User login
VAM Scholarships Available
Students and trainees can get financial help to attend the Vascular Annual Meeting in June. The Society for Vascular Surgery distributes dozens of travel scholarships to attend VAM, which is the perfect opportunity to meet other students as well as other members and leaders of the vascular surgical community. Recipients are eligible to receive complimentary meeting registration plus a travel scholarship. Two award categories are available, the General Surgery Resident/Medical Student Travel Scholarship and the Diversity Medical Student Travel Scholarship. Apply today.
Students and trainees can get financial help to attend the Vascular Annual Meeting in June. The Society for Vascular Surgery distributes dozens of travel scholarships to attend VAM, which is the perfect opportunity to meet other students as well as other members and leaders of the vascular surgical community. Recipients are eligible to receive complimentary meeting registration plus a travel scholarship. Two award categories are available, the General Surgery Resident/Medical Student Travel Scholarship and the Diversity Medical Student Travel Scholarship. Apply today.
Students and trainees can get financial help to attend the Vascular Annual Meeting in June. The Society for Vascular Surgery distributes dozens of travel scholarships to attend VAM, which is the perfect opportunity to meet other students as well as other members and leaders of the vascular surgical community. Recipients are eligible to receive complimentary meeting registration plus a travel scholarship. Two award categories are available, the General Surgery Resident/Medical Student Travel Scholarship and the Diversity Medical Student Travel Scholarship. Apply today.
Surgeons: Urge PAs to Join SVS
The SVS has a section dedicated solely to vascular physician assistants. All surgeons are encouraged to urge their PAs to join the section. This “professional home” for PAs has grown to 138 members in just one short year and more are always welcome. Benefits include PA-specific education at the Vascular Annual Meeting, leadership development, networking and mentoring opportunities, discounts on SVS events and our JVS subscription and more. Membership applications are processes quarterly. 2019 deadlines are March 1, June 1, Sep. 1 and Dec. 1. Learn more about the PA Section here.
The SVS has a section dedicated solely to vascular physician assistants. All surgeons are encouraged to urge their PAs to join the section. This “professional home” for PAs has grown to 138 members in just one short year and more are always welcome. Benefits include PA-specific education at the Vascular Annual Meeting, leadership development, networking and mentoring opportunities, discounts on SVS events and our JVS subscription and more. Membership applications are processes quarterly. 2019 deadlines are March 1, June 1, Sep. 1 and Dec. 1. Learn more about the PA Section here.
The SVS has a section dedicated solely to vascular physician assistants. All surgeons are encouraged to urge their PAs to join the section. This “professional home” for PAs has grown to 138 members in just one short year and more are always welcome. Benefits include PA-specific education at the Vascular Annual Meeting, leadership development, networking and mentoring opportunities, discounts on SVS events and our JVS subscription and more. Membership applications are processes quarterly. 2019 deadlines are March 1, June 1, Sep. 1 and Dec. 1. Learn more about the PA Section here.
Cerebral small vessel and cognitive impairment
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Nominate an Outstanding Surgeon for the Community Excellence Award
This year, the Society for Vascular Surgery will recognize a member in community practice who excels, leads and contributes. If you know of such a surgeon, make your nominations for the SVS Excellence in Community Service Award by Feb. 1. The first recipient will be announced at the 2019 Vascular Annual Meeting in June. Applicants must have a minimum of 20 years as a practicing vascular surgeon and a minimum of five years as an SVS member. Learn more about the award here.
This year, the Society for Vascular Surgery will recognize a member in community practice who excels, leads and contributes. If you know of such a surgeon, make your nominations for the SVS Excellence in Community Service Award by Feb. 1. The first recipient will be announced at the 2019 Vascular Annual Meeting in June. Applicants must have a minimum of 20 years as a practicing vascular surgeon and a minimum of five years as an SVS member. Learn more about the award here.
This year, the Society for Vascular Surgery will recognize a member in community practice who excels, leads and contributes. If you know of such a surgeon, make your nominations for the SVS Excellence in Community Service Award by Feb. 1. The first recipient will be announced at the 2019 Vascular Annual Meeting in June. Applicants must have a minimum of 20 years as a practicing vascular surgeon and a minimum of five years as an SVS member. Learn more about the award here.
Click for Credit: STIs on the rise; psoriasis & cardiac risk; more
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Flu season intensifies: High activity now in 19 states
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.
