User login
What’s it like to take Ozempic? A doctor’s own story
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Longer life after bariatric surgery, but suicide risk in young
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Death from cardiovascular disease, cancer, and diabetes was 29%, 43%, and 72% lower, respectively, in the bariatric surgery patients versus nonsurgery peers, during a mean follow-up of 13 years (all P > .001).
However, the youngest group of bariatric surgery patients – who were 18-34 years old – had a fivefold increased risk of suicide during follow-up compared with their peers who did not undergo surgery (P = .001).
These findings are from a retrospective study in Utah that matched close to 22,000 patients with severe obesity who underwent Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch from 1982 to 2018 with an equal number of nonsurgery individuals.
The study, by Ted D. Adams, PhD, MPH, and colleagues, was published online in Obesity.
‘Impressive’ data, in men too, but psychological screening important
The overall improved survival and decreased deaths from diabetes, heart disease, and cancer over this long follow-up are “impressive,” Dr. Adams, of Intermountain Surgical Specialties/Digestive Health Clinical Program, Salt Lake City, said in an interview.
Previous studies have not shown a survival benefit from bariatric surgery versus no surgery in men, he said. However, “because we had a fair number of male patients and because of the length of follow-up, we did show that the improved mortality was not only evident for the female patients but also for the male patients,” Dr. Adams stressed.
Finding increased suicide rates among bariatric surgical patients who underwent surgery at a younger age (18-34 years) shows that “we need to try and determine who is at risk for suicide,” according to Dr. Adams.
Patients with severe obesity, especially younger ones, “may need more aggressive presurgical psychological screening and postsurgery follow-up,” wrote Dr. Adams and colleagues.
The findings may also “stimulate important research related to the discovery of physiologic and biomolecular mechanisms leading to nonsurgical treatment that results in weight loss and improved mortality similar to that achieved by bariatric surgery,” they suggested.
Close to 1 in 10 Americans has severe obesity
The prevalence of severe obesity (BMI ≥ 40 kg/m2) in the United States has increased from 4.7% during 1999-2000 to 9.2% during 2017-2018, based on National Health and Nutrition Examination Survey (NHANES) data, the researchers noted.
They previously published a study of long-term mortality in 7,925 patients who had gastric bypass surgery from 1984 to 2002 matched with patients with the same BMI who did not have bariatric surgery and were followed out to 2002.
The current study extends the follow-up through 2021, doubles the number of bypass patients, and includes three newer types of bariatric surgery.
The researchers matched 21,873 patients aged 18-80 who had Roux-en-Y gastric bypass, gastric banding, sleeve gastrectomy, or duodenal switch during 1982-2018 in Utah (from the Utah Population Database) with people of the same BMI category, age category (18-34, 35-44, 45-54, and 55-80 years), and sex (from Utah driver license data).
Most patients were women (79%) and most were White (94% and 85%). They had a mean age of 42 years and a mean BMI of 46 kg/m2.
Most patients had Roux-en-Y gastric bypass (69%), and the rest had sleeve gastrectomy (14%), gastric banding (12%), and duodenal switch (4.8%).
During follow-up, 13.5% of patients in the bariatric surgery group and 14.6% of people in the nonsurgery group died.
Overall, all-cause mortality was 16% lower in patients who had bariatric surgery versus matched nonsurgical participants; it was 14% lower in women and 21% lower in men (all P < .001).
All-cause mortality was significantly lower in patients who had bariatric surgery when they were 35-44, 45-54, and 55-80 years old compared with matched peers who did not have surgery.
However, the findings “should not imply patients necessarily postpone surgery until older age,” the researchers cautioned, “as postsurgical complications have been shown to increase with increasing age at surgery and surgical postponement may result in worsened clinical status related to certain conditions such as orthopedic joint health.”
The researchers found significantly improved all-cause mortality following either type of surgery (gastric bypass, gastric banding, and sleeve gastrectomy) compared with no surgery.
Along with fewer deaths from cardiovascular disease, cancer, and diabetes, deaths from lung disease were 39% lower in the surgery group than in the nonsurgery group.
However, in the youngest group (age 18-34), deaths from cirrhosis of the liver were significantly higher in the patients who had bariatric surgery, and rates of suicide were significantly greater for both females and males, compared with similar people who did not undergo surgery.
The study was supported by grants from Ethicon Endo-Surgery (Johnson & Johnson); the National Institute of Diabetes and Digestive and Kidney Diseases, a division of the National Institutes of Health; U.S. Public Health Service; and Intermountain Research and Medical Foundation of Intermountain Healthcare. Dr. Adams disclosed ties to Ethicon Endo-Surgery and Intermountain Healthcare. A coauthor reported ties with Biomedical Research Program at Weill Cornell Medicine in Qatar, a program funded by the Qatar Foundation. The other authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM OBESITY
‘Clinical paradox’? Bariatric surgery may protect from GI cancers
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
In fact, an analysis of close to 1 million French adults suggests that the weight-loss surgery may offer some protection against these cancers.
