User login
Incidental Asymptomatic Fibular Stress Fractures Presenting as Varus Knee Osteoarthritis: A Case Report
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.
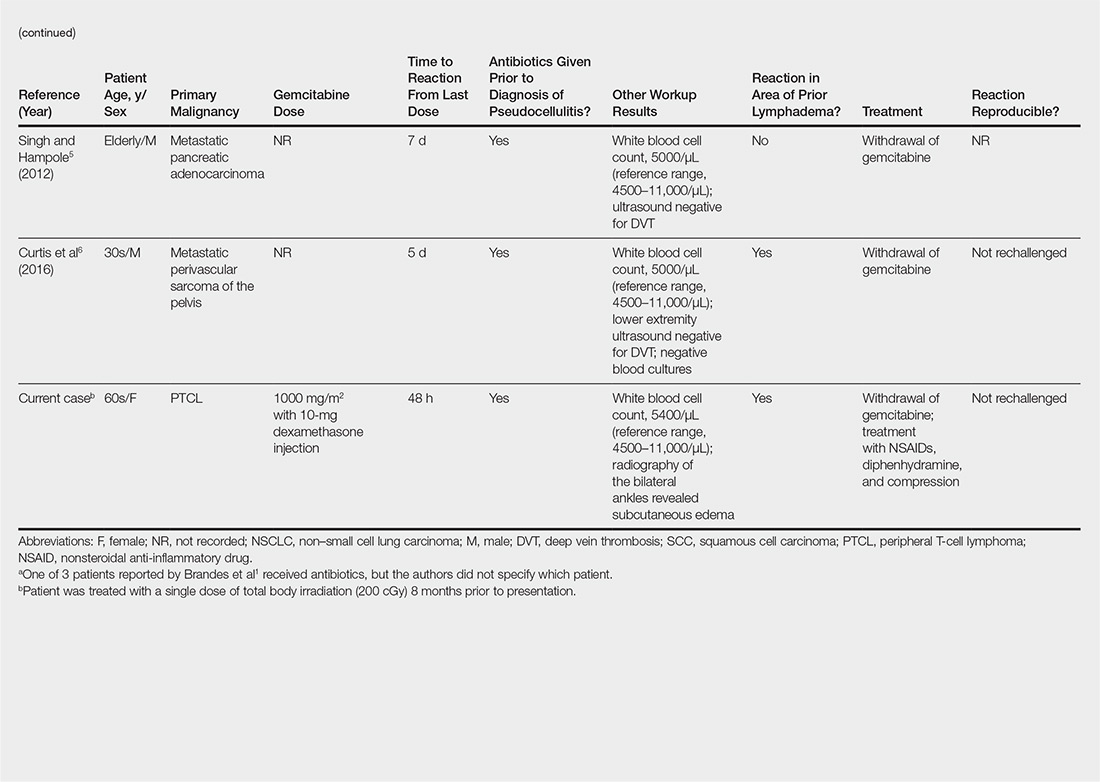
CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.
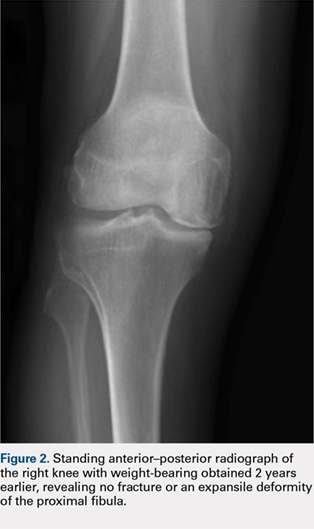
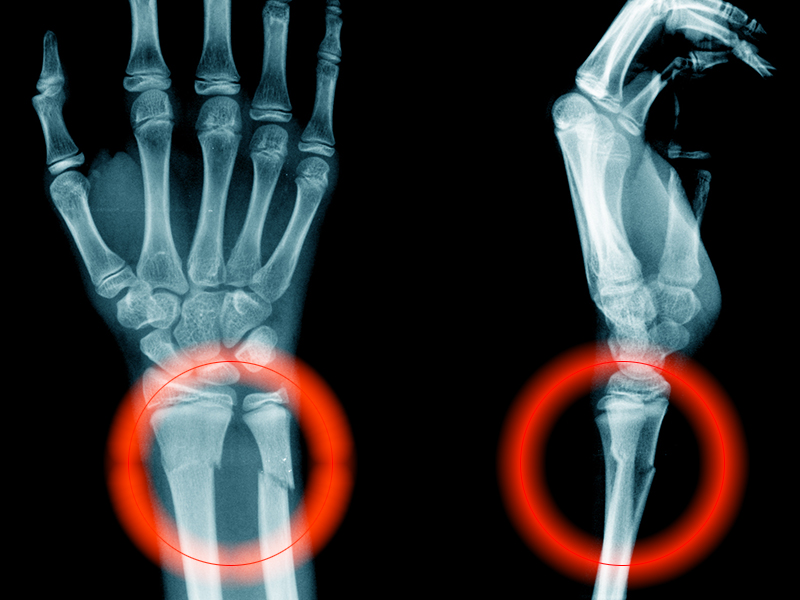
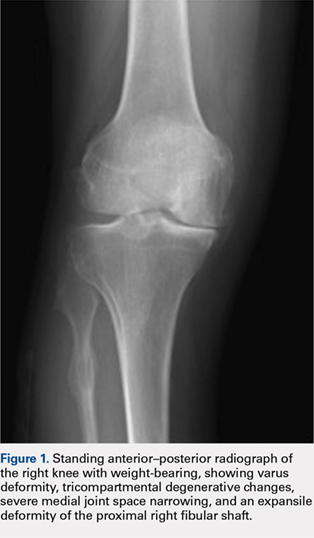
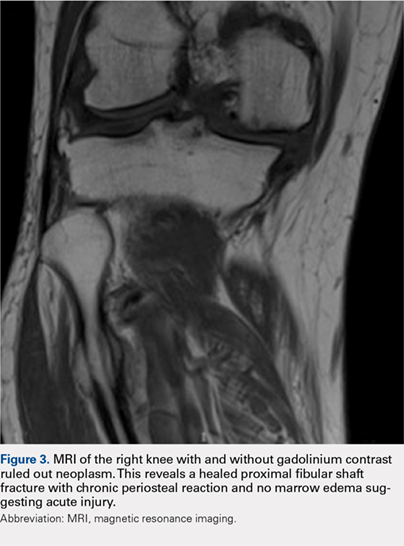
Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.
The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.
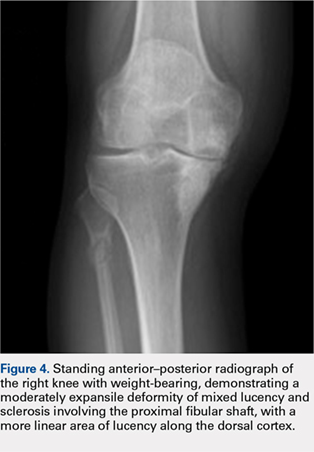
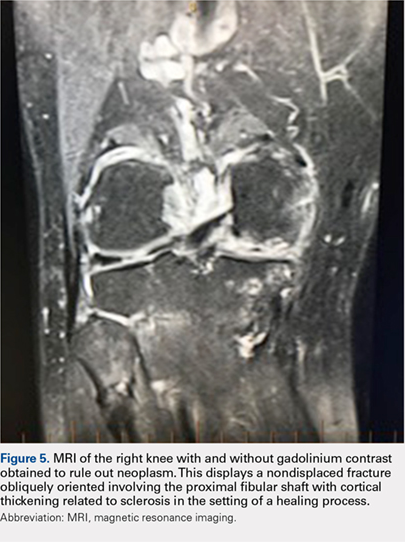
Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).

Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
TAKE-HOME POINTS
- Proximal fibular stress fractures in patients with primary osteoarthritis and fixed varus deformity have rarely been reported.
- Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.
- Proximal fibular stress fractures may present as an incidental finding of an expansile deformity on plain films in patients with varus osteoarthritic knees.
- Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease; however, it was justified in our case to rule out neoplasm.
- When present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and should not affect the plan or outcome of TKA.
To prevent fractures, treating only women with osteoporosis is not enough
The conventional bone mineral density threshold for initiating treatment to prevent fragility fractures is a T-score of less than -2.5 (the World Health Organization criteria for osteoporosis).1 However, most fractures experienced by postmenopausal women occur not in osteoporotic women but in those with low bone mass (osteopenia).2
Investigators in New Zealand recently published the results of a randomized controlled trial they conducted to determine the efficacy of zoledronate (zoledronic acid) in preventing fractures in postmenopausal women.3 They enrolled women age 65 years or older with osteopenia of the hip and randomly assigned the participants to 4 intravenous infusions of 5 mg zoledronic acid or placebo at 18-month intervals for 6 years.
Zoledronic acid reduced fracture risk
The trial included 2,000 postmenopausal women (mean age at baseline, 71 years; 94% European ethnicity) with a T-score of -1.0 to -2.5 at either the total hip or the femoral neck on either side. Both hips were assessed. The women received either zoledronic acid treatment or placebo in a 1:1 ratio. Candidates were excluded if they regularly used bone-active drugs in the previous year.
Fragility fractures were noted in 190 women in the placebo group and in 122 women treated with zoledronic acid (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.50–0.79, P<.001). The number of women that would need to be treated to prevent the occurrence of a fracture in 1 woman was 15.
Compared with placebo, zoledronic acid also lowered the risk of nonvertebral, symptomatic, and vertebral fractures as well as height loss (P≤.003 for these 4 comparisons). Relatively few adverse events occurred with zoledronic acid treatment. No atypical femoral fractures or cases of osteonecrosis of the jaw occurred in either group.
Trial closes the knowledge gap regarding treatment thresholds
This trial’s findings underscore the importance of age as a risk factor for fragility fracture and clarify that pharmacologic treatment is appropriate not only for women with osteoporosis but also for older postmenopausal women with osteopenia.
As the authors point out, administration of zoledronic acid less often than annually can be highly effective in preventing fractures; they recommend future trials of administration of this intravenous bisphosphonate at intervals less frequent than 18 months. Although the absence of atypical femoral fractures or cases of osteonecrosis of the jaw is reassuring, the authors note that their trial was underpowered to assess these uncommon events.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- World Health Organization. WHO Scientific Group on the assessment of osteoporosis at primary health care level. Summary meeting report, Brussels, Belgium, 5-7 May 2004. https://www. who.int/chp/topics/Osteoporosis.pdf. Accessed November 19, 2018.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
- Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018. doi:10.1056/NEJMoa1808082.
The conventional bone mineral density threshold for initiating treatment to prevent fragility fractures is a T-score of less than -2.5 (the World Health Organization criteria for osteoporosis).1 However, most fractures experienced by postmenopausal women occur not in osteoporotic women but in those with low bone mass (osteopenia).2
Investigators in New Zealand recently published the results of a randomized controlled trial they conducted to determine the efficacy of zoledronate (zoledronic acid) in preventing fractures in postmenopausal women.3 They enrolled women age 65 years or older with osteopenia of the hip and randomly assigned the participants to 4 intravenous infusions of 5 mg zoledronic acid or placebo at 18-month intervals for 6 years.
Zoledronic acid reduced fracture risk
The trial included 2,000 postmenopausal women (mean age at baseline, 71 years; 94% European ethnicity) with a T-score of -1.0 to -2.5 at either the total hip or the femoral neck on either side. Both hips were assessed. The women received either zoledronic acid treatment or placebo in a 1:1 ratio. Candidates were excluded if they regularly used bone-active drugs in the previous year.
Fragility fractures were noted in 190 women in the placebo group and in 122 women treated with zoledronic acid (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.50–0.79, P<.001). The number of women that would need to be treated to prevent the occurrence of a fracture in 1 woman was 15.
Compared with placebo, zoledronic acid also lowered the risk of nonvertebral, symptomatic, and vertebral fractures as well as height loss (P≤.003 for these 4 comparisons). Relatively few adverse events occurred with zoledronic acid treatment. No atypical femoral fractures or cases of osteonecrosis of the jaw occurred in either group.
Trial closes the knowledge gap regarding treatment thresholds
This trial’s findings underscore the importance of age as a risk factor for fragility fracture and clarify that pharmacologic treatment is appropriate not only for women with osteoporosis but also for older postmenopausal women with osteopenia.
As the authors point out, administration of zoledronic acid less often than annually can be highly effective in preventing fractures; they recommend future trials of administration of this intravenous bisphosphonate at intervals less frequent than 18 months. Although the absence of atypical femoral fractures or cases of osteonecrosis of the jaw is reassuring, the authors note that their trial was underpowered to assess these uncommon events.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The conventional bone mineral density threshold for initiating treatment to prevent fragility fractures is a T-score of less than -2.5 (the World Health Organization criteria for osteoporosis).1 However, most fractures experienced by postmenopausal women occur not in osteoporotic women but in those with low bone mass (osteopenia).2
Investigators in New Zealand recently published the results of a randomized controlled trial they conducted to determine the efficacy of zoledronate (zoledronic acid) in preventing fractures in postmenopausal women.3 They enrolled women age 65 years or older with osteopenia of the hip and randomly assigned the participants to 4 intravenous infusions of 5 mg zoledronic acid or placebo at 18-month intervals for 6 years.
Zoledronic acid reduced fracture risk
The trial included 2,000 postmenopausal women (mean age at baseline, 71 years; 94% European ethnicity) with a T-score of -1.0 to -2.5 at either the total hip or the femoral neck on either side. Both hips were assessed. The women received either zoledronic acid treatment or placebo in a 1:1 ratio. Candidates were excluded if they regularly used bone-active drugs in the previous year.
