User login
Pityriasis Amiantacea Following Bone Marrow Transplant
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
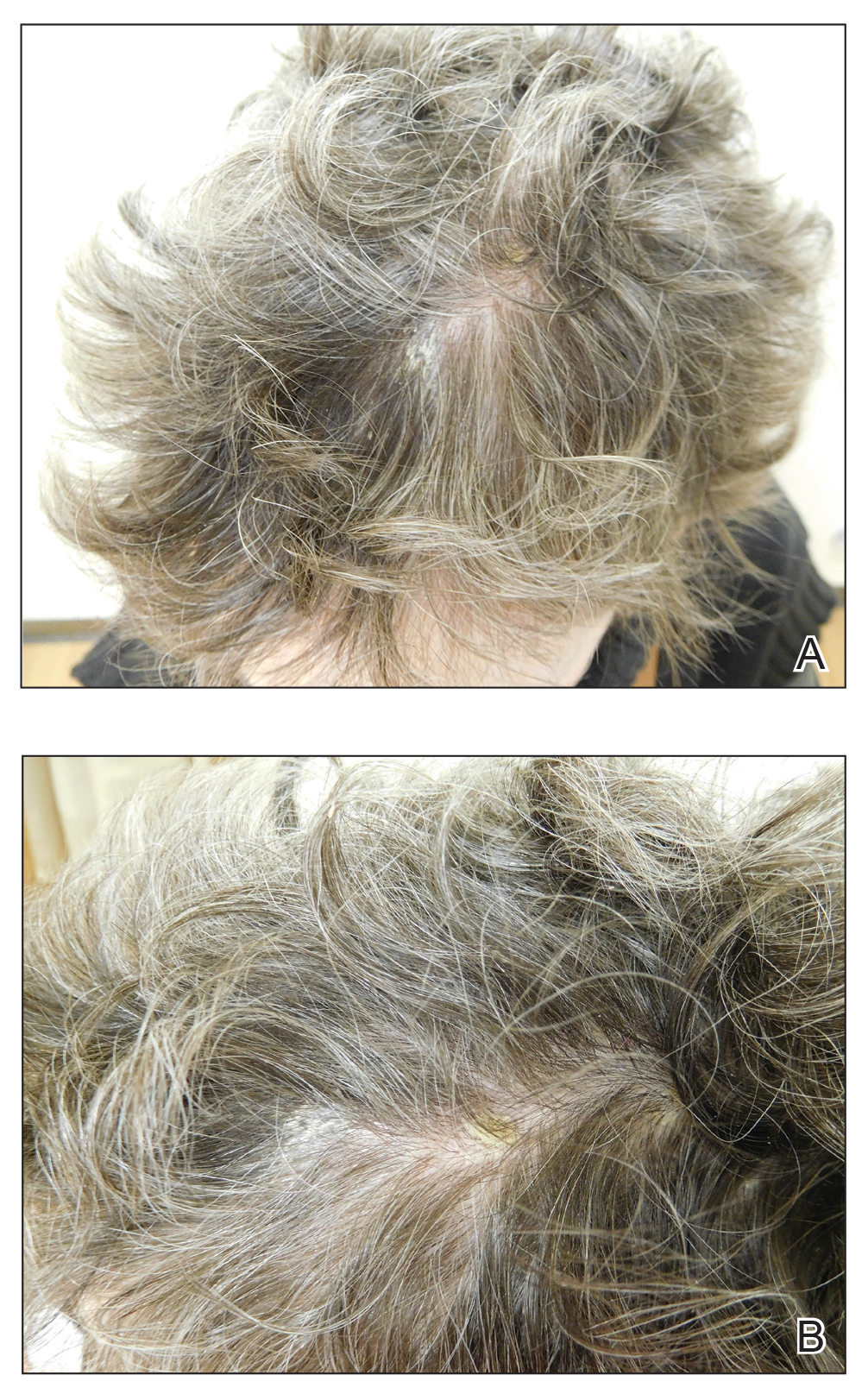
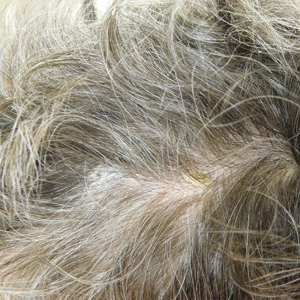
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
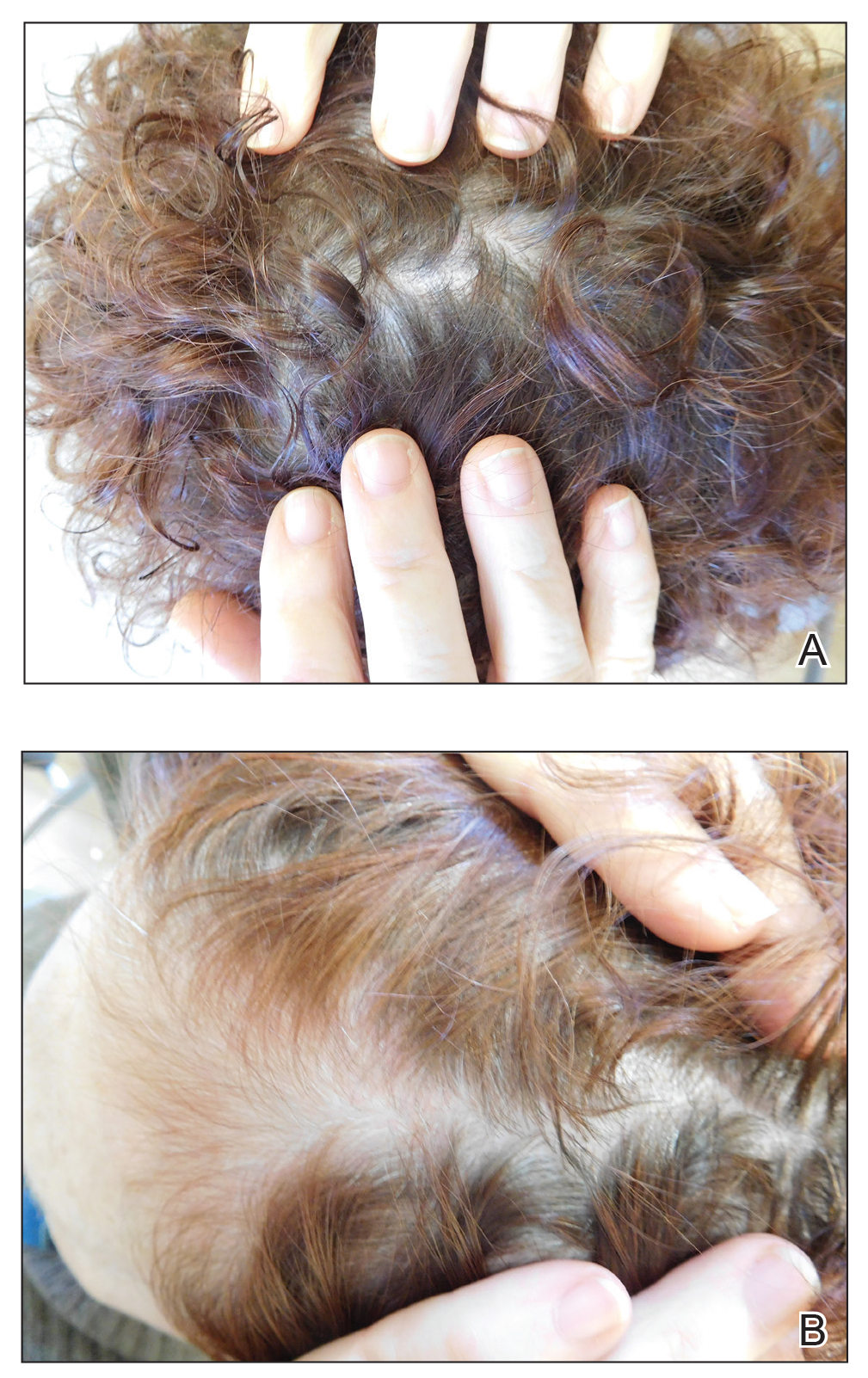
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
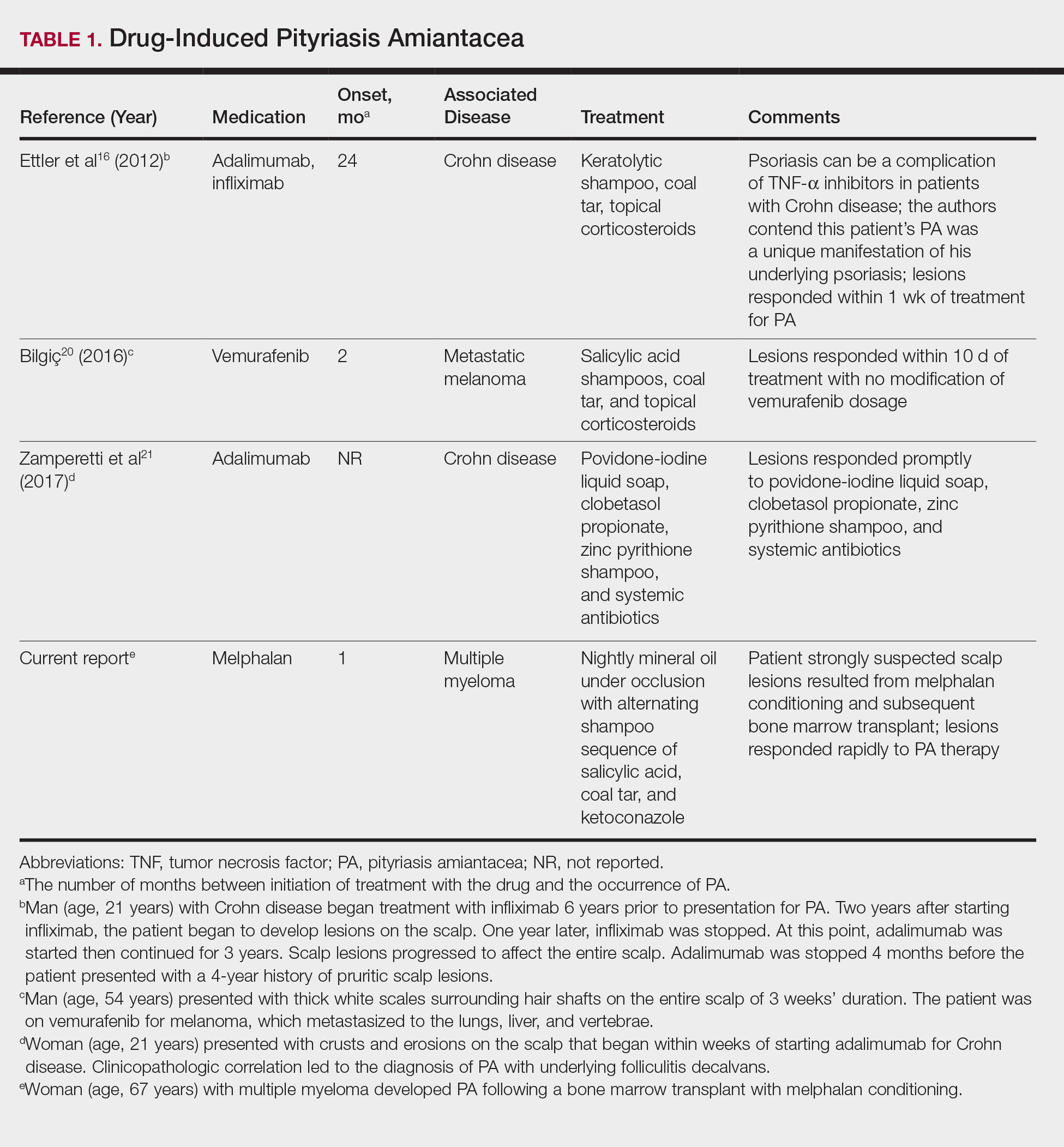
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
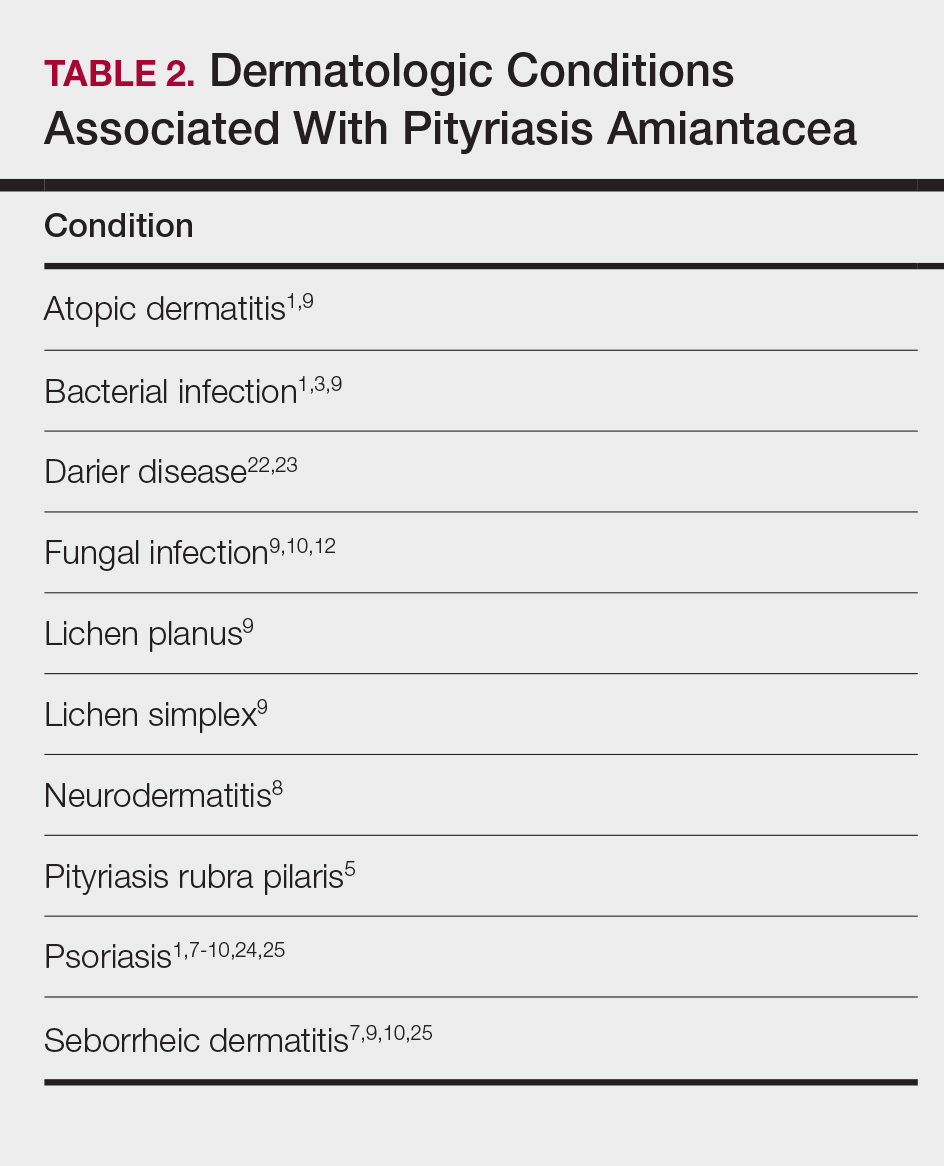
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Practice Points
- Pityriasis amiantacea (PA) is associated with several dermatologic conditions, including atopic dermatitis, bacterial and fungal infections, psoriasis, and seborrheic dermatitis.
