User login
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

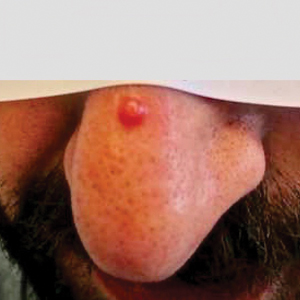
Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
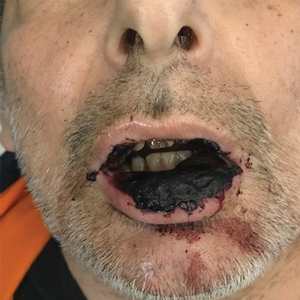
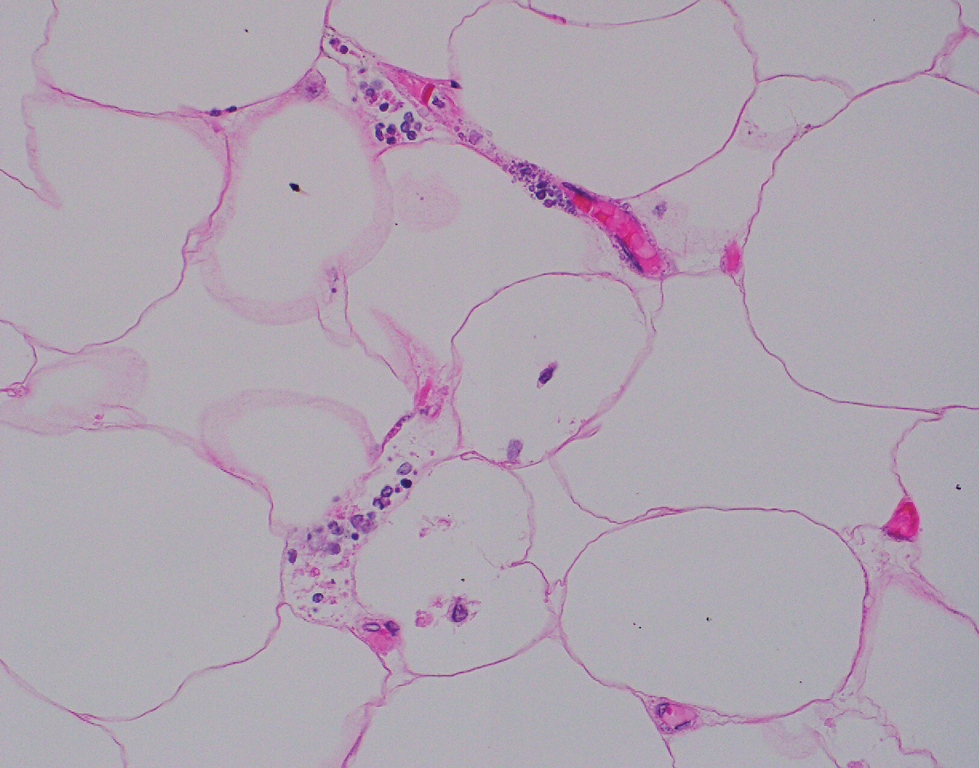
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
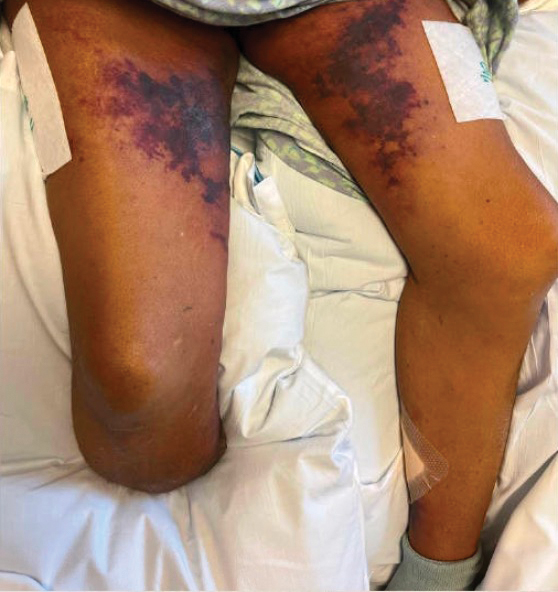
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.
Diagnosis and Treatment Options for Polycythemia Vera
Dr. Richard: The first thing we as physicians are worried about is patients with PV developing thrombosis. We start prophylaxis with aspirin, as aspirin remains the best treatment for reducing this risk. It is essential to make sure patients with PV understand the importance of taking an aspirin, even at a low dosage.
The second step is trying to control patients’ red blood cell counts. Phlebotomy has been used for this purpose for many decades and continues to be effective. You will find some experts in the field who consider phlebotomy to be the mainstay of treatment for patients with PV, and that it has benefits in and of itself.
However, despite the benefits, phlebotomy can be a little tough on patients. For instance, patients with PV cannot donate blood at a traditional blood center such as Red Cross, and therefore need to go to an actual infusion center. They also must stop their day and travel to a site to receive therapeutic phlebotomy treatment, which is most effective for patients with a blood disorder. I work in Seattle taking care of patients throughout the Northwest, and it is not always easy to find a close location to send patients for phlebotomy. Nevertheless, phlebotomy should be part of the treatment options for patients with PV, especially patients in the high-risk range who have high hemoglobin and hematocrit values.
The third step is controlling hemoglobin and hematocrit levels. Hydroxyurea is our standard of care with strong beneficial data for this purpose.
These are the 3 approaches to treatment we initially discuss with our patients during their first visit. These 3 strategies can improve a patient's life and reduce their risk for thrombosis.
Which treatment do you recommend depending on the patient’s symptoms?
Dr. Richard: The treatment that we offer can vary. The first thing I want to know is how their symptoms respond to aspirin. For instance, symptoms such as erythromelalgia oftentimes respond beautifully to aspirin. Most patients do not have massive splenomegaly—that would give me a high suspicion for myelofibrosis—but they can develop and present with some level of splenomegaly.
If the symptoms are bothersome to the patient, I will probably want to get them on some kind of cytoreduction to see if that is effective. Hydroxyurea, although not as effective as aspirin and associated with adverse effects in some patients, is a good medication to start cytoreductive therapy. The National Comprehensive Cancer Network® (NCCN®) Guidelines suggest that if a patient taking hydroxyurea experiences severe gastrointestinal (GI) toxicity, go ahead and move to a Janus kinase 2 (JAK2) inhibitor or try interferon. JAK2 inhibition with ruxolitinib, in this case, is effective, at least initially for treating splenomegaly. Unfortunately, symptoms sometimes get confused with effects from the medications. You have to use an individualized treatment approach and see what works for each patient.
What are some of the common adverse effects of treatment?
Dr. Richard: As you probably can tell, I never skip aspirin since it is such an important part of treatment for patients with PV. However, I do talk to patients about GI upset and the bleeding risks, such as the potential for GI bleed. Obviously, clotting is what causes an increase in morbidity and mortality, but bleeding can be an important adverse effect with platelets that do not function, or for other issues.
Hydroxyurea is generally well tolerated, but some patients can develop skin issues or ulcers due to GI toxicity. I have a lot of confidence in the use of hydroxyurea, and I use it without hesitation. However, you may have a patient who already has a relatively low neutrophil count or has some level of thrombocytopenia from either liver disease or some other issue, and they just cannot tolerate hydroxyurea. Some other bothersome symptoms include change of taste, skin changes, and brittle nails. It can be tricky. I will attempt hydroxyurea, if needed, but sometimes they just cannot tolerate it.
What happens when a patient cannot tolerate one or all the medications you mentioned?
Dr. Richard: Now that we have JAK2 inhibitors, ruxolitinib is generally my choice for patients who cannot tolerate hydroxyurea, which is more common than maybe we would like. Other problems with hydroxyurea include that it might not work, or phlebotomy combined with hydroxyurea results in cytopenias, or patients have a particularly aggressive form of PV.
There are numerous other JAK2 drug inhibitors on the market for myelofibrosis. The assumption is they probably work as well as ruxolitinib for PV, but right now ruxolitinib is what is approved by the US Food and Drug Administration for these patients.
The other drug that we do not use much is interferon, or now, pegylated interferon. It is a drug that has been around for a long time and worked well in chronic myelogenous leukemia before we had the tyrosine kinase inhibitors. Pegylated interferon is a well-tolerated drug that can be used for patients with PV who are pregnant or could get pregnant. It is not the interferon of our parents or grandparents. We now have options for controlling the disease and its complications, but the hardest thing is to tell patients that none of these treatments are going to cure them or reverse the overproduction of red blood cells in their bone marrow. There has always been discussion about whether there could be some effect of interferon on the actual tumor burden, but that remains to be proven.
Can you go a little bit more into your recommended approach to managing newly diagnosed patients with PV in your day-to-day practice?
Dr. Richard: Oftentimes these patients are identified through our consultation service. It can be hard on the primary care physician to identify whether a patient has secondary or primary PV.
We are lucky now that JAK2 has been identified. The JAK2-V617F and the other exon 12 mutations were identified back in 2006 by several groups and is a great test. With JAK2 along with an erythropoietin test, you can feel confident whether you have identified PV.
