User login
Mortality outcomes in hospitalized oncology patients after rapid response team activation
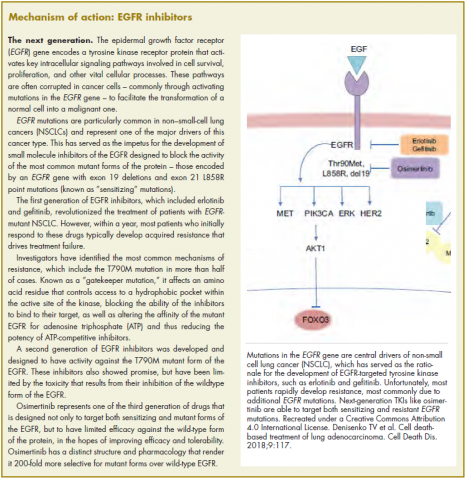
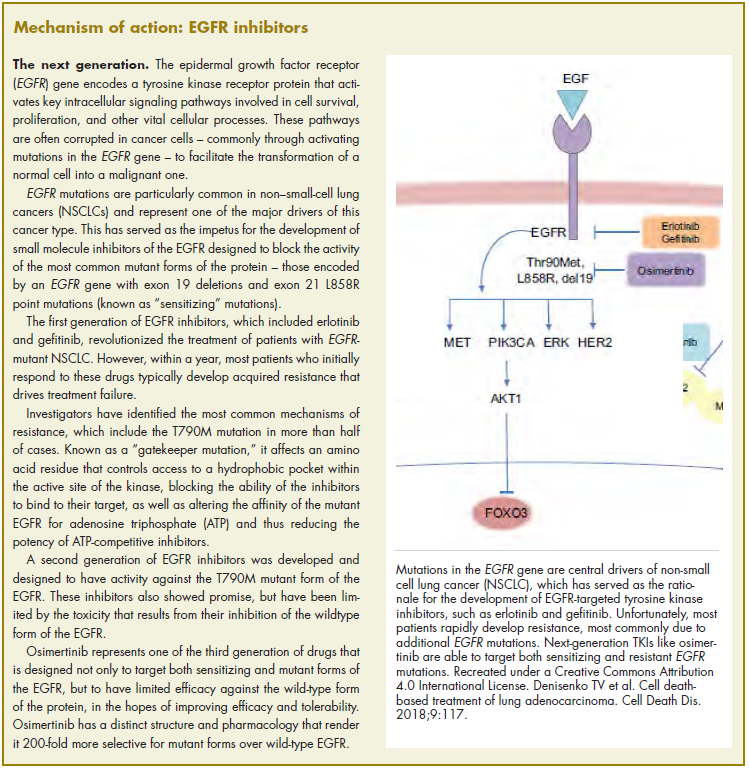
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
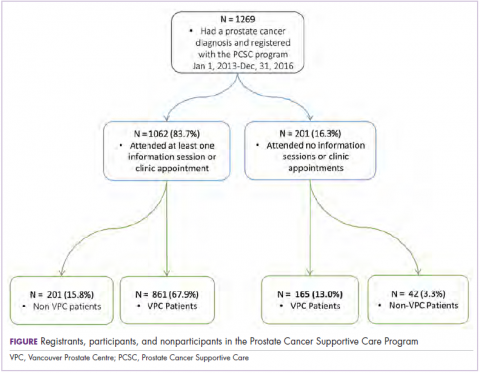
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
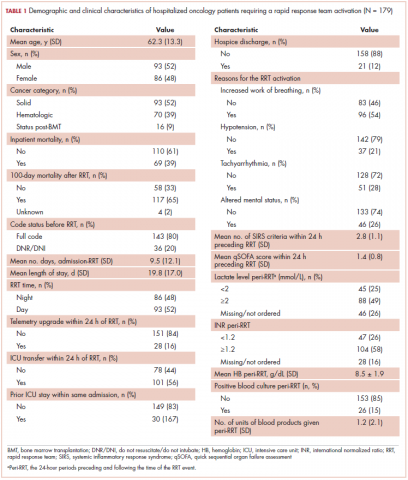
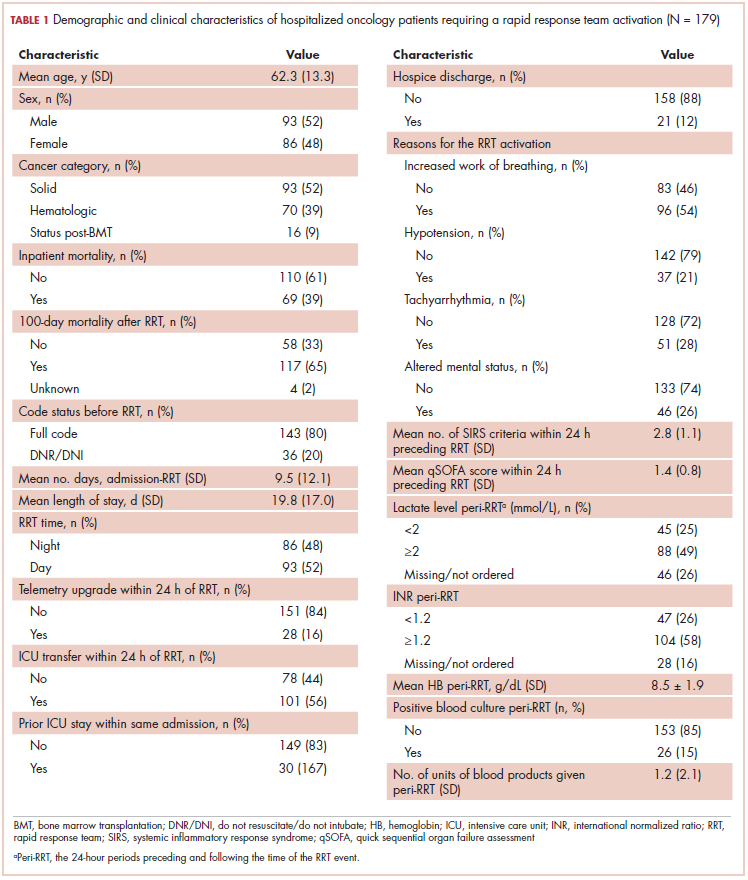
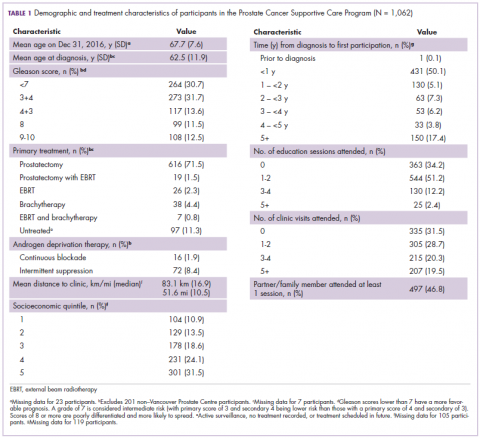
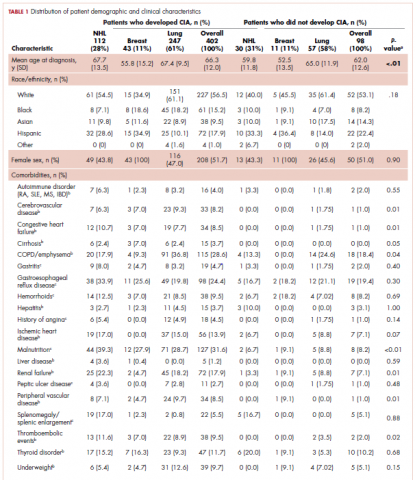
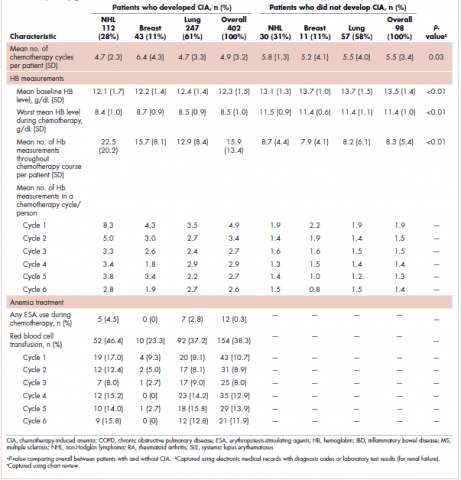
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
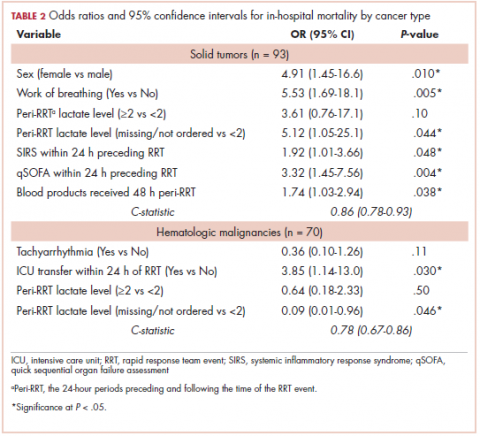
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
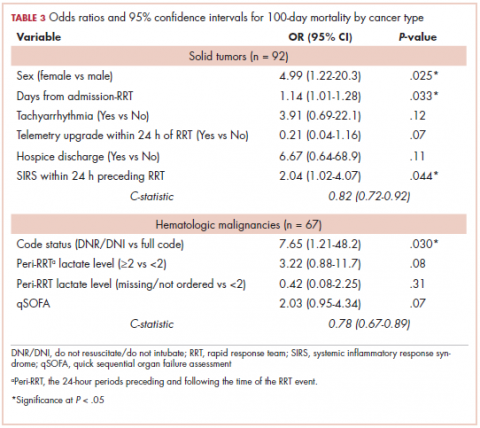
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
Effectiveness of duloxetine in treatment of painful chemotherapy-induced peripheral neuropathy: a systematic review
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
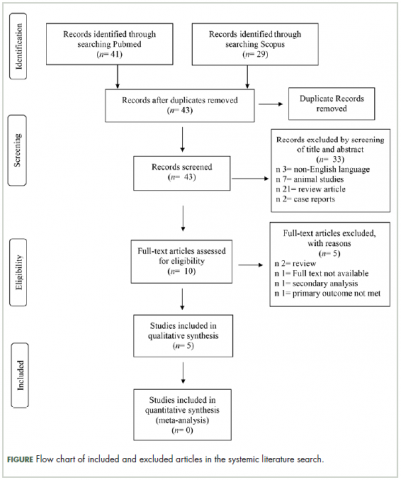
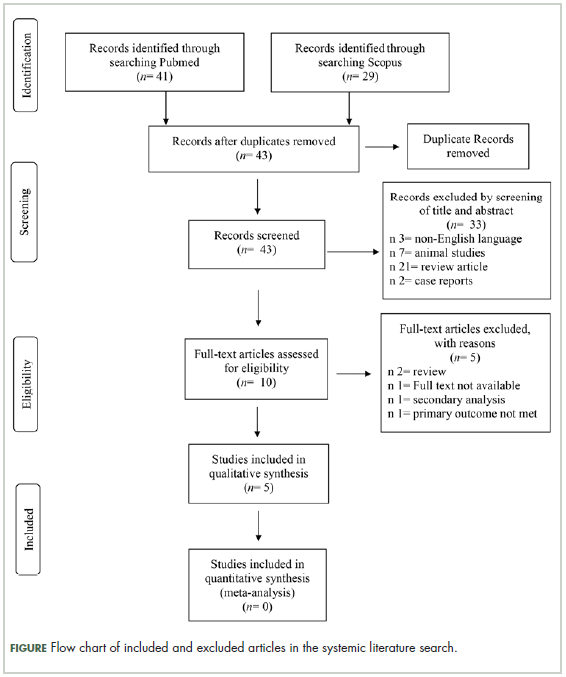
Selection of articles
The
Study characteristics
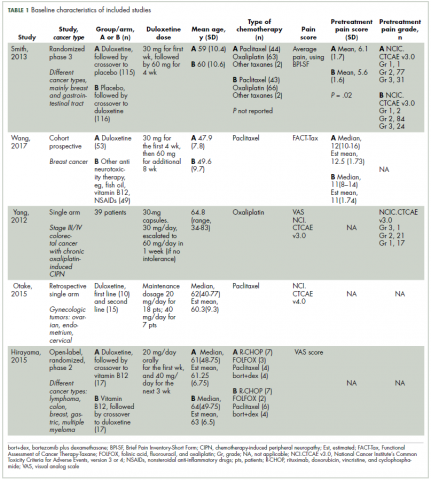
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
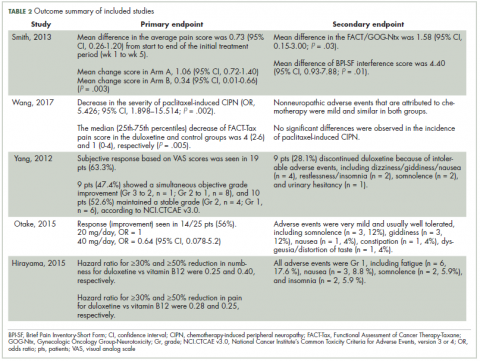
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
Selection of articles
The
Study characteristics
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious side effect that can be dose limiting and affect patient quality of life for prolonged time,1 with an overall incidence of about 38% in patients who are treated with multiple chemotherapeutic agents. 2
The most common antineoplastic agents causing peripheral neuropathy are oxaliplatin, cisplatin, taxanes, Vinca alkaloids, bortezomib, and thalidomide.3,8,9
Different components of the nervous system are targets of various chemotherapeutic agents, from dorsal root ganglion (DRG) cells to the distal axon. The DRG is the most vulnerable to neurotoxicity because it is less protected by the nervous system blood barrier, hence the predominance of sensory symptoms in CIPN.10 The pathogenesis of CIPN is not fully understood, and it is most probably multifaceted and not always related to the antineoplastic mechanism. Findings from experimental studies have shown an accumulation of chemotherapeutic compounds in the cell bodies of the DRG, resulting in decreased cellular metabolism and axoplasmic transport. Another proposed mechanism is the induction of apoptosis in sensory neuron of the posterior spinal ganglion after binding to DNA strands.7,11
Opioids had been used for managing pain in patients with cancer, but their addictive side effects limit use in the treatment of chronic pain,12 so several drugs called coanalgesics have been introduced as a treatment
The imbalance of 5HT and NE in the pain inhibitory pathways may contribute to the peripheral neuropathic pain.20 Duloxetine hydrochloride is a 5HT–NE reuptake inhibitor used to treat depression and generalized anxiety disorder.21 Duloxetine effect in decreasing pain transmission through increasing synaptic concentrations of NE and 5HT, which results in blocking input signals to the dorsal horn neurons in the spinal cord.12
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement) guidelines during the preparation of this systematic review.22
Inclusion criteria
Trial or study type. Articles publishing findings from randomized controlled trials, nonrandomized controlled trials, retrospective studies, and single-arm studies of duloxetine in the treatment of CIPN were included in our review.
