User login
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
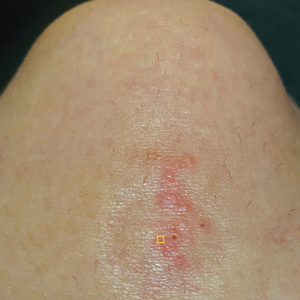
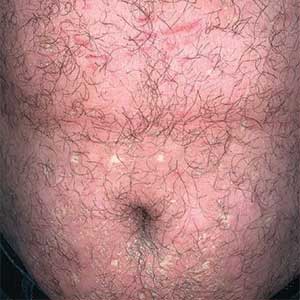
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
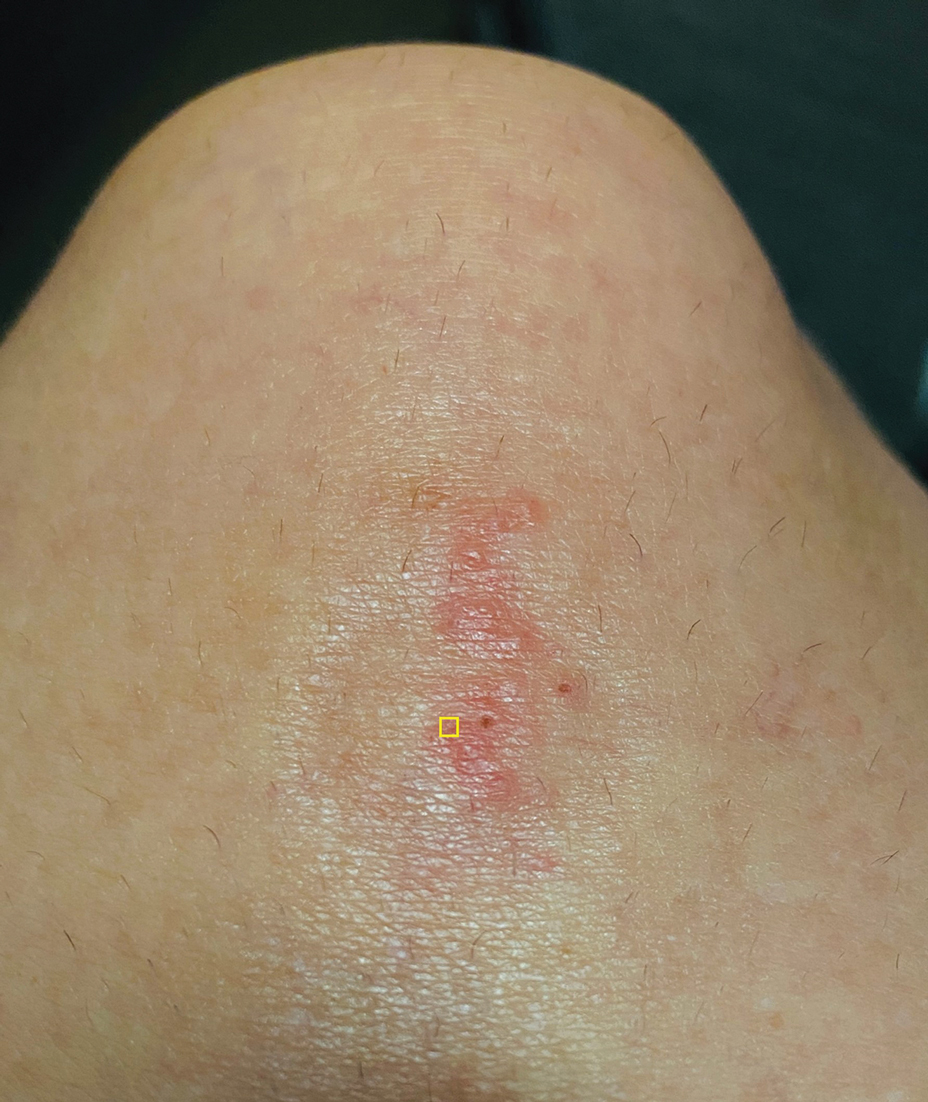
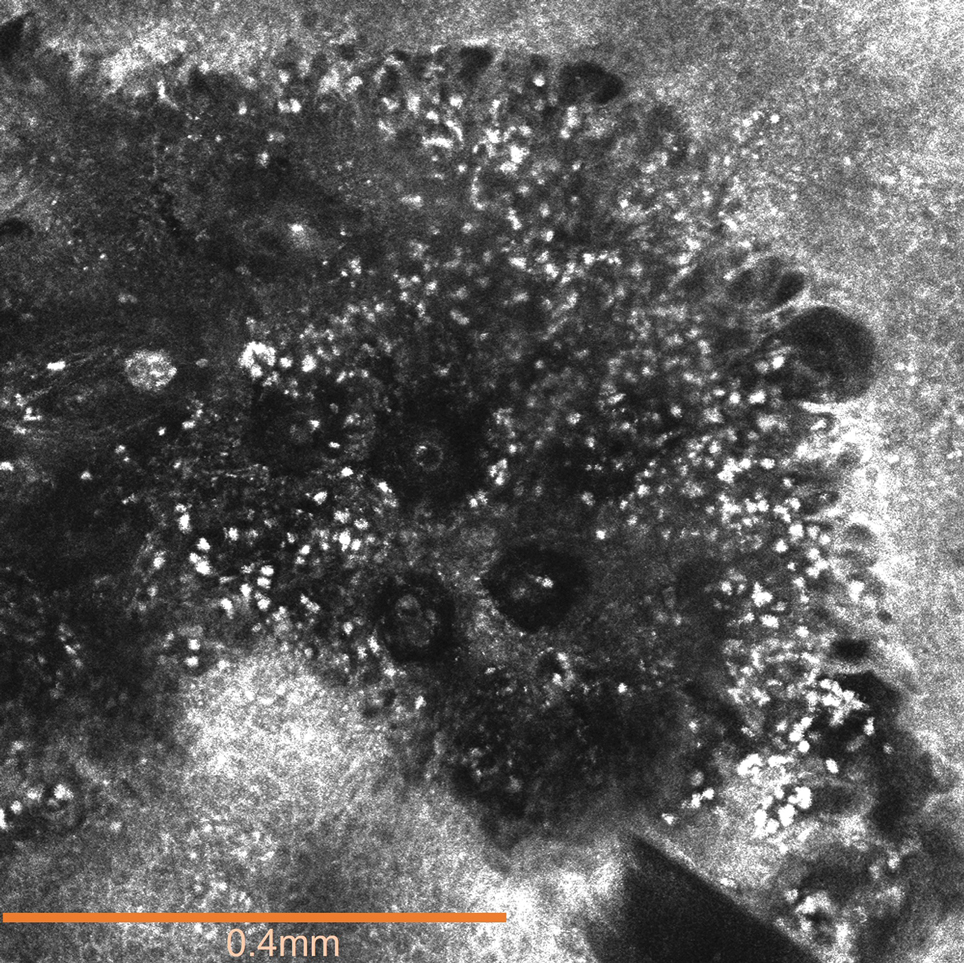
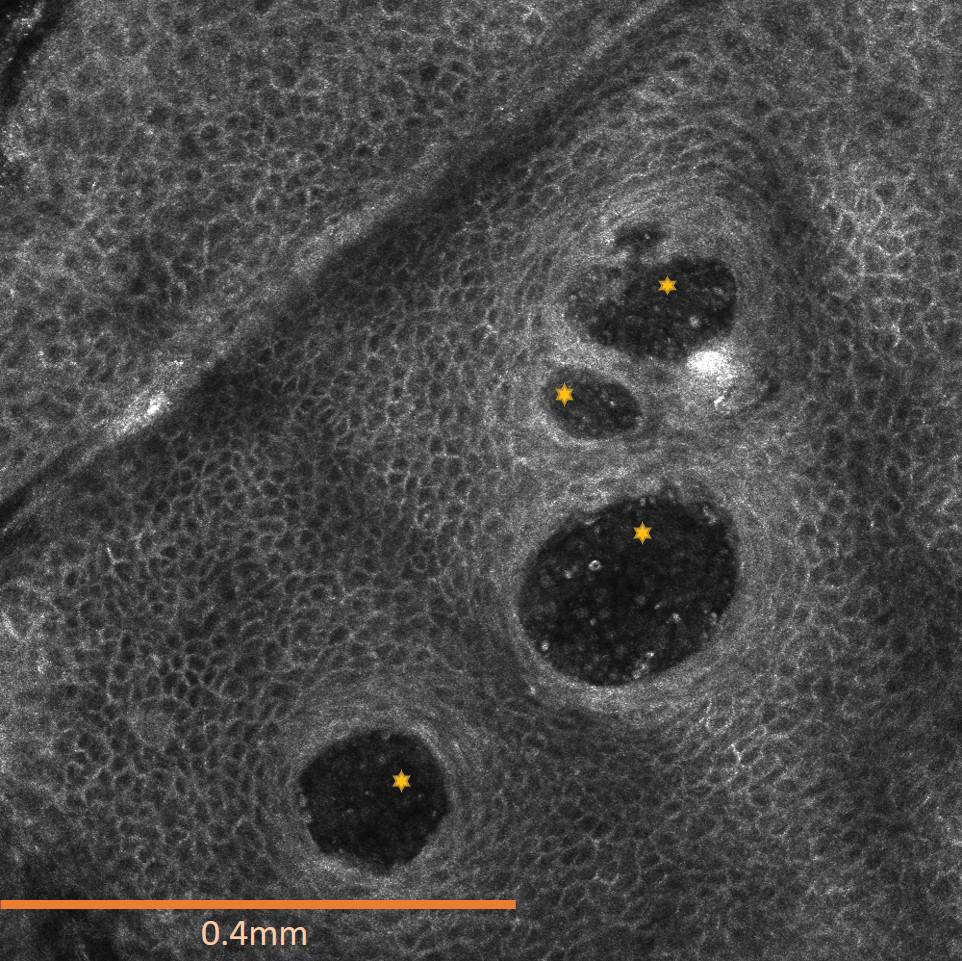
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
A Whiff of Trouble: Navigating Allergic Contact Dermatitis to Fragrance
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
Fragrances are complex organic compounds that are sufficiently volatile to produce an odor—most often a pleasant one—or at times intended to neutralize unpleasant odors. They can be further divided into natural fragrances (eg, essential oils) and synthetic ones. Fragrances are found in abundance in our daily lives: in perfumes; colognes; lotions; shampoos; and an array of other personal, household, and even industrial products (Table). These exposures include products directly applied to the skin, rinsed off, or aerosolized. A single product often contains a multitude of different fragrances to create the scents we know and love. To many, fragrances can be an important part of everyday life or even a part of one’s identity. But that once-intoxicating aroma can transform into an itchy skin nightmare; fragrances are among the most common contact allergens.

