User login
Common benign breast concerns for the primary care physician
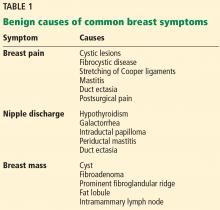
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
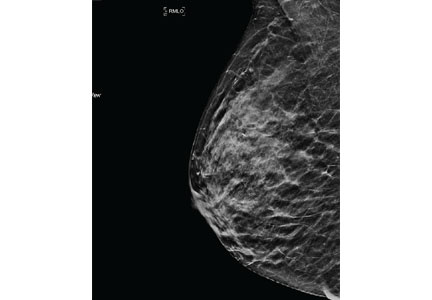
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
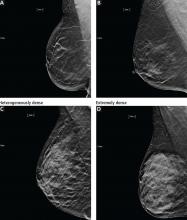
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
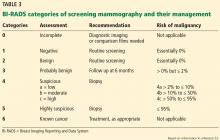
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
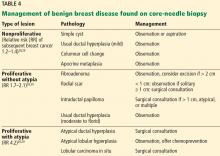
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
KEY POINTS
- The two most common breast symptoms are lumps and pain.
- Most breast problems are not caused by cancer.
- Evaluation of any breast problem begins with a focused history followed by a breast examination and, when necessary, imaging.
- If the results of the breast examination and imaging suggest a benign cause, no further follow-up is necessary.
- If there is discordance between imaging and breast examination results, or if there is a high clinical suspicion of cancer, then consider serial follow-up examinations at short intervals, referral to a breast surgeon for excision, or both.
Who needs to carry an epinephrine autoinjector?
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
Anaphylaxis is potentially fatal but can be prevented if the trigger is identified and avoided, and death can be avoided if episodes are treated promptly.
A consensus definition of anaphylaxis has been difficult to achieve, with slight variations among international guidelines. The World Allergy Organization classifies anaphylaxis as immunologic, nonimmunologic, or idiopathic.1 The National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network highlight clinical symptoms and criteria.2 The International Consensus on Food Allergy describes reactions as being immunoglobulin E (IgE)-mediated, cell-mediated, or a combination of the 2 mechanisms.3
Despite the subtle differences in these definitions, all 3 international organizations have a common recommendation for anaphylaxis: once it is diagnosed, epinephrine is the treatment of choice.
EPINEPHRINE IS THE TREATMENT OF CHOICE FOR ANAPHYLAXIS
Anaphylaxis commonly results from exposure to foods, medications, and Hymenoptera venom.4 Avoiding triggers is key in preventing anaphylaxis but is not always possible.
Although epinephrine is the cornerstone of the emergency treatment of anaphylaxis, many patients instead receive antihistamines and corticosteroids as initial therapy. Some take these medications on their own, and some receive them in emergency departments and outpatient clinics.5
Diphenhydramine, a histamine 1 receptor antagonist, is often used as a first-line medication. But diphenhydramine has a slow onset of action, taking 80 minutes after an oral dose to suppress a histamine-induced cutaneous flare by 50%, and taking 52 minutes with intramuscular administration.6 Corticosteroids also have a slow onset of action. These drugs cannot prevent death in anaphylaxis, a condition in which the median time to respiratory or cardiac arrest is 30 minutes after ingestion of food, 15 minutes after envenomation, and 5 minutes after iatrogenic reactions.7
Combination therapy with diphenhydramine and a histamine 2 receptor antagonist (eg, cimetidine, famotidine) is also commonly used,8 but this combination offers no advantage in terms of onset of action, and a Cochrane review could find no definitive evidence for or against the use of histamine 2 receptor antagonists.9
Because of their slow onset of action, all of these should be second-line therapies, given after epinephrine. Epinephrine is the first line of treatment because it has a maximal pharmacokinetic effect (time to maximal peak serum level) within 10 minutes of intramuscular injection into the thigh.10,11
In addition, epinephrine acts on numerous receptors to antagonize the multiple pathologic effects of the mediators released during an anaphylactic episode. In contrast, antihistamines block only 1 mediator, while mediators other than histamine can be responsible for severe events and deaths.12,13
It is crucial that epinephrine be given immediately, as delay has been associated with fatalities.14 In addition, guidelines recommend repeating epinephrine dosing after 5 to 15 minutes if the response to the first dose is suboptimal.1,2 From 16% to 36% of patients may need a second dose.15–18 Therefore, many physicians recommend that patients at risk of anaphylaxis keep not 1 but 2 epinephrine autoinjectors on hand at all times, and so say the US guidelines for the management of anaphylaxis.19
WHO SHOULD CARRY AN EPINEPHRINE AUTOINJECTOR?
All published guidelines recommend epinephrine as the drug of choice for anaphylaxis. And an epinephrine autoinjector is indicated for anyone who has experienced an anaphylactic event or is at risk of one, and these patients should carry it with them at all times. Such individuals include those with food allergy or Hymenoptera hypersensitivity.
Food allergy
The foods that most often cause anaphylaxis are peanuts, tree nuts, fish, shellfish, milk, and eggs, but any food can cause a reaction.
The prevalence of food allergy has increased over time, and treatments are limited. Some food desensitization protocols look promising but are still in the research stages. The best treatment at this time is to avoid the offending food, but there are accidental exposures.
Hymenoptera hypersensitivity
Patients who have had anaphylaxis after being stung by insects such as bees, wasps, yellow-faced hornets, white-faced hornets, yellow jackets, and fire ants should be evaluated by an allergist. Skin testing and serum IgE testing helps properly diagnose Hymenoptera hypersensitivity.
Once the diagnosis is confirmed, venom immunotherapy should be considered. Some patients choose only to carry an epinephrine autoinjector and to avoid these insects as much as possible. However, most patients also choose to receive venom immunotherapy, because 80% to 90% of those who receive this treatment for 3 to 5 years do not have a systemic reaction if they are stung again.20
Regardless of whether they choose to undergo immunotherapy, sensitive patients should always carry an epinephrine autoinjector. This is also the case after treatment ends, since the therapy is not 100% effective.
PATIENTS FOR WHOM THE NEED MAY BE LESS CLEAR
In other patients who may be at increased risk, the mandate for an epinephrine autoinjector is less clear, and the decision to carry one is determined on an individual basis. Such individuals are those receiving allergen immunotherapy, with large local reactions to insect stings, with oral allergy syndrome, with mastocytosis, and with drug allergy. In these cases, the benefit vs the burden of carrying an autoinjector should be discussed with the patient.
Patients on allergen immunotherapy
National guidelines recommend that all patients who receive allergen immunotherapy be monitored in the clinic under a physician’s supervision for 30 minutes after the injection. Fortunately, life-threatening reactions occurring after 30 minutes are rare. But delayed systemic reactions can occur and may account for up to 50% of such events.21
Therefore, many physicians consider it prudent for patients on immunotherapy to carry an epinephrine autoinjector, but there is no consensus. A survey22 found that 13.5% of allergists did not prescribe the autoinjector for patients on immunotherapy, while 33.3% prescribed it for all their patients on immunotherapy, and the rest prescribed based on risk.
Since there are no national guidelines on epinephrine autoinjectors for patients on immunotherapy, the decision should be based on the patient’s risks and comorbidities and informed by discussion between the individual patient and his or her allergist.
Patients with large local reactions to insect stings
From 5% to 10% of patients who have large local reactions to insect stings are at risk of systemic reactions.20
Patients with oral allergy syndrome
Oral allergy syndrome, also known as pollen-food allergy, causes itching and mild swelling of the mouth, lips, and throat after eating fresh fruits and vegetables. The prevalence ranges from 2% to 10% of patients with allergies.23
A survey of allergists found that 20% of patients with oral allergy syndrome had experienced systemic symptoms.24 The survey also showed that the decision to prescribe an epinephrine autoinjector to these patients was highly variable. Only about 30% of allergists recommend epinephrine autoinjectors to patients with oral allergy syndrome, while most believe that the decision should be based on the individual’s symptoms and risk.
More research is needed in the area of food allergy. Because data are limited, there are no national guidelines on whether these patients should carry an epinephrine autoinjector. We agree with the Joint Task Force on Practice Parameters14 recommendation that the decision be made on an individual basis following discussion between the patient and physician.
Patients with mastocytosis
Patients with mastocytosis and a history of anaphylaxis are at increased risk for systemic reactions to Hymenoptera venom.
Patients with medication allergy
Once medication allergy has been diagnosed, avoidance is usually effective, obviating the need for an epinephrine autoinjector, although the physician has the option of prescribing one.
CAUTIONS, NOT CONTRAINDICATIONS
Physicians may be reluctant to prescribe an epinephrine autoinjector because of the risk of an adverse reaction in patients with hypertension, coronary artery disease, or arrhythmias, and in elderly patients taking multiple drugs, especially drugs that can interact with epinephrine. Nevertheless, there is no absolute contraindication to the use of epinephrine in anaphylaxis.
In patients with atherosclerosis and cardiovascular disease
Epinephrine increases vasoconstriction, heart rate, and cardiac force of contraction. These effects are beneficial during anaphylaxis, but in rare cases patients have experienced myocardial infarction and acute coronary syndrome after receiving intravenous epinephrine.25 These incidents have naturally prompted reluctance to prescribe it in susceptible patients with coronary disease during anaphylaxis.
Yet epinephrine may not be solely to blame for these adverse responses. Mast cells are abundant in the heart, and their release of mediators can also result in adverse cardiac manifestations, including myocardial infarction.26
Conversely, some drugs used to treat cardiovascular disease can worsen anaphylaxis.