- Drug-induced PA is rare, but the condition has been reported in the context of treatment with tumor necrosis factor Symbol Stdα inhibitors and vemurafenib.
- Our report suggests that PA may be associated with either melphalan conditioning, bone marrow transplant, or both.
Topical antibiotic decolonizes S. aureus in NICU infants
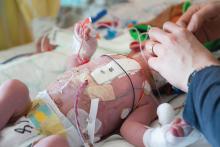
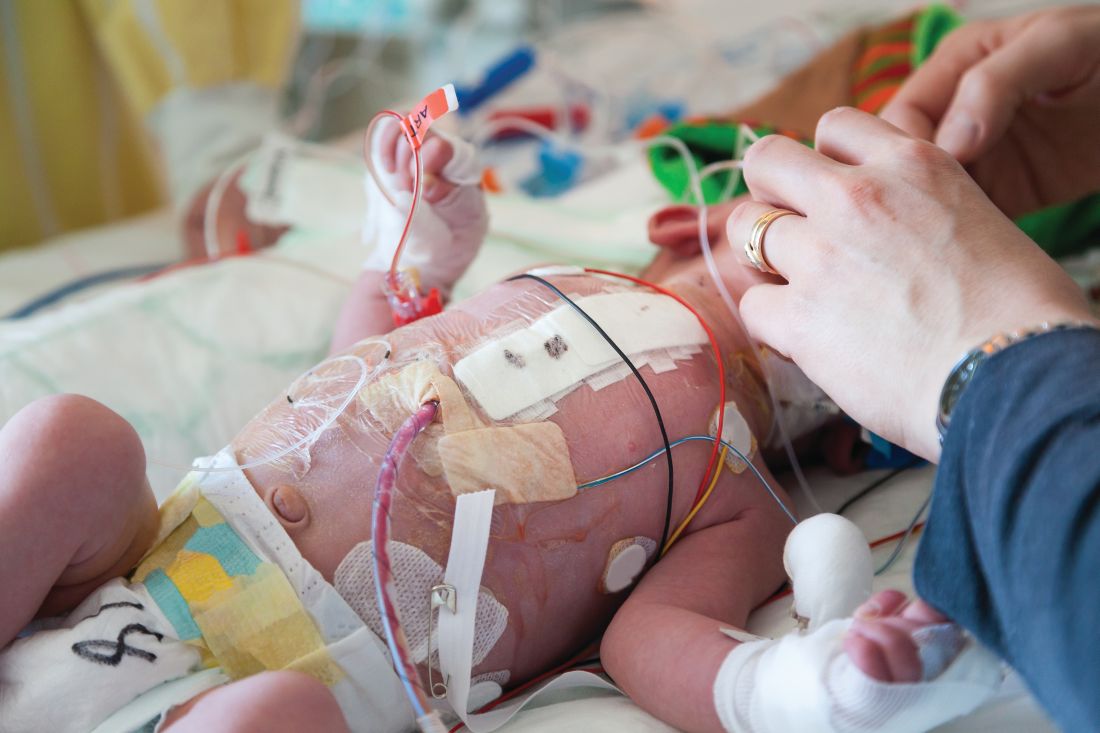
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
Application of the topical antibiotic mupirocin to multiple body sites was reported to be safe and efficacious in eradicating Staphylococcus aureus (SA) colonization on infants in the neonatal intensive care unit (NICU), according to researchers at the University of Maryland, Baltimore.
Karen L. Kotloff, MD, and her colleagues conducted a phase 2 multicenter, open-label, randomized trial to assess the safety and efficacy of intranasal plus topical mupirocin in eradicating SA colonization in critically ill infants between April 2014 and May 2016.
“Staph aureus is a leading cause of sepsis in young children admitted to the NICU. Sepsis, which is systemic infection, can be fatal in infants. Thus, preventing these infections is very important in managing risk for babies in the NICU who are fragile and struggling with multiple medical problems,” said Dr. Kotloff in a university interview.
The researchers examined infants in the NICU at eight study centers who were less than 24 months old who underwent serial screening for nasal SA. Infants colonized with SA and were randomly assigned to receive 5 days of mupirocin versus no mupirocin to the intranasal, periumbilical, and perianal areas. Treatment effects were assessed on day 8 (primary decolonization) and day 22 (persistent decolonization) for all three body areas (Pediatrics. 2019 Jan 1. doi: 10.1542/peds.2018-1565).
Primary decolonization occurred in 62/66 (93.9%) of treated infants and 3/64 (4.7%) of the control infants (P less than .001). Persistent decolonization was seen in 21/46 (45.7%) of treated infants compared with 1/48 (2.1%) of the controls (P less than .001).
“This multicenter trial supervised by Dr. Kotloff provides strong support for a safe strategy to minimize Staphylococcus aureus infections in some of the most at-risk patients in any hospital, premature babies,” E. Albert Reece, MD, dean of the University of Maryland School of Medicine, said in a university press release commenting on the study.
FROM PEDIATRICS
Too high to drive: States grapple with setting limits on weed use behind wheel
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It used to be the stuff of stoner comedies and “Just Say No” campaigns. Today, marijuana is becoming mainstream as voters across the country approve ballot questions for legalization or medical use.
In response, state governments are testing ways to ensure that the integration of this once-illicit substance into everyday life doesn’t create new public health risks. These efforts are sparking a difficult question: At what point is someone too high to get behind the wheel?
The answer is complicated. Brain scientists and pharmacologists don’t know how to measure if and to what extent marijuana causes impairment.
The reason: Existing blood and urine tests can detect marijuana use, but, because traces of the drug stay in the human body for a long time, those tests can’t specify whether the use occurred earlier that day or that month. They also don’t indicate the level at which a driver would be considered “under the influence.”
“It’s a really hard problem,” said Keith Humphreys, a psychiatry professor and drug policy expert at Stanford University (Calif.), the first state to legalize medical marijuana and where recreational pot use among adults became legal in 2016. “We don’t really have good evidence – even if we know someone has been using – [to gauge] what their level of impairment is.”
Marijuana is now legal for recreational use in 10 states and the District of Columbia – including Michigan, where a ballot initiative passed in November 2018 took effect Dec. 6. In New York, the governor said Dec. 17 that legalization would be a top priority for 2019. And nearly three dozen states have cleared the use of medical cannabis.
For alcohol, there is a clear, national standard. If your blood alcohol content is 0.08% or higher, you’re considered cognitively impaired at a level that is unsafe to drive. Extensive research supports this determination, and the clarity makes enforcement of drunken-driving laws easier.
Setting a marijuana-related impairment level is a much murkier proposition. But states that have legalized pot have to figure it out, experts said.
“You can’t legalize a substance and not have a coherent policy for controlling driving under the influence of that substance,” said Steven Davenport, an assistant policy researcher at the nonprofit Rand Corporation, who specializes in marijuana research.
Marijuana, after all, weakens a driver’s ability to maintain focus, and it slows reflexes. But regulators are “playing catch-up,” suggested Thomas Marcotte, a psychiatry professor at the University of California, San Diego, and one of a number of academics around the country who is researching driving while high.
States have put forth a bevy of approaches. At least five have what’s called a “per se” law, which outlaws driving if someone’s blood level of tetrahydrocannabinol, or THC, exceeds a set amount. THC is marijuana’s main intoxicant.
Colorado, where voters approved legalization of recreational marijuana in 2012, has this type of driving law on the books. It took 3 years to pass amid fiery debate and deems “intoxicated” any driver who tests higher than 5 ng of THC per milliliter of blood.
Indiana, Pennsylvania and Rhode Island are among states that forbid driving at any THC level. Still others say drivers should be penalized only if they are impaired by the chemical – a standard that sounds reasonable but quickly gets difficult to measure or even define.
None of these approaches offers an ideal solution, experts said.
“We’re still definitely evaluating which policies are the most effective,” said Ann Kitch, who tracks the marijuana and driving issue for the National Conference of State Legislatures.
States that set a THC-level standard confront weak technology and limited science. THC testing is imprecise at best, since the chemical can stay in someone’s bloodstream for weeks after it was ingested. Someone could legally smoke a joint and still have THC appear in blood or urine samples long after the high passes.
There’s general agreement that driving while high is bad, but there’s no linear relationship between THC levels and degree of impairment. States that have picked a number to reflect when THC in the bloodstream becomes a hazard have “made it up,” argued Dr. Humphreys.
“The ones who wrote [a number] into legislation felt they had to say something,” he said. But “we don’t know what would be the analogy. Is the legal amount [of THC] equal to a beer? Is that how impaired you are? Is it a six-pack?”
Roadside testing for THC is also logistically difficult. Blood, for instance, needs to be analyzed in a lab, and collecting urine gets ... complicated.
In Canada, which legalized recreational pot just this year, law enforcement will test drivers with a saliva test called the Dräger DrugTest 5000, but that isn’t perfect, either.
Some private companies are trying to develop a sort of breathalyzer for marijuana. But Jonathan Caulkins, a drug policy researcher at Carnegie Mellon University, Pittsburgh, said, “There are fundamental issues with the chemistry and pharmacokinetics. It’s really hard to have an objective, easy-to-administer roadside test.”
Some states rely on law enforcement to assess whether someone’s driving appears impaired and ascertain after the fact if marijuana was involved.
In California, every highway patrol member learns to administer “field sobriety tests” – undergoing an extra 16 hours of training to recognize the influence of different drugs, including marijuana. Because medical marijuana has been legal there since 1996, officers are “very used” to recognizing its influence, said Glenn Glazer, the state’s coordinator for its drug recognition expert training program.
That kind of training is taking off in other states, too, Ms. Kitch said. Lobbying groups such as Mothers Against Drunk Driving are pushing to bump up law enforcement training and rely on officers to assess whether a driver is impaired.
These tests, though, risk their own kind of error.
“They are subjective,” Mr. Davenport warned.
For one thing, officer-administered tests can be influenced by racial bias. Someone who has previously had poor experiences with law enforcement may also perform worse, not because of greater impairment but because of nervousness.
Indeed, relying on more subjective testing is in some ways the direct opposite of conventional wisdom.
“A general pattern of the last ... 40 years is to try to take human judgment out of decision making processes when possible. Because we fear exactly these issues,” Mr. Caulkins said. “The idea that you could come up with a completely objective test of performance ... is ambitious.”
Researchers like Dr. Marcotte are trying to devise some kind of test that can, in fact, gauge whether someone is showing signs of marijuana impairment. But that could take years.
In the meantime, the public health threat is real. States with legalized pot do appear to experience more car crashes, though the relationship is muddled. “This is going to be a headache of an issue for a decade,” Mr. Caulkins said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
FDA expands dasatinib indication to children with Ph+ ALL
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
The .