PV encompasses a wide variety of syndromes. Patients can come into the office with terrible symptoms, including aquagenic pruritus or erythromelalgia. I have seen young people in their 30s who happen to have a slightly elevated hematocrit and a positive JAK2 test. When this occurs, you try to understand how much the disease is affecting their life, whether they are going to be in a high-risk category or a low-risk category, whether they have had thrombosis, and how their age figures into all of it. To try to figure out this high-risk versus low-risk factor, we use a simple staging system to help determine whether they are going to need cytoreductive therapy or whether you can just start with phlebotomy and see how they do.
Oftentimes in the first visit, I recommend a bone marrow study. Is that going to be true 5 years from now? I am not sure. You know, next-generation sequencing (NGS) is turning into such an important part of determining prognosis in these patients. It helps if you have a great colleague down in hematopathology who can look at the bone marrow as it relates to megakaryocyte morphology, or whether there is early fibrosis.
Although these techniques may not be as prominent in practice today for determining diagnosis, they help us understand the prognosis. A bone marrow study or NGS is particularly useful when you have patients who you are convinced have PV, but it turns out they already have extensive fibrosis and are actually moving more toward the post-polycythemia phase a little faster than you think. While we do not use an allogeneic transplant often, in this scenario we may recommend it. Our primary goal at this point is to try to determine which patients would benefit from a transplant early on and to prepare the patient for this option.
What socioeconomic disparities have you observed in newly diagnosed patients?
Dr. Richard: There are definitely social disparities for people who have low income. I work in a veteran’s hospital where we take care of a lot of people who do not have health insurance, but who come to the VA because they do have benefits. If they are in our system, they get identified and their care is great. However, if they have been out in the regular system without health insurance, oftentimes they get diagnosed late. Identifying and treating patients from low socioeconomic backgrounds is an issue. I think everyone can agree with that.
We have a delicate situation with women veterans. Something that is incredibly painful that I think people should be aware of is the amount of military sexual trauma (MST) that has occurred over the years. These patients are in a unique place of trust with their care providers. They have spent a lot of time not being listened to in a variety of arenas. I see this in young military women who no one expects to have a stem cell disorder. We as health care providers do them a disservice if their complaints lead to referral to a psychotherapist or being prescribed a nonsteroidal anti-inflammatory drug or something like that. As health care providers, we are in a unique position to listen to and accurately evaluate these patients. We have a large population of veterans and, increasingly more women veterans, but I think we can all agree that they need better care, especially if they have suffered from MST. That is what I see in my patient population.
When I was a resident in Baltimore, it was Black people with lower income who did not trust doctors. We still have a lot of work to do.
Dr. Richard: The first thing we as physicians are worried about is patients with PV developing thrombosis. We start prophylaxis with aspirin, as aspirin remains the best treatment for reducing this risk. It is essential to make sure patients with PV understand the importance of taking an aspirin, even at a low dosage.
The second step is trying to control patients’ red blood cell counts. Phlebotomy has been used for this purpose for many decades and continues to be effective. You will find some experts in the field who consider phlebotomy to be the mainstay of treatment for patients with PV, and that it has benefits in and of itself.
However, despite the benefits, phlebotomy can be a little tough on patients. For instance, patients with PV cannot donate blood at a traditional blood center such as Red Cross, and therefore need to go to an actual infusion center. They also must stop their day and travel to a site to receive therapeutic phlebotomy treatment, which is most effective for patients with a blood disorder. I work in Seattle taking care of patients throughout the Northwest, and it is not always easy to find a close location to send patients for phlebotomy. Nevertheless, phlebotomy should be part of the treatment options for patients with PV, especially patients in the high-risk range who have high hemoglobin and hematocrit values.
The third step is controlling hemoglobin and hematocrit levels. Hydroxyurea is our standard of care with strong beneficial data for this purpose.
These are the 3 approaches to treatment we initially discuss with our patients during their first visit. These 3 strategies can improve a patient's life and reduce their risk for thrombosis.
Which treatment do you recommend depending on the patient’s symptoms?
Dr. Richard: The treatment that we offer can vary. The first thing I want to know is how their symptoms respond to aspirin. For instance, symptoms such as erythromelalgia oftentimes respond beautifully to aspirin. Most patients do not have massive splenomegaly—that would give me a high suspicion for myelofibrosis—but they can develop and present with some level of splenomegaly.
If the symptoms are bothersome to the patient, I will probably want to get them on some kind of cytoreduction to see if that is effective. Hydroxyurea, although not as effective as aspirin and associated with adverse effects in some patients, is a good medication to start cytoreductive therapy. The National Comprehensive Cancer Network® (NCCN®) Guidelines suggest that if a patient taking hydroxyurea experiences severe gastrointestinal (GI) toxicity, go ahead and move to a Janus kinase 2 (JAK2) inhibitor or try interferon. JAK2 inhibition with ruxolitinib, in this case, is effective, at least initially for treating splenomegaly. Unfortunately, symptoms sometimes get confused with effects from the medications. You have to use an individualized treatment approach and see what works for each patient.
What are some of the common adverse effects of treatment?
Dr. Richard: As you probably can tell, I never skip aspirin since it is such an important part of treatment for patients with PV. However, I do talk to patients about GI upset and the bleeding risks, such as the potential for GI bleed. Obviously, clotting is what causes an increase in morbidity and mortality, but bleeding can be an important adverse effect with platelets that do not function, or for other issues.
Hydroxyurea is generally well tolerated, but some patients can develop skin issues or ulcers due to GI toxicity. I have a lot of confidence in the use of hydroxyurea, and I use it without hesitation. However, you may have a patient who already has a relatively low neutrophil count or has some level of thrombocytopenia from either liver disease or some other issue, and they just cannot tolerate hydroxyurea. Some other bothersome symptoms include change of taste, skin changes, and brittle nails. It can be tricky. I will attempt hydroxyurea, if needed, but sometimes they just cannot tolerate it.
What happens when a patient cannot tolerate one or all the medications you mentioned?
Dr. Richard: Now that we have JAK2 inhibitors, ruxolitinib is generally my choice for patients who cannot tolerate hydroxyurea, which is more common than maybe we would like. Other problems with hydroxyurea include that it might not work, or phlebotomy combined with hydroxyurea results in cytopenias, or patients have a particularly aggressive form of PV.
There are numerous other JAK2 drug inhibitors on the market for myelofibrosis. The assumption is they probably work as well as ruxolitinib for PV, but right now ruxolitinib is what is approved by the US Food and Drug Administration for these patients.
The other drug that we do not use much is interferon, or now, pegylated interferon. It is a drug that has been around for a long time and worked well in chronic myelogenous leukemia before we had the tyrosine kinase inhibitors. Pegylated interferon is a well-tolerated drug that can be used for patients with PV who are pregnant or could get pregnant. It is not the interferon of our parents or grandparents. We now have options for controlling the disease and its complications, but the hardest thing is to tell patients that none of these treatments are going to cure them or reverse the overproduction of red blood cells in their bone marrow. There has always been discussion about whether there could be some effect of interferon on the actual tumor burden, but that remains to be proven.
Can you go a little bit more into your recommended approach to managing newly diagnosed patients with PV in your day-to-day practice?
Dr. Richard: Oftentimes these patients are identified through our consultation service. It can be hard on the primary care physician to identify whether a patient has secondary or primary PV.
We are lucky now that JAK2 has been identified. The JAK2-V617F and the other exon 12 mutations were identified back in 2006 by several groups and is a great test. With JAK2 along with an erythropoietin test, you can feel confident whether you have identified PV.
PV encompasses a wide variety of syndromes. Patients can come into the office with terrible symptoms, including aquagenic pruritus or erythromelalgia. I have seen young people in their 30s who happen to have a slightly elevated hematocrit and a positive JAK2 test. When this occurs, you try to understand how much the disease is affecting their life, whether they are going to be in a high-risk category or a low-risk category, whether they have had thrombosis, and how their age figures into all of it. To try to figure out this high-risk versus low-risk factor, we use a simple staging system to help determine whether they are going to need cytoreductive therapy or whether you can just start with phlebotomy and see how they do.
Oftentimes in the first visit, I recommend a bone marrow study. Is that going to be true 5 years from now? I am not sure. You know, next-generation sequencing (NGS) is turning into such an important part of determining prognosis in these patients. It helps if you have a great colleague down in hematopathology who can look at the bone marrow as it relates to megakaryocyte morphology, or whether there is early fibrosis.
Although these techniques may not be as prominent in practice today for determining diagnosis, they help us understand the prognosis. A bone marrow study or NGS is particularly useful when you have patients who you are convinced have PV, but it turns out they already have extensive fibrosis and are actually moving more toward the post-polycythemia phase a little faster than you think. While we do not use an allogeneic transplant often, in this scenario we may recommend it. Our primary goal at this point is to try to determine which patients would benefit from a transplant early on and to prepare the patient for this option.
What socioeconomic disparities have you observed in newly diagnosed patients?
Dr. Richard: There are definitely social disparities for people who have low income. I work in a veteran’s hospital where we take care of a lot of people who do not have health insurance, but who come to the VA because they do have benefits. If they are in our system, they get identified and their care is great. However, if they have been out in the regular system without health insurance, oftentimes they get diagnosed late. Identifying and treating patients from low socioeconomic backgrounds is an issue. I think everyone can agree with that.
