User login
Huntington’s research returns to Latin America, as scientists tread with care
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.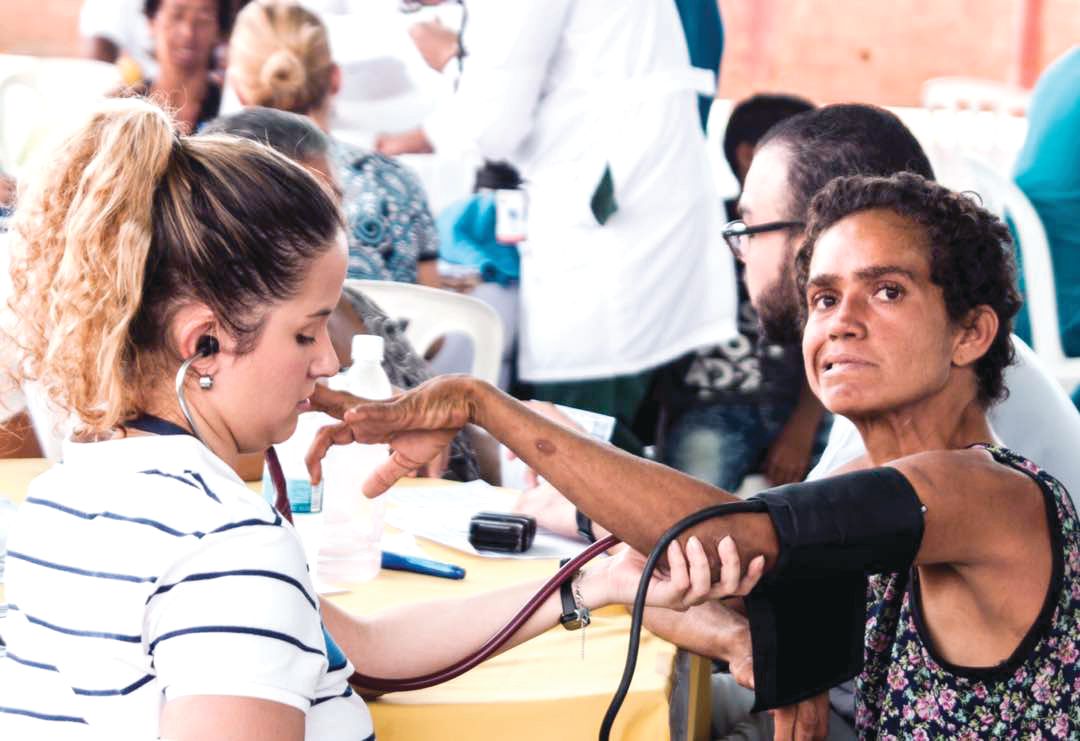
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.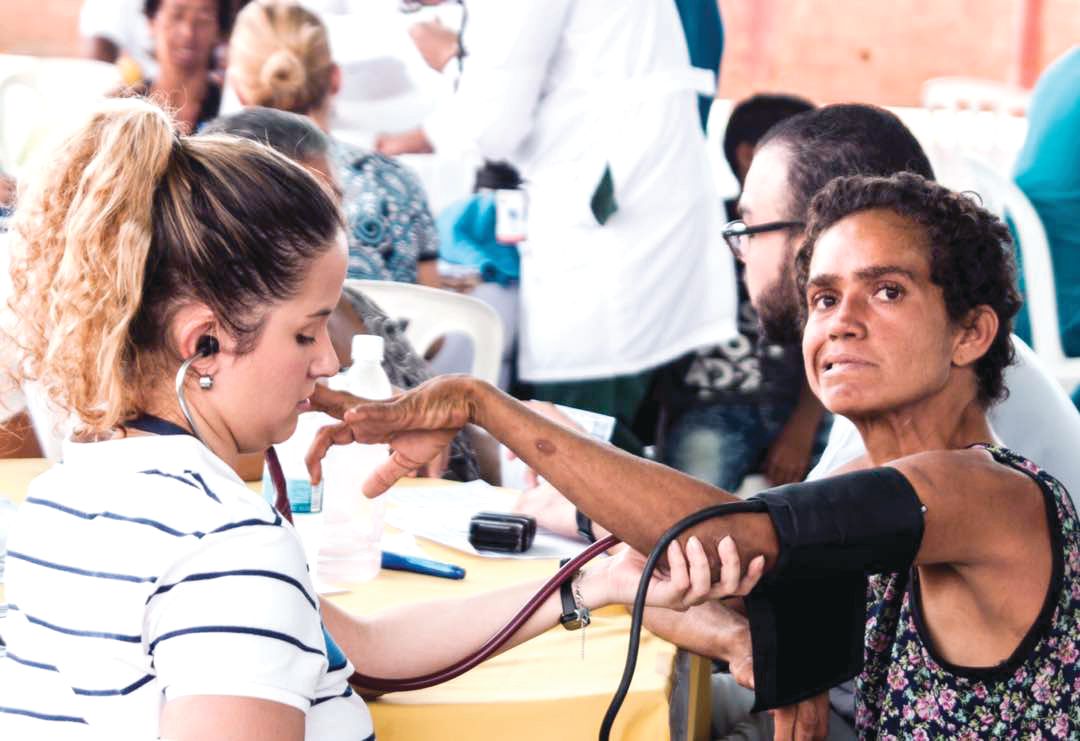
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.
Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.
While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.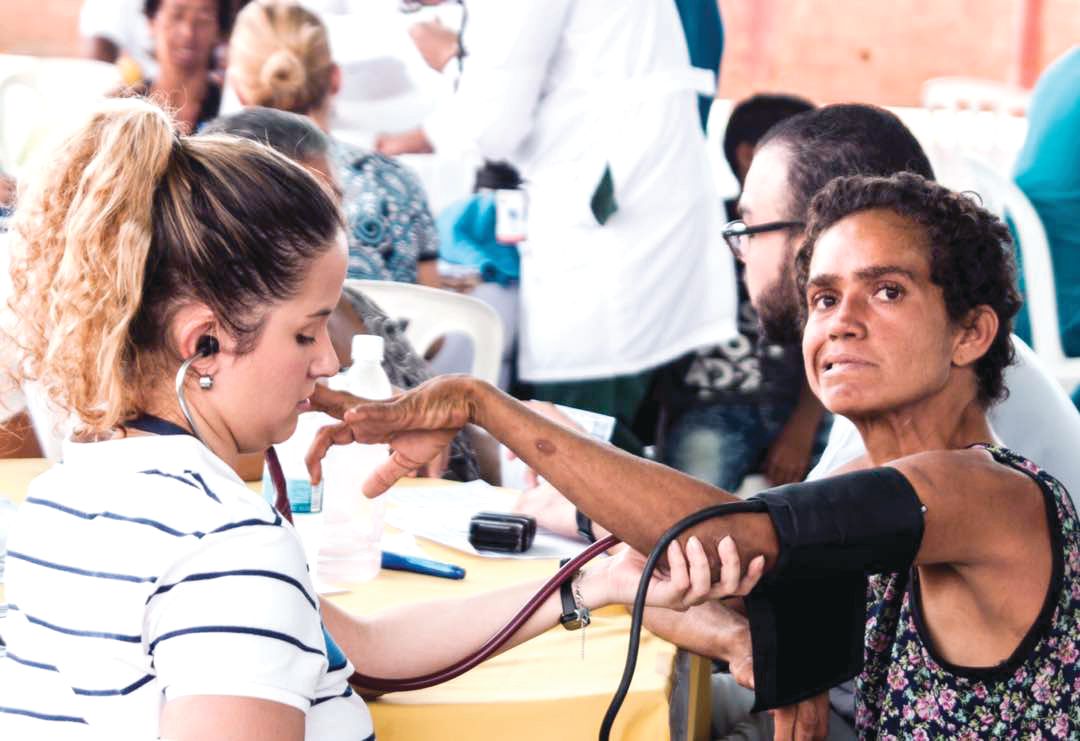
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.
Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
High prices driving insulin underuse
One in four patients at an urban diabetes center reported underusing insulin because of concerns about cost, according to a survey of patients with type 1 or type 2 diabetes mellitus who were recently prescribed the drug.
“These results highlight an urgent need to address affordability of insulin,” lead author Darby Herkert of Yale College in New Haven, Conn., and her coauthors wrote in a study published online in JAMA Internal Medicine.
In the survey of 199 diabetes patients who had an outpatient visit at the Yale Diabetes Center between June and August 2017, 25.5% reported cost-related insulin underuse. Only 60.8% of those patients discussed the prohibitive costs with their clinician, and 29.4% changed insulin types because of high prices. Patients who reported insulin underuse were also more likely to have poorer glycemic control than patients who did not, at a rate of 43.1% versus 28.1% (odds ratio, 2.96; 95% confidence interval, 1.14-8.16; P = .03).
The authors noted potential limitations in their study, including focusing on patients of just one treatment center and the inability to establish a causal relationship between cost-related underuse and poor glycemic control. Nonetheless, they strongly encouraged asking diabetes patients about potential cost issues; they also stressed the need for larger forces to step in and guarantee insulin’s availability. “Insulin is a life-saving, essential medicine, and most patients cannot act as price-sensitive buyers. Regulators and the medical community need to intervene to ensure that insulin is affordable to patients who need it,” they wrote.
This study was supported by the Global Health Field Experiences Award, the Yale College Fellowship for Research in Global Health Studies, and the Global Health Field Experiences Seed Funding Award. The corresponding author reported receiving funding from the Centers of Medicare and Medicaid Services to develop publicly reported quality measures. Another author reported receiving support from Health Action International and Alosa Health. No other disclosures were reported.
SOURCE: Herkert D et al. JAMA Internal Medicine. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5008.
This study by Herkert and colleagues reinforces that American drug makers, device manufacturers, and insurers are willing to sacrifice human lives in their quest for profit, according to Elisabeth Rosenthal, MD, of Kaiser Health News.
Diabetes is a disease that starts in childhood, she noted, which means young Americans who are often low-earning and uninsured must start managing it with insulin before they are financially stable. And if drug prices keep going up – and if competitors are sued out of the market – then that means people with chronic disease will suffer, including the 25.5% that Herkert et al. found underuse insulin because of cost.
“As drug costs have generally increased in the United States, we know that many patients are skimping on medicines, taking less than prescribed, and cutting pills in half to make every fill last longer. This is terrible, but for many diseases, it is not catastrophic,” she wrote.
“But skimping on insulin,” she added, “can be rapidly deadly in people whose bodies make none of their own and can result in a life-threatening metabolic disturbance.”
Dr. Rosenthal shared the story of a 29-year-old diabetes patient in Missouri who would consider only doctoral programs outside the United States. “My one goal in life has been to move to Europe so I don’t have to pay these staggering prices just to survive,” the patient revealed.
But others – that 25% – will quietly skimp on their insulin, taking less than they need but more, perhaps, than they can really afford. Some of them will die.
Dr. Rosenthal is the author of “An American Sickness: How Healthcare Became Big Business and How You Can Take It Back.” These comments are adapted from an accompanying editorial (JAMA Internal Med. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5007).
This study by Herkert and colleagues reinforces that American drug makers, device manufacturers, and insurers are willing to sacrifice human lives in their quest for profit, according to Elisabeth Rosenthal, MD, of Kaiser Health News.
Diabetes is a disease that starts in childhood, she noted, which means young Americans who are often low-earning and uninsured must start managing it with insulin before they are financially stable. And if drug prices keep going up – and if competitors are sued out of the market – then that means people with chronic disease will suffer, including the 25.5% that Herkert et al. found underuse insulin because of cost.
“As drug costs have generally increased in the United States, we know that many patients are skimping on medicines, taking less than prescribed, and cutting pills in half to make every fill last longer. This is terrible, but for many diseases, it is not catastrophic,” she wrote.
“But skimping on insulin,” she added, “can be rapidly deadly in people whose bodies make none of their own and can result in a life-threatening metabolic disturbance.”
Dr. Rosenthal shared the story of a 29-year-old diabetes patient in Missouri who would consider only doctoral programs outside the United States. “My one goal in life has been to move to Europe so I don’t have to pay these staggering prices just to survive,” the patient revealed.
But others – that 25% – will quietly skimp on their insulin, taking less than they need but more, perhaps, than they can really afford. Some of them will die.
Dr. Rosenthal is the author of “An American Sickness: How Healthcare Became Big Business and How You Can Take It Back.” These comments are adapted from an accompanying editorial (JAMA Internal Med. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5007).
This study by Herkert and colleagues reinforces that American drug makers, device manufacturers, and insurers are willing to sacrifice human lives in their quest for profit, according to Elisabeth Rosenthal, MD, of Kaiser Health News.
Diabetes is a disease that starts in childhood, she noted, which means young Americans who are often low-earning and uninsured must start managing it with insulin before they are financially stable. And if drug prices keep going up – and if competitors are sued out of the market – then that means people with chronic disease will suffer, including the 25.5% that Herkert et al. found underuse insulin because of cost.
“As drug costs have generally increased in the United States, we know that many patients are skimping on medicines, taking less than prescribed, and cutting pills in half to make every fill last longer. This is terrible, but for many diseases, it is not catastrophic,” she wrote.
“But skimping on insulin,” she added, “can be rapidly deadly in people whose bodies make none of their own and can result in a life-threatening metabolic disturbance.”
Dr. Rosenthal shared the story of a 29-year-old diabetes patient in Missouri who would consider only doctoral programs outside the United States. “My one goal in life has been to move to Europe so I don’t have to pay these staggering prices just to survive,” the patient revealed.
But others – that 25% – will quietly skimp on their insulin, taking less than they need but more, perhaps, than they can really afford. Some of them will die.
Dr. Rosenthal is the author of “An American Sickness: How Healthcare Became Big Business and How You Can Take It Back.” These comments are adapted from an accompanying editorial (JAMA Internal Med. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5007).