Beta-blockers can cause bronchospasm and decrease cardiac contractility. They can also blunt the pharmacologic effects of epinephrine. There is concern that epinephrine may produce dangerous elevations of blood pressure in patients taking beta-blockers by unopposed alpha-adrenergic stimulation and reflex vagotonic effects.27 And there is evidence that beta-blockers may increase the risk and severity of reactions. One study reported that patients taking beta-blockers are more than 8 times more likely to be hospitalized due to anaphylactoid reaction with bronchospasm.28
Beta-blockers and, to a lesser extent, angiotensin-converting enzyme inhibitors have been shown to increase the risk of anaphylaxis in the emergency department.29,30 However, some investigators have not found beta-blockers to be a risk factor. A study evaluating anaphylactoid reactions from contrast media found no statistically significant higher risk in patients taking beta-blockers.31 Similarly, a study of 3,178 patients on beta-blockers receiving venom immunotherapy or allergen immunotherapy found no increase in the frequency of systemic reactions.32 Nevertheless, overall, more studies support the hypothesis that beta-blockers may be an additional risk factor in anaphylaxis.33
Thus, clinicians treating patients with cardiovascular disease and anaphylaxis face a dilemma. Although there is concern in this population, epinephrine should not be withheld in patients with cardiovascular disease who are experiencing an anaphylactic event.33 If epinephrine is not administered, the patient could die.
Elderly patients on multiple medications
Older patients are also at risk of anaphylaxis. But clinicians are reluctant to treat older patients with epinephrine because of concerns about adverse effects.
Epinephrine dispensing rates vary substantially in different age groups: 1.44% for patients under age 17, 0.9% for those ages 17 to 64, and 0.32% for those age 65 or older.34 A Canadian study of 492 patients with anaphylaxis in the emergency department showed that those over age 50 received epinephrine less often than younger patients (36.1% vs 60.5%).35 Cardiovascular complications were more frequent in the older group, occurring in 4 (9.1%) of the 44 older patients who received epinephrine compared with 1 (0.4%) of the 225 younger patients who received it. On the other hand, the rate of adverse effects from subcutaneous epinephrine was no different in older asthma patients compared with younger patients.36
Many older patients take multiple medications, raising concern about adverse effects. Commonly prescribed medications in the elderly can affect the actions of epinephrine. Monoamine oxidase inhibitors retard the catabolism of epinephrine. Tricyclic antidepressants may decrease the reuptake of catecholamines by neurons and thus interfere with the degradation of epinephrine. Digoxin has a narrow therapeutic window and can potentially increase the risk of arrhythmias when given with epinephrine.
Although the clinician must be cautious in treating older patients who have comorbidities, these are not sufficient to withhold prescribing an epinephrine autoinjector to elderly patients at risk of anaphylaxis.
INJECTOR OPTIONS
Epinephrine autoinjectors come preloaded for prompt delivery of the drug. They are intended primarily for use by patients themselves in unsupervised settings in suspected anaphylaxis. Simplicity of use and safety must be considered in such a setting so that patients can use the device correctly and are not incorrectly dosed.
Several models are commercially available, with different ergonomic designs and sizes. EpiPen, the first one marketed in the United States, was introduced in 1987. One device (Auvi-Q) contains an audio chip that gives step-by-step instructions at the time of use. It is hoped that this device will reduce errors in usage during this stressful time for patients and caregivers.
In the United States, epinephrine autoinjectors contain either 0.15 or 0.30 mg of the drug, but some clinicians believe this may not be enough. The UK Resuscitation Council recommends 0.50 mg for patients over age 12,37 and an epinephrine autoinjector with that dose is available in Europe.
Subcutaneous vs intramuscular delivery
The package insert for some epinephrine autoinjectors says the injector can be used to treat anaphylaxis by both subcutaneous and intramuscular administration. However, the routes are not equivalent.
The goal in anaphylaxis is to quickly achieve high tissue and plasma epinephrine concentrations, and studies have found that injection into the vastus lateralis muscle, but not the deltoid muscle, results in faster time to peak plasma concentration: 8 minutes for injection in the vastus lateralis muscle and 34 minutes for subcutaneous delivery.10,11 In addition, injection in the vastus lateralis muscle results in a higher peak plasma concentration than the subcutaneous or deltoid route. Based on these data, intramuscular injection into the vastus lateralis muscle in the thigh appears to be the preferred route of administration of epinephrine.
Obese patients may need a longer needle
Research on the original autoinjector was conducted by the US military, which wanted a rapidly effective and easy-to-use antidote for battlefield exposure to poison gas. The resulting device had 2 separate spring-loaded syringes, 1 containing pralidoxime chloride and the other atropine sulfate. To enable its use through the thick fabric of a chemical warfare suit, the needles were 2.2 cm long.
The first commercial autoinjector to contain epinephrine was made by Survival Technology (Bethesda, MD) in the mid-1970s. The manufacturer considered a 2.2-cm needle to be too long, and the first commercially available epinephrine autoinjector, EpiPen, had a 1.43-cm needle for adult use.
Since then, needle lengths have ranged from 1.17 to 2.5 cm to accommodate different skin-to-muscle depths, with shorter needles for children and longer needles for obese adults.38
However, the prevalence of obesity is high and continues to rise.39 Obesity raises concern that the needles in epinephrine autoinjectors may be too short for the preferred intramuscular delivery, resulting in subcutaneous deposition.
A study that used computed tomography of the thigh found that 1 (2%) of 50 men and 21 (42%) of 50 women studied had a subcutaneous tissue depth greater than 1.43 cm, the needle length in EpiPen. These were not anaphylaxis patients, but the findings suggest that many patients—especially women—may be getting subcutaneous instead of intramuscular delivery with this device.40
Another study that used ultrasonography showed that the 1.43-cm EpiPen needle was too short for 36 (31%) of 116 adults.41 Women were 6.4 times more likely than men to encounter this problem. Other risk factors include higher body mass index, short height, and thicker thighs.
Emerade, an injector with a 2.5-cm needle, is available in some European countries. A longer needle may be helpful in some cases. but we do not yet have enough data to determine the optimal needle length.
Conversely, some children may need shorter needles and may in fact be at risk of having the needle penetrate bone.42 The US Food and Drug Administration recently approved a shorter needle for an epinephrine autoinjector (Auvi-Q) to be used in children weighing 7.5 kg to 15 kg.
BARRIERS TO USING EPINEPHRINE AUTOINJECTORS
Many patients do not use their epinephrine autoinjector in times of anaphylaxis or do not have one with them. Common reasons cited by respondents in a survey43 of 1,385 patients included the following:
They took an oral antihistamine instead (38%).
They never received a prescription for an epinephrine autoinjector (28%).
They thought their symptoms were mild and would resolve with time (13%).
They were afraid (6%). There are reports of accidental injection, typically into fingers, hands, and thumbs. Fortunately, most accidental injections do not require a hand surgeon evaluation or surgery.44 Conservative therapy and monitoring of the injection site are sufficient in most cases.
They could not afford an epinephrine autoinjector (1%).43 Mylan Pharmaceuticals infamously increased the price of its EpiPen to more than $600 for a package of 2 pens. Generic devices are available in the United States but are still too expensive for some patients and are cumbersome to carry.
However, even expensive epinephrine autoinjectors may be cost-effective. Epidemiologic studies have found that patients who did not use an epinephrine autoinjector incurred a higher burden of cost due to emergency department visits and inpatient hospitalizations.45
As a do-it-yourself option, some resourceful patients are obtaining autoinjectors intended for insulin injection, replacing the needle, and filling the injector with epinephrine, at a cost of about $30. (The manufacturer does not endorse this off-label use of their device—www.owenmumford.com/us/patients/if-you-need-to-inject.) Least costly of all is to prescribe multidose vials of epinephrine and regular syringes and teach patients and their caregivers how to draw up the proper dose and give themselves an injection—in essence going back to what was done before 1987.
It was past its expiration date (2%).43 Failure to refill the prescription is common. A California Kaiser Permanente study46 showed that only 46% of patients refilled their epinephrine autoinjector prescription at least once, and the refill rate decreased over time: 43% at 1 to 2 year follow-up, 35% at 3 to 4 years, and 30% at 5 years or longer. Based on these data, it is imperative to educate patients regarding the importance of replacing the epinephrine autoinjector when the old one expires.
NEED FOR PATIENT EDUCATION
Even though prompt treatment with epinephrine decreases fatalities, it continues to be underused in the community. In addition, it is often prescribed without adequate training in its use and appropriate emphasis on the need to keep the device on hand at all times and to replace it in a timely manner if it is used or has expired. Physicians need to educate patients on how to avoid triggers and how to recognize symptoms of anaphylaxis whenever they prescribe an epinephrine autoinjector.