We have a delicate situation with women veterans. Something that is incredibly painful that I think people should be aware of is the amount of military sexual trauma (MST) that has occurred over the years. These patients are in a unique place of trust with their care providers. They have spent a lot of time not being listened to in a variety of arenas. I see this in young military women who no one expects to have a stem cell disorder. We as health care providers do them a disservice if their complaints lead to referral to a psychotherapist or being prescribed a nonsteroidal anti-inflammatory drug or something like that. As health care providers, we are in a unique position to listen to and accurately evaluate these patients. We have a large population of veterans and, increasingly more women veterans, but I think we can all agree that they need better care, especially if they have suffered from MST. That is what I see in my patient population.
When I was a resident in Baltimore, it was Black people with lower income who did not trust doctors. We still have a lot of work to do.
Dr. Richard: The first thing we as physicians are worried about is patients with PV developing thrombosis. We start prophylaxis with aspirin, as aspirin remains the best treatment for reducing this risk. It is essential to make sure patients with PV understand the importance of taking an aspirin, even at a low dosage.
The second step is trying to control patients’ red blood cell counts. Phlebotomy has been used for this purpose for many decades and continues to be effective. You will find some experts in the field who consider phlebotomy to be the mainstay of treatment for patients with PV, and that it has benefits in and of itself.
However, despite the benefits, phlebotomy can be a little tough on patients. For instance, patients with PV cannot donate blood at a traditional blood center such as Red Cross, and therefore need to go to an actual infusion center. They also must stop their day and travel to a site to receive therapeutic phlebotomy treatment, which is most effective for patients with a blood disorder. I work in Seattle taking care of patients throughout the Northwest, and it is not always easy to find a close location to send patients for phlebotomy. Nevertheless, phlebotomy should be part of the treatment options for patients with PV, especially patients in the high-risk range who have high hemoglobin and hematocrit values.
The third step is controlling hemoglobin and hematocrit levels. Hydroxyurea is our standard of care with strong beneficial data for this purpose.
These are the 3 approaches to treatment we initially discuss with our patients during their first visit. These 3 strategies can improve a patient's life and reduce their risk for thrombosis.
Which treatment do you recommend depending on the patient’s symptoms?
Dr. Richard: The treatment that we offer can vary. The first thing I want to know is how their symptoms respond to aspirin. For instance, symptoms such as erythromelalgia oftentimes respond beautifully to aspirin. Most patients do not have massive splenomegaly—that would give me a high suspicion for myelofibrosis—but they can develop and present with some level of splenomegaly.
If the symptoms are bothersome to the patient, I will probably want to get them on some kind of cytoreduction to see if that is effective. Hydroxyurea, although not as effective as aspirin and associated with adverse effects in some patients, is a good medication to start cytoreductive therapy. The National Comprehensive Cancer Network® (NCCN®) Guidelines suggest that if a patient taking hydroxyurea experiences severe gastrointestinal (GI) toxicity, go ahead and move to a Janus kinase 2 (JAK2) inhibitor or try interferon. JAK2 inhibition with ruxolitinib, in this case, is effective, at least initially for treating splenomegaly. Unfortunately, symptoms sometimes get confused with effects from the medications. You have to use an individualized treatment approach and see what works for each patient.
What are some of the common adverse effects of treatment?
Dr. Richard: As you probably can tell, I never skip aspirin since it is such an important part of treatment for patients with PV. However, I do talk to patients about GI upset and the bleeding risks, such as the potential for GI bleed. Obviously, clotting is what causes an increase in morbidity and mortality, but bleeding can be an important adverse effect with platelets that do not function, or for other issues.
Hydroxyurea is generally well tolerated, but some patients can develop skin issues or ulcers due to GI toxicity. I have a lot of confidence in the use of hydroxyurea, and I use it without hesitation. However, you may have a patient who already has a relatively low neutrophil count or has some level of thrombocytopenia from either liver disease or some other issue, and they just cannot tolerate hydroxyurea. Some other bothersome symptoms include change of taste, skin changes, and brittle nails. It can be tricky. I will attempt hydroxyurea, if needed, but sometimes they just cannot tolerate it.
What happens when a patient cannot tolerate one or all the medications you mentioned?
Dr. Richard: Now that we have JAK2 inhibitors, ruxolitinib is generally my choice for patients who cannot tolerate hydroxyurea, which is more common than maybe we would like. Other problems with hydroxyurea include that it might not work, or phlebotomy combined with hydroxyurea results in cytopenias, or patients have a particularly aggressive form of PV.
There are numerous other JAK2 drug inhibitors on the market for myelofibrosis. The assumption is they probably work as well as ruxolitinib for PV, but right now ruxolitinib is what is approved by the US Food and Drug Administration for these patients.
The other drug that we do not use much is interferon, or now, pegylated interferon. It is a drug that has been around for a long time and worked well in chronic myelogenous leukemia before we had the tyrosine kinase inhibitors. Pegylated interferon is a well-tolerated drug that can be used for patients with PV who are pregnant or could get pregnant. It is not the interferon of our parents or grandparents. We now have options for controlling the disease and its complications, but the hardest thing is to tell patients that none of these treatments are going to cure them or reverse the overproduction of red blood cells in their bone marrow. There has always been discussion about whether there could be some effect of interferon on the actual tumor burden, but that remains to be proven.
Can you go a little bit more into your recommended approach to managing newly diagnosed patients with PV in your day-to-day practice?
Dr. Richard: Oftentimes these patients are identified through our consultation service. It can be hard on the primary care physician to identify whether a patient has secondary or primary PV.
We are lucky now that JAK2 has been identified. The JAK2-V617F and the other exon 12 mutations were identified back in 2006 by several groups and is a great test. With JAK2 along with an erythropoietin test, you can feel confident whether you have identified PV.
PV encompasses a wide variety of syndromes. Patients can come into the office with terrible symptoms, including aquagenic pruritus or erythromelalgia. I have seen young people in their 30s who happen to have a slightly elevated hematocrit and a positive JAK2 test. When this occurs, you try to understand how much the disease is affecting their life, whether they are going to be in a high-risk category or a low-risk category, whether they have had thrombosis, and how their age figures into all of it. To try to figure out this high-risk versus low-risk factor, we use a simple staging system to help determine whether they are going to need cytoreductive therapy or whether you can just start with phlebotomy and see how they do.
Oftentimes in the first visit, I recommend a bone marrow study. Is that going to be true 5 years from now? I am not sure. You know, next-generation sequencing (NGS) is turning into such an important part of determining prognosis in these patients. It helps if you have a great colleague down in hematopathology who can look at the bone marrow as it relates to megakaryocyte morphology, or whether there is early fibrosis.
Although these techniques may not be as prominent in practice today for determining diagnosis, they help us understand the prognosis. A bone marrow study or NGS is particularly useful when you have patients who you are convinced have PV, but it turns out they already have extensive fibrosis and are actually moving more toward the post-polycythemia phase a little faster than you think. While we do not use an allogeneic transplant often, in this scenario we may recommend it. Our primary goal at this point is to try to determine which patients would benefit from a transplant early on and to prepare the patient for this option.
What socioeconomic disparities have you observed in newly diagnosed patients?
Dr. Richard: There are definitely social disparities for people who have low income. I work in a veteran’s hospital where we take care of a lot of people who do not have health insurance, but who come to the VA because they do have benefits. If they are in our system, they get identified and their care is great. However, if they have been out in the regular system without health insurance, oftentimes they get diagnosed late. Identifying and treating patients from low socioeconomic backgrounds is an issue. I think everyone can agree with that.
We have a delicate situation with women veterans. Something that is incredibly painful that I think people should be aware of is the amount of military sexual trauma (MST) that has occurred over the years. These patients are in a unique place of trust with their care providers. They have spent a lot of time not being listened to in a variety of arenas. I see this in young military women who no one expects to have a stem cell disorder. We as health care providers do them a disservice if their complaints lead to referral to a psychotherapist or being prescribed a nonsteroidal anti-inflammatory drug or something like that. As health care providers, we are in a unique position to listen to and accurately evaluate these patients. We have a large population of veterans and, increasingly more women veterans, but I think we can all agree that they need better care, especially if they have suffered from MST. That is what I see in my patient population.
When I was a resident in Baltimore, it was Black people with lower income who did not trust doctors. We still have a lot of work to do.
Higher vitamin B6 and folate intake tied to lower severe headache or migraine risk
Key clinical point: Higher dietary intakes of vitamin B6 and folate were significantly associated with a lower risk for severe headaches or migraines, with a synergistic interaction observed between vitamin B6 and folate intake and reduced migraine risk.
Major finding: Compared with the lowest quintile of dietary vitamin B6 (≤1.13 mg/day) and folate (≤240 μg/day) intake, the risk for severe headache or migraine was lower in the highest quintile of vitamin B6 (≥2.39 mg/day; adjusted odds ratio [aOR] 0.66; P = .01) and folate (≥502.01 μg/day; aOR 0.57; P = .002) intake. A synergistic interaction was observed between the high mass of vitamin B6 and folate intake and a lower risk for severe headache or migraine (synergy index 0.58; 95% CI 0.40-0.83).
