User login
New marks of distinction for SVS members
The SVS Executive Board has announced that all Active SVS members in good standing will now be considered Fellows of the Society for Vascular Surgery™ (FSVS™). The trademarked designation is one of the benefits of SVS membership and is a public acknowledgement that a surgeon has met the high standards required by the SVS of its members and has shown and professional commitment to the field of vascular surgery. Active members in good standing may add the initials FSVS™ after their name in any usage, such as signature lines, letterhead, door signage and so on. Distinguished Fellows also may use the trademarked designation of DFSVS™. Read the official announcement here.
The SVS Executive Board has announced that all Active SVS members in good standing will now be considered Fellows of the Society for Vascular Surgery™ (FSVS™). The trademarked designation is one of the benefits of SVS membership and is a public acknowledgement that a surgeon has met the high standards required by the SVS of its members and has shown and professional commitment to the field of vascular surgery. Active members in good standing may add the initials FSVS™ after their name in any usage, such as signature lines, letterhead, door signage and so on. Distinguished Fellows also may use the trademarked designation of DFSVS™. Read the official announcement here.
The SVS Executive Board has announced that all Active SVS members in good standing will now be considered Fellows of the Society for Vascular Surgery™ (FSVS™). The trademarked designation is one of the benefits of SVS membership and is a public acknowledgement that a surgeon has met the high standards required by the SVS of its members and has shown and professional commitment to the field of vascular surgery. Active members in good standing may add the initials FSVS™ after their name in any usage, such as signature lines, letterhead, door signage and so on. Distinguished Fellows also may use the trademarked designation of DFSVS™. Read the official announcement here.
Beta-cell therapies for type 1 diabetes: Transplants and bionics
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
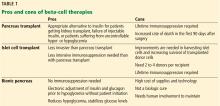
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
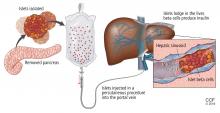
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
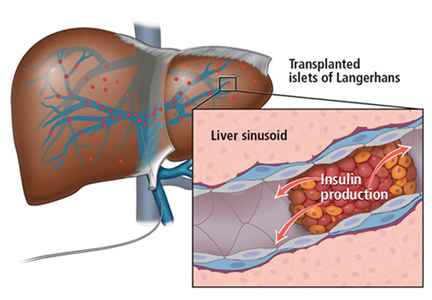
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
KEY POINTS
- Most pancreas transplant recipients become insulin-independent immediately.
- A key drawback to islet transplant is the need for multiple donors to provide enough islet cells to achieve insulin independence.
- As with other organs for transplant, the need for donor pancreases far outnumbers the supply. Stem cells or beta cells grown from stem cells may avoid this problem. Another potential solution is to use organs from animals, possibly pigs, but much more work is needed to make these procedures viable.
- While we await a breakthrough in beta-cell therapy, a bionic pancreas may be the answer for a number of patients.
Cannabis for peripheral neuropathy: The good, the bad, and the unknown
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
Marijuana, which is still illegal under federal law but legal in 30 states for medical purposes as of this writing, has shown promising results for treating peripheral neuropathy. Studies suggest that cannabis may be an option for patients whose pain responds poorly to standard treatments; however, its use may be restricted by cognitive and psychiatric adverse effects, particularly at high doses.1
In this article, we discuss the basic pharmacology of cannabis and how it may affect neuropathic pain. We review clinical trials on its use for peripheral neuropathy and provide guidance for its use.
PERIPHERAL NEUROPATHY IS COMMON AND COMPLEX
An estimated 20 million people in the United States suffer from neuropathic pain. The prevalence is higher in certain populations, with 26% of people over age 65 and 30% of patients with diabetes mellitus affected.2–4
Peripheral neuropathy is a complex, chronic state that occurs when nerve fibers are damaged, dysfunctional, or injured, sending incorrect signals to pain centers in the central nervous system.5 It is characterized by weakness, pain, and paresthesias that typically begin in the hands or feet and progress proximally.4 Symptoms depend on the number and types of nerves affected.
In many cases, peripheral neuropathy is idiopathic, but common causes include diabetes, alcoholism, human immunodeficiency virus (HIV) infection, and autoimmune disease. Others include toxicity from chemotherapy and heavy metals.
Peripheral neuropathy significantly worsens quality of life and function. Many patients experience emotional, cognitive, and functional problems, resulting in high rates of medical and psychiatric comorbidities and occupational impairment.4,6,7 Yet despite its clinical and epidemiologic significance, it is often undertreated.8
STANDARD TREATMENTS INADEQUATE
Peripheral neuropathy occurs in patients with a wide range of comorbidities and is especially difficult to treat. Mainstays of therapy include anticonvulsants, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors.9 A more invasive option is spinal cord stimulation.
These treatments can have considerable adverse effects, and response rates remain suboptimal, with pain relief insufficient to improve quality of life for many patients.9,10 Better treatments are needed to improve clinical outcomes and patient experience.11
CANNABIS: A MIX OF COMPOUNDS
Cannabis sativa has been used as an analgesic for centuries. The plant contains more than 400 chemical compounds and is often used for its euphoric properties. Long-term use may lead to addiction and cognitive impairment.12,13
Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the main components and the 2 best-studied cannabinoids with analgesic effects.
THC is the primary psychoactive component of cannabis. Its effects include relaxation, altered perception, heightened sensations, increased libido, and perceptual distortions of time and space. Temporary effects may include decreased short-term memory, dry mouth, impaired motor function, conjunctival injection, paranoia, and anxiety.
CBD is nonpsychoactive and has anti-inflammatory and antioxidant properties. It has been shown to reduce pain and inflammation without the effects of THC.14
Other compounds in the cannabis plant include phytocannabinoids, flavonoids, and tapenoids, which may produce individual, interactive, or synergistic effects.15 Different strains of cannabis have varying amounts of the individual components, making comparisons among clinical studies difficult.
THE ENDOCANNABINOID SYSTEM
The endogenous mammalian cannabinoid system plays a regulatory role in the development, homeostasis, and neuroplasticity of the central nervous system. It is also involved in modulating pain transmission in the nociceptive pathway.
Two of the most abundant cannabinoid endogenous ligands are anandamide and 2-arachidonylglycerol.9 These endocannabinoids are produced on demand in the central nervous system to reduce pain by acting as a circuit breaker.16–18 They target the G protein-coupled cannabinoid receptors CB1 and CB2, located throughout the central and peripheral nervous system and in organs and tissues.12
CB1 receptors are found primarily in the central nervous system, specifically in areas involved in movement, such as the basal ganglia and cerebellum, as well as in areas involved in memory, such as the hippocampus.12 They are also abundant in brain regions implicated in conducting and modulating pain signals, including the periaqueductal gray and the dorsal horn of the spinal cord.16–20
CB2 receptors are mostly found in peripheral tissues and organs, mainly those involved in the immune system, including splenic, tonsillar, and hematopoietic cells.12 They help regulate inflammation, allodynia, and hyperalgesia.17
Modifying response to injury
Following a nerve injury, neurons along the nociceptive pathway may become more reactive and responsive in a process known as sensitization.21 The process involves a cascade of cellular events that result in sprouting of pain-sensitive nerve endings.21,22
Cannabinoids are thought to reduce pain by modifying these cellular events. They also inhibit nociceptive conduction in the dorsal horn of the spinal cord and in the ascending spinothalamic tract.20 CB1 receptors found in nociceptive terminals along the peripheral nervous system impede pain conduction, while activation of CB2 receptors in immune cells decreases the release of nociceptive agents.
STUDIES OF CANNABIS FOR NEUROPATHIC PAIN
A number of studies have evaluated cannabis for treating neuropathic pain. Overall, available data support the efficacy of smoked or inhaled cannabis in its flower form when used as monotherapy or adjunctive therapy for relief of neuropathic pain of various etiologies. Many studies also report secondary benefits, including better sleep and functional improvement.23,24
However, adverse effects are common, especially at high doses, and include difficulty concentrating, lightheadedness, fatigue, and tachycardia. More serious reported adverse effects include anxiety, paranoia, and psychosis.
Wilsey et al, 2008: Neuropathic pain reduced
Wilsey et al25 conducted a double-blind, placebo-controlled crossover study that assessed the effects of smoking cannabis in 38 patients with central or peripheral neuropathic pain. Participants were assigned to smoke either high- or low-dose cannabis (7% or 3.5% delta-9-THC) or placebo cigarettes. Cigarettes were smoked during treatment sessions using the following regimen: 2 puffs at 60 minutes from baseline, 3 puffs at 120 minutes, and 4 puffs at 180 minutes. Patients were assessed after each set of puffs and for 2 hours afterwards. The primary outcome was spontaneous relief of pain as measured by a visual analog scale.