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014; 7(1):9. doi:10.1186/1939-4551-7-9
- NIAID-Sponsored Expert Panel; Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6 suppl):S1–S58. doi:10.1016/j.jaci.2010.10.007
- Burks AW, Tang M, Sicherer S, et al. ICON: food allergy. J Allergy Clin Immunol 2012; 129(4):906–920. doi:10.1016/j.jaci.2012.02.001
- Lieberman P, Carmago CA Jr, Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma, and Immunology. Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006; 97(5):596–602. doi:10.1016/S1081-1206(10)61086-1
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis—a statement of the World Allergy Organization. World Allergy Organ J 2008; 1(suppl 7):S18–S26. doi:10.1097/WOX.0b013e31817c9338
- Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol 2008; 100(5):452–456. doi:10.1016/S1081-1206(10)60470-X
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allerg 2000; 30(8):1144–1150. pmid:10931122
- Runge JW, Martinez JC, Caravati EM, Williamson SG, Hartsell SC. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med 1992; 21:237–242. pmid:1536481
- Sheikh A, Simons FE, Barbour V, Worth A. Adrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the community. Cochrane Database Syst Rev 2012; (8):CD008935. doi:10.1002/14651858.CD008935.pub2
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108(5):871–873. doi:10.1067/mai.2001.119409
- Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101(1 pt 1):33–37. doi:10.1016/S0091-6749(98)70190-3
- Vadas P. The platelet-activating factor pathway in food allergy and anaphylaxis. Ann Allergy Asthma Immunol 2016; 117(5):455–457. doi:10.1016/j.anai.2016.05.003
- Stone SF, Brown SG. Mediators released during human anaphylaxis. Curr Allergy Asthma Rep 2012; 12(1):33–41. doi:10.1007/s11882-011-0231-6
- Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol 2010; 126(3):477–480.e1–e42. doi:10.1016/j.jaci.2010.06.022
- Kemp SF, Lockey RF, Simons FE; World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy 2008; 63(8):1061–1070. doi:10.1111/j.1398-9995.2008.01733.x
- Oren E, Banderji A, Clark S, Camargo CA Jr. Food-induced anaphylaxis and repeated epinephrine treatments. Ann Allergy Asthma Immunol 2007; 99(5):429–432. doi:10.1016/S1081-1206(10)60568-6
- Uguz A, Lack G, Pumphrey R, et al. Allergic reactions in the community: a questionnaire survey of members of the anaphylaxis campaign. Clin Exp Allergy 2005; 35(6):746–750. doi:10.1111/j.1365-2222.2005.02257.x
- Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117(2):464–465. doi:10.1016/j.jaci.2005.11.015
- Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis—a practice parameter update 2015. Ann Allergy Asthma Immunol 2015; 115(5):341–384. doi:10.1016/j.anai.2015.07.019
- Golden BK, Demain J, Freeman T, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol 2017; 118(1):28–54. doi:10.1016/j.anai.2016.10.031
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011; 127(suppl 1):S1–S55. doi:10.1016/j.jaci.2010.09.034
- Gupta P, Gerrish PK, Silverman B, Schneider A. Current practices among allergists on writing self-injectable epinephrine prescriptions for immunotherapy patients. J Allergy Clin Immunol 2012; 129(2):571–572.e1-e2. doi:10.1016/j.jaci.2011.09.033
- Ortolani C, Pastorello EA, Farioli L, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy 1993; 71:470–476. pmid: 8250353
- Ma S, Shcherer SH, Nowak-Wegrzyn A. A survey on the management of pollen food allergy syndrome in allergy practices. J Allergy Clin Immunol 2003;112:784–788. doi:10.1016/S0091-6749(03)02008-6
- Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low dose intravenous epinephrine for anaphylaxis. CJEM 2006; 8(4):289–294. pmid:17324313
- Triggiani M, Patella V, Staiano RI, Granata F, Marone G. Allergy and the cardiovascular system. Clin Exp Immunol 2008; 153(suppl 1):7–11. doi:10.1111/j.1365-2249.2008.03714.x
- Gilman AG, Rail TW, Nies AS, Taylor P, eds. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. 8th ed. New York, NY: Pergamon Press; 1990.
- Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med 1991; 115(14):270–276. pmid:1677239
- Nassiri M, Babina M, Dölle S, Edenharter G, Ruëff F, Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol 2015; 135(2):491–499. doi:10.1016/j.jaci.2014.09.004
- Lee S, Hess EP, Nestler DM, et al. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol 2013; 131(4):1103–1108. doi:10.1016/j.jaci.2013.01.011
- Greenberger PA, Meyers SN, Kramer BL, Kramer BL. Effects of beta-adrenergic and calcium antagonists on the development of anaphylactoid reactions from radiographic contrast media during cardiac angiography. J Allergy Clin Immunol 1987; 80(5):698–702. pmid:2890682
- Hepner MJ, Ownby DR, Anderson JA, Rowe MS, Sears-Ewald D, Brown EB. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol 1990; 86(3 pt 1):407–411. pmid:1976666
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy 2015; 45(8):1288–1295. doi:10.1111/cea.12520
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol 2002; 110(4):647–651. pmid:12373275
- Kawano T, Scheuermeyer FX, Stenstrom R, Rowe BH, Grafstein E, Grunau B. Epinephrine use in older patients with anaphylaxis: clinical outcomes and cardiovascular complications. Resuscitation 2017; 112:53–58. doi:10.1016/j.resuscitation.2016.12.020
- Cydulka R, Davison R, Grammer L, Parker M, Mathews J 4th. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med 1988; 17(4):322–326. pmid:3354935
- Soar J, Pumphrey R, Cant A, et al; Working Group of the Resuscitation Council (UK). Emergency treatment of anaphylactic reactions—guidelines for healthcare providers. Resuscitation 2008; 77(2):157–169. doi:10.1016/j.resuscitation.2008.02.001
- Dreborg S, Wen X, Kim L, et al. Do epinephrine auto-injectors have an unsuitable needle length in children and adolescents at risk for anaphylaxis from food allergy? Allergy Asthma Clin Immunol 2016; 12:11. doi:10.1186/s13223-016-0110-8
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014; 311(8):806–814. doi:10.1001/jama.2014.732
- Song TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol 2005; 94(5):539–542. doi:10.1016/S1081-1206(10)61130-1
- Bhalla MC, Gable BD, Frey JA, Reichenbach MR, Wilber ST. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med 2013; 31(12):1671–1676. doi:10.1016/j.ajem.2013.09.001
- Kim H, Dinakar C, McInnis P, et al. Inadequacy of current pediatric epinephrine autoinjector needle length for use in infants and toddlers. Ann Allergy Asthma Immunol 2017; 118(6):719–725.e1. doi:10.1016/j.anai.2017.03.017
- Simons FE, Clark S, Camargo CA Jr. Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol 2009; 124(2):301–306. doi:10.1016/j.jaci.2009.03.050
- Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med 2010; 56(3):270–274. doi:10.1016/j.annemergmed.2010.02.019
- Fleming JT, Clark S, Camargo CA Jr, Rudders SA. Early treatment of food-induced anaphylaxis with epinephrine is associated with a lower risk of hospitalization. J Allergy Clin Immunol Pract 2015; 3(1):57–62. doi:10.1016/j.jaip.2014.07.004
- Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep 2011; 11(1):65–70. doi:10.1007/s11882-010-0155-6
KEY POINTS
- Based on current data, there is no absolute contraindication to epinephrine for anaphylaxis. And failure to give epinephrine promptly has resulted in deaths.
- Clinicians concerned about adverse effects of epinephrine may be reluctant to give it during anaphylaxis.
- Education about anaphylaxis and its prompt treatment with epinephrine is critical for patients and their caregivers.
CONDOR trial: Most psoriasis patients can be downshifted to reduced-dose biologics
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
PARIS – with long-term maintenance of disease control and no adverse consequences, Juul van den Reek, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.

She presented the results of the CONDOR trial, the first-ever formal, randomized, controlled trial of tightly regulated dose reduction of biologics, compared with usual care standard-dose therapy. “Our current advice is we think you can try to reduce the dose because there are a lot of patients who benefit from this,” declared Dr. van den Reek, a dermatologist at Radboud University, Nijmegen, the Netherlands.
The advantages of this strategy are twofold: lower expenditures for this costly collection of medications and less exposure to any long-term, drug-related health risks, she noted.
CONDOR was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial including 111 patients. Participants had to have stable low disease activity as defined by both Psoriasis Area and Severity Index (PASI) and Dermatology Life Quality Index (DLQI) scores of 5 or less for at least 6 months while on standard-dose etanercept (Enbrel), adalimumab (Humira), or ustekinumab (Stelara) prior to enrollment. In fact, the average baseline PASI score was less than 2, with a DLQI of 0.
Participants were randomized to usual care – the customary approved dose of biologic therapy – or a drop down to 67% of that dose, achieved through prolongation of the dosing interval. If the reduced-dose patients kept their PASI and DLQI scores at 5 or less for 3 months straight, they dropped further to 50% of their original dose. However, patients who exceeded those thresholds were immediately returned to their previously effective dose.
The primary endpoint in this noninferiority trial was the difference in mean PASI scores between the dose-reduction and usual-care groups at 12 months. The prespecified margin for noninferiority was a difference of 0.5 PASI points. And that’s where the results get dicey: The mean difference turned out to be 1.1 PASI points in favor of usual care, meaning that, according to the study ground rules, dose reduction was not statistically noninferior. In hindsight, however, that 0.5-point margin was ill-considered and too narrowly defined.
“Within the chosen margins, the dose-reduction strategy seemed inferior. But what is the clinical relevance of a mean difference of 1.1 PASI points, when the accepted minimal clinically important difference is 3.2 points?” Dr. van den Reek observed.
There was no significant between-group difference in DLQI scores at 12 months. Nor did the two study arms differ in terms of the prespecified secondary endpoint of persistent disease flares as defined by a PASI or DLQI greater than 5 for 3 consecutive months: five patients in the reduced-dose group and three in the usual-care arm experienced such flares. There were no serious adverse events or other safety signals related to the intervention.
At 12 months, 50% of patients in the dose-reduction group were well maintained on 50% of their original approved-dose biologic and another 17% were doing well on 67% of their former dose.
Session chair Dedee Murrell, MD, professor of dermatology at the University of New South Wales, Sydney, noted that neither patients nor dermatologists were blinded as to treatment status in CONDOR. She then asked the question on everybody’s minds: Was there any loss of treatment efficacy when patients in the dose-reduction arm needed to resume higher-dose therapy?
No, Dr. van den Reek replied. She added that planned future CONDOR analyses include a cost-effectiveness determination as well as measurement of serum drug levels and identification of antidrug antibodies, information that might prove helpful in identifying an enriched population of patients most likely to respond favorably to biologic dose reduction. In addition, CONDOR-X, a long-term extension study, is ongoing in order to learn how patients on reduced-dose biologics fare after the 12-month mark.
The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; Dr. van den Reek reported having no financial conflicts of interest.
REPORTING FROM THE EADV CONGRESS
Key clinical point: An attempt at dose reduction is worthwhile in psoriasis patients well controlled on full-dose biologic therapy.
Major finding: Two-thirds of psoriasis patients maintained disease control after 12 months on reduced-dose biologic therapy.
Study details: This was a Dutch six-center, 12-month, open-label, unblinded, noninferiority, randomized trial of 111 psoriasis patients with stable low disease activity on standard-dose biologics at enrollment.