One in four patients at an urban diabetes center reported underusing insulin because of concerns about cost, according to a survey of patients with type 1 or type 2 diabetes mellitus who were recently prescribed the drug.
“These results highlight an urgent need to address affordability of insulin,” lead author Darby Herkert of Yale College in New Haven, Conn., and her coauthors wrote in a study published online in JAMA Internal Medicine.
In the survey of 199 diabetes patients who had an outpatient visit at the Yale Diabetes Center between June and August 2017, 25.5% reported cost-related insulin underuse. Only 60.8% of those patients discussed the prohibitive costs with their clinician, and 29.4% changed insulin types because of high prices. Patients who reported insulin underuse were also more likely to have poorer glycemic control than patients who did not, at a rate of 43.1% versus 28.1% (odds ratio, 2.96; 95% confidence interval, 1.14-8.16; P = .03).
The authors noted potential limitations in their study, including focusing on patients of just one treatment center and the inability to establish a causal relationship between cost-related underuse and poor glycemic control. Nonetheless, they strongly encouraged asking diabetes patients about potential cost issues; they also stressed the need for larger forces to step in and guarantee insulin’s availability. “Insulin is a life-saving, essential medicine, and most patients cannot act as price-sensitive buyers. Regulators and the medical community need to intervene to ensure that insulin is affordable to patients who need it,” they wrote.
This study was supported by the Global Health Field Experiences Award, the Yale College Fellowship for Research in Global Health Studies, and the Global Health Field Experiences Seed Funding Award. The corresponding author reported receiving funding from the Centers of Medicare and Medicaid Services to develop publicly reported quality measures. Another author reported receiving support from Health Action International and Alosa Health. No other disclosures were reported.
SOURCE: Herkert D et al. JAMA Internal Medicine. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5008.
One in four patients at an urban diabetes center reported underusing insulin because of concerns about cost, according to a survey of patients with type 1 or type 2 diabetes mellitus who were recently prescribed the drug.
“These results highlight an urgent need to address affordability of insulin,” lead author Darby Herkert of Yale College in New Haven, Conn., and her coauthors wrote in a study published online in JAMA Internal Medicine.
In the survey of 199 diabetes patients who had an outpatient visit at the Yale Diabetes Center between June and August 2017, 25.5% reported cost-related insulin underuse. Only 60.8% of those patients discussed the prohibitive costs with their clinician, and 29.4% changed insulin types because of high prices. Patients who reported insulin underuse were also more likely to have poorer glycemic control than patients who did not, at a rate of 43.1% versus 28.1% (odds ratio, 2.96; 95% confidence interval, 1.14-8.16; P = .03).
The authors noted potential limitations in their study, including focusing on patients of just one treatment center and the inability to establish a causal relationship between cost-related underuse and poor glycemic control. Nonetheless, they strongly encouraged asking diabetes patients about potential cost issues; they also stressed the need for larger forces to step in and guarantee insulin’s availability. “Insulin is a life-saving, essential medicine, and most patients cannot act as price-sensitive buyers. Regulators and the medical community need to intervene to ensure that insulin is affordable to patients who need it,” they wrote.
This study was supported by the Global Health Field Experiences Award, the Yale College Fellowship for Research in Global Health Studies, and the Global Health Field Experiences Seed Funding Award. The corresponding author reported receiving funding from the Centers of Medicare and Medicaid Services to develop publicly reported quality measures. Another author reported receiving support from Health Action International and Alosa Health. No other disclosures were reported.
SOURCE: Herkert D et al. JAMA Internal Medicine. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5008.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Patients who reported cost-related insulin underuse were more likely to have poorer glycemic control than those who were able to afford the drug.
Major finding: Insulin cost accounted for its underuse by 25.5% of diabetic patients surveyed.
Study details: A survey of 199 patients with type 1 or type 2 diabetes mellitus who were prescribed insulin in the last 6 months and had an outpatient visit at the Yale Diabetes Center between June and August 2017.
Disclosures: This study was supported by the Global Health Field Experiences Award, the Yale College Fellowship for Research in Global Health Studies, and the Global Health Field Experiences Seed Funding Award. The corresponding author reported receiving funding from the Centers of Medicare and Medicaid Services to develop publicly reported quality measures. Another author reported receiving support from Health Action International and Alosa Health. No other disclosures were reported.
Source: Herkert D et al. JAMA Inter Med. 2018 Dec 3. doi: 10.1001/jamainternmed.2018.5008.
Lower-dose rituximab may be enough in acquired TTP
SAN DIEGO – Lower-than-usual doses of rituximab may be sufficient in patients with acquired thrombotic thrombocytopenic purpura (TTP), results of a recent pilot safety and efficacy study suggest.
Patients receiving just 100 mg/week for 4 weeks had rates of relapse and exacerbation that were favorable, compared with historical controls, according to investigator Jeffrey I. Zwicker, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. He presented the findings at the annual meeting of the American Society of Hematology.
However, the low-dose treatment was not without side effects, according to Dr. Zwicker, who described one case of acute respiratory failure out of the 19 patients enrolled in the ART (Adjuvant Rituximab in TTP) study.
“The likely benefit is cost savings, rather than less toxicity,” Dr. Zwicker said of the low-dose rituximab regimen.
Out of 19 patients enrolled in ART, 18 were eligible to receive the study treatment, which included low-dose rituximab plus standard plasma exchange and corticosteroids.
Following this initial therapy, all patients had a response, defined as a platelet count 150,000/mcL or greater for 2 consecutive days, with a median time to response of 5 days.
There were two exacerbations (12%) at 30 days after stopping plasma exchange and no cases of refractory TTP, which compared favorably to historical controls, Dr. Zwicker said.
The rate of relapse at 2 years was 28%, which again compared favorably with a historical control data repository in which the rate of relapse at 2 years was 51%.
One patient in the study suffered a case of acute respiratory failure requiring intubation during the third rituximab infusion and was ultimately placed on extracorporeal membrane oxygenation.
“The patient did survive, but this is just a reminder that there are potential side effects, even with lower doses of rituximab,” Dr. Zwicker said.
A few other serious adverse events – including central line infection and bacteremia in one patient – were more likely related to the plasma exchange, he added.
These results with low-dose rituximab are consistent with findings that rituximab 375 mg/m2 for four doses reduces the incidence of exacerbation and refractory disease and prevents or delays relapses, according to Dr. Zwicker and his coinvestigators, including J. Evan Sadler, MD, PhD, of Washington University, St. Louis, who initiated the study.
The typical TTP regimen of rituximab 375 mg/m2 for four weekly doses is borrowed from protocols for B-cell lymphomas; however, the B-cell mass in nonmalignant disease is likely to be much less than in lymphoproliferative disorders, Dr. Zwicker told attendees.
“The benefit, principally, of lower-dose rituximab is saving of thousands upon thousands of dollars,” Dr. Zwicker said.