Pain intensity was comparable and significantly reduced in both treatment groups compared with placebo. At the high dose, some participants experienced neurocognitive impairment in attention, learning, memory, and psychomotor speed; only learning and memory declined at the low dose.
Ellis et al, 2009: Pain reduction in HIV neuropathy
Ellis et al23 conducted a double-blind, placebo-controlled crossover trial in patients with HIV neuropathy that was unresponsive to at least 2 analgesics with different modes of action. During each treatment week, participants were randomly assigned to smoke either active cannabis or placebo, while continuing their standard therapy. Titration started at 4% THC and was adjusted based on tolerability and efficacy. Twenty-eight of the 34 enrolled patients completed both cannabis and placebo treatments. The principal outcome was change in pain intensity from baseline at the end of each week, using the Descriptor Differential Scale of Pain Intensity.
Of the 28 patients, 46% achieved an average pain reduction of 3.3 points (30%). One patient experienced cannabis-induced psychosis, and another developed an intractable cough, which resolved with smoking cessation.
Ware et al, 2010: Reduced posttraumatic or postsurgical neuropathic pain
Ware et al24 performed a randomized crossover trial in 21 patients with posttraumatic or postsurgical neuropathic pain. Participants inhaled 4 different formulations of cannabis (containing 0%, 2.5%, 6.0%, and 9.4% THC) during 4 14-day periods. They inhaled a 25-mg dose through a pipe 3 times a day for the first 5 days of each cycle, followed by a 9-day washout period. Daily average pain intensity was measured using a numeric rating scale. The investigators also assessed mood, sleep, quality of life, and adverse effects.
Patients in the 9.4% THC group reported significantly less pain and better sleep, with average pain scores decreasing from 6.1 to 5.4 on an 11-point scale. Although the benefit was modest, the authors noted that the pain had been refractory to standard treatments.
The number of reported adverse events increased with greater potency and were most commonly throat irritation, burning sensation, headache, dizziness, and fatigue. This study suggests that THC potency affects tolerability, with higher doses eliciting clinically important adverse effects, some of which may reduce the ability to perform activities of daily living, such as driving.
Wilsey et al, 2013: Use in resistant neuropathic pain
Wilsey et al26 conducted another double-blind, placebo-controlled crossover study assessing the effect of vaporized cannabis on central and peripheral neuropathic pain resistant to first-line pharmacotherapies. Dose-effect relationships were explored using medium-dose (3.5%), low-dose (1.3%), and placebo cannabis. The primary outcome measure was a 30% reduction in pain intensity based on a visual analog scale.
In the placebo group, 26% of patients achieved this vs 57% of the low-dose cannabis group and 61% of those receiving the medium dose. No significant difference was found between the 2 active doses in reducing neuropathic pain, and both were more effective than placebo. The number needed to treat to achieve a 30% reduction in pain was about 3 for both cannabis groups compared with placebo. Psychoactive effects were minimal, of short duration, and reversible.
Wallace et al, 2015: Use in diabetic peripheral neuropathy
Wallace et al27 conducted a randomized, double-blind, placebo-controlled crossover study evaluating cannabis for diabetic peripheral neuropathy in 16 patients. Each had experienced at least 6 months of neuropathic pain in their feet. The participants inhaled a single dose of 1%, 4%, or 7% THC cannabis or placebo. Spontaneous pain was reported with a visual analog scale and also tested with a foam brush and von Frey filament at intervals until 4 hours after treatment.
Pain scores were lower with treatment compared with placebo, with high-dose cannabis having the greatest analgesic effect. Pain reduction lasted for the full duration of the test. Cannabis recipients had declines in attention and working memory, with the high-dose group experiencing the greatest impact 15 minutes after treatment. High-dose recipients also had poorer scores on testing of quick task-switching, with the greatest effect at 2 hours.27
Research and market cannabis are not equal
Results of US studies must be qualified. Most have used cannabis provided by the National Institute of Drug Abuse (NIDA),23–26 which differs in potency from commercially available preparations. This limits the clinical usefulness of the analysis of benefits and risks.
Vergara et al28 found that NIDA varieties contained much lower THC levels and as much as 23 times the cannabinol content as cannabis in state-legalized markets.
Studies based on NIDA varieties likely underestimate the risks of consumer-purchased cannabis, as THC is believed to be most responsible for the risk of psychosis and impaired driving and cognition.24,28
CBD MAY PROTECT AGAINST ADVERSE EFFECTS
Studies of CBD alone are limited to preclinical data.29 Evidence suggests that CBD alone or combined with THC can suppress chronic neuropathic pain, and that CBD may have a protective effect after nerve injury.30
Nabiximols, an oromucosal spray preparation with equal amounts of THC and CBD, has been approved in Canada as well as in European countries including the United Kingdom. Although its use has not been associated with many of the adverse effects of inhaled cannabis,30–32 evidence of efficacy from clinical trials has been mixed.
Lynch et al,31 in a 2014 randomized, double-blind, placebo-controlled crossover pilot study31 evaluated nabiximols in 16 patients with neuropathic pain related to chemotherapy. No statistically significant difference was found between treatment and placebo. However, the trial was underpowered.
Serpell et al,32 in a 2014 European randomized, placebo-controlled parallel-group study, evaluated 246 patients with peripheral neuropathy with allodynia, with 128 receiving active treatment (THC-CBD oromucosal spray) and 118 receiving placebo. Over the 15-week study, participants continued their current analgesic treatments.
Pain was reduced in the treatment group, but the difference from placebo was not statistically significant. However, the treatment group reported significantly better sleep quality and Patient Global Impression of Change measures (reflecting a patient’s belief of treatment efficacy).
META-ANALYSES CONFIRM EFFECT
Three meta-analyses of available studies of the effects of cannabis on neuropathic pain have been completed.
Andreae et al, 2015: 5 trials, 178 patients
Andreae et al1 evaluated 5 randomized controlled trials in 178 patients in North America. All had had neuropathy for at least 3 months, with a pain level of at least about 3 on a scale of 10. Two studies had patients with HIV-related neuropathy; the other 3 involved patients with neuropathy related to trauma, diabetes, complex regional pain syndrome, or spinal cord injury. All trials used whole cannabis plant provided by NIDA, and the main outcomes were patient-reported pain scales. No study evaluated pain beyond 2 weeks after trial termination.
They found that 1 of every 5 to 6 patients treated with cannabis had at least a 30% pain reduction.
Nugent et al, 2017: 13 trials, 246 patients
Nugent et al33 reviewed 13 trials in 246 patients that evaluated the effects of different cannabis-based preparations on either central or peripheral neuropathic pain from various conditions. Actively treated patients were more likely to report a 30% improvement in neuropathic pain. Again, studies tended to be small and brief.
Cochrane review, 2018: 16 trials, 1,750 patients
A Cochrane review34 analyzed 16 trials (in 1,750 patients) lasting 2 to 26 weeks. Treatments included an oromucosal spray with a plant-derived combination of THC and CBD, nabilone, inhaled herbal cannabis, and plant-derived THC.
With cannabis-based treatments, significantly more people achieved 50% or greater pain relief than with placebo (21% vs 17%, number needed to treat 20); 30% pain reduction was achieved in 39% of treated patients vs 33% of patients taking placebo (number needed to treat 11).
On the other hand, significantly more participants withdrew from studies because of adverse events with cannabis-based treatments than placebo (10% vs 5%), with psychiatric disorders occurring in 17% of patients receiving active treatment vs 5% of those receiving placebo (number needed to harm 10).
The primary studies suffered from methodologic limitations including small size, short duration, and inconsistency of formulations and study designs. Further evaluation of long-term efficacy, tolerability, and addiction potential is critical to determine the risk-benefit ratio.
RISKS OF CANNABIS USE
Like any drug therapy, cannabis has effects that may limit its use. Cannabis can affect a person’s psyche, physiology, and lifestyle.
Impaired attention, task speed
Neurocognitive changes associated with cannabis use—especially dizziness, fatigue, and slowed task-switching—could affect driving and other complex tasks. Evidence indicates that such activities should be avoided in the hours after treatment.26,27,32,33
Concern over brain development
Most worrisome is the effect of long-term cannabis use on brain development in young adults. Regular use of cannabis at an early age is associated with lower IQ, decline in school performance, and lower rates of high school graduation.35
Avoid in psychiatric patients
It is unlikely that cannabis can be safely used in patients with psychiatric illnesses. Anxiety, depression, and psychotic disorders can be exacerbated by the regular use of cannabis, and the risk of developing these conditions is increased while using cannabis.36,37
High concentrations of THC (the highest concentration used in the above studies was 9.5%) can cause anxiety, paranoia, and psychosis.