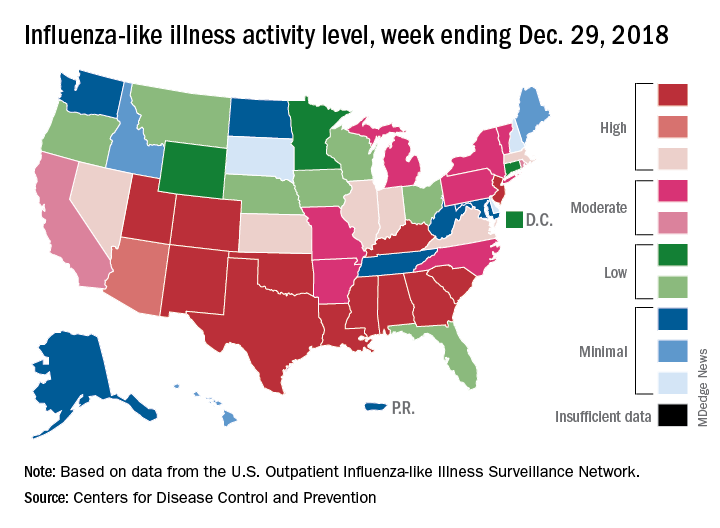
A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.

A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.

A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
Hospital Readmissions Reduction Program may be doing more harm than good
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
Evidence in this study shows that while the Hospital Readmissions Reduction Program my be succeeding in reducing hospital admissions, little evidence is available to show that it is having a positive effect on patient outcomes.
The Centers for Medicare & Medicaid Services needs to reexamine the program and find alternative methods that are both effective at reducing hospital readmissions while at the same time protect patients from unintentional harm, including death.
Gregg C. Fonarow, MD , University of California Medical Center, Los Angeles, in an editorial published in JAMA, Dec. 25, 2018. doi:10.1001/jama.2018.19325 .
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
A Medicare program aimed at lowering readmissions to hospitals could be having an adverse effect on mortality.
Results from a retrospective cohort study of hospitalizations for heart failure, acute myocardial infarction, and pneumonia among Medicare beneficiaries aged 65 years and older between April 1, 2005 and March 31, 2015 (covering the period before and after the Medicare Hospital Readmissions Reduction Program was announced in April 2010 and implemented in October 2012) found a significant increase in 30-day post discharge mortality among heart failure and pneumonia patients.
“Most concerning, however, is the possibility that the relationship between the HRRP and postdischarge mortality for heart failure and pneumonia is causal, indicating that the HRRP led to changes in quality of care that adversely affected patients,” Rishi Wadhera, MD, Harvard Medical School, Boston, and his colleagues wrote in a report published Dec. 25, 2018, in JAMA.
They looked at 8.3 million hospitalizations for heart failure, acute MI, and pneumonia, among whom 7.9 million were alive at the time of discharge. There were roughly 270,000 deaths within 30 days of discharge for heart failure; 128,000 for acute MI; and 246,000 for pneumonia.
To examine trends, the timing was divided into four periods: two prior to the announcement of the HRRP (April 2005–September 2007 and October 2007–March 2010); a third covering the time when the HRRP was announced (April 2010–September 2012); and the fourth when HRRP was implemented (October 2012–March 2015).
They found that among patients discharged with heart failure, 30-day mortality was rising even before the announcement of the HRRP, by 0.27% from the first period to the second period. That baseline trend continued when the HRRP was announced, by 0.49%, from second period to third. The difference in change between those periods was 0.22%. After implementation, 30-day mortality increased by 0.52%, with a difference in change from the third period of 0.25%. Both changes were statistically significant.
Among pneumonia patients, postdischarge mortality was stable before HRRP, but significantly increased after HRRP announcement, by 0.26%, with a difference in change from the second period to the third period of 0.22%. After implementation, the 30-day postdischarge mortality was 0.44%, with a significant difference in change of 0.40%.
Acute MI was a different story. Postdischarge mortality decreased significantly after the implementation of the HRRP, by 0.22%. The difference in change was –0.26%.
The authors suggested that “some hospitals may have focused more resources and efforts on reducing or avoiding readmissions than on prioritizing survival.” They add that the increases in heart failure morbidity could be related to patients with more severe heart conditions.
