User login
LAIV4 was less effective for children than IIV against influenza A/H1N1pdm09
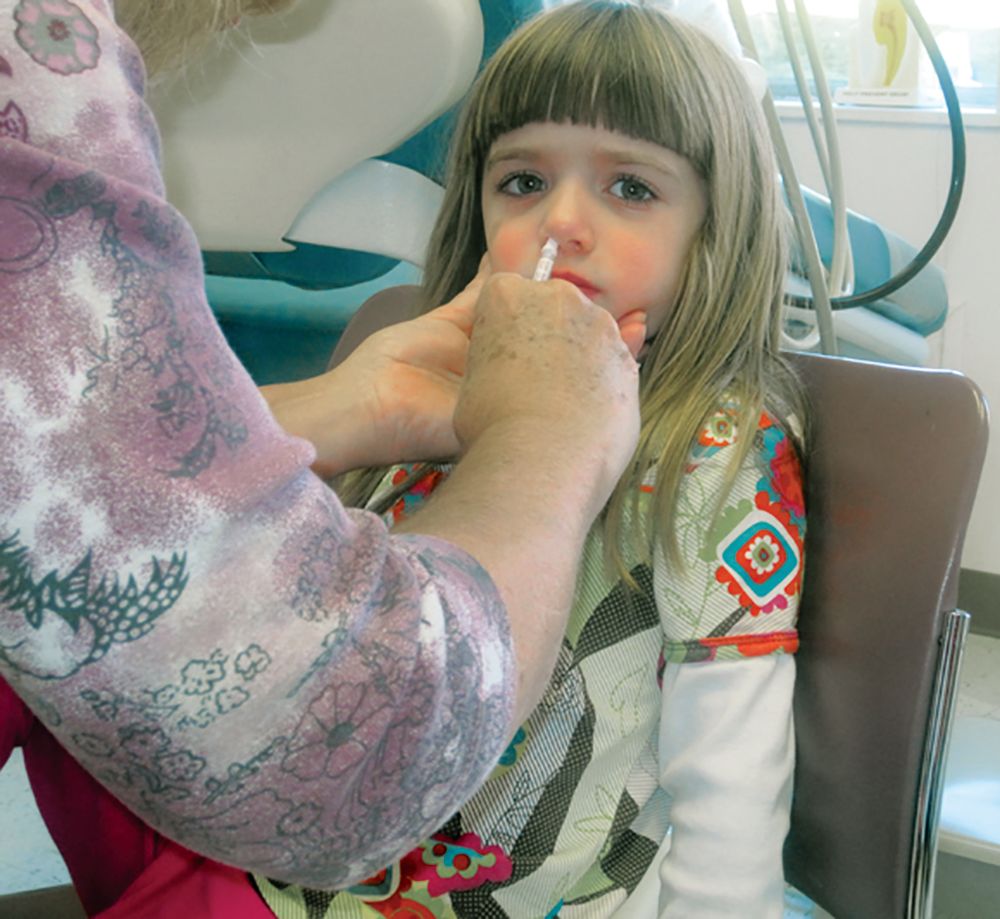
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
FROM PEDIATRICS
Key clinical point: The live attenuated influenza vaccine (LAIV4) was significantly less effective than was the inactivated influenza vaccine (IIV) for children against the influenza A/H1N1pdm09 virus across multiple flu seasons.
Major finding:
Study details: A combined analysis of five studies in the United States between the periods of 2013-2014 and 2015-2016 from the U.S. Influenza Vaccine Effectiveness Network.
Disclosures: The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
Source: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
Actionable mutations more likely in women with both breast and uterine cancer
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Women with both breast and uterine cancer are more likely to carry genetic mutations than women with either breast or uterine cancer alone, according to results of a retrospective analysis of test results.
The majority of the mutations identified were actionable, suggesting that women with breast and uterine cancer should be offered expanded genetic testing, authors of the analysis wrote in Gynecologic Oncology.
“Expanded testing for patients with breast and uterine cancer can help guide management by identifying more patients who may benefit from additional surveillance, along with at-risk family members,” said Kelly Fulk, an oncology genetic specialist at Ambry Genetics, and her coauthors.
The analysis by Ms. Fulk and her colleagues at St. Thomas Health, Nashville, and the University of California, Irvine, was based on a cohort of 52,000 women who had undergone multigene panel testing.
That cohort included 1,650 women with both breast and uterine cancer, of whom about 70% were white, and more than 94% were older than age 50 years at the time of testing. Their median age at first diagnosis was 56 years for breast cancer and 58 years for uterine cancer.
A total of 231 women with breast and uterine cancer, or 14.0%, carried at least one pathogenic mutation or likely pathogenic variant, Ms. Fulk and her colleagues reported. By comparison, mutations were seen in 9.3% of women with breast cancer only (P less than .001), 11.5% of women with uterine cancer only (P = 4.63 x 10–3), and 6.8% of women with no personal cancer history (P less than .001).
Women with both breast and uterine cancer more often had mutations in ATM, BARD1, BRCA2, MSH2, MSH6, PALB2, PMS2, and PTEN, when compared with those women with no personal cancer history, according to the report by Ms. Fulk and her coauthors.
When compared with women with just breast cancer, the women with both breast and uterine cancer more often had mutations in BRCA1, MLH1, MSH2, MSH6, PMS2 and PTEN, they added, noting that women with both cancers were twice as likely to have a BRCA1 mutation (odds ratio, 2.01; 95% confidence interval, 1.08-3.39, P = .016).
While this study had limitations, including its retrospective nature and use of genetic tests with varying numbers of genes analyzed, authors said the results nonetheless support expanded testing in women with both breast and uterine cancers to help guide therapy and cancer surveillance.
“Mutations associated with hereditary breast and ovarian cancer, Lynch syndrome, Cowden syndrome, and Li-Fraumeni syndrome have clear guidelines regarding management and surveillance for other cancers, which can benefit patients and their at-risk family members,” the investigators said.
Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics. No other disclosures were provided.
SOURCE: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
FROM GYNECOLOGIC ONCOLOGY
Key clinical point: Women with both breast and uterine cancer are more likely to carry actionable mutations than do women with breast or uterine cancer alone.
Major finding: At least one actionable mutation was seen in 14% of women with breast and uterine cancer, compared with 9.3% of women with breast cancer only, 11.5% of women with uterine cancer only, and 6.8% of women with no personal cancer history.
Study details: A retrospective analysis of a cohort of nearly 52,000 patients who underwent multigene panel testing.
Disclosures: Ms. Fulk and five coauthors reported that they are paid employees of Ambry Genetics.
Source: Fulk K et al. Gynecol Oncol. 2019 Jan 3. doi: 10.1016/j.ygyno.2018.12.021.
Appropriate use criteria for imaging in nonvalvular heart disease released
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
The American College of Cardiology, the American Heart Association, and other groups have jointly released an appropriate use criteria (AUC) document regarding the use of imaging modalities in diagnosing nonvalvular (that is, structural) heart disease.
Imaging plays an important role in diagnosing both valvular and nonvalvular heart diseases, so the goal of the document was to help clinicians provide high-quality care by standardizing the decision-making process. To do so, a committee was formed to devise scenarios that reflected situations in real-world practice; these scenarios were considered within categories to prevent the list from being too exhaustive. The scenarios were then reviewed by a rating panel in terms of how appropriate certain modalities were in each situation. The panel members first evaluated the scenarios independently then face to face as a panel before giving their final scores (from 1 to 9) independently.
For example, for the indication of nonsustained ventricular tachycardia, the panelists rated transthoracic echocardiography with or without 3-D and with contrast as needed as a 8, which means it’s an “appropriate test,” whereas they gave CT for the same indication a 3, which means “rarely appropriate.” For sustained ventricular tachycardia or ventricular fibrillation, they gave a 9 and a 6, respectively; this latter score indicates the test “may be appropriate.” These scenarios and the respective scores for any given test are organized into tables, such as initial evaluation or follow-up.