The tyrosine kinase inhibitor is now approved for use in combination with chemotherapy to treat pediatric patients aged 1 year and older who have newly diagnosed, Philadelphia-chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib is already approved for use in children aged 1 year and older who have chronic phase, Ph+ chronic myeloid leukemia (CML).
In adults, dasatinib is approved to treat newly diagnosed, Ph+, chronic phase CML; chronic, accelerated, or myeloid/lymphoid blast phase, Ph+ CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL with resistance or intolerance to prior therapy. The approval in children with Ph+ ALL is based on data from a phase 2 study (CA180-372, NCT01460160).
In this trial, researchers evaluated dasatinib in combination with the AIEOP-BFM ALL 2000 multi-agent chemotherapy protocol in patients (aged 1-17 years) with newly diagnosed, B-cell precursor, Ph+ ALL.
There were 78 patients evaluated for efficacy in cohort 1. They received dasatinib at a daily dose of 60 mg/m2 for up to 24 months.
Patients with central nervous system 3 disease received cranial irradiation, and patients were assigned to stem cell transplant based on minimal residual disease if they were thought to have a high risk of relapse.
The 3-year event-free survival rate in the 78 patients was 64.1%.
There were 81 patients evaluable for safety who received dasatinib continuously in combination with chemotherapy. Their median duration of treatment was 24 months.
The most common adverse events (AEs) in these patients were mucositis, febrile neutropenia, pyrexia, diarrhea, nausea, vomiting, musculoskeletal pain, abdominal pain, cough, headache, rash, fatigue, and constipation.
Eight patients (10%) had AEs leading to treatment discontinuation. These included fungal sepsis, hepatotoxicity in the setting of graft-versus-host disease, thrombocytopenia, cytomegalovirus infection, pneumonia, nausea, enteritis, and drug hypersensitivity.
Three patients (4%) had fatal AEs, all infections.
This trial was sponsored by Bristol-Myers Squibb. Additional data are available in the prescribing information for dasatinib.
All the Little Lesions in a Row
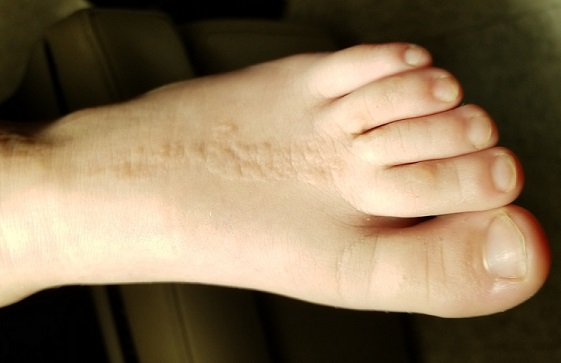
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.
EXAMINATION
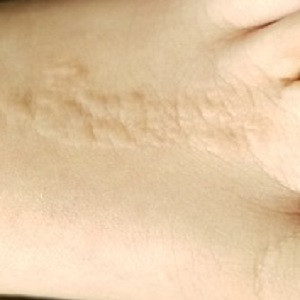
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
A 10-year-old boy has had a lesion on his left foot for almost a year. It has not responded to either topical antifungal cream (econazole, applied twice daily for weeks) or, subsequently, topical corticosteroid cream (mometazone, also applied twice daily). Frustrated by the lack of resolution, his mother brings him for evaluation.
The condition began with faint linear scaling, the area of which became gradually wider and longer. The child reports no associated symptoms, and the mother denies seeing her son manipulate, rub, or scratch the affected skin.
Aside from mild atopy—in the form of seasonal allergies and asthma—the boy is healthy.

EXAMINATION
The child is well developed, well nourished, and in no distress. He gladly permits examination of the lesion, which is located on the dorsum of the left foot, running from the lower leg to just proximal to the toes. The linear strip of skin measures 2 cm at its widest point. The lesion is tan and uniformly scaly; it exhibits no overt signs of inflammation or increased warmth or tenderness on palpation.
Examination reveals no other such lesions, or indeed any abnormalities. The adjacent toenails do not appear to be involved.
What is the diagnosis?
DISCUSSION
This child has a common condition called lichen striatus in modern times, but also known as linear lichenoid dermatitis, or (in older texts) Blaschko linear acquired inflammatory skin eruption. It has nothing to do with fungal infection.
This case illustrates a fairly typical presentation, but—as with most dermatologic conditions—there are many variants. For example, lichen striatus can present as a linear collection of scaly skin running the entire length of the leg (often beginning on the buttocks) and can even affect the toenails at its distal terminus. Although the line is usually solitary, lichen striatus can affect multiple locations simultaneously. Likewise, the lesions can run in an uninterrupted line, or stop and start at various points.
Skin type can affect the appearance of the lesions: on children with darker skin, lichen striatus usually appears lighter and on fair-skinned children, darker. The condition is more common in girls than boys (3:1) and occurs most often in those ages 5 to 15. The arms are another typical location, but it can even affect the face in rare instances. There is some support for atopy as a predisposing factor—but since almost 20% of all children are atopic, this is debatable.
Lichen striatus received its historical name because it follows Blaschko’s lines—named for Alfred Blaschko, a German dermatologist who first described the condition in 1901. These bizarre curving lines are now known to follow recognized patterns of embryonic cell migration that have nothing to do with neural, lymphatic, or vascular patterns as one might otherwise imagine. Several other skin conditions involve so-called blaschkoid features, including inflammatory linear verruciform nevi and some forms of epidermal nevi.
LS is not dangerous in any way, though it does cause considerable consternation among parents of affected children. Luckily, it causes few if any symptoms and is self-limited. A few children will complain of mild itching, for which class 4 or 5 topical steroids can be used. Within a year or two at most, the condition will resolve—albeit with occasional postinflammatory hyperpigmentation in those with darker skin.
TAKE-HOME LEARNING POINTS
- Lichen striatus is a common condition affecting children ages 5 to 15 who develop a linear, papulosquamous eruption that favors arms and leg (but can rarely involve the face).
- Not infrequently, the condition can cause dystrophy of the nails at the terminus of the lesions.
- The lesions follow Blaschko’s lines, which are thought to represent patterns of embryonic cell migration.
- The condition is seldom symptomatic, is self-limited, and rarely leaves permanent signs of damage.
Single-dose propranolol tied to ‘selective erasure’ of anxiety disorders
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
BARCELONA – A single 40-mg dose of oral propranolol, judiciously timed, constitutes an outside-the-box yet highly promising treatment for anxiety disorders, and perhaps for posttraumatic stress disorder as well, Marieke Soeter, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
The concept here is that the beta-blocker, when given with a brief therapist-led reactivation of a fear memory, blocks beta-adrenergic receptors in the brain so as to interfere with the specific proteins required for reconsolidation of that memory, thereby disrupting the reconsolidation process and neutralizing subsequent expression of that memory in its toxic form. In effect, timely administration of one dose of propranolol, a drug that readily crosses the blood/brain barrier, achieves pharmacologically induced amnesia regarding the learned fear, explained Dr. Soeter, a clinical psychologist at TNO, the Netherlands Organization for Scientific Research, an independent nonprofit translational research organization.
“It looks like permanent fear erasure. You can never say that something is erased, but we have not been able to get it back,” she said. “Propranolol achieves selective erasure: It targets the emotional component, but knowledge is intact. They know what happened, but they aren’t scared anymore. The fear association is affected, but not the innate fear response to a threat stimulus, so it doesn’t alter reactions to potentially dangerous situations, which is important. If there is a bomb, they still know to run away from it.”
This single-session therapy addressing what psychologists call fear memory reconsolidation is totally outside the box relative to contemporary psychotherapy for anxiety disorders, which typically entails gradual fear extinction learning requiring multiple treatment sessions. But contemporary psychotherapy for anxiety disorders leaves much room for improvement, given that up to 60% of patients experience relapse. That’s probably because the original fear memory remains intact and resurfaces at some point despite initial treatment success, according to Dr. Soeter.
Nearly 2 decades ago, other investigators showed in animal studies that fear memories are not necessarily permanent. Rather, they are modifiable, and even erasable, during the vulnerable period that occurs when the memories are reactivated and become labile.
Later, Dr. Soeter – then at the University of Amsterdam – and her colleagues demonstrated the same phenomenon using Pavlovian fear-conditioning techniques involving pictures and electric shocks in healthy human volunteers. They showed that a dose of propranolol given before memory reactivation blocked the fear response, while nadolol, a beta-blocker that does not cross the blood/brain barrier, did not.
However, since the fear memories they could ethically induce in the psychology laboratory are far less intense than those experienced by patients with anxiety disorders, the researchers next conducted a randomized, double-blind clinical trial in 45 individuals with arachnophobia. Fifteen received 40 mg of propranolol after spending 2 minutes in proximity to a large tarantula, 15 got placebo, and another 15 received propranolol without exposure to a tarantula. One week later, all patients who received propranolol with spider exposure were able to approach and actually pet the tarantula. Pharmacologic disruption of reconsolidation and storage of their fear memory had turned avoidance behavior into approach behavior. This benefit was maintained for at least a year after the brief treatment session (Biol Psychiatry. 2015 Dec 15;78[12]:880-6).
“Interestingly, there was no direct effect of propranolol on spider beliefs. Therefore, do we need treatment that targets the cognitive level? These findings challenge one of the fundamental tenets of cognitive-behavioral therapy that emphasizes changes in cognition as central to behavioral modification,” Dr. Soeter said.
Most recently, she and a coinvestigator have been working to pin down the precise conditions under which memory reconsolidation can be targeted to extinguish fear memories. They have shown in a 30-subject study that the process is both time- and sleep-dependent. The propranolol must be given within roughly an hour before to 1 hour after therapeutic reactivation of the fear memory to be effective. And sleep is an absolute necessity: When subjects were rechallenged 12 hours after memory reactivation and administration of propranolol earlier on the same day, with no opportunity for sleep, there was no therapeutic effect: The disturbing fear memory was elicited. However, when subjects were rechallenged 12 hours after taking propranolol the previous day – that is, after a night’s sleep – the fear memory was gone (Nat Commun. 2018 Apr 3;9[1]:1316. doi: 10.1038/s41467-018-03659-1).
“Postretrieval amnesia requires sleep to happen. ,” Dr. Soeter said. It’s still unclear, however, how much sleep is required. Perhaps a nap will turn out to be sufficient, she said.
Colleagues at the University of Amsterdam are now using single-dose propranolol-based therapy in patients with a wide range of phobias.
“The effects are pretty amazing,” Dr. Soeter said. “Everything is treatable. It’s almost too good to be true, but these are our findings.”
Based upon her favorable anecdotal experience in treating a Dutch military veteran with severe combat-related PTSD of 10 years’ duration which had proved resistant to multiple conventional and unconventional interventions, a pilot study of single-dose propranolol with traumatic memory reactivation is now being planned in patients with war-related PTSD.
“After one pill and a 20-minute session, this veteran with severe chronic PTSD has no more nightmares, insomnia, or alcohol problems, and he now travels the world,” she said.
Her research met with an enthusiastic reception from other speakers at the ECNP session on PTSD. Eric Vermetten, MD, PhD, welcomed the concept that pharmacologic therapy upon reexposure to fearful cues can impede the molecular and cellular cascade required to reestablish fearful memories. This also is the basis for the extremely encouraging, albeit preliminary, clinical data on ketamine, an N-methyl-D-aspartate receptor antagonist, as well as 3,4-Methylenedioxymethamphetamine (MDMA) for therapeutic manipulation of trauma memories.
“Targeting reconsolidation of existing fear memories is worthy of looking into further,” declared Dr. Vermetten, professor of psychiatry at Leiden (the Netherlands) University and a military mental health researcher for the Dutch Ministry of Defense.
New thinking regarding pharmacotherapy for PTSD is sorely needed, he added. He endorsed a consensus statement by the PTSD Psychopharmacology Working Group that decried what was termed a crisis in pharmacotherapy of PTSD (Biol Psychiatry. 2017 Oct 1;82[7]:e51-e59. doi: 10.1016/j.biopsych.2017.03.007. Epub 2017 Mar 14).
“We only have two [Food and Drug Administration]-approved medications for PTSD – sertraline and paroxetine – and they were approved back in 2001,” Dr. Vermetten noted. “Research has stalled, and there is a void in new drug development.”
Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: A single 40-mg dose of oral propranolol, judiciously timed, is a highly promising novel treatment for anxiety disorders.
Major finding: The beta-blocker must be given within an hour before to an hour after therapist-facilitated reactivation of the fear memory.
Study details: This study included 30 healthy volunteers who underwent a cued Pavlovian fear-conditioning program.
Disclosures: Dr. Soeter’s study of the time- and sleep-dependent nature of propranolol-induced amnesia was supported by the Netherlands Organization for Scientific Research, where she is employed.
Endometriosis surgery: Women can expect years-long benefits
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: Endometriosis excision improves quality of life for at least 7 years, even when women have conservative, fertility-sparing surgery.
Major finding: The overall score on the Endometriosis Health Profile-30 fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at the 7-year follow-up.
Study details: A review of 61 cases
Disclosures: The investigators didn’t report any disclosures.