The study results present a “clinical paradox,” according to authors of a commentary published this week along with the study in JAMA Surgery. A procedure known to increase the risk of gastroesophageal reflux disease (GERD), and potentially adenocarcinoma of the distal esophagus and gastroesophageal junction, may help shield patients from esophagogastric cancer.
The study marks “an important step toward improving the understanding of potential lifetime risks of bariatric surgery and overall major health benefits of surgically induced weight loss,” commentary authors Piotr Gorecki, MD, and Michael Zenilman, MD, with Weill Cornell Medicine in New York, write.
Recent data indicate that excess body weight is associated with nearly 8% of cancer cases and 6.5% of cancer deaths. Studies also show that bariatric surgery can reduce the risk of some cancers, but whether this extends to esophageal and gastric cancer remains unclear.
To investigate, the researchers used French national data to compare the incidence of esophageal and gastric cancer in 303,709 mostly female patients with obesity who underwent bariatric surgery and a matched group of 605,140 patients with obesity who did not undergo the surgery.
The mean age of the cohort was about 40 years. The mean period of follow-up was 6 years for the surgery group and 5.6 years for the control arm. A total of 337 patients underwent esophagogastric cancer – 83 in the surgical group and 254 in the control group. Gastric cancer was about two times more common than esophageal cancer (225 vs. 112 patients).
The incidence rate of esophagogastric cancer was higher in the control group than in the surgery group – 6.9 vs. 4.9 cases per 100,000 population per year, for an incidence rate ratio of 1.42 (P = .005).
Bariatric surgery was associated with a significant 24% lower risk of esophagogastric cancer (hazard ratio, 0.76; P = .03) and a 40% lower risk of overall in-hospital mortality, defined as “any death occurring during a hospital stay regardless of the cause” (HR, 0.60; P < .001).
The authors also found no significant difference in cancer outcomes and type of bariatric procedure, which included sleeve gastrectomy, gastric bypass, and adjustable gastric banding.
They note that key study limitations include the retrospective design, limited follow-up period, and lack of histologic data on the specific cancers. In addition, the study population was relatively young, whereas esophageal cancer is more common in older people.
But overall, the findings suggest that bariatric surgery can be performed to treat severe obesity without increasing the risk of esophageal and gastric cancer, the authors conclude.
“It seems that the balance between protective factors (weight loss, metabolic effects, and eradication of H. pylori infection) and risk factors (GERD and bile reflux) for cancer after bariatric surgery is in favor of protective factors,” the authors, led by Andrea Lazzati, MD, PhD, of Centre Hospitalier Intercommunal de Créteil, France, explain.
Although the potential protective mechanisms remain unclear, in their commentary, Dr. Gorecki and Dr. Zenilman suggest that a reduction in chronic inflammation and immunosuppression following bariatric surgery could help explain the results.
Although the study provides “reassurance of the protective clinical benefits of weight loss surgery,” more large-scale studies are needed to “better identify, elucidate, and address the pathophysiological processes of bariatric procedure,” Dr. Gorecki and Dr. Zenilman conclude.
No specific funding for the study was reported. Dr. Lazzati has received personal fees from Johnson & Johnson, Medtronic, and Gore. Dr. Zenilman has received personal fees from Academic Medical Professionals Insurance and Mohamed & Obaid Almulla Group.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
New guidelines on peds obesity call for aggressive treatment
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
and hope the problem solves itself. That’s the upshot of new guidelines from the American Academy of Pediatrics.
The authors of the guidelines also encourage primary care doctors to collaborate with other medical professionals to treat the comorbidities often linked to obesity, rather than take on the entire challenge themselves.
“It’s impossible to treat obesity within the four walls of the clinic. That’s one thing I have learned,” Ihuoma Eneli, MD, associate director of the AAP Institute for Healthy Childhood Weight, told this news organization. For example, a primary care doctor could partner with a gastroenterologist when treating a child who has nonalcoholic fatty liver disease, added Dr. Eneli, a professor of pediatrics at the Ohio State University, Columbus, who helped write the recommendations.
The new document updates 2007 recommendations from AAP about treating children and adolescents who are overweight or obese. The earlier statement focused on behavioral modification and healthy eating behaviors and paid less attention to weight-lowering medications or bariatric surgery for young people. That document did not offer specific advice to health care providers about how to address childhood overweight or obesity.
The 2023 guidelines recommend that pediatricians offer anyone aged 12 years and older with obesity – defined as a body mass index (BMI) at the 95th percentile or higher – the option of receiving weight-loss medications in addition to ongoing support for lifestyle modifications, such as exercising more and eating healthier foods.
The same approach holds for bariatric surgery once children reach age 13, and AAP stressed that no physician should ever stigmatize children or imply that they are to blame for their weight.
