User login
Status Epilepticus Scores Aren’t Specific Enough
Trying to predict morbidity and mortality among patients with status epilepticus has proven difficult, and the 2 scoring metrics designed to accomplish that feat have significant shortcomings, according to a retrospective analysis of status epilepticus patients conducted at the Ohio State University Wexner Medical Center.
- Investigators reviewed the records of 46 affected patients admitted to the hospital’s neuro-critical care unit.
- Data from the status epilepticus Severity Score (STESS) and Epidemiology-based Mortality Score in Status Epilepticus (EMSE) were analyzed.
- Sensitivity of EMSE was 100% and sensitivity of STESS was 90%.
- The specificity of both metrics was wanting, however: EMSE, 28.6% and STESS, 42.9%.
- Researchers concluded that both scoring systems may help predict clinical outcomes in status epilepticus patients who have few co-existing conditions but are less valuable in populations with several medical problems.
Yechoor A, Adeli A, Hafeez S. External validation of the epidemiology-based mortality score in status epilepticus in an American intensive care population. Epilepsy Res. 2018;148:32-36.
Trying to predict morbidity and mortality among patients with status epilepticus has proven difficult, and the 2 scoring metrics designed to accomplish that feat have significant shortcomings, according to a retrospective analysis of status epilepticus patients conducted at the Ohio State University Wexner Medical Center.
- Investigators reviewed the records of 46 affected patients admitted to the hospital’s neuro-critical care unit.
- Data from the status epilepticus Severity Score (STESS) and Epidemiology-based Mortality Score in Status Epilepticus (EMSE) were analyzed.
- Sensitivity of EMSE was 100% and sensitivity of STESS was 90%.
- The specificity of both metrics was wanting, however: EMSE, 28.6% and STESS, 42.9%.
- Researchers concluded that both scoring systems may help predict clinical outcomes in status epilepticus patients who have few co-existing conditions but are less valuable in populations with several medical problems.
Yechoor A, Adeli A, Hafeez S. External validation of the epidemiology-based mortality score in status epilepticus in an American intensive care population. Epilepsy Res. 2018;148:32-36.
Trying to predict morbidity and mortality among patients with status epilepticus has proven difficult, and the 2 scoring metrics designed to accomplish that feat have significant shortcomings, according to a retrospective analysis of status epilepticus patients conducted at the Ohio State University Wexner Medical Center.
- Investigators reviewed the records of 46 affected patients admitted to the hospital’s neuro-critical care unit.
- Data from the status epilepticus Severity Score (STESS) and Epidemiology-based Mortality Score in Status Epilepticus (EMSE) were analyzed.
- Sensitivity of EMSE was 100% and sensitivity of STESS was 90%.
- The specificity of both metrics was wanting, however: EMSE, 28.6% and STESS, 42.9%.
- Researchers concluded that both scoring systems may help predict clinical outcomes in status epilepticus patients who have few co-existing conditions but are less valuable in populations with several medical problems.
Yechoor A, Adeli A, Hafeez S. External validation of the epidemiology-based mortality score in status epilepticus in an American intensive care population. Epilepsy Res. 2018;148:32-36.
Postop Invasive Monitoring Worth Considering
Among children who have had surgical resection of epileptic lesions, it may be wise to continue postoperative invasive monitoring, suggests this investigation of 71 patients published in Epilepsy Research.
- A retrospective analysis of 5 patients with MRI-negative epilepsy and 66 patients with MRI-identified neocortical lesions, post-resection invasive monitoring yielded positive outcomes in 86%.
- In 55 of 71 patients, post-resection monitoring resulted in additional resections.
- Postop monitoring detected clinical seizures at the resection margins, subclinical seizures and interictal discharges at the resection margins, and both clinical and subclinical seizures that indicated a new epileptogenic focus.
Hidalgo ET, Frankel HG, Rodriguez C, et al. Invasive monitoring after resection of epileptogenic neocortical lesions in multi-staged epilepsy surgery in children. Epilepsy Res. 2018; 148:48-54.
Among children who have had surgical resection of epileptic lesions, it may be wise to continue postoperative invasive monitoring, suggests this investigation of 71 patients published in Epilepsy Research.
- A retrospective analysis of 5 patients with MRI-negative epilepsy and 66 patients with MRI-identified neocortical lesions, post-resection invasive monitoring yielded positive outcomes in 86%.
- In 55 of 71 patients, post-resection monitoring resulted in additional resections.
- Postop monitoring detected clinical seizures at the resection margins, subclinical seizures and interictal discharges at the resection margins, and both clinical and subclinical seizures that indicated a new epileptogenic focus.
Hidalgo ET, Frankel HG, Rodriguez C, et al. Invasive monitoring after resection of epileptogenic neocortical lesions in multi-staged epilepsy surgery in children. Epilepsy Res. 2018; 148:48-54.
Among children who have had surgical resection of epileptic lesions, it may be wise to continue postoperative invasive monitoring, suggests this investigation of 71 patients published in Epilepsy Research.
