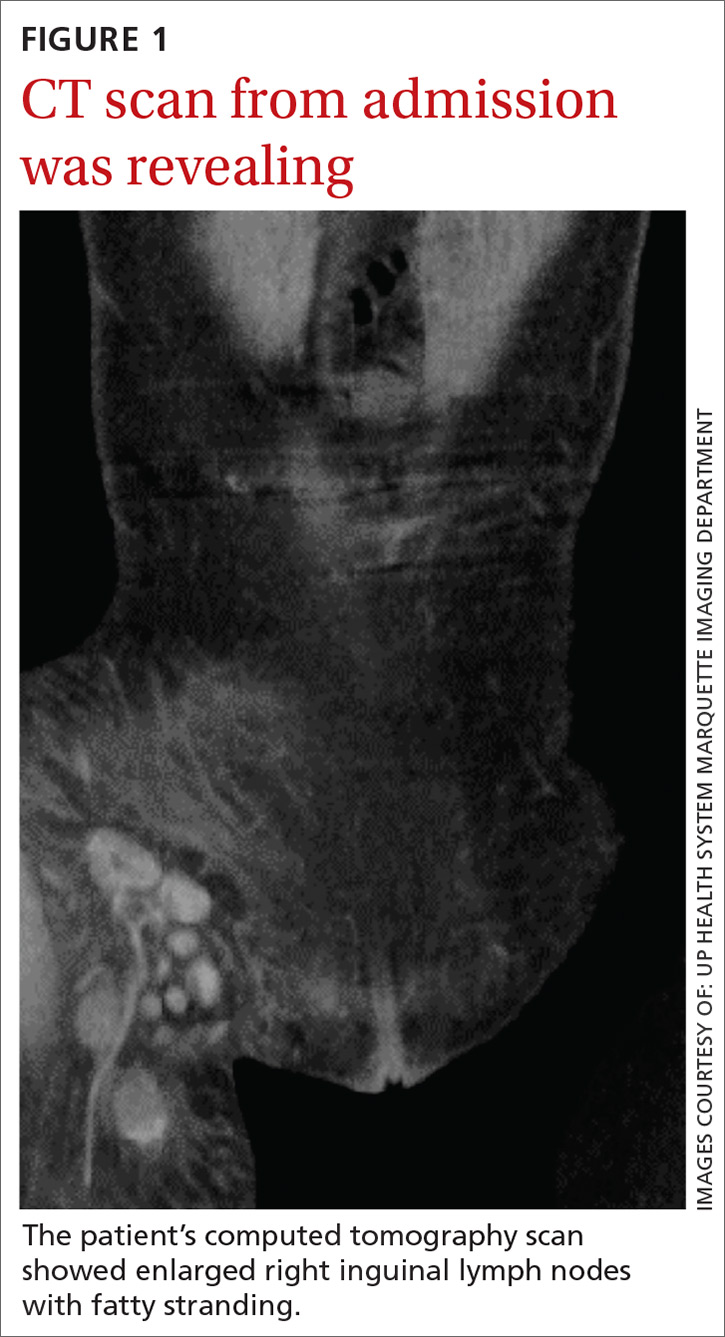
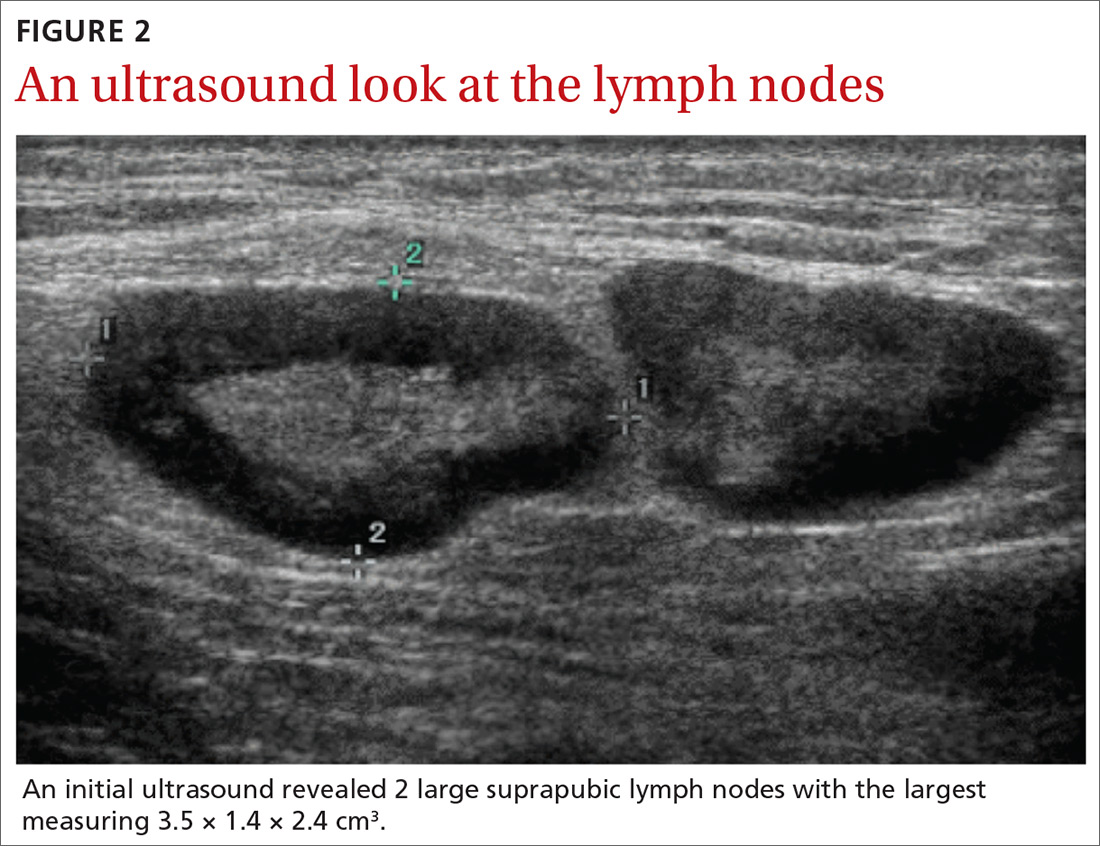
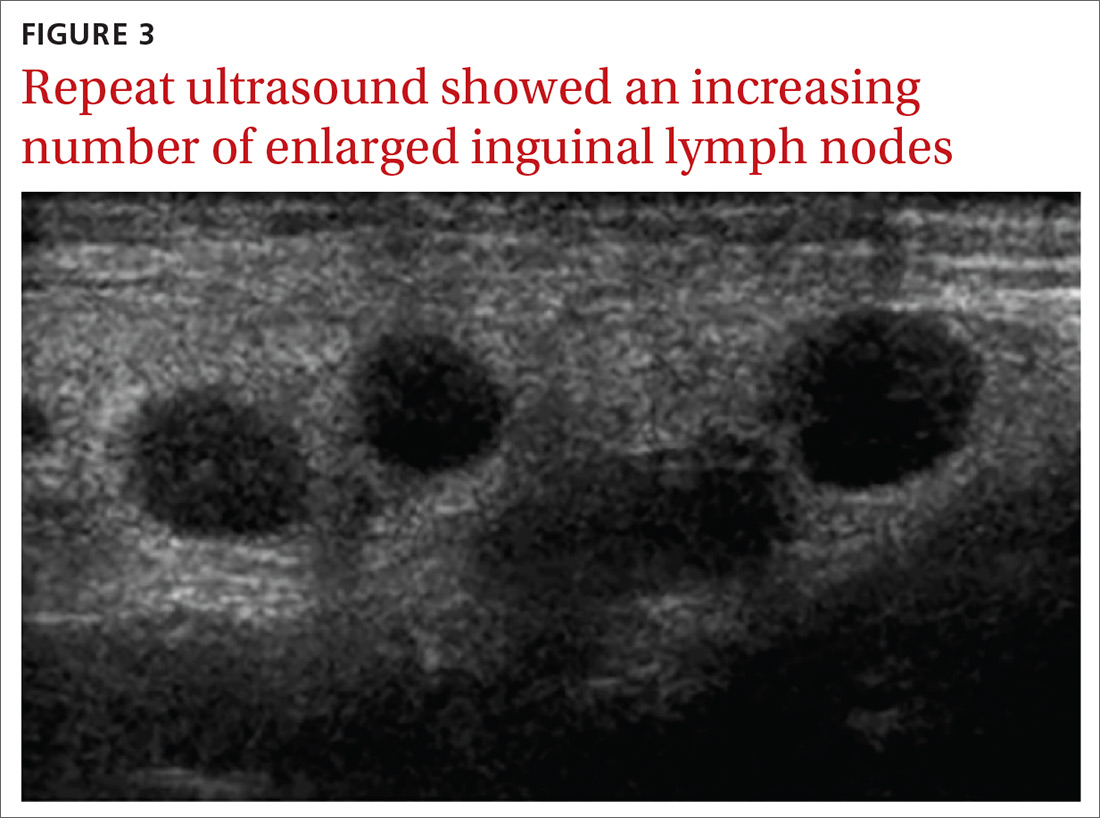
User login
Upending this country’s approach to health care
In these first decades of the 21st century, the United States is the richest, strongest, most innovative nation on the planet. Americans like to chant “We’re Number 1”—and by many measures, they’re right. But in one crucial area of human endeavor—keeping people healthy—the mighty United States is a third-rate power.
All the other industrialized democracies have significantly better health outcomes than the United States—longer life expectancy, better recovery rates from illness or injury, less infant mortality.1 Yet these nations spend, on average, half as much as the United States does for health care.1 And these other rich democracies guarantee health care for everyone—while the United States leaves 29 million people ages <65 years with no health insurance, and another 50 million with deductibles so high that they are effectively uninsured.2,3
This disgraceful state of affairs is not the fault of the nation’s physicians. Rather, the problems with health care in the United States stem from the system that American providers have to work in.
Health care has become big business. As the physician-turned-reporter Dr. Elisabeth Rosenthal notes in An American Sickness: How Healthcare Became Big Business, profits have come to matter more than patients for much of the $3.3 trillion US health care industry.4,5 And the financial winners in our system—notably the “Big Four” health insurance giants, the for-profit hospital chains, and “Big Pharma”—fight hard to protect their profits. When the Affordable Care Act (“ObamaCare”) was first proposed, one of its main goals was to cut the administrative costs of health insurance, to force the private insurers to run their business as efficiently as Medicare. The insurance industry didn’t like that; its lobbyists fought back, successfully. As passed, the law allows the insurers to add up to 20% in administrative fees to every doctor and hospital bill—which adds hundreds of billions of dollars to the nation’s total health care spending every year.
Then there’s the problem that health-care economists call “specialty distribution imbalance.” In plain English, this means that the United States has too many doctors working in narrow (but highly compensated) subspecialties and not enough in the primary care fields of family medicine, internal medicine, and pediatrics. This is one more area where our country is out of sync with other industrialized democracies.
When I traveled the world studying health care systems, economists and government health ministers regularly told me that an efficient system should have 2 primary care providers for every 1 specialist. That is, primary care should make up about 66% of the overall physician work force.
Most rich countries come close to this desired ratio. In the United Kingdom, family doctors working out of their own offices (it’s called a “surgery”) and treating patients on the local High Street (that is, Main Street), control 70% of the National Health Service (NHS) budget.6 “That’s the framework of the NHS, and of course we want to keep it,” John Reid, the UK’s former Minister of Health, told me. “If you just pop into your doctor’s surgery on the high street, that’s often just as effective, but never as expensive, as waiting to see a specialist.”
Continue to: If that 2:1 ratio is the right proportion...
If that 2:1 ratio is the right proportion for an effective health care system, the United States is upside down. For decades now, some two-thirds of new medical graduates have gone into narrower specialties, leaving our country with a serious shortage of primary care physicians.7 This situation helps to explain the higher cost and poorer overall outcomes that characterize American health care.
“Health care is often delivered according to a model that concentrates on diseases, high technology, and specialist care,” a report from the World Health Organization noted.8 “The results are...higher overall costs, and exclusion of people who cannot pay.” The report concluded that an emphasis on primary care leads to better outcomes for the same level of investment. This simple truth has been called the “Iron Law” of health care systems.
How can the United States get more primary care physicians? One answer is compensation. American primary care doctors routinely earn significantly less than specialists. But it doesn’t have to be that way. When I asked my family doctor in London, Dr. Ahmed Badat, why it is that 62% of British physicians are in family care, he was blunt: “Under the NHS, I make twice as much as a cardiac surgeon.”
If the big payers—government programs and private insurers—beef up fees for primary care (and pay for it by reducing compensation for specialists), more young American med students are likely to choose that route. Repayment plans that forgive the student loans of those in primary care fields also would attract more newly-minted physicians; these programs already are in place in several states.9
Continue to: Medical schools also have a role...
Medical schools also have a role to play. It’s no secret that the schools have emphasized specialties, with faculty members often steering their best students into narrow fields. But schools could promote an atmosphere in which primary care is treated as the most desirable destination for new doctors. Actively seeking out, and accepting, applicants who say they want to practice primary care is another key tool the medical schools could employ to deal with this national problem.
More doctors in primary care would mean better health care at lower cost for American patients. It’s long past time to take the steps needed to reach that goal.
1. World Health Organization. The World Health Report 2008 - primary Health Care (Now More Than Ever). http://www.who.int/whr/2008/en/. Accessed October 10, 2018.
2. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2018 to 2028. https://www.cbo.gov/publication/53826. Published May 23, 2018. Accessed November 5, 2018.
3. Cohen RA, Martinez ME, Zammitti EP. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January–March 2016. Division of Health Interview Statistics, National Center for Health Statistics. 2016. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201609.pdf. Accessed November 5, 2018.
4. Rosentahl E. An American Sickness: How Healthcare Became Big Business. New York, NY: Penguin Press; 2017.
5. U.S. Centers for Medicare & Medicaid Services. National Health Expenditures 2016 Highlights. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed November 5, 2018.
6. Roland M, Guthrie B, Thomé DC. Primary medical care in the United kingdom. J Am Board Fam Med. 2012;25 Suppl 1:S6-S11.
7. U.S. Department of Health & Human Services, Agency for Healthcare Research and Quality. The Number of Practicing Primary Care Physicians in the United States. https://www.ahrq.gov/research/findings/factsheets/primary/pcwork1/index.html. Accessed October 10, 2018.
8. World Health Organization. World Health Report calls for return to primary health care approach. http://www.who.int/mediacentre/news/releases/2008/pr38/en/. Published October 14, 2008. Accessed October 15, 2018.
9. Association of American Medical Colleges. Loan Repayment/Forgiveness/Scholarship and Other Programs. https://services.aamc.org/fed_loan_pub/index.cfm?fuseaction=public.welcome&CFID=255039&CFTOKEN=96604802. Accessed October 15, 2018.
In these first decades of the 21st century, the United States is the richest, strongest, most innovative nation on the planet. Americans like to chant “We’re Number 1”—and by many measures, they’re right. But in one crucial area of human endeavor—keeping people healthy—the mighty United States is a third-rate power.
All the other industrialized democracies have significantly better health outcomes than the United States—longer life expectancy, better recovery rates from illness or injury, less infant mortality.1 Yet these nations spend, on average, half as much as the United States does for health care.1 And these other rich democracies guarantee health care for everyone—while the United States leaves 29 million people ages <65 years with no health insurance, and another 50 million with deductibles so high that they are effectively uninsured.2,3
This disgraceful state of affairs is not the fault of the nation’s physicians. Rather, the problems with health care in the United States stem from the system that American providers have to work in.
Health care has become big business. As the physician-turned-reporter Dr. Elisabeth Rosenthal notes in An American Sickness: How Healthcare Became Big Business, profits have come to matter more than patients for much of the $3.3 trillion US health care industry.4,5 And the financial winners in our system—notably the “Big Four” health insurance giants, the for-profit hospital chains, and “Big Pharma”—fight hard to protect their profits. When the Affordable Care Act (“ObamaCare”) was first proposed, one of its main goals was to cut the administrative costs of health insurance, to force the private insurers to run their business as efficiently as Medicare. The insurance industry didn’t like that; its lobbyists fought back, successfully. As passed, the law allows the insurers to add up to 20% in administrative fees to every doctor and hospital bill—which adds hundreds of billions of dollars to the nation’s total health care spending every year.
Then there’s the problem that health-care economists call “specialty distribution imbalance.” In plain English, this means that the United States has too many doctors working in narrow (but highly compensated) subspecialties and not enough in the primary care fields of family medicine, internal medicine, and pediatrics. This is one more area where our country is out of sync with other industrialized democracies.
When I traveled the world studying health care systems, economists and government health ministers regularly told me that an efficient system should have 2 primary care providers for every 1 specialist. That is, primary care should make up about 66% of the overall physician work force.
Most rich countries come close to this desired ratio. In the United Kingdom, family doctors working out of their own offices (it’s called a “surgery”) and treating patients on the local High Street (that is, Main Street), control 70% of the National Health Service (NHS) budget.6 “That’s the framework of the NHS, and of course we want to keep it,” John Reid, the UK’s former Minister of Health, told me. “If you just pop into your doctor’s surgery on the high street, that’s often just as effective, but never as expensive, as waiting to see a specialist.”
Continue to: If that 2:1 ratio is the right proportion...
If that 2:1 ratio is the right proportion for an effective health care system, the United States is upside down. For decades now, some two-thirds of new medical graduates have gone into narrower specialties, leaving our country with a serious shortage of primary care physicians.7 This situation helps to explain the higher cost and poorer overall outcomes that characterize American health care.
“Health care is often delivered according to a model that concentrates on diseases, high technology, and specialist care,” a report from the World Health Organization noted.8 “The results are...higher overall costs, and exclusion of people who cannot pay.” The report concluded that an emphasis on primary care leads to better outcomes for the same level of investment. This simple truth has been called the “Iron Law” of health care systems.
How can the United States get more primary care physicians? One answer is compensation. American primary care doctors routinely earn significantly less than specialists. But it doesn’t have to be that way. When I asked my family doctor in London, Dr. Ahmed Badat, why it is that 62% of British physicians are in family care, he was blunt: “Under the NHS, I make twice as much as a cardiac surgeon.”
If the big payers—government programs and private insurers—beef up fees for primary care (and pay for it by reducing compensation for specialists), more young American med students are likely to choose that route. Repayment plans that forgive the student loans of those in primary care fields also would attract more newly-minted physicians; these programs already are in place in several states.9
Continue to: Medical schools also have a role...
Medical schools also have a role to play. It’s no secret that the schools have emphasized specialties, with faculty members often steering their best students into narrow fields. But schools could promote an atmosphere in which primary care is treated as the most desirable destination for new doctors. Actively seeking out, and accepting, applicants who say they want to practice primary care is another key tool the medical schools could employ to deal with this national problem.
More doctors in primary care would mean better health care at lower cost for American patients. It’s long past time to take the steps needed to reach that goal.
In these first decades of the 21st century, the United States is the richest, strongest, most innovative nation on the planet. Americans like to chant “We’re Number 1”—and by many measures, they’re right. But in one crucial area of human endeavor—keeping people healthy—the mighty United States is a third-rate power.
All the other industrialized democracies have significantly better health outcomes than the United States—longer life expectancy, better recovery rates from illness or injury, less infant mortality.1 Yet these nations spend, on average, half as much as the United States does for health care.1 And these other rich democracies guarantee health care for everyone—while the United States leaves 29 million people ages <65 years with no health insurance, and another 50 million with deductibles so high that they are effectively uninsured.2,3
This disgraceful state of affairs is not the fault of the nation’s physicians. Rather, the problems with health care in the United States stem from the system that American providers have to work in.
Health care has become big business. As the physician-turned-reporter Dr. Elisabeth Rosenthal notes in An American Sickness: How Healthcare Became Big Business, profits have come to matter more than patients for much of the $3.3 trillion US health care industry.4,5 And the financial winners in our system—notably the “Big Four” health insurance giants, the for-profit hospital chains, and “Big Pharma”—fight hard to protect their profits. When the Affordable Care Act (“ObamaCare”) was first proposed, one of its main goals was to cut the administrative costs of health insurance, to force the private insurers to run their business as efficiently as Medicare. The insurance industry didn’t like that; its lobbyists fought back, successfully. As passed, the law allows the insurers to add up to 20% in administrative fees to every doctor and hospital bill—which adds hundreds of billions of dollars to the nation’s total health care spending every year.
