User login
Adult atopic dermatitis is fraught with dermatologic comorbidities
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
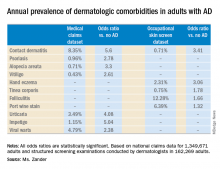
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
PARIS – : that is, from alopecia areata to vitiligo, and points in between, according to a large German national study.
These dermatologic comorbidities are in addition to already well established strong associations between AD and allergic rhinitis and bronchial asthma, as well as the emerging evidence of increased risk for depression, sleep impairment, suicidality, and other nondermatologic conditions, Nicole Zander noted at the annual congress of the European Academy of Dermatology and Venereology.
“Atopic dermatitis patients seem to be at substantial risk for a variety of comorbidities. It’s important to recognize the whole spectrum of comorbidities as a prerequisite for provision of patient-centered care,” said Ms. Zander, a research associate at the University of Hamburg Institute for Health Services Research in Dermatology and Nursing.
This is a priority in Germany, where the prevalence of AD has doubled or even tripled in the last 3 decades, she added.
She presented a snapshot of the comorbid conditions associated with AD based upon two German large national datasets: Structured skin screening examinations conducted by dermatologists in 162,269 adults working in more than 500 German companies and national medical claims data for 1,349,671 adults aged 18 years and up.
The annual point prevalence for adult AD was 1.4% in the occupational screening, while in the claims dataset it was 3.7%. The true prevalence, as well as that of the dermatologic comorbidities, probably lies somewhere in between, since both of these enormous datasets have their limitations. The claims dataset may contain coding errors, plus many of the skin disorders entombed in that database were diagnosed by nondermatologists. And the occupational dataset is vulnerable to what epidemiologists call the healthy worker effect: some people with severe AD might be absent from work or on disability, with a resultant lower point prevalence of the disorder in the workplace, she explained.
Both datasets documented a clear decline in the prevalence of AD with advancing age. In the medical claims database, for example, the prevalence was 6.6% among 18- and 19-year-olds, 5.4% for patients in their 20s, 3.9% for those in their 30s, 3.4% for those in their 40s, 3.0% for patients in their 50s, 2.7% for those in their 70s, and 2.4% for patients in their 80s.
This is the most likely explanation for the significantly reduced risks of stroke, hypertension, diabetes, hyperlipidemia, and ischemic heart disease seen in AD patients in the claims dataset. These are all conditions that become much more common with advancing age, whereas AD is skewed toward a younger population. And for purposes of this study the data weren’t age adjusted, Ms. Zander observed.
Ms. Zander reported having no financial conflicts regarding her study, conducted without commercial support.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Adult atopic dermatitis patients have higher prevalence of psoriasis, alopecia areata, vitiligo, and other dermatologic disorders.
Major finding: The risk of contact dermatitis is increased 3.4- to 5.6-fold.
Study details: This German population-based study used both a medical claims database including more than 1.3 million adults and dermatologist-conducted occupational skin screening in more than 162,000 workers.
Disclosures: The study presenter reported having no financial conflicts regarding her study, conducted without commercial support.
Ray Barfield Part II: Philosophy and Medicine
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
In part I of the conversation, Dr. Barfield and MDedge host Nick Andrews discussed physician burnout and Dr. Barfield’s journey back to medicine. In this episode, Dr. Barfield and Nick discuss philosophy and science.
You can listen to part I of this conversation here:
Apple Podcasts
Google Podcasts
Spotify
Deep sleep decreases, Alzheimer’s increases
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Adding umbralisib to ibrutinib produced responses in MCL, CLL
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
Dual B-cell receptor pathway blockade was tolerable and efficacious for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or mantle cell lymphoma (MCL) who participated in a multicenter phase 1-1b clinical study that added umbralisib to ibrutinib.
The study “is the first successful combination for two drugs targeting the B-cell receptor pathway,” Matthew S. Davids, MD, of the Dana-Farber Cancer Institute in Boston and his colleagues wrote in the Lancet Haematology.
Of the 21 patients with CLL, 90% (n = 19) achieved an overall response (OR), 62% (n = 13) achieved partial response (PR) or PR with lymphocytosis, and 29% (n = 6) achieved complete response (CR). All patients in complete response still had minimal residual disease (MRD) in bone marrow. No CLL patients had progressive disease.
Of the 21 patients with MCL, 67% (n = 14) had an OR, with 19% (n = 4) showing CR and 48% (n = 10) achieving partial response. Three MCL patients (14%) had progressive disease.
Umbralisib is a next-generation phosphoinositide-3-kinase-delta inhibitor that, when added to the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib, offers once-daily oral dosing. The combination affords the possibility of overcoming the resistance that can come with prolonged ibrutinib monotherapy.
A total of 44 patients were enrolled, and 42 patients (21 with CLL and 21 with MCL) received at least one dose of the study drug and were included in the analysis. At enrollment, patients had received a median of two previous therapies.
Diarrhea was the most frequent adverse event, seen in 22 patients (52%), and half of all patients (n = 21) had infections.