Source: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
Behavioral Therapy for Migraine and Tension-Type Headache
Steven M. Baskin, PhD, a clinical psychologist at the New England Institute for Neurology and Headache in Stamford, Connecticut, recently answered the Migraine Resource Center’s questions about the benefits of behavioral therapy in the treatment of migraine and tension-type headache.
Alan M. Rapoport, MD: Could you please give a brief description of the 5 best modalities of behavioral therapy for migraine and tension-type headache?
Steven M. Baskin, PhD: The most researched modalities that have a good evidence base for both migraine and tension-type headache (TTHA) are relaxation therapies that often combine abdominal breathing with some form of progressive relaxation, electromyography (EMG) biofeedback therapy where headache patients learn to decrease scalp and neck muscle tension utilizing muscular biofeedback, thermal biofeedback where migraine sufferers learn a way to warm their hands which often creates a low arousal state that may reduce brain hyperexcitability, and cognitive behavioral therapy (CBT) techniques to learn stress management. The combination of behavioral medicine techniques plus preventive pharmacological treatment has been showed to be more efficacious than either treatment alone. (Holroyd KA, et al. Effect of preventive (β blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ. 2010;1-12)
CBT to treat insomnia has also been shown to reverse many chronic migraine sufferers back to episodic migraine. (Smitherman TA, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache 2018;58:1052-1059)
Dr. Rapoport: How do you identify a patient who may benefit from behavioral therapy over acute medication, and what is the first step that you suggest?
Dr. Baskin: Behavioral therapies for migraine management are typically preventive therapies that can and should be combined with medications to control acute attacks. There are behavioral principles that can maximize adherence to abortive agents in order to optimize acute care.
Dr. Rapoport: Which tends to work the best for migraine?
Dr. Baskin: What works best is to first do a good behavioral assessment of the frequency, duration, intensity, and disability level of their headaches as well as current stress levels, history, and adherence to drug and nondrug therapies, and psychiatric comorbidities. A program should then be developed that includes some combination of pharmacological and behavioral interventions to address these issues. It is important to increase self-efficacy: patients’ belief in the ability to control the headache, belief in the ability to manage emotional reactivity to pain, and belief that they can achieve functionality in the presence of a significant headache disorder.
Dr. Rapoport: Who should not have biofeedback therapy?
Dr. Baskin: Biofeedback has shown to be effective in treating migraine and TTHA. It has not been shown to be effective in treating trigeminal autonomic cephalgias (TACs) such as cluster headache. Like pharmacological therapies, it is less effective in chronic migraine that is daily and constant. A patient with severe psychiatric disorder should be treated for their psychiatric disorder before beginning biofeedback therapy.
Dr. Rapoport: Some doctors see patients twice per week for several months. What is your typical routine for behavioral therapy?
Dr. Baskin: We have a variety of programs. For complicated patients, we tend to see them weekly and have a very systematic program of biofeedback and CBT for approximately 12 to 15 sessions. This may include treating psychiatric comorbidities. We see many other patients for 1 or 2 sessions of biofeedback to try to effect physiological learning and for 1 or 2 sessions of CBT to help them manage stressors and learn coping skills that they can use to help manage migraines and life stress.
Dr. Rapoport: Does behavioral medicine work best in conjunction with preventive medications, or on its own?
Dr. Baskin: Many patients do well with a behavioral treatment as a preventive therapy and a pharmacologic agent to optimize acute care. I believe that many patients with higher frequency migraine with psychological issues or ongoing stressors do best with a combination of preventive pharmacologic therapy and behavior therapy. Any migraine patient with sleep issues should learn CBT for insomnia.
Dr. Rapoport: Is there evidence that suggests behavioral therapy can help patients at various ages manage their migraines?
Dr. Baskin: There is both adult and child data on behavioral therapy for migraine. An excellent study was done in children and adolescents by Powers et al. It showed that adding 10 sessions of CBT to preventive amitriptyline therapy, compared to adding headache education, significantly reduced the number of headache days, level of disability, and kids with a better than 50% decrease in days of headache compared to amitriptyline, plus headache education control in chronic migraine patients. (Powers SW et al. Cognitive Behavioral Therapy Plus Amitriptyline for Chronic Migraine in Children and Adolescents: A Randomized Clinical Trial. JAMA 2013;310(24):2622-2630)
Dr. Rapoport: A recent MedPage Today article noted that “anxiety may complicate migraine more than depression with greater long-term persistence, greater headache-related disability, and reduced satisfaction with acute therapies.” Could you please elaborate on why this may be the case?
Dr. Baskin: Anxiety disorders are often based on feeling threat. They are always associated with avoidance behaviors. Headache sufferers with significant anxiety tend to overestimate the probability of danger (migraine) and perceive it as more unmanageable and threatening than objective reality. They are often very sensitive to medication side effects and benign somatic sensations. They sometimes take medications pre-emptively, because of their fear of getting a migraine, which may lead to medication misuse or overuse. The lifetime prevalence of anxiety disorders in migraineurs (ranging from 51-58%) is almost twice that of major depression.
Please write to us at Neurology Reviews Migraine Resource Center (mrc@mdedge.com) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
Steven M. Baskin, PhD, a clinical psychologist at the New England Institute for Neurology and Headache in Stamford, Connecticut, recently answered the Migraine Resource Center’s questions about the benefits of behavioral therapy in the treatment of migraine and tension-type headache.
Alan M. Rapoport, MD: Could you please give a brief description of the 5 best modalities of behavioral therapy for migraine and tension-type headache?
Steven M. Baskin, PhD: The most researched modalities that have a good evidence base for both migraine and tension-type headache (TTHA) are relaxation therapies that often combine abdominal breathing with some form of progressive relaxation, electromyography (EMG) biofeedback therapy where headache patients learn to decrease scalp and neck muscle tension utilizing muscular biofeedback, thermal biofeedback where migraine sufferers learn a way to warm their hands which often creates a low arousal state that may reduce brain hyperexcitability, and cognitive behavioral therapy (CBT) techniques to learn stress management. The combination of behavioral medicine techniques plus preventive pharmacological treatment has been showed to be more efficacious than either treatment alone. (Holroyd KA, et al. Effect of preventive (β blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ. 2010;1-12)
CBT to treat insomnia has also been shown to reverse many chronic migraine sufferers back to episodic migraine. (Smitherman TA, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache 2018;58:1052-1059)
Dr. Rapoport: How do you identify a patient who may benefit from behavioral therapy over acute medication, and what is the first step that you suggest?
Dr. Baskin: Behavioral therapies for migraine management are typically preventive therapies that can and should be combined with medications to control acute attacks. There are behavioral principles that can maximize adherence to abortive agents in order to optimize acute care.
Dr. Rapoport: Which tends to work the best for migraine?
Dr. Baskin: What works best is to first do a good behavioral assessment of the frequency, duration, intensity, and disability level of their headaches as well as current stress levels, history, and adherence to drug and nondrug therapies, and psychiatric comorbidities. A program should then be developed that includes some combination of pharmacological and behavioral interventions to address these issues. It is important to increase self-efficacy: patients’ belief in the ability to control the headache, belief in the ability to manage emotional reactivity to pain, and belief that they can achieve functionality in the presence of a significant headache disorder.
Dr. Rapoport: Who should not have biofeedback therapy?
Dr. Baskin: Biofeedback has shown to be effective in treating migraine and TTHA. It has not been shown to be effective in treating trigeminal autonomic cephalgias (TACs) such as cluster headache. Like pharmacological therapies, it is less effective in chronic migraine that is daily and constant. A patient with severe psychiatric disorder should be treated for their psychiatric disorder before beginning biofeedback therapy.
Dr. Rapoport: Some doctors see patients twice per week for several months. What is your typical routine for behavioral therapy?
Dr. Baskin: We have a variety of programs. For complicated patients, we tend to see them weekly and have a very systematic program of biofeedback and CBT for approximately 12 to 15 sessions. This may include treating psychiatric comorbidities. We see many other patients for 1 or 2 sessions of biofeedback to try to effect physiological learning and for 1 or 2 sessions of CBT to help them manage stressors and learn coping skills that they can use to help manage migraines and life stress.
Dr. Rapoport: Does behavioral medicine work best in conjunction with preventive medications, or on its own?
Dr. Baskin: Many patients do well with a behavioral treatment as a preventive therapy and a pharmacologic agent to optimize acute care. I believe that many patients with higher frequency migraine with psychological issues or ongoing stressors do best with a combination of preventive pharmacologic therapy and behavior therapy. Any migraine patient with sleep issues should learn CBT for insomnia.
Dr. Rapoport: Is there evidence that suggests behavioral therapy can help patients at various ages manage their migraines?
Dr. Baskin: There is both adult and child data on behavioral therapy for migraine. An excellent study was done in children and adolescents by Powers et al. It showed that adding 10 sessions of CBT to preventive amitriptyline therapy, compared to adding headache education, significantly reduced the number of headache days, level of disability, and kids with a better than 50% decrease in days of headache compared to amitriptyline, plus headache education control in chronic migraine patients. (Powers SW et al. Cognitive Behavioral Therapy Plus Amitriptyline for Chronic Migraine in Children and Adolescents: A Randomized Clinical Trial. JAMA 2013;310(24):2622-2630)
Dr. Rapoport: A recent MedPage Today article noted that “anxiety may complicate migraine more than depression with greater long-term persistence, greater headache-related disability, and reduced satisfaction with acute therapies.” Could you please elaborate on why this may be the case?
Dr. Baskin: Anxiety disorders are often based on feeling threat. They are always associated with avoidance behaviors. Headache sufferers with significant anxiety tend to overestimate the probability of danger (migraine) and perceive it as more unmanageable and threatening than objective reality. They are often very sensitive to medication side effects and benign somatic sensations. They sometimes take medications pre-emptively, because of their fear of getting a migraine, which may lead to medication misuse or overuse. The lifetime prevalence of anxiety disorders in migraineurs (ranging from 51-58%) is almost twice that of major depression.
Please write to us at Neurology Reviews Migraine Resource Center (mrc@mdedge.com) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
Steven M. Baskin, PhD, a clinical psychologist at the New England Institute for Neurology and Headache in Stamford, Connecticut, recently answered the Migraine Resource Center’s questions about the benefits of behavioral therapy in the treatment of migraine and tension-type headache.
Alan M. Rapoport, MD: Could you please give a brief description of the 5 best modalities of behavioral therapy for migraine and tension-type headache?
Steven M. Baskin, PhD: The most researched modalities that have a good evidence base for both migraine and tension-type headache (TTHA) are relaxation therapies that often combine abdominal breathing with some form of progressive relaxation, electromyography (EMG) biofeedback therapy where headache patients learn to decrease scalp and neck muscle tension utilizing muscular biofeedback, thermal biofeedback where migraine sufferers learn a way to warm their hands which often creates a low arousal state that may reduce brain hyperexcitability, and cognitive behavioral therapy (CBT) techniques to learn stress management. The combination of behavioral medicine techniques plus preventive pharmacological treatment has been showed to be more efficacious than either treatment alone. (Holroyd KA, et al. Effect of preventive (β blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ. 2010;1-12)
CBT to treat insomnia has also been shown to reverse many chronic migraine sufferers back to episodic migraine. (Smitherman TA, et al. Cognitive-Behavioral Therapy for Insomnia to Reduce Chronic Migraine: A Sequential Bayesian Analysis. Headache 2018;58:1052-1059)
Dr. Rapoport: How do you identify a patient who may benefit from behavioral therapy over acute medication, and what is the first step that you suggest?
Dr. Baskin: Behavioral therapies for migraine management are typically preventive therapies that can and should be combined with medications to control acute attacks. There are behavioral principles that can maximize adherence to abortive agents in order to optimize acute care.
Dr. Rapoport: Which tends to work the best for migraine?
Dr. Baskin: What works best is to first do a good behavioral assessment of the frequency, duration, intensity, and disability level of their headaches as well as current stress levels, history, and adherence to drug and nondrug therapies, and psychiatric comorbidities. A program should then be developed that includes some combination of pharmacological and behavioral interventions to address these issues. It is important to increase self-efficacy: patients’ belief in the ability to control the headache, belief in the ability to manage emotional reactivity to pain, and belief that they can achieve functionality in the presence of a significant headache disorder.
Dr. Rapoport: Who should not have biofeedback therapy?
Dr. Baskin: Biofeedback has shown to be effective in treating migraine and TTHA. It has not been shown to be effective in treating trigeminal autonomic cephalgias (TACs) such as cluster headache. Like pharmacological therapies, it is less effective in chronic migraine that is daily and constant. A patient with severe psychiatric disorder should be treated for their psychiatric disorder before beginning biofeedback therapy.
Dr. Rapoport: Some doctors see patients twice per week for several months. What is your typical routine for behavioral therapy?
Dr. Baskin: We have a variety of programs. For complicated patients, we tend to see them weekly and have a very systematic program of biofeedback and CBT for approximately 12 to 15 sessions. This may include treating psychiatric comorbidities. We see many other patients for 1 or 2 sessions of biofeedback to try to effect physiological learning and for 1 or 2 sessions of CBT to help them manage stressors and learn coping skills that they can use to help manage migraines and life stress.
Dr. Rapoport: Does behavioral medicine work best in conjunction with preventive medications, or on its own?
