User login
TNBC survival appears better when adjuvant chemotherapy is delivered within 30 days
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
SAN ANTONIO – The longer the delay in initiating adjuvant chemotherapy, the worse the survival in patients with triple-negative breast cancer (TNBC), findings from a review of nearly 700 cases suggest.
Delays of more than 30 days between surgery and initiation of chemotherapy were associated with lower disease-free survival (DFS), distant recurrence–free survival (DRFS), and overall survival (OS), Zaida Morante, MD, reported at the San Antonio Breast Cancer Symposium.
In 687 women with clinical stage I, II, or III TNBC who were diagnosed at the Instituto Nacional de Enfermedades Neoplasicas in Lima, Peru, during 2000-2014 and followed for a median of 8.5 years, time to chemotherapy was less than 30 days in 189 patients (27.5%), 31-60 days in 329 patients (47.9%), 61-90 days in 115 patients (16.7%), and more than 91 days in 54 patients (7.9%), said Dr. Morante, a medical oncologist at the institute.
Overall survival at 10 years was 82% in those who received chemotherapy within 30 days of surgery, compared with 67.4%, 67.1%, and 65.1% in those treated at 31-60, 61-90, and more than 91 days after surgery, respectively, she said.
“The difference was consistent across the different periods of the evaluation,” she said during a press briefing at the symposium. “Additionally, the benefit of receiving chemotherapy within 30 days exists and is statistically significant for [nodal status] N0 and N1 (hazard ratios, 1.701 and 2.498).”
In those with N2 and N3 nodal status, there was a numerical difference, but it didn’t reach statistical significance.
DFS was also significantly worse if treated later than 30 days after surgery; those treated within 30 days had 10-year DFS of 81.4%, compared with 68.8%, 70.8%, and 68.1% in the other groups, respectively. The difference was even more pronounced for 10-year DRFS, which was 80.2%, 64.9%, 67.5%, and 58.6% in the groups, respectively.
Multivariate analyses confirmed that time to adjuvant chemotherapy was an independent prognostic factor for survival, she said, noting that compared with patients treated within 30 days of surgery, those treated at 31-60 days had 1.9-fold increased risk of death, and those treated at 61-90 days had a 2.4-fold increased risk of death.
“The difference in 10-year overall survival rates between receiving chemotherapy within 30 days after surgery and after 30 days was more than 10%,” she said. “These results represent a feasible opportunity for improving outcomes in triple-negative breast cancer patients.”
Although only 28% of patients in this review received adjuvant chemotherapy within 30 days, most patients in the United States “will fall within the 30 days and under” category, said press briefing moderator Carlos Arteaga, MD, professor and director of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern Medical Center in Dallas.
However, the findings might suggest a greater role for neoadjuvant chemotherapy in these patients.
“Because this is systemic therapy ... it’s treating the systemic disease. I wonder if this is arguing ... that we need to have an impetus to deliver the systemic therapy as soon as we can – early, even before the operation,” he said.
Indeed, while timing isn’t everything, Dr. Morante’s findings and others presented at the meeting “highlight the possibility that perhaps it is more important than we previously suspected,” discussant Joseph A. Sparano, MD, said at the meeting, adding that the findings raise questions about current paradigms for management of breast cancer.
“We now have substantial data suggesting that the timing of adjuvant chemotherapy matters in triple-negative breast cancer, and that 30 days may be optimal,” said Dr. Sparano, professor at the Albert Einstein College of Medicine, New York.
“This doesn’t mean that patients who may not be ready for the chemotherapy because of complications related to the surgery should be forced into a situation where they are at higher risk from receiving the chemotherapy, but nevertheless, the results are important,” he said.
Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
SOURCE: Morante Z et al. SABCS 2018, Abstract GS2-05.
REPORTING FROM SABCS 2018
Key clinical point: Outcomes are improved with adjuvant chemotherapy within 30 days of surgery, compared with beyond 30 days, in triple-negative breast cancer.
Major finding: 10-year overall survival was 82% with chemotherapy within 30 days of surgery versus 67.4%, 67.1%, and 65.1% with chemotherapy at 31-60, 61-90, and more than 91 days after surgery, respectively.
Study details: A retrospective review of 687 cases of TNBC.
Disclosures: Dr. Morante and Dr. Arteaga each reported having no relevant conflicts of interest to declare. Dr. Sparano has received consulting fees from Roche, Eli Lilly, Novartis, Celldex, AstraZeneca, Pfizer, and Adgero. He also has ownership interests with MetaStat.
Source: Morante Z et al., SABCS 2018 Abstract GS2-05.