Fragility fractures were noted in 190 women in the placebo group and in 122 women treated with zoledronic acid (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.50–0.79, P<.001). The number of women that would need to be treated to prevent the occurrence of a fracture in 1 woman was 15.
Compared with placebo, zoledronic acid also lowered the risk of nonvertebral, symptomatic, and vertebral fractures as well as height loss (P≤.003 for these 4 comparisons). Relatively few adverse events occurred with zoledronic acid treatment. No atypical femoral fractures or cases of osteonecrosis of the jaw occurred in either group.
Trial closes the knowledge gap regarding treatment thresholds
This trial’s findings underscore the importance of age as a risk factor for fragility fracture and clarify that pharmacologic treatment is appropriate not only for women with osteoporosis but also for older postmenopausal women with osteopenia.
As the authors point out, administration of zoledronic acid less often than annually can be highly effective in preventing fractures; they recommend future trials of administration of this intravenous bisphosphonate at intervals less frequent than 18 months. Although the absence of atypical femoral fractures or cases of osteonecrosis of the jaw is reassuring, the authors note that their trial was underpowered to assess these uncommon events.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- World Health Organization. WHO Scientific Group on the assessment of osteoporosis at primary health care level. Summary meeting report, Brussels, Belgium, 5-7 May 2004. https://www. who.int/chp/topics/Osteoporosis.pdf. Accessed November 19, 2018.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
- Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018. doi:10.1056/NEJMoa1808082.
- World Health Organization. WHO Scientific Group on the assessment of osteoporosis at primary health care level. Summary meeting report, Brussels, Belgium, 5-7 May 2004. https://www. who.int/chp/topics/Osteoporosis.pdf. Accessed November 19, 2018.
- Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108-1112.
- Reid IR, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018. doi:10.1056/NEJMoa1808082.
LCAR-B38M CAR T therapy appears durable in myeloma
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
SAN DIEGO – The chimeric antigen receptor (CAR) T-cell therapy LCAR-B38M is in the race for approval in multiple myeloma following encouraging phase 1 results reported at the annual meeting of the American Society of Hematology.
In the LEGEND-2 phase 1/2 open study of 57 patients with advanced relapsed/refractory multiple myeloma treated with the investigational CAR T therapy, the overall response rate was 88% and the complete response rate was 74%. Among 42 patients who achieved complete response, 39 (68%) were negative for minimal residual disease (MRD).
With a median follow-up of 12 months, the median duration of response was 16 months and progression-free survival was 15 months. But in patients who achieved MRD-negative complete response, the median progression-free survival was extended to 24 months.
Pyrexia and cytokine release syndrome were reported in 90% or more of patients. Thrombocytopenia and leukopenia were reported in nearly half of patients.
The phase 1 study was conducted by researchers from the Second Affiliated Hospital of Xi’an Jiaotong University in Xi’an, China. The B-cell maturation antigen (BCMA)–directed CAR T-cell therapy is being jointly developed by Nanjing Legend Biotech and Janssen. A phase 2 study is currently being planned in China for LCAR-B38M. In parallel, Janssen and Legend are enrolling patients in a phase 1b/2 trial of the agent (also known as JNJ-68284528) in the United States.
The therapy joins a growing field of anti-BCMA CAR T-cell agents with promising initial trial results, including bb2121.
In a video interview at ASH, Sen Zhuang, MD, PhD, vice president of oncology clinical development at Janssen Research & Development, said this class of CAR T agents offers the potential for “very long remissions” and possibly even a “cure” for myeloma.
The LEGEND-2 study is sponsored by Nanjing Legend Biotech and two of the investigators reported employment with the company.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: The complete response rate was 74% with median progression-free survival of 15 months.
Study details: A phase 1/2 study of 57 patients with advanced relapsed/refractory multiple myeloma.
Disclosures: The study is sponsored by Nanjing Legend Biotech. Two of the investigators reported employment with the company.
PD-L1 expression best predicts response to atezolizumab + nab-paclitaxel for mTNBC
SAN ANTONIO – in patients with untreated metastatic triple-negative breast cancer, according to exploratory efficacy analyses of data from the phase 3 IMpassion130 trial.
The analyses of data for the 902 patients randomized to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-palcitaxel for the study also showed consistency between local and central estrogen-receptor, progesterone-receptor, and human epidermal growth factor–receptor 2 testing, Leisha A. Emens, MD, reported at the San Antonio Breast Cancer Symposium.
“IMpassion130 is the first phase 3 study to demonstrate a benefit from [atezolizumab + nab-paclitaxel] in metastatic triple-negative breast cancer (mTNBC),” said Dr. Emens, professor of medicine in hematology/oncology, coleader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center.
She explained that progression-free survival (PFS) was significantly better in PD-L1–positive mTNBC patients treated with the atezolizumab + nab-paclitaxel, than in those who received placebo + nab-paclitaxel (hazard ratios in the intent-to-treat population, 0.8 and 0.62, respectively).
At the first interim overall survival analysis, a clinically meaningful improvement in OS was seen in PD-L1–positive patients in the treatment group (HR, 0.62; median OS improvement from 15.5 months with placebo to 25 months), she added.
In exploratory analyses, Dr. Emens and her colleagues sought to evaluate whether preexisting immune biology is associated with clinical benefit from atezolizumab + nab-paclitaxel, as has been demonstrated in studies of other agents that target the PD-1 pathway in other cancer types of cancer. They also assessed BRCA 1/2 mutation status as a biomarker for response.
“In patients enrolled on the IMpassion130 trial we found that PD-L1 in triple-negative breast cancer was expressed primarily on tumor-infiltrating immune cells,” she said. “In contrast to this, we found a very low rate of PD-L1 expression specifically on tumor cells across the patient population.”
Looking at both of those biomarkers together showed that a majority of patients with expression of PD-L1 on tumor cells were included in the PD-L1 immune cell–positive population, with only 2% having PD-L1 expression exclusively on their tumor cells.
Data previously reported at the European Society for Medical Oncology and published in the New England Journal of Medicine showed a PFS benefit, as well as a clinically meaningful improvement in OS of nearly 10 months, specifically in patients with PD-L1 immune cell–positive lesions treated with atezolizumab + nab-paclitaxel, she noted.
“In data presented for the first time today you can see that PD-L1–negative patients derive no overall survival benefit as there was no treatment effect with this therapy combination,” she said.
A trend was seen toward an association between immune cell positivity and poor prognosis, but this was not statistically significant, she said.
“Taken together, these data definitively show that PD-L1 immune cell positivity is predictive of both progression-free and overall survival benefit with atezolizumab + nab-paclitaxel,” she said.
She and her colleagues also looked at the level of PD-L1 expression in immune cells to assess whether there is a threshold that might be required.
“As long as there was a PD-L1 expression level of 1% or more in the immune cells, there was a significant progression-free and overall survival benefit for patients treated with atezolizumab + nab-paclitaxel. This suggests that this expression of over 1% will represent a threshold for identifying those patients who are likely to benefit from this combination,” she said.
Further assessment by CD8 T-cell status showed that patients who had CD8-positive T cells but who were PD-L1 immune cell negative had no benefit from atezolizumab + nab-paclitaxel, whereas those who were positive for both CD8 and PD-L1 expression on their immune cells derived significant PFS and OS benefit (HR, 0.89 and 0.77, respectively).
“So patients with CD8-positive tumors derive clinical benefit only if their tumors are also PD-L1-positive,” she said.
Similarly, no clinical benefit was seen in patients with stromal tumor-infiltrating lymphocyte (TIL)–positive tumors but who were PD-L1-negative, whereas those with stromal TIL-positive PD-L1–positive tumors derived significant PFS and OS benefit (HRs, 0.99 and 1.53, respectively), and this was also seen in the 15% of evaluable patients who had BRCA mutations.
“In patients who were BRCA mutated, but who were PD-L1 immune cell negative, there was no association of progression-free survival or an overall survival benefit [with atezolizumab + nab-paclitaxel]. In contrast, in patients who were BRCA mutated but PD-L1 immune cell positive ... there was an association with progression-free survival and a trend toward overall survival,” she said, noting that while the BRCA mutation findings are limited by small numbers, “they do show that mutations in BRCA and PD-L1 expression in immune cells are independent biomarkers; patients with BRCA1 or 2 mutations derive clinical benefit only if their tumors are also PD-L1 positive.”
“In this phase 3 IMpassion130 study, PD-L1 expression on immune cells is a predictive biomarker for selecting patients who benefit clinically during first-line treatment with atezolizumab + nab-paclitaxel for metastatic triple-negative breast cancer,” she concluded, adding that “patients with newly diagnosed metastatic and unresectable locally advanced triple-negative breast cancer should be routinely tested for their PD-L1 immune cell status to determine if they might benefit from the combination of atezolizumab + nab-paclitaxel.
IMpassion130 was sponsored by Hoffman-La Roche. Dr. Emens reported receiving royalties and consulting fees from several companies. She has contracts with Roche/Genentech, Corvus, AstraZeneca, and EMD Serono, and ownership in Molecuvax. She receives other support from DSMB and Syndax, and has received grants from Aduro Biotech, Merck, Maxcyte, and the Breast Cancer Research Foundation. She also reported serving as a member of the Food and Drug Administration Advisory Committee on Tissue, Cell, and Gene Therapies, and is a member of the board of directors for the Society of Immunotherapy for Cancer.
SOURCE: Emens L et al. SABCS 2018, Abstract GS1-04.
SAN ANTONIO – in patients with untreated metastatic triple-negative breast cancer, according to exploratory efficacy analyses of data from the phase 3 IMpassion130 trial.
The analyses of data for the 902 patients randomized to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-palcitaxel for the study also showed consistency between local and central estrogen-receptor, progesterone-receptor, and human epidermal growth factor–receptor 2 testing, Leisha A. Emens, MD, reported at the San Antonio Breast Cancer Symposium.
“IMpassion130 is the first phase 3 study to demonstrate a benefit from [atezolizumab + nab-paclitaxel] in metastatic triple-negative breast cancer (mTNBC),” said Dr. Emens, professor of medicine in hematology/oncology, coleader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center.
She explained that progression-free survival (PFS) was significantly better in PD-L1–positive mTNBC patients treated with the atezolizumab + nab-paclitaxel, than in those who received placebo + nab-paclitaxel (hazard ratios in the intent-to-treat population, 0.8 and 0.62, respectively).
At the first interim overall survival analysis, a clinically meaningful improvement in OS was seen in PD-L1–positive patients in the treatment group (HR, 0.62; median OS improvement from 15.5 months with placebo to 25 months), she added.
In exploratory analyses, Dr. Emens and her colleagues sought to evaluate whether preexisting immune biology is associated with clinical benefit from atezolizumab + nab-paclitaxel, as has been demonstrated in studies of other agents that target the PD-1 pathway in other cancer types of cancer. They also assessed BRCA 1/2 mutation status as a biomarker for response.
“In patients enrolled on the IMpassion130 trial we found that PD-L1 in triple-negative breast cancer was expressed primarily on tumor-infiltrating immune cells,” she said. “In contrast to this, we found a very low rate of PD-L1 expression specifically on tumor cells across the patient population.”
Looking at both of those biomarkers together showed that a majority of patients with expression of PD-L1 on tumor cells were included in the PD-L1 immune cell–positive population, with only 2% having PD-L1 expression exclusively on their tumor cells.
Data previously reported at the European Society for Medical Oncology and published in the New England Journal of Medicine showed a PFS benefit, as well as a clinically meaningful improvement in OS of nearly 10 months, specifically in patients with PD-L1 immune cell–positive lesions treated with atezolizumab + nab-paclitaxel, she noted.
“In data presented for the first time today you can see that PD-L1–negative patients derive no overall survival benefit as there was no treatment effect with this therapy combination,” she said.
A trend was seen toward an association between immune cell positivity and poor prognosis, but this was not statistically significant, she said.
“Taken together, these data definitively show that PD-L1 immune cell positivity is predictive of both progression-free and overall survival benefit with atezolizumab + nab-paclitaxel,” she said.
She and her colleagues also looked at the level of PD-L1 expression in immune cells to assess whether there is a threshold that might be required.
“As long as there was a PD-L1 expression level of 1% or more in the immune cells, there was a significant progression-free and overall survival benefit for patients treated with atezolizumab + nab-paclitaxel. This suggests that this expression of over 1% will represent a threshold for identifying those patients who are likely to benefit from this combination,” she said.
Further assessment by CD8 T-cell status showed that patients who had CD8-positive T cells but who were PD-L1 immune cell negative had no benefit from atezolizumab + nab-paclitaxel, whereas those who were positive for both CD8 and PD-L1 expression on their immune cells derived significant PFS and OS benefit (HR, 0.89 and 0.77, respectively).
“So patients with CD8-positive tumors derive clinical benefit only if their tumors are also PD-L1-positive,” she said.
Similarly, no clinical benefit was seen in patients with stromal tumor-infiltrating lymphocyte (TIL)–positive tumors but who were PD-L1-negative, whereas those with stromal TIL-positive PD-L1–positive tumors derived significant PFS and OS benefit (HRs, 0.99 and 1.53, respectively), and this was also seen in the 15% of evaluable patients who had BRCA mutations.
