User login
Longterm maintenance of PASI 75 responses observed with tildrakizumab
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
PARIS – Dermatologists are likely to do a double-take when they see the long-term efficacy and safety data for tildrakizumab (Ilumya), a high-affinity humanized monoclonal antibody targeting interleukin-23 p19, relative to the performance of older and more familiar biologic agents with other targets in psoriasis, Diamant Thaçi, MD, predicted at the annual congress of the European Academy of Dermatology and Venereology.
“The time to relapse [off tildrakizumab] is very different from what we are used to with other biologics; for example, the tumor necrosis factor inhibitors,” observed Dr. Thaçi, professor and chairman of the department of dermatology at the University of Lübeck (Germany).
He presented the 148-week, follow-up results of a pooled analysis of the open-label extension studies of reSURFACE 1 and reSURFACE 2, two pivotal phase 3 randomized double-blind international trials of 1,862 patients with moderate to severe chronic plaque psoriasis. The primary outcomes through week 12, which were instrumental in gaining marketing approval for tildrakizumab for treating psoriasis in 2018 from the Food and Drug Administration and the European Medicines Agency, have been published in the Lancet (2017 Jul 15;390[10091]:276-88).
Dr. Thaçi’s analysis of the 148-week outcomes was restricted to the patients who had at least a 75% improvement from baseline in Psoriasis Area and Severity Index scores (PASI 75) at week 28. Nearly 80% of patients on tildrakizumab reached that threshold at week 28 in reSURFACE 1, as did 73% in reSURFACE 2.
The question asked in the extension study was, How do responders to tildrakizumab at 28 weeks fare after nearly 3 years on the drug? And the answer was: very well. Maintenance of at least a PASI 75 response was observed at 148 weeks in 91% of patients on tildrakizumab at the approved 100-mg dose and 92% of those on the 200-mg dose. The FDA-approved regimen is 100 mg by subcutaneous injection at weeks 0 and 4, and then every 12 weeks after that.
An intriguing feature of reSURFACE 1 was that a subset of PASI 75 responders at week 28 got taken off tildrakizumab at that point and switched to double-blind placebo, then restarted on their earlier dose of tildrakizumab upon relapse, which was defined as loss of at least 50% of the achieved on-drug PASI improvement.
At week 64, fully 48 weeks after their last dose of tildrakizumab, the relapse rate was 54% in the group formerly on 100 mg of tildrakizumab and slightly better at 47% in those formerly on 200 mg. The median time to relapse was 226 days in the 100-mg group and 258 days in the higher-dose arm. Those are exceptionally long times to relapse, and it’s useful information to file away in the event a psoriasis patient needs to discontinue biologic therapy for a period of time, Dr. Thaçi observed.
At week 64 – again, off active treatment since week 16 – 63% of the tildrakizumab 100-mg group had lost their previous PASI 75 response, as had 52% who were formerly on tildrakizumab at 200 mg.
The long-term safety profile of tildrakizumab paralleled that of placebo. For example, the exposure-adjusted adverse event rates of serious infections and major adverse cardiovascular events were closely similar in the placebo, tildrakizumab 100 mg, and tildrakizumab 200 mg groups.
There were two notable between-group differences in adverse events of interest: injection site reactions occurred at a rate of 5.36 per 100 person-years with placebo, compared with 1.94 and 2.3 per 100 person-years with tildrakizumab at 100 and 200 mg, respectively; and the incidence of nonmelanoma skin cancer was 0.97 cases per 100 person-years in the placebo arm, versus 0.5 and 0.49 cases per 100 person-years in the two tildrakizumab arms.
Dr. Thaçi did not present PASI 90 response outcomes because, at the time the reSURFACE trials were planned, PASI 75 was considered state of the art. The PASI 90 data are still being crunched but will be available soon. The 4- and 5-year follow-up data from the long-term extension studies are also on their way.
The reSURFACE 1 and reSURFACE 2 trials and their extension studies were funded by Sun Pharma and Merck. Dr. Thaçi reported receiving research grants from and serving as a consultant and paid scientific advisor to those pharmaceutical companies and more than a dozen others.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Inhibition of interleukin-23 p19 via tildrakizumab pays major long-term dividends.
Major finding: Of patients with a PASI 75 response to tildrakizumab 100 mg at 6 months, 91% maintained that level of response through 148 weeks.
Study details: This was a long-term, prospective, open-label extension study of the phase 3 reSURFACE 1 and 2 trials of 1,862 psoriasis patients.
Disclosures: The reSURFACE 1 and reSURFACE 2 trials and their extension study were funded by Sun Pharma and Merck. The presenter reported receiving research grants from and serving as a consultant to those pharmaceutical companies and more than a dozen others.
HIPAA compliance: Three cases to learn from
Data security experts say three HIPAA violations that resulted in significant fines by the Office for Civil Rights (OCR) in 2018 hold important lessons for health professionals about safeguarding records and training staff in HIPAA compliance.
Read on to learn how the cases unfolded and what knowledge practices can gain from the common HIPAA mistakes.
Who? Allergy Associates of Hartford, Conn.
What happened? A patient contacted a local television station to complain about a dispute between herself and a physician at Allergy Associates in Hartford, Conn. The disagreement stemmed from the office turning away the patient because she allegedly brought her service animal, according to a Nov. 26 announcement by the Department of Health & Human Services. The reporter contacted the doctor in question for a news story and, in responding, the physician disclosed protected patient information to the reporter.
What else? An OCR investigation determined that a privacy officer with Allergy Associates had instructed the physician not to respond to the media about the complaint or to respond with “no comment”; that advice was disregarded. The practice then failed to discipline the physician or take any corrective action following the disclosure, according to the OCR.
How much? The OCR imposed a $125,000 fine on the practice and a corrective action plan that includes 2 years of OCR monitoring.
Lessons learned: Had the practice disciplined the physician or taken corrective action after the disclosure, the OCR may not have penalized the group so severely, according to Jennifer Mitchell, a Cincinnati-based health law attorney and vice chair of the American Bar Association eHealth, Privacy, & Security Interest Group.
“In my opinion, the government levied these penalties because the provider did not sanction the doctor,” Ms. Mitchell said in an interview. “Health care entities need to take proper steps to remediate and, at a minimum, hold their workforce responsible for their behavior and ensure that it won’t happen again.”
The case emphasizes the need to train team members on media protocols and to ensure that protected health information is not mistakenly released. In addition to implementing policies and procedures, practices must also be willing to discipline health professionals when violations occur.
“A health care provider’s natural inclination is to defend themselves if they are being accused by a patient,” she said. “However, under the HIPAA rules, health care providers have to understand that they are prohibited from making such public statements about any patient.”
Who? Advanced Care Hospitalists of Lakeland, Fla.
What happened? Advanced Care Hospitalists (ACH) received billing services from an individual who represented himself to be affiliated with a Florida-based company named Doctor’s First Choice Billing. A local hospital later notified ACH that patient information, including names and Social Security numbers, were viewable on the First Choice website. ACH identified at least 400 patients affected by the breach and reported the breach to the OCR. However, ACH later determined that an additional 8,855 patients may have been affected and revised its OCR notification.
What else? During its investigation, the OCR found that the hospitalist group had never entered into a business associate agreement for billing services with First Choice, as required by HIPAA, and that the practice also failed to adopt any policies regarding business associate agreements until 2014, according to a Dec. 4 announcement from HHS.
How much? The OCR fined the practice $500,000 and also imposed a robust corrective action plan that includes an enterprise-wide risk analysis and the adoption of business associate agreements. Roger Severino, OCR director, called the case especially troubling because “the practice allowed the names and Social Security numbers of thousands of patients to be exposed on the Internet after it failed to follow basic security requirements under HIPAA.”
Lessons learned: The case illustrates the importance of having a business associate agreement in place for all third parties that may have access to protected health information, said Clinton Mikel, a Farmington Hills, Mich., health law attorney specializing in HIPAA compliance.
Under HIPAA, a business associate is defined as a person or entity, other than a member of the workforce of a covered entity, who “performs functions or activities on behalf of, or provides certain services to, a covered entity that involve access by the business associate to protected health information.”
HIPAA requires that covered entities enter into contracts with business associates to ensure appropriate safeguarding of protected health information.
“If your business associate has a breach, your practice must report the breach to OCR and your patients,” Mr. Mikel said in an interview. “The OCR will then investigate your practice and your relationship with the business associate. Just because the breach and fault clearly happened elsewhere, you will still be investigated, and could face a penalty if HIPAA requirements weren’t met.”
Who? Filefax of Northbrook, Ill.
What happened? The OCR opened an investigation after receiving an anonymous complaint that medical records obtained from Filefax, a company that provided storage, maintenance, and delivery of medical records for health professionals, were left unmonitored at a shredding and recycling facility. OCR’s investigation revealed that a person left the records of 2,150 patients at the recycling plant and that the records contained protected health information, according to an HHS announcement. It is unclear if the person worked for Filefax.
What else? The OCR discovered that, in a related incident, an individual who obtained medical records from Filefax left them unattended in an unlocked truck in the Filefax parking lot.
How much? The OCR imposed a $100,000 fine on Filefax. The company is no longer in business; however, a court-appointed liquidator has agreed to properly store and dispose of the remaining records.
Lessons learned: Although the case did not involve a health provider, the circumstances are applicable to physicians, particularly when practices move or close, Mr. Mikel said. In some cases, a former patient may contact a shuttered practice only to learn their record cannot be located, or worse, that a breach has occurred.
“[Such a case is] ripe for a patient to complain to OCR,” he said. “OCR doesn’t care if you’re closed or retired, they’re going to look.”
HIPAA requires thatcovered entities apply appropriate administrative, technical, and physical safeguards to protect the privacy of protected health information in any form when moving or closing. The safeguards must prevent prohibited uses and disclosures of protected health information in connection with the disposal of such information, according to the rule. The HHS provides guidance for the disposing of medical records; further, the American Academy of Family Physicians has created a checklist on closing a practice that addresses the transferring of medical records.
Without taking the correct measures, doctors may end up drawing scrutiny from OCR and face a potential fine if violations are found, experts said.
“Covered entities and business associates need to be aware that OCR is committed to enforcing HIPAA regardless of whether a covered entity is opening its doors or closing them,” Mr. Severino of the OCR said in a statement. “HIPAA still applies.”
Data security experts say three HIPAA violations that resulted in significant fines by the Office for Civil Rights (OCR) in 2018 hold important lessons for health professionals about safeguarding records and training staff in HIPAA compliance.
Read on to learn how the cases unfolded and what knowledge practices can gain from the common HIPAA mistakes.
Who? Allergy Associates of Hartford, Conn.
What happened? A patient contacted a local television station to complain about a dispute between herself and a physician at Allergy Associates in Hartford, Conn. The disagreement stemmed from the office turning away the patient because she allegedly brought her service animal, according to a Nov. 26 announcement by the Department of Health & Human Services. The reporter contacted the doctor in question for a news story and, in responding, the physician disclosed protected patient information to the reporter.
What else? An OCR investigation determined that a privacy officer with Allergy Associates had instructed the physician not to respond to the media about the complaint or to respond with “no comment”; that advice was disregarded. The practice then failed to discipline the physician or take any corrective action following the disclosure, according to the OCR.
How much? The OCR imposed a $125,000 fine on the practice and a corrective action plan that includes 2 years of OCR monitoring.
Lessons learned: Had the practice disciplined the physician or taken corrective action after the disclosure, the OCR may not have penalized the group so severely, according to Jennifer Mitchell, a Cincinnati-based health law attorney and vice chair of the American Bar Association eHealth, Privacy, & Security Interest Group.
“In my opinion, the government levied these penalties because the provider did not sanction the doctor,” Ms. Mitchell said in an interview. “Health care entities need to take proper steps to remediate and, at a minimum, hold their workforce responsible for their behavior and ensure that it won’t happen again.”
The case emphasizes the need to train team members on media protocols and to ensure that protected health information is not mistakenly released. In addition to implementing policies and procedures, practices must also be willing to discipline health professionals when violations occur.
“A health care provider’s natural inclination is to defend themselves if they are being accused by a patient,” she said. “However, under the HIPAA rules, health care providers have to understand that they are prohibited from making such public statements about any patient.”
Who? Advanced Care Hospitalists of Lakeland, Fla.
What happened? Advanced Care Hospitalists (ACH) received billing services from an individual who represented himself to be affiliated with a Florida-based company named Doctor’s First Choice Billing. A local hospital later notified ACH that patient information, including names and Social Security numbers, were viewable on the First Choice website. ACH identified at least 400 patients affected by the breach and reported the breach to the OCR. However, ACH later determined that an additional 8,855 patients may have been affected and revised its OCR notification.
What else? During its investigation, the OCR found that the hospitalist group had never entered into a business associate agreement for billing services with First Choice, as required by HIPAA, and that the practice also failed to adopt any policies regarding business associate agreements until 2014, according to a Dec. 4 announcement from HHS.
How much? The OCR fined the practice $500,000 and also imposed a robust corrective action plan that includes an enterprise-wide risk analysis and the adoption of business associate agreements. Roger Severino, OCR director, called the case especially troubling because “the practice allowed the names and Social Security numbers of thousands of patients to be exposed on the Internet after it failed to follow basic security requirements under HIPAA.”