ICU-acquired pneumonia mortality risk may be underestimated
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
In a large prospectively collected database, the risk of death at 30 days in ICU patients was far greater in those with hospital-acquired pneumonia (HAP) than in those with ventilator-associated pneumonia (VAP) even after adjustment for prognostic factors, according to a large study that compared mortality risk for these complications.
The data for this newly published study were drawn from an evaluation of 14,212 patients treated at 23 ICUs participating in a collaborative French network OUTCOMEREA and published Critical Care Medicine.
HAP in ICU patients “was associated with an 82% increase in the risk of death at day 30,” reported a team of investigators led by Wafa Ibn Saied, MD, of the Université Paris Diderot. Although VAP and HAP were independent risk factors (P both less than .0001) for death at 30 days, VAP increased risk by 38%, less than half of HAP, which increased risk by 82%.
From an observational but prospective database initiated in 1997, this study evaluated 7,735 ICU patients at risk for VAP and 9,747 at risk for HAP. Of those at risk, defined by several factors including an ICU stay of more than 48 hours, HAP developed in 8% and VAP developed in 1%.
The 30-day mortality rates at 30 days after pneumonia were 23.9% for HAP and 28.4% for VAP. The greater risk of death by HR was identified after an analysis that adjusted for mortality risk factors, the adequacy of initial treatment, and other factors, such as prior history of pneumonia.
In HAP patients, the rate of mortality at 30 days was 32% in the 75 who were reintubated but only 16% in the 101 who were not. Adequate empirical therapy within the first 24 hours for HAP was not associated with a reduction in the risk of death.
As in the HAP patients, mortality was not significantly higher in VAP patients who received inadequate empirical therapy, compared with those who did, according to the authors.
Previous studies have suggested that both HAP and VAP increase risk of death in ICU patients, but the authors of this study believe that the relative risk of HAP “is underappreciated.” They asserted, based on these most recent data as well as on previously published analyses, that nonventilated HAP results in “significant increases in cost, length of stay, and mortality.”
The researchers had no disclosures.
SOURCE: Saied WI et al. Crit Care Med. 2018 Nov 7. doi: 10.1097/CCM.0000000000003553.
FROM CRITICAL CARE MEDICINE
Key clinical point: Hospital-acquired pneumonia poses a greater risk of death in the ICU than ventilator-associated pneumonia.
Major finding: After prognostic adjustment, the mortality hazard ratios were 1.82 and 1.38 for HAP and VAP, respectively.
Study details: Observational cohort study.
Disclosures: The researchers had no disclosures.
Source: Saied WI et al. Crit Care Med. 2018 Nov 7; doi: 10.1097/CCM.0000000000003553.
CTC matches MD judgment for mBC therapeutic choice
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
SAN ANTONIO – For patients with estrogen-receptor positive, HER2-negative metastatic breast cancer, the use of circulating tumor cell (CTC) counts can help clinicians decide with confidence between ordering first-line hormonal therapy or chemotherapy, investigators say.
In the phase 3 STIC CTC trial, patients were randomly assigned to receive therapy based on either the clinician’s judgment of the best course of therapy for each patient; or on the CTC count with a cutoff of less than 5 CT/7.5 mL, indicating hormonal therapy; and 5 CTC/7.5 mL or above, indicating higher-risk disease requiring chemotherapy. In the clinician’s choice arm, the CTC reading was recorded but not implemented, and in the CTC arm, the clinician’s choice was dismissed.
The trial met its primary noninferiority endpoint, indicating that, in the overall population, CTC counts can provide clinician’s with confidence in the therapeutic choice, said Francois-Clement Bidard, MD, PhD, from Institut Curie in Paris.
In a video interview, Dr. Bidard discussed the trial findings, including the provocative exploratory analysis suggesting that, in patients in whom there is discordance between CTC and clinician choice, chemotherapy may be a better therapeutic option.
The study was funded by the National Cancer Institute of France, Institut Curie, and Menarini Silicon Biosystems. Dr. Bidard disclosed research funding and travel grants from Menarini.
REPORTING FROM SABCS 2018
Proposed neuroblastoma classification scheme hinges on telomere maintenance mechanisms
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Telomere maintenance mechanisms, RAS mutations, and p53 mutations can be used to mechanistically classify clinical phenotypes of neuroblastoma, according to investigators.
Genomic analysis of neuroblastomas showed that the aforementioned markers were strongly associated with outcome and other disease characteristics, reported Sandra Ackermann, MD, of the department of experimental pediatric oncology at the University Children’s Hospital of Cologne (Germany), and her colleagues.
Although previous studies have shown relationships between genetic alterations and behavior of neuroblastomas, “to date, these genomic data have not produced a coherent model of pathogenesis that can explain the extremely divergent clinical phenotypes of neuroblastoma,” the investigators wrote in Science.
