User login
Is salpingectomy (vs standard tubal ligation) for sterilization a feasible option at cesarean delivery?
Invasive strategy increased bleeding risk in frail older AMI patients
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
This analysis is important and has clinical implications beyond those of previous analyses linking frailty to poor outcomes in patients with cardiovascular disease, according to John A. Bittl, MD.
“The present study helps to transform the rote recording of frailty from a mere quality metric in the medical record into an actionable diagnosis,” Dr. Bittl said in an editorial comment on the findings.
Results of the study show an association between invasive cardiac procedures and increased bleeding in frail patients with AMI.
The benefits of an invasive procedure might be outweighed by the incremental risk added by frailty in older AMI patients at low to moderate risk of poor outcomes, Dr. Bittl suggested.
By contrast, frail older AMI patients at high risk for poor outcomes might be better candidates for an invasive procedure if they can undergo a transradial approach, he said, noting that in the study by Dodson and colleagues, only 26% of frail patients received radial access despite randomized trials showing the approach reduces risk of bleeding.
“In this way, diagnosing frailty in a patient with AMI facilitates clinical decision making and helps to personalize an approach to optimize outcomes,” Dr. Bittl concluded.
Dr. Bittl is with the Interventional Cardiology Group, Florida Hospital Ocala (Fla.) He reported no relationships relevant to his editorial comment ( JACC Cardiovasc Interv. 2018 Nov 26;11[2];2297-93 ).
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
Frail older patients with acute myocardial infarction (AMI) may be at increased bleeding risk if managed with an invasive strategy, results of a large U.S. registry study suggest.
The increased bleeding risk was seen among frail older AMI patients who underwent cardiac catheterization, but it was not seen in those treated with more conservative medical management, according to study results.
That finding highlights the conundrum with invasive management strategies for frail patients with AMI, wrote John A. Dodson, MD, MPH, of New York University and study coinvestigators.
“Awareness of vulnerability and greater utilization of evidence-based strategies to reduce bleeding, including radial access and properly dose-adjusted anticoagulant therapies, may mitigate some bleeding events,” they wrote in JACC: Cardiovascular Interventions.
Results of this study, the first large U.S. registry analysis evaluating in-hospital bleeding risk in frail older adults with AMI, confirm findings from several previous small cohort studies linking frailty in AMI patients to in-hospital bleeding, investigators reported.
The analysis included a total of 129,330 AMI patients in the ACTION (Acute Coronary Treatment and Intervention Outcomes Network) registry who were aged at least 65 years in 2015 or 2016.
About one in six of these older patients were frail, as defined by a composite score based on impaired walking, cognition, and activities of daily living, investigators reported.
The bleeding rate was significantly higher among frail patients undergoing cardiac catheterization, at 9.4% for patients rated as having vulnerable/mild frailty and 9.9% for patients with moderate to severe frailty (P less than.001), compared with fit/well patients, whose rate was 6.5%, investigators wrote. By contrast, there was no significant difference in bleeding rates for frail versus nonfrail patients managed conservatively, they said.
After adjusting for bleeding risk factors, frailty was independently associated with increased risk of bleeding, compared with fit/well status, with odds ratios of 1.33 for vulnerable/mild frailty and 1.40 for moderate to severe frailty. Again, no association was found between frailty and bleeding risk in patients managed conservatively, according to investigators.
Frail patients in the ACTION registry were more often older and female and less likely to undergo cardiac catheterization when compared with fit or well patients, they added in the report.
Like the small cohort studies that preceded it, this large U.S. registry study shows that frailty is an “important additional risk factor” among older adults with AMI who are managed with an invasive strategy, investigators said.
“When applicable, estimation of bleeding risk in frail patients before invasive care may facilitate clinical decision making and the informed consent process,” they wrote.
The ACTION registry, an ongoing quality improvement initiative sponsored by the American College of Cardiology and the American Heart Association, started collecting frailty characteristics among hospitalized AMI patients in 2015, investigators noted.
Dr. Dodson reported support from the National Institutes of Health/National Institute on Aging and from the American Heart Association. Study coauthors provided disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96
SOURCE: Dodson JA et al. JACC Cardiovasc Intv. 2018;11:2287-96.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Frail older patients with acute myocardial infarction may be at increased bleeding risk if managed with an invasive strategy.
Major finding: Frailty was associated with increased risk of bleeding, with odds ratios of 1.33 and 1.40, compared with fit or well patients.
Study details: Analysis including 129,330 AMI patients in a U.S. registry who were at least 65 years of age.
Disclosures: Researchers reported support from the National Institutes of Health/National Institute on Aging, as well as other disclosures related to Bayer, Janssen, Abbott Vascular, Jarvik Heart, LifeCuff Technologies, and Ancora Heart.
Source: Dodson JA et al. JACC Cardiovasc Interv. 2018 Nov 26;11:2287-96.
Immunotherapy-related toxicities may be more common than reported in trials
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
SAN DIEGO – Certain immune-related adverse events related to PD1/PD-L1 treatment of patients with non–small cell lung cancer (NSCLC) may be more common than reported in clinical trials, a recent analysis of administrative claims data suggests.
Pneumonitis was seen in 10.9% of patients up to 60 days after the last dose of immunotherapy, according to the analysis of data from a large, U.S. commercial insurance database, presented at the Palliative and Supportive Care in Oncology Symposium.
By comparison, pneumonitis was reported in just 5.8% of NSCLC patients during treatment with the PD-1 (programmed cell death-1) inhibitor pembrolizumab in KEYNOTE-024, a pivotal randomized phase 3 clinical trial, said Elizabeth Jane Cathcart-Rake, MD, senior study author and an oncology fellow at the Mayo Clinic, Rochester, Minn.
Rates of immune-related adverse events in this study were generally higher than in clinical trials, both for common side effects and more rare conditions such as hypophysitis, according to Dr. Cathcart-Rake.
These new claims-based data might be considered complementary to clinical trial data, the researcher said.
“Together, they may give us a better sense of the broader implications of these adverse events,” she said in an interview.
Joe Rotella, MD, a board member of the American Academy for Hospice and Palliative Care Medicine, said results of this insurance database study provide a perspective on the real-world incidence of adverse events associated with immune checkpoint inhibitors.
“We’ve only been using these therapies for a few years, so this new analysis gives us more information on the prevalence of these side effects in patients as the therapies gain wider use,” Dr. Rotella said in a news release.
In the study, Dr. Cathcart-Rake and coinvestigators queried the OptumLabs Data Warehouse to identify 3,164 patients with NSCLC who received PD-1 or PD-L1 (programmed death-ligand 1) inhibitors between 2015 and 2017. They looked at incidence of adverse events both at the time of the last immunotherapy dose and at 60 days after the last dose.