Then there’s the problem that health-care economists call “specialty distribution imbalance.” In plain English, this means that the United States has too many doctors working in narrow (but highly compensated) subspecialties and not enough in the primary care fields of family medicine, internal medicine, and pediatrics. This is one more area where our country is out of sync with other industrialized democracies.
When I traveled the world studying health care systems, economists and government health ministers regularly told me that an efficient system should have 2 primary care providers for every 1 specialist. That is, primary care should make up about 66% of the overall physician work force.
Most rich countries come close to this desired ratio. In the United Kingdom, family doctors working out of their own offices (it’s called a “surgery”) and treating patients on the local High Street (that is, Main Street), control 70% of the National Health Service (NHS) budget.6 “That’s the framework of the NHS, and of course we want to keep it,” John Reid, the UK’s former Minister of Health, told me. “If you just pop into your doctor’s surgery on the high street, that’s often just as effective, but never as expensive, as waiting to see a specialist.”
Continue to: If that 2:1 ratio is the right proportion...
If that 2:1 ratio is the right proportion for an effective health care system, the United States is upside down. For decades now, some two-thirds of new medical graduates have gone into narrower specialties, leaving our country with a serious shortage of primary care physicians.7 This situation helps to explain the higher cost and poorer overall outcomes that characterize American health care.
“Health care is often delivered according to a model that concentrates on diseases, high technology, and specialist care,” a report from the World Health Organization noted.8 “The results are...higher overall costs, and exclusion of people who cannot pay.” The report concluded that an emphasis on primary care leads to better outcomes for the same level of investment. This simple truth has been called the “Iron Law” of health care systems.
How can the United States get more primary care physicians? One answer is compensation. American primary care doctors routinely earn significantly less than specialists. But it doesn’t have to be that way. When I asked my family doctor in London, Dr. Ahmed Badat, why it is that 62% of British physicians are in family care, he was blunt: “Under the NHS, I make twice as much as a cardiac surgeon.”
If the big payers—government programs and private insurers—beef up fees for primary care (and pay for it by reducing compensation for specialists), more young American med students are likely to choose that route. Repayment plans that forgive the student loans of those in primary care fields also would attract more newly-minted physicians; these programs already are in place in several states.9
Continue to: Medical schools also have a role...
Medical schools also have a role to play. It’s no secret that the schools have emphasized specialties, with faculty members often steering their best students into narrow fields. But schools could promote an atmosphere in which primary care is treated as the most desirable destination for new doctors. Actively seeking out, and accepting, applicants who say they want to practice primary care is another key tool the medical schools could employ to deal with this national problem.
More doctors in primary care would mean better health care at lower cost for American patients. It’s long past time to take the steps needed to reach that goal.
1. World Health Organization. The World Health Report 2008 - primary Health Care (Now More Than Ever). http://www.who.int/whr/2008/en/. Accessed October 10, 2018.
2. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2018 to 2028. https://www.cbo.gov/publication/53826. Published May 23, 2018. Accessed November 5, 2018.
3. Cohen RA, Martinez ME, Zammitti EP. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January–March 2016. Division of Health Interview Statistics, National Center for Health Statistics. 2016. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201609.pdf. Accessed November 5, 2018.
4. Rosentahl E. An American Sickness: How Healthcare Became Big Business. New York, NY: Penguin Press; 2017.
5. U.S. Centers for Medicare & Medicaid Services. National Health Expenditures 2016 Highlights. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed November 5, 2018.
6. Roland M, Guthrie B, Thomé DC. Primary medical care in the United kingdom. J Am Board Fam Med. 2012;25 Suppl 1:S6-S11.
7. U.S. Department of Health & Human Services, Agency for Healthcare Research and Quality. The Number of Practicing Primary Care Physicians in the United States. https://www.ahrq.gov/research/findings/factsheets/primary/pcwork1/index.html. Accessed October 10, 2018.
8. World Health Organization. World Health Report calls for return to primary health care approach. http://www.who.int/mediacentre/news/releases/2008/pr38/en/. Published October 14, 2008. Accessed October 15, 2018.
9. Association of American Medical Colleges. Loan Repayment/Forgiveness/Scholarship and Other Programs. https://services.aamc.org/fed_loan_pub/index.cfm?fuseaction=public.welcome&CFID=255039&CFTOKEN=96604802. Accessed October 15, 2018.
1. World Health Organization. The World Health Report 2008 - primary Health Care (Now More Than Ever). http://www.who.int/whr/2008/en/. Accessed October 10, 2018.
2. Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2018 to 2028. https://www.cbo.gov/publication/53826. Published May 23, 2018. Accessed November 5, 2018.
3. Cohen RA, Martinez ME, Zammitti EP. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, January–March 2016. Division of Health Interview Statistics, National Center for Health Statistics. 2016. https://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201609.pdf. Accessed November 5, 2018.
4. Rosentahl E. An American Sickness: How Healthcare Became Big Business. New York, NY: Penguin Press; 2017.
5. U.S. Centers for Medicare & Medicaid Services. National Health Expenditures 2016 Highlights. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/highlights.pdf. Accessed November 5, 2018.
6. Roland M, Guthrie B, Thomé DC. Primary medical care in the United kingdom. J Am Board Fam Med. 2012;25 Suppl 1:S6-S11.
7. U.S. Department of Health & Human Services, Agency for Healthcare Research and Quality. The Number of Practicing Primary Care Physicians in the United States. https://www.ahrq.gov/research/findings/factsheets/primary/pcwork1/index.html. Accessed October 10, 2018.
8. World Health Organization. World Health Report calls for return to primary health care approach. http://www.who.int/mediacentre/news/releases/2008/pr38/en/. Published October 14, 2008. Accessed October 15, 2018.
9. Association of American Medical Colleges. Loan Repayment/Forgiveness/Scholarship and Other Programs. https://services.aamc.org/fed_loan_pub/index.cfm?fuseaction=public.welcome&CFID=255039&CFTOKEN=96604802. Accessed October 15, 2018.
Diffuse facial rash in a former collegiate wrestler
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
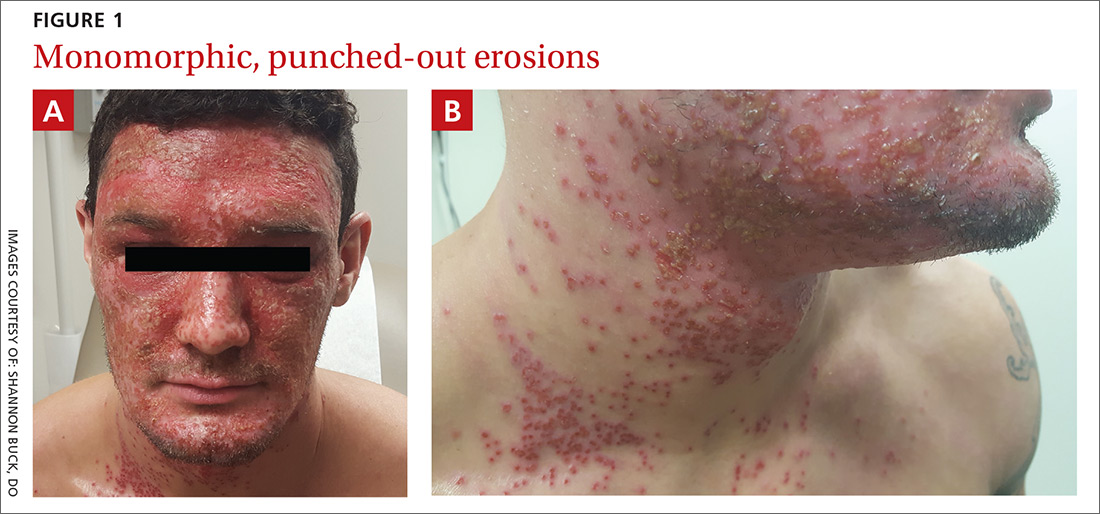
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
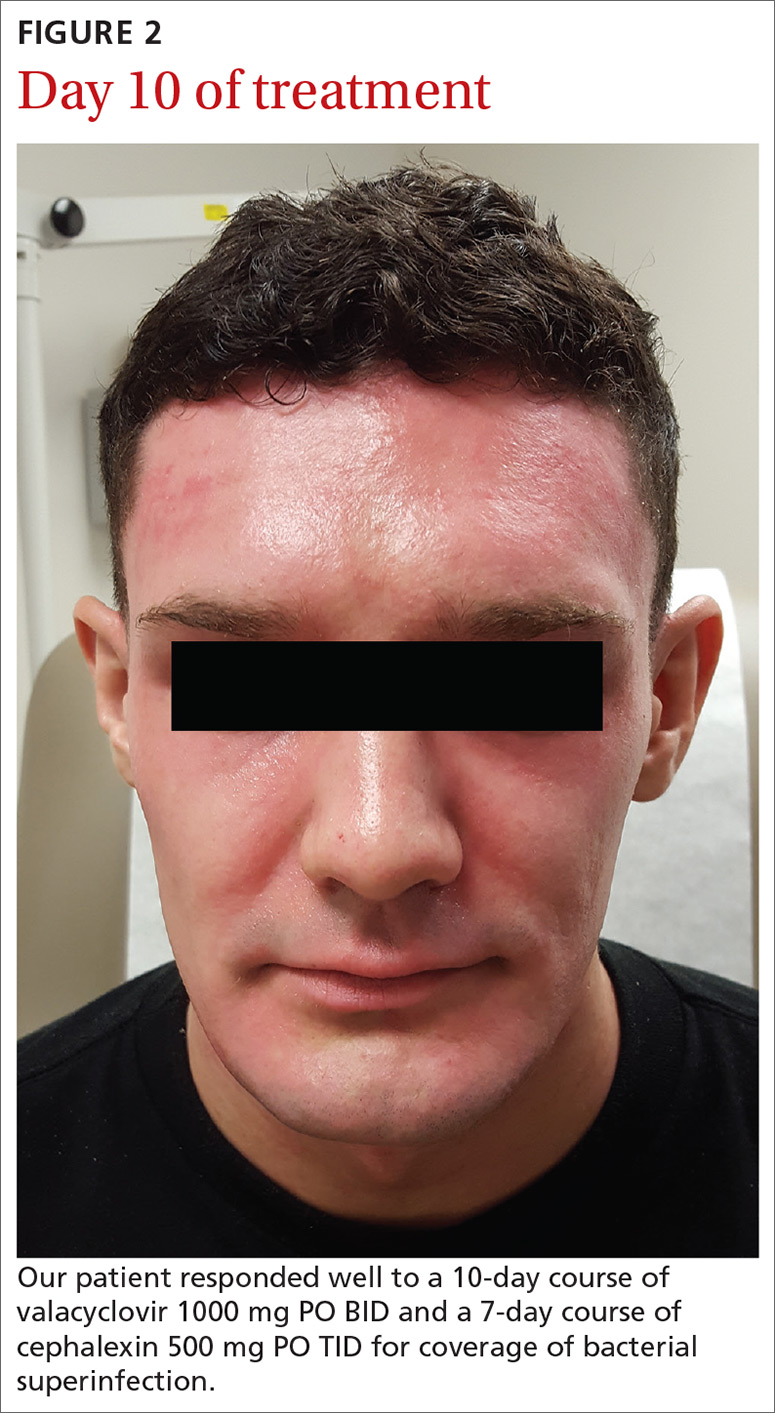
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.
CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, jonathan.f.madden.mil@mail.mil
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.
CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, jonathan.f.madden.mil@mail.mil
A 22-year-old Caucasian man with a history of atopic dermatitis (AD) was referred to our dermatology clinic for evaluation of a diffuse facial rash that had been present for the previous 7 days. The rash initially presented as erythema on the right malar cheek that rapidly spread to the entire face. Initially diagnosed as impetigo, empiric treatment with sulfamethoxazole/trimethoprim (800 mg/160 mg PO BID for 7 days), dicloxacillin (500 mg PO BID for 6 days), cephalexin (500 mg TID for 5 days), and mupirocin (2% topical cream applied TID for 6 days) failed to improve the patient’s symptoms. He reported mild pain associated with facial movements.
The patient had a history of similar (but more limited) rashes, which he described as “recurrent impetigo,” that began during his career as a high school and collegiate wrestler. These rashes were different from the rashes he described as his history of AD, which consisted of pruritic and erythematous skin in his antecubital and popliteal fossae. He denied any history of herpes simplex virus (HSV) infection.
A physical examination revealed numerous monomorphic, 1- to 3-mm, punched-out erosions and ulcers with overlying yellow-brown crust encompassing the patient’s entire face and portions of his anterior neck. Several clustered vesicles on erythematous bases also were noted (FIGUREs 1A and 1B). We used a Dermablade to unroof some of the vesicles and sent the scrapings to the lab for Tzanck, direct fluorescent antibody assay (DFA), and HSV polymerase chain reaction (PCR) testing.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Eczema herpeticum secondary to herpes gladiatorum
The patient’s laboratory results came back and the Tzanck preparation was positive for multinucleated giant cells, and both the DFA and HSV PCR were positive for HSV infection. This, paired with the widely disseminated rash observed on examination and the patient’s history of AD, was consistent with a diagnosis of eczema herpeticum (EH).
Rather than primary impetigo, the patient’s self-described history of recurrent rashes was felt to represent a history of HSV outbreaks. Given his denial of prior oral or genital HSV infection, as well as the coincident onset of these outbreaks during his career as a competitive wrestler, the most likely primary infection source was direct contact with another HSV-infected wrestler.
Herpes gladiatorum refers to a primary cutaneous HSV infection contracted by an athlete through direct skin-to-skin contact with another athlete.1 It is common in contact sports, such as rugby and wrestling, and particularly common at organized wrestling camps, where mass outbreaks are a frequent occurrence.2 Herpes gladiatorum is so common at these camps that many recommend prophylactic valacyclovir treatment for all participants to mitigate the risk of contracting HSV. In a 2016 review, Anderson et al concluded that prophylactic valacyclovir treatment at a 28-day high school wrestling camp effectively reduced outbreak incidence by 89.5%.2
The lesions of herpes gladiatorum are classically limited in distribution and reflective of the areas of direct contact with infected skin, most commonly the face, neck, and arms. Our patient’s history of more limited outbreaks on his face was consistent with this typical presentation. His current outbreak, however, had become much more widely disseminated, which led to the diagnosis of EH secondary to herpes gladiatorum.
Eczema herpeticum: Pathogenesis and diagnosis
Also known as Kaposi’s varicelliform eruption, EH is a rapid, widespread cutaneous dissemination of HSV infection in areas of dermatitis or skin barrier disruption, most commonly caused by HSV-1 infection.3 It is classically associated with AD, but also can occur in patients with impaired epidermal barrier function due to other conditions, such as burns, pemphigus vulgaris, mycosis fungoides, and Darier disease.4 It occurs in <3% of patients with AD and is more commonly observed in infants and children with AD than adults.5
Continue to: Clinically, the most common manifestations are discrete..
Clinically, the most common manifestations are discrete, monomorphic, 2- to 3-mm, punched-out erosions with hemorrhagic crusts; intact vesicles are less commonly observed.4 Involved skin is typically painful and may be pruritic. Clinical diagnosis should be confirmed by laboratory evaluation, typically Tzanck preparation, DFA, and/or HSV PCR.
Complications and the importance of rapid treatment
The most common complication of EH is bacterial superinfection (impetigo), usually by Staphylococcus aureus or group A streptococci. Signs of bacterial superinfection include weeping lesions, pustules, honey-colored/golden crusting, worsening of existing dermatitis, and failure to respond to antiviral treatment. Topical mupirocin 2% cream is generally effective for controlling limited infection. However, systemic antibiotics (cephalosporins or penicillinase-resistant penicillins) may be necessary to control widespread disease.4 Clinical improvement should be observed within a single course of an appropriate antibiotic.
In contrast to impetigo, less common but more serious complications of EH can be life threatening. Systemic dissemination of disease is of particular importance in vulnerable populations such as pediatric and immunocompromised patients. Meningoencephalitis, secondary bacteremia, and herpes keratitis can all develop secondary to EH and incur significant morbidity and mortality.1
Fever, malaise, lymphadenopathy, or eye pain should prompt immediate consideration of inpatient evaluation and treatment for these potentially deadly or debilitating complications. All patients with EH distributed near the eyes should be referred to ophthalmology to rule out ocular involvement.
Immediately treat with antivirals
Due to the potential complications discussed above, a diagnosis of EH necessitates immediate treatment with oral or intravenous antiviral medication. Acyclovir, valacyclovir, or famciclovir may be used, with typical treatment courses ranging from 10 to 14 days or until all mucocutaneous lesions are healed.4 Although typically reserved for patients with recurrent genital herpes resulting in 6 or more outbreaks annually, chronic suppressive therapy also may be considered for patients with EH who suffer from frequent or severe recurrent outbreaks.
Continue to: Our patient
Our patient. Given his otherwise excellent health and the absence of symptoms of potentially serious complications, our patient was treated as an outpatient with a 10-day course of valacyclovir 1000 mg PO BID. He was additionally prescribed a 7-day course of cephalexin 500 mg PO TID for coverage of bacterial superinfection. He responded well to treatment.
Ten days after his initial presentation to our clinic, his erosions and vesicles had completely cleared, and the associated erythema had significantly improved (FIGURE 2). Given the severity of his presentation and his history of 2 to 3 outbreaks annually, he opted to continue prophylactic valacyclovir (500 mg/d) for long-term suppression.
CORRESPONDENCE
Jonathan Madden, MD, 221 3rd Street West, JBSA-Randolph, TX 78150, jonathan.f.madden.mil@mail.mil
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
1. Shenoy R, Mostow E, Cain G. Eczema herpeticum in a wrestler. Clin J Sport Med. 2015;25:e18-e19.
2. Anderson BJ, McGuire DP, Reed M, et al. Prophylactic valacyclovir to prevent outbreaks of primary herpes gladiatorum at a 28-day wrestling camp: a 10-year review. Clin J Sport Med. 2016;26:272-278.
3. Olson J, Robles DT, Kirby P, et al. Kaposi varicelliform eruption (eczema herpeticum). Dermatol Online J. 2008;14:18.
4. Downing C, Mendoza N, Tyring S. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1400-1424.
5. Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
What’s the best treatment setting for stable PE patients?
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
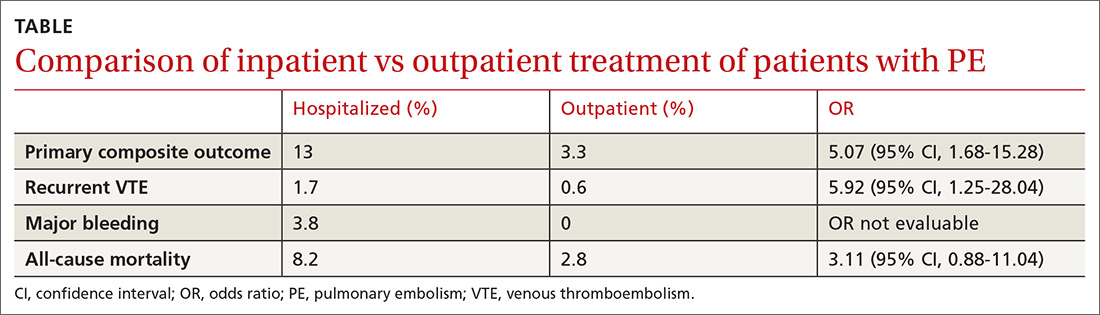
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.
WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.
WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.
WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
PRACTICE CHANGER
Manage patients with acute pulmonary embolism (PE) who are hemodynamically stable in the outpatient setting to decrease adverse events—regardless of their initial risk category.1
STRENGTH OF RECOMMENDATION
B: Based upon a good-quality retrospective cohort propensity score analysis.
Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
Dialing back opioids for chronic pain one conversation at a time
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
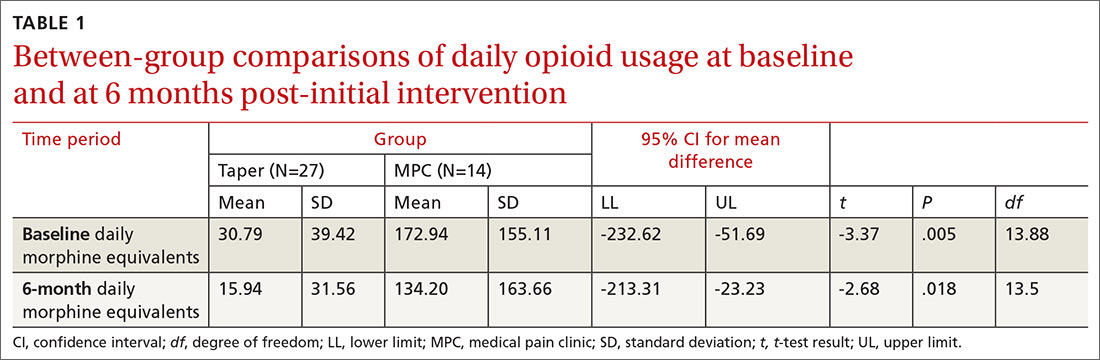
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
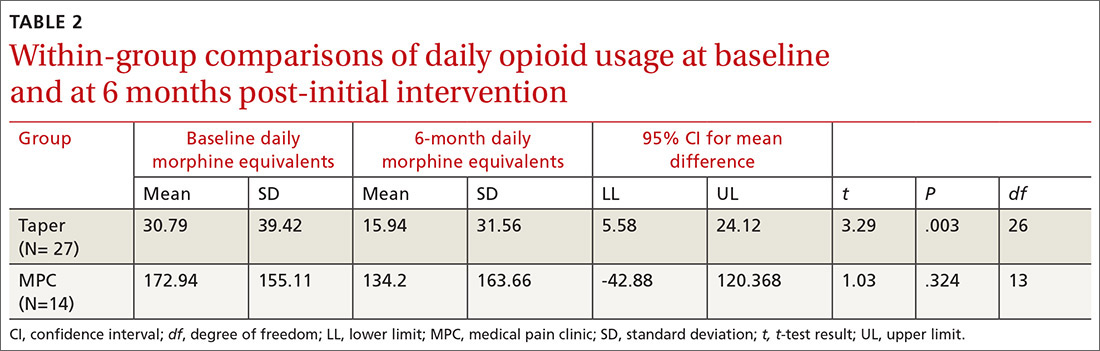
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; tpguck@creighton.edu.
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; tpguck@creighton.edu.
ABSTRACT
Purpose Our study examined the efficacy of a primary-care intervention in reducing opioid use among patients who have chronic non-cancer pain (CNCP). We also recorded the intervention’s effect on patients’ decisions to leave (or stay) with the primary-care practice.
Methods A family physician (FP) identified 41 patients in his practice who had CNCP of at least 6 month’s duration and were using opioids. The intervention with each patient involved an initial discussion of ethical principles, evidence-based practice, and current published guidelines. Following the discussion, patients self-selected to participate with their FP in a continuing tapering program or to accept referral to a pain center for management of their opioid medications. Tapering ranged from a 10% reduction per week to a more rapid 25% to 50% reduction every few days. Twenty-seven patients continued tapering with their FP, and 6 months later were retrospectively placed in the Taper Group. Fourteen patients chose not to pursue the tapering option and were referred to a single-modality medical pain clinic (MPC). All patients had the option of staying with the FP for other medical care.
Results At baseline and again at 6 months post-initial intervention, the MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group. The Taper Group at 6 months was taking significantly lower average daily narcotic doses in morphine equivalents than at baseline. No significant baseline-to-6 month differences were found in the MPC Group. Contrary to many physicians’ fear of losing patients following candid discussions about opioid use, 40 of the 41 patients continued with the FP for other health needs.
Conclusions FPs can frankly discuss opioid use with their patients based on ethical principles and evidence-based recommendations and employ a tapering protocol consistent with current opioid treatment guidelines without jeopardizing the patient-physician relationship.
[polldaddy:10180698]
Opioid prescriptions for chronic noncancer pain (CNCP) have increased significantly over the past 25 years in the United States.1 Despite methodologic concerns surrounding research on opioid harms, prescription opioid misuse among CNCP patients is estimated to be 21% to 29% and prescription addiction 8% to 12%.2 Tragically, with the overall increase in opioid use for CNCP, substance-related hospital admissions and deaths due to opioid overdose have also risen.3
Increased opioid use began in 1985 when the World Health Organization expanded its ethical mandate for pain relief in dying patients to include relief from all cancer pain.3 Opioid use then accelerated following Portenoy and Foley’s 1986 article4 and the 1997 consensus statement by the American Academy of Pain Medicine (AAPM) and the American Pain Society (APS),5 with both organizations arguing that opioids have a role in the treatment of CNCP. Increased use of opioids for CNCP continued throughout the 1990s and 2000s, as many states passed legislation removing sanctions on prescribing long-term and high-dose opioid therapy, and pharmaceutical companies aggressively marketed sustained-release opioids.3
A balanced approach to opioids. While acknowledging the serious public health problems of drug abuse, addiction, and diversion of opioids from licit to illicit uses, clinical research and regulation leaders have called for a balanced approach that recognizes the legitimate medical need for opioids for CNCP. In 2009 the APS, in partnership with the AAPM, published evidence-based guidelines on chronic opioid therapy (COT) for adults with CNCP.6 In developing these guidelines, a multidisciplinary panel of experts conducted systematic reviews of available evidence and made recommendations on formulating COT for individuals, initiating and titrating therapy, regularly monitoring patients, and managing opioid-related adverse effects. Additional recommendations addressed the use of therapies focusing on psychosocial factors. The APS-AAPM guidelines received the highest rating in a systematic review critically appraising 13 guidelines that address the use of opioids for CNCP.7
Continue to: When opioid use is prolonged...
When opioid use is prolonged. Most primary care physicians are aware of the risks of prolonged opioid use, and many have successfully tapered or discontinued opioid medications for patients in acute or pre-chronic stages of pain.8 However, many physicians face the challenge of patients who have used COT for a longer time. The APS-AAPM guidelines may help primary care physicians at any stage of treating CNCP patients.
METHODS
Purpose and design. This retrospective study, which reviewed pretest-posttest findings between and within study groups, received an exempt status from Creighton University’s institutional review board. We designed the study to determine the efficacy of an intervention protocol to reduce opioid use by patients with CNCP who had been in a family physician (FP)'s panel for quite some time. Furthermore, because a common fear among primary care providers is that raising concerns with patients about their opioid use may cause those patients to leave their panel,9 our study also recorded how many patients stayed with their FP after initiation of the opioid management protocol.
Subjects. This study tracked 41 patients with CNCP in 1 FP’s panel. Inclusion criteria for participation was: 1) presence of CNCP for at least 6 months, 2) current use of opioid medication for CNCP, 3) age of at least 16 years, and 4) ability to read and write English. Two exclusion criteria were the presence of a surgically correctable condition or an organic brain syndrome or psychosis.
Clinical intervention. The FP identified eligible patients in his practice that were taking opioids for CNCP and initiated a discussion with each of them emphasizing his desire to follow the ethical principles of beneficence, nonmaleficence, respect for autonomy, and justice.10 The FP also presented his reasons for wanting the patient to stop using opioid medication. They included his beliefs that:
1) COT was not safe for the patient based on a growing body of published evidence of harm and death from COT3;
2) long-term use of opioids could lead to misuse, abuse, or addiction2;
3) prolonged opioid use paradoxically increases pain sensitivity that does not resolve
4) the patient’s current pain medications were not in line with published guidelines for use of opioids for CNCP.6
Initially, 45 patients were eligible for the study, but 4 declined participation before the intervention discussion and were immediately referred to a single-modality medical pain clinic (MPC). These patients were not included in subsequent analyses. Of the remaining 41 patients, all had a discussion with the MD about ethical principles, practice guidelines, and the importance of opioid tapering. After the discussion, patients decided whether to continue with the plan to taper their opioid therapy or to not taper their therapy and so receive a referral to an MPC.
Continue to: The 27 patients who chose to work with...
The 27 patients who chose to work with their FP started an individually tailored opioid-tapering program and were retrospectively placed in the Taper Group 6 months later. Tapering ranged from a slow 10% reduction in dosage per week to a more rapid 25% to 50% reduction every few days. Although evidence to guide specific recommendations on the rate of reduction is lacking, a slower rate may reduce unpleasant symptoms of opioid withdrawal.6 Following the patient-FP discussion, the 14 patients who chose not to pursue the tapering option were referred to an MPC for pain management, but could opt to remain with the FP for all other medical care. At 6 months post-discussion, we retrospectively assigned these 14 patients to the MPC Group.
Measures. We obtained demographic and medical information, including age, gender, race, marital status, and medication level in morphine equivalents, from the electronic health record. Medication level in morphine equivalents was recorded at the beginning of the intervention and again 6 months later. All analyses were conducted using SPSS Version 24 (IBM Corp, Armonk, NY) with P<.05 used to indicate statistical significance.
RESULTS
Between-group differences. The Taper and MPC groups did not differ significantly on demographic variables, with mean ages, respectively, at 57 and 51 years, sex 56% and 50% female, race 74% and 79% white, and marital status 48% and 50% married.
We found significant differences between the Taper and MPC groups on total daily dose in morphine equivalents at baseline and at 6 months following initial intervention. The Levene’s test for equality of variances was statistically significant, indicating unequal variances between the groups. In our SPSS analyses, we therefore used the option “equal variances not assumed.” TABLE 1 lists resultant means, standard deviations, individual sample t-test scores, and confidence intervals. The MPC Group was taking significantly higher daily doses of morphine equivalents than the Taper Group both at baseline and at 6 months following initial intervention.
Within-group differences. Paired sample t tests indicated significant differences between baseline and 6-month average daily narcotic doses in morphine equivalents for the Taper Group. No significant difference was found between baseline and 6-month daily morphine equivalents for the MPC group. These results indicated that patients who continued opioid tapering with the FP significantly reduced their daily morphine equivalents over the 6 months of the study. Patients in the MPC Group reduced morphine equivalents over the 6 months, but the reduction was not statistically significant. Paired sample t test results are presented in TABLE 2.
Continue to: Patient retention
Patient retention. All but one of the 41 patients in the Tapering and MPC groups continued with the FP for the remainder of their health care needs. Contrary to some physicians’ fears, the patients in this study maintained continuity with their FP.
DISCUSSION
Results of this study indicate that an intervention consisting of a physician-patient discussion of ethical principles and evidence-based practice, followed by individualized opioid tapering per published guidelines, led to a significant reduction in opioid use in patients with CNCP. The Taper Group, which completed the intervention, exhibited significant morphine reductions between baseline and 6-month follow-up. This did not hold true for the MPC Group.
The MPC Group, despite participating in the discussion with the FP, chose not to complete the tapering program and was referred to a single-modality MPC where opioids were managed rather than tapered. While the MPC group reduced daily opioid dose levels, the reduction was not statistically significant. A possible reason for no difference within the MPC Group may be that they had greater dependence on opioids, as their baseline average daily dose was much higher than that in the Taper Group (173 mg vs 31 mg, respectively). Although we did not assess anxiety directly, we speculate that the MPC Group was more anxious about opioid reduction than the Taper Group, and that this anxiety potentially led 4 patients to opt out of the initial FP discussion and 14 patients to self-select out of the tapering program following the discussion.
The FP intervention was successful for the Taper Group. For MPC patients, an enhanced intervention including behavior health strategies13 might have reduced anxiety and increased motivation14 to continue tapering. Based on moderate-quality evidence, APS-AAPM guidelines strongly recommend that CNCP be viewed as a complex biopsychosocial condition. Therefore, clinicians who prescribe opioids should routinely integrate psychotherapeutic interventions, functional restoration, interdisciplinary therapy, and other adjunctive nonopioid therapies.6
Opioid tapering within multidisciplinary rehabilitation programs is possible without significant worsening of pain, mood, and function.15 Recently, an outpatient opioid-tapering support intervention showed promise for efficacy in reducing prescription opioid doses without resultant increases in pain intensity or pain interference.16
Continue to: The tapering protocol in our study...
The tapering protocol in our study and the inclusion of behavioral health co-interventions are also recommended by the 2016 guidelines published by the Center for Disease Control and Prevention.17 More information on the similarities and differences among the various guidelines is available online.18,19
Caveats with our study. Patients’ entry into the Taper or MPC groups occurred through self-selection rather than random assignment. Thus, caution is recommended in interpreting findings of the FP intervention. And, we did not measure patients’ levels of pain, so differences between groups may have been possible. In addition, the number of patients per group was relatively small, which may have accounted for the lack of significance in the MPC Group findings. Conversely, significant reductions in opioid use in the small tapering sample suggests a relatively robust intervention, despite a lack of random assignment to treatment conditions.
These findings suggest that FPs can have a frank conversation about opioid use with their patients based on ethical principles and evidence-based practice, and employ a tapering protocol consistent with current opioid treatment guidelines. Furthermore, this approach appears not to jeopardize the patient-physician relationship.
CORRESPONDENCE
Thomas P. Guck, PhD, Creighton University School of Medicine, 2412 Cuming Street, Omaha, NE 68131; tpguck@creighton.edu.
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
1. Manchikanti L, Helm S, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15:ES9-ES38.
2. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576.
3. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:S94-S100.
4. Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986;25:171-186.
5. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13:6-8.
6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130.
7. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
8. Hwang CS, Turner LW, Kruszewski SP, et al. Primary care physicians’ knowledge and attitudes regarding prescription opioid abuse and diversion. Clin J Pain. 2016;279-284.
9. Top 15 challenges facing physicians in 2015. Medical Economics. http://www.medicaleconomics.com/medical-economics/news/top-15-challenges-facing-physicians-2015?page=0,12. Accessed October 18, 2018.
10. Kotalik J. Controlling pain and reducing misuse of opioids: ethical considerations. Can Fam Physician. 2012;58:381-385.
11. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
12. Wachholtz A, Gonzalez G. Co-morbid pain and opioid addiction: long term effect of opioid maintenance on acute pain. Drug Alcohol Depend. 2014;145:143-149.
13. Hunter CL, Goodie JL, Oordt MS, Dobmeyer AC. Integrated Behavioral Health in Primary Care. 2nd ed. Washington DC: American Psychological Association; 2017.
14. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
15. Townsend CO, Kerkvliet JL, Bruce BK, et al. A longitudinal study of the efficacy of a comprehensive pain rehabilitation program with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Pain. 2008;140:177-189.
16. Sullivan MD, Turner JA, DiLodovico C, et al. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18:308-318.
17. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
18. Barth KS, Guille C, McCauley J, et al. Targeting practitioners: a review of guidelines, training, and policy in pain management. Drug Alcohol Depend. 2017;173:S22-S30.
19. CDC. Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Injury Prevention & Control: Prescription Drug Overdose 2016. http://www.cdc.gov/drugoverdose/prescribing/common-elements.html. Accessed October 18, 2018.
6-day history of fever • groin pain and swelling • recent hiking trip in Colorado • Dx?
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
The art of delivering evidence-based dual antiplatelet therapy
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
In landmark clinical research published in 1996, aspirin (ASA) and the P2Y12 inhibitor ticlopidine used after coronary artery stent implantation was compared to intravenous anticoagulation—at the time, the postprocedure standard of care for preventing thrombosis. What investigators found was a marked reduction in cardiac and hemorrhagic events in patients who were treated with this novel dual antiplatelet therapy (DAPT).1 Since publication of the results of that trial, the use of ASA plus a P2Y12 inhibitor has expanded to treating acute coronary syndrome (ACS) and stroke.
Over the past 2 decades, much research has been devoted to 1) determining the effectiveness of more potent P2Y12 inhibitors—which block chemoreceptors for adenosine diphosphate—to prevent stent thrombosis and 2) safer regimens to reduce hemorrhagic complications.
When does stent thrombosis occur?
The timing of stent thrombosis is defined as:
- acute (within 24 hours of placement),
- subacute (within 30 days),
- late (within 1 year), or
- very late (after 1 year).
Acute stent thrombosis is almost always related to technical issues surrounding stent implantation. Subacute thrombosis is almost always platelet activation within the stent with thrombus formation—the reason why antiplatelet therapy is beneficial and anticoagulation pathway inhibition is not beneficial.
Late stent complications can be caused by thrombosis, but also might be related to restenosis by 4 to 6 months—ie, tissue overgrowth as the stent becomes part of the body, not clot formation. In several studies, restenosis was a significant issue with balloon dilation alone, occurring in 33% of patients.2 Bare-metal stents (BMS) have been shown to reduce the rate of restenosis to approximately 20%; drug-eluting stents (DES) have further decreased restenosis to approximately 5%, in various reports, by impairing endothelial healing, thus limiting tissue overgrowth that leads to restenosis.3 This delay in healing caused by DES makes it necessary to administer DAPT for a longer duration—an increase that is not needed with BMS.
DAPT has well-defined benefits
As drug-eluting stents were introduced and improved, trials studying optimal duration of DAPT showed that longer duration of treatment reduced stroke incidence and the long-term risk of myocardial infarction (MI) unrelated to stent thrombosis.4 Nuances in the treatment of ischemic coronary artery disease (CAD) and secondary prevention of stroke can be perplexing, as can be P2Y12inhibitor selection. Here, we review DAPT agents and discuss current evidence and evidence-based guidelines, thus providing a framework to better understand treatment options and recommendations.
What constitutes DAPT?
Many combinations of antiplatelet therapy are possible but, in the United States, DAPT denotes ASA 81 mg/d plus any of the 3 P2Y12inhibitors: clopidogrel, prasugrel, and ticagrelor. Stimulation of the platelet P2Y12receptor causes stimulation of the platelet glycoprotein IIb/IIIa receptor, which, in turn, enhances platelet degranulation, thromboxane production, and prolonged platelet aggregation. Blocking P2Y12receptors thus impairs the thrombotic processes.5
Continue to: ASA, as a component of DAPT...
ASA, as a component of DAPT, is recommended at a dosage of 81 mg/d. In trials of ASA plus clopidogrel, lower ASA dosages had comparable ischemic event rates compared to higher ASA dosages.6,7 Patients given higher ASA dosages with ticagrelor had poorer outcomes when compared with low-dosage ASA.8 Higher dosages of ASA, alone or with DAPT, increase the risk of bleeding complications.9,10
Clopidogrel is the only P2Y12 inhibitor available as a generic medication in the United States. As a pro-drug, clopidogrel requires 2 metabolic transformations to its active metabolite after being hydrolyzed in the gut, which delays onset of platelet inhibition for several hours after ingestion.11 Furthermore, individual genetic variation in cytochrome P450 (CYP) 2C19 (CYP2C19), one of the hepatic enzymes in this metabolic process, may lead to less alteration of clinical platelet aggregation response, and increased drug interactions.12 Methods to assess platelet function have shown decreased inhibition of platelet aggregation for some CYP2C19 polymorphisms, although consistent clinical effects of this inhibition have not been identified to date; genetic testing for these polymorphisms is, therefore, not recommended routinely.13
Indications for DAPT treatment with clopidogrel are unstable angina or non-ST-segment elevation acute coronary syndrome (NSTE-ACS), whether planned treatment is medical or coronary revascularization. Other indications include acute ST-segment elevation MI (STEMI) with planned medical treatment, and recent MI, stroke, or established peripheral arterial disease.13,14
Prasugrel has faster onset of action and greater and more consistent P2Y12 inhibition than clopidogrel. After prasugrel is hydrolyzed in the gut, an intermediary metabolite is activated in the liver. Peak serum concentration is reached within 30 minutes.12 Unlike the case with clopidogrel, genetic variation in the CYP gene does not impart significant impact on forming the active metabolite.15
Indication for the use of prasugrel is ACS that is managed with percutaneous coronary intervention (PCI). Dual antiplatelet therapy with prasugrel results in reduced risk of cardiovascular death, nonfatal MI, and stroke, compared with ASA plus clopidogrel, with an increase in bleeding events.16 Thrombolysis patients and those who have a history of stroke had a greater risk of hemorrhage complications with prasugrel treatment, compared with clopidogrel. Prasugrel offered no benefit to patients older than 75 years or those who weigh <60 kg. If used in patients who weigh <60 kg, however, dosage reduction is recommended.16
Continue to: Ticagrelor
Ticagrelor. Unlike clopidogrel and prasugrel, ticagrelor is a direct oral, reversible-binding P2Y12 inhibitor. Peak serum concentration is reached within 2 to 3 hours.17 Indications are ACS or a history of MI, and those with ACS undergoing stent implantation. Ticagrelor was superior to clopidogrel in reducing the risk of death from vascular causes, MI, and stroke, and superior to clopidogrel in reducing the risk of stent thrombosis. There was no increase in the overall major bleeding rate and a decrease in fatal bleeding events compared to clopidogrel. Adverse effects unique to ticagrelor include dyspnea and, in patients with bradydysrhythmias, asymptomatic ventricular pauses. Both effects tend to resolve with continued treatment. This P2Y12 inhibitor should be avoided in patients with severe liver disease.