Dr. Baskin: Many patients do well with a behavioral treatment as a preventive therapy and a pharmacologic agent to optimize acute care. I believe that many patients with higher frequency migraine with psychological issues or ongoing stressors do best with a combination of preventive pharmacologic therapy and behavior therapy. Any migraine patient with sleep issues should learn CBT for insomnia.
Dr. Rapoport: Is there evidence that suggests behavioral therapy can help patients at various ages manage their migraines?
Dr. Baskin: There is both adult and child data on behavioral therapy for migraine. An excellent study was done in children and adolescents by Powers et al. It showed that adding 10 sessions of CBT to preventive amitriptyline therapy, compared to adding headache education, significantly reduced the number of headache days, level of disability, and kids with a better than 50% decrease in days of headache compared to amitriptyline, plus headache education control in chronic migraine patients. (Powers SW et al. Cognitive Behavioral Therapy Plus Amitriptyline for Chronic Migraine in Children and Adolescents: A Randomized Clinical Trial. JAMA 2013;310(24):2622-2630)
Dr. Rapoport: A recent MedPage Today article noted that “anxiety may complicate migraine more than depression with greater long-term persistence, greater headache-related disability, and reduced satisfaction with acute therapies.” Could you please elaborate on why this may be the case?
Dr. Baskin: Anxiety disorders are often based on feeling threat. They are always associated with avoidance behaviors. Headache sufferers with significant anxiety tend to overestimate the probability of danger (migraine) and perceive it as more unmanageable and threatening than objective reality. They are often very sensitive to medication side effects and benign somatic sensations. They sometimes take medications pre-emptively, because of their fear of getting a migraine, which may lead to medication misuse or overuse. The lifetime prevalence of anxiety disorders in migraineurs (ranging from 51-58%) is almost twice that of major depression.
Please write to us at Neurology Reviews Migraine Resource Center (mrc@mdedge.com) with your opinions.
Alan M. Rapoport, M.D.
Editor-in-Chief
Migraine Resource Center
Clinical Professor of Neurology
The David Geffen School of Medicine at UCLA
Los Angeles, California
Monoclonal gammopathy of undetermined significance: A primary care guide
MGUS is present in 3% to 4% of the population over age 50 and is more common in older men, African Americans, and Africans.1–6
The overall risk of progression to myeloma and related disorders is less than or equal to 1% per year depending on the subtype of the M protein (higher risk with IgM than non-IgM and light-chain MGUS).7,8 While the risk of malignant transformation is low, multiple myeloma is almost always preceded by the presence of an asymptomatic and often unrecognized monoclonal protein.
WHEN SHOULD WE LOOK FOR AN M PROTEIN?
An M protein is typically an incidental finding when a patient is being assessed for any of a number of presenting symptoms or conditions. A large retrospective study9 found that screening for MGUS was mostly performed by internal medicine physicians. The indications for testing were anemia, bone-related issues, elevated creatinine, elevated erythrocyte sedimentation rate, and neuropathy.
A low anion gap is not a major indicator of an M protein unless in a high concentration, in which case other manifestations would be present, such as renal failure, which would guide the diagnosis. Polyclonal hypergammaglobulinemia as a cause of low anion gap is far more common than MGUS.
HOW SHOULD WE SCREEN FOR AN M PROTEIN?
Serum protein electrophoresis is an initial test used to identify an M protein and has a key role in quantifying it (Figure 2). An M protein appears as a narrow spike on the agarose gel and should be distinguished from the broad band seen in polyclonal gammopathies associated with cirrhosis and chronic infectious and inflammatory conditions, among others.12 A major disadvantage of serum protein electrophoresis is that it cannot detect an M protein in very low concentrations or determine its identity.
Serum immunofixation is more sensitive than serum protein electrophoresis and should always be ordered in conjunction with it, mostly to ensure detecting tiny amounts of M protein and to identify the type of its heavy chain and light-chain components.13
The serum free light-chain assay is also considered an essential part of the screening process to detect light-chain MGUS and light-chain myeloma. As many as 16% of myeloma patients secrete only light chains, which may not be identified on serum immunofixation.3,6,7,10,14,15 In general, a low kappa-lambda ratio (< 0.26) indicates the overproduction of lambda light chains, and a high ratio (> 1.65) indicates the overproduction of kappa light chains.
The serum free light-chain assay helps detect abnormal secretion of monoclonal light chains before they appear in the urine once the kidney tubules become saturated and unable to reabsorb them.
Of note, the free light-chain ratio can be abnormal (< 0.26 or > 1.65) in chronic kidney disease. Thus, it may be challenging to discern whether an abnormal light-chain ratio is related to impaired light-chain clearance by the kidneys or to MGUS. In general, kappa light chains are more elevated than lambda light chains in chronic kidney disease, but the ratio should not be considerably skewed. A kappa-lambda ratio below 0.37 or above 3 is rarely seen in chronic kidney disease and should prompt workup for MGUS.16
Tests in combination. The sensitivity of screening for M proteins ranges from 82% with serum protein electrophoresis alone to 93% with the addition of serum immunofixation and to 98% with the serum free light-chain assay.15 The latter can replace urine protein electrophoresis and immunofixation when screening for M protein, given its higher sensitivity.15,17 An important caveat is that urine dipstick testing does not detect urine light chains.
Table 3 lists the initial laboratory tests required in patients with MGUS.
WHAT IS THE DIFFERENTIAL DIAGNOSIS OF MONOCLONAL GAMMOPATHIES?
that feature an M protein and would otherwise require treatment (Table 4). The differential diagnosis includes smoldering multiple myeloma, symptomatic multiple myeloma, Waldenström macroglobulinemia, light-chain amyloidosis, low-grade B-cell lymphoproliferative disorders, a variety of monoclonal protein-related kidney disorders, and plasmacytomas.10,14
MGUS
Based on the International Myeloma Working Group consensus, a formal diagnosis of MGUS is established when a serum M protein is detected and measured at a concentration less than 3 g/dL on serum protein electrophoresis along with less than 10% clonal plasma cells in the bone marrow.1–6,14,18,19 Nevertheless, bone marrow biopsy can be omitted in certain patients as discussed below. The absence of myeloma-related organ damage—particularly osteolytic bone lesions, anemia, otherwise unexplained renal failure, and hypercalcemia—is fundamental and necessary for a diagnosis of MGUS.
Smoldering multiple myeloma
Compared with patients with MGUS, patients with smoldering multiple myeloma have higher M protein concentrations (≥ 3 g/dL) or 10% or more clonal plasma cells in the marrow or both, and are at higher risk of progression to symptomatic multiple myeloma. Nevertheless, like patients with MGUS, they have no myeloma symptoms or evidence of end-organ damage.
Symptomatic multiple myeloma
By definition, patients with multiple myeloma develop organ damage related to their malignancy and need therapy to halt disease progression. Multiple myeloma causes clinical manifestations through cellular infiltration of the bone and bone marrow (anemia, osteolysis, and hypercalcemia) and light chain-induced toxicity (renal tubular damage and cast nephropathy).
In 2014, the definition of multiple myeloma was updated to include 3 new myeloma-defining events that herald a significantly higher risk of progression from smoldering to symptomatic multiple myeloma, and now constitute an integral part of the diagnosis of symptomatic multiple myeloma. These are:
- Focal lesions (> 1 lesion larger than 5 mm) visible on magnetic resonance imaging
- ≥ 60% clonal plasma cells on bone marrow biopsy
- Ratio of involved to uninvolved serum free light chains ≥ 100 (the involved light chain is the one detected on serum protein electrophoresis and immunofixation).14
Bone pain, symptoms of anemia, and decreased urine output may suggest myeloma, but are not diagnostic. Although the “CRAB” criteria (elevated calcium, renal failure, anemia, and bone lesions) define multiple myeloma, the presence of anemia, hypercalcemia, or renal dysfunction do not by themselves mark transformation from MGUS to multiple myeloma. Thus, other causes need to be considered, since the risk of transformation is so low. Importantly, hyperparathyroidism must be ruled out if hypercalcemia is present in a patient with MGUS.10
Waldenström macroglobulinemia
Waldenström macroglobulinemia, also called lymphoplasmacytic lymphoma, is an indolent non-Hodgkin B-cell lymphoma that can invade the marrow, liver, spleen, and lymph nodes, leading to anemia and organomegaly. It features a monoclonal IgM protein that can be associated with increased blood viscosity, cold agglutinin disease, peripheral neuropathy, and cryoglobulinemia.
Waldenström macroglobulinemia should be suspected in any patient with IgM type M protein and symptoms related to hyperviscosity (headache, blurry vision, lightheadedness, shortness of breath, unexplained epistaxis, gum bleeding); systemic symptoms (fever, weight loss, and night sweats); and abdominal pain (due to organomegaly).23
Monoclonal gammopathy of renal significance
Monoclonal gammopathy of renal significance (MGRS) is a newly recognized entity defined by kidney dysfunction associated with an M protein without evidence of myeloma or other lymphoid disorders.24 Multiple disorders have been included in this category with different underlying mechanisms of kidney injury. This entity is beyond the scope of this discussion.
Light-chain amyloidosis
Misfolded light-chain deposition leading to organ dysfunction is the hallmark of light-chain amyloidosis, which constitutes a subset of MGRS. An abnormal light-chain ratio, especially if skewed toward lambda should trigger an investigation for light-chain amyloidosis.10
Abnormal light chains may infiltrate any organ or tissue, but of greatest concern is infiltration of the myocardium with ensuing heart failure manifestations. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a sensitive marker for cardiac amyloidosis in the presence of suggestive features on transthoracic echocardiography (eg, left ventricular hypertrophy) but is not specific as it can be elevated in heart failure regardless of the underlying cause.10
Glomerular injury with nephrotic syndrome may also point toward renal involvement by light-chain amyloidosis and establishes a key distinctive factor from myeloma in which tubular injury is the main mechanism of kidney dysfunction.
Clinical clues for light-chain amyloidosis include heart failure symptoms, neuropathy, and macroglossia. If any of these symptoms and signs is present, we recommend electrocardiography (look for low voltage in limb leads), transthoracic echocardiography, measuring the NT-proBNP level, and urinalysis to look for albuminuria. Notably, carpal tunnel syndrome may be a very early clinical manifestation of amyloidosis, but by itself it is nonspecific. Light-chain amyloidosis is a common cause of macroglossia in adults.10,25
Neuropathy associated with M proteins is a clinical entity related to a multitude of disorders that may necessitate treating the underlying cellular clone responsible for the secretion of the toxic M protein. These disorders include light-chain amyloidosis, POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes or sclerotic bone lesions) syndrome, and IgM-related neuropathies with anti-myelin-associated glycoprotein antibodies.3,10,11,14
Notably, weight loss and fatigue in a patient with MGUS may be the first signs of light-chain amyloidosis or Waldenström macroglobulinemia and should prompt further evaluation.25
HOW ARE PATIENTS WITH MGUS RISK-STRATIFIED AND FOLLOWED?
Research has helped to refine the diagnostic workup and recognize subsets of patients with MGUS at different risks of progression to myeloma and related disorders. Factors predicting progression are 1,6,7,26,27:
- The amount of the M protein
- The type of M protein (IgG vs non-IgG)
- An abnormal free light-chain ratio.
Half of patients with MGUS fall into the low-risk category, which is defined by IgG-type serum M protein in a concentration less than 1.5 g/dL and a normal serum free light-chain ratio (kappa-lambda 0.26–1.65).5,27 The absolute risk of progression at 20 years is only 5% for patients with low-risk MGUS, compared with 58% in patients with high-risk MGUS (positive for all 3 risk factors).5
The presence of less than 10% plasma cells in the bone marrow is required to satisfy the definition of MGUS, but bone marrow biopsy can be omitted for patients with low-risk MGUS, given the slim chance of finding a significant percentage of clonal plasma cells in the marrow and the inherently low risk of progression.5,10 Skeletal surveys are often deferred for low-risk MGUS, but we obtain them in all our patients to ensure the absence of plasmacytomas, which need to be treated (typically with radiotherapy). Importantly, patients with unexplained bone pain (mostly in long bones, ribs, and spine, whereas joints are not typically involved) and a normal skeletal survey should undergo advanced imaging (whole-body magnetic resonance imaging or whole-body positron emission tomography and computed tomography) to detect bone lesions otherwise missed on plain radiography.28,29
Most of the recommendations regarding follow-up are based on expert opinion, given the lack of randomized data. Most experts agree that all patients should be reevaluated 6 months after an M protein is detected, with laboratory surveillance tests (complete blood cell count, serum creatinine, serum calcium level, serum protein electrophoresis, and serum free light chains). Low-risk patients with a stable M protein level can be followed every 2 to 3 years.
Suspect malignant progression if the serum M protein level increases by 50% or more (with an absolute increase of ≥ 0.5 g/dL); the serum M protein level is 3 g/dL or higher; the serum free light-chain ratio is more than 100; or the patient has unexplained anemia, elevated creatinine, bone pain, fracture, or hypercalcemia.
