User login
Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
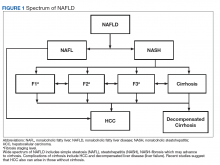
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
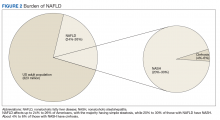
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
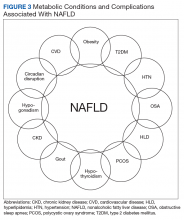
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD also is an independent risk factor for cardiovascular disease, type 2 diabetes mellitus (T2DM), chronic kidney disease, cirrhosis, liver cancer, and all-cause mortality.1-3 As the leading cause of liver disease in the US and globally, NAFLD is strongly associated with obesity and metabolic syndrome, with the rising prevalence of NAFLD closely mirroring the epidemic of obesity and T2DM.4,5 The unrelenting increase of NAFLD prevalence has led to a significant rise in associated health care and economic burdens, compounded by the boom in childhood obesity and an aging population. In this review, we will discuss the epidemiology and economic burden of NAFLD in the US and how it affects veteran health.
NAFLD Definition
NAFLD is defined as the presence of > 5% of hepatic steatosis in the absence of excessive alcohol use, steatosis-inducing medication, or other concurrent chronic liver diseases.
Compared with patients with NAFL, patients with NASH are at a much higher risk of developing fibrosis (scarring of the liver) and cirrhosis (significant scarring with distorted liver architecture). Patients with either NAFL or NASH, with or without advanced fibrosis, also can develop hepatocellular carcinoma (HCC). Severity of liver fibrosis (ie, fibrosis stage) is the most important predictor of liver-associated mortality and all-cause mortality; those with significant fibrosis (≥ F2 stage of fibrosis) are more likely to die of liver disease or to undergo a liver transplant compared with those with earlier stages of disease (ie, stages 0 to F1). Those with advanced scarring or cirrhosis (≥ F3 stage of fibrosis) exhibit an even higher risk of death or liver transplantation.6
NAFLD is a slow and often progressive disease. Time to progression between each stage of fibrosis is about 7 years; however, there has been a documented subset of patients with rapid progression to advanced fibrosis.7 The risk factors associated with this increased risk of fibrosis progression remain poorly understood.
Prevalence
The prevalence of NAFLD in the US is about 24% to 26%—about 85 million Americans. Up to 20% to 30% of these cases (about 17-25 million Americans) are thought to have NASH (Figure 2).
Although liver biopsy remains the current gold standard for diagnosis and histopathologic staging of NAFLD, alternatives to liver biopsy include elastography techniques (ie, transient elastography using Fibroscan[Paris, France], shear wave elastography using Supersonic Image Aixplorer [Weston, FL], and magnetic resonance elastography), magnetic resonance spectroscopy, liver enzymes, and noninvasive simple and complex (serologic) scoring systems such as the Fatty Liver Index. Among these, liver enzymes and serologic scores are most likely to underestimate NAFLD disease burden. Transient elastographyis widely used because the test is easy to perform, noninvasive, and reliably estimates the degree of liver fibrosis in patients with NAFLD by measuring the speed of a mechanically induced shear wave using pulse-echo ultrasonic acquisitions in a much larger portion of the tissue (about 100 times more than a liver biopsy core). Transient elastography also can objectively quantify the amount of liver fat by measuring a 3.5 MHz ultrasound coefficient of attenuation or controlled attenuation parameter (CAP). This correlates with the degree of liver fat, and a higher CAP level reflects a greater degree of steatosis.
The largest study of US veterans utilized abnormal (ie, elevated) liver enzymes as the diagnostic criteria and reviewed nearly 10 million veterans who were followed between 2003 and 2011. Investigators reported a NAFLD prevalence of 13.6% in this population and noted an overall increase in NAFLD prevalence from 6.3% in 2003 to 17.6% in 2011, which highlights the continued growth in NAFLD clinical burden.10 This study, however, is likely to have underestimated the prevalence of NAFLD among veterans because liver enzymes are often normal among those with NAFLD (ie, low sensitivity), and the prevalence of obesity and T2DM are significantly higher in the veteran population vs the general population.
Incidence
There are limited studies on NAFLD incidence. The largest study of US veterans to date used liver enzymes as its diagnostic criteria and reported an annual NAFLD incidence of 2 to 3 per 100 persons (over 9 years from 2003 to 2011).10 There are a few studies from Asia and Europe, and a recent pooled meta-analysis of these studies reported the incidence of NAFLD in Asia to be 52.3 per 1,000 person-years; the incidence in Western countries was 28 per 1,000 person-years.5 These variances may be explained, in part, to disparities in race/background. For example, Hispanics and South Asians (ie, people from Bangladesh, India, or Pakistan) are at higher risk for NAFLD/NASH.11 These findings reinforce the need for further studies to better estimate the true incidence of NAFLD among veterans.
Chronic Liver Disease, Cirrhosis, and Hepatocellular Carcinoma
The prevalence of NASH cirrhosis also has been evaluated using serologic scores, such as aspartate aminotransferase to platelet ratio index (APRI). The National Health and Nutrition Examination Survey (NHANES) database was reviewed, and data for adults in 2 separate periods were analyzed (1999-2002 and 2009-2012) and the prevalence of NASH cirrhosis was noted to have increased 2.5-fold over the period (0.072% vs 0.178%, P < .05).11 Based on data from the HealthCore Integrated Research Database from 2006 to 2014, about 15% of cirrhosis cases were attributed to NAFLD, and about 24% each were attributed to hepatitis C virus (HCV) and alcoholic liver disease.12 A review of about 60,000 veterans with cirrhosis between 2001 and 2013 revealed a prevalence of NAFLD-related cirrhosis of about 15%, while 48% were attributed to HCV.13 In contrast to the continued increase in NAFLD prevalence, the number of patients with HCV-associated liver disease has been in gradual decline since the advent of direct acting antiviral medications in 2011.12
Based on data from the United Network for Organ Sharing (UNOS), the number of patients awaiting liver transplant due to NAFLD nearly tripled from 2004 to 2013, and in 2013 NAFLD became the second leading disease among waitlisted patients for liver transplantation.14 Dynamic Markov modeling predicts that cases of decompensated NASH cirrhosis (ie, liver failure) will rise by 161%, from about 144,000 to 376,000 cases over the next 15 years.8 These projections predict that NAFLD will overtake HCV as the leading cause of chronic liver disease among patients awaiting a liver transplant and will pose a significant clinical and economic burden in the coming years.
Aside from cirrhosis due to NAFLD, NAFLD-related HCC has been on the rise and has overtaken HCV-related HCC. UNOS data from 2003 to 2015 have shown a 2-fold decline in liver transplantation for HCV-associated HCC; however, the same period saw a 10-fold increase in liver transplantation for NAFLD-associated HCC.15,16 This trend in NAFLD-related HCC is expected to grow from 5,000 to 6,000 cases in 2005 to 45,000 cases by 2025.9 More surprisingly, studies from the US veteran population have reported that patients with NAFLD-related HCC are less likely to have cirrhosis compared with patients with HCV- or alcohol-related HCC.17 Among all US veterans who developed HCC in the absence of cirrhosis between 2005 and 2010, NAFLD and metabolic syndrome seemed to be the leading risk factors for development of HCC.18 These trends raise concern for the rise in noncirrhotic HCC in the NAFLD population and highlight the need to improve current screening guidelines for this subset of patients.
Economic Burden
With such a heavy clinical burden and a projected increase in volume over the next decade, NAFLD is expected to have a similarly exponential impact on the economic burden. A review of 976 Medicare beneficiaries with NAFLD who were hospitalized from January 1, 2010 to December 31, 2010, noted a median annual total payment of about $11,000, with significantly lower payment for patients without cirrhosis compared with those with cirrhosis ($10,146 vs $18,804, P < .01).19 Another review of 29,528 Medicare beneficiaries with NAFLD who sought outpatient care between 2005 and 2010 saw a rise in mean yearly charges in 2005 of $2,624 ± 3,308 to $3,608 ± 5,132 in 2010 (P < .05).20
To place these costs in perspective, Allen and colleagues reviewed a large national claims database of individuals enrolled with private and Medicare advantage health plans.21 Comparing patients with NAFLD with a control matched group with similar metabolic comorbidities the study revealed annual median outpatient care costs of $5,363 for the patients with NAFLD with Medicare advantage plans, which was significantly higher than $4,111 for the control group. Projection models based on similar Medicare beneficiaries estimate a rise in annual US economic burden to $103 billion from direct medical care costs alone and another $188 billion in societal costs related to NAFLD.22 New NASH/antifibrotic therapies are being evaluated in clinical trials and are expected to lead to even higher costs. Given the similarities in the trends of NAFLD prevalence between veterans and the general population, it is anticipated that a similar growth and burden in health care utilization cost will affect the VHA.
Association With Other Chronic Medical Conditions
NAFLD is closely associated with metabolic syndrome (Figure 3). Concurrent diagnosis of NAFLD in patients with existing T2DM is associated with poor glycemic control, progressive diabetic retinopathy, diabetic nephropathy, increased risk of cardiovascular complications, and a 2-fold increase in all-cause mortality.1-3
Similar associations have been described between NAFLD and other metabolic conditions such as obesity, hypertension, hypothyroidism, polycystic ovarian syndrome, and chronic kidney disease.31 Identifying patients with NAFLD may help with screening for the above metabolic diseases because patients with NAFLD (by comparison with patients with non-NAFLD) are at higher risk for diabetic, cardiovascular, and pulmonary complications and may warrant a more intensive treatment approach.
