User login
The state of hospital medicine in 2018
Productivity, pay, and roles remain center stage
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.
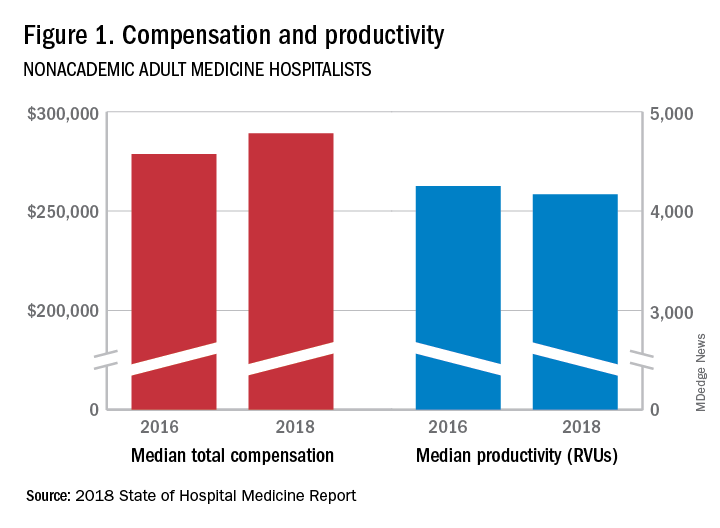
These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.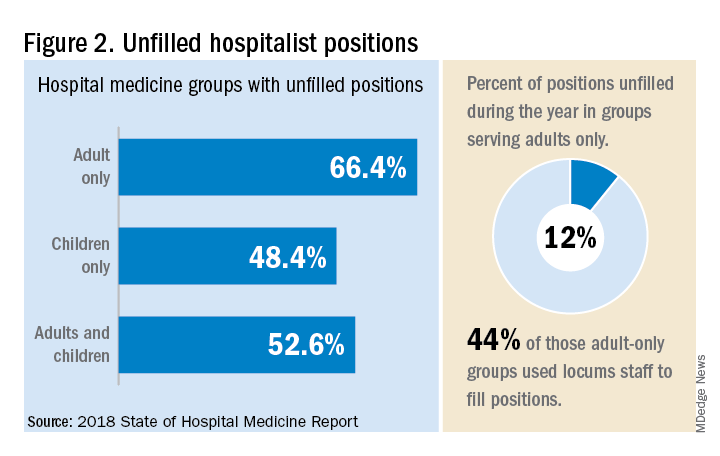
But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.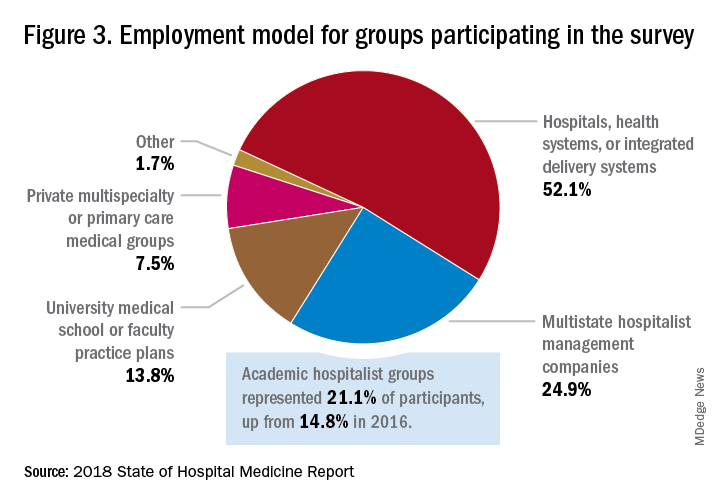
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”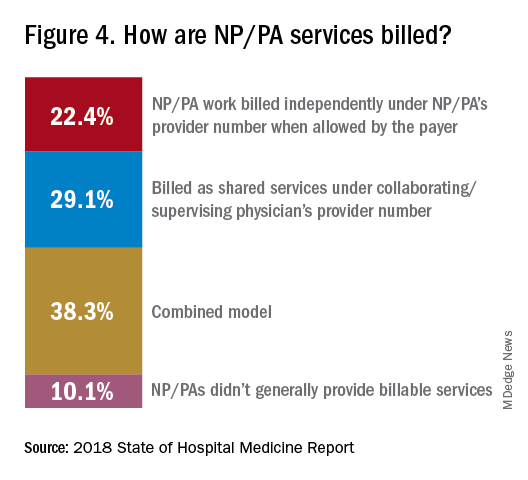
The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”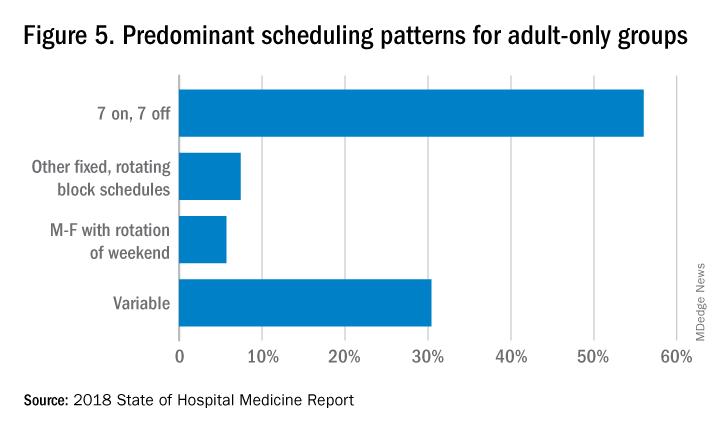
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.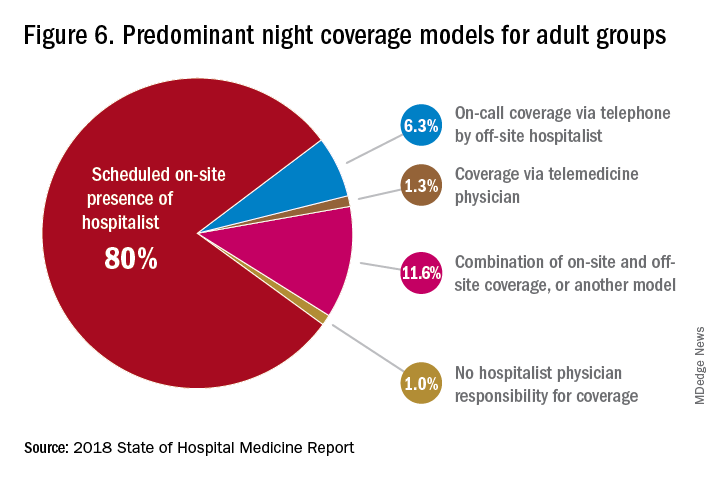
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.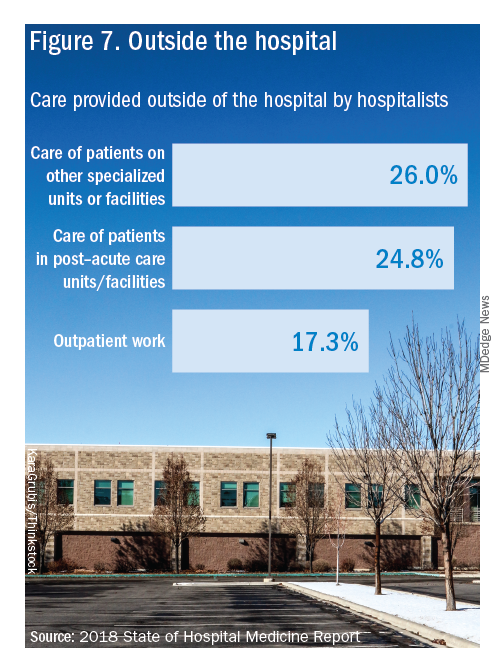
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: survey@hospitalmedicine.org or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
Productivity, pay, and roles remain center stage
Productivity, pay, and roles remain center stage
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.