- A retrospective analysis of 5 patients with MRI-negative epilepsy and 66 patients with MRI-identified neocortical lesions, post-resection invasive monitoring yielded positive outcomes in 86%.
- In 55 of 71 patients, post-resection monitoring resulted in additional resections.
- Postop monitoring detected clinical seizures at the resection margins, subclinical seizures and interictal discharges at the resection margins, and both clinical and subclinical seizures that indicated a new epileptogenic focus.
Hidalgo ET, Frankel HG, Rodriguez C, et al. Invasive monitoring after resection of epileptogenic neocortical lesions in multi-staged epilepsy surgery in children. Epilepsy Res. 2018; 148:48-54.
Surgical model to study reflux esophagitis after esophagojejunostomy
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
Agoston et al. reported that metaplastic columnar-lined esophagus develops in a wound-healing process on the distal edge of the ulcer, starting distally at the esophagojejunal anastomosis in the esophagojejunal anastomosed rat model. They also concluded that the columnar-lined esophagus was caused through migration of jejunal cells into the esophagus.
These new findings bring up a couple of issues. One is that metaplastic columnar-lined esophagus originates from jejunal crypt budding over the anastomosis. Some researchers may think this is not metaplasia, as there is no reprogramming of the stem cells. However, the definition of metaplasia is an endpoint such that a normal lineage is placed in an abnormal position, and it can be called metaplasia even it is from budding of jejunal crypt. This new finding is not denying metaplasia.
The second issue is whether these rodent models are really mimicking human metaplastic columnar-lined esophagus or not. In humans, metaplastic columnar-lined esophagus usually accompanies gastroesophageal reflux, but jejunum is not next to esophagus, and jejunal crypt budding is less likely. However, it is common to observe ulcerated lesions in the proximal front of long-segment Barrett’s esophagus in humans. In this process, the model of Agosto et al. is describing the human metaplastic columnar-lined esophagus elongation.
There would be more reprogramming happening in the body of animals under the effect of microenvironment. This is a kind of adaptation, and analyzing key factors for this reprogramming would be the path to clarifying carcinogenesis in the metaplastic field and also a way to advance regenerative medicine.
Sachiyo Nomura MD, PhD, AGAF, FACS, is an investigator in gastrointestinal carcinogenesis and epithelial biology, and a gastrointestinal surgeon, at University of Tokyo Hospital, department of stomach and esophageal surgery, as well as an associate professor, department of gastrointestinal surgery, graduate school of medicine, at the university. She has no conflicts.
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
During a wound repair process in rats, metaplastic columnar-lined esophagus was produced and increased in length following esophagojejunostomy, which may be independent of stem cell reprogramming, according to results from an anastomosed rodent study.
The investigators studied esophageal and tissue sections of 52 rats at different time points after esophagojejunostomy and samples were analyzed for length, type, and location of columnar lining. In addition, the sections were examined immunophenotypically to elucidate the molecular changes that occur during ulceration. Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital and the department of pathology at Harvard Medical School, Boston, and colleagues reported the findings in Cellular and Molecular Gastroenterology and Hepatology.
“This rodent columnar-lined esophagus has been proposed to develop from cellular reprogramming of progenitor cells, but studies on early columnar-lined esophagus development are lacking,” the researchers wrote.
In the model, ulceration was seen 2 weeks after surgery, which began distally at the esophagojejunal anastomosis. Representative of wound healing, reepithelialization of the ulcer region took place through formation of immature glands, which were found to bud directly from jejunal crypts.
After immunophenotypic analysis, the researchers reported that “immunohistochemical characterization of neoglandular epithelium located immediately proximal to the anastomosis showed features similar to those of the native nonproliferating jejunal epithelium located immediately distal to the anastomosis.” They further reported that “the columnar-lined esophagus’s immunoprofile was similar to jejunal crypt epithelium.”
Upon further examination of the ulcer segment, Dr. Agoston and colleagues found that columnar-lined esophagus elongated from 0.15 mm (standard error of the mean, ± 0.1) to 5.22 mm (SEM, ± 0.37) at 2 and 32 weeks post esophagojejunostomy, respectively.
“There was a highly significant linear relationship between the length of the neoglandular epithelium in the distal esophagus and the number of weeks after surgery (correlation coefficient, 0.94; P less than .0001),” the investigators stated.
Locational analysis revealed epithelial-mesenchymal transition markers being expressed by spindle-shaped cells at the leading edge of the columnar-lined esophagus. In addition, neoglands were identified within esophageal ulcer beds and actively dividing squamous epithelium was seen exclusively at the proximal ulcer border.
Following the systematic analysis, the authors noted that the columnar-lined esophagus was most likely the result of jejunal cell migration into the esophagus. They suggested that if compared, jejunal cells may competitively dominate squamous cells in the context of chronic gastroesophageal reflux disease. Furthermore, they observed that the region of ulceration following esophagojejunostomy in their model was more expansive than that reported in other comparable rodent models of reflux esophagitis.