The incidence of pneumonitis, just 4.9% on the last date of immunotherapy, increased to 10.9% at 60 days after the last dose, Dr. Cathcart-Rake reported.
Beyond pneumonitis, the most common immunotherapy-related toxicities at 60 days were hypothyroidism in 7.0%, arrhythmia in 6.1%, and nephritis or acute kidney injury in 5.4%, according to the investigators.
Dr. Cathcart-Rake also highlighted the incidence of some less common immunotherapy-related toxicities such as hypophysitis or hypothalamic-pituitary-adrenal axis toxicity, seen in 2.8% of patients by 60 days.
“That’s a small number, but hypophysitis can be really profound, and frequently leads to hospitalization,” she said. “I think this just gives us enough of a signal that providers really need to be on top of looking for these adverse events and to counsel patients beforehand.”
These data could also be helpful for advising hospitalists, emergency room physicians, and other providers who may not be attuned to the potential risks of cancer immunotherapy as compared with traditional cytotoxic chemotherapy, Dr. Cathcart-Rake said at the meeting cosponsored by AAHPM, ASCO, ASTRO, and MASCC.
“A patient with cancer may be on immunotherapy and their risk for infection is quite low, but they may be at a huge risk for pneumonitis, which is treated completely differently,” she said. “So I think this should just raise alarms that close clinical monitoring for these conditions is really important.”
Dr. Cathcart-Rake disclosed that her institution receives research funding from Novartis. One study coinvestigator reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
SOURCE: Cathcart-Rake EJ et al. 2018 Palliative and Supportive Care in Oncology Symposium. Abstract 184.
REPORTING FROM PALLONC 2018
Key clinical point: In non–small cell lung cancer patients treated with PD-1/PD-L1 inhibitors, immune-related adverse events may occur more frequently than has been suggested by clinical trial data.
Major finding: Pneumonitis was seen in nearly 11% of patients up to 60 days after the last immunotherapy dose, which investigators said was higher than reported in a pivotal phase 3 study.
Study details: Analysis of administrative claims data for 3,164 NSCLC patients treated between 2015 and 2017.
Disclosures: Researchers reported institutional research funding from Novartis. One researcher reported consulting or advisory roles with Trovagene, Genentech, Bristol-Myers Squibb, and Abbvie.
Source: Cathcart-Rake EJ et al. Palliative and Supportive Care in Oncology Symposium. Abstract 184.
Embolic protection devices advocated in some lower limb endovascular surgery
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
NEW YORK – Embolic protection devices to prevent debris from causing complications in percutaneous cardiac procedures also have a role when treating occlusions in the lower extremities, according to an expert who reviewed data and spoke about his experience at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
In this video interview, Peter Schneider, MD, chief of the division of vascular therapy at the Hawaii Kaiser Permanente Medical Group, Honolulu, provided an expert’s opinion about when filters can be helpful to reduce risk of complications.
According to Dr. Schneider, filters do have relative disadvantages so he does not advocate their use when risk is low. Among these disadvantages, the wire used to place the filter can make the endovascular procedure more difficult.
However, he said that there are well-documented cases in which debris in the runoff of a lower limb endovascular procedure resulted in adverse clinical consequences. Importantly, these may not be acute clinical events. Rather, occlusions in the tibial or pedal collaterals may remain asymptomatic for months or years.
Outlining those clinical situations that pose the greatest risk, Dr. Schneider identified several specific patient groups for whom he would advocate filters, particularly those with a large thrombotic burden. Based on his own experience, he provided some tips about situations in which he now employs embolic protection devices routinely.
REPORTING FROM VEITHSYMPOSIUM
BAP1 expression augurs prognosis of metastatic RCC
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
Expression in metastatic tissues of BAP1, a gene encoding for a tumor suppressor protein, is a significant marker for progression-free and overall survival in patients with metastatic clear cell renal cell carcinoma (ccRCC), investigators contended.
An analysis of tissue samples from 124 patients with metastatic ccRCC showed that patients with metastatic tissues expressing BAP1 had a 5-year overall survival (OS) rate of 53.2%, compared with 35.1% for patients whose tissues did not express the gene (P = .004), reported Walter Henriques da Costa, MD, PhD, and colleagues from the A.C. Camargo Cancer Center in Sao Paulo.
BAP1 expression was also associated with significantly better progression-free survival (PFS), but expression of a different gene thought to be associated with prognosis in metastatic ccRCC, PBRM1, was not a significant predictor of either overall survival (OS) or PFS.
“The pattern of immunohistochemical expression of both PBRM1 and BAP1 was shown to be significantly discordant when comparing the expression of primary tumor and metastatic tumor tissue. The use of prognostic biomarkers identified in the primary tumor tissue might be not reliable in the metastatic disease scenario. Patients with metastatic ccRCC that present loss of BAP1 expression in metastatic tissue demonstrated poor survival rates and represent a relevant risk group for tumor recurrence and death,” the investigators wrote in Urologic Oncology.
Both BAP1 and PBRM1 have been shown to be frequently mutated in ccRCC. These and other mutated genes recently identified through next-generation sequencing encode proteins that are involved in chromatin regulation and act as gene suppressors.
To see whether expression of the genes had prognostic value, they performed immunohistochemical studies of tissues from 124 consecutive patients in their center who underwent metastasectomy or biopsy of metastases and from 38 paired cases with tissues from the primary tumors of patients who underwent partial or radical nephrectomies.
They found that 98 of the metastatic samples (79%) stained negative for PBRM1 and 26 (21%) stained positive for expression of the gene; 62.1% of samples were negative for BAP1 expression and 37.9% were positive.
There were discordant expression patterns between primary tumors and metastases for BAP1 in 17 of the 38 (44.7%) samples from patients with both primary and metastatic tissues and for PBRM1 in 19 of 38 (50%) samples.
As noted before, the 5-year OS rate was 53.2% for patients with BAP1-positive metastases, compared with 35.1% for patients with BAP1-negative tissues (P = .004). The respective 5-year PFS rates for BAP1 expression were 14.9% and 3.9% (P = .003).
There were no significant differences associated with PBRM1 expression for either PFS or OS, however.
Negative expression of BAP1 in metastases was associated in multivariate analysis with both higher risk of death (hazard ratio, 2.017; P = .045) and with disease progression (HR, 1.586; P = .012).