These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.
But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”
The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: survey@hospitalmedicine.org or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.

These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.
But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”
The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: survey@hospitalmedicine.org or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
Solitary Nodule on the Thigh
The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
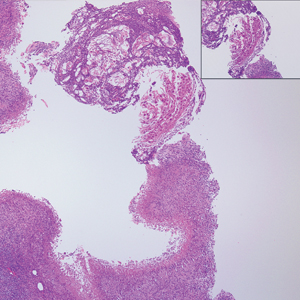
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
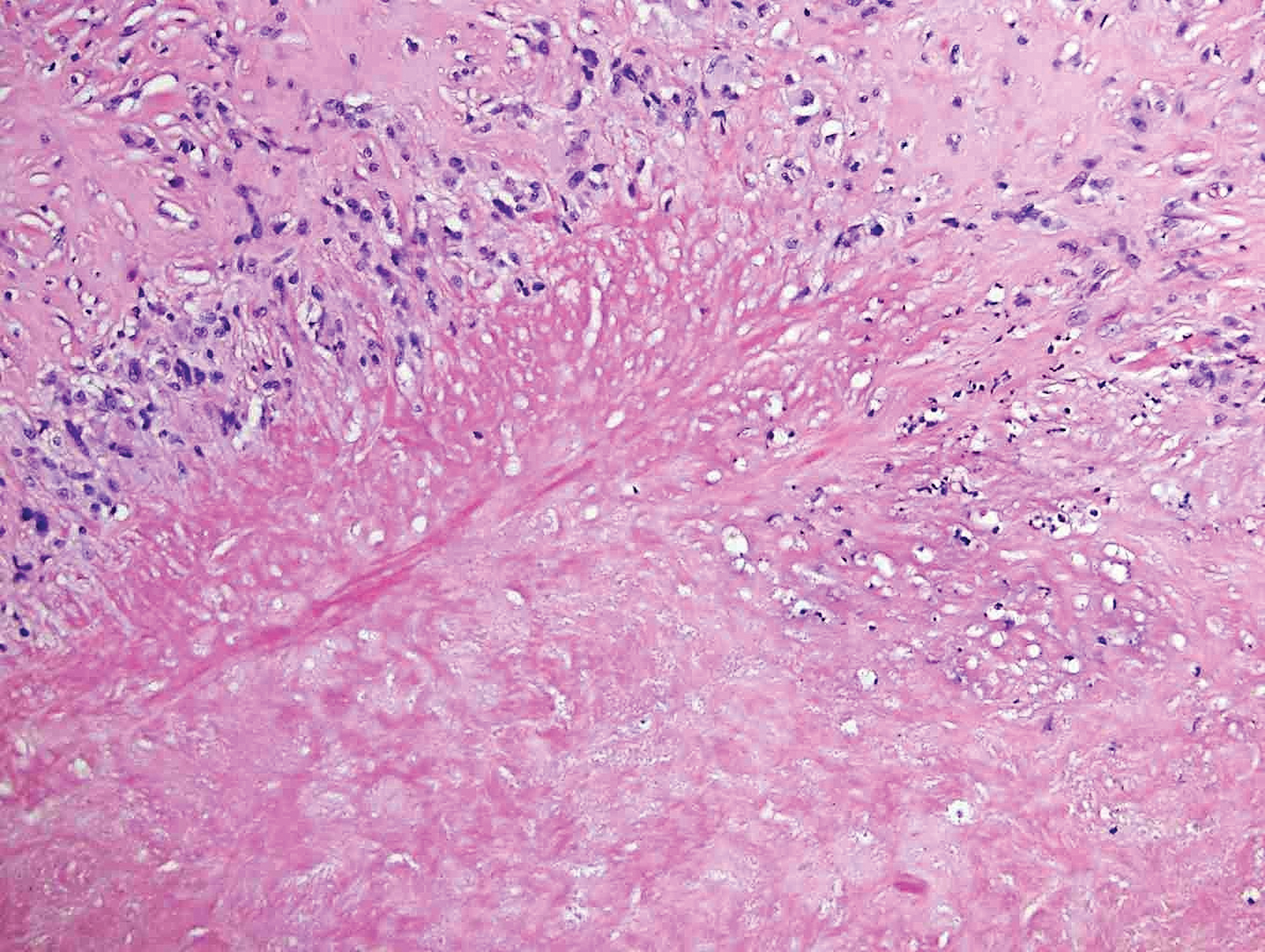
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7
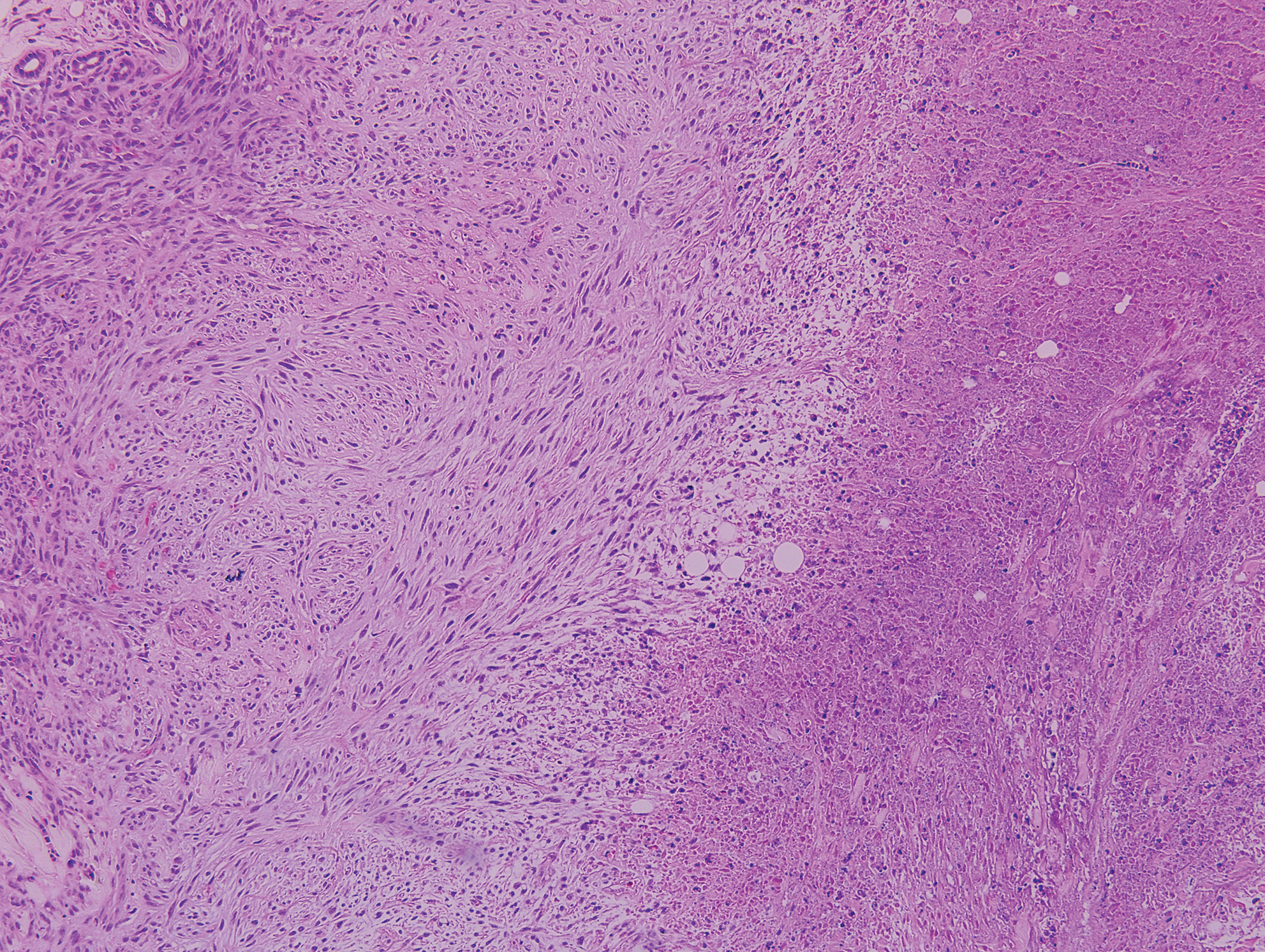
Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).