Conclusion
A leading cause of chronic liver disease and cirrhosis in the US, NAFLD is independently associated with metabolic syndrome and all-cause mortality. The number of veterans with NAFLD is expected to grow significantly over the coming years given the ongoing epidemic of adult and childhood obesity and T2DM. It is associated with many other medical conditions, including cardiovascular disease and complications, incident metabolic diseases, and may progress to liver cirrhosis and cirrhosis associated complications like HCC and liver failure. The current lack of any approved drug treatment for NASH/fibrosis and the shortage of organs for liver transplant emphasize the need for comprehensive primary prevention measures to reduce the future health and economic costs associated with NAFLD.
There is a growing need to address the epidemic of metabolic syndrome, as heralded by the World Health Organization in its 2013 global action plan. To address this multifaceted disease, initial approach should be to improve NAFLD education among veterans, beginning with the primary care teams and extending to specialty care, including hepatologists. Future steps also should include the development of a comprehensive metabolic/NAFLD clinic in all US Department of Veterans Affairs medical centers that integrates multidisciplinary care, point-of-care evaluation (eg, elastography staging of disease), and access to clinical trials, and have NAFLD care/outcome as a key performance target for all providers.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
1. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218.
2. Targher G, Bertolini L, Rodella S, et al. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia. 2008;51(3):444-450.
3. Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010. 105(7):1567-1573.
4. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686-690.
5. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84.
6. Angulo P, Machado MV, Diehl AM. Fibrosis in nonalcoholic fatty liver disease: mechanisms and clinical implications. Semin Liver Dis. 2015;35(2):132-145.
7. Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221-235.
8. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904.
9. Ahmed O, Liu L, Gayed A, et al. The changing face of hepatocellular carcinoma: forecasting prevalence of nonalcoholic steatohepatitis and hepatitis C cirrhosis. J Clin Exp Hepatol. 2018. In press.
10. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol. 2016;14(2):301-308.
11. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112(4):581-587.
12. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152(5):1090-1099.
13. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471-1482.
14. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555.
15. Belli LS, Perricone G, Adam R, et al; all the contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810-817.
16. Flemming JA, Kim WR, Brosgart CL, Terrault NA. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65(3):804-812.
17. Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594-601.
18. Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124-131.e1.
19. Sayiner M, Otgonsuren M, Cable R. Variables associated with inpatient and outpatient resource utilization among medicare beneficiaries with nonalcoholic fatty liver disease with or without cirrhosis. J Clin Gastroenterol. 2017;51(3):254-260.
20. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49(3):222-227.
21. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real-world data from a large US claims database. Hepatology. 2018;68(6):2230-2238.
22. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586.
23. Armstrong MJ, Hazlehurst JM, Parker R, et al. Severe asymptomatic non-alcoholic fatty liver disease in routine diabetes care; a multi-disciplinary team approach to diagnosis and management. QJM. 2014;107(1):33-41.
24. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865-873.
25. Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56(2):605-613.
26. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10(6):646-650.
27. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341-1350.
28. Mir HM, Stepanova M, Afendy H, Cable R, Younossi ZM. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol. 2013;3(3):181-185.
29. Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9(2):283-291.
30. Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23(11):1815-1825.
31. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174-1197.
Hospitalist movers and shakers – January 2019
The Michigan chapter of the Society of Hospital Medicine has named Peter Watson, MD, SFHM, as state Hospitalist of the Year. Dr. Watson is the vice president of care management and outcomes for Health Alliance Plan (HAP) in Detroit. The Michigan chapter cited Dr. Watson’s leadership in hospital medicine and “generosity of spirit” as reasons for his selection.
Dr. Watson oversees nurses, social workers, and support staff while also serving as HAP Midwest Health Plan’s medical director. He’s a founding member of the Michigan SHM chapter, which he formerly represented as president.
Dr. Watson spent 11 years overseeing the Henry Ford Medical Group’s hospitalist program prior to joining HAP, and still works as an attending hospitalist for Henry Ford.
Hyung (Harry) Cho, MD, was named the inaugural chief value officer for NYC Health + Hospitals, which includes 11 hospitals in New York and is the largest public health system in the United States. He will oversee systemwide initiatives in value improvement and the reduction of unnecessary testing and treatment.
Prior to this appointment, Dr. Cho served as an academic hospitalist at Mount Sinai Hospital for 7 years, leading high-value care initiatives. Currently, he is a senior fellow with the Lown Institute in Brookline, Mass., and director of quality improvement implementation for the High Value Practice Academic Alliance.
Nick Fitterman, MD, SFHM, has been promoted to executive director at Huntington (N.Y.) Hospital. Dr. Fitterman has been a long-time physician and administrator at Huntington, serving previously as vice chair of medicine as well as head of hospitalists.
Dr. Fitterman has served as president of SHM’s Long Island chapter.
Previously, Dr. Fitterman was chief resident at the State University of New York at Stony Brook, and he remains an associate professor at Hofstra University, Hempstead, N.Y.
Allen Kachalia, MD, was named director of the Armstrong Institute for Patient Safety and Quality and senior vice president of patient safety and quality for Johns Hopkins Medicine in Baltimore. Dr. Kachalia is a general internist who has been an active academic hospitalist at Brigham and Women’s Hospital in Boston.
Dr. Kachalia will oversee patient safety and quality across all of Hopkins Medicine, with a focus on ending preventable harm, improving outcomes and patient experience, and reducing waste in the system’s delivery of care. He also will guide academic efforts for the Armstrong Institute, formed recently thanks to a $10 million gift.
In addition to his hospitalist work, Dr. Kachalia comes to Hopkins after serving as chief quality officer and vice president of quality and safety at Brigham Health.
Riane Dodge, PA, has been elevated to director of clinical education in physician assistant studies at Clarkson University, Potsdam, N.Y. The veteran physician assistant previously worked as a hospitalist in the Claxton Hepburn Medical Center in Ogdensburg, N.Y. There, she cared for patients in acute rehab, mental health, and on regular medical floors.
Dodge also has a background in urgent care and family medicine, and has experience as an emergency department technician.
BUSINESS MOVES
Surgical Affiliates of Sacramento, a surgical hospitalist provider with expertise in trauma, orthopedic, neurosurgery, and general surgery for hospital systems, has added partnerships with Christus Spohn Hospital South and Christus Spohn Hospital Shoreline in Corpus Christi, Texas.
Surgical Affiliates’ hospitalist system will provide round-the-clock emergency orthopedic surgery service to adult and pediatric patients in the two hospitals. With Surgical Affiliates’ help, Christus Spohn facilities will be able to cover its own patients, as well as those requiring transfer from regional hospitals.
Hospitalist surgeons will handle emergency surgeries and patient surgery consultations. Clinics will be provided at each facility to care for patients after they are discharged.
The Michigan chapter of the Society of Hospital Medicine has named Peter Watson, MD, SFHM, as state Hospitalist of the Year. Dr. Watson is the vice president of care management and outcomes for Health Alliance Plan (HAP) in Detroit. The Michigan chapter cited Dr. Watson’s leadership in hospital medicine and “generosity of spirit” as reasons for his selection.
Dr. Watson oversees nurses, social workers, and support staff while also serving as HAP Midwest Health Plan’s medical director. He’s a founding member of the Michigan SHM chapter, which he formerly represented as president.
Dr. Watson spent 11 years overseeing the Henry Ford Medical Group’s hospitalist program prior to joining HAP, and still works as an attending hospitalist for Henry Ford.
Hyung (Harry) Cho, MD, was named the inaugural chief value officer for NYC Health + Hospitals, which includes 11 hospitals in New York and is the largest public health system in the United States. He will oversee systemwide initiatives in value improvement and the reduction of unnecessary testing and treatment.
Prior to this appointment, Dr. Cho served as an academic hospitalist at Mount Sinai Hospital for 7 years, leading high-value care initiatives. Currently, he is a senior fellow with the Lown Institute in Brookline, Mass., and director of quality improvement implementation for the High Value Practice Academic Alliance.
Nick Fitterman, MD, SFHM, has been promoted to executive director at Huntington (N.Y.) Hospital. Dr. Fitterman has been a long-time physician and administrator at Huntington, serving previously as vice chair of medicine as well as head of hospitalists.
Dr. Fitterman has served as president of SHM’s Long Island chapter.
Previously, Dr. Fitterman was chief resident at the State University of New York at Stony Brook, and he remains an associate professor at Hofstra University, Hempstead, N.Y.
Allen Kachalia, MD, was named director of the Armstrong Institute for Patient Safety and Quality and senior vice president of patient safety and quality for Johns Hopkins Medicine in Baltimore. Dr. Kachalia is a general internist who has been an active academic hospitalist at Brigham and Women’s Hospital in Boston.
Dr. Kachalia will oversee patient safety and quality across all of Hopkins Medicine, with a focus on ending preventable harm, improving outcomes and patient experience, and reducing waste in the system’s delivery of care. He also will guide academic efforts for the Armstrong Institute, formed recently thanks to a $10 million gift.
In addition to his hospitalist work, Dr. Kachalia comes to Hopkins after serving as chief quality officer and vice president of quality and safety at Brigham Health.
Riane Dodge, PA, has been elevated to director of clinical education in physician assistant studies at Clarkson University, Potsdam, N.Y. The veteran physician assistant previously worked as a hospitalist in the Claxton Hepburn Medical Center in Ogdensburg, N.Y. There, she cared for patients in acute rehab, mental health, and on regular medical floors.
Dodge also has a background in urgent care and family medicine, and has experience as an emergency department technician.