Intervention. The intervention was duloxetine with all doses, either administered alone or with other antineuropathic drugs.
Comparator. The comparator was placebo (control group) or other antineuropathic drugs or no control group.
Population. The population included cancer patients with painful CIPN.
Outcome. At least one of the following outcomes was used for pain assessment:
Exclusion criteria
Studies in a non-English language, animal studies, studies whose full-text article was not available, and thesis and conference papers were not included.
Objective and study design
The objective of this systematic review was to systematically assess the effectiveness of duloxetine in the treatment of pain in patients with CIPN.
Information sources and search
Medical electronic databases. PubMed and Scopus, from inception to January 2018, were searched using the following search queries: (((duloxetine) AND chemotherapy induced peripheral neuropathy)) OR ((((chemotherapy) AND (neuropathic pain OR peripheral neuropathy))) AND duloxetine).
Selection of studies. The authors selected eligible studies. The screening of search results was performed in the following 2 steps:
n Screen title and abstracts against the selection criteria. Articles that were unclear from their title or abstract were reviewed against the selection criteria through the full text.
n Retrieve and screen full-text articles of eligible abstracts for eligibility to systematic review.
Data extraction
Two authors extracted the following data independently: sample size, mean age, chemotherapeutic drug, duloxetine dosage, and outcomes for pain assessment using at least one score from VAS, BPI-SF, neuropathic pain score using the NCI-CTCAE v3.0 and v4.0, or FACT-Tax, and other secondary outcomes. The data was exported from the online forms as a Microsoft Excel sheet.
Statistical analysis
We calculated the mean age and associated standard deviations (SDs) for all patients by using the pooled mean and pooled SD equation, according to Cochrane handbook of systematic reviews of interventions 5.1.0 (updated March 2011).23 When data are expressed as median and interquartile range, we used Hozo and colleagues’ BMC Research Methodology equation to calculate or estimate the mean and SD.24
Data are expressed as means with SD (unless stated otherwise); statistical results were considered significant when the P-value was less than .05. Data analysis was performed using the SPSS Statistical Package, version 23 (IBM Corp., Armonk, NY).
Synthesis of data and analysis
Because of heterogeneity and low sample size of studies, no statistically justified analyses could be performed on the provided data. Instead, a descriptive analysis of published studies was performed.
Summary measures
The search strings, the list of relevant reviews, the data coding, and the quality criteria that were used can be requested from the corresponding author.
Results
Selection of articles
The
Study characteristics
Characteristics of the included studies and patient outcome are summarized in Table 1 and Table 2. A total of 5 studies from 2012 through 2017 were included in the descriptive analysis and systematic review. In all, 4 trials were prospective studies, and 1 trial was retrospective; among all trials, 2 studies were single arm and 3 were placebo-controlled and/or crossover.
There were 431 participants in the total 5 studies included in this systematic review. The number of patients per study ranged from 25 to 231. Patients were mostly older, with mean sample ages ranging from 47.9 to 63 years, and the pooled mean age for all participants in the total 5 studies was 57.7 years.
In all included studies, duloxetine was given in varying doses of 20 mg, 30 mg, 40 mg, or 60 mg. Also, different therapeutic regimens of duloxetine were used, including placebo control or crossover with vitamin B12; 80% of the studies used escalation of doses over time (only 1 trial used fixed doses for each group of patients in the study). Escalation of duloxetine by doubling the dose was done in all 4 studies, with duloxetine doses of 30 mg and 60 mg used in 75% of those studies (3 out of 4 studies).
Comparator drug was used in 4 studies (1 was single arm) in our review analysis. The comparator drug was placebo in 1 study only, and the remaining 3 studies used other antineurotoxicity or antineuropathic pain therapy, mainly vitamin B12 (as only comparator in 1 study), fish oil, pregabalin, selective 5HT reuptake inhibitors, and nonsteroidal anti-inflammatory agents.
Regarding CIPN, the chemotherapeutic agents used in the studies were as follows (after exclusion of 11 patients who never received treatment in 1 study): 224 patients (52.9%) were on paclitaxel, 168 (39.7%) on oxaliplatin, 14 (3.30%) on R-CHOP, 8 (1.89%) on combined bortezomib–dexamethasone, 5 (1.18%) on FOLFOX, and 4 (0.94%) on other taxanes.
Improvement in pain scores was the primary and/or secondary endpoint in the included studies (Table 2). Pain was assessed by 6 different scores, including the VAS, BPI-SF, neuropathic pain score using NCI-CTCAE v3.0 and v4.0, and FACT-Tax, with all reported once except the VAS score, which was reported in 2 studies. Only 1 study, by Yang and colleagues,25 measured pain by 2 scores (the VAS and NCI-CTCAE v3.0), with the rest of the studies assessing pain by just 1 of the aforementioned scores. The pretreatment pain score was reported in only 2 studies, by Smith and colleagues and Wang and colleagues, using BPI-SF and FACT-Tax scores, respectively, with total respective mean scores of 5.8 (SD, 1.7) and 11.77 (SD, 1.73).17,26
Secondary endpoints were related mainly to pain score, drug adverse effect, and assessment of quality of life (Table 2). In the study by Yang and colleagues,25 9 patients (28.1%) discontinued duloxetine because of intolerable adverse events, with dizziness or giddiness as the most common cause (44.4% of patients who discontinued treatment). Studies by Otake and colleagues12 and Hirayama and colleagues2 reported duloxetine adverse events that were very mild and usually well tolerated in collectively 22 patients, with fatigue (n = 6) and somnolence (n = 5) as the most reported adverse effects. Wang and colleagues17 reported nonneuropathic adverse events that were attributed to chemotherapy and were mild and similar in both study groups.
Discussion
To our knowledge, this is the first systematic review to discuss the effectiveness of duloxetine specifically in treatment of pain in CIPN. The administration of chemotherapeutic agents such as paclitaxel, cisplatin, oxaliplatin, and vincristine was accompanied by CIPN. The currently available treatment options for CIPN are limited. To date, no drug has been approved specifically for treatment of pain in CIPN.12
In our review, we included cancer patients with CIPN and associated pain. Several previous studies8,27,28 discussed tingling and numbness as a common adverse effect in CIPN, and usually about 20% to 42% of patients develop chronic pain.
Six different pain assessment scores were reported in the total 5 studies in our review, with VAS and NCI-CTCAE scores reported in more than 1 study. This reflects the major challenges facing the assessment of CIPN, as various scales and tools are available for pain assessment but without a standardized approach for CIPN that can be precisely implemented.8 Several other challenges regarding pain scores were observed, with Smith and colleagues as the only authors to report both pretreatment pain score and grade, while the rest of the studies failed to report either pain score or grade, or even both.
Another difficulty observed in our review was the variability in study participants in both population size and type of cancer treated. The population size in largest study included in our review was 231 patients and the smallest was 25 patients; collectively, there were only 431 patients in the included studies. Although the type of primary cancer varied in between studies, gynecologic malignancies comprised most cases (215 patients), followed by gastrointestinal tumors, and few cases of hematologic and genitourinary malignancies were reported. Similar results were observed by Geber and colleagues in their large study screening pain in cancer patients, in which gynecologic malignancies were diagnosed in 28 patients out of 61 with CIPN, representing the highest percentage (45.9%) of malignancy type in that study.26
In the study by Otake and colleagues12 examining duloxetine for CIPN in patients with gynecologic cancer, the authors concluded that duloxetine dosage either 20 mg/day or 40 mg/day was not associated with the effectiveness of duloxetine treatment by either univariate or multivariate analysis. Previous authors have provided an explanation for the difference in duloxetine response among CIPN patients and attributed those differences to the underlying pain mechanisms.14,29 In other words, pain in those patients is both peripheral nociceptive and central neuropathic, and it is likely to be caused by mixed mechanisms.
