User login
Minority cancer patient scan discussions 3 minutes shorter
SAN DIEGO – Oncologists spent on average 3 minutes less with minority patients than with nonminority patients at a critical transition visit to talk about a scan result related to advanced cancer, a study investigator reported.
The gap was most pronounced for visits where a negative scan result was communicated, said investigator Cardinale B. Smith, MD, PhD, director of Quality for Cancer Services, Mount Sinai Health System, New York.
Future work is needed to look at the content of these conversations to determine to what extent physician- or patient-specific factors may have contributed to this difference, according to Dr. Smith.
“This will be critically important to ensure that we provide high-quality care to our minority patients,” Dr. Smith said at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study included 22 oncologists from four hospitals randomized to either a usual-care group or an intervention group that included several interventions designed to improve communication techniques related to goals-of-care conversations
Sixty-nine percent of the physicians were white, while 23% were Asian, 8% were Hispanic, and 5% were black, Dr. Smith said.
Postimaging encounters were audio recorded for 142 patients, more than half of whom were minorities; 32% were black and 26% were Hispanic, according to the investigator, while 38% were white and 4% were Asian.
Overall, time spent with minorities at the scan results visit was 11.5 minutes versus 16.5 minutes for nonminorities (P = .0007), Dr. Smith reported.
When the scan was positive, there was actually no significant difference in time spent on minority versus nonminority visits, (16 versus 18 minutes; P = .59), a finding that Dr. Smith said was “encouraging” in an interview. However, there was a marked difference in length of time spent on minority versus nonminority visits when the scans showed no progression (10 versus 15 minutes; P = .0003).
“There’s something about regular, routine visits, which are still just as important as [positive] scan visits, in which there is a difference in the time spent,” Dr. Smith said in an interview. “We’re really interested in doing a more in-depth analysis of those conversations to see what’s contributing to those differences.”
After adjusting for patient insurance, education, income progression, status, and hospital, investigators found that oncologists spent 13.5 minutes (interquartile range, 12-16) with minority patients versus 16.5 minutes (IQR, 16-19) for nonminority patients (P less than .0001).
In terms of the communications intervention, investigators found no difference in time spent with minorities versus nonminorities among the intervention or control physicians. However, the intervention was aimed at improving the rate of goals of care conversations rather than improving communication based on race or ethnicity, Dr. Smith said.
By contrast, the finding of decreased time spent in communication encounters with minority patients merits further study to see how much of the disparity is mediated by differences in patients versus oncologists, she added.
In literature on communication disparities in other care settings outside of oncology, care conversations with minorities tend to be more closed ended, as opposed to open ended – so instead of asking if a patient is doing well, a physician might start out with a declarative statement that the patient’s findings are positive, she said.
On the other hand, some literature suggests that minority patients may not question the physician as much as nonminorities, or may not spend as much time asking directed questions during the visit. “That may also contribute to decreased time spent during those encounters,” Dr. Smith added.
The research was supported by the Patient Centered Outcomes Research Institute. Dr. Smith reported honoraria and a consulting or advisory role with Teva.
SOURCE: Smith CB et al. PallOnc 2018, Abstract 19.
SAN DIEGO – Oncologists spent on average 3 minutes less with minority patients than with nonminority patients at a critical transition visit to talk about a scan result related to advanced cancer, a study investigator reported.
The gap was most pronounced for visits where a negative scan result was communicated, said investigator Cardinale B. Smith, MD, PhD, director of Quality for Cancer Services, Mount Sinai Health System, New York.
Future work is needed to look at the content of these conversations to determine to what extent physician- or patient-specific factors may have contributed to this difference, according to Dr. Smith.
“This will be critically important to ensure that we provide high-quality care to our minority patients,” Dr. Smith said at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study included 22 oncologists from four hospitals randomized to either a usual-care group or an intervention group that included several interventions designed to improve communication techniques related to goals-of-care conversations
Sixty-nine percent of the physicians were white, while 23% were Asian, 8% were Hispanic, and 5% were black, Dr. Smith said.
Postimaging encounters were audio recorded for 142 patients, more than half of whom were minorities; 32% were black and 26% were Hispanic, according to the investigator, while 38% were white and 4% were Asian.
Overall, time spent with minorities at the scan results visit was 11.5 minutes versus 16.5 minutes for nonminorities (P = .0007), Dr. Smith reported.
When the scan was positive, there was actually no significant difference in time spent on minority versus nonminority visits, (16 versus 18 minutes; P = .59), a finding that Dr. Smith said was “encouraging” in an interview. However, there was a marked difference in length of time spent on minority versus nonminority visits when the scans showed no progression (10 versus 15 minutes; P = .0003).
“There’s something about regular, routine visits, which are still just as important as [positive] scan visits, in which there is a difference in the time spent,” Dr. Smith said in an interview. “We’re really interested in doing a more in-depth analysis of those conversations to see what’s contributing to those differences.”