Lessons learned: The case illustrates the importance of having a business associate agreement in place for all third parties that may have access to protected health information, said Clinton Mikel, a Farmington Hills, Mich., health law attorney specializing in HIPAA compliance.
Under HIPAA, a business associate is defined as a person or entity, other than a member of the workforce of a covered entity, who “performs functions or activities on behalf of, or provides certain services to, a covered entity that involve access by the business associate to protected health information.”
HIPAA requires that covered entities enter into contracts with business associates to ensure appropriate safeguarding of protected health information.
“If your business associate has a breach, your practice must report the breach to OCR and your patients,” Mr. Mikel said in an interview. “The OCR will then investigate your practice and your relationship with the business associate. Just because the breach and fault clearly happened elsewhere, you will still be investigated, and could face a penalty if HIPAA requirements weren’t met.”
Who? Filefax of Northbrook, Ill.
What happened? The OCR opened an investigation after receiving an anonymous complaint that medical records obtained from Filefax, a company that provided storage, maintenance, and delivery of medical records for health professionals, were left unmonitored at a shredding and recycling facility. OCR’s investigation revealed that a person left the records of 2,150 patients at the recycling plant and that the records contained protected health information, according to an HHS announcement. It is unclear if the person worked for Filefax.
What else? The OCR discovered that, in a related incident, an individual who obtained medical records from Filefax left them unattended in an unlocked truck in the Filefax parking lot.
How much? The OCR imposed a $100,000 fine on Filefax. The company is no longer in business; however, a court-appointed liquidator has agreed to properly store and dispose of the remaining records.
Lessons learned: Although the case did not involve a health provider, the circumstances are applicable to physicians, particularly when practices move or close, Mr. Mikel said. In some cases, a former patient may contact a shuttered practice only to learn their record cannot be located, or worse, that a breach has occurred.
“[Such a case is] ripe for a patient to complain to OCR,” he said. “OCR doesn’t care if you’re closed or retired, they’re going to look.”
HIPAA requires thatcovered entities apply appropriate administrative, technical, and physical safeguards to protect the privacy of protected health information in any form when moving or closing. The safeguards must prevent prohibited uses and disclosures of protected health information in connection with the disposal of such information, according to the rule. The HHS provides guidance for the disposing of medical records; further, the American Academy of Family Physicians has created a checklist on closing a practice that addresses the transferring of medical records.
Without taking the correct measures, doctors may end up drawing scrutiny from OCR and face a potential fine if violations are found, experts said.
“Covered entities and business associates need to be aware that OCR is committed to enforcing HIPAA regardless of whether a covered entity is opening its doors or closing them,” Mr. Severino of the OCR said in a statement. “HIPAA still applies.”
Data security experts say three HIPAA violations that resulted in significant fines by the Office for Civil Rights (OCR) in 2018 hold important lessons for health professionals about safeguarding records and training staff in HIPAA compliance.
Read on to learn how the cases unfolded and what knowledge practices can gain from the common HIPAA mistakes.
Who? Allergy Associates of Hartford, Conn.
What happened? A patient contacted a local television station to complain about a dispute between herself and a physician at Allergy Associates in Hartford, Conn. The disagreement stemmed from the office turning away the patient because she allegedly brought her service animal, according to a Nov. 26 announcement by the Department of Health & Human Services. The reporter contacted the doctor in question for a news story and, in responding, the physician disclosed protected patient information to the reporter.
What else? An OCR investigation determined that a privacy officer with Allergy Associates had instructed the physician not to respond to the media about the complaint or to respond with “no comment”; that advice was disregarded. The practice then failed to discipline the physician or take any corrective action following the disclosure, according to the OCR.
How much? The OCR imposed a $125,000 fine on the practice and a corrective action plan that includes 2 years of OCR monitoring.
Lessons learned: Had the practice disciplined the physician or taken corrective action after the disclosure, the OCR may not have penalized the group so severely, according to Jennifer Mitchell, a Cincinnati-based health law attorney and vice chair of the American Bar Association eHealth, Privacy, & Security Interest Group.
“In my opinion, the government levied these penalties because the provider did not sanction the doctor,” Ms. Mitchell said in an interview. “Health care entities need to take proper steps to remediate and, at a minimum, hold their workforce responsible for their behavior and ensure that it won’t happen again.”
The case emphasizes the need to train team members on media protocols and to ensure that protected health information is not mistakenly released. In addition to implementing policies and procedures, practices must also be willing to discipline health professionals when violations occur.
“A health care provider’s natural inclination is to defend themselves if they are being accused by a patient,” she said. “However, under the HIPAA rules, health care providers have to understand that they are prohibited from making such public statements about any patient.”
Who? Advanced Care Hospitalists of Lakeland, Fla.
What happened? Advanced Care Hospitalists (ACH) received billing services from an individual who represented himself to be affiliated with a Florida-based company named Doctor’s First Choice Billing. A local hospital later notified ACH that patient information, including names and Social Security numbers, were viewable on the First Choice website. ACH identified at least 400 patients affected by the breach and reported the breach to the OCR. However, ACH later determined that an additional 8,855 patients may have been affected and revised its OCR notification.
What else? During its investigation, the OCR found that the hospitalist group had never entered into a business associate agreement for billing services with First Choice, as required by HIPAA, and that the practice also failed to adopt any policies regarding business associate agreements until 2014, according to a Dec. 4 announcement from HHS.
How much? The OCR fined the practice $500,000 and also imposed a robust corrective action plan that includes an enterprise-wide risk analysis and the adoption of business associate agreements. Roger Severino, OCR director, called the case especially troubling because “the practice allowed the names and Social Security numbers of thousands of patients to be exposed on the Internet after it failed to follow basic security requirements under HIPAA.”
Lessons learned: The case illustrates the importance of having a business associate agreement in place for all third parties that may have access to protected health information, said Clinton Mikel, a Farmington Hills, Mich., health law attorney specializing in HIPAA compliance.
Under HIPAA, a business associate is defined as a person or entity, other than a member of the workforce of a covered entity, who “performs functions or activities on behalf of, or provides certain services to, a covered entity that involve access by the business associate to protected health information.”
HIPAA requires that covered entities enter into contracts with business associates to ensure appropriate safeguarding of protected health information.
“If your business associate has a breach, your practice must report the breach to OCR and your patients,” Mr. Mikel said in an interview. “The OCR will then investigate your practice and your relationship with the business associate. Just because the breach and fault clearly happened elsewhere, you will still be investigated, and could face a penalty if HIPAA requirements weren’t met.”
Who? Filefax of Northbrook, Ill.
What happened? The OCR opened an investigation after receiving an anonymous complaint that medical records obtained from Filefax, a company that provided storage, maintenance, and delivery of medical records for health professionals, were left unmonitored at a shredding and recycling facility. OCR’s investigation revealed that a person left the records of 2,150 patients at the recycling plant and that the records contained protected health information, according to an HHS announcement. It is unclear if the person worked for Filefax.
What else? The OCR discovered that, in a related incident, an individual who obtained medical records from Filefax left them unattended in an unlocked truck in the Filefax parking lot.
How much? The OCR imposed a $100,000 fine on Filefax. The company is no longer in business; however, a court-appointed liquidator has agreed to properly store and dispose of the remaining records.
Lessons learned: Although the case did not involve a health provider, the circumstances are applicable to physicians, particularly when practices move or close, Mr. Mikel said. In some cases, a former patient may contact a shuttered practice only to learn their record cannot be located, or worse, that a breach has occurred.
“[Such a case is] ripe for a patient to complain to OCR,” he said. “OCR doesn’t care if you’re closed or retired, they’re going to look.”
HIPAA requires thatcovered entities apply appropriate administrative, technical, and physical safeguards to protect the privacy of protected health information in any form when moving or closing. The safeguards must prevent prohibited uses and disclosures of protected health information in connection with the disposal of such information, according to the rule. The HHS provides guidance for the disposing of medical records; further, the American Academy of Family Physicians has created a checklist on closing a practice that addresses the transferring of medical records.
Without taking the correct measures, doctors may end up drawing scrutiny from OCR and face a potential fine if violations are found, experts said.
“Covered entities and business associates need to be aware that OCR is committed to enforcing HIPAA regardless of whether a covered entity is opening its doors or closing them,” Mr. Severino of the OCR said in a statement. “HIPAA still applies.”
How can I improve opioid safety at my hospital?
Quality improvement is essential
Case
A 67-year-old opioid-naive male with a history of obstructive sleep apnea and chronic kidney disease became unresponsive 2 days after hip replacement. Physical exam revealed a respiratory rate of 6 breaths/minute and oxygen saturation of 82%. He had received 6 doses of 6-mg IV morphine within the past 7 hours. How can I improve opioid safety at my hospital?
Background
Opioids are the most commonly prescribed class of medication in the hospital and the second–most common class causing adverse drug events (ADEs), the most serious being respiratory depression and death.1
Opioid ADEs and side effects can cause prolonged length of stay and patient suffering. These vary from potentially life-threatening events such as serotonin syndrome and adrenal insufficiency to more manageable problems still requiring intervention such as constipation, urinary retention, cognitive impairment, nausea, and vomiting. Treatment of side effects can lead to complications, including side effects from antiemetics and urinary tract infections from catheters.
A 4-year review found 700 deaths in the United States attributed to patient-controlled analgesia (PCA) use.2 Another study revealed that one out of every 200 patients has postoperative respiratory depression attributable to opioids.3
It is estimated that 2 million patients a year become chronic opioid users. Inpatient opioid prescribing contributes to this problem;4 for instance, 5.9% of patients after minor surgery and 6.5% after major surgery become chronic opioid users if discharged with an opioid.5 Calcaterra et al. found 25% of opioid-naive medical patients received an opioid at discharge from a medical service.6 Those patients had an odds ratio of 4.90 for becoming a chronic opioid user that year.6
Most hospitals have incomplete or outdated policies and procedures for safe opioid prescribing and administration.7 The Joint Commission on Accreditation of Healthcare Organizations has specific pain standards for pain assessment, pain management, and safe opioid prescribing for hospitals. Additions and revisions were developed to go into effect Jan. 1, 2018. (Table 1)8
Quality improvement
Quality improvement (QI) is an effective way to improve opioid safety. The Society of Hospital Medicine has developed a QI guide, “Reducing adverse drug events related to opioids” or “RADEO,” to increase safety and decrease serious ADEs attributable to opioids.7
The steps in the RADEO program are as follows:
1. Assemble your team
It is critical to identify and include stakeholders from multiple disciplines on your project team. This team will be essential to develop a practical project, identify barriers, create solutions, and gain buy-in from medical staff and administrative leadership.
Front-line staff will have invaluable insight and need to be team members. The majority of interventions are performed by nurses; therefore, nursing leadership and input is essential. Representatives from pharmacy, information technology, and the quality department will be extremely valuable team members to guide you through the correct approach to a successful QI project.
A project champion can keep a high profile for the project and build and lead the team.
Identify an “executive sponsor” such as your CEO, CMO, or CNO. This leader will focus the team on issues critical to your organization, such as accreditation from governmental agencies, and help you obtain dedicated time and resources. Aligning with hospital goals will make your project a priority.
Coordinate with existing opioid initiative teams in the hospital to integrate efforts. This will keep the work of different departments aligned and allow you to learn from pitfalls and barriers the other groups experienced.
Patients/families contribute a unique and valuable perspective. Consider including a member of your hospital’s patient and family advisory council on your team.
2. Perform a needs assessment
Determine the current state of your hospital including: opioid prescribers; opioids prescribed; areas with increased ADEs or naloxone use; formulary restrictions, policies, or guidelines for monitoring, prescribing, and administering opioids; order sets; safety alerts; provider education; or patient education.
Your risk management or quality department may be able to a share root cause analysis of ADEs related to opioids. Joint Commission and CMS recommendations as well as other regulatory requirements may shape your QI interventions.8
Most importantly, review all of the concerns and priorities of your diverse team, which will identify areas of most pressing need and provide insight regarding needs you have not considered.
3. Develop SMART aims
Frame your QI project into a series of well-defined, clear SMART aims.9
Specific: Who will carry out the intervention? Who is your target population? What will be improved? In what way will it be improved?
Measurable: What will be measured? How it will be measured? Does it measure the outcome that needs to be improved?
Attainable/achievable: Ensure you have the resources and time to achieve the aim.
Relevant: Ensure each aim moves your team toward the project vision.
Timely: The aim should be achieved within a realistic time frame, long enough to meet goals but not so long that interest is lost.
An example of a poor aim is “Clinicians will improve knowledge of opioids.”
An example of a SMART aim is “75% of inpatient opioid prescribers including MDs, NPs, and PAs will complete and pass the opioid safety training module by July 1, 2018.”
4. Choose metrics
Outcome metrics measure if the intervention has improved patient safety, for example, measuring a decrease in opioid related ADEs. Structure metrics are the physical and organizational properties of the health care delivery setting, for example, the presence of EMR opioid safety. Processes are communication and practice patterns, for example, adherence to policy by examining nursing documentation of pain assessments.