Hematologic toxicities included neutropenia, seen in 9 (43%) of the CLL patients and 8 (38%) of the MCL patients; thrombocytopenia, seen in 6 (29%) of the CLL patients and 10 (48%) of the MCL patients; and anemia, seen in 4 (19%) of the CLL and 9 (43%) of the MCL patients. Grade 3 and 4 hematologic toxicities of any type were less common, occurring in less than 20% of patients. One MCL patient developed febrile neutropenia. According to the study investigators, none of the hematologic toxicities were deemed related to the study drugs.
Adverse events did not appear to be dose-dependent for umbralisib, with the maximum tolerated dose not reached in the study, the investigators wrote. For phase 2 trials, the recommended dose of umbralisib is 800 mg given orally once daily in combination with ibrutinib.
“One unanticipated benefit of doublet B-cell receptor pathway inhibition in this study was the ability to continue one drug when a characteristic toxicity required the other drug to be held,” the investigators wrote.
For MCL patients, 67% achieved OR and 19% achieved CR, figures similar to those reported for ibrutinib monotherapy. However, “the 2-year progression-free survival of 49% and overall survival of 58% suggest that patients who made it to 1 year progression-free had few events during the second year on therapy,” the investigators wrote. They also noted that this MCL population was high risk; more than one-quarter of patients had relapsed after prior autologous stem cell transplantation.
The study was limited by small sample size and a short duration of follow-up, so durability of response can’t yet be assessed. Also, neither pharmacokinetics nor resistance mutations were tracked for participants.
Currently, the doublet regimen is designed to be continuous therapy, and although it’s not known whether this regimen would be effective as time-limited therapy, it’s unlikely because 100% of patients who had CR still had detectable minimal residual disease, the investigators noted.
Umbralisib and ibrutinib are also being explored as part of triplet therapy, with the type 2 CD20 antibody ublituximab, for relapsed or refractory B-cell malignancies (NCT02006485).
“These novel drug-based approaches, along with several others in development, hold promise as highly effective and well-tolerated regimens with the potential to substantially improve outcomes for patients with B-cell malignancies,” the investigators wrote.
The study was supported by TG Therapeutics and the Leukemia and Lymphoma Society Therapy Accelerator Program. The authors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
SOURCE: Davids MS et al. Lancet Haemtol. 2019;6:e38-47.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Of CLL patients, 90% achieved an overall response.
Study details: Phase 1-1b trial of umbralisib and ibrutinib in patients with relapsed or refractory MCL or CLL.
Disclosures: The study was supported by TG Therapeutics and the Leukemia and Lymphoma Therapy Accelerator Program. Dr. Davids and his coauthors reported financial relationships with several pharmaceutical companies, including TG Therapeutics.
Source: Davids MS et al. Lancet Haematol. 2019;6:e38-47.
Benralizumab maintains effectiveness in severe asthma at 2 years
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
out to 2 years, according findings of the BORA trial, an extension study of the phase 3 SIROCCO and CALIMA trials. The study follows up and reinforces previously reported 1-year data and was reported by William W. Busse, MD, of University of Wisconsin, Madison, and his colleagues in the Lancet Respiratory Medicine.
Benralizumab is a monoclonal antibody that targets interleukin-5 receptor alpha. It causes rapid deletion of eosinophils through cell-mediated cytotoxicity. A 30-mg dose of benralizumab every 8 weeks is approved for severe asthma treatment in Canada, Europe, Japan, the United States, and other countries.
In the second year of treatment, there were no new adverse events associated with depleted eosinophils, and the frequency of opportunistic infections was similar to that observed in the first year.
Eosinophilic inflammation occurs in about half of asthma cases and is associated with greater severity.
The 48-week SIROCCO trial, the 56-week CALIMA trial, and the 28-week ZONDA trial tested the effect of benralizumab 30 mg given every 4 weeks or 8 weeks, combined with high-dosage inhaled steroids and long-acting beta2-agonists. The 8-week dose of the drug reduced annual exacerbations by 51%, compared with placebo in the SIROCCO trial and by 28% in the CALIMA trial. In the ZONDA trial, benralizumab reduced oral glucocorticoid use by 75%, compared with placebo, and by 25% from baseline.
The BORA extension trial included participants in the previous three trials. In the current report, researchers presented results from the analysis from BORA participants recruited from the SIROCCO and CALIMA trials. Data from participants from all three trials will be reported in the future.
The analysis included 1,576 patients who continued to receive benralizumab after being assigned to the treatment arm in SIROCCO or CALIMA, or who had received placebo were randomized to benralizumab on the 4-week (n = 783; 265 from placebo) or 8-week dose (n = 793; 281 from placebo) schedule.
A total of 166 patients, or about 10% in each group, discontinued treatment. The frequency of any serious adverse event (SAE) ranged between 10% and 11% in all groups. SAEs associated with infections ranged from 1% to 3%, indicating that there were no significant differences in SAE frequencies between those who were originally assigned to placebo and those who originally received benralizumab. That suggests no safety differences between receiving the drug for 1 year or 2 years.
A total of 1,046 subjects had blood eosinophil counts of 300 cells per mcL or greater at baseline; 72% of these patients had no asthma exacerbations during the BORA study. This was true for 74% of patients in the 8-week treatment arm.