Given the widespread prevalence of fragrances in so many products, understanding fragrance allergy and skillful avoidance is imperative. In this review, we explore important aspects of fragrance allergic contact dermatitis (ACD), including chemistry, epidemiology, patch test considerations, and management strategies for patients, with the goal of providing valuable clinical insights for treating physicians on how patients can embrace a fragrance-free lifestyle.
How Fragrances Act as Allergens
A plethora of chemicals emit odors, of which more than 2000 are used to create the fragranced products we see on our shelves today.1 For many of these fragrances, contact allergy develops because the fragrance acts as a hapten (ie, a small molecule that combines with a carrier protein to elicit an immune response).2 Some fragrance molecules require “activation” to be able to bind to proteins; these are known as prehaptens.3 For example, the natural fragrance linalool is generally considered nonallergenic in its initial form. However, once it is exposed to air, it may undergo oxidation to become linalool hydroperoxides, a well-established contact allergen. Some fragrances can become allergenic in the skin itself, often secondary to enzymatic reactions—these are known as prohaptens.3 However, most fragrances are directly reactive to skin proteins on the basis of chemical reactions such as Michael addition and Schiff base formation.4 In either case, the end result is that fragrance allergens, including essential oils, may cause skin sensitization and subsequent ACD.5,6
Epidemiology
Contact allergy to fragrances is not uncommon; in a multicenter cross-sectional study conducted in 5 European countries, the prevalence in the general population was estimated to be as high as 2.6% and 1.9% among 3119 patients patch tested to fragrance mix I (FMI) and fragrance mix II (FMII), respectively.7 Studies in patients referred for patch testing have shown a higher 5% to 25% prevalence of fragrance allergy, largely depending on what population was evaluated.1 Factors such as sociocultural differences in frequency and types of fragrances used could contribute to this variation.
During patch testing, the primary fragrance screening allergens are FMI, FMII, and balsam of Peru (BOP)(Myroxylon pereirae resin).7 In recent years, hydroperoxides of linalool and limonene also have emerged as potentially important fragrance allergens.8 The frequencies of patch-test positivity of these allergens can be quite high in referral-based populations. In a study performed by the North American Contact Dermatitis Group (NACDG) from 2019 to 2020, frequencies of fragrance allergen positivity were 12.8% for FMI, 5.2% for FMII, 7.4% for BOP, 11.1% for hydroperoxides of linalool, and 3.5% for hydroperoxides of limonene.8 Additionally, it was noted that FMI and hydroperoxides of linalool were among the top 10 most frequently positive allergens.9 It should be kept in mind that NACDG studies are drawn from a referral population and not representative of the general population.
Allergic contact dermatitis to fragrances can manifest anywhere on the body, but certain patterns are characteristic. A study by the NACDG analyzed fragrance and botanical patch test results in 24,246 patients and found that fragrance/botanical-sensitive patients more commonly had dermatitis involving the face (odds ratio [OR], 1.12; 95% CI, 1.03-1.21), legs (OR, 1.22; 95% CI, 1.06-1.41), and anal/genital areas (OR, 1.26; 95% CI, 1.04-1.52) and were less likely to have hand dermatitis (OR, 0.88; 95% CI, 0.82-0.95) compared with non–fragrance/botanical-sensitive patients.10 However, other studies have found that hand dermatitis is common among fragrance-allergic individuals.11-13
Fragrance allergy tends to be more common in women than men, which likely is attributable to differences in product use and exposure.10 The prevalence of fragrance allergy increases with age in both men and women, peaking at approximately 50 years of age, likely due to repeat exposure or age-related changes to the skin barrier or immune system.14
Occupational fragrance exposures are important to consider, and fragrance ACD is associated with hairdressers, beauticians, office workers exposed to aromatherapy diffusers, and food handlers.15 Less-obvious professions that involve exposure to fragrances used to cover up unwanted odors—such as working with industrial and cleaning chemicals or even metalworking—also have been reported to be associated with ACD.16
Patch Test Considerations
Patch testing is essential to confirm fragrance allergy and guide treatment, but because there are so many potential fragrance allergens, there is no perfect patch test strategy. In a standard patch test series, the most important screening allergens are considered to be FMI, FMII, and BOP; tested together, they are thought to detect a large proportion of cases of fragrance allergy. Strikingly, in a large European study (N=1951), patch testing with the fragrance markers in the baseline panel failed to detect more than 40% of cases of allergy compared to testing with 26 individual fragrance allergens.17 Other studies have reported that a smaller proportion of fragrance allergies are missed by using baseline screening allergens alone.18,19 Limonene and linalool hydroperoxides also are potentially important fragrance allergens to consider adding to the patch test panel, as unoxidized limonene and linalool commonly are used in many products and could theoretically undergo auto-oxidation under use conditions.8 However, because of the high number of irritant, questionable, and potentially false-positive reactions, the Information Network of Departments of Dermatology has recommended against adding these hydroperoxides to a standard screening tray for patch testing.20 It must be remembered that a positive patch test to a fragrance does not necessarily represent ACD unless the patient has a clinically relevant exposure to the allergen.21
In patients who test negative to the baseline fragrance-screening allergens and in whom a high degree of suspicion remains, further testing with supplemental fragrance allergens (commercially available from patch test suppliers) is warranted.17 The thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice) includes FMI and BOP but not FMII or linalool or limonene hydroperoxides. More comprehensive patch test panels are available that include additional fragrances, such as the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core Allergen Series.22-24 It is important to remain vigilant and consider expanded patch testing if patients initially test negative but suspicion remains.
Furthermore, patch testing with the patient’s own products is an important consideration. Uter et al25 evaluated patch testing using patients’ perfumes, deodorants, and shaving lotions, and approximately 41% (53/129) of patients who tested positive to their own product tested negative for fragrance-screening allergens. Although it can be difficult to ascertain which exact component of a commercial product is the culprit, a positive patch test may still provide clinically relevant information for patients and treating physicians. In cases of questionable or weak-positive results, repeat testing or repeated open application tests can help re-evaluate suspected products.
Cross-reactivity should be considered when patch testing for fragrances. Atwater et al10 found that cross-reactivity between FMI, FMII, and BOP was common; for instance, approximately 40% of patients testing positive to FMII or BOP also had positive reactions to FMI (522/1182 and 768/1942, respectively). Understanding this concept is important because in some cases (as detailed below) patients will need to avoid all fragrances, not just the ones to which they have previously been exposed, given the limitations on fragrance labeling in the United States. However, this may change with the Modernization of Cosmetic Regulation Act of 2022.26
Avoiding Fragrances: Improving Patient Education and Outcomes
Once a relevant contact allergy to fragrance is established after patch testing, successful avoidance is critical but challenging, as there are numerous potential pitfalls. Missing just 1 hidden source of fragrance exposure will often be the difference between success or failure. Dermatologists play a crucial role in guiding patients through the intricate process of identifying and avoiding potential allergens.
Optimal Safety: Embracing a Fragrance-Free Lifestyle
For fragrance-allergic patients, it generally is safest to completely avoid fragrance.
First, if a patient only shows positive patch-test reactions to fragrance screening mixes (and not to the particular fragrances in these mixes), there is no way to be certain which fragrances the patient needs to avoid.