5. Development and implementation 7,10
Use PDSA for development and implementation of the QI intervention.
Plan: Determine the intervention group such as a specific unit, number of units, and if there will be a control group. Determine who will collect the data, if baseline data will be collected, and who will analyze the data. Your information technology department will be essential to determine if the data can be collected via the EMR and how. Input from your multidisciplinary team is critical to anticipate unintended consequences, such as limiting opioid prescribing at discharge inadvertently increasing emergency department visits for pain control.
Do: Start as a small pilot study to make it as easy as possible to implement the project and begin data collection. A small-scale intervention will be more manageable and allow rapid responses to unanticipated problems.
Study: Analyze the data early to determine if the intervention is improving opioid safety and if alterations are needed. At this stage both process metrics (are processes being followed?) and outcome metrics (is the process leading to a desired outcome?) are important.
Act: Based on data analysis, refine the intervention as necessary. You may have to repeat cycles of PDSA to develop the final intervention. Then implement the final intervention to the entire hospital.
The Joint Commission recommendations for opioid QI
The Joint Commission recommends7 the following to reduce opioid-related respiratory depression:
- Effective processes which include processes such as tracking and analyzing ADEs related to opioids.
- Safe technology which includes using technology such as the EMR to monitor opioid prescribing of greater than 90 morphine milligram equivalents.
- Effective tools which include valid and reliable tools to improve opioid safety, such as the Pasero Opioid Induced Sedation Scale (POSS).
- Opioid education and training which includes provider and patient education such as patient discharge education.
Education
Develop educational interventions to ensure medical and hospital staff are aware of new processes, with an emphasis on “why.”7 If possible, use web-based programs that provide CME. Improve education interventions by using multiple live, interactive, and multimedia exposures.
Principles for successful interventions
- Keep it simple for the end user. This makes it more likely that the intervention is performed. Minimize complex tasks such as calculations and if possible design automated processes.
- Build your process into current work flow. If possible simplify or streamline work flow. A project that competes with staff’s other tasks and competing priorities is doomed to fail. It is critical to have input from those performing the intervention to develop a user-friendly and less disruptive intervention.
- Design reliability into the process. Make your intervention the default action. Build prompts into the work flow. Standardize the intervention into the work flow. And, consider having the intervention at scheduled intervals.7
Opioid safety QI interventions
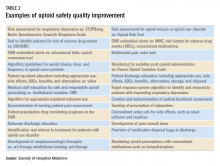
Interventions for improving opioid safety and reducing opioid -elated ADEs may be generalized into areas including risk screening and assessment, pain treatment, opioid administration, pain assessment, post opioid administration monitoring, and patient and provider education (Table 2).7
Back to the case
The patient received naloxone. His respiratory rate and oxygen saturation returned to normal. His dose of morphine was reduced and his interval increased. A multimodal approach was implemented including low-dose scheduled acetaminophen. There were no further ADEs while maintaining good pain control.
A multidisciplinary opioid task force was created and performed a hospital-wide review of opioid ADEs. Opportunities for improvement were identified and new procedures implemented. The Pasero opioid sedation scale (POSS) was added to the nursing work flow to monitor patients who received an opioid for sedation. An algorithm was developed for opioid-naive patients including guidance for opioid selection, dosing, and frequency. Multiple pain control modalities were added to pain control order sets. Annual training was developed for opioid prescribers, pharmacists, and nurses regarding safe and responsible use of opioids.
And, lastly, in-hospital and discharge patient education was developed for patients and families to be well-informed of opioid risk and benefit including how to identify and respond to ADEs.
Bottom line
Quality improvement is an effective method to improve patient safety and reduce serious adverse events related to opioids in the hospital setting.
Dr. Holmes-Maybank, is codirector, Fundamentals of Patient Care Year 1 and Internship 101, and chair, Clinical Competency Examination Committee, division of hospital medicine, Medical University of South Carolina. Dr. Frederickson is medical director, Hospital Medicine and Palliative Care at CHI Health, Omaha, Neb., and assistant professor at Creighton University School of Medicine, Omaha.
References
1. Davies EC et al. Adverse drug reactions in hospital inpatients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439. doi: 10.1371/journal.pone.0004439. Epub 2009 Feb 11.
2. Association for the Advancement of Medical Instrumentation. Infusing patients safely: Priority issues from the AAMI/FDA Infusion Device Summit. 2010;1-39.
3. Dahan Aet al. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226-238. doi: 10.1097/ALN.0b013e3181c38c25.
4. Estimate about opioid users.
5. Brummett CM et al. New persistent opioid use after minor and major surgical procedures in U.S. adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504.
6. Calcaterra SL et al. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-85. doi: 10.1007/s11606-015-3539-4.
7. Frederickson TW et al. Reducing adverse drug events related to opioids implementation guide. Philadelphia: Society of Hospital Medicine, 2015.
8. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
9. Minnesota Department of Health. SMART objectives.
10. Agency for Healthcare Research and Quality. Health Literacy Universal Precautions Toolkit, 2nd Edition.
Plan-Do-Study-Act (PDSA) Directions and Examples.
Recommended reading
Dowell D et al. CDC guideline for prescribing opioids for chronic pain – United States, 2016. Recommendations and Reports. 2016 Mar 18;65(1):1-49.
Frederickson TW et al. Using the 2018 guidelines from the Joint Commission to kickstart your hospital’s program to reduce opioid-induced ventilatory impairment. Anesthesia Patient Safety Foundation Newsletter. 2018;33(1):1-32.
Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018 Apr;13(4):256-62. doi: 10.12788/jhm.2979.
Herzig SJ et al. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018 Apr;13(4):263-71. doi: 10.12788/jhm.2980.
Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
Key points
- Quality improvement is required by the Joint Commission and is an effective method to improve opioid safety in the hospital setting.
- It is critical to the success of a QI project to develop a multidisciplinary team.
- Input from frontline users of the intervention is essential to produce an effective intervention.
- Executive sponsorship and aligning the goals of your QI project with those of your institution will prioritize your project and increase resource availability.
Quiz
1. Based on a needs assessment at your hospital you assemble a multidisciplinary team to improve education for patients discharged on opioids. You recognize the importance of multidisciplinary input to develop a successful intervention for discharge education. Essential team members include all EXCEPT the following:
a. Executive sponsor
b. Patient representative
c. Nursing
d. Medical student representative ---- CORRECT
Explanation: The assembly of a multidisciplinary team is critical to the success of a QI intervention. An executive sponsor may assist you in aligning your goals with that of the hospital and provide resources for its development and implementation. Patient input would help determine how to best deliver the education. Lastly, the individuals carrying out the intervention are essential to develop an intervention that will easy for the end user and increase the likelihood of being used, in this case nursing.
2. You performed a review of naloxone use at your hospital and find that it is greater than similar hospitals. Prior to starting the QI project, you review SHM’s “Reducing adverse events related to opioids implementation guide” and learn that keys to success for QI implementation include:
a. A team of primarily hospitalists
b. Implementing the intervention hospital wide
c. Information technology input for data collection ---- CORRECT
d. No team – it is more effective to work alone
Explanation: Successful implementation of a QI project involves a multidisciplinary team. It is critical to involve information technology early in the development of the project to determine how and if the data can be collected from the EMR. It is best to pilot the intervention on one or two units to make alterations as needed rapidly and perfect the final intervention prior to rolling it out to the entire hospital.
3. You have assembled a multidisciplinary team to respond to the newly revised JCAHO pain standards. An example of a requirement from the new and revised JCAHO standards for pain assessment and management includes:
a. Programs for physician wellness
b. No opioids for chronic pain
c. No more than 5 days of opioids for acute pain
d. Nonpharmacologic pain management options ---- CORRECT
Explanation: JCAHO released new and revised requirements for pain assessment and management including offering nonpharmacologic pain management options. (See Table 1)
4. Your multidisciplinary QI team decides to develop a project to reduce respiratory depression in patients receiving opioids by monitoring for sedation with the Pasero Opioid Induced Sedation Scale. Principles for successful QI interventions include:
a. Complex tasks
b. Make the intervention a default action ---- CORRECT
c. Avoid EMR prompts
d. Competing with other hospital priorities
Explanation: Principles for successful QI interventions include keeping tasks simple, ensuring the intervention does not compete with other priorities, making the intervention the default action, installing prompts in the EMR, and standardizing the intervention into the work flow.
Quality improvement is essential
Quality improvement is essential
Case
A 67-year-old opioid-naive male with a history of obstructive sleep apnea and chronic kidney disease became unresponsive 2 days after hip replacement. Physical exam revealed a respiratory rate of 6 breaths/minute and oxygen saturation of 82%. He had received 6 doses of 6-mg IV morphine within the past 7 hours. How can I improve opioid safety at my hospital?
Background
Opioids are the most commonly prescribed class of medication in the hospital and the second–most common class causing adverse drug events (ADEs), the most serious being respiratory depression and death.1
Opioid ADEs and side effects can cause prolonged length of stay and patient suffering. These vary from potentially life-threatening events such as serotonin syndrome and adrenal insufficiency to more manageable problems still requiring intervention such as constipation, urinary retention, cognitive impairment, nausea, and vomiting. Treatment of side effects can lead to complications, including side effects from antiemetics and urinary tract infections from catheters.
A 4-year review found 700 deaths in the United States attributed to patient-controlled analgesia (PCA) use.2 Another study revealed that one out of every 200 patients has postoperative respiratory depression attributable to opioids.3
It is estimated that 2 million patients a year become chronic opioid users. Inpatient opioid prescribing contributes to this problem;4 for instance, 5.9% of patients after minor surgery and 6.5% after major surgery become chronic opioid users if discharged with an opioid.5 Calcaterra et al. found 25% of opioid-naive medical patients received an opioid at discharge from a medical service.6 Those patients had an odds ratio of 4.90 for becoming a chronic opioid user that year.6
Most hospitals have incomplete or outdated policies and procedures for safe opioid prescribing and administration.7 The Joint Commission on Accreditation of Healthcare Organizations has specific pain standards for pain assessment, pain management, and safe opioid prescribing for hospitals. Additions and revisions were developed to go into effect Jan. 1, 2018. (Table 1)8
Quality improvement
Quality improvement (QI) is an effective way to improve opioid safety. The Society of Hospital Medicine has developed a QI guide, “Reducing adverse drug events related to opioids” or “RADEO,” to increase safety and decrease serious ADEs attributable to opioids.7
The steps in the RADEO program are as follows:
1. Assemble your team
It is critical to identify and include stakeholders from multiple disciplines on your project team. This team will be essential to develop a practical project, identify barriers, create solutions, and gain buy-in from medical staff and administrative leadership.
Front-line staff will have invaluable insight and need to be team members. The majority of interventions are performed by nurses; therefore, nursing leadership and input is essential. Representatives from pharmacy, information technology, and the quality department will be extremely valuable team members to guide you through the correct approach to a successful QI project.
A project champion can keep a high profile for the project and build and lead the team.
Identify an “executive sponsor” such as your CEO, CMO, or CNO. This leader will focus the team on issues critical to your organization, such as accreditation from governmental agencies, and help you obtain dedicated time and resources. Aligning with hospital goals will make your project a priority.
Coordinate with existing opioid initiative teams in the hospital to integrate efforts. This will keep the work of different departments aligned and allow you to learn from pitfalls and barriers the other groups experienced.
Patients/families contribute a unique and valuable perspective. Consider including a member of your hospital’s patient and family advisory council on your team.
2. Perform a needs assessment
Determine the current state of your hospital including: opioid prescribers; opioids prescribed; areas with increased ADEs or naloxone use; formulary restrictions, policies, or guidelines for monitoring, prescribing, and administering opioids; order sets; safety alerts; provider education; or patient education.
Your risk management or quality department may be able to a share root cause analysis of ADEs related to opioids. Joint Commission and CMS recommendations as well as other regulatory requirements may shape your QI interventions.8
Most importantly, review all of the concerns and priorities of your diverse team, which will identify areas of most pressing need and provide insight regarding needs you have not considered.
3. Develop SMART aims
Frame your QI project into a series of well-defined, clear SMART aims.9
Specific: Who will carry out the intervention? Who is your target population? What will be improved? In what way will it be improved?
Measurable: What will be measured? How it will be measured? Does it measure the outcome that needs to be improved?
Attainable/achievable: Ensure you have the resources and time to achieve the aim.
Relevant: Ensure each aim moves your team toward the project vision.
Timely: The aim should be achieved within a realistic time frame, long enough to meet goals but not so long that interest is lost.
An example of a poor aim is “Clinicians will improve knowledge of opioids.”
An example of a SMART aim is “75% of inpatient opioid prescribers including MDs, NPs, and PAs will complete and pass the opioid safety training module by July 1, 2018.”
4. Choose metrics
Outcome metrics measure if the intervention has improved patient safety, for example, measuring a decrease in opioid related ADEs. Structure metrics are the physical and organizational properties of the health care delivery setting, for example, the presence of EMR opioid safety. Processes are communication and practice patterns, for example, adherence to policy by examining nursing documentation of pain assessments.
5. Development and implementation 7,10
Use PDSA for development and implementation of the QI intervention.