This AUC document “signals a shift from documents evaluating a single modality in various disease states to documents evaluating multiple imaging modalities and focusing on evidence and clinical experience within a given disease category,” the authors wrote. “We believe this approach better reflects clinical decision making in real-world scenarios and offers the diagnostic choices available to the clinician.”
The full document can be viewed in JACC.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Inguinal hernia recurrence rates in women lower after laparoscopic repair
according to findings published in JAMA Surgery.
In a systematic review of 43,870 female patients in 55 studies, the recurrence rate was 1.2% after laparoscopic repair, compared with 2.4% after open repair. The recurrent hernia was a femoral hernia in 40.9% of patients after open repair, compared with no recurrences after laparoscopic repair, reported Line Schmidt of the department of surgery at Herlev (Denmark) Hospital, and coauthors.
Patients were women aged 18 years and older who had repair of a primary unilateral or bilateral inguinal hernia. The review included all retrospective cohort studies, prospective cohort studies, prospective clinical trials, and retrospective cohort studies with 20 or more women with inguinal hernias. PubMed, Embase, and the Cochrane Library databases were searched. The primary outcome was recurrence rate after primary laparoscopic and open repairs, with or without mesh.
The overall recurrence rate among women was 2.6%. The overall crude recurrence rate for studies with low risk of bias was 3.9%, the authors wrote. A femoral hernia was found in 43% of reoperations, though in one study including both open and laparoscopic repairs in women, the rate of detection of incidental femoral hernia was only 2%.
The results “support the recommendation that women with inguinal hernias should undergo laparoscopic repair, unless there are concerns where an open repair might be more clinically appropriate,” the authors wrote. “A substantial number of recurrences after open repair were femoral hernias that may have been overlooked at the primary operation.”
Authors reported receiving personal fees from Bard and Merck. No other disclosures were reported.
SOURCE: Schmidt L et al. JAMA Surg. 2018;153(12):1135-42.
according to findings published in JAMA Surgery.
In a systematic review of 43,870 female patients in 55 studies, the recurrence rate was 1.2% after laparoscopic repair, compared with 2.4% after open repair. The recurrent hernia was a femoral hernia in 40.9% of patients after open repair, compared with no recurrences after laparoscopic repair, reported Line Schmidt of the department of surgery at Herlev (Denmark) Hospital, and coauthors.
Patients were women aged 18 years and older who had repair of a primary unilateral or bilateral inguinal hernia. The review included all retrospective cohort studies, prospective cohort studies, prospective clinical trials, and retrospective cohort studies with 20 or more women with inguinal hernias. PubMed, Embase, and the Cochrane Library databases were searched. The primary outcome was recurrence rate after primary laparoscopic and open repairs, with or without mesh.
The overall recurrence rate among women was 2.6%. The overall crude recurrence rate for studies with low risk of bias was 3.9%, the authors wrote. A femoral hernia was found in 43% of reoperations, though in one study including both open and laparoscopic repairs in women, the rate of detection of incidental femoral hernia was only 2%.
The results “support the recommendation that women with inguinal hernias should undergo laparoscopic repair, unless there are concerns where an open repair might be more clinically appropriate,” the authors wrote. “A substantial number of recurrences after open repair were femoral hernias that may have been overlooked at the primary operation.”
Authors reported receiving personal fees from Bard and Merck. No other disclosures were reported.
SOURCE: Schmidt L et al. JAMA Surg. 2018;153(12):1135-42.
according to findings published in JAMA Surgery.
In a systematic review of 43,870 female patients in 55 studies, the recurrence rate was 1.2% after laparoscopic repair, compared with 2.4% after open repair. The recurrent hernia was a femoral hernia in 40.9% of patients after open repair, compared with no recurrences after laparoscopic repair, reported Line Schmidt of the department of surgery at Herlev (Denmark) Hospital, and coauthors.
Patients were women aged 18 years and older who had repair of a primary unilateral or bilateral inguinal hernia. The review included all retrospective cohort studies, prospective cohort studies, prospective clinical trials, and retrospective cohort studies with 20 or more women with inguinal hernias. PubMed, Embase, and the Cochrane Library databases were searched. The primary outcome was recurrence rate after primary laparoscopic and open repairs, with or without mesh.
The overall recurrence rate among women was 2.6%. The overall crude recurrence rate for studies with low risk of bias was 3.9%, the authors wrote. A femoral hernia was found in 43% of reoperations, though in one study including both open and laparoscopic repairs in women, the rate of detection of incidental femoral hernia was only 2%.
The results “support the recommendation that women with inguinal hernias should undergo laparoscopic repair, unless there are concerns where an open repair might be more clinically appropriate,” the authors wrote. “A substantial number of recurrences after open repair were femoral hernias that may have been overlooked at the primary operation.”
Authors reported receiving personal fees from Bard and Merck. No other disclosures were reported.
SOURCE: Schmidt L et al. JAMA Surg. 2018;153(12):1135-42.
FROM JAMA SURGERY
SHM announces National Hospitalist Day
Inaugural day of recognition to honor hospital medicine care team
The Society of Hospital Medicine is proud to announce the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019. Occurring the first Thursday in March annually, National Hospitalist Day will serve to celebrate the fastest-growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
National Hospitalist Day was recently approved by the National Day Calendar and was one of approximately 30 national days to be approved for the year out of an applicant pool of more than 18,000.
“As the only society dedicated to the specialty of hospital medicine, it is appropriate that SHM spearhead a national day to recognize the countless contributions of hospitalists to health care, from clinical, academic, and leadership perspectives and more,” said Larry Wellikson, MD, MHM, chief executive officer of SHM. “We look forward to hospitalists across the nation contributing to the festivities and making this a tradition for years to come.”
In addition to celebrating hospitalists’ contributions to patient care, SHM will also be highlighting the diverse career paths of hospital medicine professionals, from frontline hospitalist physicians, nurse practitioners, and physician assistants to practice administrators, C-suite executives, and academic hospitalists.
Highlights of SHM’s campaign include the following:
- Downloadable customizable posters and assets for hospitals and individuals’ offices to celebrate their hospital medicine team, available on SHM’s website, hospitalmedicine.org.
- A series of spotlights of hospitalists at all stages of their careers in The Hospitalist, SHM’s monthly newsmagazine.
- A social media campaign inviting hospitalists and their employers to share their success stories using the hashtag #HowWeHospitalist, including banner graphics, profile photo overlays, and more.
- A social media contest to determine the most creative ways of celebrating use of the hashtag.
- A Twitter chat for hospitalists to celebrate virtually with their colleagues and peers from around the world.
“Hospitalists innovate, lead, and push the boundaries of clinical care and deserve to be recognized for their transformative contributions to health care,” said Eric E. Howell, MD, MHM, chief operating officer of SHM. “We hope this is the beginning of a long-standing tradition in honoring hospitalists and the noteworthy work they do.”
For more information, visit www.hospitalmedicine.org/hospitalistday.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Inaugural day of recognition to honor hospital medicine care team
Inaugural day of recognition to honor hospital medicine care team
The Society of Hospital Medicine is proud to announce the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019. Occurring the first Thursday in March annually, National Hospitalist Day will serve to celebrate the fastest-growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
National Hospitalist Day was recently approved by the National Day Calendar and was one of approximately 30 national days to be approved for the year out of an applicant pool of more than 18,000.
“As the only society dedicated to the specialty of hospital medicine, it is appropriate that SHM spearhead a national day to recognize the countless contributions of hospitalists to health care, from clinical, academic, and leadership perspectives and more,” said Larry Wellikson, MD, MHM, chief executive officer of SHM. “We look forward to hospitalists across the nation contributing to the festivities and making this a tradition for years to come.”
In addition to celebrating hospitalists’ contributions to patient care, SHM will also be highlighting the diverse career paths of hospital medicine professionals, from frontline hospitalist physicians, nurse practitioners, and physician assistants to practice administrators, C-suite executives, and academic hospitalists.
Highlights of SHM’s campaign include the following:
- Downloadable customizable posters and assets for hospitals and individuals’ offices to celebrate their hospital medicine team, available on SHM’s website, hospitalmedicine.org.
