User login
Hepatitis C among the mentally ill: Review and treatment update
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
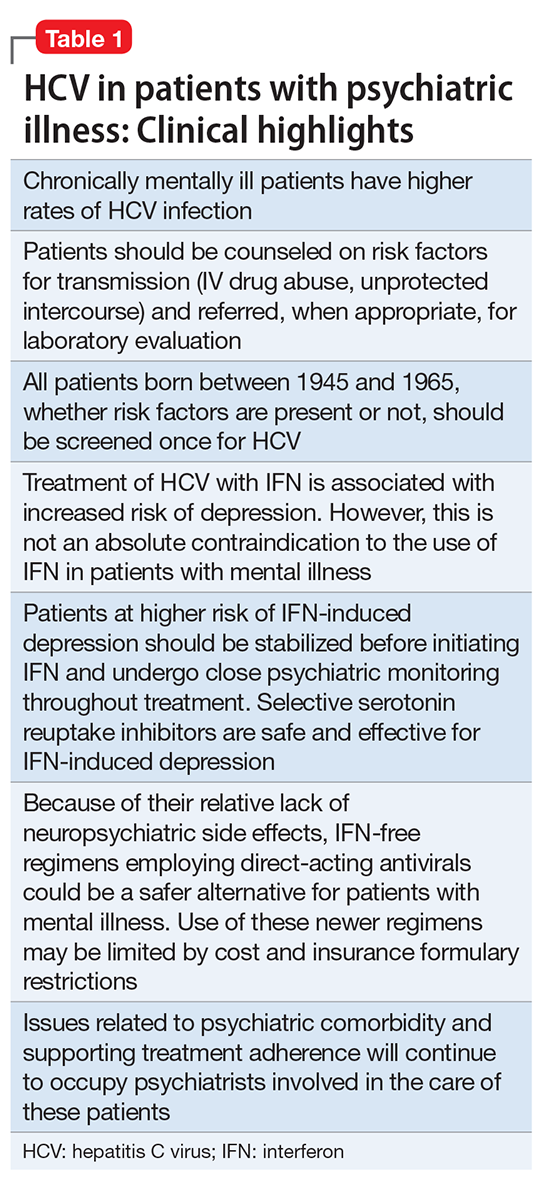
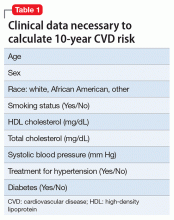
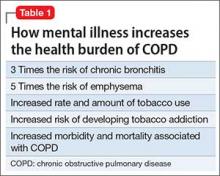
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
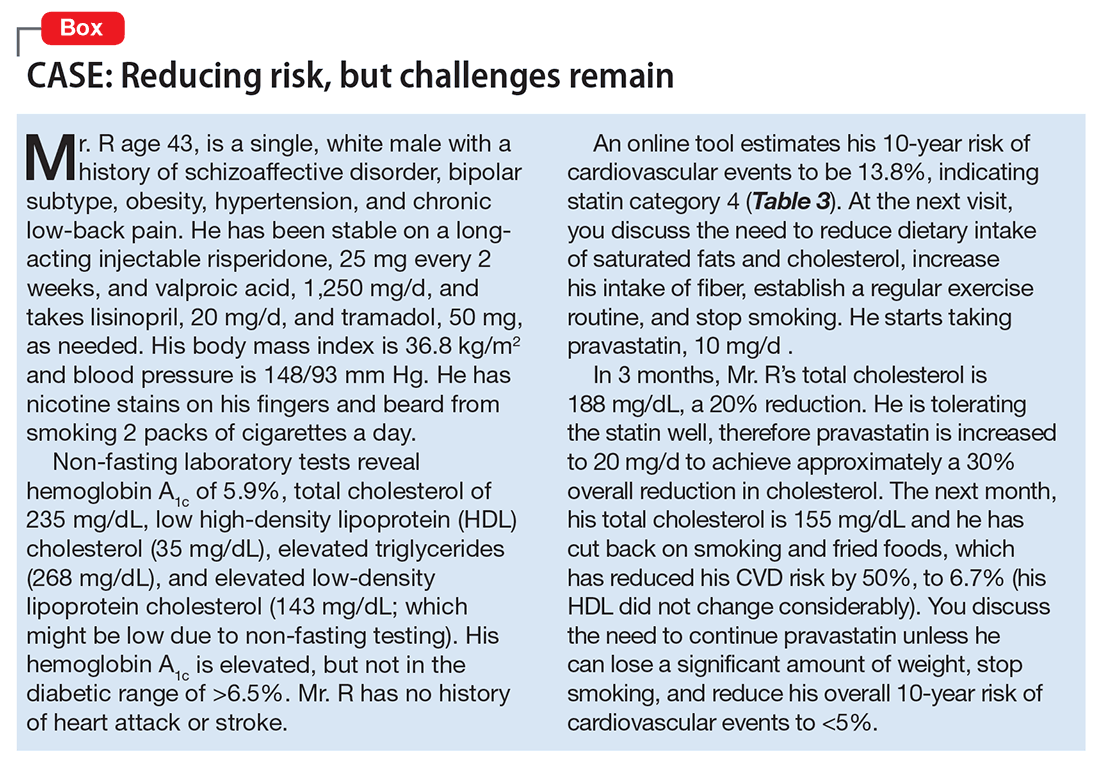
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
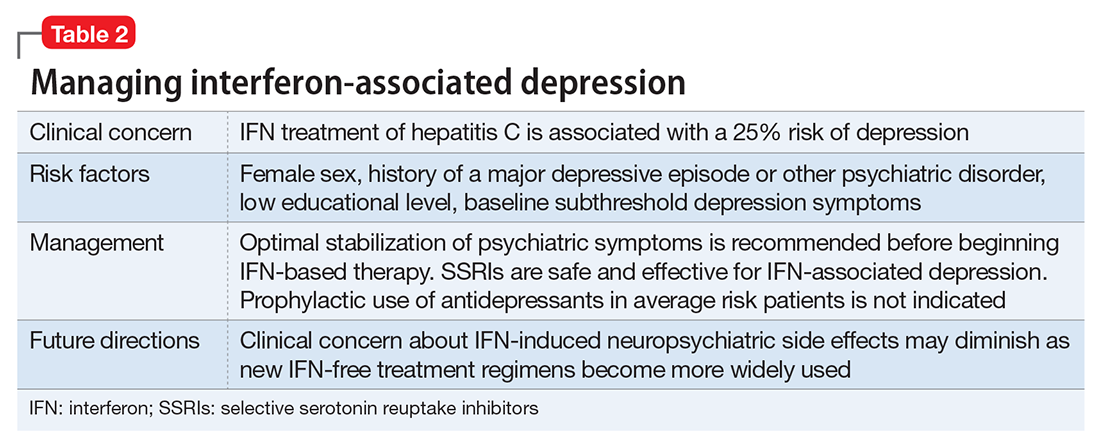
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
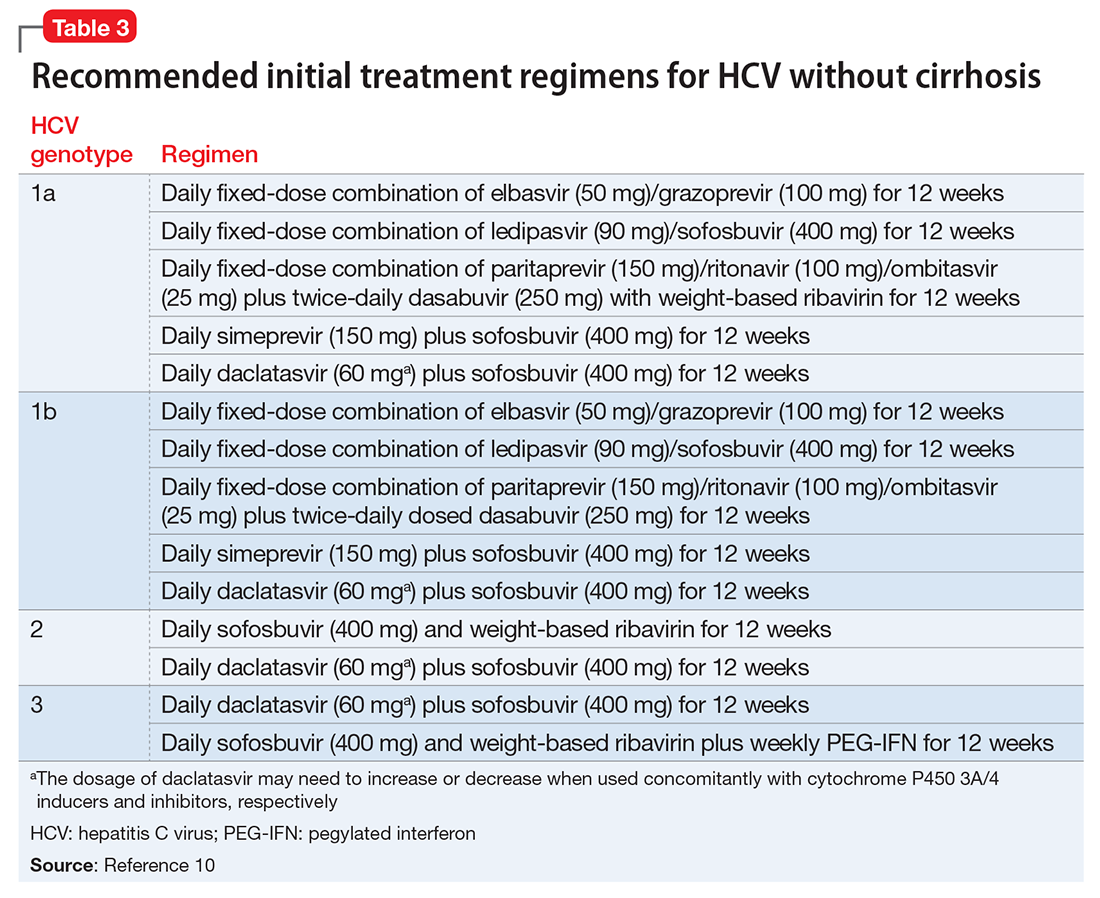
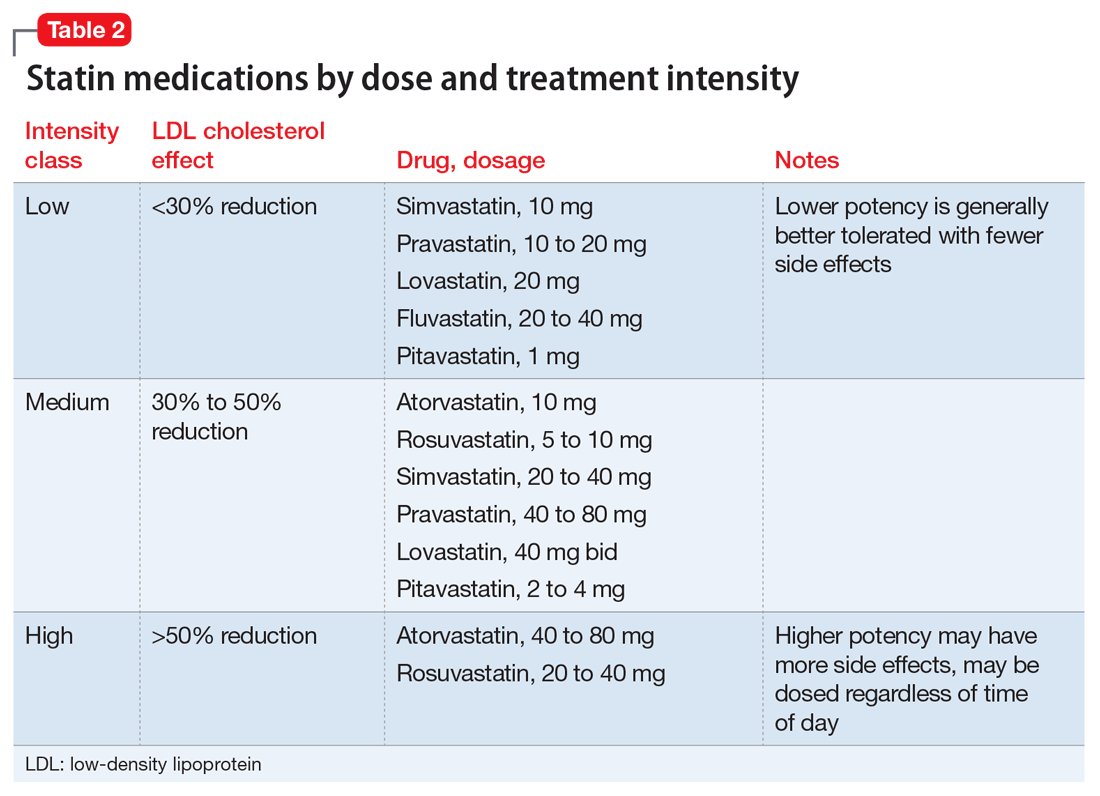
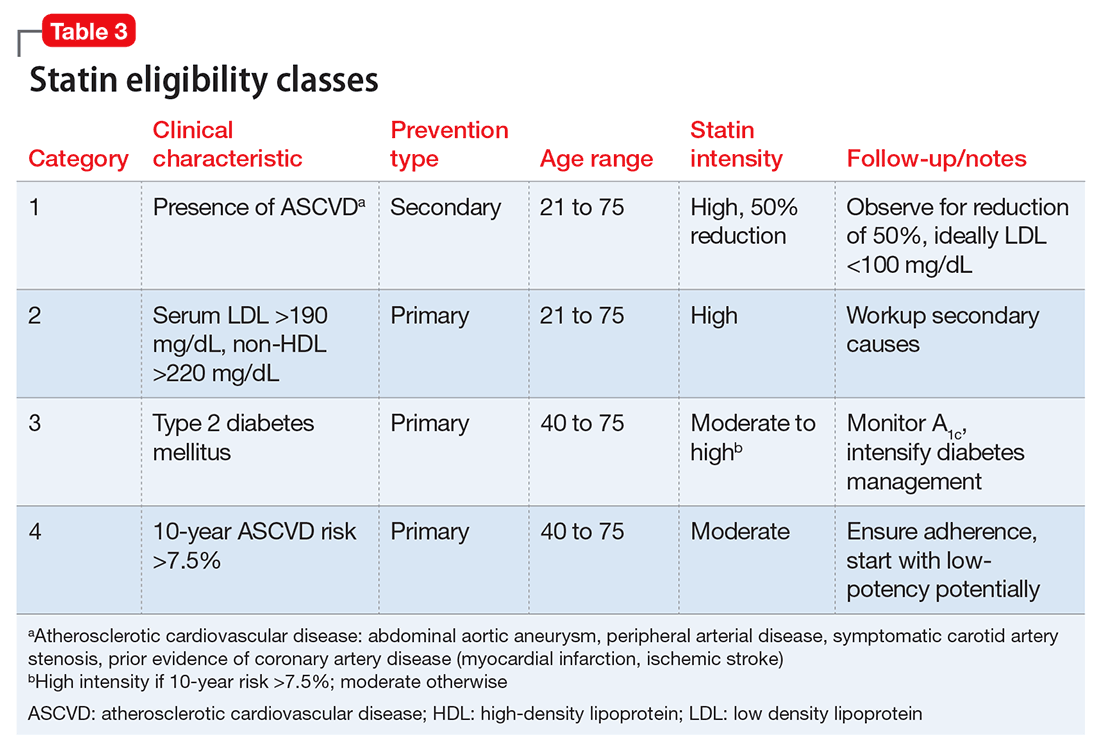
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
At approximately 3 to 4 million patients, hepatitis C virus (HCV) is the most common viral hepatitis in the United States. Patients with mental illness are disproportionately affected by HCV and the management of their disease poses particular challenges.
HCV is commonly transmitted via IV drug use and blood transfusions; transmission through sexual contact is rare. Most patients with HCV are asymptomatic, although some do develop symptoms of acute hepatitis. Most HCV infections become chronic, with a high incidence of liver failure requiring liver transplantation.
Hepatitis refers to inflammation of the liver, which could have various etiologies, including viral infections, alcohol abuse, or autoimmune disease. Viral hepatitis refers to infection from 5 distinct groups of virus, coined A through E.1 This article will focus on chronic HCV (Table 1).
CASE Bipolar disorder, stress, history of IV drug use
Ms. S, age 48, has bipolar I disorder and has been hospitalized 4 times in the past, including once for a suicide attempt. She has 3 children and works as a cashier. Her psychiatric symptoms have been stable on lurasidone, 80 mg/d, and escitalopram, 10 mg/d. Recently, Ms. S has been under more stress at her job. Sometimes she misses doses of her medication, and then becomes more irritable and impulsive. Her husband, noting that she has used IV heroin in the past, comes with her today and is concerned that she is “not acting right.” What is Ms. S’s risk for HCV?
HCV in mental illness
Compared with the general population, HCV is more prevalent among chronically mentally ill persons. In one study, HCV occurred twice as often in men vs women with chronic mental illness.2 Up to 50% of patients with HCV have a history of mental illness and nearly 90% have a history of substance use disorders.3 Among 668 chronically mentally ill patients at 4 public sector clinics, risk factors for HCV were common and included use of injection drugs (>20%), sharing needles (14%), and crack cocaine use (>20%).4 Higher rates of HCV were reported in hospitalized patients with schizophrenia and comorbid psychoactive substance abuse in Japan.5 Because of the high prevalence in this population, it is essential to assess for substance use disorders. Employing a non-judgmental approach with motivational interviewing techniques can be effective.6
Individuals with mental illness should be screened for HCV risk factors, such as unprotected intercourse with high-risk partners and sharing needles used for illicit drug use. Patients frequently underreport these activities. At-risk individuals should undergo laboratory testing for the HIV-1 antibody, hepatitis C antibodies, and hepatitis B antibodies. Mental health providers should counsel patients about risk reduction (eg, avoiding unprotected sexual intercourse and sharing of drug paraphernalia). Educating patients about complications of viral hepatitis, such as liver failure, could be motivation to change risky behaviors.
CASE continued
During your interview with Ms. S, she becomes irritable and tells you that you are asking too many questions. It is clear that she is not taking her medications consistently, but she agrees to do so because she does not want to lose custody of her children. She denies current use of heroin but her husband says, “I don’t know what she is doing.” In addition to advising her on reducing risk factors, you order appropriate screening tests, including hepatitis and HIV antibody tests.
Screening guidelines
The U.S. Preventive Services Task Force and the CDC both recommend a 1-time screening for HCV in asymptomatic or low-risk patients born between 1945 and 1965.1,7 Furthermore, both organizations recommend screening for HCV in persons at high risk, including:
- those with a history of injection drug use
- persons with recognizable exposure, such as needlesticks
- persons who received blood transfusions before 1992
- medical conditions, such as long-term dialysis.
There is no vaccine for HCV; however, patients with HCV should receive vaccination against hepatitis B.
Diagnosis
Acute symptoms include fever, fatigue, headache, cough, nausea, and vomiting. Jaundice could develop, often accompanied by pain in the right upper quadrant. If there is suspicion of viral hepatitis, psychiatrists can initiate the laboratory evaluation. Chronic hepatitis, on the other hand, often is asymptomatic, although stigmata of chronic liver disease (eg, jaundice, ascites, peripheral edema) might be detected on physical exam.8 Elevated serum transaminases are seen with acute viral hepatitis, although levels could vary in chronic cases. Serologic detection of anti-HCV antibodies establishes a HCV diagnosis.
Treatment recommendations
All patients who test positive for HCV should be evaluated and treated by a hepatologist. Goals of therapy are to reduce complications from chronic viral hepatitis, including cirrhosis and hepatic failure. Duration and optimal regimen depends on the HCV genotype.8 Treatment outcomes are measured by virological parameters, including serum aminotransferases, HCV RNA levels, and histology. The most important parameter in treating chronic HCV is the sustained virological response (SVR), which is the absence of HCV RNA 12 weeks after completing therapy.9
Treatment is recommended for all persons with chronic HCV infection, according to current treatment guidelines, which are updated regularly by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America.10 Until recently, treatment consisted of IV pegylated interferon (PEG-IFN) in combination with oral ribavirin. Success rates with this regimen are approximately 40% to 50%. The advent of direct-acting antivirals (DAAs) has revolutionized treatment of chronic HCV. These agents include simeprevir, sofosbuvir, ledipasvir, and the combination of ombitasvir-paritaprevir-ritonavir plus dasabuvir (brand name, Viekira Pak). Advantages of these agents are oral administration, high treatment success rates (>90%), shorter treatment duration (12 weeks vs up to 48 weeks with older regimens), and few serious adverse effects9-11; drawbacks include the pricing of these regimens, which could cost upward of ≥$100,000 for a 12-week course, and a lack of coverage under some health insurance plans.12 The manufacturers of 2 agents, telaprevir and boceprevir, removed them from the market because of decreased demand related to their unfavorable side-effect profile and the availability of better tolerated agents.
Treatment considerations for interferon in psychiatric patients
Various neuropsychiatric symptoms have been reported with the use of PEG-IFN. The range of reported symptoms include:
- depressed mood
- anxiety
- hostility
- slowness
- fatigue
- sleep disturbance
- lethargy
- irritability
- emotional lability
- social withdrawal
- poor concentration.13,14
Depressive symptoms can present as early as 1 month after starting treatment, but typically occur at 8 to 12 weeks. A systematic review and meta-analysis of 26 observational studies found a cumulative 25% risk of interferon (IFN)-induced depression in the general HCV population.15 Risk factors for IFN-induced depression include:
- female sex
- history of major depression or other psychiatric disorder
- low educational level
- the presence of baseline subthreshold depressive symptoms.
Because of the risk of inducing depression, there was initial hesitation with providing IFN treatment to patients with psychiatric disorders. However, there is evidence that individuals with chronic psychiatric illness can be treated safely with IFN-based regimens and achieve results similar to non-psychiatric populations.16,17 For example, patients with schizophrenia in a small Veterans Affairs database who received IFN for HCV did not experience higher rates of symptoms of schizophrenia, depression, or mania over 8 years of follow-up.18 Furthermore, those with schizophrenia were just as likely to reach SVR as patients without psychiatric illness.19 Other encouraging results have been reported in depressed patients. One study found similar rates of treatment completion and SVR in patients with a history of major depressive disorder compared with those without depression.20 No difference in frequency of neuropsychiatric side effects was found between the groups.
Presence of a psychiatric disorder is no longer an absolute contraindication to IFN treatment for HCV. Optimal control of psychiatric symptoms should be attained in all patients before starting HCV treatment, and close clinical monitoring is warranted. A review of 9 studies showed benefit of antidepressants for HCV patients with elevated baseline depression or a history of IFN-induced depression.21 The largest body of evidence supports the safety and efficacy of selective serotonin reuptake inhibitors for treating IFN-induced depression. Although no antidepressants are FDA-approved for this indication, the best-studied agents include citalopram, escitalopram, sertraline, and paroxetine.
A review of 6 studies on using antidepressants to prevent IFN-induced depression concluded there was inadequate evidence to support this approach in all patients.22 Pretreatment primarily is indicated for those with elevated depressive symptoms at baseline or those with a history of IFN-induced depression. The prevailing approach to IFN-induced depression assessment, prevention, and treatment is summarized in Table 2.
CASE continued
Ms. S tests positive for the HCV antibody but negative for HIV and hepatitis B. She immediately receives the hepatitis B vaccine series. Her sister discourages her from receiving treatment for HCV, warning her, “it will make you crazy depressed.” As a result, Ms. S avoids following up with the hepatologist. Her psychiatrist, aware that she now was taking her psychotropic medication and seeing that her mood is stable, educates her about new treatment options for HCV that do not cause depression. Ms. S finally agrees to see a hepatologist to discuss her treatment options.
IFN-free regimens
With the arrival of the DAAs, the potential now exists to use IFN-free treatment regimens,10 which could eliminate concerns about IFN-induced depression.
Clinical trials of the DAAs and real-world use so far do not indicate an elevated risk for neuropsychiatric symptoms, including depression.11 As a result, more patients with severe psychiatric illness likely will be eligible to receive treatment for HCV. However, as clinical experience builds with these new agents, it is important to monitor the experience of patients with psychiatric comorbidity. Current treatment guidelines for HCV genotype 1, which is most common in the United States, do not include IFN-based regimens.10 Treatment of genotype 3, which affects 6% of the U.S. population, still includes IFN. Therefore, the risk of IFN-induced depression still exists for some patients with HCV. Table 310 describes current treatment regimens in use for HCV without cirrhosis (see Related Resources for treating HCV with cirrhosis).