Plan: Determine the intervention group such as a specific unit, number of units, and if there will be a control group. Determine who will collect the data, if baseline data will be collected, and who will analyze the data. Your information technology department will be essential to determine if the data can be collected via the EMR and how. Input from your multidisciplinary team is critical to anticipate unintended consequences, such as limiting opioid prescribing at discharge inadvertently increasing emergency department visits for pain control.
Do: Start as a small pilot study to make it as easy as possible to implement the project and begin data collection. A small-scale intervention will be more manageable and allow rapid responses to unanticipated problems.
Study: Analyze the data early to determine if the intervention is improving opioid safety and if alterations are needed. At this stage both process metrics (are processes being followed?) and outcome metrics (is the process leading to a desired outcome?) are important.
Act: Based on data analysis, refine the intervention as necessary. You may have to repeat cycles of PDSA to develop the final intervention. Then implement the final intervention to the entire hospital.
The Joint Commission recommendations for opioid QI
The Joint Commission recommends7 the following to reduce opioid-related respiratory depression:
- Effective processes which include processes such as tracking and analyzing ADEs related to opioids.
- Safe technology which includes using technology such as the EMR to monitor opioid prescribing of greater than 90 morphine milligram equivalents.
- Effective tools which include valid and reliable tools to improve opioid safety, such as the Pasero Opioid Induced Sedation Scale (POSS).
- Opioid education and training which includes provider and patient education such as patient discharge education.
Education
Develop educational interventions to ensure medical and hospital staff are aware of new processes, with an emphasis on “why.”7 If possible, use web-based programs that provide CME. Improve education interventions by using multiple live, interactive, and multimedia exposures.
Principles for successful interventions
- Keep it simple for the end user. This makes it more likely that the intervention is performed. Minimize complex tasks such as calculations and if possible design automated processes.
- Build your process into current work flow. If possible simplify or streamline work flow. A project that competes with staff’s other tasks and competing priorities is doomed to fail. It is critical to have input from those performing the intervention to develop a user-friendly and less disruptive intervention.
- Design reliability into the process. Make your intervention the default action. Build prompts into the work flow. Standardize the intervention into the work flow. And, consider having the intervention at scheduled intervals.7
Opioid safety QI interventions
Interventions for improving opioid safety and reducing opioid -elated ADEs may be generalized into areas including risk screening and assessment, pain treatment, opioid administration, pain assessment, post opioid administration monitoring, and patient and provider education (Table 2).7
Back to the case
The patient received naloxone. His respiratory rate and oxygen saturation returned to normal. His dose of morphine was reduced and his interval increased. A multimodal approach was implemented including low-dose scheduled acetaminophen. There were no further ADEs while maintaining good pain control.
A multidisciplinary opioid task force was created and performed a hospital-wide review of opioid ADEs. Opportunities for improvement were identified and new procedures implemented. The Pasero opioid sedation scale (POSS) was added to the nursing work flow to monitor patients who received an opioid for sedation. An algorithm was developed for opioid-naive patients including guidance for opioid selection, dosing, and frequency. Multiple pain control modalities were added to pain control order sets. Annual training was developed for opioid prescribers, pharmacists, and nurses regarding safe and responsible use of opioids.
And, lastly, in-hospital and discharge patient education was developed for patients and families to be well-informed of opioid risk and benefit including how to identify and respond to ADEs.
Bottom line
Quality improvement is an effective method to improve patient safety and reduce serious adverse events related to opioids in the hospital setting.
Dr. Holmes-Maybank, is codirector, Fundamentals of Patient Care Year 1 and Internship 101, and chair, Clinical Competency Examination Committee, division of hospital medicine, Medical University of South Carolina. Dr. Frederickson is medical director, Hospital Medicine and Palliative Care at CHI Health, Omaha, Neb., and assistant professor at Creighton University School of Medicine, Omaha.
References
1. Davies EC et al. Adverse drug reactions in hospital inpatients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439. doi: 10.1371/journal.pone.0004439. Epub 2009 Feb 11.
2. Association for the Advancement of Medical Instrumentation. Infusing patients safely: Priority issues from the AAMI/FDA Infusion Device Summit. 2010;1-39.
3. Dahan Aet al. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226-238. doi: 10.1097/ALN.0b013e3181c38c25.
4. Estimate about opioid users.
5. Brummett CM et al. New persistent opioid use after minor and major surgical procedures in U.S. adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504.
6. Calcaterra SL et al. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-85. doi: 10.1007/s11606-015-3539-4.
7. Frederickson TW et al. Reducing adverse drug events related to opioids implementation guide. Philadelphia: Society of Hospital Medicine, 2015.
8. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
9. Minnesota Department of Health. SMART objectives.
10. Agency for Healthcare Research and Quality. Health Literacy Universal Precautions Toolkit, 2nd Edition.
Plan-Do-Study-Act (PDSA) Directions and Examples.
Recommended reading
Dowell D et al. CDC guideline for prescribing opioids for chronic pain – United States, 2016. Recommendations and Reports. 2016 Mar 18;65(1):1-49.
Frederickson TW et al. Using the 2018 guidelines from the Joint Commission to kickstart your hospital’s program to reduce opioid-induced ventilatory impairment. Anesthesia Patient Safety Foundation Newsletter. 2018;33(1):1-32.
Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018 Apr;13(4):256-62. doi: 10.12788/jhm.2979.
Herzig SJ et al. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018 Apr;13(4):263-71. doi: 10.12788/jhm.2980.
Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
Key points
- Quality improvement is required by the Joint Commission and is an effective method to improve opioid safety in the hospital setting.
- It is critical to the success of a QI project to develop a multidisciplinary team.
- Input from frontline users of the intervention is essential to produce an effective intervention.
- Executive sponsorship and aligning the goals of your QI project with those of your institution will prioritize your project and increase resource availability.
Quiz
1. Based on a needs assessment at your hospital you assemble a multidisciplinary team to improve education for patients discharged on opioids. You recognize the importance of multidisciplinary input to develop a successful intervention for discharge education. Essential team members include all EXCEPT the following:
a. Executive sponsor
b. Patient representative
c. Nursing
d. Medical student representative ---- CORRECT
Explanation: The assembly of a multidisciplinary team is critical to the success of a QI intervention. An executive sponsor may assist you in aligning your goals with that of the hospital and provide resources for its development and implementation. Patient input would help determine how to best deliver the education. Lastly, the individuals carrying out the intervention are essential to develop an intervention that will easy for the end user and increase the likelihood of being used, in this case nursing.
2. You performed a review of naloxone use at your hospital and find that it is greater than similar hospitals. Prior to starting the QI project, you review SHM’s “Reducing adverse events related to opioids implementation guide” and learn that keys to success for QI implementation include:
a. A team of primarily hospitalists
b. Implementing the intervention hospital wide
c. Information technology input for data collection ---- CORRECT
d. No team – it is more effective to work alone
Explanation: Successful implementation of a QI project involves a multidisciplinary team. It is critical to involve information technology early in the development of the project to determine how and if the data can be collected from the EMR. It is best to pilot the intervention on one or two units to make alterations as needed rapidly and perfect the final intervention prior to rolling it out to the entire hospital.
3. You have assembled a multidisciplinary team to respond to the newly revised JCAHO pain standards. An example of a requirement from the new and revised JCAHO standards for pain assessment and management includes:
a. Programs for physician wellness
b. No opioids for chronic pain
c. No more than 5 days of opioids for acute pain
d. Nonpharmacologic pain management options ---- CORRECT
Explanation: JCAHO released new and revised requirements for pain assessment and management including offering nonpharmacologic pain management options. (See Table 1)
4. Your multidisciplinary QI team decides to develop a project to reduce respiratory depression in patients receiving opioids by monitoring for sedation with the Pasero Opioid Induced Sedation Scale. Principles for successful QI interventions include:
a. Complex tasks
b. Make the intervention a default action ---- CORRECT
c. Avoid EMR prompts
d. Competing with other hospital priorities
Explanation: Principles for successful QI interventions include keeping tasks simple, ensuring the intervention does not compete with other priorities, making the intervention the default action, installing prompts in the EMR, and standardizing the intervention into the work flow.
Case
A 67-year-old opioid-naive male with a history of obstructive sleep apnea and chronic kidney disease became unresponsive 2 days after hip replacement. Physical exam revealed a respiratory rate of 6 breaths/minute and oxygen saturation of 82%. He had received 6 doses of 6-mg IV morphine within the past 7 hours. How can I improve opioid safety at my hospital?
Background
Opioids are the most commonly prescribed class of medication in the hospital and the second–most common class causing adverse drug events (ADEs), the most serious being respiratory depression and death.1
Opioid ADEs and side effects can cause prolonged length of stay and patient suffering. These vary from potentially life-threatening events such as serotonin syndrome and adrenal insufficiency to more manageable problems still requiring intervention such as constipation, urinary retention, cognitive impairment, nausea, and vomiting. Treatment of side effects can lead to complications, including side effects from antiemetics and urinary tract infections from catheters.
A 4-year review found 700 deaths in the United States attributed to patient-controlled analgesia (PCA) use.2 Another study revealed that one out of every 200 patients has postoperative respiratory depression attributable to opioids.3
It is estimated that 2 million patients a year become chronic opioid users. Inpatient opioid prescribing contributes to this problem;4 for instance, 5.9% of patients after minor surgery and 6.5% after major surgery become chronic opioid users if discharged with an opioid.5 Calcaterra et al. found 25% of opioid-naive medical patients received an opioid at discharge from a medical service.6 Those patients had an odds ratio of 4.90 for becoming a chronic opioid user that year.6
Most hospitals have incomplete or outdated policies and procedures for safe opioid prescribing and administration.7 The Joint Commission on Accreditation of Healthcare Organizations has specific pain standards for pain assessment, pain management, and safe opioid prescribing for hospitals. Additions and revisions were developed to go into effect Jan. 1, 2018. (Table 1)8
Quality improvement
Quality improvement (QI) is an effective way to improve opioid safety. The Society of Hospital Medicine has developed a QI guide, “Reducing adverse drug events related to opioids” or “RADEO,” to increase safety and decrease serious ADEs attributable to opioids.7
The steps in the RADEO program are as follows:
1. Assemble your team
It is critical to identify and include stakeholders from multiple disciplines on your project team. This team will be essential to develop a practical project, identify barriers, create solutions, and gain buy-in from medical staff and administrative leadership.
Front-line staff will have invaluable insight and need to be team members. The majority of interventions are performed by nurses; therefore, nursing leadership and input is essential. Representatives from pharmacy, information technology, and the quality department will be extremely valuable team members to guide you through the correct approach to a successful QI project.
A project champion can keep a high profile for the project and build and lead the team.
Identify an “executive sponsor” such as your CEO, CMO, or CNO. This leader will focus the team on issues critical to your organization, such as accreditation from governmental agencies, and help you obtain dedicated time and resources. Aligning with hospital goals will make your project a priority.
Coordinate with existing opioid initiative teams in the hospital to integrate efforts. This will keep the work of different departments aligned and allow you to learn from pitfalls and barriers the other groups experienced.
Patients/families contribute a unique and valuable perspective. Consider including a member of your hospital’s patient and family advisory council on your team.
2. Perform a needs assessment
Determine the current state of your hospital including: opioid prescribers; opioids prescribed; areas with increased ADEs or naloxone use; formulary restrictions, policies, or guidelines for monitoring, prescribing, and administering opioids; order sets; safety alerts; provider education; or patient education.
Your risk management or quality department may be able to a share root cause analysis of ADEs related to opioids. Joint Commission and CMS recommendations as well as other regulatory requirements may shape your QI interventions.8
Most importantly, review all of the concerns and priorities of your diverse team, which will identify areas of most pressing need and provide insight regarding needs you have not considered.
3. Develop SMART aims
Frame your QI project into a series of well-defined, clear SMART aims.9
Specific: Who will carry out the intervention? Who is your target population? What will be improved? In what way will it be improved?
Measurable: What will be measured? How it will be measured? Does it measure the outcome that needs to be improved?
Attainable/achievable: Ensure you have the resources and time to achieve the aim.
Relevant: Ensure each aim moves your team toward the project vision.
Timely: The aim should be achieved within a realistic time frame, long enough to meet goals but not so long that interest is lost.
An example of a poor aim is “Clinicians will improve knowledge of opioids.”
An example of a SMART aim is “75% of inpatient opioid prescribers including MDs, NPs, and PAs will complete and pass the opioid safety training module by July 1, 2018.”
4. Choose metrics
Outcome metrics measure if the intervention has improved patient safety, for example, measuring a decrease in opioid related ADEs. Structure metrics are the physical and organizational properties of the health care delivery setting, for example, the presence of EMR opioid safety. Processes are communication and practice patterns, for example, adherence to policy by examining nursing documentation of pain assessments.
5. Development and implementation 7,10
Use PDSA for development and implementation of the QI intervention.
Plan: Determine the intervention group such as a specific unit, number of units, and if there will be a control group. Determine who will collect the data, if baseline data will be collected, and who will analyze the data. Your information technology department will be essential to determine if the data can be collected via the EMR and how. Input from your multidisciplinary team is critical to anticipate unintended consequences, such as limiting opioid prescribing at discharge inadvertently increasing emergency department visits for pain control.