BUSINESS MOVES
Surgical Affiliates of Sacramento, a surgical hospitalist provider with expertise in trauma, orthopedic, neurosurgery, and general surgery for hospital systems, has added partnerships with Christus Spohn Hospital South and Christus Spohn Hospital Shoreline in Corpus Christi, Texas.
Surgical Affiliates’ hospitalist system will provide round-the-clock emergency orthopedic surgery service to adult and pediatric patients in the two hospitals. With Surgical Affiliates’ help, Christus Spohn facilities will be able to cover its own patients, as well as those requiring transfer from regional hospitals.
Hospitalist surgeons will handle emergency surgeries and patient surgery consultations. Clinics will be provided at each facility to care for patients after they are discharged.
The Michigan chapter of the Society of Hospital Medicine has named Peter Watson, MD, SFHM, as state Hospitalist of the Year. Dr. Watson is the vice president of care management and outcomes for Health Alliance Plan (HAP) in Detroit. The Michigan chapter cited Dr. Watson’s leadership in hospital medicine and “generosity of spirit” as reasons for his selection.
Dr. Watson oversees nurses, social workers, and support staff while also serving as HAP Midwest Health Plan’s medical director. He’s a founding member of the Michigan SHM chapter, which he formerly represented as president.
Dr. Watson spent 11 years overseeing the Henry Ford Medical Group’s hospitalist program prior to joining HAP, and still works as an attending hospitalist for Henry Ford.
Hyung (Harry) Cho, MD, was named the inaugural chief value officer for NYC Health + Hospitals, which includes 11 hospitals in New York and is the largest public health system in the United States. He will oversee systemwide initiatives in value improvement and the reduction of unnecessary testing and treatment.
Prior to this appointment, Dr. Cho served as an academic hospitalist at Mount Sinai Hospital for 7 years, leading high-value care initiatives. Currently, he is a senior fellow with the Lown Institute in Brookline, Mass., and director of quality improvement implementation for the High Value Practice Academic Alliance.
Nick Fitterman, MD, SFHM, has been promoted to executive director at Huntington (N.Y.) Hospital. Dr. Fitterman has been a long-time physician and administrator at Huntington, serving previously as vice chair of medicine as well as head of hospitalists.
Dr. Fitterman has served as president of SHM’s Long Island chapter.
Previously, Dr. Fitterman was chief resident at the State University of New York at Stony Brook, and he remains an associate professor at Hofstra University, Hempstead, N.Y.
Allen Kachalia, MD, was named director of the Armstrong Institute for Patient Safety and Quality and senior vice president of patient safety and quality for Johns Hopkins Medicine in Baltimore. Dr. Kachalia is a general internist who has been an active academic hospitalist at Brigham and Women’s Hospital in Boston.
Dr. Kachalia will oversee patient safety and quality across all of Hopkins Medicine, with a focus on ending preventable harm, improving outcomes and patient experience, and reducing waste in the system’s delivery of care. He also will guide academic efforts for the Armstrong Institute, formed recently thanks to a $10 million gift.
In addition to his hospitalist work, Dr. Kachalia comes to Hopkins after serving as chief quality officer and vice president of quality and safety at Brigham Health.
Riane Dodge, PA, has been elevated to director of clinical education in physician assistant studies at Clarkson University, Potsdam, N.Y. The veteran physician assistant previously worked as a hospitalist in the Claxton Hepburn Medical Center in Ogdensburg, N.Y. There, she cared for patients in acute rehab, mental health, and on regular medical floors.
Dodge also has a background in urgent care and family medicine, and has experience as an emergency department technician.
BUSINESS MOVES
Surgical Affiliates of Sacramento, a surgical hospitalist provider with expertise in trauma, orthopedic, neurosurgery, and general surgery for hospital systems, has added partnerships with Christus Spohn Hospital South and Christus Spohn Hospital Shoreline in Corpus Christi, Texas.
Surgical Affiliates’ hospitalist system will provide round-the-clock emergency orthopedic surgery service to adult and pediatric patients in the two hospitals. With Surgical Affiliates’ help, Christus Spohn facilities will be able to cover its own patients, as well as those requiring transfer from regional hospitals.
Hospitalist surgeons will handle emergency surgeries and patient surgery consultations. Clinics will be provided at each facility to care for patients after they are discharged.
Sleep disorders in children with ADHD treated with off-label medications
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
Sleep problems in children diagnosed with attention-deficit/hyperactivity disorder are treated with a variety of medications, many off label for sleep and unstudied for safety and effectiveness in children, a study of Medicaid prescriptions has found.
“Sleep disorders coexist with attention-deficit/hyperactivity disorder (ADHD) for many children and are associated with neuropsychiatric, physiologic, and medication-related outcomes,” wrote Tracy Klein, PhD, of Washington State University, Vancouver, and her colleagues. The report is in the Journal of Pediatric Health Care. These patients can have sleep disordered breathing and behavioral issues occurring around bedtime. Known adverse effects of the stimulant and nonstimulant medications used to treat ADHD can include sleep disturbance, delayed circadian rhythm, insomnia, and somnolence. Yet, research on both sleep problems in children with ADHD and prescribing patterns is scanty, according to the investigators.
Dr. Klein and her colleagues conducted a study aimed at identifying the off-label medications being prescribed to potentiate sleep in children with ADHD, and the characteristics of the children and their prescribers. They used 5 years of pharmacy claims for children in Oregon insured through Medicaid and had a provider diagnosis of ADHD during Jan. 1, 2012, to Dec. 31, 2016. The children were aged 3-18 years and the prescriptions measured were the number of 30-day prescriptions. Prescribers were identified by national provider identifier taxonomies (nurse, physician, other prescriber), and classified as either generalist or specialist. The medications were classified as controlled or uncontrolled as determined by Title 21 of the U.S. Controlled Substances Act.
The data yielded 14,567 prescriptions for 2,518 children for a 30-day supply of medication known to potentiate sleep but off-label for children. Children aged 3-11 years comprised about 38% of these patients. Some children were prescribed more than one of these medications. Medications specifically on label for sleep but not indicated for children were not included. Those medications indicated for comorbid conditions and those indicated for ADHD that specifically cause somnolence were excluded.
The uncontrolled medications prescribed in this sample were amitriptyline, doxepin, hydroxyzine, low-dose quetiapine, and trazodone. The controlled medications identified were clonazepam and lorazepam, and a few prescriptions for phenobarbital.
Most of the prescriptions (63.8%) went to older children aged 12-18 years and most prescriptions (66.3%) went to males. The most commonly prescribed noncontrolled medication was trazodone (5,190 prescriptions), followed by hydroxyzine (2,539), and quetiapine (2,402). The most frequently prescribed controlled medication was clonazepam (2,145), followed by lorazepam (534).
Specialist prescribers wrote most of the prescriptions for this patient group, but no differences were found in prescribing patterns between specialists and generalists.
Dr. Klein and her colleagues noted that 871 unique children were prescribed 5,190 30-day−supply prescriptions for trazodone, including 23 children under age 5. Trazodone is a serotonin modulator indicated for the treatment of major depressive disorder, but has not been studied for safety and efficacy in children and has no Food and Drug Administration indication for children. “Hydroxyzine, quetiapine, and amitriptyline also were prescribed for a large number of children, including some for children as young as 3 years, despite lack of approval for use to induce to sleep and increased potential for significant adverse reactions in children,” they wrote.
Dr. Klein suggested that prescribers receive pressure from families to “do something” for their children, who may be disruptive day and night. “Prescribers may be unaware that trazodone, which is commonly used in practice, has never been approved for treatment of insomnia in children or adults. Insurance may not adequately fund other options, such as extensive behavioral therapy,” she stated in an interview. These medications come with some risk for children, Dr. Klein noted.
especially if their reaction to it is behavioral.” There is also potential for unanticipated drug interactions between off-label medications prescribed for sleep and drugs prescribed to treat ADHD.
This study has limitations related to the absence of detailed clinical explanatory information found in claims data. Information on adherence to treatment and adverse events, for example, is not contained in claims data. The study does not address the overall rates of sleep disorders in children with ADHD nor the percentage of children with ADHD who are prescribed any medication to potentiate sleep but looks at which off-label drugs are being prescribed, to which children, and by whom.
“Most medications prescribed in this study, used to induce sleep or treat insomnia, have not been studied for safety and efficacy in children, and their use should not be extrapolated from adult studies,” the researchers concluded.
They reported having no disclosures.
SOURCE: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
FROM THE JOURNAL OF PEDIATRIC HEALTH CARE
Key clinical point: The most commonly prescribed off-label medications prescribed to children were trazodone (5,190), hydroxyzine (2,539), quetiapine (2,402), and clonazepam (2,145).
Major finding: Most of the prescriptions (63.8%) went to older children aged 12-18 years, and most prescriptions (66.3%) went to males.
Study details: Medicaid claims data for Jan. 1, 2012, to Dec. 31, 2016, yielding 14,567 prescriptions of off-label medications for 2,518 children.
Disclosures: The investigators reported no disclosures.
Source: Klein T et al. J Pediatr Health Care. 2018 Jan 8. doi: 10.1016/j.pedhc.2018.10.002.
Treprostinil improves function for complex PAH patients
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
Treatment with , a study based on data from 105 adults has shown.
Data on the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) with treprostinil are limited, although alternatives to surgery are needed for many patients with the condition, wrote Roela Sadushi-Koliçi, MD, of the Medical University of Vienna, and her colleagues.