Evolving role of the psychiatrist
The availability of shorter, better-tolerated regimens means that the psychiatric contraindications to HCV treatment will be eased. With the emergence of non-IFN treatment regimens, the role of mental health providers could shift toward assisting with treatment adherence, monitoring drug–drug interactions, and managing comorbid substance use disorders.10
The psychiatrist’s role might shift away from the psychosocial assessment of factors affecting treatment eligibility, such as IFN-associated depressive symptoms. Clinical focus will likely shift to supporting adherence to HCV treatment regimens.23 Because depression and substance use disorders are risk factors for non-adherence, mental health providers may be called upon to optimize treatment of these conditions before beginning DAA regimens. A multi-dose regimen might be complicated for those with severe mental illness, and increased psychiatric and community support could be needed in these patients.23 Furthermore, models of care that integrate an HCV specialist with psychiatric care have demonstrated benefits.6,23 Long-term follow-up with a mental health provider will be key to provide ongoing psychiatric support, especially for those who do not achieve SVR.
Psychotropic drug–drug interactions with DAAs
Both sofosbuvir and ledipasvir are substrates of P-glycoprotein and not metabolized by cytochrome P450 (CYP) enzymes.24 Therefore, there are no known contraindications with psychotropic medications. However, co-administration of P-glycoprotein inducers, such as St. John’s wort, could reduce sofosbuvir and ledipasvir levels leading to reduced therapeutic efficacy.
Because it has been used for many years as an HIV treatment, drug interactions with ritonavir have been well-described. This agent is a “pan-inhibitor” and inhibits the CYP3A4, 2D6, 2C9, and 2C19 enzymes and could increase levels of any psychotropic metabolized by these enzymes.25 After several weeks of treatment, it also could induce CYP3A4, which could lead to reduced efficacy of oral contraceptives because ethinylestradiol is metabolized by CYP3A4. Ritonavir is primarily metabolized by CYP3A4 (and CYP2D6 to a smaller degree). Carbamazepine induces CYP3A4, which may lead to decreased levels of ritonavir.23 This, in turn, could reduce the likelihood of attaining SVR and successful treatment of HCV.
Boceprevir, telaprevir, and simeprevir inhibit CYP3A4 to varying degrees and therefore could affect psychotropic medications metabolized by this enzyme.23,26,27 These DAAs are metabolized by CYP3A4; therefore CYP3A4 inducers, such as carbamazepine, could lower DAA blood levels, increasing risk of HCV treatment failure and viral resistance.
Daclatasvir is a substrate of CYP3A4 and an inhibitor of P-glycoprotein.28 Concomitant buprenorphine or buprenorphine/naloxone levels may be increased, although the manufacturer does not recommend dosage adjustment. Elbasvir and grazoprevir are metabolized by CYP3A4.29 Drug–drug interactions therefore may result when administered with either CYP3A4 inducers or inhibitors.
CASE Conclusion
Ms. S sees her new hepatologist, Dr. Smith. She decides to try a 12-week course of ledipasvir/sofosbuvir. Dr. Smith collaborates frequently with Ms. S’s psychiatrist to discuss her case and to help monitor her psychiatric symptoms. She follows up closely with her psychiatrist for symptom monitoring and to help ensure treatment compliance. Ms. S does well with the IFN-free treatment regimen and experiences no worsening of her psychiatric symptoms during treatment.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
1. Centers for Disease Control and Prevention. Viral hepatitis. http://www.cdc.gov/hepatitis. Updated December 9, 2016. Accessed February 9, 2017.
2. Butterfield MI, Bosworth HB, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Gender differences in hepatitis C infection and risks among persons with severe mental illness. Psychiatr Serv. 2003;54(6):848-853.
3. Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877. doi: 10.4088/PCC.09r00877whi.
4. Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172-174.
5. Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591-597.
6. Sockalingam S, Blank D, Banga CA, et al. A novel program for treating patients with trimorbidity: hepatitis C, serious mental illness, and substance abuse. Eur J Gastroenterol Hepatol. 2013;25(12):1377-1384.
7. U.S. Preventive Services Task Force. Screening for hepatitis C virus infection: recommendation summary. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening. Published June 2013. Accessed February 9, 2017.
8. Longo DL, Fauci AS, Kasper DL. Harrison’s principles of internal medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
9. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92-102.
10. American Association for the Study of Liver Diseases (AASLD); The Infectious Diseases Society of America (IDSA). HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed February 9, 2017.
11. Rowan PJ, Bhulani N. Psychosocial assessment and monitoring in the new era of non-interferon-alpha hepatitis C treatments. World J Hepatol. 2015;7(19):2209-2213.
12. Good Rx, Inc. http://www.goodrx.com. Accessed October 9, 2015.
13. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
14. Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63(2):131-135.
15. Udina M, Castellví P, Moreno-España J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2012;73(8):1128-1138.
16. Mustafa MZ, Schofield J, Mills PR, et al. The efficacy and safety of treating hepatitis C in patients with a diagnosis of schizophrenia. J Viral Hepat. 2014;21(7):e48-e51.
17. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
18. Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403-406.
19. Huckans M, Mitchell A, Ruimy S, et al. Antiviral therapy completion and response rates among hepatitis C patients with and without schizophrenia. Schizophr Bull. 2010;36(1):165-172.
20. Hauser P, Morasco BJ, Linke A, et al. Antiviral completion rates and sustained viral response in hepatitis C patient with and without preexisting major depressive disorder. Psychosomatics. 2009;50(5):500-505.
21. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
22. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
23. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis c treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
24. Harvoni [package insert]. Foster City, CA: Gilead Sciences, Inc.; 2016.
25. Wynn GH, Oesterheld, JR, Cozza KL, et al. Clinical manual of drug interactions principles for medical practice. Arlington, VA: American Psychiatric Publishing; 2009.
26. Olysio [package insert]. Titusville, NJ: Janssen Therapeutics; 2016.
27. Victrelis [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
28. Daklinza [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2016.
29. Zepatier [package insert]. Whitehouse Station, NJ: Merck & Co.; 2017.
Evaluating the risk of sexually transmitted infections in mentally ill patients
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
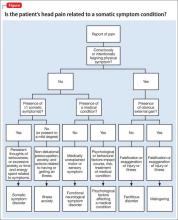
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.
Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.
Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
Sexually transmitted infections (STIs) continue to be a significant public health problem with potentially serious complications.1 The incidence of new STIs, including viral STIs, in the United States is estimated at 19 million cases per year.2Chlamydia trachomatis remains the most common bacterial STI with an estimated annual incidence of 2.8 million cases in the United States and 50 million worldwide. Second in prevalence is gonococcal infection. Herpes simplex virus is one of the most common viral STIs, but the incidence of human papillomavirus virus (HPV), which is associated with cervical cancer, has steadily increased worldwide.3 Young persons age 15 to 24 are at the highest risk of acquiring new STIs with almost 50% of new cases reported among this age group.4
STIs can have serious complications and sequelae. For example, 20% to 40% of women who have chlamydia infections and 10% to 20% of women who have gonococcal infections develop pelvic inflammatory disease (PID),2 which increases the risk for ectopic pregnancy, infertility, and chronic pelvic pain.
Patients with mental illness are at high risk of acquiring STIs. In the United States, the prevalence of HIV among patients with psychiatric illness is 10 to 20 times higher than in the general population.4,5 Factors contributing to increased vulnerability to STIs among psychiatric patients include:
- impaired autonomy
- increased impulsivity
- increased susceptibility to coerced sex.6
Furthermore, a higher incidence of poverty, placement in risky environments, and overall poor health and medical care also contribute to the high prevalence of STIs and their complications in this population (Table 1). Because of risk factors specific to psychiatric illness, standard STI prevention interventions are not always successful and novel and innovative behavioral approaches are necessary.7
Case Abdominal pain and fever
Ms. K, age 25, has a history of bipolar disorder treated with lithium and presents to the community psychiatrist with lower abdominal pain. She recently recovered from a manic episode and has started to reintegrate with the community mental health team. She refuses to see her primary care physician and is adamant that she wishes to see her psychiatrist, who is the only doctor she has rapport with.
Ms. K reports lower abdominal pain for 3 or 4 days and fever for 1 day. The pain is dull in character. She denies diarrhea, vomiting, or urinary symptoms, but on further questioning describes new-onset, foul-smelling vaginal discharge without vaginal bleeding. Her menstrual cycle usually is regular, but her last menstrual period occurred 2 months ago. Her medical history includes an appendectomy at age 10 and she is a current cigarette smoker. Chart notes taken during her manic episode describe high-risk behavior, including having unprotected sexual intercourse with several partners. On examination, she is febrile and tachycardic with a tender lower abdomen.
Diagnosing STIs
To diagnose an STI, first a clinician must consider its likelihood. Taking a thorough sexual history allows assessment of the need for further investigation and provides an opportunity to discuss risk reduction. In accordance with recent guidelines,8 all health care providers are encouraged to consider the sexual history a routine aspect of the clinical encounter. The Centers for Disease Control and Prevention’s (CDC’s) “Five Ps” approach (Table 2) is an excellent tool for guiding investigation and counseling.9
The Figure provides health care providers with an algorithm to guide testing for STIs among psychiatric patients. Note that chlamydia, gonorrhea, syphilis, chancroid, viral hepatitis, and HIV must be reported to state public health agencies and the CDC.
Modern laboratory techniques make diagnosing STIs easier. Analysis of urine or serum reduces the need for invasive sampling. If swabs are required for diagnosis, patient self-collection of urethral, vulvovaginal, rectal, or pharyngeal specimens is as accurate as clinician collected samples and is better tolerated.8 Because of variation in diagnostic assays, we recommend contacting the laboratory before sending non-standard samples to ensure accurate collection and analysis.
Guidelines for preventing and screening for STIs
There are no prevention guidelines for STIs specific to the psychiatric population, although there is a clear need for focused intervention in this vulnerable patient group.10 Rates of STI screening generally are low in the psychiatric setting,11 which results in a considerable burden of disease. All psychiatric patients should be encouraged to engage with STI screening programs that are in line with national guidelines. In the inpatient psychiatric or medical environment, clinicians have a responsibility to ensure that STI screening is considered for each patient.
Patients with mental illness should be assumed to be sexually active, even if they do not volunteer this information to clinicians. Employ a low threshold for recommending safer sex practices including condom use. Encourage women to develop a relationship with a family practitioner, internist, or gynecologist. Advise men who have sex with men (MSM) to visit a doctor regularly for screening of HIV and rectal, anal, and oral STIs as behavior and symptoms dictate.
There is general agreement about STI screening among the United States Preventive Services Task Force (USPSTF), CDC, American Academy of Family Physicians, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. USPSTF guidelines are summarized in Table 3.12
In addition to these guidelines, the CDC suggests that all adults and adolescents be tested at least once for HIV.13 The CDC also recommends annual testing of MSM for HIV, syphilis, chlamydia, and gonorrhea. In MSM who have multiple partners or who have sex while using illicit drugs, testing should occur more frequently, such as every 3 to 6 months.14
HPV. Routine HPV screening is not recommended; however, 2 vaccines are available to prevent oncogenic HPV (types 16 and 18). All females age 13 to 26 should receive 3 doses of HPV vaccine over a 6-month period. The quadrivalent vaccine (Gardasil) also protects against HPV types 6 and 11, which cause 90% of genital warts and is preferred when available. Males age 9 to 26 also can receive the vaccine, although ideally it should be administered before sexual activity begins.15 Women still should attend routine cervical cancer screening even if they have the vaccine because 30% of cervical cancers are not caused by HPV 16/18. However, this means that 70% of cervical cancers are associated with HPV 16/18, making screening and the vaccine an important public health initiative. There also is a link between HPV and oral cancers.
Treating STIs among mentally ill individuals
Treatment of STIs among mentally ill individuals is important to prevent medical complications and to reduce transmission. Here are a few additional questions to keep in mind when treating a patient with psychiatric illness:
Does the patient have a primary psychiatric disorder, or is the patient’s current psychiatric presentation a result of the infection?
Some STIs can manifest with psychiatric symptoms—for example, neurosyphilis and HIV-associated neurocognitive disorders—and pose a diagnostic challenge. Obtaining a longitudinal history of the patient’s mental health, age of onset, and family history can help clarify the cause.
Are there any psychiatric adverse effects of STI treatment?
Most drugs used for treating common STIs are not known to cause psychiatric adverse effects (See the American Psychiatric Association16 and Sockalingham et al17 for a thorough discussion of HIV and hepatitis C treatment). The exception is fluoroquinolones, which could be prescribed for PID if cephalosporin therapy is not feasible. CNS effects of fluoroquinolones include insomnia, restlessness, confusion, and, in rare cases, mania and psychosis.
What are possible medication interactions to keep in mind when treating a psychiatric patient?
Nonsteroidal anti-inflammatory drugs (NSAIDs), other than sulindac, could increase serum lithium levels. Although NSAIDs are not contraindicated in patients taking lithium, other pain relievers, such as acetaminophen, may be preferred as a first-line choice.
Carbamazepine could lower serum levels of doxycycline.18
Azithromycin and other macrolides, as well as fluoroquinolones, could have QTc prolonging effects and has been associated with torsades de pointes.19 Several psychiatric medications, in particular, atypical antipsychotics, also could prolong the QTc interval. This could be a consideration in patients with underlying long QT intervals at baseline or a family history of sudden cardiac death.
Psychiatric patients might refuse or not adhere to their medication. Refusals could be the result of grandiose delusions (“I don’t need treatment”) or paranoia (“The doctor is trying to poison me”). Consider 1-time doses of antibiotics that can be given in the clinic for uncomplicated infections when adherence is an issue. Because psychiatric patients are at higher risk for acquiring STIs, education and counseling—especially substance abuse counseling—are vital as both primary and secondary prevention strategies. Treatment of STIs should be accompanied by referrals to the social work team or a therapist when appropriate.
Finally, as with any proposed treatment, it is important to consider whether the patient has capacity to consent to or refuse treatment. To assess for capacity, a patient must be able to:
- communicate a choice
- understand the relevant information
- appreciate the medical consequences of the decision
- demonstrate the ability to reason about treatment choices.20
Case continued
In the emergency department, Ms. K’s vital signs are: temperature 39.5°C; pulse 110 beats per minute; blood pressure 96/67 mm Hg; and breathing 20 respirations per minute. She complains of nausea and has 2 episodes of emesis. She allows clinicians to perform a complete physical examination, including pelvic exam. Her cervix is inflamed, and she is noted to have adnexal and cervical motion tenderness.
Labs and imaging confirm a diagnosis of PID due to gonorrhea and she is admitted to the hospital for IV antibiotics. She continues to experience nausea and vomiting, but also complains of dizziness and diarrhea. Her speech is slurred and a coarse tremor is noticed in her hands. Renal function tests show slight impairment, probably due to dehydration. A pregnancy test is negative.
Lithium is held. Her nausea, vomiting, and diarrhea resolve quickly, and Ms. K asks to leave. When she is told that she is not ready for discharge, Ms. K becomes upset and rips out her IV yelling, “I don’t need treatment from you guys!” A psychiatry consult is called to assess for her capacity to refuse treatment. The team determines that she has capacity, but she becomes agreeable to remaining in the hospital after a phone conversation with her community mental health team.
Ms. K improves with antibiotic treatment. HIV and syphilis serology tests are negative. Before discharge, both the community psychiatrist and her primary care physicians are informed her lithium was held during hospitalization and restarted before discharge. Ms. K also is educated about the signs and symptoms of lithium toxicity, as well as common STIs.
Clinical considerations
- Physicians should have a low threshold of suspicion for PID in a sexually active young woman who presents with abdominal pain and shuffling gait, which is a natural attempt to reduce cervical irritation and is associated with PID.
- Ask about sexual history and symptoms of STIs.
- Rule out STIs in men presenting with urinary tract infections.
- If chlamydia is diagnosed, treatment for gonorrhea also is essential, and vice versa.
- Always think about HIV and hepatatis B and C in a patient with a STI.
- Treatment with single-dose medications can be effective.
- Risk of STIs is higher during episodes of mania or psychosis.
- Consider hospitalization if medically indicated or if you suspect non-adherence to therapy. It is important to remember that all kinds of systemic infections—including PID—can result in dehydration and alter renal metabolism leading to lithium accumulation.
- Mentally ill patients might require placement under involuntary commitment if they are found to be a danger to themselves or others. It is important to liaise with both the community psychiatry team and primary care physician both during hospitalization and before discharge to ensure a smooth transition.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
1. Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80(4):255-263.
2. Trigg BG, Kerndt PR, Aynalem G. Sexually transmitted infections and pelvic inflammatory disease in women. Med Clin North Am. 2008;92(5):1083-1113, x.
3. Frenkl TL, Potts J. Sexually transmitted infections. Urol Clin North Am. 2008;35(1):33-46; vi.
4. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6-10.
5. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
6. King C, Feldman J, Waithaka Y, et al. Sexual risk behaviors and sexually transmitted infection prevalence in an outpatient psychiatry clinic. Sex Transm Dis. 2008;35(10):877-882.
7. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8-12.
8. Freeman AH, Bernstein KT, Kohn RP, et al. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38(11):1036-1039.
9. Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
10. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care. 2004;10(12):917-924.
11. Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534-537.
12. Meyers D, Wolff T, Gregory K, et al. USPSTF recommendations for STI screening. Am Fam Physician. 2008;77(6):819-824.
13. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17; quiz CE1-CE 4.
14. Centers for Disease Control and Prevention. Incidence, prevalence, and cost of sexually transmitted infections in the United States. https://npin.cdc.gov/publication/incidence-prevalence-and-cost-sexually-transmitted-infections-united-states. Published February 2013. Accessed December 12, 2016.
15. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705-1708.
16. American Psychiatric Association. HIV psychiatry. https://www.psychiatry.org/psychiatrists/practice/professional-interests/hiv-psychiatry. Accessed December 13, 2016.
17. Sockalingam S, Sheehan K, Feld JJ, et al. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry. 2015;172(6):512-516.
18. Neuvonen PJ, Pentikäinen PJ, Gothoni G. Inhibition of iron absorption by tetracycline. Br J Clin Pharmacol. 1975;2(1):94-96.
19. Sears SP, Getz TW, Austin CO, et al. Incidence of sustained ventricular tachycardia in patients with prolonged QTc after the administration of azithromycin: a retrospective study. Drugs Real World Outcomes. 2016;3:99-105.
20. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
How to assess and manage high cholesterol in patients with mental illness
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16
Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18
Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16
Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18
Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).
High serum cholesterol is a leading cause of heart attack and stroke,1,2 yet remains one of the most under-screened and undertreated modifiable risk factors in persons with mental illness. Well tolerated and effective treatments can considerably lower the risk of cardiovascular events, and should be offered to psychiatric patients who are at high risk, while considering possible adverse effects and potential interactions between psychotropics and medications used to lower cholesterol.
Systematic lowering of total cholesterol and, particularly, atherogenic low-density lipoprotein (LDL) and non-high density lipoprotein (HDL) cholesterol, results in consistent and significant reduction in risk of cardiovascular events in persons at risk for developing cardiovascular disease (CVD) and in preventing reoccurrence of these events.1,3,4 Even individuals who have relatively lower levels of total cholesterol but are at high risk (such as if a cardiovascular event has occurred) could reduce their CVD risk (known as secondary prevention) through lipid lowering therapies.5,6
Adults with psychiatric illness shoulder a disproportionate burden of CVD morbidity and mortality, especially those with severe mental illness (SMI, schizophrenia, schizoaffective disorder, bipolar disorder, treatment-resistant depression).7-9 Among modifiable CVD risk factors, dyslipidemia has the highest rates of missed screenings and treatment within psychiatric populations. In one analysis, up to 90% of adults with SMI and identified lipid disorders did not receive treatment.10 Persons with SMI generally do not receive guideline-concordant, systematic quality preventive care, which contributes to a widening mortality gap for this population.11,12
This review aims to provide clinicians with practical guidance on the assessment and management of high cholesterol to improve recognition and treatment, lower CVD risk, and reduce this observed mortality gap.
Screening and diagnosis
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) released updated guidelines on diagnosing and managing high cholesterol to reduce CVD risk.1 These guidelines focus on updated 10-year CVD risk assessment models with treatment goals reliant on adherence to statin therapy rather than pre-specified cholesterol targets listed in previous guidelines.13
Updates to assessment and treatment guidelines have removed some barriers to screening and diagnosing high cholesterol—namely, fasting lipid panels are no longer required to determine 10-year CVD risk and initiate treatment.14 For adults taking a second-generation antipsychotic that is associated with weight gain and metabolic syndrome, experts generally recommend yearly non-fasting lipid panels.6,14
The United States Preventive Services Task Force recommends screening:
- men age ≥35 at average risk for CVD every 5 years
- women age ≥45 every 5 years15
- adults as young as age 20 who have accelerated risk factors, such as cigarette smoking and hypertension
- adults with a family history of heart attack or stroke in male first-degree relative age ≥50 and female first-degree relatives age ≥60.
Many adults receiving care in behavioral health settings, regardless of their medication regimen, qualify for screening at least every 5 years, if not more frequently. Although statin treatment before age 40 is less beneficial and likely not necessary for primary prevention, monitoring could help identify alternative therapies and prioritize more intensive diet and lifestyle modifications.
At a routine office visit, clinicians can collect vital signs, record smoking status, and reconcile all medications, which provides the data needed to calculate a patient’s 10-year CVD risk (Table 1). Coupled with laboratory testing, which includes a non-fasting total cholesterol, HDL, and hemoglobin A1c (representative of a 3-month blood sugar average, ≥6.5% is diagnostic of type 2 diabetes mellitus [T2DM]), all data points can be entered into online risk calculators (search “ASCVD risk calculator” or visit http://tools.acc.org/ASCVD-Risk-Estimator to access the ACC/AHA risk calculator). Persons scoring >20% 10-year risk are considered at extremely high risk, and are in the same risk category as adults with existing CVD or who have had a cardiovascular event. Persons at <5% 10-year risk generally are considered low risk, and primary prevention with a statin medication is not indicated.
Treatment and management
Dietary modification and lifestyle changes (exercise, quitting smoking), lowering high cholesterol with medications, and switching from highly metabolically active drugs to less metabolically active ones can help lower total cholesterol in patients at risk of CVD.
Statins
HMG-CoA reductase inhibitors (statins) consistently reduce total cholesterol and non-HDL cholesterol by 30% to 50%, depending on drug and dosage (potency, listed as low, medium, and high). Not all statins are equally effective at lowering cholesterol; some are more potent than others (Table 2).16
Individuals are eligible for statin therapy based on their level of CVD risk. Persons at higher risk generally benefit from greater intensity statin treatment and cholesterol reduction; highest intensity statin regimens can lower total cholesterol by approximately 50%.
There are 4 statin eligibility classes (Table 3). Most adults fall into category 4: 10-year risk of >7.5% and needing primary prevention. In addition to removing specific LDL targets as therapy goals, calculation of this risk percentage and the specific cut-off values have been the most controversial aspects of the new cholesterol guidelines. Most experts agree that, in adults age 40 to 75, 10-year risk >10% indicates daily statin use as tolerated for primary prevention, and 10-year risk <5% does not warrant statin use. Recent large studies have validated these new techniques for calculating risk, and found them to be beneficial in potential for cost savings and risk classification.17,18
Considerations in psychiatric patients. Statins have been associated with depression in case series, but larger analyses have not confirmed this association.19 Emerging evidence has identified a potential correlation between statin use and accelerated onset of T2DM, but the absolute risk is relatively low and most experts continue to recommend statin therapy despite this potential risk.13 Many statins, including atorvastatin, are available as a generic and can be taken once daily. Some, such as simvastatin, have notable interactions with commonly prescribed psychotropics including risperidone and quetiapine. Pravastatin is dually excreted by the liver and kidneys and may have fewer drug-drug interactions in patients with psychiatric illness taking common psychotropic therapies, but is not considered a high-potency statin and might not confer adequate benefits in CVD risk reduction.
Contraindications. Statins are pregnancy category X, and generally should not be prescribed for women of childbearing age without intensive counseling. The most notable adverse effects for statins include muscle aches and cramps (myalgia), but generally are not severe. If encountered, consider checking a serum creatinine kinase (CK) level, and if significantly elevated above 10 times the upper limit, stopping statin therapy would be advised. If the CK is only mildly elevated, consider lowering the dosage or switching to a lower potency agent. Lovastatin and pravastatin generally are better tolerated than atorvastatin and are considered lower potency (Table 2).
Statins can be safely used in the presence of liver conditions, such as hepatitis C and alcohol use, although periodic monitoring of transaminase levels is recommended. For adults in the general population without liver disease, regular monitoring of transaminase levels is not necessary.