“The reason for this difference is not clear, but we speculate that it is the result of technical aspects of our reflux-inducing surgery,” the researchers wrote. They further explained that “we intentionally fashioned a large anastomotic orifice between the esophagus and jejunum, perhaps larger than that fashioned by other investigators.” And they concluded, “we suspect that this larger orifice resulted in esophageal exposure to larger volumes of refluxate and, consequently, larger areas of ulceration.”
The authors acknowledged their results may not be fully applicable in the context of human Barrett’s esophagus, given the rodent model. However, they do believe the findings may provide a basis to help understand the wound repair process, particularly the distal edge of ulcers that border the columnar epithelium.
“Using a rat model of reflux esophagitis via surgical esophagojejunostomy, we have shown that a metaplastic, columnar-lined esophagus develops via a wound healing process, and not via genetic reprogramming of progenitor cells,” the researchers concluded.
The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
SOURCE: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Post esophagojejunostomy in rats, a wound repair process produces metaplastic columnar-lined esophagus likely independent of progenitor cell reprogramming.
Major finding: In an esophageal tissue section, columnar-lined esophagus length was significantly elongated from 0.15 (±0.1) mm to 5.22 (±0.37) mm at 2 and 32 weeks, respectively, following esophagojejunostomy.
Study details: Histologic and immunophenotypic analysis of 52 rats investigating the molecular characteristics of columnar-lined esophagus, specific to ulceration and wound healing, following surgery in an anastomosed rodent model.
Disclosures: The study was supported by grant funding from the National Institutes of Health and the Baylor Scott and White Research Institute. The authors reported no conflicts of interest.
Source: Agoston AT et al. Cell Mol Gastroenterol Hepatol. 2018 Jun 26. doi: 10.1016/j.jcmgh.2018.06.007.
HCV antibodies linked to poorer cardiac outcomes in ACHD patients
Among patients with adult congenital heart disease (ACHD), hepatitis C virus antibody positivity was significantly associated with a composite end point that comprised cardiac death, heart failure hospitalization, lethal ventricular arrhythmias, and cardiac reoperation, according to a report published online in the American Journal of Cardiology.
The study retrospectively enrolled 243 ACHD patients (mean age 26 years) who underwent cardiac surgery before 1992 and visited a single hospital during 1995-2015. Clinical characteristics, including cardiac function and long-term prognosis, were compared between HCV antibody–positive (48) and –negative (195) patients, according to Ryo Konno, MD, of Tohoku University, Sendai, Japan, and his colleagues.
They found that the prevalence of reduced systemic ventricular ejection fraction less than 50% was significantly higher in the HCV antibody–positive group than in the HCV antibody–negative group (17 vs. 5.4%; P = .014), and that during a mean follow-up of 10 years the composite end point occurred in 51 patients.
Overall, Kaplan-Meier analysis showed the HCV antibody–positive group had significantly poorer event-free survival than the HCV antibody–negative group (P = .002). In addition, HCV antibody positivity was significantly associated with the composite end point in both univariable and multivariable Cox regression models (hazard ratio, 2.37; P = .005 and HR, 1.96; P = .032, respectively).
“These results indicate that screening for HCV should be performed in all ACHD patients with a history of heart surgery before 1992. Further, cardiac functions should be monitored more frequently to detect [stroke volume] dysfunction earlier in case of a positive result. These management strategies may have a beneficial impact on the long-term prognosis in this population,” Dr. Konno and his colleagues concluded.
The research was supported by the Japan Agency for Medical Research and the authors had no conflicts of interest to disclose.
SOURCE: Konno R. et al. Am J Card. 2018;122:1965-71.
Among patients with adult congenital heart disease (ACHD), hepatitis C virus antibody positivity was significantly associated with a composite end point that comprised cardiac death, heart failure hospitalization, lethal ventricular arrhythmias, and cardiac reoperation, according to a report published online in the American Journal of Cardiology.
The study retrospectively enrolled 243 ACHD patients (mean age 26 years) who underwent cardiac surgery before 1992 and visited a single hospital during 1995-2015. Clinical characteristics, including cardiac function and long-term prognosis, were compared between HCV antibody–positive (48) and –negative (195) patients, according to Ryo Konno, MD, of Tohoku University, Sendai, Japan, and his colleagues.
They found that the prevalence of reduced systemic ventricular ejection fraction less than 50% was significantly higher in the HCV antibody–positive group than in the HCV antibody–negative group (17 vs. 5.4%; P = .014), and that during a mean follow-up of 10 years the composite end point occurred in 51 patients.
Overall, Kaplan-Meier analysis showed the HCV antibody–positive group had significantly poorer event-free survival than the HCV antibody–negative group (P = .002). In addition, HCV antibody positivity was significantly associated with the composite end point in both univariable and multivariable Cox regression models (hazard ratio, 2.37; P = .005 and HR, 1.96; P = .032, respectively).