The finding of discordance in expression between the primary tumor and metastases shows that the “strategy of using tissue markers of the primary tumor in the prediction of response of metastatic disease is not reliable. Such fact reinforces the imminent need for identification and validation of tumor markers in metastatic tissue,” the authors wrote.
No funding source or author disclosures were reported.
SOURCE: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
FROM UROLOGIC ONCOLOGY
Key clinical point: Metastatic clear cell renal cell carcinoma tissue expression of BAP1 is a marker for better overall and progression-free survival.
Major finding: The 5-year overall OS rate was 53.2% for patients with BAP1-positive metastases versus 35.1% for those with BAP1-negative tissues (P = .004).
Study details: A retrospective analysis of tumor samples from 124 patients with metastatic clear cell renal cell carcinoma and 38 patients with paired primary tumor and metastases samples.
Disclosures: No funding source or author disclosures were reported.
Source: da Costa WH et al. Urol Oncol. 2018 Nov 13. doi: 10.1016/j.urolonc.2018.10.017.
Risk score can predict thrombosis in ITP
Research suggests a scoring system can predict the risk of thrombosis in patients with immune thrombocytopenia (ITP) who are taking anticoagulants.
Researchers tested their Thrombosis and Thrombocytopenia (TH2) risk assessment score in a small group of ITP patients on anticoagulants, and the score was able to identify all seven patients who developed thrombosis.
The researchers also found that patients’ TH2 scores changed quickly, within a matter of days, suggesting they should be reevaluated for thrombosis risk frequently.
Amaris K. Balitsky, MD, of McMaster University in Hamilton, Ont., and her colleagues reported their findings in Blood.
To develop the TH2 score, the researchers conducted a review of the literature and existing tools used to assess the risk of thrombosis and bleeding. The score consists of two thrombosis items and two bleeding items.
The thrombosis items are:
- High thrombotic risk, which includes patients with atrial fibrillation and a CHA2DS2-VASc score greater than five; unprovoked, recurrent, or cancer-associated thrombosis; or antiphospholipid antibody syndrome.
- Receipt of ITP therapies known to increase the risk of thrombosis in the previous 14 days or splenectomy in the previous 30 days.
The score’s bleeding items are:
- Platelet count less than 20 x 109/L.
- Major bleeding (grade 2 bleeding that does not involve the skin) at the time of visit.
Each thrombosis item is assigned a score of +1, and each bleeding item is assigned a score of -1. A positive score or score of 0 suggests an increased risk of thrombosis, and a negative score suggests an increased risk of bleeding.
The researchers tested the TH2 score in patients enrolled in the McMaster ITP Registry from 2010 to 2017.
There were 314 patients enrolled, but only 13 were receiving anticoagulation and had a platelet count less than 50 x 109/L. Six of these patients were receiving anticoagulation for atrial fibrillation and seven for venous thrombosis. Four patients were taking antiplatelet agents as well.
The median follow up was 9 months. During that time, there were 41 encounters between patients and clinicians. Data on treatment decisions and clinical outcomes were available for 32 of these encounters.
Ten of the 13 patients had anticoagulation withheld during 22 encounters. Major bleeding was present at five of the encounters. At 17 encounters, patients received additional ITP treatments.
Six of the 10 patients who stopped anticoagulation had new thrombotic events, and two of these events were fatal. Three of the patients had thrombotic events even though they resumed anticoagulation.
Three of the 13 patients continued on anticoagulation during 10 encounters. The patients received additional ITP treatments at 6 of these encounters.
Major bleeding was present at 2 of the 10 encounters, and one new thrombotic event occurred in a patient with metastatic squamous cell cancer (despite continued treatment with warfarin).
The TH2 score accurately predicted all seven thrombotic events. There were four patients who initially had a negative TH2 score, which suggested an increased risk of bleeding.
However, these patients had a positive or 0 score – suggesting an increased risk of thrombosis – when they were assessed again, after their platelet counts increased above 50 x 109/L.
The remaining three patients had initial scores of 0 and subsequent positive scores, both suggesting an increased risk of thrombosis.
The researchers said these findings suggest patients should be reevaluated for thrombosis risk frequently, as ITP treatments are given and platelet counts increase.
“The results of our study suggest that the risk of thrombosis is high in patients with ITP who have a separate indication for anticoagulation, especially after ITP therapies are administered and the severe thrombocytopenia improves,” the researchers wrote. “Early resumption of anticoagulation should be considered in this population.”
The researchers also noted that this study was limited by its retrospective, single-center design and the small number patients evaluated. The TH2 score should be validated in additional, larger studies, they advised.
One researcher reported relationships with Amgen, Novartis, Rigel Pharmaceuticals, UCB, and Principia Biopharma.
SOURCE: Balitsky AK et al. Blood. 2018 Nov 8. doi: 10.1182/blood-2018-08-868406.
Research suggests a scoring system can predict the risk of thrombosis in patients with immune thrombocytopenia (ITP) who are taking anticoagulants.
Researchers tested their Thrombosis and Thrombocytopenia (TH2) risk assessment score in a small group of ITP patients on anticoagulants, and the score was able to identify all seven patients who developed thrombosis.
The researchers also found that patients’ TH2 scores changed quickly, within a matter of days, suggesting they should be reevaluated for thrombosis risk frequently.
Amaris K. Balitsky, MD, of McMaster University in Hamilton, Ont., and her colleagues reported their findings in Blood.
To develop the TH2 score, the researchers conducted a review of the literature and existing tools used to assess the risk of thrombosis and bleeding. The score consists of two thrombosis items and two bleeding items.
The thrombosis items are:
- High thrombotic risk, which includes patients with atrial fibrillation and a CHA2DS2-VASc score greater than five; unprovoked, recurrent, or cancer-associated thrombosis; or antiphospholipid antibody syndrome.
- Receipt of ITP therapies known to increase the risk of thrombosis in the previous 14 days or splenectomy in the previous 30 days.
The score’s bleeding items are:
- Platelet count less than 20 x 109/L.
- Major bleeding (grade 2 bleeding that does not involve the skin) at the time of visit.
Each thrombosis item is assigned a score of +1, and each bleeding item is assigned a score of -1. A positive score or score of 0 suggests an increased risk of thrombosis, and a negative score suggests an increased risk of bleeding.
The researchers tested the TH2 score in patients enrolled in the McMaster ITP Registry from 2010 to 2017.
There were 314 patients enrolled, but only 13 were receiving anticoagulation and had a platelet count less than 50 x 109/L. Six of these patients were receiving anticoagulation for atrial fibrillation and seven for venous thrombosis. Four patients were taking antiplatelet agents as well.
The median follow up was 9 months. During that time, there were 41 encounters between patients and clinicians. Data on treatment decisions and clinical outcomes were available for 32 of these encounters.