AAP did not receive any industry funding to develop the guidelines.
As children reach the threshold BMI levels, physicians should conduct complete physicals and order blood tests to get a fuller picture of the patients’ health.
These are the first guidelines from AAP aimed at giving pediatricians and other primary care providers concrete guidance for managing overweight and obesity in younger patients.
“Obesity is a complex, chronic disease, and that’s a frame shift here,” said Sandra S. Hassink, MD, leader of the guideline group and director of the AAP Institute for Healthy Childhood Weight.
Dr. Hassink compared obesity to asthma, another chronic disease that merits prompt attention and ongoing treatment. A physician would never let a child with asthma go untreated until their breathing problems are so severe that they turn blue, Dr. Hassink said; similarly, physicians should treat obesity in young people promptly and over time.
While some aspects of treating overweight and obesity are the same for children and adults, Dr. Hassink noted distinct differences. “Every child is embedded in a family and extended support structure,” Dr. Hassink said, which means that any obesity management technique needs the buy-in and support of the child’s family too.
AAP’s new advice reflects current understanding that excess weight or obesity in children is a result of biological and social factors, such as living in a food desert or experiencing the effects of structural racism.
The guidelines synthesize the results of hundreds of studies about the best way to treat excess weight in young people. If multiple studies were of high quality and all reached similar conclusions, they received an “A.” Less robust but still informative studies rated a “B.” In aggregate, the guideline about weight-lowering medication is based on “B” evidence that could shift with further research.
The authors recommend that clinicians calculate a child’s BMI beginning at age 2 years, with particular attention to those at the 85th percentile or higher for their age and sex (which would be defined as overweight), at the 95th percentile or higher (obesity), or at the 120th percentile and higher (severe obesity). Clinicians also should monitor blood pressure and cholesterol in their patients with overweight or obesity, particularly once they reach age 10.
Starting at age 6, providers should interview patients and their families about what would motivate them to lose weight, then tailor interventions to those factors rather than just make a blanket declaration that weight loss is necessary. This step should be coupled with intensive support – ideally, at least 26 hours of face-to-face support over the course of a year, although more is better – about effective exercise and dietary habits that result in weight loss.
The intensive support model should remain in place throughout childhood and adolescence and should be coupled with referrals for weight-loss medications or bariatric surgeries as needed once children reach age 12 or 13. Those age cutoffs are based on current evidence as to when weight-loss medications or surgery becomes effective, Dr. Hassink said, and could be shifted to lower ages if that’s what new evidence shows.
“Intensive health behavioral and lifestyle treatment is the base of all other treatment extensions,” Dr. Eneli said.
Young patients who needed weight-lowering medication used to have fewer options, according to Aaron S. Kelly, PhD, the Minnesota American Legion and Auxiliary Chair in Children’s Health at the University of Minnesota, Minneapolis.
.No longer.
Dr. Kelly was not involved in drafting the guidelines but was the lead investigator for trials of liraglutide (Saxenda), which in 2020 received U.S. Food and Drug Administration approval for treating obesity in adolescents. In 2022, the agency approved phentermine and topiramate extended-release capsules (Qsymia) for long-term weight management for patients aged 12 years and older, along with a once-weekly injection of semaglutide (Wegovy) patients in this age group. There are no weight-lowering medications for children younger than 12, Dr. Kelly said.
“Obesity is not a lifestyle problem. A lot of it is driven by the underlying biology,” Dr. Kelly said. “Really, what these medicines do is make it easier for people to make the right lifestyle choices by pushing back against the biology.”
For example, a drug can make people feel full for longer or disrupt chemical pathways that result in craving certain foods. Dr. Kelly emphasized that these drugs do not give license for people to eat as much as they want.
As for bariatric surgery, the new guidelines adhere closely to those in a 2019 AAP statement that bariatric surgery is safe and effective in pediatric settings. This is gratifying to Kirk W. Reichard, MD, MBA, a lead author of the 2019 article and director of the bariatric surgery program at Nemours Children’s Health.
Even if the information isn’t new as of 2023, Dr. Reichard said, AAP’s imprimatur could cause some eligible families to consider bariatric surgery when they may not have done so before.
Dr. Eneli, Dr. Hassink, and Dr. Reichard reported no relevant financial conflicts of interest. Dr. Kelly has relationships with Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.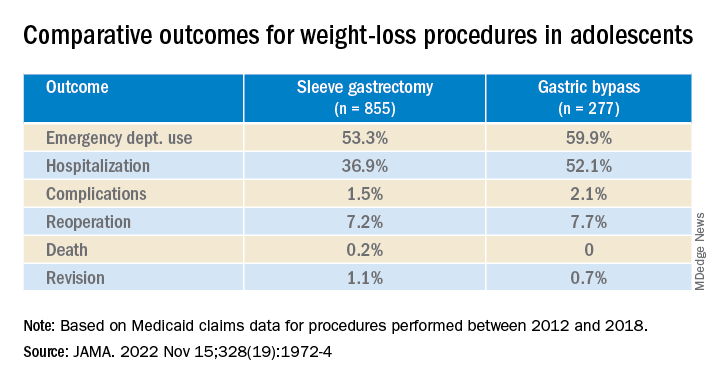
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
More weight loss with surgery than new obesity meds: meta-analysis
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – but glycemic control was similar after either treatment.