- A series of spotlights of hospitalists at all stages of their careers in The Hospitalist, SHM’s monthly newsmagazine.
- A social media campaign inviting hospitalists and their employers to share their success stories using the hashtag #HowWeHospitalist, including banner graphics, profile photo overlays, and more.
- A social media contest to determine the most creative ways of celebrating use of the hashtag.
- A Twitter chat for hospitalists to celebrate virtually with their colleagues and peers from around the world.
“Hospitalists innovate, lead, and push the boundaries of clinical care and deserve to be recognized for their transformative contributions to health care,” said Eric E. Howell, MD, MHM, chief operating officer of SHM. “We hope this is the beginning of a long-standing tradition in honoring hospitalists and the noteworthy work they do.”
For more information, visit www.hospitalmedicine.org/hospitalistday.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
The Society of Hospital Medicine is proud to announce the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019. Occurring the first Thursday in March annually, National Hospitalist Day will serve to celebrate the fastest-growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
National Hospitalist Day was recently approved by the National Day Calendar and was one of approximately 30 national days to be approved for the year out of an applicant pool of more than 18,000.
“As the only society dedicated to the specialty of hospital medicine, it is appropriate that SHM spearhead a national day to recognize the countless contributions of hospitalists to health care, from clinical, academic, and leadership perspectives and more,” said Larry Wellikson, MD, MHM, chief executive officer of SHM. “We look forward to hospitalists across the nation contributing to the festivities and making this a tradition for years to come.”
In addition to celebrating hospitalists’ contributions to patient care, SHM will also be highlighting the diverse career paths of hospital medicine professionals, from frontline hospitalist physicians, nurse practitioners, and physician assistants to practice administrators, C-suite executives, and academic hospitalists.
Highlights of SHM’s campaign include the following:
- Downloadable customizable posters and assets for hospitals and individuals’ offices to celebrate their hospital medicine team, available on SHM’s website, hospitalmedicine.org.
- A series of spotlights of hospitalists at all stages of their careers in The Hospitalist, SHM’s monthly newsmagazine.
- A social media campaign inviting hospitalists and their employers to share their success stories using the hashtag #HowWeHospitalist, including banner graphics, profile photo overlays, and more.
- A social media contest to determine the most creative ways of celebrating use of the hashtag.
- A Twitter chat for hospitalists to celebrate virtually with their colleagues and peers from around the world.
“Hospitalists innovate, lead, and push the boundaries of clinical care and deserve to be recognized for their transformative contributions to health care,” said Eric E. Howell, MD, MHM, chief operating officer of SHM. “We hope this is the beginning of a long-standing tradition in honoring hospitalists and the noteworthy work they do.”
For more information, visit www.hospitalmedicine.org/hospitalistday.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
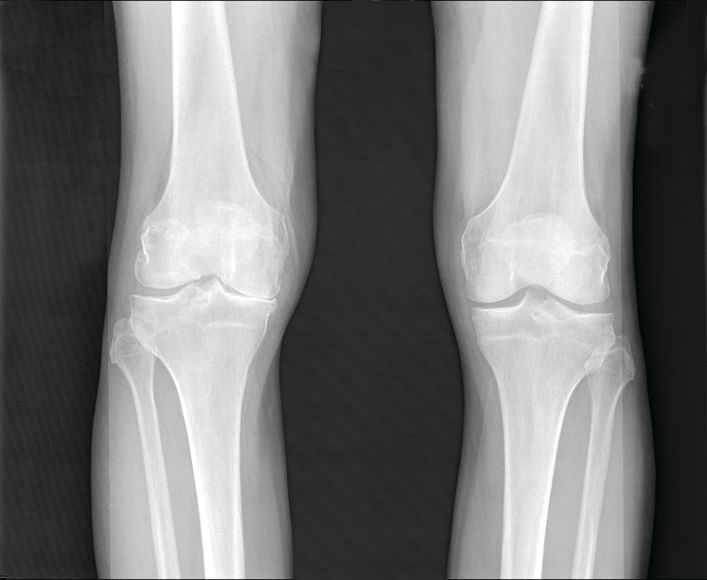
Knee pathologies, including multiple meniscal tears, predict accelerated OA
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: One year before the knee OA index visit, more than 75% of patients with accelerated knee OA had meniscal damage in at least two regions.
Study details: The data come from 375 adults with typical knee OA, accelerated knee OA, or no knee OA in the longitudinal Osteoarthritis Initiative cohort study.
Disclosures: The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
Source: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
New diabetes drugs solidify their cardiovascular and renal benefits
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
CHICAGO – When the first results from a large trial that showed profound and unexpected benefits for preventing heart failure hospitalizations associated with use of the antihyperglycemic sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin came out – a little over 3 years ago – the general reaction from clinicians was some variant of “Could this be real?”
Since then, as results from some five other large, international trials have come out showing both similar benefits from two other drugs in the same SGLT2 inhibitor class, canagliflozin and dapagliflozin, as well as results showing clear cardiovascular disease benefits from three drugs in a second class of antihyperglycemics, the glucagonlike peptide–1 receptor agonists (GLP-1 RAs), the general consensus among cardiologists became: “The cardiovascular and renal benefits are real. How can we now best use these drugs to help patients?”
This change increasingly forces cardiologists, as well as the primary care physicians who often manage patients with type 2 diabetes mellitus, to become more comfortable prescribing these two classes of antihyperglycemic drugs. During a talk at the American Heart Association scientific sessions, Eugene Braunwald, MD, arguably the top thought leader in cardiology, coined a new name for the medical subspecialty that he foresees navigating this overlap between diabetes care and cardiovascular disease prevention: diabetocardiology (although a more euphonic alternative might be cardiodiabetology, while the more comprehensive name could be cardionephrodiabetology).
“I was certainly surprised” by the first report in 2015 from the EMPA-REG OUTCOME trial (N Engl J Med. 2015 Nov 26;373[22]:2117-28), said Dr. Braunwald, who is professor of medicine at Harvard Medical School in Boston. A lot of his colleagues were surprised and said, “It’s just one trial.”
“Now we have three trials,” with the addition of the CANVAS trial for canagliflozin (N Engl J Med. 2017 Aug 17;377[7]:644-57) and the DECLARE-TIMI 58 trial (N Engl J Med. 2018 Nov 10. doi:10.1056/NEJMoa1812389) for dapagliflozin reported at the AHA meeting in November.
“We are in the midst of two pandemics: heart failure and type 2 diabetes. As cardiologists, we have to learn how to deal with this,” said Dr. Braunwald, and the evidence now clearly shows that these drugs can help with that.
As another speaker at the meeting, Javed Butler, MD, a heart failure specialist, observed in a separate talk at the meeting, “Heart failure is one of the most common, if not the most common complication, of patients with diabetes.” This tight link between heart failure and diabetes helps make cardiovascular mortality “the number one cause of death” in patients with diabetes, said Dr. Butler, professor and chairman of medicine at the University of Mississippi in Jackson.
“Thanks to the cardiovascular outcome trials, we now have a much broader and deeper appreciation of heart failure and renal disease as integral components of the cardiovascular-renal spectrum in people with diabetes,” said Subodh Verma, MD, a professor at the University of Toronto and cardiac surgeon at St. Michael’s Hospital in Toronto. Dr. Braunwald spelled out in his talk some of the interrelationships of diabetes, heart failure, and renal dysfunction that together produce a downward-spiraling vicious circle for patients, a pathophysiological process that clinicians can now short-circuit by treatment with a SGLT2 inhibitor.
Cardiovascular outcome trials show the way
In the context of antihyperglycemic drugs, the “cardiovascular outcome trials” refers to a series of large trials mandated by the Food and Drug Administration in 2008 to assess the cardiovascular disease effects of new agents coming onto the U.S. market to treat type 2 diabetes mellitus (T2DM). By the time Dr. Verma spoke at the AHA meeting, he could cite reported results from 12 of these trials: 5 different drugs in the GLP-1 RA class, 4 drugs in the dipeptidyl peptidase-4 (DPP-4) inhibitor class, and 3 drugs from the SGLT2 inhibitor class. Dr. Verma summed what the findings have shown.