Loading and maintenance doses of the 3 P2Y12 inhibitors are provided in TABLE 1.13,14
When—and when not—to initiate DAPT
Treatment recommendations for DAPT originated in the 2016 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease14 and in the 2017 European Society of Cardiology (ESC) focused update on dual antiplatelet therapy in coronary artery disease.13 Although these guidelines differ slightly, the overall approach they present is similar, with an emphasis on limiting bleeding while preventing stent thrombosis.
Stable ischemic heart disease (SIHD) is defined as confirmed obstructive CAD without either ACS or a history of PCI in the past year.18 Patients with SIHD but without a history of PCI or recent coronary artery bypass grafting (CABG) receive no benefit from DAPT (Strength of recommendation [SOR]: A).19 (See TABLE 2 for definitions of SOR and corresponding levels of evidence.)
For patients who have undergone BMS placement, minimum DAPT with clopidogrel is 1 month (SOR: A) and, if there is no significant bleeding on DAPT and no high risk of bleeding (ie, no prior bleeding while taking DAPT, coagulopathy, or oral anticoagulant use), continuation of ASA and clopidogrel beyond 1 month might be reasonable (SOR: B).
Continue to: With a drug-eluting stent...
With a drug-eluting stent, the minimum time for DAPT (using clopidogrel) is 6 months (SOR: A), with a longer duration being reasonable if the patient is not at high risk of bleeding and has had no bleeding complications (SOR: B). For DES patients who have developed a high risk of bleeding, have had significant bleeding, or require a procedure that will place them at high risk of bleeding, DAPT discontinuation can be considered at 3 months (SOR: B).
Updated guidelines allow longer therapy for patients who tolerate DAPT; for them, 12 months of therapy is preferred. In comparing longer and shorter therapy, it has been determined that longer DAPT treatment is superior for reducing the risk of MI and stent thrombosis without increasing the risk of stroke or bleeding complications.20 With increased bleeding, or where there is a need for elective surgery, shortened DAPT is an option.
When treating patients with ACS, including NSTE-ACS or STEMI, DAPT for 1 year is recommended (SOR: A). When medical therapy alone is planned, DAPT is provided with clopidogrel or ticagrelor.
When a patient has been treated with PCI (BMS or DES), DAPT with any of the P2Y12 inhibitors is recommended (SOR: A) unless there is history of stroke or transient ischemic attack (TIA) or the patient is ≥75 years of age, in which case prasugrel is contraindicated (SOR: A: Harm).
Continue to: When lytic interventions are employed in STEMI...
When lytic interventions are employed in STEMI, DAPT with clopidogrel—for a minimum of 14 days and, ideally, for 12 months—should be considered. Without high risk of bleeding, or significant bleeding on DAPT, continuing DAPT for >12 months might be reasonable (SOR: A).
TABLE 3,13,14 adapted from 2016 ACC/AHA14 and 2017 ESC13 guidelines, provides recommendations about agents and duration of therapy in the management of patients with CAD.
How long should you give DAPT?
Balancing the hemorrhagic complications of DAPT against its benefits is challenging. The use of risk scores to guide duration of DAPT may be considered (SOR: B).
The PRECISE-DAPT score21 is used at the time of coronary artery stenting to guide treatment duration. The scoring algorithm incorporates hemoglobin level, leukocyte count, age, creatinine clearance and prior bleeding to create a composite score on a 100-point scale.22 (The algorithm can be found at www.precisedaptscore.com/predapt/webcalculator.html.) If the composite is <25 points, the number needed to treat to prevent an ischemic event is 65, and standard or long-term DAPT (12 to 24 months) is recommended. When the PRECISE-DAPT score is ≥25, the number needed to harm with a hemorrhagic event is 38, and a shorter duration of therapy (3 to 6 months) is recommended.
The DAPT score,23 available from the American College of Cardiology24 (http://tools.acc.org/DAPTriskapp/#!/content/calculator) is a risk calculator for use after 12 months of DAPT in the absence of complications. Age, cigarette use, diabetes, current or previous MI, presence of congestive heart disease, and type and location of stent all factor into calculating the risk score. DAPT scores range from -2 to 10. A score ≥2 suggests less bleeding risk, with a recommendation to consider longer treatment (≤30 months); a score <2 leads to a recommendation to adhere to standard treatment duration of 12 months.
Continue to: Patients with CAD should...
Patients with CAD should continue ASA treatment when DAPT is discontinued or completed, unless contraindicated.13,14
Triple therapy: DAPT + anticoagulant
Given that the US population is aging, there are an increasing number of patients with CAD and atrial fibrillation. Stroke is prevented in patients with atrial fibrillation with anticoagulant therapy; when these patients have stent placement for coronary, carotid, vertebral, or intracranial arterial disease, they need DAPT to prevent stent thrombosis. In the immediate post-stenting period, therefore, patients are often placed on an oral anticoagulant as well as DAPT. Vitamin K antagonists (VKAs) should be discontinued after acute stroke, with individualized resumption of a VKA when clinically appropriate.
As we emphasize throughout this article, there is a balance between bleeding risk and the potential benefits of therapy of the selected anticoagulant/DAPT regimen. These complex patients are best managed in close consultation with Cardiology and Neurology because of their potential risk of 3-fold bleeding.25 The findings of a recent study addressing post-stent placement therapy in patients with nonvalvular atrial fibrillation suggests that the direct oral anticoagulant dabigatran may be preferable to warfarin in this setting, because of the lower risk of bleeding with dabigatran without increased thrombotic risk.26 In this study, 3-drug therapy was used for 1 month, followed by discontinuation of ASA and continuation of 2-drug therapy with the direct oral anticoagulant and the P2Y12 inhibitor for the 6- to 12-month time frame post-stenting (SOR: B).
Consider a PPI to reduce the risk of a GI bleed
Proton-pump inhibitors (PPIs) should be considered for patients treated with DAPT if there is a history of gastrointestinal (GI) bleeding (SOR: A). Although a potential interaction between PPIs and P2Y12 inhibition has been identified in laboratory studies, this has not been supported in clinical studies. Therefore, although warnings exist for concomitant use of clopidogrel and PPIs, a PPI is reasonable for patients who are at increased risk of GI hemorrhage, including those taking warfarin, a corticosteroid, or a nonsteroidal anti-inflammatory drug and those of advanced age (SOR: B). Risks and benefits of clopidogrel and PPIs should be discussed with patients. There is no benefit in using PPIs for low-risk patients. (SOR: A: No benefit).27,28
Perioperative management with DAPT can be thorny
Perioperative management of DAPT patients who have an indwelling coronary stent and require noncardiac surgery is complicated. Stent thrombosis is a calamity, with ≥50% risk of death. Delaying surgery for at least 4 weeks after placement of a BMS and 6 months after placement of a DES reduces the risk of thrombosis.29
Continue to: For emergent surgery...
For emergent surgery, when severe bleeding is not seen or expected, interruption of DAPT can be minimized. After cessation of DAPT components, normal platelet function will return in12:
- 7 to 10 days for ASA,
- 5 to 7 days for prasugrel,
- 5 days for clopidogrel, and
- 3 to 5 days for ticagrelor.
If significant bleeding occurs perioperatively, or is expected, platelet transfusion can be helpful, and might need to be repeated because each P2Y12inhibitor has a half-life of between 8 and 12 hours.
For urgent or time-sensitive surgery, discontinuing a P2Y12inhibitor can be considered—while continuing ASA, if possible. DAPT should be restarted as soon as safely possible. If enteral administration is not feasible, ASA can be administered rectally. In this setting, cardiology consultation is strongly encouraged.
Last, elective surgery should be delayed until DAPT is completed, but without discontinuing ASA, if feasible. Spinal, intracranial, prostate, middle-ear, and ophthalmologic surgery while taking ASA can lead to catastrophic complications; consider discontinuing ASA. Cardiology consultation can provide an estimate of thrombosis risk to guide clinical decision-making.30
Can DAPT prevent secondary stroke?
DAPT has brought improvements in the treatment of patients with cardiovascular disease; it has been hypothesized that similar benefits can be seen in patients with ischemic stroke. Knowing the cause of stroke is key to developing a secondary prevention plan; patients with stroke secondary to atherosclerotic disease are most likely to benefit from DAPT.31 Conversely, secondary prevention in patients with small-vessel disease and in studies of unselected stroke type has been shown to be harmful.32,33
Continue to: Clopidogrel and ASA initiated...
Clopidogrel and ASA initiated within 24 hours of a minor stroke (ie, National Institutes of Health Stroke Score/Scale <4 [www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf]34) or TIA and continued for a total of 21 days of DAPT, followed by clopidogrel alone to complete 90 days of treatment, have been demonstrated to reduce the risk of recurrent ischemic stroke compared to ASA alone without increasing the risk of bleeding (SOR: B).35
In a multinational trial of DAPT, stroke risk was reduced at 90 days after TIA or mild stroke but bleeding risk was higher, compared to ASA alone; continuing DAPT for 90 days might explain the higher risk of bleeding.36
For secondary prevention of stroke in patients with aspirin allergy, monotherapy with clopidogrel is an option, but use of clopidogrel or ticagrelor is not superior to ASA.37,38 Therefore, there may be benefit, in patients with TIA or minor stroke, to continue DAPT beyond 21 days but at the risk of bleeding complications. (SOR: A: Harm).33,34
Based on these data, the best duration of DAPT after TIA or mild stroke is likely 21 to 28 days.
When a patient requires VKA therapy, the benefit of using DAPT to further reduce ischemic cerebrovascular or cardiovascular events is unknown (SOR: C). In the setting of atrial fibrillation with unstable angina or CAD stent implantation, however, therapy with DAPT plus a VKA can be considered—but with increased risk of nonfatal and fatal bleeding.39
Continue to: Summing up
Summing up: Key guidance
DAPT has benefits for patients with SIHD and ACS in the setting of medical management or implantation of a coronary artery stent. Balancing the reduction in risk of ongoing ischemic events with hemorrhagic complications presents challenges, as does deciding on duration of therapy. Using a DAPT risk calculator can be helpful to present information to the health care team and the patient, thus encouraging patient-centered treatment decisions.
Patients at increased risk of ischemia, such those with an ACS presentation, multiple myocardial infarcts, extensive CAD, left-ventricular ejection fraction <40%, chronic kidney disease, or diabetes mellitus might benefit from longer DAPT. Conversely, patients with prior bleeding complications, taking oral anticoagulation, with body weight <60 kg, or on chronic steroids or nonsteroidal medications might benefit from shorter duration of DAPT.
Earlier recommendations about the duration of DAPT continue to be refined by ongoing clinical research. Current-generation DESs have improved over first-generation stents; updated guidelines from the AHA and ESC presented in this review are based on new, improved stents.
ASA should almost always be continued upon completion of DAPT or if P2Y12inhibitors are held for surgery.
Last, in patients with mild ischemic stroke or TIA, DAPT therapy, begun within 24 hours and continued for 21 to 28 days, followed by ASA, 81 mg/d, alone indefinitely, can reduce the risk of recurrent stroke.
CORRESPONDENCE
William J. Curry, MD, MS, Departments of Family and Community Medicine and Public Health Sciences, H154, 500 University Drive, Pennsylvania State University College of Medicine, Hershey, PA 17033; wcurry@pennstatehealth.psu.edu.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
1. Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
2. Ducrocq G, Serebruany V, Tanguay J.
3. Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013;128:2785-2798.
4. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791-1800.
5. Damman P, Woudstra P, Kuijt WJ, et al. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33:143-153.
6. Steinhubl SR, Bhatt DL, Brennan DM, et al; CHARISMA Investigators. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379-386.
7. Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233-1243.
8. Mahaffey KW, Wojdyla DM, Carroll K, et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2011;124:544-554.
9. Xian Y, Wang TY, McCoy LA, et al. Association of discharge aspirin dose with outcomes after acute myocardial infarction: insights from the Treatment with ADP Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2015;132:174-181.
10. Patrono C, Baigent C, Hirsh J, et al. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:199S-233S.
11. Wenaweser P, Dörffler-Melly J, Imboden K, et al. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748-1752.
12. Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126-142.
13. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Euro Heart J. 2017;39:213-260.
14. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J Am Coll Cardiol. 2016;68:1082-1115.
15. Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166-1173.
16. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
17. Debesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34:1077-1090.
18. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2014;130:1749-1767.
19. Benedetto U, Altman DG, Gerry S, et al. Impact of dual antiplatelet therapy after coronary artery bypass surgery on 1-year outcomes in the Arterial Revascularization Trial. Eur J Cardiothorac Surg. 2017;52:456-461.
20. Toyota T, Shiomi H, Morimoto T, et al. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502.
21. Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025-1034.
22. PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy (PRECISE-DAPT) WebCalculator. www.precisedaptscore.com/predapt/webcalculator.html. Accessed October 21, 2018.
23. Yeh RW, Secemsky EA, Kereiakes DJ, et al; DAPT Study Investigators. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735-1749.
24. American College of Cardiology. DAPT Risk Calculator. http://tools.acc.org/DAPTriskapp/#!/content/calculator/. Accessed October 21, 2018.
25. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
26. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524.
27. Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 Expert Consensus Document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 Expert Consensus Document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;122:2619-2633.
28. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574-e651.
29. Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e77-e137.
30. Kristensen SD, Knuuti J, Saraste A, et al; Authors/Task Force Members. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anesthesiology (ESA). Euro Heart J. 2014;35:2383-2431.
31. Wong KS, Chen C, Fu J, et al; CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010:9:489-497.
32. SPS3 Investigators; Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
33. Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared to clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331-337.
34. NIH Stroke Scale. National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/sites/default/files/NIH_Stroke_Scale_Booklet.pdf. Accessed November 14, 2018.
35. Wang Y, Wang Y, Zhao X, et al; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11-19.
36. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215-225.
37. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996:16;348:1329-1339.
38. Johnson SC, Amarenco P, Albers GW, et al; SOCRATES Steering Committee and Investigators. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35-43.
39. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441.
PRACTICE RECOMMENDATIONS
› Use a dual antiplatelet therapy (DAPT) risk calculator to encourage patient-centric decisions when presenting information to the health care team and the patient. B
› Consider the potential benefit of a shorter duration of DAPT for patients who 1) have prior bleeding complications or 2) are taking an oral anticoagulant, chronic corticosteroid, or nonsteroidal anti-inflammatory drug. B
› Continue aspirin use upon completion of DAPT or if a P2Y12 inhibitor is being held for surgery. A
› Reduce the risk of recurrent stroke in patients who have had a mild ischemic stroke or transient ischemic attack by providing DAPT for 21 to 28 days, followed by aspirin indefinitely—so long as treatment can begin within 24 hours of the event. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Adults with Congenital Heart Disease: The Critical Transition from Pediatric to Adult Care
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; mpatel@ghs.org.
Financial disclosures: None.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; mpatel@ghs.org.
Financial disclosures: None.
From the Greenville Health System, Greenville, SC.
Abstract
- Objective: To review the management of patients with congenital heart disease (CHD) transitioning from pediatric to adult care.
- Methods: Review of the literature.
- Results: Persons with CHD require close monitoring and evaluation throughout life to address the physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. During the transition from pediatric to adult cardiology, a high proportion of patients are lost to follow up or have long gaps in care after leaving pediatric cardiology, which can lead to poor outcomes. Care of the adult with CHD requires close coordination between the patient’s primary care physician), cardiologist, adult CHD specialist, and other specialists. The transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living. Successful transition programs use a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.
- Conclusion: The transition from pediatric to adult care in ACHD patients is best provided through a comprehensive transition program that begins in early adolescence and enables patients to take charge of their disease process in adulthood, allowing them to maximize their quality of life and societal contributions.
Keywords: adult; congenital heart defects; complications; disease management; patient care team.
The population of adults with congenital heart disease (CHD) in developed countries has grown at an exponential rate in the past 4 decades. With advances in medical care and surgical interventions, the proportion of pediatric patients reaching adulthood has increased from 15% in the 1930s-60s to more than 95% for patients with mild to moderate complexity CHD. The rate of survival to adulthood for patients with severely complex CHD remains lower at around 56%.1
There are now more adult than pediatric patients with CHD in the United States. Because adult CHD (ACHD) patients have increased morbidity and mortality in their young adult years, it is imperative for all providers to understand and address the long-terms needs of this population. Unfortunately, adults with CHD do not always receive adequate health care, frequently because they are lost to follow-up, particularly during their adolescent years when they are expected to gain independence in their medical management. As will be discussed, CHD is a chronic illness fraught with numerous expected and unexpected complications that require close monitoring and re-interventions. Effectively anticipating and addressing these complications requires a standardized and comprehensive process of transition from the pediatric to the adult population to ensure maximal quality of life.
Epidemiology
The actual prevalence of ACHD in the United States is unknown, as a national database of persons with CHD has not been established.2 In contrast, Europe and China have maintained databases that enable ongoing monitoring of the evolving CHD epidemiology in those regions.3,4 The best estimates of the U.S. incidence and prevalence of ACHD stem from extrapolations from Canadian data. According to this data, there were more than 1.2 million adults with ACHD in the United States in 2012, with an anticipated 5% annual increase.1,5 However, the limitations of such extrapolations must be noted, as the Canadian population does not perfectly mirror that of the United States. Canada has lower infant mortality and adult obesity rates, and the United States has larger African American and Hispanic populations.6 Also, the juxtaposition of universal access to health care in Canada and the socioeconomic class–dependent access in the United States causes variations in care and outcomes of ACHD between the 2 populations. These differing genetic and social backgrounds may change the incidence of CHD by affecting maternal-fetal health.7
The 32nd Bethesda Conference on “Care of the Adult with Congenital Heart Disease” in 2000 was tasked with characterizing the ACHD population in the United States. This project found a prevalence similar to that of the Canadian extrapolation and showed that among persons with ACHD in the United States, 45% have mild disease, 37% moderate disease, and 13% severe disease.8
Characterizing the true incidence of CHD in the United States also has proven difficult because of variations in the definitions and methods used to detect lesions across the multiple studies that have looked at this matter. The estimated incidence of CHD, grouped according to severity, is 2.5 to 3 per 1000 live births for severe CHD, and from 3 to 13 per 1000 live births for moderately severe forms.9 When all forms are considered, including minor CHD (which includes tiny muscular ventricular septal defect [VSDs] present at birth and other trivial lesions), the total incidence of CHD rises to 75 per 1000 live births.9 CHD is one of the most common chronic illnesses in young adults with special health care needs.
Complications in Adulthood
The ACHD population represents a diverse population in terms of severity of CHD, history of surgical/catheter-based interventions, and socioeconomic status. However, a unifying clinical concern for these patients is their increased risk for morbidity and mortality in the young adult years. Despite the tremendous advances in the field over previous decades, mortality in this population in adulthood is estimated to be up to 7 times higher compared to age-matched peers.10,11 For many patients, palliative CHD interventions result in a significant drop in early morbidity and mortality but frequently lead to delayed morbidity from secondary multi-organ complications as these patients transition from pediatric to adult care. For example, due to the chronic low flow and low cardiac output state created by Fontan palliations, patients are at risk for diastolic dysfunction, arrhythmias, thrombotic events, protein-losing enteropathy, and cirrhosis/congestive hepatopathy, among other chronic conditions. These patients require frequent follow up and management by a multidisciplinary team including a primary care provider and various specialty groups.