Second, even if specific fragrance allergens are identified, numerous chemically related fragrances to which the patient may be allergic are not commercially available for patch testing. One review provided evidence of 162 fragrance allergens that have been documented to cause contact allergy.1 Dermatologists generally patch test to screening mixtures and/or the 26 fragrance chemicals required on labels in European products (European Directive fragrance).27 Therefore, there are more than 100 known fragrance allergens that are not routinely tested to which patients could be allergic.
Third, certain fragrances, such as limonene and linalool, are found in many products with fragrance, and it is difficult to find products without these substances. Limonene and linalool themselves are not potent allergens; however, upon air exposure, they may auto-oxidize to hydroperoxides of limonene and linalool, which are increasingly common positive patch tests.19
Additionally, patients should be advised that many products labeled “fragrance free,” “unscented,” or “free and clear” are not truly fragrance free, and patients should not choose products based on these claims. There are no legal definitions for these claims in the United States, and industries are allowed to choose the definition they prefer. Numerous products labeled “unscented” use this term to indicate that the product had an odor, the company used a masking fragrance to hide the odor, and then the product can be considered unscented. In many holistic stores, most products labeled “fragrance free” are only free of artificial fragrances but contain essential oils. Of the 162 documented fragrance allergens, 80 are essential oils.6 Essential oils are perceived to be safe by the vast majority of the population because they are viewed as “natural” and “unprocessed” sources of fragrance.28 However, numerous allergenic terpenes have been discovered in essential oils, including functionalized variations of alcohols (eg, geraniol, bisabolol) and aldehydes (eg, citronellal).6 Essential oils also consist of nonterpenic compounds produced through the phenylpropanoids pathway, including eugenol and cinnamaldehyde. One review showed that most essential oils contain one or more European Directive fragrance.29 Therefore, many products labeled “unscented,” “fragrance free,” or “natural” are not free of fragrance and may be unsafe for fragrance-allergic patients.
Although not required, manufacturers sometimes voluntarily list one or more of the 162 currently identified fragrance allergens on product labels. Also, there are more than 50 potentially allergenic essential oils that can be listed on labels by their common names or by genus or species. In addition, there are synonyms for fragrance, such as aroma, parfum, perfume, and scent. Therefore, there are several hundred different ingredient names on labels that indicate the presence of fragrance, and patients are very unlikely to successfully identify fragrance-free products by trying to read product labels on their own.
Lastly, in the United States product labels only require products to state that they contain “fragrance” and do not mandate the listing of specific fragrances. If a patient is allergic to a specific fragrance, there is no way to determine if that fragrance is present in these products. This will change with the enactment of Modernization of Cosmetics Regulation Act of 2022, which empowers the US Food and Drug Administration to require manufacturers to disclose many, but not all, fragrance allergens on the labels of cosmetic and topical products.26
For all these reasons, patients should be advised to use a medical database to choose safe alternative products instead of trying to read labels themselves to avoid fragrance. The American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) is designed to identify safe alternative products for patients with contact allergies. When CAMP is programmed to avoid “fragrance,” it will list only “safe” products free of all fragrances found in a comprehensive fragrance cross-reactor group.30 This customizable database is available as an application that can be downloaded onto a patient’s mobile device. Fragrance-allergic patients should be encouraged to use the CAMP application or other similar applications (eg, SkinSAFE)(https://www.skinsafeproducts.com/) to find all the products they use.
Potential Pitfalls in Fragrance Avoidance
Most physicians, even dermatologists, will not know which products on the market are fragrance free from a contact allergy standpoint. Patients should instruct their physicians to use the allergen-avoidance application of choice whenever recommending new topical products, whether prescription or nonprescription. In 2009, Nardelli and colleagues31 found that 10% of topical pharmaceutical products contained a total of 66 different fragrance substances.
Individuals who are allergic to fragrance also can react to fragrances used by close contacts (ie, consort dermatitis).32 Therefore, fragrance-allergic individuals who do not improve after changing their personal products should consider urging their spouses or significant others to choose their personal care products using an allergen-avoidance application. Also, physical contact with pets can cause reactions, and the use of a fragrance-free pet shampoo is recommended. Additionally, allergic individuals who are providing care for small children should select fragrance-free products for them.
Some of the most heavily fragranced products on the market are found at hair salons. One exposure to an allergen often can keep patients broken out for up to 4 weeks and occasionally longer, a typical frequency for salon visits—even if the individual is taking great care to avoid fragrance at home. Patients should be instructed to bring their own shampoo, conditioner, and styling products to the salon. These patients also should bring safe moisturizer and nail polish remover for manicures. Additionally, aromatherapy used in most massages can cause flare-ups, and it is recommended that allergic patients purchase fragrance-free massage oil to bring to their sessions.
Fragranced soaps and cleansers can leave a residue on the palmar surface of the hands and fingers. This residue may not meet the threshold for causing a reaction on the thick skin of these surfaces, but it is sufficient to passively transfer fragrance to other more sensitive areas, such as the eyelids. Passive transfer of fragrance can be a major source of allergen exposure and should not be overlooked. Allergic patients should be instructed to bring safe hand cleansers to friends’ houses, restaurants, or work.
Airborne fragrances in a patient’s environment can reach sufficient concentration to cause airborne contact dermatitis. In one case report, an Uber driver developed facial airborne ACD from a fragrance diffuser in his vehicle and his condition improved upon removing the diffuser.33 Therefore, patients should be instructed to avoid fragranced diffusers, scented candles, room deodorizers, incense, and wax melts.
Fragrance in household products also can be an issue. Fragrance-allergic patients should be instructed to choose fragrance-free cleaning products and to avoid fragranced wipes on surfaces that may be touched. In addition, they should be instructed to use fragrance-free laundry products. It is not required for household products in the United States to list their ingredients, and the majority do not have complete ingredient lists. Therefore, it is imperative that the patient use an allergen-avoidance application that identifies products that have full ingredient disclosure and are free of fragrance.
For individuals who enjoy perfume and/or cologne, it may be possible for them to resume use of these products in some cases after their condition has fully cleared with complete fragrance avoidance. They should avoid spraying products into the air or applying them directly onto the skin and should instead dip a cotton swab into the perfume/cologne and dab a small amount onto their clothing. This technique can sometimes satisfy the patient and improve compliance.
If a patient who is allergic to fragrance does not clear after 6 weeks of complete fragrance avoidance, it is worth considering systemic contact dermatitis due to ingestion of fragrance-related substances in foods.34 A large number of fragrance materials also are food flavorings. For patients allergic to a specific fragrance(s), systemic avoidance needs to be specific to the allergen, and the Flavor and Extract Manufacturers Association’s flavor ingredient library is most helpful (https://www.femaflavor.org/flavor-library). If the patient is allergic to the complex mixture BOP, a balsam-free diet can be attempted.35,36
Final Thoughts
Dermatologists must equip themselves with the knowledge to educate fragrance-allergic patients on proper avoidance. The multifaceted nature of fragrance avoidance requires a personalized approach, combining label scrutiny, utilization of a safe-product application, and tailored recommendations for specific situations. By guiding patients through these complexities, dermatologists can empower patients to manage their fragrance allergy and enhance their quality of life.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
- de Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35.
- Uter W. Contact allergy to fragrances: current clinical and regulatory trends. Allergol Select. 2017;1:190-199.
- Karlberg AT, Börje A, Duus Johansen J, et al. Activation of non-sensitizing or low-sensitizing fragrance substances into potent sensitizers - prehaptens and prohaptens. Contact Dermatitis. 2013;69:323-334.
- Patlewicz GY, Wright ZM, Basketter DA, et al. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219-226. doi:10.1034/j.1600-0536.2002.470406
- Ward JM, Reeder M, Atwater AR. Essential oils debunked: separating fact from myth. Cutis. 2020;105:174-176.
- de Groot AC, Schmidt E. Essential oils, part IV: contact allergy. Dermatitis. 2016;27:170-175.
- Diepgen TL, Ofenloch R, Bruze M, et al. Prevalence of fragrance contact allergy in the general population of five European countries: a cross-sectional study. Br J Dermatol. 2015;173:1411-1419
- Ogueta IA, Brared Christensson J, Giménez-Arnau E, et al. Limonene and linalool hydroperoxides review: pros and cons for routine patch testing. Contact Dermatitis. 2022;87:1-12.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group Data 2007-2016. Dermatitis. 2021;32:42-52.
- Tai V, Sharifah Rosniza SNC, Tang MM. Contact sensitization to fragrance allergen: a 5-year review in the Department of Dermatology, Hospital Kuala Lumpur. Med J Malaysia. 2023;78:583-588.
- Periyasamy MK, Sekar SC, Rai R. Analysis of hypersensitivity in fragrance series by patch testing. Indian Dermatol Online J. 2019;10:657-662.
- Heydorn S, Menné T, Johansen JD. Fragrance allergy and hand eczema - a review. Contact Dermatitis. 2003;48:59-66.
- Buckley DA, Rycroft RJG, White IR, et al. The frequency of fragrance allergy in patch-tested patients increases with their age. Br J Dermatol. 2003;149:986-989.