“In patients who were BRCA mutated, but who were PD-L1 immune cell negative, there was no association of progression-free survival or an overall survival benefit [with atezolizumab + nab-paclitaxel]. In contrast, in patients who were BRCA mutated but PD-L1 immune cell positive ... there was an association with progression-free survival and a trend toward overall survival,” she said, noting that while the BRCA mutation findings are limited by small numbers, “they do show that mutations in BRCA and PD-L1 expression in immune cells are independent biomarkers; patients with BRCA1 or 2 mutations derive clinical benefit only if their tumors are also PD-L1 positive.”
“In this phase 3 IMpassion130 study, PD-L1 expression on immune cells is a predictive biomarker for selecting patients who benefit clinically during first-line treatment with atezolizumab + nab-paclitaxel for metastatic triple-negative breast cancer,” she concluded, adding that “patients with newly diagnosed metastatic and unresectable locally advanced triple-negative breast cancer should be routinely tested for their PD-L1 immune cell status to determine if they might benefit from the combination of atezolizumab + nab-paclitaxel.
IMpassion130 was sponsored by Hoffman-La Roche. Dr. Emens reported receiving royalties and consulting fees from several companies. She has contracts with Roche/Genentech, Corvus, AstraZeneca, and EMD Serono, and ownership in Molecuvax. She receives other support from DSMB and Syndax, and has received grants from Aduro Biotech, Merck, Maxcyte, and the Breast Cancer Research Foundation. She also reported serving as a member of the Food and Drug Administration Advisory Committee on Tissue, Cell, and Gene Therapies, and is a member of the board of directors for the Society of Immunotherapy for Cancer.
SOURCE: Emens L et al. SABCS 2018, Abstract GS1-04.
SAN ANTONIO – in patients with untreated metastatic triple-negative breast cancer, according to exploratory efficacy analyses of data from the phase 3 IMpassion130 trial.
The analyses of data for the 902 patients randomized to receive the PD-L1 inhibitor atezolizumab (Tecentriq) plus nanoparticle albumin-bound (nab)–paclitaxel or placebo plus nab-palcitaxel for the study also showed consistency between local and central estrogen-receptor, progesterone-receptor, and human epidermal growth factor–receptor 2 testing, Leisha A. Emens, MD, reported at the San Antonio Breast Cancer Symposium.
“IMpassion130 is the first phase 3 study to demonstrate a benefit from [atezolizumab + nab-paclitaxel] in metastatic triple-negative breast cancer (mTNBC),” said Dr. Emens, professor of medicine in hematology/oncology, coleader of the Hillman Cancer Immunology and Immunotherapy Program, and director of translational immunotherapy for the Women’s Cancer Research Center at the University of Pittsburgh Medical Center.
She explained that progression-free survival (PFS) was significantly better in PD-L1–positive mTNBC patients treated with the atezolizumab + nab-paclitaxel, than in those who received placebo + nab-paclitaxel (hazard ratios in the intent-to-treat population, 0.8 and 0.62, respectively).
At the first interim overall survival analysis, a clinically meaningful improvement in OS was seen in PD-L1–positive patients in the treatment group (HR, 0.62; median OS improvement from 15.5 months with placebo to 25 months), she added.
In exploratory analyses, Dr. Emens and her colleagues sought to evaluate whether preexisting immune biology is associated with clinical benefit from atezolizumab + nab-paclitaxel, as has been demonstrated in studies of other agents that target the PD-1 pathway in other cancer types of cancer. They also assessed BRCA 1/2 mutation status as a biomarker for response.
“In patients enrolled on the IMpassion130 trial we found that PD-L1 in triple-negative breast cancer was expressed primarily on tumor-infiltrating immune cells,” she said. “In contrast to this, we found a very low rate of PD-L1 expression specifically on tumor cells across the patient population.”
Looking at both of those biomarkers together showed that a majority of patients with expression of PD-L1 on tumor cells were included in the PD-L1 immune cell–positive population, with only 2% having PD-L1 expression exclusively on their tumor cells.
Data previously reported at the European Society for Medical Oncology and published in the New England Journal of Medicine showed a PFS benefit, as well as a clinically meaningful improvement in OS of nearly 10 months, specifically in patients with PD-L1 immune cell–positive lesions treated with atezolizumab + nab-paclitaxel, she noted.
“In data presented for the first time today you can see that PD-L1–negative patients derive no overall survival benefit as there was no treatment effect with this therapy combination,” she said.
A trend was seen toward an association between immune cell positivity and poor prognosis, but this was not statistically significant, she said.
“Taken together, these data definitively show that PD-L1 immune cell positivity is predictive of both progression-free and overall survival benefit with atezolizumab + nab-paclitaxel,” she said.
She and her colleagues also looked at the level of PD-L1 expression in immune cells to assess whether there is a threshold that might be required.
“As long as there was a PD-L1 expression level of 1% or more in the immune cells, there was a significant progression-free and overall survival benefit for patients treated with atezolizumab + nab-paclitaxel. This suggests that this expression of over 1% will represent a threshold for identifying those patients who are likely to benefit from this combination,” she said.
Further assessment by CD8 T-cell status showed that patients who had CD8-positive T cells but who were PD-L1 immune cell negative had no benefit from atezolizumab + nab-paclitaxel, whereas those who were positive for both CD8 and PD-L1 expression on their immune cells derived significant PFS and OS benefit (HR, 0.89 and 0.77, respectively).
“So patients with CD8-positive tumors derive clinical benefit only if their tumors are also PD-L1-positive,” she said.
Similarly, no clinical benefit was seen in patients with stromal tumor-infiltrating lymphocyte (TIL)–positive tumors but who were PD-L1-negative, whereas those with stromal TIL-positive PD-L1–positive tumors derived significant PFS and OS benefit (HRs, 0.99 and 1.53, respectively), and this was also seen in the 15% of evaluable patients who had BRCA mutations.
“In patients who were BRCA mutated, but who were PD-L1 immune cell negative, there was no association of progression-free survival or an overall survival benefit [with atezolizumab + nab-paclitaxel]. In contrast, in patients who were BRCA mutated but PD-L1 immune cell positive ... there was an association with progression-free survival and a trend toward overall survival,” she said, noting that while the BRCA mutation findings are limited by small numbers, “they do show that mutations in BRCA and PD-L1 expression in immune cells are independent biomarkers; patients with BRCA1 or 2 mutations derive clinical benefit only if their tumors are also PD-L1 positive.”
“In this phase 3 IMpassion130 study, PD-L1 expression on immune cells is a predictive biomarker for selecting patients who benefit clinically during first-line treatment with atezolizumab + nab-paclitaxel for metastatic triple-negative breast cancer,” she concluded, adding that “patients with newly diagnosed metastatic and unresectable locally advanced triple-negative breast cancer should be routinely tested for their PD-L1 immune cell status to determine if they might benefit from the combination of atezolizumab + nab-paclitaxel.
IMpassion130 was sponsored by Hoffman-La Roche. Dr. Emens reported receiving royalties and consulting fees from several companies. She has contracts with Roche/Genentech, Corvus, AstraZeneca, and EMD Serono, and ownership in Molecuvax. She receives other support from DSMB and Syndax, and has received grants from Aduro Biotech, Merck, Maxcyte, and the Breast Cancer Research Foundation. She also reported serving as a member of the Food and Drug Administration Advisory Committee on Tissue, Cell, and Gene Therapies, and is a member of the board of directors for the Society of Immunotherapy for Cancer.
SOURCE: Emens L et al. SABCS 2018, Abstract GS1-04.
REPORTING FROM SABCS 2018
Key clinical point: Treatment-naive mTNBC patients should be tested for PD-L1 expression as a biomarker of potential benefit from atezolizumab + nab-paclitaxel.
Major finding: PD-L1 expression of at least 1% confers a significant PFS and OS benefit in patients treated with atezolizumab + nab-paclitaxel.
Study details: Exploratory efficacy analyses of a phase 3 study of 902 patients.
Disclosures: IMpassion130 was sponsored by Hoffman-La Roche. Dr. Emens reported receiving royalties from and consulting fees from several companies. She has contracts with Roche/Genentech, Corvus, AstraZeneca, and EMD Serono, and ownership in Molecuvax. She receives other support from DSMB and Syndax, and has received grants from Aduro Biotech, Merck, Maxcyte, and the Breast Cancer Research Foundation. She also reported serving as a member of the FDA Advisory Committee on Tissue, Cell, and Gene Therapies, and is a member of the board of directors for the Society of Immunotherapy for Cancer.
Source: Emens L et al. SABCS 2018, Abstract GS1-04.
Ice Pack–Induced Perniosis: A Rare and Underrecognized Association
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
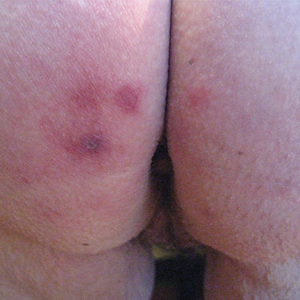
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).
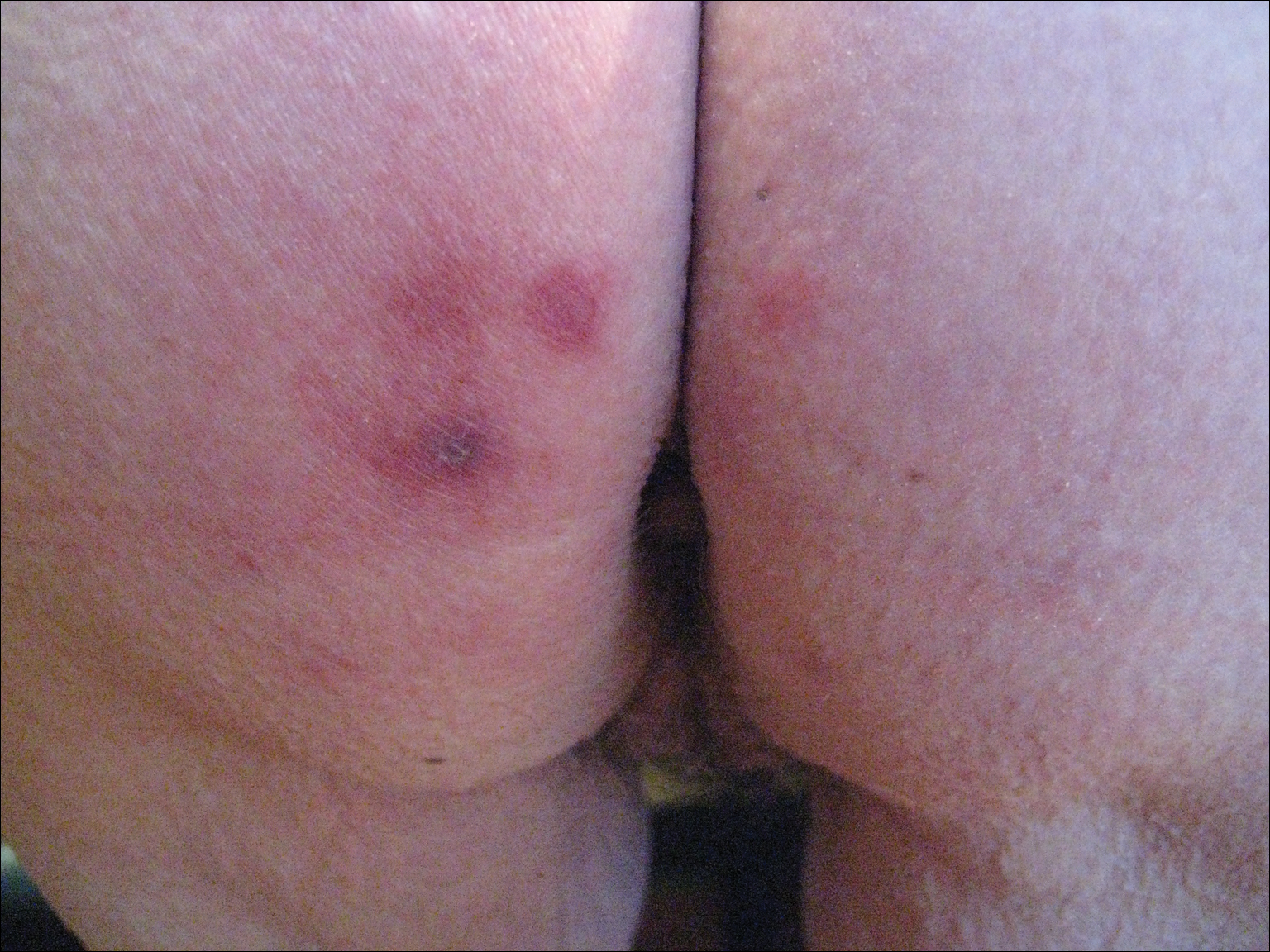
After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.