Cardiac Disease
The most common causes of death in ACHD patients are heart failure (27%) and sudden cardiac death (19%), which occur at mean ages of 48 years and 39 years, respectively.10 The form of heart failure in ACHD patients is related to subsystemic right ventricle (RV) dysfunction, coronary under-perfusion, residual shunts, and residual progressive valve regurgitation. One of the more common examples of this is seen in palliated Tetralogy of Fallot patients who have undergone a transannular patch as a neonate. These patients are frequently left with significant pulmonary regurgitation leading to RV dilation, RV failure, and subsequent left ventricle (LV) failure. Another common example is the patient with dextro-transposition of the great arteries (DTGA) status post atrial switch who has a subsystemic morphologic RV. These patients will often develop significant RV dysfunction related to the chronic high pressures associated with systemic circulation.
Arrhythmias are a major contributor to morbidity and mortality in this population and are the most common reason patients present back into care. Difficult to control, multifocal intra-atrial re-entrant tachycardia is extremely common in ACHD, with an estimated 50% of all patients developing atrial arrhythmia by age 55. A recent study determined that the risk of atrial fibrillation in individuals with CHD was 22 times higher than that in age-matched controls, with the highest risk being seen in patients with conotruncal defects. Furthermore 10% of these patients develop heart failure.12 The risk for, incidence of, and type of arrhythmia is associated with the severity of the congenital heart lesions, as well as the type and timing of surgical interventions. Later age of repair has been associated with an increased likelihood of arrhythmias.13 Tetralogy of Fallot is an example of a moderately complex congenital heart lesion and is the most common cyanotic congenital heart lesion. In these individuals, the risk for atrial tachycardias, ventricular tachycardias, and need for a pacemaker is much higher than in age-matched peers.14 This includes an increased risk of sudden cardiac death, with many of these patients requiring placement of an implantable cardioverter defibrillator.
Pulmonary Disease
There is a 44% to 56% prevalence of restrictive pulmonary disease in the ACHD population, compared to 9% in the general non-CHD adult population. The incidence of pulmonary hypertension is also significantly higher in the ACHD population. The etiology for development of pulmonary hypertension is multifactorial, including chronic thromboembolic disease, left-sided heart disease, longstanding left to right shunts, and obstructive sleep apnea. These conditions have a significant impact on survival, as moderate/severe lung function impairment is an independent predictor of survival. Patients with shunt lesions are at risk of developing pulmonary arterial hypertension later in life,1 which quadruples the risk of all-cause mortality and more than triples the risk of cardiovascular mortality.7
Liver Disease
Hepatic morbidity associated with palliated CHD is often related to prior surgical interventions. The most common morbidities include chronic hepatitis C and liver failure from chronic under-perfusion and passive congestion, especially following Fontan palliation. Long term, these complications can lead to cirrhosis and hepatocellular carcinoma.15-18 Unfortunately, hepatic morbidity often precludes patients from having a surgical intervention, complicating the management of a population with baseline significantly increased need for surgical re-intervention.
Renal Disease
Approximately 50% of the ACHD population has some degree of renal dysfunction, with a higher incidence in cyanotic CHD.19 The American College of Cardiology/American Heart Association (ACC/AHA) recommends routine assessment of renal function in all adults with moderate and severe CHD due to its association with a poor prognosis in the ACHD population.1 In the immediate cardiac postoperative period, acute kidney injury leads to an eightfold increase in mortality.20 Over the longer term, there is a fivefold increase in mortality with moderate to severe renal impairment and a twofold increase with mild renal impairment compared to those with normal renal function.21
Acquired Cardiovascular Disease
As the ACHD patient ages, acquired cardiovascular disease becomes a significant issue. Approximately 80% of adults with CHD have at least 1 cardiovascular risk factor,22 though overall there is a relative lack of specific data regarding the U.S. population. Surveillance of the Canadian CHD population older than 65 years shows a 47% prevalence of hypertension,23 with increased risk in certain conditions such as aortic coarctation and renal disease associated with CHD. Although studies on the increased risk of diabetes mellitus in the ACHD population have yielded conflicting results,22,24 there is evidence of abnormal glucose metabolism in ACHD patients, which is a predictor of cardiac morbidity and mortality.25,26 The incidence of hyperlipidemia in U.S. ACHD patients is estimated to be at least as high as that of the general population.1 These factors combine with abnormalities in the myocardial substrate, hemodynamic abnormalities, arrhythmias, and sequelae of surgical repairs to confer an increased risk of ischemic heart disease and cerebrovascular disease in the ACHD population.15,27 One large case-control cohort study showed that the risk for ischemic heart disease was 16.5 times higher in patients with CHD as compared with non-CHD patients, with the highest incidence being in those with conotruncal defects and severe non-conotruncal defects. Interestingly, hypertension and diabetes were less common among CHD patients with ischemic heart disease than among non-CHD patients with ischemic heart disease.28
Adults with CHD have an increased risk for cerebrovascular disease compared with the general population, and cerebrovascular disease appears to occur at a younger age.29 The risk of ischemic stroke in individuals with ACHD younger than 55 years is 9 to 12 times higher than that in the general population. As in the general population, the incidence of ischemic stroke in ACHD patients increases with age, and in those older than 55 years, the incidence remains 2 to 4 times higher than in the general population.30,31
Clearly, complications arising from therapeutic interventions in CHD patients contribute significantly to morbidity/mortality in adult life, which underscores the need for life-long follow up and prevention of lapses in care.
The Transition from Pediatric to Adult Care
The monitoring and evaluation of CHD patients throughout life requires close coordination between the patient’s primary care physician, cardiologist, ACHD specialist, and other specialists, as appropriate. The timing of routine follow-up appointments is largely dependent on the severity of the congenital heart lesion and clinical status of the individual patient. Routine surveillance often includes cardiac imaging, preconception/genetic counseling, Holter screenings for arrhythmia, laboratory testing, and titration of medication. Unfortunately, only 30% of adults with CHD receive the recommended cardiac care.32
Children with chronic conditions transitioning to adulthood frequently experience a drop off in coordinated services as they transition from pediatric to adult medicine. Adult institutions often have less multidisciplinary support staff in the form of social workers and case management.33 Furthermore, a recent systematic review of articles that outlined the transition process from pediatric to adult cardiology in the CHD population showed that a high proportion of patients were either lost to follow up or had long gaps in care after leaving pediatric cardiology, with the first lapse in care commonly occurring at approximately age 19 years.28,34 A 2004 study showed that only 48% of adolescents with CHD underwent successful transition.35 A multicenter study of 922 ACHD patients found a gap in care lasting longer than 3 years in 42%, with 8% having gaps exceeding 10 years.36 Another study showed that lapses exceeding 2 years occurred in 63% of patients, with a median duration of lapse of medical care of 10 years. The most common reasons for lapse in care were: being told that cardiac follow up was not required (33%); being discharged from a children’s hospital without appropriate follow up plans in place (23%); being aware of need for follow up but having no symptoms (19%); lack of insurance (18%); and ignoring follow up recommendations for fear of receiving bad news (7%).37 Moreover, living independently from one’s parents was independently associated with a lapse in care, and patients with moderate complexity defects were more likely to experience a lapse than those with high complexity defects.
In the absence of a structured transition program, there is often delayed or inadequate care, which can result in significant emotional and financial stress on families and increased stress on the health care system.38 Inadequate, incomplete, or nonexistent transition and transfer for care has been shown to lead to poor health outcomes. Patients who experienced a lapse in care were 3 times more likely to require urgent cardiac intervention and to have an adverse outcome.37 The urgent interventions required by these patients included pulmonary valve replacement, mitral and tricuspid valve repair/replacement, VSD closure, pulmonary artery stenting, Fontan revision, and pacemaker/defibrillator placement.37 Clearly, there is significant room for improvement in the transition process of patients with CHD.
Best Practices in Transitioning CHD Patients to Adulthood
The overarching goal of pediatric to adult care CHD transition programs is to empower the patient and their support system to assume ownership of the disease process in order to maximize quality of life, life expectancy, and productivity.39 This involves ensuring that the patient has a thorough understanding of their diagnosis, heart anatomy, prior cardiac interventions, limitations imposed upon them by their condition, and the frequency of their anticipated follow-up care. The components of a successful transition program include a systematic approach to addressing the medical, psychosocial, and educational/vocational needs of the adolescent as he or she moves from the family-centered pediatric to the patient-centered adult health care system.40 The visits during the transition period are also an opportunity to discuss reproductive issues and the need for planning pregnancies for women with CHD. The goal is to encourage autonomy and promote ownership of their medical condition to the best of their social-cognitive ability. Adolescents should be encouraged to speak alone with their doctor to foster independence and self-management in their disease process; this has been shown to be protective against failure in transition.32 They should be encouraged to start calling their doctors, requesting refills, and making appointments.
The ACC/AHA appropriately recommend that the transition process for CHD patients begin at 12 years of age, with a goal of discussing future expectations of the child’s education, employment, and independent living.41 As part of this process, it is important that the practitioner educate the child and the family of the need for lifelong surveillance. The exact timing of the transition process is heavily influenced by a number of factors, including the degree of dependence of the child on their guardians, the severity of the congenital heart lesion, and the anticipated short- and long-term prognosis. However, regardless of these circumstances a reasonable age of transition into adult services should be established early on so that an expectation remains in place and the family is adequately prepared.
The challenge of learning how to navigate the adult health care system is as daunting for the transitioning patient as the medical consequences of their disease process. It is critical for patients to have easy access to social workers and case managers, ideally in the setting of a medical home, to connect them to community resources as needed. It is incredibly important that patients consider vocational options and training along with planning their insurance and/or disability qualifications as they move into adulthood. Establishing guardianship is also an important consideration for young adults with CHD who have remained dependent on their guardians.
Towards this end, the AHA/ACC has developed a curriculum that outlines the core principles that should be addressed before the patient moves to the ACHD clinic.27 The transition program should be flexible to accommodate for the patient’s degree of development, and the transfer should not occur before the adolescent has demonstrated the ability to independently manage their own health care to the greatest possible extent.
The ideal transition occurs through the auspices of a medical home that can coordinate the multiple subspecialists involved in the patient’s care. However, what often occurs is that a patient transitions from the pediatric cardiologist’s care before transitioning from pediatric to adult primary care. Prior to transition, the pediatric cardiologist should identify a cardiac destination at an ACHD center. This must be done in conjunction with the pediatrician, who will help identify an internist to take over the patient’s primary care and continue the coordination via the medical home. Information regarding the patient’s complete medical history, medication lists, exercise prescriptions, dietary restrictions, anesthetic issues, functional status, diagnostic studies, and comorbidities should be compiled in a health summary.40 To aid the process of transitioning, the ACC has developed several tools that may be used during the transition process, including self-knowledge assessments and medical summary templates.42
The Primary Care Provider’s Role and the Medical Home
Ensuring adequate care during the transition period requires close coordination between the patient’s various subspecialists. It is vital to avoid multiple subspecialists providing care without knowledge of each other’s treatments, as the treatment course for each ACHD patient is dependent on their unique history of prior therapies.27 The role of the primary care physician in establishing a “medical home” in this setting, as defined by the American Academy of Pediatrics Policy Statement, is exceedingly important.43 In this structure, the primary care physician maintains an easily accessible, centralized, and comprehensive record of the patient’s entire medical history, including surgical and medical treatments of both cardiac and noncardiac issues. Establishing the medical home framework is crucial, as it has been shown to lead to better outcomes in transitioning youth with special health care needs.44
With the establishment of this centralized care, the primary care physician must be able to negotiate the various medications prescribed by subspecialists and monitor for drug levels, adverse effects, and drug-drug interactions. ACHD patients also need regular monitoring and care aside from the care related to their chronic disease. Medical issues of particular importance to the ACHD patient include vaccinations, cholesterol and hypertension screening, cancer screening, and nutritional counseling. The primary care physician is responsible for addressing both the cardiac and noncardiac needs of the patient, ensuring that the patient truly receives comprehensive care. Thorough knowledge of a patient’s unique medical/surgical history will enable the primary care physician to adequately triage and appropriately refer for the development of a new symptom in an ACHD patient. On the other end of the spectrum, the patient’s subspecialists must maintain accurate and up to date information regarding their patient and transmit this to the patient’s medical home.
ACHD Centers
ACHD centers are an important part of any ACHD patient’s clinical team. Regardless of the complexity of the heart defect, there is tremendous value in the education and anticipatory guidance ACHD centers provide for their patients. The providers at these centers are often board-certified ACHD physicians who will work within a multidisciplinary team that includes mid-level practitioners, electrophysiology physicians, high-risk obstetrics/gynecology physicians, pulmonologists, and hepatologists. Each center differs in terms of their on-site interventional capacity and experience. However, the ACHD provider community is highly capable in directing patients who require interventions to centers of excellence, where there is proven quality in congenital surgical and interventional outcomes. ACHD centers often serve as the portals of reentry into care and are critical for providing and coordinating the complex care of each patient. Regular follow-up at these centers will ensure that patients receive adequate management of complications as they arise and preventive care against acquired heart disease.
The timing of follow-up at ACHD centers varies according to the complexity of heart disease. Individuals with simple CHD should be evaluated at an ACHD center at least once to determine the need for further follow-up. Patients with moderate and complex CHD must be monitored at a minimum of every 12 to 24 months, whereas very complex CHD should be monitored every 6 to 12 months.23 The frequency with which the young adult population moves may hinder adequate continuity of care and long-term follow up; a searchable directory of ACHD clinics in the United States and Canada can be found at www.achaheart.org/your-heart/clinic-directory/clinic-listings/.
Managing Specific Issues in the Transitioning Patient
Arrhythmias and Heart Failure
As mentioned, arrhythmias in the ACHD population are extremely common, the most frequent being atrial arrhythmias, especially in patients who have undergone single-ventricle repairs. Patients with late repair of an atrial septal defect have a high incidence of supraventricular tachycardia, which can be treated with catheter ablation procedures.45,46 Pacemaker implantation is another therapeutic option, especially in those who have undergone atrial surgery (ie, Mustard or Senning repairs). In these individuals, particularly in adolescents, abdominal implantation of a pacemaker generator may lessen the psychological impact of the external appearance of the pacemaker. In this population avoiding blunt contact sports (ie, tackle football, wrestling) is also important.28 It is critical that adult and pediatric electrophysiologists work together in the care and management of these complex, recurrent arrhythmias.
As noted above, many ACHD patients will require surgical or catheter-based interventions (as high as 40% in 1 study),47 and many encounter late-onset morbidity as a sequela of interventions earlier in life or as a result of failure of these interventions. The key for adult cardiologists and ACHD providers is delineating the reversible causes (eg, residual shunts, progressive valve regurgitation, and recoarctation) through routine intermittent surveillance, including echocardiograms, magnetic resonance imaging, and cardiac catherization, so that heart failure and arrhythmias in these patients can be identified, treated, and even prevented.
Pregnancy
Pregnancy is the most common reason for women to reenter care. Pregnancy is associated with significant hemodynamic changes, resulting in an increase in cardiac output to up to 150% of pre-pregnancy levels at 32 weeks, and up to 180% during labor. The outcome of pregnancy in patients with CHD is favorable in most instances provided that functional class systemic ventricular function is good. Accordingly, pregnancy is contraindicated in instances of severe pulmonary arterial hypertension (eg, Eisenmenger’s physiology), systemic ventricular dysfunction, and severe left-sided obstructions (eg, aortic or mitral stenosis). It is therefore imperative for health care providers to address the risks of pregnancy and the need for contraception with women who have CHD and are of reproductive age. The AHA advises beginning this conversation at 12 years of age and recommends that counseling be provided by health care providers knowledgeable in both CHD and adolescent health.27 Given the thrombotic potential of estrogen-containing contraception, the selection of contraception for women with ACHD who are seeking birth control requires discussion between the health care provider and patient. Though there have been limited studies performed on the use of contraception in women with CHD, a British working group has developed a consensus statement regarding contraceptive use in women with heart disease based on the World Health Organization format.48,49
Surgical Procedures
The need for operative interventions and re-interventions, both cardiac and noncardiac, in many CHD populations is considerable. Regardless of the type of procedure, these patients should receive a comprehensive preoperative risk assessment as well as appropriate intraoperative and postoperative management, ideally at a center equipped to meet their unique needs. Approaching the surgical procedure under the guidance of an interdisciplinary team that includes an ACHD specialist, anesthesiologist, and surgeon ensures that critical issues for appropriate management are not overlooked.
The preoperative risk assessment should be aimed at identifying and minimizing major risk factors. Historical factors to consider include the congenital lesion, outcomes of prior surgeries, history of syncope or arrhythmias, and the presence of pulmonary disease, among others.27 If the patient has a pacemaker or defibrillator, this should be interrogated prior to the planned procedure to ensure proper functioning. The preoperative evaluation should include consultation with a cardiologist experienced in the care of adolescents with CHD. Cardiac medications should be continued until the time of surgery and restarted as soon after the procedure as possible. Periods without anticoagulation should be minimized if indicated at baseline, and may require substituting warfarin with heparin in the preoperative period. The need for endocarditis prophylaxis must be considered as well; antibacterial prophylaxis prior to dental surgery, respiratory tract procedures, and procedures on infected skin and musculoskeletal structures is recommended in individuals with prosthetic heart valves, previous infective endocarditis, unrepaired CHD, repaired CHD with prosthetic material for the first 6 months after surgery, repaired CHD with residual defects, and valvulopathy after cardiac transplantation.50
Fluid management is important intraoperatively and post procedure, particularly in individuals who are preload dependent at baseline (eg, patients who have had Fontan palliation). Mechanical ventilation strategies with high positive end-expiratory pressure and tidal volume may decrease systemic venous return and should be monitored closely. Early mobilization and pulmonary toilet post extubation is advised to avoid pulmonary infection.
Exercise Capacity and Restrictions
The ability to exercise is an important factor in the quality of life of ACHD patients, especially in the adolescent period when participation in school and recreational athletics oftentimes functions as a social institution. Exercise ability is influenced by both real limitations imposed by limited cardiopulmonary reserve as a result of underlying pathology and by misconceptions of and anxiety about their ability to safely participate in these activities. There is evidence of diminished aerobic activity in all groups with CHD. However, symptomatic restrictions account for only approximately 30% of all barriers to exercise,51 and some studies have shown that exercise training programs can improve functional capacity and some standards of quality of life in CHD patients, in addition to the general health benefits associated with obesity prevention.52
Recommendations regarding exercise capacity are often addressed at primary care visits, and should be reinforced by the patient’s cardiologist. In general, most patients with repaired or mild defects can engage in moderate- to high-intensity exercise; those with more complex defects, cyanosis, or arrhythmias should be evaluated by an ACHD specialist to determine an appropriate level of activity.27 The “exercise prescription” provided to the patient should include type of exercise tolerated as well as heart rate goals and limits. In patients with extremely limited exercise capacity, a cardiac rehabilitation program can be beneficial. The presence of significant pulmonary hypertension, cyanosis or aortic stenosis, symptomatic arrhythmias, or evidence of myocardial dysfunction usually restricts the degree of exercise; full recommendations by activity and lesion type can be found in the guidelines proposed by the 36th Bethesda Conference.53 The importance of serial and regular evaluations is emphasized in these guidelines due to changing hemodynamic status of the patient over time as their cardiac lesions evolve and new complications arise.
Social and Psychological Impact of Chronic Illness
Living with a chronic disease can have a psychological impact on the child and transitioning adolescent. Frequent hospitalizations, physician visits, medical tests, and management of medical emergencies take a toll on the patient’s self-image and self-esteem, particularly during their formative adolescent years. Adolescents with CHD often feel “different” from their peers due to their condition,54 causing them to withhold disclosures about their heart disease to others out of fear of its impact on personal and professional relationships. Recent studies have shown that children and adolescents with CHD are at risk of internalizing problems and exhibiting behavior problems;55 they are also more likely to have impaired quality of life secondary to their increased incidence of psychosocial difficulties.56 The social and physical debility often experienced by patients with ACHD leads to a higher incidence of depression and anxiety in this population.57 Studies have shown that ACHD patients are interested in psychological treatment and peer support of their mood and anxiety disorders.58
At least some degree of the mental health issues ACHD patients experience is thought to have a physiological basis and be related to early cyanosis and neonatal surgical bypass duration. Prolonged duration of deep hypothermic circulatory arrest (DHCA) during corrective surgery is associated with reduced social competence, and has been found to be an independent risk factor for anxiety, depression, aggressive behavior, and attention deficiencies.59 In other studies, DHCA has been associated with decreased intellectual ability and worse fine motor skills, memory, and visuospatial skills, among other neurodevelopmental outcomes.60-62 Psychiatric disorders have also been associated with genetic syndromes like DiGeorge syndrome.63 This impacts executive function, leading to missed appointments, delay in clinical visits, and medication noncompliance. Given the potential for worse outcomes and risk of transition failure, primary care providers should routinely evaluate CHD patients for mood disorders and neurocognitive delay.