After adjusting for patient insurance, education, income progression, status, and hospital, investigators found that oncologists spent 13.5 minutes (interquartile range, 12-16) with minority patients versus 16.5 minutes (IQR, 16-19) for nonminority patients (P less than .0001).
In terms of the communications intervention, investigators found no difference in time spent with minorities versus nonminorities among the intervention or control physicians. However, the intervention was aimed at improving the rate of goals of care conversations rather than improving communication based on race or ethnicity, Dr. Smith said.
By contrast, the finding of decreased time spent in communication encounters with minority patients merits further study to see how much of the disparity is mediated by differences in patients versus oncologists, she added.
In literature on communication disparities in other care settings outside of oncology, care conversations with minorities tend to be more closed ended, as opposed to open ended – so instead of asking if a patient is doing well, a physician might start out with a declarative statement that the patient’s findings are positive, she said.
On the other hand, some literature suggests that minority patients may not question the physician as much as nonminorities, or may not spend as much time asking directed questions during the visit. “That may also contribute to decreased time spent during those encounters,” Dr. Smith added.
The research was supported by the Patient Centered Outcomes Research Institute. Dr. Smith reported honoraria and a consulting or advisory role with Teva.
SOURCE: Smith CB et al. PallOnc 2018, Abstract 19.
SAN DIEGO – Oncologists spent on average 3 minutes less with minority patients than with nonminority patients at a critical transition visit to talk about a scan result related to advanced cancer, a study investigator reported.
The gap was most pronounced for visits where a negative scan result was communicated, said investigator Cardinale B. Smith, MD, PhD, director of Quality for Cancer Services, Mount Sinai Health System, New York.
Future work is needed to look at the content of these conversations to determine to what extent physician- or patient-specific factors may have contributed to this difference, according to Dr. Smith.
“This will be critically important to ensure that we provide high-quality care to our minority patients,” Dr. Smith said at the 2018 Palliative and Supportive Care in Oncology Symposium.
The study included 22 oncologists from four hospitals randomized to either a usual-care group or an intervention group that included several interventions designed to improve communication techniques related to goals-of-care conversations
Sixty-nine percent of the physicians were white, while 23% were Asian, 8% were Hispanic, and 5% were black, Dr. Smith said.
Postimaging encounters were audio recorded for 142 patients, more than half of whom were minorities; 32% were black and 26% were Hispanic, according to the investigator, while 38% were white and 4% were Asian.
Overall, time spent with minorities at the scan results visit was 11.5 minutes versus 16.5 minutes for nonminorities (P = .0007), Dr. Smith reported.
When the scan was positive, there was actually no significant difference in time spent on minority versus nonminority visits, (16 versus 18 minutes; P = .59), a finding that Dr. Smith said was “encouraging” in an interview. However, there was a marked difference in length of time spent on minority versus nonminority visits when the scans showed no progression (10 versus 15 minutes; P = .0003).
“There’s something about regular, routine visits, which are still just as important as [positive] scan visits, in which there is a difference in the time spent,” Dr. Smith said in an interview. “We’re really interested in doing a more in-depth analysis of those conversations to see what’s contributing to those differences.”
After adjusting for patient insurance, education, income progression, status, and hospital, investigators found that oncologists spent 13.5 minutes (interquartile range, 12-16) with minority patients versus 16.5 minutes (IQR, 16-19) for nonminority patients (P less than .0001).
In terms of the communications intervention, investigators found no difference in time spent with minorities versus nonminorities among the intervention or control physicians. However, the intervention was aimed at improving the rate of goals of care conversations rather than improving communication based on race or ethnicity, Dr. Smith said.
By contrast, the finding of decreased time spent in communication encounters with minority patients merits further study to see how much of the disparity is mediated by differences in patients versus oncologists, she added.
In literature on communication disparities in other care settings outside of oncology, care conversations with minorities tend to be more closed ended, as opposed to open ended – so instead of asking if a patient is doing well, a physician might start out with a declarative statement that the patient’s findings are positive, she said.
On the other hand, some literature suggests that minority patients may not question the physician as much as nonminorities, or may not spend as much time asking directed questions during the visit. “That may also contribute to decreased time spent during those encounters,” Dr. Smith added.
The research was supported by the Patient Centered Outcomes Research Institute. Dr. Smith reported honoraria and a consulting or advisory role with Teva.
SOURCE: Smith CB et al. PallOnc 2018, Abstract 19.
REPORTING FROM PALLONC 2018
Key clinical point: Oncologists spent on average 3 minutes less with minority patients than with nonminority patients discussing a scan result related to an advanced cancer.
Major finding: Adjusted discussion time was 13.5 minutes for minority patients versus 16.5 minutes for nonminority patients.
Study details: Analysis of audio recordings of discussions between 142 patients and 22 oncologists at four hospitals.
Disclosures: The research was supported by the Patient Centered Outcomes Research Institute. The presenting author reported honoraria and a consulting or advisory role with Teva.
Source: Smith CB et al. PallOnc 2018, Abstract 19.