Patients at intermediate or high risk should be followed annually after the initial 6-month visit.5,7,10
A recent study highlighted the importance of risk stratification in reducing the costs associated with an overzealous diagnostic workup of patients with low-risk MGUS.30 These savings are in addition to a reduction in patient anticipation and anxiety that universally occur before invasive procedures.
THE ROLE OF THE PRIMARY CARE PROVIDER AND THE HEMATOLOGIST
Once an M protein is identified, a comprehensive history, physical examination, and laboratory tests (serum protein electrophoresis to quantify the protein, serum immunofixation, serum free light chains, complete blood cell count, calcium, and creatinine) should be done, taking into consideration the differential diagnosis of monoclonal gammopathies discussed above. After MGUS is confirmed, the patient should be risk-stratified to determine the need for bone marrow biopsy and to predict the risk of progression to more serious conditions.
Referral to a hematologist is warranted for patients with intermediate- and high-risk MGUS, patients with abnormal serum free light-chain ratios, and those who show evidence of malignant progression. Patients with intermediate- and high-risk MGUS could be referred for bone marrow biopsy before assessment by a hematologist. The primary care provider may continue to follow patients with low-risk MGUS who do not display clinical or laboratory evidence of myeloma or related disorders.
The importance of educating patients to report any new worrisome symptom (eg, fatigue, neuropathy, weight loss, night sweats, bone pain) cannot be overemphasized, as some patients may progress to myeloma or other disorders between follow-up visits.
- van de Donk NW, Palumbo A, Johnsen HE, et al; European Myeloma Network. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica 2014; 99(6):984–996. doi:10.3324/haematol.2013.100552
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121(5):749–757. pmid:12780789
- Rajan AM, Rajkumar SV. Diagnostic evaluation of monoclonal gammopathy of undetermined significance. Eur J Haematol 2013; 91(6):561–562. doi:10.1111/ejh.12198
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134(6):573–589. doi:10.1111/j.1365-2141.2006.06235.x
- Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010; 24(6):1121–1127. doi:10.1038/leu.2010.60
- Bird J, Behrens J, Westin J, et al; Haemato-oncology Task Force of the British Committee for Standards in Haematology, UK Myeloma Forum and Nordic Myeloma Study Group. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2009; 147(1):22–42. doi:10.1111/j.1365-2141.2009.07807.x
- Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc 2010; 85(10):945–948. doi:10.4065/mcp.2010.0520
- Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346(8):564–569. doi:10.1056/NEJMoa01133202
- Doyle LM, Gundrum JD, Farnen JP, Wright LJ, Kranig JAI, Go RS. Determining why and which clinicians order serum protein electrophoresis (SPEP), subsequent diagnoses based on indications, and clinical significance of routine follow-up: a study of patients with monoclonal gammopathy of undetermined significance (MGUS). Blood 2009; 114(22):Abstr 4883. www.bloodjournal.org/content/114/22/4883. Accessed December 4, 2018.
- Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am Soc Hematol Educ Program 2012; 2012:595–603. doi:10.1182/asheducation-2012.1.595
- Glavey SV, Leung N. Monoclonal gammopathy: the good, the bad and the ugly. Blood Rev 2016; 30(3):223–231. doi:10.1016/j.blre.2015.12.001
- Dispenzieri A, Gertz MA, Therneau TM, Kyle RA. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc 2001; 76(5):476–487. doi:10.4065/76.5.476
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 2006; 108(8):2520–2530. doi:10.1182/blood-2006-03-001164
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78(1):21–33. doi:10.4065/78.1.21
- Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3(6):1684–1690. doi:10.2215/CJN.02290508
- Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81(12):1575–1578. doi:10.4065/81.12.1575
- Berenson JR, Anderson KC, Audell RA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol 2010; 150(1):28–38. doi:10.1111/j.1365-2141.2010.08207.x
- Mangiacavalli S, Cocito F, Pochintesta L, et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol 2013; 91(4):356–360. doi:10.1111/ejh.12172
- Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood 2010;116:2019–2025. doi:10.1182/blood-2010-04-277566
- Rosiñol L, Cibeira MT, Montoto S, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc 2007; 82(4):428–434. doi:10.4065/82.4.428
- Vanderschueren S, Mylle M, Dierickx D, et al. Monoclonal gammopathy of undetermined significance: significant beyond hematology. Mayo Clin Proc 2009; 84(9):842–845. doi:10.4065/84.9.842
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol 2007; 139(5):730–743. doi:10.1111/j.1365-2141.2007.06873.x
- Leung N, Bridoux F, Hutchison CA, et al; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012; 120(22):4292–4295. doi:10.1182/blood-2012-07-445304
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood 2013; 121(26):5124–5130. doi:10.1182/blood-2013-01-453001
- Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 2010; 375(9727):1721–1728. doi:10.1016/S0140-6736(10)60482-5
- Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106(3):812–817. doi:10.1182/blood-2005-03-1038
- Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 2015; 33(6):657–664. doi:10.1200/JCO.2014.57.9961
- Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood 2011; 117(18):4701–4705. doi:10.1182/blood-2010-10-299529
- Pompa T, Maddox M, Woodard A, et al. Cost effectiveness in low risk MGUS patients. Blood 2016; 128:2360. http://www.bloodjournal.org/content/128/22/2360. Accessed December 4, 2018.
MGUS is present in 3% to 4% of the population over age 50 and is more common in older men, African Americans, and Africans.1–6
The overall risk of progression to myeloma and related disorders is less than or equal to 1% per year depending on the subtype of the M protein (higher risk with IgM than non-IgM and light-chain MGUS).7,8 While the risk of malignant transformation is low, multiple myeloma is almost always preceded by the presence of an asymptomatic and often unrecognized monoclonal protein.
WHEN SHOULD WE LOOK FOR AN M PROTEIN?
An M protein is typically an incidental finding when a patient is being assessed for any of a number of presenting symptoms or conditions. A large retrospective study9 found that screening for MGUS was mostly performed by internal medicine physicians. The indications for testing were anemia, bone-related issues, elevated creatinine, elevated erythrocyte sedimentation rate, and neuropathy.
A low anion gap is not a major indicator of an M protein unless in a high concentration, in which case other manifestations would be present, such as renal failure, which would guide the diagnosis. Polyclonal hypergammaglobulinemia as a cause of low anion gap is far more common than MGUS.
HOW SHOULD WE SCREEN FOR AN M PROTEIN?
Serum protein electrophoresis is an initial test used to identify an M protein and has a key role in quantifying it (Figure 2). An M protein appears as a narrow spike on the agarose gel and should be distinguished from the broad band seen in polyclonal gammopathies associated with cirrhosis and chronic infectious and inflammatory conditions, among others.12 A major disadvantage of serum protein electrophoresis is that it cannot detect an M protein in very low concentrations or determine its identity.
Serum immunofixation is more sensitive than serum protein electrophoresis and should always be ordered in conjunction with it, mostly to ensure detecting tiny amounts of M protein and to identify the type of its heavy chain and light-chain components.13
The serum free light-chain assay is also considered an essential part of the screening process to detect light-chain MGUS and light-chain myeloma. As many as 16% of myeloma patients secrete only light chains, which may not be identified on serum immunofixation.3,6,7,10,14,15 In general, a low kappa-lambda ratio (< 0.26) indicates the overproduction of lambda light chains, and a high ratio (> 1.65) indicates the overproduction of kappa light chains.
The serum free light-chain assay helps detect abnormal secretion of monoclonal light chains before they appear in the urine once the kidney tubules become saturated and unable to reabsorb them.
Of note, the free light-chain ratio can be abnormal (< 0.26 or > 1.65) in chronic kidney disease. Thus, it may be challenging to discern whether an abnormal light-chain ratio is related to impaired light-chain clearance by the kidneys or to MGUS. In general, kappa light chains are more elevated than lambda light chains in chronic kidney disease, but the ratio should not be considerably skewed. A kappa-lambda ratio below 0.37 or above 3 is rarely seen in chronic kidney disease and should prompt workup for MGUS.16
Tests in combination. The sensitivity of screening for M proteins ranges from 82% with serum protein electrophoresis alone to 93% with the addition of serum immunofixation and to 98% with the serum free light-chain assay.15 The latter can replace urine protein electrophoresis and immunofixation when screening for M protein, given its higher sensitivity.15,17 An important caveat is that urine dipstick testing does not detect urine light chains.
Table 3 lists the initial laboratory tests required in patients with MGUS.
WHAT IS THE DIFFERENTIAL DIAGNOSIS OF MONOCLONAL GAMMOPATHIES?
that feature an M protein and would otherwise require treatment (Table 4). The differential diagnosis includes smoldering multiple myeloma, symptomatic multiple myeloma, Waldenström macroglobulinemia, light-chain amyloidosis, low-grade B-cell lymphoproliferative disorders, a variety of monoclonal protein-related kidney disorders, and plasmacytomas.10,14
MGUS
Based on the International Myeloma Working Group consensus, a formal diagnosis of MGUS is established when a serum M protein is detected and measured at a concentration less than 3 g/dL on serum protein electrophoresis along with less than 10% clonal plasma cells in the bone marrow.1–6,14,18,19 Nevertheless, bone marrow biopsy can be omitted in certain patients as discussed below. The absence of myeloma-related organ damage—particularly osteolytic bone lesions, anemia, otherwise unexplained renal failure, and hypercalcemia—is fundamental and necessary for a diagnosis of MGUS.
Smoldering multiple myeloma
Compared with patients with MGUS, patients with smoldering multiple myeloma have higher M protein concentrations (≥ 3 g/dL) or 10% or more clonal plasma cells in the marrow or both, and are at higher risk of progression to symptomatic multiple myeloma. Nevertheless, like patients with MGUS, they have no myeloma symptoms or evidence of end-organ damage.
Symptomatic multiple myeloma
By definition, patients with multiple myeloma develop organ damage related to their malignancy and need therapy to halt disease progression. Multiple myeloma causes clinical manifestations through cellular infiltration of the bone and bone marrow (anemia, osteolysis, and hypercalcemia) and light chain-induced toxicity (renal tubular damage and cast nephropathy).
In 2014, the definition of multiple myeloma was updated to include 3 new myeloma-defining events that herald a significantly higher risk of progression from smoldering to symptomatic multiple myeloma, and now constitute an integral part of the diagnosis of symptomatic multiple myeloma. These are:
- Focal lesions (> 1 lesion larger than 5 mm) visible on magnetic resonance imaging
- ≥ 60% clonal plasma cells on bone marrow biopsy
- Ratio of involved to uninvolved serum free light chains ≥ 100 (the involved light chain is the one detected on serum protein electrophoresis and immunofixation).14
Bone pain, symptoms of anemia, and decreased urine output may suggest myeloma, but are not diagnostic. Although the “CRAB” criteria (elevated calcium, renal failure, anemia, and bone lesions) define multiple myeloma, the presence of anemia, hypercalcemia, or renal dysfunction do not by themselves mark transformation from MGUS to multiple myeloma. Thus, other causes need to be considered, since the risk of transformation is so low. Importantly, hyperparathyroidism must be ruled out if hypercalcemia is present in a patient with MGUS.10
Waldenström macroglobulinemia
Waldenström macroglobulinemia, also called lymphoplasmacytic lymphoma, is an indolent non-Hodgkin B-cell lymphoma that can invade the marrow, liver, spleen, and lymph nodes, leading to anemia and organomegaly. It features a monoclonal IgM protein that can be associated with increased blood viscosity, cold agglutinin disease, peripheral neuropathy, and cryoglobulinemia.
Waldenström macroglobulinemia should be suspected in any patient with IgM type M protein and symptoms related to hyperviscosity (headache, blurry vision, lightheadedness, shortness of breath, unexplained epistaxis, gum bleeding); systemic symptoms (fever, weight loss, and night sweats); and abdominal pain (due to organomegaly).23
Monoclonal gammopathy of renal significance
Monoclonal gammopathy of renal significance (MGRS) is a newly recognized entity defined by kidney dysfunction associated with an M protein without evidence of myeloma or other lymphoid disorders.24 Multiple disorders have been included in this category with different underlying mechanisms of kidney injury. This entity is beyond the scope of this discussion.
Light-chain amyloidosis
Misfolded light-chain deposition leading to organ dysfunction is the hallmark of light-chain amyloidosis, which constitutes a subset of MGRS. An abnormal light-chain ratio, especially if skewed toward lambda should trigger an investigation for light-chain amyloidosis.10
Abnormal light chains may infiltrate any organ or tissue, but of greatest concern is infiltration of the myocardium with ensuing heart failure manifestations. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a sensitive marker for cardiac amyloidosis in the presence of suggestive features on transthoracic echocardiography (eg, left ventricular hypertrophy) but is not specific as it can be elevated in heart failure regardless of the underlying cause.10
Glomerular injury with nephrotic syndrome may also point toward renal involvement by light-chain amyloidosis and establishes a key distinctive factor from myeloma in which tubular injury is the main mechanism of kidney dysfunction.