Respiratory effects
Long-term cannabis smoking may cause wheezing, cough, dyspnea, and exacerbations of chronic bronchitis. There is some evidence that symptoms improve after stopping smoking.33,38
SHOULD WE RECOMMEND CANNABIS?
Where cannabis can be legally used, doctors should be familiar with the literature and its limitations so that they can counsel patients on the best use and potential risks and benefits of cannabis treatment.
A recent conceptualization of pain suggests that a pain score reflects a composite of sensory factors (eg, tissue damage), cognitive factors (eg, beliefs about pain), and affective factors (eg, anxiety, depression).39 Physicians should keep this in mind when evaluating patients to better assess the risks and benefits of cannabis. While pharmacotherapy may address sensory factors, cognitive behavioral therapy may help alter beliefs about the pain as well as anxiety and depressive symptoms that might influence subjective reports.
Ideally, patients being considered for cannabis treatment would have a type of neuropathic pain proven to respond to cannabis in randomized, controlled studies, as well as evidence of failed first-line treatments.
Relative contraindications include depression, anxiety, substance use, psychotic disorders, and respiratory conditions, and these should also be considered.
Although current research shows an analgesic benefit of cannabis on neuropathic pain comparable to that of gabapentin,40 further investigation is needed to better evaluate long-term safety, efficacy, and interactions with standard therapies. Until we have a more complete picture, we should use the current literature, along with a thorough knowledge of each patient, to determine if the benefits of cannabis therapy outweigh the risks.
Acknowledgments: We thank Camillo Ferrari, BS, and Christina McMahon, BA, for their helpful comments.
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
- Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain 2015; 16(12):1221–1232. doi:10.1016/j.jpain.2015.07.009
- National Institute of Neurological Disorders and Stroke. Peripheral Neuropathy Fact Sheet. www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet. Accessed November 14, 2018.
- Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older adults. J Am Board Fam Med 2004; 17(5):308–318. doi:10.3122/jabfm.17.5.309
- Bansal D, Gudala K, Muthyala H, Esam HP, Nayakallu R, Bhansali A. Prevalence and risk factors of developing peripheral diabetic neuropathy in type 2 diabetes mellitus in a tertiary care setting. J Diabetes Investig 2014; 5(6):714–721. doi:10.1111/jdi.12223
- Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
- Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain 2016; 157(suppl 1):S23–S32. doi:10.1097/j.pain.0000000000000428
- Zeng L, Alongkronrusmee D, van Rijn RM. An integrated perspective on diabetic, alcoholic, and drug-induced neuropathy, etiology, and treatment in the US. J Pain Res 2017; 10:219–228. doi:10.2147/JPR.S125987
- Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy: a review. JAMA 2015; 314(20):2172–2181. doi:10.1001/jama.2015.13611
- Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthc (Amst) 2017; 5(4):171–173. doi:10.1016/j.hjdsi.2016.10.003
- Gwak YS, Kim HY, Lee BH, Yang CH. Combined approaches for the relief of spinal cord injury-induced neuropathic pain. Complement Ther Med 2016; 25:27–33. doi:10.1016/j.ctim.2015.12.021
- Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016; 30(11):1020–1029. pmid:27854104
- Grotenhermen F. Cannabinoids and the endocannabinoid system. Cannabinoids 2006; 1(1):10–14.
- Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313(24):2474–2483. doi:10.1001/jama.2015.6199
- Campos AC, Fogaça MV, Scarante FF, et al. Plastic and neuroprotective mechanisms involved in the therapeutic effects of cannabidiol in psychiatric disorders. Front Pharmacol 2017; 8:269. doi:10.3389/fphar.2017.00269
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
- Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci 2017; Jul 7: 1–20. doi:10.1080/1028415X.2017.1347373
- Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018; 43(1):155–172. doi:10.1038/npp.2017.130
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991; 11(2):563–583. pmid:1992016
- Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience1998; 83(2):393–411. pmid:9460749
- Russo EB, Hohmann AG. Role of cannabinoids in pain management. In: Deer TR, Leong MS, ed. Comprehensve Treatment of Chronic Pain by Medical, Interventional, and Integrative Approaches. New York, NY: Springer; 2013:181–193.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012; 12(4):304–314. pmid:23033930
- Yamanaka H, Noguchi K. Pathophysiology of neuropathic pain: molecular mechanisms underlying central sensitization in the dorsal horn in neuropathic pain. Brain Nerve 2012; 64(11):1255–1265. Japanese. pmid:23131736
- Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34(3):672–680. doi:10.1038/npp.2008.120
- Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182(14):E694–E701. doi:10.1503/cmaj.091414
- Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008; 9(6):506–521. doi:10.1016/j.jpain.2007.12.010
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013; 14(2):136–148. doi:10.1016/j.jpain.2012.10.009
- Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16(7):616–627. doi:10.1016/j.jpain.2015.03.008
- Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Scientific Reports. 2017; 7(1):1-8. doi:10.1038/srep46528
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterized by allodynia: a randomized, double-blind, placebo-controlled clinical trial. Pain 2007; 133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
- Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017; 158(12):2442–2451. doi:10.1097/j.pain.0000000000001052
- Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage 2014; 47(1):166–173. doi:10.1016/j.jpainsymman.2013.02.018
- Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
- Nugent SM, Morasco BJ, O’Neil ME, et al. The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167(5):319–331. doi:10.7326/M17-0155
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018; 3:CD012182. doi:10.1002/14651858.CD012182.pub2
- Castellanos-Ryan N, Pingault JB, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol 2017; 29(4):1253–1266. doi:10.1017/S0954579416001280
- Karila L, Roux P, Benyamina A, et al. Acute and long-term effects of cannabis use: a review. Curr Pharm Des 2014; 20(25):4112–4118. pmid:24001294
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122. pmid:11157424
- National Academies of Science, Engineering, and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academy Press; 2017. doi:10.17226/24625
- Modesto-Lowe V, Griard L, Chaplin M. Cancer pain in the opioid-addicted patient: can we treat it right? J Opioid Manag 2012; 8(3):167–175. doi:10.5055/jom.2012.0113
- Grant I. Medicinal cannabis and painful sensory neuropathy. Virtual Mentor 2013; 15(5):466–469. doi:10.1001/virtualmentor.2013.15.5.oped1-1305
KEY POINTS
- Small clinical studies have found that cannabis provides benefits for peripheral neuropathy, including pain reduction, better sleep, and improved function, even in patients with symptoms refractory to standard therapies.
- Adverse effects such as throat irritation, headache, and dizziness are common, and serious neuropsychiatric effects can occur at high doses.
- Safety may not be adequately assessed in US trials because cannabis supplied by the National Institute of Drug Abuse is less potent than commercially available products.
Geriatrics update 2018: Challenges in mental health, mobility, and postdischarge care
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
Unfortunately, recent research has not unveiled a breakthrough for preventing or treating cognitive impairment or Alzheimer disease. But several studies from the last 2 years are helping to drive the field of geriatrics forward, providing evidence of what does and does not help a variety of issues specific to the elderly.
Based on a search of the 2017 and 2018 literature, this article presents new evidence on preventing and treating cognitive impairment, managing dementia-associated behavioral disturbances and delirium, preventing falls, and improving inpatient mobility and posthospital care transitions.
COGNITIVE IMPAIRMENT, DEMENTIA: STILL NO SILVER BULLET
With the exception of oral anticoagulation treatment for atrial fibrillation, there is little evidence that pharmacologic or nonpharmacologic interventions slow the onset or progression of Alzheimer disease.
Nonpharmacologic interventions
Home occupational therapy. A 2-year home-based occupational therapy intervention1 showed no evidence of slowing functional decline in patients with Alzheimer disease. The randomized controlled trial involving 180 participants consisted of monthly sessions of an intensive, well-established collaborative-care management model that included fall prevention and other safety strategies, personalized training in activities of daily living, exercise, and education. Outcome measures for activities of daily living did not differ significantly between the treatment and control groups.1
Physical activity. Whether physical activity interventions slow cognitive decline and prevent dementia in cognitively intact adults was examined in a systematic review of 32 trials.2 Most of the trials followed patients for 6 months; a few stretched for 1 or 2 years.