The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm, compared with placebo (95% confidence interval, 0.42-0.56) and 0.46 in the 8-week arm (95% CI, 0.39-0.53). For patients who started out on placebo, the crude exacerbation rate during BORA was 0.53 in the 4-week group (95% CI, 0.43-0.65) and 0.57 in the 8-week group (95% CI, 0.47-0.68).
Patients who started on benralizumab had similar exacerbation frequencies during year 1 and year 2.
AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
SOURCE: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: The antibody had similar safety, efficacy in year 2 as in year 1.
Major finding: The crude asthma exacerbation rate for patients who received benralizumab in SIROCCO or CALIMA was 0.48 in the 4-week arm and 0.46 in the 8-week arm; the crude exacerbation rate during BORA was 0.53 in the 4-week group and 0.57 in the 8-week group.
Study details: Extension of randomized, clinical trial (n = 1,576).
Disclosures: AstraZeneca and Kyowa Hakko Kirin funded the studies. The authors have received fees from AstraZeneca and other pharmaceutical companies, and some are employees of AstraZeneca.
Source: Busse WW et al. Lancet Respir Med. 2019 Jan 1;7(1):46-59.
Biodegradable stent polymer offers no long-term protection
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
This week from MDedge Cardiology, a biodegradable polymer shows no long-term benefit in heart stents, appropriate use criteria for imaging in nonvalvular heart disease are released, ACOG updates guidance on hypertension in pregnancy, and more losartan lots are recalled.
Subscribe to Cardiocast wherever you get your podcasts.
Amazon AlexaApple Podcasts
Annular Elastolytic Giant Cell Granuloma: Mysterious Enlarging Scarring Lesions
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
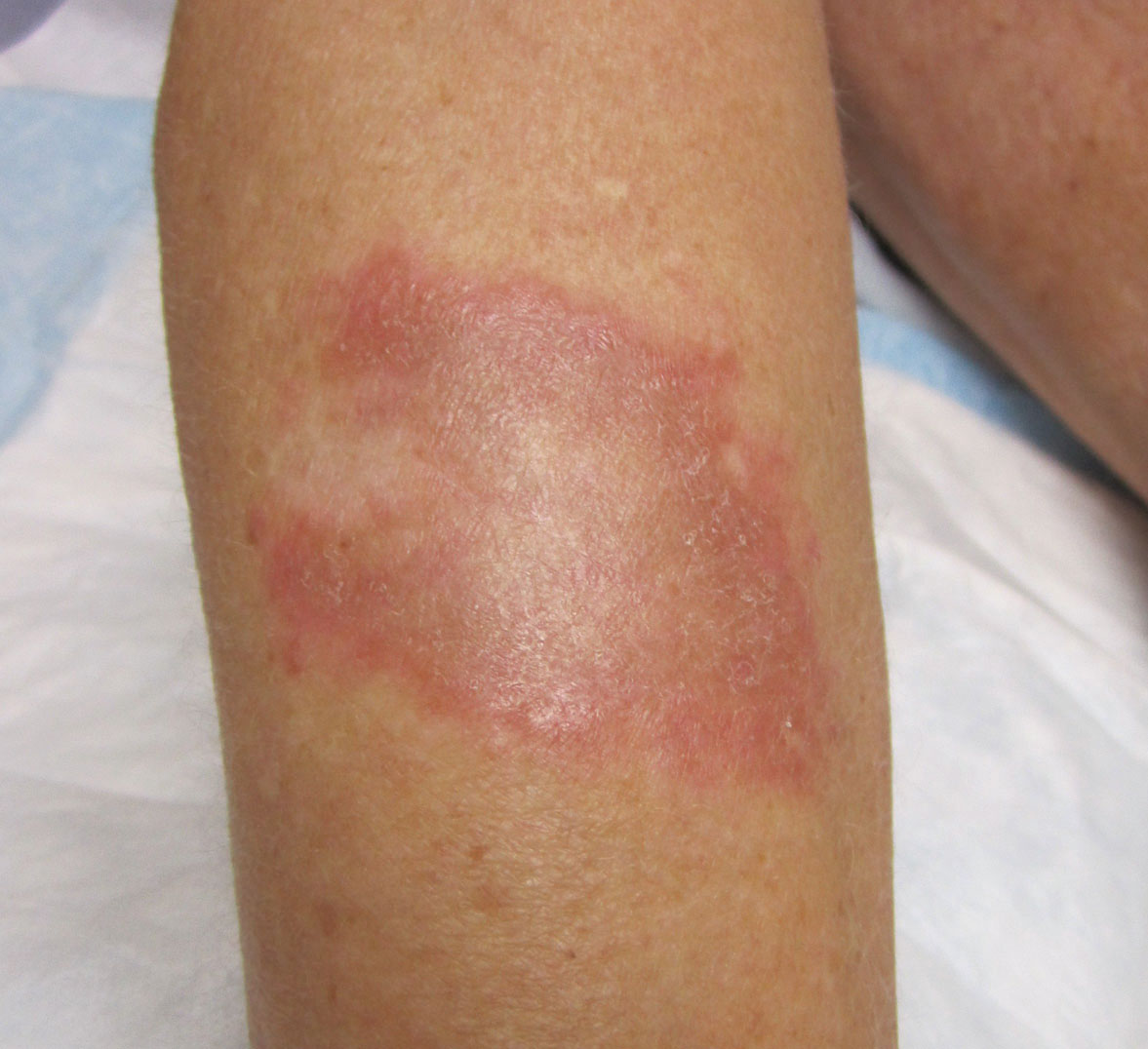
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.