The four tested DDP-4 inhibitors (alogliptin, linagliptin, saxagliptin, and sitagliptin) consistently showed neutrality for the primary outcome of major adverse cardiovascular disease events (MACE), constituted by cardiovascular disease death, MI, or stroke.
The five tested GLP-1 RAs (albiglutide, exenatide, liraglutide, lixisenatide, and semaglutide) showed a mixed pattern of MACE results that seemed to be linked with the subclass the drug fell into. The two exedin-4–based drugs, exenatide and lixisenatide, each showed a statistically neutral effect for MACE, as well as collectively in a combined analysis. In contrast, three human GLP-1–based drugs, albiglutide, liraglutide, and semaglutide, each showed a consistent, statistically-significant MACE reduction in their respective outcome trials, and collectively they showed a highly significant 18% reduction in MACE, compared with placebo, Dr. Verma said. Further, recent analysis by Dr. Verma that used data from liraglutide treatment in the LEADER trial showed the MACE benefit occurred only among enrolled patients treated with liraglutide who had established atherosclerotic cardiovascular disease (ASCVD). Patients enrolled in the trial with only multiple risk factors (in addition to having T2DM) but without established ASCVD showed no significant benefit from liraglutide treatment for the MACE endpoint, compared with control patients.
Recently a press-release announcement of results from a sixth GLP-1 RA, dulaglutide, in the REWIND trial of MACE outcomes suggested that a drug in this class could have broader effect. The majority, 69%, of the 9,901 patients with T2DM enrolled in REWIND had risk factors but not established ASCVD at enrollment. A Nov. 5, 2018, statement from the company developing this drug, Lilly, reported that the study overall produced a statistically significant reduction in MACE, although it provided no additional details. As the released noted, this made REWIND the first trial to show a MACE benefit from a drug in the GLP-1 RA class in patients without established ASCVD.
The MACE outcome results from the three SGLT2 inhibitor trials showed a similar pattern as liraglutide: In patients with established ASCVD, the drugs individually each produced a MACE reduction, although dapagliflozin just missed having a statistically significant reduction. Collectively, the three drugs showed a statistically significant, 14% relative risk reduction for MACE, compared with control patients. But among patients with multiple risk factors only, but without established ASCVD, included in two of the three trials (CANVAS and DECLARE-TIMI 58), the results showed both individually and collectively a neutral MACE effect.
But unlike the other antihyperglycemic drugs tested in the cardiovascular outcome trials, the SGLT2 inhibitors have shown two additional, highly important secondary outcomes: a consistent reduction in hospitalization for heart failure and a consistent reduction in renal-disease progression.
A meta-analysis of the three SGLT2 inhibitor trials published coincident with the release of the DECLARE-TIMI 58 results showed that, for the outcome of either cardiovascular death or hospitalization for heart failure, the SGLT2 inhibitors collectively showed a significant 29% relative decrease in this incidence among patients with a history of heart failure, and a significant 21% relative decrease among patients without history of heart failure (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32590-X). Among the subset of patients with established ASCVD, treatment with a SGLT2 inhibitor across all three trials showed a significant 16% relative risk reduction, and in the subset with multiple risk factors but no established ASCVD, the two SGLT2 inhibitors collectively produced a 16% relative cut in cardiovascular death or heart failure hospitalization with a P value of .06. Finally, the Lancet meta-analysis showed that, for a combined endpoint that reflected renal worsening, the SGLT2 inhibitors showed a significant relative reduction of about 45% in both the subgroup of patients with established ASCVD and in the subgroup of those with just risk factors.
“This is a big step forward for patients with multiple risk factors and diabetes but without ASCVD, that both renal disease and hospitalization for heart failure are sensitive” to the SGLT2 inhibitors, Dr. Verma noted. “We see renal protection and reduction of heart failure hospitalization across both primary and secondary prevention patients, with no need to distinguish them based on ASCVD.” In contrast, he noted, the MACE benefit from the SGLT2 inhibitors seems limited to patients with ASCVD. The day before making this point in a talk during the meeting, Dr. Verma had published the same message in a commentary (Lancet. 2018 Nov 10. doi: 10.1016/S0140-6736(18)32824-1).
Although the “nomenclature of primary versus secondary prevention is appropriate for atherosclerotic outcomes, it is likely to be inappropriate for a person with type 2 diabetes who is at risk of hospitalization for heart failure and renal disease,” Dr. Verma wrote with his associates in the commentary.
What it means for clinicians
The upshot of all of these cardiovascular outcome trial results that came out over the past 3 years has been a new appreciation of how antihyperglycemic drugs can have cardiovascular and renal benefits that transcend their effects on glycemia. The evidence has put the SGLT2 inhibitors and GLP-1 RAs on track to challenge, and potentially displace, metformin as the top drug to prescribe for patients with T2DM.
Clinicians should realize that they should prescribe SGLT2 inhibitors and selected GLP-1 RAs “as early as metformin in patients with established ASCVD,” said Dr. Verma. “For patients with recalcitrant atherosclerotic disease and a history of MI and ischemia, I’d primarily treat with a GLP-1 RA. In a patient with left ventricular dysfunction or evidence of heart failure, I’d use an SGLT2 inhibitor. But it’s not a fight between these two. You could treat a patients with type 2 diabetes with both classes,” although the practicality of this approach is limited by the high cost of these drugs.
The SGLT2 inhibitors “should now be considered as first-line therapy after metformin in most people with type 2 diabetes, irrespective of whether or not they have established atherosclerotic vascular disease, chronic kidney disease, or heart failure,” he and his associates wrote in their recent commentary.
“What I struggle with the most is how we prioritize and individualize secondary-prevention therapies based on risk for ischemia and heart failure. Some therapies [the SGLT2 inhibitors] are predominantly for heart failure prevention, and some [the GLP-1 RAs] are primarily for ischemia. How do we choose when a patient cannot afford to take both? Does a combination of a SGLT2 inhibitor and a GLP-1 RA offer the greatest CVD benefit? We need to test this in a trial. And will metformin be displaced as first-line treatment?” Dr. Verma asked.
“The day will probably come when, for maximal protection, you treat with both classes. But right now we’re forced to choose because of the cost,” said John McMurray, MD, professor of cardiology at the University of Glasgow, in a talk during the meeting.
As to specifically which SGLT2 inhibitor to prescribe, “they all look pretty much the same” in the newly published meta-analysis, Dr. McMurray said, although he noted that safety differences among agents in the class remain possible.
“For patients similar to those studied in the three SGLT2 inhibitor trials, clinicians should use one of these drugs to reduce the risk for incident heart failure, irrespective of their effect on MACE,” said Dr. Butler. Reducing the risk for incident heart failure and of progressive renal dysfunction are two new goals for antihyperglycemic therapy that now overlay the long-standing goals of controlling glycemia and reducing cardiovascular disease risk and the more recent goals of cutting cardiovascular disease mortality and cutting the risk for a MACE event.
A current limitation for practice is that the none of the three drug companies that market the tested SGLT2 inhibitor drugs has sought regulatory approval for an indication of reducing the risk for heart failure hospitalization. Despite that, “these drugs should be used for renal protection and reducing heart failure hospitalizations,” Dr. Butler said. “We need to start thinking about this and not get lost thinking about only their MACE effect because, when you focus on MACE, there is a competition between the SGLT2 inhibitors and the GLP-1 RA. If we think of GLP-1 RAs as drugs to prevent MACE, and SGLT2 inhibitors as drugs that primarily prevent heart failure and renal dysfunction, then there is no competition. Perhaps combined treatment is where we need to go,” he said in an interview.