Evidence was insufficient to prove cognitive benefit for short-term, single-component or multicomponent physical activity interventions. However, a multidomain physical activity intervention that also included dietary modifications and cognitive training did show a delay in cognitive decline, but only “low-strength” evidence.2
Nutritional supplements. The antioxidants vitamin E and selenium were studied for their possible cognitive benefit in the double-blind randomized Prevention of Alzheimer Disease by Vitamin E and Selenium trial3 in 3,786 asymptomatic men ages 60 and older. Neither supplement was found to prevent dementia over a 7-year follow-up period.
A review of 38 trials4 evaluated the effects on cognition of omega-3 fatty acids, soy, ginkgo biloba, B vitamins, vitamin D plus calcium, vitamin C, beta-carotene, and multi-ingredient supplements. It found insufficient evidence to recommend any over-the-counter supplement for cognitive protection in adults with normal cognition or mild cognitive impairment.
Pharmacologic treatments
Testosterone supplementation. The Testosterone Trials tested the effects of testosterone gel vs placebo for 1 year on 493 men over age 65 with low testosterone (< 275 ng/mL) and with subjective memory complaints and objective memory performance deficits. Treatment was not associated with improved memory or other cognitive functions compared with placebo.5
Antiamyloid drugs. A randomized, double-blind, placebo-controlled trial in nearly 2,000 patients evaluated verubecestat, an oral beta-site amyloid precursor protein-cleaving enzyme-1 inhibitor that reduces the amyloid-beta level in cerebrospinal fluid.6
Verubecestat did not reduce cognitive or functional decline in patients with mild-to-moderate Alzheimer disease, while adverse events including rashes, falls, injuries, sleep disturbances, suicidal ideation, weight loss, and hair color change were more common in the treatment groups. The trial was terminated early because of futility at 50 months.
And in a placebo-controlled trial of solanezumab, a monoclonal antibody directed against the amyloid beta peptide, no benefit was demonstrated at 80 weeks in more than 2,000 patients with Alzheimer disease.7
Multiple common agents. A well-conducted systematic review8 of 51 trials of at least a 6-month duration did not support the use of antihypertensive agents, diabetes medications, nonsteroidal anti-inflammatory drugs, aspirin, hormones, or lipid-lowering drugs for cognitive protection for people with normal cognition or mild cognitive impairment.
However, some studies found reassuring evidence that standard therapies for other conditions do not worsen cognitive decline and are protective for atrial fibrillation.8
Proton-pump inhibitors. Concern exists for a potential link between dementia risk and proton-pump inhibitors, which are widely used to treat acid-related gastrointestinal disorders.9
A prospective population-based cohort study10 of nearly 3,500 people ages 65 and older without baseline dementia screened participants for dementia every 2 years over a mean period of 7.5 years and provided further evaluation for those who screened positive. Use of proton-pump inhibitors was not found to be associated with dementia risk, even with high cumulative exposure.
Results from this study do not support avoiding proton-pump inhibitors out of concern for dementia risk, although long-term use is associated with other safety concerns.
Oral anticoagulation. The increased risk of dementia with atrial fibrillation is well documented.11
A retrospective study12 based on a Swedish health registry and using more than 444,000 patients covering more than 1.5 million years at risk found that oral anticoagulant treatment at baseline conferred a 29% lower risk of dementia in an intention-to-treat analysis and a 48% lower risk in on-treatment analysis compared with no oral anticoagulation therapy. No difference was found between new oral anticoagulants and warfarin.
Transcatheter aortic valve implantation is not associated with cognitive decline
For patients with severe aortic stenosis who are not surgical candidates, transcatheter aortic valve implantation is superior to standard medical therapy,13 but there are concerns of neurologic and cognitive changes after the procedure.14 A meta-analysis of 18 studies assessing cognitive performance in more than 1,000 patients (average age ≥ 80) after undergoing the procedure for severe aortic stenosis found no significant cognitive performance changes from baseline perioperatively or 3 or 6 months later.15
TREATING DEMENTIA-ASSOCIATED BEHAVIORAL DISTURBANCES
Behavioral and psychiatric symptoms often accompany dementia, but no drugs have yet been approved by the US Food and Drug Administration (FDA) to address them in this population. Nonpharmacologic interventions are recommended as first-line therapy.
Antipsychotics are not recommended
Antipsychotics are often prescribed,16 although they are associated with metabolic syndrome17 and increased risks of stroke and death.18 The FDA has issued black box warnings against using antipsychotics for behavioral management in patients with dementia. Further, the American Geriatrics Society and the American Psychiatric Association do not endorse using them as initial therapy for behavioral and psychological symptoms of dementia.16,19
The Centers for Medicare and Medicaid Services partnered with nursing homes to improve the quality of care for patients with dementia, with results measured as the rate of prescribing antipsychotic medications. Although the use of psychotropic medications declined after initiating the partnership, the use of mood stabilizers increased, possibly as a substitute for antipsychotics.20
Dextromethorphan-quinidine use is up, despite modest evidence of benefit
A consumer news report in 2017 stated that the use of dextromethorphan-quinidine in long-term care facilities increased by nearly 400% between 2012 and 2016.21
Evidence for its benefits comes from a 10-week, phase 2, randomized controlled trial conducted at 42 US study sites with 194 patients with probable Alzheimer disease. Compared with the placebo group, the active treatment group had mildly reduced agitation but an increased risk of falls, dizziness, and diarrhea. However, rates of adverse effects were low, and the authors concluded that treatment was generally well tolerated.22
Pimavanserin: No long-term benefit for psychosis
In a phase 2, randomized, double-blind, placebo-controlled trial in 181 patients with possible or probable Alzheimer disease and psychotic symptoms, pimavanserin was associated with improved symptoms as measured by the Neuropsychiatric Inventory–Nursing Home Version psychosis score at 6 weeks, but no difference was found compared with placebo at 12 weeks. The treatment group had more adverse events, including agitation, aggression, peripheral edema, anxiety, and symptoms of dementia, although the differences were not statistically significant.23
DELIRIUM: AVOID ANTIPSYCHOTICS
Delirium is common in hospitalized older adults, especially those who have baseline cognitive or functional impairment and are exposed to precipitating factors such as treatment with anticholinergic or narcotic medications, infection, surgery, or admission to an intensive care unit.24
Delirium at discharge predicts poor outcomes
In a prospective study of 152 hospitalized patients with delirium, those who either did not recover from delirium or had only partially recovered at discharge were more likely to visit the emergency department, be rehospitalized, or die during the subsequent 3 months than those who had fully recovered from delirium at discharge.25
Multicomponent, patient-centered approach can help
A randomized trial in 377 patients in Taiwan evaluated the use of a modified Hospital Elder Life Program, consisting of 3 protocols focused on orienting communication, oral and nutritional assistance, and early mobilization. Patients were at least 65 years old and undergoing elective abdominal surgery with expected length of hospital stay longer than 6 days. The program, administered daily during hospitalization, significantly lowered postoperative delirium by 56% and hospital stay by 2 days compared with usual care.26
Prophylactic haloperidol does not improve outcomes
In a multicenter randomized, double-blind, placebo-controlled trial, van den Boogaard et al studied prophylactic intravenous haloperidol in nearly 1,800 critically ill patients at high risk of delirium.27 Haloperidol did not improve survival at 28 days compared with placebo. For secondary outcomes, including delirium incidence, delirium-free and coma-free days, duration of mechanical ventilation, and hospital and intensive care department length of stay, treatment was not found to differ statistically from placebo.
Antipsychotics may worsen delirium
A double-blind, parallel-arm, dose-titrated randomized trial, conducted at 11 Australian hospices or hospitals with palliative care services, administered oral risperidone, haloperidol, or placebo to 247 patients with life-limiting illness and delirium. Both treatment groups had higher delirium symptom scores than the placebo group.28
In addition, a systematic review and meta-analysis of 19 studies found no benefit of antipsychotic medications for preventing or treating delirium in hospitalized adults.29
Antipsychotics are often continued indefinitely
A retrospective chart review at a US academic health system found30 that among 487 patients with a new antipsychotic medication prescribed during hospitalization, 147 (30.2%) were discharged on an antipsychotic. Of these, 121 (82.3%) had a diagnosis of delirium. Only 15 (12.4%) had discharge summaries that included instructions for discontinuing the drug.
Another US health system retrospectively reviewed antipsychotic use and found31 that out of 260 patients who were newly exposed to an antipsychotic drug during hospitalization, 146 (56.2%) were discharged on an antipsychotic drug, and 65% of these patients were still on the drug at the time of the next hospital admission.