Another variation observed among CIPN patients in our review was the chemotherapeutic agents used. That was noted by Smith and colleagues,26 who reported that patients with cancer who received platinum therapies (oxaliplatin) experienced more benefit from duloxetine in terms of pain improvement than those who received taxanes (P = .13). We found no other published studies on the response to duloxetine among different chemotherapeutic agents used. However, 2 studies of duloxetine response in patients with other pain-related disorders (painful diabetic peripheral neuropathy and fibromyalgia) showed significant improvement in pain symptoms compared with placebo. In a study of pain in chemotherapy-induced neuropathy (CIN) by Geber and colleagues,29 the preexisting pain medication was not reported, but the authors concluded that treatment for CIN-related neuropathic pain differs from that for nonneuropathic (ie, musculoskeletal) pain, with the former being treated mainly with pharmacotherapy and the latter with physiotherapy and behavioral exercises. They asserted that different pain patterns could help flag neuropathic or musculoskeletal pain so that the selected treatments would be more specific. Differences in pain improvement related to duloxetine may be attributed to the underlying pain mechanism, and whether it is mixed or centrally or peripherally related was also discussed by Geber and colleagues.29
In the study by
Findings from studies on the effect of duloxetine in treatment of pain in diabetic peripheral neuropathy have shown that duloxetine at a dose of 60 mg/day effectively improves pain in 43% to 68% of patients.15,16,30 Similarly, in our review, the study by Yang and colleagues25 showed a 63% subjective reduction in pain severity by VAS score in CIPN patients but lower improvement of 47.4% by NCI-CTCAE v3.0; this can be attributed to the simplistic 4-grade rating scale of the latter.
During our analysis of studies, we noticed that no diagnostic criteria were implemented for diagnosis or inclusion of CIPN patients in any of the included studies, and this represents a major challenge in any analysis of studies with neuropathic pain patients. In 2016, Finnerup and colleagues updated the previous 2008 grading system for diagnosis of neuropathic pain, which is intended to determine the level of certainty with which the pain in question is neuropathic.31 The system defines the diagnostic certainty into 3 levels: Possible, Probable, and Definite. Although the number of studies used the grading system during the inclusion of neuropathic pain patients increased from 5% in 2009 to 30% in 2014, still more than two-thirds of studies do not use a standardized system for diagnosis and/or inclusion of neuropathic pain in patients.
Strength and limitations
The first strength of this review is that it identifies gaps in our current knowledge about duloxetine in the treatment of pain in cancer patients with CIPN. Second, we collected all available articles from inception until January 2018. Third, this review can serve as a model for future studies investigating the effectiveness of duloxetine in treatment of CIPN.
There are also limitations to this review that should be discussed. First, the studies vary greatly in samples, methodologies, and outcomes measured. Second, the diagnostic criteria for CIPN and the pain assessment tools vary among the studies. Third, there is also variability in the duloxetine doses and administration regimens among the studies, and some articles did not report the precise outcome in pain scores. Furthermore, the articles reviewed included retrospective, single-arm, or nonrandomized controlled studies with relatively small numbers of participants.
To improve the results, more placebo-controlled or head-to-head trials (with other agents used in treatment of CIPN) with large sample sizes would be needed.
Conclusions
Our purpose was to describe the effectiveness of duloxetine in improving pain scores among CIPN patients
Acknowledgments
That authors express their sincere gratitude to Nahla A Merghany and Sarah M Abd Elfadel for helping them retrieve all the relevant articles for this review.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
1. Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27-46.
2. Hirayama Y, Ishitani K, Sato Y, et al. Effect of duloxetine in Japanese patients with chemotherapy-induced peripheral neuropathy: a pilot randomized trial. Int J Clin Oncol. 2015;20(5):866-871.
3. Stubblefield MD, McNeely ML, Alfano CM, Mayer DK. A prospective surveillance model for physical rehabilitation of women with breast cancer: chemotherapy-induced peripheral neuropathy. Cancer. 2012;118(suppl 8):2250-2260.
4. Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419-437.
5. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014;6(1):135-147.
6. Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323-331.
7. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
8. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15-49.
9. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094.
10. Caponero R, Montarroyos ES, Tahamtani SMM. Post-chemotherapy neuropathy. Rev Dor. Sao Paulo. 2016;17(suppl 1):S56-S58.
11. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116-131.
12. Otake A, Yoshino K, Ueda Y, et al. Usefulness of duloxetine for paclitaxel-induced peripheral neuropathy treatment in gynecological cancer patients. Anticancer Res. 2015;35(1):359-363.
13. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967.
14. Smith EM, Pang H, Ye C, et al. Predictors of duloxetine response in patients with oxaliplatin-induced painful chemotherapy-induced peripheral neuropathy (CIPN): a secondary analysis of randomised controlled trial – CALGB/alliance 170601 [published online November 25, 2015]. Eur J Cancer Care (Engl). 2017;26(2). doi:10.1111/ecc.12421
15. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118.
16. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356.
17. Wang J, Li Q, Xu B, Zhang T, Chen S, Luo Y. Efficacy and safety of duloxetine in Chinese breast cancer patients with paclitaxel-induced peripheral neuropathy. Chin J Cancer Res. 2017;29(5):411-418.
18. Irving G, Tanenberg RJ, Raskin J, Risser RC, Malcolm S. Comparative safety and tolerability of duloxetine vs pregabalin vs duloxetine plus gabapentin in patients with diabetic peripheral neuropathic pain. Int J Clin Pract. 2014;68(9):1130-1140.
19. Esin E, Yalcin S. Neuropathic cancer pain: what we are dealing with? How to manage it? Onco Targets Ther. 2014;7:599-618.
20. Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25(12):613-617.
21. Mancini M, Perna G, Rossi A, Petralia A. Use of duloxetine in patients with an anxiety disorder, or with comorbid anxiety and major depressive disorder: a review of the literature. Expert Opin Pharmacother. 2010;11(7):1167-1181.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269.
23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://handbook-5-1.cochrane.org/. Updated March 2011. Accessed November 19, 2018.
24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
25. Yang YH, Lin JK, Chen WS, et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: an open-label pilot study. Support Care Cancer. 2012;20(7):1491-1497.
26. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359-1367.
27. Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18(6):343-349.
28. Cavenagh J, Good P, Ravenscroft P. Neuropathic pain: are we out of the woods yet? Intern Med J. 2006;36(4):251-255.
29. Geber C, Breimhorst M, Burbach B, et al. Pain in chemotherapy-induced neuropathy—more than neuropathic? Pain. 2013;154(12):2877-2887.
30. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420.
31. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. 2016;157(8):1599-1606.
Development, implementation, and evaluation of a prostate cancer supportive care program
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
Paradigm-changing osimertinib approval in front-line for advanced NSCLC
The US Food and Drug Administration awarded regulatory approval this spring to the third-generation epidermal growth factor receptor (EGFR) inhibitor osimertinib for the treatment of patients with exon 19 deletion- or exon21 L858R mutation-positive advanced non–small-cell lung cancer (NSCLC) not previously treated for advanced disease.
Osimertinib is designed to target both sensitizing and resistant mutant forms of EGFR, but not the wildtype protein, in an effort to improve safety and efficacy compared with other standard of care (SoC) EGFR inhibitors. It was previously approved in the second-line setting in NSCLC following failure of prior EGFR inhibitor therapy in 2015. The current approval represents a paradigm shift in the front-line treatment of advanced NSCLC, reinforcing the role of osimertinib, which has been recommended in this setting by the National Comprehensive Cancer Network Guidelines in Oncology for more than a year.
Approval was based on the phase 3, multicenter, international, randomized, double-blind, active-controlled FLAURA trial. A total of 556 patients were randomized 1:1 to receive an oral daily dose of 80 mg osimertinib or gefitinib 250 mg or erlotinib 150 mg. The trial was conducted during December 2014 through March 2016 at 132 sites in 29 countries.
Eligible patients were aged 18 or over and had locally advanced or metastatic NSCLC, had not previously received treatment for advanced disease, were eligible for first-line treatment with erlotinib or gefitinib, had locally or centrally confirmed EGFR exon 19 deletion or L858R mutations alone or concurrently with other EGFR mutations, and a World Health Organization Performance Status of 0 (fully active, able to carry on all predisease performance without restriction) or 1 (restricted in strenuous activity but ambulatory and able to carry out light work), and a minimum life expectancy of 12 weeks.
Patients with central nervous system metastases were eligible if their condition was neurologically stable. Patients who had previous definitive treatment or glucocorticoid therapy had to have completed it at least 2 weeks before the start of the trial. Patients were excluded from the trial if they had any previous treatment with any systemic anticancer therapy for advanced NSCLC, had major surgery within 4 weeks of the first dose of the study drug, had radiation therapy to more than 30% of the bone marrow or a wide field of radiation within 4 weeks of the first dose of the study drug, or were currently receiving potent inhibitors or inducers of cytochrome P450 3A4.
Osimertinib cut the risk of disease progression or death by more than 50% compared with standard TKI therapy. The estimated median progression-free survival (PFS) was 18.9 months with osimertinib, compared with 10.2 months for erlotinib or gefitinib (hazard ratio [HR]: 0.46; P < .0001). PFS benefit extended across all prespecified subgroups, including patients with CNS metastases (median PFS: 15.2 months vs 9.6 months; HR: 0.47; P = .0009). Confirmed overall response rate was 77% and 69% in the study and SoC groups, respectively, and estimated duration of response (DoR) was 17.6 months and 9.6 months. At the time of analysis, there were too few deaths to compare overall survival.
The most common adverse events (AEs) experienced by patients treated with osimertinib were diarrhea, rash, dry skin, nail toxicity, stomatitis, and reduced appetite. Serious AEs occurred in 4% of patients treated with osimertinib, most commonly involving pneumonia, interstitial lung disease/pneumonitis, and pulmonary embolism (PE). The rate of grade 3/4 AEs was 33.7% in the osimertinib group and 44.8% in the SoC group. Patients treated with osimertinib were less likely to discontinue treatment due to AEs (13.3% vs 18.1% of those receiving SoC).
Osimertinib is marketed as Tagrisso by AstraZeneca and the recommended dose is 80 mg orally once daily, with or without food. The prescribing information details warnings and precautions relating to interstitial lung disease and pneumonitis, QTc interval prolongation, cardiomyopathy, keratitis, and embryofetal toxicity.
Treatment with osimertinib should be withheld in patients presenting with worsening of respiratory symptoms indicative of ILD and permanently discontinued if ILD is confirmed. Electrocardiograms and electrolytes should be monitored periodically in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities or in patients taking medications known to prolong QTc interval. Treatment should be permanently discontinued in those who develop QTc interval prolongation with signs and symptoms of life-threatening arrhythmia.
Cardiac monitoring, including assessment of left ventricular ejection fraction should be performed at baseline and throughout treatment in patients with cardiac risk factors and treatment should be permanently discontinued in patients who develop symptomatic congestive heart failure. Patients with signs and symptoms of keratitis should be referred to an ophthalmologist. Osimertinib can cause fetal harm and patients should be advised of the potential risk and the need for effective contraception use during treatment and for 6 weeks after the final dose is administered.
1. US Food and Drug Administration Website. FDA approves osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm605113.htm. Last updated April 18, 2018. Accessed October 6, 2018.
2. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-
3. Tagrisso (osimertinib tablets) for oral use. Prescribing information. AstraZeneca. https://www.azpicentral.com/tagrisso/tagrisso.pdf#page=1. August 2018. Accessed October 6, 2018.
The US Food and Drug Administration awarded regulatory approval this spring to the third-generation epidermal growth factor receptor (EGFR) inhibitor osimertinib for the treatment of patients with exon 19 deletion- or exon21 L858R mutation-positive advanced non–small-cell lung cancer (NSCLC) not previously treated for advanced disease.
Osimertinib is designed to target both sensitizing and resistant mutant forms of EGFR, but not the wildtype protein, in an effort to improve safety and efficacy compared with other standard of care (SoC) EGFR inhibitors. It was previously approved in the second-line setting in NSCLC following failure of prior EGFR inhibitor therapy in 2015. The current approval represents a paradigm shift in the front-line treatment of advanced NSCLC, reinforcing the role of osimertinib, which has been recommended in this setting by the National Comprehensive Cancer Network Guidelines in Oncology for more than a year.