- Montgomery RL, Agius R, Wilkinson SM, et al. UK trends of allergic occupational skin disease attributed to fragrances 1996-2015. Contact Dermatitis. 2018;78:33-40.
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377.
- Mann J, McFadden JP, White JML, et al. Baseline series fragrance markers fail to predict contact allergy. Contact Dermatitis. 2014;70:276-281.
- Vejanurug P, Tresukosol P, Sajjachareonpong P, et al. Fragrance allergy could be missed without patch testing with 26 individual fragrance allergens. Contact Dermatitis. 2016;74:230-235.
- Sukakul T, Bruze M, Mowitz M, et al. Simultaneous patch testing with fragrance markers in the baseline series and the ingredients of fragrance mixes: an update from southern Sweden. Contact Dermatitis. 2022;86:514-523.
- Schubert S, Geier J, Brans R, et al; IVDK. Patch testing hydroperoxides of limonene and linalool in consecutive patients-results of the IVDK 2018-2020. Contact Dermatitis. 2023;89:85-94. doi:10.1111/cod.14332
- Storrs FJ. Fragrance. Dermatitis. 2007;18:3-7.
- T.R.U.E. test. SmartPractice website. Accessed July 24, 2024. https://www.smartpractice.com/shop/category?id=581719&m=SPA ACDS
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. https://pubmed.ncbi.nlm.nih.gov/32947457/
- North American 80 Comprehensive Series NAC-80. Chemotechnique MB Diagnostics AB website. Accessed July 24, 2024. https://www.chemotechnique.se/products/national-series/north-american-80-comprehensive-series/
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998-2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Filley AR, Woodruff CM. The Modernization of Cosmetics Regulation Act of 2022: what dermatologists need to know. J Am Acad Dermatol. 2023;89:629-631.
- European Parliament and the Council of the European Union. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products (text with EEA relevance). November 3, 2003. Accessed June 7, 2024. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF
- Sharmeen JB, Mahomoodally FM, Zengin G, et al. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules. 2021;26:666.
- Scheman A, Scheman N, Rakowski EM. European Directive fragrances in natural products. Dermatitis. 2014;25:51-55.
- Scheman A, Hipolito R, Severson D, et al. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28:128-140.
- Nardelli A, D’Hooghe E, Drieghe J, et al. Allergic contact dermatitis from fragrance components in specific topical pharmaceutical products in Belgium. Contact Dermatitis. 2009;60:303-313.
- Lee J, Guo S, Dinalo J, et al. Consort allergic contact dermatitis: a systematic review. Dermatitis. 2022;33:181-186.
- Perper M, Cervantes J, Eber AE, et al. Airborne contact dermatitis caused by fragrance diffusers in Uber cars. Contact Dermatitis. 2017;77:116-117.
- Nijhawan RI, Molenda M, Zirwas MJ, et al. Systemic contact dermatitis. Dermatol Clin. 2009;27:355-364.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Scheman A, Rakowski EM, Chou V, et al. Balsam of Peru: past and future. Dermatitis. 2013;24:153-160.
Practice Points
- Fragrance allergy is common due to daily exposure from many sources, ranging from personal care products and cosmetics to cleaning products, foods/spices, and workplace materials.
- More than 100 different fragrances can cause contact allergy, but patch testing in routine practice usually is limited to a few key screening allergens with important limitations.
- Fragrance avoidance is challenging, and comprehensive patient education is critical, including the provision of a list of safe products that are truly fragrance free.
Brazilian Peppertree: Watch Out for This Lesser-Known Relative of Poison Ivy
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
Brazilian peppertree (Schinus terebinthifolia), a member of the Anacardiaceae family, is an internationally invasive plant that causes allergic contact dermatitis (ACD) in susceptible individuals. This noxious weed has settled into the landscape of the southern United States and continues to expand. Its key identifying features include its year-round white flowers as well as a peppery and turpentinelike aroma created by cracking its bright red berries. The ACD associated with contact—primarily with the plant’s sap—stems from known alkenyl phenols, cardol and cardanol. Treatment of Brazilian peppertree–associated ACD parallels that for poison ivy. As this pest increases its range, dermatologists living in endemic areas should familiarize themselves with Brazilian peppertree and its potential for harm.
Brazilian Peppertree Morphology and Geography
Plants in the Anacardiaceae family contribute to more ACD than any other family, and its 80 genera include most of the urushiol-containing plants, such as Toxicodendron (poison ivy, poison oak, poison sumac, Japanese lacquer tree), Anacardium (cashew tree), Mangifera (mango fruit), Semecarpus (India marking nut tree), and Schinus (Brazilian peppertree). Deciduous and evergreen tree members of the Anacardiaceae family grow primarily in tropical and subtropical locations and produce thick resins, 5-petalled flowers, and small fruit known as drupes. The genus name for Brazilian peppertree, Schinus, derives from Latin and Greek words meaning “mastic tree,” a relative of the pistachio tree that the Brazilian peppertree resembles.1 Brazilian peppertree leaves look and smell similar to Pistacia terebinthus (turpentine tree or terebinth), from which the species name terebinthifolia derives.2
Brazilian peppertree originated in South America, particularly Brazil, Paraguay, and Argentina.3 Since the 1840s,4 it has been an invasive weed in the United States, notably in Florida, California, Hawaii, Alabama, Georgia,5 Arizona,6 Nevada,3 and Texas.5,7 The plant also grows throughout the world, including parts of Africa, Asia, Central America, Europe,6 New Zealand,8 Australia, and various islands.9 The plant expertly outcompetes neighboring plants and has prompted control and eradication efforts in many locations.3
Identifying Features and Allergenic Plant Parts
Brazilian peppertree can be either a shrub or tree up to 30 feet tall.4 As an evergreen, it retains its leaves year-round. During fruiting seasons (primarily December through March7), bright red or pink (depending on the variety3) berries appear (Figure 1A) and contribute to its nickname “Florida holly.” Although generally considered an unwelcome guest in Florida, it does display white flowers (Figure 1B) year-round, especially from September to November.9 It characteristically exhibits 3 to 13 leaflets per leaf.10 The leaflets’ ovoid and ridged edges, netlike vasculature, shiny hue, and aroma can help identify the plant (Figure 2A). For decades, the sap of the Brazilian peppertree has been associated with skin irritation (Figure 2B).6 Although the sap of the plant serves as the main culprit of Brazilian peppertree–associated ACD, it appears that other parts of the plant, including the fruit, can cause irritating effects to skin on contact.11,12 The leaves, trunk, and fruit can be harmful to both humans and animals.6 Chemicals from flowers and crushed fruit also can lead to irritating effects in the respiratory tract if aspirated.13


Urushiol, an oily resin present in most plants of the Anacardiaceae family,14 contains many chemicals, including allergenic phenols, catechols, and resorcinols.15 Urushiol-allergic individuals develop dermatitis upon exposure to Brazilian peppertree sap.6 Alkenyl phenols found in Brazilian peppertree lead to the cutaneous manifestations in sensitized patients.11,12 In 1983, Stahl et al11 identified a phenol, cardanol (chemical name 3-pentadecylphenol16) C15:1, in Brazilian peppertree fruit. The group further tested this compound’s effect on skin via patch testing, which showed an allergic response.11 Cashew nut shells (Anacardium occidentale) contain cardanol, anacardic acid (a phenolic acid), and cardol (a phenol with the chemical name 5-pentadecylresorcinol),15,16 though Stahl et al11 were unable to extract these 2 substances (if present) from Brazilian peppertree fruit. When exposed to cardol and anacardic acid, those allergic to poison ivy often develop ACD,15 and these 2 substances are more irritating than cardanol.11 A later study did identify cardol in addition to cardanol in Brazilian peppertree.12
Cutaneous Manifestations
Brazilian peppertree–induced ACD appears similar to other plant-induced ACD with linear streaks of erythema, juicy papules, vesicles, coalescing erythematous plaques, and/or occasional edema and bullae accompanied by intense pruritus.
Treatment
Avoiding contact with Brazilian peppertree is the first line of defense, and treatment for a reaction associated with exposure is similar to that of poison ivy.17 Application of cool compresses, calamine lotion, and topical astringents offer symptom alleviation, and topical steroids (eg, clobetasol propionate 0.05% twice daily) can improve mild localized ACD when given prior to formation of blisters. For more severe and diffuse ACD, oral steroids (eg, prednisone 1 mg/kg/d tapered over 2–3 weeks) likely are necessary, though intramuscular options greatly alleviate discomfort in more severe cases (eg, intramuscular triamcinolone acetonide 1 mg/kg combined with betamethasone 0.1 mg/kg). Physicians should monitor sites for any signs of superimposed bacterial infection and initiate antibiotics as necessary.17
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
- Zona S. The correct gender of Schinus (Anacardiaceae). Phytotaxa. 2015;222:075-077.
- Terebinth. Encyclopedia.com website. Updated May 17, 2018. Accessed July 9, 2024. https://www.encyclopedia.com/plants-and-animals/plants/plants/terebinth
- Brazilian pepper tree. iNaturalist website. Accessed July 1, 2024. https://www.inaturalist.org/guide_taxa/841531#:~:text=Throughout% 20South%20and%20Central%20America,and%20as%20a%20topical%20antiseptic
- Center for Aquatic and Invasive Plants. Schinus terebinthifolia. Brazilian peppertree. Accessed July 1, 2024. https://plants.ifas.ufl.edu/plant-directory/schinus-terebinthifolia/#:~:text=Species%20Overview&text=People%20sensitive%20to%20poison%20ivy,associated%20with%20its%20bloom%20period
- Brazilian peppertree (Schinus terebinthifolia). Early Detection & Distribution Mapping System. Accessed July 4, 2024. https://www.eddmaps.org/distribution/usstate.cfm?sub=78819
- Morton F. Brazilian pepper: its impact on people, animals, and the environment. Econ Bot. 1978;32:353-359.
- Fire Effects Information System. Schinus terebinthifolius. US Department of Agriculture website. Accessed July 4, 2024. https://www.fs.usda.gov/database/feis/plants/shrub/schter/all.html
- New Zealand Plant Conservation Network. Schinus terebinthifolius. Accessed July 1, 2024. https://www.nzpcn.org.nz/flora/species/schinus-terebinthifolius
- Rojas-Sandoval J, Acevedo-Rodriguez P. Schinus terebinthifolius (Brazilian pepper tree). CABI Compendium. July 23, 2014. Accessed July 1, 2024. https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.49031
- Patocka J, Diz de Almeida J. Brazilian peppertree: review of pharmacology. Mil Med Sci Lett. 2017;86:32-41.
- Stahl E, Keller K, Blinn C. Cardanol, a skin irritant in pink pepper. Plant Medica. 1983;48:5-9.
- Skopp G, Opferkuch H-J, Schqenker G. n-Alkylphenols from Schinus terebinthifolius Raddi (Anacardiaceae). In German. Zeitschrift für Naturforschung C. 1987;42:1-16. https://doi.org/10.1515/znc-1987-1-203.
- Lloyd HA, Jaouni TM, Evans SL, et al. Terpenes of Schinus terebinthifolius. Phytochemistry. 1977;16:1301-1302.
- Goon ATJ, Goh CL. Plant dermatitis: Asian perspective. Indian J Dermatol. 2011;56:707-710.
- Rozas-Muñoz E, Lepoittevin JP, Pujol RM, et al. Allergic contact dermatitis to plants: understanding the chemistry will help our diagnostic approach. Actas Dermosifiliogr. 2012;103:456-477.
- Caillol S. Cardanol: a promising building block for biobased polymers and additives. Curr Opin Green Sustain Chem. 2018;14: 26-32.
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated June 21, 2024. Accessed July 7, 2024. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis#
Practice Points
- The Anacardiaceae family contains several plants, including Brazilian peppertree and poison ivy, that have the potential to cause allergic contact dermatitis (ACD).
- Hot spots for Brazilian peppertree include Florida and California, though it also has been reported in Texas, Hawaii, Georgia, Alabama, Arkansas, Nevada, and Arizona.
- Alkenyl phenols (eg, cardol, cardanol) are the key sensitizers found in Brazilian peppertree.
- Treatment consists of supportive care and either topical, oral, or intramuscular steroids depending on the extent and severity of the ACD.
Cosmetic Tattoo Ingredients Associated With Contact Dermatitis
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, but the ability to identify these allergies in patients is limited.
METHODOLOGY:
- While the allergenic potential of pigments in traditional tattoos has been documented, there is less clarity about pigments used in inks contained in cosmetic tattoos, also known as permanent makeup, and their association with ACD.
- Researchers conducted an Internet search and identified 974 individual permanent makeup ink products sold in the United States and also identified 79 unique pigments in those products.
- They evaluated the safety data sheets of these products and performed a PubMed search to identify documented ACD cases related to these pigments.
TAKEAWAY:
- Of the 79 pigments, 20 contained inorganic metals, which included iron, aluminum, silicone, chromium, copper, titanium, molybdenum, and manganese.
- Organic pigments were more common: 59 of the remaining pigments were organic compounds, mostly azo, quinacridone, or anthraquinone dyes, including 4 black pigments made from carbon only.
- A literature search identified 29 cases where patients had developed ACD thought to be caused by at least one of the 79 pigments identified by the authors of the current study and included 10 of the 79 pigments (12%).
- In 18 of the 29 cases in the literature, patch testing to the suspected pigment had been performed; in 3 cases, ACD was suspected without confirmatory testing.
IN PRACTICE:
Permanent makeup is becoming more popular, and there have been reports of ACD related to pigments contained in the inks, the authors wrote. “Traditional patch testing methods may not be useful in confirming the presence of a pigment allergy, even if one is suspect,” they added. “Consumers and patch testing physicians would benefit from better labeling of tattoo inks and the development of protocols designed to specifically test for tattoo pigment allergies.”
SOURCE:
The study was led by Sarah Rigali, MS, of Rosalind Franklin University, Chicago Medical School, Chicago, and coauthors from the Department of Dermatology, Northwestern University, Chicago, published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study is limited by incomplete safety data sheets. So, many brands of permanent makeup ink could not be investigated. In addition, some pigments may not be fully disclosed in ingredient lists and precise ink content measurements were not available.
DISCLOSURES:
The study reported receiving no funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10
magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).
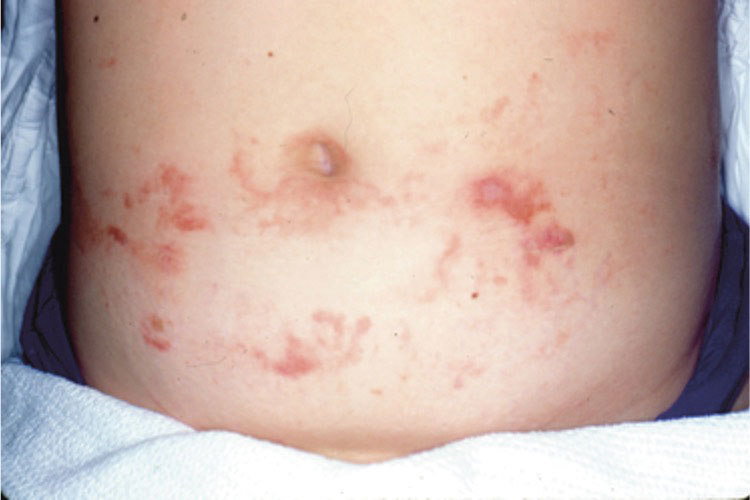
Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
PRACTICE POINTS
- Lyngbya majuscula causes seaweed dermatitis in swimmers and can be prevented by avoiding rough turbid waters in areas known to have L majuscula blooms.
- Seaweed dermatitis should be included in the differential diagnosis for erythematous papulovesicular rashes manifesting in patients who recently have spent time in the ocean.
Hypopigmented Cutaneous Langerhans Cell Histiocytosis in a Hispanic Infant
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.
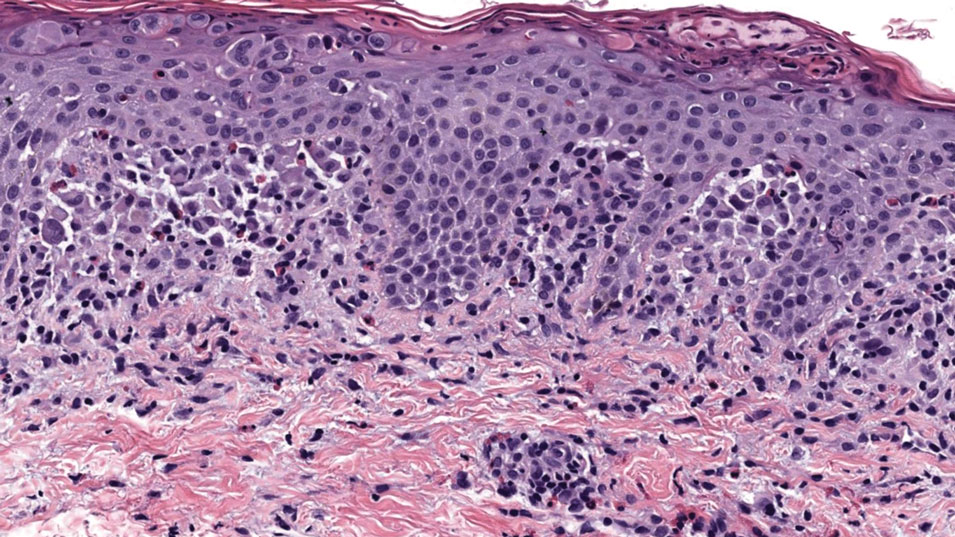
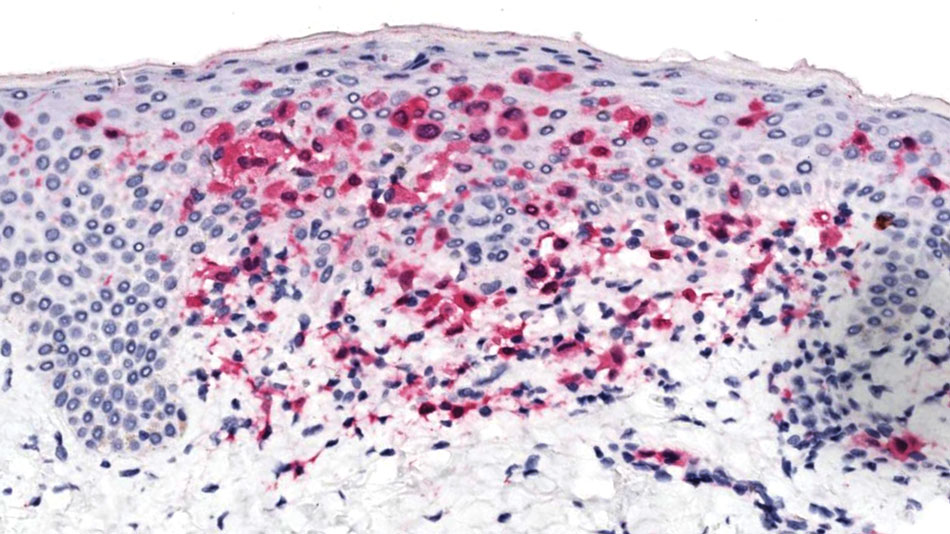
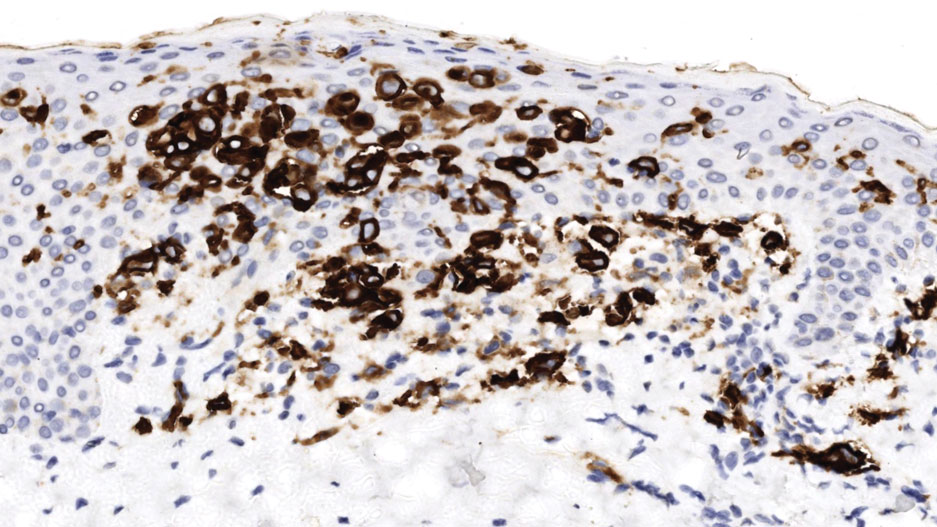
The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
Practice Points
- Dermatologists should be aware of the hypopigmented variant of cutaneous Langerhans cell histiocytosis (LCH), which has been reported exclusively in patients with skin of color.
- Langerhans cell histiocytosis should be included in the differential diagnosis of hypopigmented macules, which may be the only cutaneous manifestation or may coincide with typical lesions of LCH.
- Hypopigmented cutaneous LCH may be more common in newborns and associated with a better prognosis.
Reactive Granulomatous Dermatitis: Variability of the Predominant Inflammatory Cell Type
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.
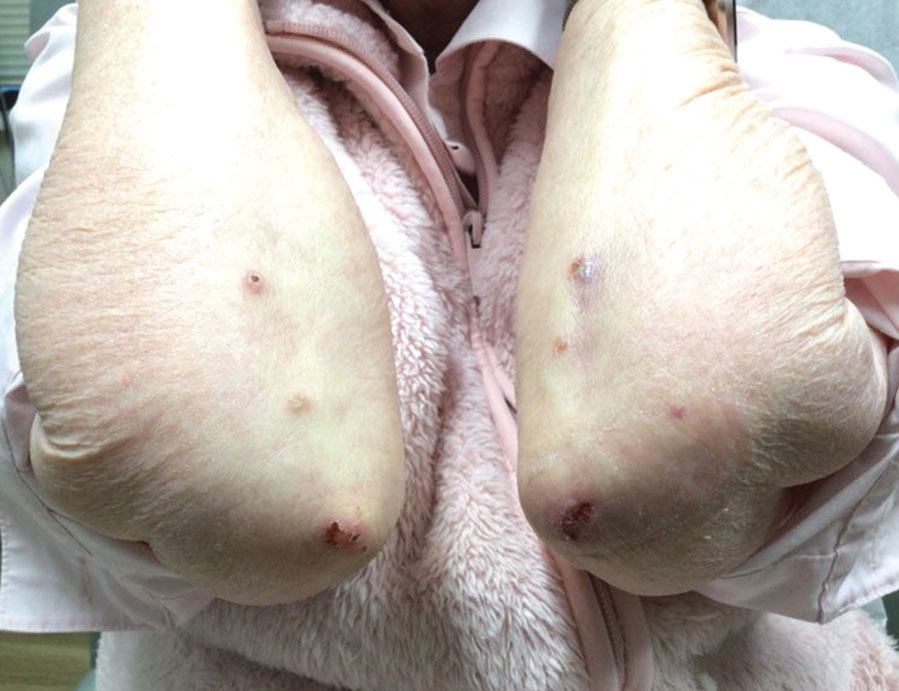
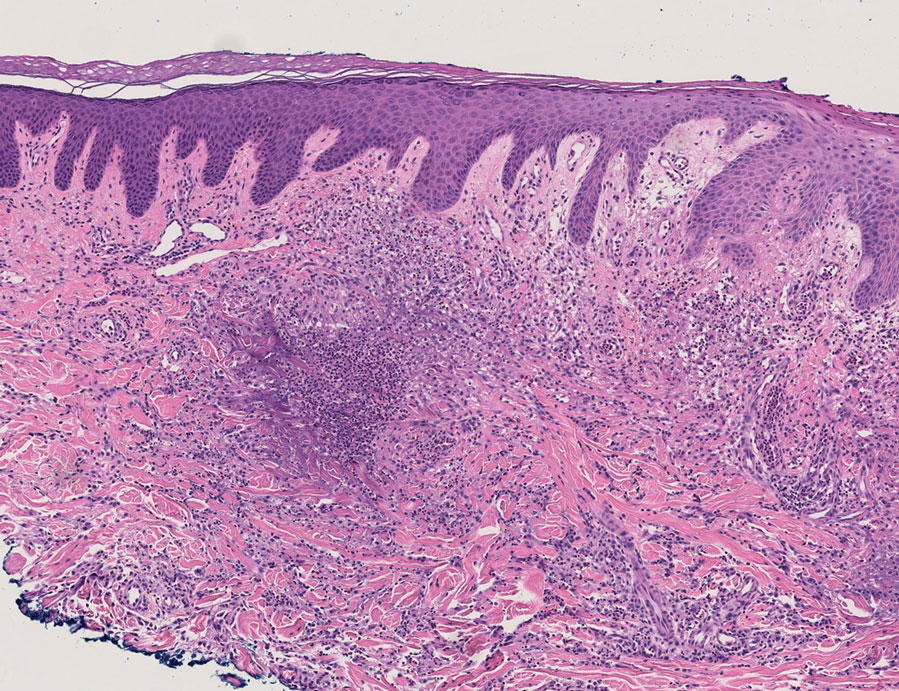
The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.
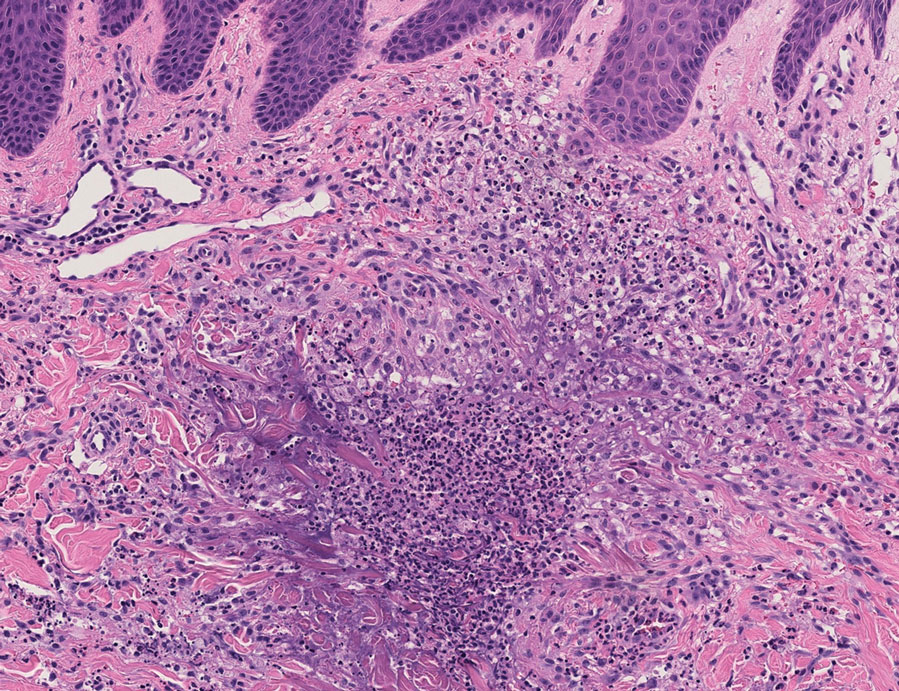
In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.
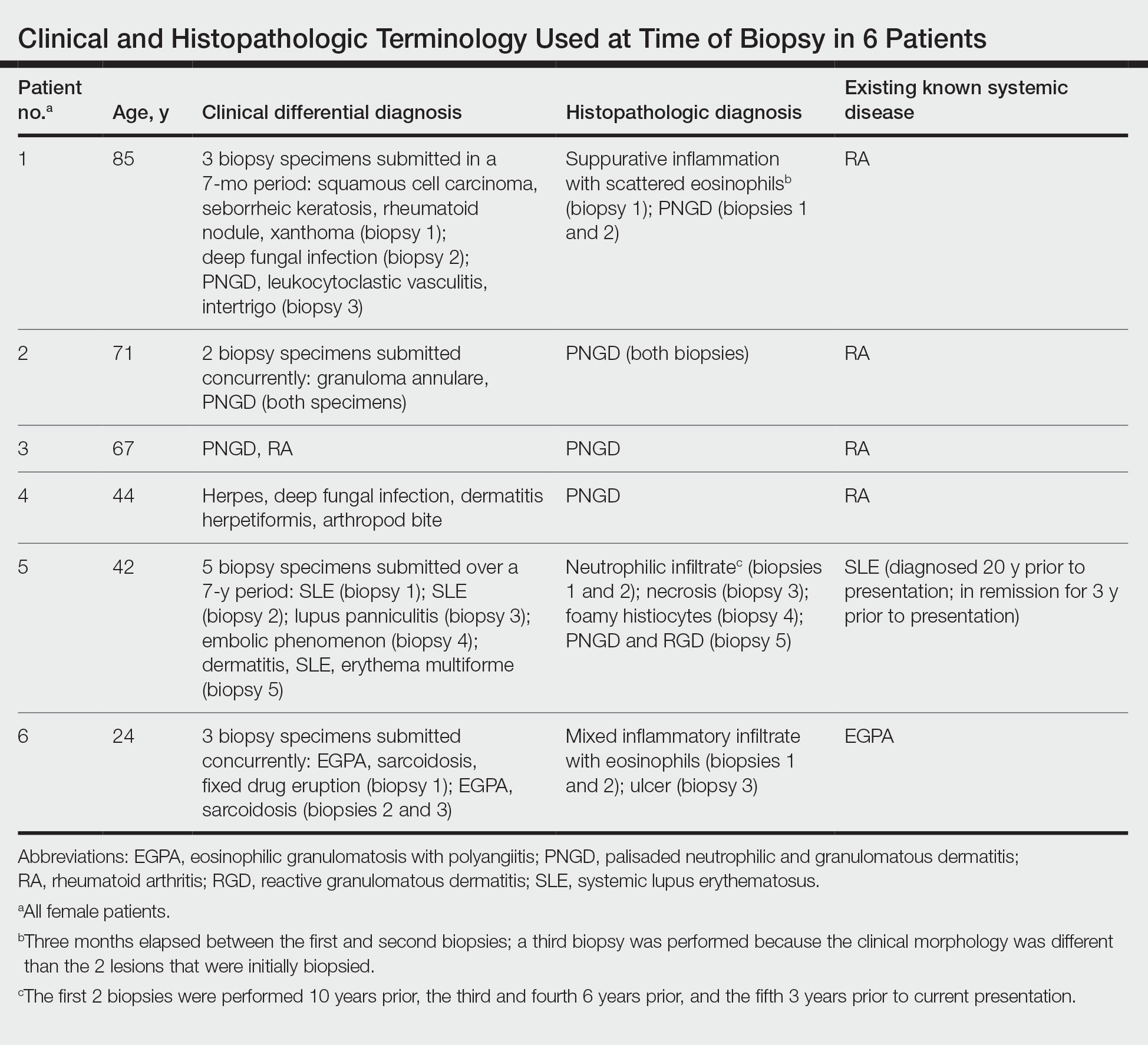
The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
Practice Points
- The term reactive granulomatous dermatitis (RGD) provides a unifying rubric for palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction.
- Reactive granulomatous dermatitis can have a variable infiltrate that includes neutrophils, histiocytes, and/or eosinophils.
- Awareness of the variability in inflammatory cell type is important for the diagnosis of RGD.
Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed
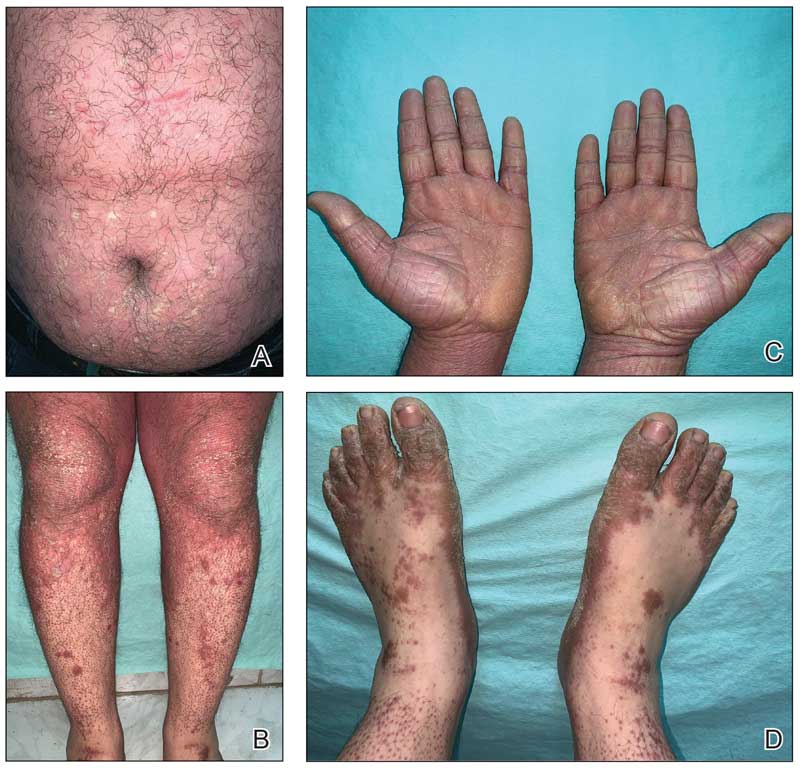
Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed
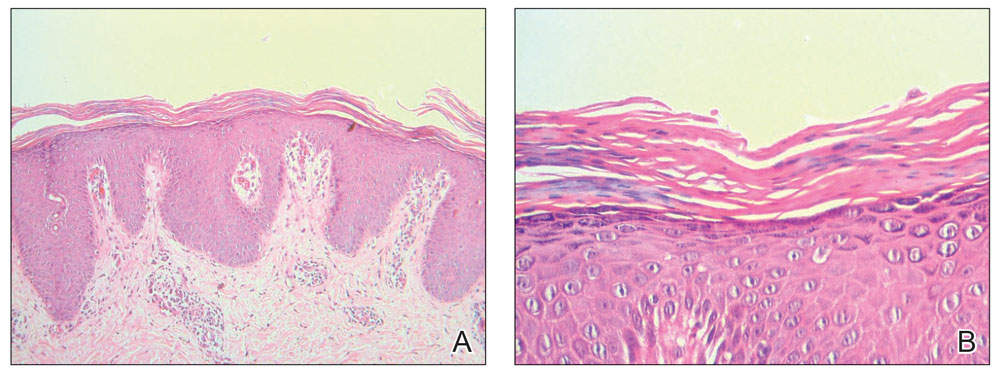
Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
Practice Points
- Dermatologists must be aware of the possibility of COVID-19 vaccine–induced pityriasis rubra pilaris (PRP), especially in de novo cases.
- Management of these cases usually follows similar standards for PRP cases.
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
Botanical Briefs: Fig Phytophotodermatitis (Ficus carica)
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
Plant Parts and Nomenclature
Ficus carica (common fig) is a deciduous shrub or small tree with smooth gray bark that can grow up to 10 m in height (Figure 1). It is characterized by many spreading branches, but the trunk rarely grows beyond a diameter of 7 in. Its hairy leaves are coarse on the upper side and soft underneath with 3 to 7 deep lobes that can extend up to 25 cm in length or width; the leaves grow individually, alternating along the sides of the branches. Fig trees often can be seen adorning yards, gardens, and parks, especially in tropical and subtropical climates. Ficus carica should not be confused with Ficus benjamina (weeping fig), a common ornamental tree that also is used to provide shade in hot climates, though both can cause phototoxic skin eruptions.

The common fig tree originated in the Mediterranean and western Asia1 and has been cultivated by humans since the second and third millennia

Ficus carica is a member of the Moraceae family (derived from the Latin name for the mulberry tree), which includes 53 genera and approximately 1400 species, of which about 850 belong to the genus Ficus (the Latin name for a fig tree). The term carica likely comes from the Latin word carricare (to load) to describe a tree loaded with figs. Family members include trees, shrubs, lianas, and herbs that usually contain laticifers with a milky latex.
Traditional Uses
For centuries, components of the fig tree have been used in herbal teas and pastes to treat ailments ranging from sore throats to diarrhea, though there is no evidence to support their efficacy.4 Ancient Indians and Egyptians used plants such as the common fig tree containing furocoumarins to induce hyperpigmentation in vitiligo.5
Phototoxic Components
The leaves and sap of the common fig tree contain psoralens, which are members of the furocoumarin group of chemical compounds and are the source of its phototoxicity. The fruit does not contain psoralens.6-9 The tree also produces proteolytic enzymes such as protease, amylase, ficin, triterpenoids, and lipodiastase that enhance its phototoxic effects.8 Exposure to UV light between 320 and 400 nm following contact with these phototoxic components triggers a reaction in the skin over the course of 1 to 3 days.5 The psoralens bind in epidermal cells, cross-link the DNA, and cause cell-membrane destruction, leading to edema and necrosis.10 The delay in symptoms may be attributed to the time needed to synthesize acute-phase reaction proteins such as tumor necrosis factor α and IL-1.11 In spring and summer months, an increased concentration of psoralens in the leaves and sap contribute to an increased incidence of phytophotodermatitis.9 Humidity and sweat also increase the percutaneous absorption of psoralens.12,13
Allergens
Fig trees produce a latex protein that can cause cross-reactive hypersensitivity reactions in those allergic to F benjamina latex and rubber latex.6 The latex proteins in fig trees can act as airborne respiratory allergens. Ingestion of figs can produce anaphylactic reactions in those sensitized to rubber latex and F benjamina latex.7 Other plant families associated with phototoxic reactions include Rutaceae (lemon, lime, bitter orange), Apiaceae (formerly Umbelliferae)(carrot, parsnip, parsley, dill, celery, hogweed), and Fabaceae (prairie turnip).
Cutaneous Manifestations
Most cases of fig phytophotodermatitis begin with burning, pain, and/or itching within hours of sunlight exposure in areas of the skin that encountered components of the fig tree, often in a linear pattern. The affected areas become erythematous and edematous with formation of bullae and unilocular vesicles over the course of 1 to 3 days.12,14,15 Lesions may extend beyond the region of contact with the fig tree as they spread across the skin due to sweat or friction, and pain may linger even after the lesions resolve.12,13,16 Adults who handle fig trees (eg, pruning) are susceptible to phototoxic reactions, especially those using chain saws or other mechanisms that result in spray exposure, as the photosensitizing sap permeates the wood and bark of the entire tree.17 Similarly, children who handle fig leaves or sap during outdoor play can develop bullous eruptions. Severe cases have resulted in hospital admission after prolonged exposure.16 Additionally, irritant dermatitis may arise from contact with the trichomes or “hairs” on various parts of the plant.

Patients who use natural remedies containing components of the fig tree without the supervision of a medical provider put themselves at risk for unsafe or unwanted adverse effects, such as phytophotodermatitis.12,15,16,18 An entire family presented with burns after they applied fig leaf extract to the skin prior to tanning outside in the sun.19 A 42-year-old woman acquired a severe burn covering 81% of the body surface after topically applying fig leaf tea to the skin as a tanning agent.20 A subset of patients ingesting or applying fig tree components for conditions such as vitiligo, dermatitis, onychomycosis, and motor retardation developed similar cutaneous reactions.13,14,21,22 Lesions resembling finger marks can raise concerns for potential abuse or neglect in children.22
The differential diagnosis for fig phytophotodermatitis includes sunburn, chemical burns, drug-related photosensitivity, infectious lesions (eg, herpes simplex, bullous impetigo, Lyme disease, superficial lymphangitis), connective tissue disease (eg, systemic lupus erythematosus), contact dermatitis, and nonaccidental trauma.12,15,18 Compared to sunburn, phytophotodermatitis tends to increase in severity over days following exposure and heals with dramatic hyperpigmentation, which also prompts visits to dermatology.12
Treatment
Treatment of fig phytophotodermatitis chiefly is symptomatic, including analgesia, appropriate wound care, and infection prophylaxis. Topical and systemic corticosteroids may aid in the resolution of moderate to severe reactions.15,23,24 Even severe injuries over small areas or mild injuries to a high percentage of the total body surface area may require treatment in a burn unit. Patients should be encouraged to use mineral-based sunscreens on the affected areas to reduce the risk for hyperpigmentation. Individuals who regularly handle fig trees should use contact barriers including gloves and protective clothing (eg, long-sleeved shirts, long pants).
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
- Ikegami H, Nogata H, Hirashima K, et al. Analysis of genetic diversity among European and Asian fig varieties (Ficus carica L.) using ISSR, RAPD, and SSR markers. Genetic Resources and Crop Evolution. 2009;56:201-209.
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319-327.
- Young R. Young’s Analytical Concordance. Thomas Nelson; 1982.
- Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
- Pathak MA, Fitzpatrick TB. Bioassay of natural and synthetic furocoumarins (psoralens). J Invest Dermatol. 1959;32:509-518.
- Focke M, Hemmer W, Wöhrl S, et al. Cross-reactivity between Ficus benjamina latex and fig fruit in patients with clinical fig allergy. Clin Exp Allergy. 2003;33:971-977.
- Hemmer W, Focke M, Götz M, et al. Sensitization to Ficus benjamina: relationship to natural rubber latex allergy and identification of foods implicated in the Ficus-fruit syndrome. Clin Exp Allergy. 2004;34:1251-1258.
- Bonamonte D, Foti C, Lionetti N, et al. Photoallergic contact dermatitis to 8-methoxypsoralen in Ficus carica. Contact Dermatitis. 2010;62:343-348.
- Zaynoun ST, Aftimos BG, Abi Ali L, et al. Ficus carica; isolation and quantification of the photoactive components. Contact Dermatitis. 1984;11:21-25.
- Tessman JW, Isaacs ST, Hearst JE. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985;24:1669-1676.
- Geary P. Burns related to the use of psoralens as a tanning agent. Burns. 1996;22:636-637.
- Redgrave N, Solomon J. Severe phytophotodermatitis from fig sap: a little known phenomenon. BMJ Case Rep. 2021;14:E238745.
- Ozdamar E, Ozbek S, Akin S. An unusual cause of burn injury: fig leaf decoction used as a remedy for a dermatitis of unknown etiology. J Burn Care Rehabil. 2003;24:229-233; discussion 228.
- Berakha GJ, Lefkovits G. Psoralen phototherapy and phototoxicity. Ann Plast Surg. 1985;14:458-461.
- Papazoglou A, Mantadakis E. Fig tree leaves phytophotodermatitis. J Pediatr. 2021;239:244-245.
- Imen MS, Ahmadabadi A, Tavousi SH, et al. The curious cases of burn by fig tree leaves. Indian J Dermatol. 2019;64:71-73.
- Rouaiguia-Bouakkaz S, Amira-Guebailia H, Rivière C, et al. Identification and quantification of furanocoumarins in stem bark and wood of eight Algerian varieties of Ficus carica by RP-HPLC-DAD and RP-HPLC-DAD-MS. Nat Prod Commun. 2013;8:485-486.
- Oliveira AA, Morais J, Pires O, et al. Fig tree induced phytophotodermatitis. BMJ Case Rep. 2020;13:E233392.
- Bassioukas K, Stergiopoulou C, Hatzis J. Erythrodermic phytophotodermatitis after application of aqueous fig-leaf extract as an artificial suntan promoter and sunbathing. Contact Dermatitis. 2004;51:94-95.
- Sforza M, Andjelkov K, Zaccheddu R. Severe burn on 81% of body surface after sun tanning. Ulus Travma Acil Cerrahi Derg. 2013;19:383-384.
- Son JH, Jin H, You HS, et al. Five cases of phytophotodermatitis caused by fig leaves and relevant literature review. Ann Dermatol. 2017;29:86-90.
- Abali AE, Aka M, Aydogan C, et al. Burns or phytophotodermatitis, abuse or neglect: confusing aspects of skin lesions caused by the superstitious use of fig leaves. J Burn Care Res. 2012;33:E309-E312.
- Picard C, Morice C, Moreau A, et al. Phytophotodermatitis in children: a difficult diagnosis mimicking other dermatitis. 2017;5:1-3.
- Enjolras O, Soupre V, Picard A. Uncommon benign infantile vascular tumors. Adv Dermatol. 2008;24:105-124.
Practice Points
- Exposure to the components of the common fig tree (Ficus carica) can induce phytophotodermatitis.
- Notable postinflammatory hyperpigmentation typically occurs in the healing stage of fig phytophotodermatitis.