The present study involved genomic sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Based on existing data, the investigators first devised a panel based on 17 genes related to the RAS pathway (11 genes included ALK) and 6 related to the p53 pathway. In 198 cases, 28 tested positive for RAS- or p53-pathway abnormalities (17.8%). Positivity was more common in high-risk tumors than non–high-risk tumors (21.3% vs. 13.3%; P = .048), and in both risk groups, positivity was associated with poor outcome (hazard ratio, 2.056; P = .001).
However, because clinical courses varied widely among non–high-risk patients with RAS/p53 mutations, the investigators recognized that a piece of the puzzle was missing. They hypothesized that telomere maintenance mechanisms could also be playing a role. Following several intervening experiments, the investigators devised telomere maintenance mechanism testing, defined by MYCN amplification or TERT rearrangements, elevated TERT expression if negative for these abnormalities, or presence of ALT-associated promyelocytic leukemia nuclear bodies. Subsequent testing revealed that positivity for these parameters was associated with a HR of 5.184 (P less than .001), thereby confirming that telomere maintenance mechanisms could independently predict survival.
“Together, our findings demonstrate that the divergent clinical phenotypes of human neuroblastoma are driven by molecular alterations affecting telomere maintenance and RAS or p53 pathways, suggesting a mechanistic classification of this malignancy,” the authors concluded.
The proposed classification scheme also includes associations with other genetic features (tumor cell ploidy, segmental copy number alterations, MYCN/TERT/ATRX alterations, and gene expression favorability) and clinical characteristics (stage of disease and age at diagnosis).
The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
SOURCE: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
FROM SCIENCE
Key clinical point: A proposed mechanistic classification of clinical phenotypes in neuroblastoma is based on presence of telomere maintenance mechanisms, along with RAS and p53 mutations.
Major finding: The presence of telomere maintenance mechanisms was associated with a hazard ratio of 5.184 (P less than .001).
Study details: A genome sequencing of 416 pretreatment neuroblastomas, with tests for telomere maintenance mechanisms, RAS-pathway mutations, and p53-pathway mutations.
Disclosures: The study was funded by the German Cancer Aid, the German Ministry of Science and Education, the MYC-NET, the Deutsche Forschungsgemeinschaft, the Berlin Institute of Health, the European Union, and others. One coauthor reported financial relationships with Biogazelle and pxlence, and another reported consulting fees from NEO New Oncology.
Source: Ackermann S et al. Science. 2018 Dec 7. doi: 10.1126/science.aat6768.
Potty pathogens in space, fundus photos, and ethnic microbiomes
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
The earth is not enough
Earthly competitors have proved to be unworthy, so this week, Bacteria vs. the World visits the International Space Station, which – and we double-checked this – is in space. It’s a pretty exclusive location, and admission is by invitation only. Unless, of, course, you happen to be the ultimate hitchhiker. Four samples taken from the toilet of the ISS (and one from a piece of exercise equipment) were found to contain unknown strains of antibiotic-resistant Enterobacter bugandensis, investigators reported (BMC Microbiol. 2018 Nov 23;18[1]:175).
These bacterial stowaways were not virulent, lead author Nitin Singh, PhD, of the Jet Propulsion Laboratory said in a separate statement. But an analysis conducted by the team “reveals that the ISS isolates have a 79% probability of being a human pathogen.”
So, what does this mean for future space exploration? Cue the “Star Trek” music: “Space … the final frontier. These are the voyages of the bacterial transport ship Enterprise.”
Putting the FUN in fundus photos
You just got even more dependent on your phone: The American Academy of Opthalmology has published guidelines on how to use smartphones to take fundus photography, a.k.a. photographs of the back of the eye.
Advancement in smartphone optical quality has turned them into an important clinical tool, especially for specialists in low-funded or rural areas who don’t have access to imaging systems. Doctors can purchase special lenses and phone software to take these photos and then can easily upload the images to their Instagram accounts. (Even doctors need likes.)
An eye hospital in India has taken fundus accessibility a step further and posted a video on YouTube showing how to make a functional fundus camera that costs only 100 rupees. All you need in some cardboard, a water bottle, and a lens. “MacGyver: Chennai Edition.”
I feel it in my gut
Whoever said “inside, we’re all the same” clearly wasn’t considering the gut. A study from Vanderbilt University comprising 1,700 American subjects found that differences in gut microbiomes are most consistently linked with ethnicity. Vanderbilt biologist Seth Bordenstein emphasized how changing the gut microbiome can lead to curing illness but that it’s imperative that medical professionals understand how the gut differs across ethnicities.
Researchers found 12 types of bacteria that vary in abundancy by ethnicity. No comment on whether this was linked to differences in cuisine, but this writer fervently hopes new research arrives proving that tacos produce the healthiest gut microbiome.