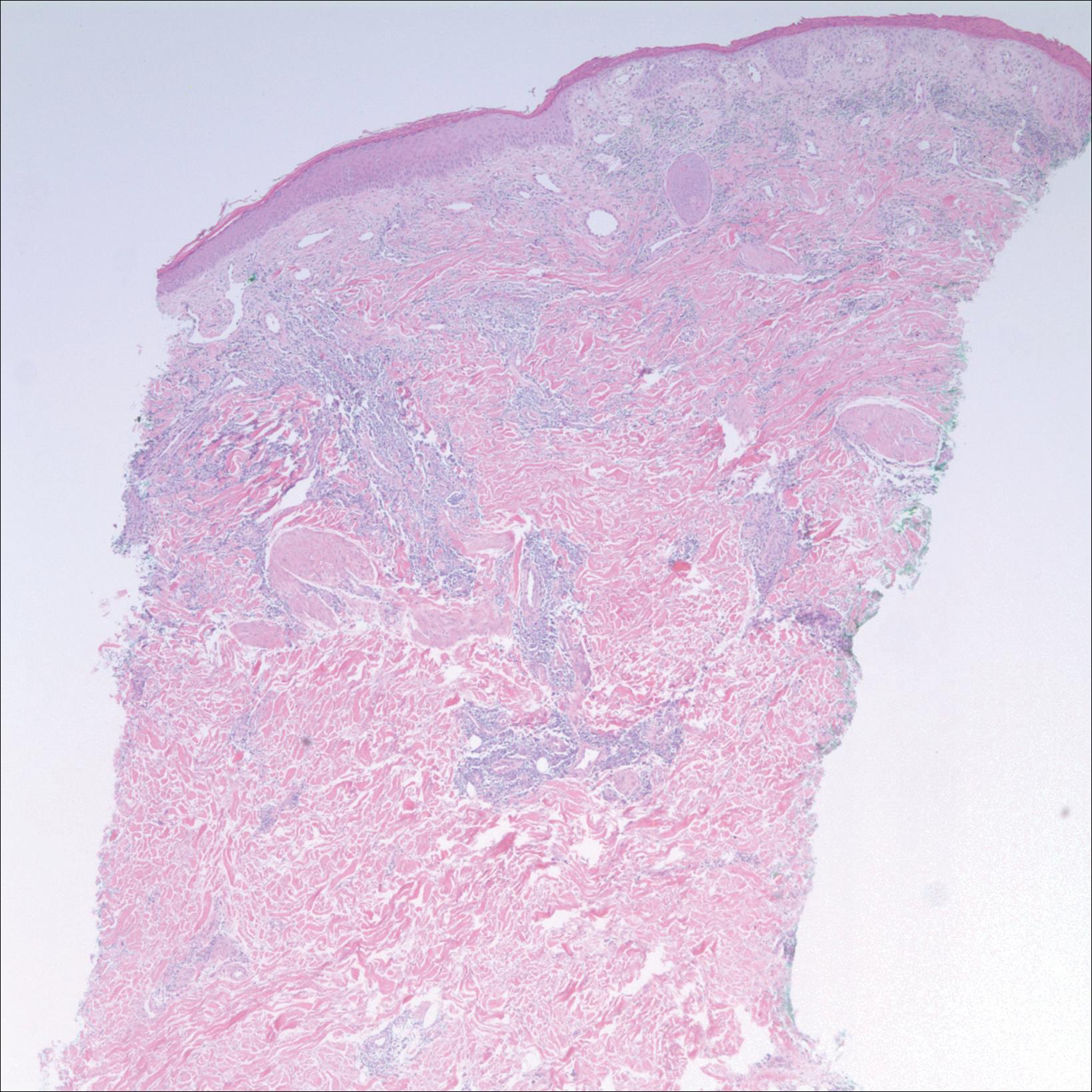
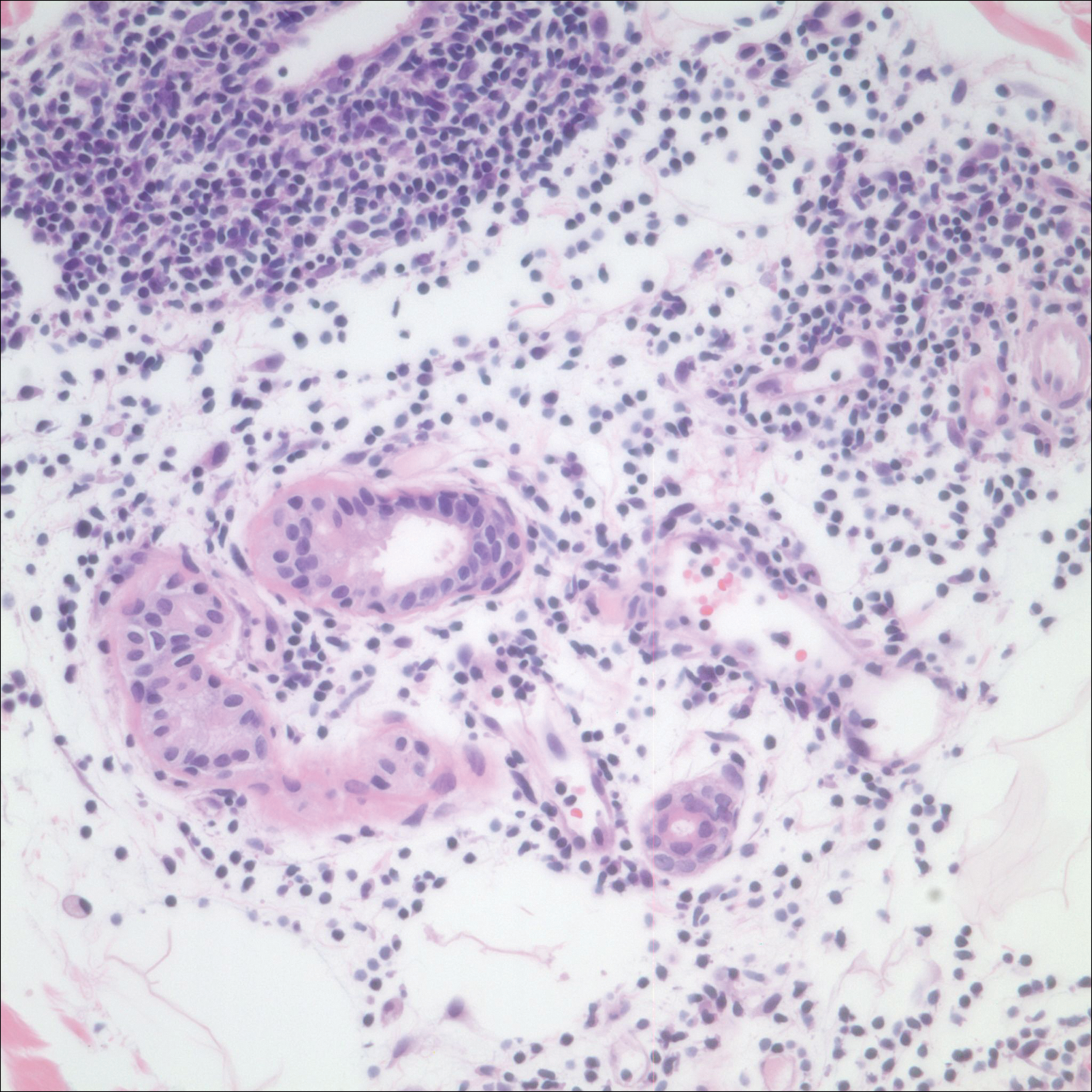
Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Practice Points
- Ice pack-induced perniosis is a rare condition that can occur in patients using long-term ice pack therapy.
- This entity histopathologically mimics cutaneous lupus erythematosus and can present a diagnostic challenge.
- A thorough clinical history and awareness of this diagnosis is essential for diagnostic accuracy.
Gemcitabine-Induced Pseudocellulitis
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
Practice Points
- Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies.
- Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy.
- Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics.
Biologics options for pediatric asthma continue to grow
ORLANDO – The goal of treatment is the same for all asthma cases, regardless of severity: “to enable a patient to achieve and maintain control over their asthma,” according to Stanley J. Szefler, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora.
That goal includes “reducing the risk of exacerbations, emergency department visits, hospitalizations, and progression as well as reducing impairments, including symptoms, functional limitations, poor quality of life, and other manifestations of asthma,” Dr. Szefler, also director of the Children’s Hospital of Colorado pediatric asthma research program, told colleagues at the annual meeting of the American Academy of Pediatrics.
Severe asthma challenges
These aims are more difficult with severe asthma, defined by the World Health Organization as “the current level of clinical control and risks which can result in frequent severe exacerbations and/or adverse reactions to medications and/or chronic morbidity,” Dr. Szefler explained. Severe asthma includes untreated severe asthma, difficult-to-treat asthma, and treatment-resistant severe asthma, whether controlled on high-dose medication or not.
Allergen sensitization, viral respiratory infections, and respiratory irritants (such as air pollution and smoking) are common features of severe asthma in children. Also common are challenges specific to management: poor medication adherence, poor technique for inhaled medications, and undertreatment. Poor management can lead to repeated exacerbations, adverse effects from drugs, disease progression, possible development of chronic obstructive pulmonary disease (COPD), and early mortality.
The National Heart, Lung, and Blood Institute EPR-3 guidelines for treatment of pediatric asthma recommend a stepwise approach to therapy, starting with short-acting beta2-agonists as needed (SABA p.r.n.). The clinician then assesses the patient’s symptoms, exacerbations, side effects, quality of life, and lung function to determine whether the asthma is well managed or requires inhaled corticosteroids, or another therapy in moving through the steps. Each step also involves patient education, environmental control, and management of the child’s comorbidities.
It is not until steps 5 and 6 that the guidelines advise considering the biologic omalizumab for patients who have allergies. But other biologic options exist as well. Four biologics currently approved for treating asthma include omalizumab, mepolizumab, benralizumab, and reslizumab, but reslizumab is approved only for patients at least 18 years old.
Biologics for pediatric asthma
Omalizumab, which targets IgE, is appropriate for patients at least 6 years old in whom inhaled corticosteroids could not adequately control the symptoms of moderate to-severe persistent asthma. Dosing of omalizumab is a subcutaneous injection every 2-4 weeks based on pretreatment serum IgE and body weight using a dosing table that starts at 0.016 mg/kg/IgE (IU/mL). Maximum dose is 375 mg every 2 weeks in the United States and 600 mg every 2 weeks in the European Union.
The advantages of an anti-IgE drug are its use only once a month and its substantial effect on reducing exacerbations in a clearly identified population. However, these drugs are costly and require supervised administration, Dr. Szefler noted. They also carry a risk of anaphylaxis in less than 0.2% of patients, requiring the patient to be monitored after first administration and to carry an injectable epinephrine after omalizumab administration as a precaution for late-occurring anaphylaxis.
Mepolizumab is an anti–interleukin (IL)–5 drug used in patients at least 12 years old with severe persistent asthma that’s inadequately controlled with inhaled corticosteroids. Peripheral blood counts of eosinophilia determine if a patient has an eosinophilic phenotype, which has the best response to mepolizumab. People with at least 150 cells per microliter at baseline or at least 300 cells per microliter within the past year have shown a good response to mepolizumab. Dosing is 100 mg subcutaneously every 4 weeks.
For patients with atopic asthma, mepolizumab is effective in reducing the daily oral corticosteroid dose and the number of both annual exacerbations and exacerbations requiring hospitalization or an emergency visit. Other benefits of mepolizumab include increasing the time to a first exacerbation, the pre- and postbronchodilator forced expiratory volume in one second (FEV1) and overall quality of life.
Patient reductions in exacerbations while taking mepolizumab were associated with eosinophil count but not IgE, atopic status, FEV1 or bronchodilator response in the DREAM study (Lancet. 2012 Aug 18;380[9842]:651-9.).
Two safety considerations with mepolizumab include an increased risk of shingles and the risk of a preexisting helminth infection getting worse. Providers should screen for helminth infection and might consider a herpes zoster vaccination prior to starting therapy, Dr. Szefler said.
Benralizumab is an anti-IL5Ra for use in people at least 12 years old with severe persistent asthma and an eosinophilic phenotype (at least 300 cells per microliter). Dosing begins with three subcutaneous injections of 30 mg every 4 weeks, followed by administration every 8 weeks thereafter.
Benralizumab’s clinical effects include reduced exacerbations and oral corticosteroid use, and improved asthma symptom scores and prebronchodilator FEV1. Higher serum eosinophils and a history of more frequent exacerbations are both biomarkers for reduced exacerbations with benralizumab treatment.
Dupilumab: New kid on the block
The newest biologic for asthma is dupilumab, approved Oct. 19, 2018, by the Food and Drug Administration as the only asthma biologic that patients can administer at home. Dupilumab is an anti–IL-4 and anti–IL-13 biologic whose most recent study results showed a severe exacerbations rate 50% lower than placebo (N Engl J Med. 2018 Jun 28;378[26]:2486-96.). Patients with higher baseline levels of eosinophils had the best response, although some patients showed hypereosinophilia following dupilumab therapy.
The study had a low number of adolescents enrolled, however, and more data on predictive biomarkers are needed. Dupilumab also requires a twice-monthly administration.
“It could be potentially better than those currently available due to additional effect on FEV1,” Dr. Szefler said, but cost and safety may determine how dupilumab is recommended and used, including possible use for early intervention.
As development in biologics for pediatric asthma continues to grow, questions about best practices for management remain, such as what age is best for starting biologics, what strategies are most safe and effective, and what risks and benefits exist for each strategy. Questions also remain regarding the risk factors for asthma and what early intervention strategies might change the disease’s natural history.
“Look at asthma in children as a chronic disease that can result in potentially preventable adverse respiratory outcomes in adulthood,” Dr. Szefler said. He recommended monitoring children’s lung function over time and using “measures of clinical outcomes, lung function, and biomarkers to assess potential benefits of biologic therapy.”
Dr. Szefler has served on the advisory board for Regeneron and Sanofi, and he has consulted for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Novartis, and Propeller Health.
ORLANDO – The goal of treatment is the same for all asthma cases, regardless of severity: “to enable a patient to achieve and maintain control over their asthma,” according to Stanley J. Szefler, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora.
