User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Studies Reinforce JAK Inhibitor Efficacy for Most Challenging Alopecia Types
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
Cognitive Deficits After Most Severe COVID Cases Associated With 9-Point IQ Drop
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
A new study from the United Kingdom provides greater clarity on how SARS-CoV-2 infection can affect cognition and memory, including novel data on how long brain fog may last after the illness resolves and which cognitive functions are most vulnerable.
In a large community sample, researchers found that on average, people who had recovered from COVID-19 showed small cognitive deficits equivalent to a 3-point loss in IQ for up to 1 year or more after recovering from the acute illness compared with peers who never had COVID-19.
However, people who had more severe cases, requiring treatment in a hospital intensive care unit, had cognitive deficits equivalent to a 9-point drop in IQ.
“People with ongoing persistent symptoms, indicative of long COVID, had larger cognitive deficits than people whose symptoms had resolved,” first author Adam Hampshire, PhD, with Imperial College London, London, England, told this news organization.
The largest deficits among cognitive tasks were in memory, reasoning, and executive function, he added.
“That is, people who had had COVID-19 were both slower and less accurate when performing tasks that measure those abilities,” Dr. Hampshire said. “The group with the largest cognitive deficits were patients who had been in intensive care for COVID-19.”
The study was published online in The New England Journal of Medicine.
Lingering Brain Fog
Cognitive symptoms after SARS-CoV-2 infection are well recognized, but whether objectively measurable cognitive deficits exist and how long they persist remains unclear.
To investigate, researchers invited 800,000 adults from the REACT study of SARS-CoV-2 transmission in England to complete an online assessment for cognitive function with eight domains.
Altogether, 141,583 participants started the cognitive battery by completing at least one task, and 112,964 completed all eight tasks.
The researchers estimated global cognitive scores among participants who had been previously infected with SARS-CoV-2 with symptoms that persisted for at least 12 weeks, whether or not resolved, and among uninfected participants.
Compared with uninfected adults, those who had COVID-19 that resolved had a small cognitive deficit, corresponding to a 3-point loss in IQ, the researchers found.
Adults with unresolved persistent COVID-19 symptoms had the equivalent of a 6-point loss in IQ, and those who had been admitted to the intensive care unit had the equivalent of a 9-point loss in IQ, in line with previous findings of cognitive deficits in patients hospitalized in a critical care unit, the researchers report.
Larger cognitive deficits were evident in adults infected early in the pandemic by the original SARS-CoV-2 virus or the B.1.1.7 variant, whereas peers infected later in the pandemic (eg., in the Omicron period), showed smaller cognitive deficits. This finding is in line with other studies suggesting that the association between COVID-19–associated cognitive deficits attenuated as the pandemic progressed, the researchers noted.
They also found that people who had COVID-19 after receiving two or more vaccinations showed better cognitive performance compared with those who had not been vaccinated.
The memory, reasoning, and executive function tasks were among the most sensitive to COVID-19–related cognitive differences and performance on these tasks differed according to illness duration and hospitalization.
Dr. Hampshire said that more research is needed to determine whether the cognitive deficits resolve with time.
“The implications of longer-term persistence of cognitive deficits and their clinical relevance remain unclear and warrant ongoing surveillance,” he said.
Larger Cognitive Deficits Likely?
These results are “a concern and the broader implications require evaluation,” wrote Ziyad Al-Aly, MD, with Washington University School of Medicine in St. Louis, Missouri, and Clifford Rosen, MD, with Tufts University School of Medicine in Boston, Massachusetts, in an accompanying editorial.
In their view, several outstanding questions remain, including what the potential functional implications of a 3-point loss in IQ may be and whether COVID-19–related cognitive deficits predispose to a higher risk for dementia later in life.
“A deeper understanding of the biology of cognitive dysfunction after SARS-CoV-2 infection and how best to prevent and treat it are critical for addressing the needs of affected persons and preserving the cognitive health of populations,” Drs. Al-Aly and Rosen concluded.