Clinical clues for light-chain amyloidosis include heart failure symptoms, neuropathy, and macroglossia. If any of these symptoms and signs is present, we recommend electrocardiography (look for low voltage in limb leads), transthoracic echocardiography, measuring the NT-proBNP level, and urinalysis to look for albuminuria. Notably, carpal tunnel syndrome may be a very early clinical manifestation of amyloidosis, but by itself it is nonspecific. Light-chain amyloidosis is a common cause of macroglossia in adults.10,25
Neuropathy associated with M proteins is a clinical entity related to a multitude of disorders that may necessitate treating the underlying cellular clone responsible for the secretion of the toxic M protein. These disorders include light-chain amyloidosis, POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes or sclerotic bone lesions) syndrome, and IgM-related neuropathies with anti-myelin-associated glycoprotein antibodies.3,10,11,14
Notably, weight loss and fatigue in a patient with MGUS may be the first signs of light-chain amyloidosis or Waldenström macroglobulinemia and should prompt further evaluation.25
HOW ARE PATIENTS WITH MGUS RISK-STRATIFIED AND FOLLOWED?
Research has helped to refine the diagnostic workup and recognize subsets of patients with MGUS at different risks of progression to myeloma and related disorders. Factors predicting progression are 1,6,7,26,27:
- The amount of the M protein
- The type of M protein (IgG vs non-IgG)
- An abnormal free light-chain ratio.
Half of patients with MGUS fall into the low-risk category, which is defined by IgG-type serum M protein in a concentration less than 1.5 g/dL and a normal serum free light-chain ratio (kappa-lambda 0.26–1.65).5,27 The absolute risk of progression at 20 years is only 5% for patients with low-risk MGUS, compared with 58% in patients with high-risk MGUS (positive for all 3 risk factors).5
The presence of less than 10% plasma cells in the bone marrow is required to satisfy the definition of MGUS, but bone marrow biopsy can be omitted for patients with low-risk MGUS, given the slim chance of finding a significant percentage of clonal plasma cells in the marrow and the inherently low risk of progression.5,10 Skeletal surveys are often deferred for low-risk MGUS, but we obtain them in all our patients to ensure the absence of plasmacytomas, which need to be treated (typically with radiotherapy). Importantly, patients with unexplained bone pain (mostly in long bones, ribs, and spine, whereas joints are not typically involved) and a normal skeletal survey should undergo advanced imaging (whole-body magnetic resonance imaging or whole-body positron emission tomography and computed tomography) to detect bone lesions otherwise missed on plain radiography.28,29
Most of the recommendations regarding follow-up are based on expert opinion, given the lack of randomized data. Most experts agree that all patients should be reevaluated 6 months after an M protein is detected, with laboratory surveillance tests (complete blood cell count, serum creatinine, serum calcium level, serum protein electrophoresis, and serum free light chains). Low-risk patients with a stable M protein level can be followed every 2 to 3 years.
Suspect malignant progression if the serum M protein level increases by 50% or more (with an absolute increase of ≥ 0.5 g/dL); the serum M protein level is 3 g/dL or higher; the serum free light-chain ratio is more than 100; or the patient has unexplained anemia, elevated creatinine, bone pain, fracture, or hypercalcemia.
Patients at intermediate or high risk should be followed annually after the initial 6-month visit.5,7,10
A recent study highlighted the importance of risk stratification in reducing the costs associated with an overzealous diagnostic workup of patients with low-risk MGUS.30 These savings are in addition to a reduction in patient anticipation and anxiety that universally occur before invasive procedures.
THE ROLE OF THE PRIMARY CARE PROVIDER AND THE HEMATOLOGIST
Once an M protein is identified, a comprehensive history, physical examination, and laboratory tests (serum protein electrophoresis to quantify the protein, serum immunofixation, serum free light chains, complete blood cell count, calcium, and creatinine) should be done, taking into consideration the differential diagnosis of monoclonal gammopathies discussed above. After MGUS is confirmed, the patient should be risk-stratified to determine the need for bone marrow biopsy and to predict the risk of progression to more serious conditions.
Referral to a hematologist is warranted for patients with intermediate- and high-risk MGUS, patients with abnormal serum free light-chain ratios, and those who show evidence of malignant progression. Patients with intermediate- and high-risk MGUS could be referred for bone marrow biopsy before assessment by a hematologist. The primary care provider may continue to follow patients with low-risk MGUS who do not display clinical or laboratory evidence of myeloma or related disorders.
The importance of educating patients to report any new worrisome symptom (eg, fatigue, neuropathy, weight loss, night sweats, bone pain) cannot be overemphasized, as some patients may progress to myeloma or other disorders between follow-up visits.
MGUS is present in 3% to 4% of the population over age 50 and is more common in older men, African Americans, and Africans.1–6
The overall risk of progression to myeloma and related disorders is less than or equal to 1% per year depending on the subtype of the M protein (higher risk with IgM than non-IgM and light-chain MGUS).7,8 While the risk of malignant transformation is low, multiple myeloma is almost always preceded by the presence of an asymptomatic and often unrecognized monoclonal protein.
WHEN SHOULD WE LOOK FOR AN M PROTEIN?
An M protein is typically an incidental finding when a patient is being assessed for any of a number of presenting symptoms or conditions. A large retrospective study9 found that screening for MGUS was mostly performed by internal medicine physicians. The indications for testing were anemia, bone-related issues, elevated creatinine, elevated erythrocyte sedimentation rate, and neuropathy.
A low anion gap is not a major indicator of an M protein unless in a high concentration, in which case other manifestations would be present, such as renal failure, which would guide the diagnosis. Polyclonal hypergammaglobulinemia as a cause of low anion gap is far more common than MGUS.
HOW SHOULD WE SCREEN FOR AN M PROTEIN?
Serum protein electrophoresis is an initial test used to identify an M protein and has a key role in quantifying it (Figure 2). An M protein appears as a narrow spike on the agarose gel and should be distinguished from the broad band seen in polyclonal gammopathies associated with cirrhosis and chronic infectious and inflammatory conditions, among others.12 A major disadvantage of serum protein electrophoresis is that it cannot detect an M protein in very low concentrations or determine its identity.
Serum immunofixation is more sensitive than serum protein electrophoresis and should always be ordered in conjunction with it, mostly to ensure detecting tiny amounts of M protein and to identify the type of its heavy chain and light-chain components.13
The serum free light-chain assay is also considered an essential part of the screening process to detect light-chain MGUS and light-chain myeloma. As many as 16% of myeloma patients secrete only light chains, which may not be identified on serum immunofixation.3,6,7,10,14,15 In general, a low kappa-lambda ratio (< 0.26) indicates the overproduction of lambda light chains, and a high ratio (> 1.65) indicates the overproduction of kappa light chains.
The serum free light-chain assay helps detect abnormal secretion of monoclonal light chains before they appear in the urine once the kidney tubules become saturated and unable to reabsorb them.
Of note, the free light-chain ratio can be abnormal (< 0.26 or > 1.65) in chronic kidney disease. Thus, it may be challenging to discern whether an abnormal light-chain ratio is related to impaired light-chain clearance by the kidneys or to MGUS. In general, kappa light chains are more elevated than lambda light chains in chronic kidney disease, but the ratio should not be considerably skewed. A kappa-lambda ratio below 0.37 or above 3 is rarely seen in chronic kidney disease and should prompt workup for MGUS.16
Tests in combination. The sensitivity of screening for M proteins ranges from 82% with serum protein electrophoresis alone to 93% with the addition of serum immunofixation and to 98% with the serum free light-chain assay.15 The latter can replace urine protein electrophoresis and immunofixation when screening for M protein, given its higher sensitivity.15,17 An important caveat is that urine dipstick testing does not detect urine light chains.
Table 3 lists the initial laboratory tests required in patients with MGUS.
WHAT IS THE DIFFERENTIAL DIAGNOSIS OF MONOCLONAL GAMMOPATHIES?
that feature an M protein and would otherwise require treatment (Table 4). The differential diagnosis includes smoldering multiple myeloma, symptomatic multiple myeloma, Waldenström macroglobulinemia, light-chain amyloidosis, low-grade B-cell lymphoproliferative disorders, a variety of monoclonal protein-related kidney disorders, and plasmacytomas.10,14
MGUS
Based on the International Myeloma Working Group consensus, a formal diagnosis of MGUS is established when a serum M protein is detected and measured at a concentration less than 3 g/dL on serum protein electrophoresis along with less than 10% clonal plasma cells in the bone marrow.1–6,14,18,19 Nevertheless, bone marrow biopsy can be omitted in certain patients as discussed below. The absence of myeloma-related organ damage—particularly osteolytic bone lesions, anemia, otherwise unexplained renal failure, and hypercalcemia—is fundamental and necessary for a diagnosis of MGUS.
Smoldering multiple myeloma
Compared with patients with MGUS, patients with smoldering multiple myeloma have higher M protein concentrations (≥ 3 g/dL) or 10% or more clonal plasma cells in the marrow or both, and are at higher risk of progression to symptomatic multiple myeloma. Nevertheless, like patients with MGUS, they have no myeloma symptoms or evidence of end-organ damage.
Symptomatic multiple myeloma
By definition, patients with multiple myeloma develop organ damage related to their malignancy and need therapy to halt disease progression. Multiple myeloma causes clinical manifestations through cellular infiltration of the bone and bone marrow (anemia, osteolysis, and hypercalcemia) and light chain-induced toxicity (renal tubular damage and cast nephropathy).
In 2014, the definition of multiple myeloma was updated to include 3 new myeloma-defining events that herald a significantly higher risk of progression from smoldering to symptomatic multiple myeloma, and now constitute an integral part of the diagnosis of symptomatic multiple myeloma. These are:
- Focal lesions (> 1 lesion larger than 5 mm) visible on magnetic resonance imaging
- ≥ 60% clonal plasma cells on bone marrow biopsy
- Ratio of involved to uninvolved serum free light chains ≥ 100 (the involved light chain is the one detected on serum protein electrophoresis and immunofixation).14
Bone pain, symptoms of anemia, and decreased urine output may suggest myeloma, but are not diagnostic. Although the “CRAB” criteria (elevated calcium, renal failure, anemia, and bone lesions) define multiple myeloma, the presence of anemia, hypercalcemia, or renal dysfunction do not by themselves mark transformation from MGUS to multiple myeloma. Thus, other causes need to be considered, since the risk of transformation is so low. Importantly, hyperparathyroidism must be ruled out if hypercalcemia is present in a patient with MGUS.10
Waldenström macroglobulinemia
Waldenström macroglobulinemia, also called lymphoplasmacytic lymphoma, is an indolent non-Hodgkin B-cell lymphoma that can invade the marrow, liver, spleen, and lymph nodes, leading to anemia and organomegaly. It features a monoclonal IgM protein that can be associated with increased blood viscosity, cold agglutinin disease, peripheral neuropathy, and cryoglobulinemia.
Waldenström macroglobulinemia should be suspected in any patient with IgM type M protein and symptoms related to hyperviscosity (headache, blurry vision, lightheadedness, shortness of breath, unexplained epistaxis, gum bleeding); systemic symptoms (fever, weight loss, and night sweats); and abdominal pain (due to organomegaly).23
Monoclonal gammopathy of renal significance
Monoclonal gammopathy of renal significance (MGRS) is a newly recognized entity defined by kidney dysfunction associated with an M protein without evidence of myeloma or other lymphoid disorders.24 Multiple disorders have been included in this category with different underlying mechanisms of kidney injury. This entity is beyond the scope of this discussion.
Light-chain amyloidosis
Misfolded light-chain deposition leading to organ dysfunction is the hallmark of light-chain amyloidosis, which constitutes a subset of MGRS. An abnormal light-chain ratio, especially if skewed toward lambda should trigger an investigation for light-chain amyloidosis.10
Abnormal light chains may infiltrate any organ or tissue, but of greatest concern is infiltration of the myocardium with ensuing heart failure manifestations. N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a sensitive marker for cardiac amyloidosis in the presence of suggestive features on transthoracic echocardiography (eg, left ventricular hypertrophy) but is not specific as it can be elevated in heart failure regardless of the underlying cause.10
Glomerular injury with nephrotic syndrome may also point toward renal involvement by light-chain amyloidosis and establishes a key distinctive factor from myeloma in which tubular injury is the main mechanism of kidney dysfunction.
Clinical clues for light-chain amyloidosis include heart failure symptoms, neuropathy, and macroglossia. If any of these symptoms and signs is present, we recommend electrocardiography (look for low voltage in limb leads), transthoracic echocardiography, measuring the NT-proBNP level, and urinalysis to look for albuminuria. Notably, carpal tunnel syndrome may be a very early clinical manifestation of amyloidosis, but by itself it is nonspecific. Light-chain amyloidosis is a common cause of macroglossia in adults.10,25
Neuropathy associated with M proteins is a clinical entity related to a multitude of disorders that may necessitate treating the underlying cellular clone responsible for the secretion of the toxic M protein. These disorders include light-chain amyloidosis, POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes or sclerotic bone lesions) syndrome, and IgM-related neuropathies with anti-myelin-associated glycoprotein antibodies.3,10,11,14
Notably, weight loss and fatigue in a patient with MGUS may be the first signs of light-chain amyloidosis or Waldenström macroglobulinemia and should prompt further evaluation.25
HOW ARE PATIENTS WITH MGUS RISK-STRATIFIED AND FOLLOWED?