Hep C–infected livers are safe for transplant
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
SAN FRANCISCO – A new analysis shows that hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality. The work confirms that readily available direct-acting antiviral therapy can protect organ recipients and open a source of organs that is typically overlooked.
The work should encourage both physicians and patients to take a closer look at hepatitis C–infected organs, especially for sicker patients, according to Sonali Paul, MD, who presented the study at the annual meeting of the American Association for the Study of Liver Disease 2018.
“A lot of people have an ethical issue with it because we’re actively transplanting a virus into someone. We’re giving someone a disease. My take on it is that we give people Epstein Barr virus or cytomegalovirus all the time – we just [provide] prophylaxis against it, and we don’t even bat an eye. Hepatitis C can be devastating, but we have totally effective treatments for it,” said Dr. Paul, who is an assistant professor of medicine at the University of Chicago.
She cited one colleague at the University of Chicago who several years ago transplanted an organ that had been passed over 700 times, though times have changed since then. “I think people more and more are doing this practice because we know it’s so successful,” said Dr. Paul.
It’s also cost effective. Another study, presented during the same session by Jag Chhatwal, PhD, assistant professor at Harvard Medical School, Boston, showed that accepting a hepatitis C–positive liver is cost effective in patients with Model for End-Stage Liver Disease (MELD) scores ranging from 22 to 40.
“I think we’re going to find across all organ systems, if we can transplant patients rather than keep them on dialysis or keep them on wait lists, it’s got to be cost effective, especially if you think of the health care–associated costs – like a heart transplant patient waiting on the list in the ICU. That’s a huge health care cost,” said Dr. Paul.
Dr. Paul’s team performed an analysis of the Scientific Registry of Transplant Recipients, including single organ transplants from deceased donors, during 2014-2018. Over that period, the number of transplants from hepatitis C–positive donors to hepatitis C–positive recipients rose from 8 in 2014 to 269, and the number of transplants from hepatitis C–positive donors to hepatitis C–negative recipients rose from 0 to 46.
The researchers compared trends from hepatitis C–negative donors with hepatitis C–negative recipients (n = 11,270), negative donors with positive recipients (n = 4,748), positive donors with negative recipients (n = 87), and positive donors with positive recipients (n = 753). Donor status had no effect on graft survival times at 1 or 2 years, with values ranging from 92.6% (negative to negative) to 94.3% (positive to positive) at 1 year and between 85.7% (positive to negative) and 89.7% (positive to positive) at 2 years.
“For someone who has a MELD score of over 20, who has a declining quality of life and really can’t do anything, I think this is a great opportunity. And most patients are absolutely willing to take these organs. We haven’t had many people say no, especially if they feel poorly,” said Dr. Paul.
She also underscored the importance of ensuring that the patient is informed of the status of the donor liver and the need to complete treatment: “The patient has to know what’s happening, and the hospital has to have a safety net if the insurance doesn’t pay for hepatitis C treatment.”
SOURCE: AASLD 2018, Abstract 0249.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: Use of hepatitis C–positive livers can significantly increase the donor organ pool.
Major finding: Hepatitis C–infected livers can be safely transplanted into recipients with no effect on graft survival, retransplantation, or mortality.
Study details: Retrospective analysis of 16,858 liver transplants.
Disclosures: The study was funded internally. Dr. Paul has no financial disclosures.
Source: AASLD 2018, Abstract 0249.
Novel topical JAK inhibitor shows promise for atopic dermatitis
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.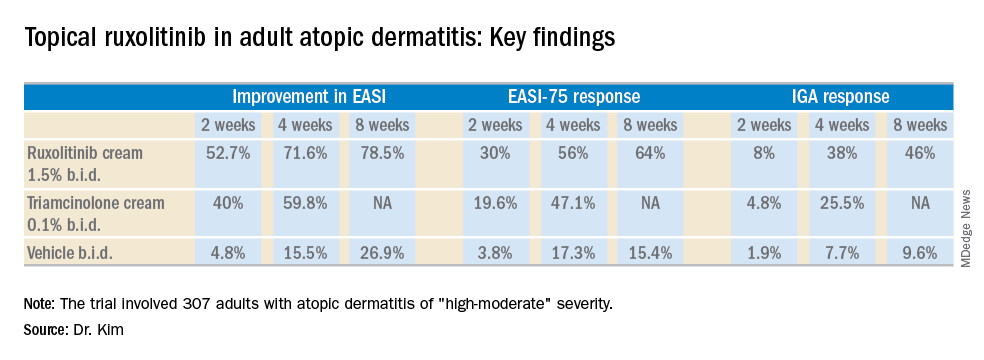
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
PARIS – A cream formulation of ruxolitinib, a selective inhibitor of Janus kinase (JAK) 1 and 2, outperformed triamcinolone cream 0.1% and vehicle control in a large, phase 2, dose-ranging, randomized trial in patients with atopic dermatitis (AD), Brian S. Kim, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
This novel topical JAK inhibitor not only modulates inflammatory cytokines involved in the pathogenesis of AD, including interleukin-4, -5, -13, and -31, but Dr. Kim and his coinvestigators also demonstrated that ruxolitinib has antipruritic effects achieved by acting directly on sensory nerve fibers.