Approval was based on the phase 3, multicenter, international, randomized, double-blind, active-controlled FLAURA trial. A total of 556 patients were randomized 1:1 to receive an oral daily dose of 80 mg osimertinib or gefitinib 250 mg or erlotinib 150 mg. The trial was conducted during December 2014 through March 2016 at 132 sites in 29 countries.
Eligible patients were aged 18 or over and had locally advanced or metastatic NSCLC, had not previously received treatment for advanced disease, were eligible for first-line treatment with erlotinib or gefitinib, had locally or centrally confirmed EGFR exon 19 deletion or L858R mutations alone or concurrently with other EGFR mutations, and a World Health Organization Performance Status of 0 (fully active, able to carry on all predisease performance without restriction) or 1 (restricted in strenuous activity but ambulatory and able to carry out light work), and a minimum life expectancy of 12 weeks.
Patients with central nervous system metastases were eligible if their condition was neurologically stable. Patients who had previous definitive treatment or glucocorticoid therapy had to have completed it at least 2 weeks before the start of the trial. Patients were excluded from the trial if they had any previous treatment with any systemic anticancer therapy for advanced NSCLC, had major surgery within 4 weeks of the first dose of the study drug, had radiation therapy to more than 30% of the bone marrow or a wide field of radiation within 4 weeks of the first dose of the study drug, or were currently receiving potent inhibitors or inducers of cytochrome P450 3A4.
Osimertinib cut the risk of disease progression or death by more than 50% compared with standard TKI therapy. The estimated median progression-free survival (PFS) was 18.9 months with osimertinib, compared with 10.2 months for erlotinib or gefitinib (hazard ratio [HR]: 0.46; P < .0001). PFS benefit extended across all prespecified subgroups, including patients with CNS metastases (median PFS: 15.2 months vs 9.6 months; HR: 0.47; P = .0009). Confirmed overall response rate was 77% and 69% in the study and SoC groups, respectively, and estimated duration of response (DoR) was 17.6 months and 9.6 months. At the time of analysis, there were too few deaths to compare overall survival.
The most common adverse events (AEs) experienced by patients treated with osimertinib were diarrhea, rash, dry skin, nail toxicity, stomatitis, and reduced appetite. Serious AEs occurred in 4% of patients treated with osimertinib, most commonly involving pneumonia, interstitial lung disease/pneumonitis, and pulmonary embolism (PE). The rate of grade 3/4 AEs was 33.7% in the osimertinib group and 44.8% in the SoC group. Patients treated with osimertinib were less likely to discontinue treatment due to AEs (13.3% vs 18.1% of those receiving SoC).
Osimertinib is marketed as Tagrisso by AstraZeneca and the recommended dose is 80 mg orally once daily, with or without food. The prescribing information details warnings and precautions relating to interstitial lung disease and pneumonitis, QTc interval prolongation, cardiomyopathy, keratitis, and embryofetal toxicity.
Treatment with osimertinib should be withheld in patients presenting with worsening of respiratory symptoms indicative of ILD and permanently discontinued if ILD is confirmed. Electrocardiograms and electrolytes should be monitored periodically in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities or in patients taking medications known to prolong QTc interval. Treatment should be permanently discontinued in those who develop QTc interval prolongation with signs and symptoms of life-threatening arrhythmia.
Cardiac monitoring, including assessment of left ventricular ejection fraction should be performed at baseline and throughout treatment in patients with cardiac risk factors and treatment should be permanently discontinued in patients who develop symptomatic congestive heart failure. Patients with signs and symptoms of keratitis should be referred to an ophthalmologist. Osimertinib can cause fetal harm and patients should be advised of the potential risk and the need for effective contraception use during treatment and for 6 weeks after the final dose is administered.
The US Food and Drug Administration awarded regulatory approval this spring to the third-generation epidermal growth factor receptor (EGFR) inhibitor osimertinib for the treatment of patients with exon 19 deletion- or exon21 L858R mutation-positive advanced non–small-cell lung cancer (NSCLC) not previously treated for advanced disease.
Osimertinib is designed to target both sensitizing and resistant mutant forms of EGFR, but not the wildtype protein, in an effort to improve safety and efficacy compared with other standard of care (SoC) EGFR inhibitors. It was previously approved in the second-line setting in NSCLC following failure of prior EGFR inhibitor therapy in 2015. The current approval represents a paradigm shift in the front-line treatment of advanced NSCLC, reinforcing the role of osimertinib, which has been recommended in this setting by the National Comprehensive Cancer Network Guidelines in Oncology for more than a year.
Approval was based on the phase 3, multicenter, international, randomized, double-blind, active-controlled FLAURA trial. A total of 556 patients were randomized 1:1 to receive an oral daily dose of 80 mg osimertinib or gefitinib 250 mg or erlotinib 150 mg. The trial was conducted during December 2014 through March 2016 at 132 sites in 29 countries.
Eligible patients were aged 18 or over and had locally advanced or metastatic NSCLC, had not previously received treatment for advanced disease, were eligible for first-line treatment with erlotinib or gefitinib, had locally or centrally confirmed EGFR exon 19 deletion or L858R mutations alone or concurrently with other EGFR mutations, and a World Health Organization Performance Status of 0 (fully active, able to carry on all predisease performance without restriction) or 1 (restricted in strenuous activity but ambulatory and able to carry out light work), and a minimum life expectancy of 12 weeks.
Patients with central nervous system metastases were eligible if their condition was neurologically stable. Patients who had previous definitive treatment or glucocorticoid therapy had to have completed it at least 2 weeks before the start of the trial. Patients were excluded from the trial if they had any previous treatment with any systemic anticancer therapy for advanced NSCLC, had major surgery within 4 weeks of the first dose of the study drug, had radiation therapy to more than 30% of the bone marrow or a wide field of radiation within 4 weeks of the first dose of the study drug, or were currently receiving potent inhibitors or inducers of cytochrome P450 3A4.
Osimertinib cut the risk of disease progression or death by more than 50% compared with standard TKI therapy. The estimated median progression-free survival (PFS) was 18.9 months with osimertinib, compared with 10.2 months for erlotinib or gefitinib (hazard ratio [HR]: 0.46; P < .0001). PFS benefit extended across all prespecified subgroups, including patients with CNS metastases (median PFS: 15.2 months vs 9.6 months; HR: 0.47; P = .0009). Confirmed overall response rate was 77% and 69% in the study and SoC groups, respectively, and estimated duration of response (DoR) was 17.6 months and 9.6 months. At the time of analysis, there were too few deaths to compare overall survival.
The most common adverse events (AEs) experienced by patients treated with osimertinib were diarrhea, rash, dry skin, nail toxicity, stomatitis, and reduced appetite. Serious AEs occurred in 4% of patients treated with osimertinib, most commonly involving pneumonia, interstitial lung disease/pneumonitis, and pulmonary embolism (PE). The rate of grade 3/4 AEs was 33.7% in the osimertinib group and 44.8% in the SoC group. Patients treated with osimertinib were less likely to discontinue treatment due to AEs (13.3% vs 18.1% of those receiving SoC).
Osimertinib is marketed as Tagrisso by AstraZeneca and the recommended dose is 80 mg orally once daily, with or without food. The prescribing information details warnings and precautions relating to interstitial lung disease and pneumonitis, QTc interval prolongation, cardiomyopathy, keratitis, and embryofetal toxicity.
Treatment with osimertinib should be withheld in patients presenting with worsening of respiratory symptoms indicative of ILD and permanently discontinued if ILD is confirmed. Electrocardiograms and electrolytes should be monitored periodically in patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities or in patients taking medications known to prolong QTc interval. Treatment should be permanently discontinued in those who develop QTc interval prolongation with signs and symptoms of life-threatening arrhythmia.
Cardiac monitoring, including assessment of left ventricular ejection fraction should be performed at baseline and throughout treatment in patients with cardiac risk factors and treatment should be permanently discontinued in patients who develop symptomatic congestive heart failure. Patients with signs and symptoms of keratitis should be referred to an ophthalmologist. Osimertinib can cause fetal harm and patients should be advised of the potential risk and the need for effective contraception use during treatment and for 6 weeks after the final dose is administered.
1. US Food and Drug Administration Website. FDA approves osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm605113.htm. Last updated April 18, 2018. Accessed October 6, 2018.
2. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-
3. Tagrisso (osimertinib tablets) for oral use. Prescribing information. AstraZeneca. https://www.azpicentral.com/tagrisso/tagrisso.pdf#page=1. August 2018. Accessed October 6, 2018.
1. US Food and Drug Administration Website. FDA approves osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm605113.htm. Last updated April 18, 2018. Accessed October 6, 2018.
2. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-
3. Tagrisso (osimertinib tablets) for oral use. Prescribing information. AstraZeneca. https://www.azpicentral.com/tagrisso/tagrisso.pdf#page=1. August 2018. Accessed October 6, 2018.
BRAF-MEK inhibitor combo approved for adjuvant melanoma therapy
On April 30, 2018, the US Food and Drug Administration expanded the indication for the combined use of dabrafenib and trametinib to include adjuvant treatment of BRAF-mutant melanoma following complete surgical resection. Dabrafenib is an inhibitor of the BRAF kinase, and trametinib is an inhibitor of the MEK kinase, both of which are components of the mitogen-activated protein kinase (MAPK) signaling pathway. The 2 drugs are already approved as both single agents and in combination for the treatment of BRAF-mutated metastatic melanoma.
The current approval was based on data from a phase 3, international, multicenter, randomized, double-blind, placebo-controlled trial. The COMBI-AD trial was carried out from January 2013 through December 2014 at 169 sites in 26 countries. A total of 870 patients with stage III melanoma and BRAF V600E/K mutations and pathologic involvement of regional lymph nodes following complete resection were randomly assigned to receive dabrafenib 150 mg twice daily in combination with trametinib 2 mg once daily, or 2 matched placebos for up to 1 year. Randomization was stratified according to BRAF mutation status (V600E or V600K) and disease stage (IIIA, IIIB or IIIC).
Eligible patients were aged 18 years or older and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (on a scale of 1-5, with higher scores indicating greater disability). Patients who had undergone previous systemic anticancer therapy or radiotherapy were excluded from the study.
The primary endpoint was relapse-free survival (RFS), defined as the time from randomization to disease recurrence or death from any cause. Secondary endpoints included overall survival (OS), distant metastasis-free survival (DMFS), freedom from relapse (FFR), and safety. Clinical examination and imaging by computed tomography, magnetic resonance imaging, or both was performed every 3 months for the first 2 years and then every 6 months until disease recurrence or trial completion.
As of the data cut-off, the combination of dabrafenib and trametinib reduced the risk of disease recurrence or death by 53% compared with placebo (hazard ratio [HR], 0.47; P < .001). Median RFS was not yet reached in the combination arm, compared with 16.6 months in the placebo arm. The RFS benefit was observed across all prespecified subgroups, and the combination was also found to improve OS, DMFS, and FFR.
The most common adverse events (AEs) included pyrexia, fatigue, nausea, rash, vomiting, diarrhea, chills, and myalgia. Overall, 97% of patients experienced an AE, 41% experienced a grade 3/4 AE, and 26% had an AE that led to treatment discontinuation. In patients treated with placebo, those numbers were 88%, 14%, and 3%, respectively.
The separate prescribing information for dabrafenib and trametinib detail warnings and precautions relating to their combined use, including the need to confirm BRAF status before starting therapy (because use in BRAF wildtype tumors can promote tumor cell proliferation), new primary malignancies, hemorrhage, cardiomyopathy, uveitis, serious febrile reactions, serious skin toxicity, hyperglycemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, colitis and gastrointestinal perforation, venous thromboembolism, ocular toxicities, interstitial lung disease, and embryofetal toxicity.