Study details: This cross-sectional study included 7017 adult participants (age ≥ 20 years), of whom 1350 (19.23%) had migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangxi Province, and others. No conflicts of interests were declared.
Source: Tian S et al. Vitamin B6 and folate intake are associated with lower risk of severe headache or migraine in adults: An analysis based on NHANES 1999-2004. Nutr Res. 2023;121:51-60 (Nov 15). doi: 10.1016/j.nutres.2023.11.008
Key clinical point: Higher dietary intakes of vitamin B6 and folate were significantly associated with a lower risk for severe headaches or migraines, with a synergistic interaction observed between vitamin B6 and folate intake and reduced migraine risk.
Major finding: Compared with the lowest quintile of dietary vitamin B6 (≤1.13 mg/day) and folate (≤240 μg/day) intake, the risk for severe headache or migraine was lower in the highest quintile of vitamin B6 (≥2.39 mg/day; adjusted odds ratio [aOR] 0.66; P = .01) and folate (≥502.01 μg/day; aOR 0.57; P = .002) intake. A synergistic interaction was observed between the high mass of vitamin B6 and folate intake and a lower risk for severe headache or migraine (synergy index 0.58; 95% CI 0.40-0.83).
Study details: This cross-sectional study included 7017 adult participants (age ≥ 20 years), of whom 1350 (19.23%) had migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangxi Province, and others. No conflicts of interests were declared.
Source: Tian S et al. Vitamin B6 and folate intake are associated with lower risk of severe headache or migraine in adults: An analysis based on NHANES 1999-2004. Nutr Res. 2023;121:51-60 (Nov 15). doi: 10.1016/j.nutres.2023.11.008
Key clinical point: Higher dietary intakes of vitamin B6 and folate were significantly associated with a lower risk for severe headaches or migraines, with a synergistic interaction observed between vitamin B6 and folate intake and reduced migraine risk.
Major finding: Compared with the lowest quintile of dietary vitamin B6 (≤1.13 mg/day) and folate (≤240 μg/day) intake, the risk for severe headache or migraine was lower in the highest quintile of vitamin B6 (≥2.39 mg/day; adjusted odds ratio [aOR] 0.66; P = .01) and folate (≥502.01 μg/day; aOR 0.57; P = .002) intake. A synergistic interaction was observed between the high mass of vitamin B6 and folate intake and a lower risk for severe headache or migraine (synergy index 0.58; 95% CI 0.40-0.83).
Study details: This cross-sectional study included 7017 adult participants (age ≥ 20 years), of whom 1350 (19.23%) had migraine.
Disclosures: This study was supported by the National Natural Science Foundation of China, Natural Science Foundation of Jiangxi Province, and others. No conflicts of interests were declared.
Source: Tian S et al. Vitamin B6 and folate intake are associated with lower risk of severe headache or migraine in adults: An analysis based on NHANES 1999-2004. Nutr Res. 2023;121:51-60 (Nov 15). doi: 10.1016/j.nutres.2023.11.008
Commentary: Allergies, EDN, and the Psychosocial Burden of EoE, February 2024
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
A significant gap in our understanding of eosinophilic esophagitis (EoE) lies in how environmental factors, such as allergens or food, influence the response to proton pump inhibitor (PPI) therapy. While PPI achieve histologic remission in approximately 50% of patients, the response in the remaining 50% remains unclear. Addressing this, Muftah and colleagues conducted a study to evaluate the relationship between environmental and food allergies and PPI response in newly diagnosed EoE patients.
Between 2012 and 2016, adult patients newly diagnosed with EoE were tested for environmental and food allergies. Following diagnosis, patients participated in an 8-week trial of twice-daily PPI therapy. The treatment's effectiveness was assessed through repeated upper endoscopies with esophageal biopsies.
The study's primary outcome was the histologic remission of EoE, defined as a decrease in eosinophils to < 15 eosinophils/high-powered field (eos/hpf) in all esophageal biopsy samples during repeat endoscopy. Out of 61 patients, 21 achieved histologic remission, while 40 were classified as having PPI-nonresponding EoE. Among PPI-nonresponding EoE patients, positive food allergen testing was significantly more prevalent compared with PPI-responding EoE patients (82.5% vs 42.9%; P = .0003). Additionally, patients with >10 positive environmental allergen tests were significantly less likely to be PPI-responding EoE patients than those with <10 positive results (21% vs 53.9%; P = .03). A similar trend was observed in patients with >5 positive environmental allergens.
This study is not without limitations. It may exhibit a selection bias toward more severe cases and has a relatively small sample size, affecting its statistical power and generalizability.
This research supports the idea of more tailored management for EoE patients, focusing on their allergen profile, potentially leading to more effective treatment strategies and reducing unnecessary PPI trials. The statistically significant results pave the way for further research, providing an additional tool to predict PPI responsiveness and prevent delays in achieving remission.
Clinicians should consider patient characteristics, particularly positive food allergen tests, that might affect treatment response. More studies are needed, however, to understand the effect of environmental allergies on PPI response fully. A notable finding is that specific aeroallergens, such as oak, birch, Hormodendrum mold, dust mite (Dermatophagoides pteronyssinus), tree mix, and grass mix allergens, are associated with a lack of PPI response. This raises questions about whether exposure to these allergens during peak seasons could worsen PPI response in allergic EoE patients.
Key takeaways from this study include: (1) the importance of integrating allergen testing in EoE patients, especially those unresponsive to standard PPI therapy or suspected as having allergic phenotypes; (2) the need to monitor and adjust therapy based on clinical and histologic responses; and (3) the necessity of staying abreast of emerging research in this area.
EoE diagnosis presents unique challenges, particularly when patients exhibit exclusive distal esophageal eosinophilia or when discrepancies arise between endoscopic and histologic findings. Eosinophil-derived neurotoxin (EDN), a molecule previously studied for its role in monitoring allergy-mediated inflammatory diseases such as asthma and eczema, can shed light on these diagnostic difficulties.
Thomas and coworkers conducted a retrospective study in which they reviewed 231 pediatric patients, obtaining a minimum of four biopsies from at least two different levels of the esophagus. The study aimed to evaluate whether EDN concentrations, determined through esophageal epithelial brushing at the time of biopsy, could serve as an adjunctive diagnostic tool for EoE.
EDN levels proved sensitive (84.4%) and specific (94.6%) in evaluating active EoE when several measures of EoE were used in patients with active EoE compared with those with inactive EoE and the control group. Previous studies at the same institution had found EDN useful for differentiating EoE patients from non-EoE patients. Moreover, an EDN concentration > 10 μg/mL, when collected through esophageal epithelial brushing, was highly sensitive (97%) and specific (89%) for active EoE. This finding suggests the potential for using EDN as a biochemical marker, enhancing diagnostic accuracy and reducing the need for additional interventions in complex cases.
EDN as a biomarker could be invaluable for distinguishing difficult cases, such as those involving distal eosinophilia, active vs nonactive EoE, or non-EoE conditions, such as gastroesophageal reflux disease. Of note, lower EDN levels were observed in pediatric EoE patients who responded to PPI, suggesting EDN's potential utility in predicting PPI responsiveness. Incorporating the measurement of eosinophilic activity could add a new dimension to existing criteria, equipping clinicians with more precise diagnostic tools and reducing the reliance on multiple procedures. This approach would strengthen the correlation between symptomatic, endoscopic, and histologic data.
The study by Jensen and colleagues sheds light on a crucial aspect of EoE management: the psychosocial burden. A recent EoE diagnosis can be associated with increased symptom burden, somatization, and anxiety in patients and families, underscoring the need for a multidisciplinary approach to patient care that considers both physical and mental health. To date, numerous studies have focused on understanding the disease, its follow-up, and treatment. However, there has been limited exploration of the psychosocial burden and patient-associated factors in EoE.
In this context, this team aimed to enhance our understanding of the burden of EoE by evaluating psychosocial comorbidities, such as disordered sleep, anxiety, and somatization, in a pediatric population with EoE. The study included 87 patients of age 8-18 years who completed validated assessments during routine clinic visits, encompassing EoE symptoms (Pediatric Eosinophilic Esophagitis Symptom Scores, PEESSv2.0), quality of life (PedsQL-EoE), anxiety state and trait (State-Trait Anxiety Inventory for Children, STAI-C), somatization (Children's Somatic Symptoms Inventory-24, CSSI-24), and sleep-disordered breathing (University of Michigan Pediatric Sleep Questionnaire, PSQ).
The mean age of the participants was 12.8 years, highlighting the importance of addressing psychosocial distress in this age group, which undergoes crucial developmental stages. Most patients (82%, 71) had been diagnosed with EoE at least 12 months prior, and 60% (52) were treated with multiple approaches. Additionally, 34% (29) had undergone seven or more esophagogastroduodenoscopies, and nearly one third (33%, 27) had experienced a gastrointestinal-related emergency department visit. These factors potentially increase patient stress due to the continuous need for repeat procedures and hospital visits. An intriguing finding was that patients with shorter disease durations (6-12 months since diagnosis) experienced higher symptom burdens (P = .03). Patients with public insurance had less favorable scores for sleep-disordered breathing (P = .01).