Commenting on the study for this news organization, Jacqueline Becker, PhD, clinical neuropsychologist and assistant professor of medicine, Icahn School of Medicine at Mount Sinai, New York City, noted that “one important caveat” is that the study used an online assessment tool for cognitive function and therefore the findings should be taken with “a grain of salt.”
“That said, this is a large sample, and the findings are generally consistent with what we’ve seen in terms of cognitive deficits post-COVID,” Dr. Becker said.
It’s likely that this study “underestimates” the degree of cognitive deficits that would be seen on validated neuropsychological tests, she added.
In a recent study, Dr. Becker and her colleagues investigated rates of cognitive impairment in 740 COVID-19 patients who recovered and were treated in outpatient, emergency department, or inpatient hospital settings.
Using validated neuropsychological measures, they found a relatively high frequency of cognitive impairment several months after patients contracted COVID-19. Impairments in executive functioning, processing speed, category fluency, memory encoding, and recall were predominant among hospitalized patients.
Dr. Becker noted that in her experience, cognition typically will improve in some patients 12-18 months post COVID.
Support for the study was provided by the National Institute for Health and Care Research and UK Research and Innovation and by the Department of Health and Social Care in England and the Huo Family Foundation. Disclosures for authors and editorial writers are available at NEJM.org. Dr. Becker has no relevant disclosures.
A version of this article appeared on Medscape.com.
Medtronic’s Duet EDMS Catheter Tubing Under Class I Recall
If this happens, potential harm to patients may include infections, cerebrospinal fluid (CSF) leakage, overdrainage of CSF, and abnormality of the ventricles. Uncontrolled overdrainage of CSF could lead to neurological injury or death if the disconnection is undetected.
The Food and Drug Administration has identified this as a Class I recall — the most serious type — due to the risk for serious injury or death. To date, there have been 26 reported injuries and no deaths related to this issue.
The recall includes 45,176 devices distributed in the United States between May 3, 2021, and January 9, 2024, with model numbers 46913, 46914, 46915, 46916, and 46917.
The Duet EDMS is used for temporary CSF drainage or sampling in patients who have surgery for open descending thoracic aortic aneurysm (TAA) or descending thoraco-abdominal aortic aneurysm (TAAA) or patients who have TAA/TAAA repair surgery and develop symptoms such as paraplegia.
Medtronic has sent an urgent medical device recall letter to all affected customers asking them to identify, quarantine, and return any unused recalled products.
Customers are also advised to check all Duet EDMS components for damage and ensure that all connections are secure and leak-free.
If a patient is currently connected to an impacted Duet EDMS and a leak or disconnection is detected, the device should be changed to a new alternative device utilizing a sterile technique.
It is not recommended that a Duet system device that is connected to a patient and working as intended be removed or replaced.
Customers in the United States with questions about this recall should contact Medtronic at 1-800-874-5797.
A version of this article appeared on Medscape.com.
If this happens, potential harm to patients may include infections, cerebrospinal fluid (CSF) leakage, overdrainage of CSF, and abnormality of the ventricles. Uncontrolled overdrainage of CSF could lead to neurological injury or death if the disconnection is undetected.
The Food and Drug Administration has identified this as a Class I recall — the most serious type — due to the risk for serious injury or death. To date, there have been 26 reported injuries and no deaths related to this issue.
The recall includes 45,176 devices distributed in the United States between May 3, 2021, and January 9, 2024, with model numbers 46913, 46914, 46915, 46916, and 46917.
The Duet EDMS is used for temporary CSF drainage or sampling in patients who have surgery for open descending thoracic aortic aneurysm (TAA) or descending thoraco-abdominal aortic aneurysm (TAAA) or patients who have TAA/TAAA repair surgery and develop symptoms such as paraplegia.
Medtronic has sent an urgent medical device recall letter to all affected customers asking them to identify, quarantine, and return any unused recalled products.
Customers are also advised to check all Duet EDMS components for damage and ensure that all connections are secure and leak-free.
If a patient is currently connected to an impacted Duet EDMS and a leak or disconnection is detected, the device should be changed to a new alternative device utilizing a sterile technique.
It is not recommended that a Duet system device that is connected to a patient and working as intended be removed or replaced.
