopnea, and dyspnea with exertion. She denied fever, rhinorrhea, congestion, hemoptysis, or paroxysmal nocturnal dyspnea.
Her age and past medical history of asthma are incongruent with her new symptoms, as asthma is typified by intermittent exacerbations, not progressive symptoms. Thus, another process, in addition to asthma, is most likely present; it is also important to question the accuracy of previous diagnoses in light of new information. Her symptoms may signify an underlying cardiopulmonary process, such as infiltrative diseases (eg, lymphoma or sarcoidosis), atypical infections, genetic conditions (eg, variant cystic fibrosis), autoimmune conditions, or cardiomyopathy. A detailed symptom history, family history, and careful physical examination will help expand and then refine the differential diagnosis. At this stage, typical infections are less likely.
She had presented two months prior with nonproductive cough and dyspnea. At that presentation, her temperature was 36.3°C, heart rate 110 beats per minute, blood pressure 119/63 mm Hg, respiratory rate 43 breaths per minute, and oxygen saturation 86% while breathing ambient air. A chest CT with contrast demonstrated diffuse patchy multifocal ground-glass opacities in the bilateral lungs as well as a mixture of atelectasis and lobular emphysema in the dependent lobes bilaterally (Figure 1). Her main pulmonary artery was dilated at 3.6 cm (mean of 2.42 cm with SD 0.22). She was diagnosed with atypical pneumonia. She was administered azithromycin, weaned off oxygen, and discharged after a seven-day hospitalization.
Two months prior, she had marked tachypnea, tachycardia, and hypoxemia, and imaging revealed diffuse ground-glass opacities. The differential diagnosis for this constellation of symptoms is extensive and includes many conditions that have an inflammatory component, such as atypical pneumonia caused by Mycoplasma or Chlamydia pneumoniae or a common respiratory virus such as rhinovirus or human metapneumovirus. However, two findings make an acute pneumonia unlikely to be the sole cause of her symptoms: underlying emphysema and an enlarged pulmonary artery. Emphysema is an uncommon finding in children and can be related to congenital or acquired causes; congenital lobar emphysema most often presents earlier in life and is focal, not diffuse. Alpha-1-anti-trypin deficiency and mutations in connective tissue genes such as those encoding for elastin and fibrillin can lead to pulmonary disease. While not diagnostic of pulmonary hypertension, her dilated pulmonary artery, coupled with her history, makes pulmonary hypertension a strong possibility. While her pulmonary hypertension is most likely secondary to chronic lung disease based on the emphysematous changes on CT, it could still be related to a cardiac etiology.
The patient had a history of seasonal allergies and well-controlled asthma. She was hospitalized at age six for an asthma exacerbation associated with a respiratory infection. She was discharged with an albuterol inhaler, but seldom used it. Her parents denied any regular coughing during the day or night. She was morbidly obese. Her tonsils and adenoids were removed to treat obstructive sleep apnea (OSA) at age seven, and a subsequent polysomnography was normal. Her medications included intranasal fluticasone propionate and oral iron supplementation. She had no known allergies or recent travels. She had never smoked. She had two pet cats and a dog. Her mother had a history of obesity, OSA, and eczema. Her father had diabetes and eczema.
The patient’s history prior to the recent few months sheds little light on the cause of her current symptoms. While it is possible that her current symptoms are related to the worsening of a process that had been present for many years which mimicked asthma, this seems implausible given the long period of time between her last asthma exacerbation and her present symptoms. Similarly, while tonsillar and adenoidal hypertrophy can be associated with infiltrative diseases (such as lymphoma), this is less common than the usual (and normal) disproportionate increase in size of the adenoids compared to other airway structures during growth in children.
She was admitted to the hospital. On initial examination, her temperature was 37.4°C, heart rate 125 beats per minute, blood pressure 143/69 mm Hg, respiratory rate 48 breaths per minute, and oxygen saturation 86% breathing ambient air. Her BMI was 58 kg/m2. Her exam demonstrated increased work of breathing with accessory muscle use, and decreased breath sounds at the bases. There were no wheezes or crackles. Cardiovascular, abdominal, and skin exams were normal except for tachycardia. At rest, later in the hospitalization, her oxygen saturation was 97% breathing ambient air and heart rate 110 bpm. After two minutes of walking, her oxygen saturation was 77% and heart rate 132 bpm. Two minutes after resting, her oxygen saturation increased to 91%.
Her white blood cell count was 11.9 x 10 9 /L (67% neutrophils, 24.2% lymphocytes, 6% monocytes, and 2% eosinophils), hemoglobin 11.2 g/dL, and platelet count 278,000/mm 3 . Her complete metabolic panel was normal. The C-reactive protein (CRP) was 24 mg/L (normal range, < 4.9) and erythrocyte sedimentation rate (ESR) 103 mm/hour (normal range, 0-32). A venous blood gas (VBG) showed a pH of 7.42 and pCO2 39. An EKG demonstrated sinus tachycardia.
The combination of the patient’s tachypnea, hypoxemia, respiratory distress, and obesity is striking. Her lack of adventitious lung sounds is surprising given her CT findings, but the sensitivity of chest auscultation may be limited in obese patients. Her laboratory findings help narrow the diagnostic frame: she has mild anemia and leukocytosis along with significant inflammation. The normal CO2 concentration on VBG is concerning given the degree of her tachypnea and reflects significant alveolar hypoventilation.
This marked inflammation with diffuse lung findings again raises the possibility of an inflammatory or, less likely, infectious disorder. Sjogren’s syndrome, systemic lupus erythematosus (SLE), and juvenile dermatomyositis can present in young women with interstitial lung disease. She does have exposure to pets and hypersensitivity pneumonitis can worsen rapidly with continued exposure. Another possibility is that she has an underlying immunodeficiency such as common variable immunodeficiency, although a history of recurrent infections such as pneumonia, bacteremia, or sinusitis is lacking.
An echocardiogram should be performed. In addition, laboratory evaluation for the aforementioned autoimmune causes of interstitial lung disease, immunoglobulin levels, pulmonary function testing (if available as an inpatient), and potentially a bronchoscopy with bronchoalveolar lavage (BAL), and biopsy should be pursued. The BAL and biopsy would be helpful in evaluating for infection and interstitial lung disease in an expeditious manner.