Significantly, patients with neurodevelopmental comorbidities had higher scores for somatic symptoms, trait anxiety, and sleep-disordered breathing, and lower quality-of-life scores, compared with those without such comorbidities (P < .01 for all), suggesting that patients with neurodevelopmental issues might particularly benefit from tailored treatments addressing these aspects of the disease. Furthermore, patients with shorter disease durations since diagnosis exhibited higher somatic symptoms and trait anxiety (both P < .01). The study also revealed that patients with fewer esophagogastroduodenoscopies (1-3) had higher somatic symptom scores (P < .01), state anxiety (P = .02), and trait anxiety (P = .03). EoE-associated symptom burden was significantly correlated with increased somatic symptoms (0.34; 95% CI 0.23-0.45) and decreased quality of life (-0.42; 95% CI -0.59 to -0.25). Concerns about eating food and EoE-associated symptoms were both linked to the EoE-associated symptom burden.
This study has several limitations, including a relatively small sample size, which decreases the power and limits inferences for smaller groups within the sample. There was also an imbalance in gender distribution, with only 26% of patients being female, potentially limiting the generalizability of the findings. Moreover, the study included only EoE patients, lacking a control group for comparison to the general pediatric population.
Highlighting a significant aspect of pediatric EoE treatment, this study illuminates an area that might affect patients' long-term quality of life. It underscores the need for multidisciplinary care for EoE patients, where mental health professionals, such as psychologists or psychiatrists, can play a vital role in improving mental health through early identification and intervention for anxiety and somatization disorders. They can also provide education for patients and families on coping strategies. Peer support groups for children and adolescents could be another beneficial tool, allowing them to share experiences and reduce feelings of isolation.
Physicians who treat chronic diseases such as EoE should consider psychosocial factors, as they can affect both physical and mental quality of life. Using screening tools (such as PEESSv2.0, PedsQL-EoE, STAI-C, CSSI-24, or PSQ) during clinic visits can facilitate a more comprehensive evaluation.
Commentary: Gut Microbiota and CGRP in Migraine, February 2024
The authors of a study published in the December 2023 issue of the Scandinavian Journal of Gastroenterology analyzed data to examine the potential link between gut microbiota and migraine. According to the statistical analysis, the researchers found that "a greater abundance of genus Lactobacillus was associated with a higher risk of migraine and a higher abundance of family Prevotellaceae was related to a decreased risk of migraine." Furthermore, they noted that these gut microbial patterns could be due to a genetic predisposition. The authors suggested that stool sampling could potentially be helpful in the diagnosis of migraine, and that measures to modify gut microbiota in the context of migraine therapy could be identified with future research.
While it is not clear whether migraine is a cause or effect of these alterations, or whether there is another confounding variable, the idea of using diet as a means of reducing migraine risk would be appealing for many patients. This offers hope, but it also leaves a window open for exaggeration and excessive reliance on certain foods or supplements before reliable links are established.
Further examining the genetic factors that might play a role in migraine, a large Korean data analysis published in the December 2023 issue of Epidemiology and Health described a link between migraine and Parkinson's disease. The researchers included 214,193 patients with migraine and 5,879,711 individuals without migraine. According to the statistical analysis, the patients who had migraine with aura showed a 1.35-fold higher risk for Parkinson's disease than individuals without migraine. However, the researchers did not note a statistically significant difference between the risk for Parkinson's disease among patients who had migraine without aura and individuals without migraine.
They also examined other factors, and noted that among individuals with migraine, those who had preexisting dyslipidemia had a higher risk for Parkinson's disease than those who did not have dyslipidemia. Other factors that were not correlated with an association between migraine and Parkinson's disease included cardiovascular risk factors, hypertension, diabetes, smoking status, and high body mass index.
The study authors noted that factors associated with the activity of calcitonin gene-related peptide (CGRP), which is known to play a significant role in the pathophysiology of migraine, could play a role in the link between migraine and Parkinson's disease. They pointed to previous studies that found evidence of elevated CGRP levels in the cerebrospinal fluid of patients with Parkinson's disease as possible evidence of a pathophysiologic link.
An earlier commentary, published in the April 2020 issue of Headache, suggested an implication of CGRP antagonists in the development of neurodegenerative disorders such as Parkinson's disease. The commentary authors noted that previous research correlated midlife migraine to late-life parkinsonism, suggesting a conceivable common pathology, which could include a genetic or environmental predisposition.[1] They also noted that studies suggest a possible link between CGRP and multiple system atrophy, a parkinsonian disorder.[1] They considered the possibility that one of the ways that CGRP could contribute to these disorders is through its role in the recruitment of inflammatory mediators, which can alter the function of nicotinic receptors in the dopaminergic system in Parkinson's disease pathogenesis.[2]
Recent research published in the December 2023 issue of Headache suggests that CGRP responsiveness in migraine therapy could be mediated by genetics. The study included 198 patients who had been typed for genes involved in CGRP signaling or pharmacologic response and were given genetic and polygenic risk scores. Responders were defined as patients who experienced ≥ 50% reduction in migraine days per month at 5.7-month follow-up.
The analysis revealed an association between nonresponder status and rs12615320-G in RAMP1, a gene that encodes a component of high-affinity CGRP receptors, which increased the risk for nonresponder status. The researchers also identified an association between nonresponder status and rs4680-A in COMT, a gene that has been associated with lower COMT enzymatic activity, chronic pain/fibromyalgia, and a "worrier" phenotype. Nonresponders also had a lower mean genetic risk score than responders. These genetic associations could help identify which patients would be most likely to benefit from anti-CGRP therapies.
Given that CGRP responsiveness may have a genetic component, it is possible that one of the contributors to the link between migraine and Parkinson's disease could lie in patients' genetic predisposition to CGRP activity. Yet, the association between these two common conditions is not thoroughly established, and the role of CGRP in the pathogenesis of Parkinson's disease is not fully validated. Nevertheless, the new developments in treatments that modify CGRP activity could have implications beyond migraine.
Additional References
1. Alexoudi A, Deftereos S. CGRP antagonists: side effects and potential Parkinson's disease development. Headache. 2020;60:789-790. doi: 10.1111/head.13770 Source
2. Blumenfeld A, Durham PL, Feoktistov A, et al. Hypervigilance, allostatic load, and migraine prevention: Antibodies to CGRP or receptor. Neurol Ther. 2021;10:469-497. doi:10.1007/s40120-021-00250-7 Source
The authors of a study published in the December 2023 issue of the Scandinavian Journal of Gastroenterology analyzed data to examine the potential link between gut microbiota and migraine. According to the statistical analysis, the researchers found that "a greater abundance of genus Lactobacillus was associated with a higher risk of migraine and a higher abundance of family Prevotellaceae was related to a decreased risk of migraine." Furthermore, they noted that these gut microbial patterns could be due to a genetic predisposition. The authors suggested that stool sampling could potentially be helpful in the diagnosis of migraine, and that measures to modify gut microbiota in the context of migraine therapy could be identified with future research.
While it is not clear whether migraine is a cause or effect of these alterations, or whether there is another confounding variable, the idea of using diet as a means of reducing migraine risk would be appealing for many patients. This offers hope, but it also leaves a window open for exaggeration and excessive reliance on certain foods or supplements before reliable links are established.
Further examining the genetic factors that might play a role in migraine, a large Korean data analysis published in the December 2023 issue of Epidemiology and Health described a link between migraine and Parkinson's disease. The researchers included 214,193 patients with migraine and 5,879,711 individuals without migraine. According to the statistical analysis, the patients who had migraine with aura showed a 1.35-fold higher risk for Parkinson's disease than individuals without migraine. However, the researchers did not note a statistically significant difference between the risk for Parkinson's disease among patients who had migraine without aura and individuals without migraine.
They also examined other factors, and noted that among individuals with migraine, those who had preexisting dyslipidemia had a higher risk for Parkinson's disease than those who did not have dyslipidemia. Other factors that were not correlated with an association between migraine and Parkinson's disease included cardiovascular risk factors, hypertension, diabetes, smoking status, and high body mass index.
The study authors noted that factors associated with the activity of calcitonin gene-related peptide (CGRP), which is known to play a significant role in the pathophysiology of migraine, could play a role in the link between migraine and Parkinson's disease. They pointed to previous studies that found evidence of elevated CGRP levels in the cerebrospinal fluid of patients with Parkinson's disease as possible evidence of a pathophysiologic link.
An earlier commentary, published in the April 2020 issue of Headache, suggested an implication of CGRP antagonists in the development of neurodegenerative disorders such as Parkinson's disease. The commentary authors noted that previous research correlated midlife migraine to late-life parkinsonism, suggesting a conceivable common pathology, which could include a genetic or environmental predisposition.[1] They also noted that studies suggest a possible link between CGRP and multiple system atrophy, a parkinsonian disorder.[1] They considered the possibility that one of the ways that CGRP could contribute to these disorders is through its role in the recruitment of inflammatory mediators, which can alter the function of nicotinic receptors in the dopaminergic system in Parkinson's disease pathogenesis.[2]
Recent research published in the December 2023 issue of Headache suggests that CGRP responsiveness in migraine therapy could be mediated by genetics. The study included 198 patients who had been typed for genes involved in CGRP signaling or pharmacologic response and were given genetic and polygenic risk scores. Responders were defined as patients who experienced ≥ 50% reduction in migraine days per month at 5.7-month follow-up.