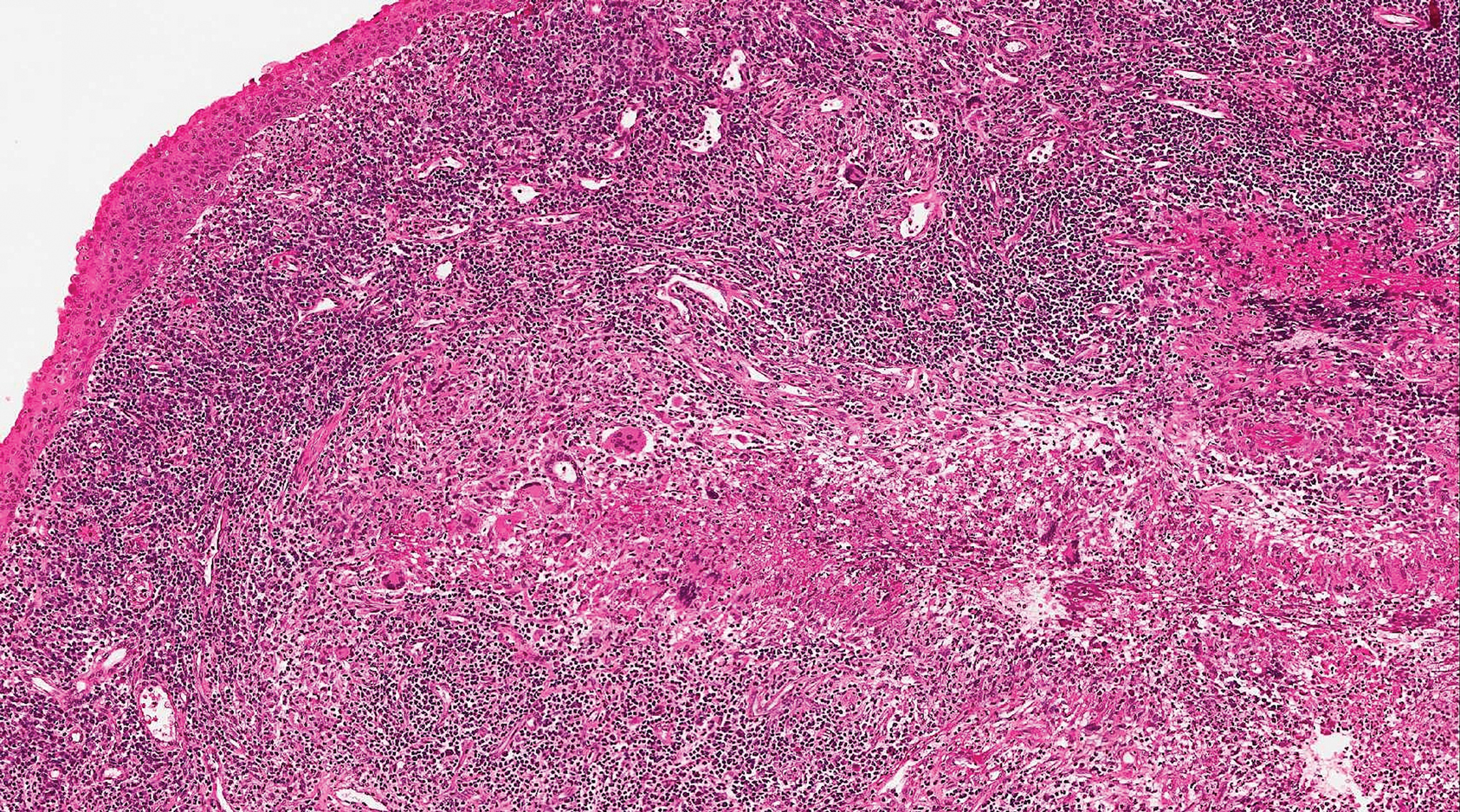
Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13
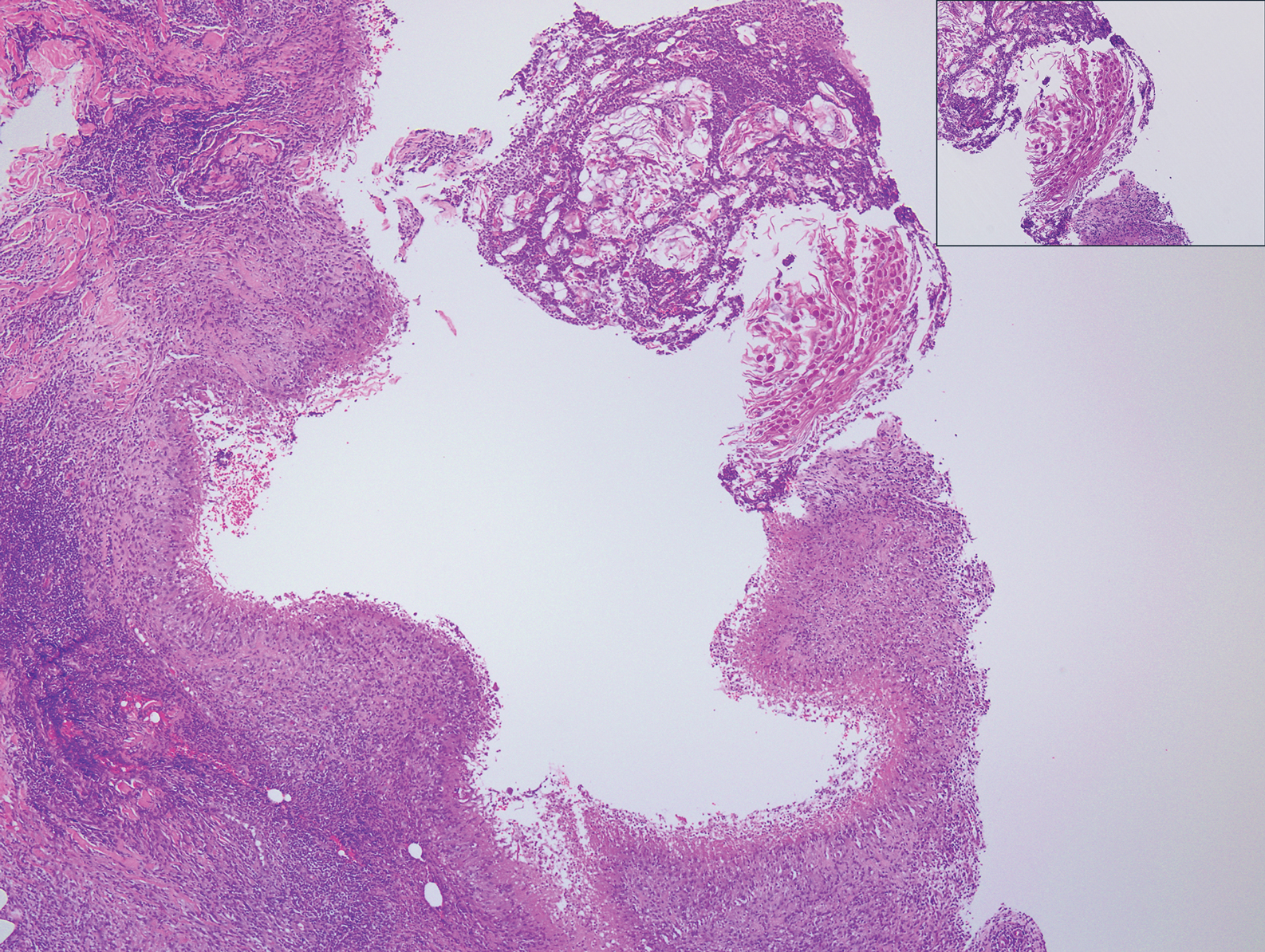
Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17
1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7
Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).
Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13
Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17
The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7
Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).
Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13
Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17
1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
A 17-year-old adolescent girl presented with a discrete nodule on the thigh.
Researchers exploring ways to mitigate aging’s impact on diabetes
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2018
Proposed triple I criteria may overlook febrile women at risk post partum
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The sensitivity and specificity of the suspected triple I criteria to predict an adverse clinical infectious outcome were 68% for the suspected triple I group and 38% for the isolated maternal fever group.
Study details: A retrospective cohort study of 339 women with intrapartum fever from June 2015 to September 2017.
Disclosures: The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
Source: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
2019 Update on Obstetrics
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1
Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
How does HT in recent and 10+ years past menopause affect atherosclerosis progression?
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
Health care costs matter to patients, and we can do something about it
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.
Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.

Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
CASE 1 Huge out-of-pocket cost makes patient forego treatment
Ms. M. is a 28-year-old patient who recently posted this on her Facebook page: “I went to the drugstore this morning to pick up a prescription, and as the pharmacist handed it to me she said, ‘That will be $180.00.’ And that’s after insurance coverage! Wow! I think I’ll pass!”
Our patients probably experience this type of situation more commonly than we know.
CASE 2 Catastrophic medical costs bankrupt family
A middle-class couple who had college degrees and full-time jobs with health insurance had twins at 24 weeks’ gestation. They accrued $450,000 in medical debt after exceeding the $2 million cap of their insurance policy. Having premature twins cost them everything. They liquidated their retirement and savings accounts, sold everything they had, and still ended up filing for bankruptcy.1
Costs indeed matter to patients, and we have a professional responsibility to help our patients navigate the murky waters of health care so that they can maintain financial as well as physical health.
Rising costs, lower yield,and opportunities for change
Rising health care costs are unsustainable for both our patients and our society. Although the United States spends more on health care than any other developed country, our health outcomes are actually worse—ranking at or near the bottom in both prevalence and mortality for multiple diseases, risk factors, and injuries.2