F-bombing blood cancer
Call it a tale of two Toms.
TV newsman Tom Brokaw, who has multiple myeloma, says he’s become the “poster boy” for blood cancer. At first, though, he kept his diagnosis secret from just about everyone. But occasionally he let his emotions get the best of him. Especially when he’d see a Manhattan bus stop ad spotlighting the chiseled body of another Tom: the quarterback named Brady.
As he explained in a presentation at the annual meeting of the American Society of Hematology, he found it harder to get around because of back problems, which are common in multiple myeloma. As a result, he couldn’t manage to get to the office.
Still, “every day I’d force myself to leave the walker at home,” he recalled. “In that cold and sleety fall, I’d walk half a block to the coffee shop to get a bagel. There was this enormous new bus stop, with an animated advertisement board. Looking right at me was Tom Brady, advertising Ugg boots. I’d look down 79th Street at every inch of Tom Brady, and all the little old ladies were mooning over him as they were getting on the bus.”
Brokaw knew just what to do to make himself feel better. “I’d hobble over and look at him and drop the F-bomb on him every morning. Frankly, it was therapeutic for me.”
Later, he met the New England Patriots quarterback and told him the story, replacing “F-bomb” with the real word. “He had this little posse with him, and they roared. They said nobody talks to Tom like that.”
Brokaw still resists pleas to slow down from concerned loved ones, such as his emergency physician daughter. “My birth certificate says I’m 78 years old,” he said, “but I still think I’m 38 anchoring the news.” And still tossing tight-spiral F-bombs at cancer and gridiron G.O.A.T.s alike.