But the enthusiasm that experts like Dr. Butler, Dr. McMurray, and Dr. Verma have for wider use of both classes of drugs in appropriate patients is not necessarily matched right now among many community physicians. Cardiologist David J. Becker, MD, is an example of the clinicians who appreciate the growing evidence that supports wider use of these antihyperglycemic drugs but remain uneasy about applying this evidence in their practice.
Dr. Becker, associate director of the Preventive and Integrative Heart Health Program of the Temple Heart and Vascular Institute in Philadelphia, writes a column for the Philadelphia Inquirer on medical care. In a December 2018 piece, he said “like most cardiologists, I ‘don’t do diabetes’ – because it’s not my expertise. The new drugs, however, mean I need to learn more” about treating these patients. “The problem: There are so many of these medications that they present a bewildering choice for patients and doctors.”
Dr. Becker cited several barriers he sees for himself and his nonendocrinologist colleagues to prescribe these drugs – and for patients to take them:
- High cost, with prices that run close to $20/day for each medication.
- A thicket of names and choices that “lead to confusion and paralysis,” which has been exacerbated by “advertising wars” among competing drug companies.
- Cardiologists and primary care physicians usually defer to endocrinologists to prescribe these drugs, but most patients with T2DM aren’t seen by endocrinologists. The result: “Few doctors prescribe them.”
The cardiovascular disease benefits of these drugs have not been adequately promoted. Until that changes, “cardiologists like me will not realize their importance,” Dr. Becker concluded.
While christening the new diabetocardiology subspecialty, Dr. Braunwald placed the onus for managing this emerging facet of diabetes largely outside the scope of endocrinology.
“We can’t call in a consultant every time we have a patient with diabetes; it would bankrupt the system,” he said. Training of cardiologists now needs to include several months of treating patients with diabetes, Dr. Braunwald advised, just like 30 or so years ago when cardiologists like himself had to become more familiar with blood clotting to better manage thrombotic disease.
Dr. Braunwald has been a consultant to Cardurion, Myokardia, and Sanofi; an advisor to Endcardia; and has received research funding from AstraZeneca, Daiishi Sankyo, and Novartis. Dr. Butler has been a consultant or advisor to AstraZeneca, Amgen, Bayer, Boehringer Ingelheim, Janssen, Merck, Novartis, Novo Nordisk, and Sanofi. Dr. Verma has received honoraria and research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, NovoNordisk, Sanofi, and Valeant. Dr. McMurray has received research funding from 12 companies. Dr. Becker had no disclosures.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Ask about family military service, says new AAP guidance
Children in military families face unique challenges and stressors related to deployment and frequent relocation. However, these children also have access to an array of services of which civilian pediatricians and other care providers may be unaware.
New guidance from the American Academy of Pediatrics’ Section on Uniformed Services details needs, challenges, and opportunities for military-connected children and points clinicians to resources for these families.
For clinicians who care for military-connected children, key suggestions outlined in the report include attainment of cultural competency; this begins with simply asking about the military status of families, wrote Cmdr. Chadley R. Huebner, MD, MPH, the lead author of the report published in Pediatrics.
A Veteran’s Affairs Community Provider Toolkit gives good grounding in military culture, he added.
The behavioral and emotional screening recommended by the AAP as part of routine pediatric practice also will provide clinicians with valuable information to help guide care of military-connected children; asking about prior or current parental military service and deployment also will help guide care.
Up to half of children in military families receive care in civilian settings, according to Dr. Huebner. “Many children in military families live in settings remote from a military community, and civilian health providers are faced with caring for military children in their practices.”
Dr. Huebner, an active duty commander in the U.S. Navy, led the revision of a 2013 report on the health and mental health needs of children in U.S. military families.
The broad category of military-connected children includes not just the estimated 1.3 million children of active duty service members, but also children of 818,000 National Guard and Reserve members and more than 2 million military retirees. All told, about 4 million children are in military-connected families, with about a third of these aged 5 years or younger.
In an era where the United States has been involved in multiple conflicts, deployment is the best-known stressor for military families. “The stressors associated with deployment, including prolonged family separation, potential injury or death of a service member, and traumatic experiences, can have a cumulative negative effect on the entire family unit,” wrote Dr. Huebner.
Even in young children aged 8 years and under, mental and behavioral health visits increase during deployment; older children experience more psychosocial morbidity as parental stress increases, an effect that can be mitigated by military support systems. Much existing research focuses on “the immediate effects of wartime deployment, and more longitudinal studies are needed to assess the long-term effects,” he wrote.
Still, neglect and child maltreatment increase in military families that have experienced deployment, with the risk increasing at the time of redeployment.
In addition to the known family stresses of deployment, children in military families face frequent relocation, with transitions occurring every 2-4 years and an average of nine schools attended by high school graduation, according to government data cited by Dr. Huebner.
Relocation within the past year is associated with increased use of mental health services, and adolescents in this group saw more psychiatric hospitalizations and ED visits. However, increased resilience among children in military families has been seen in some studies, with frequent relocation associated with fewer problems in school and a positive attitude about the changes associated with moves.
And families turn to each other for support with frequent moves. “Because families often move away from extended family support, they often refer to the military community as a surrogate family that provides a support network,” wrote Dr. Huebner.
Reservists don’t relocate as frequently as active duty service members but are more likely to live in areas without military resources, and their children’s peers, teachers, and caregivers may not be aware of the special challenges of military life. Similar isolation may occur when veterans make the transition to civilian life, with changes in eligibility for and access to military services and benefits.
Military programs can help
Military families, their children, and care providers and educators can turn to the military for help in many areas, whether families are receiving mental and physical health care through the military or from civilian facilities.
A key resource for neglect and abuse prevention is the military’s Family Advocacy Program (FAP), which engages families by means of workshops and other support programs. When child maltreatment is alleged, FAP also conducts its own investigation, so health care professionals should include the local FAP office in the reporting process when there are concerns.
For new parents, home visits and other support programs are available through the New Parent Support program, which will connect families to resources within the community and the Department of Defense (DOD).
Families living near or on military facilities may access DOD-sponsored infant and preschool child development programs, as well as school-aged care programs; subsidies for civilian childcare are also available. Although these programs constitute the country’s largest employer-sponsored childcare program, they serve just a small minority of military families, noted Dr. Huebner, citing a 2008 study by RAND.
DOD schools are attended by 72,000 students, but DOD resources stretch into civilian schools: School liaison offices assist civilian schools and military families located near military installations, and grant funding helps the DOD partner with civilian schools serving military-connected children.
Turning to health care, the military health system provides care globally to service members, retirees, and their families. Tricare is a single-payer, government-managed insurance program that is managed through regional contracts; some care is also delivered through the centralized Military Health System.
Whether Tricare participants receive care at military facilities or from civilian network providers, they generally do not have out-of-pocket costs unless they enroll in the Tricare Select program, a fee-for-service plan that involved cost sharing with deductibles. A link to information about how to connect patients to a Tricare provider or how to become on is available in the full report in Pediatrics.
About 20% of military-connected children have special health care needs and may receive specialty care through civilian providers. To help these families navigate multiple systems of care, the DOD provides a publication called Special Needs Tool Kit: Birth to 18. This toolkit guides families through early intervention and special education, and also provides military-specific information about relocation, Tricare benefits, and military support services.
A program available to all family members with special education or chronic medical needs is the Exceptional Family Members Program (EFMP). Children with autism spectrum disorders and ADHD, for example, are eligible for EFMP enrollment.
Additional supplemental benefits, with rank-adjusted sliding fees, are available for children with serious developmental and physical problems; children with autism spectrum disorders are eligible for additional therapy through an autism care demonstration program.
Forms to document chronic medical conditions (DD Form 2792) and special educational needs, if needed (DD Form 2791-1), are required for EFMP enrollment, which is mandatory for children of active duty personnel. Guidance for completing the forms can be found at www.militaryonesource.mil.
When overseas posts are imminent, clinicians should know that certain medical conditions may disqualify children from accompanying their service member parent. Overseas screening coordinators within the military medical system serve as the point of contact for the family and pediatrician in such circumstances, and clinicians can help families by providing appropriate documentation early in the process.