Disclosures: The CONDOR trial was funded by the Netherlands Organization for Health Research and Development; the presenter reported having no financial conflicts of interest.
MGUS: It’s about the protein, not just the marrow
In the past decade, it has been increasingly recognized that these clonally produced proteins—entire immunoglobulins or free light chains—may be directly pathogenic, independent of any pathologic effect of cellular clonal expansion and infiltration. Brouet class 1 cryoglobulinemia (in which a monoclonal paraprotein precipitates in cooler temperatures and acts as a source of complement, activating the immune complex) and light chain (usually lambda)-related amyloidosis have been recognized for much longer. But a newer concept, monoclonal gammopathy of renal significance (MGRS), has attracted significant attention and to some extent has modified our approach to patients with either known MGUS or unexplained chronic kidney disease.
Finding MGUS still warrants a parsimonious evaluation for possible progression to myeloma or other proliferative disorder, as discussed by Khouri et al in this issue of the Journal. But it should also prompt a thoughtful assessment of renal function, including estimating the glomerular filtration rate and looking for proteinuria, hematuria, and unexplained glucosuria or inappropriate urine pH. While typical light chain-induced renal tubular injury is usually associated with high levels of proteins such as those seen with myeloma, other patterns of glomerular, vascular, and mixed renal disease are associated with deposition of proteins that, once considered in the differential diagnosis, warrant renal biopsy to diagnose and direct appropriate therapy. That MGUS and MGRS occur more frequently in older patients, who are already at greater risk of multiple common causes of kidney disease, complicates clinical decision-making.1 Some of these disorders are associated with other initially subtle or seemingly disconnected clinical symptoms such as polyneuropathy, rash, and carpal tunnel syndrome, but many are at least initially limited to the kidneys.
As we enter a new calendar year, we at the Journal send our best wishes to all of our readers, authors, and peer reviewers, and we thank you for sharing in our medical education ventures. I personally hope that we have added some joy, enthusiasm—and some knowledge—to your professional activities, and I hope that we all can participate in some way to refashion a more civil and peaceful world in 2019.
- Rosner MH, Edeani A, Yanagita M, et al. Paraprotein-related kidney disease: diagnosing and treating monoclonal gammopathy of renal significance. Clin J Am Soc Neph 2016; 11(12):2280–2287. doi:10.2215/CJN.02920316
In the past decade, it has been increasingly recognized that these clonally produced proteins—entire immunoglobulins or free light chains—may be directly pathogenic, independent of any pathologic effect of cellular clonal expansion and infiltration. Brouet class 1 cryoglobulinemia (in which a monoclonal paraprotein precipitates in cooler temperatures and acts as a source of complement, activating the immune complex) and light chain (usually lambda)-related amyloidosis have been recognized for much longer. But a newer concept, monoclonal gammopathy of renal significance (MGRS), has attracted significant attention and to some extent has modified our approach to patients with either known MGUS or unexplained chronic kidney disease.
Finding MGUS still warrants a parsimonious evaluation for possible progression to myeloma or other proliferative disorder, as discussed by Khouri et al in this issue of the Journal. But it should also prompt a thoughtful assessment of renal function, including estimating the glomerular filtration rate and looking for proteinuria, hematuria, and unexplained glucosuria or inappropriate urine pH. While typical light chain-induced renal tubular injury is usually associated with high levels of proteins such as those seen with myeloma, other patterns of glomerular, vascular, and mixed renal disease are associated with deposition of proteins that, once considered in the differential diagnosis, warrant renal biopsy to diagnose and direct appropriate therapy. That MGUS and MGRS occur more frequently in older patients, who are already at greater risk of multiple common causes of kidney disease, complicates clinical decision-making.1 Some of these disorders are associated with other initially subtle or seemingly disconnected clinical symptoms such as polyneuropathy, rash, and carpal tunnel syndrome, but many are at least initially limited to the kidneys.
As we enter a new calendar year, we at the Journal send our best wishes to all of our readers, authors, and peer reviewers, and we thank you for sharing in our medical education ventures. I personally hope that we have added some joy, enthusiasm—and some knowledge—to your professional activities, and I hope that we all can participate in some way to refashion a more civil and peaceful world in 2019.
In the past decade, it has been increasingly recognized that these clonally produced proteins—entire immunoglobulins or free light chains—may be directly pathogenic, independent of any pathologic effect of cellular clonal expansion and infiltration. Brouet class 1 cryoglobulinemia (in which a monoclonal paraprotein precipitates in cooler temperatures and acts as a source of complement, activating the immune complex) and light chain (usually lambda)-related amyloidosis have been recognized for much longer. But a newer concept, monoclonal gammopathy of renal significance (MGRS), has attracted significant attention and to some extent has modified our approach to patients with either known MGUS or unexplained chronic kidney disease.
Finding MGUS still warrants a parsimonious evaluation for possible progression to myeloma or other proliferative disorder, as discussed by Khouri et al in this issue of the Journal. But it should also prompt a thoughtful assessment of renal function, including estimating the glomerular filtration rate and looking for proteinuria, hematuria, and unexplained glucosuria or inappropriate urine pH. While typical light chain-induced renal tubular injury is usually associated with high levels of proteins such as those seen with myeloma, other patterns of glomerular, vascular, and mixed renal disease are associated with deposition of proteins that, once considered in the differential diagnosis, warrant renal biopsy to diagnose and direct appropriate therapy. That MGUS and MGRS occur more frequently in older patients, who are already at greater risk of multiple common causes of kidney disease, complicates clinical decision-making.1 Some of these disorders are associated with other initially subtle or seemingly disconnected clinical symptoms such as polyneuropathy, rash, and carpal tunnel syndrome, but many are at least initially limited to the kidneys.
As we enter a new calendar year, we at the Journal send our best wishes to all of our readers, authors, and peer reviewers, and we thank you for sharing in our medical education ventures. I personally hope that we have added some joy, enthusiasm—and some knowledge—to your professional activities, and I hope that we all can participate in some way to refashion a more civil and peaceful world in 2019.
- Rosner MH, Edeani A, Yanagita M, et al. Paraprotein-related kidney disease: diagnosing and treating monoclonal gammopathy of renal significance. Clin J Am Soc Neph 2016; 11(12):2280–2287. doi:10.2215/CJN.02920316
- Rosner MH, Edeani A, Yanagita M, et al. Paraprotein-related kidney disease: diagnosing and treating monoclonal gammopathy of renal significance. Clin J Am Soc Neph 2016; 11(12):2280–2287. doi:10.2215/CJN.02920316
Acute-onset quadriplegia with hyperreflexia
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
A 79-year-old man presented with sudden-onset bilateral weakness in the lower and upper extremities that had started 6 hours earlier. He reported no vision disturbances or urinary incontinence. He was afebrile, with a blood pressure of 148/94 mm Hg, heart rate 98 bpm, and oxygen saturation of 95% on room air.
Physical examination revealed quadriplegia with hyperreflexia, sustained ankle clonus, and bilateral Babinski reflex, as well as spontaneous adductor and extensor spasms of the lower extremities.
Funduscopy was negative for optic neuritis. Results of a complete blood cell count and renal and liver function testing were within normal limits.
The patient was admitted to the intensive care unit. Methylprednisolone 1 g was given intravenously once daily for 5 days, with plasma exchange every other day for 5 sessions. A workup for neoplastic, autoimmune, and infectious disease was negative, as was testing for serum aquaporin-4 antibody (ie, neuromyelitis optica immunoglobulin G antibody).
Over the course of 7 days, the patient’s motor strength improved, and he was able to walk without assistance. Steroid therapy was tapered, and he was prescribed rituximab to prevent recurrence.
LONGITUDINALLY EXTENSIVE TRANSVERSE MYELITIS
A subtype of transverse myelitis, LETM is defined by partial or complete spinal cord dysfunction due to a lesion extending 3 or more vertebrae as confirmed on MRI. The clinical presentation can include paraparesis, sensory disturbances, and gait, bladder, bowel, or sexual dysfunction.1 Identifying the cause requires an extensive workup, as the differential diagnosis includes a wide range of conditions2:
- Autoimmune disorders such as Behçet disease, systemic lupus erythematosus, and Sjögren syndrome
- Infectious disorders such as syphilis, tuberculosis, and viral and parasitic infections
- Demyelinating disorders such as multiple sclerosis and neuromyelitis optica
- Neoplastic conditions such as intramedullary metastasis and lymphoma
- Paraneoplastic syndromes.
In our patient, the evaluation did not identify a specific underlying condition, and testing for serum aquaporin-4 antibody was negative. Therefore, the LETM was ruled an isolated idiopathic episode.
Idiopathic seronegative LETM has been associated with fewer recurrences than seropositive LETM.3 Management consists of high-dose intravenous steroids and plasma exchange. Rituximab can be used to prevent recurrence.4
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
- Trebst C, Raab P, Voss EV, et al. Longitudinal extensive transverse myelitis—it’s not all neuromyelitis optica. Nat Rev Neurol 2011; 7(12):688–698. doi:10.1038/nrneurol.2011.176
- Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord 2017; 10(7):265–289. doi:10.1177/1756285617709723
- Kitley J, Leite MI, Küker W, et al. Longitudinally extensive transverse myelitis with and without aquaporin 4 antibodies. JAMA Neurol 2013; 70(11):1375–1381. doi:10.1001/jamaneurol.2013.3890
- Tobin WO, Weinshenker BG, Lucchinetti CF. Longitudinally extensive transverse myelitis. Curr Opin Neurol 2014; 27(3):279–289. doi:10.1097/WCO.0000000000000093
Emphysematous cystitis
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
A 59-year-old woman with a history of chronic kidney disease and atonic bladder was brought to the hospital by emergency medical services. She had fallen in her home 2 days earlier and remained on the floor until neighbors eventually heard her cries and called 911. She complained of abdominal pain and distention along with emesis.
On presentation, she had tachycardia and tachypnea. The examination was notable for pronounced abdominal distention, diminished bowel sounds, and costovertebral angle tenderness.