This is not the only data set to suggest a potential role for lower-dose rituximab, he added, noting that a recently published retrospective analysis showed “fairly similar” treatment-free survival rates for standard rituximab and a reduced-dose regimen. There also are case series in other autoimmune cytopenias, namely idiopathic thrombocytopenic purpura and pure red cell aplasia, that provide evidence in support of low-dose rituximab, he added.
Dr. Zwicker reported research funding with Incyte and Quercegen, and consultancy with Parexel. Dr. Sadler reported consultancy with Ablynx.
SOURCE: Zwicker JI et al. ASH 2018, Abstract 374.
SAN DIEGO – Lower-than-usual doses of rituximab may be sufficient in patients with acquired thrombotic thrombocytopenic purpura (TTP), results of a recent pilot safety and efficacy study suggest.
Patients receiving just 100 mg/week for 4 weeks had rates of relapse and exacerbation that were favorable, compared with historical controls, according to investigator Jeffrey I. Zwicker, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. He presented the findings at the annual meeting of the American Society of Hematology.
However, the low-dose treatment was not without side effects, according to Dr. Zwicker, who described one case of acute respiratory failure out of the 19 patients enrolled in the ART (Adjuvant Rituximab in TTP) study.
“The likely benefit is cost savings, rather than less toxicity,” Dr. Zwicker said of the low-dose rituximab regimen.
Out of 19 patients enrolled in ART, 18 were eligible to receive the study treatment, which included low-dose rituximab plus standard plasma exchange and corticosteroids.
Following this initial therapy, all patients had a response, defined as a platelet count 150,000/mcL or greater for 2 consecutive days, with a median time to response of 5 days.
There were two exacerbations (12%) at 30 days after stopping plasma exchange and no cases of refractory TTP, which compared favorably to historical controls, Dr. Zwicker said.
The rate of relapse at 2 years was 28%, which again compared favorably with a historical control data repository in which the rate of relapse at 2 years was 51%.
One patient in the study suffered a case of acute respiratory failure requiring intubation during the third rituximab infusion and was ultimately placed on extracorporeal membrane oxygenation.
“The patient did survive, but this is just a reminder that there are potential side effects, even with lower doses of rituximab,” Dr. Zwicker said.
A few other serious adverse events – including central line infection and bacteremia in one patient – were more likely related to the plasma exchange, he added.
These results with low-dose rituximab are consistent with findings that rituximab 375 mg/m2 for four doses reduces the incidence of exacerbation and refractory disease and prevents or delays relapses, according to Dr. Zwicker and his coinvestigators, including J. Evan Sadler, MD, PhD, of Washington University, St. Louis, who initiated the study.
The typical TTP regimen of rituximab 375 mg/m2 for four weekly doses is borrowed from protocols for B-cell lymphomas; however, the B-cell mass in nonmalignant disease is likely to be much less than in lymphoproliferative disorders, Dr. Zwicker told attendees.
“The benefit, principally, of lower-dose rituximab is saving of thousands upon thousands of dollars,” Dr. Zwicker said.
This is not the only data set to suggest a potential role for lower-dose rituximab, he added, noting that a recently published retrospective analysis showed “fairly similar” treatment-free survival rates for standard rituximab and a reduced-dose regimen. There also are case series in other autoimmune cytopenias, namely idiopathic thrombocytopenic purpura and pure red cell aplasia, that provide evidence in support of low-dose rituximab, he added.
Dr. Zwicker reported research funding with Incyte and Quercegen, and consultancy with Parexel. Dr. Sadler reported consultancy with Ablynx.
SOURCE: Zwicker JI et al. ASH 2018, Abstract 374.
SAN DIEGO – Lower-than-usual doses of rituximab may be sufficient in patients with acquired thrombotic thrombocytopenic purpura (TTP), results of a recent pilot safety and efficacy study suggest.
Patients receiving just 100 mg/week for 4 weeks had rates of relapse and exacerbation that were favorable, compared with historical controls, according to investigator Jeffrey I. Zwicker, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston. He presented the findings at the annual meeting of the American Society of Hematology.
However, the low-dose treatment was not without side effects, according to Dr. Zwicker, who described one case of acute respiratory failure out of the 19 patients enrolled in the ART (Adjuvant Rituximab in TTP) study.
“The likely benefit is cost savings, rather than less toxicity,” Dr. Zwicker said of the low-dose rituximab regimen.
Out of 19 patients enrolled in ART, 18 were eligible to receive the study treatment, which included low-dose rituximab plus standard plasma exchange and corticosteroids.
Following this initial therapy, all patients had a response, defined as a platelet count 150,000/mcL or greater for 2 consecutive days, with a median time to response of 5 days.
There were two exacerbations (12%) at 30 days after stopping plasma exchange and no cases of refractory TTP, which compared favorably to historical controls, Dr. Zwicker said.
The rate of relapse at 2 years was 28%, which again compared favorably with a historical control data repository in which the rate of relapse at 2 years was 51%.
One patient in the study suffered a case of acute respiratory failure requiring intubation during the third rituximab infusion and was ultimately placed on extracorporeal membrane oxygenation.
“The patient did survive, but this is just a reminder that there are potential side effects, even with lower doses of rituximab,” Dr. Zwicker said.
A few other serious adverse events – including central line infection and bacteremia in one patient – were more likely related to the plasma exchange, he added.
These results with low-dose rituximab are consistent with findings that rituximab 375 mg/m2 for four doses reduces the incidence of exacerbation and refractory disease and prevents or delays relapses, according to Dr. Zwicker and his coinvestigators, including J. Evan Sadler, MD, PhD, of Washington University, St. Louis, who initiated the study.
The typical TTP regimen of rituximab 375 mg/m2 for four weekly doses is borrowed from protocols for B-cell lymphomas; however, the B-cell mass in nonmalignant disease is likely to be much less than in lymphoproliferative disorders, Dr. Zwicker told attendees.
“The benefit, principally, of lower-dose rituximab is saving of thousands upon thousands of dollars,” Dr. Zwicker said.
This is not the only data set to suggest a potential role for lower-dose rituximab, he added, noting that a recently published retrospective analysis showed “fairly similar” treatment-free survival rates for standard rituximab and a reduced-dose regimen. There also are case series in other autoimmune cytopenias, namely idiopathic thrombocytopenic purpura and pure red cell aplasia, that provide evidence in support of low-dose rituximab, he added.
Dr. Zwicker reported research funding with Incyte and Quercegen, and consultancy with Parexel. Dr. Sadler reported consultancy with Ablynx.
SOURCE: Zwicker JI et al. ASH 2018, Abstract 374.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: After low-dose rituximab plus standard plasma exchange and corticosteroids, the rate of relapse at 2 years was 28%, versus 51% in a historical control data set.
Study details: Findings of the ART (Adjuvant Rituximab in TTP) study including 19 patients with acquired TTP.
Disclosures: Dr. Zwicker reported research funding with Incyte and Quercegen, and consultancy with Parexel. Dr. Sadler reported consultancy with Ablynx.
Source: Zwicker JI et al. ASH 2018, Abstract 374.