RT of lymph nodes as good as dissection for the long-term
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
SAN ANTONIO – Both axillary radiation therapy and axillary lymph node dissection provide excellent, comparable locoregional control in patients with early-stage breast cancer who have a positive sentinel node, according to updated results of the European Organisation for Research and Treatment of Cancer’s AMAROS trial.
The 10-year cumulative incidence rate of axillary recurrence was 1.82% with radiation and 0.93% with lymph node dissection, a nonsignificant difference (hazard ratio, 1.71; P = .365). Distant metastasis–free survival and overall survival also were statistically on par. The findings reinforce the trial’s 5-year results, which additionally showed a markedly lower incidence of lymphedema with axillary radiation therapy. Lead investigator Emiel J. T. Rutgers, MD, PhD, reflected on hesitation in the uptake of axillary radiation therapy among oncologists and discussed the AMAROS results in the context of the ACOSOG Z11 trial. Dr. Rutgers, the principal investigator of the AMAROS trial and a surgical oncologist at the Netherlands Cancer Institute in Amsterdam, also described how the trial’s findings have altered practice at his institution.
Dr. Rutgers disclosed that he had no relevant conflicts of interest. The study was supported by the EORTC Charitable Trust.
REPORTING FROM SABCS 2018
Oral Bowenoid Papulosis
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
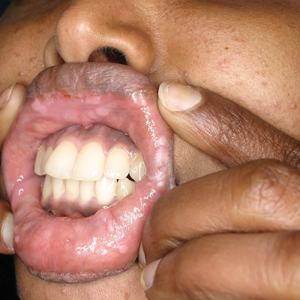
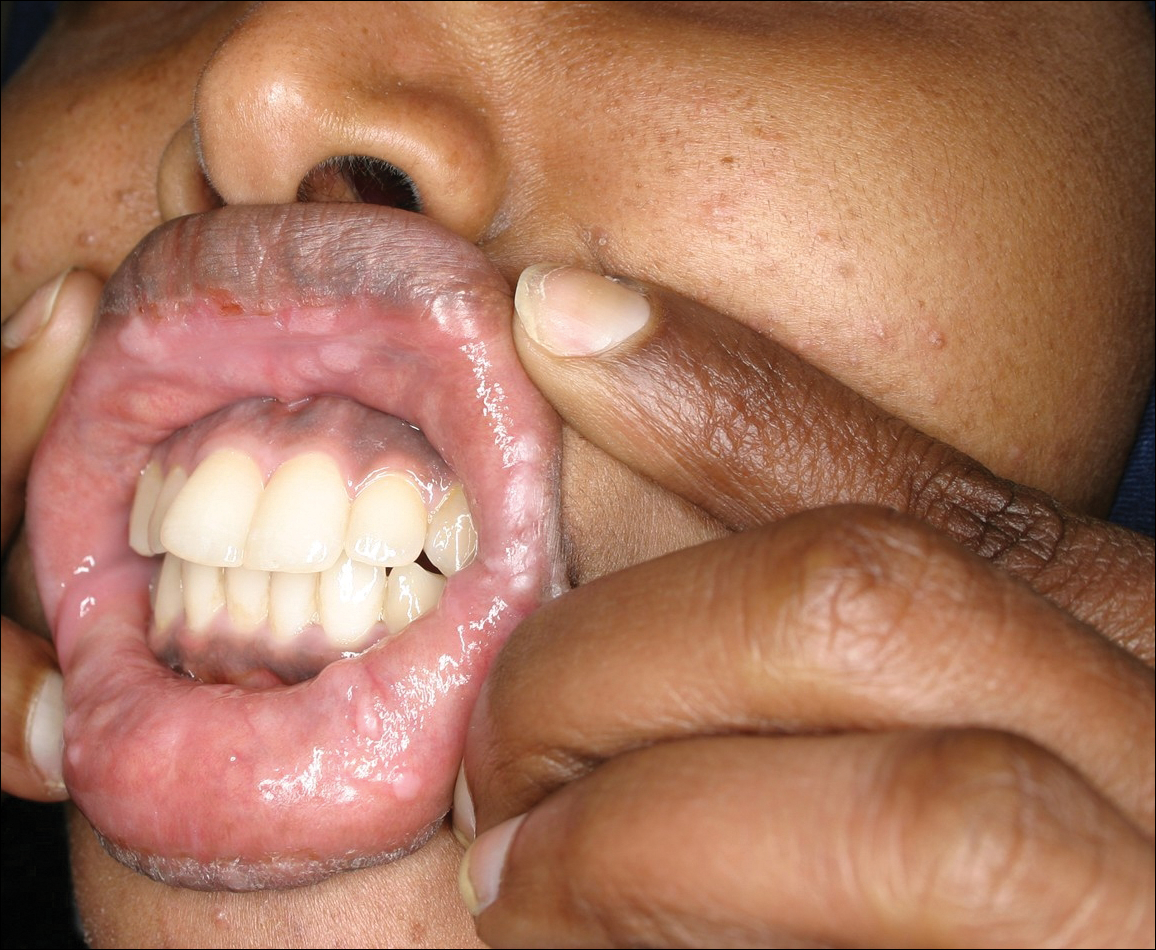
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.
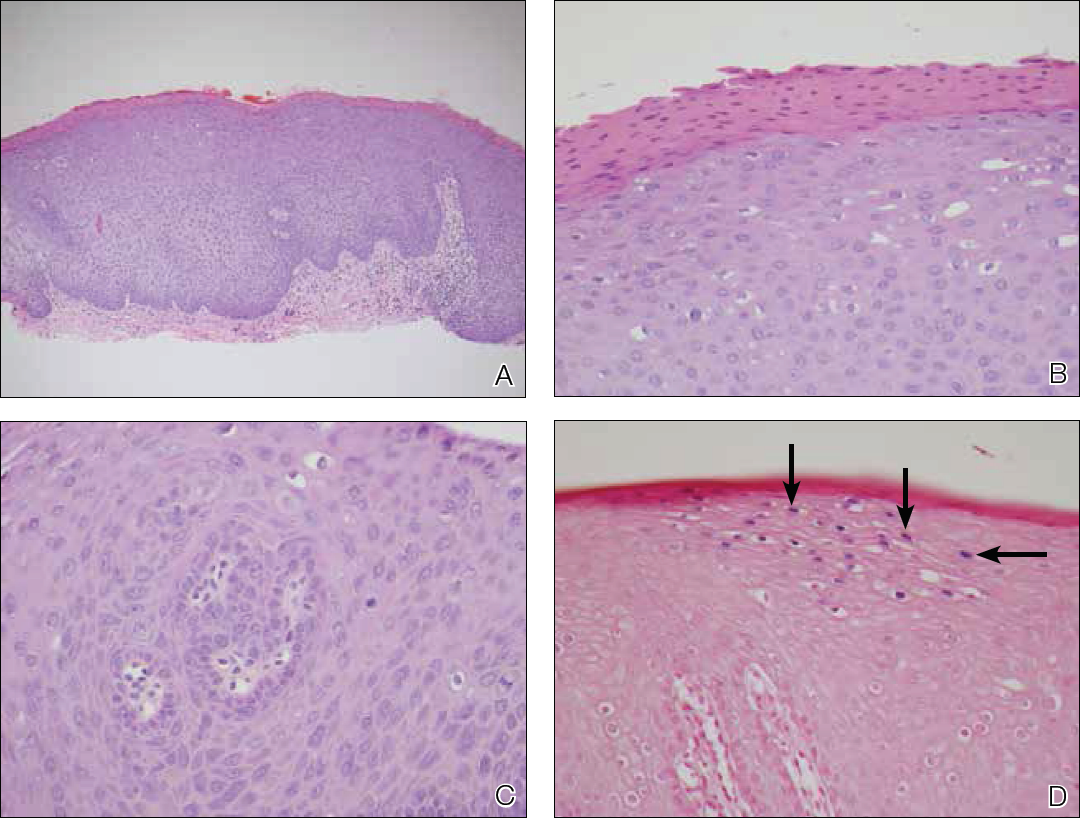
Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.