A chest CT without contrast was done and compared to the scan from two months prior. New diffuse, ill-defined centrilobular ground-glass opacities were evident throughout the lung fields; dilation of the main pulmonary artery was unchanged, and previously seen ground-glass opacities had resolved. There were patchy areas of air-trapping and mosaic attenuation in the lower lobes (Figure 2).
Transthoracic echocardiogram demonstrated a right ventricular systolic pressure of 58 mm Hg with flattened intraventricular septum during systole. Left and right ventricular systolic function were normal. The left ventricular diastolic function was normal. Pulmonary function testing demonstrated a FEV1/FVC ratio of 100 (112% predicted), FVC 1.07 L (35 % predicted) and FEV1 1.07 L (39% predicted), and total lung capacity was 2.7L (56% predicted) (Figure 3). Single-breath carbon monoxide uptake in the lung was not interpretable based on 2017 European Respiratory Society (ERS)/American Thoracic Society (ATS) technical standards.
This information is helpful in classifying whether this patient’s primary condition is cardiac or pulmonary in nature. Her normal left ventricular systolic and diastolic function make a cardiac etiology for her pulmonary hypertension less likely. Further, the combination of pulmonary hypertension, a restrictive pattern on pulmonary function testing, and findings consistent with interstitial lung disease on cross-sectional imaging all suggest a primary pulmonary etiology rather than a cardiac, infectious, or thromboembolic condition. While chronic thromboembolic hypertension can result in nonspecific mosaic attenuation, it typically would not cause centrilobular ground-glass opacities nor restrictive lung disease. Thus, it seems most likely that this patient has a progressive pulmonary process resulting in hypoxia, pulmonary hypertension, centrilobular opacities, and lower-lobe mosaic attenuation. Considerations for this process can be broadly categorized as one of the childhood interstitial lung disease (chILD). While this differential diagnosis is broad, strong consideration should be given to hypersensitivity pneumonitis, chronic aspiration, sarcoidosis, and Sjogren’s syndrome. An intriguing possibility is that the patient’s “response to azithromycin” two months prior was due to the avoidance of an inhaled antigen while she was in the hospital; a detailed environmental history should be explored. The normal polysomnography after tonsilloadenoidectomy makes it unlikely that OSA is a major contributor to her current presentation. However, since the surgery was seven years ago, and her BMI is presently 58 kg/m2 she remains at risk for OSA and obesity-hypoventilation syndrome. Polysomnography should be done after her acute symptoms improve.
She was started on 5 mm Hg of continuous positive airway pressure (CPAP) at night after a sleep study on room air demonstrated severe OSA with a respiratory disturbance index of 13 events per hour. Antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibody (ANCA), anti-Jo-1 antibody, anti-RNP antibody, anti-Smith antibody, anti-Ro/SSA and anti-La/SSB antibody were negative as was the histoplasmin antibody. Serum angiotensin-converting enzyme (ACE) level was normal. Mycoplasma IgM and IgG were negative. IgE was 529 kU/L (normal range, <114).
This evaluation reduces the likelihood the patient has Sjogren’s syndrome, SLE, dermatomyositis, or ANCA-associated pulmonary disease. While many patients with dermatomyositis may have negative serologic evaluations, other findings usually present such as rash and myositis are lacking. The negative ANCA evaluation makes granulomatosis with polyangiitis and microscopic polyangiitis very unlikely given the high sensitivity of the ANCA assay for these conditions. ANCA assays are less sensitive for eosinophilic granulomatosis with polyangiitis (EGPA), but the lack of eosinophilia significantly decreases the likelihood of EGPA. ACE levels have relatively poor operating characteristics in the evaluation of sarcoidosis; however, sarcoidosis seems unlikely in this case, especially as patients with sarcoidosis tend to have low or normal IgE levels. Patients with asthma can have elevated IgE levels. However, very elevated IgE levels are more common in other conditions, including allergic bronchopulmonary aspergillosis (ABPA) and the Hyper-IgE syndrome. The latter manifests with recurrent infections and eczema, and is inherited in an autosomal dominant manner. However, both the Hyper-IgE syndrome and ABPA have much higher IgE levels than seen in this case. Allergen-specific IgE testing (including for antibodies to Aspergillus) should be sent. It seems that an interstitial lung disease is present; the waxing and waning pattern and clinical presentation, along with the lack of other systemic findings, make hypersensitivity pneumonitis most likely.
The family lived in an apartment building. Her symptoms started when the family’s neighbor recently moved his outdoor pigeon coop into his basement. The patient often smelled the pigeons and noted feathers coming through the holes in the wall.
One of the key diagnostic features of hypersensitivity pneumonitis (HP) is the history of exposure to a potential offending antigen—in this case likely bird feathers—along with worsening upon reexposure to that antigen. HP is primarily a clinical diagnosis, and testing for serum precipitants has limited value, given the high false negative rate and the frequent lack of clinical symptoms accompanying positive testing. Bronchoalveolar lavage fluid may reveal lymphocytosis and reduced CD4:CD8 ratio. Crackles are commonly heard on examination, but in this case were likely not auscultated due to her obese habitus. The most important treatment is withdrawal of the offending antigen. Limited data suggest that corticosteroid therapy may be helpful in certain HP cases, including subacute, chronic and severe cases as well as patients with hypoxemia, significant imaging findings, and those with significant abnormalities on pulmonary function testing (PFT).
A hypersensitivity pneumonitis precipitins panel was sent with positive antibodies to M. faeni, T. Vulgaris, A. Fumigatus 1 and 6, A. Flavus, and pigeon serum. Her symptoms gradually improved within five days of oral prednisone (60 mg). She was discharged home without dyspnea and normal oxygen saturation while breathing ambient air. A repeat echocardiogram after nighttime CPAP for 1 week demonstrated a right ventricular systolic pressure of 17 mm Hg consistent with improved pulmonary hypertension.
Three weeks later, she returned to clinic for follow up. She had re-experienced dyspnea, cough, and wheezing, which improved when she was outdoors. She was afebrile, tachypneic, tachycardic, and her oxygen saturation was 92% on ambient air.