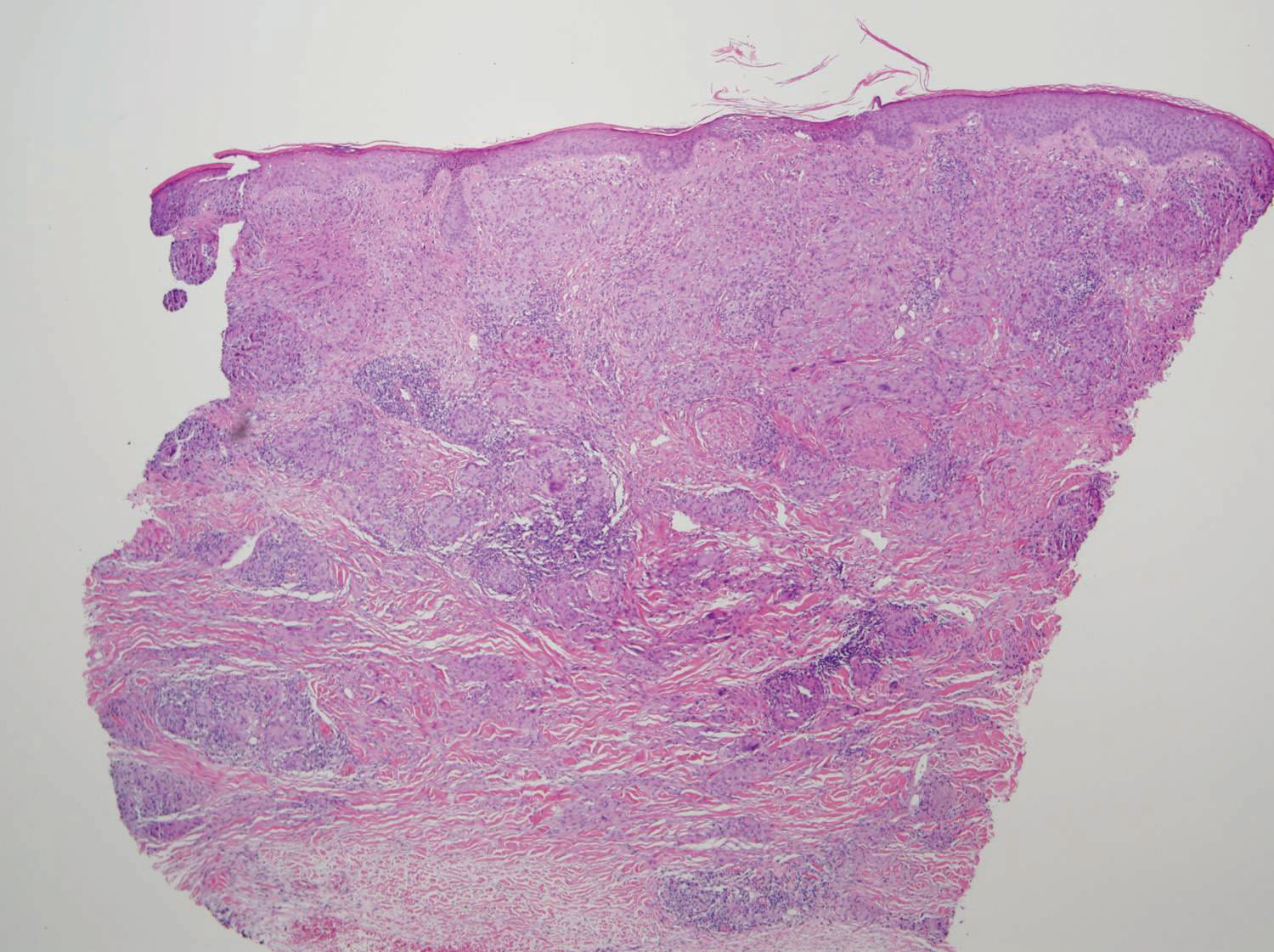
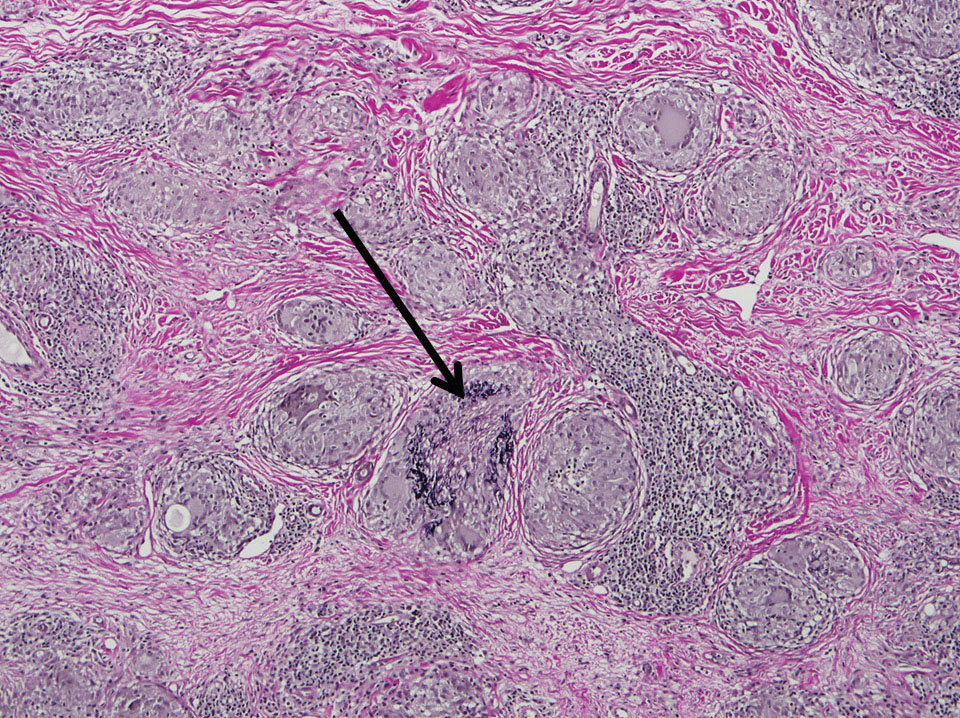
Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.
The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.
Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.
The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
To the Editor:
A 52-year-old woman with a medical history of migraines and cervicalgia presented with lesions on the right arm, back, and right calf. The patient stated that the lesions began as small papules that had grown over 13 months, with the largest papule on the right forearm. She reported no itching, bleeding, pain, discharge, or other symptoms associated with the lesions. She had a multiple-year history of similar lesions that did not respond to treatment with antifungals, moderate-potency steroids, and other over-the-counter creams. The lesions would resolve spontaneously with scarring and subsequently recur. Prior skin biopsies were inconclusive. The patient did not report any systemic symptoms or a personal or family history of connective tissue diseases.
Physical examination revealed a 4-cm asymmetric, annular, erythematous plaque with central clearing on the right dorsal forearm with defined margins except over the distal aspect (Figure 1). She also had several 1- to 2-cm erythematous, nummular, asymmetric plaques on the right upper arm with well-defined margins. She had several lesions over the central and left sides of the upper back that were similar to the lesions on the upper arm.