Do: Start as a small pilot study to make it as easy as possible to implement the project and begin data collection. A small-scale intervention will be more manageable and allow rapid responses to unanticipated problems.
Study: Analyze the data early to determine if the intervention is improving opioid safety and if alterations are needed. At this stage both process metrics (are processes being followed?) and outcome metrics (is the process leading to a desired outcome?) are important.
Act: Based on data analysis, refine the intervention as necessary. You may have to repeat cycles of PDSA to develop the final intervention. Then implement the final intervention to the entire hospital.
The Joint Commission recommendations for opioid QI
The Joint Commission recommends7 the following to reduce opioid-related respiratory depression:
- Effective processes which include processes such as tracking and analyzing ADEs related to opioids.
- Safe technology which includes using technology such as the EMR to monitor opioid prescribing of greater than 90 morphine milligram equivalents.
- Effective tools which include valid and reliable tools to improve opioid safety, such as the Pasero Opioid Induced Sedation Scale (POSS).
- Opioid education and training which includes provider and patient education such as patient discharge education.
Education
Develop educational interventions to ensure medical and hospital staff are aware of new processes, with an emphasis on “why.”7 If possible, use web-based programs that provide CME. Improve education interventions by using multiple live, interactive, and multimedia exposures.
Principles for successful interventions
- Keep it simple for the end user. This makes it more likely that the intervention is performed. Minimize complex tasks such as calculations and if possible design automated processes.
- Build your process into current work flow. If possible simplify or streamline work flow. A project that competes with staff’s other tasks and competing priorities is doomed to fail. It is critical to have input from those performing the intervention to develop a user-friendly and less disruptive intervention.
- Design reliability into the process. Make your intervention the default action. Build prompts into the work flow. Standardize the intervention into the work flow. And, consider having the intervention at scheduled intervals.7
Opioid safety QI interventions
Interventions for improving opioid safety and reducing opioid -elated ADEs may be generalized into areas including risk screening and assessment, pain treatment, opioid administration, pain assessment, post opioid administration monitoring, and patient and provider education (Table 2).7
Back to the case
The patient received naloxone. His respiratory rate and oxygen saturation returned to normal. His dose of morphine was reduced and his interval increased. A multimodal approach was implemented including low-dose scheduled acetaminophen. There were no further ADEs while maintaining good pain control.
A multidisciplinary opioid task force was created and performed a hospital-wide review of opioid ADEs. Opportunities for improvement were identified and new procedures implemented. The Pasero opioid sedation scale (POSS) was added to the nursing work flow to monitor patients who received an opioid for sedation. An algorithm was developed for opioid-naive patients including guidance for opioid selection, dosing, and frequency. Multiple pain control modalities were added to pain control order sets. Annual training was developed for opioid prescribers, pharmacists, and nurses regarding safe and responsible use of opioids.
And, lastly, in-hospital and discharge patient education was developed for patients and families to be well-informed of opioid risk and benefit including how to identify and respond to ADEs.
Bottom line
Quality improvement is an effective method to improve patient safety and reduce serious adverse events related to opioids in the hospital setting.
Dr. Holmes-Maybank, is codirector, Fundamentals of Patient Care Year 1 and Internship 101, and chair, Clinical Competency Examination Committee, division of hospital medicine, Medical University of South Carolina. Dr. Frederickson is medical director, Hospital Medicine and Palliative Care at CHI Health, Omaha, Neb., and assistant professor at Creighton University School of Medicine, Omaha.
References
1. Davies EC et al. Adverse drug reactions in hospital inpatients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439. doi: 10.1371/journal.pone.0004439. Epub 2009 Feb 11.
2. Association for the Advancement of Medical Instrumentation. Infusing patients safely: Priority issues from the AAMI/FDA Infusion Device Summit. 2010;1-39.
3. Dahan Aet al. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226-238. doi: 10.1097/ALN.0b013e3181c38c25.
4. Estimate about opioid users.
5. Brummett CM et al. New persistent opioid use after minor and major surgical procedures in U.S. adults. JAMA Surg. 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504.
6. Calcaterra SL et al. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-85. doi: 10.1007/s11606-015-3539-4.
7. Frederickson TW et al. Reducing adverse drug events related to opioids implementation guide. Philadelphia: Society of Hospital Medicine, 2015.
8. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
9. Minnesota Department of Health. SMART objectives.
10. Agency for Healthcare Research and Quality. Health Literacy Universal Precautions Toolkit, 2nd Edition.
Plan-Do-Study-Act (PDSA) Directions and Examples.
Recommended reading
Dowell D et al. CDC guideline for prescribing opioids for chronic pain – United States, 2016. Recommendations and Reports. 2016 Mar 18;65(1):1-49.
Frederickson TW et al. Using the 2018 guidelines from the Joint Commission to kickstart your hospital’s program to reduce opioid-induced ventilatory impairment. Anesthesia Patient Safety Foundation Newsletter. 2018;33(1):1-32.
Herzig SJ et al. Safe opioid prescribing for acute noncancer pain in hospitalized adults: a systematic review of existing guidelines. J Hosp Med. 2018 Apr;13(4):256-62. doi: 10.12788/jhm.2979.
Herzig SJ et al. Improving the safety of opioid use for acute noncancer pain in hospitalized adults: a consensus statement from the society of hospital medicine. J Hosp Med. 2018 Apr;13(4):263-71. doi: 10.12788/jhm.2980.
Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):2-4.
Key points
- Quality improvement is required by the Joint Commission and is an effective method to improve opioid safety in the hospital setting.
- It is critical to the success of a QI project to develop a multidisciplinary team.
- Input from frontline users of the intervention is essential to produce an effective intervention.
- Executive sponsorship and aligning the goals of your QI project with those of your institution will prioritize your project and increase resource availability.
Quiz
1. Based on a needs assessment at your hospital you assemble a multidisciplinary team to improve education for patients discharged on opioids. You recognize the importance of multidisciplinary input to develop a successful intervention for discharge education. Essential team members include all EXCEPT the following:
a. Executive sponsor
b. Patient representative
c. Nursing
d. Medical student representative ---- CORRECT
Explanation: The assembly of a multidisciplinary team is critical to the success of a QI intervention. An executive sponsor may assist you in aligning your goals with that of the hospital and provide resources for its development and implementation. Patient input would help determine how to best deliver the education. Lastly, the individuals carrying out the intervention are essential to develop an intervention that will easy for the end user and increase the likelihood of being used, in this case nursing.
2. You performed a review of naloxone use at your hospital and find that it is greater than similar hospitals. Prior to starting the QI project, you review SHM’s “Reducing adverse events related to opioids implementation guide” and learn that keys to success for QI implementation include:
a. A team of primarily hospitalists
b. Implementing the intervention hospital wide
c. Information technology input for data collection ---- CORRECT
d. No team – it is more effective to work alone
Explanation: Successful implementation of a QI project involves a multidisciplinary team. It is critical to involve information technology early in the development of the project to determine how and if the data can be collected from the EMR. It is best to pilot the intervention on one or two units to make alterations as needed rapidly and perfect the final intervention prior to rolling it out to the entire hospital.
3. You have assembled a multidisciplinary team to respond to the newly revised JCAHO pain standards. An example of a requirement from the new and revised JCAHO standards for pain assessment and management includes:
a. Programs for physician wellness
b. No opioids for chronic pain
c. No more than 5 days of opioids for acute pain
d. Nonpharmacologic pain management options ---- CORRECT
Explanation: JCAHO released new and revised requirements for pain assessment and management including offering nonpharmacologic pain management options. (See Table 1)
4. Your multidisciplinary QI team decides to develop a project to reduce respiratory depression in patients receiving opioids by monitoring for sedation with the Pasero Opioid Induced Sedation Scale. Principles for successful QI interventions include:
a. Complex tasks
b. Make the intervention a default action ---- CORRECT
c. Avoid EMR prompts
d. Competing with other hospital priorities
Explanation: Principles for successful QI interventions include keeping tasks simple, ensuring the intervention does not compete with other priorities, making the intervention the default action, installing prompts in the EMR, and standardizing the intervention into the work flow.
Reducing heart failure readmissions raises mortality
This week from MDedge Cardiology, the Hospital Readmissions Reduction Program may be doing more harm than good, ticagrelor holds no edge over aspirin in CABG patients, weight-loss apps lack evidence, and the Surgeon General sends out an alarm.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week from MDedge Cardiology, the Hospital Readmissions Reduction Program may be doing more harm than good, ticagrelor holds no edge over aspirin in CABG patients, weight-loss apps lack evidence, and the Surgeon General sends out an alarm.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
This week from MDedge Cardiology, the Hospital Readmissions Reduction Program may be doing more harm than good, ticagrelor holds no edge over aspirin in CABG patients, weight-loss apps lack evidence, and the Surgeon General sends out an alarm.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon Alexa
Apple Podcasts
Childhood inflammatory bowel disease linked to increased mortality
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
FROM GASTROENTEROLOGY
Key clinical point: Childhood-onset inflammatory bowel disease is associated with an increased risk of death.
Major finding: The risk was approximately threefold higher than in the general population in those with childhood-onset inflammatory bowel disease aged under 25 years (aHR, 3.2; 95% CI, 2.8-3.7).
Study details: Retrospective cohort study of 9,442 patients with childhood-onset inflammatory bowel disease and 93,180 individuals from the general population.
Disclosures: Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
Source: Olén O et al. Gastroenterology. 2018 Oct 17.
Metachronous advanced neoplasia linked to diminutive polyp number, not histology
Among patients with diminutive (1-5 mm) colonic polyps, multiplicity was a significant risk factor for advanced metachronous colonic neoplasia, while advanced histologic features alone were not, according to the results of a pooled analysis of data from 64,344 patients.
Metachronous advanced neoplasia affected similar proportions of patients with and without high-risk diminutive polyps (17.6% vs. 14.6%, respectively; relative risk, 1.13; 95% confidence interval, 0.79-1.61), reported Jasper L.A. Vleugels, MD, of the University of Amsterdam, together with his associates. However, patients with at least three nonadvanced (diminutive or small) adenomas were at significantly increased risk of metachronous advanced neoplasia, compared with low-risk patients (overall risk ratio, 2.12; 95% CI, 1.89-2.38), the investigators wrote in the February issue of Gastroenterology.
This multicenter study spanned 12 prospectively evaluated cohorts of patients in the United States and Europe. All patients underwent colonoscopy because of a positive fecal immunochemical test result or for the purpose of screening, surveillance, or evaluation of symptoms. The researchers defined low-risk patients as individuals with one or two diminutive or small nonadvanced adenomas. In contrast, “high-risk” patients had a polyp with advanced histology (at least a 25% villous component, high-grade dysplasia, or colonic rectal carcinoma), at least three diminutive (1-5 mm) or small (6-9 mm) nonadvanced adenomas, or an adenomatous or sessile serrated lesion measuring at least 10 mm.
Among more than 50,000 diminutive polyps in the dataset, the prevalence of advanced histologic features was 7.1% among patients who underwent colonoscopy because of a positive fecal immunochemical test and 1.5% among those who had a colonoscopy for other reasons (P = .04). However, statistically similar proportions of patients in each of these subgroups were classified as “high risk” because of advanced histology (0.8% and 0.4%, respectively) or multiplicity (3.8% and 3.0%, respectively). Because metachronous advanced neoplasia was detected in similar proportions of patients with and without diminutive polyps with advanced histologic features (17.6% vs. 14.6%, respectively), the presence of such features did not independently predict metachronous advanced neoplasia, either overall (relative risk, 1.13; 95% CI, 0.79-1.61), or in either subgroup.
“On the other hand, multiplicity of diminutive adenomas was associated with increased risk of metachronous advanced neoplasia,” the researchers wrote. Among these patients, nearly 24% of those in the fecal immunochemical subgroup developed metachronous advanced neoplasia, as did nearly 30% of those who had a colonoscopy for other reasons, yielding risk ratios of 2.45 (95% CI, 1.67-3.58) and 1.92 (95% CI, 1.68-2.20), respectively.
“While multiplicity has been described as a risk factor of metachronous advanced adenomas, we were surprised to find that even if all adenomas are diminutive, the risk was increased,” the investigators commented. Taken together, the findings “underline the importance of correctly classifying diminutive adenomatous lesions, preventing misclassification of patients with at least three adenomas to a low-risk status.”
Partial funding for this study came from PERIS and Fundción Científica de la Asociación Española contra el Cáncer. Dr. Vleugels reported having no conflicts of interest. Three coinvestigators disclosed ties to Fujifilm, Olympus, Norgine, Clinical Genomics, and Boston Scientific.
SOURCE: Vleugels J et al. Gastroenterology. 2018 Nov 2. doi: 10.1053/j.gastro.2018.10.050.
When to perform surveillance colonoscopy in patients previously diagnosed with colorectal neoplasms is one of the most significant questions facing experts creating guidelines for colorectal cancer (CRC) screening. This study by Vleugels et al. provides important information that should better inform this issue.