“These results indicate that screening for HCV should be performed in all ACHD patients with a history of heart surgery before 1992. Further, cardiac functions should be monitored more frequently to detect [stroke volume] dysfunction earlier in case of a positive result. These management strategies may have a beneficial impact on the long-term prognosis in this population,” Dr. Konno and his colleagues concluded.
The research was supported by the Japan Agency for Medical Research and the authors had no conflicts of interest to disclose.
SOURCE: Konno R. et al. Am J Card. 2018;122:1965-71.
Among patients with adult congenital heart disease (ACHD), hepatitis C virus antibody positivity was significantly associated with a composite end point that comprised cardiac death, heart failure hospitalization, lethal ventricular arrhythmias, and cardiac reoperation, according to a report published online in the American Journal of Cardiology.
The study retrospectively enrolled 243 ACHD patients (mean age 26 years) who underwent cardiac surgery before 1992 and visited a single hospital during 1995-2015. Clinical characteristics, including cardiac function and long-term prognosis, were compared between HCV antibody–positive (48) and –negative (195) patients, according to Ryo Konno, MD, of Tohoku University, Sendai, Japan, and his colleagues.
They found that the prevalence of reduced systemic ventricular ejection fraction less than 50% was significantly higher in the HCV antibody–positive group than in the HCV antibody–negative group (17 vs. 5.4%; P = .014), and that during a mean follow-up of 10 years the composite end point occurred in 51 patients.
Overall, Kaplan-Meier analysis showed the HCV antibody–positive group had significantly poorer event-free survival than the HCV antibody–negative group (P = .002). In addition, HCV antibody positivity was significantly associated with the composite end point in both univariable and multivariable Cox regression models (hazard ratio, 2.37; P = .005 and HR, 1.96; P = .032, respectively).
“These results indicate that screening for HCV should be performed in all ACHD patients with a history of heart surgery before 1992. Further, cardiac functions should be monitored more frequently to detect [stroke volume] dysfunction earlier in case of a positive result. These management strategies may have a beneficial impact on the long-term prognosis in this population,” Dr. Konno and his colleagues concluded.
The research was supported by the Japan Agency for Medical Research and the authors had no conflicts of interest to disclose.
SOURCE: Konno R. et al. Am J Card. 2018;122:1965-71.
FROM THE AMERICAN JOURNAL OF CARDIOLOGY
Key clinical point: Adults with congenital heart disease and HCV antibody positivity had significantly worse cardiac outcomes than those without.
Major finding: The HCV antibody–positive group had significantly poorer event-free survival (P = .002).
Study details: A retrospective study of 243 ACHD patients; 48 had HCV antibody positivity.
Disclosures: The research was supported by the Japan Agency for Medical Research; the authors had no conflicts of interest to disclose.
Source: Konno R et al. Am J Card. 2018;122:1965-71.
SVS guidelines address scope of practice concerns
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
NEW YORK – Vascular surgeons are the only specialty qualified to treat all vascular disorders with open surgery and/or endovascular treatment, including the thoracic aorta, according to the updated “Guidelines for hospital privileges in vascular surgery and endovascular interventions: Recommendations of the Society for Vascular Surgery.”
The guidelines, published in May’s Journal of Vascular Surgery, were last updated in 2008, said Keith D. Calligaro, MD, who spoke on their importance and potential benefits to vascular surgeons during his presentation at the VEITHsymposium.
The thoracic aorta component of the guidelines addresses scope of practice concerns between vascular and thoracic surgeons, said Dr. Calligaro, who is a clinical professor of surgery, University of Pennsylvania, and chief of vascular surgery and endovascular therapy at Pennsylvania Hospital, both in Philadelphia.
The guidelines relied on training requirements to provide some of the data to define vascular surgeons and privileges. The open vascular surgery training requirements still are defined by the Residency Review Committee for surgery, and those requirements include 250 major open vascular cases during training, including 30 open abdominal operations, 25 carotid, 45 peripheral open surgery cases, and 10 complex vascular surgeries, said Dr. Calligaro. “In terms of endovascular treatment, the training requirements are over 100 diagnostic caths and over 80 therapeutic interventions, and during training you would have had to have done more than 20 EVARs [endovascular aneurysm repairs].” That number jumped up from five EVARs in the previous guidelines.
Ultimately, “the SVS is basically saying ‘you need to be a vascular surgeon to perform vascular surgery,’ and you need have to have completed an Accreditation Council for Graduate Medical Education–accredited vascular residency.
“So if you are a general surgeon or a heart surgeon and you go to a new hospital and say ‘I want to do vascular,’ the vascular surgeon at that institution can refer to this document and say ‘no, the SVS is saying [the surgeon doesn’t] have the training.’ And I think that’s a pretty gutsy and important call,” said Dr. Calligaro.
It is a different case for endovascular surgery, he said. In this case, the requirement is to have completed an ACGME-accredited program in either vascular surgery, interventional radiology, or interventional cardiology to indicate the appropriate level of training. But SVS agreed with the recommendation by the American College of Cardiology that cardiologists not only had to complete 1 year of coronary interventions but also 1 year of peripheral intervention training, as well.