Of the 171 countries included in a study by the United Nations Maternal Mortality Estimation Inter-Agency Group, the United States was 1 of 13 countries that had an increasing maternal mortality and the only developed nation with an increasing maternal mortality rate.3 This tells us that, as our country spends more on health care, our patients’ health is not improving. For individuals, medical bills are now the leading cause of personal bankruptcy in the United States, even for those who are insured.4
ObGyns play an important leadership role in the practice of cost-conscious health care, as 25% of hospitalizations in the United States are pregnancy related.5,6 In addition, the wide scope of ObGyn practice reaches beyond pregnancy-related conditions and provides multiple opportunities to decrease the use of unnecessary tests and treatments.
The good news is that approximately 30% of health care costs are wasted on unnecessary care that could be eliminated without decreasing the quality of care.7
High-value change #1: Eliminate use of expensive products
Embarking on a high-value care improvement project, experts at Greenville Health System examined the cost of different topical pain medications for perineal pain after a vaginal delivery. They found that Epifoam (hydrocortisone acetate/pramoxine hydrochloride) was ordered 2,287 times over the course of a year.
The study intervention consisted of an educational grand rounds and discussion of a Cochrane review, which concluded there was no difference in pain relief with topical anesthetics compared with placebo.8 Less expensive options for pain relief were discussed, and the department agreed to remove Epifoam as a standing order.
After the intervention, Epifoam was ordered 228 times, a 90% reduction. Over the period of a year, this translated to a cost savings of $92,655 for the hospital, with reduced charges passed on to patients.9 Thus, a seemingly small individual cost ($45.00 per can of Epifoam) can add up to a substantial sum in a large health care system.

Similarly, practitioners were educated about options for cervical ripening and were given information on the cost and efficacy of various cervical ripening agents. A Cochrane review found that oral misoprostol is as effective as vaginal misoprostol and results in fewer cesarean deliveries than vaginal dinoprostone (Cervidil).10 Practitioners were asked to consider making the transition to oral misoprostol. This action resulted in a 50.5% decrease in Cervidil use, from 384 to 194 cases, producing a cost savings of $66,500. The following year, the department removed Cervidil from the formulary as a high-value decision.9
Both of these examples illustrate what a value-minded department can accomplish by implementing performance improvement projects that focus on high-value care.
Continue to: High-value change #2: Stop ordering unnecessary lab work...
High-value change #2: Stop ordering unnecessary lab work
Another high-value change to consider: Examine each laboratory test order to understand if the test results will really alter the care of a patient. Providers vary, and ordering lab tests to “make sure” can add up as financial expense.