Customers in the United States with questions about this recall should contact Medtronic at 1-800-874-5797.
A version of this article appeared on Medscape.com.
If this happens, potential harm to patients may include infections, cerebrospinal fluid (CSF) leakage, overdrainage of CSF, and abnormality of the ventricles. Uncontrolled overdrainage of CSF could lead to neurological injury or death if the disconnection is undetected.
The Food and Drug Administration has identified this as a Class I recall — the most serious type — due to the risk for serious injury or death. To date, there have been 26 reported injuries and no deaths related to this issue.
The recall includes 45,176 devices distributed in the United States between May 3, 2021, and January 9, 2024, with model numbers 46913, 46914, 46915, 46916, and 46917.
The Duet EDMS is used for temporary CSF drainage or sampling in patients who have surgery for open descending thoracic aortic aneurysm (TAA) or descending thoraco-abdominal aortic aneurysm (TAAA) or patients who have TAA/TAAA repair surgery and develop symptoms such as paraplegia.
Medtronic has sent an urgent medical device recall letter to all affected customers asking them to identify, quarantine, and return any unused recalled products.
Customers are also advised to check all Duet EDMS components for damage and ensure that all connections are secure and leak-free.
If a patient is currently connected to an impacted Duet EDMS and a leak or disconnection is detected, the device should be changed to a new alternative device utilizing a sterile technique.
It is not recommended that a Duet system device that is connected to a patient and working as intended be removed or replaced.
Customers in the United States with questions about this recall should contact Medtronic at 1-800-874-5797.
A version of this article appeared on Medscape.com.
Vitamin D Supplements May Be a Double-Edged Sword
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Imagine, if you will, the great Cathedral of Our Lady of Correlation. You walk through the majestic oak doors depicting the link between ice cream sales and shark attacks, past the rose window depicting the cardiovascular benefits of red wine, and down the aisles frescoed in dramatic images showing how Facebook usage is associated with less life satisfaction. And then you reach the altar, the holy of holies where, emblazoned in shimmering pyrite, you see the patron saint of this church: vitamin D.
Yes, if you’ve watched this space, then you know that I have little truck with the wildly popular supplement. In all of clinical research, I believe that there is no molecule with stronger data for correlation and weaker data for causation.
Low serum vitamin D levels have been linked to higher risks for heart disease, cancer, falls, COVID, dementia, C diff, and others. And yet, when we do randomized trials of vitamin D supplementation — the thing that can prove that the low level was causally linked to the outcome of interest — we get negative results.
Trials aren’t perfect, of course, and we’ll talk in a moment about a big one that had some issues. But we are at a point where we need to either be vitamin D apologists, saying, “Forget what those lying RCTs tell you and buy this supplement” — an $800 million-a-year industry, by the way — or conclude that vitamin D levels are a convenient marker of various lifestyle factors that are associated with better outcomes: markers of exercise, getting outside, eating a varied diet.
Or perhaps vitamin D supplements have real effects. It’s just that the beneficial effects are matched by the harmful ones. Stay tuned.
The Women’s Health Initiative remains among the largest randomized trials of vitamin D and calcium supplementation ever conducted — and a major contributor to the negative outcomes of vitamin D trials.
But if you dig into the inclusion and exclusion criteria for this trial, you’ll find that individuals were allowed to continue taking vitamins and supplements while they were in the trial, regardless of their randomization status. In fact, the majority took supplements at baseline, and more took supplements over time.
That means, of course, that people in the placebo group, who were getting sugar pills instead of vitamin D and calcium, may have been taking vitamin D and calcium on the side. That would certainly bias the results of the trial toward the null, which is what the primary analyses showed. To wit, the original analysis of the Women’s Health Initiative trial showed no effect of randomization to vitamin D supplementation on improving cancer or cardiovascular outcomes.
But the Women’s Health Initiative trial started 30 years ago. Today, with the benefit of decades of follow-up, we can re-investigate — and perhaps re-litigate — those findings, courtesy of this study, “Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women” appearing in Annals of Internal Medicine.