In addition to attaining military cultural competence, being aware of resources available to military families, and working closely with school personnel to support military-connected children, local and national advocacy efforts can make a difference, noted Dr. Huebner. And in all cases, “health care professional, schools, and communities should proactively reach out to military families.”
Dr. Huebner reported no conflicts of interest and no outside sources of funding. The full report contains hyperlinks to all resources named.
SOURCE: Huebner CR. Pediatrics. 2019;143(1):e20183258.
Children in military families face unique challenges and stressors related to deployment and frequent relocation. However, these children also have access to an array of services of which civilian pediatricians and other care providers may be unaware.
New guidance from the American Academy of Pediatrics’ Section on Uniformed Services details needs, challenges, and opportunities for military-connected children and points clinicians to resources for these families.
For clinicians who care for military-connected children, key suggestions outlined in the report include attainment of cultural competency; this begins with simply asking about the military status of families, wrote Cmdr. Chadley R. Huebner, MD, MPH, the lead author of the report published in Pediatrics.
A Veteran’s Affairs Community Provider Toolkit gives good grounding in military culture, he added.
The behavioral and emotional screening recommended by the AAP as part of routine pediatric practice also will provide clinicians with valuable information to help guide care of military-connected children; asking about prior or current parental military service and deployment also will help guide care.
Up to half of children in military families receive care in civilian settings, according to Dr. Huebner. “Many children in military families live in settings remote from a military community, and civilian health providers are faced with caring for military children in their practices.”
Dr. Huebner, an active duty commander in the U.S. Navy, led the revision of a 2013 report on the health and mental health needs of children in U.S. military families.
The broad category of military-connected children includes not just the estimated 1.3 million children of active duty service members, but also children of 818,000 National Guard and Reserve members and more than 2 million military retirees. All told, about 4 million children are in military-connected families, with about a third of these aged 5 years or younger.
In an era where the United States has been involved in multiple conflicts, deployment is the best-known stressor for military families. “The stressors associated with deployment, including prolonged family separation, potential injury or death of a service member, and traumatic experiences, can have a cumulative negative effect on the entire family unit,” wrote Dr. Huebner.
Even in young children aged 8 years and under, mental and behavioral health visits increase during deployment; older children experience more psychosocial morbidity as parental stress increases, an effect that can be mitigated by military support systems. Much existing research focuses on “the immediate effects of wartime deployment, and more longitudinal studies are needed to assess the long-term effects,” he wrote.
Still, neglect and child maltreatment increase in military families that have experienced deployment, with the risk increasing at the time of redeployment.
In addition to the known family stresses of deployment, children in military families face frequent relocation, with transitions occurring every 2-4 years and an average of nine schools attended by high school graduation, according to government data cited by Dr. Huebner.
Relocation within the past year is associated with increased use of mental health services, and adolescents in this group saw more psychiatric hospitalizations and ED visits. However, increased resilience among children in military families has been seen in some studies, with frequent relocation associated with fewer problems in school and a positive attitude about the changes associated with moves.
And families turn to each other for support with frequent moves. “Because families often move away from extended family support, they often refer to the military community as a surrogate family that provides a support network,” wrote Dr. Huebner.
Reservists don’t relocate as frequently as active duty service members but are more likely to live in areas without military resources, and their children’s peers, teachers, and caregivers may not be aware of the special challenges of military life. Similar isolation may occur when veterans make the transition to civilian life, with changes in eligibility for and access to military services and benefits.
Military programs can help
Military families, their children, and care providers and educators can turn to the military for help in many areas, whether families are receiving mental and physical health care through the military or from civilian facilities.
A key resource for neglect and abuse prevention is the military’s Family Advocacy Program (FAP), which engages families by means of workshops and other support programs. When child maltreatment is alleged, FAP also conducts its own investigation, so health care professionals should include the local FAP office in the reporting process when there are concerns.
For new parents, home visits and other support programs are available through the New Parent Support program, which will connect families to resources within the community and the Department of Defense (DOD).
Families living near or on military facilities may access DOD-sponsored infant and preschool child development programs, as well as school-aged care programs; subsidies for civilian childcare are also available. Although these programs constitute the country’s largest employer-sponsored childcare program, they serve just a small minority of military families, noted Dr. Huebner, citing a 2008 study by RAND.
DOD schools are attended by 72,000 students, but DOD resources stretch into civilian schools: School liaison offices assist civilian schools and military families located near military installations, and grant funding helps the DOD partner with civilian schools serving military-connected children.
Turning to health care, the military health system provides care globally to service members, retirees, and their families. Tricare is a single-payer, government-managed insurance program that is managed through regional contracts; some care is also delivered through the centralized Military Health System.
Whether Tricare participants receive care at military facilities or from civilian network providers, they generally do not have out-of-pocket costs unless they enroll in the Tricare Select program, a fee-for-service plan that involved cost sharing with deductibles. A link to information about how to connect patients to a Tricare provider or how to become on is available in the full report in Pediatrics.
About 20% of military-connected children have special health care needs and may receive specialty care through civilian providers. To help these families navigate multiple systems of care, the DOD provides a publication called Special Needs Tool Kit: Birth to 18. This toolkit guides families through early intervention and special education, and also provides military-specific information about relocation, Tricare benefits, and military support services.
A program available to all family members with special education or chronic medical needs is the Exceptional Family Members Program (EFMP). Children with autism spectrum disorders and ADHD, for example, are eligible for EFMP enrollment.
Additional supplemental benefits, with rank-adjusted sliding fees, are available for children with serious developmental and physical problems; children with autism spectrum disorders are eligible for additional therapy through an autism care demonstration program.
Forms to document chronic medical conditions (DD Form 2792) and special educational needs, if needed (DD Form 2791-1), are required for EFMP enrollment, which is mandatory for children of active duty personnel. Guidance for completing the forms can be found at www.militaryonesource.mil.
When overseas posts are imminent, clinicians should know that certain medical conditions may disqualify children from accompanying their service member parent. Overseas screening coordinators within the military medical system serve as the point of contact for the family and pediatrician in such circumstances, and clinicians can help families by providing appropriate documentation early in the process.
In addition to attaining military cultural competence, being aware of resources available to military families, and working closely with school personnel to support military-connected children, local and national advocacy efforts can make a difference, noted Dr. Huebner. And in all cases, “health care professional, schools, and communities should proactively reach out to military families.”
Dr. Huebner reported no conflicts of interest and no outside sources of funding. The full report contains hyperlinks to all resources named.
SOURCE: Huebner CR. Pediatrics. 2019;143(1):e20183258.
Children in military families face unique challenges and stressors related to deployment and frequent relocation. However, these children also have access to an array of services of which civilian pediatricians and other care providers may be unaware.
New guidance from the American Academy of Pediatrics’ Section on Uniformed Services details needs, challenges, and opportunities for military-connected children and points clinicians to resources for these families.
For clinicians who care for military-connected children, key suggestions outlined in the report include attainment of cultural competency; this begins with simply asking about the military status of families, wrote Cmdr. Chadley R. Huebner, MD, MPH, the lead author of the report published in Pediatrics.
A Veteran’s Affairs Community Provider Toolkit gives good grounding in military culture, he added.
The behavioral and emotional screening recommended by the AAP as part of routine pediatric practice also will provide clinicians with valuable information to help guide care of military-connected children; asking about prior or current parental military service and deployment also will help guide care.
Up to half of children in military families receive care in civilian settings, according to Dr. Huebner. “Many children in military families live in settings remote from a military community, and civilian health providers are faced with caring for military children in their practices.”
Dr. Huebner, an active duty commander in the U.S. Navy, led the revision of a 2013 report on the health and mental health needs of children in U.S. military families.
The broad category of military-connected children includes not just the estimated 1.3 million children of active duty service members, but also children of 818,000 National Guard and Reserve members and more than 2 million military retirees. All told, about 4 million children are in military-connected families, with about a third of these aged 5 years or younger.