Best practices from the American College of Obstetricians and Gynecologists (ACOG) and other professional societies can help guide decision-making as we order lab tests. Think twice, for example, about whether every evaluation for preeclampsia requires a uric acid test, since ACOG does not endorse that as part of the diagnostic criteria. While a single uric acid test costs only $8.00 to $38.00 (according to Healthcare Bluebook), testing uric acid in many patients over the course of a year can add up to significant dollars.11
High-value change #3: Consider care redesign
In addition to seeking opportunities to use more cost-effective products and reduce the use of unnecessary tests, “care redesign” is an innovative way to provide high-quality care (and increased patient satisfaction) at a lower cost for both the health care system and the patient. A prime example of care redesign is using telehealth to enhance prenatal care.
Several health systems around the country are piloting and implementing remote blood pressure monitoring, app-based prenatal education, and telehealth visits to enhance prenatal care.12,13 Use of a home blood pressure monitor can reduce in-person visits for low-risk prenatal care and open up access for other patients. Additionally, allowing the patient to participate in her own care at home or work can eliminate drives to and waits in the office and reduce absence from work because of a doctor visit.
A systematic review of more than 60,000 women showed that low-risk women who attend 5 to 9 prenatal visits have the same outcomes as women who attend the standard schedule of 13 to 15 visits.14 Although patient satisfaction was higher with more visits, when a bidirectional app or a telehealth visit is offered as an option, then patient satisfaction is equivalent to that in the standard schedule group.12 So why not expand the choice for patients?
The challenge of teaching high-value care: Medical education responds
In a 2010 article in the New England Journal of Medicine, Dr. Molly Cooke commented on medical education’s responsibility regarding cost consciousness in patient care, and she highlighted the importance of teaching medical students and residents about considering cost in treating patients.15 Similarly, the Accreditation Council for Graduate Medical Education asks residents to consider cost and stewardship of medical resources as one of its system-based practice competencies.16 In 2012, the Choosing Wisely campaign, initiated by the American Board of Internal Medicine Foundation, asked specialty society members to identify tests or procedures commonly used in their field whose necessity should be questioned and discussed.17 ACOG and other women’s health specialty societies participate in this campaign.
From an educational standpoint, ACOG’s Council on Resident Education in Obstetrics and Gynecology has developed a curriculum resource, “Cases in High Value Care,” that can be used by any women’s health department to start the conversation on high-value care.18 The web program encourages medical students and residents to submit clinical vignettes that demonstrate examples of low- and high-value care. These cases can be used for discussion and debate and can serve as high-value care performance improvement projects in your own department.
Other useful publications are available outside the ObGyn specialty. Consider the Society of Hospital Medicine’s article series in the Journal of Hospital Medicine, “Choosing Wisely: Things We Do for No Reason”and “Choosing Wisely: Next Steps in Improving Healthcare Value.”19 The former focuses on discussing practices (tests, procedures, supplies, and prescriptions) that may be poorly supported by evidence or are part of standard practice even though other less expensive, higher-value alternatives may be available. The latter highlights perspective pieces that describe health care value initiatives relating to the practice of hospital medicine.
Continue to: The bottom line...
The bottom line
ObGyns and other health care providers are concerned about providing high-value care to patients and are working toward improving performance in this area. We really do care about the health care–related financial burdens that confront Ms. M., the premature twins’ parents, and all our patients.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
- Sinconis J. Bankrupted by giving birth: having premature twins cost me everything. The Guardian. January 17, 2018. https://www.theguardian.com/us-news/commentisfree/2018/jan/16/bankrupted-by-giving-birth-having-premature-twins-cost-me-everything. Accessed December 20, 2018.
- Woolf SH, Aron LY. The US health disadvantage relative to other high-income countries: findings from a National Research Council/Institute of Medicine report. JAMA. 2013;309:771-772.
- Ozimek JA, Kilpatrick SJ. Maternal mortality in the twenty-first century. Obstet Gynecol Clin North Am. 2018;45:175-186.
- Himmelstein DU, Thorne D, Warren E, et al. Medical bankruptcy in the United States, 2007: results of a national study. Am J Med. 2009;122:741-746.
- Healthy babies healthy business. March of Dimes website. http://www.marchofdimes.org/hbhb/index.asp. Accessed December 20, 2018.
- Werner EF. Cost matters. Obstet Gynecol. 2014;123:919-920.
- Institute of Medicine (US) Roundtable on Evidence-Based Medicine; Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Hedayati H, Parsons J, Crowther CA. Topically applied anaesthetics for treating perineal pain after childbirth. Cochrane Database Syst Rev. 2005;2:CD004223.
- Demosthenes LD, Lane AS, Blackhurst DW. Implementing high-value care. South Med J. 2015;108:645-648.
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2014;6:CD001338.
- Lane A. Preeclampsia evaluation. American College of Obstetricians and Gynecologists website. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care/Example-2. Published July 14, 2015. Accessed July 10, 2018.
- Clark EN. Evidence-based prenatal care. University of Utah Health website. https://physicians.utah.edu/echo/pdfs/2018-06-29_evidence-based_prenatal_care.pdf. Accessed August 6, 2018.
- Marko KI, Krapf JM, Meltzer AC, et al. Testing the feasibility of remote patient monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial. JMIR Res Protoc. 2016;5:e200.
- Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010;10:CD000934.
- Cooke M. Cost consciousness in patient care—what is medical education’s responsibility? N Engl J Med. 2010;362:1253-1255.
- Accreditation Council for Graduate Medical Education. ACGME Common program requirements (residency).https://www.acgme.org/Portals/0/PFAssets/Program Requirements/CPRs_2017-07-01.pdf. Accessed December 19, 2018.
- Choosing Wisely. American Board of Internal Medicine Foundation website. http://www.choosingwisely.org/. Accessed August 7, 2018.
- American College of Obstetricians and Gynecologists Council on Resident Education in Obstetrics and Gynecology. Cases in high value care. https://www.acog.org/About-ACOG/ACOG-Departments/CREOG/CREOG-Search/Cases-in-High-Value-Care. Accessed August 8, 2018.
- Journal of Hospital Medicine website. https://www.journalofhospitalmedicine.com/jhospmed/page/author-guidelines. Accessed August 8, 2018.
Managing menopausal vasomotor and genitourinary symptoms after breast cancer
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.
The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
Breast cancer survivors entering menopause face the risk of several menopausal symptoms:
- Hot flashes, the most common symptom, occur in more than 75% of women during menopause and have the potential to persist for as long as 15 years.1 That lengthy interval becomes a major issue for patients, especially when hot flashes are associated with other menopausal symptoms, including sleep disruption, difficulty concentrating, and emotional instability (crying, irritability).
Painful intercourse and loss of interest in sexual activity often develop as a result of vaginal atrophy and dryness.
- Urinary tract symptoms include urgency and, compared to the patient’s history, more frequent infections.
- Bone loss is a concern for many women after breast cancer, especially if they are, or have been, on aromatase inhibitor therapy.
Depression might be related to hormonal changes due to menopause or hormonal therapies, a consequence of merely having a diagnosis of cancer, or an adverse effect of chemotherapy.
In this brief review, I’ll examine options for treating symptoms of menopause by strategy—lifestyle modifications, over-the-counter treatments, and prescription drugs. Separately, I’ll look at options for managing genitourinary syndrome of menopause (GSM).