“So if you are at your hospital and have a cardiologist who is starting to do peripheral vascular stuff, now at least you can wave part of this document and say ‘Hey, look, the most important vascular society in the country is saying that, unless this individual had a year of peripheral training, this cardiologist should not be allowed to do endovascular peripheral interventions,’ ” Dr. Calligaro said.
SOURCE: Calligaro KD et al. J Vasc Surg. 2018 May;67(5):1337-44.
REPORTING FROM THE VEITHSYMPOSIUM
FDA expands approval of brentuximab vedotin to PTCL
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
The , marking the first FDA approval of a treatment for newly-diagnosed PTCL.
The drug, which is marketed by Seattle Genetics as Adcetris, is a monoclonal antibody that binds to CD30 protein found on some cancer cells.
It was previously approved for adult patients with untreated stage III or IV classical Hodgkin lymphoma (cHL), cHL after relapse, cHL after stem cell transplant in patients at high risk for relapse or progression, systemic anaplastic large cell lymphoma (ALCL) after other treatments fail, and primary cutaneous ALCL or CD30-expressing mycosis fungoides after other treatments fail.
The expanded approval, which followed the granting of Priority Review and Breakthrough Therapy designations for the supplemental Biologic License Application, was made using the FDA’s new Real-Time Oncology Review pilot program (RTOR). This program allows for data review and communication with a sponsor prior to official application submission with the goal of speeding up the review process.
The brentuximab vedotin approval now extends to previously untreated systemic ALCL and other CD30-expressing PTCLs in combination with chemotherapy.
Approval was based on the ECHELON-2 clinical trial involving 452 patients, which demonstrated improved progression-free survival (PFS) in patients with certain types of PTCL who were treated first-line with either brentuximab vedotin plus chemotherapy with cyclophosphamide, doxorubicin, prednisone (CHP), or standard chemotherapy with CHP and vincristine (CHOP). Median PFS was 48 months vs. 21 months in the groups, respectively (hazard ratio, 0.71).
The FDA advises health care providers to “monitor patients for infusion reactions, life-threatening allergic reactions (anaphylaxis), neuropathy, fever, gastrointestinal complications, and infections,” according to a press release announcing the approval, which also states that patients should be monitored for tumor lysis syndrome, serious skin reactions, pulmonary toxicity, and hepatotoxicity.
The drug may cause harm to a developing fetus or newborn and should not be used in women who are pregnant or breastfeeding. A Boxed Warning regarding risk of progressive multifocal leukoencephalopathy is also included in the prescribing information.
The current standard of care for initial treatment of PTCL is multiagent chemotherapy – a treatment that “has not significantly changed in decades and is too often unsuccessful in leading to long-term remissions, underscoring the need for new treatments, ” Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a statement issued by Seattle Genetics.
“With this approval, clinicians have the opportunity to transform the way newly diagnosed CD30-expressing PTCL patients are treated,” Dr. Horwitz said.
The ECHELON-2 data will be presented at the American Society of Hematology annual meeting in San Diego on Monday, Dec. 3, 2018.
Fear of blindness hobbles hydroxychloroquine treatment of SLE
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
REPORTING FROM THE ACR ANNUAL MEETING
Quick FDA approval for brentuximab vedotin in PTCL
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
The U.S. Food and Drug Administration (FDA) has approved a new indication for brentuximab vedotin (ADCETRIS®) less than 2 weeks after receiving the completed supplemental biologics license application (sBLA).
Brentuximab vedotin is now approved for use in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) to treat adults with previously untreated systemic anaplastic large-cell lymphoma or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma and PTCL not otherwise specified.
The FDA granted priority review and breakthrough therapy designation to the sBLA for brentuximab vedotin in this indication.
The FDA reviewed the sBLA under the Real-Time Oncology Review Pilot Program, which led to approval less than 2 weeks after the application was submitted in full.
“The Real-Time Oncology Review program allows the FDA to access key data prior to the official submission of the application, allowing the review team to begin their review earlier and communicate with the sponsor prior to the application’s actual submission,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Hematology and Oncology Products.
“When the sponsor submits the completed application, the review team will already be familiar with the data and be able to conduct a more efficient, timely, and thorough review.”
Trial data
The FDA’s approval is based on results from the phase 3 ECHELON-2 trial (NCT01777152).
Seattle Genetics, Inc. and Takeda Pharmaceutical Company Limited announced results from this trial last month. Additional results are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 997).
The trial enrolled 452 patients with CD30-positive PTCL. The patients were randomized to receive brentuximab vedotin (BV) plus CHP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
The objective response rate was 83% in the BV-CHP arm and 72% in the CHOP arm (P=0.003). The complete response rate was 68% and 56%, respectively (P=0.007).
Progression-free survival was significantly better in the BV-CHP arm than in the CHOP arm (hazard ratio=0.71; P=0.011), as was overall survival (hazard ratio=0.66; P=0.024).