EXERCISE, EXERCISE, EXERCISE
Exercise recommended, but not vitamin D, to prevent falls
In 2018, the US Preventive Services Task Force updated its recommendations for preventing falls in community-dwelling older adults.32 Based on the findings of several trials, the task force recommends exercise interventions for adults age 65 and older who are at increased risk for falls. Gait, balance, and functional training were studied in 17 trials, resistance training in 13, flexibility in 8, endurance training in 5, and tai chi in 3, with 5 studies including general physical activity. Exercise interventions most commonly took place for 3 sessions per week for 12 months (range 2–42 months).
The task force also recommends against vitamin D supplementation for fall prevention in community-dwelling adults age 65 or older who are not known to have osteoporosis or vitamin D deficiency.
Early mobilization helps inpatients
Hospitalized older adults usually spend most of their time in bed. Forty-five previously ambulatory patients (age ≥ 65 without dementia or delirium) in a Veterans Affairs hospital were monitored with wireless accelerometers and were found to spend, on average, 83% of the measured hospital stay in bed. Standing or walking time ranged from 0.2% to 21%, with a median of only 3% (43 minutes a day).33
Since falls with injury became a Centers for Medicare and Medicaid Services nonreimbursable hospital-acquired condition, tension has arisen between promoting mobility and preventing falls.34 Two studies evaluating the adoption of mobility-restricting approaches such as bed-alarms, “fall-alert” signs, supervision of patients in the bathroom, and ensuring patients’ walking aids are within reach, did not find a significant reduction in falls or fall-related injuries.35,36
A clinically significant loss of community mobility is common after hospitalization in older adults.37 Older adults who developed mobility impairment during hospitalization had a higher risk of death in a large, retrospective study.38 A large Canadian multisite intervention trial39 that promoted early mobilization in older patients who were admitted to general medical wards resulted in increased mobilization and significantly shorter hospital stays.
POSTHOSPITAL CARE NEEDS IMPROVEMENT
After hospitalization, older adults who have difficulty with activities of daily living or complex medical needs often require continued care.
About 20% of hospitalized Medicare beneficiaries in the United States are discharged to skilled nursing facilities.40 This is often a stressful transition, and most people have little guidance on selecting a facility and simply choose one based on its proximity to home.41
A program of frequent visits by hospital-employed physicians and advanced practice professionals at skilled nursing facilities resulted in a significantly lower 30-day readmission rate compared with nonparticipating skilled nursing facilities in the same geographic area.42
Home healthcare is recommended after hospital discharge at a rapidly increasing rate. Overall referral rates increased from 8.6% to 14.1% between 2001 and 2012, and from 14.3% to 24.0% for patients with heart failure.43 A qualitative study of home healthcare nurses found a need for improved care coordination between home healthcare agencies and discharging hospitals, including defining accountability for orders and enhancing communication.44
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
- Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med 2017; 166(3):164–171. doi:10.7326/M16-0830
- Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):30–38. doi:10.7326/M17-1528
- Kryscio RJ, Abner EL, Caban-Holt A, et al. Association of antioxidant supplement use and dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol 2017; 74(5):567–573. doi:10.1001/jamaneurol.2016.5778
- Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):52–62. doi:10.7326/M17-1530
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, et al. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017; 317(7):717–727. doi:10.1001/jama.2016.21044
- Egan MF, Kost J, Tariot PN, et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 2018; 378(18):1691–1703. doi:10.1056/NEJMoa1706441
- Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med 2018; 378(4):321–330. doi:10.1056/NEJMoa1705971
- Fink HA, Jutkowitz E, McCarten JR, et al. Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 2018; 168(1):39–51. doi:10.7326/M17-1529
- Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016; 73(4):410–416. doi:10.1001/jamaneurol.2015.4791
- Gray SL, Walker RL, Dublin S, et al. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc 2018; 66(2):247–253. doi:10.1111/jgs.15073
- de Bruijn RF, Heeringa J, Wolters FJ, et al. Association between atrial fibrillation and dementia in the general population. JAMA Neurol 2015; 72(11):1288–1294. doi:10.1001/jamaneurol.2015.2161
- Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J 2018; 39(6):453–460. doi:10.1093/eurheartj/ehx579
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363(17):1597–1607. doi:10.1056/NEJMoa1008232
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA 2016; 316(6):592–601. doi:10.1001/jama.2016.10302
- Khan MM, Herrmann N, Gallagher D, et al. Cognitive outcomes after transcatheter aortic valve implantation: a metaanalysis. J Am Geriatr Soc 2018; 66(2):254–262. doi:10.1111/jgs.15123
- Choosing Wisely; ABIM Foundation. American Geriatrics Society: ten things physicians and patients should question. www.choosingwisely.org/societies/american-geriatrics-society. Accessed November 6, 2018.
- Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry 2004; 6(suppl 2):8–13. pmid:16001095
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294(15):1934–1943. doi:10.1001/jama.294.15.1934
- Choosing Wisely; ABIM Foundation. American Psychiatric Association: five things physicians and patients should question. www.choosingwisely.org/societies/american-psychiatric-association. Accessed November 6, 2018.
- Maust DT, Kim HM, Chiang C, Kales HC. Association of the Centers for Medicare & Medicaid Services’ National Partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med 2018; 178(5):640–647. doi:10.1001/jamainternmed.2018.0379
- CNN. The little red pill being pushed on the elderly. www.cnn.com/2017/10/12/health/nuedexta-nursing-homes-invs/index.html. Accessed November 6, 2018.
- Cummings JL, Lyketsos CG, Peskind ER, et al. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA 2015; 314(12):1242–1254. doi:10.1001/jama.2015.10214
- Ballard C, Banister C, Khan Z, et al; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer’s disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol 2018; 17(3):213–222. doi:10.1016/S1474-4422(18)30039-5
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354(11):1157–1165. doi:10.1056/NEJMra052321
- Cole MG, McCusker J, Bailey R, et al. Partial and no recovery from delirium after hospital discharge predict increased adverse events. Age Ageing 2017; 46(1):90–95. doi:10.1093/ageing/afw153
- Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 2017; 152(9):827–834. doi:10.1001/jamasurg.2017.1083
- van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 2018; 319(7):680–690. doi:10.1001/jama.2018.0160
- Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med 2017; 177(1):34–42. doi:10.1001/jamainternmed.2016.7491
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64(4):705–714. doi:10.1111/jgs.14076
- Johnson KG, Fashoyin A, Madden-Fuentes R, Muzyk AJ, Gagliardi JP, Yanamadala M. Discharge plans for geriatric inpatients with delirium: a plan to stop antipsychotics? J Am Geriatr Soc 2017; 65(10):2278–2281. doi:10.1111/jgs.15026
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11(8):550–555. doi:10.1002/jhm.2585
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force Recommendation statement. JAMA 2018; 319(16):1696–1704. doi:10.1001/jama.2018.3097
- Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57(9):1660–1665. doi:10.1111/j.1532-5415.2009.02393.x
- Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med 2017; 177(6):759–760. doi:10.1001/jamainternmed.2017.0840
- Barker AL, Morello RT, Wolfe R, et al. 6-PACK programme to decrease fall injuries in acute hospitals: cluster randomised controlled trial. BMJ 2016; 352:h6781. doi:10.1136/bmj.h6781
- Shorr RI, Chandler AM, Mion LC, et al. Effects of an intervention to increase bed alarm use to prevent falls in hospitalized patients: a cluster randomized trial. Ann Intern Med 2012; 157(10):692–699. doi:10.7326/0003-4819-157-10-201211200-00005
- Loyd C, Beasley TM, Miltner RS, Clark D, King B, Brown CJ. Trajectories of community mobility recovery after hospitalization in older adults. J Am Geriatr Soc 2018; 66(7):1399–1403. doi:10.1111/jgs.15397
- Valiani V, Chen Z, Lipori G, Pahor M, Sabbá C, Manini TM. Prognostic value of Braden Activity subscale for mobility status in hospitalized older adults. J Hosp Med 2017; 12(6):396–401. doi:10.12788/jhm.2748
- Liu B, Moore JE, Almaawiy U, et al; MOVE ON Collaboration. Outcomes of mobilisation of vulnerable elders in Ontario (MOVE ON): a multisite interrupted time series evaluation of an implementation intervention to increase patient mobilisation. Age Ageing 2018; 47(1):112–119. doi:10.1093/ageing/afx128
- Report to Congress: Medicare Payment Policy. Medicare Payment Advisory Commission 2016. www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed November 6, 2018.