Dermatologic evaluations should be completed before starting therapy, every 2 months during and for up to 6 months after completion of therapy, and patients should be monitored closely for the signs and symptoms of noncutaneous primary malignancies. Treatment should be discontinued for all grade 4 hemorrhagic events and for any grade 3 events that do not improve, and withheld for grade 3 events until they resolve, at which point treatment can be resumed at the next lowest dose as described in the prescribing information.
Left ventricular ejection fraction (LVEF) values should be assessed before initiating therapy, after 1 month, and then at intervals of 2-3 months. Treatment should be withheld for up to 4 weeks if absolute LVEF values decrease by 10% and are less than the lower limit of normal (LLN) and it should be permanently discontinued for symptomatic cardiomyopathy or persistent, asymptomatic left ventricular dysfunction of >20% from baseline that is below LLN and does not resolve within 4 weeks.
Treatment should be withheld for fevers higher than 104°F or for serious febrile reactions or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure. Serum creatinine levels should be monitored, along with other evidence of renal function, during, and after severe pyrexia. Antipyretics should be administered as secondary prophylaxis when treatment is resumed if the patient had previous episodes of severe febrile reaction or if fever was associated with complications. Corticosteroids should be administered for at least 5 days for second or subsequent pyrexia if the body temperature dose not return to baseline within 3 days of fever onset or for pyrexia associated with complications and no evidence of active infection.
Treatment should also be withheld for intolerable or severe skin toxicity and resumed at a lower dose as per guidelines in patients who improve or recover within 3 weeks. Serum glucose levels should be monitored at the start of treatment and as clinically appropriate in patients with pre-existing diabetes or hyperglycemia. Patients with G6PD deficiency should be monitored closely for signs of hemolytic anemia.
Patients should be monitored closely for signs and symptoms of colitis and gastrointestinal
Ophthalmological evaluations should be performed periodically and within 24 hours of patient-reported loss of vision or other visual disturbances. Treatment should be permanently discontinued in patients with documented retinal vein occlusion and withheld for retinal pigment epithelial detachment. Treatment should also be withheld in patients presenting with new or progressive pulmonary symptoms and findings and permanently discontinued for treatment-related interstitial lung disease or pneumonitis.
Both dabrafenib and trametinib can cause fetal harm and patients should be warned of this risk and the need for adequate contraceptive measures. Dabrafenib and trametinib are marketed as Tafinlar and Mekinist by Novartis.
1. US Food and Drug Administration Website. FDA approves dabrafenib plus trametinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm606165.htm. Last updated April 30, 2018. Accessed October 6, 2018.
2. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1913-1823.
3. Tafinlar (dabrafenib) capsules, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tafinlar.pdf. May 2018. Accessed October 6, 2018.
4. Mekinist (trametinib) tablets, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mekinist.pdf. May 2018. Accessed October 6th, 2018.
On April 30, 2018, the US Food and Drug Administration expanded the indication for the combined use of dabrafenib and trametinib to include adjuvant treatment of BRAF-mutant melanoma following complete surgical resection. Dabrafenib is an inhibitor of the BRAF kinase, and trametinib is an inhibitor of the MEK kinase, both of which are components of the mitogen-activated protein kinase (MAPK) signaling pathway. The 2 drugs are already approved as both single agents and in combination for the treatment of BRAF-mutated metastatic melanoma.
The current approval was based on data from a phase 3, international, multicenter, randomized, double-blind, placebo-controlled trial. The COMBI-AD trial was carried out from January 2013 through December 2014 at 169 sites in 26 countries. A total of 870 patients with stage III melanoma and BRAF V600E/K mutations and pathologic involvement of regional lymph nodes following complete resection were randomly assigned to receive dabrafenib 150 mg twice daily in combination with trametinib 2 mg once daily, or 2 matched placebos for up to 1 year. Randomization was stratified according to BRAF mutation status (V600E or V600K) and disease stage (IIIA, IIIB or IIIC).
Eligible patients were aged 18 years or older and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (on a scale of 1-5, with higher scores indicating greater disability). Patients who had undergone previous systemic anticancer therapy or radiotherapy were excluded from the study.
The primary endpoint was relapse-free survival (RFS), defined as the time from randomization to disease recurrence or death from any cause. Secondary endpoints included overall survival (OS), distant metastasis-free survival (DMFS), freedom from relapse (FFR), and safety. Clinical examination and imaging by computed tomography, magnetic resonance imaging, or both was performed every 3 months for the first 2 years and then every 6 months until disease recurrence or trial completion.
As of the data cut-off, the combination of dabrafenib and trametinib reduced the risk of disease recurrence or death by 53% compared with placebo (hazard ratio [HR], 0.47; P < .001). Median RFS was not yet reached in the combination arm, compared with 16.6 months in the placebo arm. The RFS benefit was observed across all prespecified subgroups, and the combination was also found to improve OS, DMFS, and FFR.
The most common adverse events (AEs) included pyrexia, fatigue, nausea, rash, vomiting, diarrhea, chills, and myalgia. Overall, 97% of patients experienced an AE, 41% experienced a grade 3/4 AE, and 26% had an AE that led to treatment discontinuation. In patients treated with placebo, those numbers were 88%, 14%, and 3%, respectively.
The separate prescribing information for dabrafenib and trametinib detail warnings and precautions relating to their combined use, including the need to confirm BRAF status before starting therapy (because use in BRAF wildtype tumors can promote tumor cell proliferation), new primary malignancies, hemorrhage, cardiomyopathy, uveitis, serious febrile reactions, serious skin toxicity, hyperglycemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, colitis and gastrointestinal perforation, venous thromboembolism, ocular toxicities, interstitial lung disease, and embryofetal toxicity.
Dermatologic evaluations should be completed before starting therapy, every 2 months during and for up to 6 months after completion of therapy, and patients should be monitored closely for the signs and symptoms of noncutaneous primary malignancies. Treatment should be discontinued for all grade 4 hemorrhagic events and for any grade 3 events that do not improve, and withheld for grade 3 events until they resolve, at which point treatment can be resumed at the next lowest dose as described in the prescribing information.
Left ventricular ejection fraction (LVEF) values should be assessed before initiating therapy, after 1 month, and then at intervals of 2-3 months. Treatment should be withheld for up to 4 weeks if absolute LVEF values decrease by 10% and are less than the lower limit of normal (LLN) and it should be permanently discontinued for symptomatic cardiomyopathy or persistent, asymptomatic left ventricular dysfunction of >20% from baseline that is below LLN and does not resolve within 4 weeks.
Treatment should be withheld for fevers higher than 104°F or for serious febrile reactions or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure. Serum creatinine levels should be monitored, along with other evidence of renal function, during, and after severe pyrexia. Antipyretics should be administered as secondary prophylaxis when treatment is resumed if the patient had previous episodes of severe febrile reaction or if fever was associated with complications. Corticosteroids should be administered for at least 5 days for second or subsequent pyrexia if the body temperature dose not return to baseline within 3 days of fever onset or for pyrexia associated with complications and no evidence of active infection.
Treatment should also be withheld for intolerable or severe skin toxicity and resumed at a lower dose as per guidelines in patients who improve or recover within 3 weeks. Serum glucose levels should be monitored at the start of treatment and as clinically appropriate in patients with pre-existing diabetes or hyperglycemia. Patients with G6PD deficiency should be monitored closely for signs of hemolytic anemia.
Patients should be monitored closely for signs and symptoms of colitis and gastrointestinal
Ophthalmological evaluations should be performed periodically and within 24 hours of patient-reported loss of vision or other visual disturbances. Treatment should be permanently discontinued in patients with documented retinal vein occlusion and withheld for retinal pigment epithelial detachment. Treatment should also be withheld in patients presenting with new or progressive pulmonary symptoms and findings and permanently discontinued for treatment-related interstitial lung disease or pneumonitis.
Both dabrafenib and trametinib can cause fetal harm and patients should be warned of this risk and the need for adequate contraceptive measures. Dabrafenib and trametinib are marketed as Tafinlar and Mekinist by Novartis.
On April 30, 2018, the US Food and Drug Administration expanded the indication for the combined use of dabrafenib and trametinib to include adjuvant treatment of BRAF-mutant melanoma following complete surgical resection. Dabrafenib is an inhibitor of the BRAF kinase, and trametinib is an inhibitor of the MEK kinase, both of which are components of the mitogen-activated protein kinase (MAPK) signaling pathway. The 2 drugs are already approved as both single agents and in combination for the treatment of BRAF-mutated metastatic melanoma.
The current approval was based on data from a phase 3, international, multicenter, randomized, double-blind, placebo-controlled trial. The COMBI-AD trial was carried out from January 2013 through December 2014 at 169 sites in 26 countries. A total of 870 patients with stage III melanoma and BRAF V600E/K mutations and pathologic involvement of regional lymph nodes following complete resection were randomly assigned to receive dabrafenib 150 mg twice daily in combination with trametinib 2 mg once daily, or 2 matched placebos for up to 1 year. Randomization was stratified according to BRAF mutation status (V600E or V600K) and disease stage (IIIA, IIIB or IIIC).
Eligible patients were aged 18 years or older and had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (on a scale of 1-5, with higher scores indicating greater disability). Patients who had undergone previous systemic anticancer therapy or radiotherapy were excluded from the study.
The primary endpoint was relapse-free survival (RFS), defined as the time from randomization to disease recurrence or death from any cause. Secondary endpoints included overall survival (OS), distant metastasis-free survival (DMFS), freedom from relapse (FFR), and safety. Clinical examination and imaging by computed tomography, magnetic resonance imaging, or both was performed every 3 months for the first 2 years and then every 6 months until disease recurrence or trial completion.
As of the data cut-off, the combination of dabrafenib and trametinib reduced the risk of disease recurrence or death by 53% compared with placebo (hazard ratio [HR], 0.47; P < .001). Median RFS was not yet reached in the combination arm, compared with 16.6 months in the placebo arm. The RFS benefit was observed across all prespecified subgroups, and the combination was also found to improve OS, DMFS, and FFR.
The most common adverse events (AEs) included pyrexia, fatigue, nausea, rash, vomiting, diarrhea, chills, and myalgia. Overall, 97% of patients experienced an AE, 41% experienced a grade 3/4 AE, and 26% had an AE that led to treatment discontinuation. In patients treated with placebo, those numbers were 88%, 14%, and 3%, respectively.
The separate prescribing information for dabrafenib and trametinib detail warnings and precautions relating to their combined use, including the need to confirm BRAF status before starting therapy (because use in BRAF wildtype tumors can promote tumor cell proliferation), new primary malignancies, hemorrhage, cardiomyopathy, uveitis, serious febrile reactions, serious skin toxicity, hyperglycemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, colitis and gastrointestinal perforation, venous thromboembolism, ocular toxicities, interstitial lung disease, and embryofetal toxicity.
Dermatologic evaluations should be completed before starting therapy, every 2 months during and for up to 6 months after completion of therapy, and patients should be monitored closely for the signs and symptoms of noncutaneous primary malignancies. Treatment should be discontinued for all grade 4 hemorrhagic events and for any grade 3 events that do not improve, and withheld for grade 3 events until they resolve, at which point treatment can be resumed at the next lowest dose as described in the prescribing information.
Left ventricular ejection fraction (LVEF) values should be assessed before initiating therapy, after 1 month, and then at intervals of 2-3 months. Treatment should be withheld for up to 4 weeks if absolute LVEF values decrease by 10% and are less than the lower limit of normal (LLN) and it should be permanently discontinued for symptomatic cardiomyopathy or persistent, asymptomatic left ventricular dysfunction of >20% from baseline that is below LLN and does not resolve within 4 weeks.