While laboratory work was being done, the patient’s tachypnea progressed to respiratory distress, and she ultimately required intubation. Vasopressors were started, as the patient was hemodynamically unstable. A Foley catheter was placed, which yielded about 1,100 mL of purulent urine.
Laboratory workup showed:
- Procalcitonin 189 ng/mL (reference range < 2.0 ng/mL)
- White blood cell count 10.7 × 109/L (4.5–10.0)
- Myoglobin 20,000 ng/mL (< 71)
- Serum creatinine 4.8 mg/dL (0.06–1.10).
Urinalysis was positive for infection; blood and urine cultures later were positive for Escherichia coli.
The patient went into shock that was refractory to pressors, culminating in cardiac arrest despite resuscitative measures.
EMPHYSEMATOUS CYSTITIS, A FORM OF URINARY TRACT INFECTION
Emphysematous cystitis is a rare form of complicated urinary tract infection characterized by gas inside the bladder and in the bladder wall. While the exact mechanisms underlying gas formation are not clear, gas-producing pathogens are clearly implicated in severe infection. E coli and Klebsiella pneumoniae are the most common organisms associated with emphysematous cystitis; others include Proteus mirabilis, and Enterobacter and Streptococcus species.1,2
More than 50% of patients with emphysematous cystitis have diabetes mellitus. Other risk factors include bladder outlet obstruction, neurogenic bladder, and female sex.3 The severity of disease ranges from asymptomatic pneumaturia (up to 7% of cases)2 to fulminant emphysematous cystitis, as in our patient.
The clinical presentation of emphysematous cystitis is nonspecific and can range from minimally symptomatic urinary tract infection to acute abdomen and septic shock.4
Some patients present with pneumaturia (the passing of gas through the urethra with micturition). Pneumaturia arises from 3 discrete causes: urologic instrumentation, fistula between the bladder and large or small bowel, and gas-producing bacteria in the bladder (emphysematous cystitis).5 Pneumaturia should always raise the suspicion of emphysematous cystitis.
The diagnosis can be made with either radiographic or computed tomographic evidence of gas within the bladder and bladder wall, in the absence of both bladder fistula and history of iatrogenic pneumaturia. Emphysematous cystitis should prompt urine and blood cultures to direct antimicrobial therapy, as 50% of patients with emphysematous cystitis have concomitant bacteremia.6
Our patient had an elevated serum level of procalcitonin, a marker of bacterial infection. Procalcitonin is a more specific biomarker of bacterial infection than acute-phase reactants such as the erythrocyte sedimentation rate or the C-reactive protein level. Measuring procalcitonin may help physicians make the diagnosis earlier, differentiate infectious from sterile causes of severe systemic inflammation, assess the severity of systemic inflammation caused by bacterial infections, and decide whether to start or discontinue antibiotic therapy.7
Most cases of emphysematous cystitis can be treated with antibiotics, though early diagnosis is crucial to a favorable outcome. Delay in diagnosis may contribute to the 20% mortality rate associated with this condition.6
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
- Stein JP, Spitz A, Elmajian DA, et al. Bilateral emphysematous pyelonephritis: a case report and review of the literature. Urology 1996; 47(1):129–134. pmid:8560648
- Amano M, Shimizu T. Emphysematous cystitis: a review of the literature. Intern Med 2014; 53(2):79–82. pmid:24429444
- Wang JH. Emphysematous cystitis. Urol Sci 2010; 21(4):185–186. doi:10.1016/S1879-5226(10)60041-3
- Thomas AA, Lane BR, Thomas AZ, Remer EM, Campbell SC, Shoskes DA. Emphysematous cystitis: a review of 135 cases. BJU Int 2007; 100(1):17–20. doi:10.1111/j.1464-410X.2007.06930.x
- Arthur LM, Johnson HW. Pneumaturia: a case report and review of the literature. J Urol 1948; 60(4):659–665. pmid:18885959
- Grupper M, Kravtsov A, Potasman I. Emphysematous cystitis: illustrative case report and review of the literature. Medicine (Baltimore) 2007; 86(1):47–53. doi:10.1097/MD.0b013e3180307c3a
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28(3):285–291. doi:10.3904/kjim.2013.28.3.285
When can I stop dual antiplatelet therapy in patients with drug-eluting stents?
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1
THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1

THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
Stopping dual antiplatelet therapy (DAPT) (eg, clopidogrel plus aspirin) after 3 months is reasonable in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and a high bleeding risk, with stable ischemic disease defined as at least 1 year free of acute coronary syndromes. However, these patients should continue lifelong aspirin monotherapy. Current guidelines suggest that in stable ischemic disease, the risk-benefit ratio may favor an even shorter duration of DAPT than the 6 months currently recommended.1
STABLE ISCHEMIC HEART DISEASE VS ACUTE CORONARY SYNDROME
Percutaneous coronary intervention for stable ischemic heart disease is indicated primarily in patients with angina that persists despite optimal antianginal therapy.
The prognostic implications of DAPT are different in stable ischemic disease than in acute coronary syndromes. The substrate treated by percutaneous intervention in stable ischemic disease is primarily fibrofatty plaque, as opposed to thrombus in acute coronary syndromes.
Percutaneous intervention significantly improves the prognosis in acute coronary syndromes, whereas its impact on overall survival in stable ischemic heart disease is not well documented. Given these differences, our discussion about DAPT in stable ischemic disease cannot be extrapolated to acute coronary syndromes.
BENEFITS OF DAPT
DAPT is mandatory early after drug-eluting stent placement, when the stent continuously releases medication, inhibiting tissue growth within the lumen of the stent.
Endothelialization of the stent normally occurs during the first 7 to 30 days after placement. During this period, the nonendothelialized stent poses a risk of thrombosis, a life-threatening, catastrophic condition with a mortality rate between 9% and 45%.1

THERAPY BEYOND 12 MONTHS
Although guidelines have traditionally recommended 12 months of DAPT, the optimal duration is still debated.
A duration beyond 12 months in patients with a history of myocardial infarction was shown to be reasonable in 2 large trials,2,3 while a 2016 review by Bittl et al4 suggested that therapy beyond 12 months in patients with a newer-generation drug-eluting stent could increase the incidence of major bleeding. A detailed discussion of DAPT longer than 12 months is beyond the scope of this article.
EVIDENCE FOR SHORTER DURATION
The results of 5 major trials support shorter duration of DAPT in stable ischemic disease.
The OPTIMIZE5 and RESET6 trials found that 3 months of DAPT was not inferior to 12 months in terms of ischemic and safety end points.
The ISAR-SAFE,7 EXCELLENT,8 and SECURITY9 trials also reported that 6 months of DAPT was not inferior to 12 months for the primary composite end point of death, stent thrombosis, myocardial infarction, stroke, or major bleeding.
However, these trials may have been underpowered to detect a difference in rates of stent thrombosis with shorter-duration DAPT.
CURRENT GUIDELINES
For patients at high bleeding risk, the current guidelines of the American College of Cardiology and American Heart Association, updated in 2016, suggest that it may be reasonable to discontinue DAPT 3 months after drug-eluting stent placement in patients with stable ischemic heart disease, and at 6 months in patients with acute coronary syndrome (class IIb recommendation, level of evidence C).1 These recommendations are based on results of randomized controlled trials showing no difference in the rate of stent thrombosis and composite ischemic events with a shorter duration than with 12 months of therapy.5–10
The evidence for DAPT in stable ischemic disease is based on clopidogrel, with only limited data on ticagrelor.1 To our knowledge, no study to date has evaluated DAPT in this setting for less than 3 months, and further study is needed to address shorter-duration approaches with current-generation drug-eluting stents Since 2017, all coronary stents implanted in the United States have been second-generation stents.
TOOLS TO HELP DECISION-MAKING
The decision to stop DAPT in a patient at high risk of bleeding requires a careful assessment of the risks and benefits. Risk factors for bleeding include advanced age, history of major bleeding, anticoagulation, chronic kidney disease (serum creatinine level ≥ 2 mg/dL), platelet count 100 × 109/L or lower, and history of stroke.11
- Age 75 or older: −2 points
- Ages 65 to 74: −1
- Age under 65: 0
- Diabetes mellitus: 1
- Myocardial infarction at presentation: 1
- History of percutaneous coronary intervention or myocardial infarction: 1
- Stent diameter less than 3 mm: 1
- Paclitaxel drug-eluting stent: 1
- Current smoker: 2
- Percutaneous coronary intervention with saphenous vein graft: 2
- Congestive heart failure or left ventricular ejection fraction less than 30%: 2.
A score of 2 or greater favors continuing DAPT, as it indicates higher ischemic risk. A score less than 2 favors discontinuing DAPT, as it indicates higher bleeding risk.1,2
IF BLEEDING RISK IS HIGH
Preventing and controlling bleeding associated with DAPT is important. The gastrointestinal tract is the most common site of bleeding.
Aspirin inhibits prostaglandin synthesis, leading to disruption of the protective mucous membrane. Therefore, a proton pump inhibitor should be started along with DAPT in patients at high risk of gastrointestinal bleeding.
If a patient’s bleeding risk significantly outweighs the risk of stent thrombosis, or if active hemorrhage makes a patient hemodynamically unstable, antiplatelet therapy must be stopped.1
FACING SURGERY
For patients with a drug-eluting stent who are on DAPT and are to undergo elective noncardiac surgery, 3 considerations must be kept in mind:
- The risk of stent thrombosis if DAPT needs to be interrupted
- The consequences of delaying the surgical procedure
- The risk and consequences of periprocedural and intraprocedural bleeding if DAPT is continued.
Because clinical evidence for bridging therapy with intravenous antiplatelet or anticoagulant agents is limited, it is difficult to make recommendations about stopping DAPT. However, once bleeding risk is stabilized, DAPT should be restarted as soon as possible.1
CURRENT RESEARCH
Several trials are under way to further evaluate ways to minimize bleeding risk and shorten the duration of DAPT.