Dr Cynthia Thomson, of the Mel and Enid Zuckerman College of Public Health at the University of Arizona, and colleagues led this updated analysis focused on two findings that had been hinted at, but not statistically confirmed, in other vitamin D studies: a potential for the supplement to reduce the risk for cancer, and a potential for it to increase the risk for heart disease.
The randomized trial itself only lasted 7 years. What we are seeing in this analysis of 36,282 women is outcomes that happened at any time from randomization to the end of 2023 — around 20 years after the randomization to supplementation stopped. But, the researchers would argue, that’s probably okay. Cancer and heart disease take time to develop; we see lung cancer long after people stop smoking. So a history of consistent vitamin D supplementation may indeed be protective — or harmful.
Here are the top-line results. Those randomized to vitamin D and calcium supplementation had a 7% reduction in the rate of death from cancer, driven primarily by a reduction in colorectal cancer. This was statistically significant. Also statistically significant? Those randomized to supplementation had a 6% increase in the rate of death from cardiovascular disease. Put those findings together and what do you get? Stone-cold nothing, in terms of overall mortality.
Okay, you say, but what about all that supplementation that was happening outside of the context of the trial, biasing our results toward the null?
The researchers finally clue us in.
First of all, I’ll tell you that, yes, people who were supplementing outside of the trial had higher baseline vitamin D levels — a median of 54.5 nmol/L vs 32.8 nmol/L. This may be because they were supplementing with vitamin D, but it could also be because people who take supplements tend to do other healthy things — another correlation to add to the great cathedral.
To get a better view of the real effects of randomization, the authors restricted the analysis to just those who did not use outside supplements. If vitamin D supplements help, then these are the people they should help. This group had about a 11% reduction in the incidence of cancer — statistically significant — and a 7% reduction in cancer mortality that did not meet the bar for statistical significance.
There was no increase in cardiovascular disease among this group. But this small effect on cancer was nowhere near enough to significantly reduce the rate of all-cause mortality.
Among those using supplements, vitamin D supplementation didn’t really move the needle on any outcome.
I know what you’re thinking: How many of these women were vitamin D deficient when we got started? These results may simply be telling us that people who have normal vitamin D levels are fine to go without supplementation.
Nearly three fourths of women who were not taking supplements entered the trial with vitamin D levels below the 50 nmol/L cutoff that the authors suggest would qualify for deficiency. Around half of those who used supplements were deficient. And yet, frustratingly, I could not find data on the effect of randomization to supplementation stratified by baseline vitamin D level. I even reached out to Dr Thomson to ask about this. She replied, “We did not stratify on baseline values because the numbers are too small statistically to test this.” Sorry.
In the meantime, I can tell you that for your “average woman,” vitamin D supplementation likely has no effect on mortality. It might modestly reduce the risk for certain cancers while increasing the risk for heart disease (probably through coronary calcification). So, there might be some room for personalization here. Perhaps women with a strong family history of cancer or other risk factors would do better with supplements, and those with a high risk for heart disease would do worse. Seems like a strategy that could be tested in a clinical trial. But maybe we could ask the participants to give up their extracurricular supplement use before they enter the trial. F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now.
Long-Term Calcium and Vitamin D: Cancer Deaths Down, CVD Deaths Up in Older Women?