That goal includes “reducing the risk of exacerbations, emergency department visits, hospitalizations, and progression as well as reducing impairments, including symptoms, functional limitations, poor quality of life, and other manifestations of asthma,” Dr. Szefler, also director of the Children’s Hospital of Colorado pediatric asthma research program, told colleagues at the annual meeting of the American Academy of Pediatrics.
Severe asthma challenges
These aims are more difficult with severe asthma, defined by the World Health Organization as “the current level of clinical control and risks which can result in frequent severe exacerbations and/or adverse reactions to medications and/or chronic morbidity,” Dr. Szefler explained. Severe asthma includes untreated severe asthma, difficult-to-treat asthma, and treatment-resistant severe asthma, whether controlled on high-dose medication or not.
Allergen sensitization, viral respiratory infections, and respiratory irritants (such as air pollution and smoking) are common features of severe asthma in children. Also common are challenges specific to management: poor medication adherence, poor technique for inhaled medications, and undertreatment. Poor management can lead to repeated exacerbations, adverse effects from drugs, disease progression, possible development of chronic obstructive pulmonary disease (COPD), and early mortality.
The National Heart, Lung, and Blood Institute EPR-3 guidelines for treatment of pediatric asthma recommend a stepwise approach to therapy, starting with short-acting beta2-agonists as needed (SABA p.r.n.). The clinician then assesses the patient’s symptoms, exacerbations, side effects, quality of life, and lung function to determine whether the asthma is well managed or requires inhaled corticosteroids, or another therapy in moving through the steps. Each step also involves patient education, environmental control, and management of the child’s comorbidities.
It is not until steps 5 and 6 that the guidelines advise considering the biologic omalizumab for patients who have allergies. But other biologic options exist as well. Four biologics currently approved for treating asthma include omalizumab, mepolizumab, benralizumab, and reslizumab, but reslizumab is approved only for patients at least 18 years old.
Biologics for pediatric asthma
Omalizumab, which targets IgE, is appropriate for patients at least 6 years old in whom inhaled corticosteroids could not adequately control the symptoms of moderate to-severe persistent asthma. Dosing of omalizumab is a subcutaneous injection every 2-4 weeks based on pretreatment serum IgE and body weight using a dosing table that starts at 0.016 mg/kg/IgE (IU/mL). Maximum dose is 375 mg every 2 weeks in the United States and 600 mg every 2 weeks in the European Union.
The advantages of an anti-IgE drug are its use only once a month and its substantial effect on reducing exacerbations in a clearly identified population. However, these drugs are costly and require supervised administration, Dr. Szefler noted. They also carry a risk of anaphylaxis in less than 0.2% of patients, requiring the patient to be monitored after first administration and to carry an injectable epinephrine after omalizumab administration as a precaution for late-occurring anaphylaxis.
Mepolizumab is an anti–interleukin (IL)–5 drug used in patients at least 12 years old with severe persistent asthma that’s inadequately controlled with inhaled corticosteroids. Peripheral blood counts of eosinophilia determine if a patient has an eosinophilic phenotype, which has the best response to mepolizumab. People with at least 150 cells per microliter at baseline or at least 300 cells per microliter within the past year have shown a good response to mepolizumab. Dosing is 100 mg subcutaneously every 4 weeks.
For patients with atopic asthma, mepolizumab is effective in reducing the daily oral corticosteroid dose and the number of both annual exacerbations and exacerbations requiring hospitalization or an emergency visit. Other benefits of mepolizumab include increasing the time to a first exacerbation, the pre- and postbronchodilator forced expiratory volume in one second (FEV1) and overall quality of life.
Patient reductions in exacerbations while taking mepolizumab were associated with eosinophil count but not IgE, atopic status, FEV1 or bronchodilator response in the DREAM study (Lancet. 2012 Aug 18;380[9842]:651-9.).
Two safety considerations with mepolizumab include an increased risk of shingles and the risk of a preexisting helminth infection getting worse. Providers should screen for helminth infection and might consider a herpes zoster vaccination prior to starting therapy, Dr. Szefler said.
Benralizumab is an anti-IL5Ra for use in people at least 12 years old with severe persistent asthma and an eosinophilic phenotype (at least 300 cells per microliter). Dosing begins with three subcutaneous injections of 30 mg every 4 weeks, followed by administration every 8 weeks thereafter.
Benralizumab’s clinical effects include reduced exacerbations and oral corticosteroid use, and improved asthma symptom scores and prebronchodilator FEV1. Higher serum eosinophils and a history of more frequent exacerbations are both biomarkers for reduced exacerbations with benralizumab treatment.
Dupilumab: New kid on the block
The newest biologic for asthma is dupilumab, approved Oct. 19, 2018, by the Food and Drug Administration as the only asthma biologic that patients can administer at home. Dupilumab is an anti–IL-4 and anti–IL-13 biologic whose most recent study results showed a severe exacerbations rate 50% lower than placebo (N Engl J Med. 2018 Jun 28;378[26]:2486-96.). Patients with higher baseline levels of eosinophils had the best response, although some patients showed hypereosinophilia following dupilumab therapy.
The study had a low number of adolescents enrolled, however, and more data on predictive biomarkers are needed. Dupilumab also requires a twice-monthly administration.
“It could be potentially better than those currently available due to additional effect on FEV1,” Dr. Szefler said, but cost and safety may determine how dupilumab is recommended and used, including possible use for early intervention.
As development in biologics for pediatric asthma continues to grow, questions about best practices for management remain, such as what age is best for starting biologics, what strategies are most safe and effective, and what risks and benefits exist for each strategy. Questions also remain regarding the risk factors for asthma and what early intervention strategies might change the disease’s natural history.
“Look at asthma in children as a chronic disease that can result in potentially preventable adverse respiratory outcomes in adulthood,” Dr. Szefler said. He recommended monitoring children’s lung function over time and using “measures of clinical outcomes, lung function, and biomarkers to assess potential benefits of biologic therapy.”
Dr. Szefler has served on the advisory board for Regeneron and Sanofi, and he has consulted for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Novartis, and Propeller Health.
ORLANDO – The goal of treatment is the same for all asthma cases, regardless of severity: “to enable a patient to achieve and maintain control over their asthma,” according to Stanley J. Szefler, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora.
That goal includes “reducing the risk of exacerbations, emergency department visits, hospitalizations, and progression as well as reducing impairments, including symptoms, functional limitations, poor quality of life, and other manifestations of asthma,” Dr. Szefler, also director of the Children’s Hospital of Colorado pediatric asthma research program, told colleagues at the annual meeting of the American Academy of Pediatrics.
Severe asthma challenges
These aims are more difficult with severe asthma, defined by the World Health Organization as “the current level of clinical control and risks which can result in frequent severe exacerbations and/or adverse reactions to medications and/or chronic morbidity,” Dr. Szefler explained. Severe asthma includes untreated severe asthma, difficult-to-treat asthma, and treatment-resistant severe asthma, whether controlled on high-dose medication or not.
Allergen sensitization, viral respiratory infections, and respiratory irritants (such as air pollution and smoking) are common features of severe asthma in children. Also common are challenges specific to management: poor medication adherence, poor technique for inhaled medications, and undertreatment. Poor management can lead to repeated exacerbations, adverse effects from drugs, disease progression, possible development of chronic obstructive pulmonary disease (COPD), and early mortality.
The National Heart, Lung, and Blood Institute EPR-3 guidelines for treatment of pediatric asthma recommend a stepwise approach to therapy, starting with short-acting beta2-agonists as needed (SABA p.r.n.). The clinician then assesses the patient’s symptoms, exacerbations, side effects, quality of life, and lung function to determine whether the asthma is well managed or requires inhaled corticosteroids, or another therapy in moving through the steps. Each step also involves patient education, environmental control, and management of the child’s comorbidities.
It is not until steps 5 and 6 that the guidelines advise considering the biologic omalizumab for patients who have allergies. But other biologic options exist as well. Four biologics currently approved for treating asthma include omalizumab, mepolizumab, benralizumab, and reslizumab, but reslizumab is approved only for patients at least 18 years old.
Biologics for pediatric asthma
Omalizumab, which targets IgE, is appropriate for patients at least 6 years old in whom inhaled corticosteroids could not adequately control the symptoms of moderate to-severe persistent asthma. Dosing of omalizumab is a subcutaneous injection every 2-4 weeks based on pretreatment serum IgE and body weight using a dosing table that starts at 0.016 mg/kg/IgE (IU/mL). Maximum dose is 375 mg every 2 weeks in the United States and 600 mg every 2 weeks in the European Union.
The advantages of an anti-IgE drug are its use only once a month and its substantial effect on reducing exacerbations in a clearly identified population. However, these drugs are costly and require supervised administration, Dr. Szefler noted. They also carry a risk of anaphylaxis in less than 0.2% of patients, requiring the patient to be monitored after first administration and to carry an injectable epinephrine after omalizumab administration as a precaution for late-occurring anaphylaxis.
Mepolizumab is an anti–interleukin (IL)–5 drug used in patients at least 12 years old with severe persistent asthma that’s inadequately controlled with inhaled corticosteroids. Peripheral blood counts of eosinophilia determine if a patient has an eosinophilic phenotype, which has the best response to mepolizumab. People with at least 150 cells per microliter at baseline or at least 300 cells per microliter within the past year have shown a good response to mepolizumab. Dosing is 100 mg subcutaneously every 4 weeks.
For patients with atopic asthma, mepolizumab is effective in reducing the daily oral corticosteroid dose and the number of both annual exacerbations and exacerbations requiring hospitalization or an emergency visit. Other benefits of mepolizumab include increasing the time to a first exacerbation, the pre- and postbronchodilator forced expiratory volume in one second (FEV1) and overall quality of life.
Patient reductions in exacerbations while taking mepolizumab were associated with eosinophil count but not IgE, atopic status, FEV1 or bronchodilator response in the DREAM study (Lancet. 2012 Aug 18;380[9842]:651-9.).
Two safety considerations with mepolizumab include an increased risk of shingles and the risk of a preexisting helminth infection getting worse. Providers should screen for helminth infection and might consider a herpes zoster vaccination prior to starting therapy, Dr. Szefler said.
Benralizumab is an anti-IL5Ra for use in people at least 12 years old with severe persistent asthma and an eosinophilic phenotype (at least 300 cells per microliter). Dosing begins with three subcutaneous injections of 30 mg every 4 weeks, followed by administration every 8 weeks thereafter.
Benralizumab’s clinical effects include reduced exacerbations and oral corticosteroid use, and improved asthma symptom scores and prebronchodilator FEV1. Higher serum eosinophils and a history of more frequent exacerbations are both biomarkers for reduced exacerbations with benralizumab treatment.
Dupilumab: New kid on the block
The newest biologic for asthma is dupilumab, approved Oct. 19, 2018, by the Food and Drug Administration as the only asthma biologic that patients can administer at home. Dupilumab is an anti–IL-4 and anti–IL-13 biologic whose most recent study results showed a severe exacerbations rate 50% lower than placebo (N Engl J Med. 2018 Jun 28;378[26]:2486-96.). Patients with higher baseline levels of eosinophils had the best response, although some patients showed hypereosinophilia following dupilumab therapy.
The study had a low number of adolescents enrolled, however, and more data on predictive biomarkers are needed. Dupilumab also requires a twice-monthly administration.
“It could be potentially better than those currently available due to additional effect on FEV1,” Dr. Szefler said, but cost and safety may determine how dupilumab is recommended and used, including possible use for early intervention.
As development in biologics for pediatric asthma continues to grow, questions about best practices for management remain, such as what age is best for starting biologics, what strategies are most safe and effective, and what risks and benefits exist for each strategy. Questions also remain regarding the risk factors for asthma and what early intervention strategies might change the disease’s natural history.
“Look at asthma in children as a chronic disease that can result in potentially preventable adverse respiratory outcomes in adulthood,” Dr. Szefler said. He recommended monitoring children’s lung function over time and using “measures of clinical outcomes, lung function, and biomarkers to assess potential benefits of biologic therapy.”
Dr. Szefler has served on the advisory board for Regeneron and Sanofi, and he has consulted for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Novartis, and Propeller Health.
EXPERT ANALYSIS FROM AAP 18
Meaningful endometriosis treatment requires a holistic approach and an understanding of chronic pain
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Although it has been more than 100 years since endometriosis was first described in the literature, deciphering the mechanisms that cause pain in women with this enigmatic disease is an ongoing pursuit.
Pain is the most debilitating symptom of endometriosis.1,2 In many cases, it has a profoundly negative impact on a patient’s quality of life, and contributes significantly to disease burden, as well as to personal and societal costs from lost productivity.3,4 Women with endometriosis often experience chronic pelvic pain, deep dyspareunia, dysmenorrhea, and subfertility.5 The majority of women with the disease also have one or more comorbidities, including adenomyosis, adhesive disease, and other pelvic pain conditions such as interstitial cystitis, irritable bowel disease, inflammatory bowel disease, and pelvic floor myalgia.6-8
Recent studies have yielded new insights into the development of endometriosis-associated pelvic pain. The role of peritoneal inflammation, de novo innervation of endometriosis implants, and changes in the central nervous system are becoming increasingly clear.5,9,10 These discoveries have important treatment implications.
In this article, Andrea J. Rapkin, MD, Professor of Obstetrics and Gynecology at the University of California, Los Angeles, and Founder and Director of the UCLA Pelvic Pain Center, offers her expert opinion on the findings of key studies and their clinical implications, including the importance of a multidisciplinary treatment approach that focuses on the whole patient.
Q What mechanisms underlie the chronic pain that many women with endometriosis feel?