A prospective multicenter trial is evaluating 3-month DAPT in patients at high bleeding risk who undergo placement of an everolimus-eluting stent.11 This study is expected to be completed in August 2019.
Another strategy for patients at high bleeding risk is use of a polymer-free drug-coated coronary stent. In a 2015 trial comparing a biolimus A9-coated stent vs a bare-metal stent, patients received DAPT for 1 month after stent placement. The drug-coated stent was found to be superior in terms of the primary safety end point (cardiac death, myocardial infarction, or stent thrombosis).12 This stent is not yet approved by the US Food and Drug Administration at the time of this writing.
Further study is needed to evaluate DAPT durations of less than 3 months and to establish the proper timing for safely discontinuing DAPT in difficult clinical scenarios.
WHEN STOPPING MAY BE REASONABLE
According to current guidelines, in patients at high bleeding risk with a second-generation or newer drug-eluting stent for stable ischemic heart disease, discontinuing DAPT 3 months after stent placement may be reasonable.1 The decision to stop DAPT in these patients requires a careful assessment of the risks and benefits and may be aided by a tool such as the DAPT risk score. However, these recommendations cannot be extrapolated to patients with an acute coronary syndrome within the past year, as they are at higher risk.
TAKE-HOME MESSAGES
- A cardiologist should be consulted before discontinuing DAPT in patients with a drug-eluting stent, especially if the stent was recently placed.
- The duration of therapy depends on the indication for stent placement (stable ischemic heart disease vs acute coronary syndrome) and on stent location.
- Based on the 2016 American College of Cardiology/American Heart Association guidelines,1 in patients at high bleeding risk with a second-generation drug-eluting stent, discontinuing DAPT is safe after 3 months in patients with stable ischemic heart disease, and after 6 months in patients with an acute coronary syndrome.
- When prescribing DAPT, available evidence favors clopidogrel in patients with stable ischemic heart disease who have a second-generation drug-eluting stent and are at high bleeding risk.
- In these patients, the risk-benefit ratio based on the DAPT score may be useful when considering stopping clopidogrel.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016; 134(10):e123–e155. doi:10.1161/CIR.0000000000000404 [correction in doi:10.1161/CIR.0000000000000452]
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371(23):2155–2166. doi:10.1056/NEJMoa1409312
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372(19):1791–1800. doi:10.1056/NEJMoa1500857
- Bittl JA, Baber U, Bradley SM, Wijeysundera DN. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68(10):1116–1139. doi:10.1016/j.jacc.2016.03.512
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310(23):2510–2522. doi:10.1001/jama.2013.282183
- Kubo T, Akasaka T, Kozuma K, et al. Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of RESET. EuroIntervention 2015. doi:10.4244/EIJV11I5A109
- Schulz-Schupke S, Byrne RA, ten Berg JM, et al; Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015; 36(20):1252–1263. doi:10.1093/eurheartj/ehu523
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus vs Cypher to reduce late loss after stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125(3):505–513. doi:10.1161/CIRCULATIONAHA.111.059022
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- vs 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64(20):2086–2097. doi:10.1016/j.jacc.2014.09.008
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60(15):1340–1348. doi:10.1016/j.jacc.2012.06.043
- US National Library of Medicine. ClinicalTrials.gov. EVOLVE Short DAPT Study. https://clinicaltrials.gov/ct2/show/NCT02605447. Accessed December 3, 2018.
- Urban P, Meredith IT, Abizaid A, et al; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373(21):2038–2047. doi:10.1056/NEJMoa1503943
Should metformin be used in every patient with type 2 diabetes?
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
Most patients should receive it, with exceptions as noted below. Metformin is the cornerstone of diabetes therapy and should be considered in all patients with type 2 diabetes. Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE)1,2 recommend it as first-line treatment for type 2 diabetes. It lowers blood glucose levels by inhibiting hepatic glucose production, and it does not tend to cause hypoglycemia.
However, metformin is underused. A 2012 study showed that only 50% to 70% of patients with type 2 diabetes treated with a sulfonylurea, dipeptidyl peptidase-4 (DPP-4) inhibitor, thiazolidinedione, or glucagon-like peptide-1 analogue also received metformin.3 This occurred despite guidelines recommending continuing metformin when starting other diabetes drugs.4
EVIDENCE METFORMIN IS EFFECTIVE
The United Kingdom Prospective Diabetes Study (UKPDS)5 found that metformin significantly reduced the incidence of:
- Any diabetes-related end point (hazard ratio [HR] 0.68, 95% confidence interval [CI] 0.53–0.87)
- Myocardial infarction (HR 0.61, 95% CI 0.41–0.89)
- Diabetes-related death (HR 0.58, 95% CI 0.37–0.91)
- All-cause mortality (HR 0.64; 95% CI 0.45–0.91).
The Hyperinsulinemia: Outcomes of Its Metabolic Effects (HOME) trial,6 a multicenter trial conducted in the Netherlands, evaluated the effect of adding metformin (vs placebo) to existing insulin regimens. Metformin recipients had a significantly lower rate of macrovascular mortality (HR 0.61, 95% CI 0.40–0.94, P = .02), but not of the primary end point, an aggregate of microvascular and macrovascular morbidity and mortality.
The Study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease trial,7 a multicenter trial conducted in China, compared the effects of metformin vs glipizide on cardiovascular outcomes. At about 3 years of treatment, the metformin group had a significantly lower rate of the composite primary end point of recurrent cardiovascular events (HR 0.54, 95% CI 0.30–0.90). This end point included nonfatal myocardial infarction, nonfatal stroke, arterial revascularization by percutaneous transluminal coronary angioplasty or by coronary artery bypass graft, death from a cardiovascular cause, and death from any cause.
These studies prompted the ADA to emphasize that metformin can reduce the risk of cardiovascular events or death. Metformin also has been shown to be weight-neutral or to induce slight weight loss. Furthermore, it is inexpensive.
WHAT ABOUT THE RENAL EFFECTS?
Because metformin is renally cleared, it has caused some concern about nephrotoxicity, especially lactic acidosis, in patients with impaired renal function. But the most recent guidelines have relaxed the criteria for metformin use in this patient population.
Revised labeling
Metformin’s labeling,8 revised in 2016, states the following:
- If the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m2, metformin is contraindicated
- If the eGFR is between 30 and 45 mL/min/1.73 m2, metformin is not recommended
- If the eGFR is below 45 mL/min/1.73 m2 in a patient taking metformin, the risks and benefits of continuing treatment should be assessed, the dosage may need to be adjusted, and renal function should be monitored more frequently.8
These labeling revisions were based on a systematic review by Inzucchi et al9 that found metformin is not associated with increased rates of lactic acidosis in patients with mild to moderate kidney disease. Subsequently, an observational study published in 2018 by Lazarus et al10 showed that metformin increases the risk of acidosis only at eGFR levels below 30 mL/min/1.73 m2. Also, a Cochrane review published in 2003 did not find a single case of lactic acidosis in 347 trials with 70,490 patient-years of metformin treatment.11
Previous guidelines used serum creatinine levels, with metformin contraindicated at levels of 1.5 mg/dL or above for men and 1.4 mg/dL for women, or with abnormal creatinine clearance. The ADA and the AACE now use the eGFR1,2 instead of the serum creatinine level to measure kidney function because it better accounts for factors such as the patient’s age, sex, race, and weight.
Despite the evidence, the common patient perception is that metformin is nephrotoxic, and it is important for practitioners to dispel this myth during clinic visits.
What about metformin use with contrast agents?
Labeling has a precautionary note stating that metformin should be held at the time of, or prior to, any imaging procedure involving iodinated contrast agents in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will receive intra-arterial iodinated contrast. The eGFR should be reevaluated 48 hours after the imaging procedure.8
Additionally, if the iodinated contrast agent causes acute kidney injury, metformin could accumulate, with resultant lactate accumulation.
The American College of Radiology (ACR) has proposed less stringent guidelines for metformin during radiocontrast imaging studies. This change is based on evidence that lactic acidosis is rare—about 10 cases per 100,000 patient-years—and that there are no reports of lactic acidosis after intravenously administered iodinated contrast in properly selected patients.12,13
The ACR divides patients taking metformin into 2 categories:
- No evidence of acute kidney injury and eGFR greater than 30 mL/min/1.73 m2
- Either acute kidney injury or chronic kidney disease with eGFR below 30 mL/min/1.73 m2 or undergoing arterial catheter studies with a high chance of embolization to the renal arteries.14
For the first group, they recommend against discontinuing metformin before or after giving iodinated contrast or checking kidney function after the procedure.
For the second group, they recommend holding metformin before and 48 hours after the procedure. It should not be restarted until renal function is confirmed to be normal.
METFORMIN AND INSULIN
The ADA recommends1 continuing metformin after initiating insulin. However, in clinical practice, it is often not done.
Clinical trials have shown that combining metformin with insulin significantly improves glycemic control, prevents weight gain, and decreases insulin requirements.15,16 One trial16 also looked at cardiovascular end points during a 4-year follow-up period; combining metformin with insulin decreased the macrovascular disease-related event rate compared with insulin alone.
In the HOME trial,6 which added metformin to the existing insulin regimen, both groups gained weight, but the metformin group had gained about 3 kg less than the placebo group at the end of the 4.3-year trial. Metformin did not increase the risk of hypoglycemia, but it also did not reduce the risk of microvascular disease.
Concomitant metformin reduces costs
These days, practitioners can choose from a large selection of diabetes drugs. These include insulins with better pharmacokinetic profiles, as well as newer classes of noninsulin agents such as sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 analogues.
Metformin is less expensive than these newer drugs, and using it concomitantly with other diabetes drugs can decrease their dosage requirements, which in turn decreases their monthly costs.