- Gadbois EA, Tyler DA, Mor V. Selecting a skilled nursing facility for postacute care: individual and family perspectives. J Am Geriatr Soc 2017; 65(11):2459–2465. doi:10.1111/jgs.14988
- Kim LD, Kou L, Hu B, Gorodeski EZ, Rothberg MB. Impact of a connected care model on 30-day readmission rates from skilled nursing facilities. J Hosp Med 2017; 12(4):238–244. doi:10.12788/jhm.2710
- Jones CD, Ginde AA, Burke RE, Wald HL, Masoudi FA, Boxer RS. Increasing home healthcare referrals upon discharge from U.S. hospitals: 2001-2012. J Am Geriatr Soc 2015; 63(6):1265–1266. doi:10.1111/jgs.13467
- Jones CD, Jones J, Richard A, et al. “Connecting the dots”: a qualitative study of home health nurse perspectives on coordinating care for recently discharged patients. J Gen Intern Med 2017; 32(10):1114–1121. doi:10.1007/s11606-017-4104-0
KEY POINTS
- Oral anticoagulant treatment for atrial fibrillation helps preserve cognitive function.
- Antipsychotics are not recommended as initial therapy for dementia-associated behavioral disturbances or for hospitalization-induced delirium.
- A multicomponent inpatient program can help prevent postoperative delirium in hospitalized patients.
- The US Preventive Services Task Force recommends exercise to prevent falls.
- Early mobility should be encouraged for hospitalized patients.
- Better continuity of care between hospitals and skilled nursing facilities can reduce hospital readmission rates.
Narcolepsy: Diagnosis and management
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
KEY POINTS
- Features of narcolepsy include daytime sleepiness, sleep attacks, cataplexy (in narcolepsy type 1), sleep paralysis, and sleep-related hallucinations.
- People with narcolepsy feel sleepy and can fall asleep quickly, but they do not stay asleep for long. They go into rapid eye movement sleep soon after falling asleep. Total sleep time is normal, but sleep is fragmented.
- Scheduled naps lasting 15 to 20 minutes can improve alertness. A consistent sleep schedule with good sleep hygiene is also important.
- Modafinil, methylphenidate, and amphetamines are used to manage daytime sleepiness, and sodium oxybate and antidepressants are used for cataplexy.
A new reason to reconsider that antibiotic prescription: The microbiome
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
Lung complications of prescription drug abuse
A 39-year-old woman presented to the emergency department with a 2-day history of exertional dyspnea, left-sided chest pain with pleuritic characteristics, and cough without fever or chills. She had a history of severe postprandial nausea and vomiting, weight loss, and malnutrition, which had necessitated placement of a peripherally inserted central catheter in her right arm for total parenteral nutrition.
On physical examination, the patient was afebrile but tachycardic and tachypneic. Her oxygen saturation on room air by pulse oximetry was 91%, though she was not in significant distress. Breath sounds were equal bilaterally and clear, with symmetrical chest wall expansion.
Her white blood cell count was 18.5 × 109/L (reference range 3.5–10.5), with 19.3% eosinophils (reference range 1%–7%); her D-dimer level was also elevated.
Conditions to consider in a patient with these imaging findings in the setting of leukocytosis and eosinophilia include mycobacterial infection, hypersensitivity reaction, diffuse fungal infiltrates, and possibly metastatic disease such as thyroid carcinoma or melanoma. The patient reported having had a purified protein derivative test that was positive for tuberculosis, but she denied having had active disease.
She underwent bronchosocopy. Bronchoalveolar lavage specimen study showed an elevated eosinophil count of 17%. Acid-fast staining detected no organisms. Transbronchial biopsy study revealed foreign-body granulomas from microcrystalline cellulose microemboli deposited in the microvasculature of the patient’s lungs. Upon further questioning the patient admitted she had crushed oral tablets of prescribed opioids and injected them intravenously.
A COMPLICATION OF INJECTING ORAL TABLETS
Oral tablets typically contain talc, cellulose, cornstarch, or combinations of these substances as binding agents. When pulverized, the powder can be combined with water to form an injectable solution with higher and more rapid bioavailability.1,2 The binders, however, are insoluble and accumulate in various tissues.
Intravenous injection of microcellulose has been shown to produce pulmonary and peripheral eosinophilia in birds. In humans, the immune response in foreign body granulomatosis can vary, and case reports have not mentioned eosinophils in the lungs or serum.3,4
Deposition of these particles in pulmonary vessels is common and can trigger a potentially fatal reaction, presenting as acute onset of cough, chest pain, dyspnea, fever, and pulmonary hypertension. The severity of these clinical findings is relative to the degree of pulmonary hypertension created by the arteriolar involvement of these emboli.2,5
Our patient’s exertional dyspnea and hypoxemia resolved during 1 week of hospitalization with conservative management and supplemental oxygen. She was referred to our pain rehabilitation clinic, where she was successfully weaned from narcotics. Her pulmonary findings on computed tomography were still present 3 years after her initial images, though less prominent.
- Nguyen VT, Chan ES, Chou SH, et al. Pulmonary effects of IV injection of crushed oral tablets: “excipient lung disease.” AJR Am J Roentgenol 2014; 203(5):W506–W515. doi:10.2214/AJR.14.12582
- Bendeck SE, Leung AN, Berry GJ, Daniel D, Ruoss SJ. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am J Roentgenol 2001; 177(5):1151–1153. doi:10.2214/ajr.177.5.1771151
- Radow SK, Nachamkin I, Morrow C, et al. Foreign body granulomatosis. Clinical and immunologic findings. Am Rev Respir Dis 1983; 127(5):575–580. doi:10.1164/arrd.1983.127.5.575
- Wang W, Wideman RF Jr, Bersi TK, Erf GF. Pulmonary and hematological inflammatory responses to intravenous cellulose micro-particles in broilers. Poult Sci 2003; 82(5):771–780. doi:10.1093/ps/82.5.771
- Marchiori E, Lourenco S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188(2):165–171. doi:10.1007/s00408-010-9230-y
A 39-year-old woman presented to the emergency department with a 2-day history of exertional dyspnea, left-sided chest pain with pleuritic characteristics, and cough without fever or chills. She had a history of severe postprandial nausea and vomiting, weight loss, and malnutrition, which had necessitated placement of a peripherally inserted central catheter in her right arm for total parenteral nutrition.
On physical examination, the patient was afebrile but tachycardic and tachypneic. Her oxygen saturation on room air by pulse oximetry was 91%, though she was not in significant distress. Breath sounds were equal bilaterally and clear, with symmetrical chest wall expansion.
Her white blood cell count was 18.5 × 109/L (reference range 3.5–10.5), with 19.3% eosinophils (reference range 1%–7%); her D-dimer level was also elevated.
Conditions to consider in a patient with these imaging findings in the setting of leukocytosis and eosinophilia include mycobacterial infection, hypersensitivity reaction, diffuse fungal infiltrates, and possibly metastatic disease such as thyroid carcinoma or melanoma. The patient reported having had a purified protein derivative test that was positive for tuberculosis, but she denied having had active disease.
She underwent bronchosocopy. Bronchoalveolar lavage specimen study showed an elevated eosinophil count of 17%. Acid-fast staining detected no organisms. Transbronchial biopsy study revealed foreign-body granulomas from microcrystalline cellulose microemboli deposited in the microvasculature of the patient’s lungs. Upon further questioning the patient admitted she had crushed oral tablets of prescribed opioids and injected them intravenously.
A COMPLICATION OF INJECTING ORAL TABLETS
Oral tablets typically contain talc, cellulose, cornstarch, or combinations of these substances as binding agents. When pulverized, the powder can be combined with water to form an injectable solution with higher and more rapid bioavailability.1,2 The binders, however, are insoluble and accumulate in various tissues.
Intravenous injection of microcellulose has been shown to produce pulmonary and peripheral eosinophilia in birds. In humans, the immune response in foreign body granulomatosis can vary, and case reports have not mentioned eosinophils in the lungs or serum.3,4
Deposition of these particles in pulmonary vessels is common and can trigger a potentially fatal reaction, presenting as acute onset of cough, chest pain, dyspnea, fever, and pulmonary hypertension. The severity of these clinical findings is relative to the degree of pulmonary hypertension created by the arteriolar involvement of these emboli.2,5
Our patient’s exertional dyspnea and hypoxemia resolved during 1 week of hospitalization with conservative management and supplemental oxygen. She was referred to our pain rehabilitation clinic, where she was successfully weaned from narcotics. Her pulmonary findings on computed tomography were still present 3 years after her initial images, though less prominent.
A 39-year-old woman presented to the emergency department with a 2-day history of exertional dyspnea, left-sided chest pain with pleuritic characteristics, and cough without fever or chills. She had a history of severe postprandial nausea and vomiting, weight loss, and malnutrition, which had necessitated placement of a peripherally inserted central catheter in her right arm for total parenteral nutrition.