Her steroid-responsive interstitial lung disease and rapid improvement upon avoidance of the offending antigen is consistent with HP. The positive serum precipitins assay lends further credence to the diagnosis of HP, although serologic analysis with such antibody assays is limited by false positives and false negatives; further, individuals exposed to pigeons often have antibodies present without evidence of HP. History taking at this visit should ask specifically about further pigeon exposure: were the pigeons removed from the home completely, were heating-cooling filters changed, carpets cleaned, and bedding laundered? An in-home evaluation may be helpful before conducting further diagnostic testing.
She was admitted for oxygen therapy and a bronchoscopy, which showed mucosal friability and cobblestoning, suggesting inflammation. BAL revealed a normal CD4:CD8 ratio of 3; BAL cultures were sterile. Her shortness of breath significantly improved following a prolonged course of systemic steroids and removal from the triggering environment. PFTs improved with a FEV1/FVC ratio of 94 (105% predicted), FVC of 2.00 L (66% predicted), FEV1 of 1.88L (69% predicted) (Figure 3B). Her presenting symptoms of persistent cough and progressive dyspnea on exertion, characteristic CT, sterile BAL cultures, positive serum precipitants against pigeon serum, and resolution of her symptoms with withdrawal of the offending antigen were diagnostic of hypersensitivity pneumonitis due to pigeon exposure, also known as bird fancier’s disease.
COMMENTARY
The patient’s original presentation of dyspnea, tachypnea, and hypoxia is commonly associated with pediatric pneumonia and asthma exacerbations.1 However, an alternative diagnosis was suggested by the lack of wheezing, absence of fever, and recurrent presentations with progressive symptoms.
Hypersensitivity pneumonitis (HP) represents an exaggerated T-cell meditated immune response to inhalation of an offending antigen that results in a restrictive ventilatory defect and interstitial infiltrates.2 Bird pneumonitis (also known as bird fancier’s disease) is a frequent cause of HP, accounting for approximately 65-70% of cases.3 HP, however, only manifests in a small number of subjects exposed to culprit antigens, suggesting an underlying genetic susceptibility.4 Prevalence estimates vary depending on bird species, county, climate, and other possible factors.
There are no standard criteria for the diagnosis of HP, though a combination of findings is suggestive. A recent prospective multicenter study created a scoring system for HP based on factors associated with the disease to aid in accurate diagnosis. The most relevant criteria included antigen exposure, recurrent symptoms noted within 4-8 hours after antigen exposure, weight loss, presence of specific IgG antibodies to avian antigens, and inspiratory crackles on exam. Using this rule, the probability that our patient has HP based on clinical characteristics was 93% with an area under the receiver operating curve of 0.93 (96% confidence interval: 0.90-0.95)5. Chest imaging (high resolution CT) often consists of a mosaic pattern of air trapping, as seen in this patient in combination with ground-glass opacities6. Bronchoalveolar lavage (BAL) is sensitive in detecting lung inflammation in a patient with suspected HP. On BAL, a lymphocytic alveolitis can be seen, but absence of this finding does not exclude HP.5,7,8 Pulmonary function tests (PFTs) may be normal in acute HP. When abnormal, PFTs may reveal a restrictive pattern and reduction in carbon monoxide diffusing capacity.7 However, BAL and PFT results are neither specific nor diagnostic of HP; it is important to consider results in the context of the clinical picture.
The respiratory response to inhalation of the avian antigen has traditionally been classified as acute, subacute, or chronic.9 The acute response occurs within hours of exposure to the offending agent and usually resolves within 24 hours after antigen withdrawal. The subacute presentation involves cough and dyspnea over several days to weeks, and can progress to chronic and permanent lung damage if unrecognized and untreated. In chronic presentations, lung abnormalities may persist despite antigen avoidance and pharmacologic interventions.4,10 The patient’s symptoms occurred over a six-month period which coincided with pigeon exposure and resolved during each hospitalization with steroid treatment and removal from the offending agent. Her presentation was consistent with a subacute time course of HP.
The dilated pulmonary artery, elevated right systolic ventricular pressure, and normal right ventricular function in our patient suggested pulmonary hypertension of chronic duration. Her risk factors for pulmonary hypertension included asthma, sleep apnea, possible obesity-hypoventilation syndrome, and HP-associated interstitial lung disease.11
The most important intervention in HP is avoidance of the causative antigen. Medical therapy without removal of antigen is inadequate. Systemic corticosteroids can help ameliorate acute symptoms though dosing and duration remains unclear. For chronic patients unresponsive to steroid therapy, lung transplantation can be considered.4
The key to diagnosis of HP in this patient—and to minimizing repeat testing upon the patient’s recrudescence of symptoms—was the clinician’s consideration that the major impetus for the patient’s improvement in the hospital was removal from the offending antigen in her home environment. As in this case, taking time to delve deeply into a patient’s environment—even by descending the basement stairs—may lead to the diagnosis.
LEARNING POINTS
- Consider hypersensitivity pneumonitis (HP) in patients with recurrent respiratory distress, offending exposure, and resolution of symptoms with removal of culprit antigen.
- The most important treatment of HP is removal of offending antigen; systemic and/or inhaled corticosteroids are indicated until the full resolution of respiratory symptoms.
- Prognosis is dependent on early diagnosis and removal of offending exposures.
- Failure to treat HP might result in end-stage lung disease from pulmonary fibrosis secondary to long-term inflammation.
Disclosures
Dr. Manesh is supported by the Jeremiah A. Barondess Fellowship in the Clinical Transaction of the New York Academy of Medicine, in collaboration with the Accreditation Council for Graduate Medical Education (ACGME). The authors declare no conflicts of interests.
1. Ebell MH. Clinical diagnosis of pneumonia in children. Am Fam Physician. 2010;82(2):192-193. PubMed
2. Cormier Y, Lacasse Y. Hypersensitivity pneumonitis and organic dust toxic syndrome. In: Malo J-L, Chan-Yeung M, Bernstein DI, eds. Asthma in the Workplace. Vol 32. Boca Raton, FL: Fourth Informa Healthcare; 2013:392-405.