Although pain is the primary symptom experienced by women with endometriosis, the disease burden and symptom severity do not often correlate.11,12 “This was the first conundrum presented to clinicians,” noted Dr. Rapkin. “In fact, we do not know the true prevalence of endometriosis because women with endometriosis only come to diagnosis either based on pain or infertility. When infertility is the problem, very often we are surprised by how much disease is present in an individual with either no pain or minimal pain. Conversely, in other individuals with very severe pain, upon laparoscopic surgery, have minimal or mild endometriosis.”
Efforts to solve this clinical puzzle began decades ago. “Dr. Michael Vernon discovered that the small, red, endometriosis implants that looked like petechial hemorrhages produced more prostaglandin E2 (PGE2) in vitro than the older black-brown lesions. PGE2 is a pain-producing (algesic) chemical produced after cytokines stimulation,” said Dr. Rapkin. “This was the first evidence that, yes, there is a reason for pain in many individuals with lower-stage disease.”
“Prostaglandins are known to be a major cause of dysmenorrhea. Prostaglandins induce uterine cramping, sensitize nerve endings, and promote other inflammatory factors responsible for attracting monocytes that become macrophages, further contributing to inflammation,” Dr. Rapkin continued. “PGE2 also stimulates the enzyme aromatase, which allows androgens to be converted to estrogen, which promotes growth of endometriotic lesions. This is a self-feeding aspect of endometriosis.”
Continue to: These discoveries were followed by the realization that deeply infiltrating endometriosis...
These discoveries were followed by the realization that deeply infiltrating endometriosis (defined by disease infiltration of more than 5 mm, often in the uterosacral ligaments) was more likely to be painful than superficial disease, said Dr. Rapkin. “In some women with endometriosis, the disease we see laparoscopically is really the tip of the iceberg.”
In 2005, landmark studies performed by Karen J. Berkley, PhD, were summarized in a paper coauthored by Dr. Berkley, Dr. Rapkin, and Raymond E. Papka, PhD.13 “In a rodent model where endometriosis was developed by suturing pieces of endometrium in the mesentery, the endometriosis implants developed a vascular supply and a nerve supply. These nerves were not just functioning to govern the dilation and contraction of the blood vessels (in other words the sympathetic type nerves), but these nerves stained for neurotransmitters associated with pain (algesic agents, such as substance P and CGRP),” said Dr. Rapkin. “At UCLA, we acquired tissue from women with endometriosis and analyzed in Dr. Papka’s lab. Those tissues also showed nerves staining for pain-producing chemicals.” Other studies performed worldwide also demonstrated nerve endings with neurotrophic and algesic chemicals in endometriotic tissues. In addition to prostaglandins and cytokines, increased expression of various neuropeptides, neurotrophins, and alterations in ion channels contribute to hypersensitivity and pain.
Q What other chronic pain conditions might women with endometriosis experience?
Overlapping chronic pain conditions are common in women with endometriosis. “There is a very high co-occurrence of interstitial cystitis/painful bladder syndrome,” said Dr. Rapkin. “Irritable bowel syndrome is more common in women with endometriosis, as is vulvodynia. Fibromyalgia, migraine headache, temporo-mandibular joint pain (TMJ), anxiety, and depression also commonly co-occur in women with endometriosis.”
“Two concepts may be relevant to why these overlapping pain conditions develop,” Dr. Rapkin continued. “First, visceral sensitization: If one organ or tissue is inflamed and becomes hyperalgesic then other organs in the adjacent region with shared thoracolumbar and sacral innervation can become sensitized through shared cell bodies in the spinal cord, cross-sensitization in the cord, or at higher regions of the CNS. In addition, visceral somatic conversion occurs, whereby somatic tissues such as muscles and subcutaneous tissues with the same nerve supply as the affected organs become sensitized. This process may explain why abdominal wall and pelvic floor muscles become painful. The involvement of surrounding musculature is an important contributor to the pain in many women with endometriosis.”
“Finally, genetic studies of alterations in genes that encode for chemicals affecting the sensitivity and perception of pain are shedding light on the development of chronic pain. Ultimately these studies will advance our understanding of pain related to endometriosis.”
Continue to: Q How have new understandings about the pain mechanisms...
Q How have new understandings about the pain mechanisms involved with endometriosis-caused pelvic pain improved treatment?
According to Dr. Rapkin, the increased understanding of the mechanisms involved in endometriosis-associated pain gained from these key studies led to a paradigm shift, with endometriosis being viewed not just as a condition with mechanical hypersensitivity due to altered anatomy and inflammation but also as a neurologic condition, or a nerve pain condition with peripheral and central sensitization. “This means there is upregulation or hyperactivity both in the periphery (in the pelvis) and centrally (in the spinal cord and brain),” said Dr. Rapkin.
“In the periphery, the endometriotic lesions develop an afferent sensory innervation and communicate with the brain. Stimulation of these nerves by the inflammatory milieu contributes to pain.” Dr. Rapkin noted research by Maria Adele Giamberardino, which demonstrated that women with endometriosis and pain have a lower threshold for feeling pain in the tissues overlying the pelvis (the abdominal wall and back).14 This also has been shown by Dr. Berkley in rodents given endometriosis.
“The muscles develop trigger points and tender hyperalgesic points as part of the sensitization process. In addition, distant sensitization develops—women with pelvic pain and endometriosis have a lower threshold for sensing experimental pain in areas outside the pelvis, for example the back, leg, or shoulder. These discoveries clearly reflect up regulation for pain processing in the central nervous system.”
Dr. Rapkin also pointed to research published in 2016 by Sawson As-Sanie, MD, MPH, that showed an association between endometriosis-associated pelvic pain and altered brain chemistry and function.16 “Dr. As-Sanie demonstrated a decrease in gray matter volume in key neural pain processing areas in the brain in women with pain with endometriosis. This was not found in women with endometriosis who did not have pain,” she said. “Altered connectivity in brain areas related to perception and inhibition of pain is important in maintaining pain. Dr. As-Sanie’s studies also found that these changes are correlated with anxiety, depression, and pain intensity in patients with endometriosis and chronic pain.”
Continue to: Q What are some newer treatment approaches to chronic pain with endometriosis?
Q What are some newer treatment approaches to chronic pain with endometriosis?
“Multidisciplinary approaches to endometriosis-related pain are important,” said Dr. Rapkin. “Although it is important to excise or cauterize endometriosis lesions, or debulk as much as can safely be removed during laparoscopic surgery, it is now standard of care that medical therapy, not surgery, is the first approach to treatment. Endometriosis is a chronic condition. Inflammatory factors will continue to proliferate in patients who menstruate and produce high levels of estrogen with ovulation. The goal of medical therapy is to decrease the levels of estrogen that contribute to maintenance and proliferation of the implants. We want to suppress estrogen in a way that is compatible with long-term quality of life for our patients. Wiping out estrogen and placing patients into a chemical or surgical menopause for most of their reproductive years is not desirable.”
Approaches to hormonally modulate endometriosis include combined hormonal contraceptives and progestin-only medications, such as the levonogestrol-containing IUD, progestin-containing contraceptive implants, injections, or tablets. Second-line medical therapy consists of gonadotropin-releasing hormone agonists and antagonists that can be used for 6 months to 2 years and allows for further lowering of estrogen levels. These may not provide sufficient pain relief for some patients. “There is some evidence from Dr. Giamberadino’s studies that after women with dysmenorrhea were treated with oral contraceptives, the abdominal wall hyperalgesia decreased,” said Dr. Rapkin. “The question is, why don’t we see this in all patients? We come to the realization that endometriosis has to be treated as a neurologically mediated disorder. We have to treat the peripheral and central sensitization in a multidisciplinary way.”
A holistic approach to endometriosis is a new and exciting area for the field, said Dr. Rapkin. “We have to treat ‘bottom-up’, and ‘top-down.’ Bottom-up means we are addressing the peripheral factors that contribute to pain: endometriotic lesions, other pelvic organ pain, myofascial pain, trigger points, the tender points, and the muscle dysfunction in the abdominal wall, the back, and the pelvic floor. Pelvic floor physical therapists help women with pain and endometriosis. Often, women with endometriosis have myofascial pain and pain related to the other comorbid pain conditions they may have developed. Peripheral nerve blocks and medications used for neuropathic pain that alter nerve firing can be helpful in many situations. Pain can be augmented by cognitions and beliefs about pain, and by anxiety and depression. So the top-down approach addresses the cognitions, depression, and anxiety. We do not consider endometriosis a psychosomatic condition, but we know that if you do not address the central upregulation, including anxiety and depression, we may not get anywhere.”
“Interestingly, neurotransmitters and brain regions governing mood contribute to nerve pain. Medications such as tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants, and calcium channel blocking agents may prove fruitful. Cognitive behavioral therapy is another approach—to stimulate the prefrontal cortex, the area that is involved in pain inhibition, and other areas of the brain that may produce endogenous opioids to help with inhibiting pain. Bringing in complementary approaches is very important—for example, mindfulness-based meditation or yoga. There is growing evidence for acupuncture as well. Physical therapists, pain psychologists, anesthesiologists, or gynecologists who are facile with nerve blocks, to help tone down hyperalgesic tissues, in addition to medical and surgical therapy, have the possibility of really improving the lives of women with endometriosis.”
Q What key pearls would you like to share with readers?
“It is important to evaluate the entire individual,” she said. “Do not just viscerally focus on the uterus, the ovaries, fallopian tubes, and the peritoneum; investigate the adjacent organs and somatic tissues. Think about the abdominal wall, think about the pelvic floor. Learn how to evaluate these structures. There are simple evaluation techniques that gynecologists can learn and should include with every patient with pelvic pain, whether or not they are suspected of having endometriosis. You also want to get a complete history to determine if there are other co-occurring pain conditions. If there are, it is already a sign that there may be central sensitization.”
“Very often, it is necessary to bring in a pain psychologist—not because the disease is psychosomatic but because therapy can help the patient to learn how to use their brain to erase pain memory, and of course to address the concomitant anxiety, depression, and social isolation that happens with pain.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
- Olive DL, Lindheim SR, Pritts EA. New medical treatments for endometriosis. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):319-328.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447): 1789-1799.
- Nnoaham KE, Hummelshoj L, Webster P, et al; World Endometriosis Research Foundation Global Study of Women’s Health consortium. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
- Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299.
- Bruner-Tran KL, Mokshagundam S, Herington JL. Rodent models of experimental endometriosis: identifying mechanisms of disease and therapeutic targets. Curr Womens Health Rev. 2018;14(2):173-188.
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164-185.
- Tirlapur SA, Kuhrt K, Chaliha C. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233-237.
- Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: a review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol. 2018 Feb 15. pii: S1521-6934(18)30032-4. doi: 10.1016/j.bpobgyn.2018.01.014. [Epub ahead of print]
- Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14-24.
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
- Fedele L, Parazzini F, Bianchi S. Stage and localization of pelvic endometriosis and pain. Fertil Steril. 1990;53(1):155-158.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587-1589.
- Giamberardino MA, Tana C, Costantini R. Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol. 2014;26(4):253-259.
- Giamberardino MA, Berkley KJ, Affaitati G. Influence of endometriosis on pain behaviors and muscle hyperalgesia induced by a ureteral calculosis in female rats. Pain. 2002;95(3):247-257.
- As-Sanie S, Kim J, Schmidt-Wilcke T. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1-13.
Coding and reimbursement 101: How to maximize your payments
While reimbursement for ObGyn services seemingly should be a simple matter of putting codes on a claim form, the reality is that it is complex, and it requires a team approach to accomplish timely filing to receive fair and accurate reimbursement.
Reimbursement occurs over the length of the revenue cycle for a patient encounter and involves many steps. It starts when the patient makes an appointment for services and ends when the practice receives payment. Along the way, there must be good clinician documentation and sound knowledge about the billing process (including the Current Procedural Terminology [CPT] or Healthcare Common Procedure Coding System [HCPCS] codes for services), the International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes that establish medical necessity, the modifiers that alter the meaning of the codes, and, of course, the bundling issues that now accompany many coding situations.
In addition, ObGyn practices must contend with a multitude of payers—from federal to commercial—and must understand and adhere to each payer’s rules and policies to maximize and retain reimbursement.
In this article, I detail stumbling blocks to maximizing reimbursement and how to avoid them.
Coding considerations for office services
Good documentation before, during, and after a patient’s office visit is essential, along with accurate codes, modifiers, and order of services on the claims you submit.
Prep paperwork before the patient encounter
Once a patient makes an appointment, the front-end staff can handle some of the tasks in the cycle. This includes ensuring that the patient’s insurance coverage information is current, informing the patient of any additional information to bring at the time of the visit (such as a patient history form for a new patient visit or a list of current prescriptions), or, if an established patient will be having a procedure, making sure that prior authorization is complete. This streamlines the process, assists the clinician with documentation housekeeping, and ensures that incorrect or missing information does not cause a claim to be denied or not be filed in a timely manner (many payers require submission of an initial claim 30 days from the date of service).
Continue to: Document details of the clinician-patient interaction
Document details of the clinician-patient interaction
At the time of the encounter, you are responsible for documenting your contact with the patient in enough detail to support billing a CPT evaluation and management (E/M) code at the level selected and/or any procedures or other services performed. The TABLE provides an overview of the requirements for each level of office service.
If both an E/M and a procedure are performed on the same date of service, the E/M must be documented to show it was separate from the procedure and that the work was significantly more than would be required to accomplish the procedure. Documentation of the procedure should include the indication, steps performed, findings, the patient’s condition afterward, and instructions for aftercare or follow-up.