Two 4-mm punch biopsies of the right dorsal forearm and left side of the upper back revealed similar histologic features with a predominantly unremarkable epidermis. The dermis revealed a lymphohistiocytic infiltrate with prominent multinucleated giant cells organized into foreign body–type granulomas that extended into the deep dermis and subcutaneous tissue (Figure 2). In the granulomatous areas, there was a near-complete loss of elastic fibers with focal elastophagocytosis highlighted with Verhoeff-van Gieson (elastin) stain (Figure 3). Grocott-Gomori methenamine-silver and Fite stains for microorganisms were negative, and there was an absence of necrobiosis, lipids, and mucin.
The histologic findings of a granulomatous dermatitis with loss of elastic fibers and elastophagocytosis in addition to the patient’s clinical presentation and history were consistent with the diagnosis of annular elastolytic giant cell granuloma (AEGCG). Infectious and other granulomatous diseases including sarcoidosis were ruled out via clinical history, unremarkable laboratory analysis (ie, complete blood cell count, chemistry panel, antinuclear antibody, urinalysis), and a normal chest radiograph. The histologic findings via the various stains were instrumental to the diagnosis. The patient was treated with fluocinonide and subsequently lost to follow-up.
Annular elastolytic giant cell granuloma is an uncommon cutaneous disease that presents with recurring annular plaques with raised erythematous borders and subsequent residual scarring.1 O’Brien2 originally described this condition in 1975 as an actinic granuloma due to similar histologic findings in areas of the patient’s sun-exposed skin. Ragaz and Ackerman3 disputed O’Brien’s2 description, claiming granulomatous inflammation was a primary pathologic process and not a consequence to damaged elastotic material. In 1979, Hanke et al4 termed the lesions as AEGCG because he did not find a correlation to the sun-exposed areas of the patients and did not see solar elastosis.