3. Chan AL, Juarez MM, Leslie KO, Ismail HA, Albertson TE. Bird fancier’s lung: a state-of-the-art review. Clin Rev Allergy Immunol. 2012;43(1-2):69-83. doi: 10.1007/s12016-011-8282-y. PubMed
4. Camarena A, Juárez A, Mejía M, et al. Major histocompatibility complex and tumor necrosis factor-α polymorphisms in pigeon breeder’s disease. Am J Respir Crit Care Med. 2001;163(7):1528-1533. https:/doi.org/10.1164/ajrccm.163.7.2004023. PubMed
5. Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168(8):952-958. doi: 10.1164/rccm.200301-137OC. PubMed
6. Glazer CS, Rose CS, Lynch DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging. 2002;17(4):261-272. PubMed
7. Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314-324. doi: 10.1164/rccm.201203-0513CI. PubMed
8. Calillad DM, Vergnon, JM, Madroszyk A, et al. Bronchoalveolar lavage in hypersensitivity pneumonitis: a series of 139 patients. Inflamm Allergy Drug Targets. 2012;11(1):15-19. doi: 10.2174/187152812798889330. PubMed
9. Richerson HB, Bernstein IL, Fink JN, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol. 1989;84(5 Pt 2):839-844. doi: 10.1016/0091-6749(89)90349-7. PubMed
10. Zacharisen MC, Schlueter DP, Kurup VP, Fink JN. The long-term outcome in acute, subacute, and chronic forms of pigeon breeder’s disease hypersensitivity pneumonitis. Ann Allergy Asthma Immunol. 2002;88(2):175-182. doi: 10.1016/S1081-1206(10)61993-X. PubMed
11. Raymond TE, Khabbaza JE, Yadav R, Tonelli AR. Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc. 2014;11(10):1623-1632. doi: 10.1513/AnnalsATS.201406-253PP. PubMed
1. Ebell MH. Clinical diagnosis of pneumonia in children. Am Fam Physician. 2010;82(2):192-193. PubMed
2. Cormier Y, Lacasse Y. Hypersensitivity pneumonitis and organic dust toxic syndrome. In: Malo J-L, Chan-Yeung M, Bernstein DI, eds. Asthma in the Workplace. Vol 32. Boca Raton, FL: Fourth Informa Healthcare; 2013:392-405.
3. Chan AL, Juarez MM, Leslie KO, Ismail HA, Albertson TE. Bird fancier’s lung: a state-of-the-art review. Clin Rev Allergy Immunol. 2012;43(1-2):69-83. doi: 10.1007/s12016-011-8282-y. PubMed
4. Camarena A, Juárez A, Mejía M, et al. Major histocompatibility complex and tumor necrosis factor-α polymorphisms in pigeon breeder’s disease. Am J Respir Crit Care Med. 2001;163(7):1528-1533. https:/doi.org/10.1164/ajrccm.163.7.2004023. PubMed
5. Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168(8):952-958. doi: 10.1164/rccm.200301-137OC. PubMed
6. Glazer CS, Rose CS, Lynch DA. Clinical and radiologic manifestations of hypersensitivity pneumonitis. J Thorac Imaging. 2002;17(4):261-272. PubMed
7. Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314-324. doi: 10.1164/rccm.201203-0513CI. PubMed
8. Calillad DM, Vergnon, JM, Madroszyk A, et al. Bronchoalveolar lavage in hypersensitivity pneumonitis: a series of 139 patients. Inflamm Allergy Drug Targets. 2012;11(1):15-19. doi: 10.2174/187152812798889330. PubMed
9. Richerson HB, Bernstein IL, Fink JN, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol. 1989;84(5 Pt 2):839-844. doi: 10.1016/0091-6749(89)90349-7. PubMed
10. Zacharisen MC, Schlueter DP, Kurup VP, Fink JN. The long-term outcome in acute, subacute, and chronic forms of pigeon breeder’s disease hypersensitivity pneumonitis. Ann Allergy Asthma Immunol. 2002;88(2):175-182. doi: 10.1016/S1081-1206(10)61993-X. PubMed
11. Raymond TE, Khabbaza JE, Yadav R, Tonelli AR. Significance of main pulmonary artery dilation on imaging studies. Ann Am Thorac Soc. 2014;11(10):1623-1632. doi: 10.1513/AnnalsATS.201406-253PP. PubMed
© 2019 Society of Hospital Medicine
Random Drug Testing of Physicians: A Complex Issue Framed in 7 Questions
Should physicians be subject to random drug testing? It’s a controversial topic. One in 10 Americans suffer from a drug use disorder at some point in their lives.1 Although physicians engaging in drug diversion is very rare, we recognize, in the context of rising rates of opiate use, that drug misuse and addiction can involve physicians.2,3 When it occurs, addiction can drive behaviors that endanger both clinicians and patients. Media reports on drug diversion describe an anesthesiologist who died of overdose from diverted fentanyl and a surgical technician with HIV who used and replaced opioids in the operating room, resulting in thousands of patients needing to be tested for infection.4 Multiple outbreaks of hepatitis C involving more than a dozen hospitals in eight states were traced to a single health care provider diverting narcotics.5 An investigation of outbreaks at various medical centers in the United States over a 10-year period identified nearly 30,000 patients that were potentially exposed and more than 100 iatrogenic infections.6
The profession of medicine holds a special place in the esteem of the public, with healthcare providers being among the most trusted professions. Patients rely on us to keep them safe when they are at their most vulnerable. This trust is predicated on the belief that the profession of medicine will self-regulate. Drug diversion by clinicians is a violation of this trust.
Our hospital utilizes existing structures to address substance use disorder; such structures include regular education on recognizing impairment for the medical staff, an impaired clinician policy for suspicion of impairment, and a state physician health program that provides nonpunitive evaluation and treatment for substance use by clinicians. In response to the imperative to mitigate the potential for drug diversion, our health system undertook a number of additional initiatives. These initiatives, included inventory control and tracking of controlled substances, and random testing and trigger-based audits of returned medications to ensure the entire amount had been accounted for. As part of this system-wide initiative, UCHealth began random drug testing of employees in safety-sensitive positions (for whom impairment would represent the potential for harm to others). Medical staff are not employees of the health system and were not initially subject to testing. The key questions at the time included the following:
- Is our organization doing everything possible to prevent drug diversion?
- If nurses and other staff are subject to random drug testing, why would physicians be exempt?