“Ultimately, said Dr. Kim, a dermatologist and codirector of the Center for the Study of Itch at Washington University, St. Louis.
The trial included 307 adults, mean age 35 years, with a median 21-year disease history and a mean of 7.3 flares within the past 12 months. Dr. Kim characterized the study population as having AD of “high-moderate” severity, with a mean involved body surface area of 9.7%, half of patients having a baseline Eczema Area and Severity Index (EASI) score greater than 7, and having a mean itch numeric rating scale of 7. Two-thirds of patients had an Investigator’s Global Assessment (IGA) score of 3 and the rest had scores of 2.
Patients were randomized to one of six study arms entailing 8 weeks of double-blind therapy: ruxolitinib cream 1.5% once daily, 1.5% twice daily; 0.5% once daily; 0.15% once daily; twice-daily vehicle; or triamcinolone cream 0.1% twice a day for 4 weeks followed by 4 weeks of vehicle.
All the ruxolitinib regimens provided dose- and time-dependent efficacy, compared with vehicle. The best results were seen with ruxolitinib 1.5% twice daily, which outperformed triamcinolone cream.
The primary study endpoint was change in EASI score from baseline to week 4, but the week 2 and week 8 data were also informative. Key secondary endpoints included the proportion of subjects achieving an EASI-75 response and/or an IGA response, which required improvement to an IGA score of 0 or 1 with at least a 2-point reduction from baseline.
As for itch, ruxolitinib cream provided rapid and sustained improvement, said Dr. Kim. Indeed, within the first 2 days of the study, the ruxolitinib 1.5% twice-daily group had a mean 1.8-point reduction on the numeric rating scale, compared with a 0.2-point drop with vehicle and a 1-point drop with triamcinolone cream twice a day. By week 4, the twice-daily ruxolitinib 1.5% group had about a 4-point drop from baseline, the once-daily ruxolitinib 1.5% group had a 3.5-point drop, and the triamcinolone-treated patients had a 2.5-point drop.
Topical ruxolitinib was not associated with any significant safety or tolerability issues, and there were no clinically significant application site reactions, according to the dermatologist.
Session cochair Konstantine Buxtorf Friedli, MD, a Swiss dermatologist, commented that she could easily imagine this topical JAK inhibitor also being useful in other diseases with itch.
Dr. Kim reported serving as a consultant to and recipient of research funding from Incyte, which sponsored the study.
REPORTING FROM THE EADV CONGRESS
Key clinical point: A novel topical Janus kinase inhibitor may provide a valuable alternative to potent topical steroids in atopic dermatitis.
Major finding: At week 4, the mean improvement in Eczema Area and Severity Index score was 72% with ruxolitinib cream 1.5% twice a day, compared with 60% with triamcinolone cream 0.1% twice a day.
Study details: This 8-week, phase 2 clinical trial included 307 adult atopic dermatitis patients randomized to ruxolitinib cream, triamcinolone cream, or vehicle.
Disclosures: The study was sponsored by Incyte. The presenter reported serving as a consultant to and recipient of research funding from the company.
Topical treatments remain a good option for psoriasis
LAS VEGAS – in mild cases of psoriasis.
Topicals often are a worthwhile complement to even the most advanced systemic medications, according to Linda Stein Gold, MD, director of clinical research in the department of dermatology at the Henry Ford Health System, Detroit.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, she pointed out that as the variety of vehicles for topical treatments has grown, so has the need to pay attention to the potency of these treatments. “Traditionally, we had thought we had to use a thick ointment to drive the drug in and get the best efficacy,” she said. “But we’ve changed our thought process.”
For example, betamethasone dipropionate 0.05%, now comes in multiple types of ointments and creams, with different potency classes, including Diprolene ointment, 0.05%, Diprosone cream, 0.05%, Diprolene cream AF, 0.05%, and Diprolene cream, 0.05%, as well as a lotion and an emollient spray.
“It’s the same active drug, but different vehicles absolutely change the potency of the drug,” Dr. Stein Gold said.
So which is the most potent? She said you can’t tell just by the vehicle. In this case, the most potent forms – in the “superpotent” class 1 – are Diprolene cream, 0.05%, and Diprolene ointment, 0.05%. (The National Psoriasis Foundation has a potency chart for topical psoriasis medications.)
She also recommended considering combination therapy with tazarotene. Tazarotene, a vitamin A derivative, is associated with a variety of side effects in 10%-30% of patients, including pruritus, erythema, irritation, skin pain, psoriasis worsening, and burning/stinging. But combination therapy with topical corticosteroids can reduce adverse effects, and it boosts efficacy as well, Dr. Stein Gold said.
She added that tazarotene can be a tool against acne. The 0.1% cream and gel formulations are approved by the Food and Drug Administration for treating acne; the 0.05% cream and gel forms are approved only for psoriasis. “Both concentrations work well and hit the different pillars of the pathogenesis of acne,” she said.