Alternate lipid-lowering pharmacotherapies unfortunately have fallen out of favor. Fibrates, niacin, ezetimibe, and omega-3 fatty acids once were recommended to lower triglycerides or raise HDL cholesterol levels, but since have been shown to have little effect on cardiovascular morbidity or mortality. Adding further medications, other than statins, to lower cholesterol values to pre-defined targets is not the current standard of care.
High triglyceride concentrations traditionally have been addressed directly, but failure to improve CVD mortality or morbidity by treating triglycerides alone has resulted in refocusing clinical efforts in dyslipidemia management on atherogenic cholesterol, including LDL and non-HDL fractions.20 Non-fasting triglycerides >500 mg/dL should be retested when fasting, and levels that remain >500 mg/dL could place the patient at risk for pancreatitis and might warrant intervention with fibrates at that time. This scenario is not common, and referral to a primary care physician or endocrinologist may be warranted.
Lifestyle changes
With or without statin therapies, diet and lifestyle changes are the cornerstone of healthy living and should be encouraged in all patients. Most overweight or obese patients will benefit from exercise and dietary modifications. Such interventions have shown potential for reducing total cholesterol and non-HDL and HDL cholesterol, but rarely are these interventions sustained long enough to produce meaningful reduction in CVD risk through lipid lowering. Diets rich in isocaloric tree nuts and red-yeast rice extract—a form of a statin—have shown promise in reducing cholesterol, but typically take excessive personal resources and are not sustained to the degree necessary to reduce CVD risk over time.21 Similarly, regular exercise routines can help lower overall cholesterol numbers, but rarely reduce total cholesterol by >10%.
Because individuals with SMI smoke at a higher rate than the general population, it should be noted that smoking cessation is associated with a reduction in total cholesterol and a trial of smoking cessation therapy is warranted before initiating a statin medication for primary prevention of CVD. Many patients would discover that their 10-year ASCVD risk would fall under the level needed for statin therapy if they could successfully stop smoking.
Switching pharmacotherapies
Switching antipsychotic agents from highly metabolically risky compounds, such as risperidone and olanzapine, to less metabolically active compounds, such as aripiprazole, ziprasidone, or haloperidol, have been associated with improvements in lipid profiles.22-24 Clinicians must weigh the potential benefits of switching therapies against the risk of psychiatric destabilization and long-term adherence, keeping in mind that changes in lipids seen with switching could be mild (approximately 10% reduction in total cholesterol).
Summing up
Cholesterol management is considered part of a program to systematically lower CVD risk. Statin therapy usually is indicated for life, or until the age of 75, at which point treatment risks and benefits change because of life expectancy. Other components of CVD risk reduction include a focus on blood pressure control, smoking cessation, T2DM management, and weight loss. Tracking lipid profiles over time to ensure broad targets of 30% to 50% reduction in total cholesterol, approximately 3 months after initiation and yearly thereafter, can help ensure adherence to therapy. With systematic lowering of modifiable CVD risk factors, we can hope to gradually improve the quality of life for our patients with mental illnesses (see the Box for a case example illustrating successful use of these strategies).
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 suppl B):S1-S45.
2. LaRosa JC, Hunninghake D, Bush D, et al. The cholesterol facts. A summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease. A joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. The Task Force on Cholesterol Issues, American Heart Association. Circulation. 1990;81(5):1721-1733.
3. Albert MA, Glynn RJ, Fonseca FA, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Am Heart J. 2011;162(1):106-114.e2.
4. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1):CD004816. doi: 10.1002/14651858.CD004816.pub5.
5. Gaziano JM, Gaziano TA. What’s new with measuring cholesterol? JAMA. 2013;310(19):2043-2044.
6. Emerging Risk Factors Collaboration; Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000.
7. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
8. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324-333.
9. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states [published online March 15, 2006]. Prev Chronic Dis. 2006;3(2):A42.
10. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
11. Osby U, Correia N, Brandt L, et al. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321(7259):483-484.
12. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(suppl 4):69-80.
13. Ganda OP. Deciphering cholesterol treatment guidelines: a clinician’s perspective. JAMA. 2015;313(10):1009-1010.
14. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv. 2014;65(5):573-576.
15. U.S. Preventive Services Task Force. Lipid disorders in adults (cholesterol, dyslipidemia). http://www.uspreventiveservicestaskforce.org/uspstf/uspschol.htm. Published June 2008. Accessed October 12, 2016.
16. Cupp M. Characteristics of the various statins. Pharmacist’s Letter. 2012;28(6):280606.
17. Pursnani A, Massaro JM, D’Agostino RB Sr, et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314(2):134-141.
18. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314(2):142-150.
19. You H, Lu W, Zhao S, et al. The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother. 2013;14(11):1467-1476.
20. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician. 2010;81(9):1097-1102.
22. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205(1):23-32.
23. Weiden PJ. Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry. 2007;68(suppl 4):34-39.
24. Stroup TS, McEvoy JP, Ring KD, et al; Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. 2011;168(9):947-956.
High-value intervention: Providing colorectal cancer screening
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
Cancer screening is an important example of secondary prevention—the aim being to detect disease at an early stage, when treatment can prevent symptomatic disease. Over the years, screening tests for breast cancer, colorectal cancer (CRC), cervical cancer, and, most recently, lung cancer have been developed and recommended by the U.S. Preventive Services Task Force (USPSTF). Among breast cancer, cervical cancer, and CRC, the screening rate for CRC remains lowest, at 58.6%.1
The importance of screening for CRC is highlighted by the facts that:
- CRC is the third most commonly diagnosed form of cancer in the United States among both men and women
- CRC is the second leading cause of cancer-related death.2
The overall decrease in the incidence of CRC in the United States has been credited to improvements in screening and removal of potentially precancerous lesions.3
Harmful disparity puts the mentally ill at exceptional risk
Screening patterns for CRC among patients with mental illness are poorly characterized, but it is known that the overall cancer screening rate among patients with severe psychiatric illness lags significantly behind the rate in the general population.4,5 In addition, studies have shown that mortality among patients with CRC who have a mental disorder is elevated, compared with CRC patients who do not have a psychiatric diagnosis.6
Why this disparity? It might be that CRC is more likely to be diagnosed at an advanced stage among these patients, or that they are less likely to receive cancer treatment after diagnosis, or are more likely to have a longer delay between diagnosis and initial treatment than patients who do not have a psychiatric diagnosis.7
Regardless, psychiatric practitioners can make a significant impact on reducing this health disparity by leveraging their unique therapeutic relationship to educate patients about screening options and dispel myths about cancer screening. In this article, we outline practical strategies for CRC screening and weigh the advantages and disadvantages for the use of several tools and guidelines in psychiatric patients.
What is the pathogenesis of colorectal cancer?
Most cases of CRC evolve from polyps, abnormal growths on the lining of the colon or rectum. Constituting an estimated 96% of all polyps, adenomas are by far the most common form in the colon and rectum.
Adenomas also are most likely to transform over time to dysplasia, and then to progress to cancer.8 Although all adenomas have malignant potential, <10% evolve to adenocarcinoma. This proposed adenoma➝carcinoma sequence is not well understood; however, it is known that CRC usually develops slowly—over 10 to 15 years.9 Detection and removal of adenomas and treatable, localized carcinomas form the basis of screening for CRC.
Risk factors for colorectal cancer
A number of risk factors for CRC have been identified.
Specific heritable conditions, such as Lynch syndrome and familial adenomatous polyposis, pose the greatest risk of CRC, particularly at younger ages and compared with people without such a history.10
Family history. One of the strongest risk factors for CRC remains a family history of the disease. People who have a first-degree relative with a diagnosis of CRC are at 2 to 3 times the risk of CRC, compared with people without a family history of the disease. This risk increases further if multiple family members are affected or if the diagnosis was made in a relative at a young age.11,12
Other non-modifiable risk factors include a personal history of inflammatory bowel disease, type 2 diabetes mellitus, male sex, African American heritage, and increasing age.13-15
Common modifiable risk factors include obesity, smoking, and alcohol consumption.16-18
What is the role of screening?
CRC screening is only appropriate for patients who are asymptomatic. CRC generally is asymptomatic in early stages. Prognosis also is most favorable when CRC is detected in the asymptomatic stage.
As lesions of CRC grow, the presentation might include hematochezia, melena, abdominal pain, weight loss, occult anemia, constipation or diarrhea, and changes in stool caliber.19 These signs and symptoms are not highly specific for CRC, however, and might be indicative of other gastrointestinal pathology, including inflammatory bowel disease, diverticulitis, irritable bowel syndrome, infectious colitis, hemorrhoids, and mesenteric ischemia.
Symptomatic patients should be referred directly for diagnostic evaluation. Colonoscopy with biopsy is the standard for diagnosing CRC. Once a diagnosis of CRC is made, patients should be referred to a specialist to discuss treatment; options largely depend on the stage of the cancer at diagnosis.
What screening tests are available?
Unlike screening for other cancers, there are a number of reasonable options for CRC screening; Table 115 compares their relative pros and cons. Each test has its benefits and drawbacks, allowing the screening strategy to be customized based on patient preference and characteristics, but this variability also can lead to confusion by patient and provider about those options.
Stool-based tests detect trace amounts of blood from early-stage treatable cancers. Highly sensitive fecal occult blood testing (FOBT) has been shown specifically to decrease mortality from CRC.20 Stool-based tests are inexpensive and noninvasive, but require:
- more frequent testing
- that the patient collect the stool specimen
- follow-up colonoscopy when test results are positive.
Endoscopic and imaging tests detect polyps and early-stage treatable cancers; all require some degree of bowel preparation, and some require sedation. Testing intervals vary but, as a group, are longer than the interval between stool-based tests because polyps grow slowly. Because colonoscopy with biopsy is the preferred screening method for diagnosing CRC, it is the only screening option that also is a diagnostic procedure.
Where can screening guidelines be found?
Several professional organizations have developed guidelines for CRC screening. The 2 major
An update to both guidelines was released in 2008. Table 221,22 summarizes their recommendations.
Both guidelines recommend that screening begin at age 50 (Box). The primary differences between the 2 guidelines lie in the scope of recommended options for screening and the time frame for discontinuing screening:
- USPSTF requires a higher level of evidence for screening options and limits recommended options to FOBT, sigmoidoscopy combined with FOBT, and colonoscopy.
- ACS-MSTF-ACR emphasizes options that detect premalignant polyps, and generally is more inclusive of testing options; it also delineates tests as useful for either (1) early detection of cancer (stool-based studies) or (2) cancer prevention (endoscopic and imaging tests).
On the question of when to stop screening, ACS-MSTF-ACR bases its recommendations on life expectancy; USPSTF sets a specific age for ending screening.21,22
Recommendations of a third entity, the American College of Gastroenterology (ACG), are similar to those of ACS-MSTF-ACR; however, ACG (1) recommends beginning screening African American patients at age 45 because of their increased risk of CRC and (2) gives preference to colonoscopy as the preferred screening modality.23
Guidelines vary for high-risk patients (those with a history of familial adenomatous polyposis or another inherited syndrome associated with CRC; those with a family history of CRC in the young; those with a history of radiation exposure, history of CRC, or inflammatory bowel disease; and those with several first-degree relatives with CRC). Patients who fall into any of these categories should be referred for specialty care to establish the time of initial screening and the interval of subsequent screening.
CRC screening in the presence of psychiatric illness
Psychiatrists have an opportunity to support their patients when considering potentially confusing CRC screening recommendations. This opportunity might occur during a discussion about general preventive care, or a patient might come to an appointment after visiting a primary care provider, and ask for advice about screening options.
The potential benefits of CRC screening are negated if a patient is unable or unwilling to complete the test or undergo timely follow-up of positive results. It is important, therefore, to individualize screening recommendations—keeping in mind the degree of impairment from mental illness and the patient’s preferences and reliability to engage in follow-up. To date, there are no agreed-on screening guidelines specifically for patients with comorbid mental illness.
Adapting USPSTF guidelines for CRC screening of average-risk patients with mental illness, we offer the following recommendations:
Recommend screening. Begin routine screening at age 50. Patients with well-controlled or mild symptoms should be screened with a stool study with or without flexible sigmoidoscopy. Stool studies are safe, noninvasive, and require no bowel preparation; when used alone, however, they need to be performed yearly.
Screening accuracy is increased when a stool-based test is combined with flexible sigmoidoscopy; screening then can be performed less often. Unlike colonoscopy, flexible sigmoidoscopy does not involve sedation; a high-functioning patient might find this appealing and tolerate the greater frequency of screening. On the other hand, some patients might not accept the inconvenience of collecting the stool sample with the kit provided and returning it to the lab for processing.
Manage psychiatric illness optimally. For a patient with moderate or severe psychiatric symptoms, first attempt to optimize treatment of the underlying psychiatric condition before establishing a CRC screening program. If control of symptoms is likely to improve over the next 1 or 2 visits, it might be reasonable to defer screening until symptoms are better controlled and then reassess the patient before making specific screening recommendations. Screening should not be delayed, however, if significant improvement in symptoms is not expected in the near future. Lengthy delay might lead to failure in initiating screening at all.
We recommend that patients with persistent moderate or severe symptoms be screened with traditional colonoscopy. The sedation associated with colonoscopy (1) may be preferable to some patients with more severe illness and (2) allows for screening and diagnostic biopsy if needed during the same procedure. Screening with colonoscopy also:
- avoids the yearly adherence to a screening program that is needed with stool cards alone
- does not rely on patients collecting and returning stool kits for processing.
A potential challenge for patients with limited social support is the requirement to have someone accompany the patient on the day of colonoscopy.
Take steps to improve the screening rate. In addition to specific recommendations based on symptom severity, there are systems-level interventions that should be considered to improve the screening rate. These include:
- addressing transportation issues that are a barrier to screening
- considering the use of health navigators or peer advocates to help guide patients through the sometimes complex systems of care.
A more comprehensive systems-level intervention for mental health clinics that work primarily with persistent and severe mentally ill populations might include employing a care coordinator to organize referrals to primary care or even exploring reverse integration. In reverse integration, primary care providers co-locate within the mental health clinic, (1) allowing for “one-stop shopping” of mental health and primary care needs and (2) facilitating collaboration and shared treatment planning between primary care and mental health for complex patients.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
3. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573.
4. Miller E, Lasser KE, Becker AE. Breast and cervical cancer screening for women with mental illness: patient and provider perspectives on improving linkages between primary care and mental health. Arch Womens Ment Health. 2007;10(5):189-197.
5. Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11(8):797-804.
6. Baillargeon J, Kuo YF, Lin YL, et al. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268-1273.
7. Robertson R, Campbell NC, Smith S, et al. Factors influencing time from presentation to treatment of colorectal and breast cancer in urban and rural areas. Br J Cancer. 2004;90(8):1479-1485.
8. Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998-2001. Cancer. 2006;107(suppl 5):1128-1141.
9. Levine JS, Ahnen DJ. Clinical practice. Adenomatous polyps of the colon. N Engl J Med. 2006;355(24):2551-2557.
10. Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932.
11. Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216-227.
12. Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992-3003.
13. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323(18):1228-1233.
14. Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3(6):587-594.
15. American Cancer Society. Colorectal cancer facts & figures 2011-2013. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. Published 2011. Accessed July 5, 2016.
16. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765-2778.
17. Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140(8):603-613.
18. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86(3):556-565.
19. Speights VO, Johnston MW, Stoltenberg PH, et al. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84(5):575-578.
20. Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106-1114.
21. U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627-637.
22. Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130-160.
23. Rex DK, Johnson DA, Anderson JC, et al; American College of Gastroenterology. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [corrected] [Erratum in: Am J Gastroenetrol. 2009;104(6):1613]. Am J Gastroenterol. 2009;104(3):739-750.
COPD comorbid with mental illness: What psychiatrists can do
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Chronic pain and psychiatric illness: Managing comorbid conditions
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
Pain is one of the most common symptoms for which patients seek medical care, with an associated estimated annual cost of $600 billion.1 Using a multimodal approach to care—thorough evaluation, cognitive-behavioral and psychophysiological therapy, physical therapy, medications, and other interventions—can help patients effectively manage their condition and achieve healthier outcomes.
Evaluating a patient with pain
When developing a safe, comprehensive, and effective treatment plan for patients with chronic pain, first perform a thorough history and physical exam using the following elements:
Pain history. The PQRST mnemonic (Table 1) can help you obtain critical information and assist in determining the appropriate diagnosis and cause of the patient’s pain complaints.
Psychiatric history. Document the mental health history of the patient and first-degree relatives.
Medical history. Knowing the medical history could reveal comorbidities contributing to a patient’s pain complaint.
Treatment history. Listing past and current treatments for pain, including effectiveness, helps the clinician understand if an existing treatment plan should be modified.
Functional status. Document current level of daily activity, how life activities are affected by pain; strategies used to help cope with pain; level of physical and emotional support provided in home, work, and school environments; and active stressors (eg, financial, interpersonal).
Psychosocial history. Document historical information related to coping skills, trauma history, family of origin, abuse, interpersonal relationships, social support, and academic and vocational functioning.
Substance use or abuse. Assess for use of controlled substances (ie, early refills; lost medications; obtaining medications from multiple prescribers, friends, families, or strangers; use of prescribed and non-prescribed medications for non-medical and medical purposes), nicotine, alcohol, illicit substances, and caffeine. A thorough inventory can help to identify substances a patient is using that could affect daily functioning and pain level.
Behavioral observations. Assessing mental status (eg, insight, pain behavior, cooperation) can be useful. Paying attention to pain behaviors, such as complaints of pain, decreased activity, increased medication intake, or altered facial expressions or body posture, can help the clinician gain insight to the extent that pain affects the patient’s quality of life.
The information gathered in the patient evaluation can be used to design a multimodal treatment plan to achieve maximum effectiveness.
Assessing psychiatric illness
Current approaches to pain evaluation and treatment recommend use of a biopsychosocial orientation because psychological, behavioral, and social factors can influence the experience and impact of pain, regardless of the primary cause.2 A comprehensive psychiatric evaluation, diagnosis, and treatment plan should consider the broader context in which a patient’s pain occurs.
Regarding psychiatric illness, past and current symptoms, treatment history, and risk assessment should all be included. Using the “AMPS approach” (Figure)3—assessing Anxiety, Mood (depression and mania), Psychotic symptoms (paranoid ideation and hallucinations), and Substance use—helps screen for comorbid psychiatric conditions in patients with chronic pain.
Sleep assessment
Chronic pain patients often experience significant sleep disturbance that could be caused by physiological aspects of the pain condition, environmental factors (eg, uncomfortable bedding), a comorbid sleep disorder (eg, sleep apnea), a psychiatric disorder, or a combination of the above.
Obstructive and central sleep apnea are characterized by nighttime hypoxia, which leads to frequent disruption of the sleep-wake cycle and often manifests as daytime fatigue, irritability, depression, drowsiness, headaches, and increased pain sensitivity. Changes in sleep arousal can lead to neuropsychological changes during the day, such as decreased attention, memory problems, impaired executive functioning, and reduced impulse control.
Screen patients for central and obstructive sleep apnea before prescribing opioids or benzodiazepines for pain because these medications can cause or exacerbate underlying sleep apnea. Although many screening tools, such as the Epworth Sleepiness Scale, assess daytime somnolence,4 the STOP-BANG questionnaire is a quick, validated, and efficient screening tool that often is used to assess sleep apnea risk5,6 (Table 2). The presence of ≥3 risk factors identifies patients at increased risk and warrants consideration for further workup by a sleep specialist.7,8
Pharmacotherapy for chronic pain
Non-opioid medications. Pain can be broadly categorized as neuropathic or nociceptive. Neuropathic pain can be described by patients as numbness, burning, electric-like, and tingling, and is associated with nerve damage. Nociceptive pain commonly is described as similar to a toothache with descriptors such as stabbing, sharp, or a dull aching sensation; it is often, but not always, associated with acute injury or ongoing trauma to tissue. Drug treatment is most successful when the appropriate class of medication is matched to the specific type of pain.
Nociceptive pain often is successfully treated with non-steroidal anti-inflammatory drugs and acetaminophen. Non-selective COX inhibitors (eg, ibuprofen, indomethacin, ketorolac) and COX-2 selective inhibitors (eg, celecoxib) have been associated with cardiovascular, gastrointestinal, and renal disease; acetaminophen is associated with liver dysfunction.9-11 However, the absolute risk for complications in healthy patients is low.12 To minimize risk, use these agents for the shortest duration and at the lowest effective dosage possible.
Neuropathic pain can be addressed with certain antidepressants13—specifically, those that increase serotonin and norepinephrine (eg, tricyclic antidepressants [TCAs] and serotonin-norepinephrine reuptake inhibitors [SNRIs]), or medications that block ion channels (eg, anticonvulsants). TCAs (eg, desipramine, nortriptyline, amitriptyline) are among the best studied and most cost effective medications for treating neuropathic pain,14,15 but they can have sedating and anticholinergic effects, as well as cardiac adverse effects (ie, prolongation of the QTc interval). SNRIs (eg, venlafaxine, desvenlafaxine, duloxetine, and milnacipran) can be effective and often are better tolerated than TCAs.14
Some newer anticonvulsants (eg, gabapentin and pregabalin) have been found to be more effective than placebo for a variety of neuropathic pain conditions.16,17 Although they have few drug-drug interactions, anticonvulsants can cause dizziness, forgetfulness, and sedation. These adverse effects can be minimized by starting at a low dosage and titrating carefully. Because hepatic or renal impairment can affect metabolism or excretion of these drugs, review the prescribing information to determine safe dosing.
Targeted injection of medications to major pain generators (eg, an epidural steroid for radicular neck and back pain; facet injections for facet-related neck and back pain; trigger point injections for myofascial pain; occipital nerve blocks for occipital neuralgia; and botulinum toxin A injections for chronic migraine headache) can be effective in reducing discomfort and increasing function in patients with chronic pain. A detailed discussion of such therapies is beyond the scope of this article, but have been reviewed extensively elsewhere.18,19
Opioids. Although there is little evidence of long-term efficacy with chronic opioid therapy for most patients, a trial of opioids might be warranted for select patients who do not respond to other medications. Because the risk–benefit ratio for chronic opioid therapy is high,20-22 a decision to initiate a trial of a low-dosage opioid should be made only after careful consideration of those risks. It is generally agreed that treatment of chronic pain with low-dosage opioid therapy is more likely to be successful when it is used as an adjuvant to non-opioid modalities (eg, physical reconditioning, injection therapies, spinal cord stimulation, neurobehavioral interventions, non-opioid medications).
The Federation of State Medical Boards has stated that excessive reliance on opioid medications for treating chronic pain is a deviation from best practices.23 To maximize benefit and minimize risk, clinicians should carefully select appropriate patients, establish functional goals, and regularly monitor for efficacy and compliance. Thoroughly document these steps in the patient’s record for later reference.23
After establishing a clinical diagnosis for the cause of the pain, you should determine the risk of opioid abuse or misuse by using any one of the available risk assessment tools (Box). Understand, however, that no single tool has been shown to be more effective than others.
Although patients and some clinicians tend to overvalue the benefits of chronic opioid therapy, many do not fully appreciate the risks (eg, respiratory depression and death), which can be exacerbated if the patient is using other substance that suppress respiration (eg, benzodiazepines, alcohol, and illicit substances). Written informed consent and treatment agreement is highly recommended; components of such a document are listed in Table 3.23
Develop a treatment plan that emphasizes functional goals based on the patient’s physical limitations and that incorporates some type of daily, atraumatic physical activity. This plan should be documented and reviewed regularly to help monitor treatment effectiveness.
After an initial trial of a few weeks, the patient and clinician should meet to review the 5 “A”s (Table 4)24 to determine the success of the opioid regimen. Consulting your state’s prescription drug monitoring program (if one is available), obtaining a random urine drug test, and doing a pill count can provide useful, objective data. If the patient has not made progress but has experienced no adverse effects, then a small dosage increase might be warranted. If any of the 5 “A”s indicates lack of improvement or increased risk, consider stopping opioid therapy and exploring non-opioid options to manage chronic pain.
Referrals to a pain specialist or an addiction specialist, or both, might be needed, depending on the patient’s condition at any given follow-up visit. Such referral decisions, as well as all treatment plans, should be documented clearly in the medical record to prevent any misunderstanding, false accusations, or medicolegal repercussions regarding the rationale for continuing or terminating opioid-based treatment.
Non-pharmaceutical therapy for treating pain
The pain management field has successfully integrated the biopsychosocial model into regular practice. This model advocates the use of multimodal non-drug interventions in conjunction with opioid and non-opioid medications. Such interventions address behavioral, cognitive, sociocultural (psychosocial), lifestyle, and physiological dimensions of pain. A partial list of non-drug interventions is provided in Table 5.