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
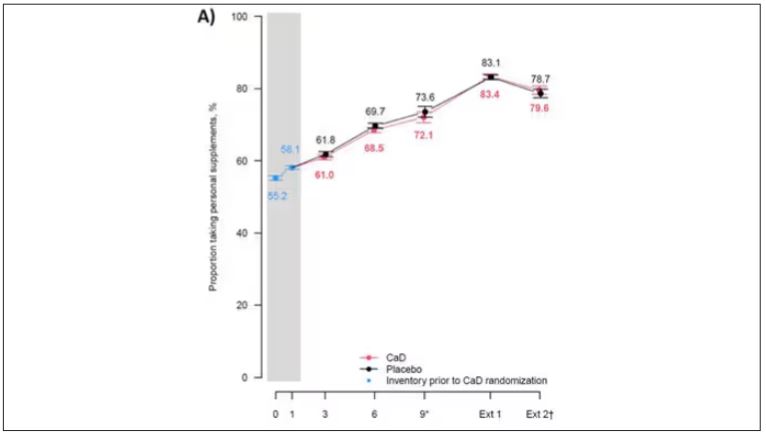
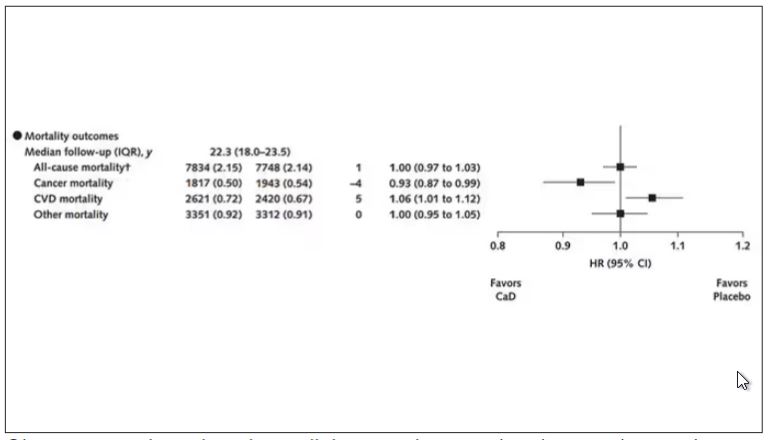
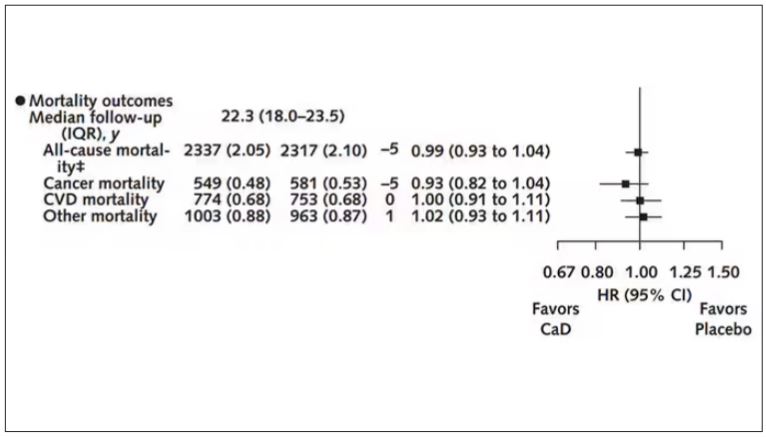
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
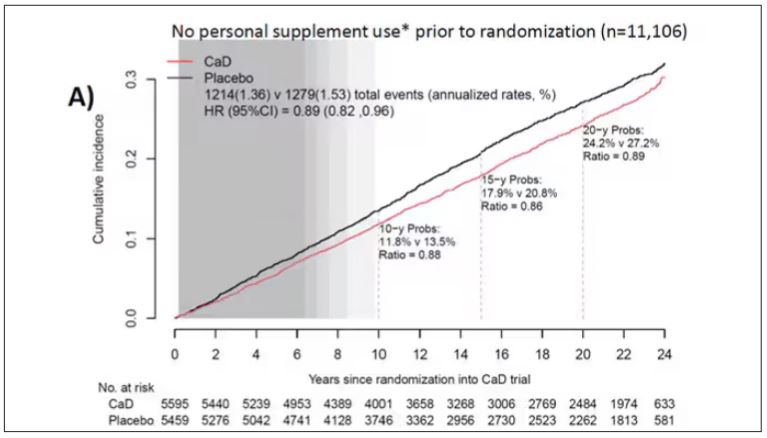
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
Some doctors may be scratching their heads over a new analysis reporting that combined calcium and vitamin D (CaD) supplements appear to be associated with a slight 6% increase in cardiovascular (CVD) mortality, a slight 7% decrease in cancer risk, and no effect on osteoporotic fracture in postmenopausal women.
The study, in Annals of Internal Medicine, found no effect of supplementation on all-cause mortality.
The findings emerged from an analysis of more than 20 years’ follow-up data on a randomized trial in postmenopausal women conducted as part of the Women’s Health Initiative (WHI).