However, researchers have yet to directly compare bariatric surgery with new dual and even triple agonists that are in development.
The review by Shohinee Sarma, MD, MPH, and Patricia Palcu, MD, from the University of Toronto, was published in Obesity. Dr. Sarma also presented the findings virtually at the Obesity journal symposium at ObesityWeek® 2022.
Eric Ravussin, PhD, outgoing editor-in-chief of Obesity, explained to in an interview that this is one of five articles the editors chose from about 20 papers submitted for consideration for the symposium, and it was selected because it is a first review and meta-analysis of this direct comparison.
It showed that in “a straight head-to-head comparison, weight loss is larger by about 20 kg (44 lb) with bariatric surgery versus a GLP-1 agonist, but the improvement in glycemia (carbohydrate metabolism) was similar,” said Dr. Ravussin, from Pennington Biomedical Research Center, Louisiana State University, Baton Rouge.
Study limitations, which the authors also acknowledge, include that this was a small review of small studies: There were only six studies and 322 patients.
Moreover, the data are from 2007 to 2017, and newer weight-loss drugs are more potent.
Most studies in the review compared bariatric surgery with liraglutide, Dr. Ravussin noted, whereas, “we have now better GLP-1 agonists like semaglutide,” as well as drugs that are combinations of a GLP-1 agonist with another agonist or agonists.
“Tirzepatide, for example, which is a combination of a GLP-1 agonist and a [glucose-dependent insulinotropic polypeptide (GIP) agonist], is showing results that are very close to weight loss with bariatric surgery,” he observed.
There are quite a few other drugs in development, too, he continued, which are going to approach the weight loss obtained with bariatric surgery.
Novo Nordisk is coming out with a combination of an amylin analog (cagrilintide) and a GLP-1 agonist (semaglutide), he noted. “There are others coming in with GLP-1 and glucagon [dual agonists], and there is even a ... combo called triple G, which is a glucagon, GLP-1, and GIP [agonist].”
We now need a head-to-head comparison between bariatric surgery versus a combination drug like tirzepatide in a large population, he said.
“This is an exciting period,” Dr. Ravussin summarized, “because, 10 years ago, nobody thought that [results with] pharmacotherapy can approach bariatric surgery. Now we have other drugs that are still in development that are going to approach really close bariatric surgery.”
In an email to this news organization, Dr. Sarma noted that “due to the potent weight loss and glycemic benefits of GLP-1 agonists, patients who wish to avoid the risks of bariatric surgery may wish to discuss the option of medical therapy with their health professionals.”
“For next steps,” she said, “we need long-term studies comparing the weight-lowering, glycemic, and cardiovascular benefits of GLP-1 agonists in comparison to bariatric surgery for better counseling in obesity treatment.”
Three RCTs, three observational studies
The researchers searched the literature for randomized controlled trials (RCTs) and observational studies up to April 21, 2021, which directly compared absolute weight loss with a GLP-1 agonist – liraglutide, dulaglutide, semaglutide, exenatide, lixisenatide, and albiglutide (which are approved by the U.S. Food and Drug Administration or Health Canada) – versus any type of bariatric surgery including Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy, gastric banding, and biliopancreatic diversion.
The studies included patients aged 18 and older with a body mass index (BMI) greater than 25 kg/m2.
Secondary outcomes included change in BMI, and for patients with type 2 diabetes, change in A1c.
The researchers identified three RCTs and three observational studies, with diverse drugs and diverse types of bariatric surgery, which enrolled 13 to 134 patients, with follow-up from 6 months to 10 years.
During follow-up, the overall mean weight loss was 22.7 kg greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 25.1 kg greater in the two non-RCTs with these data (Capristo et al. and Cotugno et al.).
The overall mean decrease in BMI was 8.2 kg/m2 greater in the bariatric surgery groups than in the GLP-1 agonist groups in the two RCTs with these data (Migrone et al. and Schauer et al.), and it was 10.6 kg/m2 greater in the three non-RCTs with these data.
The overall mean decrease in A1c was 1.28% lower in the three RCTs with these data, and it was 0.9% lower in the one non-RCT with these data.
“In adults with obesity, bariatric surgery still confers the highest reductions in weight and BMI but confers similar effects in glycemic control when compared with GLP-1 agonists,” the researchers summarize.