GASTROINTESTINAL EFFECTS
Metformin’s gastrointestinal adverse effects such as diarrhea, flatulence, nausea, and vomiting are a barrier to its use. The actual incidence rate of diarrhea varies widely in randomized trials and observational studies, and gastrointestinal effects are worse in metformin-naive patients, as well as those who have chronic gastritis or Helicobacter pylori infection.17
We have found that starting metformin at a low dose and up-titrating it over several weeks increases tolerability. We often start patients at 500 mg/day and increase the dosage by 1 500-mg tablet every 1 to 2 weeks. Also, we have noticed that intolerance is more likely in patients who eat a high-carbohydrate diet, but there is no high-level evidence to back this up because patients in clinical trials all undergo nutrition counseling and are therefore more likely to adhere to the low-carbohydrate diet.
Also, the extended-release formulation is more tolerable than the immediate-release formulation and has similar glycemic efficacy. It may be an option as first-line therapy or for patients who have significant adverse effects from immediate-release metformin.18 For patients on the immediate-release formulation, taking it with meals helps lessen some gastrointestinal effects, and this should be emphasized at every visit.
Finally, we limit the metformin dose to 2,000 mg/day, rather than the 2,550 mg/day allowed on labeling. Garber et al19 found that the lower dosage still provides the maximum clinical efficacy.
OTHER CAUTIONS
Metformin should be avoided in patients with acute or unstable heart failure because of the increased risk of lactic acidosis.
It also should be avoided in patients with hepatic impairment, according to the labeling. But this remains controversial in practice. Zhang et al20 showed that continuing metformin in patients with diabetes and cirrhosis decreases the mortality risk by 57% compared with those taken off metformin.
Diet and lifestyle measures need to be emphasized at each visit. Wing et al21 showed that calorie restriction regardless of weight loss is beneficial for glycemic control and insulin sensitivity in obese patients with diabetes.
TAKE-HOME POINTS
Metformin improves glycemic control without tending to cause weight gain or hypoglycemia. It may also have cardiovascular benefits. Metformin is an inexpensive agent that should be continued, if tolerated, in those who need additional agents for glycemic control. It should be considered in all adult patients with type 2 diabetes.
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018; 24(1):91–120. doi:10.4158/CS-2017-0153
- Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care 2014; 37(5):1367–1374. doi:10.2337/dc13-2289
- Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35(6):1364–1379. doi:10.2337/dc12-0413
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131):854–865. pmid:9742977
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013; 36(5):1304–1311. doi:10.2337/dc12-0719
- Glucophage (metformin hydrochloride) and Glucophage XR (extended-release) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company. www.accessdata.fda.gov/drugsatfda_docs/label/2018/020357s034,021202s018lbl.pdf. Accessed December 5, 2018.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 2014; 312(24):2668–2675. doi:10.1001/jama.2014.15298
- Lazarus B, Wu A, Shin JI, et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med 2018; 178(7):903–910. doi:10.1001/jamainternmed.2018.0292
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003; (2):CD002967. doi:10.1002/14651858.CD002967
- Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014; 37(8):2218–2224. doi:10.2337/dc13-3023
- Richy FF, Sabidó-Espin M, Guedes S, Corvino FA, Gottwald-Hostalek U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: a retrospective cohort study. Diabetes Care 2014; 37(8):2291–2295. doi:10.2337/dc14-0464
- American College of Radiology (ACR). Manual on Contrast Media. Version 10.3. www.acr.org/Clinical-Resources/Contrast-Manual. Accessed December 5, 2018.
- Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002; 25(12):2133–2140. pmid:12453950
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009; 169(6):616–625. doi:10.1001/archinternmed.2009.20
- Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance, Diabetes Obes Metab 2017; 19(4):473–481. doi:10.1111/dom.12854
- Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123(1):15–23. doi:10.3810/pgm.2011.01.2241
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103(6):491–497. pmid:9428832
- Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology 2014; 60(6):2008–2016. doi:10.1002/hep.27199
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17(1):30–36. pmid:8112186
Rapidly progressive pleural effusion
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
A 33-year-old male nonsmoker with no significant medical history presented to the pulmonary clinic with severe left-sided pleuritic chest pain and mild breathlessness for the past 5 days. He denied fever, chills, cough, phlegm, runny nose, or congestion.
Five days before this visit, he had been seen in the emergency department with mild left-sided pleuritic chest pain. His vital signs at that time had been as follows:
- Blood pressure 141/77 mm Hg
- Heart rate 77 beats/minute
- Respiratory rate 17 breaths/minute
- Temperature 36.8°C (98.2°F)
- Oxygen saturation 98% on room air.
- White blood cell count 6.89 × 109/L (reference range 3.70–11.00)
- Neutrophils 58% (40%–70%)
- Lymphocytes 29.6% (22%–44%)
- Monocytes 10.7% (0–11%)
- Eosinophils 1% (0–4%)
- Basophils 0.6% (0–1%)
- Troponin T and D-dimer levels normal.
DIFFERENTIAL DIAGNOSIS OF PLEURITIC CHEST PAIN
1. What is the most likely cause of his pleuritic chest pain?
- Pleuritis
- Pneumonia
- Pulmonary embolism
- Malignancy
The differential diagnosis of pleuritic chest pain is broad.
The patient’s symptoms at presentation to the emergency department did not suggest an infectious process. There was no fever, cough, or phlegm, and his white blood cell count was normal. Nonetheless, pneumonia could not be ruled out, as the lung parenchyma was not normal on radiography, and the findings could have been consistent with an early or resolving infectious process.
Pulmonary embolism was a possibility, but his normal D-dimer level argued against it. Further, the patient subsequently underwent CT angiography, which ruled out pulmonary embolism.
Malignancy was unlikely in a young nonsmoker, but follow-up imaging would be needed to ensure resolution and rule this out.
The emergency department physician diagnosed inflammatory pleuritis and discharged him home on a nonsteroidal anti-inflammatory drug.
CLINIC VISIT 5 DAYS LATER
At his pulmonary clinic visit 5 days later, the patient reported persistent but stable left-sided pleuritic chest pain and mild breathlessness on exertion. His blood pressure was 137/81 mm Hg, heart rate 109 beats per minute, temperature 37.1°C (98.8°F), and oxygen saturation 97% on room air.
Auscultation of the lungs revealed rales and slightly decreased breath sounds at the left base. No dullness to percussion could be detected.
Because the patient had developed mild tachycardia and breathlessness along with clinical signs that suggested worsening infiltrates, consolidation, or the development of pleural effusion, he underwent further investigation with chest radiography, a complete blood cell count, and measurement of serum inflammatory markers.
- White blood cell count 13.08 × 109/L
- Neutrophils 81%
- Lymphocytes 7.4%
- Monocytes 7.2%
- Eeosinophils 0.2%
- Basophils 0.2%
- Procalcitonin 0.34 µg/L (reference range < 0.09).
Bedside ultrasonography to assess the effusion’s size and characteristics and the need for thoracentesis indicated that the effusion was too small to tap, and there were no fibrinous strands or loculations to suggest empyema.
FURTHER TREATMENT
2. What was the best management strategy for this patient at this time?
- Admit to the hospital for thoracentesis and intravenous antibiotics
- Give oral antibiotics with close follow-up
- Perform thoracentesis on an outpatient basis and give oral antibiotics
- Repeat chest CT
The patient had worsening pleuritic pain with development of a small left pleural effusion. His symptoms had not improved on a nonsteroidal anti-inflammatory drug. He now had an elevated white blood cell count with a “left shift” (ie, an increase in neutrophils, indicating more immature cells in circulation) and elevated procalcitonin. The most likely diagnosis was pneumonia with a resulting pleural effusion, ie, parapneumonic effusion, requiring appropriate antibiotic therapy. Ideally, the pleural effusion should be sampled by thoracentesis, with management on an outpatient or inpatient basis.
5 DAYS LATER, THE EFFUSION HAD BECOME MASSIVE
On follow-up 5 days later, the patient’s chest pain was better, but he was significantly more short of breath. His blood pressure was 137/90 mm Hg, heart rate 117 beats/minute, respiratory rate 16 breaths/minute, oxygen saturation 97% on room air, and temperature 36.9°C (98.4°F). Chest auscultation revealed decreased breath sounds over the left hemithorax, with dullness to percussion and decreased fremitus.
RAPIDLY PROGRESSIVE PLEURAL EFFUSIONS
A rapidly progressive pleural effusion in a healthy patient suggests parapneumonic effusion. The most likely organism is streptococcal.2
Explosive pleuritis is defined as a pleural effusion that increases in size in less than 24 hours. It was first described by Braman and Donat3 in 1986 as an effusion that develops within hours of admission. In 2001, Sharma and Marrie4 refined the definition as rapid development of pleural effusion involving more than 90% of the hemithorax within 24 hours, causing compression of pulmonary tissue and a mediastinal shift. It is a medical emergency that requires prompt investigation and treatment with drainage and antibiotics. All reported cases of explosive pleuritis have been parapneumonic effusion.
The organisms implicated in explosive pleuritis include gram-positive cocci such as Streptococcus pneumoniae, S pyogenes, other streptococci, staphylococci, and gram-negative cocci such as Neisseria meningitidis and Moraxella catarrhalis. Gram-negative bacilli include Haemophilus influenzae, Klebsiella pneumoniae, Pseudomonas species, Escherichia coli, Proteus species, Enterobacter species, Bacteroides species, and Legionella species.4,5 However, malignancy is the most common cause of massive pleural effusion, accounting for 54% of cases; 17% of cases are idiopathic, 13% are parapneumonic, and 12% are hydrothorax related to liver cirrhosis.6
CASE CONTINUED
Our patient’s massive effusion needed drainage, and he was admitted to the hospital for further management. Samples of blood and sputum were sent for culture. Intravenous piperacillin-tazobactam was started, and an intercostal chest tube was inserted into the pleural cavity under ultrasonographic guidance to drain turbid fluid.
Multiple pleural fluid samples sent for bacterial, fungal, and acid-fast bacilli culture were negative. Blood and sputum cultures also showed no growth. The administration of oral antibiotics for 5 days on an outpatient basis before pleural fluid culture could have led to sterility of all cultures.