In an era where the United States has been involved in multiple conflicts, deployment is the best-known stressor for military families. “The stressors associated with deployment, including prolonged family separation, potential injury or death of a service member, and traumatic experiences, can have a cumulative negative effect on the entire family unit,” wrote Dr. Huebner.
Even in young children aged 8 years and under, mental and behavioral health visits increase during deployment; older children experience more psychosocial morbidity as parental stress increases, an effect that can be mitigated by military support systems. Much existing research focuses on “the immediate effects of wartime deployment, and more longitudinal studies are needed to assess the long-term effects,” he wrote.
Still, neglect and child maltreatment increase in military families that have experienced deployment, with the risk increasing at the time of redeployment.
In addition to the known family stresses of deployment, children in military families face frequent relocation, with transitions occurring every 2-4 years and an average of nine schools attended by high school graduation, according to government data cited by Dr. Huebner.
Relocation within the past year is associated with increased use of mental health services, and adolescents in this group saw more psychiatric hospitalizations and ED visits. However, increased resilience among children in military families has been seen in some studies, with frequent relocation associated with fewer problems in school and a positive attitude about the changes associated with moves.
And families turn to each other for support with frequent moves. “Because families often move away from extended family support, they often refer to the military community as a surrogate family that provides a support network,” wrote Dr. Huebner.
Reservists don’t relocate as frequently as active duty service members but are more likely to live in areas without military resources, and their children’s peers, teachers, and caregivers may not be aware of the special challenges of military life. Similar isolation may occur when veterans make the transition to civilian life, with changes in eligibility for and access to military services and benefits.
Military programs can help
Military families, their children, and care providers and educators can turn to the military for help in many areas, whether families are receiving mental and physical health care through the military or from civilian facilities.
A key resource for neglect and abuse prevention is the military’s Family Advocacy Program (FAP), which engages families by means of workshops and other support programs. When child maltreatment is alleged, FAP also conducts its own investigation, so health care professionals should include the local FAP office in the reporting process when there are concerns.
For new parents, home visits and other support programs are available through the New Parent Support program, which will connect families to resources within the community and the Department of Defense (DOD).
Families living near or on military facilities may access DOD-sponsored infant and preschool child development programs, as well as school-aged care programs; subsidies for civilian childcare are also available. Although these programs constitute the country’s largest employer-sponsored childcare program, they serve just a small minority of military families, noted Dr. Huebner, citing a 2008 study by RAND.
DOD schools are attended by 72,000 students, but DOD resources stretch into civilian schools: School liaison offices assist civilian schools and military families located near military installations, and grant funding helps the DOD partner with civilian schools serving military-connected children.
Turning to health care, the military health system provides care globally to service members, retirees, and their families. Tricare is a single-payer, government-managed insurance program that is managed through regional contracts; some care is also delivered through the centralized Military Health System.
Whether Tricare participants receive care at military facilities or from civilian network providers, they generally do not have out-of-pocket costs unless they enroll in the Tricare Select program, a fee-for-service plan that involved cost sharing with deductibles. A link to information about how to connect patients to a Tricare provider or how to become on is available in the full report in Pediatrics.
About 20% of military-connected children have special health care needs and may receive specialty care through civilian providers. To help these families navigate multiple systems of care, the DOD provides a publication called Special Needs Tool Kit: Birth to 18. This toolkit guides families through early intervention and special education, and also provides military-specific information about relocation, Tricare benefits, and military support services.
A program available to all family members with special education or chronic medical needs is the Exceptional Family Members Program (EFMP). Children with autism spectrum disorders and ADHD, for example, are eligible for EFMP enrollment.
Additional supplemental benefits, with rank-adjusted sliding fees, are available for children with serious developmental and physical problems; children with autism spectrum disorders are eligible for additional therapy through an autism care demonstration program.
Forms to document chronic medical conditions (DD Form 2792) and special educational needs, if needed (DD Form 2791-1), are required for EFMP enrollment, which is mandatory for children of active duty personnel. Guidance for completing the forms can be found at www.militaryonesource.mil.
When overseas posts are imminent, clinicians should know that certain medical conditions may disqualify children from accompanying their service member parent. Overseas screening coordinators within the military medical system serve as the point of contact for the family and pediatrician in such circumstances, and clinicians can help families by providing appropriate documentation early in the process.
In addition to attaining military cultural competence, being aware of resources available to military families, and working closely with school personnel to support military-connected children, local and national advocacy efforts can make a difference, noted Dr. Huebner. And in all cases, “health care professional, schools, and communities should proactively reach out to military families.”
Dr. Huebner reported no conflicts of interest and no outside sources of funding. The full report contains hyperlinks to all resources named.
SOURCE: Huebner CR. Pediatrics. 2019;143(1):e20183258.
FROM PEDIATRICS
Quincy the (diabetic) koala leaves behind more than memories
SAN DIEGO – A miracle of marsupial medicine is no more.
An endocrinologist is no longer checking his blood sugar levels on her smartphone a couple times a day, and zookeepers have stopped responding to glucose alerts by preparing tiny doses of insulin. But Quincy, the recipient of a continuous glucose monitor, has provided valuable insight that may benefit a variety of creatures beyond our furry, eucalyptus-eating cousins.
“Through this experience, I am hopeful that we’ll be able to offer better treatment in the future for any animals that are found to have diabetes,” the endocrinologist, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, said in an interview.
And, she added, the experience of working with Quincy “provided an indication of where remote management of diabetes is going for the future, whether this is humans or animals.”
Quincy, a Queensland koala, reportedly died at the San Diego Zoo on Dec. 13 of pneumonia at the age of about 3 years. (Koalas can live into their teens.)
It’s not clear if his death was related to his diabetes. Dr. Philis-Tsimikas said. “Although infection can worsen with poor glucose control, my understanding from the veterinarian was that his diabetes had stabilized and was being successfully treated with a small dose of daily basal insulin,” she said. “He was not having wide fluctuations in glucose control, and the CGM had been removed to make it easier for him to get around his enclosures.”
Nine months before his death, Quincy was diagnosed with type 1 diabetes and transferred from the Los Angeles Zoo for medical reasons. Last June, after veterinarians consulted with Dr. Philis-Tsimikas, Quincy underwent an operation to fit him with a CGM so zookeepers could avoid having to wake him multiple times a day for skin pricks.
Koalas are among many species that can develop the equivalent of human diabetes. Dogs, cats, pigs, apes, horses, and even dolphins can become diabetic.
“The providers and caretakers could all respond with appropriate interventions based on the real-time readings. Improved treatment decisions were made despite not having any verbal communication,” Dr. Philis-Tsimikas said.
“I found it amazing that the CGM device could be placed on such a small body with very little subcutaneous fat,” she said. “It stayed in place and functioned successfully despite movement of the koala around his enclosure.”
In light of his small body and lack of body fat, could Quincy’s experience offer insight into the use of CGM technology in fragile humans such as babies and the elderly? Absolutely, Dr. Philis-Tsimikas said, noting that babies have been diagnosed with diabetes at as young as 9 months.
She said Quincy’s story, which got extensive media attention, provided another benefit. “His story was very relatable to many people with newly diagnosed type 1 diabetes and how difficult it can be to manage the highs and lows,” she said. “Quincy helped show us how this could be addressed with the new technology of a CGM and new types of basal insulin and pens that deliver half units.”
Dr. Philis-Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk.
SAN DIEGO – A miracle of marsupial medicine is no more.
An endocrinologist is no longer checking his blood sugar levels on her smartphone a couple times a day, and zookeepers have stopped responding to glucose alerts by preparing tiny doses of insulin. But Quincy, the recipient of a continuous glucose monitor, has provided valuable insight that may benefit a variety of creatures beyond our furry, eucalyptus-eating cousins.
“Through this experience, I am hopeful that we’ll be able to offer better treatment in the future for any animals that are found to have diabetes,” the endocrinologist, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, said in an interview.
And, she added, the experience of working with Quincy “provided an indication of where remote management of diabetes is going for the future, whether this is humans or animals.”