Although AEGCG has an unclear pathogenesis, cellular immunologic reactions induced by modified function of elastic fibers’ antigenicity contribute to AEGCG formation.5 Therefore, environmental and host factors may play a role in its etiopathogenesis. In one study, 37% of 38 Japanese patients with AEGCG were found to have definitive or latent diabetes mellitus, raising the possible role of diabetes in the structural damage of the elastic fibers.6
Patients typically are middle-aged women who present clinically with red or atrophic plaques that have slightly elevated borders. They have centripetal spread with a resulting atrophic center.7 Clinically, the differential diagnosis of this condition includes actinic granuloma, granuloma annulare, and granuloma multiforme.8
Histologically, AEGCG has a granulomatous component with multinucleated giant cells in the upper and mid dermis. This component typically is distributed peripherally to a central zone that lacks elastic tissue. Elastophagocytosis, a classic finding in AEGCG, is the phagocytosis of elastic fibers that can microscopically be seen in the cytoplasm of histiocytes and multinucleated giant cells. There also is an absence of necrobiosis, lipids, mucin, and a palisading arrangement of the granulomas. These findings distinguish AEGCG from granuloma annulare and necrobiosis lipoidica, the primary histologic differential diagnoses.9 In addition, consideration of entities consistently exhibiting elastophagocytosis such as mid-dermal elastolysis, papillary dermal elastolysis, actinic granuloma, and granulomatous slack skin should be considered.5,10,11
Therapy for AEGCG is broad and includes topical, intralesional, and systemic corticosteroids. Hydroxychloroquine, isotretinoin, clofazimine, dapsone, photochemotherapy, and cyclosporine also have been utilized with varying results. Other reports show improvement with surgical excision, cryotherapy, or cauterization of small lesions.12-15
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
1. Tock CL, Cohen PR. Annular elastolytic giant cell granuloma. Cutis. 1998;62:181-187.
2. O’Brien JP. Actinic granuloma: an annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111:460-466.
3. Ragaz A, Ackerman AB. Is actinic granuloma a specific condition? Am J Dermatopathol. 1979;1:43-50.
4. Hanke CW, Bailin PL, Roenigk HH Jr. Annular elastolytic giant cell granuloma. a clinicopathologic study of five cases and a review of similar entities. J Am Acad Dermatol. 1979;1:413-421.
5. El-Khoury J, Kurban M, Abbas O. Elastophagocytosis: underlying mechanisms and associated cutaneous entities. J Am Acad Dermatol. 2014;70:934-44.
6. Aso Y, Izaki Y, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919.
7. Pestoni C, Pereiro M Jr, Toribio J. Annular elastolytic giant cell granuloma produced on an old burn scar and spreading after a mechanical trauma. Acta Derm Venereol. 2003;83:312-313.
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40:220-222.
9. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282.
10. McGrae JD Jr. Actinic granuloma: a clinical, histopathologic, and immunocytochemical study. Arch Dermatol. 1986;122:43-47.
11. Shah A, Safaya A. Granulomatous slack skin disease: a review, in comparison with mycosis fungoides. J Eur Acad Dermatol Venereol. 2012;26:1472-1478.
12. Chou WT, Tsai TF, Hung CM, et al. Multiple annular erythematous plaques on the back. Annular elastolytic giant cell granuloma (AEGCG). Indian J Dermatol Venereol Leprol. 2011;77:727-728.
13. Pérez-Pérez L, Garcia-Gavin J, Alleque F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralen-ultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266.
14. Babuna G, Buyukbabani N, Yazganoglu KD, et al. Effective treatment with hydroxychloroquine in a case of annular elastolytic giant cell granuloma. Indian J Dermatol Venereol Leprol. 2011;77:110-111.
15. Can B, Kavala M, Türkoglu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxylchloroquine. Int J Dermatol. 2013;52:509-511.
Practice Points
- Annular elastolytic giant cell granuloma (AEGCG) should be kept in the differential diagnosis when assessing a middle-aged woman with recurring annular plaques with a raised border and an atrophic center on both sun-exposed and sun-protected areas of the body.
- Histologically, AEGCG classically has a granulomatous component in the dermis that lacks elastic tissue and has no necrobiosis, lipids, or mucin. Staining with elastin may be necessary to highlight these areas as well as demonstrate elastophagocytosis.
Low-normal thyroid function tied to advanced fibrosis
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
Advanced fibrosis affected 5.9% of adults with low-normal thyroid function or subclinical hypothyroidism – more than double the prevalence among adults with strict-normal thyroid function (2.8%; P less than .001), according to the results of a large survey study.
Based on these findings, therapy to improve low thyroid function might help prevent advanced fibrosis secondary to nonalcoholic fatty liver disease, wrote Donghee Kim, MD, PhD, of Stanford University (Calif.), together with his associates in Clinical Gastroenterology and Hepatology.
Prior research has linked low-normal thyroid function with obesity, cardiometabolic diseases, and fractures. For this study, Dr. Kim and his coinvestigators analyzed data from 7,259 adults who lacked major etiologies of chronic liver disease and were included in the National Health and Nutrition Examination Survey between 2007 and 2012.
After accounting for demographic, socioeconomic, and clinical variables, the odds of biopsy-confirmed advanced fibrosis were 100% higher in adults with low-normal thyroid function or subclinical hypothyroidism, compared with adults with strict-normal thyroid function (odds ratio, 2.0; 95% confidence interval, 1.2-3.3). The prevalence and odds of advanced fibrosis was similar in each of these two subgroups. Furthermore, low thyroid function remained strongly linked with advanced fibrosis after accounting for insulin resistance using data from fasting subjects (OR, 2.3; 95% CI, 1.2-4.4).