Treatment should be withheld for fevers higher than 104°F or for serious febrile reactions or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure. Serum creatinine levels should be monitored, along with other evidence of renal function, during, and after severe pyrexia. Antipyretics should be administered as secondary prophylaxis when treatment is resumed if the patient had previous episodes of severe febrile reaction or if fever was associated with complications. Corticosteroids should be administered for at least 5 days for second or subsequent pyrexia if the body temperature dose not return to baseline within 3 days of fever onset or for pyrexia associated with complications and no evidence of active infection.
Treatment should also be withheld for intolerable or severe skin toxicity and resumed at a lower dose as per guidelines in patients who improve or recover within 3 weeks. Serum glucose levels should be monitored at the start of treatment and as clinically appropriate in patients with pre-existing diabetes or hyperglycemia. Patients with G6PD deficiency should be monitored closely for signs of hemolytic anemia.
Patients should be monitored closely for signs and symptoms of colitis and gastrointestinal
Ophthalmological evaluations should be performed periodically and within 24 hours of patient-reported loss of vision or other visual disturbances. Treatment should be permanently discontinued in patients with documented retinal vein occlusion and withheld for retinal pigment epithelial detachment. Treatment should also be withheld in patients presenting with new or progressive pulmonary symptoms and findings and permanently discontinued for treatment-related interstitial lung disease or pneumonitis.
Both dabrafenib and trametinib can cause fetal harm and patients should be warned of this risk and the need for adequate contraceptive measures. Dabrafenib and trametinib are marketed as Tafinlar and Mekinist by Novartis.
1. US Food and Drug Administration Website. FDA approves dabrafenib plus trametinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm606165.htm. Last updated April 30, 2018. Accessed October 6, 2018.
2. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1913-1823.
3. Tafinlar (dabrafenib) capsules, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tafinlar.pdf. May 2018. Accessed October 6, 2018.
4. Mekinist (trametinib) tablets, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mekinist.pdf. May 2018. Accessed October 6th, 2018.
1. US Food and Drug Administration Website. FDA approves dabrafenib plus trametinib for adjuvant treatment of melanoma with BRAF V600E or V600K mutations. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm606165.htm. Last updated April 30, 2018. Accessed October 6, 2018.
2. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1913-1823.
3. Tafinlar (dabrafenib) capsules, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tafinlar.pdf. May 2018. Accessed October 6, 2018.
4. Mekinist (trametinib) tablets, for oral use. Prescribing information. Novartis. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mekinist.pdf. May 2018. Accessed October 6th, 2018.
HU could save millions of lives in Africa, speaker says
SAN DIEGO—Daily hydroxyurea (HU) treatment is feasible, safe, and effective for children with sickle cell disease (SCD) in sub-Saharan Africa, according to a phase 1/2 trial.
During HU treatment, children experienced less vaso-occlusive pain, fewer cases of malaria and other infections, and lower rates of transfusions and death, compared to rates observed in the pretreatment screening phase of the trial.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” said Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, Democratic Republic of the Congo.
Dr. Tshilolo reported the data, from the REACH trial (NCT01966731), during the plenary session at the 2018 ASH Annual Meeting (abstract 3*). Data were simultaneously published in The New England Journal of Medicine.
Use of HU has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to researchers.
Moreover, most of the efficacy data on HU come from studies conducted in the United States, Europe, and other high-income settings, said senior study author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center in Ohio.
“Now that there’s data in an African setting, I think this will go a long way to advancing [HU therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said.
To collect the data, Drs. Ware and Tshilolo and their colleagues evaluated SCD patients, ages 1 to 10, living in four sub-Saharan African countries—Angola, Democratic Republic of the Congo, Kenya, and Uganda.
The children completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data.
The children were started at 15 mg/kg to 20 mg/kg of HU for 6 months, followed by escalation to the maximum-tolerated dose.
A total of 606 children were treated, 600 of them for 3 months. Treatment is ongoing, but the mean treatment duration at the time of analysis was 29 months.
Results
The average maximum tolerated dose was 22.5 mg/kg/day. Dose-limiting toxicities occurred in 5.1% of the children, which was below the 20% protocol-specified threshold for safety, Dr. Tshilolo said.
Dose-limiting toxicities included severe anemia, reticulocytopenia, neutropenia, and thrombocytopenia. However, there were similar rates of these events during the screening period and the treatment period.
The rate of vaso-occlusive pain during HU treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio [IRR], 0.45; 95% confidence interval [CI], 0.37-0.56).
The rate of malaria infection was 22.9 events per 100 patient-years in the HU treatment period, compared to 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66).
The rate of nonmalaria infections was 90.0 events per 100 patient-years in the HU treatment period, compared to 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said the researchers were “encouraged” by the reduced infection rates, particularly in light of previous concerns that HU could suppress the immune system and put children at risk for malaria.
The rate of transfusion during HU treatment was 14.2 events per 100 patient-years, compared to 43.3 events per 100 patient-years (IRR, 0.33; 95% CI, 0.23 to 0.47).
Death rates were 1.1 per 100 patient-years in the HU treatment period and 3.6 per 100 patient-years in the pretreatment period (IRR, 0.30; 95% CI, 0.10-0.88).
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Bristol-Myers Squibb.
*Data in the abstract differ from the presentation and the article.
SAN DIEGO—Daily hydroxyurea (HU) treatment is feasible, safe, and effective for children with sickle cell disease (SCD) in sub-Saharan Africa, according to a phase 1/2 trial.
During HU treatment, children experienced less vaso-occlusive pain, fewer cases of malaria and other infections, and lower rates of transfusions and death, compared to rates observed in the pretreatment screening phase of the trial.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” said Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, Democratic Republic of the Congo.
Dr. Tshilolo reported the data, from the REACH trial (NCT01966731), during the plenary session at the 2018 ASH Annual Meeting (abstract 3*). Data were simultaneously published in The New England Journal of Medicine.
Use of HU has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to researchers.
Moreover, most of the efficacy data on HU come from studies conducted in the United States, Europe, and other high-income settings, said senior study author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center in Ohio.
“Now that there’s data in an African setting, I think this will go a long way to advancing [HU therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said.
To collect the data, Drs. Ware and Tshilolo and their colleagues evaluated SCD patients, ages 1 to 10, living in four sub-Saharan African countries—Angola, Democratic Republic of the Congo, Kenya, and Uganda.
The children completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data.
The children were started at 15 mg/kg to 20 mg/kg of HU for 6 months, followed by escalation to the maximum-tolerated dose.
A total of 606 children were treated, 600 of them for 3 months. Treatment is ongoing, but the mean treatment duration at the time of analysis was 29 months.
Results
The average maximum tolerated dose was 22.5 mg/kg/day. Dose-limiting toxicities occurred in 5.1% of the children, which was below the 20% protocol-specified threshold for safety, Dr. Tshilolo said.
Dose-limiting toxicities included severe anemia, reticulocytopenia, neutropenia, and thrombocytopenia. However, there were similar rates of these events during the screening period and the treatment period.
The rate of vaso-occlusive pain during HU treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio [IRR], 0.45; 95% confidence interval [CI], 0.37-0.56).
The rate of malaria infection was 22.9 events per 100 patient-years in the HU treatment period, compared to 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66).
The rate of nonmalaria infections was 90.0 events per 100 patient-years in the HU treatment period, compared to 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said the researchers were “encouraged” by the reduced infection rates, particularly in light of previous concerns that HU could suppress the immune system and put children at risk for malaria.
The rate of transfusion during HU treatment was 14.2 events per 100 patient-years, compared to 43.3 events per 100 patient-years (IRR, 0.33; 95% CI, 0.23 to 0.47).
Death rates were 1.1 per 100 patient-years in the HU treatment period and 3.6 per 100 patient-years in the pretreatment period (IRR, 0.30; 95% CI, 0.10-0.88).
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Bristol-Myers Squibb.
*Data in the abstract differ from the presentation and the article.
SAN DIEGO—Daily hydroxyurea (HU) treatment is feasible, safe, and effective for children with sickle cell disease (SCD) in sub-Saharan Africa, according to a phase 1/2 trial.
During HU treatment, children experienced less vaso-occlusive pain, fewer cases of malaria and other infections, and lower rates of transfusions and death, compared to rates observed in the pretreatment screening phase of the trial.
“Based on that data, we believe that wider access to hydroxyurea for sickle cell anemia has the potential to save millions of lives in Africa,” said Léon Tshilolo, MD, PhD, of Centre Hospitalier Monkole in Kinshasa, Democratic Republic of the Congo.
Dr. Tshilolo reported the data, from the REACH trial (NCT01966731), during the plenary session at the 2018 ASH Annual Meeting (abstract 3*). Data were simultaneously published in The New England Journal of Medicine.
Use of HU has been limited in Africa because of cost, access issues, and challenges associated with laboratory monitoring, according to researchers.
Moreover, most of the efficacy data on HU come from studies conducted in the United States, Europe, and other high-income settings, said senior study author Russell E. Ware, MD, PhD, of Cincinnati Children’s Hospital Center in Ohio.
“Now that there’s data in an African setting, I think this will go a long way to advancing [HU therapy] and encouraging governments, organizations, and pharmaceutical companies to bring it in,” Dr. Ware said.
To collect the data, Drs. Ware and Tshilolo and their colleagues evaluated SCD patients, ages 1 to 10, living in four sub-Saharan African countries—Angola, Democratic Republic of the Congo, Kenya, and Uganda.
The children completed a 2-month pretreatment screening phase designed to capture baseline clinical and laboratory data.
The children were started at 15 mg/kg to 20 mg/kg of HU for 6 months, followed by escalation to the maximum-tolerated dose.
A total of 606 children were treated, 600 of them for 3 months. Treatment is ongoing, but the mean treatment duration at the time of analysis was 29 months.
Results
The average maximum tolerated dose was 22.5 mg/kg/day. Dose-limiting toxicities occurred in 5.1% of the children, which was below the 20% protocol-specified threshold for safety, Dr. Tshilolo said.
Dose-limiting toxicities included severe anemia, reticulocytopenia, neutropenia, and thrombocytopenia. However, there were similar rates of these events during the screening period and the treatment period.
The rate of vaso-occlusive pain during HU treatment was 44.6 events per 100 patient-years, compared with 98.3 events per 100 patient-years in the pretreatment period (incidence rate ratio [IRR], 0.45; 95% confidence interval [CI], 0.37-0.56).
The rate of malaria infection was 22.9 events per 100 patient-years in the HU treatment period, compared to 46.9 events in the pretreatment period (IRR, 0.49; 95% CI, 0.37-0.66).
The rate of nonmalaria infections was 90.0 events per 100 patient-years in the HU treatment period, compared to 142.5 events per 100 patient-years in the pretreatment period (IRR, 0.62; 95% CI, 0.53-0.72).
Dr. Tshilolo said the researchers were “encouraged” by the reduced infection rates, particularly in light of previous concerns that HU could suppress the immune system and put children at risk for malaria.
The rate of transfusion during HU treatment was 14.2 events per 100 patient-years, compared to 43.3 events per 100 patient-years (IRR, 0.33; 95% CI, 0.23 to 0.47).
Death rates were 1.1 per 100 patient-years in the HU treatment period and 3.6 per 100 patient-years in the pretreatment period (IRR, 0.30; 95% CI, 0.10-0.88).
Dr. Tshilolo reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Cincinnati Children’s Research Foundation, along with nonfinancial support from Bristol-Myers Squibb. Dr. Ware reported grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and Bristol-Myers Squibb.
*Data in the abstract differ from the presentation and the article.
Triplet demonstrates activity in relapsed/refractory MM
SAN DIEGO—A three-drug combination produced “deep and durable” responses in patients with relapsed/refractory multiple myeloma (MM), according to a speaker at the 2018 ASH Annual Meeting.
Selinexor, dexamethasone, and daratumumab produced a response rate of 73% when given at the recommended dosing schedule to MM patients who had received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
Most responders had a very good partial response (VGPR), but there were no complete responses. At a median follow-up of 7.7 months, the median progression-free survival had not been reached.