The analysis revealed an association between nonresponder status and rs12615320-G in RAMP1, a gene that encodes a component of high-affinity CGRP receptors, which increased the risk for nonresponder status. The researchers also identified an association between nonresponder status and rs4680-A in COMT, a gene that has been associated with lower COMT enzymatic activity, chronic pain/fibromyalgia, and a "worrier" phenotype. Nonresponders also had a lower mean genetic risk score than responders. These genetic associations could help identify which patients would be most likely to benefit from anti-CGRP therapies.
Given that CGRP responsiveness may have a genetic component, it is possible that one of the contributors to the link between migraine and Parkinson's disease could lie in patients' genetic predisposition to CGRP activity. Yet, the association between these two common conditions is not thoroughly established, and the role of CGRP in the pathogenesis of Parkinson's disease is not fully validated. Nevertheless, the new developments in treatments that modify CGRP activity could have implications beyond migraine.
Additional References
1. Alexoudi A, Deftereos S. CGRP antagonists: side effects and potential Parkinson's disease development. Headache. 2020;60:789-790. doi: 10.1111/head.13770 Source
2. Blumenfeld A, Durham PL, Feoktistov A, et al. Hypervigilance, allostatic load, and migraine prevention: Antibodies to CGRP or receptor. Neurol Ther. 2021;10:469-497. doi:10.1007/s40120-021-00250-7 Source
The authors of a study published in the December 2023 issue of the Scandinavian Journal of Gastroenterology analyzed data to examine the potential link between gut microbiota and migraine. According to the statistical analysis, the researchers found that "a greater abundance of genus Lactobacillus was associated with a higher risk of migraine and a higher abundance of family Prevotellaceae was related to a decreased risk of migraine." Furthermore, they noted that these gut microbial patterns could be due to a genetic predisposition. The authors suggested that stool sampling could potentially be helpful in the diagnosis of migraine, and that measures to modify gut microbiota in the context of migraine therapy could be identified with future research.
While it is not clear whether migraine is a cause or effect of these alterations, or whether there is another confounding variable, the idea of using diet as a means of reducing migraine risk would be appealing for many patients. This offers hope, but it also leaves a window open for exaggeration and excessive reliance on certain foods or supplements before reliable links are established.
Further examining the genetic factors that might play a role in migraine, a large Korean data analysis published in the December 2023 issue of Epidemiology and Health described a link between migraine and Parkinson's disease. The researchers included 214,193 patients with migraine and 5,879,711 individuals without migraine. According to the statistical analysis, the patients who had migraine with aura showed a 1.35-fold higher risk for Parkinson's disease than individuals without migraine. However, the researchers did not note a statistically significant difference between the risk for Parkinson's disease among patients who had migraine without aura and individuals without migraine.
They also examined other factors, and noted that among individuals with migraine, those who had preexisting dyslipidemia had a higher risk for Parkinson's disease than those who did not have dyslipidemia. Other factors that were not correlated with an association between migraine and Parkinson's disease included cardiovascular risk factors, hypertension, diabetes, smoking status, and high body mass index.
The study authors noted that factors associated with the activity of calcitonin gene-related peptide (CGRP), which is known to play a significant role in the pathophysiology of migraine, could play a role in the link between migraine and Parkinson's disease. They pointed to previous studies that found evidence of elevated CGRP levels in the cerebrospinal fluid of patients with Parkinson's disease as possible evidence of a pathophysiologic link.
An earlier commentary, published in the April 2020 issue of Headache, suggested an implication of CGRP antagonists in the development of neurodegenerative disorders such as Parkinson's disease. The commentary authors noted that previous research correlated midlife migraine to late-life parkinsonism, suggesting a conceivable common pathology, which could include a genetic or environmental predisposition.[1] They also noted that studies suggest a possible link between CGRP and multiple system atrophy, a parkinsonian disorder.[1] They considered the possibility that one of the ways that CGRP could contribute to these disorders is through its role in the recruitment of inflammatory mediators, which can alter the function of nicotinic receptors in the dopaminergic system in Parkinson's disease pathogenesis.[2]
Recent research published in the December 2023 issue of Headache suggests that CGRP responsiveness in migraine therapy could be mediated by genetics. The study included 198 patients who had been typed for genes involved in CGRP signaling or pharmacologic response and were given genetic and polygenic risk scores. Responders were defined as patients who experienced ≥ 50% reduction in migraine days per month at 5.7-month follow-up.
The analysis revealed an association between nonresponder status and rs12615320-G in RAMP1, a gene that encodes a component of high-affinity CGRP receptors, which increased the risk for nonresponder status. The researchers also identified an association between nonresponder status and rs4680-A in COMT, a gene that has been associated with lower COMT enzymatic activity, chronic pain/fibromyalgia, and a "worrier" phenotype. Nonresponders also had a lower mean genetic risk score than responders. These genetic associations could help identify which patients would be most likely to benefit from anti-CGRP therapies.
Given that CGRP responsiveness may have a genetic component, it is possible that one of the contributors to the link between migraine and Parkinson's disease could lie in patients' genetic predisposition to CGRP activity. Yet, the association between these two common conditions is not thoroughly established, and the role of CGRP in the pathogenesis of Parkinson's disease is not fully validated. Nevertheless, the new developments in treatments that modify CGRP activity could have implications beyond migraine.
Additional References
1. Alexoudi A, Deftereos S. CGRP antagonists: side effects and potential Parkinson's disease development. Headache. 2020;60:789-790. doi: 10.1111/head.13770 Source
2. Blumenfeld A, Durham PL, Feoktistov A, et al. Hypervigilance, allostatic load, and migraine prevention: Antibodies to CGRP or receptor. Neurol Ther. 2021;10:469-497. doi:10.1007/s40120-021-00250-7 Source
Methotrexate-Induced Mucositis in a Patient With Angioimmunoblastic T-cell Lymphoma
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.
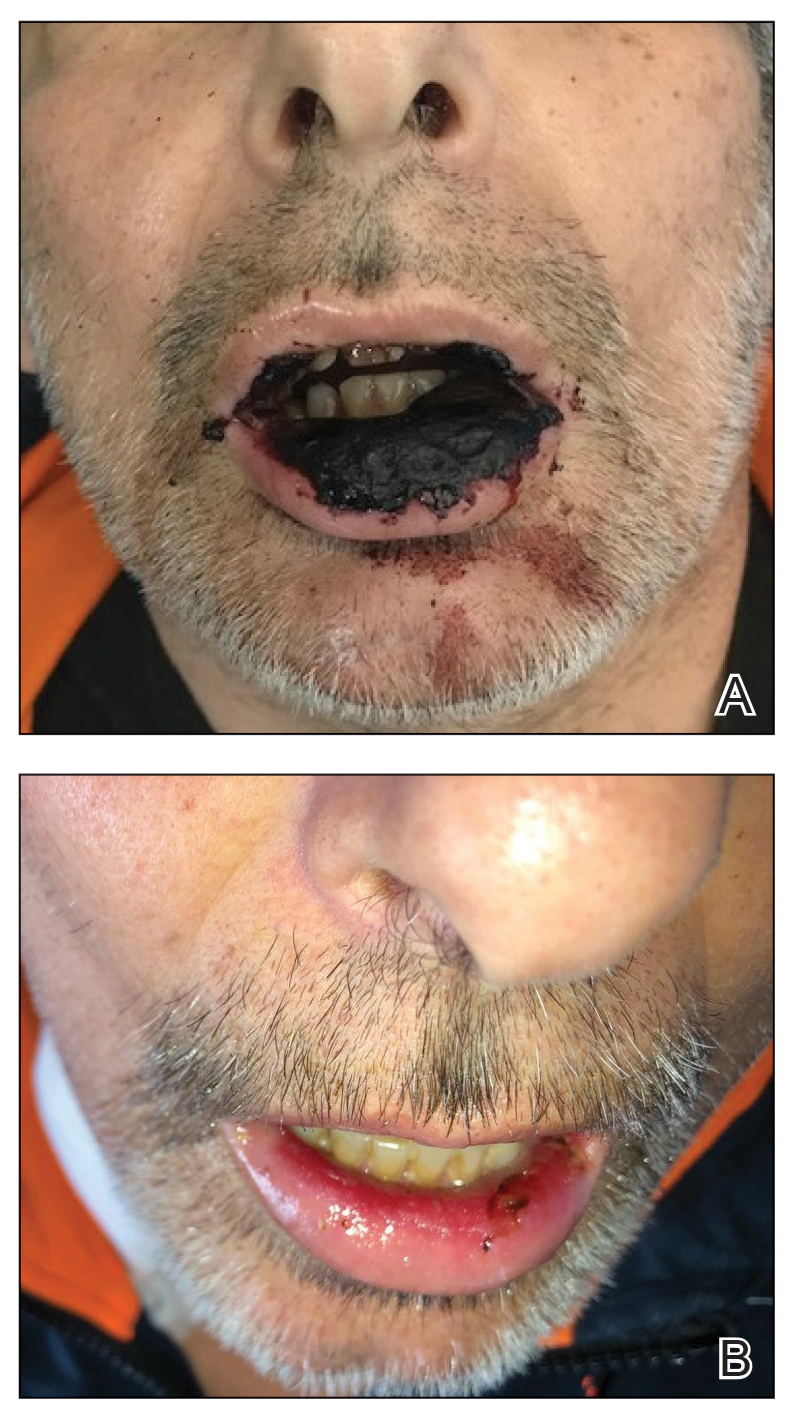
Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
PRACTICE POINTS
- Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed to manage cancers such as refractory angioimmunoblastic T-cell lymphoma.