TRED-HF: Despite recovery, dilated cardiomyopathy returns after halting HF drugs
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
CHICAGO – Phased withdrawal of guideline-directed medical therapy in patients who seemed to have recovered from dilated cardiomyopathy resulted in relapses in 40% of patients within 6 months in the TRED-HF trial.
The clinical implications of this small pilot randomized trial are clear: “Withdrawal of therapy should not usually be attempted, at least until we can predict who’s going to relapse and who’s not,” Brian P. Halliday, MD, PhD, said at the American Heart Association scientific sessions.
“Improvement in function represents remission rather than permanent recovery for many patients,” added Dr. Halliday of Imperial College London.
The study was performed to address a question that arises with increasing frequency in clinical practice as a result of the impressive advances in heart failure therapy in recent years, he said. “Patients frequently come to us in clinic and ask us, ‘Do I need to continue to take these medications forever?’ They’re frequently young, and they want to know if they really need to be subject to 40 or 50 years of medication. Some are concerned about side effects, others are interested in pregnancy, and then there is the financial cost.”
Simultaneously published in The Lancet, TRED-HF was a single-center, open-label study of 51 patients who had prior dilated cardiomyopathy (DCM) and a median left ventricular ejection fraction (LVEF) of 25% at the time of diagnosis 4.9 years earlier and who subsequently recovered in response to therapy. That is, they became symptom-free with an LVEF greater than 50%, a normal left ventricular end diastolic volume index, and a reassuringly low median N-terminal pro b-type natriuretic peptide (NP-pro-BNP) level of 72 ng/L.
For the study, 25 patients were randomized to phased withdrawal of their heart failure drugs over a 16-week period: First they reduced or stopped loop diuretics, then mineralocorticoid antagonists, then beta-blockers, and finally their ACE inhibitor or angiotensin receptor blocker. The other 26 participants continued therapy during the first 6 months of the study, then 25 of the 26 crossed over to phased withdrawal. The outlier didn’t cross over because of atrial fibrillation.
The primary endpoint was relapse of DCM within 6 months of the start of the study. Relapse was defined as either a drop in LVEF of more than 10% to a level below 50%, at least a doubling of NT-pro-BNP to greater than 400 ng/L, clinical evidence of heart failure, or a greater than 10% increase in LV end diastolic volume as assessed by cardiac MRI.
The results
During the first half of the study, 11 of 25 patients (44%) relapsed during or after medication withdrawal. None of the controls relapsed. In the crossover phase, 9 of 25 patients (35%) relapsed in response to treatment withdrawal. Of the 20 patients who relapsed, 13 did so within 16 weeks of beginning medication withdrawal. Indeed, most patients relapsed within 8 weeks of their last medication. Ten of the twenty fulfilled multiple criteria for relapse.
Medication withdrawal was accompanied not only by a mean 9.5% reduction in LVEF, compared with baseline, but by a 15.4-bpm rise in heart rate, a 7.0–mm Hg increase in diastolic blood pressure, and 5.1-point deterioration in Kansas City Cardiomyopathy Questionnaire scores, demonstrating that what happened off treatment was true DCM recurrence and not simply an imaging artifact.
Everyone who relapsed immediately restarted treatment. At their next follow-up visit, all were once again asymptomatic, and 17 of the 20 (85%) had an LVEF greater than 50%. Two of the other three had an LVEF of 45%-50%, and the other had an LVEF of 43%.
“So they did seem to recover when they went back on medication,” Dr. Halliday observed.
Underpowered exploratory analyses designed for hypothesis generation identified several potential baseline predictors of DCM relapse, including older age, being on three or more heart failure drugs, and use of a mineralocorticoid antagonist.
Experts react
Designated discussant Jane E. Wilcox, MD, commented, “Currently, in 2018, we have no true signature of recovery. These patients are indeed in cardiac remission and have an indefinite indication for continuing their evidence-based medical therapy without interruption.”
“The clinical implication here is, I think, we should TRED-lightly,” quipped Dr. Wilcox of Northwestern University in Chicago.
Her own research indicates that even patients who have recovered their LVEF and no longer seem to have a heart failure phenotype still have an abnormal myocardial substrate as evidenced by persistent dysfunctional cardiac mechanics on echocardiography. Nonetheless, she remains optimistic.
“I don’t think [TRED-HF] squelches the future of myocardial recovery. I think it actually invigorates the field for an assessment of genomics, proteomics, and metabolomics looking for that true signature of cardiac recovery,” she said.
Donald Lloyd-Jones, MD, who chaired a press conference where Dr. Halliday presented the TRED-HF results, complimented the investigators for tackling what he termed “an incredibly important clinical question that comes up all the time.”
“I really want to commend the investigators for taking on what, on its face, might be an ethically challenging question by taking treatment away when we don’t know what the answer is likely to be. But they really checked all the boxes to make sure this was done in a very safe and monitored way, so that even though the outcome was what it turned out to be, the harm to patients was minimalized,” said Dr. Lloyd-Jones, professor and chair of the department of preventive medicine and director of the Northwestern University Clinical and Translational Sciences Institute, Chicago.
“No patient wants to be on more medication than they need to be, but I think for the time being this class of patients is going to have to be maintained on medications until we understand a little more,” Dr. Lloyd-Jones concluded.
Dr. Halliday reported having no financial conflicts regarding the study, funded by the British Heart Foundation.
SOURCE: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The heart failure relapse rate is high after medication withdrawal.
Major finding: Of patients who were seemingly recovered from dilated cardiomyopathy, 40% experienced early relapse following structured medication withdrawal.
Study details: This randomized crossover trial included 51 patients whose medications were withdrawn after their apparent recovery from dilated cardiomyopathy.
Disclosures: The study was funded by the British Heart Foundation. The presenter reported having no financial conflicts.
Source: Halliday BP. AHA scientific sessions, Abstract 18621. Simulpub The Lancet. 2018 Nov 11. doi: 10.1016/S0140-6736(18)32484-X.
Model bests IPSS-R for predicting survival, risk for AML in myelodysplastic syndrome
SAN DIEGO – A newly developed personalized model that “harnesses the power of artificial intelligence” to predict overall survival and transformation to acute myeloid leukemia (AML) in patients with myelodysplastic syndromes outperforms both the original and revised International Prognostic Scoring Systems (IPSS, IPSS-R), according to Aziz Nazha, MD.
The machine learning model, which was built using clinical and genomic data derived from myelodysplastic syndrome (MDS) patients diagnosed according to 2008 World Health Organization criteria, incorporates information beyond that included in the IPSS and IPSS-R, and provides patient-specific survival probabilities at different time points, Dr. Nazha of Cleveland Clinic reported during a press briefing at the annual meeting of the American Society of Hematology.
The model was developed in a combined training cohort of 1,471 patients from the Cleveland Clinic and Munich Leukemia Laboratory and was validated in a separate cohort of 831 patients from the Moffitt Cancer Center in Tampa, Fla.