Integration of these interventions within a biopsychosocial framework can assist you in making a comprehensive treatment plan. For example, patients with focal myofascial shoulder and back pain might derive only transient benefit from trigger point injection. However, concurrent referral to a pain psychologist and physical therapist could substantially improve functional outcomes by addressing factors that directly and indirectly influence myofascial pain. Inclusion of cognitive-behavioral therapy (addressing psychosocial and lifestyle dimensions), surface electromyography, psychophysiological interventions/biofeedback (addressing psychosocial, lifestyle, and physiological dimensions), and physical therapy (addressing lifestyle and physiological dimensions) allows the patient to learn coping skills, decrease physiological arousal that can lead to unnecessary tensing of muscles, and strengthen core muscle groups.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
1. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://www.iom.edu/~/media/Files/Report%20 Files/2011/Relieving-Pain-in-America-A-Blueprint-for- Transforming-Prevention-Care-Education-Research/ Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed April 15, 2015.
2. Jensen MP, Moore MR, Bockow TB, et al. Psychosocial factors and adjustment to chronic pain in persons with physical disabilities: a systematic review. Arch Phys Med Rehabil. 2011;92(1):146-160.
3. McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
4. Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423-438.
5. Boynton G, Vahabzadeh A, Hammoud S, et al. Validation of the STOP-BANG questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4). doi: 10.4172/2325-9639.1000121.
6. Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84-94.
7. Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol. 2009;22(3):405-411.
8. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812-821.
9. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med. 2007;167(4):394-399.
10. Sudano I, Flammer AJ, Périat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789-1796.
11. U.S. Food and Drug Administration. Questions and answers about oral prescription acetaminophen products to be limited to 325 mg per dosage unit. http://www.fda.gov/ drugs/drugsafety/informationbydrugclass/ucm239871. htm. Updated December 11, 2014. Accessed February 23, 2015.
12. Bhala N, Emberson J, Merhi A, et al; Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769-779.
13. Sullivan MD, Robinson JP. Antidepressant and anticonvulsant medication for chronic pain. Phys Med Rehabil Clin N Am. 2006;17(2):381-400, vi-vii.
14. Sindrup SH, Otto M, Finnerup NB, et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
15. Pilowsky I, Hallett EC, Bassett DL, et al. A controlled study of amitriptyline in the treatment of chronic pain. Pain. 1982;14(2):169-179.
16. Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573-581.
17. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251.
18. Manchikanti L, Abdi S, Atluri S, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(suppl 2):S49-S283.
19. Singh V, Trescot A, Nishio I. Injections for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):249-261.
20. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers— United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492.
21. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
22. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
23. Federation of State Medical Boards. Model policy for the use of opioid analgesics in the treatment of chronic pain. http:// www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/ pain_policy_july2013.pdf. Published July 2013. Accessed December 18, 2015.
24. Passik SD, Weinreb HJ. Managing chronic nonmalignant pain: overcoming obstacles to the use of opioids. Adv Ther. 2000;17(2):70-83.
Head pain and psychiatric illness: Applying the biopsychosocial model to care
More than 45% of people worldwide suffer from headache at some point in their life.1 Head pain can lead to disability and functional decline, yet headache disorders often are underdiagnosed and poorly assessed. For example, 60% of migraine and tension-type headaches go undiagnosed and 50% of persons suffering from migraine have severe functional disability or require bed rest.2-4
Because head pain can be associated with secondary medical and psychiatric conditions, diagnosis can be challenging. This article reviews the medical and psychological aspects of major headaches and assists with clinical assessment. We present clinical interviewing tools and a diagram to enhance focused, efficient assessment and inform treatment plans.
Classification of headache
Headache is a common complaint, yet it is often underdiagnosed and ineffectively treated. The World Health Organization estimates that, globally, 50% of people with headache self-treat their pain.5 The International Headache Society classifies headache as primary or secondary; approximately 90% of complaints are from primary headache.6
Assessment and diagnosis of headache can be complex because of overlapping, subjective symptoms. It is important to have a general understanding of primary and secondary causes of headache so that interrelated symptoms do not obscure the most accurate diagnosis and effective treatment course. Although most headache complaints are benign, ruling out secondary causes helps gauge the likelihood of developing severe sequelae from underlying pathology.
By definition, primary headaches are idiopathic and commonly include migraine, tension-type, cluster, and hemicrania continua headache. Secondary headaches have an underlying pathology, which could improve by targeting the disorder. Common secondary causes of headache include:
• trauma
• vascular abnormalities
• structural abnormalities
• chemical (including medications)
• inflammation or infection
• metabolic conditions
• diseases of the neck and pericranial and intracranial structures
• psychiatric conditions.
Table 1 illustrates common causes of head pain. More definitive criteria for symptoms and diagnosis can be found in the International Classification of Headache Disorders.7
Primary headache
Tension-type is the most common primary headache, accounting for more than one-half of all headaches.7 Patients usually describe a tight pain in a bilateral band-like distribution, which could be caused by sustained neck muscle contraction. Pain usually builds in intensity and can last 30 minutes to several days. There is a well-established association between emotional stress or depression and the development of tension-type headaches.8
Migraine typically causes pulsating pain in a localized area of the head that lasts as long as 72 hours and can be associated with nausea, vomiting, photophobia, phono-phobia, and aura. Patients report varying precipitating factors but commonly cite certain foods, menstruation, and sleep deprivation. Although rare, migraine with aura has been linked to ischemic stroke; most cases have been reported in female smokers and oral contraceptive users age <45.9
Because migraines can be debilitating, some patients—typically those with ≥4 attacks a month—opt for prophylactic medication. Effective prophylactics include amitriptyline, propranolol, divalproex sodium, and topiramate, which should be monitored closely and given a trial for several months before switching to another drug. Commonly used abortive treatments include triptans and anti-emetics such as metoclopramide.
Meperidine and ketorolac are popular second-line agents for migraine. Botulinum toxin A also has been used in severe cases to reduce the number of headache days in chronic migraine patients.6
Cluster headache is rare, but typically exhibits repeated burning and intense unilateral periorbital or retro-orbital pain that lasts 15 minutes to 3 hours over several weeks. Men are predominantly affected. Cluster headaches typically improve with oxygen treatment.
Biopsychosocial model of head pain
The biomedical model has helped iden tify pathophysiological pain mechanisms and pharmacotherapeutic agents for headache. However, during assessment, limiting one’s attention to the linear relationship between pathology, mechanism of action, and pain oversimplifies common questions clinicians face when assessing chronic head pain.
Advancements in the last 3 decades have expanded the conceptualization of head pain to integrate sociocultural, environmental, behavioral, affective, cognitive, and biological variables—otherwise known as the biopsychosocial model.10,11 The biopsychosocial model is a multidimensional theory that helps answer difficult clinical assessment questions and complex patient presentations (Table 2).10-13 Many unusual responses to pain treatment, questionable validity of pain behavior, and disproportionate pain perception and functional decline are explained by non-pathophysiological and non-biomechanical models.
Psychiatric comorbidity and head pain
Psychiatric conditions are highly prevalent among persons with primary headache. Verri et al14 found that 90% of chronic daily headache patients had ≥1 psychiatric condition; depression and anxiety were most common. Of concern, 1 study found that headache is associated with increased frequency of suicidal ideation among patients with chronic pain.15 It is critical for clinicians to screen for psychiatric comorbidities in patients with chronic headache. Conversely, clinicians might want to screen for headache in their patients with psychiatric illness.
Migraine. Mood disorders are common among patients who suffer from migraine. The rate of depression is 2 to 4 times higher in those with migraine compared with healthy controls.16,17 In a large-scale study, patients with migraine had a 1.9-fold higher risk (compared with controls) of having a comorbid depressive episode; a 2-fold higher risk of manic episodes; and a 3-fold higher risk of both mania and depression.18 In a study of 62 inpatients, Fasmer19 reported that 46% of patients with unipolar depression and 44% of patients with bipolar disorder experienced migraine (77% of the bipolar disorder patients with migraine had bipolar II disorder). Patients with migraine are at increased risk of suicide attempts (odds ratio 4.3; 95% CI, 1.2-15.7).20
Tension-type headache. The relationship between psychiatric comorbidity in tension-type headache is well established. In contrast to what is seen with migraines, Puca et al21 found a higher prevalence of anxiety disorders (52.5%) than depressive disorders (36.4%) in patients with tension-type headache. Generalized anxiety disorder was one of the most prevalent anxiety conditions (83.3%), and dysthymia was the most prevalent mood disorder (45.6%). In the same study, 21.7% of patients were found to have a comorbid somatoform disorder.21
Emotional and cognitive factors can co-occur in patients with tension-type headache and a comorbid psychiatric condition. For example, difficulty identifying or recognizing emotions—commonly referred to as alexithymia—has been linked to tension-type headache.22 Additionally, maladaptive cognitive appraisal of stress is more common among patients with tension-type headache when compared with those without headaches.23 Being mindful of and recognizing these co-occurring emotional and cognitive factors will help clinicians construct a more accurate assessment and effective behavioral treatment plan.
Clinical assessment with a useful mnemonic
Clinical assessment of psychiatric illness is essential when evaluating chronic pain patients. Using the acronym AMPS (Anxiety, Mood, Psychosis, and Substance use disorders) (Table 3) is an efficient way for the clinician to ask pertinent questions regarding common psychiatric conditions that could have a direct effect on chronic pain.24 Head pain can be more intense when combined with untreated anxiety, depression, psychosis, or a substance use disorder. Untreated anxiety, for example, can amplify sympathetic response to pain and complicate treatment.
Investigating head pain patients for an underlying mood disorder is essential to providing successful treatment. Consider:
• starting psychotherapy modalities that address both pain and psychiatric illness, such as cognitive-behavioral therapy (CBT)
• reframing unhelpful pain beliefs
• managing activity-rest levels
• biofeedback
• supportive group therapy
• reducing family members’ reinforcement of the patient’s pain behavior or sick role.25
Assessing for somatic symptom disorders
In addition to using the AMPS approach for psychiatric assessment, clinicians should evaluate for somatization, which can present as head pain. Somatic symptom disorders (SSD) are a class of conditions that are impacted by affective, cognitive, and reinforcing factors that might or might not be consciously or intentionally produced. Patients with an SSD have somatic symptoms that are distressing or cause significant disruption of daily life because of excessive thoughts, feelings, or behaviors related to the somatic symptoms, for ≥6 months. The Figure outlines SSD, related conditions, and their respective prominent symptoms to assist in the differential diagnosis.26
Note that some headache conditions present with severe distress because of their abrupt onset and severity of symptoms (eg, cluster headaches). Therefore, the expectation and likelihood of psychological disturbance should be factored into a diagnosis of SSD and related conditions as seen in the Figure.
Secondary factors of unusual pain behavior or treatment response. The role of thoughts, affect, and behaviors is clinically meaningful in understanding SSD and similar conditions. Specific questions about cultural beliefs and rituals as they relate to exacerbations of head pain are of value. Table 413,27 lists behavioral, cognitive, and affective dimensions of head pain using the biopsychosocial model, and further clarifies common questions that arise with unusual pain response and complex patient presentations, which were outlined in the beginning of the article.
Because depression and anxiety can be comorbid with head pain, it is important to recognize psychological factors that contribute to pain perception. Indifference or denial of emotional stress as a result of severe pain and disability can imply a somatization process, which could suggest emotional disconnection or dissociation from somatic functioning.28 This finding can be a component of alexithymia, in which a person is disconnected from emotions and how emotions impact the body. Therefore, recognizing alexithymia assists in identifying psychological factors when patients deny mood symptoms, particularly in tension-type headache.
Functional assessment to rule out the disproportional impact of pain on daily activities is helpful in understanding the somatization process. Neurocognitive functioning should be assessed, particularly because frontal and subcortical dysregulation has been observed in head pain sufferers.29,30 Patients with cognitive changes as a result of a medical illness (eg, stroke, head concussion, brain tumor, or seizures) are especially at risk for neurocognitive dysfunction.
Neuropsychological assessment can be useful, not only to assess neurocognitive functioning (eg, Repeated Battery for the Assessment of Neuropsychological Status) but to identify objective test profiles associated with altered motivation (eg, Rey 15-Item Test, Minnesota Multiphasic Personality Inventory-2-Restructured Form F Scale, Personality Assessment Inventory [PAI] Negative Impression Management) and somatization processes (eg, PAI Somatization Scale). These instruments help to identify the severity of psychiatric and neurocognitive symptoms by comparing scores to normative (eg, healthy control group), clinical (eg, somatization, traumatic brain injury, mild cognitive impairment), and altered motivation (eg, persons instructed to exaggerate symptoms) databases.
If the clinician pursues neurocognitive assessment, direct referral to a neuropsychologist, referral to neurologist, or administration of a cognitive screening tool such as the Montreal Cognitive Assessment, Saint Louis University Mental Status, or Cognitive Log is recommended. If the cognitive screening is positive, next steps include: referring for full neuropsychological assessment, which includes complete cognitive and motor testing, personality testing, and integration of neuroimaging data (eg, MRI, CT scans, and/or EEG).
Assessing the patients’ self-talk or thought patterns as they describe their head pain will help clinicians understand belief systems that may be distorting the reality of the medical condition. For example, a patient might report that “my pain feels like someone is hitting me with an axe”; this is a catastrophic thought that can distort the clarity and perceptibility of pain. Encouraging patients to monitor and analyze their anxiety and associated negative thoughts is an important strategy for improving mood and decreasing somatization. Recording daily thoughts and CBT can help the patient identify and appropriately address his (her) cognitive distortions and futile thinking.
When implementing a treatment plan for somatization disorder, we propose the mnemonic device CARE MD:
• CBT
• Assess (by ruling out a medical cause for somatic complaints)
• Regular visits
• Empathy
• Med-psych interface (help the patient connect physical complaints and emotional stressors)
• Do no harm.
Clinical recommendations
Chronic head pain can be debilitating; psychodiagnostic assessment should therefore be considered an important part of the diagnosis and treatment plan. After ruling out common and emergent primary or secondary causes of head pain, consider psychiatric comorbidities. Depression and anxiety have a strong bidirectional relationship with chronic headache; therefore, we suggest evaluating patients with the intention of alleviating both psychiatric symptoms and head pain.
It is important to diligently assess for common psychiatric comorbidities; using the AMPS and CARE MD mnemonics, along with screening for somatization disorders, is an easy and effective way to evaluate for relevant psychiatric conditions associated with chronic head pain. Because many patients have unusual and complicated responses to head pain that can be explained by non-pathophysiological and non-biomechanical models, using the biopsychosocial model is essential for effective diagnosis, assessment, and treatment. Abortive and prophylactic medical interventions, as well as behavioral, sociocultural, and cognitive assessment, are vital to a comprehensive treatment approach.
Bottom Line
The psychodiagnostic assessment can help the astute clinician identify comorbid psychiatric conditions, psychological factors, and somatic symptoms to develop a comprehensive biopsychosocial treatment plan for patients with chronic head pain. Rule out primary and secondary causes of pain and screen for somatization disorders. Consider medication and psychotherapeutic treatment options.
Related Resources
• Pompili M, Di Cosimo D, Innamorati M, et al. Psychiatric comorbidity in patients with chronic daily headache and migraine: a selective overview including personality traits and suicide risk. J Headache Pain. 2009;10(4):283-290.
• Sinclair AJ, Sturrock A, Davies B, et al. Headache management: pharmacological approaches [published online July 3, 2015]. Pract Neurol. doi: 10.1136/practneurol-2015-001167.
Drug Brand Names
Amitriptyline • Elavil Meperidine • Demerol
Botulinum toxin A • Botox Metoclopramide • Reglan
Divalproex sodium • Depakote Propranolol • Inderide
Ketorolac • Toradol Topiramate • Topamax
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193-210.
2. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657.
3. The World Health Report 2001: Mental health: new understanding new hope. Geneva, Switzerland: World Health Organization; 2001.
4. World Health Organization. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_ disease/GBD_report_2004update_full.pdf. Published 2004. Accessed July 31, 2015.
5. World Health Organization. Headache disorders. http:// www.who.int/mediacentre/factsheets/fs277/en/. Published October 2012. Accessed July 31, 2015.
6. Clinch C. Evaluation & management of headache. In: South- Paul JE, Matheny SC, Lewis EL. eds. Current diagnosis & treatment in family medicine, 4th ed. New York, NY: McGraw-Hill; 2015:293-297.
7. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
8. Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia. 2015;35(2):165-181.
9. Janke EA, Holroyd KA, Romanek K. Depression increases onset of tension-type headache following laboratory stress. Pain. 2004;111(3):230-238.
10. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
11. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544.
12. Turk DC, Flor H. Chronic pain: a biobehavioral perspective. In: Gatchel RJ, Turk DC, eds. Psychosocial factors in pain: critical perspectives. New York, NY: Guilford Press; 1999:18-34.
13. Andrasik F, Flor H, Turk DC. An expanded view of psychological aspects in head pain: the biopsychosocial model. Neurol Sci. 2005;26(suppl 2):s87-s91.
14. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998; 18(suppl 21):45-49.
15. Ilgen MA, Zivin K, McCammon RJ, et al. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30(6):521-527.
16. Kowacs F, Socal MP, Ziomkowski SC, et al. Symptoms of depression and anxiety, and screening for mental disorders in migrainous patients. Cephalalgia. 2003;23(2):79-89.
17. Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache. 2006;46(9):1327-1333.
18. Nguyen TV, Low NC. Comorbidity of migraine and mood episodes in a nationally representative population-based sample. Headache. 2013;53(3):498-506.
19. Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001; 21(9):894-899.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology. 1992;42(2):392-395.
21. Puca F, Genco S, Prudenzano MP, et al. Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. Cephalalgia. 1999;19(3):159-164.
22. Yücel B, Kora K, Ozyalçín S, et al. Depression, automatic thoughts, alexithymia, and assertiveness in patients with tension-type headache. Headache. 2002;42(3):194-199.
23. Wittrock DA, Myers TC. The comparison of individuals with recurrent tension-type headache and headache-free controls in physiological response, appraisal, and coping with stressors: a review of the literature. Ann Behav Med. 1998;20(2):118-134.
24. Onate J, Xiong G, McCarron R. The primary care psychiatric interview. In: McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott, Williams and Wilkins; 2009:3-4.
25. Songer D. Psychotherapeutic approaches in the treatment of pain. Psychiatry (Edgmont). 2005;2(5):19-24.
26. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
27. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(pt 9):2751-2768.
28. Packard RC. Conversion headache. Headache. 1980;20(5):266-268.
29. Mongini F, Keller R, Deregibus A, et al. Frontal lobe dysfunction in patients with chronic migraine: a clinical-neuropsychological study. Psychiatry Res. 2005;133(1):101-106.
30. Martelli MF, Grayson RL, Zasler ND. Posttraumatic headache: neuropsychological and psychological effects and treatment implications. J Head Trauma Rehabil. 1999;14(1):49-69.
More than 45% of people worldwide suffer from headache at some point in their life.1 Head pain can lead to disability and functional decline, yet headache disorders often are underdiagnosed and poorly assessed. For example, 60% of migraine and tension-type headaches go undiagnosed and 50% of persons suffering from migraine have severe functional disability or require bed rest.2-4
Because head pain can be associated with secondary medical and psychiatric conditions, diagnosis can be challenging. This article reviews the medical and psychological aspects of major headaches and assists with clinical assessment. We present clinical interviewing tools and a diagram to enhance focused, efficient assessment and inform treatment plans.
Classification of headache
Headache is a common complaint, yet it is often underdiagnosed and ineffectively treated. The World Health Organization estimates that, globally, 50% of people with headache self-treat their pain.5 The International Headache Society classifies headache as primary or secondary; approximately 90% of complaints are from primary headache.6
Assessment and diagnosis of headache can be complex because of overlapping, subjective symptoms. It is important to have a general understanding of primary and secondary causes of headache so that interrelated symptoms do not obscure the most accurate diagnosis and effective treatment course. Although most headache complaints are benign, ruling out secondary causes helps gauge the likelihood of developing severe sequelae from underlying pathology.
By definition, primary headaches are idiopathic and commonly include migraine, tension-type, cluster, and hemicrania continua headache. Secondary headaches have an underlying pathology, which could improve by targeting the disorder. Common secondary causes of headache include:
• trauma
• vascular abnormalities
• structural abnormalities
• chemical (including medications)
• inflammation or infection
• metabolic conditions
• diseases of the neck and pericranial and intracranial structures
• psychiatric conditions.
Table 1 illustrates common causes of head pain. More definitive criteria for symptoms and diagnosis can be found in the International Classification of Headache Disorders.7
Primary headache
Tension-type is the most common primary headache, accounting for more than one-half of all headaches.7 Patients usually describe a tight pain in a bilateral band-like distribution, which could be caused by sustained neck muscle contraction. Pain usually builds in intensity and can last 30 minutes to several days. There is a well-established association between emotional stress or depression and the development of tension-type headaches.8
Migraine typically causes pulsating pain in a localized area of the head that lasts as long as 72 hours and can be associated with nausea, vomiting, photophobia, phono-phobia, and aura. Patients report varying precipitating factors but commonly cite certain foods, menstruation, and sleep deprivation. Although rare, migraine with aura has been linked to ischemic stroke; most cases have been reported in female smokers and oral contraceptive users age <45.9
Because migraines can be debilitating, some patients—typically those with ≥4 attacks a month—opt for prophylactic medication. Effective prophylactics include amitriptyline, propranolol, divalproex sodium, and topiramate, which should be monitored closely and given a trial for several months before switching to another drug. Commonly used abortive treatments include triptans and anti-emetics such as metoclopramide.
Meperidine and ketorolac are popular second-line agents for migraine. Botulinum toxin A also has been used in severe cases to reduce the number of headache days in chronic migraine patients.6
Cluster headache is rare, but typically exhibits repeated burning and intense unilateral periorbital or retro-orbital pain that lasts 15 minutes to 3 hours over several weeks. Men are predominantly affected. Cluster headaches typically improve with oxygen treatment.
Biopsychosocial model of head pain
The biomedical model has helped iden tify pathophysiological pain mechanisms and pharmacotherapeutic agents for headache. However, during assessment, limiting one’s attention to the linear relationship between pathology, mechanism of action, and pain oversimplifies common questions clinicians face when assessing chronic head pain.
Advancements in the last 3 decades have expanded the conceptualization of head pain to integrate sociocultural, environmental, behavioral, affective, cognitive, and biological variables—otherwise known as the biopsychosocial model.10,11 The biopsychosocial model is a multidimensional theory that helps answer difficult clinical assessment questions and complex patient presentations (Table 2).10-13 Many unusual responses to pain treatment, questionable validity of pain behavior, and disproportionate pain perception and functional decline are explained by non-pathophysiological and non-biomechanical models.
Psychiatric comorbidity and head pain
Psychiatric conditions are highly prevalent among persons with primary headache. Verri et al14 found that 90% of chronic daily headache patients had ≥1 psychiatric condition; depression and anxiety were most common. Of concern, 1 study found that headache is associated with increased frequency of suicidal ideation among patients with chronic pain.15 It is critical for clinicians to screen for psychiatric comorbidities in patients with chronic headache. Conversely, clinicians might want to screen for headache in their patients with psychiatric illness.
Migraine. Mood disorders are common among patients who suffer from migraine. The rate of depression is 2 to 4 times higher in those with migraine compared with healthy controls.16,17 In a large-scale study, patients with migraine had a 1.9-fold higher risk (compared with controls) of having a comorbid depressive episode; a 2-fold higher risk of manic episodes; and a 3-fold higher risk of both mania and depression.18 In a study of 62 inpatients, Fasmer19 reported that 46% of patients with unipolar depression and 44% of patients with bipolar disorder experienced migraine (77% of the bipolar disorder patients with migraine had bipolar II disorder). Patients with migraine are at increased risk of suicide attempts (odds ratio 4.3; 95% CI, 1.2-15.7).20
Tension-type headache. The relationship between psychiatric comorbidity in tension-type headache is well established. In contrast to what is seen with migraines, Puca et al21 found a higher prevalence of anxiety disorders (52.5%) than depressive disorders (36.4%) in patients with tension-type headache. Generalized anxiety disorder was one of the most prevalent anxiety conditions (83.3%), and dysthymia was the most prevalent mood disorder (45.6%). In the same study, 21.7% of patients were found to have a comorbid somatoform disorder.21
Emotional and cognitive factors can co-occur in patients with tension-type headache and a comorbid psychiatric condition. For example, difficulty identifying or recognizing emotions—commonly referred to as alexithymia—has been linked to tension-type headache.22 Additionally, maladaptive cognitive appraisal of stress is more common among patients with tension-type headache when compared with those without headaches.23 Being mindful of and recognizing these co-occurring emotional and cognitive factors will help clinicians construct a more accurate assessment and effective behavioral treatment plan.