CASE 1
Rose is a 56-year-old woman who presents to clinic with a new breast mass, felt on breast self exam. The mass is about 1 cm, mobile, and firm. Diagnostic mammogram and ultrasound confirm a worrisome mass; biopsy returns positive with a 9-mm invasive, estrogen-receptor positive, ductal carcinoma with negative sentinel nodes at the time of lumpectomy. Radiation therapy was completed. She then met with oncology and decided against chemotherapy. Instead, she began an aromatase inhibitor 3 months ago. Bone density showed osteopenia. She presents to your office reporting frequent bothersome hot flashes and disrupted sleep.
Strategy #1: Lifestyle adaptations
First-line interventions for menopausal women who have had breast cancer usually involve taking a critical look at lifestyle and undertaking modifications that can alleviate discomfort. Because overall health is important for women who have had breast cancer, you should, across the spectrum of patients, encourage them to:
- increase physical activity
- reduce body weight by approximately 10% (if overweight or obese)
- reduce alcohol consumption
- stop smoking
- ensure adequate intake of calcium (1,200 mg, preferably by diet)
- optimize the level of vitamin D, including by increasing intake of fresh fish, eggs, and numerous other fortified foods.

The value of nondrug therapy for hot flashes is difficult to prove. Certain lifestyle changes are sensible, even if not evidence-based, and will help some women (but not others). We suggest that patients try lowering the temperature in the home (65–68˚ at night); running a fan; wearing clothing that can be removed in layers; and avoiding triggers such as spicy food, alcohol, cigarettes, and hot drinks. Hypnosis and cognitive behavioral therapy (CBT) have been shown to help in clinical trials. Measures with benefit and minimal risks, but effectiveness not established, include acupuncture (sham worked as well as traditional), exercise, yoga, paced respiration, relaxation training, and mindfulness-based stress reduction.
Continue to: Strategy #2: OTC compounds...
Strategy #2: OTC compounds
Over-the-counter products—from soy products to black cohosh to flax seed, and including dong quai, evening primrose oil, maca, omegas, pollen extract, ginseng, and red clover,2 or several compounds formulated in combination—have not been proven to be of more benefit for relieving symptoms of menopause than placebo in randomized trials, and thus might or might not be effective in a given patient. S-equol, a metabolite of a soy isoflavone taken by women who are non-equol producers, is available under the trade name Equelle and has shown some benefit. Note: There is concern that supplements that contain estrogen-like compounds, like soy products, might actually increase the risk of breast cancer. Dietary soy is not felt to be a concern.
Ask questions about the severity of a patient’s hot flashes. When a patient reports hot flashes, and is requesting help to relieve her discomfort, inquire 1) how often she has hot flashes, 2) how severe they are, and 3) how bothered she is by them (not all women are equally troubled, of course). The patient’s answers to these questions will help you decide which treatment option to offer, based on evidence and your experience.
CASE 1 Continued
Rose tried black cohosh OTC without improvement. She was interested in hypnosis but did not find it effective for her. She returned 3 months later stating that she is miserable, exhausted, not getting enough sleep, and her hot flashes and night sweats are affecting both her work and her relationship.
Strategy #3: Prescription medication
When addressing hot flashes, consider whether they occur more at night or during the day, or do not follow a day–night pattern. For women whose hot flashes occur mostly at night, and might therefore make sleeping difficult and cause fatigue and irritability, gabapentin, taken approximately 1 hour before bed, can be helpful. If tolerated without excessive somnolence the next day, the dose can be increased at night or additional doses provided during the day depending on hot flash response. For women who have hot flashes day and night, we often prescribe a low-dose antidepressant from the selective serotonin-reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) class.
When prescribing an antidepressant, we make a distinction between breast cancer patients who are taking tamoxifen and those who are not, to avoid cytochrome P450 2D6 inhibitors in women taking tamoxifen.3 Better choices for women taking tamoxifen include desvenlafaxine, venlafaxine, escitalopram, or gabapentin or pregabalin.
For women with breast cancer who are taking an aromatase inhibitor, and who are also experiencing mood changes with their hot flashes, we often choose a trial of a low-dose antidepressant, either an SSRI or SNRI. One drug is approved by the US Food and Drug Administration (FDA) for the treatment of hot flashes (but not for mood disorder). This is low-dose salt of paroxetine, 7.5 mg/d, which has the advantage of exerting no adverse effect on libido or weight (but is sometimes difficult to obtain because it is a branded product that might not be covered, or not covered fully, by a given patient’s insurance plan). Other antidepressants can be used in doses lower than needed for depression, with more rapid onset of effect on hot flashes, often within 2 weeks.
Last, transdermal clonidine, an antihypertensive, also has been found to relieve hot flashes.
Continue to: Not a recommended strategy: Systemic hormone therapy
Not a recommended strategy: Systemic hormone therapy
Although hormone therapy is, in general, the gold standard for alleviating hot flashes, it is contraindicated in most women with breast cancer.4 At our institution, we avoid systemic hormone therapy for hot flashes in almost all breast cancer patients.
CASE 2
Sarah first presented with hot flashes that improved while taking escitalopram 10 mg. Her night sweats persisted, however. Gabapentin 300 mg was added to take nightly. With this regimen, she finally felt that she was coping better. Six months later, she reported that she and her long-term partner had not been able to resume vaginal intercourse post–breast cancer treatment because of pain.
The challenge of managing GSM
What if your patient says, “Doctor, I’m really doing OK with my hot flashes, but sex has become painful. I don’t have any interest. I have vaginal dryness, and it’s affecting my quality of life”?
Studies have shown that GSM affects up to 50% of women, and even more than that among women who have had breast cancer.5 The condition interferes with sexual intimacy, disrupts quality of life, and can sour a partnership—significant quality-of-life concerns for breast cancer survivors.
For mild symptoms, encourage patients to apply a lubricant just before intercourse or a vaginal moisturizer twice weekly; moisturizers improve vaginal pH, too. These treatments do not fix the problem of a lack of superficial cells due to estrogen loss, however; to accomplish that, consider prescribing low-dose vaginal estrogen therapy or intravaginal dehydroepiandrosterone (DHEA). This strategy is felt to be safe for many breast cancer survivors, as systemic absorption of estrogen is minimal if dosed low, keeping levels in the postmenopausal range.
The American College of Obstetricians and Gynecologists (ACOG), the North American Menopause Society (NAMS), and the Endocrine Society agree that vaginal estrogen therapy may be a good option for many women with breast cancer for whom moisturizers and lubricants are inadequate.6 Delivery options include vaginal creams, tablets, suppositories used 2 or 3 times per week, or the low-dose vaginal estrogen ring, replaced every 3 months. We are concerned about using vaginal estrogen in women who have had aromatase inhibitor (AI) therapy; their estrogen levels are so low that absorbing even a small amount might make a difference in terms of effectiveness of AI. For women who need more than lubricants or vaginal moisturizers, particularly those taking anti-estrogen therapy (aromatase therapy), the use of low-dose vaginal hormones may be considered on an individual basis, but should include the oncologist in decision making.1,3
Beyond low-dose vaginal estrogen therapies, there are additional options that can be considered but with less supporting data for treating GSM in women with breast cancer.