Dr. Sarma received funding from the Clinical Investigator Program. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT OBESITYWEEK®
Bariatric surgery prompts visceral fat reduction, cardiac changes
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
Weight loss after bariatric surgery was linked with visceral fat reduction as well as reduced blood pressure, fasting glucose, and left ventricular remodeling, based an imaging study in 213 patients.
“We found that ventricular function measured by strain imaging improved in both the left and right sides of the heart, but function measured in the traditional method using endocardial motion [in other words, ejection fraction] actually worsened,” senior investigator Barry A. Borlaug, MD, said in an interview.
Although previous studies have shown positive effects of weight loss on the heart after bariatric surgery, most have been short term and have not specifically examined the effects of visceral fat reduction, wrote the investigators.
“We are in the middle of an increasing epidemic of obesity worldwide, but particularly in the United States, where it is currently projected that one in two adults will be obese by 2030,” added Dr. Borlaug of Mayo Clinic, Rochester, Minn. “Heart failure with preserved ejection fraction (HFpEF) is growing in tandem, and numerous recent studies have shown that obesity is one of the strongest risk factors for developing HFpEF, and that the severity of HFpEF is intimately linked to excess body fat. This suggests that therapies to reduce body fat could improve the cardiac abnormalities that cause HFpEF, which was our focus in this study,” he explained.
In the study, published in the Journal of the American College of Cardiology, the researchers reviewed echocardiography data from 213 obese patients before and more than 180 days after bariatric surgery. They also measured abdominal visceral adipose tissue (VAT) of 52 patients via computed tomography. The average age of the patients was 54 years, the average body mass index was 45 kg/m2, and 67% were women. Comorbidities included hypertension, diabetes, dyslipidemia, and obstructive sleep apnea.
The primary outcome was changes in cardiac structure and function.
After a median follow-up of 5.3 years, patients overall averaged a 23% reduction in body weight and a 22% reduction in BMI. In the 52 patients with abdominal scans, the VAT area decreased by 30% overall. Changes in left ventricular mass were significantly correlated to changes in the VAT.
Epicardial adipose thickness decreased by 14% overall. Left and right ventricular longitudinal strains improved at follow-up, but left atrial strain deteriorated, the researchers noted.
Although the mechanism of action remains unclear, the results suggest that left ventricular remodeling was associated with visceral adiposity rather than subcutaneous fat, the researchers wrote.
They also found that right ventricular strain was negatively correlated with VAT, but not with body weight or BMI.
“These findings suggest that weight loss, particularly reduction in visceral adiposity, benefits [right ventricular] structure and function in a manner akin to that observed in the [left ventricle],” the researchers noted.
Some surprises and limitations
Dr. Borlaug said he found some, but not all, of the results surprising. “Earlier studies had shown evidence for benefit from weight loss on cardiac structure and function, but had been limited by smaller sample sizes, shorter durations of evaluation, and variable methods used,” he said in an interview.
The findings that strain imaging showed both left and right ventricular function improved while EF declined “shows some of the problems with using EF, as it is affected by chamber size and geometry. We have previously shown that patients with HFpEF display an increase in fat around the heart, and this affects cardiac function and interaction between the left and right sides of the heart, so we expected to see that this fat depot would be reduced, and this was indeed the case,” Dr. Borlaug added.
In the current study, “visceral fat was most strongly tied to the heart remodeling in obesity, and changes in visceral fat were most strongly tied to improvements in cardiac structure following weight loss,” Dr. Borlaug told this news organization. “This further supports this concept that excess visceral fat plays a key role in HFpEF, especially in the abdomen and around the heart,” he said.
However, “The biggest surprise was the discordant effects in the left atrium,” Dr. Borlaug said. “Left atrial remodeling and dysfunction play a crucial role in HFpEF as well, and we expected that this would improve following weight loss, but in fact we observed that left atrial function deteriorated, and other indicators of atrial myopathy worsened, including higher estimates of left atrial pressures and increased prevalence of atrial fibrillation,” he said.
This difference emphasizes that weight loss may not address all abnormalities that lead to HFpEF, although a key limitation of the current study was the lack of a control group of patients with the same degree of obesity and no weight-loss intervention, and the deterioration in left atrial function might have been even greater in the absence of weight loss, Dr. Borlaug added.
Larger numbers support effects
Previous research shows that structural heart changes associated with obesity can be reversed through weight loss, but the current study fills a gap by providing long-term data in a larger sample than previously studied, wrote Paul Heidenreich, MD, of Stanford (Calif.) University in an accompanying editorial).
“There has been uncertainty regarding the prolonged effect of weight loss on cardiac function; this study was larger than many prior studies and provided a longer follow-up,” Dr. Heidenreich said in an interview.
“One unusual finding was that, while weight loss led to left ventricle reverse remodeling (reduction in wall thickness), the same effect was not seen for the left atrium; the left atrial size continued to increase,” he said. “I would have expected the left atrial changes to mirror the changes in the left ventricle,” he noted.