- Dermatologists should be aware of the potential mucocutaneous adverse effects of high-dosage MTX.
- To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy.
My Kidney Is Fine, Can’t You Cystatin C?
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCrby assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCysreassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cyswas 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCrby assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCysreassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cyswas 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCrby assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCysreassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cyswas 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
A Cross-sectional Analysis of Regional Trends in Medicare Reimbursement for Phototherapy Services From 2010 to 2023
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.
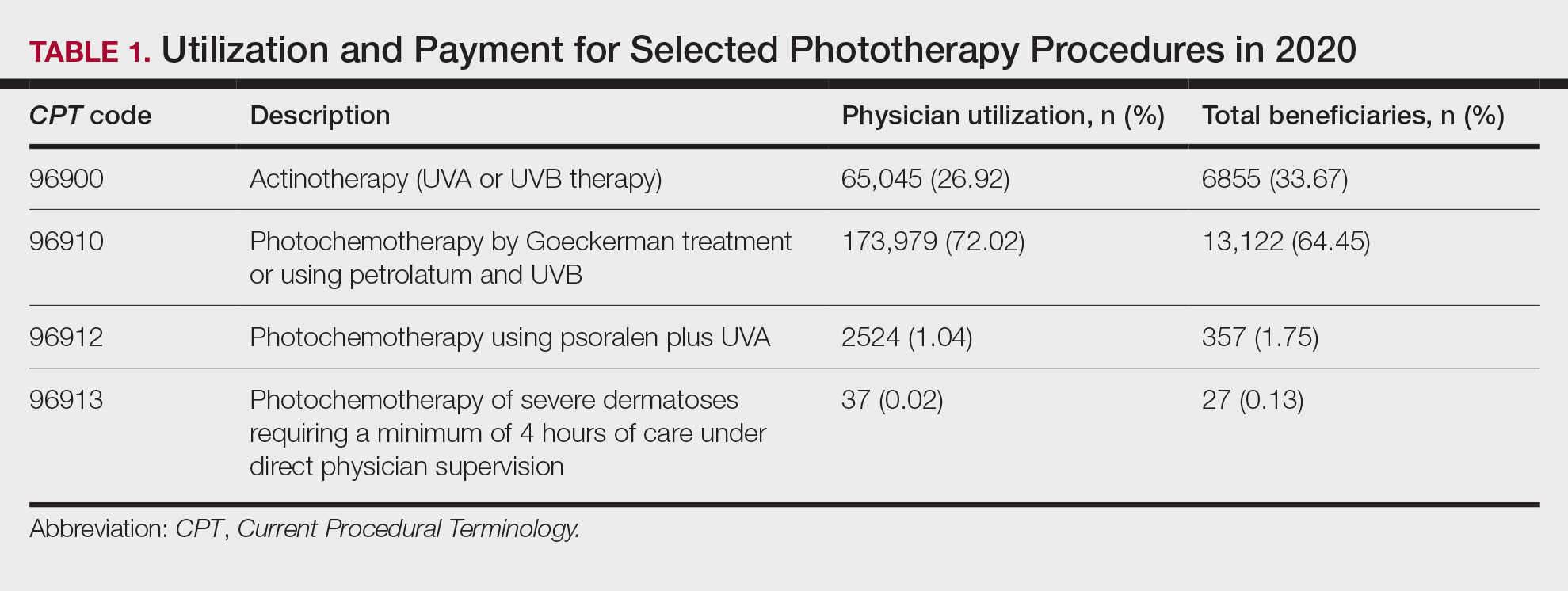
On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).
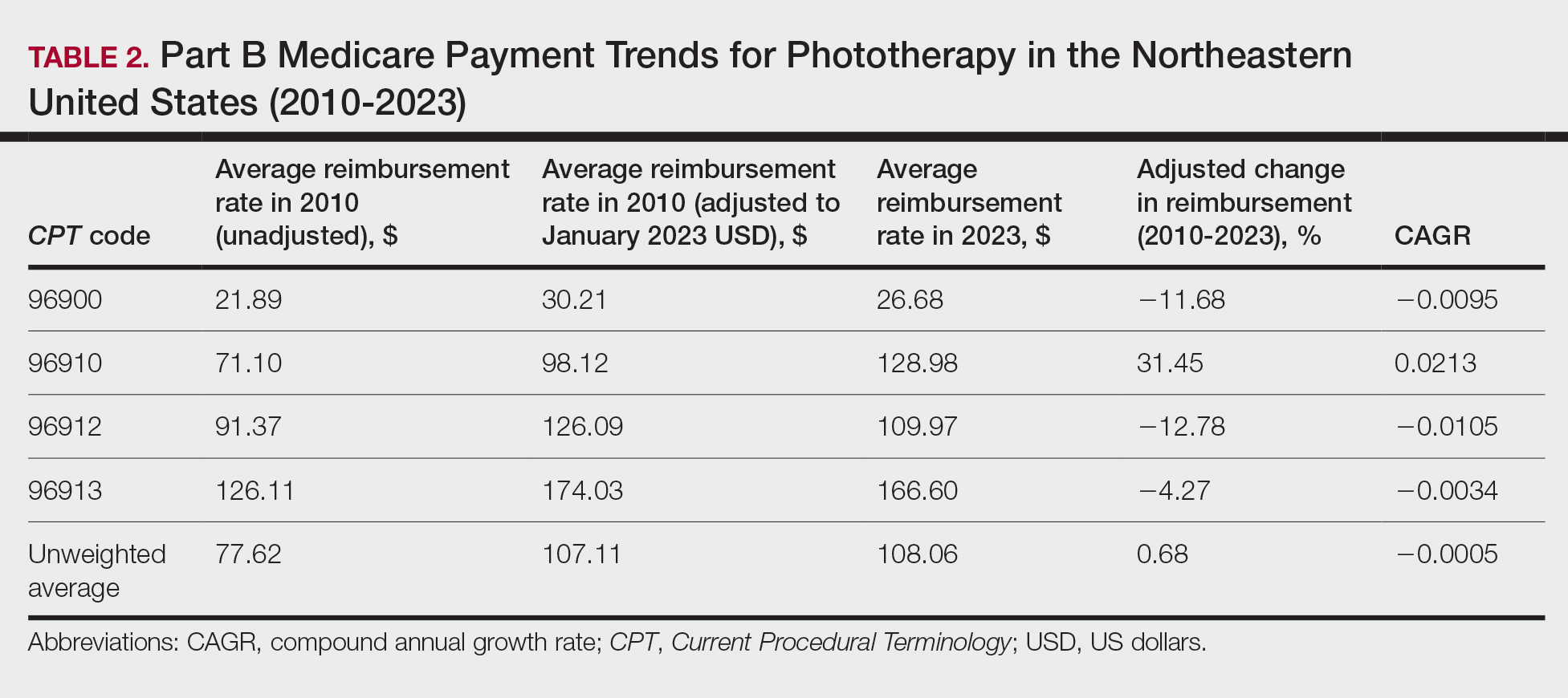
On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).