Social Determinants of Health and Medical Legal Partnerships
Social determinants of health and workplace discrimination play a large role in determining the ability of individuals with CHD to achieve adequate health care and maintain gainful employment. Individuals with CHD often face significant challenges as they prepare to enter the workforce, including discrimination within the workplace and maintaining employment through medical emergencies. Studies have shown that while educational milestones are similar between patients with and without CHD, those with CHD are much less likely to be employed.64 Challenges facing adolescents as they enter the workforce include hiring discrimination, physical challenges imposed by functional limitations, and misunderstanding of disease process and actual functional capacity. Career counseling is therefore an integral part of the transitioning process and should be started in early adolescence to allow for full assessment of mental, physical, and social abilities.65
Medical-legal partnerships (MLPs) can be extremely beneficial to the CHD population adversely affected by social determinants of health and workplace discrimination. These partnerships integrate lawyers into health care to address legal problems that create and perpetuate poor health; on a broader scale, these partnerships can advance and support public policy changes that improve population health.66
The major social determinants of health addressed by MLPs are income supports/insurance, housing/utilities, employment/education, legal status, and personal/family stability (summarized in the mnemonic I-HELP).67 Some of the more specific areas in which MLPs may assist in the delivery of care to CHD patients include case management, translation services, health literacy, and legal aid/legal services. ACHD patients also often experience a significant loss of services, including physical, occupational, and speech therapy and nutrition services, as adult clinics may not be prepared to provide these services. While physicians can best address the individual patient’s health, members of the legal system can address the systemic ailments that propagate that patient’s recurrent hospitalizations and other use of medical resources. Members of the legal system are present onsite in health care settings and participate in clinical meetings, which allows a coordinated and comprehensive screening for social needs that may harm a patient’s health.
Loss of insurance coverage is a major issue for transitioning patients; while adolescents with complex medical conditions are eligible for Medicaid to help cover the significant cost of their health care that goes beyond the abilities of private insurance, this eligibility ends when the patient turns 21. Additionally, the Social Security Administration re-determines supplemental security income (SSI) eligibility when the patient turns 18, and about one-third of patients lose their SSI benefits. Without appropriate guidance in navigating the nuances of insurance, many patients are at risk of losing coverage for their health care expenditures as they transition. Uninsured adults with a chronic condition are 8 times more likely to have unmet medical needs and 6 times more likely to have no access to routine care than insured young adults, with a 35% likelihood of the unmet medical need being due to cost.68 Undoubtedly, linability to pay for health care contributes to the lack of follow-up in the adult population, and MLPs may be a valuable tool to aid in ameliorating this problem.
Studies have shown that when legal services are used to address the social determinants of health, patients with chronic illnesses such as asthma and sickle cell disease have reduced hospital admissions.69,70 Other studies have shown utilization of MLPs has reduced spending on the care of high-need, high-use patients.71 According to a 2016 national survey of health care organizations conducted by the National Center for Medical-Legal Partnership, 39% clinicians reported improved compliance with medical treatment and 66% reported improved health outcomes after their patients received MLP services.72 Families referred to MLPs have shown increased access to health care, food, and income resources, and two-thirds reported improved child health and well-being.73 Given the numerous challenges faced by patients with CHD, involving MLPs as a part of both the transition process and the patient-centered medical home benefits these patients greatly and allows them to maximize their quality of life.
Conclusion
As more patients are living to adulthood with CHD, there is an increasing need for long-term care and adequate follow up, especially regarding the need for re-intervention and management of physiologic consequences of acquired cardiopulmonary, gastrointestinal, and renal disease in the setting of underlying congenital heart lesions. Beyond the purely medical aspects of the individual’s long-term management, psychosocial issues must be addressed, including preparing the individual for future employment and family counseling. Crucial to this process is the implementation of a comprehensive transition that begins in early adolescence and enables patients to take charge of their disease process in adulthood and ultimately enables them to maximize their quality of life and societal contributions. Towards this end, the role of MLPs may be important in ensuring that local, state, and federal policies that promote health harming norms are addressed.
Acknowledgments: We thank Dr. Frances ‘Kitty’ O’Hare and Bobbie Lewis for inviting us to submit this review; Dr. Russ Kolarik, Current Med-Peds Residency Program Director and Former President of the National Med-Peds Program Directors Association; and Dr. Peter Tilkemeier, Chairman, Department of Internal Medicine at Greenville Health System, for his unending support of our ACHD program. We also thank our patients, whose resounding resilience in the face of ongoing medical and psychosocial challenges remains our daily inspiration.
Corresponding author: Manisha S. Patel, MD, Department of Medicine and Pediatrics, Division of Cardiology, University of South Carolina School of Medicine, Columbia, SC; mpatel@ghs.org.
Financial disclosures: None.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
1. Bhatt AB, Foster E, Kuehl K, et al. Congenital heart disease in the older adult: a scientific Statement from the American Heart Association. Circulation. 2015;26;131:1884-1931.
2. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation. 2016;134:101-109.
3. Dolk H, Loane M, Garne E. Congenital heart defects in Europe. Circulation. 2011;123:841-849.
4. Qu Y, Liu X, Zhuang J, et al. Incidence of congenital heart disease: the 9-year experience of the Guangdog Registry of Congenital Heart Disease, China. PloS One. 2016;11:e0159257.
5. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med.
6. McGee Banks C. Variations in diversity in the United States and Canada. 2010. http://www.canadianstudies.isp.msu.edu/docs/Cherry%20McGee%20Banks.pdf.
7. van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241-2247.
8. Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170-1175.
9. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39;1890-1900.
10. Verheugt CL, Uiterwaal CS, van der Velde ET, et al. Mortality in adult congenital heart disease. Eur Heart J. 2010;31:1220-1229.
11. Greutmann M, Tobler D, Kovacs AH, et al. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis. 2015;10:117-127.
12. Mandalenakis Z, Rosengren A, Lappas G, et al. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928-937.
13. Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg. 1997;113:80–86.
14. Khairy P, Aboulhosn J, Gurvitz M; AARC. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868-875.
15. Ewe SH, Tan JL. Hepatotocellular carcinoma—a rare complication post Fontan operation. Congenit Heart Dis. 2009;4:103-106.
16. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348-1352.
17. Saliba T, Dorkhom S, O’Reilly EM, et al. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010;22:889-891.
18. Wang A, Book W, McConnell M, et al. Prevalence of hepatitis C infection in adult patients who underwent congenital heart surgery prior to screening in 1992. Am J Cardiol. 2006;100:1307-1309.
19. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
20. Karkouti K, Wijeysundera DN, Yau TM, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495-502.
21. Dimopoulos K, Diller GP, Koltsida E, et al. Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation. 2008;117:2320-2328.
22. Moons P, Van Deyk K, Dedroog D, et al. Prevalence of cardiovascular risk factors in adults with congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:612-616.
23. Afilalo J, Therrien J, Pilote L, et al. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol. 2011;58:1509-1515.
24. Billett J, Cowie MR, Gatzoulis MA, et al. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross-sectional, population-based study with case-control analysis. Heart. 2008;94:1194-1199.
25. Hait G, Corpus M, Lamarre FR, et al. Alteration of glucose and insulin metabolism in congenital heart disease. Circulation. 1972;46:333-346.
26. Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J. 2009;158:30-39.
27. Lui GK, Fernandes S, McElhinney DB. Management of cardiovascular risk factors in adults with congenital heart disease. J Am Heart Assoc. 2014;3(6):e001076.
28. Fedchenko M, Mandalenakis Z, Rosegren A, et al. Ischemic heart disease in children and young adults with congenital heart disease in Sweden. Int J Cardiol. 2017;248:143-148.
29. Mandalenakis Z, Rosengren A, Lappas G, et al. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016. 23;5(2):e003071..
30. Hoffmann A, Chockalingam P, Balint OH, et al. Cerebrovascular accidents in adult patients with congenital heart disease. Heart. 2010;96:1223-1226.
31. Lanz J, Brophy JM, Therrien J, et al. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385-2394
32. Sable C, Foster E, Uzark K, et al; on behalf of the American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues: a scientific statement from the American Heart Association. Circulation. 2011;123:1454-1485.
33. Steinway C, Gable J, Jan S. Transitioning to adult care: supporting youth with special health care needs. Children’s Hospital of Philadelphia: Policylab Evidence to Action in Brief. Spring 2017.
34. Heery E, Sheehan AM, While AE, Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10413-427.
35. Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(part 1):e197-e205.
36. Gurvitz M, Valente AM, Broberg C, et al; AARCC. Prevalence and predictors of gaps in care among adult congenital heart disease patients (The Health, Education and Access Research Trial). J Am Coll Cardiol. 2013;61:2180-2184.
37. Yeung E, Kay J, Roosevelt GE, et al. Lapse of care as a predictor for morbidity in adults in congenital heart disease. Int J Cardiol. 2008;125:62-65.
38. Meadows AK, Bosco V, Tong E, et al. Transition and transfer from pediatric to adult care of young adults with complex congenital heart disease. Current Cardiol Rep. 2009; 11;4;291-297.
39. lum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 1993;14:570-576.
40. Rosen DS, Blum RW, Britto M, et al; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309-311.
41. Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115:112-120.
42. Congenital heart disease transition tools. American College of Cardiology. www.acc.org/membership/sections-and-councils/adult-congenital-and-pediatric-cardiology-section/resources/chdtransitiontools. Accessed November 1, 2018.
43. American Academy of Pediatrics Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(suppl):1545-1547.
44. Lotstein DS, McPherson M, Strickland B, Newacheck PW. Transition planning for youth with special health care needs: results from the National Survey of Children with Special Health Care Needs. Pediatrics. 2005;115:1562-1568.
45. Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839-846.
46. Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032-1038.
47. Zomer AC, Verheugt CL, Vaartjes I, et al. Surgery in adults with congenital heart disease. Circulation. 2011;124:2195-2201.
48. Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11:298-305.
49. Thorne S, Nelson-Piercy C, MacGregor A, et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J Fam Plann Reprod Health Care. 2006;32:75-81.
50. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group [published correction appears in Circulation. 2007;116:e376–e377]. Circulation. 2007;116:1736-1754.
51. Warnes CA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Adults With Congenital Heart Disease): developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:2395-2451.
52. Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol. 2007;28:276-279.
53. Graham TP Jr, Driscoll DJ, Gersony WM, et al Task force 2: congenital heart disease. J Am Coll Cardiol. 2005;45:1326-1333.
54. Tong EM, Sparacino PS, Messias DK, et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
55. Karsdorp PA, Everaerd W, Kindt M, Mulder BJ. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32:527-541.
56. Kovacs AH, Moons P. Psychosocial functioning and quality of life in adults with congenital heart disease and heart failure. Heart Fail Clin. 2014;10:35-42.
57. Bromberg JI, Beasley PJ, D’Angelo EJ, et al. Depression and anxiety in adults with congenital heart disease: a pilot study. Heart Lung. 2003;32:105–10.
58. Kovacs AH, Bendell KL, Colman J, et al. Adults with congenital heart disease: psychological needs and treatment preferences. Congenit Heart Dis. 2009;4:139-146
59. Hovels-Gurich HH, Konrad K, Wiesner M, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–510.
60. Forbess JM, Visconti KJ, Hancock-Friesen C, et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:195-102.
61. Bellinger DC, Wypij D, duPlessis AJ, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385-1396.
62. Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397-1403.
63. Tang SX, Yi JJ, Calkins ME, et al. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Pscychol Med 2017;44:1267-1277.
64. Simko LC, McGinnis KA, Schembri J. Educational needs of adults with congenital heart disease. J Cardiovasc Nurs. 2006;21:85-94.
65. Foster E, Graham TP Jr, Driscoll DJ, et al. Task force 2: special health care needs of adults with congenital heart disease. J Am Coll Cardiol. 2001;37:1176-1183.
66. Sandel M, Hansen M, Kahn R, et al. Medical-legal partnerships: transforming primary care by addressing the legal needs of vulnerable populations. Health Aff. 2010;29:1697-1705.
67. The National Center for Medical-Legal Partnership. medical-legalpartnership.org. Accessed November 1, 2018.
68. Callahan ST, Cooper WO. Access to health care for young adults with disabling chronic conditions. Arch Pediatr Adolesc Med. 2006;160:178-182.
69. Pettignano R, Caley SB, Bliss LR. Medical-legal partnership: impact on patients with sickle cell disease. Pediatrics. 2011;128:1482-1488.
70. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children--outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24:1063-1073.
71. Martin J, Martin A, Schultz C, Sandel M. Embedding civil legal aid in care of high-utilizing patients using medical-legal partnership. Health Affairs blog. 22 April 2015. www.healthaffairs.org/do/10.1377/hblog20150422.047143/full. Accessed November 1, 2018.
72. Regenstein M, Sharac J, Williamson A. The state of the medical legal partnership field: findings from the 2016 National Center for Medical-Legal Partnership Surveys. August 2017.
73. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(2 Suppl):157-168.
Sexually Transmitted Infections Caused by Mycoplasma genitalium and Neisseria gonorrhoeae: Diagnosis and Treatment
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
From the Fargo Veterans Affairs Health Care System, Fargo, ND (Dr. Dietz, Dr. Hammer, Dr. Zegarra, and Dr. Lo), and the Queen Elizabeth Hospital, Hong Kong, China (Dr. Cho).
Abstract
- Objective: To review the management of patients with Mycoplasma genitalium and Neisseria gonorrhoeae infections.
- Methods: Review of the literature.
- Results: Mycoplasma genitalium and Neisseria gonorrhoeae are organisms that cause urethritis, cervicitis, and pelvic inflammatory disease. There is increasing antibiotic resistance to both organisms, which poses significant challenges to clinicians. Additionally, diagnostic tests for M. genitalium are not widely available, and commonly used tests for both organisms do not provide antibiotic sensitivity information. The increasing resistance of both M. genitalium and N. gonorrhoeae to currently used antimicrobial agents is alarming and warrants cautious monitoring.
- Conclusion: As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to difficult treatment scenarios for sexually transmitted infections caused by these 2 organisms.
Keywords: Mycoplasma genitalium, Neisseria gonorrhoeae, antibiotic resistance, sexually transmitted infections, STIs.
The World Health Organization (WHO) estimates that more than 1 million cases of sexually transmitted Infections (STIs) are acquired every day worldwide,1 and that the majority of STIs have few or no symptoms, making diagnosis difficult. Two organisms of interest are Mycoplasma genitalium and Neisseria gonorrhoeae. In contrast to Chlamydia trachomatis, which is rarely resistant to treatment regimens, M. genitalium and N. gonorrhoeae are becoming increasingly resistant to antibiotic treatment and pose an impending threat. These bacteria can cause urethritis, cervicitis, and pelvic inflammatory disease (PID). Whereas antibiotic resistance to M. genitalium is emerging, resistance to N. gonorrhea has been a continual problem for decades. Drug resistance, especially for N. gonorrhoeae, is listed as a major threat to efforts to reduce the impact of STIs worldwide.2 In 2013, the U.S. Centers for Disease Control and Prevention (CDC) classified N. gonorrhoeae drug resistance as an urgent threat.3 As the yield of new or effective antibiotic therapies has decreased over the past few years, increasing antibiotic resistance will lead to challenging treatment scenarios for STIs caused by these 2 organisms.
Epidemiology and Pathogenesis
M. genitalium
M. genitalium is an emerging pathogen that is an etiologic agent of upper and lower genital tract STIs, such as urethritis, cervicitis, and PID.4-13 In addition, it is thought to be involved in tubal infertility and acquisition of other sexually transmitted pathogens, including HIV.7,8,13 The prevalence of M. genitalium in the general U.S. population in 2016 was reported to be approximately 17.2% for males and 16.1% for females.14 Infections are more common in patients aged 30 years and younger than in older populations.15 Also, patients self-identifying as black were found to have a higher prevalence of M. genitalium.14 This organism was first reported as being isolated from the urethras of 2 men with non-gonococcal urethritis (NGU) in London in 1980.15,16 It is a significant cause of acute and chronic NGU in males, and is estimated to account for 6% to 50% of cases of NGU.17,18M. genitalium in females has been associated with cervicitis4,9 and PID.8,10 A meta-analysis by Lis et al showed that M. genitalium infection was associated with an increased risk for preterm birth and spontaneous abortion.11 In addition, M. genitalium infections occur frequently in HIV-positive patients.19,20 M. genitalium increases susceptibility for passage of HIV across the epithelium by reducing epithelial barrier integrity.19
Beta lactams are ineffective against M. genitalium because mycoplasmas lack a cell wall and thus cell wall penicillin-binding proteins.21M. genitalium’s abilty to invade host epithelial cells is another mechanism that can protect the bacteria from antibiotic exposure.20 One of the first reports of antibiotic sensitivity testing for M. genitalium, published in 1997, noted that the organism was not susceptible to nalidixic acid, cephalosporins, penicillins, and rifampicin.22 In general, mycoplasmas are normally susceptible to antibiotics that inhibit protein synthesis,23 and initial good sensitivity to doxycycline and erythromycin was noted but this has since decreased. New antibiotics are on the horizon, but they have not been extensively tested in vivo.23
N. gonorrhoeae
Gonorrhea is the second most common STI of bacterial origin following C. trachomatis,24-26 which is rarely resistant to conventional regimens. In 2008, the World Health Organization (WHO) estimated that 106 million cases of N. gonorrhoeae infection were acquired annually and that 36.4 million adults were infected with N. gonorrhoeae.27 In the United States, the CDC estimates that gonorrhea cases are under-reported. An estimated 800,000 or more new cases are reported per year.28
The most common clinical presentations are urethritis in men and cervicitis in women.29 While urethritis is most likely to be symptomatic, only 50% of women with acute gonorrhea are symptomatic.29 In addition to lower urogenital tract infection, N. gonorrhoeae can also cause PID, ectopic pregnancy, infertility in women, and epididymitis in men.29,30 Rare complications can develop from the spread of N. gonorrhoeae to other parts of the body including the joints, eyes, cardiovascular system, and skin.29
N. gonorrhoeae can attach to the columnar epithelium and causes host innate immune-driven inflammation with neutrophil influx.29 It can avoid the immune response by varying its outer membrane protein expression. The organism is also able to acquire DNA from other Neisseria species30 and genera, which results in reduced susceptibility to therapies.
The Gonococcal Isolate Surveillance Project (GISP), established in 1986, is a collaborative project involving the CDC and STI clinics in 26 cities in the United States along with 5 regional laboratories.31 The GISP monitors susceptibilities in N. gonorrhoeae isolates obtained from roughly 6000 symptomatic men each year.31 Data collected from the GISP allows clinicians to treat infections with the correct antibiotic. Just as they observed patterns of fluoroquinolone-resistant N. gonorrhoeae, there has been a geographic progression of decreasing susceptibility to cephalosporins in recent years.31
The ease with which N. gonorrhoeae can develop resistance is particularly alarming. Sulfonamide use began in the 1930s, but resistance developed within approximately 10 years.30,32N. gonorrhoeae has acquired resistance to each therapeutic agent used for treatment over the course of its lifetime. One hypothesis is that use of single-dose therapy to rapidly treat the infection has led to treatment failure and allows for selective pressure where organisms with decreased antibiotic susceptibility are more likely to survive.30 However, there is limited evidence to support monotherapy versus combination therapy in treating N. gonorrhoeae.33,34 It is no exaggeration to say gonorrhea is now at risk of becoming an untreatable disease because of the rapid emergence of multidrug resistant N. gonorrhoeae strains worldwide.35
Diagnosis
Whether the urethritis, cervicitis, or PID is caused by N. gonorrhoeae, M. genitalium, or other non-gonococcal microorganisms (eg, C. trachomatis), no symptoms are specific to any of the microorganisms. Therefore, clinicians rely on laboratory tests to diagnose STIs caused by N. gonorrhoeae or M. genitalium.