In addition, Dr. Stein Gold noted that she led two 2018 studies that found a fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate to severe plaque psoriasis was associated with significant reductions in the severity of the clinical signs of psoriasis, and minimal safety concerns (J Am Acad Dermatol. 2018 Aug;79[2]:287-93).
As for the future in topical treatment for psoriasis, she said researchers are exploring phosphodiesterase-4 inhibitors, Janus kinase inhibitors, and aryl hydrocarbon receptor agonists.
Dr. Stein Gold disclosed speaker bureau relationships with Galderma, Leo, Valeant, Novartis, Celgene and Allergan; consulting for Sol‐Gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor and Medimetriks; receiving grant/research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan and Foamix; and serving on scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix and Promius.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – in mild cases of psoriasis.
Topicals often are a worthwhile complement to even the most advanced systemic medications, according to Linda Stein Gold, MD, director of clinical research in the department of dermatology at the Henry Ford Health System, Detroit.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, she pointed out that as the variety of vehicles for topical treatments has grown, so has the need to pay attention to the potency of these treatments. “Traditionally, we had thought we had to use a thick ointment to drive the drug in and get the best efficacy,” she said. “But we’ve changed our thought process.”
For example, betamethasone dipropionate 0.05%, now comes in multiple types of ointments and creams, with different potency classes, including Diprolene ointment, 0.05%, Diprosone cream, 0.05%, Diprolene cream AF, 0.05%, and Diprolene cream, 0.05%, as well as a lotion and an emollient spray.
“It’s the same active drug, but different vehicles absolutely change the potency of the drug,” Dr. Stein Gold said.
So which is the most potent? She said you can’t tell just by the vehicle. In this case, the most potent forms – in the “superpotent” class 1 – are Diprolene cream, 0.05%, and Diprolene ointment, 0.05%. (The National Psoriasis Foundation has a potency chart for topical psoriasis medications.)
She also recommended considering combination therapy with tazarotene. Tazarotene, a vitamin A derivative, is associated with a variety of side effects in 10%-30% of patients, including pruritus, erythema, irritation, skin pain, psoriasis worsening, and burning/stinging. But combination therapy with topical corticosteroids can reduce adverse effects, and it boosts efficacy as well, Dr. Stein Gold said.
She added that tazarotene can be a tool against acne. The 0.1% cream and gel formulations are approved by the Food and Drug Administration for treating acne; the 0.05% cream and gel forms are approved only for psoriasis. “Both concentrations work well and hit the different pillars of the pathogenesis of acne,” she said.
In addition, Dr. Stein Gold noted that she led two 2018 studies that found a fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate to severe plaque psoriasis was associated with significant reductions in the severity of the clinical signs of psoriasis, and minimal safety concerns (J Am Acad Dermatol. 2018 Aug;79[2]:287-93).
As for the future in topical treatment for psoriasis, she said researchers are exploring phosphodiesterase-4 inhibitors, Janus kinase inhibitors, and aryl hydrocarbon receptor agonists.
Dr. Stein Gold disclosed speaker bureau relationships with Galderma, Leo, Valeant, Novartis, Celgene and Allergan; consulting for Sol‐Gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor and Medimetriks; receiving grant/research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan and Foamix; and serving on scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix and Promius.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – in mild cases of psoriasis.
Topicals often are a worthwhile complement to even the most advanced systemic medications, according to Linda Stein Gold, MD, director of clinical research in the department of dermatology at the Henry Ford Health System, Detroit.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, she pointed out that as the variety of vehicles for topical treatments has grown, so has the need to pay attention to the potency of these treatments. “Traditionally, we had thought we had to use a thick ointment to drive the drug in and get the best efficacy,” she said. “But we’ve changed our thought process.”
For example, betamethasone dipropionate 0.05%, now comes in multiple types of ointments and creams, with different potency classes, including Diprolene ointment, 0.05%, Diprosone cream, 0.05%, Diprolene cream AF, 0.05%, and Diprolene cream, 0.05%, as well as a lotion and an emollient spray.
“It’s the same active drug, but different vehicles absolutely change the potency of the drug,” Dr. Stein Gold said.
So which is the most potent? She said you can’t tell just by the vehicle. In this case, the most potent forms – in the “superpotent” class 1 – are Diprolene cream, 0.05%, and Diprolene ointment, 0.05%. (The National Psoriasis Foundation has a potency chart for topical psoriasis medications.)
She also recommended considering combination therapy with tazarotene. Tazarotene, a vitamin A derivative, is associated with a variety of side effects in 10%-30% of patients, including pruritus, erythema, irritation, skin pain, psoriasis worsening, and burning/stinging. But combination therapy with topical corticosteroids can reduce adverse effects, and it boosts efficacy as well, Dr. Stein Gold said.