Clinical assessment with a useful mnemonic
Clinical assessment of psychiatric illness is essential when evaluating chronic pain patients. Using the acronym AMPS (Anxiety, Mood, Psychosis, and Substance use disorders) (Table 3) is an efficient way for the clinician to ask pertinent questions regarding common psychiatric conditions that could have a direct effect on chronic pain.24 Head pain can be more intense when combined with untreated anxiety, depression, psychosis, or a substance use disorder. Untreated anxiety, for example, can amplify sympathetic response to pain and complicate treatment.
Investigating head pain patients for an underlying mood disorder is essential to providing successful treatment. Consider:
• starting psychotherapy modalities that address both pain and psychiatric illness, such as cognitive-behavioral therapy (CBT)
• reframing unhelpful pain beliefs
• managing activity-rest levels
• biofeedback
• supportive group therapy
• reducing family members’ reinforcement of the patient’s pain behavior or sick role.25
Assessing for somatic symptom disorders
In addition to using the AMPS approach for psychiatric assessment, clinicians should evaluate for somatization, which can present as head pain. Somatic symptom disorders (SSD) are a class of conditions that are impacted by affective, cognitive, and reinforcing factors that might or might not be consciously or intentionally produced. Patients with an SSD have somatic symptoms that are distressing or cause significant disruption of daily life because of excessive thoughts, feelings, or behaviors related to the somatic symptoms, for ≥6 months. The Figure outlines SSD, related conditions, and their respective prominent symptoms to assist in the differential diagnosis.26
Note that some headache conditions present with severe distress because of their abrupt onset and severity of symptoms (eg, cluster headaches). Therefore, the expectation and likelihood of psychological disturbance should be factored into a diagnosis of SSD and related conditions as seen in the Figure.
Secondary factors of unusual pain behavior or treatment response. The role of thoughts, affect, and behaviors is clinically meaningful in understanding SSD and similar conditions. Specific questions about cultural beliefs and rituals as they relate to exacerbations of head pain are of value. Table 413,27 lists behavioral, cognitive, and affective dimensions of head pain using the biopsychosocial model, and further clarifies common questions that arise with unusual pain response and complex patient presentations, which were outlined in the beginning of the article.
Because depression and anxiety can be comorbid with head pain, it is important to recognize psychological factors that contribute to pain perception. Indifference or denial of emotional stress as a result of severe pain and disability can imply a somatization process, which could suggest emotional disconnection or dissociation from somatic functioning.28 This finding can be a component of alexithymia, in which a person is disconnected from emotions and how emotions impact the body. Therefore, recognizing alexithymia assists in identifying psychological factors when patients deny mood symptoms, particularly in tension-type headache.
Functional assessment to rule out the disproportional impact of pain on daily activities is helpful in understanding the somatization process. Neurocognitive functioning should be assessed, particularly because frontal and subcortical dysregulation has been observed in head pain sufferers.29,30 Patients with cognitive changes as a result of a medical illness (eg, stroke, head concussion, brain tumor, or seizures) are especially at risk for neurocognitive dysfunction.
Neuropsychological assessment can be useful, not only to assess neurocognitive functioning (eg, Repeated Battery for the Assessment of Neuropsychological Status) but to identify objective test profiles associated with altered motivation (eg, Rey 15-Item Test, Minnesota Multiphasic Personality Inventory-2-Restructured Form F Scale, Personality Assessment Inventory [PAI] Negative Impression Management) and somatization processes (eg, PAI Somatization Scale). These instruments help to identify the severity of psychiatric and neurocognitive symptoms by comparing scores to normative (eg, healthy control group), clinical (eg, somatization, traumatic brain injury, mild cognitive impairment), and altered motivation (eg, persons instructed to exaggerate symptoms) databases.
If the clinician pursues neurocognitive assessment, direct referral to a neuropsychologist, referral to neurologist, or administration of a cognitive screening tool such as the Montreal Cognitive Assessment, Saint Louis University Mental Status, or Cognitive Log is recommended. If the cognitive screening is positive, next steps include: referring for full neuropsychological assessment, which includes complete cognitive and motor testing, personality testing, and integration of neuroimaging data (eg, MRI, CT scans, and/or EEG).
Assessing the patients’ self-talk or thought patterns as they describe their head pain will help clinicians understand belief systems that may be distorting the reality of the medical condition. For example, a patient might report that “my pain feels like someone is hitting me with an axe”; this is a catastrophic thought that can distort the clarity and perceptibility of pain. Encouraging patients to monitor and analyze their anxiety and associated negative thoughts is an important strategy for improving mood and decreasing somatization. Recording daily thoughts and CBT can help the patient identify and appropriately address his (her) cognitive distortions and futile thinking.
When implementing a treatment plan for somatization disorder, we propose the mnemonic device CARE MD:
• CBT
• Assess (by ruling out a medical cause for somatic complaints)
• Regular visits
• Empathy
• Med-psych interface (help the patient connect physical complaints and emotional stressors)
• Do no harm.
Clinical recommendations
Chronic head pain can be debilitating; psychodiagnostic assessment should therefore be considered an important part of the diagnosis and treatment plan. After ruling out common and emergent primary or secondary causes of head pain, consider psychiatric comorbidities. Depression and anxiety have a strong bidirectional relationship with chronic headache; therefore, we suggest evaluating patients with the intention of alleviating both psychiatric symptoms and head pain.
It is important to diligently assess for common psychiatric comorbidities; using the AMPS and CARE MD mnemonics, along with screening for somatization disorders, is an easy and effective way to evaluate for relevant psychiatric conditions associated with chronic head pain. Because many patients have unusual and complicated responses to head pain that can be explained by non-pathophysiological and non-biomechanical models, using the biopsychosocial model is essential for effective diagnosis, assessment, and treatment. Abortive and prophylactic medical interventions, as well as behavioral, sociocultural, and cognitive assessment, are vital to a comprehensive treatment approach.
Bottom Line
The psychodiagnostic assessment can help the astute clinician identify comorbid psychiatric conditions, psychological factors, and somatic symptoms to develop a comprehensive biopsychosocial treatment plan for patients with chronic head pain. Rule out primary and secondary causes of pain and screen for somatization disorders. Consider medication and psychotherapeutic treatment options.
Related Resources
• Pompili M, Di Cosimo D, Innamorati M, et al. Psychiatric comorbidity in patients with chronic daily headache and migraine: a selective overview including personality traits and suicide risk. J Headache Pain. 2009;10(4):283-290.
• Sinclair AJ, Sturrock A, Davies B, et al. Headache management: pharmacological approaches [published online July 3, 2015]. Pract Neurol. doi: 10.1136/practneurol-2015-001167.
Drug Brand Names
Amitriptyline • Elavil Meperidine • Demerol
Botulinum toxin A • Botox Metoclopramide • Reglan
Divalproex sodium • Depakote Propranolol • Inderide
Ketorolac • Toradol Topiramate • Topamax
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
More than 45% of people worldwide suffer from headache at some point in their life.1 Head pain can lead to disability and functional decline, yet headache disorders often are underdiagnosed and poorly assessed. For example, 60% of migraine and tension-type headaches go undiagnosed and 50% of persons suffering from migraine have severe functional disability or require bed rest.2-4
Because head pain can be associated with secondary medical and psychiatric conditions, diagnosis can be challenging. This article reviews the medical and psychological aspects of major headaches and assists with clinical assessment. We present clinical interviewing tools and a diagram to enhance focused, efficient assessment and inform treatment plans.
Classification of headache
Headache is a common complaint, yet it is often underdiagnosed and ineffectively treated. The World Health Organization estimates that, globally, 50% of people with headache self-treat their pain.5 The International Headache Society classifies headache as primary or secondary; approximately 90% of complaints are from primary headache.6
Assessment and diagnosis of headache can be complex because of overlapping, subjective symptoms. It is important to have a general understanding of primary and secondary causes of headache so that interrelated symptoms do not obscure the most accurate diagnosis and effective treatment course. Although most headache complaints are benign, ruling out secondary causes helps gauge the likelihood of developing severe sequelae from underlying pathology.
By definition, primary headaches are idiopathic and commonly include migraine, tension-type, cluster, and hemicrania continua headache. Secondary headaches have an underlying pathology, which could improve by targeting the disorder. Common secondary causes of headache include:
• trauma
• vascular abnormalities
• structural abnormalities
• chemical (including medications)
• inflammation or infection
• metabolic conditions
• diseases of the neck and pericranial and intracranial structures
• psychiatric conditions.
Table 1 illustrates common causes of head pain. More definitive criteria for symptoms and diagnosis can be found in the International Classification of Headache Disorders.7
Primary headache
Tension-type is the most common primary headache, accounting for more than one-half of all headaches.7 Patients usually describe a tight pain in a bilateral band-like distribution, which could be caused by sustained neck muscle contraction. Pain usually builds in intensity and can last 30 minutes to several days. There is a well-established association between emotional stress or depression and the development of tension-type headaches.8
Migraine typically causes pulsating pain in a localized area of the head that lasts as long as 72 hours and can be associated with nausea, vomiting, photophobia, phono-phobia, and aura. Patients report varying precipitating factors but commonly cite certain foods, menstruation, and sleep deprivation. Although rare, migraine with aura has been linked to ischemic stroke; most cases have been reported in female smokers and oral contraceptive users age <45.9
Because migraines can be debilitating, some patients—typically those with ≥4 attacks a month—opt for prophylactic medication. Effective prophylactics include amitriptyline, propranolol, divalproex sodium, and topiramate, which should be monitored closely and given a trial for several months before switching to another drug. Commonly used abortive treatments include triptans and anti-emetics such as metoclopramide.
Meperidine and ketorolac are popular second-line agents for migraine. Botulinum toxin A also has been used in severe cases to reduce the number of headache days in chronic migraine patients.6
Cluster headache is rare, but typically exhibits repeated burning and intense unilateral periorbital or retro-orbital pain that lasts 15 minutes to 3 hours over several weeks. Men are predominantly affected. Cluster headaches typically improve with oxygen treatment.
Biopsychosocial model of head pain
The biomedical model has helped iden tify pathophysiological pain mechanisms and pharmacotherapeutic agents for headache. However, during assessment, limiting one’s attention to the linear relationship between pathology, mechanism of action, and pain oversimplifies common questions clinicians face when assessing chronic head pain.
Advancements in the last 3 decades have expanded the conceptualization of head pain to integrate sociocultural, environmental, behavioral, affective, cognitive, and biological variables—otherwise known as the biopsychosocial model.10,11 The biopsychosocial model is a multidimensional theory that helps answer difficult clinical assessment questions and complex patient presentations (Table 2).10-13 Many unusual responses to pain treatment, questionable validity of pain behavior, and disproportionate pain perception and functional decline are explained by non-pathophysiological and non-biomechanical models.
Psychiatric comorbidity and head pain
Psychiatric conditions are highly prevalent among persons with primary headache. Verri et al14 found that 90% of chronic daily headache patients had ≥1 psychiatric condition; depression and anxiety were most common. Of concern, 1 study found that headache is associated with increased frequency of suicidal ideation among patients with chronic pain.15 It is critical for clinicians to screen for psychiatric comorbidities in patients with chronic headache. Conversely, clinicians might want to screen for headache in their patients with psychiatric illness.
Migraine. Mood disorders are common among patients who suffer from migraine. The rate of depression is 2 to 4 times higher in those with migraine compared with healthy controls.16,17 In a large-scale study, patients with migraine had a 1.9-fold higher risk (compared with controls) of having a comorbid depressive episode; a 2-fold higher risk of manic episodes; and a 3-fold higher risk of both mania and depression.18 In a study of 62 inpatients, Fasmer19 reported that 46% of patients with unipolar depression and 44% of patients with bipolar disorder experienced migraine (77% of the bipolar disorder patients with migraine had bipolar II disorder). Patients with migraine are at increased risk of suicide attempts (odds ratio 4.3; 95% CI, 1.2-15.7).20
Tension-type headache. The relationship between psychiatric comorbidity in tension-type headache is well established. In contrast to what is seen with migraines, Puca et al21 found a higher prevalence of anxiety disorders (52.5%) than depressive disorders (36.4%) in patients with tension-type headache. Generalized anxiety disorder was one of the most prevalent anxiety conditions (83.3%), and dysthymia was the most prevalent mood disorder (45.6%). In the same study, 21.7% of patients were found to have a comorbid somatoform disorder.21
Emotional and cognitive factors can co-occur in patients with tension-type headache and a comorbid psychiatric condition. For example, difficulty identifying or recognizing emotions—commonly referred to as alexithymia—has been linked to tension-type headache.22 Additionally, maladaptive cognitive appraisal of stress is more common among patients with tension-type headache when compared with those without headaches.23 Being mindful of and recognizing these co-occurring emotional and cognitive factors will help clinicians construct a more accurate assessment and effective behavioral treatment plan.
Clinical assessment with a useful mnemonic
Clinical assessment of psychiatric illness is essential when evaluating chronic pain patients. Using the acronym AMPS (Anxiety, Mood, Psychosis, and Substance use disorders) (Table 3) is an efficient way for the clinician to ask pertinent questions regarding common psychiatric conditions that could have a direct effect on chronic pain.24 Head pain can be more intense when combined with untreated anxiety, depression, psychosis, or a substance use disorder. Untreated anxiety, for example, can amplify sympathetic response to pain and complicate treatment.
Investigating head pain patients for an underlying mood disorder is essential to providing successful treatment. Consider:
• starting psychotherapy modalities that address both pain and psychiatric illness, such as cognitive-behavioral therapy (CBT)
• reframing unhelpful pain beliefs
• managing activity-rest levels
• biofeedback
• supportive group therapy
• reducing family members’ reinforcement of the patient’s pain behavior or sick role.25
Assessing for somatic symptom disorders
In addition to using the AMPS approach for psychiatric assessment, clinicians should evaluate for somatization, which can present as head pain. Somatic symptom disorders (SSD) are a class of conditions that are impacted by affective, cognitive, and reinforcing factors that might or might not be consciously or intentionally produced. Patients with an SSD have somatic symptoms that are distressing or cause significant disruption of daily life because of excessive thoughts, feelings, or behaviors related to the somatic symptoms, for ≥6 months. The Figure outlines SSD, related conditions, and their respective prominent symptoms to assist in the differential diagnosis.26
Note that some headache conditions present with severe distress because of their abrupt onset and severity of symptoms (eg, cluster headaches). Therefore, the expectation and likelihood of psychological disturbance should be factored into a diagnosis of SSD and related conditions as seen in the Figure.
Secondary factors of unusual pain behavior or treatment response. The role of thoughts, affect, and behaviors is clinically meaningful in understanding SSD and similar conditions. Specific questions about cultural beliefs and rituals as they relate to exacerbations of head pain are of value. Table 413,27 lists behavioral, cognitive, and affective dimensions of head pain using the biopsychosocial model, and further clarifies common questions that arise with unusual pain response and complex patient presentations, which were outlined in the beginning of the article.
Because depression and anxiety can be comorbid with head pain, it is important to recognize psychological factors that contribute to pain perception. Indifference or denial of emotional stress as a result of severe pain and disability can imply a somatization process, which could suggest emotional disconnection or dissociation from somatic functioning.28 This finding can be a component of alexithymia, in which a person is disconnected from emotions and how emotions impact the body. Therefore, recognizing alexithymia assists in identifying psychological factors when patients deny mood symptoms, particularly in tension-type headache.
Functional assessment to rule out the disproportional impact of pain on daily activities is helpful in understanding the somatization process. Neurocognitive functioning should be assessed, particularly because frontal and subcortical dysregulation has been observed in head pain sufferers.29,30 Patients with cognitive changes as a result of a medical illness (eg, stroke, head concussion, brain tumor, or seizures) are especially at risk for neurocognitive dysfunction.
Neuropsychological assessment can be useful, not only to assess neurocognitive functioning (eg, Repeated Battery for the Assessment of Neuropsychological Status) but to identify objective test profiles associated with altered motivation (eg, Rey 15-Item Test, Minnesota Multiphasic Personality Inventory-2-Restructured Form F Scale, Personality Assessment Inventory [PAI] Negative Impression Management) and somatization processes (eg, PAI Somatization Scale). These instruments help to identify the severity of psychiatric and neurocognitive symptoms by comparing scores to normative (eg, healthy control group), clinical (eg, somatization, traumatic brain injury, mild cognitive impairment), and altered motivation (eg, persons instructed to exaggerate symptoms) databases.
If the clinician pursues neurocognitive assessment, direct referral to a neuropsychologist, referral to neurologist, or administration of a cognitive screening tool such as the Montreal Cognitive Assessment, Saint Louis University Mental Status, or Cognitive Log is recommended. If the cognitive screening is positive, next steps include: referring for full neuropsychological assessment, which includes complete cognitive and motor testing, personality testing, and integration of neuroimaging data (eg, MRI, CT scans, and/or EEG).
Assessing the patients’ self-talk or thought patterns as they describe their head pain will help clinicians understand belief systems that may be distorting the reality of the medical condition. For example, a patient might report that “my pain feels like someone is hitting me with an axe”; this is a catastrophic thought that can distort the clarity and perceptibility of pain. Encouraging patients to monitor and analyze their anxiety and associated negative thoughts is an important strategy for improving mood and decreasing somatization. Recording daily thoughts and CBT can help the patient identify and appropriately address his (her) cognitive distortions and futile thinking.
When implementing a treatment plan for somatization disorder, we propose the mnemonic device CARE MD:
• CBT
• Assess (by ruling out a medical cause for somatic complaints)
• Regular visits
• Empathy
• Med-psych interface (help the patient connect physical complaints and emotional stressors)
• Do no harm.
Clinical recommendations
Chronic head pain can be debilitating; psychodiagnostic assessment should therefore be considered an important part of the diagnosis and treatment plan. After ruling out common and emergent primary or secondary causes of head pain, consider psychiatric comorbidities. Depression and anxiety have a strong bidirectional relationship with chronic headache; therefore, we suggest evaluating patients with the intention of alleviating both psychiatric symptoms and head pain.
It is important to diligently assess for common psychiatric comorbidities; using the AMPS and CARE MD mnemonics, along with screening for somatization disorders, is an easy and effective way to evaluate for relevant psychiatric conditions associated with chronic head pain. Because many patients have unusual and complicated responses to head pain that can be explained by non-pathophysiological and non-biomechanical models, using the biopsychosocial model is essential for effective diagnosis, assessment, and treatment. Abortive and prophylactic medical interventions, as well as behavioral, sociocultural, and cognitive assessment, are vital to a comprehensive treatment approach.
Bottom Line
The psychodiagnostic assessment can help the astute clinician identify comorbid psychiatric conditions, psychological factors, and somatic symptoms to develop a comprehensive biopsychosocial treatment plan for patients with chronic head pain. Rule out primary and secondary causes of pain and screen for somatization disorders. Consider medication and psychotherapeutic treatment options.
Related Resources
• Pompili M, Di Cosimo D, Innamorati M, et al. Psychiatric comorbidity in patients with chronic daily headache and migraine: a selective overview including personality traits and suicide risk. J Headache Pain. 2009;10(4):283-290.
• Sinclair AJ, Sturrock A, Davies B, et al. Headache management: pharmacological approaches [published online July 3, 2015]. Pract Neurol. doi: 10.1136/practneurol-2015-001167.
Drug Brand Names
Amitriptyline • Elavil Meperidine • Demerol
Botulinum toxin A • Botox Metoclopramide • Reglan
Divalproex sodium • Depakote Propranolol • Inderide
Ketorolac • Toradol Topiramate • Topamax
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193-210.
2. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657.
3. The World Health Report 2001: Mental health: new understanding new hope. Geneva, Switzerland: World Health Organization; 2001.
4. World Health Organization. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_ disease/GBD_report_2004update_full.pdf. Published 2004. Accessed July 31, 2015.
5. World Health Organization. Headache disorders. http:// www.who.int/mediacentre/factsheets/fs277/en/. Published October 2012. Accessed July 31, 2015.
6. Clinch C. Evaluation & management of headache. In: South- Paul JE, Matheny SC, Lewis EL. eds. Current diagnosis & treatment in family medicine, 4th ed. New York, NY: McGraw-Hill; 2015:293-297.
7. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
8. Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia. 2015;35(2):165-181.
9. Janke EA, Holroyd KA, Romanek K. Depression increases onset of tension-type headache following laboratory stress. Pain. 2004;111(3):230-238.
10. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
11. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544.
12. Turk DC, Flor H. Chronic pain: a biobehavioral perspective. In: Gatchel RJ, Turk DC, eds. Psychosocial factors in pain: critical perspectives. New York, NY: Guilford Press; 1999:18-34.
13. Andrasik F, Flor H, Turk DC. An expanded view of psychological aspects in head pain: the biopsychosocial model. Neurol Sci. 2005;26(suppl 2):s87-s91.
14. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998; 18(suppl 21):45-49.
15. Ilgen MA, Zivin K, McCammon RJ, et al. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30(6):521-527.
16. Kowacs F, Socal MP, Ziomkowski SC, et al. Symptoms of depression and anxiety, and screening for mental disorders in migrainous patients. Cephalalgia. 2003;23(2):79-89.
17. Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache. 2006;46(9):1327-1333.
18. Nguyen TV, Low NC. Comorbidity of migraine and mood episodes in a nationally representative population-based sample. Headache. 2013;53(3):498-506.
19. Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001; 21(9):894-899.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology. 1992;42(2):392-395.
21. Puca F, Genco S, Prudenzano MP, et al. Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. Cephalalgia. 1999;19(3):159-164.
22. Yücel B, Kora K, Ozyalçín S, et al. Depression, automatic thoughts, alexithymia, and assertiveness in patients with tension-type headache. Headache. 2002;42(3):194-199.
23. Wittrock DA, Myers TC. The comparison of individuals with recurrent tension-type headache and headache-free controls in physiological response, appraisal, and coping with stressors: a review of the literature. Ann Behav Med. 1998;20(2):118-134.
24. Onate J, Xiong G, McCarron R. The primary care psychiatric interview. In: McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott, Williams and Wilkins; 2009:3-4.
25. Songer D. Psychotherapeutic approaches in the treatment of pain. Psychiatry (Edgmont). 2005;2(5):19-24.
26. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
27. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(pt 9):2751-2768.
28. Packard RC. Conversion headache. Headache. 1980;20(5):266-268.
29. Mongini F, Keller R, Deregibus A, et al. Frontal lobe dysfunction in patients with chronic migraine: a clinical-neuropsychological study. Psychiatry Res. 2005;133(1):101-106.
30. Martelli MF, Grayson RL, Zasler ND. Posttraumatic headache: neuropsychological and psychological effects and treatment implications. J Head Trauma Rehabil. 1999;14(1):49-69.
1. Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193-210.
2. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-657.
3. The World Health Report 2001: Mental health: new understanding new hope. Geneva, Switzerland: World Health Organization; 2001.
4. World Health Organization. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_ disease/GBD_report_2004update_full.pdf. Published 2004. Accessed July 31, 2015.
5. World Health Organization. Headache disorders. http:// www.who.int/mediacentre/factsheets/fs277/en/. Published October 2012. Accessed July 31, 2015.
6. Clinch C. Evaluation & management of headache. In: South- Paul JE, Matheny SC, Lewis EL. eds. Current diagnosis & treatment in family medicine, 4th ed. New York, NY: McGraw-Hill; 2015:293-297.
7. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
8. Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia. 2015;35(2):165-181.
9. Janke EA, Holroyd KA, Romanek K. Depression increases onset of tension-type headache following laboratory stress. Pain. 2004;111(3):230-238.
10. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129-136.
11. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544.
12. Turk DC, Flor H. Chronic pain: a biobehavioral perspective. In: Gatchel RJ, Turk DC, eds. Psychosocial factors in pain: critical perspectives. New York, NY: Guilford Press; 1999:18-34.
13. Andrasik F, Flor H, Turk DC. An expanded view of psychological aspects in head pain: the biopsychosocial model. Neurol Sci. 2005;26(suppl 2):s87-s91.
14. Verri AP, Proietti Cecchini A, Galli C, et al. Psychiatric comorbidity in chronic daily headache. Cephalalgia. 1998; 18(suppl 21):45-49.