The researchers conducted a phase 3 randomized, controlled trial of the safety and efficacy of subcutaneous treprostinil for nonoperable CTEPH or persistent or recurrent pulmonary hypertension after pulmonary endarterectomy; the findings were published online in the Lancet Respiratory Medicine. The patients received continuous subcutaneous treprostinil at either 30 ng/kg per min (high dose) or 3 ng/kg per min (low dose) and all patients were assessed at weeks 6, 12, 18, and 24.
Overall, 6-minute walk distance, hemodynamics, and functional status significantly improved in the high-dose patients, compared with the low-dose patients.
The primary outcome of 6-minute walk distance increased by 44.98 m from baseline in the high-dose group, compared with an increase of 4.29 m from baseline in the low-dose group.
In addition, “changes in pulmonary vascular resistance, one of the most important prognostic indicators of CTEPH, were significant in favour of high-dose subcutaneous treprostinil, as were improvements of WHO functional class and N-terminal probrain natriuretic peptide,” the researchers noted.
Rates of serious adverse events were similar between the groups; a total of 12 serious adverse events were reported in 10 of 52 patients in the low-dose group (19%) and 16 serious adverse events were reported in 9 of 53 patients in the high-dose group (17%). In both groups, the most common treatment-related adverse events were infusion site pain and other infusion site reactions.
The findings were limited by the small sample size and the possibility that the 6-minute walk test might not translate to long-term outcomes for PAH and CTEPH, the researchers wrote. However, the data support the safety and efficacy of subcutaneous treprostinil for CTEPH patients who do not tolerate riociguat, the other approved option for nonoperable CTEPH, or those who need combination therapy, they said.
The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
SOURCE: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: Treprostinil is a safe and effective nonsurgical treatment option for severe CTEPH patients.
Major finding: After 24 weeks, 6-minute walk distance improved by 44.98 m from baseline in the high-dose group compared with an increase of 4.29 m from baseline in the low-dose group.
Study details: The data come from a randomized trial of 105 adults with confirmed CTEPH.
Disclosures: The study was supported in part by SciPharm Sàrl and United Therapeutics, which provided the medication for part of the study. Dr. Sadushi-Koliçi disclosed relationships with Actelion, AOP Orphan Pharmaceuticals, Bayer Schering Pharma, GlaxoSmithKline, and SciPharm Sàrl, among others.
Source: Sadushi-Koliçi R et al. Lancet Respir Med. 2018 Nov 23. doi: 10.1016/S2213-2600(18)30367-9.
A prescription for ‘deprescribing’: A case report
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
In 2016, Swapnil Gupta, MD, and John Daniel Cahill, MD, PhD, challenged the field of psychiatry to reexamine our prescribing patterns. They warned against our use of polypharmacy when not attached to improvement in functioning for our patients.1 They were concerned about the lack of evidence for those treatment regimens and for our diagnostic criteria. In their inspiring article, they described how psychiatrists might proceed in the process of “deprescribing” – which they define as a process of pharmacologic regimen optimization through reducing or ending medications for which “benefits no longer outweigh risks.”1
In my practice, I routinely confront medication regimens that I have never encountered in the literature. The evidence for two psychotropics is limited but certainly available, in particular adjunct treatment of antidepressants2 and mood stabilizers.3 The evidence supporting the use of more than two psychotropics, however, is quite sparse. Yet, patients often enter my office on more than five psychotropics. I am also confronted with poorly defined diagnostic labels – which present more as means to justify polypharmacy than a thorough review of the patient’s current state.
Dr. Gupta and Dr. Cahill recommend a series of steps aimed at attempting the deprescription of psychotropics. Those steps include timeliness, knowledge of the patient’s current regimen, discussion about the risk of prescriptions, discussion about deprescribing, choosing the right medications to stop, a plan for describing, and monitoring. In the case presented below, I used some of those steps in an effort to provide the best care for the patient. Key details of the case have been changed, including the name, to protect the patient’s confidentiality.
Overview of the case
Rosalie Bertin is a 54-year-old female who has been treated for depression by a variety of primary care physicians for the better part of the last 30 years. She had tried an array of antidepressants, including sertraline, citalopram, duloxetine, and mirtazapine, over that time. Each seemed to provide some benefit when reviewing the notes, but there is no mention of why she was continued on those medications despite the absence of continuing symptoms. Occasionally, Rosalie would present to her clinician tearful and endorsing sadness, though the record did not comment on reports of energy, concentration, sleep, appetite, and interest.
In 2014, Rosalie’s husband passed away from lung cancer. His death was fairly quick, and initially, Rosalie did not mention any significant emotional complaints. However, when visiting her primary care physician 4 months later, she was noted to experience auditory hallucinations. “Sometimes I hear my husband when I am alone in my home,” she said. Rosalie was referred to a psychiatrist with a diagnosis of “psychosis not otherwise specified.”
When discussing her condition with the psychiatrist, Rosalie mentioned experiencing low mood, and having diminished interest in engaging in activities. “I miss Marc when I go places; I used to do everything with him.” She reported hearing him often but only when at her home. He would say things like, “I miss you,” or ask her about her day. She was diagnosed with “major depressive disorder with psychotic features.” Risperidone was added to the escitalopram, buspirone, and gabapentin that had been started by her primary care physician.
After several months of psychotropic management, the dose of risperidone was titrated to 8 mg per day. Her mood symptoms were unchanged, but she now was complaining of poor concentration and memory. The psychiatrist performed a Mini-Mental State Examination (MMSE). It was noted that taking the MMSE engendered significant anxiety for the patient. Rosalie received a score suggesting mild cognitive impairment. She was started on donepezil for the memory complaint, quetiapine for the continued voices, and recommended for disability.
Once on short-term disability, the patient relocated to live closer to her mother in San Diego and subsequently contacted me about continuing psychiatric care.
Initial visit
Rosalie is a petite white woman, raised in the Midwest, who married her high school sweetheart, and subsequently became an administrative assistant. Rosalie and Marc were unable to have children. Marc was an engineer, and a longtime smoker. She describes their lives as simple – “few friends, few vacations, few problems, few regrets.” She states she misses her husband and often cries when thinking about him.
When asked about psychiatric diagnoses, she answered: “I have psychosis. … My doctor said maybe schizophrenia, but he is not sure yet.” She described schizophrenia as hearing voices. Rosalie also mentioned having memory problems: “They cannot tell if it is Alzheimer’s disease until I die and they look at my brain, but the medication should delay the progression.”
She reported no significant effect from her prior antidepressant trials: “I am not sure if or how they helped.” Rosalie could not explain the role of the medication. “I take medications as prescribed by my doctor,” she said.
When discussing her antipsychotics, she mentioned: “Those are strong medications; it is hard for me to stay awake with them.” She declared having had no changes in the voices while on the risperidone but said they went away since also being on the quetiapine: “I wonder if the combination of the two really fixed my brain imbalance.”
Assessment
I admit that I have a critical bias against the overuse of psychotropics, and this might have painted how I interpreted Rosalie’s story. Nonetheless, I was honest with her and told her of my concerns. I informed her that her diagnosis was not consistent with my understanding of mood and thought disorders. Her initial reports of depression neither met the DSM criteria for depression nor felt consistent with my conceptualization of the illness. She had retained appropriate functioning and seemed to be responding with the sadness expected when facing difficult challenges like grief.
Her subsequent reports of auditory hallucinations were not associated with delusions or forms of disorganization that I would expect in someone with a thought disorder. Furthermore, the context of the onset gave me the impression that this was part of her process of grief. Her poor result in the dementia screen was most surprising and inconsistent with my evaluation. I told her that I suspected that she was not suffering from Alzheimer’s but from being overmedicated and from anxiety at the time of the testing.
She was excited and hesitant about my report. She was surprised by the length of our visit and interested in hearing more from me. Strangely, I wished she had challenged my different approach. I think that I was hoping she would question my conceptualization, the way I hoped she would have done with her prior clinicians. Nonetheless, she agreed to make a plan with me.
Treatment plan
We decided to review each of her medications and discuss their benefits and risks over a couple of visits. She was most eager to discontinue the donepezil, which had caused diarrhea. She was concerned when I informed her of the potential side effects of antipsychotics. “My doctor asked me if I had any side effects at each visit; I answered that I felt nothing wrong; I had not realized that side effects could appear later.”
She was adamant about staying on buspirone, as she felt it helped her the most with her anxiety at social events. She voiced concern about discontinuing the antipsychotics despite being unsettled by my review of their risks. She asked that we taper them slowly.
In regard to receiving psychosocial support throughout this period of deprescribing, Rosalie declined weekly psychotherapy. She reported having a good social network in San Diego that she wanted to rely on.
Outcome
I often worry about consequences of stopping a medication, especially when I was not present at the time of its initiation. I agonize that the patient might relapse from my need to carry out my agenda on deprescribing. I try to remind myself that the evidence supports my decision making. The risks of psychotropics often are slow to show up, making the benefit of deprescribing less tangible. However, this case was straightforward.
Rosalie quickly improved. Tapering the antipsychotics was astonishing to her: “I can think clearly again.” Within 6 months, she was on buspirone only – though willing to discuss its discontinuation. She had a lead for a job and was hoping to return to work soon. Rosalie continued to miss her husband but had not heard him in some time. She has not had symptoms of psychosis or depression. Her cognition and mood were intact on my clinical assessment.
Discussion
Sadly and shockingly, cases like that of Rosalie are common. In my practice, I routinely see patients on multiple psychotropics – often on more than one antipsychotic. Their diagnoses are vague and dubious, and include diagnoses such as “unspecified psychosis” and “cognitive impairments.” Clinicians occasionally worry about relapse and promote a narrative that treatment must be not only long term but lifelong.4 There is some evidence for this perspective in a research context, but the clinical world also is filled with patients like Rosalie.