Ten of the 13 patients had anticoagulation withheld during 22 encounters. Major bleeding was present at five of the encounters. At 17 encounters, patients received additional ITP treatments.
Six of the 10 patients who stopped anticoagulation had new thrombotic events, and two of these events were fatal. Three of the patients had thrombotic events even though they resumed anticoagulation.
Three of the 13 patients continued on anticoagulation during 10 encounters. The patients received additional ITP treatments at 6 of these encounters.
Major bleeding was present at 2 of the 10 encounters, and one new thrombotic event occurred in a patient with metastatic squamous cell cancer (despite continued treatment with warfarin).
The TH2 score accurately predicted all seven thrombotic events. There were four patients who initially had a negative TH2 score, which suggested an increased risk of bleeding.
However, these patients had a positive or 0 score – suggesting an increased risk of thrombosis – when they were assessed again, after their platelet counts increased above 50 x 109/L.
The remaining three patients had initial scores of 0 and subsequent positive scores, both suggesting an increased risk of thrombosis.
The researchers said these findings suggest patients should be reevaluated for thrombosis risk frequently, as ITP treatments are given and platelet counts increase.
“The results of our study suggest that the risk of thrombosis is high in patients with ITP who have a separate indication for anticoagulation, especially after ITP therapies are administered and the severe thrombocytopenia improves,” the researchers wrote. “Early resumption of anticoagulation should be considered in this population.”
The researchers also noted that this study was limited by its retrospective, single-center design and the small number patients evaluated. The TH2 score should be validated in additional, larger studies, they advised.
One researcher reported relationships with Amgen, Novartis, Rigel Pharmaceuticals, UCB, and Principia Biopharma.
SOURCE: Balitsky AK et al. Blood. 2018 Nov 8. doi: 10.1182/blood-2018-08-868406.
Research suggests a scoring system can predict the risk of thrombosis in patients with immune thrombocytopenia (ITP) who are taking anticoagulants.
Researchers tested their Thrombosis and Thrombocytopenia (TH2) risk assessment score in a small group of ITP patients on anticoagulants, and the score was able to identify all seven patients who developed thrombosis.
The researchers also found that patients’ TH2 scores changed quickly, within a matter of days, suggesting they should be reevaluated for thrombosis risk frequently.
Amaris K. Balitsky, MD, of McMaster University in Hamilton, Ont., and her colleagues reported their findings in Blood.
To develop the TH2 score, the researchers conducted a review of the literature and existing tools used to assess the risk of thrombosis and bleeding. The score consists of two thrombosis items and two bleeding items.
The thrombosis items are:
- High thrombotic risk, which includes patients with atrial fibrillation and a CHA2DS2-VASc score greater than five; unprovoked, recurrent, or cancer-associated thrombosis; or antiphospholipid antibody syndrome.
- Receipt of ITP therapies known to increase the risk of thrombosis in the previous 14 days or splenectomy in the previous 30 days.
The score’s bleeding items are:
- Platelet count less than 20 x 109/L.
- Major bleeding (grade 2 bleeding that does not involve the skin) at the time of visit.
Each thrombosis item is assigned a score of +1, and each bleeding item is assigned a score of -1. A positive score or score of 0 suggests an increased risk of thrombosis, and a negative score suggests an increased risk of bleeding.
The researchers tested the TH2 score in patients enrolled in the McMaster ITP Registry from 2010 to 2017.
There were 314 patients enrolled, but only 13 were receiving anticoagulation and had a platelet count less than 50 x 109/L. Six of these patients were receiving anticoagulation for atrial fibrillation and seven for venous thrombosis. Four patients were taking antiplatelet agents as well.
The median follow up was 9 months. During that time, there were 41 encounters between patients and clinicians. Data on treatment decisions and clinical outcomes were available for 32 of these encounters.
Ten of the 13 patients had anticoagulation withheld during 22 encounters. Major bleeding was present at five of the encounters. At 17 encounters, patients received additional ITP treatments.
Six of the 10 patients who stopped anticoagulation had new thrombotic events, and two of these events were fatal. Three of the patients had thrombotic events even though they resumed anticoagulation.
Three of the 13 patients continued on anticoagulation during 10 encounters. The patients received additional ITP treatments at 6 of these encounters.
Major bleeding was present at 2 of the 10 encounters, and one new thrombotic event occurred in a patient with metastatic squamous cell cancer (despite continued treatment with warfarin).
The TH2 score accurately predicted all seven thrombotic events. There were four patients who initially had a negative TH2 score, which suggested an increased risk of bleeding.
However, these patients had a positive or 0 score – suggesting an increased risk of thrombosis – when they were assessed again, after their platelet counts increased above 50 x 109/L.
The remaining three patients had initial scores of 0 and subsequent positive scores, both suggesting an increased risk of thrombosis.
The researchers said these findings suggest patients should be reevaluated for thrombosis risk frequently, as ITP treatments are given and platelet counts increase.
“The results of our study suggest that the risk of thrombosis is high in patients with ITP who have a separate indication for anticoagulation, especially after ITP therapies are administered and the severe thrombocytopenia improves,” the researchers wrote. “Early resumption of anticoagulation should be considered in this population.”
The researchers also noted that this study was limited by its retrospective, single-center design and the small number patients evaluated. The TH2 score should be validated in additional, larger studies, they advised.
One researcher reported relationships with Amgen, Novartis, Rigel Pharmaceuticals, UCB, and Principia Biopharma.
SOURCE: Balitsky AK et al. Blood. 2018 Nov 8. doi: 10.1182/blood-2018-08-868406.
FROM BLOOD
Key clinical point:
Major finding: The score predicted all seven thrombotic events in the study.
Study details: Retrospective study of 13 patients with ITP.
Disclosures: One researcher reported relationships with Amgen, Novartis, Rigel Pharmaceuticals, UCB, and Principia Biopharma.
Source: Balitsky AK et al. Blood. 2018 Nov 8. doi: 10.1182/blood-2018-08-868406.
Frozen embryo transfer benefits limited to subgroups
Elective frozen embryo transfer for fertility treatment is only associated with an higher live birth rate among individuals with polycystic ovarian syndrome or hyper-responders, or those who underwent preimplantation genetic testing for aneuploidy, research suggests.
Use of frozen embryo transfer also appeared to lower the risk of moderate to severe ovarian hyperstimulation syndrome, although there may be an increased risk of preeclampsia, compared with use of fresh embryos.