15. Ilgen MA, Zivin K, McCammon RJ, et al. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30(6):521-527.
16. Kowacs F, Socal MP, Ziomkowski SC, et al. Symptoms of depression and anxiety, and screening for mental disorders in migrainous patients. Cephalalgia. 2003;23(2):79-89.
17. Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache. 2006;46(9):1327-1333.
18. Nguyen TV, Low NC. Comorbidity of migraine and mood episodes in a nationally representative population-based sample. Headache. 2013;53(3):498-506.
19. Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001; 21(9):894-899.
20. Breslau N. Migraine, suicidal ideation, and suicide attempts. Neurology. 1992;42(2):392-395.
21. Puca F, Genco S, Prudenzano MP, et al. Psychiatric comorbidity and psychosocial stress in patients with tension-type headache from headache centers in Italy. Cephalalgia. 1999;19(3):159-164.
22. Yücel B, Kora K, Ozyalçín S, et al. Depression, automatic thoughts, alexithymia, and assertiveness in patients with tension-type headache. Headache. 2002;42(3):194-199.
23. Wittrock DA, Myers TC. The comparison of individuals with recurrent tension-type headache and headache-free controls in physiological response, appraisal, and coping with stressors: a review of the literature. Ann Behav Med. 1998;20(2):118-134.
24. Onate J, Xiong G, McCarron R. The primary care psychiatric interview. In: McCarron R, Xiong G, Bourgeois J. Lippincott’s primary care psychiatry. Philadelphia, PA: Lippincott, Williams and Wilkins; 2009:3-4.
25. Songer D. Psychotherapeutic approaches in the treatment of pain. Psychiatry (Edgmont). 2005;2(5):19-24.
26. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
27. Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(pt 9):2751-2768.
28. Packard RC. Conversion headache. Headache. 1980;20(5):266-268.
29. Mongini F, Keller R, Deregibus A, et al. Frontal lobe dysfunction in patients with chronic migraine: a clinical-neuropsychological study. Psychiatry Res. 2005;133(1):101-106.
30. Martelli MF, Grayson RL, Zasler ND. Posttraumatic headache: neuropsychological and psychological effects and treatment implications. J Head Trauma Rehabil. 1999;14(1):49-69.
Cannabinoid hyperemesis syndrome: A result of chronic, heavy Cannabis use
Cannabis is the most commonly abused drug in the United States. Since 2008, Cannabis use has significantly increased,1 in part because of legalization for medicinal and recreational use. Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily Cannabis use, recurrent nausea, vomiting and abdominal pain, compulsive bathing for symptom relief, and symptom resolution with cessation of use.
Prompt recognition of CHS can reduce costs associated with unnecessary workups, emergency department (ED) and urgent care visits, and hospital admissions.2,3 This article provides a review of CHS with discussion of diagnostic and management considerations.
CASE REPORT Nauseated and vomiting—and stoned
Mr. M, age 24, self-presents to the ED complaining of two days of severe nausea, colicky abdominal pain, and nonbloody, nonbilious vomiting, as often as 20 times a day. His symptoms become worse with food, and he has difficulty eating and drinking because of his vomiting. Mr. M reports transient symptom relief when he takes hot showers, and has been taking more than 14 showers a day. He reports similar episodes, occurring every two or three months over the last two years, resulting in several ED visits and three hospital admissions.
Mr. M has smoked two to three joints a day for seven years; he has increased his Cannabis use in an attempt to alleviate his symptoms, but isn’t sure if doing so was helpful. He denies use of tobacco and other illicit drugs, and reports drinking one to three drinks no more than twice a month. He reports dizziness when standing, but no other symptoms. He does not take any medications, and medical and psychiatric histories are unremarkable.
Physical exam reveals a thin, uncomfortable, young man. Vital signs were significant for tachycardia and mild orthostatic hypotension. His abdomen was diffusely tender, soft, and nondistended. Urine toxicology is positive for delta-9-tetrahydrocannabinol (THC) only. Labs, including a complete blood count (CBC), basic metabolic panel, liver function tests, and lipase, are within normal limits. Prior workup included abdominal radiographs, abdominal ultrasonography, abdominal CT, and gastric biopsy; all are normal. He has mild gastritis and esophagitis on esophagogastroduodenoscopy and mildly delayed gastric emptying. HIV and hepatitis screenings are negative. Six months ago he received antibiotic therapy for Helicobacter pylori infection.
Mr. M is admitted to the hospital and seen by the psychiatric consultation service. He is treated with IV ondansetron and prochlorperazine, with little effect. He showers frequently until his symptoms begin to abate within 36 hours of stopping Cannabis use, and is discharged soon after. Psychiatric clinicians provide brief motivational interviewing while Mr. M is in the hospital, and refer him to outpatient psychiatric care and Narcotics Anonymous. Mr. M is then lost to follow up.
In 2011, 18.1 million people reported Cannabis use in the previous month; 39% reported use in 20 of the last 30 days.1 A high rate of use and a relatively low number of cases suggests that CHS is rare. However, it is likely that CHS is under-recognized and under-reported.2,4,5 CHS symptoms may be misattributed to cyclic vomiting syndrome,3 because 50% of patients diagnosed with cyclic vomiting syndrome report daily Cannabis use.6 There is no epidemiological data on the incidence or prevalence of CHS among regular Cannabis users.7
Allen and colleagues first described this syndrome in 2004.4 Since then, CHS has been documented in a growing number of case reports and reviews,2,3,5,7-13 yet it continues to be under-recognized. Many CHS patients experience delays in diagnosis—often years—resulting in prolonged suffering, and costs incurred by frequent ED and urgent care visits, hospital admissions, and unnecessary workups.2,3,7
Clinical characteristics
CHS is characterized by recurrent, hyperemetic episodes in the context of chronic, daily Cannabis use.4 The average age of onset is 25.6 years (range: 16 to 51 years).3 Ninety-five percent of CHS patients used Cannabis daily, for, on average, 9.8 years before symptom onset.3 The amount of Cannabis used, although generally high, is difficult to quantify, and has been described as heavy and hourly in units of blunts, cones, joints, bongs, etc. Patients are most likely to present during acute hyperemetic episodes, which occur in a cyclic pattern, every four to eight weeks,3 interspersed with symptom-free periods. Three phases have been described:
- prodromal or pre-emetic phase
- hyperemetic phase
- recovery phase.4,10
Many patients report a prodromal phase, with one or two weeks of morning nausea, food aversion, preserved eating patterns, possible weight loss, and occasional vomiting. The acute, hyperemetic phase is characterized by severe nausea, frequent vomiting, abdominal pain, and compulsive bathing for temporary symptom relief. In the recovery phase, symptom improvement and resolution occur with cessation of Cannabis use.4,10 Symptom improvement can occur within 12 hours of Cannabis cessation, but can take as long as three weeks.3 Patients remain symptom-free while abstinent, but symptoms rapidly recur when they resume use.3,4
Cannabis is used as an antiemetic and appetite stimulant for chemotherapy-associated nausea and for anorexia in HIV infection. The pathogenesis of paradoxical hyperemetic symptoms of CHS remain unclear, but several mechanisms have been proposed. The principle active cannabinoid in Cannabis is the highly lipophilic compound THC, which binds to cannabinoid type 1 (CB1) and type 2 (CB2) receptors in the CNS and other tissues. It is thought that the antiemetic and appetite-stimulating effects of Cannabis are mediated by CB1 receptor activation in the hypothalamus. Nausea and vomiting are thought to be mediated by CB1 receptor activation in the enteric nervous system, which causes slowed peristalsis, delayed gastric emptying, and splanchnic vasodilation.4,14
In sensitive persons, chronic heavy Cannabis use can cause THC to accumulate to a toxic level in fatty tissues, causing enteric receptor binding effects to override the CNS receptor-binding effects.4 This is supported by case studies describing severe vomiting with IV injection of crude marijuana extract.15 Nearly 100 different THC metabolites have been identified. The Cannabis plant contains more than 400 chemicals, with 60 cannabinoid structures, any of which could cause CHS in toxic concentrations.4,7 Among them, cannabidiol, a 5-HT1A partial agonist, was shown to cause vomiting at higher doses in animal studies.4,7
Mechanisms of action
Cannabis has been used for centuries, so it is unclear why CHS is only recently being recognized. It may be because of higher THC content through selective breeding of plants and a more selective use of female buds that contain more concentrated THC levels than leaves and stems.3 Alternately, CHS may be caused by exogenous substances, such as pesticides, additives, preservatives, or other chemicals used in marijuana preparation, although there is little evidence to support this.3
The mechanism of symptom relief with hot bathing also is unclear. Patients report consistent, global symptom improvement with hot bathing.3 Relief is rapid, transient, and temperature dependent.4 CB1 receptors are located near the thermoregulatory center of the hypothalamus. Increased body temperature with hot bathing may counteract the thermoregulatory dysregulation associated with Cannabis use.4,9 It has been proposed that splanchnic vasodilation might contribute to CHS symptoms. Thus, redistribution of blood from the gut to the skin with warm bathing causes a “cutaneous steal syndrome,” resulting in symptom relief.11
Diagnostic approach
Four key features should be present when making a diagnosis of CHS:
- heavy marijuana use
- recurrent episodes of severe nausea, vomiting, and abdominal cramping
- compulsive bathing for transient symptom relief
- resolution of symptoms with cessation of Cannabis use.2,4,8
Compulsive, hot bathing for symptom relief was described in 98% of all reported cases,3 and should be considered pathognomonic.2 CHS patients can present with other symptoms, including polydipsia, mild fever, weight loss, and orthostasis.3 Although lab studies usually are normal, mild leukocytosis, hypokalemia, hypochloremia, elevated salivary amylase, mild gastritis on esophagogastroduodenoscopy, and delayed gastric emptying have been described during acute episodes (Table 1).2-4,7,8
Diagnosis starts with a history and physical exam, followed by a basic workup geared towards ruling out other causes of acute nausea and vomiting.2,7 Establish temporal relationships between symptoms, Cannabis use (onset, frequency, amount, duration), and bathing behaviors. A positive urine toxicology screen supports a CHS diagnosis and can facilitate discussion of Cannabis use.2 If you suspect CHS, rule out potentially life-threatening causes of acute nausea, vomiting, and abdominal pain, such as intestinal obstruction or perforation, pancreaticobiliary disease, and pregnancy. The initial workup should include a CBC, basic metabolic panel, liver function tests, amylase, lipase, pregnancy test, urinalysis, urine toxicology screen, and abdominal radiographs (Table 2).2,4,7 The differential diagnosis of recurrent vomiting is broad and should be considered (Table 3).2,4,7,16 Further workup can proceed non-emergently, and should be prompted by clinical suspicion.2,7
Supportive treatment, education
Treatment of acute hyperemetic episodes in CHS primarily is supportive; address dehydration with IV fluids and electrolyte replenishment as needed.2,4,7 Standard antiemetics, including 5-HT3 receptor antagonists, D2 receptor antagonists, and H1 receptor antagonists, are largely ineffective.5,9 Although narcotics have been used to treat abdominal pain, use caution when prescribing because they can exacerbate nausea and vomiting.7 Case reports have described symptom relief with inpatient treatment with lorazepam12 and self-medication with alprazolam,4 but more evidence is needed. A recent case report described prompt resolution of symptoms with IV haloperidol.13 Treating gastritis symptoms with acid suppression therapy, such as a proton pump inhibitor, has been suggested.7 Symptoms abate during hospitalization regardless of treatment, marking the progression into the recovery phase with abstinence. There are no proven treatments for CHS, aside from cessation of Cannabis use. Treatment should focus on motivating your patient to stop using Cannabis.
Acute, hyperemetic episodes are ideal teachable moments because of the acuity of symptoms and clear association with Cannabis use. However, some patients may be skeptical about CHS because of the better-known antiemetic effects of Cannabis. For such patients, provide informational materials describing CHS and take time to address their concerns or doubts.
Motivational interviewing can help provoke behavior change by exploring patient ambivalence in a directive, patient-focused manner. Randomized controlled trials have documented significant reductions in Cannabis use with single-session motivational interviewing, with greater effect among heavy users.17 Single-session motivational interviewing showed results comparable to providing drug information and advice, suggesting that education and information are useful interventions.18 Although these single-session studies appear promising, they focus on younger users who have not been using Cannabis as long as typical CHS patients. Multi-session interventions may be needed to address longstanding, heavy Cannabis use in adult CHS patients.
Cognitive-behavioral therapy. In a series of randomized controlled trials,
motivational enhancement training and cognitive-behavioral therapy (CBT) were effective for Cannabis use cessation and maintenance of abstinence.19
Although these interventions take more time—six to 14 sessions for CBT and one to four sessions for motivational enhancement training—they should be considered for CHS patients with persistent use.
Bottom Line
Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily, heavy Cannabis use, cyclic nausea and vomiting, and compulsive bathing. Symptoms resolve with Cannabis cessation. Workup of suspected CHS should rule out life- threatening causes of nausea and vomiting. Acute hyperemetic episodes should be managed supportively. Motivational enhancement therapy or cognitive-behavioral therapy should be considered for persistent Cannibis use.
Related Resources
- Motivational interviewing for substance use disorders. www.motivationalinterview.org.
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35(2):309-326.
Drug Brand Names
Alprazolam • Xanax Haloperidol • Haldol Lorazepam • Ativan
Ondansetron • Zofran Prochlorperazine • Compazine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2011 National Survey on Drug Use and Health: Mental health findings. http://www.samhsa.gov/data/NSDUH/2k11MH_FindingsandDetTables/2K11MHFR/NSDUHmhfr2011.htm. Published November 2012. Accessed April 18, 2013.
2. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011; 104(9):659-664.
3. Nicolson SE, Denysenko L, Mulcare JL, et al. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53(3):212-219.
4. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Cut. 2004;53(11):1566-1570.
5. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264-1266.
6. Fajardo NR, Cremonini F, Talley NJ. Cyclic vomiting syndrome and chronic cannabis use. Am J Gastroenterol. 2005;100:S343.
7. Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid hyperemesis syndrome. Curr Drug Abuse Rev. 2011;4(4):241-249.
8. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285.
9. Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc. 2009;84(1):76-78.
10. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci. 2010;55(11):3113-3119.
11. Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23(6):790-793.
12. Cox B, Chhabra A, Adler M, et al. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med. 2012;2012:757696.
13. Hickey JL, Witsil JC, Mycyk MB. Haloperidol for treatment of cannabinoid hyperemesis syndrome [published online April 10, 2013]. Am J Emerg Med. 2013;31(6):1003.e5-6. doi: 10.1016/j.ajem.2013.02.021.
14. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13(1):77-80.
15. Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol. 1981;18(3):353-366.
16. Abell TL, Adams KA, Boles RG, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008; 20(4):269-284.
17. McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction. 2004;99(1):39-52.
18. McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction. 2008;103(11):1809-1818.
19. Elkashef A, Vocci F, Huestis M, et al. Marijuana neurobiology and treatment. Subst Abus. 2008;29(3):17-29.
Cannabis is the most commonly abused drug in the United States. Since 2008, Cannabis use has significantly increased,1 in part because of legalization for medicinal and recreational use. Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily Cannabis use, recurrent nausea, vomiting and abdominal pain, compulsive bathing for symptom relief, and symptom resolution with cessation of use.
Prompt recognition of CHS can reduce costs associated with unnecessary workups, emergency department (ED) and urgent care visits, and hospital admissions.2,3 This article provides a review of CHS with discussion of diagnostic and management considerations.
CASE REPORT Nauseated and vomiting—and stoned
Mr. M, age 24, self-presents to the ED complaining of two days of severe nausea, colicky abdominal pain, and nonbloody, nonbilious vomiting, as often as 20 times a day. His symptoms become worse with food, and he has difficulty eating and drinking because of his vomiting. Mr. M reports transient symptom relief when he takes hot showers, and has been taking more than 14 showers a day. He reports similar episodes, occurring every two or three months over the last two years, resulting in several ED visits and three hospital admissions.
Mr. M has smoked two to three joints a day for seven years; he has increased his Cannabis use in an attempt to alleviate his symptoms, but isn’t sure if doing so was helpful. He denies use of tobacco and other illicit drugs, and reports drinking one to three drinks no more than twice a month. He reports dizziness when standing, but no other symptoms. He does not take any medications, and medical and psychiatric histories are unremarkable.
Physical exam reveals a thin, uncomfortable, young man. Vital signs were significant for tachycardia and mild orthostatic hypotension. His abdomen was diffusely tender, soft, and nondistended. Urine toxicology is positive for delta-9-tetrahydrocannabinol (THC) only. Labs, including a complete blood count (CBC), basic metabolic panel, liver function tests, and lipase, are within normal limits. Prior workup included abdominal radiographs, abdominal ultrasonography, abdominal CT, and gastric biopsy; all are normal. He has mild gastritis and esophagitis on esophagogastroduodenoscopy and mildly delayed gastric emptying. HIV and hepatitis screenings are negative. Six months ago he received antibiotic therapy for Helicobacter pylori infection.
Mr. M is admitted to the hospital and seen by the psychiatric consultation service. He is treated with IV ondansetron and prochlorperazine, with little effect. He showers frequently until his symptoms begin to abate within 36 hours of stopping Cannabis use, and is discharged soon after. Psychiatric clinicians provide brief motivational interviewing while Mr. M is in the hospital, and refer him to outpatient psychiatric care and Narcotics Anonymous. Mr. M is then lost to follow up.
In 2011, 18.1 million people reported Cannabis use in the previous month; 39% reported use in 20 of the last 30 days.1 A high rate of use and a relatively low number of cases suggests that CHS is rare. However, it is likely that CHS is under-recognized and under-reported.2,4,5 CHS symptoms may be misattributed to cyclic vomiting syndrome,3 because 50% of patients diagnosed with cyclic vomiting syndrome report daily Cannabis use.6 There is no epidemiological data on the incidence or prevalence of CHS among regular Cannabis users.7
Allen and colleagues first described this syndrome in 2004.4 Since then, CHS has been documented in a growing number of case reports and reviews,2,3,5,7-13 yet it continues to be under-recognized. Many CHS patients experience delays in diagnosis—often years—resulting in prolonged suffering, and costs incurred by frequent ED and urgent care visits, hospital admissions, and unnecessary workups.2,3,7
Clinical characteristics
CHS is characterized by recurrent, hyperemetic episodes in the context of chronic, daily Cannabis use.4 The average age of onset is 25.6 years (range: 16 to 51 years).3 Ninety-five percent of CHS patients used Cannabis daily, for, on average, 9.8 years before symptom onset.3 The amount of Cannabis used, although generally high, is difficult to quantify, and has been described as heavy and hourly in units of blunts, cones, joints, bongs, etc. Patients are most likely to present during acute hyperemetic episodes, which occur in a cyclic pattern, every four to eight weeks,3 interspersed with symptom-free periods. Three phases have been described:
- prodromal or pre-emetic phase
- hyperemetic phase
- recovery phase.4,10
Many patients report a prodromal phase, with one or two weeks of morning nausea, food aversion, preserved eating patterns, possible weight loss, and occasional vomiting. The acute, hyperemetic phase is characterized by severe nausea, frequent vomiting, abdominal pain, and compulsive bathing for temporary symptom relief. In the recovery phase, symptom improvement and resolution occur with cessation of Cannabis use.4,10 Symptom improvement can occur within 12 hours of Cannabis cessation, but can take as long as three weeks.3 Patients remain symptom-free while abstinent, but symptoms rapidly recur when they resume use.3,4
Cannabis is used as an antiemetic and appetite stimulant for chemotherapy-associated nausea and for anorexia in HIV infection. The pathogenesis of paradoxical hyperemetic symptoms of CHS remain unclear, but several mechanisms have been proposed. The principle active cannabinoid in Cannabis is the highly lipophilic compound THC, which binds to cannabinoid type 1 (CB1) and type 2 (CB2) receptors in the CNS and other tissues. It is thought that the antiemetic and appetite-stimulating effects of Cannabis are mediated by CB1 receptor activation in the hypothalamus. Nausea and vomiting are thought to be mediated by CB1 receptor activation in the enteric nervous system, which causes slowed peristalsis, delayed gastric emptying, and splanchnic vasodilation.4,14
In sensitive persons, chronic heavy Cannabis use can cause THC to accumulate to a toxic level in fatty tissues, causing enteric receptor binding effects to override the CNS receptor-binding effects.4 This is supported by case studies describing severe vomiting with IV injection of crude marijuana extract.15 Nearly 100 different THC metabolites have been identified. The Cannabis plant contains more than 400 chemicals, with 60 cannabinoid structures, any of which could cause CHS in toxic concentrations.4,7 Among them, cannabidiol, a 5-HT1A partial agonist, was shown to cause vomiting at higher doses in animal studies.4,7
Mechanisms of action
Cannabis has been used for centuries, so it is unclear why CHS is only recently being recognized. It may be because of higher THC content through selective breeding of plants and a more selective use of female buds that contain more concentrated THC levels than leaves and stems.3 Alternately, CHS may be caused by exogenous substances, such as pesticides, additives, preservatives, or other chemicals used in marijuana preparation, although there is little evidence to support this.3
The mechanism of symptom relief with hot bathing also is unclear. Patients report consistent, global symptom improvement with hot bathing.3 Relief is rapid, transient, and temperature dependent.4 CB1 receptors are located near the thermoregulatory center of the hypothalamus. Increased body temperature with hot bathing may counteract the thermoregulatory dysregulation associated with Cannabis use.4,9 It has been proposed that splanchnic vasodilation might contribute to CHS symptoms. Thus, redistribution of blood from the gut to the skin with warm bathing causes a “cutaneous steal syndrome,” resulting in symptom relief.11
Diagnostic approach
Four key features should be present when making a diagnosis of CHS:
- heavy marijuana use
- recurrent episodes of severe nausea, vomiting, and abdominal cramping
- compulsive bathing for transient symptom relief
- resolution of symptoms with cessation of Cannabis use.2,4,8
Compulsive, hot bathing for symptom relief was described in 98% of all reported cases,3 and should be considered pathognomonic.2 CHS patients can present with other symptoms, including polydipsia, mild fever, weight loss, and orthostasis.3 Although lab studies usually are normal, mild leukocytosis, hypokalemia, hypochloremia, elevated salivary amylase, mild gastritis on esophagogastroduodenoscopy, and delayed gastric emptying have been described during acute episodes (Table 1).2-4,7,8
Diagnosis starts with a history and physical exam, followed by a basic workup geared towards ruling out other causes of acute nausea and vomiting.2,7 Establish temporal relationships between symptoms, Cannabis use (onset, frequency, amount, duration), and bathing behaviors. A positive urine toxicology screen supports a CHS diagnosis and can facilitate discussion of Cannabis use.2 If you suspect CHS, rule out potentially life-threatening causes of acute nausea, vomiting, and abdominal pain, such as intestinal obstruction or perforation, pancreaticobiliary disease, and pregnancy. The initial workup should include a CBC, basic metabolic panel, liver function tests, amylase, lipase, pregnancy test, urinalysis, urine toxicology screen, and abdominal radiographs (Table 2).2,4,7 The differential diagnosis of recurrent vomiting is broad and should be considered (Table 3).2,4,7,16 Further workup can proceed non-emergently, and should be prompted by clinical suspicion.2,7
Supportive treatment, education
Treatment of acute hyperemetic episodes in CHS primarily is supportive; address dehydration with IV fluids and electrolyte replenishment as needed.2,4,7 Standard antiemetics, including 5-HT3 receptor antagonists, D2 receptor antagonists, and H1 receptor antagonists, are largely ineffective.5,9 Although narcotics have been used to treat abdominal pain, use caution when prescribing because they can exacerbate nausea and vomiting.7 Case reports have described symptom relief with inpatient treatment with lorazepam12 and self-medication with alprazolam,4 but more evidence is needed. A recent case report described prompt resolution of symptoms with IV haloperidol.13 Treating gastritis symptoms with acid suppression therapy, such as a proton pump inhibitor, has been suggested.7 Symptoms abate during hospitalization regardless of treatment, marking the progression into the recovery phase with abstinence. There are no proven treatments for CHS, aside from cessation of Cannabis use. Treatment should focus on motivating your patient to stop using Cannabis.
Acute, hyperemetic episodes are ideal teachable moments because of the acuity of symptoms and clear association with Cannabis use. However, some patients may be skeptical about CHS because of the better-known antiemetic effects of Cannabis. For such patients, provide informational materials describing CHS and take time to address their concerns or doubts.