The findings support the greater cardiac risk of visceral vs. subcutaneous adipose tissue, and although body mass index will retain prognostic value, measures of central obesity are more likely predictors of cardiac structural changes and events and should be reported in clinical studies, Dr. Heidenreich wrote.
However, “We need a better understanding of the factors that influence left atrial remodeling and reverse remodeling,” Dr. Heidenreich told this news organization. “While left ventricular compliance and pressure play a role, there are other factors that need to be elucidated,” he said.
Studies in progress may inform practice
The current data call for further study to test novel treatments to facilitate weight loss in patients with HFpEF and those at risk for HFpEF, and some of these studies with medicines are underway, Dr. Borlaug said in the interview.
“Until such studies are completed, we will not truly understand the effects of weight loss on the heart, but the present data certainly provide strong support that patients who have obesity and HFpEF or are at risk for HFpEF should try to lose weight through lifestyle interventions,” he said.
Whether the cardiac changes seen in the current study would be different with nonsurgical weight loss remains a key question because many obese patients are reluctant to undergo bariatric surgery, Dr. Borlaug said. “We cannot assess whether the effects would differ with nonsurgical weight loss, and this requires further study,” he added.
As for additional research, “Randomized, controlled trials of weight-loss interventions, with appropriate controls and comprehensive assessments of cardiac structure, function, and hemodynamics will be most informative,” said Dr. Borlaug. “Larger trials powered to evaluate cardiovascular outcomes such as heart failure hospitalization or cardiovascular death also are critically important to better understand the role of weight loss to treat and prevent HFpEF, the ultimate form of obesity-related heart disease,” he emphasized.
The study was supported in part by grants to lead author Dr. Hidemi Sorimachi of the Mayo Clinic from the Uehara Memorial Foundation, Japan, and to corresponding author Dr. Borlaug from the National Institutes of Health. Dr. Borlaug also disclosed previous grants from National Institutes of Health/National Heart, Lung, and Blood Institute, AstraZeneca, Corvia, Medtronic, GlaxoSmithKline, Mesoblast, Novartis, and Tenax Therapeutics; and consulting fees from Actelion, Amgen, Aria, Axon Therapies, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, and VADovations. Dr. Heidenreich had no financial disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Bariatric surgery may up risk for epilepsy
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Analyzing health records, investigators compared almost 17,000 patients who had undergone bariatric surgery with more than 620,000 individuals with obesity who had not undergone the surgery.
During a minimum 3-year follow-up period, the surgery group had a 45% higher risk of developing epilepsy than the nonsurgery group. Moreover, patients who had a stroke after their bariatric surgery were 14 times more likely to develop epilepsy than those who did not have a stroke.
“When considering having bariatric surgery, people should talk to their doctors about the benefits and risks,” senior investigator Jorge Burneo, MD, professor of neurology, biostatistics, and epidemiology and endowed chair in epilepsy at Western University, London, told this news organization.
“While there are many health benefits of weight loss, our findings suggest that epilepsy is a long-term risk of bariatric surgery for weight loss,” Dr. Burneo said.
The findings were published online in Neurology.
Unrecognized risk factor?
Bariatric surgery has become more common as global rates of obesity have increased. The surgery has been shown to reduce the risk for serious obesity-related conditions, the researchers note.
However, “in addition to the positive outcomes of bariatric surgery, several long-term neurological complications have also been identified,” they write.
One previous study reported increased epilepsy risk following gastric bypass. Those findings “suggest that bariatric surgery may be an unrecognized epilepsy risk factor; however, this possible association has not been thoroughly explored,” write the investigators.
Dr. Burneo said he conducted the study because he has seen patients with epilepsy in his clinic who were “without risk factors, with normal MRIs, who shared the history of having bariatric surgery before the development of epilepsy.”
The researchers’ primary objective was to “assess whether epilepsy risk is elevated following bariatric surgery for weight loss relative to a nonsurgical cohort of patients who are obese,” he noted.
The study used linked administrative health databases in Ontario, Canada. Patients were accrued from July 1, 2010, to Dec. 31, 2016, and were followed until Dec. 31, 2019. The analysis included 639,472 participants, 2.7% of whom had undergone bariatric surgery.
The “exposed” cohort consisted of all Ontario residents aged 18 years or older who had undergone bariatric surgery during the 6-year period (n = 16,958; 65.1% women; mean age, 47.4 years), while the “unexposed” cohort consisted of patients hospitalized with a diagnosis of obesity who had not undergone bariatric surgery (n = 622,514; 62.8% women; mean age, 47.6 years).
Patients with a history of seizures, epilepsy, epilepsy risk factors, prior brain surgery, psychiatric disorders, or drug or alcohol abuse/dependence were excluded from the analysis.