The concordance index – a measure for comparing the accuracy of the various models – was 0.80 for overall survival (OS), and 0.78 for AML transformation vs. 0.66 and 0.73, respectively, for IPSS, and 0.67 and 0.73, respectively, for IPSS-R, Dr. Nazha said. The new “geno-clinical” model also outperformed mutations-only analysis, mutations plus cytogenetics analysis, and mutations plus cytogenetics plus age analyses for both OS and AML transformation.
Adding mutational variant allelic frequency did not significantly improve prediction accuracy, he noted.
Dr. Nazha and his colleagues are developing a web application tool that can be used to run the trained model to calculate patient-specific, time-specific OS and AML transformation probabilities. He discussed the new model and its implications for personalized prognosis and treatment in this video interview.
Improved risk assessment helps patients understand their disease and “establish expectations about their journey with their disease,” and it is also extremely important for treating physicians, he said.
“All of our consensus guidelines and treatment recommendations are based on risk,” he explained, noting that the approach varies greatly for higher- and lower-risk patients.
This model represents a potential new focus on “personalized prediction” in addition to the increasing focus on personalized treatment and takes into account the heterogeneous outcomes seen in patients with MDS, he said.
Dr. Nazha reported consultancy for Karyopharma and Tolero, and data-monitoring committee membership for MEI.
SOURCE: Nazha A et al. ASH 2018, Abstract 793.
SAN DIEGO – A newly developed personalized model that “harnesses the power of artificial intelligence” to predict overall survival and transformation to acute myeloid leukemia (AML) in patients with myelodysplastic syndromes outperforms both the original and revised International Prognostic Scoring Systems (IPSS, IPSS-R), according to Aziz Nazha, MD.
The machine learning model, which was built using clinical and genomic data derived from myelodysplastic syndrome (MDS) patients diagnosed according to 2008 World Health Organization criteria, incorporates information beyond that included in the IPSS and IPSS-R, and provides patient-specific survival probabilities at different time points, Dr. Nazha of Cleveland Clinic reported during a press briefing at the annual meeting of the American Society of Hematology.
The model was developed in a combined training cohort of 1,471 patients from the Cleveland Clinic and Munich Leukemia Laboratory and was validated in a separate cohort of 831 patients from the Moffitt Cancer Center in Tampa, Fla.
The concordance index – a measure for comparing the accuracy of the various models – was 0.80 for overall survival (OS), and 0.78 for AML transformation vs. 0.66 and 0.73, respectively, for IPSS, and 0.67 and 0.73, respectively, for IPSS-R, Dr. Nazha said. The new “geno-clinical” model also outperformed mutations-only analysis, mutations plus cytogenetics analysis, and mutations plus cytogenetics plus age analyses for both OS and AML transformation.
Adding mutational variant allelic frequency did not significantly improve prediction accuracy, he noted.
Dr. Nazha and his colleagues are developing a web application tool that can be used to run the trained model to calculate patient-specific, time-specific OS and AML transformation probabilities. He discussed the new model and its implications for personalized prognosis and treatment in this video interview.
Improved risk assessment helps patients understand their disease and “establish expectations about their journey with their disease,” and it is also extremely important for treating physicians, he said.
“All of our consensus guidelines and treatment recommendations are based on risk,” he explained, noting that the approach varies greatly for higher- and lower-risk patients.
This model represents a potential new focus on “personalized prediction” in addition to the increasing focus on personalized treatment and takes into account the heterogeneous outcomes seen in patients with MDS, he said.
Dr. Nazha reported consultancy for Karyopharma and Tolero, and data-monitoring committee membership for MEI.
SOURCE: Nazha A et al. ASH 2018, Abstract 793.
SAN DIEGO – A newly developed personalized model that “harnesses the power of artificial intelligence” to predict overall survival and transformation to acute myeloid leukemia (AML) in patients with myelodysplastic syndromes outperforms both the original and revised International Prognostic Scoring Systems (IPSS, IPSS-R), according to Aziz Nazha, MD.
The machine learning model, which was built using clinical and genomic data derived from myelodysplastic syndrome (MDS) patients diagnosed according to 2008 World Health Organization criteria, incorporates information beyond that included in the IPSS and IPSS-R, and provides patient-specific survival probabilities at different time points, Dr. Nazha of Cleveland Clinic reported during a press briefing at the annual meeting of the American Society of Hematology.
The model was developed in a combined training cohort of 1,471 patients from the Cleveland Clinic and Munich Leukemia Laboratory and was validated in a separate cohort of 831 patients from the Moffitt Cancer Center in Tampa, Fla.
The concordance index – a measure for comparing the accuracy of the various models – was 0.80 for overall survival (OS), and 0.78 for AML transformation vs. 0.66 and 0.73, respectively, for IPSS, and 0.67 and 0.73, respectively, for IPSS-R, Dr. Nazha said. The new “geno-clinical” model also outperformed mutations-only analysis, mutations plus cytogenetics analysis, and mutations plus cytogenetics plus age analyses for both OS and AML transformation.
Adding mutational variant allelic frequency did not significantly improve prediction accuracy, he noted.
Dr. Nazha and his colleagues are developing a web application tool that can be used to run the trained model to calculate patient-specific, time-specific OS and AML transformation probabilities. He discussed the new model and its implications for personalized prognosis and treatment in this video interview.
Improved risk assessment helps patients understand their disease and “establish expectations about their journey with their disease,” and it is also extremely important for treating physicians, he said.
“All of our consensus guidelines and treatment recommendations are based on risk,” he explained, noting that the approach varies greatly for higher- and lower-risk patients.
This model represents a potential new focus on “personalized prediction” in addition to the increasing focus on personalized treatment and takes into account the heterogeneous outcomes seen in patients with MDS, he said.
Dr. Nazha reported consultancy for Karyopharma and Tolero, and data-monitoring committee membership for MEI.
SOURCE: Nazha A et al. ASH 2018, Abstract 793.
REPORTING FROM ASH 2018
Physical-Mental Comorbidity of Pediatric Migraine
Comorbidity between headaches with a range of physical conditions that have been associated with adult migraine demonstrates that multimorbidity occurs early in development. This according to a recent study that examined the associations between headaches and migraine with physical and mental disorders in a large pediatric registry. The study included 9,329 youth aged 8-21 years from the Philadelphia Neurodevelopmental Cohort. Physical conditions, including headache, were ascertained from electronic medical records and in-person interviews. Modified International Classification of Headache Disorders (ICHD-II) criteria were used to classify migraine symptoms. Forty-two other physical conditions were classified into 14 classes of medical disorders. Researchers found:
- Lifetime prevalence of any headache was 45.5%, and of migraine was 22.6%.
- Any headache was associated with a broad range of physical disorders, attention-deficit/hyperactivity disorder (odds ratio [OR] 1.2), and behavior disorders (1.3).
- Youth with migraine had greater odds of specific physical conditions and mental disorders, including respiratory, neurologic/central nervous system, developmental, anxiety, behavior, and mood disorders than those with non-migraine headache (OR ranged from 1.3 to 1.9).