Previously, Dr. Kim and his coinvestigators found a strong link between biopsy-proven advanced fibrosis and low-normal thyroid function or subclinical hypothyroidism among adults in Korea. “These [new] results are consistent with our previous observations in [an] Asian population, and show their generalizability to the Western world across all ethnicities.”
The researchers did not acknowledge external funding sources. They reported having no conflicts of interest.
SOURCE: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Low-normal thyroid function correlates significantly with advanced fibrosis.
Major finding: In all, 5.9% of adults with low-normal thyroid function had advanced fibrosis, compared with 2.8% of individuals with strict-normal thyroid function (P less than .001).
Study details: A study of 7,259 adults from the National Health and Nutrition Examination Survey (2007-2012).
Disclosures: The investigators did not acknowledge funding sources. They reported having no conflicts of interest.
Source: Kim D et al. Clin Gastroenterol Hepatol. 2018 Nov 17. doi: 10.1016/j.cgh.2018.11.024.
ICYMI: Caplacizumab benefits patients with acquired TTP
and were 1.55 times more likely to achieve platelet normalization, compared with placebo, according to results of the double-blind, controlled HERCULES trial published in the New England Journal of Medicine (2019 Jan 9. doi: 10.1056/NEJMoa1806311).
Patients taking caplacizumab also had a 74% lower incidence of a composite outcome that included TTP-related deaths, recurrence of TTP, or a major thromboembolic event.
We covered this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
and were 1.55 times more likely to achieve platelet normalization, compared with placebo, according to results of the double-blind, controlled HERCULES trial published in the New England Journal of Medicine (2019 Jan 9. doi: 10.1056/NEJMoa1806311).
Patients taking caplacizumab also had a 74% lower incidence of a composite outcome that included TTP-related deaths, recurrence of TTP, or a major thromboembolic event.
We covered this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
and were 1.55 times more likely to achieve platelet normalization, compared with placebo, according to results of the double-blind, controlled HERCULES trial published in the New England Journal of Medicine (2019 Jan 9. doi: 10.1056/NEJMoa1806311).
Patients taking caplacizumab also had a 74% lower incidence of a composite outcome that included TTP-related deaths, recurrence of TTP, or a major thromboembolic event.
We covered this story at the annual meeting of the American Society of Hematology before it was published in the journal. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Secondhand vaping aerosols linked to childhood asthma exacerbations
Just like exposure to secondhand smoke, , according to a review of the 11,830 kids with asthma in the 2016 Florida Youth Tobacco survey.
Every year, the Florida Department of Health surveys public school children aged 11-17 years about various tobacco issues. In 2016, almost 12% of the asthmatic children in the survey said they vaped. Almost half were exposed to secondhand smoke, and a third reported exposure to secondhand vaping aerosols within the past 30 days. Overall, 21% reported an asthma attack in the past 12 months.
Using data from the Florida survey, the investigators crunched the numbers and found that secondhand aerosol exposure increased the odds of an asthma attack by 27%, independent of exposure to secondhand smoke and whether children smoked or vaped themselves (adjusted odds ratio, 1.27; 95% confidence interval, 1.11-1.47).
“Health professionals may wish to counsel asthmatic youth and their families regarding the potential risks of ENDS [electronic nicotine delivery system] use and exposure to ENDS aerosols.” Providers “may also consider including ENDS aerosol exposure as a possible trigger in asthma self-management/action plans and updating asthma home environment assessments to include exposure to ENDS aerosols,” said investigators led by medical student Jennifer Bayly, a research fellow at the National Institute on Minority Health and Health Disparities in Bethesda, Md.
About 4% of adults in the United States and 11% of high school students vape, and almost 10% of U.S. adolescents reported living with an ENDS user in 2014. Given the data, “it is likely that a substantial number of asthmatic youth are exposed,” the investigators said.
The study adds to a growing body of evidence linking e-cigarettes to asthma. There’s moderate evidence for increased cough and wheezing in adolescents who use e-cigarettes, plus an association with e-cigarette use and increased asthma exacerbations. The new study, however, is likely the first to look specifically at secondhand exposure among asthmatic children. Ingredients in vaping aerosols, including flavorings, propylene glycol, and vegetable glycerin, are physiologically active in the lungs, and may be lung irritants.
Overall, about half of the respondents were female, and two-thirds were 11-13 years old. About a third identified as Hispanic, a third as white, and just over a fifth as black. Three-quarters of the sample lived in large or midsized metropolitan areas, and close to two-thirds in stand-alone homes. Participants were considered exposed to secondhand aerosols if they reported that in the past month they were in a room or car with someone who was vaping.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Bayly JE et al. CHEST®. 2018 Oct 22. doi: 10.1016/j.chest.2018.10.005.
Just like exposure to secondhand smoke, , according to a review of the 11,830 kids with asthma in the 2016 Florida Youth Tobacco survey.
Every year, the Florida Department of Health surveys public school children aged 11-17 years about various tobacco issues. In 2016, almost 12% of the asthmatic children in the survey said they vaped. Almost half were exposed to secondhand smoke, and a third reported exposure to secondhand vaping aerosols within the past 30 days. Overall, 21% reported an asthma attack in the past 12 months.