If you use an electronic health record for reporting, you may be the one responsible for selecting both the CPT code for services performed and an ICD-10-CM code(s) to establish the medical need for them. Select the most accurate CPT codes, and clearly link them to a supporting diagnosis for each service that will be billed. If more than one diagnosis is applicable, the first one linked to any given service should represent the most important justification, as not all payers will accept more than one diagnosis code on the claim per service billed.
If the billing staff is assigned the task of selecting the CPT and/or ICD-10-CM diagnostic codes based on your documentation, they should be well versed in the services, procedures, and diagnoses reported for their ObGyn practice.
The actual code selection may end up being a joint venture between the clinician and the staff to ensure that accurate information will be entered on the claim. Good and frequent clinician-staff communication on billing of services can transform average reimbursement into maximized reimbursement.
Be aware of bundles
Sometimes more than one service or procedure is listed on a claim on the same date of service. However, it is important to identify all potential bundles before billing to ensure correct payment. For instance, payers like to bundle an E/M service and a procedure, or you may be in the global period (defined below) of a surgery but need to report an unrelated service.
You and your staff must work together to ensure the claim is submitted with the correct modifiers; on the other hand, you may decide that a better method of coding is in order. Some payers, for example, will not reimburse both an insertion and a removal of an intrauterine device (IUD) on the same date of service. If that does happen, a modifier on the removal code might save the day, rather than billing 2 codes.
Continue to: Manage the modifiers
Manage the modifiers
Sometimes the code billed requires a modifier to ensure payment. Typical modifiers used in an ObGyn office setting include the following:
- 22, Increased procedural services (the clinician must assign a fee that is higher than the usual fee for the procedure and be able to document CPT equivalents to the work involved)
- 24, Unrelated E/M during the postoperative period (note that this modifier does not apply during the antepartum period for pregnancy)
- 25, Significant and separate E/M on the same date as another service or minor procedure
- 52, Reduced services (generally, the payer will expect an explanation of the reduced service and will determine payment accordingly)
- 57, Decision to perform major surgery the day of or the day before the surgery
- 59, Distinct procedural service (used when 2 procedures are bundled and a modifier is allowed). Note that payment reductions for multiple procedures will still apply.
- 79, Unrelated procedure during the postoperative period (usually paid at the full allowable).
Organize the order of services on the claim
For an outpatient claim that includes both an E/M service and procedures, the order of the services—not the order in which they were performed—may be important to obtaining maximum reimbursement. In general, payers will pay in full for a supported E/M service no matter where it appears on the claim, but they apply reductions only for multiple procedures.
For instance, if you insert levonorgestrel implants on the same date as you remove a large polyp from the cervix, you would want to report the code with the highest relative value unit (RVU) first. In this case, it would be 11981 (4.05 RVUs), 57500 (3.61 RVUs).
In the IUD case mentioned earlier (removal and insertion of IUDs on the same date), the order of the codes, assuming the payer reimburses for both, will be even more important since removal usually has a higher payment: 58301 (2.70 RUVs), 58300 (1.54 RVUs).
Coding considerations for surgical services
Surgical services performed in a hospital or ambulatory surgical center present another set of must-dos to ensure timely and fair reimbursement.
Grasp the ‘global package’ concept
Understanding this concept can be crucial to getting paid for additional services during this time period and correct billing for any E/M services performed prior to surgery. In general, the routine history and physical examination performed prior to a major surgery is considered included in the work and should not be billed separately. Surgical clearance for a patient’s condition, such as hypertension, a heart condition, or lung issues, can be billed separately, but these generally are performed by someone other than the operating surgeon.
Procedures performed in the hospital setting generally will have a 10- or 90-day global period. During this time, any related E/M service should not be billed separately, and the use of modifiers becomes even more important than with office services.
Applicable modifiers for use with hospital surgery can include all those for outpatient services plus:
- 50, Bilateral procedure (for which you may be paid up to 150% of the allowable)
- 58, Staged or related procedure during the postoperative period (this may be paid at the full allowable)
- 62, Co-surgeons (both surgeons bill the same CPT code and both document their involvement in the surgery). Medicare will reimburse each surgeon 62.5% of the allowable.
- 78, Return to the operating room for an unplanned related procedure (the full allowable may be reduced by some payers owing to their belief that this is soon after the original procedure so intraoperative time only is considered).
Be savvy about surgical bundles
Here, it is important to understand all published bundling edits for multiple procedures performed by the same surgeon at the same surgical session. If a code combination is never allowed but the surgery is more intense due to additional work required, a modifier -22 may be your only option. Again, clear, concise documentation of the additional work is imperative to receive the additional payment.
When a modifier is allowed, it generally will be one that denotes a procedure done on bilateral organs (such as the ovaries) when there is no extensive code to cover all of the work or when the additional procedure is “distinct” and meets the criteria for using a modifier 59.
Medicare has expanded the modifier -59 into additional modifiers to further explain the situation. These additional modifiers are:
- XE, A service that is distinct because it occurred during a separate encounter on the same date of service
- XS, A service that is distinct because it was performed on a separate organ/structure
- XP, A service that is distinct because it was performed by a different practitioner
- XU, The use of a service that is distinct because it does not overlap usual components of the main service.
Standards of care: Some steps are inherent to the surgery
Expect to receive claim denials if you bill separately for adhesiolysis during a surgical procedure. Every payer considers this procedure related to access to the surgical site and will deny separate coding. If the lysis was truly significant in terms of work, try reporting the modifier 22 and provide adequate documentation.
Other procedures at the time of surgery that generally are not paid for include 1) examination under anesthesia, 2) any procedure done to check the surgeon’s work (for example, cystoscopy, especially when done after urinary or pelvic reconstruction procedures, or chromotubation following extensive ovariolysis), 3) placement of catheters, and 4) placement of devices to alleviate postsurgical pain.
Bottom line
Maximizing reimbursement involves good documentation, correct CPT codes linked to specific and accurate medical indications, the use of appropriate modifiers, and listing codes in order of their relative values from highest to lowest.
Should a denial or unfair reduction in payment come your way, analyze the rejection to determine the cause and make billing and reporting changes as needed to improve your future reimbursements.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
While reimbursement for ObGyn services seemingly should be a simple matter of putting codes on a claim form, the reality is that it is complex, and it requires a team approach to accomplish timely filing to receive fair and accurate reimbursement.
Reimbursement occurs over the length of the revenue cycle for a patient encounter and involves many steps. It starts when the patient makes an appointment for services and ends when the practice receives payment. Along the way, there must be good clinician documentation and sound knowledge about the billing process (including the Current Procedural Terminology [CPT] or Healthcare Common Procedure Coding System [HCPCS] codes for services), the International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes that establish medical necessity, the modifiers that alter the meaning of the codes, and, of course, the bundling issues that now accompany many coding situations.
In addition, ObGyn practices must contend with a multitude of payers—from federal to commercial—and must understand and adhere to each payer’s rules and policies to maximize and retain reimbursement.
In this article, I detail stumbling blocks to maximizing reimbursement and how to avoid them.
Coding considerations for office services
Good documentation before, during, and after a patient’s office visit is essential, along with accurate codes, modifiers, and order of services on the claims you submit.
Prep paperwork before the patient encounter
Once a patient makes an appointment, the front-end staff can handle some of the tasks in the cycle. This includes ensuring that the patient’s insurance coverage information is current, informing the patient of any additional information to bring at the time of the visit (such as a patient history form for a new patient visit or a list of current prescriptions), or, if an established patient will be having a procedure, making sure that prior authorization is complete. This streamlines the process, assists the clinician with documentation housekeeping, and ensures that incorrect or missing information does not cause a claim to be denied or not be filed in a timely manner (many payers require submission of an initial claim 30 days from the date of service).
Continue to: Document details of the clinician-patient interaction
Document details of the clinician-patient interaction
At the time of the encounter, you are responsible for documenting your contact with the patient in enough detail to support billing a CPT evaluation and management (E/M) code at the level selected and/or any procedures or other services performed. The TABLE provides an overview of the requirements for each level of office service.
If both an E/M and a procedure are performed on the same date of service, the E/M must be documented to show it was separate from the procedure and that the work was significantly more than would be required to accomplish the procedure. Documentation of the procedure should include the indication, steps performed, findings, the patient’s condition afterward, and instructions for aftercare or follow-up.
If you use an electronic health record for reporting, you may be the one responsible for selecting both the CPT code for services performed and an ICD-10-CM code(s) to establish the medical need for them. Select the most accurate CPT codes, and clearly link them to a supporting diagnosis for each service that will be billed. If more than one diagnosis is applicable, the first one linked to any given service should represent the most important justification, as not all payers will accept more than one diagnosis code on the claim per service billed.
If the billing staff is assigned the task of selecting the CPT and/or ICD-10-CM diagnostic codes based on your documentation, they should be well versed in the services, procedures, and diagnoses reported for their ObGyn practice.
The actual code selection may end up being a joint venture between the clinician and the staff to ensure that accurate information will be entered on the claim. Good and frequent clinician-staff communication on billing of services can transform average reimbursement into maximized reimbursement.
Be aware of bundles
Sometimes more than one service or procedure is listed on a claim on the same date of service. However, it is important to identify all potential bundles before billing to ensure correct payment. For instance, payers like to bundle an E/M service and a procedure, or you may be in the global period (defined below) of a surgery but need to report an unrelated service.
You and your staff must work together to ensure the claim is submitted with the correct modifiers; on the other hand, you may decide that a better method of coding is in order. Some payers, for example, will not reimburse both an insertion and a removal of an intrauterine device (IUD) on the same date of service. If that does happen, a modifier on the removal code might save the day, rather than billing 2 codes.
Continue to: Manage the modifiers
Manage the modifiers
Sometimes the code billed requires a modifier to ensure payment. Typical modifiers used in an ObGyn office setting include the following:
- 22, Increased procedural services (the clinician must assign a fee that is higher than the usual fee for the procedure and be able to document CPT equivalents to the work involved)
- 24, Unrelated E/M during the postoperative period (note that this modifier does not apply during the antepartum period for pregnancy)
- 25, Significant and separate E/M on the same date as another service or minor procedure
- 52, Reduced services (generally, the payer will expect an explanation of the reduced service and will determine payment accordingly)
- 57, Decision to perform major surgery the day of or the day before the surgery
- 59, Distinct procedural service (used when 2 procedures are bundled and a modifier is allowed). Note that payment reductions for multiple procedures will still apply.
- 79, Unrelated procedure during the postoperative period (usually paid at the full allowable).
Organize the order of services on the claim
For an outpatient claim that includes both an E/M service and procedures, the order of the services—not the order in which they were performed—may be important to obtaining maximum reimbursement. In general, payers will pay in full for a supported E/M service no matter where it appears on the claim, but they apply reductions only for multiple procedures.
For instance, if you insert levonorgestrel implants on the same date as you remove a large polyp from the cervix, you would want to report the code with the highest relative value unit (RVU) first. In this case, it would be 11981 (4.05 RVUs), 57500 (3.61 RVUs).
In the IUD case mentioned earlier (removal and insertion of IUDs on the same date), the order of the codes, assuming the payer reimburses for both, will be even more important since removal usually has a higher payment: 58301 (2.70 RUVs), 58300 (1.54 RVUs).
Coding considerations for surgical services
Surgical services performed in a hospital or ambulatory surgical center present another set of must-dos to ensure timely and fair reimbursement.
Grasp the ‘global package’ concept
Understanding this concept can be crucial to getting paid for additional services during this time period and correct billing for any E/M services performed prior to surgery. In general, the routine history and physical examination performed prior to a major surgery is considered included in the work and should not be billed separately. Surgical clearance for a patient’s condition, such as hypertension, a heart condition, or lung issues, can be billed separately, but these generally are performed by someone other than the operating surgeon.
Procedures performed in the hospital setting generally will have a 10- or 90-day global period. During this time, any related E/M service should not be billed separately, and the use of modifiers becomes even more important than with office services.
Applicable modifiers for use with hospital surgery can include all those for outpatient services plus:
- 50, Bilateral procedure (for which you may be paid up to 150% of the allowable)
- 58, Staged or related procedure during the postoperative period (this may be paid at the full allowable)
- 62, Co-surgeons (both surgeons bill the same CPT code and both document their involvement in the surgery). Medicare will reimburse each surgeon 62.5% of the allowable.
- 78, Return to the operating room for an unplanned related procedure (the full allowable may be reduced by some payers owing to their belief that this is soon after the original procedure so intraoperative time only is considered).
Be savvy about surgical bundles
Here, it is important to understand all published bundling edits for multiple procedures performed by the same surgeon at the same surgical session. If a code combination is never allowed but the surgery is more intense due to additional work required, a modifier -22 may be your only option. Again, clear, concise documentation of the additional work is imperative to receive the additional payment.
When a modifier is allowed, it generally will be one that denotes a procedure done on bilateral organs (such as the ovaries) when there is no extensive code to cover all of the work or when the additional procedure is “distinct” and meets the criteria for using a modifier 59.
Medicare has expanded the modifier -59 into additional modifiers to further explain the situation. These additional modifiers are:
- XE, A service that is distinct because it occurred during a separate encounter on the same date of service
- XS, A service that is distinct because it was performed on a separate organ/structure
- XP, A service that is distinct because it was performed by a different practitioner
- XU, The use of a service that is distinct because it does not overlap usual components of the main service.
Standards of care: Some steps are inherent to the surgery
Expect to receive claim denials if you bill separately for adhesiolysis during a surgical procedure. Every payer considers this procedure related to access to the surgical site and will deny separate coding. If the lysis was truly significant in terms of work, try reporting the modifier 22 and provide adequate documentation.