A systematic review and meta-analysis, published in Human Reproduction Update, looked at 11 randomized, controlled trials (RCTs) involving 5,379 patients comparing the reproductive, obstetric, and perinatal outcomes of fresh versus frozen embryo transfer for in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).
Matheus Roque, MD, from the ORIGEN–Center for Reproductive Medicine in Rio de Janiero, and his coauthors found that, overall, elective frozen embryo transfer was associated with a 12% higher chance of live birth than fresh (P = .04), although the quality of evidence was low.
When the researchers looked at the effects in different subgroups, they found hyper-responders and individuals with polycystic ovary syndrome had a 16% higher chance of live birth with frozen rather than fresh transfer (P = .004). But among individuals who were normal responders or without polycystic ovary syndrome, there was no significant improvement in live birth rate in the frozen embryo group, compared with the fresh embryo group.
There also was a significant 33% higher live birth rate with frozen embryos, compared with fresh when blastocysts were transferred, although this was deemed very low quality evidence.
In patients who underwent preimplantation genetic testing for aneuploidy, the use of frozen embryos was associated with a 55% higher chance of live birth, compared with the use of fresh embryos (P = .005), but no difference was seen in patients who didn’t have preimplantation genetic testing.
Another subanalysis examined the effects of different routes of progesterone administration and found patients who received intramuscular injections as luteal phase support had a 20% higher chance of live birth with frozen rather than fresh embryos. But no differences were seen for vaginal or oral progesterone.
Researchers also found that the method of embryo cryopreservation, whether slow freezing or vitrification, did not have any impact on live birth rates.
However, patients who underwent frozen embryo transfer showed a 58% lower risk of moderate to severe ovarian hyperstimulation syndrome, compared with those who had fresh embryo transfer. At the same time, the risk of preeclampsia was 79% higher with frozen, compared with fresh transfer.
“There are currently no clinical data supporting the indiscriminate use of eFET [elective frozen embryo transfer] for all patients submitted to IVF/ICSI,” Dr. Roque and his coauthors wrote. “Based on the available RCTs, it seems appropriate to implement this strategy in patients at risk of OHSS [ovarian hyperstimulation syndrome], in hyper-responders, and in those undergoing PGT-A [preimplantation genetic testing for aneuploidy] at the blastocyst stage.”
No funding or conflicts of interest were reported.
SOURCE: Roque M et al. Hum Reprod Update. 2018 Nov 2. doi: 10.1093/humupd/dmy033.
Elective frozen embryo transfer for fertility treatment is only associated with an higher live birth rate among individuals with polycystic ovarian syndrome or hyper-responders, or those who underwent preimplantation genetic testing for aneuploidy, research suggests.
Use of frozen embryo transfer also appeared to lower the risk of moderate to severe ovarian hyperstimulation syndrome, although there may be an increased risk of preeclampsia, compared with use of fresh embryos.
A systematic review and meta-analysis, published in Human Reproduction Update, looked at 11 randomized, controlled trials (RCTs) involving 5,379 patients comparing the reproductive, obstetric, and perinatal outcomes of fresh versus frozen embryo transfer for in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).
Matheus Roque, MD, from the ORIGEN–Center for Reproductive Medicine in Rio de Janiero, and his coauthors found that, overall, elective frozen embryo transfer was associated with a 12% higher chance of live birth than fresh (P = .04), although the quality of evidence was low.
When the researchers looked at the effects in different subgroups, they found hyper-responders and individuals with polycystic ovary syndrome had a 16% higher chance of live birth with frozen rather than fresh transfer (P = .004). But among individuals who were normal responders or without polycystic ovary syndrome, there was no significant improvement in live birth rate in the frozen embryo group, compared with the fresh embryo group.
There also was a significant 33% higher live birth rate with frozen embryos, compared with fresh when blastocysts were transferred, although this was deemed very low quality evidence.
In patients who underwent preimplantation genetic testing for aneuploidy, the use of frozen embryos was associated with a 55% higher chance of live birth, compared with the use of fresh embryos (P = .005), but no difference was seen in patients who didn’t have preimplantation genetic testing.
Another subanalysis examined the effects of different routes of progesterone administration and found patients who received intramuscular injections as luteal phase support had a 20% higher chance of live birth with frozen rather than fresh embryos. But no differences were seen for vaginal or oral progesterone.
Researchers also found that the method of embryo cryopreservation, whether slow freezing or vitrification, did not have any impact on live birth rates.
However, patients who underwent frozen embryo transfer showed a 58% lower risk of moderate to severe ovarian hyperstimulation syndrome, compared with those who had fresh embryo transfer. At the same time, the risk of preeclampsia was 79% higher with frozen, compared with fresh transfer.
“There are currently no clinical data supporting the indiscriminate use of eFET [elective frozen embryo transfer] for all patients submitted to IVF/ICSI,” Dr. Roque and his coauthors wrote. “Based on the available RCTs, it seems appropriate to implement this strategy in patients at risk of OHSS [ovarian hyperstimulation syndrome], in hyper-responders, and in those undergoing PGT-A [preimplantation genetic testing for aneuploidy] at the blastocyst stage.”
No funding or conflicts of interest were reported.
SOURCE: Roque M et al. Hum Reprod Update. 2018 Nov 2. doi: 10.1093/humupd/dmy033.
Elective frozen embryo transfer for fertility treatment is only associated with an higher live birth rate among individuals with polycystic ovarian syndrome or hyper-responders, or those who underwent preimplantation genetic testing for aneuploidy, research suggests.
Use of frozen embryo transfer also appeared to lower the risk of moderate to severe ovarian hyperstimulation syndrome, although there may be an increased risk of preeclampsia, compared with use of fresh embryos.
A systematic review and meta-analysis, published in Human Reproduction Update, looked at 11 randomized, controlled trials (RCTs) involving 5,379 patients comparing the reproductive, obstetric, and perinatal outcomes of fresh versus frozen embryo transfer for in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI).
Matheus Roque, MD, from the ORIGEN–Center for Reproductive Medicine in Rio de Janiero, and his coauthors found that, overall, elective frozen embryo transfer was associated with a 12% higher chance of live birth than fresh (P = .04), although the quality of evidence was low.
When the researchers looked at the effects in different subgroups, they found hyper-responders and individuals with polycystic ovary syndrome had a 16% higher chance of live birth with frozen rather than fresh transfer (P = .004). But among individuals who were normal responders or without polycystic ovary syndrome, there was no significant improvement in live birth rate in the frozen embryo group, compared with the fresh embryo group.
There also was a significant 33% higher live birth rate with frozen embryos, compared with fresh when blastocysts were transferred, although this was deemed very low quality evidence.