The most common adverse events of any grade that occurred in at least 20% of patients in the BV-CHP arm were peripheral neuropathy, nausea, diarrhea, neutropenia, lymphopenia, fatigue, mucositis, constipation, alopecia, pyrexia, vomiting, and anemia.
Serious adverse events occurring in at least 2% of patients in the BV-CHP arm included febrile neutropenia, pneumonia, pyrexia, and sepsis.
Results of this trial suggest prophylactic growth factors should be given to previously untreated PTCL patients receiving BV-CHP (starting at cycle 1).
AEDs strongly linked to rare serious skin reactions
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
Antiepileptic drugs were found to be linked with almost ninefold increased odds for two adverse skin reactions, Steven‐Johnson syndrome and toxic epidermal necrolysis, compared with non-AED medication classes in an analysis of adverse-event data from the Food and Drug Administration Adverse Event Reporting System.
Researchers at the University of Rhode Island College of Pharmacy in Kingston, who performed the retrospective study, also found that six drugs within the antiepileptic drug (AED) class had a reporting odds ratio estimate of more than 20, compared with other non-AEDs.
“Although several antiepileptic drugs have been associated with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), the class effect and impact of other AEDs are not well described,” Eric P. Borrelli, PharmD, and his colleagues reported in Epilepsia.
The investigators examined rates of SJS and TEN for several AEDs using adverse event data from the Food and Drug Administration Adverse Event Reporting System between July 2014 and December 2017. The study investigators examined 198 adverse reaction reports related to AEDs, which was greater than any other drug class.
Overall, AEDs as a group had a reporting odds ratio risk estimate of 8.7 (95% confidence interval, 7.5-10.2), compared with non-AEDs. Similarly, the proportional reporting ratio was found to be 8.7 (95% CI, 7.5-10.2) in the AED group.
Within the class, the medications with the highest risk were zonisamide, rufinamide, and clorazepate, which had about 70-, 60-, and 56-fold higher odds for SJS and TEN, compared with all other medications. Other high-risk AEDs in the group included lamotrigine (reporting odds ratio, 53.0), carbamazepine (reporting OR, 24.5), and phenytoin (reporting OR, 26.3).
“Greater than 90% of SJS [and] TEN reactions associated with AEDs occur within the first 2 months of treatment initiation, although some AEDs have been associated with such reactions during long‐term use,” the researchers wrote.
The authors acknowledged that measures of prevalence and incidence could not be determined from these data since the number of patients taking AEDs is unknown.
“Increased awareness of this risk among both prescribers and patients, particularly variations in risk among different AEDs, along with education on early recognition of SJS [and] TEN signs [and] symptoms, may help mitigate the number and severity of these adverse events,” the researchers concluded.
The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
SOURCE: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
FROM EPILEPSIA
Key clinical point:
Major finding: The reporting odds ratio risk estimate for SJS and TEN in the AED group was 8.7 (95% CI, 7.5-10.2), compared with non-AEDs.
Study details: A retrospective analysis of 198 adverse reaction reports from the Food and Drug Administration Adverse Event Reporting System.
Disclosures: The study was partially funded by the Department of Veterans Affairs. One coauthor reported receiving research funding from Pfizer, Merck (Cubist), and The Medicines Company.
Source: Borrelli EP et al. Epilepsia. 2018 Nov 5. doi: 10.1111/epi.14591.
How do hospital medicine groups deal with staffing shortages?
Persistent demand for hospitalists nationally
During the last two decades, the United States health care labor market had an almost insatiable appetite for hospitalists, driving the specialty from nothing to over 50,000 members. Evidence of persistent demand for hospitalists abounds in the freshly released 2018 State of Hospital Medicine (SoHM) report: rising salaries, growing responsibility for the overall hospital census, and a diversifying scope of services.
The SoHM offers fascinating and detailed insights into these trends, as well as hundreds of other aspects of the field’s growth. Unfortunately, this expanding and dynamic labor market has a challenging side for hospitals, management companies, and hospitalist group leaders – we are constantly recruiting and dealing with open positions!
As a multisite leader at an academic health system, I’m looking toward the next season of recruitment with excitement. In the fall and winter we’re fortunate to receive applications from the best and brightest graduating residents and hospitalists. I realize this is a blessing, particularly compared with programs in rural areas that may not hear from many applicants. However, even when we succeed at filling the openings, there is an inevitable trickle of talent out of our clinical labor pool during the spring and summer. One person is invited to spend 20% of their time leading a teaching program, another secures a highly coveted grant, and yet another has to move because their spouse is relocated. By then, we don’t have a packed roster of applicants and have to solve the challenge in other ways. What does the typical hospital medicine program do when faced with this circumstance?
The 2018 SoHM survey first asked program leaders whether they had open and unfilled physician positions during the last year because of turnover, growth, or other factors. On average, 66% of groups serving adults and 48% of groups serving children said “yes.” For the job seekers out there, take note of some important regional differences: The regions with the highest percentage of programs dealing with unfilled positions were the East and West Coasts at 79% and 73%, respectively.