Oral ospemifene, a selective estrogen-receptor modulator (SERM; Osphena), might be neutral or even protective in its effect on the breast, as demonstrated in preclinical trials.7 In human trials, the drug is approved only for painful intercourse, not for loss of libido, and has not been tested in breast cancer patients.
Intravaginal DHEA (Prasterone), has been on the market for almost 1 year. The drug is approved for treating painful intercourse, but it also reverses vaginal atrophy and alleviates urinary symptoms. Because DHEA is a prohormone, it is converted to estrogen and androgen in the vagina. Again, absorption appears minimal. Intravaginal DHEA does not have the US Food and Drug Administration (FDA) black-box warning that vaginal estrogen products do, but it is accompanied by a warning that it has not been tested in women with breast cancer.
Tissue selective estrogen receptor modulator is a conjugated estrogen combined with a third-generation SERM bazedoxifene, which treats hot flashes and reverses vaginal atrophy. This new systemic agent is probably neutral on the breast (at least that is the finding in clinical trials at 2 years8); again, however, it has not been tested in patients with breast cancer.
Continue to: Nonhormone therapies...
Nonhormone therapies
Topical lidocaine for insertional dyspareunia has been studied in postmenopausal women with breast cancer with severe GSM, dyspareunia, increased sexual distress scores, or abnormal sexual function with improvement seen using 4% aqueous lidocaine versus saline applied with a cotton ball to the vestibule for 3 minutes before vaginal penetration.9
Vaginal laser therapy has the potential to ameliorate distressing GSM without the need for local hormone intervention; however, placebo or active-controlled trials and long-term safety follow-up are needed.5
Newly arrived and on the horizon
Where does this review of available treatments leave us? Regrettably, with many women who experience painful intercourse and vaginal dryness despite what is available for treating their problems, and who continue looking to medical science and women’s health care for new options. So, what is coming next for these suffering patients? Here is a quick and selective run-through:
KNDy neurons. For hot flashes, there is the promise of nonhormonal treatment using these neurons, believed to be involved in reproduction by triggering expression of various compounds— particularly neurokinin B, which mediates hot flashes.1
Estetrol. In testing for use in treating hot flashes and its effect on GSM is this pregnancy-associated natural hormone that, importantly, did not stimulate breast cancer in a rat model.2 More evidence of efficacy is needed.
Lasers. For vaginal atrophy, many women are choosing treatment with the laser. Keep in mind, however, that, although lasers are FDA-approved devices, they do not have the FDA’s endorsement for use in vaginal atrophy, and have not been well-tested for their effectiveness for this indication in women with breast cancer who have taken an aromatase inhibitor. ACOG, NAMS, and the Endocrine Society have urged that additional trials be conducted, and have stated that the laser for vaginal atrophy cannot be recommended until there are more data on safety and efficacy.2
Lower-dose soft-gel vaginal estrogen suppositories have recently been approved by the FDA at 4 and 10 µg.3 The formulations are only minimally absorbed, potentially making them a good option for women who have had breast cancer.
Lasofoxifene, a selective estrogen-receptor modulator not yet approved by the FDA, has been shown to ameliorate vaginal changes.4 The drug is neutral or protective on the breast, but is now being tested in women with resistant breast cancer and unlikely to become available for GSM.
References
1. Anderson RA, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2018;1-4.
2. Gérard C, Mestdagt M, Tskitishvili E, et al. Combined estrogenic and anti-estrogenic properties of estetrol on breast cancer may provide a safe therapeutic window for the treatment of menopausal symptoms. Oncotarget. 2015;6(19):17621–17636.
3. Simon JA, Archer DF, Constantine GD, et al. A vaginal estradiol softgel capsule, TX-004HR, has negligible to very low systemic absorption of estradiol: efficacy and pharmacokinetic data review. Maturitas. 2017;99:51-58.
4. Bachmann G, Gass M, Kagan R, et al. Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM), improves dyspareunia in postmenopausal women with vaginal atrophy (VA). Menopause. 2005;12:238.
Treatment begins with a conversation
Most importantly, we need to listen to our patients in discomfort because of their menopausal symptoms. Consider proceeding along these lines: “You’ve been treated for breast cancer; now, let’s look at the medical issues that are affecting your quality of life. Are you depressed? Are you having hot flashes? Are you getting enough sleep? Have you stopped having sex or not restarted after your breast cancer treatment? Are you having painful sex or avoiding sex due to fear of pain? Let’s discuss options and work with your oncologist to try to relieve your symptoms and make your life better.”
First-line therapy for the treatment of menopausal symptoms in women with a history of breast cancer should start with lifestyle changes and nonhormone therapies. For GSM, lubricants and vaginal moisturizers should be tried first and may be effective. Reassure patients that there are many treatment options, even though not all of them have been well-tested in breast cancer patients, and that new modalities are under investigation and review (see “Newly arrived and on the horizon,”). Become familiar with published data on the safety and effectiveness of the range of available treatments; guide patients through the process of finding what works best for them; and invite their oncologist into the therapeutic partnership. If you do not feel comfortable with these issues in women who are breast cancer survivors, find a menopause specialist to help, available by zip code at Find a Provider, http://www.menopause.org.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. McGarry K, Geary M, Gopinath V. Beyond estrogen: treatment options for hot flashes. Clin Ther. 2018;40(10):1778-1786.
3. Santen RJ, Stuenkel CA, Davis SR, et al. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab. 2017;102:3647-3661.
4. Faubion SS, Loprinzi CL, Ruddy KJ. Management of hormone deprivation symptoms after cancer. Mayo Clin Proc. 2016;91:1133-1146.
5. Faubion SS, Larkin LC, Stuenkel, et al. 2018;25(6):596-608.
6. American College of Obstertricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:e93-e96.
7. Simon JA, Altomare C, Cort S, Jiang W. Overall safety of ospemifene in postmenopausal women from placebocontrolled Phase 2 and 3 trials. J Womens Health (Larchmt). 2018;27(1):14-23.
8. Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. Steroid Biochem Mol Biol. 2014;142:142-54.
9. Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394-3400.
Financial returns more than cover cancer drug R&D
Cancer pharmaceutical manufacturers are making profits that more than cover their research and development costs, calling into question the need for these products to carry a high price tag, limiting access.
“Based on a risk-adjusted R&D cost of $794 million [$219 million to $2.8 billion], by the end of 2017, $1 (risk-adjusted) invested for R&D of the 99 drugs had generated a median of $14.50 (range, $3.30-$55.10) in sales income for the originator companies” wrote Kiu Tay-Teo, PhD, of the World Health Organization, Geneva, Switzerland, and colleagues. The report is in JAMA Network Open.
“Assuming the upper threshold R&D cost of [$2.8 billion], rituximab, trastuzumab, bevacizumab, pegfilgrastim, and imatinib have brought in for the originator companies, $33.20, $31.20, $29.50, $22.60, and $22.60, respectively, for every risk adjusted dollar that went into their R&D.”
The study authors looked at all cancer drugs approved by the Food and Drug Administration from 1989 to 2017 (156 total) and narrowed the analysis down to the 99 products that had sales data on more than half of the years since being approved.