The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
Practice Points
- Bowenoid papulosis is triggered by human papillomavirus infection and manifests clinically as solitary or multiple verrucous papules and plaques that usually are located on the genitalia.
- Oral bowenoid papulosis is rare, and recognition of this unusual presentation is important for the diagnosis and management of this disease.
Nothing to gain from chemo after pCR achieved
SAN ANTONIO – Women with localized breast cancer who achieve a pathological complete response (pCR) after neoadjuvant chemotherapy may have little to gain from subsequent adjuvant chemotherapy except toxicity, according to a patient-level meta-analysis of more than 27,000 women. The analysis, reported by lead investigator Laura M. Spring, MD, at the San Antonio Breast Cancer Symposium, confirmed that, compared with residual disease, pCR was associated with significantly reduced risks of event-free survival events (hazard ratio, 0.31) and death (HR, 0.22). Moreover, the EFS benefit of a pCR was similar whether women went on to receive adjuvant chemotherapy (HR, 0.36) or not (HR, 0.36) (P = .60 for difference). Dr. Spring discussed overall and subgroup findings, implications for use of neoadjuvant chemotherapy, and how these new data may inform escalation and de-escalation of adjuvant therapy.
Dr. Spring disclosed that she has a consulting or advisory role with Novartis and that she receives institutional research funding from Tesaro. The study was supported by grants from the National Cancer Institute and Susan G. Komen.
SAN ANTONIO – Women with localized breast cancer who achieve a pathological complete response (pCR) after neoadjuvant chemotherapy may have little to gain from subsequent adjuvant chemotherapy except toxicity, according to a patient-level meta-analysis of more than 27,000 women. The analysis, reported by lead investigator Laura M. Spring, MD, at the San Antonio Breast Cancer Symposium, confirmed that, compared with residual disease, pCR was associated with significantly reduced risks of event-free survival events (hazard ratio, 0.31) and death (HR, 0.22). Moreover, the EFS benefit of a pCR was similar whether women went on to receive adjuvant chemotherapy (HR, 0.36) or not (HR, 0.36) (P = .60 for difference). Dr. Spring discussed overall and subgroup findings, implications for use of neoadjuvant chemotherapy, and how these new data may inform escalation and de-escalation of adjuvant therapy.
Dr. Spring disclosed that she has a consulting or advisory role with Novartis and that she receives institutional research funding from Tesaro. The study was supported by grants from the National Cancer Institute and Susan G. Komen.
SAN ANTONIO – Women with localized breast cancer who achieve a pathological complete response (pCR) after neoadjuvant chemotherapy may have little to gain from subsequent adjuvant chemotherapy except toxicity, according to a patient-level meta-analysis of more than 27,000 women. The analysis, reported by lead investigator Laura M. Spring, MD, at the San Antonio Breast Cancer Symposium, confirmed that, compared with residual disease, pCR was associated with significantly reduced risks of event-free survival events (hazard ratio, 0.31) and death (HR, 0.22). Moreover, the EFS benefit of a pCR was similar whether women went on to receive adjuvant chemotherapy (HR, 0.36) or not (HR, 0.36) (P = .60 for difference). Dr. Spring discussed overall and subgroup findings, implications for use of neoadjuvant chemotherapy, and how these new data may inform escalation and de-escalation of adjuvant therapy.
Dr. Spring disclosed that she has a consulting or advisory role with Novartis and that she receives institutional research funding from Tesaro. The study was supported by grants from the National Cancer Institute and Susan G. Komen.
REPORTING FROM SABCS 2018
Uterine cancer incidence and mortality on the rise in the U.S.
Uterine cancer incidence and mortality in the United States increased significantly from 1999 to 2015, with the greatest increases among nonwhite women, according to data published in the Morbidity and Mortality Weekly Report.
Uterine cancer is the fourth most common cancer diagnosed and the seventh most common cause of cancer death among women in the United States, wrote S. Jane Henley and her colleagues from the division of cancer prevention and control at the National Center for Chronic Disease Prevention and Health Promotion. Its incidence is thought to be on the rise because of the increasing prevalence of overweight and obesity.
In this report, researchers analyzed data from population-based cancer registries to find new cases of invasive uterine cancer from 1999 to 2015.