They noted that “although hospitals that reduce readmissions also appear to reduce mortality, this hospital-level concordance does not reflect the change in readmissions and mortality at the level of the patient population, which is arguably of greater importance to individual patients and to public health.”
Further research is needed to understand whether the increase in 30-day postdischarge mortality is a result of the HRRP, the authors concluded.
SOURCE: Wadhera R et al. JAMA. 2018 Dec 25. doi: 10.1001/jama.2018.19232.
FROM JAMA
Key clinical point:
Major finding: Heart failure patients saw mortality increase 0.52% after HRRP launched.
Study details: A retrospective cohort study across 10 years, including time before and after the implementation of the HRRP.
Disclosures: The Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology funded the study. No relevant conflicts of interest were disclosed.
Source: Wadhera R et al. JAMA 2018 Dec 25. doi: 10.1001/jama.2018.19232.
Tests can identify leukemia risk in newborns with Down syndrome
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
SAN DIEGO – Research into hundreds of babies with Down syndrome is providing valuable insight into the genetic roots of leukemia and offering a route to identify newborns at high risk.
“We can now identify children at high risk of developing myeloid leukemia within 4 years” through blood or genetic tests, Irene Roberts, MD, a pediatric hematologist at the University of Oxford’s (England) MRC Weatherall Institute of Molecular Medicine, said at the annual meeting of the American Society of Hematology.
About 2%-3% of children with Down syndrome will develop acute lymphocytic leukemia (ALL) or acute myeloid leukemia (AML), according to the National Cancer Institute, rates that are much higher than in the general population.
Research suggests that among children aged 0-4 years with Down syndrome, the standardized incidence ratio (SIR) of AML is 114, compared with other children, Dr. Roberts said. The SIR of ALL is 27 in children aged 1-4 years, she said.
For people with Down syndrome aged 0-60 years, the SIRs are 12 and 13 in AML and ALL, respectively, she said.
In her presentation, Dr. Roberts focused on AML that appears before age 4 years and is preceded by a neonatal preleukemia – transient abnormal myelopoiesis (TAM) – that only occurs in Down syndrome. In most cases, TAM, which occurs with GATA1 mutations, resolves on its own after birth, she said. But in others, the GATA1 mutations continue and cause AML to develop.
Dr. Roberts highlighted her institution’s Oxford Down Syndrome Cohort Study and offered an update to a 2013 report (Blood. 2013 Dec 5;122[24]:3908–17). The study recruited 471 neonates with Down syndrome and followed them for up to 4 years: 341 with no GATA1 mutation and 130 (28%) with the mutation. Dr. Roberts called the latter number a “very high frequency.”
Of those with the mutation, 7 patients (5%) developed AML at a median age of 16 months. None of those without the mutation developed AML.
Also, among the 130 neonates with the mutation, 42% were considered to have “clinical” TAM (more than 10% blasts) and 58% were considered to have “silent” TAM (fewer than 10% blasts).
“We predicted that these babies with clinical TAM would have more severe clinical disease ... and that in fact turned out to be the case,” Dr. Roberts said.
Why is the GATA1 mutation so significant? Research suggests that platelet production is abnormal in neonates with Down syndrome, compared with neonates without it, regardless of whether they have the mutation, Dr. Roberts said.
The mutation doesn’t reduce further platelet count, but does disrupt megakaryopoiesis – the process of the production of platelets. As a result, giant platelets and megakaryocyte fragments are more common, she explained.
Moving forward, research data can be used to identify which children are most at risk, Dr. Roberts said. Newborns with Down syndrome are more likely to survive without leukemia if they have silent TAM, compared with those who have clinical TAM, and if they have an estimated variant allele frequency above 15%, according to findings from the Oxford study.
Children at high risk of AML before age 4 years can be identified by analyzing the percentage of blasts on a smear and/or by analyzing mutation of GATA1, according to Dr. Roberts. However, this cannot be accomplished by the use of a complete blood count (CBC) test, she said, which is used to check for leukemia.
Dr. Roberts called for the development of more guidelines for screening newborns with Down syndrome for leukemia risk. The British Society for Haematology issued testing guidelines, coauthored by Dr. Roberts, in 2018 (Br J Haematol. 2018 Jul;182[2]:200-11).
Dr. Roberts reported having no financial disclosures.