In patients who underwent preimplantation genetic testing for aneuploidy, the use of frozen embryos was associated with a 55% higher chance of live birth, compared with the use of fresh embryos (P = .005), but no difference was seen in patients who didn’t have preimplantation genetic testing.
Another subanalysis examined the effects of different routes of progesterone administration and found patients who received intramuscular injections as luteal phase support had a 20% higher chance of live birth with frozen rather than fresh embryos. But no differences were seen for vaginal or oral progesterone.
Researchers also found that the method of embryo cryopreservation, whether slow freezing or vitrification, did not have any impact on live birth rates.
However, patients who underwent frozen embryo transfer showed a 58% lower risk of moderate to severe ovarian hyperstimulation syndrome, compared with those who had fresh embryo transfer. At the same time, the risk of preeclampsia was 79% higher with frozen, compared with fresh transfer.
“There are currently no clinical data supporting the indiscriminate use of eFET [elective frozen embryo transfer] for all patients submitted to IVF/ICSI,” Dr. Roque and his coauthors wrote. “Based on the available RCTs, it seems appropriate to implement this strategy in patients at risk of OHSS [ovarian hyperstimulation syndrome], in hyper-responders, and in those undergoing PGT-A [preimplantation genetic testing for aneuploidy] at the blastocyst stage.”
No funding or conflicts of interest were reported.
SOURCE: Roque M et al. Hum Reprod Update. 2018 Nov 2. doi: 10.1093/humupd/dmy033.
BY BIANCA NOGRADY FROM HUMAN REPRODUCTION UPDATE
Key clinical point: Certain subgroups of women may benefit from frozen versus fresh embryo transfer for in vitro fertilization/intracytoplasmic sperm injection.
Major finding:
Study details: A systematic review and meta-analysis of 11 randomized, controlled trials involving 5,379 patients.
Disclosures: No funding or conflicts of interest were reported.
Source: Roque M et al. Hum Reprod Update. 2018 Nov 2. doi: 10.1093/humupd/dmy033.
Lesions With a Distinct Black Pigment
The Diagnosis: Black-Spot Poison Ivy
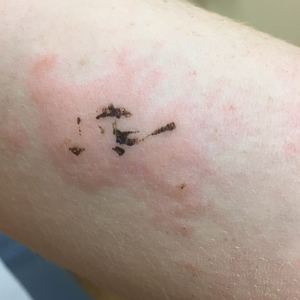
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.
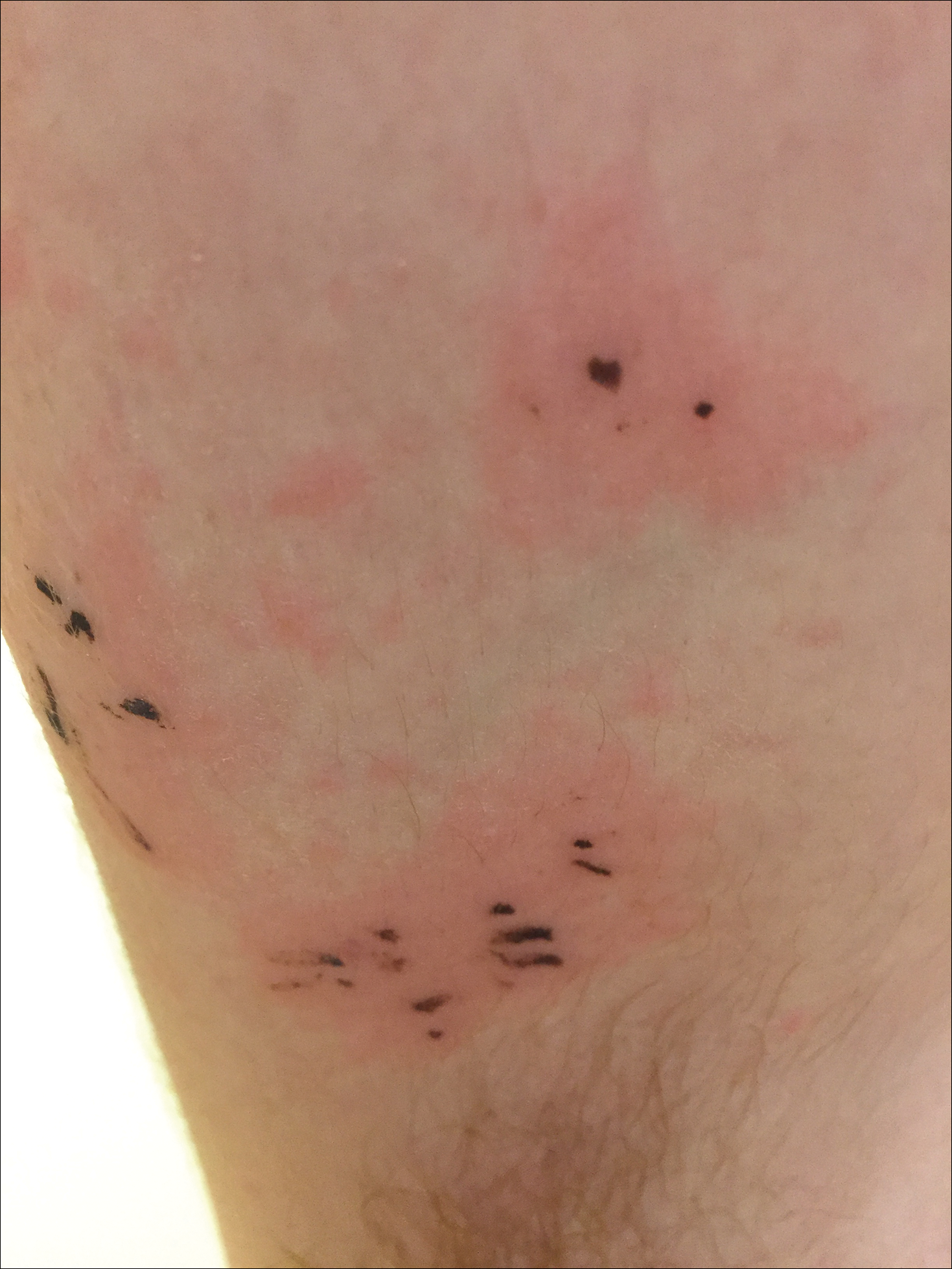
Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
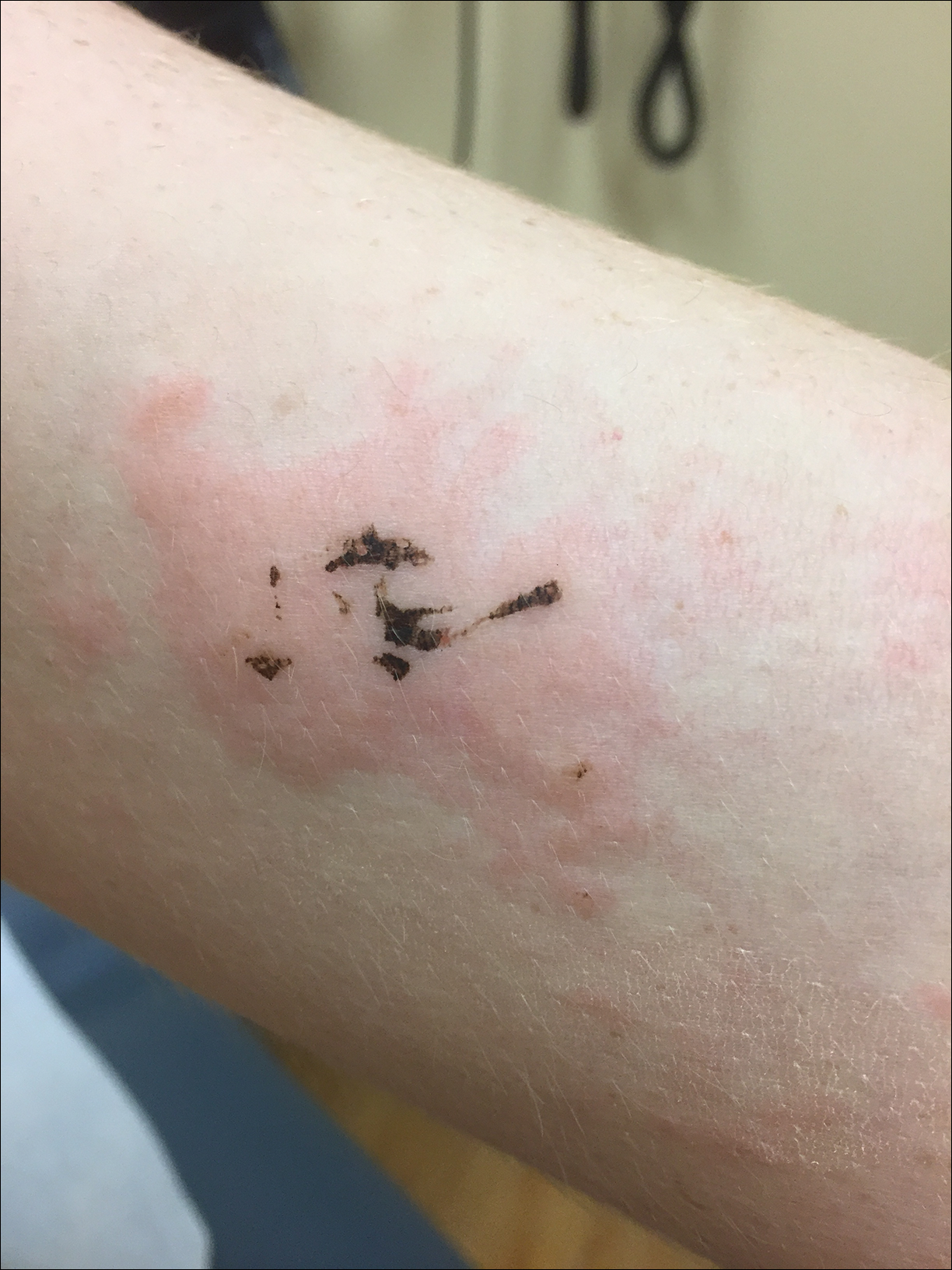
A 17-year-old adolescent boy presented to urgent care with a pruritic eruption on the bilateral arms of 1 day's duration. He was camping in the woods the night prior to presentation. On physical examination linear, erythematous, edematous plaques were observed bilaterally with overlying brown and black pigment on the arms. The pigment could not be removed with alcohol or vigorous scrubbing. The patient's condition improved with prednisone.
In the Literature - Short Takes
Sepsis Campaign bundles shortened to 1 hour