The University of Colorado Hospital (UCH) is the academic medical center within UCHealth. The structure of the relationship between the hospital and its medical staff requires the question of drug testing for physicians to be addressed by the UCH Medical Board (Medical Executive Committee). Medical staff leadership and key opinion leaders were engaged in the process of considering random drug testing of the medical staff. In the process, medical staff leadership raised additional questions about the process of decision making:
- “How should this issue be handled in the context of physician autonomy?”
- “How do we assure the concerns of the medical staff are heard and addressed?”
The guiding principles considered by the medical staff leadership in the implementation of random drug testing included the following: (1) as a matter of medical professionalism, for random drug testing to be implemented, the medical staff must elect to submit to mandatory testing; (2) the random drug testing program must be designed to minimize harm; and (3) the process for random drug testing program design needs to engage front-line clinicians. This resulted in a series of communications, meetings, and outreach to groups within the medical staff.
From front-line medical staff members, we heard overwhelming consensus for the moral case to prevent patient harm resulting from drug diversion, our professional duty to address the issue, and the need to maintain public trust in the institution of medicine. At the same time, medical staff members often expressed skepticism regarding the efficacy of random drug testing as a tactic, concerns about operational implementation, and fears regarding the unintended consequences:
- How strong is the evidence that random drug testing prevents drug diversion?
- How can we be confident that false-positive tests will not cause innocent clinicians to be incorrectly accused of drug use?
The efficacy of random drug testing in preventing drug diversion is not settled. The discussion of how to proceed in the absence of well-designed studies on the tactic was robust. One common principle we heard from members of the medical staff was that our response be driven by an authentic organizational desire to reduce patient harm. They expressed that the process of testing needs to respect the boundaries between work and home life and to avoid the disruption of clinical responsibilities. Whether targeting testing to “higher risk” groups of clinicians is appropriate and whether or not alcohol and/or marijuana would be tested came up often.
Other concerns expressed also included the intrusion of the institution into the private medical conditions of the medical staff members, breach of confidentiality, or accessibility of the information obtained as a result of the program for unrelated legal proceedings. One of the most prominent fears expressed was the possible impact of false-positive tests on the clinicians’ careers.
Following the listening tour by the medical staff and hospital leadership and extensive discussions, the Medical Board voted to approve a policy to implement random drug testing. The deliberative process lasted for approximately eight months. We sought input from other healthcare systems, such as the Veterans Administration and Cleveland Clinic, that conduct random drug tests on employed physicians. A physician from Massachusetts General Hospital who led the 2004 implementation of random drug testing for anesthesiologists was invited to come to Colorado to give grand rounds about the experience in his department and answer questions about the implementation of random drug testing at a Medical Board meeting.7 The policy went into effect January 2017.
The design of the program sought to explicitly address the issues raised by the front-line clinicians. In the interest of equity, all specialties, including Radiology and Pathology, are subject to testing. Medical staff are selected for testing using a random number generator and retained in the random selection pool at all times, regardless of previous selection for testing. Consistent with the underlying objective of identifying drug diversion, testing is limited to drugs at higher risk for diversion (eg, amphetamine, barbiturate, benzodiazepine, butorphanol, cocaine metabolite, fentanyl, ketamine, meperidine, methadone, nalbuphine, opiates, oxycodone, and tramadol). Although alcohol and marijuana are substances of abuse, they are not substances of healthcare diversion and thus are excluded from random drug testing (although included in testing for impairment). Random drug testing is conducted only for medical staff who are onsite and providing clinical services. The individuals selected for random drug testing are notified by Employee Health, or their clinical supervisor, to present to Employee Health that day to provide a urine sample. The involvement of the clinical supervisor in specific departments and the flexibility in time of presentation was implemented to address the concerns of the medical staff regarding harm from the disruption of acute patient care.
To address the concern regarding false-positive tests, an external medical laboratory that performs testing compliant with Substance Abuse and Mental Health Services and governmental standards is used. Samples are split providing the ability to perform independent testing of two samples. The thresholds are set to minimize false-positive tests. Positive results are sent to an independent medical review officer who confidentially contacts the medical staff member to assess for valid prescriptions to explain the test results. Unexplained positive test results trigger the testing of the second half of the split sample.
To address issues of dignity, privacy, and confidentiality, Employee Health discretely oversees the urine collection. The test results are not part of the individual’s medical record. Only the coordinator for random drug testing in Human Resources compliance can access the test results, which are stored in a separate, secure database. The medical review officer shares no information about the medical staff members’ medical conditions. A positive drug assay attributable to a valid medical explanation is reported as a negative test.
Positive test results, which would be reported to the President of the Medical Staff, would trigger further investigation, potential Medical Board action consistent with medical staff bylaws, and reporting to licensing bodies as appropriate. We recognize that most addiction is not associated with diversion, and all individuals struggling with substance use need support. The medical staff and hospital leadership committed through this process to connecting medical staff members who are identified by random drug testing to help for substance use disorder, starting with the State Physician Health Program.
The Medical Executive Committees of all hospitals within UCHealth have also approved random drug testing of medical staff. We are not the first healthcare organization to tackle the potential for drug diversion by healthcare workers. To our knowledge, we are the largest health system to have nonemployed medical staff leadership vote for the entire medical staff to be subject to random drug testing. Along the journey, the approach of random drug testing for physicians was vigorously debated. In this regard, we proffer one final question:
- How would you have voted?
Disclosures
The authors have nothing to disclose.
1. Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73(1):39-47. doi: 10.1001/jamapsychiatry.2015.2132. PubMed
2. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38. doi: 10.1111/ajad.12173. PubMed
3. Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267(17):2333-2339. doi:10.1001/jama.1992.03480170059029. PubMed
4. Olinger D, Osher CN. Denver Post- Drug-addicted, dangerous and licensed for the operating room. https://www.denverpost.com/2016/04/23/drug-addicted-dangerous-and-licensed-for-the-operating-room/ Published April 23, 2016. Updated June 2, 2016. Accessed June 7, 2018.
5. Federal Bureau of Investigations. Press Release. Former Employee of Exeter Hospital Pleads Guilty to Charges Related to Multi-State Hepatitis C Outbreak. https://archives.fbi.gov/archives/boston/press-releases/2013/former-employee-of-exeter-hospital-pleads-guilty-to-charges-related-to-multi-state-hepatitis-c-outbreak. Accessed June 7, 2018.