The most common grade 3/4 adverse events (AEs) in this trial were hematologic toxicities.
Cristina J. Gasparetto, MD, of Duke University Medical Center in Durham, North Carolina, presented these results, from the phase 1/2 STOMP trial (NCT02343042), as abstract 599.*
Patients
As of November 15, the trial had enrolled 28 MM patients. At baseline, their median age was 68 (range, 44-77). There were 14 males and 14 females. The median time from diagnosis to study treatment was 5.9 years (range, <1 to 12.9 years).
Patients had received a median of 3 (range, 2 to 10) prior treatment regimens.
All 28 patients had received a proteasome inhibitor, and 61% of them (n=17) were refractory to the treatment. All 28 patients had also received an immunomodulatory drug, and 64% of them (n=18) were refractory to it.
Seventy-nine percent (n=22) of patients had undergone an autologous transplant, and 7% (n=2) had received prior daratumumab.
Treatment
Patients were treated in two concurrent cohorts.
One cohort included 25 patients who received selinexor at 100 mg once-weekly (QW), dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
The other cohort included three patients who received selinexor at 60 mg twice-weekly (BIW), dexamethasone at 20 mg BIW, and daratumumab at 16 mg/kg QW.
The recommended phase 2 dose and schedule was selinexor at 100 mg QW, dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
Safety
Among patients who received the recommended phase 2 dosing schedule, common treatment-related AEs included:
- Nausea (60%)
- Diarrhea (32%)
- Anorexia (28%)
- Vomiting (24%)
- Dysgeusia (20%)
- Fatigue (48%)
- Hyponatremia (28%)
- Insomnia (24%)
- Blurred vision (24%)
- Thrombocytopenia (64%)
- Anemia (48%)
- Leukopenia (44%)
- Neutropenia (44%)
- Lymphopenia (20%).
“[T]he weekly dose was better tolerated [with] only a couple of patients with grade 3 [gastrointestinal] toxicity,” Dr Gasparetto noted.
The most common grade 3/4 AEs were thrombocytopenia (44%), anemia (28%), leukopenia (28%), and neutropenia (24%). There were no grade 5 AEs.
Efficacy
The median follow-up was 7.7 months, and the median time on study was 5.8 months.
Twenty-six patients were evaluable for response, as two patients withdrew consent prior to follow-up.
The overall response rate was 73% (n=19), which includes seven very good partial responses (VGPRs) and 12 partial responses (PRs). Two patients had a minimal response, four had stable disease, and one progressed.
Among patients with a PR or better, the median time on treatment was 7.3 months. The median time to response was 1 month.
Three VGPRs are ongoing, but four patients who achieved a VGPR progressed.
Six PRs are ongoing, and one patient with a PR progressed. Other reasons for treatment discontinuation among patients with a PR included transplant (n=1), AE (n=1), patient decision (n=2), and hospice (n=1).
One patient with a minimal response progressed, and one discontinued treatment due to an AE.
The median progression-free survival was not reached.
“Selinexor in combination with dara and dexa appears to be highly active, producing deep and durable responses in the relapsed setting,” Dr. Gasparetto said.
She reported relationships with Takeda, Janssen, Celgene, and Bristol-Myers Squibb. The trial is sponsored by Karyopharm Therapeutics.
*Data in the presentation differ from the abstract.
SAN DIEGO—A three-drug combination produced “deep and durable” responses in patients with relapsed/refractory multiple myeloma (MM), according to a speaker at the 2018 ASH Annual Meeting.
Selinexor, dexamethasone, and daratumumab produced a response rate of 73% when given at the recommended dosing schedule to MM patients who had received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
Most responders had a very good partial response (VGPR), but there were no complete responses. At a median follow-up of 7.7 months, the median progression-free survival had not been reached.
The most common grade 3/4 adverse events (AEs) in this trial were hematologic toxicities.
Cristina J. Gasparetto, MD, of Duke University Medical Center in Durham, North Carolina, presented these results, from the phase 1/2 STOMP trial (NCT02343042), as abstract 599.*
Patients
As of November 15, the trial had enrolled 28 MM patients. At baseline, their median age was 68 (range, 44-77). There were 14 males and 14 females. The median time from diagnosis to study treatment was 5.9 years (range, <1 to 12.9 years).
Patients had received a median of 3 (range, 2 to 10) prior treatment regimens.
All 28 patients had received a proteasome inhibitor, and 61% of them (n=17) were refractory to the treatment. All 28 patients had also received an immunomodulatory drug, and 64% of them (n=18) were refractory to it.
Seventy-nine percent (n=22) of patients had undergone an autologous transplant, and 7% (n=2) had received prior daratumumab.
Treatment
Patients were treated in two concurrent cohorts.
One cohort included 25 patients who received selinexor at 100 mg once-weekly (QW), dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
The other cohort included three patients who received selinexor at 60 mg twice-weekly (BIW), dexamethasone at 20 mg BIW, and daratumumab at 16 mg/kg QW.
The recommended phase 2 dose and schedule was selinexor at 100 mg QW, dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
Safety
Among patients who received the recommended phase 2 dosing schedule, common treatment-related AEs included:
- Nausea (60%)
- Diarrhea (32%)
- Anorexia (28%)
- Vomiting (24%)
- Dysgeusia (20%)
- Fatigue (48%)
- Hyponatremia (28%)
- Insomnia (24%)
- Blurred vision (24%)
- Thrombocytopenia (64%)
- Anemia (48%)
- Leukopenia (44%)
- Neutropenia (44%)
- Lymphopenia (20%).
“[T]he weekly dose was better tolerated [with] only a couple of patients with grade 3 [gastrointestinal] toxicity,” Dr Gasparetto noted.
The most common grade 3/4 AEs were thrombocytopenia (44%), anemia (28%), leukopenia (28%), and neutropenia (24%). There were no grade 5 AEs.
Efficacy
The median follow-up was 7.7 months, and the median time on study was 5.8 months.
Twenty-six patients were evaluable for response, as two patients withdrew consent prior to follow-up.
The overall response rate was 73% (n=19), which includes seven very good partial responses (VGPRs) and 12 partial responses (PRs). Two patients had a minimal response, four had stable disease, and one progressed.
Among patients with a PR or better, the median time on treatment was 7.3 months. The median time to response was 1 month.
Three VGPRs are ongoing, but four patients who achieved a VGPR progressed.
Six PRs are ongoing, and one patient with a PR progressed. Other reasons for treatment discontinuation among patients with a PR included transplant (n=1), AE (n=1), patient decision (n=2), and hospice (n=1).
One patient with a minimal response progressed, and one discontinued treatment due to an AE.
The median progression-free survival was not reached.
“Selinexor in combination with dara and dexa appears to be highly active, producing deep and durable responses in the relapsed setting,” Dr. Gasparetto said.
She reported relationships with Takeda, Janssen, Celgene, and Bristol-Myers Squibb. The trial is sponsored by Karyopharm Therapeutics.
*Data in the presentation differ from the abstract.
SAN DIEGO—A three-drug combination produced “deep and durable” responses in patients with relapsed/refractory multiple myeloma (MM), according to a speaker at the 2018 ASH Annual Meeting.
Selinexor, dexamethasone, and daratumumab produced a response rate of 73% when given at the recommended dosing schedule to MM patients who had received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
Most responders had a very good partial response (VGPR), but there were no complete responses. At a median follow-up of 7.7 months, the median progression-free survival had not been reached.
The most common grade 3/4 adverse events (AEs) in this trial were hematologic toxicities.
Cristina J. Gasparetto, MD, of Duke University Medical Center in Durham, North Carolina, presented these results, from the phase 1/2 STOMP trial (NCT02343042), as abstract 599.*
Patients
As of November 15, the trial had enrolled 28 MM patients. At baseline, their median age was 68 (range, 44-77). There were 14 males and 14 females. The median time from diagnosis to study treatment was 5.9 years (range, <1 to 12.9 years).
Patients had received a median of 3 (range, 2 to 10) prior treatment regimens.
All 28 patients had received a proteasome inhibitor, and 61% of them (n=17) were refractory to the treatment. All 28 patients had also received an immunomodulatory drug, and 64% of them (n=18) were refractory to it.
Seventy-nine percent (n=22) of patients had undergone an autologous transplant, and 7% (n=2) had received prior daratumumab.
Treatment
Patients were treated in two concurrent cohorts.
One cohort included 25 patients who received selinexor at 100 mg once-weekly (QW), dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
The other cohort included three patients who received selinexor at 60 mg twice-weekly (BIW), dexamethasone at 20 mg BIW, and daratumumab at 16 mg/kg QW.
The recommended phase 2 dose and schedule was selinexor at 100 mg QW, dexamethasone at 40 mg QW, and daratumumab at 16 mg/kg QW.
Safety
Among patients who received the recommended phase 2 dosing schedule, common treatment-related AEs included:
- Nausea (60%)
- Diarrhea (32%)
- Anorexia (28%)
- Vomiting (24%)
- Dysgeusia (20%)
- Fatigue (48%)
- Hyponatremia (28%)
- Insomnia (24%)
- Blurred vision (24%)
- Thrombocytopenia (64%)
- Anemia (48%)
- Leukopenia (44%)
- Neutropenia (44%)
- Lymphopenia (20%).
“[T]he weekly dose was better tolerated [with] only a couple of patients with grade 3 [gastrointestinal] toxicity,” Dr Gasparetto noted.
The most common grade 3/4 AEs were thrombocytopenia (44%), anemia (28%), leukopenia (28%), and neutropenia (24%). There were no grade 5 AEs.
Efficacy
The median follow-up was 7.7 months, and the median time on study was 5.8 months.
Twenty-six patients were evaluable for response, as two patients withdrew consent prior to follow-up.
The overall response rate was 73% (n=19), which includes seven very good partial responses (VGPRs) and 12 partial responses (PRs). Two patients had a minimal response, four had stable disease, and one progressed.
Among patients with a PR or better, the median time on treatment was 7.3 months. The median time to response was 1 month.
Three VGPRs are ongoing, but four patients who achieved a VGPR progressed.
Six PRs are ongoing, and one patient with a PR progressed. Other reasons for treatment discontinuation among patients with a PR included transplant (n=1), AE (n=1), patient decision (n=2), and hospice (n=1).
One patient with a minimal response progressed, and one discontinued treatment due to an AE.
The median progression-free survival was not reached.
“Selinexor in combination with dara and dexa appears to be highly active, producing deep and durable responses in the relapsed setting,” Dr. Gasparetto said.
She reported relationships with Takeda, Janssen, Celgene, and Bristol-Myers Squibb. The trial is sponsored by Karyopharm Therapeutics.
*Data in the presentation differ from the abstract.
Two-drug combo deemed ‘very promising’ for PMBCL
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
SAN DIEGO—Nivolumab plus brentuximab vedotin may be a new treatment option for patients with relapsed/refractory primary mediastinal large B-cell lymphoma (PMBCL), according to investigators from the CheckMate 436 trial.
Interim results from this phase 1/2 trial revealed an overall response rate of 70%, including a complete response rate of 27%.
“It’s very promising . . . to see this level of activity in this advanced, relapsed/refractory population,” said Joseph E. Eid, MD, senior vice president and head of medical at Bristol-Myers Squibb, which is sponsoring CheckMate 436 in collaboration with Seattle Genetics.
Dr. Eid also noted that adverse events (AEs) observed with this regimen were consistent with the safety profiles of nivolumab and brentuximab vedotin alone.
These results were presented as a poster at the 2018 ASH Annual Meeting (abstract 1691).
Rationale
Dr. Eid noted that patients with relapsed or refractory PMBCL have limited treatment options.