The analysis shows that compared with the risk-adjusted R&D cost of $794 million (with a range of $219 million to $2.8 billion) per medicine, by the end of 2017, the median cumulative sales income was $14.50 (range, $3.30-$55.10) per dollar invested for R&D. “Median time to fully recover the maximum possible risk-adjusted cost of R&D [$2.8 billion] was 5 years (range, 2-10 years; n = 56),” the authors stated.
“By the end of 2017, the cumulative sales of 73 cancer drugs (73.7%) included in the analysis had fully recovered the median R&D costs of $794 million,” Dr. Tay-Teo and colleagues reported.
The authors argue that such high prices and high profits, while far exceeding even the upper bound of R&D costs, could also be hindering access to drugs.
“The high incomes and supernormal returns would be less worrying if patients were able to access cancer drugs at affordable prices,” Dr. Tay-Teo and colleagues stated. “However, the existing evidence suggests otherwise: Access to cancer drugs globally remains low and the number of cancer drugs with annual costs at least in the tens of thousands is increasing fast. The resulting expenditure effects have compelled insurance schemes to exclude patients from coverage, restrict access, or impose high out-of-pocket costs.”
They suggest that for these reasons, the high price tags on cancer drugs is reducing patient access worldwide.
The analysis was limited to the information on sales and R&D costs that was made public by manufacturers in annual reports and did not estimate cumulative profits due to lack of information on year-over-year variations on production costs of cancer drugs.
SOURCE: Kiu Tay-Teo, PhD, et al. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.6875.
Cancer pharmaceutical manufacturers are making profits that more than cover their research and development costs, calling into question the need for these products to carry a high price tag, limiting access.
“Based on a risk-adjusted R&D cost of $794 million [$219 million to $2.8 billion], by the end of 2017, $1 (risk-adjusted) invested for R&D of the 99 drugs had generated a median of $14.50 (range, $3.30-$55.10) in sales income for the originator companies” wrote Kiu Tay-Teo, PhD, of the World Health Organization, Geneva, Switzerland, and colleagues. The report is in JAMA Network Open.
“Assuming the upper threshold R&D cost of [$2.8 billion], rituximab, trastuzumab, bevacizumab, pegfilgrastim, and imatinib have brought in for the originator companies, $33.20, $31.20, $29.50, $22.60, and $22.60, respectively, for every risk adjusted dollar that went into their R&D.”
The study authors looked at all cancer drugs approved by the Food and Drug Administration from 1989 to 2017 (156 total) and narrowed the analysis down to the 99 products that had sales data on more than half of the years since being approved.
The analysis shows that compared with the risk-adjusted R&D cost of $794 million (with a range of $219 million to $2.8 billion) per medicine, by the end of 2017, the median cumulative sales income was $14.50 (range, $3.30-$55.10) per dollar invested for R&D. “Median time to fully recover the maximum possible risk-adjusted cost of R&D [$2.8 billion] was 5 years (range, 2-10 years; n = 56),” the authors stated.
“By the end of 2017, the cumulative sales of 73 cancer drugs (73.7%) included in the analysis had fully recovered the median R&D costs of $794 million,” Dr. Tay-Teo and colleagues reported.
The authors argue that such high prices and high profits, while far exceeding even the upper bound of R&D costs, could also be hindering access to drugs.
“The high incomes and supernormal returns would be less worrying if patients were able to access cancer drugs at affordable prices,” Dr. Tay-Teo and colleagues stated. “However, the existing evidence suggests otherwise: Access to cancer drugs globally remains low and the number of cancer drugs with annual costs at least in the tens of thousands is increasing fast. The resulting expenditure effects have compelled insurance schemes to exclude patients from coverage, restrict access, or impose high out-of-pocket costs.”
They suggest that for these reasons, the high price tags on cancer drugs is reducing patient access worldwide.
The analysis was limited to the information on sales and R&D costs that was made public by manufacturers in annual reports and did not estimate cumulative profits due to lack of information on year-over-year variations on production costs of cancer drugs.
SOURCE: Kiu Tay-Teo, PhD, et al. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.6875.
Cancer pharmaceutical manufacturers are making profits that more than cover their research and development costs, calling into question the need for these products to carry a high price tag, limiting access.
“Based on a risk-adjusted R&D cost of $794 million [$219 million to $2.8 billion], by the end of 2017, $1 (risk-adjusted) invested for R&D of the 99 drugs had generated a median of $14.50 (range, $3.30-$55.10) in sales income for the originator companies” wrote Kiu Tay-Teo, PhD, of the World Health Organization, Geneva, Switzerland, and colleagues. The report is in JAMA Network Open.
“Assuming the upper threshold R&D cost of [$2.8 billion], rituximab, trastuzumab, bevacizumab, pegfilgrastim, and imatinib have brought in for the originator companies, $33.20, $31.20, $29.50, $22.60, and $22.60, respectively, for every risk adjusted dollar that went into their R&D.”
The study authors looked at all cancer drugs approved by the Food and Drug Administration from 1989 to 2017 (156 total) and narrowed the analysis down to the 99 products that had sales data on more than half of the years since being approved.
The analysis shows that compared with the risk-adjusted R&D cost of $794 million (with a range of $219 million to $2.8 billion) per medicine, by the end of 2017, the median cumulative sales income was $14.50 (range, $3.30-$55.10) per dollar invested for R&D. “Median time to fully recover the maximum possible risk-adjusted cost of R&D [$2.8 billion] was 5 years (range, 2-10 years; n = 56),” the authors stated.
“By the end of 2017, the cumulative sales of 73 cancer drugs (73.7%) included in the analysis had fully recovered the median R&D costs of $794 million,” Dr. Tay-Teo and colleagues reported.
The authors argue that such high prices and high profits, while far exceeding even the upper bound of R&D costs, could also be hindering access to drugs.
“The high incomes and supernormal returns would be less worrying if patients were able to access cancer drugs at affordable prices,” Dr. Tay-Teo and colleagues stated. “However, the existing evidence suggests otherwise: Access to cancer drugs globally remains low and the number of cancer drugs with annual costs at least in the tens of thousands is increasing fast. The resulting expenditure effects have compelled insurance schemes to exclude patients from coverage, restrict access, or impose high out-of-pocket costs.”
They suggest that for these reasons, the high price tags on cancer drugs is reducing patient access worldwide.
The analysis was limited to the information on sales and R&D costs that was made public by manufacturers in annual reports and did not estimate cumulative profits due to lack of information on year-over-year variations on production costs of cancer drugs.
SOURCE: Kiu Tay-Teo, PhD, et al. JAMA Netw Open. doi: 10.1001/jamanetworkopen.2018.6875.
FROM JAMA NETWORK OPEN
Key clinical point: Profits from cancer drugs far exceed R&D costs.
Major finding: $1 of R&D investment generated a median $14.50 in sales income for originator companies.
Study details: Researchers did an analysis of 99 cancer drugs approved by the FDA between 1989 and 2017 that had sales data for more than half of the years the drugs were approved.
Disclosures: The researchers reported no conflicts of interest.
Source: Kiu Tay-Teo, PhD, et al. JAMA Netw Open. 2019 Jan 4. doi: 10.1001/jamanetworkopen.2018.6875.