Her reports of auditory hallucinations were better explained by her grief than by a psychotic process.5 Her memory complaints were better explained by anxiety at the time of her testing while suffering from the side effects from her numerous psychotropics.6 Her depressive complaints were better explained by appropriate sadness in response to stressors. Several months later with fewer diagnoses and far fewer psychotropics, she is functioning better.
Take-home points
- Polypharmacy can lead to psychiatric symptoms and functional impairment.
- Patients often are unaware of the complete risks of psychotropics.
- Psychiatric symptoms are not always associated with a psychiatric disorder.
- Deprescribing can be performed safely and effectively.
- Deprescribing can be performed with the patient’s informed consent and agreement.
References
1. Psychiatr Serv. 2016 Aug 1;67(8):904-7.
2. Focus. 2016 Apr 13; doi: 10.1176/appi.focus.20150041.
3. Bipolar Disord. 2016 Dec;18(8):684-91.
4. Am J Psychiatry. 2017 Sep 1;174(9):840-9.
5. World Psychiatry. 2009 Jun;8(2):67-74.
6. Hosp Community Psychiatry. 1983 Sep;34(9):830-5.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Among his writings is chapter 7 in the new book “Critical Psychiatry: Controversies and Clinical Implications” (Springer, 2019).
*This column was updated 1/11/2019.
Dealing with difficult people
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
Dealing with difficult people is not a new problem. As long as there are at least two people, the potential for conflict will arise. Unfortunately, the workplace or hospital is not immune from tragedies that are born out of poor conflict resolution. Before we go further, please do not ignore the fact that more than 1 million workers are assaulted each year, and more than 60% of Americans are aware of some type of abusive conduct occurring on the job.
Who are those difficult people we may encounter? Anyone and everyone. Difficult people may include our significant others, family members, supervisors, department chairs, colleagues, competitors, trainees, patients and their families, and ancillary personnel. Looking at this list, it is amazing that we aren’t either stymied by never-ending conflict resolution seminars, or rendered completely ineffective in all aspects of life. Daily conflicts can vary in intensity and degree. At one end one can be disgruntled at the person who secured the last doughnut in the break room, and at the other extreme end one is committed to moving forward with a multimillion dollar lawsuit against the company.
Conflicts arise because of a multiplicity of reasons – work style differences, background differences, attitude difference, personality types, and competitive versus cooperative differences. To be effective, each of us must realize that we are more alike than different, and it is our differences that should fuel our passion for providing excellent patient care and customer service.
In particular, be aware of things that can accelerate the potential for conflicts – performance ratings, evaluations, recommendation for promotion, absence of role models or mentors, lack of support amongst colleagues, and failures on the part of leadership to keep promises, appreciate people, maintain personal integrity, or take responsibility for their own errors.
When conflict arises – deal with it! Identify the problem, and if it is legitimate address it as soon as possible. Always remember to document the details in writing; never forget the old adage most of us learned during training: “If it’s not written/documented it wasn’t done or didn’t happen.” More than likely you won’t need to retrieve your written documents concerning a particular conflict, but if the conflict escalates, this type of documentation will prove invaluable.
Communicate with the person or persons with whom you have the conflict – it is essential that you have the “difficult” conversation. This conversation must be done face-to-face and in private. Never communicate by email, social media, or through gossip. Remain calm, professional, and show respect even if the other person does not. At this meeting detail the problem, but also come prepared with suggestions as to how the conflict might be resolved.
Take responsibility – you can’t control situations or people – but you can choose how you will respond to every situation. This is the appropriate time to establish boundaries; avoid any behavior that might be considered bullying or harassment. Redirect negativity that emanates from the person with whom you have the conflict as well as any potentially self-imposed negativity. Make every effort to avoid statements that include “you never” and “you always,” as there are very few absolutes in life. Consider the other person’s perspective as well; try to see it from their point of view because your “personal truth” is not the only “truth.” Our individual personal life experiences form the foundation for much of our opinions and views; therefore, it should be obvious that persons from widely varied backgrounds and cultures will differ in their approaches. If at all possible, give the person another chance; even the most difficult person has good attributes.
Once you have had the “difficult” conversation and there is still no resolution in sight you should take it to management. Everyone has a boss – even the Boss! There is much to gain from involving an impartial party or mediator. This impartial individual is able to understand the viewpoint of all parties involved and frequently that person’s solution may be considered acceptable because it is coming from someone not directly affected by the conflict.
Unresolved conflicts result in many negative effects – interference with one’s career is foremost – and that alone can be a source of undue stress. Other negative effects are the development of a hostile work environment, diminished productivity, low morale, and high employee turnover. Physicians in particular are prone to experiencing an increase in medical errors, litigation claims, and poor patient care when there are unresolved conflicts on the table.
In an ideal world, there are no difficult people; there are either no conflicts or all conflicts are resolved immediately without any lasting deleterious effects. Unfortunately, the world abounds in conflict at varying stages of resolution. As a final bit of advice, in dealing with difficult persons, do not allow conflicts to obscure your goals for successful patient care and/or customer service. Focus on why you decided to join your place of employment and realize that everyone has a role in making the team work! If you are dedicated to addressing conflicts as they arise, and utilizing the strategies outlined, you will often find that foes can truly become friends.
Dr. Cole is associate section chief, gastroenterology, and chief, GI endoscopy, Michael E. DeBakey VA Medical Center; and associate professor, internal medicine, Baylor College of Medicine, Houston.
Glucocorticoid Treatment of Symptomatic Sarcoidosis in 2 Morbidly Obese Patients
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Corticosteroid management for patients with sarcoidosis requires the need for close monitoring to detect and manage any complications that may arise during treatment.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
Sarcoidosis is a systemic inflammatory condition with pulmonary and extrapulmonary manifestations. The etiology of sarcoidosis remains unknown. Iannuzzi and colleagues hypothesize that an unknown antigen sets off a cycle of chronic granulomatous inflammation in a genetically susceptible host.1
Diagnosis
A diagnosis of sarcoidosis is typically based on a patient having an appropriate clinical presentation and a biopsy, often of lungs or skin, showing noncaseating granulomas.
Symptoms
Of the protean manifestations of sarcoidosis, respiratory symptoms are the most common and typically include subacute or chronic cough and progressive dyspnea on exertion.2 Chest imaging may show only hilar or mediastinal lymphadenopathy, diffuse micronodular lung disease, or signs of chronic inflammation and fibrosis.2 Upper airway involvement and progressive lung disease may lead to increased risk of sleep-disordered breathing, particularly obstructive sleep apnea (OSA).3
Sarcoidosis also can develop in the skin, neurologic system, heart, and other systems. It typically presents as areas of patchy, infiltrative inflammation. In the heart, this can lead to heart failure, often with reduced ejection fraction (EF) and ventricular arrhythmias.1 Pulmonary hypertension (PH) may result from multiple possible mechanisms, including left-heart disease, parenchymal lung disease, sleep-disordered breathing, and possibly direct inflammation and compression of the pulmonary vasculature.2-4
Sarcoidosis in Obese Patients
Emerging evidence shows that sarcoidosis occurs at higher rates in obese patients, suggesting that obesity may be a risk factor for the disease.5-7 Rates of morbid obesity are increasing in the US. From 2000 to 2010, the prevalence of morbid obesity, defined as body mass index (BMI) > 40, increased by 70%, with even larger relative increases in the number of patients with BMI > 50.8 Among veterans who receive health care at the US Department of Veterans Affairs (VA) medical centers, 28% are obese.9 As a result, VA physicians will encounter more patients with morbid obesity and another significant comorbid condition.
Managing symptomatic sarcoidosis in patients with morbid obesity poses a dilemma. Typical treatment for symptomatic pulmonary sarcoidosis is prednisone 20 mg to 40 mg daily.10,11 Higher doses are suggested for involvement of other organs, such as the heart.2,12 Associated weight gain from corticosteroid treatment with possible sleep-disordered breathing increases an already high risk of metabolic complications in morbidly obese patients.13 No clear consensus exists on how corticosteroid doses should be adjusted. We present 2 cases that highlight the complexity of corticosteroid management in the obese sarcoidosis patient.
Case 1: Pulmonary Sarcoidosis
A 43-year-old morbidly obese man presented to his primary care provider with subacute onset of dyspnea. He had a history of OSA that was diagnosed empirically at another institution without polysomnogram and treated with autotitrating continuous positive airway pressure (CPAP).
The patient was admitted for expedited evaluation. His BMI was 63.2 with declining exercise tolerance and hypoxemia on ambulation. His oxyhemoglobin saturation rate was 85% after walking a short distance. Ongoing CPAP therapy for sleep-disordered breathing made laboratory evaluation for obesity hypoventilation syndrome (OHS) challenging. The patient’s serum bicarbonate test result was normal. Serum markers as well as induced sputum stains and cultures were negative for evidence of mycobacterial or fungal infections. A chest radiograph showed bilateral hilar adenopathy and miliary nodularity. Pulmonary function testing revealed severe obstruction and restriction as well as a moderate diffusion impairment. Bronchoscopy with biopsy revealed noncaseating granulomas consistent with sarcoidosis. An electrocardiogram (ECG) was normal. Transthoracic echocardiogram showed evidence of diastolic dysfunction and a mildly dilated right ventricle with normal function, suggestive of possible PH. We were unable to assess his pulmonary artery pressure.