Research has helped to refine the diagnostic workup and recognize subsets of patients with MGUS at different risks of progression to myeloma and related disorders. Factors predicting progression are 1,6,7,26,27:
- The amount of the M protein
- The type of M protein (IgG vs non-IgG)
- An abnormal free light-chain ratio.
Half of patients with MGUS fall into the low-risk category, which is defined by IgG-type serum M protein in a concentration less than 1.5 g/dL and a normal serum free light-chain ratio (kappa-lambda 0.26–1.65).5,27 The absolute risk of progression at 20 years is only 5% for patients with low-risk MGUS, compared with 58% in patients with high-risk MGUS (positive for all 3 risk factors).5
The presence of less than 10% plasma cells in the bone marrow is required to satisfy the definition of MGUS, but bone marrow biopsy can be omitted for patients with low-risk MGUS, given the slim chance of finding a significant percentage of clonal plasma cells in the marrow and the inherently low risk of progression.5,10 Skeletal surveys are often deferred for low-risk MGUS, but we obtain them in all our patients to ensure the absence of plasmacytomas, which need to be treated (typically with radiotherapy). Importantly, patients with unexplained bone pain (mostly in long bones, ribs, and spine, whereas joints are not typically involved) and a normal skeletal survey should undergo advanced imaging (whole-body magnetic resonance imaging or whole-body positron emission tomography and computed tomography) to detect bone lesions otherwise missed on plain radiography.28,29
Most of the recommendations regarding follow-up are based on expert opinion, given the lack of randomized data. Most experts agree that all patients should be reevaluated 6 months after an M protein is detected, with laboratory surveillance tests (complete blood cell count, serum creatinine, serum calcium level, serum protein electrophoresis, and serum free light chains). Low-risk patients with a stable M protein level can be followed every 2 to 3 years.
Suspect malignant progression if the serum M protein level increases by 50% or more (with an absolute increase of ≥ 0.5 g/dL); the serum M protein level is 3 g/dL or higher; the serum free light-chain ratio is more than 100; or the patient has unexplained anemia, elevated creatinine, bone pain, fracture, or hypercalcemia.
Patients at intermediate or high risk should be followed annually after the initial 6-month visit.5,7,10
A recent study highlighted the importance of risk stratification in reducing the costs associated with an overzealous diagnostic workup of patients with low-risk MGUS.30 These savings are in addition to a reduction in patient anticipation and anxiety that universally occur before invasive procedures.
THE ROLE OF THE PRIMARY CARE PROVIDER AND THE HEMATOLOGIST
Once an M protein is identified, a comprehensive history, physical examination, and laboratory tests (serum protein electrophoresis to quantify the protein, serum immunofixation, serum free light chains, complete blood cell count, calcium, and creatinine) should be done, taking into consideration the differential diagnosis of monoclonal gammopathies discussed above. After MGUS is confirmed, the patient should be risk-stratified to determine the need for bone marrow biopsy and to predict the risk of progression to more serious conditions.
Referral to a hematologist is warranted for patients with intermediate- and high-risk MGUS, patients with abnormal serum free light-chain ratios, and those who show evidence of malignant progression. Patients with intermediate- and high-risk MGUS could be referred for bone marrow biopsy before assessment by a hematologist. The primary care provider may continue to follow patients with low-risk MGUS who do not display clinical or laboratory evidence of myeloma or related disorders.
The importance of educating patients to report any new worrisome symptom (eg, fatigue, neuropathy, weight loss, night sweats, bone pain) cannot be overemphasized, as some patients may progress to myeloma or other disorders between follow-up visits.
- van de Donk NW, Palumbo A, Johnsen HE, et al; European Myeloma Network. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica 2014; 99(6):984–996. doi:10.3324/haematol.2013.100552
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121(5):749–757. pmid:12780789
- Rajan AM, Rajkumar SV. Diagnostic evaluation of monoclonal gammopathy of undetermined significance. Eur J Haematol 2013; 91(6):561–562. doi:10.1111/ejh.12198
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134(6):573–589. doi:10.1111/j.1365-2141.2006.06235.x
- Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010; 24(6):1121–1127. doi:10.1038/leu.2010.60
- Bird J, Behrens J, Westin J, et al; Haemato-oncology Task Force of the British Committee for Standards in Haematology, UK Myeloma Forum and Nordic Myeloma Study Group. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2009; 147(1):22–42. doi:10.1111/j.1365-2141.2009.07807.x
- Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc 2010; 85(10):945–948. doi:10.4065/mcp.2010.0520
- Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346(8):564–569. doi:10.1056/NEJMoa01133202
- Doyle LM, Gundrum JD, Farnen JP, Wright LJ, Kranig JAI, Go RS. Determining why and which clinicians order serum protein electrophoresis (SPEP), subsequent diagnoses based on indications, and clinical significance of routine follow-up: a study of patients with monoclonal gammopathy of undetermined significance (MGUS). Blood 2009; 114(22):Abstr 4883. www.bloodjournal.org/content/114/22/4883. Accessed December 4, 2018.
- Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am Soc Hematol Educ Program 2012; 2012:595–603. doi:10.1182/asheducation-2012.1.595
- Glavey SV, Leung N. Monoclonal gammopathy: the good, the bad and the ugly. Blood Rev 2016; 30(3):223–231. doi:10.1016/j.blre.2015.12.001
- Dispenzieri A, Gertz MA, Therneau TM, Kyle RA. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc 2001; 76(5):476–487. doi:10.4065/76.5.476
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 2006; 108(8):2520–2530. doi:10.1182/blood-2006-03-001164
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78(1):21–33. doi:10.4065/78.1.21
- Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3(6):1684–1690. doi:10.2215/CJN.02290508
- Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81(12):1575–1578. doi:10.4065/81.12.1575
- Berenson JR, Anderson KC, Audell RA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol 2010; 150(1):28–38. doi:10.1111/j.1365-2141.2010.08207.x
- Mangiacavalli S, Cocito F, Pochintesta L, et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol 2013; 91(4):356–360. doi:10.1111/ejh.12172
- Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood 2010;116:2019–2025. doi:10.1182/blood-2010-04-277566
- Rosiñol L, Cibeira MT, Montoto S, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc 2007; 82(4):428–434. doi:10.4065/82.4.428
- Vanderschueren S, Mylle M, Dierickx D, et al. Monoclonal gammopathy of undetermined significance: significant beyond hematology. Mayo Clin Proc 2009; 84(9):842–845. doi:10.4065/84.9.842
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol 2007; 139(5):730–743. doi:10.1111/j.1365-2141.2007.06873.x
- Leung N, Bridoux F, Hutchison CA, et al; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012; 120(22):4292–4295. doi:10.1182/blood-2012-07-445304
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood 2013; 121(26):5124–5130. doi:10.1182/blood-2013-01-453001
- Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 2010; 375(9727):1721–1728. doi:10.1016/S0140-6736(10)60482-5
- Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106(3):812–817. doi:10.1182/blood-2005-03-1038
- Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 2015; 33(6):657–664. doi:10.1200/JCO.2014.57.9961
- Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood 2011; 117(18):4701–4705. doi:10.1182/blood-2010-10-299529
- Pompa T, Maddox M, Woodard A, et al. Cost effectiveness in low risk MGUS patients. Blood 2016; 128:2360. http://www.bloodjournal.org/content/128/22/2360. Accessed December 4, 2018.
- van de Donk NW, Palumbo A, Johnsen HE, et al; European Myeloma Network. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica 2014; 99(6):984–996. doi:10.3324/haematol.2013.100552
- International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003; 121(5):749–757. pmid:12780789
- Rajan AM, Rajkumar SV. Diagnostic evaluation of monoclonal gammopathy of undetermined significance. Eur J Haematol 2013; 91(6):561–562. doi:10.1111/ejh.12198
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br J Haematol 2006; 134(6):573–589. doi:10.1111/j.1365-2141.2006.06235.x
- Kyle RA, Durie BG, Rajkumar SV, et al; International Myeloma Working Group. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010; 24(6):1121–1127. doi:10.1038/leu.2010.60
- Bird J, Behrens J, Westin J, et al; Haemato-oncology Task Force of the British Committee for Standards in Haematology, UK Myeloma Forum and Nordic Myeloma Study Group. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M-proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 2009; 147(1):22–42. doi:10.1111/j.1365-2141.2009.07807.x
- Rajkumar SV, Kyle RA, Buadi FK. Advances in the diagnosis, classification, risk stratification, and management of monoclonal gammopathy of undetermined significance: implications for recategorizing disease entities in the presence of evolving scientific evidence. Mayo Clin Proc 2010; 85(10):945–948. doi:10.4065/mcp.2010.0520
- Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346(8):564–569. doi:10.1056/NEJMoa01133202
- Doyle LM, Gundrum JD, Farnen JP, Wright LJ, Kranig JAI, Go RS. Determining why and which clinicians order serum protein electrophoresis (SPEP), subsequent diagnoses based on indications, and clinical significance of routine follow-up: a study of patients with monoclonal gammopathy of undetermined significance (MGUS). Blood 2009; 114(22):Abstr 4883. www.bloodjournal.org/content/114/22/4883. Accessed December 4, 2018.
- Merlini G, Palladini G. Differential diagnosis of monoclonal gammopathy of undetermined significance. Hematology Am Soc Hematol Educ Program 2012; 2012:595–603. doi:10.1182/asheducation-2012.1.595
- Glavey SV, Leung N. Monoclonal gammopathy: the good, the bad and the ugly. Blood Rev 2016; 30(3):223–231. doi:10.1016/j.blre.2015.12.001
- Dispenzieri A, Gertz MA, Therneau TM, Kyle RA. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc 2001; 76(5):476–487. doi:10.4065/76.5.476
- Merlini G, Stone MJ. Dangerous small B-cell clones. Blood 2006; 108(8):2520–2530. doi:10.1182/blood-2006-03-001164
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15(12):e538–e548. doi:10.1016/S1470-2045(14)70442-5
- Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78(1):21–33. doi:10.4065/78.1.21
- Hutchison CA, Harding S, Hewins P, et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3(6):1684–1690. doi:10.2215/CJN.02290508
- Katzmann JA, Dispenzieri A, Kyle RA, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clin Proc 2006; 81(12):1575–1578. doi:10.4065/81.12.1575
- Berenson JR, Anderson KC, Audell RA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol 2010; 150(1):28–38. doi:10.1111/j.1365-2141.2010.08207.x
- Mangiacavalli S, Cocito F, Pochintesta L, et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol 2013; 91(4):356–360. doi:10.1111/ejh.12172
- Bianchi G, Kyle RA, Colby CL, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood 2010;116:2019–2025. doi:10.1182/blood-2010-04-277566
- Rosiñol L, Cibeira MT, Montoto S, et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc 2007; 82(4):428–434. doi:10.4065/82.4.428
- Vanderschueren S, Mylle M, Dierickx D, et al. Monoclonal gammopathy of undetermined significance: significant beyond hematology. Mayo Clin Proc 2009; 84(9):842–845. doi:10.4065/84.9.842
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol 2007; 139(5):730–743. doi:10.1111/j.1365-2141.2007.06873.x
- Leung N, Bridoux F, Hutchison CA, et al; International Kidney and Monoclonal Gammopathy Research Group. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012; 120(22):4292–4295. doi:10.1182/blood-2012-07-445304
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: an update for treating physicians. Blood 2013; 121(26):5124–5130. doi:10.1182/blood-2013-01-453001
- Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet 2010; 375(9727):1721–1728. doi:10.1016/S0140-6736(10)60482-5
- Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005; 106(3):812–817. doi:10.1182/blood-2005-03-1038
- Dimopoulos MA, Hillengass J, Usmani S, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 2015; 33(6):657–664. doi:10.1200/JCO.2014.57.9961
- Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood 2011; 117(18):4701–4705. doi:10.1182/blood-2010-10-299529
- Pompa T, Maddox M, Woodard A, et al. Cost effectiveness in low risk MGUS patients. Blood 2016; 128:2360. http://www.bloodjournal.org/content/128/22/2360. Accessed December 4, 2018.
KEY POINTS
- MGUS is the most common of the monoclonal gammopathies.
- The overall risk of MGUS progressing to myeloma and other lymphoproliferative disorders is 1% per year.
- Low-risk MGUS is defined by an immunoglobulin G monoclonal protein at a concentration less than 1.5 g/dL and a normal serum free light-chain ratio.
- Low-risk MGUS carries a much lower risk of progression than intermediate- and high-risk MGUS, may not require subspecialty referral, and can be followed by the outpatient provider.
Hypertension guidelines: Treat patients, not numbers
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
KEY POINTS
- The 2017 ACC/AHA guidelines lowered the definition of hypertension to 130/80 mm Hg or higher, thereby in-creasing the number of US adults with hypertension from 31.9% to 45.6%.
- For patients with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, drug treatment “is recommended” if the average blood pressure is 130/80 mm Hg or higher. For those without cardiovascular disease and at lower risk, drug treatment is recommended if the aver-age blood pressure is 140/90 mm Hg or higher.
- A treatment goal of less than 130/80 mm Hg “is recommended” for patients with hypertension and known car-diovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, and “may be reasonable” for those without additional markers of increased cardiovascular risk.
- Intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease, at the price of causing more adverse effects.