The authors pooled data from 12 different study cohorts of patients undergoing colonoscopy in either the United States or Europe. The cohorts included patients who underwent colonoscopy to follow up a positive fecal immunochemical test (FIT) or as a primary test for screening, surveillance, or symptom management. The authors found that diminutive adenomas (1-5 mm) rarely contained advanced histology (CRC, high-grade dysplasia, or more than 25% villous features) and that these lesions seldom defined patients regarding risk for metachronous neoplasms. They also found that high-risk patients defined by 1-2 diminutive adenomas with advanced histology were no more likely to develop metachronous advanced neoplasia (adenoma containing advanced histology, at least three diminutive or small nonadvanced adenomas, or an adenoma or sessile serrated lesion at least 10 mm) than were patients defined as low risk by their initial adenoma histology. Interestingly, multiplicity (more than three) of diminutive or small adenomas regardless of histology did predict a significantly increased risk of metachronous advanced neoplasia. These data support a resect-and-discard strategy (not sending the resected polyp to pathology) for diminutive polyps and polyp surveillance guidelines that employ less frequent colonoscopy to follow patients whose most significant finding at initial colonoscopy is a diminutive adenoma.
Future studies should examine the risk for metachronous neoplasms posed by diminutive adenoma within the milieu of other patient characteristics informing colorectal cancer risk.
Reid M. Ness, MD, MPH, AGAF, is an associate professor of medicine in the division compliance and a quality expert in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. He has no financial conflicts to disclose.
When to perform surveillance colonoscopy in patients previously diagnosed with colorectal neoplasms is one of the most significant questions facing experts creating guidelines for colorectal cancer (CRC) screening. This study by Vleugels et al. provides important information that should better inform this issue.
The authors pooled data from 12 different study cohorts of patients undergoing colonoscopy in either the United States or Europe. The cohorts included patients who underwent colonoscopy to follow up a positive fecal immunochemical test (FIT) or as a primary test for screening, surveillance, or symptom management. The authors found that diminutive adenomas (1-5 mm) rarely contained advanced histology (CRC, high-grade dysplasia, or more than 25% villous features) and that these lesions seldom defined patients regarding risk for metachronous neoplasms. They also found that high-risk patients defined by 1-2 diminutive adenomas with advanced histology were no more likely to develop metachronous advanced neoplasia (adenoma containing advanced histology, at least three diminutive or small nonadvanced adenomas, or an adenoma or sessile serrated lesion at least 10 mm) than were patients defined as low risk by their initial adenoma histology. Interestingly, multiplicity (more than three) of diminutive or small adenomas regardless of histology did predict a significantly increased risk of metachronous advanced neoplasia. These data support a resect-and-discard strategy (not sending the resected polyp to pathology) for diminutive polyps and polyp surveillance guidelines that employ less frequent colonoscopy to follow patients whose most significant finding at initial colonoscopy is a diminutive adenoma.
Future studies should examine the risk for metachronous neoplasms posed by diminutive adenoma within the milieu of other patient characteristics informing colorectal cancer risk.
Reid M. Ness, MD, MPH, AGAF, is an associate professor of medicine in the division compliance and a quality expert in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. He has no financial conflicts to disclose.
When to perform surveillance colonoscopy in patients previously diagnosed with colorectal neoplasms is one of the most significant questions facing experts creating guidelines for colorectal cancer (CRC) screening. This study by Vleugels et al. provides important information that should better inform this issue.
The authors pooled data from 12 different study cohorts of patients undergoing colonoscopy in either the United States or Europe. The cohorts included patients who underwent colonoscopy to follow up a positive fecal immunochemical test (FIT) or as a primary test for screening, surveillance, or symptom management. The authors found that diminutive adenomas (1-5 mm) rarely contained advanced histology (CRC, high-grade dysplasia, or more than 25% villous features) and that these lesions seldom defined patients regarding risk for metachronous neoplasms. They also found that high-risk patients defined by 1-2 diminutive adenomas with advanced histology were no more likely to develop metachronous advanced neoplasia (adenoma containing advanced histology, at least three diminutive or small nonadvanced adenomas, or an adenoma or sessile serrated lesion at least 10 mm) than were patients defined as low risk by their initial adenoma histology. Interestingly, multiplicity (more than three) of diminutive or small adenomas regardless of histology did predict a significantly increased risk of metachronous advanced neoplasia. These data support a resect-and-discard strategy (not sending the resected polyp to pathology) for diminutive polyps and polyp surveillance guidelines that employ less frequent colonoscopy to follow patients whose most significant finding at initial colonoscopy is a diminutive adenoma.
Future studies should examine the risk for metachronous neoplasms posed by diminutive adenoma within the milieu of other patient characteristics informing colorectal cancer risk.
Reid M. Ness, MD, MPH, AGAF, is an associate professor of medicine in the division compliance and a quality expert in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. He has no financial conflicts to disclose.
Among patients with diminutive (1-5 mm) colonic polyps, multiplicity was a significant risk factor for advanced metachronous colonic neoplasia, while advanced histologic features alone were not, according to the results of a pooled analysis of data from 64,344 patients.
Metachronous advanced neoplasia affected similar proportions of patients with and without high-risk diminutive polyps (17.6% vs. 14.6%, respectively; relative risk, 1.13; 95% confidence interval, 0.79-1.61), reported Jasper L.A. Vleugels, MD, of the University of Amsterdam, together with his associates. However, patients with at least three nonadvanced (diminutive or small) adenomas were at significantly increased risk of metachronous advanced neoplasia, compared with low-risk patients (overall risk ratio, 2.12; 95% CI, 1.89-2.38), the investigators wrote in the February issue of Gastroenterology.
This multicenter study spanned 12 prospectively evaluated cohorts of patients in the United States and Europe. All patients underwent colonoscopy because of a positive fecal immunochemical test result or for the purpose of screening, surveillance, or evaluation of symptoms. The researchers defined low-risk patients as individuals with one or two diminutive or small nonadvanced adenomas. In contrast, “high-risk” patients had a polyp with advanced histology (at least a 25% villous component, high-grade dysplasia, or colonic rectal carcinoma), at least three diminutive (1-5 mm) or small (6-9 mm) nonadvanced adenomas, or an adenomatous or sessile serrated lesion measuring at least 10 mm.
Among more than 50,000 diminutive polyps in the dataset, the prevalence of advanced histologic features was 7.1% among patients who underwent colonoscopy because of a positive fecal immunochemical test and 1.5% among those who had a colonoscopy for other reasons (P = .04). However, statistically similar proportions of patients in each of these subgroups were classified as “high risk” because of advanced histology (0.8% and 0.4%, respectively) or multiplicity (3.8% and 3.0%, respectively). Because metachronous advanced neoplasia was detected in similar proportions of patients with and without diminutive polyps with advanced histologic features (17.6% vs. 14.6%, respectively), the presence of such features did not independently predict metachronous advanced neoplasia, either overall (relative risk, 1.13; 95% CI, 0.79-1.61), or in either subgroup.
“On the other hand, multiplicity of diminutive adenomas was associated with increased risk of metachronous advanced neoplasia,” the researchers wrote. Among these patients, nearly 24% of those in the fecal immunochemical subgroup developed metachronous advanced neoplasia, as did nearly 30% of those who had a colonoscopy for other reasons, yielding risk ratios of 2.45 (95% CI, 1.67-3.58) and 1.92 (95% CI, 1.68-2.20), respectively.
“While multiplicity has been described as a risk factor of metachronous advanced adenomas, we were surprised to find that even if all adenomas are diminutive, the risk was increased,” the investigators commented. Taken together, the findings “underline the importance of correctly classifying diminutive adenomatous lesions, preventing misclassification of patients with at least three adenomas to a low-risk status.”
Partial funding for this study came from PERIS and Fundción Científica de la Asociación Española contra el Cáncer. Dr. Vleugels reported having no conflicts of interest. Three coinvestigators disclosed ties to Fujifilm, Olympus, Norgine, Clinical Genomics, and Boston Scientific.
SOURCE: Vleugels J et al. Gastroenterology. 2018 Nov 2. doi: 10.1053/j.gastro.2018.10.050.
Among patients with diminutive (1-5 mm) colonic polyps, multiplicity was a significant risk factor for advanced metachronous colonic neoplasia, while advanced histologic features alone were not, according to the results of a pooled analysis of data from 64,344 patients.
Metachronous advanced neoplasia affected similar proportions of patients with and without high-risk diminutive polyps (17.6% vs. 14.6%, respectively; relative risk, 1.13; 95% confidence interval, 0.79-1.61), reported Jasper L.A. Vleugels, MD, of the University of Amsterdam, together with his associates. However, patients with at least three nonadvanced (diminutive or small) adenomas were at significantly increased risk of metachronous advanced neoplasia, compared with low-risk patients (overall risk ratio, 2.12; 95% CI, 1.89-2.38), the investigators wrote in the February issue of Gastroenterology.
This multicenter study spanned 12 prospectively evaluated cohorts of patients in the United States and Europe. All patients underwent colonoscopy because of a positive fecal immunochemical test result or for the purpose of screening, surveillance, or evaluation of symptoms. The researchers defined low-risk patients as individuals with one or two diminutive or small nonadvanced adenomas. In contrast, “high-risk” patients had a polyp with advanced histology (at least a 25% villous component, high-grade dysplasia, or colonic rectal carcinoma), at least three diminutive (1-5 mm) or small (6-9 mm) nonadvanced adenomas, or an adenomatous or sessile serrated lesion measuring at least 10 mm.
Among more than 50,000 diminutive polyps in the dataset, the prevalence of advanced histologic features was 7.1% among patients who underwent colonoscopy because of a positive fecal immunochemical test and 1.5% among those who had a colonoscopy for other reasons (P = .04). However, statistically similar proportions of patients in each of these subgroups were classified as “high risk” because of advanced histology (0.8% and 0.4%, respectively) or multiplicity (3.8% and 3.0%, respectively). Because metachronous advanced neoplasia was detected in similar proportions of patients with and without diminutive polyps with advanced histologic features (17.6% vs. 14.6%, respectively), the presence of such features did not independently predict metachronous advanced neoplasia, either overall (relative risk, 1.13; 95% CI, 0.79-1.61), or in either subgroup.
“On the other hand, multiplicity of diminutive adenomas was associated with increased risk of metachronous advanced neoplasia,” the researchers wrote. Among these patients, nearly 24% of those in the fecal immunochemical subgroup developed metachronous advanced neoplasia, as did nearly 30% of those who had a colonoscopy for other reasons, yielding risk ratios of 2.45 (95% CI, 1.67-3.58) and 1.92 (95% CI, 1.68-2.20), respectively.
“While multiplicity has been described as a risk factor of metachronous advanced adenomas, we were surprised to find that even if all adenomas are diminutive, the risk was increased,” the investigators commented. Taken together, the findings “underline the importance of correctly classifying diminutive adenomatous lesions, preventing misclassification of patients with at least three adenomas to a low-risk status.”
Partial funding for this study came from PERIS and Fundción Científica de la Asociación Española contra el Cáncer. Dr. Vleugels reported having no conflicts of interest. Three coinvestigators disclosed ties to Fujifilm, Olympus, Norgine, Clinical Genomics, and Boston Scientific.
SOURCE: Vleugels J et al. Gastroenterology. 2018 Nov 2. doi: 10.1053/j.gastro.2018.10.050.
FROM GASTROENTEROLOGY
Key clinical point: Among patients with diminutive polyps, multiplicity was a significant risk factor for advanced metachronous colonic neoplasia, while high-risk histologic features alone were not.
Major finding: Metachronous advanced neoplasia was found in similar proportions of patients with and without high-risk diminutive polyps (17.6% vs. 14.6%, respectively; relative risk, 1.13; 95% confidence interval, 0.79-1.61). Having at least three nonadvanced adenomas was, however, a significant correlate of metachronous advanced neoplasia (risk ratio, 2.12; 95% CI, 1.89-2.38).
Study details: Pooled analysis of data from 12 international cohorts (64,344 patients).
Disclosures: Partial funding came from PERIS and Fundación Científica de la Asociación Española contra el Cáncer. Dr. Vleugels reported having no conflicts of interest. Three coinvestigators disclosed ties to Fujifilm, Olympus, Norgine, Clinical Genomics, and Boston Scientific.
Source: Vleugels J et al. Gastroenterology. 2018 Nov 2. doi: 10.1053/j.gastro.2018.10.050.
Putting up with abusive patients? That’s not for me.
I’ll put up with a lot in this practice, but I will not tolerate mistreatment of my staff.
Rudeness, while never pleasant, is generally tolerated. Some people just have that sort of personality. Others may be having a crappy day for unrelated reasons. We all have those.
But those who are intentionally abusive of my hardworking assistants aren’t going to get very far here. I have no problem telling them to go elsewhere. (This doesn’t include those with neurologic reasons for such behavior.)
Some doctors are more willing to put up with this than I am. I once shared space with one who routinely told his staff to ignore abusive behaviors. He didn’t want to turn away any potential revenue or risk angering a referring doctor.
I take another view. Life is short, and medical practice is, by nature, hectic. I have little enough time to care for the patients who genuinely appreciate what my staff and I are trying to do for them. People who are abusive and belligerent can find another doctor who’s willing to put up with it. I won’t.