Quincy, a Queensland koala, reportedly died at the San Diego Zoo on Dec. 13 of pneumonia at the age of about 3 years. (Koalas can live into their teens.)
It’s not clear if his death was related to his diabetes. Dr. Philis-Tsimikas said. “Although infection can worsen with poor glucose control, my understanding from the veterinarian was that his diabetes had stabilized and was being successfully treated with a small dose of daily basal insulin,” she said. “He was not having wide fluctuations in glucose control, and the CGM had been removed to make it easier for him to get around his enclosures.”
Nine months before his death, Quincy was diagnosed with type 1 diabetes and transferred from the Los Angeles Zoo for medical reasons. Last June, after veterinarians consulted with Dr. Philis-Tsimikas, Quincy underwent an operation to fit him with a CGM so zookeepers could avoid having to wake him multiple times a day for skin pricks.
Koalas are among many species that can develop the equivalent of human diabetes. Dogs, cats, pigs, apes, horses, and even dolphins can become diabetic.
“The providers and caretakers could all respond with appropriate interventions based on the real-time readings. Improved treatment decisions were made despite not having any verbal communication,” Dr. Philis-Tsimikas said.
“I found it amazing that the CGM device could be placed on such a small body with very little subcutaneous fat,” she said. “It stayed in place and functioned successfully despite movement of the koala around his enclosure.”
In light of his small body and lack of body fat, could Quincy’s experience offer insight into the use of CGM technology in fragile humans such as babies and the elderly? Absolutely, Dr. Philis-Tsimikas said, noting that babies have been diagnosed with diabetes at as young as 9 months.
She said Quincy’s story, which got extensive media attention, provided another benefit. “His story was very relatable to many people with newly diagnosed type 1 diabetes and how difficult it can be to manage the highs and lows,” she said. “Quincy helped show us how this could be addressed with the new technology of a CGM and new types of basal insulin and pens that deliver half units.”
Dr. Philis-Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk.
SAN DIEGO – A miracle of marsupial medicine is no more.
An endocrinologist is no longer checking his blood sugar levels on her smartphone a couple times a day, and zookeepers have stopped responding to glucose alerts by preparing tiny doses of insulin. But Quincy, the recipient of a continuous glucose monitor, has provided valuable insight that may benefit a variety of creatures beyond our furry, eucalyptus-eating cousins.
“Through this experience, I am hopeful that we’ll be able to offer better treatment in the future for any animals that are found to have diabetes,” the endocrinologist, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, said in an interview.
And, she added, the experience of working with Quincy “provided an indication of where remote management of diabetes is going for the future, whether this is humans or animals.”
Quincy, a Queensland koala, reportedly died at the San Diego Zoo on Dec. 13 of pneumonia at the age of about 3 years. (Koalas can live into their teens.)
It’s not clear if his death was related to his diabetes. Dr. Philis-Tsimikas said. “Although infection can worsen with poor glucose control, my understanding from the veterinarian was that his diabetes had stabilized and was being successfully treated with a small dose of daily basal insulin,” she said. “He was not having wide fluctuations in glucose control, and the CGM had been removed to make it easier for him to get around his enclosures.”
Nine months before his death, Quincy was diagnosed with type 1 diabetes and transferred from the Los Angeles Zoo for medical reasons. Last June, after veterinarians consulted with Dr. Philis-Tsimikas, Quincy underwent an operation to fit him with a CGM so zookeepers could avoid having to wake him multiple times a day for skin pricks.
Koalas are among many species that can develop the equivalent of human diabetes. Dogs, cats, pigs, apes, horses, and even dolphins can become diabetic.
“The providers and caretakers could all respond with appropriate interventions based on the real-time readings. Improved treatment decisions were made despite not having any verbal communication,” Dr. Philis-Tsimikas said.
“I found it amazing that the CGM device could be placed on such a small body with very little subcutaneous fat,” she said. “It stayed in place and functioned successfully despite movement of the koala around his enclosure.”
In light of his small body and lack of body fat, could Quincy’s experience offer insight into the use of CGM technology in fragile humans such as babies and the elderly? Absolutely, Dr. Philis-Tsimikas said, noting that babies have been diagnosed with diabetes at as young as 9 months.
She said Quincy’s story, which got extensive media attention, provided another benefit. “His story was very relatable to many people with newly diagnosed type 1 diabetes and how difficult it can be to manage the highs and lows,” she said. “Quincy helped show us how this could be addressed with the new technology of a CGM and new types of basal insulin and pens that deliver half units.”
Dr. Philis-Tsimikas reports that her center conducts research with Dexcom and Novo Nordisk.
REPORTING FROM THE DIABETIC KOALA BEAT
Nuedexta mainly prescribed for dementia, Parkinson’s
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
Only 15% of patients prescribed dextromethorphan hydrobromide plus quinidine sulfate had pseudobulbar affect due to multiple sclerosis or amyotrophic lateral sclerosis, the condition for which this drug is labeled, according to an analysis of two national commercial insurance claims databases published online Jan. 7 in JAMA Internal Medicine.
Conversely, 57% of patients prescribed dextromethorphan-quinidine (Nuedexta) had a diagnosis of Parkinson’s disease or dementia. Furthermore, according to Medicare Part D data, prescriptions for dextromethorphan-quinidine rose 15-fold during a recent 6-year period, with a concurrent 50-fold rise in reimbursement. “In response to findings such as ours, further attention should be paid to educating prescribers about the actual benefits and risks of this costly drug combination,” Michael Fralick, MD, and his associates at Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote in their paper.
The Food and Drug Administration approved Nuedexta in 2010 for the treatment of pseudobulbar affect after it produced modest improvements in laughing or crying episodes in a 12-week, placebo-controlled trial of patients with multiple sclerosis (MS) or amyotrophic lateral sclerosis (ALS). The initial FDA label noted: “Nuedexta has not been shown to be safe or effective in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.” Then, in 2015, patients with Alzheimer’s disease showed modest improvements in agitation scores when they received dextromethorphan-quinidine in a 10-week, placebo-controlled, industry-designed and sponsored trial. Although the dextromethorphan-quinidine arm also had higher rates of falls, urinary tract infections, and serious adverse events, the prescribing information was updated in 2015 to remove the statement about patients with dementia.
To assess real-world prescribing patterns for dextromethorphan-quinidine, Dr. Fralick and his associates analyzed data from 12,858 patients who filled a prescription for this medication between 2010 and 2017 and were recorded in the Optum Clinformatics Data Mart or Truven Health MarketScan databases. Only 8.4% of patients had a diagnosis of MS and only 6.8% had ALS, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis. The number of patients prescribed dextromethorphan-quinidine rose from nearly 3,300 in 2011 to more than 50,000 in 2016, while spending on this medication by the Centers for Medicare & Medicaid Services increased from $3.9 million to $200.4 million during the same time period.
Current treatments for behavioral symptoms of dementia “are largely ineffective, and thus clinicians may want to prescribe dextromethorphan-quinidine to see if it helps their patients,” the researchers wrote. “Yet the absence of data showing efficacy, coupled with the demonstrated risks of falls and possible cardiac effects, calls this strategy into question.
“Further studies should be required to evaluate the safety and effectiveness of this medication as it is currently being used,” the authors suggested.
Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
SOURCE: Fralick M et al. JAMA Inter Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Only 8.4% of patients had a diagnosis of multiple sclerosis and only 6.8% had amyotrophic lateral sclerosis, while 57% had dementia and/or Parkinson’s disease and 28% had an unknown diagnosis.
Study details: Population-based cohort study of 12,858 patients prescribed dextromethorphan-quinidine between 2010 and 2017.
Disclosures: Study funders included the Laura and John Arnold Foundation, the Harvard Program in Therapeutic Science, the Engelberg Foundation, and the University of Toronto Clinician Scientist Training Program. One author disclosed grants from the Food and Drug Administration Office of Generic Drugs and Division of Health Communication unrelated to the study topic.
Source: Fralick M et al. JAMA Intern Med. 2019 Jan 7. doi: 10.1001/jamainternmed.2018.6112.