Other procedures at the time of surgery that generally are not paid for include 1) examination under anesthesia, 2) any procedure done to check the surgeon’s work (for example, cystoscopy, especially when done after urinary or pelvic reconstruction procedures, or chromotubation following extensive ovariolysis), 3) placement of catheters, and 4) placement of devices to alleviate postsurgical pain.
Bottom line
Maximizing reimbursement involves good documentation, correct CPT codes linked to specific and accurate medical indications, the use of appropriate modifiers, and listing codes in order of their relative values from highest to lowest.
Should a denial or unfair reduction in payment come your way, analyze the rejection to determine the cause and make billing and reporting changes as needed to improve your future reimbursements.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
While reimbursement for ObGyn services seemingly should be a simple matter of putting codes on a claim form, the reality is that it is complex, and it requires a team approach to accomplish timely filing to receive fair and accurate reimbursement.
Reimbursement occurs over the length of the revenue cycle for a patient encounter and involves many steps. It starts when the patient makes an appointment for services and ends when the practice receives payment. Along the way, there must be good clinician documentation and sound knowledge about the billing process (including the Current Procedural Terminology [CPT] or Healthcare Common Procedure Coding System [HCPCS] codes for services), the International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes that establish medical necessity, the modifiers that alter the meaning of the codes, and, of course, the bundling issues that now accompany many coding situations.
In addition, ObGyn practices must contend with a multitude of payers—from federal to commercial—and must understand and adhere to each payer’s rules and policies to maximize and retain reimbursement.
In this article, I detail stumbling blocks to maximizing reimbursement and how to avoid them.
Coding considerations for office services
Good documentation before, during, and after a patient’s office visit is essential, along with accurate codes, modifiers, and order of services on the claims you submit.
Prep paperwork before the patient encounter
Once a patient makes an appointment, the front-end staff can handle some of the tasks in the cycle. This includes ensuring that the patient’s insurance coverage information is current, informing the patient of any additional information to bring at the time of the visit (such as a patient history form for a new patient visit or a list of current prescriptions), or, if an established patient will be having a procedure, making sure that prior authorization is complete. This streamlines the process, assists the clinician with documentation housekeeping, and ensures that incorrect or missing information does not cause a claim to be denied or not be filed in a timely manner (many payers require submission of an initial claim 30 days from the date of service).
Continue to: Document details of the clinician-patient interaction
Document details of the clinician-patient interaction
At the time of the encounter, you are responsible for documenting your contact with the patient in enough detail to support billing a CPT evaluation and management (E/M) code at the level selected and/or any procedures or other services performed. The TABLE provides an overview of the requirements for each level of office service.
If both an E/M and a procedure are performed on the same date of service, the E/M must be documented to show it was separate from the procedure and that the work was significantly more than would be required to accomplish the procedure. Documentation of the procedure should include the indication, steps performed, findings, the patient’s condition afterward, and instructions for aftercare or follow-up.
If you use an electronic health record for reporting, you may be the one responsible for selecting both the CPT code for services performed and an ICD-10-CM code(s) to establish the medical need for them. Select the most accurate CPT codes, and clearly link them to a supporting diagnosis for each service that will be billed. If more than one diagnosis is applicable, the first one linked to any given service should represent the most important justification, as not all payers will accept more than one diagnosis code on the claim per service billed.
If the billing staff is assigned the task of selecting the CPT and/or ICD-10-CM diagnostic codes based on your documentation, they should be well versed in the services, procedures, and diagnoses reported for their ObGyn practice.
The actual code selection may end up being a joint venture between the clinician and the staff to ensure that accurate information will be entered on the claim. Good and frequent clinician-staff communication on billing of services can transform average reimbursement into maximized reimbursement.
Be aware of bundles
Sometimes more than one service or procedure is listed on a claim on the same date of service. However, it is important to identify all potential bundles before billing to ensure correct payment. For instance, payers like to bundle an E/M service and a procedure, or you may be in the global period (defined below) of a surgery but need to report an unrelated service.
You and your staff must work together to ensure the claim is submitted with the correct modifiers; on the other hand, you may decide that a better method of coding is in order. Some payers, for example, will not reimburse both an insertion and a removal of an intrauterine device (IUD) on the same date of service. If that does happen, a modifier on the removal code might save the day, rather than billing 2 codes.
Continue to: Manage the modifiers
Manage the modifiers
Sometimes the code billed requires a modifier to ensure payment. Typical modifiers used in an ObGyn office setting include the following:
- 22, Increased procedural services (the clinician must assign a fee that is higher than the usual fee for the procedure and be able to document CPT equivalents to the work involved)
- 24, Unrelated E/M during the postoperative period (note that this modifier does not apply during the antepartum period for pregnancy)
- 25, Significant and separate E/M on the same date as another service or minor procedure
- 52, Reduced services (generally, the payer will expect an explanation of the reduced service and will determine payment accordingly)
- 57, Decision to perform major surgery the day of or the day before the surgery
- 59, Distinct procedural service (used when 2 procedures are bundled and a modifier is allowed). Note that payment reductions for multiple procedures will still apply.
- 79, Unrelated procedure during the postoperative period (usually paid at the full allowable).
Organize the order of services on the claim
For an outpatient claim that includes both an E/M service and procedures, the order of the services—not the order in which they were performed—may be important to obtaining maximum reimbursement. In general, payers will pay in full for a supported E/M service no matter where it appears on the claim, but they apply reductions only for multiple procedures.
For instance, if you insert levonorgestrel implants on the same date as you remove a large polyp from the cervix, you would want to report the code with the highest relative value unit (RVU) first. In this case, it would be 11981 (4.05 RVUs), 57500 (3.61 RVUs).
In the IUD case mentioned earlier (removal and insertion of IUDs on the same date), the order of the codes, assuming the payer reimburses for both, will be even more important since removal usually has a higher payment: 58301 (2.70 RUVs), 58300 (1.54 RVUs).
Coding considerations for surgical services
Surgical services performed in a hospital or ambulatory surgical center present another set of must-dos to ensure timely and fair reimbursement.
Grasp the ‘global package’ concept
Understanding this concept can be crucial to getting paid for additional services during this time period and correct billing for any E/M services performed prior to surgery. In general, the routine history and physical examination performed prior to a major surgery is considered included in the work and should not be billed separately. Surgical clearance for a patient’s condition, such as hypertension, a heart condition, or lung issues, can be billed separately, but these generally are performed by someone other than the operating surgeon.
Procedures performed in the hospital setting generally will have a 10- or 90-day global period. During this time, any related E/M service should not be billed separately, and the use of modifiers becomes even more important than with office services.
Applicable modifiers for use with hospital surgery can include all those for outpatient services plus:
- 50, Bilateral procedure (for which you may be paid up to 150% of the allowable)
- 58, Staged or related procedure during the postoperative period (this may be paid at the full allowable)
- 62, Co-surgeons (both surgeons bill the same CPT code and both document their involvement in the surgery). Medicare will reimburse each surgeon 62.5% of the allowable.
- 78, Return to the operating room for an unplanned related procedure (the full allowable may be reduced by some payers owing to their belief that this is soon after the original procedure so intraoperative time only is considered).
Be savvy about surgical bundles
Here, it is important to understand all published bundling edits for multiple procedures performed by the same surgeon at the same surgical session. If a code combination is never allowed but the surgery is more intense due to additional work required, a modifier -22 may be your only option. Again, clear, concise documentation of the additional work is imperative to receive the additional payment.
When a modifier is allowed, it generally will be one that denotes a procedure done on bilateral organs (such as the ovaries) when there is no extensive code to cover all of the work or when the additional procedure is “distinct” and meets the criteria for using a modifier 59.
Medicare has expanded the modifier -59 into additional modifiers to further explain the situation. These additional modifiers are:
- XE, A service that is distinct because it occurred during a separate encounter on the same date of service
- XS, A service that is distinct because it was performed on a separate organ/structure
- XP, A service that is distinct because it was performed by a different practitioner
- XU, The use of a service that is distinct because it does not overlap usual components of the main service.
Standards of care: Some steps are inherent to the surgery
Expect to receive claim denials if you bill separately for adhesiolysis during a surgical procedure. Every payer considers this procedure related to access to the surgical site and will deny separate coding. If the lysis was truly significant in terms of work, try reporting the modifier 22 and provide adequate documentation.
Other procedures at the time of surgery that generally are not paid for include 1) examination under anesthesia, 2) any procedure done to check the surgeon’s work (for example, cystoscopy, especially when done after urinary or pelvic reconstruction procedures, or chromotubation following extensive ovariolysis), 3) placement of catheters, and 4) placement of devices to alleviate postsurgical pain.
Bottom line
Maximizing reimbursement involves good documentation, correct CPT codes linked to specific and accurate medical indications, the use of appropriate modifiers, and listing codes in order of their relative values from highest to lowest.
Should a denial or unfair reduction in payment come your way, analyze the rejection to determine the cause and make billing and reporting changes as needed to improve your future reimbursements.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Facing a lawsuit? Take the right steps early
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”
It’s happened. A patient is suing you. Now what? Legal experts warn that a doctor’s first steps after a lawsuit can dramatically impact the outcome of the case – for better or worse.
Below, medical malpractice defense attorneys share the most important do’s and don’ts for physicians after they receive a lawsuit notice. Spoiler: Whatever you do, don’t ignore the summons.
• Do contact your insurer and/or risk manager. Once you receive notice of a lawsuit, the first step is calling your medical malpractice insurer and/or risk manager, said Steven Fitzer, a medical liability defense attorney based in Tacoma, Wash. The insurer and risk manager will take the matter from there and advise your next moves. Resist the urge to disregard the notice and hope that the challenge goes away when the patient is no longer angry, he said. Failing to notify the insurer in a timely manner could be a policy violation and affect current or future coverage.
• Don’t contact the plaintiff/patient or patient’s family. Instinctively, many physicians feel compelled to call the patient and attempt to settle the conflict verbally, particularly if they have had a longstanding relationship, Mr. Fitzer said in an interview. Don’t do it.
“In 42 years, I’ve never come across a physician who successfully talked somebody out of a lawsuit, once it was started,” he said. “It’s a pipe dream.”
Keep in mind that conversations with patients after a lawsuit filing can be used against doctors in court and certain words can easily be misconstrued as admissions of guilt.
• Do secure all medical records pertaining to the case. Obtain and print copies of all information relevant to the patient’s suit, such as history, billing records, letters, and medical chart. Store the data in a secure location in preparation for transferring to the insurer and/or attorney, said Michael Moroney, a medical liability defense attorney based in Teaneck, N.J.
• Don’t access or change the record. It may seem tempting to review the plaintiff’s medical record and fix any errors found. However, accessing the patient’s electronic data can appear as an attempt to manipulate or delete relevant data, said Joshua R. Cohen, a medical liability defense attorney based in New York.
“Avoid accessing [the] EMR or PAC system [and] leaving a digital fingerprint,” he said in an interview. “For example, if a radiologist is sued for an alleged failure to diagnose breast cancer, they should not open that study on their computer as an audit trail will show that. Worse is when they start making measurements after the lawsuit which are now discoverable as part of the lawsuit.”
Leave the record alone and let the attorneys handle the data from here on out, he advised.
• Do discuss the patient case openly with your attorney and risk manager. Honesty about all aspects of a medical case from the start sets the right tone for a positive relationship between doctor and attorney, experts say. Help your attorney understand the medicine so that they can speak intelligently about the details to the court and any retained experts, Mr. Fitzer recommended. If disagreements continually arise among physicians and attorneys, and the match fails, consider speaking to the insurer about a change in attorneys.
• Don’t discuss the case. As Mr. Fitzer puts it, “loose lips sink ships.” Physicians lose confidentiality protections when they talk about lawsuit details with third parties, and those conversations could come back to haunt them. This includes colleagues and staff members in the patient’s care loop, said Catherine Flynn, a medical liability defense attorney based in Teaneck, N.J. The third parties could later be questioned by the plaintiff’s attorney about the case, which could harm your defense.
"It’s like that kid game of telephone where you may something to the nurses and then a year later, they’re deposed, and their recollection is very different,” Ms. Flynn said in an interview. “It turns into something that you did not say.”
Your spouse is the exception. Most states protect conversations among spouses and bar husbands and wives from having to testify against their spouse.
• Do alert staff to the lawsuit and track any document requests. Following a lawsuit notice, inform staff that a claim has been filed by a patient – without going into detail. Be alert to document requests by nonpatients and make sure your attorney is aware of such requests. For example, some plaintiffs hire a private investigator to contact the medical practice and attempt to obtain records, Mr. Moroney said. In other cases, the plaintiff’s attorney or their paralegal tries to get copies of the medical chart or billing records.
• Don’t release any patient data to third parties. Ensure that staff members do not provide any patient information to the plaintiff’s attorney or other third parties, Mr. Moroney said. All relevant records should go through your attorney. No questions about the patient or the circumstances of the complaint should be divulged by the doctor or staff members to any third party, he said.
• Do seek emotional support from family and friends. Facing a lawsuit can be draining, both physically and mentally. Make time for self-care and lean on loved ones when needed, Mr. Fitzer said. Sharing your feelings – without going into detail about the case – can help relieve stress and reduce the emotional strain of being sued.
• Don’t isolate yourself. “This can be an isolating experience,” Mr. Fitzer said. “You need support. You need reinforcements. Take care of yourself and your family – they are your biggest source of support.”