Using data from the Florida survey, the investigators crunched the numbers and found that secondhand aerosol exposure increased the odds of an asthma attack by 27%, independent of exposure to secondhand smoke and whether children smoked or vaped themselves (adjusted odds ratio, 1.27; 95% confidence interval, 1.11-1.47).
“Health professionals may wish to counsel asthmatic youth and their families regarding the potential risks of ENDS [electronic nicotine delivery system] use and exposure to ENDS aerosols.” Providers “may also consider including ENDS aerosol exposure as a possible trigger in asthma self-management/action plans and updating asthma home environment assessments to include exposure to ENDS aerosols,” said investigators led by medical student Jennifer Bayly, a research fellow at the National Institute on Minority Health and Health Disparities in Bethesda, Md.
About 4% of adults in the United States and 11% of high school students vape, and almost 10% of U.S. adolescents reported living with an ENDS user in 2014. Given the data, “it is likely that a substantial number of asthmatic youth are exposed,” the investigators said.
The study adds to a growing body of evidence linking e-cigarettes to asthma. There’s moderate evidence for increased cough and wheezing in adolescents who use e-cigarettes, plus an association with e-cigarette use and increased asthma exacerbations. The new study, however, is likely the first to look specifically at secondhand exposure among asthmatic children. Ingredients in vaping aerosols, including flavorings, propylene glycol, and vegetable glycerin, are physiologically active in the lungs, and may be lung irritants.
Overall, about half of the respondents were female, and two-thirds were 11-13 years old. About a third identified as Hispanic, a third as white, and just over a fifth as black. Three-quarters of the sample lived in large or midsized metropolitan areas, and close to two-thirds in stand-alone homes. Participants were considered exposed to secondhand aerosols if they reported that in the past month they were in a room or car with someone who was vaping.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Bayly JE et al. CHEST®. 2018 Oct 22. doi: 10.1016/j.chest.2018.10.005.
Just like exposure to secondhand smoke, , according to a review of the 11,830 kids with asthma in the 2016 Florida Youth Tobacco survey.
Every year, the Florida Department of Health surveys public school children aged 11-17 years about various tobacco issues. In 2016, almost 12% of the asthmatic children in the survey said they vaped. Almost half were exposed to secondhand smoke, and a third reported exposure to secondhand vaping aerosols within the past 30 days. Overall, 21% reported an asthma attack in the past 12 months.
Using data from the Florida survey, the investigators crunched the numbers and found that secondhand aerosol exposure increased the odds of an asthma attack by 27%, independent of exposure to secondhand smoke and whether children smoked or vaped themselves (adjusted odds ratio, 1.27; 95% confidence interval, 1.11-1.47).
“Health professionals may wish to counsel asthmatic youth and their families regarding the potential risks of ENDS [electronic nicotine delivery system] use and exposure to ENDS aerosols.” Providers “may also consider including ENDS aerosol exposure as a possible trigger in asthma self-management/action plans and updating asthma home environment assessments to include exposure to ENDS aerosols,” said investigators led by medical student Jennifer Bayly, a research fellow at the National Institute on Minority Health and Health Disparities in Bethesda, Md.
About 4% of adults in the United States and 11% of high school students vape, and almost 10% of U.S. adolescents reported living with an ENDS user in 2014. Given the data, “it is likely that a substantial number of asthmatic youth are exposed,” the investigators said.
The study adds to a growing body of evidence linking e-cigarettes to asthma. There’s moderate evidence for increased cough and wheezing in adolescents who use e-cigarettes, plus an association with e-cigarette use and increased asthma exacerbations. The new study, however, is likely the first to look specifically at secondhand exposure among asthmatic children. Ingredients in vaping aerosols, including flavorings, propylene glycol, and vegetable glycerin, are physiologically active in the lungs, and may be lung irritants.
Overall, about half of the respondents were female, and two-thirds were 11-13 years old. About a third identified as Hispanic, a third as white, and just over a fifth as black. Three-quarters of the sample lived in large or midsized metropolitan areas, and close to two-thirds in stand-alone homes. Participants were considered exposed to secondhand aerosols if they reported that in the past month they were in a room or car with someone who was vaping.
The work was funded by the National Institutes of Health. The investigators had no disclosures.
SOURCE: Bayly JE et al. CHEST®. 2018 Oct 22. doi: 10.1016/j.chest.2018.10.005.
FROM CHEST®
Key clinical point: It’s important to screen asthmatic children for exposure to secondhand vaping aerosols, and minimize exposure.
Major finding: Secondhand aerosols increased the odds of an asthma attack 27%, independent of exposure to secondhand smoke and whether children smoked or vaped themselves.
Study details: Analysis of 11,830 children with asthma in the 2016 Florida Youth Tobacco survey.
Disclosures: The work was funded by the National Institutes of Health. The investigators had no disclosures.
Source: Bayly JE et al. CHEST®. 2018 Oct 22. doi: 10.1016/j.chest.2018.10.005.