EXPERT ANALYSIS FROM ASH 2018
VHA Suicide Prevention Media Outreach Is Falling Short—But Not for Lack of Money
The VHA’s suicide prevention media outreach activities—including social media postings, public service announcements, paid media, and Suicide Prevention Month activities—shrank markedly in fiscal years 2017 and 2018, according to the Government Accountability Office (GAO).
Since 2010, the primary focus of the outreach campaign has been to raise awareness of the Veterans Crisis Line (VCL), with output falling into 2 main categories: unpaid (eg, social media, public service announcements [PSAs], website) and paid (digital media, such as online keyword searches, and “out-of-home” media, such as billboards).
But between 2016 and the first 10 months of 2018, social media content dropped from 339 pieces to only 47. VHA also had not aired a suicide prevention PSA in more than 1 year, the first time there has been a gap of more than 1 month since June 2012.
In 2015, with a budget of > $4 million, VHA ran 58 advertisements on Google, Bing, and Facebook; 30 billboards; 180 bus advertisements; > 19,000 radio advertisements; 252 print advertisements; and 39 movie theater placements across the US. Fiscal years 2013, 2014, and 2016 were similarly productive.
Meanwhile, in FY 2017, the VHA spent < 10% of its approximately $1.7 million on paid ads on Google and Bing. And as of September 2018, VHA said it had spent only $57,000 of its $6.2 million paid media budget.
The waning outreach is “inconsistent with VA’s strategic goals,” the GAO says, which identify suicide prevention as the agency’s top clinical priority for FY 2018 through 2024.
VHA officials said they had not spent all the available funds due to changes in leadership and organizational realignment of the suicide prevention program. The position of National Director for Suicide Prevention position, for example, was vacant from July 2017 to April 2018. It was filled temporarily for 6 months; the interim director was then hired permanently in April 2018.
Since 2016, the VHA says, the plan has been to have a national strategy for preventing veteran suicides—an average of 20 per day, according to the VA—using a public health approach, focusing less on raising awareness of the VCL and more on reaching veterans before the point of crisis. However, in May 2018, VHA officials told the GAO that they “were just beginning to conceptualize what the suicide prevention outreach campaign should look like moving forward.”
One problem, the GAO says, is that the VHA has not established targets for the majority of the metrics it uses to help gauge the effectiveness of the outreach campaign. As a result, the VHA does not have the information it needs for a full evaluation. The only target the VHA has set is for each PSA to rank in the top 10% of the Nielsen ratings because, it says, that is the only meaningful target available that is accepted industry-wide. (The GAO notes, however: “VHA could use information about how its metrics performed in the past to develop reasonable and meaningful targets for future performance.”)
The GAO has made 2 recommendations to the VA based on its audit:
- The Under Secretary for Health should establish an approach for overseeing its suicide prevention media outreach efforts that includes “clear delineation of roles and responsibilities for those in leadership and contract oversight roles, including during periods of staff turnover or program changes.”
- Officials within the Office of Mental Health and Suicide Prevention (OMHSP) must establish targets for the metrics used to evaluate the campaign’s effectiveness.
In written comments, the VA concurred with the GAO recommendations and provided a time line for addressing them. VA said OMHSP has made organizational improvements, including creating a new organizational structure. It also has plans to work with communications experts to develop metrics, targets, and an evaluation strategy, expected to be completed by April 2019.
The VHA’s suicide prevention media outreach activities—including social media postings, public service announcements, paid media, and Suicide Prevention Month activities—shrank markedly in fiscal years 2017 and 2018, according to the Government Accountability Office (GAO).
Since 2010, the primary focus of the outreach campaign has been to raise awareness of the Veterans Crisis Line (VCL), with output falling into 2 main categories: unpaid (eg, social media, public service announcements [PSAs], website) and paid (digital media, such as online keyword searches, and “out-of-home” media, such as billboards).
But between 2016 and the first 10 months of 2018, social media content dropped from 339 pieces to only 47. VHA also had not aired a suicide prevention PSA in more than 1 year, the first time there has been a gap of more than 1 month since June 2012.
In 2015, with a budget of > $4 million, VHA ran 58 advertisements on Google, Bing, and Facebook; 30 billboards; 180 bus advertisements; > 19,000 radio advertisements; 252 print advertisements; and 39 movie theater placements across the US. Fiscal years 2013, 2014, and 2016 were similarly productive.
Meanwhile, in FY 2017, the VHA spent < 10% of its approximately $1.7 million on paid ads on Google and Bing. And as of September 2018, VHA said it had spent only $57,000 of its $6.2 million paid media budget.