The researchers collected data on patients’ sociodemographic characteristics at the index date, as well as Charlson Comorbidity Index scores during the 2 years prior to index, and data regarding several specific comorbidities, such as diabetes mellitus, hypertension, sleep apnea, depression/anxiety, and cardiovascular factors.
The exposed and unexposed cohorts were followed for a median period of 5.8 and 5.9 person-years, respectively.
‘Unclear’ mechanisms
Before weighting, 0.4% of participants in the exposed cohort (n = 73) developed epilepsy, versus 0.2% of participants in the unexposed cohort (n = 1,260) by the end of the follow-up period.
In the weighted cohorts, there were 50.1 epilepsy diagnoses per 100,000 person-years, versus 34.1 per 100,000 person-years (rate difference, 16 per 100,000 person-years).
The multivariable analysis of the weighted cohort showed the hazard ratio for epilepsy cases that were associated with bariatric surgery was 1.45 (95% confidence interval, 1.35-1.56), after adjusting for sleep apnea and including stroke as a time-varying covariate.
Having a stroke during the follow-up period increased epilepsy 14-fold in the exposed cohort (HR, 14.03; 95% CI, 4.25-46.25).
The investigators note that they were unable to measure obesity status or body mass index throughout the study and that some obesity-related comorbidities “may affect epilepsy risk.”
In addition, Dr. Burneo reported that the study did not investigate potential causes and mechanisms of the association between bariatric surgery and epilepsy risk.
Hypotheses “include potential nutritional deficiencies, receipt of general anesthesia, or other unclear causes,” he said.
“Future research should investigate epilepsy as a potential long-term complication of bariatric surgery, exploring the possible effects of this procedure,” Dr. Burneo added.
Risk-benefit discussion
In a comment, Jacqueline French, MD, professor of neurology at NYU Grossman School of Medicine, and director of NYU’s Epilepsy Study Consortium, said she was “not 100% surprised by the findings” because she has seen in her clinical practice “a number of patients who developed epilepsy after bariatric surgery or had a history of bariatric surgery at the time they developed epilepsy.”
On the other hand, she has also seen patients who did not have a history of bariatric surgery and who developed epilepsy.
“I’m unable to tell if there is an association, although I’ve had it at the back of my head as a thought and wondered about it,” said Dr. French, who is also the chief medical and innovation officer at the Epilepsy Foundation. She was not involved with the study.
She noted that possible mechanisms underlying the association are that gastric bypass surgery leads to a “significant alteration” in nutrient absorption. Moreover, “we now know that the microbiome is associated with epilepsy” and that changes occur in the gut microbiome after bariatric surgery, Dr. French said.
There are two take-home messages for practicing clinicians, she added.
“Although the risk [of developing epilepsy] is very low, it should be presented as part of the risks and benefits to patients considering bariatric surgery,” she said.
“It’s equally important to follow up on the potential differences in these patients who go on to develop epilepsy following bariatric surgery,” said Dr. French. “Is there a certain metabolic profile or some nutrient previously absorbed that now is not absorbed that might predispose people to risk?”
This would be “enormously important to know because it might not just pertain to these people but to a whole other cohort of people who develop epilepsy,” Dr. French concluded.
The study was funded by the Ontario Ministry of Health and Ministry of Long-Term Care and by the Jack Cowin Endowed Chair in Epilepsy Research at Western University. Dr. Burneo holds the Jack Cowin Endowed Chair in Epilepsy Research at Western University. The other investigators and Dr. French have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Weight-loss surgery has a big effect on marriage
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
Kristal was only in her mid-30s when she decided to have surgery. Her doctor said it was too early. But the Oregon mom of three had found herself in the hospital twice for obesity-related lung complications before her 35th birthday. So she got the gastric sleeve.
And at first it seemed like the best decision for her and her family. She was losing weight – 100 pounds in 16 months – and so was her husband. The whole family was more active and seemed to have more energy. But then her husband’s weight began to creep back up.
While she joined a running group and signed up for half-marathons, her husband’s depression and drinking worsened. The healthier lifestyle they’d shared was now an unspoken wedge between them.
And the added attention Kristal was getting from men and women because of her thinner size only added to the tension. After 30 years together and 22 years of marriage, the high school sweethearts divorced in June 2021. Kristal’s weight loss wasn’t the only problem, but she and her ex-husband believe it was the beginning of the end.
An unexpected outcome?
New research from the University of Pittsburgh found that Kristal’s experience is a common one. The study looked at data from 1,441 bariatric surgery patients and found that never-married patients were over 50% more likely to get married, and married patients were more than twice as likely to get divorced, compared to the general U.S. population.
This U.S. data follows two Scandinavian studies from 2018 and 2020 that found similar relationship changes after bariatric surgery. But the postsurgery divorce rate in the United States was only about half that found in the Danish and Swedish studies, according to the new study published in Annals of Surgery.