On physical examination, the patient was afebrile but tachycardic and tachypneic. Her oxygen saturation on room air by pulse oximetry was 91%, though she was not in significant distress. Breath sounds were equal bilaterally and clear, with symmetrical chest wall expansion.
Her white blood cell count was 18.5 × 109/L (reference range 3.5–10.5), with 19.3% eosinophils (reference range 1%–7%); her D-dimer level was also elevated.
Conditions to consider in a patient with these imaging findings in the setting of leukocytosis and eosinophilia include mycobacterial infection, hypersensitivity reaction, diffuse fungal infiltrates, and possibly metastatic disease such as thyroid carcinoma or melanoma. The patient reported having had a purified protein derivative test that was positive for tuberculosis, but she denied having had active disease.
She underwent bronchosocopy. Bronchoalveolar lavage specimen study showed an elevated eosinophil count of 17%. Acid-fast staining detected no organisms. Transbronchial biopsy study revealed foreign-body granulomas from microcrystalline cellulose microemboli deposited in the microvasculature of the patient’s lungs. Upon further questioning the patient admitted she had crushed oral tablets of prescribed opioids and injected them intravenously.
A COMPLICATION OF INJECTING ORAL TABLETS
Oral tablets typically contain talc, cellulose, cornstarch, or combinations of these substances as binding agents. When pulverized, the powder can be combined with water to form an injectable solution with higher and more rapid bioavailability.1,2 The binders, however, are insoluble and accumulate in various tissues.
Intravenous injection of microcellulose has been shown to produce pulmonary and peripheral eosinophilia in birds. In humans, the immune response in foreign body granulomatosis can vary, and case reports have not mentioned eosinophils in the lungs or serum.3,4
Deposition of these particles in pulmonary vessels is common and can trigger a potentially fatal reaction, presenting as acute onset of cough, chest pain, dyspnea, fever, and pulmonary hypertension. The severity of these clinical findings is relative to the degree of pulmonary hypertension created by the arteriolar involvement of these emboli.2,5
Our patient’s exertional dyspnea and hypoxemia resolved during 1 week of hospitalization with conservative management and supplemental oxygen. She was referred to our pain rehabilitation clinic, where she was successfully weaned from narcotics. Her pulmonary findings on computed tomography were still present 3 years after her initial images, though less prominent.
- Nguyen VT, Chan ES, Chou SH, et al. Pulmonary effects of IV injection of crushed oral tablets: “excipient lung disease.” AJR Am J Roentgenol 2014; 203(5):W506–W515. doi:10.2214/AJR.14.12582
- Bendeck SE, Leung AN, Berry GJ, Daniel D, Ruoss SJ. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am J Roentgenol 2001; 177(5):1151–1153. doi:10.2214/ajr.177.5.1771151
- Radow SK, Nachamkin I, Morrow C, et al. Foreign body granulomatosis. Clinical and immunologic findings. Am Rev Respir Dis 1983; 127(5):575–580. doi:10.1164/arrd.1983.127.5.575
- Wang W, Wideman RF Jr, Bersi TK, Erf GF. Pulmonary and hematological inflammatory responses to intravenous cellulose micro-particles in broilers. Poult Sci 2003; 82(5):771–780. doi:10.1093/ps/82.5.771
- Marchiori E, Lourenco S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188(2):165–171. doi:10.1007/s00408-010-9230-y
- Nguyen VT, Chan ES, Chou SH, et al. Pulmonary effects of IV injection of crushed oral tablets: “excipient lung disease.” AJR Am J Roentgenol 2014; 203(5):W506–W515. doi:10.2214/AJR.14.12582
- Bendeck SE, Leung AN, Berry GJ, Daniel D, Ruoss SJ. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am J Roentgenol 2001; 177(5):1151–1153. doi:10.2214/ajr.177.5.1771151
- Radow SK, Nachamkin I, Morrow C, et al. Foreign body granulomatosis. Clinical and immunologic findings. Am Rev Respir Dis 1983; 127(5):575–580. doi:10.1164/arrd.1983.127.5.575
- Wang W, Wideman RF Jr, Bersi TK, Erf GF. Pulmonary and hematological inflammatory responses to intravenous cellulose micro-particles in broilers. Poult Sci 2003; 82(5):771–780. doi:10.1093/ps/82.5.771
- Marchiori E, Lourenco S, Gasparetto TD, Zanetti G, Mano CM, Nobre LF. Pulmonary talcosis: imaging findings. Lung 2010; 188(2):165–171. doi:10.1007/s00408-010-9230-y
Acute necrotizing esophagitis
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
An 82-year-old man with poorly controlled diabetes mellitus presented to our emergency department with a 1-day history of confusion and coffee-ground emesis.
Biopsy study revealed necrosis of the esophageal mucosa. A diagnosis of acute necrotizing esophagitis was made.
ACUTE NECROTIZING ESOPHAGITIS
Acute necrotizing esophagitis is thought to arise from a combination of an ischemic insult to the esophagus, an impaired mucosal barrier system, and a backflow injury from chemical contents of gastric secretions.1 The tissue hypoperfusion derives from vasculopathy, hypotension, or malnutrition. It is associated with diabetes mellitus, diabetic ketoacidosis, lactic acidosis, alcohol abuse, cirrhosis, renal insufficiency, malignancy, antibiotic use, esophageal infections, and aortic dissection.
The esophagus has a diverse blood supply. The upper esophagus is supplied by the inferior thyroid arteries, the mid-esophagus by the bronchial, proper esophageal, and intercostal arteries, and the distal esophagus by the left gastric and left inferior phrenic arteries.1
KEY FEATURES AND DIAGNOSTIC CLUES
The necrotic changes are prominent in the distal esophagus, which is more susceptible to ischemia and mucosal injury. The characteristic endoscopic finding is a diffuse black esophagus with a sharp transition to normal mucosa at the gastroesophageal junction.
The differential diagnosis includes melanosis, pseudomelanosis, malignant melanoma, acanthosis nigricans, coal dust deposition, caustic ingestion, radiation esophagitis, and infectious esophagitis caused by cytomegalovirus, herpes simplex virus, Candida albicans, or Klebsiella pneumoniae.2–4
TREATMENT AND OUTCOME
Avoidance of oral intake and gastric acid suppression with intravenous proton pump inhibitors are recommended to prevent additional injury of the esophageal mucosa.
The condition generally resolves with restored blood flow and treatment of any coexisting illness. However, it may be complicated by perforation (6.8%), mediastinitis (5.7%), or subsequent development of esophageal stricture (10.2%).5 Patients with esophageal stricture require endoscopic dilation after mucosal recovery.
The overall risk of death in acute necrotizing esophagitis is high (31.8%) and most often due to the underlying disease, such as sepsis, malignancy, cardiogenic shock, or hypovolemic shock.5 The mortality rate directly attributed to complications of acute necrotizing esophagitis is much lower (5.7%).5
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
- Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol 2010; 16(26):3219–3225. pmid:20614476
- Khan HA. Coal dust deposition—rare cause of “black esophagus.” Am J Gastroenterol 1996; 91(10):2256. pmid:8855776
- Ertekin C, Alimoglu O, Akyildiz H, Guloglu R, Taviloglu K. The results of caustic ingestions. Hepatogastroenterology 2004; 51(59):1397–1400. pmid:15362762
- Kozlowski LM, Nigra TP. Esophageal acanthosis nigricans in association with adenocarcinoma from an unknown primary site. J Am Acad Dermatol 1992; 26(2 pt 2):348–351. pmid:1569256
- Gurvits GE, Shapsis A, Lau N, Gualtieri N, Robilotti JG. Acute esophageal necrosis: a rare syndrome. J Gastroenterol 2007; 42(1):29–38. doi:10.1007/s00535-006-1974-z
What can I do when first-line measures are not enough for vasovagal syncope?
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
Vasovagal syncope is usually benign, and although it often recurs, increasing fluid and salt intake and performing counter-pressure maneuvers are usually sufficient.1 However, if patients continue to have syncopal episodes despite these first-line measures, other options include drug therapy with midodrine, fludrocortisone, beta-blockers, or selective serotonin reuptake inhibitors; orthostatic training; and, in some cases, pacemaker implantation. The 2017 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society (ACC/AHA/HRS) are helpful in the management of these patients.1
RATIONALE
Although vasovagal syncope is considered benign, it can result in injury and can significantly affect quality of life.