The waning outreach is “inconsistent with VA’s strategic goals,” the GAO says, which identify suicide prevention as the agency’s top clinical priority for FY 2018 through 2024.
VHA officials said they had not spent all the available funds due to changes in leadership and organizational realignment of the suicide prevention program. The position of National Director for Suicide Prevention position, for example, was vacant from July 2017 to April 2018. It was filled temporarily for 6 months; the interim director was then hired permanently in April 2018.
Since 2016, the VHA says, the plan has been to have a national strategy for preventing veteran suicides—an average of 20 per day, according to the VA—using a public health approach, focusing less on raising awareness of the VCL and more on reaching veterans before the point of crisis. However, in May 2018, VHA officials told the GAO that they “were just beginning to conceptualize what the suicide prevention outreach campaign should look like moving forward.”
One problem, the GAO says, is that the VHA has not established targets for the majority of the metrics it uses to help gauge the effectiveness of the outreach campaign. As a result, the VHA does not have the information it needs for a full evaluation. The only target the VHA has set is for each PSA to rank in the top 10% of the Nielsen ratings because, it says, that is the only meaningful target available that is accepted industry-wide. (The GAO notes, however: “VHA could use information about how its metrics performed in the past to develop reasonable and meaningful targets for future performance.”)
The GAO has made 2 recommendations to the VA based on its audit:
- The Under Secretary for Health should establish an approach for overseeing its suicide prevention media outreach efforts that includes “clear delineation of roles and responsibilities for those in leadership and contract oversight roles, including during periods of staff turnover or program changes.”
- Officials within the Office of Mental Health and Suicide Prevention (OMHSP) must establish targets for the metrics used to evaluate the campaign’s effectiveness.
In written comments, the VA concurred with the GAO recommendations and provided a time line for addressing them. VA said OMHSP has made organizational improvements, including creating a new organizational structure. It also has plans to work with communications experts to develop metrics, targets, and an evaluation strategy, expected to be completed by April 2019.
The VHA’s suicide prevention media outreach activities—including social media postings, public service announcements, paid media, and Suicide Prevention Month activities—shrank markedly in fiscal years 2017 and 2018, according to the Government Accountability Office (GAO).
Since 2010, the primary focus of the outreach campaign has been to raise awareness of the Veterans Crisis Line (VCL), with output falling into 2 main categories: unpaid (eg, social media, public service announcements [PSAs], website) and paid (digital media, such as online keyword searches, and “out-of-home” media, such as billboards).
But between 2016 and the first 10 months of 2018, social media content dropped from 339 pieces to only 47. VHA also had not aired a suicide prevention PSA in more than 1 year, the first time there has been a gap of more than 1 month since June 2012.
In 2015, with a budget of > $4 million, VHA ran 58 advertisements on Google, Bing, and Facebook; 30 billboards; 180 bus advertisements; > 19,000 radio advertisements; 252 print advertisements; and 39 movie theater placements across the US. Fiscal years 2013, 2014, and 2016 were similarly productive.
Meanwhile, in FY 2017, the VHA spent < 10% of its approximately $1.7 million on paid ads on Google and Bing. And as of September 2018, VHA said it had spent only $57,000 of its $6.2 million paid media budget.
The waning outreach is “inconsistent with VA’s strategic goals,” the GAO says, which identify suicide prevention as the agency’s top clinical priority for FY 2018 through 2024.
VHA officials said they had not spent all the available funds due to changes in leadership and organizational realignment of the suicide prevention program. The position of National Director for Suicide Prevention position, for example, was vacant from July 2017 to April 2018. It was filled temporarily for 6 months; the interim director was then hired permanently in April 2018.
Since 2016, the VHA says, the plan has been to have a national strategy for preventing veteran suicides—an average of 20 per day, according to the VA—using a public health approach, focusing less on raising awareness of the VCL and more on reaching veterans before the point of crisis. However, in May 2018, VHA officials told the GAO that they “were just beginning to conceptualize what the suicide prevention outreach campaign should look like moving forward.”
One problem, the GAO says, is that the VHA has not established targets for the majority of the metrics it uses to help gauge the effectiveness of the outreach campaign. As a result, the VHA does not have the information it needs for a full evaluation. The only target the VHA has set is for each PSA to rank in the top 10% of the Nielsen ratings because, it says, that is the only meaningful target available that is accepted industry-wide. (The GAO notes, however: “VHA could use information about how its metrics performed in the past to develop reasonable and meaningful targets for future performance.”)