Motivational interviewing can help provoke behavior change by exploring patient ambivalence in a directive, patient-focused manner. Randomized controlled trials have documented significant reductions in Cannabis use with single-session motivational interviewing, with greater effect among heavy users.17 Single-session motivational interviewing showed results comparable to providing drug information and advice, suggesting that education and information are useful interventions.18 Although these single-session studies appear promising, they focus on younger users who have not been using Cannabis as long as typical CHS patients. Multi-session interventions may be needed to address longstanding, heavy Cannabis use in adult CHS patients.
Cognitive-behavioral therapy. In a series of randomized controlled trials,
motivational enhancement training and cognitive-behavioral therapy (CBT) were effective for Cannabis use cessation and maintenance of abstinence.19
Although these interventions take more time—six to 14 sessions for CBT and one to four sessions for motivational enhancement training—they should be considered for CHS patients with persistent use.
Bottom Line
Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily, heavy Cannabis use, cyclic nausea and vomiting, and compulsive bathing. Symptoms resolve with Cannabis cessation. Workup of suspected CHS should rule out life- threatening causes of nausea and vomiting. Acute hyperemetic episodes should be managed supportively. Motivational enhancement therapy or cognitive-behavioral therapy should be considered for persistent Cannibis use.
Related Resources
- Motivational interviewing for substance use disorders. www.motivationalinterview.org.
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35(2):309-326.
Drug Brand Names
Alprazolam • Xanax Haloperidol • Haldol Lorazepam • Ativan
Ondansetron • Zofran Prochlorperazine • Compazine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Cannabis is the most commonly abused drug in the United States. Since 2008, Cannabis use has significantly increased,1 in part because of legalization for medicinal and recreational use. Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily Cannabis use, recurrent nausea, vomiting and abdominal pain, compulsive bathing for symptom relief, and symptom resolution with cessation of use.
Prompt recognition of CHS can reduce costs associated with unnecessary workups, emergency department (ED) and urgent care visits, and hospital admissions.2,3 This article provides a review of CHS with discussion of diagnostic and management considerations.
CASE REPORT Nauseated and vomiting—and stoned
Mr. M, age 24, self-presents to the ED complaining of two days of severe nausea, colicky abdominal pain, and nonbloody, nonbilious vomiting, as often as 20 times a day. His symptoms become worse with food, and he has difficulty eating and drinking because of his vomiting. Mr. M reports transient symptom relief when he takes hot showers, and has been taking more than 14 showers a day. He reports similar episodes, occurring every two or three months over the last two years, resulting in several ED visits and three hospital admissions.
Mr. M has smoked two to three joints a day for seven years; he has increased his Cannabis use in an attempt to alleviate his symptoms, but isn’t sure if doing so was helpful. He denies use of tobacco and other illicit drugs, and reports drinking one to three drinks no more than twice a month. He reports dizziness when standing, but no other symptoms. He does not take any medications, and medical and psychiatric histories are unremarkable.
Physical exam reveals a thin, uncomfortable, young man. Vital signs were significant for tachycardia and mild orthostatic hypotension. His abdomen was diffusely tender, soft, and nondistended. Urine toxicology is positive for delta-9-tetrahydrocannabinol (THC) only. Labs, including a complete blood count (CBC), basic metabolic panel, liver function tests, and lipase, are within normal limits. Prior workup included abdominal radiographs, abdominal ultrasonography, abdominal CT, and gastric biopsy; all are normal. He has mild gastritis and esophagitis on esophagogastroduodenoscopy and mildly delayed gastric emptying. HIV and hepatitis screenings are negative. Six months ago he received antibiotic therapy for Helicobacter pylori infection.
Mr. M is admitted to the hospital and seen by the psychiatric consultation service. He is treated with IV ondansetron and prochlorperazine, with little effect. He showers frequently until his symptoms begin to abate within 36 hours of stopping Cannabis use, and is discharged soon after. Psychiatric clinicians provide brief motivational interviewing while Mr. M is in the hospital, and refer him to outpatient psychiatric care and Narcotics Anonymous. Mr. M is then lost to follow up.
In 2011, 18.1 million people reported Cannabis use in the previous month; 39% reported use in 20 of the last 30 days.1 A high rate of use and a relatively low number of cases suggests that CHS is rare. However, it is likely that CHS is under-recognized and under-reported.2,4,5 CHS symptoms may be misattributed to cyclic vomiting syndrome,3 because 50% of patients diagnosed with cyclic vomiting syndrome report daily Cannabis use.6 There is no epidemiological data on the incidence or prevalence of CHS among regular Cannabis users.7
Allen and colleagues first described this syndrome in 2004.4 Since then, CHS has been documented in a growing number of case reports and reviews,2,3,5,7-13 yet it continues to be under-recognized. Many CHS patients experience delays in diagnosis—often years—resulting in prolonged suffering, and costs incurred by frequent ED and urgent care visits, hospital admissions, and unnecessary workups.2,3,7
Clinical characteristics
CHS is characterized by recurrent, hyperemetic episodes in the context of chronic, daily Cannabis use.4 The average age of onset is 25.6 years (range: 16 to 51 years).3 Ninety-five percent of CHS patients used Cannabis daily, for, on average, 9.8 years before symptom onset.3 The amount of Cannabis used, although generally high, is difficult to quantify, and has been described as heavy and hourly in units of blunts, cones, joints, bongs, etc. Patients are most likely to present during acute hyperemetic episodes, which occur in a cyclic pattern, every four to eight weeks,3 interspersed with symptom-free periods. Three phases have been described:
- prodromal or pre-emetic phase
- hyperemetic phase
- recovery phase.4,10
Many patients report a prodromal phase, with one or two weeks of morning nausea, food aversion, preserved eating patterns, possible weight loss, and occasional vomiting. The acute, hyperemetic phase is characterized by severe nausea, frequent vomiting, abdominal pain, and compulsive bathing for temporary symptom relief. In the recovery phase, symptom improvement and resolution occur with cessation of Cannabis use.4,10 Symptom improvement can occur within 12 hours of Cannabis cessation, but can take as long as three weeks.3 Patients remain symptom-free while abstinent, but symptoms rapidly recur when they resume use.3,4
Cannabis is used as an antiemetic and appetite stimulant for chemotherapy-associated nausea and for anorexia in HIV infection. The pathogenesis of paradoxical hyperemetic symptoms of CHS remain unclear, but several mechanisms have been proposed. The principle active cannabinoid in Cannabis is the highly lipophilic compound THC, which binds to cannabinoid type 1 (CB1) and type 2 (CB2) receptors in the CNS and other tissues. It is thought that the antiemetic and appetite-stimulating effects of Cannabis are mediated by CB1 receptor activation in the hypothalamus. Nausea and vomiting are thought to be mediated by CB1 receptor activation in the enteric nervous system, which causes slowed peristalsis, delayed gastric emptying, and splanchnic vasodilation.4,14
In sensitive persons, chronic heavy Cannabis use can cause THC to accumulate to a toxic level in fatty tissues, causing enteric receptor binding effects to override the CNS receptor-binding effects.4 This is supported by case studies describing severe vomiting with IV injection of crude marijuana extract.15 Nearly 100 different THC metabolites have been identified. The Cannabis plant contains more than 400 chemicals, with 60 cannabinoid structures, any of which could cause CHS in toxic concentrations.4,7 Among them, cannabidiol, a 5-HT1A partial agonist, was shown to cause vomiting at higher doses in animal studies.4,7
Mechanisms of action
Cannabis has been used for centuries, so it is unclear why CHS is only recently being recognized. It may be because of higher THC content through selective breeding of plants and a more selective use of female buds that contain more concentrated THC levels than leaves and stems.3 Alternately, CHS may be caused by exogenous substances, such as pesticides, additives, preservatives, or other chemicals used in marijuana preparation, although there is little evidence to support this.3
The mechanism of symptom relief with hot bathing also is unclear. Patients report consistent, global symptom improvement with hot bathing.3 Relief is rapid, transient, and temperature dependent.4 CB1 receptors are located near the thermoregulatory center of the hypothalamus. Increased body temperature with hot bathing may counteract the thermoregulatory dysregulation associated with Cannabis use.4,9 It has been proposed that splanchnic vasodilation might contribute to CHS symptoms. Thus, redistribution of blood from the gut to the skin with warm bathing causes a “cutaneous steal syndrome,” resulting in symptom relief.11
Diagnostic approach
Four key features should be present when making a diagnosis of CHS:
- heavy marijuana use
- recurrent episodes of severe nausea, vomiting, and abdominal cramping
- compulsive bathing for transient symptom relief
- resolution of symptoms with cessation of Cannabis use.2,4,8
Compulsive, hot bathing for symptom relief was described in 98% of all reported cases,3 and should be considered pathognomonic.2 CHS patients can present with other symptoms, including polydipsia, mild fever, weight loss, and orthostasis.3 Although lab studies usually are normal, mild leukocytosis, hypokalemia, hypochloremia, elevated salivary amylase, mild gastritis on esophagogastroduodenoscopy, and delayed gastric emptying have been described during acute episodes (Table 1).2-4,7,8
Diagnosis starts with a history and physical exam, followed by a basic workup geared towards ruling out other causes of acute nausea and vomiting.2,7 Establish temporal relationships between symptoms, Cannabis use (onset, frequency, amount, duration), and bathing behaviors. A positive urine toxicology screen supports a CHS diagnosis and can facilitate discussion of Cannabis use.2 If you suspect CHS, rule out potentially life-threatening causes of acute nausea, vomiting, and abdominal pain, such as intestinal obstruction or perforation, pancreaticobiliary disease, and pregnancy. The initial workup should include a CBC, basic metabolic panel, liver function tests, amylase, lipase, pregnancy test, urinalysis, urine toxicology screen, and abdominal radiographs (Table 2).2,4,7 The differential diagnosis of recurrent vomiting is broad and should be considered (Table 3).2,4,7,16 Further workup can proceed non-emergently, and should be prompted by clinical suspicion.2,7
Supportive treatment, education
Treatment of acute hyperemetic episodes in CHS primarily is supportive; address dehydration with IV fluids and electrolyte replenishment as needed.2,4,7 Standard antiemetics, including 5-HT3 receptor antagonists, D2 receptor antagonists, and H1 receptor antagonists, are largely ineffective.5,9 Although narcotics have been used to treat abdominal pain, use caution when prescribing because they can exacerbate nausea and vomiting.7 Case reports have described symptom relief with inpatient treatment with lorazepam12 and self-medication with alprazolam,4 but more evidence is needed. A recent case report described prompt resolution of symptoms with IV haloperidol.13 Treating gastritis symptoms with acid suppression therapy, such as a proton pump inhibitor, has been suggested.7 Symptoms abate during hospitalization regardless of treatment, marking the progression into the recovery phase with abstinence. There are no proven treatments for CHS, aside from cessation of Cannabis use. Treatment should focus on motivating your patient to stop using Cannabis.
Acute, hyperemetic episodes are ideal teachable moments because of the acuity of symptoms and clear association with Cannabis use. However, some patients may be skeptical about CHS because of the better-known antiemetic effects of Cannabis. For such patients, provide informational materials describing CHS and take time to address their concerns or doubts.
Motivational interviewing can help provoke behavior change by exploring patient ambivalence in a directive, patient-focused manner. Randomized controlled trials have documented significant reductions in Cannabis use with single-session motivational interviewing, with greater effect among heavy users.17 Single-session motivational interviewing showed results comparable to providing drug information and advice, suggesting that education and information are useful interventions.18 Although these single-session studies appear promising, they focus on younger users who have not been using Cannabis as long as typical CHS patients. Multi-session interventions may be needed to address longstanding, heavy Cannabis use in adult CHS patients.
Cognitive-behavioral therapy. In a series of randomized controlled trials,
motivational enhancement training and cognitive-behavioral therapy (CBT) were effective for Cannabis use cessation and maintenance of abstinence.19
Although these interventions take more time—six to 14 sessions for CBT and one to four sessions for motivational enhancement training—they should be considered for CHS patients with persistent use.
Bottom Line
Cannabinoid hyperemesis syndrome (CHS) is characterized by years of daily, heavy Cannabis use, cyclic nausea and vomiting, and compulsive bathing. Symptoms resolve with Cannabis cessation. Workup of suspected CHS should rule out life- threatening causes of nausea and vomiting. Acute hyperemetic episodes should be managed supportively. Motivational enhancement therapy or cognitive-behavioral therapy should be considered for persistent Cannibis use.
Related Resources
- Motivational interviewing for substance use disorders. www.motivationalinterview.org.
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35(2):309-326.
Drug Brand Names
Alprazolam • Xanax Haloperidol • Haldol Lorazepam • Ativan
Ondansetron • Zofran Prochlorperazine • Compazine
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2011 National Survey on Drug Use and Health: Mental health findings. http://www.samhsa.gov/data/NSDUH/2k11MH_FindingsandDetTables/2K11MHFR/NSDUHmhfr2011.htm. Published November 2012. Accessed April 18, 2013.
2. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011; 104(9):659-664.
3. Nicolson SE, Denysenko L, Mulcare JL, et al. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53(3):212-219.
4. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Cut. 2004;53(11):1566-1570.
5. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264-1266.
6. Fajardo NR, Cremonini F, Talley NJ. Cyclic vomiting syndrome and chronic cannabis use. Am J Gastroenterol. 2005;100:S343.
7. Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid hyperemesis syndrome. Curr Drug Abuse Rev. 2011;4(4):241-249.
8. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285.
9. Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc. 2009;84(1):76-78.
10. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci. 2010;55(11):3113-3119.
11. Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23(6):790-793.
12. Cox B, Chhabra A, Adler M, et al. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med. 2012;2012:757696.
13. Hickey JL, Witsil JC, Mycyk MB. Haloperidol for treatment of cannabinoid hyperemesis syndrome [published online April 10, 2013]. Am J Emerg Med. 2013;31(6):1003.e5-6. doi: 10.1016/j.ajem.2013.02.021.
14. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13(1):77-80.
15. Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol. 1981;18(3):353-366.
16. Abell TL, Adams KA, Boles RG, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008; 20(4):269-284.
17. McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction. 2004;99(1):39-52.
18. McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction. 2008;103(11):1809-1818.
19. Elkashef A, Vocci F, Huestis M, et al. Marijuana neurobiology and treatment. Subst Abus. 2008;29(3):17-29.
1. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. Results from the 2011 National Survey on Drug Use and Health: Mental health findings. http://www.samhsa.gov/data/NSDUH/2k11MH_FindingsandDetTables/2K11MHFR/NSDUHmhfr2011.htm. Published November 2012. Accessed April 18, 2013.
2. Wallace EA, Andrews SE, Garmany CL, et al. Cannabinoid hyperemesis syndrome: literature review and proposed diagnosis and treatment algorithm. South Med J. 2011; 104(9):659-664.
3. Nicolson SE, Denysenko L, Mulcare JL, et al. Cannabinoid hyperemesis syndrome: a case series and review of previous reports. Psychosomatics. 2012;53(3):212-219.
4. Allen JH, de Moore GM, Heddle R, et al. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Cut. 2004;53(11):1566-1570.
5. Sontineni SP, Chaudhary S, Sontineni V, et al. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264-1266.
6. Fajardo NR, Cremonini F, Talley NJ. Cyclic vomiting syndrome and chronic cannabis use. Am J Gastroenterol. 2005;100:S343.
7. Galli JA, Sawaya RA, Friedenberg FK. Cannabinoid hyperemesis syndrome. Curr Drug Abuse Rev. 2011;4(4):241-249.
8. Sullivan S. Cannabinoid hyperemesis. Can J Gastroenterol. 2010;24(5):284-285.
9. Chang YH, Windish DM. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc. 2009;84(1):76-78.
10. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci. 2010;55(11):3113-3119.
11. Patterson DA, Smith E, Monahan M, et al. Cannabinoid hyperemesis and compulsive bathing: a case series and paradoxical pathophysiological explanation. J Am Board Fam Med. 2010;23(6):790-793.
12. Cox B, Chhabra A, Adler M, et al. Cannabinoid hyperemesis syndrome: case report of a paradoxical reaction with heavy marijuana use. Case Rep Med. 2012;2012:757696.
13. Hickey JL, Witsil JC, Mycyk MB. Haloperidol for treatment of cannabinoid hyperemesis syndrome [published online April 10, 2013]. Am J Emerg Med. 2013;31(6):1003.e5-6. doi: 10.1016/j.ajem.2013.02.021.
14. McCallum RW, Soykan I, Sridhar KR, et al. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13(1):77-80.
15. Vaziri ND, Thomas R, Sterling M, et al. Toxicity with intravenous injection of crude marijuana extract. Clin Toxicol. 1981;18(3):353-366.
16. Abell TL, Adams KA, Boles RG, et al. Cyclic vomiting syndrome in adults. Neurogastroenterol Motil. 2008; 20(4):269-284.
17. McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: results from a multi-site cluster randomized trial. Addiction. 2004;99(1):39-52.
18. McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction. 2008;103(11):1809-1818.
19. Elkashef A, Vocci F, Huestis M, et al. Marijuana neurobiology and treatment. Subst Abus. 2008;29(3):17-29.
Assessing and treating depression in palliative care patients
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression is highly prevalent in hospice and palliative care settings—especially among cancer patients, in whom the prevalence of depression may be 4 times that of the general population.1 Furthermore, suicide is a relatively common, unwanted consequence of depression among cancer patients.2 Whereas the risk of suicide among advanced cancer patients may be twice that of the general population,3 in specific cancer populations (male patients with pancreatic adenocarcinoma) the risk of suicide may be 11 times that of the general population.4
Mental health professionals often are consulted when treating depressed patients with advanced illness, especially when suicidal thoughts or wishes for a hastened death are expressed to oncologists or primary care physicians. To mitigate the effects of depression among seriously ill patients (Box),5,6 mental health professionals must be able to assess and manage depression in patients with progressive, incurable illnesses such as advanced malignancy.
Diagnostic challenges
Assessing depression in seriously ill patients can be a challenge for mental health professionals. Cardinal neurovegetative symptoms of depression, such as anergia, anorexia, impaired concentration, and sleep disturbances, also are common manifestations of advanced medical illness.7 Furthermore, it can be difficult to gauge the significance of psychological distress among cancer patients. Although depressive thoughts and symptoms may be present in 15% to 50% of cancer patients, only 5% to 20% will meet diagnostic criteria for major depressive disorder (MDD).8,9 You may find it challenging to determine whether to use pharmacotherapy for depressive symptoms or whether engaging in reflective listening and exploring the patient’s concerns is the appropriate therapeutic intervention.
Side effects from commonly used therapeutics for cancer patients—chemotherapeutic agents, opioids, benzodiazepines, glucocorticoids—can mimic depressive symptoms. Clinicians should include hypoactive delirium in the differential diagnosis of depressive symptoms in cancer patients. Delirium is an important consideration in the final days of life because the condition has been shown to occur in as many as 90% of these patients.10 A mistaken diagnosis of depression in a patient who has hypoactive delirium (see “Hospitalized, elderly, and delirious: What should you do for these patients?” page 10) might lead to a prescription for an antidepressant or a psychostimulant, which can exacerbate delirium rather than alleviate depressive symptoms.
Significant attitudinal barriers from both clinicians and patients can lead to under-
recognition and undertreatment of depression. Clinicians may believe the patient’s depression is an appropriate response to the dying process; indeed, feeling sad or depressed may be an appropriate response to bad news or a medical setback, but meeting MDD criteria should be viewed as a pathologic process that has adverse medical, psychological, and social consequences. Time constraints or personal discomfort with existential concerns may prevent a clinician from exploring a patient’s distress out of fear that such discussions may cause the patient to become more depressed.11 Patients may underreport or consciously disguise depressive symptoms in their final weeks of life.12
Responding to these challenges
The Science Committee of the Association of Palliative Medicine performed a thorough assessment of available screening tools and rating scales for depressive symptoms in palliative care. Although the committee found that commonly used tools such as the Edinburgh Depression Scale and the Hospital Anxiety and Depression Scale have validated cutoff thresholds for palliative care patients, the depression screening tool with the highest sensitivity, specificity, and positive predictive value was the question: “Are you feeling down, depressed, or hopeless most of the time over the last 2 weeks?”13,14
Other short screening algorithms have been validated among palliative care patients (Table 1).15 Endicott proposed a structured approach to help clinicians differentiate MDD from common physical ailments of progressive cancer in which physical criteria for an MDD diagnosis are substituted by affective symptoms (Table 2).16 The improved risk-benefit ratio of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), coupled with the potential significant morbidity associated with MDD and subsyndromal depressive symptoms, makes it necessary to recognize and treat those symptoms even when the cause of the depressive symptoms is unclear.
Psychotherapy in palliative care
Psychotherapeutic interventions such as dignity therapy, which invites patients to utilize a meaning-centered life review to address his (her) existential concerns, may help depressed palliative care patients.17 Evidence suggests a strong association between diminished dignity and depression in patients with advanced illness.18 Individualized psychotherapeutic interventions that provide a framework for addressing dignity-related issues and existential distress among terminally ill patients could help preserve a sense of purpose throughout the dying process. Surveys of dignity therapy have been encouraging: 91% of participants reported being satisfied with dignity therapy and more than two-thirds reported an improved sense of meaning.18
Other promising psychotherapeutic interventions include supportive-expressed group therapy, in which a group of advanced cancer patients meets with a mental health professional and discusses goals of building bonds, refining life’s priorities, and “detoxifying” the experience of death and dying.19 A primary purpose of this therapy is not just to foster improved relationships within a group of cancer patients, but also within their family and oncology team, with the aim of improving compliance with anticancer therapies. Nurse-delivered, one-on-one sessions focusing on depression education, problem-solving, coping techniques, and telecare management of pain and depression also improves outcomes among depressed cancer patients.20
Hospital-based inpatient and outpatient palliative care consultation teams are becoming more common. A randomized controlled trial of early palliative care outpatient consultation for patients with incurable lung cancer showed improved depression outcomes, better quality of life, and a modest improvement in survival.21 Although the most effective elements of a palliative care consult remain unspecified and require further research, improvement in outcomes may result from more effective symptom management, better acknowledgement of the burden of illness on the patient or family, or reduced need for hospitalization. Therefore, mental health professionals should consider palliative care consultation for advanced cancer patients with signs of psychological distress.
Pharmacotherapy options
Antidepressants. Patients with excessive guilt, anhedonia, hopelessness, or ruminative thinking along with a related impairment in quality of life may benefit from pharmacotherapy regardless of whether they meet diagnostic criteria for MDD. Although SSRIs and SNRIs have become a mainstay in managing depression, placebo-controlled trials have yielded mixed results in depressed cancer patients. Furthermore, differences in efficacy among these antidepressants may not be significant, according to a recent meta-analysis.22
Select an antidepressant based on the patient’s past treatment response, target symptoms, and potential for adverse events. Mirtazapine has relatively few drug interactions; the side effects of sedation and weight gain may be welcome among patients with insomnia and impaired appetite.23 Furthermore, mirtazapine is a 5-HT3 receptor antagonist,24 which suggests it might act as an effective antiemetic.25 Other SNRIs, such as venlafaxine and duloxetine, have demonstrated benefits in managing neuropathic pain in patients who do not have cancer.26
Psychostimulants. Patients with a prognosis of days or weeks might not have enough time for an antidepressant to achieve full effect. Open prospective trials and pilot studies have shown that psychostimulants can improve cancer-related fatigue and quality of life while also augmenting the action of antidepressants.27 Psychostimulants, such as methylphenidate, have been used for treating cancer-related fatigue and depressive symptoms in medically ill patients. Their rapid onset of action, coupled with minimal side effect profile, make them a good choice for seriously ill patients with significant neurovegetative symptoms of a depressive disorder. Note: Avoid psychostimulants in patients with delirium and use with caution in patients with heart disease.28
Novel agents. A growing body of preclinical research suggests that glutamate may be involved in the pathophysiology of MDD. Ketamine modulates glutamate neurotransmission as an N-methyl-d-aspartate receptor antagonist. A recent evaluation of a single dose IV of ketamine in a placebo-controlled, double-blind trial found that depressed patients receiving ketamine experienced significant improvement their depressive symptoms.29 Irwin and Iglewicz30 describe 2 hospice patients administered a single oral dose of ketamine, which provided rapid relief of depressive symptoms and was well tolerated.
Transdermal selegiline may help patients who have trouble taking oral medications, including antidepressants. Inability to tolerate or absorb medications may be related to several conditions such as head and neck cancer, severe mucositis, and dysphagia. The dose-related dietary requirements—tyramine restriction—and careful monitoring for drug interactions may limit the use of selegiline in medically ill patients.31Table 3 features a list of dosing recommendations for pharmacotherapeutic options.32
Use the strategy of “start low, go slow” when initiating and adjusting antidepressants because patients with cancer and other advanced illnesses often have concomitant organ failure and are at risk of drug interactions. Carefully review your patient’s medication list for agents that are no longer beneficial or possibly contributing to depressive symptoms to help reduce the risk of adverse pharmacokinetic and pharmaco-dynamic interactions.