M. genitalium
Gram Stain. Because M. genitalium lacks a cell wall, it cannot be identified by routine Gram stain.
Culture. Culturing of this fastidious bacterium might offer the advantage of assessing antibiotic susceptibility;36 however, the procedure is labor intensive and time consuming, and only a few labs in the world have the capability to perform this culture.12 Thus, this testing method is primarily undertaken for research purposes.
Serological Testing. Because of serologic cross-reactions between Mycoplasma pneumoniae and M. genitalium, there are no standardized serological tests for M. genitalium.37
Nucleic Acid Amplification Tests. M. genitalium diagnosis currently is made based exclusively on nucleic acid amplification testing (NAAT) methodology (polymerase chain reaction [PCR] or transcription-mediated amplification [TMA]), which is the only clinically useful method to detect M. genitalium. TMA for M. genitalium is commercially available in an analyte-specific reagent (ASR) format, but this has not been approved by the Food and Drug Administration (FDA).38 A study analyzing urogenital specimens from female patients via this TMA product found a 98.7% true-positive result when confirmed with repeat testing or alternative-target TMA, and only a 0.5% false-negative rate.38 There is evidence that this TMA product can be used to identify M. genitalium in urine, stool, and pharyngeal samples.39 These assays are currently available in some reference labs and large medical centers but are not widely available. Table 1 summarizes the diagnostic methods for M. genitalium.
N. gonorrhoeae
Gonococcal infection can involve the urogenital tract, but can also be extra-urogenital. The method of diagnoses of urogenital infections has expanded from Gram stain of urethral or cervical discharge and the use of selective media culture (usually Thayer-Martin media)40 to molecular methods such as NAATs, which have a higher sensitivity than cultures.41,42
Gram Stain. A Gram stain that shows polymorphonuclear leukocytes with intracellular gram-negative diplococci can be considered diagnostic for N. gonorrhoeae urethritis infection in symptomatic men when samples are obtained from the urethra.43 A retrospective study of 1148 women with gonorrhea revealed that of 1049 cases of cervical gonorrhea, only 6.4% were positive by smear alone; and of 841 cases of urethral gonorrhea, only 5.1% were positive by smear alone; therefore, other diagnostic methods are generally preferred in women.44 Because Gram stain of vaginal specimens is positive in only 50% to 60% of females, its use in women and in suspected extragenital gonococcal infections is not recommended.43-45 When Gram stain was performed in asymptomatic men, the sensitivity was around 80%.39 Thus, in asymptomatic men with a high pre-test probability of having the infection, the use of other additional testing would increase the rate of detection.43
Culture. Urethral swab specimens from males with symptomatic urethritis and cervical swab samples from females with endocervical infection must be inoculated onto both a selective medium (eg, modified Thayer-Martin medium or Martin Lewis medium) and a nonselective medium (eg, chocolate agar). A selective medium is used because it can suppress the growth of contaminating organisms, and a nonselective medium is used because some strains of N. gonorrhoeae are inhibited by the vancomycin present in the selective medium.40 Specimens collected from sterile sites, such as blood, synovial fluid, and cerebrospinal fluid, should be streaked on nonselective medium such as chocolate agar. The material used for collection is critical; the preferred swabs should have plastic or wire shafts and rayon, Dacron, or calcium alginate tips. Materials such as wooden shafts or cotton tips can be toxic to N. gonorrhoeae.40 The specimen should be inoculated immediately onto the appropriate medium and transported rapidly to the laboratory, where it should be incubated at 35º to 37ºC with 5% CO2 and examined at 24 and 48 hours post collection.40 If the specimens cannot be inoculated immediately onto the appropriate medium, the specimen swab should be delivered to the lab in a special transport system that can keep the N. gonorrhoeae viable for up to 48 hours at room temperature.46
The following specimen collection techniques are recommended by the CDC:40
- In males, the cotton swab should be inserted about 2 to 3 cm into the urethral meatus and rotated 360° degrees 2 or 3 times.
- In females, collection of cervical specimens requires inserting the tip of the swab 1 to 2 centimeters into the cervical os and rotating 360° 2 or 3 times.
- Samples obtained outside of the urogenital tract: rectal specimens may be obtained by inserting the swab 3 to 4 cm into the rectal vault. Pharyngeal specimens are to be obtained from the posterior pharynx with a swab.
Culture tests allow the clinician to assess antimicrobial susceptibility and are relatively low cost when compared with nucleic acid detection tests. The sensitivity of culture ranges from 72% to 95% for symptomatic patients, but drops to 65% to 85% for asymptomatic patients.45-47 This low sensitivity is a major disadvantage of culture tests when compared to NAATs. Other disadvantages are the need for the specimens to be transported under conditions adequate to maintain the viability of organisms and the fact that 24 to 72 hours is required to report presumptive culture results.42 Antimicrobial sensitivity testing generally is not recommended; however, it is advisable to perform antimicrobial sensitivity in cases of treatment failure or disseminated gonococcal infection.12
Nucleic Acid Amplification Tests. NAATs use techniques that allow the amplification and detection of N. gonorrhoeae DNA or RNA sequences through various methods, which include assays such as PCR (eg, Amplicor; Roche, Nutley, NJ), TMA (eg, APTIMA; Gen-Probe, San Diego, CA), and strand-displacement amplification (SDA; Probe-Tec; Becton Dickinson, Franklin Lake, NJ). While PCR and SDA methods amplify bacterial DNA, TMA amplifies bacterial rRNA.41
The FDA has cleared NAATs to test endocervical, vaginal, and urethral (men) swab specimens and urine for both men and women. There are several NAATs available to test rectal, oropharyngeal, and conjunctival specimens; however, none of them are FDA-cleared. Some local and commercial laboratories have validated the reliability of these extra-urogenital NAATs.12,48 Compared to cultures, NAATs have the advantages of being more sensitive and requiring less strict collection and transport conditions. However, they are costlier than cultures, do not provide any antimicrobial susceptibility information, and have varying specificity.49,50
Rapid Tests. NAAT results are usually available in approximately 1 to 2 days, so there has been significant interest in creating technologies that would allow for a more rapid turnaround time. The GeneXpert CT/NG is a newly developed real-time PCR-based assay that can simultaneously detect C. trachomatis and N. gonorrhoeae. The advantage of this technique is the 90-minute turnaround time and its ability to process more than 90 samples at a time. The specificity of this test for N. gonorrhoeae is similar to that of other NAATs (> 99.3%), suggesting that cross-reactivity is not a significant problem.51 Table 2 summarizes the test methods used for diagnosing N. gonorrhoeae.
Treatment
M. genitalium
M. genitalium, Mycoplasma hominis, and the ureaplasmas (U. urealyticum and U. parvum) are generally transmitted sexually, and the natural habitat of this Mycoplasmataceae family of bacteria is the genitourinary tract. All the mycoplasmas can cause NGU, cervicitis, and PID. Presently, multiple-drug resistant M. hominis and ureaplasmas remain uncommon, but the prevalence of M. genitalium resistant to multiple antibiotics has increased significantly in recent years.23,52
In the 1990s, M. genitalium was highly sensitive to the tetracyclines in vitro,53 and doxycycline was the drug of choice for treating NGU. However, it later became apparent that doxycycline was largely ineffective in treating urethritis caused by M. genitalium.54,55
Subsequently, azithromycin, a macrolide, became popular in treating urethritis in males and cervicitis in females because it was highly active against C. trachomatis54 and M. genitalium56 and it can be given orally as a single 1-g dose, thus increasing patients’ compliance. However, azithromycin-resistant M. genitalium has rapidly emerged and rates of treatment failure with azithromycin as high as 40% have been reported in recent studies.57,58 The resistance was found to be mediated by mutations in the 23S rRNA gene upon exposure of M. genitalium to azithromycin.15,57-59 Multiple studies conducted in various countries (including the United States, Netherlands, England, and France) all found high rates of 23S rRNA gene mutations.15,57-59M. genitalium samples were analyzed using reverse transcription-PCR and Sanger sequencing of the 23S tRNA to assess rates of macrolide resistance markers. The study found that 50.8% of female participants and 42% of male participants harbored mutations indicating macrolide resistance.15
An in vitro study conducted in France showed that the respiratory fluoroquinolone moxifloxacin was highly active against mycoplasmas, including M. genitalium.60 This study and others led to the use of moxifloxacin in treating infections caused by azithromycin-resistant M. genitalium. Moxifloxacin initially was successful in treating previous treatment failure cases.61 Unfortunately, the success has been short-lived, as researchers from Japan and Australia have reported moxifloxacin treament failures.62-64 These treatment failures were related to mutations in the parC and gyrA genes.62
Because M. genitalium exhibits significantly increased resistance to the tetracyclines, macrolides, and fluoroquinolones, leading to treatment failures associated with the resistance, the recently published CDC sexually transmitted diseases guidelines (2015) do not specifically recommend or endorse one class of antibiotics over another to treat M. genitalium infections; this contrasts with their approach for other infections in which they make specific recommendations for treatment.12 The lack of clear recommendations from the CDC makes standardized treatment for this pathogen difficult. The CDC guidelines do identify M. genitalium as an emerging issue, and mention that a single 1-g dose of azithromycin should likely be recommended over doxycycline due to the low cure rate of 31% seen with doxycycline. Moxifloxacin is mentioned as a possible alternative, but it is noted that the medication has not been evaluated in clinical trials and several studies have shown failures.12
Although the existing antibiotics to treat M. genitalium infections are far from desirable, treatment approaches have been recommended:65
- Azithromycin or doxycycline should be considered for empiric treatment without documented M. genitalium infection.
- Azithromycin is suggested as the first choice in documented M. genitalium infections.
- In patients with urethritis, azithromycin is recommended over doxycycline based on multiple studies. A single 1-g dose of azithromycin is preferred to an extended regimen due to increased compliance despite the extended regimen being slightly superior in effectiveness. The single-dose regimen is associated with selection of macrolide-resistant strains.65
- Women with cervicitis and PID with documented M. genitalium infection should receive an azithromycin-containing regimen.
Although the existing antibiotics on the market could not keep up with the rapid mutations of M. genitalium, a few recent studies have provided a glimmer of hope to tackle this wily microorganism. Two recent studies from Japan demonstrated that sitafloxacin, a novel fluoroquinolone, administered 100 mg twice a day to patients with M. genitalium was superior to other older fluoroquinolones.66,67 This fluoroquinolone could turn out to be a promising first-line antibiotic for treatment of STIs caused by M. genitalium. Bissessor and colleagues conducted a prospective cohort study of M. genitalium-infected male and female patients attending a STI clinic in Melbourne, Australia, and found that oral pristinamycin is highly effective in treating the M. genitalium strains that are resistant to azithromycin and moxifloxacin.68 Jensen et al reported on the novel fluoroketolide solithromycin, which demonstrated superior in vitro activity against M. genitalium compared with doxycycline, fluoroquinolones, and other macrolides.69 Solithromycin could potentially become a new antibiotic to treat infection caused by multi-drug resistant M. genitalium.
N. gonorrhoeae
Because of increasing resistance of N. gonorrhoeae to fluoroquinolones in the United States, the CDC recommended against their routine use for all cases of gonorrhea in August 2007.70 In some countries, penicillin-, tetracycline-, and ciprofloxacin-resistance rates could be as high as 100%, and these antibacterial agents are no longer treatment options for gonorrhea. The WHO released new N. gonorrhoeae treatment guidelines in 2016 due to high-level of resistance to previously recommended fluoroquinolones and decreased susceptibility to the third-generation cephalosporins, which were a first-line recommendation in the 2003 guidelines.45 The CDC’s currently recommended regimens for the treatment of uncomplicated and disseminated gonorrheal infections are summarized in Table 3 and Table 4.12 Recommendations from the WHO guidelines are very similar to the CDC recommendations.45
In light of the increasing resistance of N. gonorrhoeae to cephalosporins, 1 g of oral azithromycin should be added to ceftriaxone 250 mg intramuscularly in treating all cases of gonorrhea. The rationale for adding azithromycin to ceftriaxone is that azithromycin is active against N. gonorrhoeae at a different molecular target at a high dose, and it can also cover other co-pathogens.71 Unfortunately, susceptibility to cephalosporins has been decreasing rapidly.72 The greatest concern is the potential worldwide spread of the strain isolated in Kyoto, Japan, in 2009 from a patient with pharyngeal gonorrhea that was highly resistant to ceftriaxone (minimum inhibitory concentration of 2.0 to 4.0 µg/mL).73 At this time, N. gonorrhoeae isolates that are highly resistant to ceftriaxone are still rare globally.
Although cefixime is listed as an alternative treatment if ceftriaxone is not available, the 2015 CDC gonorrhea treatment guidelines note that N. gonorrhoeae is becoming more resistant to this oral third-generation cephalosporin; this increasing resistance is due in part to the genetic exchange between N. gonorrhoeae and other oral commensals actively taking place in the oral cavity, creating more resistant species. Another possible reason for cefixime resistance is that the concentration of cefixime used in treating gonococcal pharyngeal infection is subtherapeutic.74 A recent randomized multicenter trial in the United States compared 2 non-cephalosporin regimens: a single 240-mg dose of intramuscular gentamicin plus a single 2-g dose of oral azithromycin, and a single 320-mg dose of oral gemifloxacin plus a single 2-g dose of oral azithromycin. These combinations achieved 100% and 99.5% microbiological cure rates, respectively, in 401 patients with urogenital gonorrhea.75 Thus, these combination regimens can be considered as alternatives when the N. gonorrhoeae is resistant to cephalosporins or the patient is intolerant or allergic to cephalosporins.
Because N. gonorrhoeae has evolved into a “superbug,” becoming resistant to all currently available antimicrobial agents, it is important to focus on developing new agents with unique mechanisms of action to treat N. gonorrhoeae–related infections. Zoliflodacin (ETX0914), a novel topoisomerase II inhibitor, has the potential to become an effective agent to treat multi-drug resistant N. gonorrhoeae. A recent phase 2 trial demonstrated that a single oral 2000-mg dose of zoliflodacin microbiologically cleared 98% of gonorrhea patients, and some of the trial participants were infected with ciprofloxacin- or azithromycin-resistant strains.76 An additional phase 2 clinical trial compared oral zoliflodacin and intramuscular ceftriaxone. For uncomplicated urogential infections, 96% of patients in the zoliflodacin group achieved microbiologic cure versus 100% in the ceftriaxone group; however, zoliflodacin was less efficacious for pharyngeal infections.77 Gepotidacin (GSK2140944) is another new antimicrobial agent in the pipeline that looks promising. It is a novel first-in-class triazaacenaphthylene that inhibits bacterial DNA replication. A recent phase 2 clinical trial demonstrated that 1.5-g and 3-g single oral doses eradicated urogenital N. gonorrhoeae with microbiological success rates of 97% and 95%, respectively.78
Test of Cure
Because of the decreasing susceptibility of M. genitalium and N. gonorrhoeae to recommended treatment regimens, the European Guidelines consider test of cure essential in STIs caused by these 2 organisms to ensure eradication of infection and identify emerging resistance.79 However, test of cure is not routinely recommended by the CDC for these organisms in asymptomatic patients.12
Sexual Risk-Reduction Counseling
Besides aggressive treatment with appropriate antimicrobial agents, it is also essential that patients and their partners receive counseling to reduce the risk of STI. A recently published systematic review demonstrated that high-intensity counseling could decrease STI incidents in adolescents and adults.80
Conclusion
It is clear that these 2 sexually transmitted ”superbugs” are increasingly resistant to antibiotics and pose an increasing threat. Future epidemiological research and drug development studies need to be devoted to these 2 organisms, as well as to the potential development of a vaccine. This is especially important considering that antimicrobials may no longer be recommended when the prevalence of resistance to a particular antimicrobial reaches 5%, as is the case with WHO and other agencies that set the standard of ≥ 95% effectiveness for an antimicrobial to be considered as a recommended treatment.32 With current resistance rates for penicillin, ciprofloxacin, and tetracycline at close to 100% for N. gonorrhoeae in some countries,30,79 it is important to remain cognizant about current and future treatment options.
Because screening methods for M. genitalium are not available in most countries and there is not an FDA-approved screening method in the United States, M. genitalium poses a significant challenge for clinicians treating urethritis, cervicitis, and PID. Thus, the development of an effective screening method and established screening guidelines for M. genitalium is urgently needed. Better surveillance, prudent use of available antibiotics, and development of novel compounds are necessary to eliminate the impending threat caused by M. genitalium and N. gonorrhoeae.
This article is the result of work supported with resources and the use of facilities at the Fargo VA Health Care System. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Corresponding author: Tze Shien Lo, MD, Veterans Affairs Medical Center, 2101 Elm Street N, Fargo, ND 58102.
Financial disclosures: None.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
1. World Health Organization. Sexually transmitted infections (STIs). www.who.int/mediacentre/factsheets/fs110/en/. Fact Sheet #110. Updated August 2016. Accessed December 16, 2017.
2. World Health Organization. Growing antibiotic resistance forces updates to recommended treatment for sexually transmitted infections www.who.int/en/news-room/detail/30-08-2016-growing-antibiotic-resistance-forces-updates-to-recommended-treatment-for-sexually-transmitted-infections. Released August 30, 2016.
3. Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance biggest threats. www.cdc.gov/drugresistance/biggest_threats.html. Released February 27, 2018.
4. Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24:498-514.
5. Jensen JS. Mycoplasma genitalium: The aetiological agent of urethritis and other sexually transmitted diseases. J Eur Acad Dermatol Venereol. 2004;18:1-11.
6. Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61-70.
7. McGowin CL, Anderson-Smits C. Mycoplasma genitalium: An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324.
8. Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: Should we treat and how? Clin Infect Dis. 2011;53 Suppl 3:S129-42.
9. Gaydos C, Maldeis NE, Hardick A, et al. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36(1SE0):598-606.
10. Wiesenfeld HC, Hillier SL, Meyn L, et al. O04.6 Mycoplasma genitalium-Is it a pathogen in acute pelvic inflammatory disease (PID)? Sex Transm Infect. 2013 89:A34 http://sti.bmj.com/content/89/Suppl_1/A34.2. Accessed February 1, 2018.
11. Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis. 2015;61:418-426.
12. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
13. Davies N. Mycoplasma genitalium: The need for testing and emerging diagnostic options. MLO Med Lab Obs. 2015;47:8,10-11.
14. Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016;54:2278-2283.
15. Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288-1291.
16. Taylor-Robinson D. The Harrison Lecture. The history and role of Mycoplasma genitalium in sexually transmitted diseases. Genitourin Med. 1995;71:1-8.
17. Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32:995-1003.
18. Horner P, Blee K, O’Mahony C, et al. Clinical Effectiveness Group of the British Association of Sexual Health and HIV. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27:85-96.
19. Das K, De la Garza G, Siwak EB, et al. Mycoplasma genitalium promotes epithelial crossing and peripheral blood mononuclear cell infection by HIV-1. Int J Infect Dis. 2014;23:31-38.
20. McGowin CL, Annan RS, Quayle AJ, et al. Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect Immun. 2012;80:3842-3849.
21. Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis. 2014;59:24-30.
22. Taylor-Robinson D, Bebear C. Antibiotic susceptibilities of mycoplasmas and treatment of mycoplasmal infections. J Antimicrob Chemother. 1997;40:622-630.
23. Taylor-Robinson D. Diagnosis and antimicrobial treatment of Mycoplasma genitalium infection: Sobering thoughts. Expert Rev Anti Infect Ther. 2014;12:715-722.
24. Ison CA. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G, Tyring SK, eds. Sexually Transmitted Infections and Sexually Transmitted Diseases.1st ed. Berlin, Heidelberg: Springer-Verlag; 2011:77-90.
25. Criss AK, Seifert HS. A bacterial siren song: Intimate interactions between neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178-190.
26. Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687-1696.
27. World Health Organization, Dept. of Reproductive Health and Research. Global incidence and prevalence of selected curable sexually transmitted infections - 2008. www.who.int/reproductivehealth/publications/rtis/stisestimates/en/. Published 2012. Accessed February 6, 2018.
28. Centers for Disease Control and Prevention 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/emerging.htm. Updated June 4, 2015.
29. Skerlev M, Culav-Koscak I. Gonorrhea: New challenges. Clin Dermatol. 2014;32:275-281.
30. Kirkcaldy RD, Ballard RC, Dowell D. Gonococcal resistance: Are cephalosporins next? Curr Infect Dis Rep. 2011;13:196-204.