“The initial therapy works well in 70% to 80% of patients, but the patients who fail don’t have good options,” he said.
Prior research has shown that PMBCL is often characterized by overexpression of the PD-1 ligands PD-L1 and PD-L2, and most PMBCL expresses CD30.
Dr. Eid said CheckMate 436 (NCT02581631) was designed to “take advantage” of these characteristics by employing the anti-PD-1 checkpoint inhibitor nivolumab and the anti-CD30 antibody-drug conjugate brentuximab vedotin.
Patients and treatment
The interim analysis of this trial included 30 patients with relapsed/refractory PMCBL. Their median age at enrollment was 35.5 (range, 19 to 83), and 57% of patients were female.
Sixty percent of patients had refractory disease, 23% had relapsed disease, and 17% had both.
The median number of prior therapies was 2 (range, 1-5). Thirteen percent of patients had prior autologous stem cell transplant.
The patients received nivolumab at 240 mg and brentuximab vedotin at 1.8 mg/kg every 3 weeks until progression or unacceptable toxicity.
At a median follow-up of 6.1 months, 10 patients were still on treatment. Reasons for discontinuation included maximum clinical benefit (n=9), disease progression (n=7), AEs unrelated to treatment (n=2), patient request (n=1), and “other” reasons (n=1).
Safety
“There were no new safety signals,” Dr. Eid said. “The adverse events reflected the two agents’ profiles.”
The rate of treatment-related AEs was 83%. The most common of these were neutropenia (27%), peripheral neuropathy (20%), hyperthyroidism (13%), rash (10%), and thrombocytopenia (10%).
Grade 3-4 treatment-related AEs included neutropenia (27%), thrombocytopenia (7%), decreased neutrophil count (7%), hypersensitivity (3%), diarrhea (3%), and maculopapular rash (3%).
The rate of serious treatment-related AEs was 10%. This included grade 3-4 diarrhea and maculopapular rash and grade 5 acute kidney injury.
The acute kidney injury was the only fatal AE considered treatment-related. There were three other deaths in the trial, but they were considered unrelated to treatment.
Response
The complete response rate was 27% (n=8), and the partial response rate was 43% (n=13), for an overall response rate of 70% (n=21).
“The early indication is that 70% response is a pretty good outcome in a relapsed/refractory population that, otherwise, their outcome is pretty dismal,” Dr. Eid said.
Ten percent of patients (n=3) had stable disease, 13% (n=4) progressed, and investigators were unable to determine the status for 7% of patients (n=2).
The median time to response was 1.3 months, and the median time to complete response was 3.0 months. The median duration of response and complete response were not reached.
Overall and progression-free survival data are not yet mature.
Still, the investigators concluded that nivolumab and brentuximab vedotin “may provide a new treatment option” for patients with relapsed/refractory PMBCL.
“The results are very, very promising,” Dr. Eid said.
This trial is supported by Bristol-Myers Squibb in collaboration with Seattle Genetics.
Symptom burdens related to chemotherapy-induced anemia in stage IV cancer
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
All-or-none approach boosts adherence to stroke treatments
CHICAGO – Stroke patients in low to middle income care settings may frequently fail to get timely evidence-based treatments when they’re admitted to the hospital and even when they’re discharged, but a large South American study found that an “all-or-none” approach to a multistep quality-improvement program led to a significant increase in therapy adherence and smoking cessation. The results were reported at the American Heart Association scientific sessions.
“A multifaceted quality-improvement intervention did not result in a significant increase in the composite adherence score for evidence-based therapies in patients with acute ischemic stroke [AIS] or transient ischemic attack [TIA],” said M. Julia Machline-Carrion, MD, PhD, principal investigator of the BRIDGE-Stroke study and a cardiologist at the Hospital for Heart in São Paulo. “However, when using a more conservative ‘all-or-none’ approach of complete adherence, the intervention resulted in improved adherence to evidence-based therapies.”
The quality-improvement program also resulted in a significant increase in the use of thrombolysis and uptake in smoking cessation education by study participants, Dr. Machline-Carrion added.
The study randomized 1,624 patients with AIS or TIA to the multifaceted quality-improvement intervention or routine practice. The intervention consisted of a patient identification system (wristband and printed reminders), a therapeutic plan road map and checklist, case management, educational materials, interactive workshops, and periodic audit and feedback reports to each participating cluster. Colored wristbands were to help promptly identify AIS or TIA patients in the emergency department and other departments they may have been sent to later on, such as the ICU, to avoid delays in initiating recommended therapies.
On average, the composite adherence score was 85.3% for those in the intervention group vs. 77.8% for controls, Dr. Machline-Carrion said. The composite adherence score consisted of 10 quality measures, ranging from early antithrombotics and prophylaxis for deep vein thrombosis to anticoagulation for atrial fibrillation or flutter, and smoking cessation education. “There was no statistically significant difference in the composite adherence score between the intervention group and the usual-care group,” she said.
However, when the researchers applied the all-or-none model – that is, complete adherence to all 10 in-hospital quality measures – the results were strikingly different, Dr. Machline-Carrion said. “Patients in the intervention group were more likely to receive all eligible therapies,” she said: 49.2% vs. 25.3%.
“Despite the established efficacy of several interventions for the management of patients with acute ischemic stroke and transient ischemic attack, the uptake of evidence-based measures remains suboptimal, especially in low- and middle-income countries,” Dr. Machline-Carrion said.
The BRIDGE-Stroke study involved 36 hospitals in Brazil, Argentina, and Peru with full emergency department coverage, central nervous system imaging, and access to recombinant tissue plasminogen activator therapies.
Dr. Machline-Carrion disclosed financial relationships with Amgen and Boehringer Ingelheim. The Brazil Ministry of Health was the lead sponsor of the study.
SOURCE: Machline-Carrion MJ et al. AHA scientific sessions, Abstract 19361.
CHICAGO – Stroke patients in low to middle income care settings may frequently fail to get timely evidence-based treatments when they’re admitted to the hospital and even when they’re discharged, but a large South American study found that an “all-or-none” approach to a multistep quality-improvement program led to a significant increase in therapy adherence and smoking cessation. The results were reported at the American Heart Association scientific sessions.
“A multifaceted quality-improvement intervention did not result in a significant increase in the composite adherence score for evidence-based therapies in patients with acute ischemic stroke [AIS] or transient ischemic attack [TIA],” said M. Julia Machline-Carrion, MD, PhD, principal investigator of the BRIDGE-Stroke study and a cardiologist at the Hospital for Heart in São Paulo. “However, when using a more conservative ‘all-or-none’ approach of complete adherence, the intervention resulted in improved adherence to evidence-based therapies.”
The quality-improvement program also resulted in a significant increase in the use of thrombolysis and uptake in smoking cessation education by study participants, Dr. Machline-Carrion added.
The study randomized 1,624 patients with AIS or TIA to the multifaceted quality-improvement intervention or routine practice. The intervention consisted of a patient identification system (wristband and printed reminders), a therapeutic plan road map and checklist, case management, educational materials, interactive workshops, and periodic audit and feedback reports to each participating cluster. Colored wristbands were to help promptly identify AIS or TIA patients in the emergency department and other departments they may have been sent to later on, such as the ICU, to avoid delays in initiating recommended therapies.
On average, the composite adherence score was 85.3% for those in the intervention group vs. 77.8% for controls, Dr. Machline-Carrion said. The composite adherence score consisted of 10 quality measures, ranging from early antithrombotics and prophylaxis for deep vein thrombosis to anticoagulation for atrial fibrillation or flutter, and smoking cessation education. “There was no statistically significant difference in the composite adherence score between the intervention group and the usual-care group,” she said.
However, when the researchers applied the all-or-none model – that is, complete adherence to all 10 in-hospital quality measures – the results were strikingly different, Dr. Machline-Carrion said. “Patients in the intervention group were more likely to receive all eligible therapies,” she said: 49.2% vs. 25.3%.
“Despite the established efficacy of several interventions for the management of patients with acute ischemic stroke and transient ischemic attack, the uptake of evidence-based measures remains suboptimal, especially in low- and middle-income countries,” Dr. Machline-Carrion said.
The BRIDGE-Stroke study involved 36 hospitals in Brazil, Argentina, and Peru with full emergency department coverage, central nervous system imaging, and access to recombinant tissue plasminogen activator therapies.
Dr. Machline-Carrion disclosed financial relationships with Amgen and Boehringer Ingelheim. The Brazil Ministry of Health was the lead sponsor of the study.
SOURCE: Machline-Carrion MJ et al. AHA scientific sessions, Abstract 19361.
CHICAGO – Stroke patients in low to middle income care settings may frequently fail to get timely evidence-based treatments when they’re admitted to the hospital and even when they’re discharged, but a large South American study found that an “all-or-none” approach to a multistep quality-improvement program led to a significant increase in therapy adherence and smoking cessation. The results were reported at the American Heart Association scientific sessions.
“A multifaceted quality-improvement intervention did not result in a significant increase in the composite adherence score for evidence-based therapies in patients with acute ischemic stroke [AIS] or transient ischemic attack [TIA],” said M. Julia Machline-Carrion, MD, PhD, principal investigator of the BRIDGE-Stroke study and a cardiologist at the Hospital for Heart in São Paulo. “However, when using a more conservative ‘all-or-none’ approach of complete adherence, the intervention resulted in improved adherence to evidence-based therapies.”
The quality-improvement program also resulted in a significant increase in the use of thrombolysis and uptake in smoking cessation education by study participants, Dr. Machline-Carrion added.
The study randomized 1,624 patients with AIS or TIA to the multifaceted quality-improvement intervention or routine practice. The intervention consisted of a patient identification system (wristband and printed reminders), a therapeutic plan road map and checklist, case management, educational materials, interactive workshops, and periodic audit and feedback reports to each participating cluster. Colored wristbands were to help promptly identify AIS or TIA patients in the emergency department and other departments they may have been sent to later on, such as the ICU, to avoid delays in initiating recommended therapies.
On average, the composite adherence score was 85.3% for those in the intervention group vs. 77.8% for controls, Dr. Machline-Carrion said. The composite adherence score consisted of 10 quality measures, ranging from early antithrombotics and prophylaxis for deep vein thrombosis to anticoagulation for atrial fibrillation or flutter, and smoking cessation education. “There was no statistically significant difference in the composite adherence score between the intervention group and the usual-care group,” she said.
However, when the researchers applied the all-or-none model – that is, complete adherence to all 10 in-hospital quality measures – the results were strikingly different, Dr. Machline-Carrion said. “Patients in the intervention group were more likely to receive all eligible therapies,” she said: 49.2% vs. 25.3%.
“Despite the established efficacy of several interventions for the management of patients with acute ischemic stroke and transient ischemic attack, the uptake of evidence-based measures remains suboptimal, especially in low- and middle-income countries,” Dr. Machline-Carrion said.
The BRIDGE-Stroke study involved 36 hospitals in Brazil, Argentina, and Peru with full emergency department coverage, central nervous system imaging, and access to recombinant tissue plasminogen activator therapies.
Dr. Machline-Carrion disclosed financial relationships with Amgen and Boehringer Ingelheim. The Brazil Ministry of Health was the lead sponsor of the study.
SOURCE: Machline-Carrion MJ et al. AHA scientific sessions, Abstract 19361.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Intervention group received evidence-based treatments at a rate of 49.2% vs. 25.3% for controls.
Data source: BRIDGE-Stroke, a cluster-randomized trial among 36 hospitals in Brazil, Argentina, and Peru with 1,624 patients enrolled.
Disclosures: Dr. Machline-Carrion disclosed financial relationships with Amgen and Boehringer Ingelheim. The Brazil Ministry of Health was the lead sponsor of the study.
Source: Machline-Carrion MJ et al. AHA scientific sessions, Abstract 19361.