Next, the survey asked respondents to describe the percentage of total approved physician staffing that was open or unfilled during the year. On average, 12% of positions went unfilled, with important variation between different types of employers. For a typical HM group with 15 full-time equivalents, that means constantly working short two physicians!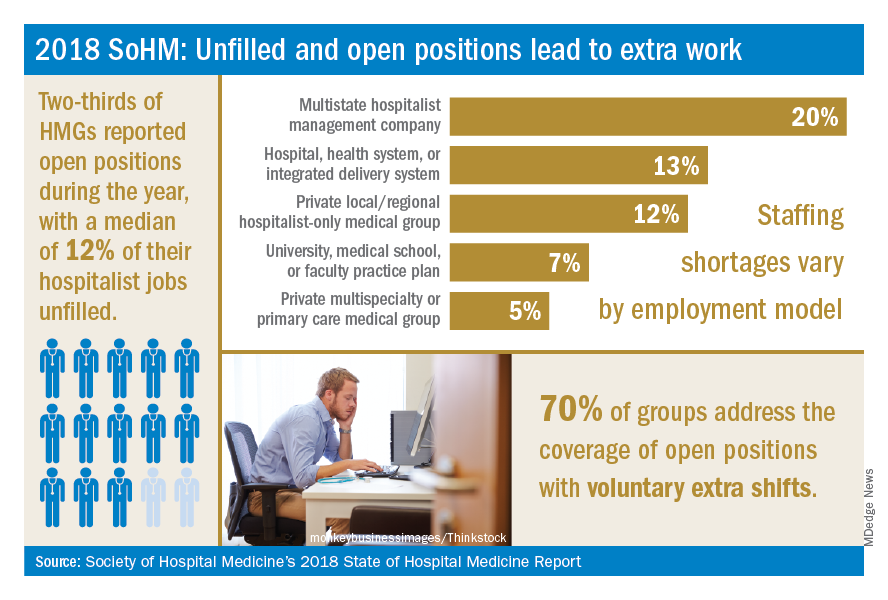
Not only is it hard for group leaders to manage chronic understaffing, it definitely takes a toll on the group. We asked leaders to describe all of the ways their groups address coverage of the open positions. The most common tactics were for existing hospitalists to perform voluntary extra shifts (70%) and the use of moonlighters (57%). Also important were the use of locum tenens physicians (44%) and just leaving some shifts uncovered (31%).
The last option might work in a large group, where everyone can pick up an extra couple of patients, but it nonetheless degrades continuity and care progression. In a small group, leaving shifts uncovered sounds like a recipe for burnout and unsafe care – hopefully subsequent surveys will find that we can avoid that approach! Obviously, the solutions must be tailored to the group, their resources, and the alternative sources of labor available in that locality.
The SoHM report provides insight into how this is commonly handled by different employers and in different regions – we encourage anyone who is interested to purchase the report (www.hospitalmedicine.org/sohm) to dig deeper. For better or worse, the issue of unfilled positions looks likely to persist for the intermediate future. The exciting rise of hospital medicine against the backdrop of an aging population means job security, rising income, and opportunities for many to live where they choose. Until the job market saturates, though, we’ll all find ourselves looking at email inboxes with a request or two to pick up an extra shift!
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
Reference
Society of Hospital Medicine. 2018 State of Hospital Medicine Report. pp. 89, 90, 181, 152.
Persistent demand for hospitalists nationally
Persistent demand for hospitalists nationally
During the last two decades, the United States health care labor market had an almost insatiable appetite for hospitalists, driving the specialty from nothing to over 50,000 members. Evidence of persistent demand for hospitalists abounds in the freshly released 2018 State of Hospital Medicine (SoHM) report: rising salaries, growing responsibility for the overall hospital census, and a diversifying scope of services.
The SoHM offers fascinating and detailed insights into these trends, as well as hundreds of other aspects of the field’s growth. Unfortunately, this expanding and dynamic labor market has a challenging side for hospitals, management companies, and hospitalist group leaders – we are constantly recruiting and dealing with open positions!
As a multisite leader at an academic health system, I’m looking toward the next season of recruitment with excitement. In the fall and winter we’re fortunate to receive applications from the best and brightest graduating residents and hospitalists. I realize this is a blessing, particularly compared with programs in rural areas that may not hear from many applicants. However, even when we succeed at filling the openings, there is an inevitable trickle of talent out of our clinical labor pool during the spring and summer. One person is invited to spend 20% of their time leading a teaching program, another secures a highly coveted grant, and yet another has to move because their spouse is relocated. By then, we don’t have a packed roster of applicants and have to solve the challenge in other ways. What does the typical hospital medicine program do when faced with this circumstance?
The 2018 SoHM survey first asked program leaders whether they had open and unfilled physician positions during the last year because of turnover, growth, or other factors. On average, 66% of groups serving adults and 48% of groups serving children said “yes.” For the job seekers out there, take note of some important regional differences: The regions with the highest percentage of programs dealing with unfilled positions were the East and West Coasts at 79% and 73%, respectively.