6. Schaefer MK, Perz JF. Outbreaks of infections associated with drug diversion by US healthcare personnel. Mayo Clin Proc. 2014;89(7):878-887. doi: 10.1016/j.mayocp.2014.04.007. PubMed
7. Fitzsimons MG, Baker K, Malhotra R, Gottlieb A, Lowenstein E, Zapol WM. Reducing the incidence of substance use disorders in anesthesiology residents: 13 years of comprehensive urine drug screening. Anesthesiology. 2018;129:821-828. doi: 10.1097/ALN.0000000000002348. In press. PubMed
Should physicians be subject to random drug testing? It’s a controversial topic. One in 10 Americans suffer from a drug use disorder at some point in their lives.1 Although physicians engaging in drug diversion is very rare, we recognize, in the context of rising rates of opiate use, that drug misuse and addiction can involve physicians.2,3 When it occurs, addiction can drive behaviors that endanger both clinicians and patients. Media reports on drug diversion describe an anesthesiologist who died of overdose from diverted fentanyl and a surgical technician with HIV who used and replaced opioids in the operating room, resulting in thousands of patients needing to be tested for infection.4 Multiple outbreaks of hepatitis C involving more than a dozen hospitals in eight states were traced to a single health care provider diverting narcotics.5 An investigation of outbreaks at various medical centers in the United States over a 10-year period identified nearly 30,000 patients that were potentially exposed and more than 100 iatrogenic infections.6
The profession of medicine holds a special place in the esteem of the public, with healthcare providers being among the most trusted professions. Patients rely on us to keep them safe when they are at their most vulnerable. This trust is predicated on the belief that the profession of medicine will self-regulate. Drug diversion by clinicians is a violation of this trust.
Our hospital utilizes existing structures to address substance use disorder; such structures include regular education on recognizing impairment for the medical staff, an impaired clinician policy for suspicion of impairment, and a state physician health program that provides nonpunitive evaluation and treatment for substance use by clinicians. In response to the imperative to mitigate the potential for drug diversion, our health system undertook a number of additional initiatives. These initiatives, included inventory control and tracking of controlled substances, and random testing and trigger-based audits of returned medications to ensure the entire amount had been accounted for. As part of this system-wide initiative, UCHealth began random drug testing of employees in safety-sensitive positions (for whom impairment would represent the potential for harm to others). Medical staff are not employees of the health system and were not initially subject to testing. The key questions at the time included the following:
- Is our organization doing everything possible to prevent drug diversion?
- If nurses and other staff are subject to random drug testing, why would physicians be exempt?
The University of Colorado Hospital (UCH) is the academic medical center within UCHealth. The structure of the relationship between the hospital and its medical staff requires the question of drug testing for physicians to be addressed by the UCH Medical Board (Medical Executive Committee). Medical staff leadership and key opinion leaders were engaged in the process of considering random drug testing of the medical staff. In the process, medical staff leadership raised additional questions about the process of decision making:
- “How should this issue be handled in the context of physician autonomy?”
- “How do we assure the concerns of the medical staff are heard and addressed?”
The guiding principles considered by the medical staff leadership in the implementation of random drug testing included the following: (1) as a matter of medical professionalism, for random drug testing to be implemented, the medical staff must elect to submit to mandatory testing; (2) the random drug testing program must be designed to minimize harm; and (3) the process for random drug testing program design needs to engage front-line clinicians. This resulted in a series of communications, meetings, and outreach to groups within the medical staff.
From front-line medical staff members, we heard overwhelming consensus for the moral case to prevent patient harm resulting from drug diversion, our professional duty to address the issue, and the need to maintain public trust in the institution of medicine. At the same time, medical staff members often expressed skepticism regarding the efficacy of random drug testing as a tactic, concerns about operational implementation, and fears regarding the unintended consequences:
- How strong is the evidence that random drug testing prevents drug diversion?
- How can we be confident that false-positive tests will not cause innocent clinicians to be incorrectly accused of drug use?
The efficacy of random drug testing in preventing drug diversion is not settled. The discussion of how to proceed in the absence of well-designed studies on the tactic was robust. One common principle we heard from members of the medical staff was that our response be driven by an authentic organizational desire to reduce patient harm. They expressed that the process of testing needs to respect the boundaries between work and home life and to avoid the disruption of clinical responsibilities. Whether targeting testing to “higher risk” groups of clinicians is appropriate and whether or not alcohol and/or marijuana would be tested came up often.
Other concerns expressed also included the intrusion of the institution into the private medical conditions of the medical staff members, breach of confidentiality, or accessibility of the information obtained as a result of the program for unrelated legal proceedings. One of the most prominent fears expressed was the possible impact of false-positive tests on the clinicians’ careers.
Following the listening tour by the medical staff and hospital leadership and extensive discussions, the Medical Board voted to approve a policy to implement random drug testing. The deliberative process lasted for approximately eight months. We sought input from other healthcare systems, such as the Veterans Administration and Cleveland Clinic, that conduct random drug tests on employed physicians. A physician from Massachusetts General Hospital who led the 2004 implementation of random drug testing for anesthesiologists was invited to come to Colorado to give grand rounds about the experience in his department and answer questions about the implementation of random drug testing at a Medical Board meeting.7 The policy went into effect January 2017.
The design of the program sought to explicitly address the issues raised by the front-line clinicians. In the interest of equity, all specialties, including Radiology and Pathology, are subject to testing. Medical staff are selected for testing using a random number generator and retained in the random selection pool at all times, regardless of previous selection for testing. Consistent with the underlying objective of identifying drug diversion, testing is limited to drugs at higher risk for diversion (eg, amphetamine, barbiturate, benzodiazepine, butorphanol, cocaine metabolite, fentanyl, ketamine, meperidine, methadone, nalbuphine, opiates, oxycodone, and tramadol). Although alcohol and marijuana are substances of abuse, they are not substances of healthcare diversion and thus are excluded from random drug testing (although included in testing for impairment). Random drug testing is conducted only for medical staff who are onsite and providing clinical services. The individuals selected for random drug testing are notified by Employee Health, or their clinical supervisor, to present to Employee Health that day to provide a urine sample. The involvement of the clinical supervisor in specific departments and the flexibility in time of presentation was implemented to address the concerns of the medical staff regarding harm from the disruption of acute patient care.