She added that tazarotene can be a tool against acne. The 0.1% cream and gel formulations are approved by the Food and Drug Administration for treating acne; the 0.05% cream and gel forms are approved only for psoriasis. “Both concentrations work well and hit the different pillars of the pathogenesis of acne,” she said.
In addition, Dr. Stein Gold noted that she led two 2018 studies that found a fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate to severe plaque psoriasis was associated with significant reductions in the severity of the clinical signs of psoriasis, and minimal safety concerns (J Am Acad Dermatol. 2018 Aug;79[2]:287-93).
As for the future in topical treatment for psoriasis, she said researchers are exploring phosphodiesterase-4 inhibitors, Janus kinase inhibitors, and aryl hydrocarbon receptor agonists.
Dr. Stein Gold disclosed speaker bureau relationships with Galderma, Leo, Valeant, Novartis, Celgene and Allergan; consulting for Sol‐Gel, Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Promius, Anacor and Medimetriks; receiving grant/research support from Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan and Foamix; and serving on scientific advisory boards for Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix and Promius.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Lenvatinib/Pembrolizumab shows promise in previously treated metastatic NSCLC
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
WASHINGTON – (NSCLC), according to interim findings from a phase 1b/2 study.
Of note, the 21 patients enrolled in the multicenter, open-label study as of March 2018 were not preselected for programmed death-ligand 1 (PD-L1) tumor expression status, Marcia S. Brose, MD, reported at the annual meeting of the Society for the Immunotherapy of Cancer.
They were treated with 20 mg of oral lenvatinib daily and 200 mg of intravenous pembrolizumab every 3 weeks, and the overall response rate at 24 weeks – the primary endpoint of the study – was 33.3%, said Dr. Brose of Abramson Cancer Center of the University of Pennsylvania, Philadelphia.
One patient had a complete response, six had a partial response, 10 had stable disease, two progressed on treatment, and the outcome in two was unknown or not evaluable, for an overall clinical benefit rate of 66%, she said, adding that the median duration of response was 10.9 months and median progression-free survival (PFS) was 5.9 months.
All patients had good performance status (ECOG score of 0-1), and nine (43%) were PD-L1–positive as defined by a tumor proportion score of at least 1%, five (24%) were PD-L1-negative, and seven (33%) were not tested for PD-L1 status. Three (14%) were treatment naive, while seven (33%), 10 (48%), and one (5%) had received one, two, or three or more prior lines of systemic therapy, respectively. No prior nivolumab or pembrolizumab treatment was allowed.
“At least one of the patients who was PD-L1–negative remained on study after 40 weeks and still continuing to respond, and ... the PD-L1–positive patients were also doing well,” Dr. Brose said.
Tumor assessments were performed by study investigators using immune-related Response Evaluation Criteria in Solid Tumors (irRECIST).
Grade 3 or greater treatment-related adverse events occurred in 10 patients (48%), and mainly included hypertension, fatigue, and diarrhea, but only four were considered serious treatment-related adverse events. Nineteen patients had treatment adjustments because of adverse events, four discontinued treatment due to adverse events, and one patient died from a pulmonary hemorrhage that was thought to possibly be treatment related, Dr. Brose said.
“The toxicity is really what you would have expected from either of these drugs on their own; it didn’t seem like there was anything that happened in synergy from the two that was unexpected,” she noted.
Lenvatinib is a multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor receptors (FGFR) 1-4, platelet-derived growth factor receptor (PDGFR) alpha, and the RET and c-KIT proto-oncogenes. Pembrolizumab is an anti–PD-1 antibody approved as a monotherapy for previously treated patients with metastatic PD-L1–positive NSCLC, and it has been shown to be associated with an overall response rate of 18%, she explained.
The current results are from the NSCLC cohort of an ongoing trial of lenvatinib plus pembrolizumab in patients with solid tumors.
“Further investigation of this study drug combination in patients is warranted, but we will have to think carefully about what point in the treatment paradigm these patients should be treated in order to maximize the benefit from this combination therapy,” she concluded.
Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.
SOURCE: Brose M et al. SITC 2018, Abstract P392.
REPORTING FROM SITC 2018
Key clinical point: Lenvatinib/pembrolizumab shows promise in metastatic NSCLC.
Major finding: Overall response rate at 24 weeks was 33.3%.
Study details: Interim findings in 21 patients from a phase 1b/2 study.
Disclosures: Dr. Brose has received consulting fees, research grants, and honorarium from Eisai.Source: Brose M et al. SITC 2018, Abstract P392.
A guide to talking with patients about probiotics
Two recent studies published in Cell, “Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features” and “Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT,” have received significant media coverage and are causing questions and concern among physicians and patients who use probiotic supplements.
Talking to patients about probiotics
1. Probiotics are generally thought to be safe for healthy individuals, but we don’t know the long-term consequences. For individuals who have a chronic disease, are immunocompromised, or otherwise vulnerable (such as the elderly), patients should seek guidance from physicians on whether probiotics may be appropriate. In general, probiotics should not be used indiscriminately; potential risk and benefit should be considered as for all human interventions.