The diagnosis can often be established in the initial evaluation with a structured history, physical examination, and electrocardiography. If the diagnosis is still unclear, tilt-table testing can be useful and has an ACC/AHA/HRS class IIa (moderate) recommendation.1 Once the diagnosis of vasovagal syncope is made, first-line measures can be instituted.
FIRST-LINE MEASURES
An explanation of the diagnosis, education on avoiding triggers such as prolonged standing and warm environments, coping with potentially stressful visits to the doctor or dentist, and reassurance that the condition is benign are all strongly recommended (class I).1
Initial measures include performing physical counter-pressure maneuvers (class IIa), increasing salt and fluid intake (class IIb) in the absence of contraindications, and, in selected patients, reducing or withdrawing hypotensive medications when appropriate (class IIb).
Physical counter-pressure maneuvers are recommended for patients whose syncopal episodes have a sufficiently long prodromal period. Maneuvers include the following:
- Leg crossing: crossing the legs while tensing leg, abdominal, and buttock muscles
- Handgrip: maximally contracting a rubber ball or other object in the dominant hand
- Squatting
- Limb or abdominal contractions
- Arm tensing: contracting both arms by gripping one hand with the other and abducting both arms.2
The effectiveness of counter-pressure maneuvers was studied by van Dijk et al2 in a multicenter prospective randomized clinical trial that included 223 patients with recurrent vasovagal syncope associated with prodromal symptoms. They concluded that these maneuvers decreased the recurrence of syncopal episodes, with a relative risk reduction of 0.36 (95% confidence interval 0.11–0.53, P < .005) and were low-cost and risk-free.
Confirming the diagnosis of vasovagal syncope with tilt-table testing may reassure the patient. It can also help the patient learn to identify the symptoms associated with a vasovagal episode, which in turn may encourage timely use of physical counter-pressure maneuvers at the onset.
The evidence for increasing salt and fluid intake for patients with vasovagal syncope is limited. But in the absence of a contraindication such as hypertension, renal disease, or heart failure, it may be reasonable to encourage the ingestion of 2 L to 3 L of fluid per day and a total of 6 g to 9 g of salt per day (around 1 to 2 heaping teaspoons of salt).1
MEDICAL THERAPY
In patients who continue to have syncopal episodes despite adequate use of first-line measures, medical therapy can be considered. Unfortunately, evidence supporting drug therapy for recurrent syncope is limited.3 Options include midodrine (class IIa), fludrocortisone (class IIb), beta-blockers (class IIb), and selective serotonin reuptake inhibitors (class IIb).1
Midodrine has the strongest recommendation and is a reasonable option if there is no history of hypertension, heart failure, or urinary retention. It is a peripheral alpha-agonist that ameliorates the reduction in peripheral sympathetic neural outflow responsible for venous pooling and vasodepression in vasovagal syncope.4–6
Fludrocortisone results in increased blood volume due to mineralocorticoid activity. In the Prevention of Syncope Trial 2 of fludrocortisone vs placebo, patients on fludrocortisone had a “marginally nonsignificant” reduction in recurrence of syncope over 1 year (hazard ratio 0.69, P = .069).7
Overall, beta-blockers have failed to prevent syncope in randomized controlled trials. But in a meta-analysis that included patients from the Prevention of Syncope Trial,8 an age-dependent benefit of beta-blockers was noted in patients age 42 and older.9 Therefore, a beta-blocker may be a reasonable option in patients in this age group with recurrent vasovagal syncope.1
Serotonin has central effects on blood pressure and heart rate that can induce syncope. However, evidence for the effectiveness of selective serotonin reuptake inhibitors in the prevention of recurrent vasovagal syncope has been contradictory in small trials.10,11
When choosing a drug, contraindications should be considered, including possible effects during pregnancy in women of childbearing age.
OTHER MEASURES
Orthostatic training, with repetitive tilt-table testing until a test is negative, or with daily standing quietly against a wall for prolonged periods of time, has not been shown to have sustained benefit in reducing the recurrence of syncopal episodes (class IIb recommendation).1
Dual-chamber pacing can be considered in carefully selected patients age 40 or older with syncope and documented asystole of at least 3 seconds or spontaneous pauses of at least 6 seconds without syncope on implantable loop recorder monitoring (class IIb recommendation).1,12,13 Strict patient selection increases the likelihood that pacing will be effective.1 For example, patients with documented asystole during syncope and a tilt-table test that induces minimal or no vasodepressor response are more likely to respond than patients with a positive tilt-table test with a vasodepressor (hypotensive) response.13
Tilt-table testing may also be considered to identify patients with a hypotensive response who would be less likely to respond to permanent cardiac pacing.14
Compression garments carry a class IIa recommendation for orthostatic hypotension,1 but they have not been adequately studied in vasovagal syncope.
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
- Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017; 136(5):e60–e122. doi:10.1161/CIR.0000000000000499
- van Dijk N, Quartieri F, Blanc JJ, Garcia-Civera R, Brignole M, Moya A, Wieling W; PC-Trial Investigators. Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the Physical Counterpressure Manoeuvres Trial (PC-Trial). J Am Coll Cardiol 2006; 48(8):1652–1657. doi:10.1016/j.jacc.2006.06.059
- Romme JJ, Reitsma JB, Black CN, et al. Drugs and pacemakers for vasovagal, carotid sinus and situational syncope. Cochrane Database Syst Rev 2011; (10):CD004194. doi:10.1002/14651858.CD004194.pub3
- Perez-Lugones A, Schweikert R, Pavia S, et al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001; 12(8):935–938. pmid:11513446
- Romme JJ, van Dijk N, Go-Schön IK, Reitsma JB, Wieling W. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011; 13(11):1639–1647. doi:10.1093/europace/eur200
- Samniah N, Sakaguchi S, Lurie KG, Iskos D, Benditt DG. Efficacy and safety of midodrine hydrochloride in patients with refractory vasovagal syncope. Am J Cardiol 2001; 88(1):A7, 80–83. pmid:11423066
- Sheldon R, Raj SR, Rose MS, et al; POST 2 Investigators. Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016; 68(1):1–9. doi:10.1016/j.jacc.2016.04.030
- Sheldon R, Connolly S, Rose S, et al; POST Investigators. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation 2006; 113(9):1164–1170. doi:10.1161/CIRCULATIONAHA.105.535161
- Sheldon RS, Morillo CA, Klingenheben T, Krahn AD, Sheldon A, Rose MS. Age-dependent effect of beta-blockers in preventing vasovagal syncope. Circ Arrhythm Electrophysiol 2012; 5(5):920–926. doi:10.1161/CIRCEP.112.974386
- Takata TS, Wasmund SL, Smith ML, et al. Serotonin reuptake inhibitor (Paxil) does not prevent the vasovagal reaction associated with carotid sinus massage and/or lower body negative pressure in healthy volunteers. Circulation 2002; 106(12):1500–1504. pmid:12234955
- Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C, Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999; 33(5):1227–1230. pmid:10193720
- Brignole M, Menozzi C, Moya A, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third International Study on Syncope of Uncertain Etiology (ISSUE-3): a randomized trial. Circulation 2012; 125(21):2566–2571. doi:10.1161/CIRCULATIONAHA.111.082313
- Brignole M, Donateo P, Tomaino M, et al; International Study on Syncope of Uncertain Etiology 3 (ISSUE-3) Investigators. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1):10–16. doi:10.1161/CIRCEP.113.001103
- Sheldon RS, Grubb BP, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015; 12(6):e41–e63. doi:10.1016/j.hrthm.2015.03.029
Make a Difference Today – Give to the SVS Foundation
This final month of the year is the giving season, and many people delay their charitable contributions until then. Please consider making a difference in the world of vascular surgery this holiday season by donating to the SVS Foundation. The mission of the Foundation has expanded, and your dollars are needed now, more than ever. From research, to disaster relief efforts, to patient awareness and disease prevention, all contributions are ultimately aimed at improving patient health. Please give today.
This final month of the year is the giving season, and many people delay their charitable contributions until then. Please consider making a difference in the world of vascular surgery this holiday season by donating to the SVS Foundation. The mission of the Foundation has expanded, and your dollars are needed now, more than ever. From research, to disaster relief efforts, to patient awareness and disease prevention, all contributions are ultimately aimed at improving patient health. Please give today.
This final month of the year is the giving season, and many people delay their charitable contributions until then. Please consider making a difference in the world of vascular surgery this holiday season by donating to the SVS Foundation. The mission of the Foundation has expanded, and your dollars are needed now, more than ever. From research, to disaster relief efforts, to patient awareness and disease prevention, all contributions are ultimately aimed at improving patient health. Please give today.