Requests for a hastened death
As many as 8.5% of terminally ill patients have a sustained and pervasive wish for an early death.33 Although requests for a hastened death may evoke strong emotional reactions and compel many clinicians to recoil or harshly reject such requests, consider such requests as an opportunity to gain insight into the patient’s narrative of his (her) suffering. The clinician’s role in such cases is to identify suicidality and perform a thorough suicide risk assessment. Interventions to prevent suicide should attempt to balance the seriousness of self-harm threats with restrictions on the patient’s liberty.34
Clinicians also need to consider the patient’s prognosis in their decision-making. For example, an extremely depressed or suicidal patient may not benefit from psychiatric hospitalization if she (he) has progressive neurovegetative symptoms and a prognosis of only a few weeks to live. These situations often are challenging and require a careful, informed discussion of the risks and benefits of all proposed interventions.
Clinicians also should be familiar with distinctions among ethical issues in end-of-life care, including physician-assisted suicide, euthanasia, and palliative sedation (Table 4).35,36
In Oregon, requests for physician-assisted suicide and hastened death through the state’s Death with Dignity Act often are short lived, and may not persist when clinicians offer patients good symptom management and psychological support.37 Requests for a hastened death often are motivated by loss of control, inability to find meaning in death, indignity from being dependent, and concern for future suffering and burden on loved ones.37
Carefully evaluate requests for hastened death in a manner that balances your personal and professional integrity. To preserve personal integrity, clearly communicate therapeutic interventions that you can and cannot provide. To ensure the patient does not feel abandoned, identify factors that contribute to the patient’s suffering and express a desire to search for alternative care approaches that will be mutually acceptable to the patient and to you.
Advance care planning and palliative care consultations may help in these circumstances. A randomized trial comparing advance care planning vs standard care in hospitalized geriatric patients found that advance care planning was more likely to lead to end-of-life wishes that were recognized by clinicians, and was associated with less distress, anxiety, and depression as reported by bereaved family members.38
Clinicians can assist patients with advanced care planning by helping them fill out advance directives, such as durable health care power of attorney documents and a living will. Palliative care clinicians can offer specialty-level assistance in advance care planning, provide focused assessments of physical and psychosocial symptoms, develop appropriate clinical goals, and assist in coordinating individualized care plans for seriously ill patients.2
Bottom Line
Depression commonly is encountered in hospice and palliative care patients and is associated with morbidity and distress. Validated screening tools can help you distinguish major depressive disorder from depressive symptoms in this population. Several psychotherapeutic techniques have been shown to be beneficial. In addition to traditional antidepressants, psychostimulants or ketamine may help address acute depressive symptoms in patients who have days or weeks to live.
Related Resources
- American Academy of Hospice and Palliative Medicine. www.aahpm.org.
- Death with Dignity National Center. www.deathwithdignity.org.
- National Hospice and Palliative Care Organization. www.nhpco.org.
- Oregon Health Authority. Death with Dignity Act. http://public.health.oregon.gov/ProviderPartnerResources/Evaluationresearch/deathwithdignityact/Pages/index.aspx.
Drug Brand Names
Duloxetine • Cymbalta Modafinil • Provigil
Ketamine • Ketalar Selegiline (transdermal) • EMSAM
Methylphenidate • Concerta, Ritalin Mirtazipine • Remeron
Venlafaxine • Effexor
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
1. Irwin SA, Rao S, Bower K, et al. Psychiatric issues in palliative care: recognition of depression in patients enrolled in hospice care. J Palliat Med. 2008;11(2):158-163.
2. Misono S, Weiss NS, Fann JR, et al. Incidence of suicide in persons with cancer. J Clin Oncol. 2008;26(29):4731-4738.
3. Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284(22):2907-2911.
4. Turaga KK, Malafa MP, Jacobsen PB, et al. Suicide in patients with pancreatic cancer. Cancer. 2011;117(3):642-647.
5. Rosenstein DL. Depression and end-of-life care for patients with cancer. Dialogues Clin Neurosci. 2011;13(1):101-108.
6. King DA, Heisel MJ, Lyness JM. Assessment and psychological treatment of depression in older adults with terminal or life-threatening illness. Clin Psychol (New York). 2005;12(3):339-353.
7. Block SD. Assessing and managing depression in the terminally ill patient. ACP-ASIM End-of-Life Care Consensus Panel. American College of Physicians - American Society of Internal Medicine. Ann Intern Med. 2000;132(3):209-218.
8. Chochinov HM, Wilson KG, Enns M, et al. Prevalence of depression in the terminally ill: effects of diagnostic criteria and symptom threshold judgments. Am J Psychiatry. 1994;151(4):537-540.
9. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
10. Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliat Med. 2006;20(1):17-23.
11. Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med. 1985;20(8):819-823.
12. Hinton J. Can home care maintain an acceptable quality of life for patients with terminal cancer and their relatives? Palliat Med. 1994;8(3):183-196.
13. Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care? Palliat Med. 2003;17(1):40-43.
14. Chochinov HM, Wilson KG, Enns M, et al. “Are you depressed?” Screening for depression in the terminally ill. Am J Psychiatry. 1997;154(5):674-676.
15. Robinson JA, Crawford GB. Identifying palliative care patients with symptoms of depression: an algorithm. Palliat Med. 2005;19(4):278-287.
16. Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(10 suppl):2243-2249.
17. Chochinov HM, Hack T, Hassard T, et al. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J Clin Oncol. 2005;23(24):5520-5525.
18. Chochinov HM. Dignity-conserving care-a new model for palliative care: helping the patient feel valued. JAMA. 2002;287(17):2253-2260.
19. Kissane DW, Grabsch B, Clarke DM, et al. Supportive-expressive group therapy: the transformation of existential ambivalence into creative living while enhancing adherence to anti-cancer therapies. Psychooncology. 2004;13(11):
755-768.
20. Strong V, Waters R, Hibberd C, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet. 2008;372(9632):40-48.
21. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
22. Gartlehner G, Hansen RA, Morgan LC, et al. Comparative benefits and harms of second-generation antidepressants for treating major depressive disorder: an updated meta-analysis. Ann Intern Med. 2011;155(11):772-785.
23. Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl). 2007; 16(4):351-354.
24. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001; 7(3):249-264.
25. Pae CU. Low-dose mirtazapine may be successful treatment option for severe nausea and vomiting. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):
1143-1145.
26. Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454.
27. Pereira J, Bruera E. Depression with psychomotor retardation: diagnostic challenges and the use of psychostimulants. J Palliat Med. 2001;4(1):15-21.
28. Jackson V, Block S. # 061 Use of Psycho-Stimulants in Palliative Care, 2nd ed. End of Life/Palliative Education Resource Center. Medical College of Wisconsin. http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_061.htm. Accessed December 28, 2012.
29. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354.
30. Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13(7):903-908.
31. Attard A, Ranjith G, Taylor D. Alternative routes to oral antidepressant therapy: case vignette and literature review. J Psychopharmacol. 2010;24(4):449-454.
32. Rozans M, Dreisbach A, Lertora JJ, et al. Palliative uses of methylphenidate in patients with cancer: a review. J Clin Oncol. 2002;20(1):335-339.
33. Chochinov HM, Wilson KG, Enns M, et al. Desire for death in the terminally ill. Am J Psychiatry. 1995;152(8):1185-1191.
34. Marks S, Heinrich TW, Rosielle D. Case report: are clinicians obligated to medically treat a suicide attempt in a patient with a prognosis of weeks? J Palliat Med. 2012;15(1):134-137.
35. Materstvedt LJ, Clark D, Ellershaw J, et al. Euthanasia and physician-assisted suicide: a view from an EAPC Ethics Task Force. Palliat Med. 2003;17(2):97-101; discussion 102-179.
36. Kirk TW, Mahon MM; Palliative Sedation Task Force of the National Hospice and Palliative Care Organization Ethics Committee. National Hospice and Palliative Care Organization (NHPCO) position statement and commentary on the use of palliative sedation in imminently dying terminally ill patients. J Pain Symptom Manage. 2010; 39(5):914-923.
37. Okie S. Physician-assisted suicide--Oregon and beyond. N Engl J Med. 2005;352(16):1627-1630.
38. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c134
Chronic non-cancer pain and substance use disorders: Challenges and strategies
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
Patients with chronic non-cancer pain (CNCP) and a comorbid substance use disorder (SUD) are difficult to treat. There is a lack of high-quality clinical trials to guide management. This article focuses on current research, guidelines, and recommendations to best manage these patients. We present an analysis of recent statistics, patient characteristics, screening methods, as well as a discussion of changes to DSM-5 regarding substance abuse and addiction (Box 1).1
Opioid use and opioid-related overdoses have increased dramatically over the last decade (Box 2).2-5 Opioids are the primary medication used to treat CNCP, but their use in patients with comorbid SUDs is controversial. It is crucial for psychiatrists and other clinicians to know how to best identify, manage, and treat patients with CNCP/SUD.
Risk factors for CNCP/SUD
Evidence regarding the efficacy of screening methods to identify patients with chronic pain who are at high risk for substance misuse is insufficient. Key risk factors for developing chronic pain may include:
• elevated psychological distress
• negative beliefs and expectations about pain
• pain fear and avoidance
• disability
• anger or hostility
• maladaptive coping strategies
• catastrophic behaviors.5
In addition, these individuals may have a spouse who enables the sick role behavior.
Risk factors for developing a SUD related to prescribed opioids include:
• a history of problematic substance use
• sedative-hypnotic use
• positive family history for substance abuse
• legal problems
• heavy tobacco use
• age <50
• major depressive disorder or anxiety.5
In a review of 38 articles, Morasco et al6 found low-grade evidence with mixed results in attempt to find a correlation among sex, depression, anxiety, and tobacco use with CNCP/SUD. Other data suggest that the risk of addiction once opioids have been started increases with long refill periods and opioid morphine equivalents >120 mg.7 A history of childhood sexual abuse also may be a risk factor for chronic pain and addiction.5
Prevalence
The prevalence of opioid abuse among CNCP patients ranges from 3% to 48%; the highest rates are found among patients visiting the emergency room for opioid refills.7 These patients are more likely to exhibit aberrant behavior with their medications and may be prescribed higher opioid doses than patients who have CNCP only. Adherent CNCP/SUD patients show no difference in response to pain treatment compared with those with CNCP alone.6 Approximately 11.5% of CNCP patients taking opioids demonstrate aberrant medication use.6
Screening: Which method is best?
Data are scarce regarding the best screening methods to identify patients with CNCP/SUD. A survey of 48 patients by Moore et al8 found the combination of a clinical interview and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is 90% sensitive in detecting CNCP/SUD. However, a systematic review by Chou et al9 found only 2 well-designed studies showing that the SOAPP-R weakly predicts future aberrant drug behavior and only 1 study showed that a high risk categorization on the Opioid Risk Tool (ORT) strongly increased the likelihood of predicting future abnormal drug-related behavior. Another well-designed study showed that the Current Opioid Misuse Measure (COMM) weakly raised the likelihood of detecting current aberrant drug behavior. No reliable data supported the efficacy of urine drug screens (UDS), pill counts, or prescription drug monitoring programs (PDMPs) for improving clinical outcomes.9 In a systematic review Starrels et al10 found only low-quality evidence supporting the effectiveness of opioid agreement contracts and UDS.
Treatment strategies
Once a patient with CNCP/SUD has been identified, it is important to categorize the severity of his (her) pain and substance use by using the decision tree (Figure) and screening tools such as SOAPP-R, ORT, and COMM. In a Veterans Administration (VA) study, only 35% of patients with an SUD received substance abuse treatment.11 The 2009 American Pain Society/American Academy of Pain Medicine guidelines recommended that opioids should considered for patients with substance abuse, serious aberrant drug-related behaviors, or psychiatric comorbidities only if frequent monitoring and treatment plan and mental health or addiction consultation were in place.12 These guidelines also recommended discontinuing opioids if repeated atypical behavior, substance abuse, diversion, lack of progress, or intolerable side effects occur. Repeated and more serious behaviors require a multidisciplinary team, expert consultation, therapy restructuring, and possibly discontinuation of opioids.12
The U.S. Office of National Drug Control Policy has created a council of federal agencies to spearhead the Prescription Drug Abuse Prevention Plan, which includes 4 major categories to reduce prescription drug abuse: education, monitoring, proper disposal, and enforcement.13 FDA commissioner Margaret Hamburg supports legislation to combine opioid education with Drug Enforcement Administration registration.14 The FDA began developing the risk evaluation and mitigation strategies in 2007 to educate physicians on proper prescribing of potentially dangerous medications.
Gourlay and Heit proposed a universal precautions method of opioid treatment for all pain patients.15 That includes:
• seeking differential diagnoses and comorbidities
• doing a baseline addiction assessment with UDS and PDMP evaluations
• obtaining informed consent for pain management
• creating pre- and post-treatment goals for pain and function
• evaluating the 4 “As” (analgesic response, increased activity, adverse events, and aberrant behavior)
• reviewing the evolution of the pain and comorbidities
• continuous documentation.5
Other helpful strategies include the Oregon’s SMART (Specific, Measurable, Action-oriented, Realistic, Time-Dependent) goal-setting, which helps physicians negotiate functional goals with patients and plan an exit strategy for those whose quality of life does not improve with opioids.5 Clinicians also can consider a sequential treatment model where patients with severe substance abuse and pain are detoxified of illicit drugs and alcohol before starting pain management. This approach is more effective if the pain is secondary to a more severe substance abuse problem that is not correlated to physical pain and acute rather than chronic.16
Psychotherapeutic interventions
In another VA study, a collaborative care intervention (CCI) combining education, self-efficacy, pain management, and feedback was not impeded by a history of SUD. The authors recommended CCI, stepped care, integrated interventions, and relapse prevention and stressed the importance of social support.17
A 10-week cognitive-behavioral therapy (CBT) program involving 44 patients enrolled in an integrated pain management program for recovering substance abusers found 50% of CNCP/SUD patients were opioid-free at 12 months.16 A combination of medication reduction and education resulted in less pain, increased functioning, decreased emotional distress, and less self-medicating. Additionally, patients reported 35% overall reduction in pain severity based on the McGill Pain Questionnaire but only 25% of patients showed a reliable improvement in their pain. Treatment changes lasted 1 year.16
A meta-analysis of psychological interventions such as CBT, behavioral treatment (BT), and self-regulated treatment (SRT) indicated that CBT and BT are moderately effective at lowering work-related disability and pain intensity for chronic low back pain alone or with multidisciplinary care and moderately lowered work-related disability. CBT had a moderate to large effect, while SRT with biofeedback and relaxation techniques had a large effect on lowering pain intensity. SRT also was shown to lower depression. Return-to-work rates were better with multidisciplinary care that included psychological interventions. These psychological interventions for chronic low back pain lowered self-reported pain, pain interference, depression, and disability while increasing quality of life; the largest effect was on pain intensity.18
A review by Williams et al19 analyzing the effects of BT and CBT on various outcome measures, including chronic pain, found small to moderate benefits for disability, mood, and catastrophic thinking with CBT, which lasted up to 6 months. Only weak improvements in pain were seen with CBT immediately after treatment. BT had a beneficial effect on catastrophic thinking but only right after treatment. CBT’s overall effect in these patients was positive, and changes lasted up to 6 months.
Pharmacologic treatments
Before and during opioid therapy, psychotherapy, physical therapy, and occupational therapy should be used with adjuvant medications appropriate to the pain condition, such as anticonvulsants (gabapentin, pregabalin, topiramate) and antidepressants including tricyclic antidepressants (amitriptyline, desipramine) and serotonin-norepinephrine reuptake inhibitors (duloxetine, venlafaxine, milnacipran).12 When considering opioids for patients with CNCP/SUD, adverse effects and safety is a primary consideration. Benzodiazepines generally should not be used with opioids because of their synergistic sedating effects.5
Opioids are misused more often by overingestion than by altering the delivery route, yet most efforts to create tamper-resistant medications has focused on
snorting or injection, which are considered more dangerous. Current tamper-resistance strategies include:
• creating a hard shell to prevent crushing and altering the medications
• chemical combinations, using agonists and antagonists such as buprenorphine combined with naloxone
• prodrugs, which become activated only in the GI system
• implants or patches.20,21
One prodrug in phase-I testing, compound PF329, becomes activated only in the GI tract by exposure to trypsin. Because it also contains trypsin inhibitors, overingestion will not lead to toxicity.20 These types of technologies may take years to develop and integrate into our therapeutic armamentarium.
If choosing opioid treatment for patients with CNCP/SUD, initially consider weak opioids such as codeine and tramadol.22 Tramadol, a partial μ agonist and weak inhibitor of serotonin and norepinephrine reuptake, is not a controlled substance and is indicated for moderate to severe pain; however, reports of its abuse potential are beginning to emerge. Tramadol has a frequency of abuse and withdrawal of approximately 2/100,000 patients taking the drug.23
Tapentadol has a dual mechanism of action—it combines a potent opioid agonist with a norepinephrine reuptake inhibitor—and is a schedule II medication. The norepinephrine and serotonin reuptake inhibition properties of tramadol and tapentadol can lead to undesired side effects and are less likely to be abused. Dart et al24 found tapentadol immediate release has the lowest abuse rate of all the opioids they studied, well below oxycodone and hydrocodone.
Methadone is a potent analgesic primarily used to treat opioid addiction, but it also is used for CNCP and cancer pain. With chronic use, methadone lacks the euphoric effect of other μ opioids; however, it can increase the QTc interval and has a long, variable half-life. As a result, methadone conversion tables are considered unreliable.
Methadone also has been associated with a disproportionate number of prescription opioid overdoses and deaths; it is present in 30% of all overdoses treated in emergency departments.4 Although methadone constitutes 5% of all opioid prescriptions in the United States, it is associated with one-third of opioid-related deaths, which is more than heroin and cocaine combined.14 Most methadone deaths occur within the first 7 days of initiating therapy, which suggests that patients were started on too high a dosage, were titrated too quickly, or had overestimated their tolerance.4 Reasons for methadone-related deaths are multifactorial and include:
• physician error and lack of knowledge
• patient nonadherence
• unanticipated comorbidities
• polypharmacy
• obstructive sleep apnea
• third-party payer policies listing it as first tier because of its low cost.4
In a Swedish study of 60 patients taking methadone, 75% had good pain relief on an average dose of 81.5 mg/d, whereas 25% had only moderate pain relief at a higher average dose of 157.5 mg/d. The authors described a methadone syndrome that included sedation, weakness, lethargy, weight gain, sweating, and sexual dysfunction, and that decreased the quality of life in 50% of patients.25 Another study found that among patients who died from sudden cardiac death and had methadone present in the toxicology screen, 45% were taking other psychotropics.26 Researchers also found a synergistic effect with benzodiazepines and an independent risk of sudden cardiac death and recommended obtaining pulmonary function tests and an electrocardiogram before starting methadone therapy, especially at higher doses.
Buprenorphine is a schedule III partial ì agonist opioid with a bell-shaped dose-response curve with a ceiling effect on respiratory depression, making it safe with an overdose. Although it is indicated for opioid dependence maintenance, it has been used off-label to treat chronic pain. It causes less euphoria than many other opioids including methadone. Buprenorphine is 25 to 50 times more potent than morphine and has a half-life of 20 to 44 hours but can be abused.27 It is available as a tablet, an injectable, and a 7-day patch. A combination of buprenorphine and naltrexone has a lower abuse potential,28 is administered sublingually and can be prescribed only by certified physicians.29 A subcutaneous implantable form of buprenorphine, which lasts 6 months, is under FDA review.30
Bottom Line
Multidisciplinary care paired with psychological interventions and a treatment plan has some evidence of efficacy in treating pain in patients with chronic non-cancer pain at high risk of substance abuse. Physician education in both pain and addiction is paramount. Frequent supervision, screening, monitoring and careful selection of medications will help physicians optimize outcomes and reduce risks.
Related Resources
- Agency Medical Directors Group. Intra-agency guideline on opioid dosing for chronic non-cancer pain. http://agencymeddirectors.wa.gov/files/opioidgdline.pdf.
- Stevenson E, Cole J, Walker R, et al. Association of chronic noncancer pain with substance abuse treatment outcomes among a community mental health center sample [published online January 3, 2013]. Addictive Disorders and their Treatment. doi: 10.1097/ADT.0b013e31827b0cd9.
Drug Brand Names
Amitriptyline • Elavil Buprenorphine • Subutex
Buprenorphine/naloxone • Suboxone Codeine • Tylenol with Codeine, others
Desipramine • Norpramin Duloxetine • Cymbalta
Gabapentin • Neurontin Methadone • Dolophine
Milnacipran • Savella Morphine • Roxanol
Oxycodone • Percolone, OxyContin Pregabalin • Lyrica
Tapentadol • Nucynta Topiramate • Topamax
Tramadol • Ultram Venlafaxine • Effexor
Hydrocodone/acetaminophen • Vicodin, Lorcet, others
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Zita Juska for her editorial assistance with this article.
Featured Audio
Mark Juska, MD, discusses strategies for treating patients with comorbid pain and substance use disorders. Dr. Juska is a Fellow, Department of Anesthesiology, Wayne State University, Detroit, Michigan.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.
1. Giordano J. Pain and addiction: words, meanings, actions in the age of DSM-5. Practical Pain Management. http://www.practicalpainmanagement.com/resources/ethics/pain-addiction-words-meanings-actions-age-dsm-5. November 1, 2010. Accessed May 28, 2013.
2. The Joint Commission. Facts about pain management. http://www.jointcommission.org/pain_management. Updated February 27, 2013. Accessed May 28, 2013.
3. Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305(13):1346-1347.
4. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-35.
5. Miotto K, Kaufman A, Kong A, et al. Managing co-occurring substance use and pain disorders. Psychiatr Clin North Am. 2012;35(2):393-410.
6. Morasco BJ, Gritzner S, Lewis L, et al. Systematic review of prevention, correlates and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorders. Pain. 2011;152:488-497.
7. Edlund MJ, Martin BC, Fan MY, et al. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP Study. Drug Alcohol Depend. 2010;112(1-2):90-98.
8. Moore TM, Jones T, Browder JH, et al. A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med. 2009;10(8):1426-1433.
9. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic non cancer pain: prediction and identification of aberrant drug-related behaviors. A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guidelines. J Pain. 2009;10(2):131-146.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152(11):712-720.
11. Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011; 26(9):965-971.
12. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):
113-130.
13. Office of National Drug Control Policy. Epidemic: responding to America’s prescription drug abuse crisis. http://www.whitehouse.gov/sites/default/files/ondcp/policy-and-research/rx_abuse_plan.pdf. Accessed May 28, 2013.
14. Kuehn BM. Methadone overdose deaths rise with increased prescribing for pain. JAMA. 2012;308(8):749-750.
15. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112.
16. Currie SR, Hodgins DC, Crabtree A, et al. Outcome from integrated pain management treatment for recovering substance abusers. J Pain. 2003;4(2):91-100.
17. Morasco BJ, Corson K, Turk DC, et al. Association between substance use disorder status and pain-related function following 12 months of treatment in primary care patients with musculoskeletal pain. J Pain. 2011;12(3):352-359.
18. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26(1):1-9.
19. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3.
20. Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37(7):412-418.
21. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clinic Proc. 2012;87(7):683-694.
22. Substance Abuse and Mental Health Services Administration. Managing chronic pain in adults with or in recovery from substance use disorders. Treatment Improvement Protocol (TIP) Series 54. HHS Publication No. (SMA) 12-4671. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
23. Senay EC, Adams EH, Geller A, et al. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233-241.
24. Dart RC, Cicero TJ, Surratt HL, et al. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8(6):395-402.
25. Rhodin A, Grönbladh L, Nilsson LH, et al. Methadone treatment of chronic non-malignant pain and opioid dependence—a long-term follow-up. Eur J Pain. 2006; 10(3):271-278.
26. Chuh SS, Socoteanu C, Reinier K, et al. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121(1):66-71.
27. Drug Enforcement Administration. Buprenorphine. http://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf. Accessed June 6, 2013.
28. Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15(3):169-178.
29. Substance Abuse and Mental Health Administration. Buprenorphine. http://buprenorphine.samhsa.gov/about.html. Accessed May 28, 2013.
30. Ling W, Casadonte P, Bigelow G, et al. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA. 2010;304(14):1576-1583.