2. This research does not conclude that probiotics are unsafe or useless for everyone. However, the results suggest that individuals may respond very differently to the same probiotic product depending on their diet, genetics, microbiome and other aspects of their health. Experts are trying to better understand which bacteria are best for whom, under which conditions as we transition from an era of empiric medicine to precision medicine.
3. Probiotics currently on the market are foods or dietary supplements. To date, no probiotic products have been approved by the FDA to treat, mitigate, cure, or prevent specific diseases.
AGA has recently developed educational materials for patients on probiotics, which can be accessed at www.gastro.org/probiotics in English and Spanish. Share this resource with your patients by printing it out, emailing it, or uploading it to your patient portal.
Two recent studies published in Cell, “Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features” and “Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT,” have received significant media coverage and are causing questions and concern among physicians and patients who use probiotic supplements.
Talking to patients about probiotics
1. Probiotics are generally thought to be safe for healthy individuals, but we don’t know the long-term consequences. For individuals who have a chronic disease, are immunocompromised, or otherwise vulnerable (such as the elderly), patients should seek guidance from physicians on whether probiotics may be appropriate. In general, probiotics should not be used indiscriminately; potential risk and benefit should be considered as for all human interventions.
2. This research does not conclude that probiotics are unsafe or useless for everyone. However, the results suggest that individuals may respond very differently to the same probiotic product depending on their diet, genetics, microbiome and other aspects of their health. Experts are trying to better understand which bacteria are best for whom, under which conditions as we transition from an era of empiric medicine to precision medicine.
3. Probiotics currently on the market are foods or dietary supplements. To date, no probiotic products have been approved by the FDA to treat, mitigate, cure, or prevent specific diseases.
AGA has recently developed educational materials for patients on probiotics, which can be accessed at www.gastro.org/probiotics in English and Spanish. Share this resource with your patients by printing it out, emailing it, or uploading it to your patient portal.
Two recent studies published in Cell, “Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features” and “Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT,” have received significant media coverage and are causing questions and concern among physicians and patients who use probiotic supplements.
Talking to patients about probiotics
1. Probiotics are generally thought to be safe for healthy individuals, but we don’t know the long-term consequences. For individuals who have a chronic disease, are immunocompromised, or otherwise vulnerable (such as the elderly), patients should seek guidance from physicians on whether probiotics may be appropriate. In general, probiotics should not be used indiscriminately; potential risk and benefit should be considered as for all human interventions.
2. This research does not conclude that probiotics are unsafe or useless for everyone. However, the results suggest that individuals may respond very differently to the same probiotic product depending on their diet, genetics, microbiome and other aspects of their health. Experts are trying to better understand which bacteria are best for whom, under which conditions as we transition from an era of empiric medicine to precision medicine.
3. Probiotics currently on the market are foods or dietary supplements. To date, no probiotic products have been approved by the FDA to treat, mitigate, cure, or prevent specific diseases.
AGA has recently developed educational materials for patients on probiotics, which can be accessed at www.gastro.org/probiotics in English and Spanish. Share this resource with your patients by printing it out, emailing it, or uploading it to your patient portal.
Total knee replacement risk soars after arthroscopic surgery for meniscal tear
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
CHICAGO – A 5-year follow-up of a major randomized trial comparing methods of meniscal tear management in patients with osteoarthritis showed the risk of total knee replacement was 400% greater in patients who underwent arthroscopic partial meniscectomy than in those who received physical therapy alone, Jeffrey N. Katz, MD, reported at the annual meeting of the American College of Rheumatology.
At 5 years, however, the two divergent initial treatment strategies – arthroscopic surgical repair versus physical therapy – resulted in similar degrees of long-term pain improvement, noted Dr. Katz, a rheumatologist who is professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
“Because that’s the case, a reasonable recommendation – and one that most folks around the world who are thinking about this problem would make – is to have the first choice initially be nonoperative; that is, physical therapy, with surgery reserved for those who don’t improve and who have an interest in undertaking the risks of surgery,” he said.
Dr. Katz presented 5-year follow-up data on 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy. A lot rides on the outcomes of this study, as there is a longstanding debate over the balance of risks and benefits of arthroscopic surgery in this common clinical scenario.
Of the 351 participants, 164 were randomized to and received arthroscopic partial meniscectomy, 109 were randomized to and received a standardized program of physical therapy, and 68 were initially randomized to physical therapy but crossed over to arthroscopic surgery within the first few months because of lack of improvement.
At 5 years of follow-up, all three groups showed similar degrees of improvement in Knee Osteoarthritis and Injury Outcome Score Pain Scale scores, from 40-50 out of a possible 100 at baseline to 20-25 at 6 months, with little change thereafter through 5 years.
The eye-catching finding was the difference in the incidence of total knee replacement (TKR) through 5 years: 10% in those who underwent arthroscopic partial meniscectomy, either as initial therapy or after crossing over from the physical therapy group, compared with 2% in patients who underwent physical therapy alone. Given that more than 400,000 arthroscopic partial meniscectomies are done annually in the United States in patients with knee osteoarthritis, extrapolation from the MeTeOR results suggests an excess of 40,000 total knee replacements in surgically treated patients.