The GAO has made 2 recommendations to the VA based on its audit:
- The Under Secretary for Health should establish an approach for overseeing its suicide prevention media outreach efforts that includes “clear delineation of roles and responsibilities for those in leadership and contract oversight roles, including during periods of staff turnover or program changes.”
- Officials within the Office of Mental Health and Suicide Prevention (OMHSP) must establish targets for the metrics used to evaluate the campaign’s effectiveness.
In written comments, the VA concurred with the GAO recommendations and provided a time line for addressing them. VA said OMHSP has made organizational improvements, including creating a new organizational structure. It also has plans to work with communications experts to develop metrics, targets, and an evaluation strategy, expected to be completed by April 2019.
Armored CAR protects T cells, induces remissions
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
SAN DIEGO – A second-generation CD19-specific “armored” chimeric antigen receptor (CAR) T-cell construct was associated with high complete remission rates in diffuse large B-cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1 trial.
The CAR T construct – labeled 1928z-41BBL – also induced “encouraging” complete remission rates in patients with chronic lymphocytic leukemia (CLL) with Richter’s transformation, reported Jae H. Park, MD, of Memorial Sloan Kettering Cancer Center (MSKCC), New York, and his colleagues.
“Interestingly and encouragingly, severe [cytokine release syndrome] was not seen and grade 3 neurotoxicity was observed in less than 10%, with no grade 4 neurotoxicity, so there appears to be a favorable side effect profile,” Dr. Park said at the annual meeting of the American Society of Hematology.
Just as armored cars are designed to protect their valuable contents from people with bad intent, armored CAR T cells are engineered to protect the modified T-cells from a hostile tumor microenvironment and simultaneously recruit non-modified T cells to the target to produce a more robust immune response against malignant cells.
MSKCC investigators had previously shown that in contrast to other CAR T-cell constructs, the 1928z-41BBL configuration, which consists of two signaling domains (CD28 and CD3zeta) and the 4-1BB ligand, hit the sweet spot between tumor-killing function and T-cell persistence (Cancer Cell. 2015 Oct 12;28[4]:415-28).
In the current study, they enrolled 35 adults with relapsed or refractory CD19-positive hematologic malignancies, 29 of whom eventually underwent CAR T-cell infusions. The treated population comprised 14 patients with CLL (4 of whom had Richter’s transformation), 9 with DLBCL, 5 with indolent NHL, and 1 with acute lymphoblastic leukemia.
The patients with CLL had received a median of 5.5 prior lines of therapy, including ibrutinib (Imbruvica) and venetoclax (Venclexta).
There were 15 complete remissions (CR), with CR rates of 78% in DLBCL, 20% in CLL, 67% in CLL with Richter’s transformation, 60% in patients with indolent NHL, as well as CR in the single patient with ALL.
There were eight partial remissions. One patient with CLL had stable disease, and four patients had disease progression (one patient each with DLBCL, CLL, CLL with Richter’s, and indolent NHL).
Dr. Park noted that T cells are being detected in peripheral blood more than 6 months after T-cell infusion.
There were no cases of severe cytokine release syndrome, defined as requiring vasopressors and/or mechanical ventilation for hypoxia, and just three cases of grade 3 neurotoxicity. There were no cases of grade 4 neurotoxicity, no deaths related to neurotoxicity, and no cases of cerebral edema – a serious complication that has been seen in earlier CAR T-cell studies.
Split or multiple infusions of CAR T cells or incorporation of the technique into earlier lines of therapy might generate higher response rates, Dr. Park said.
The study was supported by Juno Therapeutics. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
SOURCE: Park JH et al. ASH 2018, Abstract 224.
REPORTING FROM ASH 2018
Key clinical point: The 1928z-41BBL CAR T-cell construct induced high rates of complete remissions.
Major finding: The CAR T product was associated with a 78% complete remission rate in patients with heavily pretreated diffuse large B-cell lymphoma.
Study details: A phase 1 trial in 29 patients with CD19-positive hematologic malignancies.
Disclosures: Juno Therapeutics supported the study. Dr. Park reported consulting for and research funding from Juno, and financial relationships with other companies.
Source: Park JH et al. ASH 2018, Abstract 224.