My staff and I don’t expect to be thanked. We all signed up to work here. But we also try to treat patients with concern and respect, and ask the same courtesy in return. Isn’t that the golden rule?
Abusive patients are difficult to deal with, time consuming, and contribute to staff burnout. The two awesome women who work here deserve better than that. If they’re not happy, I’m not happy. All it takes is one bad person to throw the day off kilter and sometimes affect the care of the next patient in line. That person deserves better, too.
Some will argue that, as a doctor, I should care for all who need my help. In the hospital, I do. I understand that people there generally are scared and hurting and do not want to be there. But in my office I expect at least some degree of civility. We have to be at our best for each person who comes in, and having patients we can work with on a polite level helps.
There’s enough insanity in this job on a good day. People who intentionally try to make it worse aren’t welcome in my little world.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ll put up with a lot in this practice, but I will not tolerate mistreatment of my staff.
Rudeness, while never pleasant, is generally tolerated. Some people just have that sort of personality. Others may be having a crappy day for unrelated reasons. We all have those.
But those who are intentionally abusive of my hardworking assistants aren’t going to get very far here. I have no problem telling them to go elsewhere. (This doesn’t include those with neurologic reasons for such behavior.)
Some doctors are more willing to put up with this than I am. I once shared space with one who routinely told his staff to ignore abusive behaviors. He didn’t want to turn away any potential revenue or risk angering a referring doctor.
I take another view. Life is short, and medical practice is, by nature, hectic. I have little enough time to care for the patients who genuinely appreciate what my staff and I are trying to do for them. People who are abusive and belligerent can find another doctor who’s willing to put up with it. I won’t.
My staff and I don’t expect to be thanked. We all signed up to work here. But we also try to treat patients with concern and respect, and ask the same courtesy in return. Isn’t that the golden rule?
Abusive patients are difficult to deal with, time consuming, and contribute to staff burnout. The two awesome women who work here deserve better than that. If they’re not happy, I’m not happy. All it takes is one bad person to throw the day off kilter and sometimes affect the care of the next patient in line. That person deserves better, too.
Some will argue that, as a doctor, I should care for all who need my help. In the hospital, I do. I understand that people there generally are scared and hurting and do not want to be there. But in my office I expect at least some degree of civility. We have to be at our best for each person who comes in, and having patients we can work with on a polite level helps.
There’s enough insanity in this job on a good day. People who intentionally try to make it worse aren’t welcome in my little world.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’ll put up with a lot in this practice, but I will not tolerate mistreatment of my staff.
Rudeness, while never pleasant, is generally tolerated. Some people just have that sort of personality. Others may be having a crappy day for unrelated reasons. We all have those.
But those who are intentionally abusive of my hardworking assistants aren’t going to get very far here. I have no problem telling them to go elsewhere. (This doesn’t include those with neurologic reasons for such behavior.)
Some doctors are more willing to put up with this than I am. I once shared space with one who routinely told his staff to ignore abusive behaviors. He didn’t want to turn away any potential revenue or risk angering a referring doctor.
I take another view. Life is short, and medical practice is, by nature, hectic. I have little enough time to care for the patients who genuinely appreciate what my staff and I are trying to do for them. People who are abusive and belligerent can find another doctor who’s willing to put up with it. I won’t.
My staff and I don’t expect to be thanked. We all signed up to work here. But we also try to treat patients with concern and respect, and ask the same courtesy in return. Isn’t that the golden rule?
Abusive patients are difficult to deal with, time consuming, and contribute to staff burnout. The two awesome women who work here deserve better than that. If they’re not happy, I’m not happy. All it takes is one bad person to throw the day off kilter and sometimes affect the care of the next patient in line. That person deserves better, too.
Some will argue that, as a doctor, I should care for all who need my help. In the hospital, I do. I understand that people there generally are scared and hurting and do not want to be there. But in my office I expect at least some degree of civility. We have to be at our best for each person who comes in, and having patients we can work with on a polite level helps.
There’s enough insanity in this job on a good day. People who intentionally try to make it worse aren’t welcome in my little world.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Higher AML, MDS risk linked to solid tumor chemotherapy
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
Possibly the most clinical relevant finding of the study by Lindsay M. Morton, PhD, and her colleagues is that patients who received chemotherapy for solid tumor treatment at a younger age were at the highest relative risk for tMDS/AML.
The incidence of tMDS/AML was highest among patients treated with chemotherapy for bone, soft-tissue, and testicular cancers, where the median age of onset is often by 30 years, and the mean onset occurs before age 50.
The researchers also noted an increased risk for tMDS/AML associated with prolonged survival from primary tumors.
Going forward, research should consider those patients at highest risk for tMDS/AML and risk-assessment models for these therapy-related myeloid neoplasms should take into account the clonal evolution of subclinical mutations into overt disease.
The study findings point to the unanswered question of how best to perform risk assessment of chemotherapy in solid tumors. That risk stratification could include the probability of the specific chemotherapy agent initiating disease, the benefit of tumor regression from chemotherapy, and the potential consequences of tumor progression if chemotherapy is not administered.
Shyam A. Patel, MD, PhD, is with the department of medicine at Stanford (Calif.) University. Dr. Patel reported having no financial disclosures. These comments are adapted from his accompanying editorial (JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5617 ).
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
There is an increased risk for therapy-related myelodysplastic syndrome or acute myeloid leukemia (tMDS/AML) following chemotherapy for the majority of solid tumor types, according to an analysis of cancer registry data.
These findings suggest a substantial expansion in the patients at risk for tMDS/AML because, in the past, excess risks were established only after chemotherapy for cancers of the lung, ovary, breast, soft tissue, testis, and brain or central nervous system,” Lindsay M. Morton, PhD, of the National Institutes of Health, and her colleagues wrote in JAMA Oncology.
The researchers retrospectively analyzed data from 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013. Data came from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program and Medicare claims.
Study participants were given initial chemotherapy and lived for at least 1 year after treatment. Subsequently, Dr. Morton and her colleagues linked patient database records with Medicare insurance claim information to confirm the accuracy of chemotherapy data.
“Because registry data [does] not include treatment details, we used an alternative database to provide descriptive information on population-based patterns of chemotherapeutic drug use,” the researchers wrote in JAMA Oncology.
After statistical analysis, the researchers found that the risk of developing tMDS/AML was significantly elevated following chemotherapy administration for 22 of 23 solid tumor types, excluding colon cancer. They reported a 1.5-fold to more than 10-fold increased relative risk for tMDS/AML in those patients who received chemotherapy for those 22 solid cancer types, compared with the general population.
The relative risks were highest after chemotherapy for bone, soft-tissue, and testis cancers.
The researchers found that the absolute risk of developing tMDS/AML was low. Excess absolute risks ranged from 1.4 to greater than 15 cases per 10,000 person-years, compared with the general population, in those 22 solid cancer types. The greatest absolute risks were for peritoneum, small-cell lung, bone, soft-tissue, and fallopian tube cancers.
“For patients treated with chemotherapy at the present time, approximately three-quarters of tMDS/AML cases expected to occur within the next 5 years will be attributable to chemotherapy,” they added.
The researchers acknowledged a key limitation of the study was the limited data on dosing and patient-specific chemotherapy. As a result, Dr. Morton and her colleagues called for a cautious interpretation of the magnitude of the risk.
The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
SOURCE: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Treatment with chemotherapy was linked with a 1.5-fold to more than 10-fold increased risk for tMDS/AML.
Study details: A retrospective analysis of 1,619 patients with tMDS/AML who were diagnosed with an initial primary solid tumor from 2000 to 2013.
Disclosures: The study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and the California Department of Public Health. The authors reported having no conflicts of interest.
Source: Morton LM et al. JAMA Oncol. 2018 Dec 20. doi: 10.1001/jamaoncol.2018.5625.
Immediate acting inhibitors complicate hemophilia A diagnosis
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
A small but substantial proportion of patients with hemophilia A develop immediate acting factor VIII inhibitors, the diversity and complexity of which create a diagnostic challenge in the laboratory, according to authors of a recent observational study.
The great majority of the inhibitor-positive patients in the 4,900-patient study had classical FVIII inhibitors, which are typically time- and temperature-dependent and react more slowly in mixing studies, the researchers reported.
By contrast, about 1 in 10 patients demonstrated immediate acting inhibitors, and of those, some had lupus anticoagulants, some had factor VIII inhibitors, and some had both, according to Shrimati Shetty, PhD, of the National Institute of Immunohaematology in Mumbai, India, and her colleagues.
“There is a possibility of misdiagnosis of the patient when they present for the first time,” the researchers wrote. The report is in Thrombosis Research.
In the case of immediate-acting inhibitors, use of ELISA or chromogenic assays alongside lupus anticoagulant testing may help clarify the diagnosis; however, those tests are costly and may not be routinely available.
In the study by Dr. Shetty and her colleagues, patients in India with congenital hemophilia were initially screened for inhibitors. A total of 451 were found to be positive, and of those, 398 were observed to have classical factor VIII inhibitors, while the remaining 53 had immediate-acting inhibitors.
Looking specifically at hemophilia A patients with immediate-acting inhibitors, which comprised 48 of those 53 patients, the majority, or 42 patients, were positive for lupus anticoagulants, and of those, 38 were positive for both lupus anticoagulants and factor VIII inhibitors, while 4 patients were positive for lupus anticoagulants only.
“These are a heterogeneous group of antibodies interfering with all phospholipid dependent reactions,” the researchers wrote.
Properly interpreting factor inhibitor assays is an important step that helps guide later management of inhibitor-positive patients, according to Dr. Shetty and her coauthors.
“Once the patients become positive for inhibitors, they have to opt for alternate modalities of treatment, i.e. bypassing agents like activated prothrombin complex concentrate and activated recombinant factor VII, which are much more expensive,” they wrote.
In light of the diagnostic difficulties they highlighted, Dr. Shetty and her coauthors recommended a “systematic approach” to testing. Both factor VIII and factor IX assays need to be conducted, along with a lupus anticoagulant test. For inhibitor titer, either chromogenic assays or ELISA tests are recommended, they wrote.
Dr. Shetty and her coauthors reported that they had no conflicts of interest.
SOURCE: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
FROM THROMBOSIS RESEARCH
Key clinical point:
Major finding: Of 48 inhibitor-positive hemophilia A patients with immediate acting inhibitors, 42 were positive for lupus anticoagulants.
Study details: An analysis of 4,900 patients in India with confirmed or suspected congenital hemophilia.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Patil R et al. Thromb Res. 2018 Dec;172:29-35.
Patient-reported outcomes for patients with chronic liver disease
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).
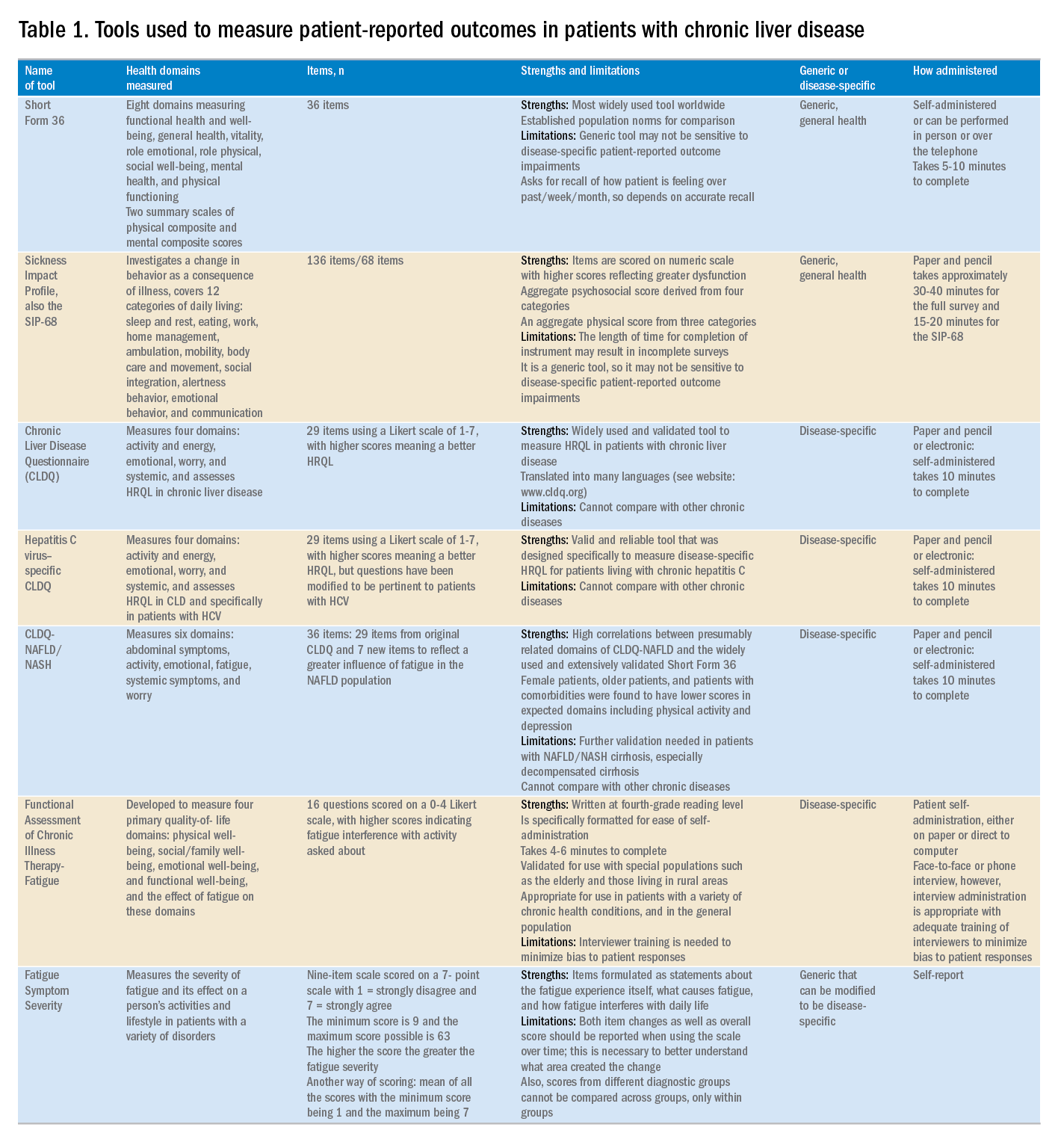
Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.