Next, the survey asked respondents to describe the percentage of total approved physician staffing that was open or unfilled during the year. On average, 12% of positions went unfilled, with important variation between different types of employers. For a typical HM group with 15 full-time equivalents, that means constantly working short two physicians!
Not only is it hard for group leaders to manage chronic understaffing, it definitely takes a toll on the group. We asked leaders to describe all of the ways their groups address coverage of the open positions. The most common tactics were for existing hospitalists to perform voluntary extra shifts (70%) and the use of moonlighters (57%). Also important were the use of locum tenens physicians (44%) and just leaving some shifts uncovered (31%).
The last option might work in a large group, where everyone can pick up an extra couple of patients, but it nonetheless degrades continuity and care progression. In a small group, leaving shifts uncovered sounds like a recipe for burnout and unsafe care – hopefully subsequent surveys will find that we can avoid that approach! Obviously, the solutions must be tailored to the group, their resources, and the alternative sources of labor available in that locality.
The SoHM report provides insight into how this is commonly handled by different employers and in different regions – we encourage anyone who is interested to purchase the report (www.hospitalmedicine.org/sohm) to dig deeper. For better or worse, the issue of unfilled positions looks likely to persist for the intermediate future. The exciting rise of hospital medicine against the backdrop of an aging population means job security, rising income, and opportunities for many to live where they choose. Until the job market saturates, though, we’ll all find ourselves looking at email inboxes with a request or two to pick up an extra shift!
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
Reference
Society of Hospital Medicine. 2018 State of Hospital Medicine Report. pp. 89, 90, 181, 152.
During the last two decades, the United States health care labor market had an almost insatiable appetite for hospitalists, driving the specialty from nothing to over 50,000 members. Evidence of persistent demand for hospitalists abounds in the freshly released 2018 State of Hospital Medicine (SoHM) report: rising salaries, growing responsibility for the overall hospital census, and a diversifying scope of services.
The SoHM offers fascinating and detailed insights into these trends, as well as hundreds of other aspects of the field’s growth. Unfortunately, this expanding and dynamic labor market has a challenging side for hospitals, management companies, and hospitalist group leaders – we are constantly recruiting and dealing with open positions!
As a multisite leader at an academic health system, I’m looking toward the next season of recruitment with excitement. In the fall and winter we’re fortunate to receive applications from the best and brightest graduating residents and hospitalists. I realize this is a blessing, particularly compared with programs in rural areas that may not hear from many applicants. However, even when we succeed at filling the openings, there is an inevitable trickle of talent out of our clinical labor pool during the spring and summer. One person is invited to spend 20% of their time leading a teaching program, another secures a highly coveted grant, and yet another has to move because their spouse is relocated. By then, we don’t have a packed roster of applicants and have to solve the challenge in other ways. What does the typical hospital medicine program do when faced with this circumstance?
The 2018 SoHM survey first asked program leaders whether they had open and unfilled physician positions during the last year because of turnover, growth, or other factors. On average, 66% of groups serving adults and 48% of groups serving children said “yes.” For the job seekers out there, take note of some important regional differences: The regions with the highest percentage of programs dealing with unfilled positions were the East and West Coasts at 79% and 73%, respectively.
Next, the survey asked respondents to describe the percentage of total approved physician staffing that was open or unfilled during the year. On average, 12% of positions went unfilled, with important variation between different types of employers. For a typical HM group with 15 full-time equivalents, that means constantly working short two physicians!
Not only is it hard for group leaders to manage chronic understaffing, it definitely takes a toll on the group. We asked leaders to describe all of the ways their groups address coverage of the open positions. The most common tactics were for existing hospitalists to perform voluntary extra shifts (70%) and the use of moonlighters (57%). Also important were the use of locum tenens physicians (44%) and just leaving some shifts uncovered (31%).
The last option might work in a large group, where everyone can pick up an extra couple of patients, but it nonetheless degrades continuity and care progression. In a small group, leaving shifts uncovered sounds like a recipe for burnout and unsafe care – hopefully subsequent surveys will find that we can avoid that approach! Obviously, the solutions must be tailored to the group, their resources, and the alternative sources of labor available in that locality.
The SoHM report provides insight into how this is commonly handled by different employers and in different regions – we encourage anyone who is interested to purchase the report (www.hospitalmedicine.org/sohm) to dig deeper. For better or worse, the issue of unfilled positions looks likely to persist for the intermediate future. The exciting rise of hospital medicine against the backdrop of an aging population means job security, rising income, and opportunities for many to live where they choose. Until the job market saturates, though, we’ll all find ourselves looking at email inboxes with a request or two to pick up an extra shift!
Dr. White is associate professor of medicine at the University of Washington, Seattle. He is the chair of SHM’s Practice Analysis Committee.
Reference
Society of Hospital Medicine. 2018 State of Hospital Medicine Report. pp. 89, 90, 181, 152.