Upon release, the patient began a course of 50 mg (0.24 mg/kg actual body weight) oral prednisone daily and home oxygen.
Six weeks after initiation of steroids, the patient reported that his dyspnea had improved. However, after 6 months of steroid treatment, his weight increased from 462 pounds to 503 pounds. He was evaluated for possible neurosarcoidosis with hypothalamic or pituitary involvement as a possible cause for the weight gain. Brain magnetic resonance imaging and hormonal testing were normal. We considered starting him on a steroid-sparing agent. However, after early efficacy, prednisone was gradually tapered and, after 1 year of treatment, discontinued. At that time, symptoms had substantially improved: His pulmonary function tests had normalized, and he was weaned off oxygen; repeat chest imaging showed only residual enlargement of the hilar lymph nodes. After cessation of steroids, the patient was able to lose 20 pounds.
Case 2: Cardiac Sarcoidosis
A 57-year-old morbidly obese man presented to the emergency department with subacute increasing dyspnea on exertion. He had a known history of sarcoidosis diagnosed by skin biopsy 28 years earlier but had been without treatment for decades. His history also included prediabetes, heart failure with preserved ejection fraction (HFpEF), OSA with an apnea hypopnea index (AHI) of 114.7 per hour, PH diagnosed by prior echocardiogram, and paroxysmal atrial fibrillation (AF). He required 2 L/m home oxygen and bilevel positive airway pressure (PAP) of 22/17 cm H2O while sleeping.
On physical examination, the patient’s BMI was 54.6. He was tachycardic and hypoxemic on his usual oxygen flow rate. His serum bicarbonate, arterial blood pH, and PaCO2 blood levels were normal. We heard bibasilar crackles over the lungs. Chest radiograph revealed an enlarged cardiac silhouette and bilateral infiltrates concerning for cardiogenic pulmonary edema. An echocardiogram showed a restrictive filling pattern with preserved EF and moderate dilation and dysfunction of the right ventricle, consistent with PH. A positron emission tomography (PET)/computed tomography scan, the preferred study for cardiac sarcoidosis, suggested active infiltrative septal cardiac disease and active hilar and mediastinal adenopathy. This was concerning for both cardiac and pulmonary sarcoidosis. Ongoing treatment of sleep-disordered breathing made laboratory assessment for OHS challenging. Given his intact EF, the absence of ventricular arrhythmias, and improvement with diuretics and bilevel PAP, specific treatment of sarcoidosis was not initiated. He was discharged home with a plan to re-evaluate sarcoidosis symptoms and initiate treatment as an outpatient.
The patient was readmitted 2 weeks later with worsened dyspnea, hypoxemia, and volume overload. A right heart catheterization confirmed PH with a mean pulmonary artery pressure of 44 mm Hg (68/32 mm Hg) and pulmonary vascular resistance of 4.6 Wood units. We also found evidence of left-heart dysfunction with a pulmonary capillary wedge pressure of 16 mm Hg.
Given his recurrent symptoms, evidence of active myocardial inflammation on recent PET, and prior biopsy-proven sarcoidosis, we made the decision to pursue treatment for symptomatic sarcoidosis. He began a course of 40 mg (0.20 mg/kg actual body weight) oral prednisone daily. He now required 6 L/m supplemental oxygen. After IV diuretic therapy during his hospitalization, the patient was discharged on his preadmission oral diuretic dose. Pulmonary vasodilator therapy was not initiated for PH as left heart disease and sleep-disordered breathing needed to be managed first.
One month after steroid initiation, the patient reported that the dyspnea and hypoxemia had markedly improved. His oxygen flow rate was reduced to 2 L/m. He remained normotensive and had no further difficulties with fluid retention or volume overload on a stable dose of oral diuretics. He had elevated blood glucose with a glycated hemoglobin (HbA1c) of 6.4%. He began treatment with glipizide 5 mg daily.
After 3 months, he returned to the emergency department with hyperosmolar nonketotic hyperglycemia due to steroid-induced diabetes mellitus (DM). His HbA1c was now 17.1%. The patient was started on a home insulin regimen, and his blood sugar values subsequently improved. He remained symptomatically better and lost 40 pounds with a guided weight management program and a stable diuretic regimen. He underwent arrhythmia evaluation with a Holter monitor that showed AF without ventricular arrhythmias.
Unfortunately, he did not return for cardiac or pulmonary reevaluation, and was lost to follow-up. Nine months after initiation of treatment, the patient died after an out-of-hospital cardiac arrest.
Discussion
These 2 cases highlight therapeutic challenges that may arise in the management of sarcoidosis with symptomatic vital organ involvement and coexistent extreme obesity. Both patients showed symptom improvement with moderate doses of prednisone (40 mg to 50 mg daily), but serious treatment-related complications developed: further weight gain in the first patient, and severe DM in the second. Although DM may have been a direct treatment complication in our second patient, his HFpEF and PH were high-risk comorbidities; he did not present with acute symptomatic worsening after treatment initiation. His symptoms were never reassessed when he was lost to follow-up.
Sarcoidosis/Obesity Relationship
Recent evidence suggests that patients with obesity are at increased risk of developing sarcoidosis.5-7 Although the mechanism of association is unclear, several possibilities have been proposed.
Neurosarcoidosis. One known but rare cause of obesity is neurosarcoidosis of the hypothalamus or pituitary.14 This was investigated in one of our patients.
Proinflammatory responses. Another possible mechanism for the association of sarcoidosis and obesity is the proinflammatory properties of increased fat and adipose tissue.15 Obesity has been linked to an aberrant expansion of inflammatory cells and mediators, including macrophages, proinflammatory cytokines, T cells, and B cells.15 Leptin, produced primarily by adipocytes, also is higher in obese patients and has been found to be proinflammatory.16 These seem to underlie the link between obesity and other inflammatory diseases, including type 2 DM, gout, and atherosclerosis.15
Behavioral link. There also is a possible behavioral link between sarcoidosis and obesity: A patient might develop symptomatic sarcoidosis and later become less active due to dyspnea, which could predispose to weight gain.5
Management of Comorbid Sarcoidosis and Obesity
Regardless of the exact mechanism of this association, management of the co-occurrence of sarcoidosis and obesity poses a clinical problem, especially in cases of extreme obesity. Corticosteroids are generally considered the treatment of choice for symptomatic sarcoidosis. The initial treatment of symptomatic pulmonary sarcoidosis is 20 mg to 40 mg prednisone daily.10,11 Higher daily doses such as 60 mg to 80 mg or 0.5 mg/kg are typically used to treat cardiac sarcoidosis, although no clear consensus exists on the appropriate dose.12,17 One recent study showed no difference in cardiac outcomes in patients treated with high- and low-dose prednisone.18
For patients who are obese and require steroids to treat a medical condition, there is conflicting evidence on whether steroid doses should be increased in proportion to total body weight. Milsap and colleagues found clearance of prednisolone correlated strongly with degree of obesity, suggesting steroid dose should be increased in accordance with actual weight.19 In contrast, Dunn and colleagues found decreased clearance of methylprednisolone in obese patients, suggesting that ideal body weight dosing is appropriate.20
Identifying the appropriate steroid dose is important because corticosteroids place obese patients at higher risk of developing complications. Treatment-related comorbidities include DM, hypertension, fluid retention, osteoporosis, and infection. Further weight gain due to steroid use is a risk for progressive OSA and, even though not generally associated with sarcoidosis alone, OHS. For patients with sarcoidosis, these complications (DM, fluid retention, hypertension, sleep-disordered breathing) may increase the risk of cardiovascular disease and PH.21-23 Cardiomyopathy, especially with reduced EF and increased PH, can be associated with a poor prognosis in sarcoidosis.4,24-26 PH also can be challenging to treat patients with sarcoidosis because the response of PH to steroids is unclear.27 Small trials have shown the benefit of pulmonary vasodilators on hemodynamics, but these have generally been used in patients with stable sarcoidosis who do not have left-heart disease.28-30
Our Prescription Model
We empirically prescribed moderate total doses of prednisone—although low on a mg/kg basis—to balance efficacy and the risk of adverse effects in these 2 morbidly obese patients. We also managed treatment-related complications with guided weight-management programs, CPAP, or noninvasive ventilation for sleep-disordered breathing, and DM treatment.
Our cases demonstrate the need for close monitoring of weight, blood pressure, and blood glucose to detect and treat any complications that may arise during corticosteroid treatment. Aggressive treatment of hyperglycemia with insulin or oral alternatives associated with weight loss such as metformin, sulfonylureas, dipeptidyl peptidase 4 inhibitors, or glucagon-like peptide 1 receptor agonists, may help prevent further DM complications. Sleep-disordered breathing should be assessed and treated. Bariatric surgery may be an option to treat obesity and minimize resultant complications. In our patients, and likely many others, the degree of respiratory and cardiac disease coupled with poor wound healing due to chronic prednisone, may increase the procedural risks.
Conclusion
Our experiences with these patients illustrate that symptomatic and objective improvement in sarcoidosis may be achieved in morbidly obese patients with doses of prednisone that are generally considered moderate, though quite low on a mg/kg basis.
We believe ours is the first report to describe the use of corticosteroids for the treatment of sarcoidosis in patients with morbid obesity. That 2 patients were treated at a single VA medical center within 1-year likely reflects the rising incidence of morbid obesity in the US veteran population and suggests that other federal practitioners might encounter similar patients.
Further study may show that, as an alternative to initial moderate-dose prednisone, patients with symptomatic sarcoidosis and extreme obesity might be started on antimetabolite or antitumor necrosis factor medication or on low-dose prednisone and a second steroid-sparing agent.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165.
2. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383 (9923):1155-1167.
3. Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105-1114.
4. Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278-285.
5. Cozier YC, Coogan PF, Govender P, Berman JS, Palmer JR, Rosenberg L. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women’s Health Study. Chest. 2015;147(4):1086-1093.
6. Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855.
7. Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: a population-based nested case-control study. Respir Med. 2016;120:87-90.
8. Sturm R, Hattori A. Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond). 2013;37(6):889-891.
9. Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006; 21(9):915-919.
10. Moller DR, Chen ES. Systemic sarcoidosis. In: Grippi MA, Elias JA, Fishman et al, eds. Fishman’s Pulmonary Diseases and Disorders. 5th ed. New York, NY: McGraw-Hill; 2015: 823-841
11. Judson MA, Morgenthau AS, Baughman RP. Sarcoidosis. In: Broaddus VC, Mason RJ, Ernst JD, et al, eds. Murray and Nadel’s Textbook of Respiratory Medicine. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:1188-1206.
12. Patel D, Hamzeh NY. Immunosuppressive management of cardiac sarcoidosis. In: Freeman AM, Weinberger HD, eds. Cardiac Sarcoidosis. New York, NY: Springer; 2015:103-112.
13. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309-319.
14. Anthony J, Esper GJ, Ioachimescu A. Hypothalamic-pituitary sarcoidosis with vision loss and hypopituitarism: case series and literature review. Pituitary. 2016;19(1):19-29.
15. Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707-712.
16. Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70(2):87-95.
17. Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006;92(2):282-288.
18. Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88(9):1006-1010.
19. Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824-831.
20. Dunn TE, Ludwig EA, Slaughter RL, Camara DS, Jusko WJ. Pharmacokinetics and pharmacodynamics of methylprednisolone in obesity. Clin Pharmacol Ther. 1991;49(5):536-549.
21. Eastwood PR, Malhotra A, Palmer LJ, et al. Obstructive sleep apnoea: from pathogenesis to treatment: current controversies and future directions. Respirology. 2010;15(4):587-595.
22. Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med. 2017;23(6):517-521.
23. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82-93.
24. Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246-1252.
25. Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138(5):1078-1085.
26. Birnie DH, Kandolin R, Nery PB, Kupari M. Cardiac manifestations of sarcoidosis: diagnosis and management. Eur Heart J. 2017;38(35):2663-2670.
27. Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61(1):68-74.
28. Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):139-145.
29. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810-817.
30. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):110-120.
New CHEST expert panel advice on cough diagnosis
The CHEST Expert Cough Panel has released two new in adults and children.
Upper and lower respiratory tract infections are a common reason for primary care visits. A cough caused by influenza or pneumonia represents an opportunity to intervene for a significant benefit. The recommendations were published in CHEST®. The panel drafted recommendations based on available evidence and graded them using the CHEST grading system. The grading is based on the strength of the recommendation (either strong or weak) and a rating of the overall quality of the body of evidence. Where available evidence was weak, but guidance was still warranted, a weak suggestion was developed and graded 2C. Recommendations based on consensus in cases of insufficient clinical evidence are labeled “ungraded consensus-based statement.”
Suspected pneumonia or influenza
In adult outpatients with acute cough, the clinical signs of pneumonia include cough, dyspnea, pleural pain, sweating/fevers/shivers, aches and pains, temperature greater than or equal to 38°C, tachypnea, and new and localizing chest examination signs. When pneumonia is suspected to cause acute cough, C-reactive protein (CRP) should be measured. A CRP value higher than 30 mg/L bolsters the case for pneumonia, whereas a CRP value of lower than 10 mg/L, or between 10 mg/L and 50 mg/L in the absence of dyspnea and daily fever, makes pneumonia less likely.
The guidelines recommend against routine measurement of procalcitonin for outpatient adults suspected to have pneumonia. For adults with acute cough and abnormal vital signs believed to be secondary to pneumonia, the guidelines call for a chest x-ray.
Routine microbiological testing need not be performed in suspected pneumonia, but it should be considered if the results could guide or lead to a change in therapy.
When pneumonia is suspected but imaging is unavailable, empiric antibiotics should be used in concordance with local and national guidelines. If imaging turns up negative, antibiotics should not be used. However, if there is no clinical or radiographic evidence of pneumonia, antibiotics should not be used routinely.
Finally, adult patients with acute cough and suspected influenza should begin antiviral treatment within 48 hours of the start of symptoms.
Pertussis
Pertussis has significant morbidity and mortality, with infants being particularly vulnerable, and it is highly contagious. Although antibiotics will not affect the course of the disease, they should be administered as quickly as possible in order to prevent further spread. This puts pressure on the clinician to make a treatment decision before further testing is available.
A prespecified meta-analysis found high sensitivity and low specificity for paroxysmal cough (sensitivity, 93.2%; specificity, 20.6%) and absence of fever (sensitivity, 81.8%; specificity, 18.8%). The study found low sensitivity and high specificity for inspiratory whoop (sensitivity, 29.8%; specificity, 79.5%) and posttussive vomiting (sensitivity, 32.5%; specificity, 77.7%). In children, the review found that posttussive vomiting was moderately sensitive (60.0%) and specific (66.0%).
In adult patients with acute cough (less than 3 weeks’ duration) or subacute cough (3-8 weeks), the new guidelines recommend that physicians consider four key characteristics: the presence of recurrent, prolonged coughing episodes with an inability to breathe during the spell (paroxysmal); posttussive vomiting; inspiratory whooping; and presence of fever.
In acute or subacute cough, if the patient has a fever (body temperature greater than 98.6° F or 37.6° C) or does not have a paroxysmal cough, pertussis is unlikely. On the other hand, posttussive vomiting or an associated inspiratory whooping sound suggests pertussis.
Children with a cough lasting fewer than 4 weeks (acute) should be assessed for paroxysmal cough, posttussive vomiting, and inspiratory whooping. A cough associated with any of these characteristics may be caused by pertussis.
SOURCES: Moore A et al. CHEST. 2019 Jan;155:147-154; Hill A et al. CHEST. 2019 Jan;155:155-167.
The CHEST Expert Cough Panel has released two new in adults and children.
Upper and lower respiratory tract infections are a common reason for primary care visits. A cough caused by influenza or pneumonia represents an opportunity to intervene for a significant benefit. The recommendations were published in CHEST®. The panel drafted recommendations based on available evidence and graded them using the CHEST grading system. The grading is based on the strength of the recommendation (either strong or weak) and a rating of the overall quality of the body of evidence. Where available evidence was weak, but guidance was still warranted, a weak suggestion was developed and graded 2C. Recommendations based on consensus in cases of insufficient clinical evidence are labeled “ungraded consensus-based statement.”
Suspected pneumonia or influenza
In adult outpatients with acute cough, the clinical signs of pneumonia include cough, dyspnea, pleural pain, sweating/fevers/shivers, aches and pains, temperature greater than or equal to 38°C, tachypnea, and new and localizing chest examination signs. When pneumonia is suspected to cause acute cough, C-reactive protein (CRP) should be measured. A CRP value higher than 30 mg/L bolsters the case for pneumonia, whereas a CRP value of lower than 10 mg/L, or between 10 mg/L and 50 mg/L in the absence of dyspnea and daily fever, makes pneumonia less likely.
The guidelines recommend against routine measurement of procalcitonin for outpatient adults suspected to have pneumonia. For adults with acute cough and abnormal vital signs believed to be secondary to pneumonia, the guidelines call for a chest x-ray.
Routine microbiological testing need not be performed in suspected pneumonia, but it should be considered if the results could guide or lead to a change in therapy.
When pneumonia is suspected but imaging is unavailable, empiric antibiotics should be used in concordance with local and national guidelines. If imaging turns up negative, antibiotics should not be used. However, if there is no clinical or radiographic evidence of pneumonia, antibiotics should not be used routinely.
Finally, adult patients with acute cough and suspected influenza should begin antiviral treatment within 48 hours of the start of symptoms.
Pertussis
Pertussis has significant morbidity and mortality, with infants being particularly vulnerable, and it is highly contagious. Although antibiotics will not affect the course of the disease, they should be administered as quickly as possible in order to prevent further spread. This puts pressure on the clinician to make a treatment decision before further testing is available.
A prespecified meta-analysis found high sensitivity and low specificity for paroxysmal cough (sensitivity, 93.2%; specificity, 20.6%) and absence of fever (sensitivity, 81.8%; specificity, 18.8%). The study found low sensitivity and high specificity for inspiratory whoop (sensitivity, 29.8%; specificity, 79.5%) and posttussive vomiting (sensitivity, 32.5%; specificity, 77.7%). In children, the review found that posttussive vomiting was moderately sensitive (60.0%) and specific (66.0%).
In adult patients with acute cough (less than 3 weeks’ duration) or subacute cough (3-8 weeks), the new guidelines recommend that physicians consider four key characteristics: the presence of recurrent, prolonged coughing episodes with an inability to breathe during the spell (paroxysmal); posttussive vomiting; inspiratory whooping; and presence of fever.
In acute or subacute cough, if the patient has a fever (body temperature greater than 98.6° F or 37.6° C) or does not have a paroxysmal cough, pertussis is unlikely. On the other hand, posttussive vomiting or an associated inspiratory whooping sound suggests pertussis.
Children with a cough lasting fewer than 4 weeks (acute) should be assessed for paroxysmal cough, posttussive vomiting, and inspiratory whooping. A cough associated with any of these characteristics may be caused by pertussis.