Over that period, incidence rates of uterine cancer increased around 0.7% per year on average, with an overall 12% increase. However, among American Indian and Alaskan Native women, the incidence rate during that time increased by 53%, among black women it increased by 46%, among Asian/Pacific Island women the incidence rate increased by 38%, and among Hispanic women it increased by 32%.
Uterine cancer death rates also increased by 21% from 1999 to 2015, representing approximately a 1.1% average increase per year. Again, the greatest increases were seen in American Indian and Alaskan Native women (52%), Hispanic women (33%), and black women (29%). Death rates increased by 18% among white women but were stable among Asian/Pacific Island women.
The most common type of uterine cancer was the endometrioid carcinomas, which accounted for 68% of uterine cancers overall. However black women had a higher percentage of other carcinomas, carcinosarcomas, and sarcomas, compared with women from other ethnic groups.
Two-thirds of cancer overall were diagnosed at a localized stage, but this was less common in black women than women of other ethnicities, while the proportion of cancers diagnosed at distant stage was higher among black women.
“This report found that black women were more likely to receive a diagnosis at distant stage and with more aggressive histologic types than were other women, which might in part account for the higher death rate among black women,” the authors wrote.
Despite the increasing incidence and mortality, the authors wrote that population-based screening tests are not recommended for uterine cancer, partly because around 90% of women with uterine cancer report abnormal vaginal bleeding.
“Uterine cancer outcomes could be improved by increasing awareness among women that abnormal vaginal bleeding should be evaluated promptly by a health care provider,” they wrote.
No conflicts of interest were reported.
SOURCE: Henley SJ et al. MMWR Morb Mortal Wkly Rep. 2018 Dec 7;67:1333-8.
Uterine cancer incidence and mortality in the United States increased significantly from 1999 to 2015, with the greatest increases among nonwhite women, according to data published in the Morbidity and Mortality Weekly Report.
Uterine cancer is the fourth most common cancer diagnosed and the seventh most common cause of cancer death among women in the United States, wrote S. Jane Henley and her colleagues from the division of cancer prevention and control at the National Center for Chronic Disease Prevention and Health Promotion. Its incidence is thought to be on the rise because of the increasing prevalence of overweight and obesity.
In this report, researchers analyzed data from population-based cancer registries to find new cases of invasive uterine cancer from 1999 to 2015.
Over that period, incidence rates of uterine cancer increased around 0.7% per year on average, with an overall 12% increase. However, among American Indian and Alaskan Native women, the incidence rate during that time increased by 53%, among black women it increased by 46%, among Asian/Pacific Island women the incidence rate increased by 38%, and among Hispanic women it increased by 32%.
Uterine cancer death rates also increased by 21% from 1999 to 2015, representing approximately a 1.1% average increase per year. Again, the greatest increases were seen in American Indian and Alaskan Native women (52%), Hispanic women (33%), and black women (29%). Death rates increased by 18% among white women but were stable among Asian/Pacific Island women.
The most common type of uterine cancer was the endometrioid carcinomas, which accounted for 68% of uterine cancers overall. However black women had a higher percentage of other carcinomas, carcinosarcomas, and sarcomas, compared with women from other ethnic groups.
Two-thirds of cancer overall were diagnosed at a localized stage, but this was less common in black women than women of other ethnicities, while the proportion of cancers diagnosed at distant stage was higher among black women.
“This report found that black women were more likely to receive a diagnosis at distant stage and with more aggressive histologic types than were other women, which might in part account for the higher death rate among black women,” the authors wrote.
Despite the increasing incidence and mortality, the authors wrote that population-based screening tests are not recommended for uterine cancer, partly because around 90% of women with uterine cancer report abnormal vaginal bleeding.
“Uterine cancer outcomes could be improved by increasing awareness among women that abnormal vaginal bleeding should be evaluated promptly by a health care provider,” they wrote.
No conflicts of interest were reported.
SOURCE: Henley SJ et al. MMWR Morb Mortal Wkly Rep. 2018 Dec 7;67:1333-8.
Uterine cancer incidence and mortality in the United States increased significantly from 1999 to 2015, with the greatest increases among nonwhite women, according to data published in the Morbidity and Mortality Weekly Report.
Uterine cancer is the fourth most common cancer diagnosed and the seventh most common cause of cancer death among women in the United States, wrote S. Jane Henley and her colleagues from the division of cancer prevention and control at the National Center for Chronic Disease Prevention and Health Promotion. Its incidence is thought to be on the rise because of the increasing prevalence of overweight and obesity.
In this report, researchers analyzed data from population-based cancer registries to find new cases of invasive uterine cancer from 1999 to 2015.
Over that period, incidence rates of uterine cancer increased around 0.7% per year on average, with an overall 12% increase. However, among American Indian and Alaskan Native women, the incidence rate during that time increased by 53%, among black women it increased by 46%, among Asian/Pacific Island women the incidence rate increased by 38%, and among Hispanic women it increased by 32%.