Cynthia A. Thomson, PhD, RD, first author and cancer prevention scientist at the Arizona Cancer Center and a professor of health promotion sciences at the University of Arizona in Tucson said the findings recommend individualized assessment of the need for supplements for older women as they consider them in hopes of preventing fractures.
“Evaluate your patients individually and understand that there are some who may benefit from supplementation, for example, in terms of reducing colorectal cancer mortality,” Dr. Thomson said in an interview. The approach should be nuanced. “If you check the adequacy of vitamin D and calcium in their diets, supplementation may not be needed.” She added that supplementation is best considered in the context of a woman’s overall health profile, including risk factors for fracture, heart disease, and cancer, especially colorectal cancer (CRC).
Study Details
The investigators conducted postintervention follow-up of the WHI’s 7-year multicenter randomized intervention trial of CaD vs placebo.
Since existing evidence of long-term health outcomes was limited, the trial, begun in 1999 and closed in 2005, enrolled 36,282 postmenopausal women (mean age 62) with no history of breast or colorectal cancer. They were randomly assigned 1:1 to supplementation with 1000 mg of calcium carbonate (400 mg elemental calcium) plus 400 IU of vitamin D3 daily or placebo, taken twice daily in half doses.
Study outcomes were incidence of CRC, total and invasive breast cancer; disease-specific and all-cause mortality; total CVD; and hip fracture measured through December 2020, with analyses stratified by personal supplement usage.
Cancer. CaD was associated with reduced incident total cancer, CRC, and invasive breast cancer — notably among participants not taking CaD before randomization. Cancer incidence estimates varied widely, the authors noted, when stratified by supplement use before randomization. Noting that CaD seemed to have more cancer-related impact in those without prior supplementation, the authors suggested supplementation may affect cancer biology primarily by augmenting nutrient insufficiency.
An estimated 7% reduction in cancer mortality was observed after a median cumulative follow-up of 22.3 years: 1817 vs 1943 deaths (hazard ratio, 0.93; 95% CI, 0.87-0.99).
CVD. An estimated 6% increase in CVD mortality was seen in the CaD group: 2621 vs 2420 deaths (HR, 1.06; 95% CI, 1.01-1.12). Pretrial supplement users were found to be at higher CVD risk.
Hip fracture. No effect on hip fracture risk was measured, but the authors cautioned that hip fracture and CVD outcomes were available only for a subset of participants, and the effects of calcium alone vs vitamin D alone vs the combination could not be disentangled.
In a small subgroup analysis, some CaD users were seen to respond in terms of bone mineral density but since only 4 of the study’s 40 sites collected such information, the study was underpowered to examine the effect. ”Many other studies, however, show a response to supplementation in women who already have bone mineral deficits,” Dr. Thomson said.
The Calcification Question
One of the possible mechanisms of harm is that high-dose calcium supplements can increase the rate of blood coagulation and promote vascular calcification, said Emma Laing, PhD, RD, director of dietetics at the University of Georgia in Athens and a spokesperson for the Chicago-based Academy of Nutrition and Dietetics.
“Other factors that should be considered when determining a patient’s CVD risk are race, genetic predisposition, medical and social history, response to stress, and lifestyle behaviors, as well as the length of time supplements have been consumed,” added Dr. Laing, who was not involved in the WHI analysis.
“We asked ourselves if CaD supplements might contribute to calcification of the coronary arteries, since some believe this to be the case, although the literature is mixed,” said Dr. Thomson.
“So we did a shorter ancillary study in a small sample of several hundred [women] to see if there was any increase in calcification” and no difference was seen on imaging across the two arms. “However, women who were already on supplements before entering the study seemed to be at higher CVD risk,” she said.
Added study coauthor JoAnn E. Manson, MD, DrPH, chief of the division of preventive medicine at Brigham and Women’s Hospital and professor of women’s health at Harvard Medical School, both in Boston: “With no increase or decrease in coronary artery calcium at the end of the trial, we don’t believe starting or continuing calcium/vitamin D supplements should require screening for coronary artery disease.”