31. Kidd S, Kirkcaldy R, Weinstock H, Bolan G. Tackling multidrug-resistant gonorrhea: How should we prepare for the untreatable? Expert Rev Anti Infect Ther. 2012;10:831-833.
32. Wang SA, Harvey AB, Conner SM, et al. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: The spread of fluoroquinolone resistance. Ann Intern Med. 2007;147:81-88.
33. Barbee LA, Kerani RP, Dombrowski JC, et al. A retrospective comparative study of 2-drug oral and intramuscular cephalosporin treatment regimens for pharyngeal gonorrhea. Clin Infect Dis. 2013;56:1539-434.
34. Sathia L, Ellis B, Phillip S, et al. Pharyngeal gonorrhoea - is dual therapy the way forward? Int J STD AIDS. 2007;18:647–8.
35. Tanaka M. Emergence of multidrug-resistant Neisseria gonorrhoeae strains circulating worldwide. Int J Urol. 2012;19:98-99.
36. Hamasuna R, Osada Y, Jensen JS. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with vero cells. J Clin Microbiol. 2007;45:847-850.
37. Razin S. Mycoplasma. In: Boricello SP, Murray PR, Funke G, eds. Topley & Wilson’s Microbiology and Microbial Infections. London, UK: Hodder Arnold; 2005:1957-2005.
38. Munson E, Bykowski H, Munson K, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-medicated ampliflication using primary female urogenital specimens. J Clin Microbiol. 2016;54:432-437.
39. Munson E, Wenten D, Jhansale S, et al. Expansion of comprehensive screening of male-sexually transmitted infection clinic attendees with Mycoplasma genitalium and Trichomonas vaginalis molecule assessment: a restrospective analysis. J Clin Microbiol. 2016;55:321-325.
40. Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae--2014. MMWR Recomm Rep. 2014;63(RR-02):1-19.
41. Boyadzhyan B, Yashina T, Yatabe JH, et al. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2004;42:3089-3093.
42. Graseck AS, Shih SL, Peipert JF. Home versus clinic-based specimen collection for Chlamydia trachomatis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9:183-194.
43. Sherrard J, Barlow D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin Med. 1996;72:422-426.
44. Goh BT, Varia KB, Ayliffe PF, Lim FK Diagnosis of gonorrhea by gram-stained smears and cultures in men and women: role of the urethral smear. Sex Transm Dis. 1985;12:135-139.
45. World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae. www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. Published 2016. Accessed December 16, 2017.
46. Arbique JC, Forward KR, LeBlanc J. Evaluation of four commercial transport media for the survival of Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2000;36:163-168.
47. Schink JC, Keith LG. Problems in the culture diagnosis of gonorrhea. J Reprod Med. 1985;30(3 Suppl):244-249.
48. Marrazzo JM, Apicella MA. Neisseria gonorrhoeae (gonorrhea). In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2015:2446-2462.
49. Barry PM, Klausner JD. The use of cephalosporins for gonorrhea: The impending problem of resistance. Expert Opin Pharmacother. 2009;10:555-577.
50. Tabrizi SN, Unemo M, Limnios AE, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49:3610-3615.
51. Goldenberg SD, Finn J, Sedudzi E, et al. Performance of the GeneXpert CT/NG assay compared to that of the Aptima AC2 assay for detection of rectal Chlamydia trachomatis and Neisseria gonorrhoeae by use of residual Aptima Samples. J Clin Microbiol. 2012;50:3867-3869.
52. Martin D. Mycoplasma genitalium, Mycoplasma hominis, and Ureaplasma species. In: Bennet J, Dolin R, Blaser M, eds. Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier Sauders; 2015:2190-2193.
53. Hannan PC. Comparative susceptibilities of various AIDS-associated and human urogenital tract mycoplasmas and strains of Mycoplasma pneumoniae to 10 classes of antimicrobial agent in vitro. J Med Microbiol. 1998;47:1115-1122.
54. Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649-1654.
55. Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52:163-170.
56. Bjornelius E, Anagrius C, Bojs G, et al. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: A controlled clinical trial. Sex Transm Infect. 2008;84:72-76.
57. Nijhuis RH, Severs TT, Van der Vegt DS, et al. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70:2515-2518.
58. Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: The need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58:631-637.
59. Touati A, Peuchant O, Jensen JS, et al. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52:1549-1555.
60. Bebear CM, de Barbeyrac B, Pereyre S, et al. Activity of moxifloxacin against the urogenital Mycoplasmas ureaplasma spp., Mycoplasma hominis and Mycoplasma genitalium and Chlamydia trachomatis. Clin Microbiol Infect. 2008;14:801-805.
61. Jernberg E, Moghaddam A, Moi H. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: An open study. Int J STD AIDS. 2008;19:676-679.
62. Tagg KA, Jeoffreys NJ, Couldwell DL, et al. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51:2245-2249.
63. Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int J STD AIDS. 2013;24:822-828.
64. Shimada Y, Deguchi T, Nakane K, et al. Emergence of clinical strains of Mycoplasma genitalium harbouring alterations in ParC associated with fluoroquinolone resistance. Int J Antimicrob Agents. 2010;36:255-258.
65. Mobley V, Seña A. Mycoplasma genitalium infection in men and women. In: UpToDate. www.uptodate.com. Last updated March 8, 2017. Accessed February 13, 2018.
66. Takahashi S, Hamasuna R, Yasuda M, et al. Clinical efficacy of sitafloxacin 100 mg twice daily for 7 days for patients with non-gonococcal urethritis. J Infect Chemother. 2013;19:941-945.
67. Ito S, Yasuda M, Seike K, et al. Clinical and microbiological outcomes in treatment of men with non-gonococcal urethritis with a 100-mg twice-daily dose regimen of sitafloxacin. J Infect Chemother. 2012;18:414-418.
68. Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2014;60:1228-1236.
69. Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151-3156.
70. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
71. Sexually transmitted diseases treatment guidelines, 2010. www.cdc.gov/std/treatment/default.htm. Published 2015. Accessed February13, 2016.
72. Centers for Disease Control and Prevention (CDC). Cephalosporin susceptibility among Neisseria gonorrhoeae isolates--United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
73. Ohnishi M, Saika T, Hoshina S, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg Infect Dis. 2011;17:148-149.
74. Centers for Disease Control and Prevention (CDC). Update to CDC’s sexually transmitted diseases treatment guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590-594.
75. Kirkcaldy RD, Weinstock HS, Moore PC, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis. 2014;59:1083-1091.
76. Seña AC, Taylor SN, Marrazzo J, et al. Microbiological cure rates and antimicrobial susceptibility of Neisseria gonorrhoeae to ETX0914 (AZD0914) in a phase II treatment trial for urogenital gonorrhea. (Poster 1308) Program and Abstract of ID Week 2016. New Orleans, LA, . October 25-30, 2016.
77. Taylor S, Marrazzo J, Batteiger B, et al. Single-dose zoliflodacin (ETX0914) for treatment of urogential gonorrhea. N Engl J Med. 2018;379:1835-1845.
78. Perry C, Dumont E, Raychaudhuri A. O05.3 A phase II, randomised, stdy in adults subjects evaluating the efficacy, safety, and tolerability of single doses of gepotidacin (GSK2140944) for treatment of uncomplicated urogenital gonorrhea. Sex Transm Infect. 2017;93(Suppl 2).
79. Bignell C, Unemo M, European STI Guidelines Editorial Board. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24:85-92.
80. O’Connor EA, Lin JS, Burda BU, et al. Behavioral sexual risk-reduction counseling in primary care to prevent sexually transmitted infections: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:874-883.
Screening for Lynch Syndrome Among Patients with Colorectal Cancer: Experiences from a Multihospital Health System
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
From the Hartford HealthCare Cancer Institute, Hartford, CT (Dr. Salner and Dr. Yu), and the Albert Einstein College of Medicine, New York, NY (Mr. Sekerak).
Abstract
- Objective: To explore the extent to which patients with newly diagnosed colorectal cancer (CRC) received standard of care screening for Lynch syndrome (LS), with testing of specimens for loss of expression of mismatch repair (MMR) genes and referral of patients with positive results to a genetic counselor.
- Methods: We conducted a retrospective study using cancer registry data from the Hartford HealthCare Cancer Institute, which is part of a 5-hospital urban health care system. Measures that were included in this study were patient age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical test, and presence of genetic counseling and testing for MMR-positive patients
- Results: 432 patients diagnosed with CRC during calendar years 2014 and 2015 were identified. The average age of the patients was 68.2 years and overall 81.3% of patients were screened (range, 30.8%–94.5%). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test. Seven patients (70%) tested positive for LS. Patients who were diagnosed with LS were younger, and the majority were male.
- Conclusion: This study showed that improved implementation strategies for LS screening at HHC hospitals were needed, as MMR testing was not fully implemented across all of our sites. Strategies that led to improved compliance included consensus building, comprehensive communications, embedding the new standard in a series of steps, and subsequent audits with feedback.
Keywords: Lynch syndrome; colorectal cancer; quality; screening; standard of care.
Colorectal cancer (CRC) is the third most common cancer in men and women, accounting for as many as 135,000 new cases and 50,000 cancer deaths per year in the United States.1 These cancers appear to be heterogeneous with multiple molecular subtypes, including chromosomal instability and microsatellite instability (MSI) pathways.2,3 MSI tumors may result from sporadic mutations or constitutional mutations. Lynch syndrome (LS), formerly known as hereditary non-polyposis colorectal cancer, is caused by a germline mutation in 1 of several DNA mismatch repair (MMR) genes or loss of expression of MSH2 due to deletions in the EPCAM gene.4 The MMR genes that have been identified in LS are MLH1, MSH2, MSH6, and PMS2.5-8 The protein products of these genes are essential to maintaining the integrity of the DNA sequence. Importantly for clinical practice, patients who carry gene mutations indicative of LS have a higher risk of certain cancers, namely CRC, pancreatic cancer, and endometrial cancer, among others.8,9
While most occurrences of CRC are sporadic, accounting for roughly 90% of all cases, approximately 5% to 10% of CRCs are caused by inherited genes.10 LS is the most common cause of inherited CRC, accounting for 1% to 3% of all CRC cases.8,10,11 Individuals with LS are likely to have onset of disease at an earlier age and also have a much higher risk for developing CRC, with a lifetime risk of CRC of approximately 70% for men and 45% for women.12,13 Thus, it is important to identify patients who have LS so that they can receive proper surveillance and care (ie, frequency of follow-up and treatment options). It is additionally important for family members of patients with LS to receive proper genetic counseling and genetic testing to better understand their possible predisposition and risk for CRC. CRC screening for LS helps clinicians appropriately personalize patient care, as the adjuvant therapy selection may be influenced by MMR results.3
The National Comprehensive Cancer Network guidelines recommend screening all patients with newly diagnosed CRC for Lynch syndrome. Hartford HealthCare (HHC), a large health care system located in Hartford, CT, has adopted these guidelines at the 5 hospitals within its cancer institute. According to the standard of care, a positive MMR pathology report should result in a referral to a genetic counselor for consultation, and the genetic counselor would recommend genetic testing for germline MMR genes. This quality improvement project sought to evaluate the performance of each of the 5 hospitals in implementing the standard of care for screening for LS in patients with CRC and to determine if the appropriate genetic referrals were made for patients with positive screening results. This study focused on LS screening in patients diagnosed only with CRC.
Data Collection and Analysis
We conducted a retrospective study examining all cases of patients diagnosed with invasive colon or rectal cancer at each of the 5 HHC Cancer Institute hospitals during calendar years 2014 and 2015. The study was developed as a quality improvement project for the HHC cancer centers. The database of patients diagnosed with colon and rectal cancer at HHC was obtained from our cancer registry.
Patients were stratified by hospital and surgeon. The study analyzed multiple factors, including age and gender, date of surgery, pathologic grade, pathologic stage, presence of MMR immunohistochemical (IHC) test, and presence of genetic counseling and testing for MMR-positive patients. Data was extracted from patient charts, pathology reports, and genetic reports. Only patients with primary adenocarcinomas were included in the study. In total, the study comprised 423 cases among the 5 hospitals. Results were tabulated and simple descriptive statistics were utilized to analyze the data.
Results
Of the 423 CRC patients treated at HHC during the study period, 45% were male and 55% were female, with an average age of 68.2 years (Table 1). The HHC Cancer Institute performed MMR IHC testing on 81.3% of all patients diagnosed in 2014 and 2015 (range, 30.8% to 94.5%). While the percentage of patients tested overall did not change from 2014 to 2015, it appreciably increased for the lower performing hospitals (Table 1). This improvement resulted from enhanced communication and establishment of pathology protocols for handling the tissue of patients with a cancer diagnosis.
Twenty-six (7.6%) of the 344 specimens tested were IHC abnormal, revealing a loss of 1 or more MMR gene products (Table 2). Of the patients with MMR-positive results, 15 (57.7%) received a genetic consult and 10 of these had a germline test of their MMR genes. Of note, 1 patient had been diagnosed with LS at an outside facility and therefore did not receive a genetic consult; 1 patient was unable to be reached for scheduling of a consult; 2 patients declined genetic testing; and 1 patient did not have their genetic test ordered.
Of the patients who underwent germline testing, 7 (70%) tested positive for LS (Table 2). Five LS patients tested positive for an MLH1 gene mutation, 1 tested positive for an MSH2 mutation, and 1 had a pathogenic variant of unknown significance (VUS) in their MLH1 gene.
The stage of cancer at diagnosis for MMR-negative, MMR-positive, and LS-positive groups was similar; nearly all patients were stage I, II, or III (Table 3). Compared to patients who were MMR-negative or MMR-positive, LS patients were younger (68.3, 60.9, and 47.6 years, respectively), and the majority were male (44.8%, 42.3%, and 57.1%, respectively).
Discussion
The shifting paradigm of health care delivery in America has led to increasing consolidation of hospitals into larger health care organizations. Consolidation creates a challenge when trying to implement a unified standard of care within distinct hospitals that comprise a health care system. In 2014, HHC integrated 2 additional hospitals into its system, for a total of 5 hospitals. As part of our quality improvement process, we wanted to explore the effect this had on universal MMR tumor screening for CRC patients among the 5 separate pathology departments, recognizing that implementation might take some time as protocols change. Although our Cancer Institute and Pathology Council had approved the universal MMR testing standard for all CCR patients, it was not clear that the standard had been embedded into pathology department standard practice.
The project reported here revealed substantial variance in MMR IHC testing among the 5 hospitals, suggesting the difficulty of implementing a unified standard of care among hospitals with separate groups of pathologists. This variance could result from several issues: lack of embedding the new standard in a series of steps to assure universal compliance; lack of agreement by pathologists on submitting every case; lack of follow-up by pathology staff to forward slides/tissue to the central lab for processing; and concern about privacy issues associated with conducting an unconsented genetic test.
There has been some debate as to whether CRC tumor screening requires consent from the patient.14 Without a clear standard of care for CRC cases, MMR IHC testing might not be ordered if a pathologist deems it necessary for the surgeon to obtain patient consent to the test. When the discrepancy in MMR IHC testing among the hospitals was investigated, we learned that one pathologist performed MMR testing only if a signed patient consent was provided. This revealed a deviation from our CRC protocol and a deficiency of communication within the HHC network. In addition, only 3 of our 5 hospitals routinely had genetic counselors present during the study period, requiring travel for patients at the other 2 hospitals and thus creating a potential barrier to the genetic consultation.
Based on the results of this study and other studies in the literature, we estimated that approximately 7 to 10 MMR-positive cases and 5 to 7 patients with LS may have been missed within the HHC network during the 2 study years as a result of suboptimal MMR testing, genetic counseling, and genetic testing.14-18 These potentially missed cases and diagnoses underscore the importance of implementing a unified standard of care across all large health care organizations. Individualized care, genetic testing, and counseling for patients and families affected by LS lead to more effective monitoring of these patients for disease.
However, our project showed that effective implementation of a standard of care for universal tumor screening for patients with CRC can modify institutional cancer care.15 Notably, hospitals that tested a lower percentage of patients overall improved their MMR testing drastically from 2014 to 2015. This significant increase in MMR testing shows the impact of measuring and disseminating compliance performance information following the institution of a new quality standard within a health care system. Further audits have revealed universal acceptance and use of this testing.
General patient perception of universal tumor screening is positive, and patients understand and endorse the benefits of screening for LS.16 In our study, patients with LS were on average 21 years younger at diagnosis compared to patients who were MMR-negative. Because LS patients are younger at diagnosis of CRC compared to patients who do not have MMR gene mutations and because colonoscopy typically is not initiated until age 50 years, molecular screening and genetic testing of MMR-positive patients is important. Identifying the presence of LS is important for both the patient and their family. Specifically, patients with LS are recommended to receive a screening colonoscopy every 1 to 2 years beginning at age 20 to 25 years.13 Personalizing care and increasing surveillance for patients with LS can help to reduce the morbidity and mortality of CRC and potentially other cancers.
Conclusion
As a result of this study, we recognized that inclusion of pathologists in the discussion is essential but not enough to ensure that all cases will be screened. Rather, a much more detailed series of steps is necessary to ensure compliance, including:
- Gain consensus among clinical leadership in CRC (including surgery, medical oncology, and pathology) that universal screening is necessary.
- Bring the appropriate strategy to pathology department operational managers to ensure that policy is transmitted to all appropriate staff.
- Ensure that involved individuals at newer hospitals in the system have access to the details of cultural discussions that have occurred to develop consensus and the policies and procedures that followed.
- Develop policies and procedures to assure that all appropriate patients are tested, including those who present outside normal hours for emergency surgery (ie, bowel obstruction).
- Develop an audit process to ensure that all patients have been screened and determine where any exceptions might be present.
- Present audit data back to the pathology team and Cancer Institute leadership team, and consider any strategy or operational modifications if needed.
The results of this study also highlight the important role quality studies play in informing health care organizations and improving clinical care. Quality studies assist in changing the culture and practice of institutions and guide the development and implementation of a unified standard of care.
Corresponding author: Andrew L. Salner, MD, Hartford HealthCare Cancer Institute, 80 Seymour Street, Hartford, CT 06102; andrew.salner@hhchealth.org.
Financial disclosures: None.
Funding: This study was funded internally as a quality improvement study.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Guinney J, Dientsmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350-1356.
3. Ryan E, Sheehan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol. 2017;116:38-57.
4. Koessler T, Oestergaard MZ, Song H, et al. Common variants in mismatch repair genes and risk of colorectal cancer. Gut. 2008;57:1097-101.
5. Quehenberger F, Vasen HF, van Houwelingen HC. Risk of colorectal and endometrial cancer for carriers of mutations of the hMLH1 and hMSH2 gene: correction for ascertainment. J Med Genet. 2005;42:491-496.
6. Senter L, Clendenning M, Sotamaa K, et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology. 2008;135:419-428.
7. Talseth-Palmer BA, McPhillips M, Groombridge C, et al. MSH6 and PMS2 mutation positive Australian Lynch syndrome families: novel mutations, cancer risk and age of diagnosis of colorectal cancer. Hered Cancer Clin Pract. 2010;8(1):5.
8. Bondona V, Bonaiti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304-2310.
9. Barrow E, Alduaij W, Robinson L, et al. Colorectal cancer in HNPCC: cumulative lifetime incidence, survival and tumour distribution. A report of 121 families with proven mutations. Clin Genet 2008;74:233-242.
10. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96:2992–3003.
11. Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783-5788.
12. Kohlmann W, Gruber SB. Lynch syndrome. 2004 Feb 5 [Updated 2014 May 22]. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016.
13. Stoffel E, Mukherjee B, Raymond VM, et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. Gastroenterology. 2009;137:1621-1627.
14. Beamer LC, Grant ML, Espenshied CR, et al. Reflex immunohistochemical and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol. 2012;30:1058-1063.
15. Cohen SA, Laurino M, Bowen DJ, et al. Initiation of universal tumor screening for Lynch syndrome in colorectal cancer patients as a model for the implementation of genetic information into clinical oncology practice. Cancer. 2016;122:393-401.
16. Hunter JE, Zepp JM, Gilmore MJ, et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281-3289.
17. Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
18. Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group (2009). Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome relatives. Genet. Med. 2009;11:35–41