Lateef T, He J-P, Nelson K, et al. Physical–mental comorbidity of pediatric migraine in the Philadelphia Neurodevelopmental Cohort. [Published online ahead of print October 29, 2018]. J Pediatr. doi:10.1016/j.jpeds.2018.09.033.
Comorbidity between headaches with a range of physical conditions that have been associated with adult migraine demonstrates that multimorbidity occurs early in development. This according to a recent study that examined the associations between headaches and migraine with physical and mental disorders in a large pediatric registry. The study included 9,329 youth aged 8-21 years from the Philadelphia Neurodevelopmental Cohort. Physical conditions, including headache, were ascertained from electronic medical records and in-person interviews. Modified International Classification of Headache Disorders (ICHD-II) criteria were used to classify migraine symptoms. Forty-two other physical conditions were classified into 14 classes of medical disorders. Researchers found:
- Lifetime prevalence of any headache was 45.5%, and of migraine was 22.6%.
- Any headache was associated with a broad range of physical disorders, attention-deficit/hyperactivity disorder (odds ratio [OR] 1.2), and behavior disorders (1.3).
- Youth with migraine had greater odds of specific physical conditions and mental disorders, including respiratory, neurologic/central nervous system, developmental, anxiety, behavior, and mood disorders than those with non-migraine headache (OR ranged from 1.3 to 1.9).
Lateef T, He J-P, Nelson K, et al. Physical–mental comorbidity of pediatric migraine in the Philadelphia Neurodevelopmental Cohort. [Published online ahead of print October 29, 2018]. J Pediatr. doi:10.1016/j.jpeds.2018.09.033.
Comorbidity between headaches with a range of physical conditions that have been associated with adult migraine demonstrates that multimorbidity occurs early in development. This according to a recent study that examined the associations between headaches and migraine with physical and mental disorders in a large pediatric registry. The study included 9,329 youth aged 8-21 years from the Philadelphia Neurodevelopmental Cohort. Physical conditions, including headache, were ascertained from electronic medical records and in-person interviews. Modified International Classification of Headache Disorders (ICHD-II) criteria were used to classify migraine symptoms. Forty-two other physical conditions were classified into 14 classes of medical disorders. Researchers found:
- Lifetime prevalence of any headache was 45.5%, and of migraine was 22.6%.
- Any headache was associated with a broad range of physical disorders, attention-deficit/hyperactivity disorder (odds ratio [OR] 1.2), and behavior disorders (1.3).
- Youth with migraine had greater odds of specific physical conditions and mental disorders, including respiratory, neurologic/central nervous system, developmental, anxiety, behavior, and mood disorders than those with non-migraine headache (OR ranged from 1.3 to 1.9).
Lateef T, He J-P, Nelson K, et al. Physical–mental comorbidity of pediatric migraine in the Philadelphia Neurodevelopmental Cohort. [Published online ahead of print October 29, 2018]. J Pediatr. doi:10.1016/j.jpeds.2018.09.033.
Vestibular Migraine and Upright Perception Errors
Recent findings suggest an abnormal sensory integration for spatial orientation in vestibular migraine (VM), related to daily dizziness in these patients. Researchers investigated the effect of static head tilts on errors of upright perception in a group of 27 patients with VM in comparison with a group of 27 healthy controls. Perception of upright was measured in a dark room using a subjective visual vertical (SVV) paradigm at 3 head tilt positions (upright, ±20°). VM patients were also surveyed about the quality of their dizziness and spatial symptoms during daily activities. Researchers found:
- In the upright head position, SVV errors were within the normal range for VM patients and healthy controls (within 2° from true vertical).
- During the static head tilts of 20° to the right, VM patients showed larger SVV errors consistent with overestimation of the tilt magnitude (ie, as if they felt further tilted toward the right side) (VM: −3.21° ± 0.93 vs control: 0.52° ± 0.70).
- During the head tilt to the left, SVV errors in VM patients did not differ significantly from controls (VM: 0.77° ± 1.05 vs control: −0.04° ± 0.68).
Winnick A, Sadeghpour S, Otero-Millan J, Chang T-P, Kheradmand A. Errors of upright perception in patients with vestibular migraine. [Published online ahead of print October 30, 2018]. Front Neurol. doi:10.3389/fneur.2018.00892.
Recent findings suggest an abnormal sensory integration for spatial orientation in vestibular migraine (VM), related to daily dizziness in these patients. Researchers investigated the effect of static head tilts on errors of upright perception in a group of 27 patients with VM in comparison with a group of 27 healthy controls. Perception of upright was measured in a dark room using a subjective visual vertical (SVV) paradigm at 3 head tilt positions (upright, ±20°). VM patients were also surveyed about the quality of their dizziness and spatial symptoms during daily activities. Researchers found:
- In the upright head position, SVV errors were within the normal range for VM patients and healthy controls (within 2° from true vertical).
- During the static head tilts of 20° to the right, VM patients showed larger SVV errors consistent with overestimation of the tilt magnitude (ie, as if they felt further tilted toward the right side) (VM: −3.21° ± 0.93 vs control: 0.52° ± 0.70).
- During the head tilt to the left, SVV errors in VM patients did not differ significantly from controls (VM: 0.77° ± 1.05 vs control: −0.04° ± 0.68).
Winnick A, Sadeghpour S, Otero-Millan J, Chang T-P, Kheradmand A. Errors of upright perception in patients with vestibular migraine. [Published online ahead of print October 30, 2018]. Front Neurol. doi:10.3389/fneur.2018.00892.
Recent findings suggest an abnormal sensory integration for spatial orientation in vestibular migraine (VM), related to daily dizziness in these patients. Researchers investigated the effect of static head tilts on errors of upright perception in a group of 27 patients with VM in comparison with a group of 27 healthy controls. Perception of upright was measured in a dark room using a subjective visual vertical (SVV) paradigm at 3 head tilt positions (upright, ±20°). VM patients were also surveyed about the quality of their dizziness and spatial symptoms during daily activities. Researchers found:
- In the upright head position, SVV errors were within the normal range for VM patients and healthy controls (within 2° from true vertical).
- During the static head tilts of 20° to the right, VM patients showed larger SVV errors consistent with overestimation of the tilt magnitude (ie, as if they felt further tilted toward the right side) (VM: −3.21° ± 0.93 vs control: 0.52° ± 0.70).
- During the head tilt to the left, SVV errors in VM patients did not differ significantly from controls (VM: 0.77° ± 1.05 vs control: −0.04° ± 0.68).
Winnick A, Sadeghpour S, Otero-Millan J, Chang T-P, Kheradmand A. Errors of upright perception in patients with vestibular migraine. [Published online ahead of print October 30, 2018]. Front Neurol. doi:10.3389/fneur.2018.00892.