It’s important to note that even with an increase in the divorce rate, most marriages in the study were unchanged, said epidemiologist and lead author Wendy King, PhD. In fact, 81% of couples were still married 5 years after surgery. But where the U.S. population has a divorce rate of 3.5%, bariatric patients in the study had an 8% divorce rate. Likewise, those who’d never been married before the surgery had a marriage rate of 18%, compared to 7% in the U.S. population.
Surgery certainly isn’t a death sentence for a patient’s love life. But the uptick in marriage and divorce suggests bariatric surgery significantly impacts how people engage in relationships.
“It makes sense,” said clinical psychologist Rachel Goldman, PhD, who specializes in health and wellness issues and bariatric surgery cases in New York City. “People are changing their lifestyle.” And those changes don’t start or stop the day of surgery, they begin as soon as someone decides to have surgery and continue as a lifelong process, she said.
For some patients, these healthy habits may offer a “new lease on life,” said Dr. King. According to the study, patients who had better physical health after surgery were more likely to get married.
But the continual lifestyle changes can dramatically impact the rituals of existing relationships, said Dr. Goldman. Maybe a couple loved to go out and enjoy an extravagant meal before surgery, or they had ice cream and watched a movie every Friday. The habit changes that come with bariatric surgery can require one partner to focus less on those rituals.
These sorts of changes may leave one or both people feeling like their partner is turning away from them, said Don Cole, DMin, a relationship therapist and clinical director at the Gottman Institute in Seattle, a think tank focused on the science of relationships. The person who had surgery may feel unsupported in the new journey if the partner keeps advocating for unhealthy habits, he said. And the person who didn’t have surgery may feel cast aside by the partner’s new health priorities.
Changes, even those that are positive and healthy, create a kind of crisis for relationships, Dr. Cole said. It’s not just bariatric surgery. Bringing a baby into the home, infertility treatments, and substance abuse recovery are all considered positive changes that are also predictors of relationship dissatisfaction and divorce, he said.
A couple could have a range of emotions after one partner gets bariatric surgery, Dr. Cole said. Unfortunately, “my experience as a therapist says they aren’t that good [at talking about it],” he said.
But bariatric surgery isn’t the only thing at play in these relationship changes, according to the study. Married patients had a much lower chance of separation or divorce (13%) than patients who were unmarried but living together (44%) by 5 years after surgery. Similarly, most people who were already separated either got divorced or resumed being married. It’s as if the surgery and lifestyle changes served as a catalyst for people who already had one foot out of (or in) the door, Dr. Goldman said.
A high sexual desire after surgery was also a predictor of divorce. In fact, there were more things before surgery that impacted divorce than surgery-related changes. It’s possible that many of these patients are “on the path toward change already,” Dr. King said. “Who knows how much the surgery had to do with it.”
Dr. Goldman recalled a patient who, before surgery, had very low self-worth. She wasn’t satisfied with her relationship but admitted to staying because she didn’t believe she could do any better than her current partner. After surgery, her perspective radically changed. She started to get healthier, invested in her education, and changed jobs. And when her partner refused to join her in making changes, she left. Maybe some of these patients “were already thinking about leaving but just didn’t have the confidence,” Dr. Goldman said.
Still, it’s critical that patients receive more counseling on how choosing to have bariatric surgery can impact their relationship before and after their weight loss procedure, Dr. King said. It should be the standard of care.
Currently, relationship-specific counseling isn’t required, Dr. Goldman said. Most programs do require a psychosocial evaluation before surgery, “but they are quite varied.” And even in programs where relationships are mentioned, there often isn’t a psychologist or licensed mental health professional on the team.
Since Dr. King’s previous research on substance abuse after bariatric surgery changed common practice in the field, Dr. Goldman said she hopes the new data will have a similar influence and relationship counseling will become the norm.
Dr. Cole actually had bariatric surgery. He recalled potential relationship issues were briefly mentioned. Someone at the clinic said if his marriage felt challenged, he should seek help from a professional, and that was it.
For Dr. Cole, there were unexpected negative feelings of shame and disappointment after surgery. He felt the extreme weight loss was all his colleagues could talk about and was very disappointed when there was no change in his chronic pain, a primary reason he had the procedure.
Fortunately, he could talk to his wife – also is a relationship therapist at Gottman – about the range of emotions. “One of the things that we know that creates a deep sense of trust is [when] I know my partner is there for me when I’m not well,” Dr. Cole said.
But these negative emotions can be the very things that feel most difficult to talk about or hear from a partner. It’s hard to share our own negative feelings and to hear someone else’s, Dr. Cole said.
He advises creating a new “ritual of connection: moments in time when you plan to turn toward one another.”
That could be a daily walk, where you intentionally talk about the surgery-related changes that both of you have had. Dr. Cole said to ask yourself, “Are we intentional about turning toward one another in those [challenging] moments?”
A version of this article first appeared on WebMD.com.
FROM ANNALS OF SURGERY