To address the concern regarding false-positive tests, an external medical laboratory that performs testing compliant with Substance Abuse and Mental Health Services and governmental standards is used. Samples are split providing the ability to perform independent testing of two samples. The thresholds are set to minimize false-positive tests. Positive results are sent to an independent medical review officer who confidentially contacts the medical staff member to assess for valid prescriptions to explain the test results. Unexplained positive test results trigger the testing of the second half of the split sample.
To address issues of dignity, privacy, and confidentiality, Employee Health discretely oversees the urine collection. The test results are not part of the individual’s medical record. Only the coordinator for random drug testing in Human Resources compliance can access the test results, which are stored in a separate, secure database. The medical review officer shares no information about the medical staff members’ medical conditions. A positive drug assay attributable to a valid medical explanation is reported as a negative test.
Positive test results, which would be reported to the President of the Medical Staff, would trigger further investigation, potential Medical Board action consistent with medical staff bylaws, and reporting to licensing bodies as appropriate. We recognize that most addiction is not associated with diversion, and all individuals struggling with substance use need support. The medical staff and hospital leadership committed through this process to connecting medical staff members who are identified by random drug testing to help for substance use disorder, starting with the State Physician Health Program.
The Medical Executive Committees of all hospitals within UCHealth have also approved random drug testing of medical staff. We are not the first healthcare organization to tackle the potential for drug diversion by healthcare workers. To our knowledge, we are the largest health system to have nonemployed medical staff leadership vote for the entire medical staff to be subject to random drug testing. Along the journey, the approach of random drug testing for physicians was vigorously debated. In this regard, we proffer one final question:
- How would you have voted?
Disclosures
The authors have nothing to disclose.
Should physicians be subject to random drug testing? It’s a controversial topic. One in 10 Americans suffer from a drug use disorder at some point in their lives.1 Although physicians engaging in drug diversion is very rare, we recognize, in the context of rising rates of opiate use, that drug misuse and addiction can involve physicians.2,3 When it occurs, addiction can drive behaviors that endanger both clinicians and patients. Media reports on drug diversion describe an anesthesiologist who died of overdose from diverted fentanyl and a surgical technician with HIV who used and replaced opioids in the operating room, resulting in thousands of patients needing to be tested for infection.4 Multiple outbreaks of hepatitis C involving more than a dozen hospitals in eight states were traced to a single health care provider diverting narcotics.5 An investigation of outbreaks at various medical centers in the United States over a 10-year period identified nearly 30,000 patients that were potentially exposed and more than 100 iatrogenic infections.6
The profession of medicine holds a special place in the esteem of the public, with healthcare providers being among the most trusted professions. Patients rely on us to keep them safe when they are at their most vulnerable. This trust is predicated on the belief that the profession of medicine will self-regulate. Drug diversion by clinicians is a violation of this trust.
Our hospital utilizes existing structures to address substance use disorder; such structures include regular education on recognizing impairment for the medical staff, an impaired clinician policy for suspicion of impairment, and a state physician health program that provides nonpunitive evaluation and treatment for substance use by clinicians. In response to the imperative to mitigate the potential for drug diversion, our health system undertook a number of additional initiatives. These initiatives, included inventory control and tracking of controlled substances, and random testing and trigger-based audits of returned medications to ensure the entire amount had been accounted for. As part of this system-wide initiative, UCHealth began random drug testing of employees in safety-sensitive positions (for whom impairment would represent the potential for harm to others). Medical staff are not employees of the health system and were not initially subject to testing. The key questions at the time included the following:
- Is our organization doing everything possible to prevent drug diversion?
- If nurses and other staff are subject to random drug testing, why would physicians be exempt?
The University of Colorado Hospital (UCH) is the academic medical center within UCHealth. The structure of the relationship between the hospital and its medical staff requires the question of drug testing for physicians to be addressed by the UCH Medical Board (Medical Executive Committee). Medical staff leadership and key opinion leaders were engaged in the process of considering random drug testing of the medical staff. In the process, medical staff leadership raised additional questions about the process of decision making:
- “How should this issue be handled in the context of physician autonomy?”
- “How do we assure the concerns of the medical staff are heard and addressed?”
The guiding principles considered by the medical staff leadership in the implementation of random drug testing included the following: (1) as a matter of medical professionalism, for random drug testing to be implemented, the medical staff must elect to submit to mandatory testing; (2) the random drug testing program must be designed to minimize harm; and (3) the process for random drug testing program design needs to engage front-line clinicians. This resulted in a series of communications, meetings, and outreach to groups within the medical staff.
From front-line medical staff members, we heard overwhelming consensus for the moral case to prevent patient harm resulting from drug diversion, our professional duty to address the issue, and the need to maintain public trust in the institution of medicine. At the same time, medical staff members often expressed skepticism regarding the efficacy of random drug testing as a tactic, concerns about operational implementation, and fears regarding the unintended consequences:
- How strong is the evidence that random drug testing prevents drug diversion?
- How can we be confident that false-positive tests will not cause innocent clinicians to be incorrectly accused of drug use?
The efficacy of random drug testing in preventing drug diversion is not settled. The discussion of how to proceed in the absence of well-designed studies on the tactic was robust. One common principle we heard from members of the medical staff was that our response be driven by an authentic organizational desire to reduce patient harm. They expressed that the process of testing needs to respect the boundaries between work and home life and to avoid the disruption of clinical responsibilities. Whether targeting testing to “higher risk” groups of clinicians is appropriate and whether or not alcohol and/or marijuana would be tested came up often.
Other concerns expressed also included the intrusion of the institution into the private medical conditions of the medical staff members, breach of confidentiality, or accessibility of the information obtained as a result of the program for unrelated legal proceedings. One of the most prominent fears expressed was the possible impact of false-positive tests on the clinicians’ careers.
Following the listening tour by the medical staff and hospital leadership and extensive discussions, the Medical Board voted to approve a policy to implement random drug testing. The deliberative process lasted for approximately eight months. We sought input from other healthcare systems, such as the Veterans Administration and Cleveland Clinic, that conduct random drug tests on employed physicians. A physician from Massachusetts General Hospital who led the 2004 implementation of random drug testing for anesthesiologists was invited to come to Colorado to give grand rounds about the experience in his department and answer questions about the implementation of random drug testing at a Medical Board meeting.7 The policy went into effect January 2017.