“The higher TKR rates that we observed in surgically treated patients are unexplained, concerning, and require further study. The finding is consistent with the observation in the Osteoarthritis Initiative that TKR rates were higher in patients with arthroscopy as opposed to those treated nonsurgically,” the rheumatologist said.
He proposed two possible explanations for the finding. “It does appear that people who have arthroscopic surgery are then, over the next 5 years, more likely to have total knee replacement. We don’t know whether that is because performing arthroscopic surgery is actually damaging the knee further, leading it to deteriorate more quickly and therefore go on to total knee replacement, or whether when patients develop a relationship with a surgeon and have arthroscopic surgery, they get over some of their apprehension about surgery and may become more likely to accept subsequent surgery for total knee replacement. We hope to find the answer. I think this story is still unfolding because 5 years is a relatively brief period of time in the course of osteoarthritis.
“Arthroscopic surgery certainly offers greater shorter-term improvement, and for some patients that’s worth trading off some downstream risk of joint damage, and for others, they would not want to make that trade-off. So I see it ultimately as a matter of patient choice,” Dr. Katz said.
Knee osteoarthritis affects an estimated 15 million Americans. More than one-half of them have a meniscal tear, the majority of which don’t cause symptoms.
Dr. Katz reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
SOURCE: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Risk of total knee replacement is five times higher after arthroscopic partial meniscectomy.
Major finding: Patients randomized to arthroscopic partial meniscectomy were 400% more likely to subsequently undergo total knee replacement than were those randomized to physical therapy alone.
Study details: This was a presentation of the 5-year follow-up results in 341 participants in the MeTeOR trial, a seven-center study in which middle-age or older subjects with knee pain, a meniscal tear, and osteoarthritic changes on x-ray were randomized to arthroscopic repair or physical therapy.
Disclosures: The presenter reported having no financial conflicts regarding MeTeOR, which was funded by the National Institutes of Health.
Source: Katz JN et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1816.
AHA 2018: Part II
MDedge reporters Mitchel Zoler and Bruce Jancin join MDedge Cardiology Editor Catherine Hackett to continue their recap of the important highlights of the 2018 annual Scientific Sessions of the American Heart Association. You can click here to find more coverage from AHA 2018.
MDedge reporters Mitchel Zoler and Bruce Jancin join MDedge Cardiology Editor Catherine Hackett to continue their recap of the important highlights of the 2018 annual Scientific Sessions of the American Heart Association. You can click here to find more coverage from AHA 2018.
MDedge reporters Mitchel Zoler and Bruce Jancin join MDedge Cardiology Editor Catherine Hackett to continue their recap of the important highlights of the 2018 annual Scientific Sessions of the American Heart Association. You can click here to find more coverage from AHA 2018.
FDA approves emapalumab for primary HLH
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
The (HLH).
Emapalumab, an interferon gamma-blocking antibody, is approved to treat patients of all ages (newborn and older) with primary HLH who have refractory, recurrent, or progressive disease or who cannot tolerate conventional HLH therapy. Emapalumab is the first treatment to be FDA approved for primary HLH, and it is expected to be available in the United States in the first quarter of 2019. The FDA previously granted emapalumab priority review, breakthrough therapy designation, orphan drug designation, and rare pediatric disease designation. The FDA’s approval of emapalumab is based on results from a phase 2/3 trial (NCT01818492).
The trial included 34 patients, 27 of whom had refractory, recurrent, or progressive disease or could not tolerate conventional HLH therapy. Patients received emapalumab in combination with dexamethasone. At the end of treatment, 63% (17/27) of patients had achieved a response, which was defined as complete response (n = 7), partial response (n=8), or HLH improvement (n = 2). A total of 70% (n=19) of patients went on to hematopoietic stem cell transplant. The most common adverse events were infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%).
Results also are scheduled to be presented at the 2018 annual meeting of the American Society of Hematology in the late-breaker abstract session (Abstract LBA-6).
Emapalumab was developed by Novimmune SA. Sobi acquired global rights to the drug in August 2018.
‘Phenomenal’ REDUCE-IT establishes triglyceride theory
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
CHICAGO – REDUCE-IT is a phenomenal trial and a game changer because it has shown for the first time that triglyceride reduction with an appropriate therapy – in this case icosapent ethyl – when used in appropriate doses can make a significant difference.
That’s according to Prakash C. Deedwania, MD, chief of the cardiology division at the Veterans Affairs Medical Center/University of California San Francisco Program in Fresno, who joined MDedge reporter Richard Mark Kirkner for a video interview at the American Heart Association scientific sessions.
In the large, placebo-controlled REDUCE-IT trial in patients with or at high risk for cardiovascular disease received who received 2 g of icosapent ethyl (Vascepa) twice daily or placebo saw a 25% lower risk of cardiovascular death or an ischemic event, compared with placebo.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS