User login
FDA labeling templates smooth way for OTC naloxone
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Patient-centric pain management decision aid reduces opioid use posthysterectomy
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
Rural suicidality and resilience
Overall U.S. life expectancy decreased from 78.7 years to 78.6 years from 2016 to 2017. Researchers from the Centers for Disease Control and Prevention noted that, along with drug overdose deaths, suicide also drove the decrease in average lifespan over that time period. Addressing suicide in rural communities presents unique challenges.
Dr. Bonham is vice chair of community behavioral health in the department of psychiatry and behavioral sciences at the University of New Mexico, Albuquerque. Dr. Kriechman is a child, adolescent and family psychiatrist at the university, where he serves as principal investigator on ASPYR – Alliance-building for Suicide Prevention & Youth Resilience.
You can hear more on resilience and suicide from the MDedge Psychcast in these past episodes:
- Find the MDedge Psychcast where ever you listen:
Overall U.S. life expectancy decreased from 78.7 years to 78.6 years from 2016 to 2017. Researchers from the Centers for Disease Control and Prevention noted that, along with drug overdose deaths, suicide also drove the decrease in average lifespan over that time period. Addressing suicide in rural communities presents unique challenges.
Dr. Bonham is vice chair of community behavioral health in the department of psychiatry and behavioral sciences at the University of New Mexico, Albuquerque. Dr. Kriechman is a child, adolescent and family psychiatrist at the university, where he serves as principal investigator on ASPYR – Alliance-building for Suicide Prevention & Youth Resilience.
You can hear more on resilience and suicide from the MDedge Psychcast in these past episodes:
- Find the MDedge Psychcast where ever you listen:
Overall U.S. life expectancy decreased from 78.7 years to 78.6 years from 2016 to 2017. Researchers from the Centers for Disease Control and Prevention noted that, along with drug overdose deaths, suicide also drove the decrease in average lifespan over that time period. Addressing suicide in rural communities presents unique challenges.
Dr. Bonham is vice chair of community behavioral health in the department of psychiatry and behavioral sciences at the University of New Mexico, Albuquerque. Dr. Kriechman is a child, adolescent and family psychiatrist at the university, where he serves as principal investigator on ASPYR – Alliance-building for Suicide Prevention & Youth Resilience.
You can hear more on resilience and suicide from the MDedge Psychcast in these past episodes:
- Find the MDedge Psychcast where ever you listen:
App aims to detect respiratory failure in opioid overdoses
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
A new smartphone app under development seeks to detect the first moments of an overdose-related respiratory crisis and summon help before it’s too late.
“We’re hoping a device that most people carry around could be transformed into technology that could save your life in an overdose,” said anesthesiologist Jacob (Jake) E. Sunshine, MD, an assistant professor with the University of Washington, Seattle, and coauthor of a study about the app’s development.
The ultimate goal is “to provide a harm reduction system that can automatically connect naloxone-equipped friends and family or emergency medical services to help prevent fatal overdose events,” Rajalakshmi Nandakumar, and her associates wrote in the study, published in Science Translational Medicine.
An estimated 70,000 people in the United States died from drug overdoses in 2017, according to a 2018 data brief from the Centers for Disease Control and Prevention. On an age-adjusted basis, the overdose death rate in 2017 was more than three times higher than in 1999.
The app, which builds on previous work aimed at detecting disordered breathing in sleep apnea, uses a “short-range active sonar system” to detect respiration in a person within the distance of about 3 feet. The approach is similar to the echolocation strategy used by a dolphin or bat, Dr. Sunshine said, and relies on sending out an audio tone that humans cannot hear.
The app’s microphone detects an “audio reflection” of the tone after it bounces off a nearby person’s body and then analyzes it to calculate the distance to the person’s chest. “We’re able to use those distances to measure when someone is taking a breath, and when they’re not taking a breath,” said Dr. Sunshine, who conceptualized the study.
If a disordered breathing pattern is detected, the app is designed to send a text message with a GPS-pinpointed location to a prespecified contact, Dr. Sunshine said. Or the app could be set to call 911.
In the study, the investigators tested the app’s algorithm at a supervised injection facility – a space designed to allow users to inject illicit drugs safely – in Vancouver. They tested the app on 94 drug users as they injected themselves; half of the users “experienced clinically important respiratory depression,” and two needed to be treated by clinic staff for overdose. Both users survived, reported Ms. Nandakumar, a PhD candidate at the University of Washington, Seattle; Shyamnath Gollakota, PhD, an associate professor at the university; and Dr. Sunshine.
(95% confidence interval, 86.0%-99.5%) with 97.7% specificity (95% confidence interval, 88.2%-99.9%). However, the app was less adept at identifying respiratory depression (respiratory rate equal to or less than 7 breaths per minute): The investigators reported 87.2% sensitivity (95% CI, 74.2%-95.1%) and 89.3% specificity (95% CI, 76.9%-96.4%).
Ms. Nandakumar and her associates also tested the app’s algorithm on patients undergoing anesthesia. It correctly detected disordered breathing in 19 of 20 patients.
It’s not clear how the app would work in environments full of breathing people and, potentially, breathing animals such as pets. And the app has clear limitations. Since it needs to be able to bounce audio signals off a user’s chest, it will not work if a phone is in a pocket or if a user is face down, turns around, or wanders off.
However, the app can detect sudden changes in motion, Dr. Sunshine said, and investigators are developing a way to require users to check in with the app in certain situations that might signal trouble.
“For harm reduction interventions to be efficacious, further studies with participant feedback and human factor testing are needed to ensure that the system meets the needs, values, and preferences of people who use opioids, in addition to establishing the system’s safety vis-à-vis its potential to encourage moral hazard,” the investigators wrote in the article.
The next steps are to refine the app’s user interface and figure out how to connect it to the 911 emergency-response system, Dr. Sunshine said. Meanwhile, researchers have created a company to develop the product. “We’re going to do additional development through that entity and seek [Food and Drug Administration] approval,” Dr. Sunshine said. The investigators do not plan to charge users for the product, which can be used on iPhones and Androids, he said.
The study was funded by the Foundation for Anesthesia Education and Research, the National Science Foundation, and the University of Washington’s Alcohol and Drug Abuse Institute. Dr. Sunshine, Ms. Nandakumar, and Dr. Gollakota are inventors on a provisional patient application related to the project, and all have equity stakes in a company that is developing the technology. Dr. Gollakota is a paid consultant to Jeeva Wireless and Edus Health.
SOURCE: Nandakumar R et al. Sci Transl Med. 2019 Jan 9;11(474). doi: 10.1126/scitranslmed.aau8914.
Think outside lower body for pelvic pain
Also today, treating obstructive sleep apnea with positive airway pressure decreased amyloid levels, spending on medical marketing increased by more than $12 billion over that past two decades, and one expert has advice on how you can get your work published.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, treating obstructive sleep apnea with positive airway pressure decreased amyloid levels, spending on medical marketing increased by more than $12 billion over that past two decades, and one expert has advice on how you can get your work published.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, treating obstructive sleep apnea with positive airway pressure decreased amyloid levels, spending on medical marketing increased by more than $12 billion over that past two decades, and one expert has advice on how you can get your work published.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Opioid clinic physicians report lack of competency in managing patients with HCV
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
A survey of clinicians who provide opioid agonist therapy (OAT) to people who inject drugs (PWID), showed several areas where self-reported competency in the management and treatment of hepatitis C virus (HCV) could be improved.
The C-SCOPE study consisted of a self-administered survey among physicians practicing at clinics providing OAT in Australia, Canada, Europe, and the United States during April-May of 2017. Among 203 physicians – 40% in the United States, 45% in Europe, and 14% in Australia/Canada – 21% were addiction medicine specialists, and 29% were psychiatrists.
The majority reported that HCV testing (86%) and treatment (82%) among PWID were important.
The minority reported less than average competence with respect to regular screening (12%) and interpretation of HCV test results (14%), while greater proportions reported less than average competence in advising patients about new HCV therapies (28%), knowledge of new treatments (37%), and treatment/management of HCV (40%). Although a minority of participants self-reported average or less competency related to the ability to ensure regular screening for HCV (34%) and in the ability to interpret HCV test results (39%), more than half of the participants self-reported average or less competency in other areas. These areas included the ability to assess liver disease (52%), the ability to treat HCV and manage side effects (65%), and knowledge of new HCV treatments (64%). This trend was consistent with findings from previous studies among competency related to HCV infection among primary care providers, according to the authors (Int J Drug Policy. 2019;63:29-38).
“These low levels of reported competency in HCV management and treatment highlight a critical need for improved HCV education and training in how to manage and treat HCV among PWID,” the researchers concluded.
The authors reported grant funding and consultancy with a number of pharmaceutical companies. Funding was provided by Merck Sharp & Dohme and the Australian government.
SOURCE: Grebely J et al. Int J Drug Policy. 2019;63:29-38.
FROM THE INTERNATIONAL JOURNAL OF DRUG POLICY
Prenatal valproate and ADHD
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, one expert calls for better ways to preserve beta cell function in youth, synthetic opioids drive a spike in the number of fatal overdoses, and mothers may play a role in the link between depression in fathers and daughters.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Synthetic opioids drive spike in U.S. fatal drug overdoses
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
New federal statistics suggest that the opioid epidemic in the United States is evolving as physicians crack down on the use of prescription painkillers: Fatal drug overdose deaths rose by 12% from 2016 to 2017, boosted by a wave of fatalities linked to illicit synthetic opioids like fentanyl that are now linked to an estimated 60% of opioid-related deaths.
“Overall, the overdose epidemic continues to worsen, and it has grown increasingly complex by coinvolvement of prescription and illicit drugs,” Lawrence Scholl, PhD, MPH, and his associates at the Centers for Disease Control & Prevention wrote in the Morbidity and Mortality Weekly Report.
The new statistics provide more evidence that 2017 marked “a sharp increase in what has characterized as the third wave of the opioid epidemic,” said drug and health policy researcher Stephen Crystal, PhD, of Rutgers University, New Brunswick, N.J., in an interview. He was referring to a wave that experts believe started in 2013 amid a spike in U.S. overdose deaths from fentanyl and other synthetic opioids.
The new report analyzes fatal drug overdose data from 2013 to 2017. According to the findings, the total number of those overdoses rose to 70,237 in 2017, up from 63,632 in 2016. The highest drug overdose death rates in 2017 were in West Virginia, followed by Ohio, Pennsylvania, and the District of Columbia.
Some statistics did not change much from 2016 to 2017: About two-thirds of the drug overdose deaths were linked to opioids in both years, and the death rate of cases linked to prescription drugs and heroin remained steady. (Death rates in the report were age adjusted.)
However, the percentage of fatal overdose cases linked to synthetic opioids grew 45% from 2016 to 2017. Overall, 60% of opioid-related fatal overdoses in 2017 involved synthetic opioids.
The report identifies increases in several areas from 2016 to 2017. Opioid-related drug overdose deaths among black people rose by 25%, and an analysis of data from 34 states and the District of Columbia found the highest increases in death rates in North Carolina (29%), Ohio (19%), and Maine (19%).
In regard to deaths linked to synthetic opioids specifically, the highest death rates in 2017 were in West Virginia (37 per 100,000), Ohio (32 per 100,000), and New Hampshire (30 per 100,000).
“Part of what we’re seeing in these increased numbers are individuals who have pain, can’t get prescribed opioids, and turn to street drugs,” Dr. Crystal said, adding that “abruptly cutting patients off is not good, and leaving patients with a lot of untreated pain is not good. If people are going to be discontinued [from opioids] or have their doses reduced, the taper needs to be done very slowly and carefully.”
Synthetic opioids were not the only drugs that are driving up fatal overdoses, as the death rates of cases linked to cocaine and psychostimulants (such as methamphetamine) jumped by more than a third in 2017.
“The most important thing these numbers are telling me is that it’s becoming more and more attractive to drug dealers to put fentanyl in the heroin, cocaine, and other drugs they sell,” Dr. Crystal said. “When that happens, dependence on street drugs becomes much more deadly. It’s almost impossible to get the dose right. Every time you shoot up, you’re taking a chance that you’ll overdose.”
The report had limitations, including the fact that details about drug use were missing from 12% (2016) and 15% (2017) of death certificates in fatal overdose cases. By state, the percentages of those death certificates that included drug information ranged from as little as 55% to 99%.
There’s some possible positive news: The report points to preliminary data from 2018 suggesting that the number of annual drug overdose deaths may be leveling off – although it says more analysis is needed to confirm the trend.
Dr. Crystal, however, is not celebrating. “I don’t see this as a good news story, really,” he said, adding that there’s “a little too much of people patting themselves on the back” because they’re proud of cutbacks in opioid prescriptions.
“This doesn’t have to do with the huge number of people who got started with opioids years ago” and are now at risk of using street drugs, he said. “We haven’t engaged that population at the rate we need to. And flattening out at 70,000 drug overdoses a year is not a good news story.”
Dr. Crystal reported no relevant disclosures.
SOURCE: Scholl L et al. MMWR. 2019 Jan 4;67(5152):1419-27.
FROM MMWR
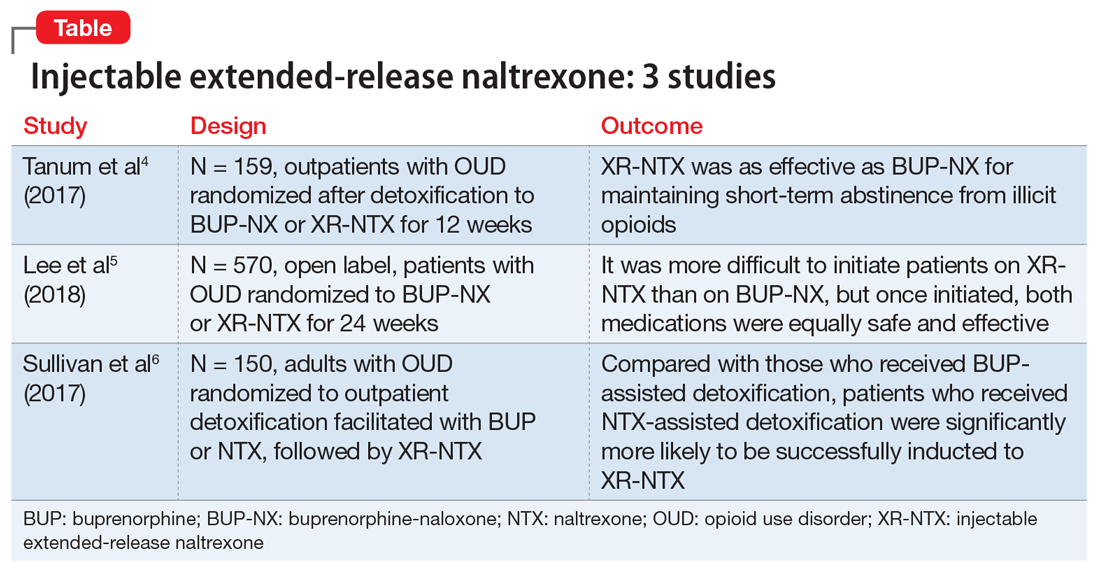
Injectable extended-release naltrexone for opioid dependence: 3 studies
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
Death by drug overdose is the number one cause of death in Americans 50 years of age and younger.1 In 2016, there were 63,632 drug overdose deaths in the United States2 Opioids were involved in 42,249 of these deaths, which represents 66.4% of all drug overdose deaths.2 From 2015 to 2016, the age-adjusted rate of overdose deaths increased significantly by 21.5% from 16.3 per 100,000 to 19.8 per 100,000.2 This means that every day, more than 115 people in the United States die after overdosing on opioids. The misuse of and addiction to opioids—including prescription pain relievers, heroin, and synthetic opioids such as fentanyl—is a serious national crisis that affects public health as well as social and economic welfare.
The gold standard treatment is medication-assisted treatment (MAT)—the use of FDA-approved medications, in combination with counseling and behavioral therapies, to provide a “whole-patient” approach.3 When it comes to MAT options for opioid use disorder (OUD), there are 3 medications, each with its own caveats.
Methadone is an opioid mu-receptor full agonist that prevents withdrawal but does not block other narcotics. It requires daily dosing as a liquid formulation that is dispensed only in regulated clinics.
Buprenorphine is a mu-receptor high affinity partial agonist/antagonist that blocks the majority of other narcotics while reducing withdrawal risk. It requires daily dosing as either a dissolving tablet or cheek film. Recently it has also become available as a 6-month implant as well as a 1-month subcutaneous injection. Buprenorphine is also available as a combined medication with naloxone; naloxone is an opioid antagonist
Naltrexone is a mu-receptor antagonist that blocks the effects of most narcotics. It does not lead to dependence, and is administered daily as a pill or monthly as a deep IM injection of its extended-release formulation.
The first 2 medications are tightly regulated options that are not available in many areas of the United States. Naltrexone, when provided as a daily pill, has adherence issues. As with any illness, lack of adherence to treatment is problematic; in the case of patients with OUD, this includes a high risk of overdose and death.
The use of injectable extended-release naltrexone (XR-NTX) may be a way to address nonadherence and thus prevent relapse. One of the challenges limiting naltrexone’s applicability has been the length of time required for an “opioid washout” of the mu receptors prior to administering naltrexone, which is a mu blocker. The washout can take as long as 7 to 10 days. This interval is not feasible for patients receiving inpatient treatment, and patients receiving treatment as outpatients are vulnerable to relapse during this time. Recently, there have been several attempts to shorten this gap through various experimental protocols based on incremental doses of NTX to facilitate withdrawal while managing symptoms.
Continue to: When selecting appropriate candidates for NTX treatment...
When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes
Outcomes
- Comparable treatment retention between groups
- Comparable opioid-negative urine drug screens (UDS)
- Significantly lower opioid use in the XR-NTX group.
Conclusion
- XR-NTX was as effective as BUP-NX in maintaining short-term abstinence from heroin and other illicit opioids, and thus should be considered as a treatment option for opioid-dependent individuals.
While this study showed similar efficacy for XR-NTX and BUP-NX, it is important to note that the randomization occurred after patients were detoxified. As a full opioid antagonist, XR-NTX can precipitate severe withdrawal, so patients need to be completely detoxified before starting XR-NTX, in contrast to BUP-NX, which patients can start even while still in mild withdrawal. Additional studies are needed in which individuals are randomized before detoxification, which would make it possible to measure the success of induction.
2. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
This study evaluated XR-NTX vs BUP-NX among adults with OUD who were actively using heroin at baseline and were admitted to community detoxification and treatment programs. Although the study began on inpatient units, it aimed to replicate usual community outpatient conditions across a 24-week outpatient treatment phase of this open-label, comparative effectiveness trial. Researchers assessed the effects on relapse-free survival, opioid use rates, and overdose events.
Study design
- N = 570, multicenter, randomized, 24-week study in the United States
- Detoxification methods: no opioids (clonidine or adjunctive medications), 3- to 5-day methadone taper, and 3- to 14-day BUP taper
- Protocol requirement: opioid-negative UDS before XR-NTX induction
- XR-NTX induction success ranged from 50% at a short-methadone-taper unit to 95% at an extended-opioid-free inpatient program. Nearly all induction failures quickly relapsed
- More participants inducted on BUP-NX group than XR-NTX group (94% vs 72%, respectively).
Continue to: Outcomes
Outcomes (once successfully inducted to treatment [n = 474])
- Comparable relapse events
- Comparable opioid-negative urine drug screens and opioid-abstinent days
- Opioid craving initially less with XR-NTX.
Conclusion
- It was more difficult to initiate patients on XR-NTX than BUP-NX, which negatively affected overall relapse rates. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Regarding induction on NTX, patients must be detoxified and opioid-free for at least 7 days. If this medication is given to patients who are physically dependent and/or have opioids in their system, NTX will displace opioids off the receptor and precipitate a severe withdrawal (rather than a slow and gradual spontaneous withdrawal).
Several studies have examined the severity of opioid withdrawal (using Self Opioid Withdrawal Scale scoring) of patients undergoing detoxification with symptomatic management (eg, clonidine, loperamide, etc.), agonist-managed (eg, with a BUP taper), and without any assistance. As expected, the latter yielded the highest scoring and most uncomfortable experiences. Using scores from the first 2 groups, a threshold of symptom tolerability was established where patients remained somewhat comfortable during the process. During detoxification from heroin, administering any dose of NTX during the first 48 to 72 hours after the last use placed patients in a withdrawal of a magnitude above the limit of tolerability. At 48 to 72 hours, however, a very low NTX dose (3 to 6 mg) was found to be well tolerated, and withdrawal symptoms were easily managed supportively to accelerate the detoxification process. Several studies have attempted to devise protocols based on these findings in order to facilitate rapid induction onto NTX. The following study offers encouragement:
Continue to: 3. Sullivan M, Bisaga A, Pavlicova M...
3. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
Study design
- N = 150 adults with OUD, randomized to outpatient opioid detoxification
- Patients were randomized to BUP- or NTX-facilitated detoxification, followed by XR-NTX
- BUP detoxification group underwent a 7-day BUP taper followed by a opioid-free week
- NTX group received a 1-day BUP dose followed by 6 days of ascending doses of oral NTX, along with clonidine and other adjunctive medications.
Outcomes
- NTX-assisted detoxification was significantly more successful for XR-NTX induction (56.1% vs 32.7%).
Conclusion
- Compared with the BUP-assisted detoxification group, NTX-assisted detoxification appears to make it significantly more likely for patients to be successfully inducted to XR-NTX.
The evidence discussed here holds promise in addressing some of the major issues surrounding MAT. For suitable candidates, XR-NTX seems to be as efficacious an option as agonist (BUP) MAT, and its induction limitations could be overcome by using NTX-facilitated detoxification protocols.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
2. Centers for Disease Control and Prevention. Drug overdose death data. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Updated December 19, 2017. Accessed October 24, 2018.
3. Substance Abuse and Mental Health Services Administration. Medication-assisted treatment (MAT). https://www.samhsa.gov/medication-assisted-treatment. Updated February 7, 2018. Accessed October 23, 2018.
4. Tanum L, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
5. Lee JD, Nunes, EV, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
6. Sullivan M, Bisaga A, Pavlicova M, et al. Long-acting injectable naltrexone induction: a randomized trial of outpatient opioid detoxification with naltrexone versus buprenorphine. Am J Psychiatry. 2017;174:459-467.
No change in postoperative pain with restrictive opioid protocol
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
The ultrarestrictive postoperative opioid prescribing protocol described in this study is a promising strategy for reducing opioid prescribing without increasing pain and limiting the potential for diversion and misuse of opioids. An important element of this protocol is the preoperative counseling, because setting patient expectations is likely to be an important factor in improving postoperative outcomes.
It is also worth noting that this study focused on patients undergoing major and minor gynecologic surgery, so more research is needed to explore these outcomes particularly among patients undergoing procedures that may be associated with a higher risk of persistent postoperative pain and/or opioid use. It is also a management strategy explored in patients at low risk of chronic postoperative opioid use, but a similar pathway should be developed and explored in more high-risk patients.
Dr. Jennifer M. Hah is from the department of anesthesiology, perioperative, and pain management at Stanford University (Calif.). These comments are taken from an accompanying editorial (JAMA Network Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5432). No conflicts of interest were reported.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Opioid prescriptions after gynecologic surgery can be significantly reduced without impacting postoperative pain scores or complication rates, according to a paper published in JAMA Network Open.
A tertiary care comprehensive care center implemented an ultrarestrictive opioid prescription protocol (UROPP) then evaluated the outcomes in a case-control study involving 605 women undergoing gynecologic surgery, compared with 626 controls treated before implementation of the new protocol.
The ultrarestrictive protocol was prompted by frequent inquiries from patients who had used very little of their prescribed opioids after surgery and wanted to know what to do with the unused pills.
The new protocol involved a short preoperative counseling session about postoperative pain management. Following that, ambulatory surgery, minimally invasive surgery, or laparotomy patients were prescribed a 7-day supply of nonopioid pain relief. Laparotomy patients were also prescribed a 3-day supply of an oral opioid.
Any patients who required more than five opioid doses in the 24 hours before discharge were also prescribed a 3-day supply of opioid pain medication as needed, and all patients had the option of requesting an additional 3-day opioid refill.
Researchers saw no significant differences between the two groups in mean postoperative pain scores 2 weeks after surgery, and a similar number of patients in each group requested an opioid refill. There was also no significant difference in the number of postoperative complications between groups.
Implementation of the ultrarestrictive protocol was associated with significant declines in the mean number of opioid pills prescribed dropped from 31.7 to 3.5 in all surgical cases, from 43.6 to 12.1 in the laparotomy group, from 38.4 to 1.3 in the minimally invasive surgery group, and from 13.9 to 0.2 in patients who underwent ambulatory surgery.
“These data suggest that the implementation of a UROPP in a large surgical service is feasible and safe and was associated with a significantly decreased number of opioids dispensed during the perioperative period, particularly among opioid-naive patients,” wrote Jaron Mark, MD, of the department of gynecologic oncology at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and his coauthors. “The opioid-sparing effect was also marked and statistically significant in the laparotomy group, where most patients remained physically active and recovered well with no negative sequelae or elevated pain score after surgery.”
The researchers also noted that patients who were discharged home with an opioid prescription were more likely to call and request a refill within 30 days, compared with patients who did not receive opioids at discharge.
The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
SOURCE: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.
Key clinical point: A ultrarestrictive postoperative opioid protocol is not associated with higher postoperative pain scores.
Major finding: The protocol achieves significant reductions in opioid use.
Study details: A case-control study in 1,231 women undergoing gynecologic surgery.
Disclosures: The study was supported by the Roswell Park Comprehensive Cancer Center, the National Cancer Institute, and the Roswell Park Alliance Foundation. Two authors reported receiving fees and nonfinancial support from the private sector unrelated to the study.
Source: Mark J et al. JAMA Netw Open. 2018 Dec 7. doi: 10.1001/jamanetworkopen.2018.5452.