Migraine with Visual Aura Risk Factor for AF
Migraine with aura was associated with increased risk of incident atrial fibrillation (AF), according to a recent study, and this may potentially lead to ischemic strokes. In the Atherosclerosis Risk in Communities (ARIC) study, a longitudinal, community-based cohort study, participants were interviewed for migraine history between 1993 and 1995 and were followed for incident AF through 2013. AF was adjudicated using electrocardiographs (ECGs), discharge codes, and death certificates. Researchers found:
- Of 11,939 participants assessed for headache and without prior AF or stroke, 426 reported migraines with visual aura, 1090 with migraine without visual aura, 1018 non-migraine headache, and 9405 no headache.
- Over a 20-year follow-up period, incident AF was noted in 232 (15%) of 1516 with migraine and 1623 (17%) of 9405 without headache.
- After adjustment for multiple confounders, migraine with visual aura was associated with increased risk of AF compared to no headache (hazard ratio 1.30) as well as when compared to migraine without visual aura (hazard ratio 1.39).
- The data suggest that AF may be a potential mediator of migraine with visual aura–stroke risk.
Sen S, Androulakis XM, Duda V, et al. Migraine with visual aura a risk factor for incident atrial fibrillation. A cohort study. [Published online ahead of print November 14, 2018]. Neurology. doi:10.1212/WNL.0000000000006650.
Migraine with aura was associated with increased risk of incident atrial fibrillation (AF), according to a recent study, and this may potentially lead to ischemic strokes. In the Atherosclerosis Risk in Communities (ARIC) study, a longitudinal, community-based cohort study, participants were interviewed for migraine history between 1993 and 1995 and were followed for incident AF through 2013. AF was adjudicated using electrocardiographs (ECGs), discharge codes, and death certificates. Researchers found:
- Of 11,939 participants assessed for headache and without prior AF or stroke, 426 reported migraines with visual aura, 1090 with migraine without visual aura, 1018 non-migraine headache, and 9405 no headache.
- Over a 20-year follow-up period, incident AF was noted in 232 (15%) of 1516 with migraine and 1623 (17%) of 9405 without headache.
- After adjustment for multiple confounders, migraine with visual aura was associated with increased risk of AF compared to no headache (hazard ratio 1.30) as well as when compared to migraine without visual aura (hazard ratio 1.39).
- The data suggest that AF may be a potential mediator of migraine with visual aura–stroke risk.
Sen S, Androulakis XM, Duda V, et al. Migraine with visual aura a risk factor for incident atrial fibrillation. A cohort study. [Published online ahead of print November 14, 2018]. Neurology. doi:10.1212/WNL.0000000000006650.
Migraine with aura was associated with increased risk of incident atrial fibrillation (AF), according to a recent study, and this may potentially lead to ischemic strokes. In the Atherosclerosis Risk in Communities (ARIC) study, a longitudinal, community-based cohort study, participants were interviewed for migraine history between 1993 and 1995 and were followed for incident AF through 2013. AF was adjudicated using electrocardiographs (ECGs), discharge codes, and death certificates. Researchers found:
- Of 11,939 participants assessed for headache and without prior AF or stroke, 426 reported migraines with visual aura, 1090 with migraine without visual aura, 1018 non-migraine headache, and 9405 no headache.
- Over a 20-year follow-up period, incident AF was noted in 232 (15%) of 1516 with migraine and 1623 (17%) of 9405 without headache.
- After adjustment for multiple confounders, migraine with visual aura was associated with increased risk of AF compared to no headache (hazard ratio 1.30) as well as when compared to migraine without visual aura (hazard ratio 1.39).
- The data suggest that AF may be a potential mediator of migraine with visual aura–stroke risk.
Sen S, Androulakis XM, Duda V, et al. Migraine with visual aura a risk factor for incident atrial fibrillation. A cohort study. [Published online ahead of print November 14, 2018]. Neurology. doi:10.1212/WNL.0000000000006650.
Frontal lobe epilepsy elevates seizure risk during pregnancy
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
based on a study reported by Paula E. Voinescu, MD, PhD, at the annual meeting of the American Epilepsy Society.
The single center study included data on 76 pregnancies in women with focal epilepsy –17 of them in patients with frontal lobe epilepsy – and 38 pregnancies in women with generalized epilepsy. Seizures were more frequent during pregnancy, compared with baseline, in 5.5% of women with generalized epilepsy, 22.6% of women with focal epilepsies, and 53.0% of women with frontal lobe epilepsy, said Dr. Voinescu, lead author of the study and a neurologist at Brigham and Women’s Hospital in Boston.
“Frontal lobe epilepsy is known to be difficult to manage in general and often resistant to therapy, but it isn’t clear why the seizures got worse among pregnant women because the levels of medication in their blood was considered adequate. Until more research provides treatment guidance, doctors should carefully monitor their pregnant patients who have focal epilepsy to see if their seizures increase despite adequate blood levels and then adjust their medication if necessary,” she advised. “As we know from other research, seizures during pregnancy can increase the risk of distress and neurodevelopmental delays for the baby, as well as the risk of miscarriage.”
For the study, Dr. Voinescu and her colleagues analyzed prospectively collected clinical data from 99 pregnant women followed at Brigham and Women’s Hospital between 2013 and 2018.
The researchers excluded patients with abortions, seizure onset during pregnancy, poorly defined preconception seizure frequency, nonepileptic seizures, antiepileptic drug (AED) noncompliance, and pregnancies that were enrolled in other studies. The investigators documented patients’ seizure types and AED regimens and recorded seizure frequency during the 9 months before conception, during pregnancy, and 9 months postpartum. The researchers summed all seizures for each individual for each interval. They defined seizure frequency worsening as any increase above the preconception baseline, and evaluated differences between focal and generalized epilepsy and between frontal lobe and other focal epilepsies.
Increased seizure activity tended to occur in women on more than one AED, according to Dr. Voinescu. In women with frontal lobe epilepsy, seizure worsening during pregnancy was most likely to begin in the second trimester.
The gap in seizure frequency between the groups narrowed in the 9-month postpartum period. Seizures were more frequent during the postpartum period, compared with baseline, in 12.12% of women with generalized epilepsy, 20.14% of women with focal epilepsies, and 20.00% of women with frontal lobe epilepsy.
Future analyses will evaluate the influence of AED type and concentration and specific timing on seizure control during pregnancy and the postpartum period, Dr. Voinescu said. Future studies should also include measures of sleep, which may be a contributory mechanism to the differences found between these epilepsy types.
Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
SOURCE: Voinescu PE et al. AES 2018, Abstract 3.236.
REPORTING FROM AES 2018
Key clinical point: Women with focal epilepsy, especially frontal lobe epilepsy, may need closer monitoring during pregnancy.
Major finding: Compared with baseline, seizures were more frequent during pregnancy in 53% of women with frontal lobe epilepsy.
Study details: An analysis of prospectively collected data from 114 pregnancies.
Disclosures: Dr. Voinescu reported receiving funding from the American Brain Foundation, the American Epilepsy Society, and the Epilepsy Foundation through the Susan Spencer Clinical Research Fellowship.
Source: Voinescu PE et al. AES 2018, Abstract 3.236.
Our missing microbes: Short-term antibiotic courses have long-term consequences
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003