SOURCES: Moore A et al. CHEST. 2019 Jan;155:147-154; Hill A et al. CHEST. 2019 Jan;155:155-167.
The CHEST Expert Cough Panel has released two new in adults and children.
Upper and lower respiratory tract infections are a common reason for primary care visits. A cough caused by influenza or pneumonia represents an opportunity to intervene for a significant benefit. The recommendations were published in CHEST®. The panel drafted recommendations based on available evidence and graded them using the CHEST grading system. The grading is based on the strength of the recommendation (either strong or weak) and a rating of the overall quality of the body of evidence. Where available evidence was weak, but guidance was still warranted, a weak suggestion was developed and graded 2C. Recommendations based on consensus in cases of insufficient clinical evidence are labeled “ungraded consensus-based statement.”
Suspected pneumonia or influenza
In adult outpatients with acute cough, the clinical signs of pneumonia include cough, dyspnea, pleural pain, sweating/fevers/shivers, aches and pains, temperature greater than or equal to 38°C, tachypnea, and new and localizing chest examination signs. When pneumonia is suspected to cause acute cough, C-reactive protein (CRP) should be measured. A CRP value higher than 30 mg/L bolsters the case for pneumonia, whereas a CRP value of lower than 10 mg/L, or between 10 mg/L and 50 mg/L in the absence of dyspnea and daily fever, makes pneumonia less likely.
The guidelines recommend against routine measurement of procalcitonin for outpatient adults suspected to have pneumonia. For adults with acute cough and abnormal vital signs believed to be secondary to pneumonia, the guidelines call for a chest x-ray.
Routine microbiological testing need not be performed in suspected pneumonia, but it should be considered if the results could guide or lead to a change in therapy.
When pneumonia is suspected but imaging is unavailable, empiric antibiotics should be used in concordance with local and national guidelines. If imaging turns up negative, antibiotics should not be used. However, if there is no clinical or radiographic evidence of pneumonia, antibiotics should not be used routinely.
Finally, adult patients with acute cough and suspected influenza should begin antiviral treatment within 48 hours of the start of symptoms.
Pertussis
Pertussis has significant morbidity and mortality, with infants being particularly vulnerable, and it is highly contagious. Although antibiotics will not affect the course of the disease, they should be administered as quickly as possible in order to prevent further spread. This puts pressure on the clinician to make a treatment decision before further testing is available.
A prespecified meta-analysis found high sensitivity and low specificity for paroxysmal cough (sensitivity, 93.2%; specificity, 20.6%) and absence of fever (sensitivity, 81.8%; specificity, 18.8%). The study found low sensitivity and high specificity for inspiratory whoop (sensitivity, 29.8%; specificity, 79.5%) and posttussive vomiting (sensitivity, 32.5%; specificity, 77.7%). In children, the review found that posttussive vomiting was moderately sensitive (60.0%) and specific (66.0%).
In adult patients with acute cough (less than 3 weeks’ duration) or subacute cough (3-8 weeks), the new guidelines recommend that physicians consider four key characteristics: the presence of recurrent, prolonged coughing episodes with an inability to breathe during the spell (paroxysmal); posttussive vomiting; inspiratory whooping; and presence of fever.
In acute or subacute cough, if the patient has a fever (body temperature greater than 98.6° F or 37.6° C) or does not have a paroxysmal cough, pertussis is unlikely. On the other hand, posttussive vomiting or an associated inspiratory whooping sound suggests pertussis.
Children with a cough lasting fewer than 4 weeks (acute) should be assessed for paroxysmal cough, posttussive vomiting, and inspiratory whooping. A cough associated with any of these characteristics may be caused by pertussis.
SOURCES: Moore A et al. CHEST. 2019 Jan;155:147-154; Hill A et al. CHEST. 2019 Jan;155:155-167.
FROM THE JOURNAL CHEST®
Managing the Silent Epidemic of Nonalcoholic Fatty Liver Disease
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.
For many years, viral hepatitis and particularly hepatitis C have been the bread and butter for clinicians dealing with chronic liver diseases. Over the past few years the Veterans Health Administration (VHA) has been incredibly successful in identifying, treating, and curing a significant proportion of veterans of this viral disease. However, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease worldwide and will soon overtake hepatitis C virus as the leading cause of liver transplantation. NAFLD covers a disease spectrum ranging from nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis (NASH) to liver cirrhosis and liver cancer or liver failure. In the absence of effective treatment approaches, it is not surprising that NAFLD will create financial challenges for the VHA and US health care budgets. It is thus appropriate that Federal Practitioner has decided to publish a series of articles highlighting NAFLD and how it affects millions of Americans on its way to reaching quietly epidemic proportions within the VHA and across the globe.
Although NAFLD seems to have quietly and quickly reached epidemic proportions, its obscurity should not be surprising. NAFLD does not cause obvious symptoms in most patients, there is no simple test available for diagnosis of NASH, and disease-specific medications have not yet been approved for treatment. Primary care providers (PCPs) are the first point of medical contact for a majority of patients with or at risk for NAFLD; shockingly, NAFLD is greatly underrecognized, resulting in delayed diagnoses, which impact both health-related and quality-of-life outcomes in these patients. As emphasized in “Identifying and Treating Nonalcoholic Fatty Liver Disease” by Hunt and colleagues (page 20), PCPs should focus on 4 main aspects related to NAFLD: (1) Does my patient have NAFL? (2) Is my patient at risk for NASH and its ensuing manifestations? (3) Do simple noninvasive serum liver fibrosis markers suggest presence of clinically relevant liver fibrosis? and (4) Does my patient benefit from being referred to a specialist. The PCP is integral in optimally managing medical comorbidities and metabolic abnormalities as well as coordinating intense lifestyle and exercise interventions.
“Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans” by Shetty and Syn (page 14) discusses the epidemiology and economic burden of NAFLD in the US and how it will affect the health of veterans. Chronic liver disease is a major cause of mortality, morbidity, and health care resource utilization worldwide. Over the past 3 decades, NAFLD has gone from obscure liver diseases to the most common cause of chronic liver disease affecting 25% of the world’s population. Patients with NAFL who have advanced to NASH have an increased risk of liver-specific death. NASH is among the top etiologies for hepatocellular cancer and the fastest growing indication for liver transplantation, projected to overtake hepatitis C virus as the leading cause of liver transplantation. Most disturbing though is the fact that patients with NASH are the least likely to be surveyed for hepatocellular cancer development and the most likely to die while awaiting liver transplantation. Recent modeling estimates a 178% increase in liver deaths related to NASH by 2030.
The clinical burden of all stages of NAFLD is related to its prevalence, incidence, and progressiveness and has to be coupled with its tremendous economic burden based on inpatient, outpatient, professional services, emergency department, and pharmacy costs. It is thus not surprising that we are heading toward a serious health care crisis in the next few decades with the cost of managing NAFLD complications alone approaching an estimated 10-year economic burden of nearly $1 trillion.
The third article by Glass and colleagues (In press) puts the spectrum of NAFLD in the context of a disrupted systemic metabolic environment related to overnutrition alongside reduced physical activity. It is not surprising that type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease are frequent comorbidities present in a high proportion of patients with NAFLD. The prevalence of NAFLD among people with T2DM exceeds 60%. Importantly, convincing evidence has accumulated supporting the concept that interactions between these metabolic syndrome components and NAFLD are complex and bidirectional. Evidence from cross-sectional and longitudinal studies favors the presence of NAFLD and its severity preceding and/or promoting the development of metabolic comorbidities such as T2DM. Concomitantly, the presence of T2DM seems to accelerate the clinical course of NAFLD and is a predictor of advanced liver fibrosis and mortality. Compared with diseases that have a single etiology, such as viral hepatitis, NAFLD is a very complex disease with multiple interacting metabolic pathways that operate in an individual, leading to the clinical manifestation. Clearly, our present understanding of NAFLD/NASH as a single conglomerate disease is overly simplistic, and further study is warranted.
NAFLD and its variations comprise an increasing number and proportion of referral to hepatologists or providers with experience treating patients with chronic liver disease for the management of advanced disease stages; similarly, PCPs face the challenge to manage early stages of NAFLD. Given the magnitude of the problem of NAFLD, it is imperative that dedicated control efforts at the population level must intensify. As is emphasized in the fourth article of this series (In press), Puri and Fuchs call for a replacement of the traditional health care model of office visits with individual specialist working in silos. To overcome the narrow focus of a subspecialty outpatient clinic, time constraints, and gaps in NAFLD awareness, a patient-centered multidisciplinary approach to the treatment and coordination of care for the medically complex NAFLD patients is needed. The VHA is the largest integrated health care system in the US and is well positioned to implement an organizational strategy to facilitate standardized NAFLD care. The proposed model is centered on a broad assessment of the patient, involving the input from several disciplines; on completion of the assessment, a multidisciplinary team will formulate a personalized intervention plan.
The composition of this multidisciplinary team will vary based on expertise and resources available in each clinical setting. Once an intervention has been started, tracking and monitoring of intermediate and long-term functional outcomes will be helpful to modify the intervention in case outcomes are not achieved. Patient education, from the initial assessment until the intervention phase, plays a critical element to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals set with their health care team.
Ultimately, integration of health care services will lead to better quality of care, increased patient satisfaction, and importantly to improved health care service utilization that will reduce health care resources and costs. Although such a proposal may seem ambitious, it is now the time for innovative thinking that will create sustainable solutions for the silent epidemic of NAFLD. Without advancing a proactive vision, the VA and the world will soon become saddled with an unmanageable economic and health care burden.