Uterine cancer death rates also increased by 21% from 1999 to 2015, representing approximately a 1.1% average increase per year. Again, the greatest increases were seen in American Indian and Alaskan Native women (52%), Hispanic women (33%), and black women (29%). Death rates increased by 18% among white women but were stable among Asian/Pacific Island women.
The most common type of uterine cancer was the endometrioid carcinomas, which accounted for 68% of uterine cancers overall. However black women had a higher percentage of other carcinomas, carcinosarcomas, and sarcomas, compared with women from other ethnic groups.
Two-thirds of cancer overall were diagnosed at a localized stage, but this was less common in black women than women of other ethnicities, while the proportion of cancers diagnosed at distant stage was higher among black women.
“This report found that black women were more likely to receive a diagnosis at distant stage and with more aggressive histologic types than were other women, which might in part account for the higher death rate among black women,” the authors wrote.
Despite the increasing incidence and mortality, the authors wrote that population-based screening tests are not recommended for uterine cancer, partly because around 90% of women with uterine cancer report abnormal vaginal bleeding.
“Uterine cancer outcomes could be improved by increasing awareness among women that abnormal vaginal bleeding should be evaluated promptly by a health care provider,” they wrote.
No conflicts of interest were reported.
SOURCE: Henley SJ et al. MMWR Morb Mortal Wkly Rep. 2018 Dec 7;67:1333-8.
FROM MMWR
Key clinical point: Uterine cancer incidence and mortality rose from 1999 to 2015 in the United States.
Major finding: The incidence of uterine cancer in the United States increased 12% from 1999 to 2015.
Study details: An analysis of cancer registry data.
Disclosures: No conflicts of interest were reported.
Source: Henley SJ et al. MMWR Morb Mortal Wkly Rep. 2018 Dec 7;67:1333-8.
Small absolute difference between partial and whole breast irradiation
SAN ANTONIO – A phase 3 randomized NRG Oncology trial (NSABP B-39/RTOG 0413) was unable to rule out the possibility that, after lumpectomy, partial breast irradiation is inferior to whole breast irradiation when it comes to the ipsilateral breast tumor recurrences (invasive disease or ductal carcinoma in situ), reported Frank Vicini, MD, of MHP Radiation Oncology Institute, Pontiac, Mich.
The hazard ratio for this event with the former versus latter modality was 1.22, with the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5). However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was just 0.7% (4.6% vs. 3.9%). In a video interview, Dr. Vicini discussed whether this difference is clinically important, and the implications of the trial’s findings, taken together, for offering partial breast irradiation to patients.
Dr. Vicini disclosed that he is a research adviser for ImpediMed. The study was sponsored by the National Cancer Institute.
SAN ANTONIO – A phase 3 randomized NRG Oncology trial (NSABP B-39/RTOG 0413) was unable to rule out the possibility that, after lumpectomy, partial breast irradiation is inferior to whole breast irradiation when it comes to the ipsilateral breast tumor recurrences (invasive disease or ductal carcinoma in situ), reported Frank Vicini, MD, of MHP Radiation Oncology Institute, Pontiac, Mich.
The hazard ratio for this event with the former versus latter modality was 1.22, with the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5). However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was just 0.7% (4.6% vs. 3.9%). In a video interview, Dr. Vicini discussed whether this difference is clinically important, and the implications of the trial’s findings, taken together, for offering partial breast irradiation to patients.
Dr. Vicini disclosed that he is a research adviser for ImpediMed. The study was sponsored by the National Cancer Institute.
SAN ANTONIO – A phase 3 randomized NRG Oncology trial (NSABP B-39/RTOG 0413) was unable to rule out the possibility that, after lumpectomy, partial breast irradiation is inferior to whole breast irradiation when it comes to the ipsilateral breast tumor recurrences (invasive disease or ductal carcinoma in situ), reported Frank Vicini, MD, of MHP Radiation Oncology Institute, Pontiac, Mich.
The hazard ratio for this event with the former versus latter modality was 1.22, with the 90% confidence interval (0.94-1.58) falling just outside the predefined range to declare the two modalities equivalent (0.667-1.5). However, the absolute difference in the 10-year cumulative incidence of ipsilateral breast tumor recurrences was just 0.7% (4.6% vs. 3.9%). In a video interview, Dr. Vicini discussed whether this difference is clinically important, and the implications of the trial’s findings, taken together, for offering partial breast irradiation to patients.
Dr. Vicini disclosed that he is a research adviser for ImpediMed. The study was sponsored by the National Cancer Institute.
REPORTING FROM SABCS 2018