The design of the program sought to explicitly address the issues raised by the front-line clinicians. In the interest of equity, all specialties, including Radiology and Pathology, are subject to testing. Medical staff are selected for testing using a random number generator and retained in the random selection pool at all times, regardless of previous selection for testing. Consistent with the underlying objective of identifying drug diversion, testing is limited to drugs at higher risk for diversion (eg, amphetamine, barbiturate, benzodiazepine, butorphanol, cocaine metabolite, fentanyl, ketamine, meperidine, methadone, nalbuphine, opiates, oxycodone, and tramadol). Although alcohol and marijuana are substances of abuse, they are not substances of healthcare diversion and thus are excluded from random drug testing (although included in testing for impairment). Random drug testing is conducted only for medical staff who are onsite and providing clinical services. The individuals selected for random drug testing are notified by Employee Health, or their clinical supervisor, to present to Employee Health that day to provide a urine sample. The involvement of the clinical supervisor in specific departments and the flexibility in time of presentation was implemented to address the concerns of the medical staff regarding harm from the disruption of acute patient care.
To address the concern regarding false-positive tests, an external medical laboratory that performs testing compliant with Substance Abuse and Mental Health Services and governmental standards is used. Samples are split providing the ability to perform independent testing of two samples. The thresholds are set to minimize false-positive tests. Positive results are sent to an independent medical review officer who confidentially contacts the medical staff member to assess for valid prescriptions to explain the test results. Unexplained positive test results trigger the testing of the second half of the split sample.
To address issues of dignity, privacy, and confidentiality, Employee Health discretely oversees the urine collection. The test results are not part of the individual’s medical record. Only the coordinator for random drug testing in Human Resources compliance can access the test results, which are stored in a separate, secure database. The medical review officer shares no information about the medical staff members’ medical conditions. A positive drug assay attributable to a valid medical explanation is reported as a negative test.
Positive test results, which would be reported to the President of the Medical Staff, would trigger further investigation, potential Medical Board action consistent with medical staff bylaws, and reporting to licensing bodies as appropriate. We recognize that most addiction is not associated with diversion, and all individuals struggling with substance use need support. The medical staff and hospital leadership committed through this process to connecting medical staff members who are identified by random drug testing to help for substance use disorder, starting with the State Physician Health Program.
The Medical Executive Committees of all hospitals within UCHealth have also approved random drug testing of medical staff. We are not the first healthcare organization to tackle the potential for drug diversion by healthcare workers. To our knowledge, we are the largest health system to have nonemployed medical staff leadership vote for the entire medical staff to be subject to random drug testing. Along the journey, the approach of random drug testing for physicians was vigorously debated. In this regard, we proffer one final question:
- How would you have voted?
Disclosures
The authors have nothing to disclose.
1. Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73(1):39-47. doi: 10.1001/jamapsychiatry.2015.2132. PubMed
2. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38. doi: 10.1111/ajad.12173. PubMed
3. Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267(17):2333-2339. doi:10.1001/jama.1992.03480170059029. PubMed
4. Olinger D, Osher CN. Denver Post- Drug-addicted, dangerous and licensed for the operating room. https://www.denverpost.com/2016/04/23/drug-addicted-dangerous-and-licensed-for-the-operating-room/ Published April 23, 2016. Updated June 2, 2016. Accessed June 7, 2018.
5. Federal Bureau of Investigations. Press Release. Former Employee of Exeter Hospital Pleads Guilty to Charges Related to Multi-State Hepatitis C Outbreak. https://archives.fbi.gov/archives/boston/press-releases/2013/former-employee-of-exeter-hospital-pleads-guilty-to-charges-related-to-multi-state-hepatitis-c-outbreak. Accessed June 7, 2018.
6. Schaefer MK, Perz JF. Outbreaks of infections associated with drug diversion by US healthcare personnel. Mayo Clin Proc. 2014;89(7):878-887. doi: 10.1016/j.mayocp.2014.04.007. PubMed
7. Fitzsimons MG, Baker K, Malhotra R, Gottlieb A, Lowenstein E, Zapol WM. Reducing the incidence of substance use disorders in anesthesiology residents: 13 years of comprehensive urine drug screening. Anesthesiology. 2018;129:821-828. doi: 10.1097/ALN.0000000000002348. In press. PubMed
1. Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73(1):39-47. doi: 10.1001/jamapsychiatry.2015.2132. PubMed
2. Oreskovich MR, Shanafelt T, Dyrbye LN, et al. The prevalence of substance use disorders in American physicians. Am J Addict. 2015;24(1):30-38. doi: 10.1111/ajad.12173. PubMed
3. Hughes PH, Brandenburg N, Baldwin DC Jr., et al. Prevalence of substance use among US physicians. JAMA. 1992;267(17):2333-2339. doi:10.1001/jama.1992.03480170059029. PubMed
4. Olinger D, Osher CN. Denver Post- Drug-addicted, dangerous and licensed for the operating room. https://www.denverpost.com/2016/04/23/drug-addicted-dangerous-and-licensed-for-the-operating-room/ Published April 23, 2016. Updated June 2, 2016. Accessed June 7, 2018.
5. Federal Bureau of Investigations. Press Release. Former Employee of Exeter Hospital Pleads Guilty to Charges Related to Multi-State Hepatitis C Outbreak. https://archives.fbi.gov/archives/boston/press-releases/2013/former-employee-of-exeter-hospital-pleads-guilty-to-charges-related-to-multi-state-hepatitis-c-outbreak. Accessed June 7, 2018.
6. Schaefer MK, Perz JF. Outbreaks of infections associated with drug diversion by US healthcare personnel. Mayo Clin Proc. 2014;89(7):878-887. doi: 10.1016/j.mayocp.2014.04.007. PubMed
7. Fitzsimons MG, Baker K, Malhotra R, Gottlieb A, Lowenstein E, Zapol WM. Reducing the incidence of substance use disorders in anesthesiology residents: 13 years of comprehensive urine drug screening. Anesthesiology. 2018;129:821-828. doi: 10.1097/ALN.0000000000002348. In press. PubMed
© 2019 Society of Hospital Medicine