In response to the 2016 Surviving Sepsis Campaign guidelines, the 3-hour and 6-hour bundles have been combined and shortened into a 1-hour bundle to emphasize the emergent nature of sepsis. Elements of the bundle remain to same: measuring lactate, obtaining blood cultures, administration of broad-spectrume antibiotics, administration of 30mL/kg crystalloid, and vasopressors when necessary.
Citation: Levy MM et al. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997-1000
Early ED discharge for PE
This randomized trial showed that low-risk emergency department patients diagnosed with pulmonary embolism who were discharged from the ED with rivaroxaban within 24 hours of presentation had decreased length of stay and nearly $2,500 in cost savings, compared with patients treated according to standard of care (often including hospitalization) without increased risk of bleeding, recurrent venous thromboembolism, or death.
Citation: Peacock WF et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018. doi: 10.1111/acem.13451.
Outbreak of coagulopathy associated with synthetic cannabinoids
The Centers for Disease Control and Prevention reports a recent outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use, because of contamination with vitmin K antagonist agents. All patients who report synthetic cannabinoid use should be screened with INR testing, especially prior to procedures.
Citation: Outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use. CDC Health Alert Network. June 27, 2018.
Lumbar puncture safe when performed on patients on dual-antiplatelet therapy
In a retrospective review of 100 adult patients who underwent lumbar puncture procedures while taking dual-antiplatelet therapy with aspirin and clopidogrel, Mayo clinic investigators found no epidural hematomas or other serious complications with at least 3 months of follow-up. This study was nto powered to detect the risk of spinal hematoma so caution and open discussion with patients still is recommended.
Citation: Carabenciov ID et al. Safety of lumbar puncture performed on dual anti-platelet therapy. Mayo Clinic Proc. 2018;93(5):627-9.
Sepsis Campaign bundles shortened to 1 hour
In response to the 2016 Surviving Sepsis Campaign guidelines, the 3-hour and 6-hour bundles have been combined and shortened into a 1-hour bundle to emphasize the emergent nature of sepsis. Elements of the bundle remain to same: measuring lactate, obtaining blood cultures, administration of broad-spectrume antibiotics, administration of 30mL/kg crystalloid, and vasopressors when necessary.
Citation: Levy MM et al. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997-1000
Early ED discharge for PE
This randomized trial showed that low-risk emergency department patients diagnosed with pulmonary embolism who were discharged from the ED with rivaroxaban within 24 hours of presentation had decreased length of stay and nearly $2,500 in cost savings, compared with patients treated according to standard of care (often including hospitalization) without increased risk of bleeding, recurrent venous thromboembolism, or death.