Our patient had inadequate pleural fluid output through his chest tube, and radiography showed that the pleural collections failed to clear. In fact, an apical locule did not appear to be connecting with the lower aspect of the pleural collection. In such cases, instillation of intrapleural agents through the chest tube has become common practice in an attempt to lyse adhesions, to connect various locules or pockets of pleural fluid, and to improve drainage.
LOCULATED EMPYEMA: MANAGEMENT
3. What was the best management strategy for this loculated empyema?
- Continue intravenous antibiotics and existing chest tube drainage for 5 to 7 days, then reassess
- Continue intravenous antibiotics and instill intrapleural fibrinolytics (eg, tissue plasminogen activator [tPA]) through the existing chest tube
- Continue intravenous antibiotics and instill intrapleural fibrinolytics with deoxyribonuclease (DNase) into the existing chest tube
- Continue intravenous antibiotics, insert a second chest tube into the apical pocket under imaging guidance, and instill tPA and DNase
- Surgical decortication
Continuing antibiotics with existing chest tube drainage and the two options of using single-agent intrapleural fibrinolytics have been shown to be less effective than combining tPA and DNase when managing a loculated empyema. As such, surgical decortication, attempting intrapleural instillation of fibrinolytics and DNase (with or without further chest tube insertion into noncommunicating locules), or both were the most appropriate options at this stage.
MANAGEMENT OF PARAPNEUMONIC PLEURAL EFFUSION IN ADULTS
There are several options for managing parapneumonic effusion, and clinicians can use the classification system in Table 1 to assess the risk of a poor outcome and to plan the management. Based on radiographic findings and pleural fluid sampling, a pleural effusion can be either observed or drained.
Options for drainage of the pleural space include repeat thoracentesis, surgical insertion of a chest tube, or image-guided insertion of a small-bore catheter. Although no randomized trial has been done to compare tube sizes, a large retrospective series showed that small-bore tubes (< 14 F) perform similarly to standard large-bore tubes.8 However, in another study, Keeling et al9 reported higher failure rates when tubes smaller than 12 F were used. Regular flushing of the chest tube (ideally twice a day) is recommended to keep it patent, particularly with small-bore tubes. Multiloculated empyema may require multiple intercostal chest tubes to drain completely, and therefore small-bore tubes are recommended.
In cases that do not improve radiographically and clinically, one must consider whether the antibiotic choice is adequate, review the position of the chest tube, and assess for loculations. As such, repeating chest CT within 24 to 48 hours of tube insertion and drainage is recommended to confirm adequate tube positioning, assess effective drainage, look for different locules and pockets, and determine the degree of communication between them.
The largest well-powered randomized controlled trials of intrapleural agents in the management of pleural infection, the Multicentre Intrapleural Sepsis Trial (MIST1)10 and MIST2,11 clearly demonstrated that intrapleural fibrinolytics were not beneficial when used alone compared with placebo. However, in MIST2, the combination of tPA and DNase led to clinically significant benefits including radiologic improvement, shorter hospital stay, and less need for surgical decortication.
At our hospital, we follow the MIST2 protocol using a combination of tPA and DNase given intrapleurally twice daily for 3 days. In our patient, we inserted a chest tube into the apical pocket under ultrasonographic guidance, as 2 instillations of intrapleural tPA and DNase did not result in drainage of the apical locule.
Success rates with intrapleural tPA-DNase for complicated pleural effusion and empyema range from 68% to 92%.12–15 Pleural thickening and necrotizing pneumonia and abscess are important predictors of failure of tPA-DNase therapy and of the need for surgery.13,14
Early surgical intervention was another reasonable option in this case. The decision to proceed with surgery is based on need to debride multiloculated empyemas or uniloculated empyemas that fail to resolve with antibiotics and tube thoracostomy drainage. Nonetheless, the decision must be individualized and based on factors such as the patient’s risks vs possible benefit from a surgical procedure under general anesthesia, the patient’s ability to tolerate multiple thoracentesis procedures and chest tubes for a potentially lengthy period, the patient’s pain threshold, the patient’s wishes to avoid a surgical procedure balanced against a longer hospital stay, and cultural norms and beliefs.
Surgical options include video-assisted thoracoscopy, thoracotomy, and open drainage. Decortication can be considered early to control pleural sepsis, or late (after 3 to 6 months) if the lung does not expand. Debate continues on the optimal timing for video-assisted thoracoscopy, with data suggesting that when the procedure is performed later in the course of the disease there is a greater chance of complications and of the need to convert to thoracotomy.
A 2017 Cochrane review16 of surgical vs nonsurgical management of empyema identified 8 randomized trials, 6 in children and 2 in adults, with a total of 391 patients. The authors compared video-assisted thoracoscopy vs tube thoracotomy, with and without intrapleural fibrinolytics. They noted no difference in rates of mortality or procedural complications. However, the mean length of hospital stay was shorter with video-assisted thoracoscopy than with tube thoracotomy (5.9 vs 15.4 days). They could not assess the impact of fibrinolytic therapy on total cost of treatment in the 2 groups.
A randomized trial is planned to compare early video-assisted thoracoscopy vs treatment with chest tube drainage and t-PA-DNase.17
At our institution, we use a multidisciplinary approach, discussing cases at weekly meetings with thoracic surgeons, pulmonologists, infectious disease specialists, and interventional radiologists. We generally try conservative management first, with chest tube drainage and intrapleural agents for 5 to 7 days, before considering surgery if the response is unsatisfactory.
THE PATIENT RECOVERED
In our patient, the multiloculated empyema was successfully cleared after intrapleural instillation of 4 doses of tPA and DNAse over 3 days and insertion of a second intercostal chest tube into the noncommunicating apical locule. He completed 14 days of intravenous piperacillin-tazobactam treatment and, after discharge home, completed another 4 weeks of oral amoxicillin-clavulanate. He made a full recovery and was back at work 2 weeks after discharge. Chest radiography 10 weeks after discharge showed normal results.
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000; 118(4):1158–1171. pmid:11035692
- Bryant RE, Salmon CJ. Pleural empyema. Clin Infect Dis 1996; 22(5):747–762. pmid:8722927
- Braman SS, Donat WE. Explosive pleuritis. Manifestation of group A beta-hemolytic streptococcal infection. Am J Med 1986; 81(4):723–726. pmid:3532794
- Sharma JK, Marrie TJ. Explosive pleuritis. Can J Infect Dis 2001; 12(2):104–107. pmid:18159325
- Johnson JL. Pleurisy, fever, and rapidly progressive pleural effusion in a healthy, 29-year-old physician. Chest 2001; 119(4):1266–1269. pmid:11296198
- Jimenez D, Diaz G, Gil D, et al. Etiology and prognostic significance of massive pleural effusions. Respir Med 2005; 99(9):1183–1187. doi:10.1016/j.rmed.2005.02.022
- Light RW, MacGregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77:507–513. pmid:4642731
- Rahman NM, Maskell NA, Davies CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest 2010; 137(3):536–543. doi:10.1378/chest.09-1044
- Keeling AN, Leong S, Logan PM, Lee MJ. Empyema and effusion: outcome of image-guided small-bore catheter drainage. Cardiovasc Intervent Radiol 2008; 31(1):135–141. doi:10.1007/s00270-007-9197-0
- Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005; 352(9):865–874. doi:10.1056/NEJMoa042473
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365(6):518–526. doi:10.1056/NEJMoa1012740
- Piccolo F, Pitman N, Bhatnagar R, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection. An effective and safe alternative to surgery. Ann Am Thorac Soc 2014; 11(9):1419–1425. doi:10.1513/AnnalsATS.201407-329OC
- Khemasuwan D, Sorensen J, Griffin DC. Predictive variables for failure in administration of intrapleural tissue plasminogen activator/deoxyribonuclease in patients with complicated parapneumonic effusions/empyema. Chest 2018; 154(3):550–556. doi:10.1016/j.chest.2018.01.037
- Abu-Daff S, Maziak DE, Alshehab D, et al. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 2013; 3(2):e001887. doi:10.1136/bmjopen-2012-001887
- Bishwakarma R, Shah S, Frank L, Zhang W, Sharma G, Nishi SP. Mixing it up: coadministration of tPA/DNase in complicated parapneumonic pleural effusions and empyema. J Bronchology Interv Pulmonol 2017; 24(1):40–47. doi:10.1097/LBR.0000000000000334
- Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev 2017; 3:CD010651. doi:10.1002/14651858.CD010651.pub2
- Feller-Kopman D, Light R. Pleural disease. N Engl J Med 2018; 378(8):740–751. doi:10.1056/NEJMra1403503
Progress in diagnosing and managing cardiac amyloidosis
From the Cleveland Clinic Journal of Medicine
This article has been removed from the website. The article was prepared by the editorial staff based on a transcript of the proceedings of a conference, and errors occurred during this process that were subsequently published. A clarification of the errors will be published in a future issue.
A review of this topic was published in the December 2017 issue of the Journal (Donnelly JP, Hanna M. Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med 2017;84[12suppl 3]:12–26). doi:10.3949/ccjm.84.s3.02
From the Cleveland Clinic Journal of Medicine
This article has been removed from the website. The article was prepared by the editorial staff based on a transcript of the proceedings of a conference, and errors occurred during this process that were subsequently published. A clarification of the errors will be published in a future issue.
A review of this topic was published in the December 2017 issue of the Journal (Donnelly JP, Hanna M. Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med 2017;84[12suppl 3]:12–26). doi:10.3949/ccjm.84.s3.02
From the Cleveland Clinic Journal of Medicine
This article has been removed from the website. The article was prepared by the editorial staff based on a transcript of the proceedings of a conference, and errors occurred during this process that were subsequently published. A clarification of the errors will be published in a future issue.
A review of this topic was published in the December 2017 issue of the Journal (Donnelly JP, Hanna M. Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med 2017;84[12suppl 3]:12–26). doi:10.3949/ccjm.84.s3.02