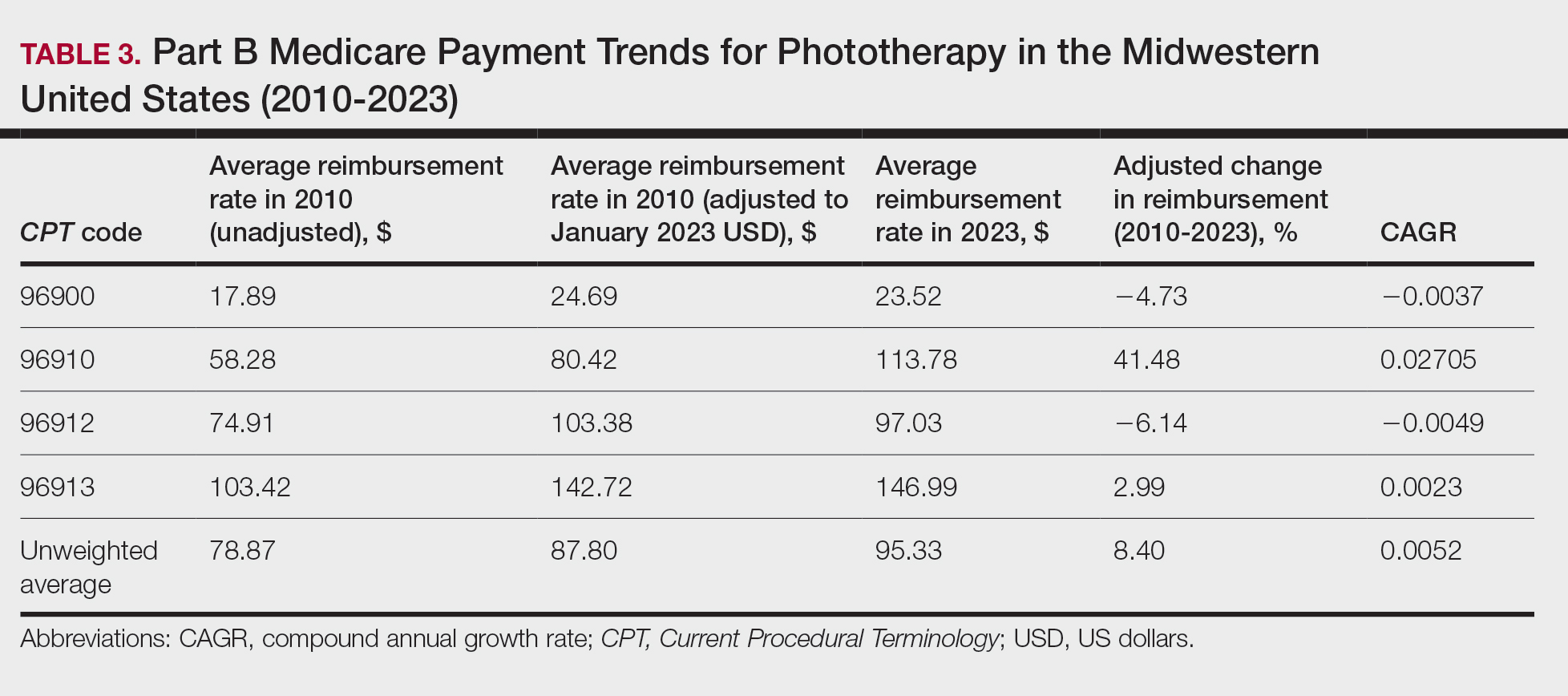
On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).
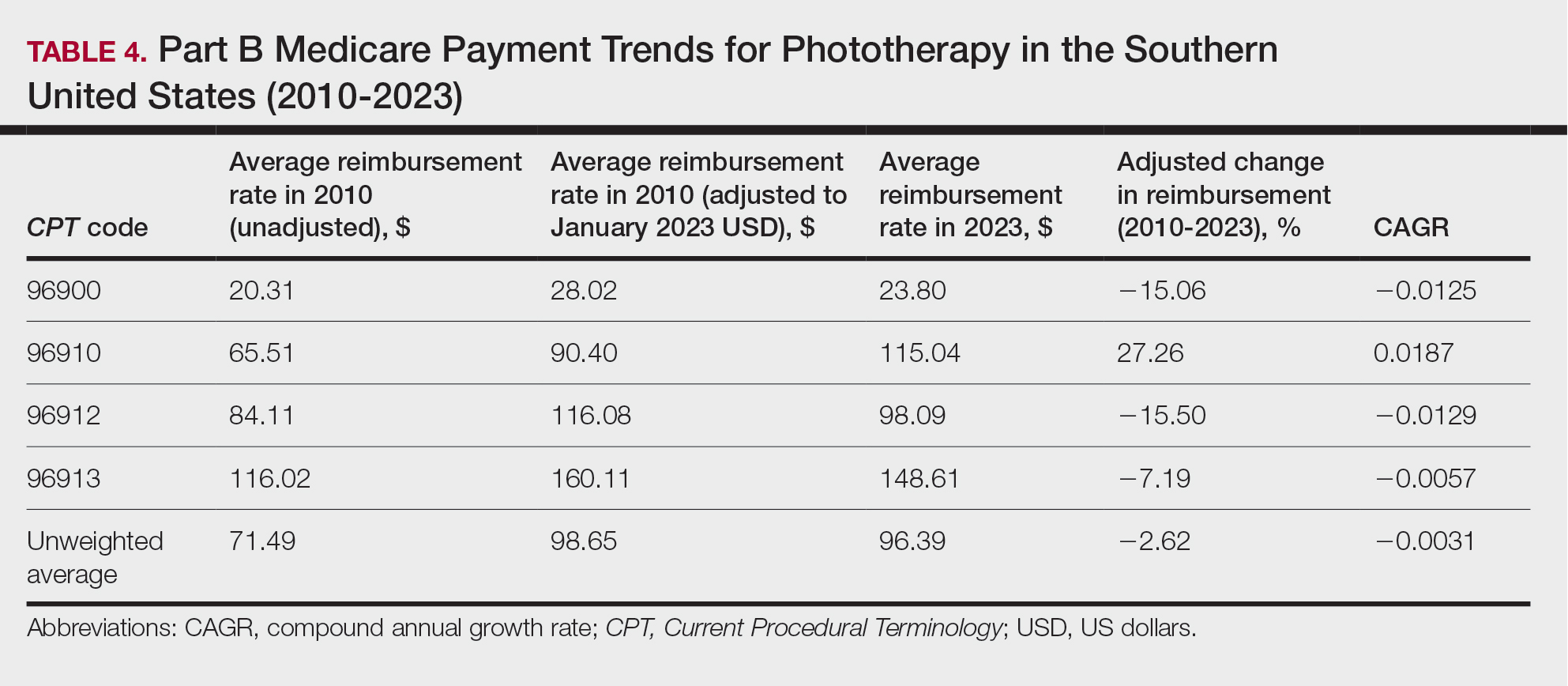
On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).
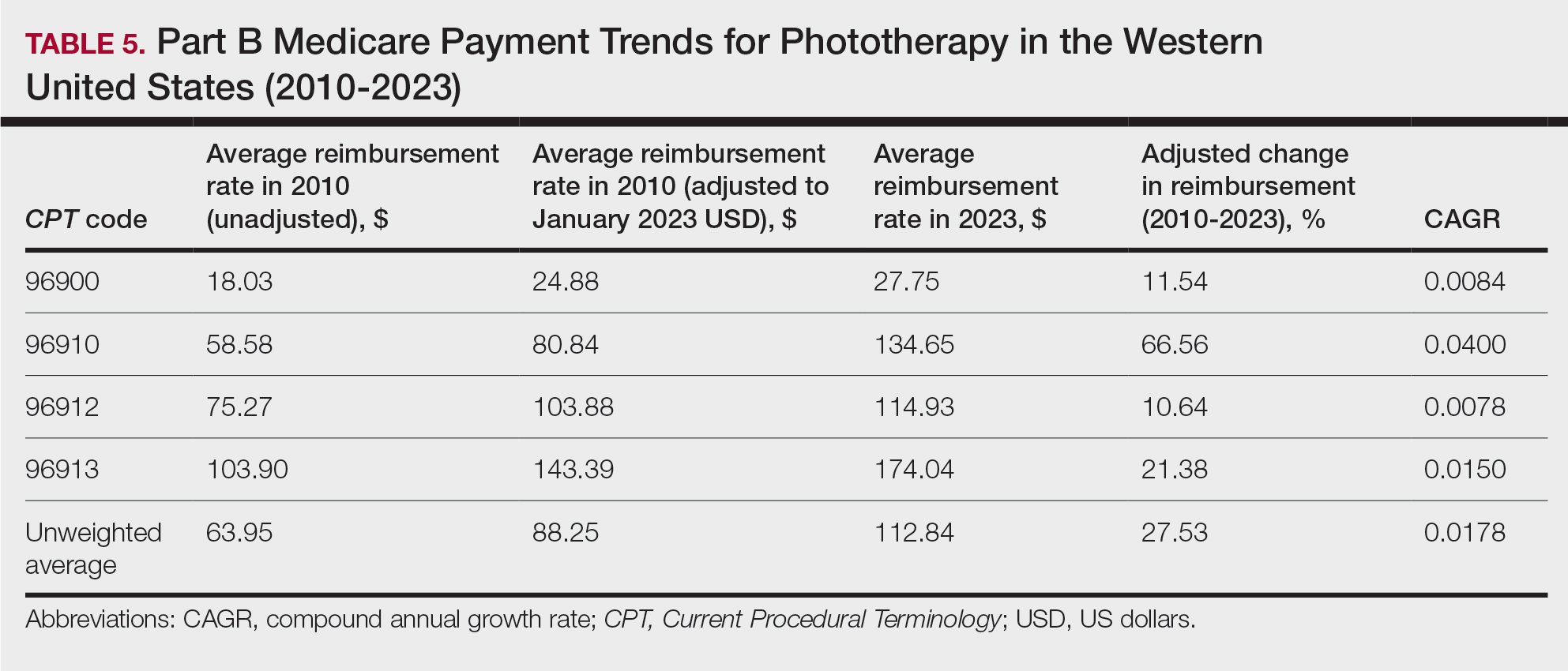
In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
Practice Points
- After weighting for procedure utilization, mean reimbursement for phototherapy increased across all US regions from 2010 to 2023 (mean change, +28.62%), yet with marked regional diversity.
- The southern United States reported the least growth in weighted mean reimbursement (+15.41%), and the western United States reported the greatest growth in weighted mean reimbursement (+51.16%).
- Region- and procedure-specific payment changes are especially valuable to dermatologists and policymakers alike, potentially reinvigorating payment reform discussions.
Thiazide-Induced Hyponatremia Presenting as a Fall in an Older Adult
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
Hypertension is a major risk factor for heart disease, stroke, and kidney disease.The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658