Citation: Peacock WF et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018. doi: 10.1111/acem.13451.
Outbreak of coagulopathy associated with synthetic cannabinoids
The Centers for Disease Control and Prevention reports a recent outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use, because of contamination with vitmin K antagonist agents. All patients who report synthetic cannabinoid use should be screened with INR testing, especially prior to procedures.
Citation: Outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use. CDC Health Alert Network. June 27, 2018.
Lumbar puncture safe when performed on patients on dual-antiplatelet therapy
In a retrospective review of 100 adult patients who underwent lumbar puncture procedures while taking dual-antiplatelet therapy with aspirin and clopidogrel, Mayo clinic investigators found no epidural hematomas or other serious complications with at least 3 months of follow-up. This study was nto powered to detect the risk of spinal hematoma so caution and open discussion with patients still is recommended.
Citation: Carabenciov ID et al. Safety of lumbar puncture performed on dual anti-platelet therapy. Mayo Clinic Proc. 2018;93(5):627-9.
Sepsis Campaign bundles shortened to 1 hour
In response to the 2016 Surviving Sepsis Campaign guidelines, the 3-hour and 6-hour bundles have been combined and shortened into a 1-hour bundle to emphasize the emergent nature of sepsis. Elements of the bundle remain to same: measuring lactate, obtaining blood cultures, administration of broad-spectrume antibiotics, administration of 30mL/kg crystalloid, and vasopressors when necessary.
Citation: Levy MM et al. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997-1000
Early ED discharge for PE
This randomized trial showed that low-risk emergency department patients diagnosed with pulmonary embolism who were discharged from the ED with rivaroxaban within 24 hours of presentation had decreased length of stay and nearly $2,500 in cost savings, compared with patients treated according to standard of care (often including hospitalization) without increased risk of bleeding, recurrent venous thromboembolism, or death.
Citation: Peacock WF et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018. doi: 10.1111/acem.13451.
Outbreak of coagulopathy associated with synthetic cannabinoids
The Centers for Disease Control and Prevention reports a recent outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use, because of contamination with vitmin K antagonist agents. All patients who report synthetic cannabinoid use should be screened with INR testing, especially prior to procedures.
Citation: Outbreak of life-threatening coagulopathy associated with synthetic cannabinoid use. CDC Health Alert Network. June 27, 2018.
Lumbar puncture safe when performed on patients on dual-antiplatelet therapy
In a retrospective review of 100 adult patients who underwent lumbar puncture procedures while taking dual-antiplatelet therapy with aspirin and clopidogrel, Mayo clinic investigators found no epidural hematomas or other serious complications with at least 3 months of follow-up. This study was nto powered to detect the risk of spinal hematoma so caution and open discussion with patients still is recommended.
Citation: Carabenciov ID et al. Safety of lumbar puncture performed on dual anti-platelet therapy. Mayo Clinic Proc. 2018;93(5):627-9.
Rituximab biosimilar looks equivalent in follicular lymphoma
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
The rituximab biosimilar CT-P10 has equivalent efficacy, compared with rituximab, and is well tolerated in the treatment of low–tumor-burden follicular lymphoma, according to results from a multinational, randomized, phase 3 study.
Overall response after 7 months of treatment exceeded 80% for patients assigned to CT-P10 and for those assigned to rituximab, investigators reported in the Lancet Haematology.
Adverse event profiles were comparable for rituximab and the biosimilar over that time period, while pharmacokinetics, pharmacodynamics, and immunogenicity were likewise comparable between arms, according to investigators.
“Thus, CT-P10 monotherapy is suggested as a new therapeutic option for patients with low–tumor-burden follicular lymphoma,” wrote senior author Larry W Kwak, MD, PhD, of the Comprehensive Cancer Center, City of Hope, Duarte, Calif., and his colleagues.
CT-P10, the first rituximab biosimilar to be authorized by the European Medicines Agency, has been recommended for approval in the United States by the Food and Drug Administration’s Oncologic Drugs Advisory Committee.
If approved by the FDA, CT-P10 would be the first rituximab biosimilar available in the United States, according to the company, which noted three proposed indications in non-Hodgkin lymphoma.
In the current randomized, double-blind, parallel-group, phase 3 trial, 258 patients with stage II-IV low–tumor-burden follicular lymphoma were randomly assigned to CT-P10 (130 patients) or rituximab sourced in the United States (128 patients).
Treatment consisted of an induction period of intravenous CT-P10 or rituximab weekly for 4 weeks, while patients experiencing disease control went on to a maintenance phase with their assigned treatment given every 8 weeks for six cycles, followed by another year of maintenance therapy with CT-P10 for those still on study.
The primary endpoint of the study was overall response at 7 months, defined as a complete response, unconfirmed complete response, or partial response.
Overall response was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab at 7 months, Dr. Kwak and his colleagues reported.
The two treatments were deemed therapeutically equivalent, as illustrated by 90% confidence intervals within a prespecified equivalence margin of 17%, investigators said.
The most common treatment-emergent adverse events in either group were infusion-related reactions, which were of grade 1-2, except for one grade 3 reaction reported in the CT-P10 group, according to the report. Other common adverse events were upper respiratory tract infections and fatigue.
Serious adverse events were reported in six patients in the CT-P10 arm and three patients in the rituximab arm.
The availability of a rituximab biosimilar is anticipated to reduce the cost of treatment and improve patient access, according to investigators.
Introduction of CT-P10 in the European Union was projected to save between 90 and 150 million euros over a year, enabling more than 12,500 new patients to be treated with the biosimilar, according to results of a budget impact analysis investigators cited in their report.
“Widespread adoption of a rituximab biosimilar could have a substantial effect on health care budgets and might also have effects at a societal level,” Dr. Kwak and his coauthors said in the report.
The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Dr. Kwak and several other coinvestigators not employed by Celltrion reported disclosures related to the company. Other disclosures provided related to Novartis, Roche, AbbVie, Celgene, and Takeda, among other entities.
SOURCE: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: Overall response after 7 months of treatment was seen in 83% of patients randomized to CT-P10 and 81% of patients randomized to rituximab.
Study details: Analysis of 258 patients randomized to CT-P10 or rituximab in a phase 3, double-blind, parallel-group trial.
Disclosures: The trial was sponsored by Celltrion and three coauthors of the study were employees of the company. Other study coauthors reported disclosures related to Celltrion, Novartis, Roche, AbbVie, Celgene, and Takeda, among other companies.
Source: Ogura M et al. Lancet Haematol. 2018 Nov;5(11):e543-53.