Chronic liver disease (CLD) and its complications such as decompensated cirrhosis and hepatocellular carcinoma are major causes of mortality and morbidity worldwide.1,2 In addition to its clinical impact, CLD causes impairment of health-related quality of life (HRQL) and other patient-reported outcomes (PROs).1 Furthermore, patients with CLD use a substantial amount of health care resources, making CLD responsible for tremendous economic burden to the society.1,2
Although CLD encompasses a number of liver diseases, globally, hepatitis B virus (HBV) and hepatitis C virus (HCV), as well as alcoholic and nonalcoholic steatohepatitis (NASH), are the most important causes of liver disease.1,2 In this context, recently developed treatment of HBV and HCV are highly effective. In contrast, there is no effective treatment for NASH and treatment of alcoholic steatohepatitis remains suboptimal.3 In the context of the growing burden of obesity and diabetes, the prevalence of NASH and its related complications are expected to grow.4
In recent years, a comprehensive approach to assessing the full burden of chronic diseases such as CLD has become increasingly recognized. In this context, it is important to evaluate not only the clinical burden of CLD (survival and mortality) but also its economic burden and its impact on PROs. PROs are defined as reports that come directly from the patient about their health without amendment or interpretation by a clinician or anyone else.5,6 Therefore, this commentary focuses on reviewing the assessment and interpretation of PROs in CLD and why they are important in clinical practice.
Assessment of patient-reported outcomes
Although a number of PRO instruments are available, three different categories are most relevant for patients with CLD. In this context, PRO instruments can be divided into generic tools, disease-/condition-specific tools, or other instruments that specifically measure outcomes such as work or activity impairment (Table 1).

Generic HRQL tools measure overall health and its impact on patients’ quality of life. One of the most commonly used generic HRQL tools in liver disease is the Short Form-36 (SF-36) version 2. The SF-36 version 2 tool measures eight domains (scores, 0–100; with a higher score indicating less impairment) and provides two summary scores: one for physical functioning and one for mental health functioning. The SF-36 has been translated into multiple languages and provides age group– and disease-specific norms to use in comparison analysis.7 In addition to the SF-36, the Sickness Impact Profile also has been used to assess a change in behavior as a consequence of illness. The Sickness Impact Profile consists of 136 items/12 categories covering activities of daily living (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, and communication). Items are scored on a numeric scale, with higher scores reflecting greater dysfunction as well as providing two aggregate scores: the psychosocial score, which is derived from four categories, and an aggregate physical score, which is calculated from three categories.8 Although generic instruments capture patients’ HRQL with different disease states (e.g., CLD vs. congestive heart failure), they may not have sufficient responsiveness to detect clinically important changes that can occur as a result of the natural history of disease or its treatment.9
For better responsiveness of HRQL instruments, disease-specific or condition-specific tools have been developed. These tools assess those aspects of HRQL that are related directly to the underlying disease. For patients with CLD, several tools have been developed and validated.10-12 One of the more popular tools is the Chronic Liver Disease Questionnaire (CLDQ), which was developed and validated for patients with CLD.10 The CLDQ has 29 items and 6 domains covering fatigue, activity, emotional function, abdominal symptoms, systemic symptoms, and worry.10 More recently, HCV-specific and NASH-specific versions of the CLDQ have been developed and validated (CLDQ-HCV and CLDQ–nonalcoholic fatty liver disease [NAFLD]/NASH). The CLDQ-HCV instrument has some items from the original CLDQ with additional items specific to patients suffering from HCV. The CLDQ-HCV has 29 items that measure 4 domains: activity and energy, emotional, worry, and systemic, with high reliability and validity.11 Finally, the CLDQ-NAFLD/NASH was developed in a similar fashion to the CLDQ and CLDQ-HCV. The CLDQ-NAFLD/NASH has 36 items grouped into 6 domains: abdominal symptoms, activity, emotional, fatigue, systemic symptoms, and worry.12 All versions of the CLDQ are scored on a Likert scale of 1-7 nd domain scores are presented in the same manner. In addition, each version of the CLDQ can provide a total score, which also ranges from 1 to 7. In this context, the higher scores represent a better HRQL.10-12In addition to generic and disease-specific instruments, some investigators may elect to include other instruments that are designed specifically to capture fatigue, a very common symptom of CLD. These include the Functional Assessment of Chronic Illness Therapy-Fatigue, Fatigue Symptom Severity, and Fatigue Assessment Inventory.13,14
Finally, work productivity can be influenced profoundly by CLD and can be assessed by self-reports or questionnaires. One of these is the Work Productivity Activity Impairment: Specific Health Problem questionnaire, which evaluates impairment in patients’ daily activities and work productivity associated with a specific health problem, and for patients with liver disease, patients are asked to think about how their disease state impacts their life. Higher impairment scores indicate a poorer health status and range from 0 to 1.15 An important aspect of the PRO assessment that is utilized in economic analysis measures health utilities. Health utilities are measured directly (time-trade off) or indirectly (SF6D, EQ5D, Health Utility Index). These assessment are from 0 (death) to 1 (perfect health). Utility adjustments are used to combine qualty of life with quantity of life such as quality-adjusted years of life (QALY).16
Patient-reported outcome results for patients with chronic liver disease
Over the years, studies using these instruments have shown that patients with CLD suffer significant impairment in their PROs in all domains measured when compared with the population norms or with individuals without liver disease. Regardless of the cause of their CLD, patients with cirrhosis, especially with decompensated cirrhosis, have the most significant impairments.16,17 On the other hand, there is substantial evidence that standard treatment for decompensated cirrhosis (i.e., liver transplantation) can significantly improve HRQL and other PROs in patients with advanced cirrhosis.18
In addition to the data for patients with advanced liver disease, there is a significant amount of PRO data that has been generated for patients with early liver disease. In this context, treatment of HCV with the new interferon-free direct antiviral agents results in substantial PRO gains during treatment and after achieving sustained virologic response.19 In fact, these improvements in PROs have been captured by disease-specific, generic, fatigue-specific, and work productivity instruments.19
In contrast to HCV, PRO data for patients with HBV are limited. Nevertheless, recent data have suggested that HBV patients who have viral suppression with a nucleoside/nucleotide analogue have a better HRQL.20 Finally, PRO assessments in subjects with NASH are in their early stages. In this context, HRQL data from patients with NASH show significant impairment, which worsens with advanced liver disease.21,22 In addition, preliminary data suggest that improvement of fibrosis with medication can lead to improvement of some aspects of PROs in NASH.23,24
Clinical practice and patient-reported outcomes
The first challenge in the implementation of PRO assessment in clinical practice is the appreciation and understanding of the practicing gastroenterologists and hepatologists about its importance and relevance to clinicians. Generally, clinicians are more focused on the classic markers of disease activity and severity (laboratory tests, and so forth), rather than those that measure patient experiences (PROs). Given that patient experience increasingly has become an important indicator of quality of care, this issue may become increasingly important in clinical practice. In addition, it is important to remember that PROs are the most important outcomes from the patient’s perspective. Another challenge in implementation of PROs in clinical practice is to choose the correct validated tool and to implement PRO assessment during an office visit. In fact, completing long questionnaires takes time and resources, which may not be feasible for a busy clinic. Furthermore, these assessments are not reimbursed by payers, which leave the burden of the PRO assessment and counseling of patients about their interpretation to the clinicians or their clinical staff. Although the other challenges are easier to solve, covering the cost of administration and counseling patients about interventions to improve their PROs can be substantial. In liver disease, the best and easiest tool to use is a validated disease-specific instrument (such as the CLDQ), which takes no more than 10 minutes to complete. In fact, these instruments can be completed electronically either during the office visit or before the visit through secure web access. Nevertheless, all of these efforts require strong emphasis and desire to assess the patient’s perspective about their disease and its treatment and to manage their quality of life accordingly.
In summary, the armamentarium of PRO tools used in multiple studies of CLD have provided excellent insight into the PRO burden of CLD, and their treatments from the patient’s perspective thus are an important part of health care workers’ interaction with patients. Work continues in understanding the impact of other liver diseases on PROs but with the current knowledge about PROs, clinicians should be encouraged to use this information when formulating their treatment plan.25 Finally, seamless implementation of PRO assessments in the clinical setting in a cost-effective manner remains a challenge and should be addressed in the future.
References
1. Afendy A, Kallman JB, Stepanova M, et al. Predictors of health-related quality of life in patients with chronic liver disease. Aliment Pharmacol Ther, 2009;30:469-76.
2. Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies. Available from: http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articles-collection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-economies. Accessed: August 31, 2017.
3. Younossi Z, Henry L. Contribution of alcoholic and nonalcoholic fatty liver disease to the burden of liver-related morbidity and mortality. Gastroenterology. 2016;150:1778-85.
4. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84.
5. Younossi ZM, Park H, Dieterich D, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality-adjusted cost of care. Medicine. (Baltimore). 2016;95:e5048.
6. Centers for Disease Control–Health Related Quality of Life. Available from: http://www.cdc.gov/HRQoL/concept.htm. Accessed: August 31, 2017.
7. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10:405-20.
8. De Bruin A, Diederiks J, De Witte L, et al. The development of a short generic version of the Sickness Impact Profile. J Clin Epidemiol. 1994;47:407-12.
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trial. 1989;10:407-15.
10. Younossi ZM, Guyatt G, Kiwia M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300.
11. Younossi ZM, Stepanova M, Henry L. Performance and validation of Chronic Liver Disease Questionnaire-Hepatitis C Version (CLDQ-HCV) in clinical trials of patients with chronic hepatitis C. Value Health. 2016;19:544-51.
12. Younossi ZM, Stepanova M, Henry L, et al. A disease-specific quality of life instrument for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: CLDQ-NAFLD. Liver Int. 2017;37:1209-18.
13. Webster K, Odom L, Peterman A, et al. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res. 1999;8:604.
14. Golabi P, Sayiner M, Bush H, et al. Patient-reported outcomes and fatigue in patients with chronic hepatitis C infection. Clin Liver Dis. 2017;21:565-78.
15. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353-65.
16. Loria A, Escheik C, Gerber NL, et al. Quality of life in cirrhosis. Curr Gastroenterol Rep. 2012;15:301.
17. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
18. Pérez-San-Gregorio MÁ, Martín-Rodríguez A, Domínguez-Cabello E, et al. Quality of life and mental health comparisons among liver transplant recipients and cirrhotic patients with different self-perceptions of health. J Clin Psychol Med Settings. 2013;20:97-106.
19. Younossi ZM, Stepanova M, Henry L, et al. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol. 2016;111:808-16.
20. Weinstein AA, Price Kallman J, Stepanova M, et al. Depression in patients with nonalcoholic fatty liver disease and chronic viral hepatitis B and C. Psychosomatics. 2011;52:127-32.
21. Younossi ZM, Stepanova M, Jacobson IM, et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259-67.
22. Sayiner M, Stepanova M, Pham H, et al. Assessment of health utilities and quality of life in patients with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000106.
23. Younossi ZM, Stepanova M, Gordon S, et al. Patient-reported outcomes following treatment of chronic hepatitis C virus infection with Sofosbuvir and Velpatasvir, with or without Voxilaprevir. Clin Gastroenterol Hepatol. 2018;16:567-74.
24. Younossi ZM, Stepanova M, Charlton M, et al. Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol. 2016;1:122-32.
25. Younossi Z. What Is the ethical responsibility of a provider when prescribing the new direct-acting antiviral agents to patients with hepatitis C infection? Clin Liver Dis. 2015;6:117-9.
Dr. Younossi is at the Center for Liver Diseases, chair, Department of Medicine, professor of medicine at Inova Fairfax Hospital, Falls Church, Va; and the Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church. He has received research funding and is a consultant with Abbvie, Intercept, BMS, Allergan, Bristol-Myers Squibb, Gilead Sciences, Novartis, Novo Nordisk, Shinogi, Terns, and Viking.