Some randomized trials and systematic reviews, however, have observed an increased risk of CVD in healthy patients on calcium supplements, with one Korean meta-analysis reporting a 15% increase in CVD risk in healthy postmenopausal women taking calcium supplements. Another meta-analysis found a link between calcium supplements and a greater risk of various cardiovascular outcomes, especially myocardial infarction.
Vitamin D Supplementation
As for vitamin D only supplementation, an updated meta-analysis including more than 83,000 individuals showed that it confers no cardiovascular protection and is therefore not indicated for this purpose.
Practice Considerations
Offering an outsider’s perspective, Sarah G. Candler, MD, MPH, an internist in Houston specializing in primary care for older high-risk adults, said: “Unfortunately, this latest study continues the trend of creating more questions than answers. If the adverse outcome of CVD death is a result of supplementation, it is unclear if this is due to the vitamin D, the calcium, or both. And it is unclear if this is dose dependent, time dependent, or due to concurrent risk factors unique to certain populations.
“It is recommended that patients at risk of osteoporosis based on age, sex, medications, and lifestyle be screened for osteoporosis and treated accordingly, including supplementation with CaD,” Dr. Candler said. “It remains unclear whether supplementation with CaD in the absence of osteoporosis and osteopenia is net beneficial or harmful, and at this time I would not recommend it to my patients.”
Added Dr. Manson: “The very small increase seen in cardiovascular mortality wouldn’t be a reason to discontinue supplementation among women who have been advised by their healthcare providers to take these supplements for bone health or other purposes.
“Among those at usual risk of fracture, we recommend trying to obtain adequate calcium and vitamin D from food sources first and to use supplements only for the purpose of filling gaps in intake,” Dr. Manson continued. Overall, the findings support the national recommended dietary allowances for daily calcium intake of 1200 mg and daily vitamin D intake of 600-800 IU among postmenopausal women for maintenance of bone health, she said.
While a 2022 study found that vitamin D supplementation alone did not prevent fractures in healthy adults, other research has shown that a calcium/vitamin D combination is more likely to protect the skeleton.
“Patients at risk for fractures will probably benefit from calcium and/or vitamin D supplementation if they do not meet dietary intake requirements, have malabsorption syndromes, are taking medications that affect nutrient absorption, or if they are older and not regularly exposed to sunlight,” said Dr. Laing. “A combination of biochemical, imaging, functional, and dietary intake data can help determine if a supplement is warranted.”
She stressed that additional research is needed in more diverse populations before changing practice guidelines. “However, doctors should continue to weigh the risks and benefits of prescribing supplements for each patient.”
The WHI program is funded by the National Heart, Lung, and Blood Institute. Dr. Thomson disclosed no competing interests. Dr. Manson reported a relationship with Mars Edge. Multiple authors reported grant support from government funding agencies. The outside commentators had no relevant competing interests to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Look Beyond BMI: Metabolic Factors’ Link to Cancer Explained
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
The new research finds that adults with persistent metabolic syndrome that worsens over time are at increased risk for any type of cancer.
The conditions that make up metabolic syndrome (high blood pressure, high blood sugar, increased abdominal adiposity, and high cholesterol and triglycerides) have been associated with an increased risk of diseases, including heart disease, stroke, and type 2 diabetes, wrote Li Deng, PhD, of Capital Medical University, Beijing, and colleagues.
However, a single assessment of metabolic syndrome at one point in time is inadequate to show an association with cancer risk over time, they said. In the current study, the researchers used models to examine the association between trajectory patterns of metabolic syndrome over time and the risk of overall and specific cancer types. They also examined the impact of chronic inflammation concurrent with metabolic syndrome.
What We Know About Metabolic Syndrome and Cancer Risk
A systematic review and meta-analysis published in Diabetes Care in 2012 showed an association between the presence of metabolic syndrome and an increased risk of various cancers including liver, bladder, pancreatic, breast, and colorectal.
More recently, a 2020 study published in Diabetes showed evidence of increased risk for certain cancers (pancreatic, kidney, uterine, cervical) but no increased risk for cancer overall.
In addition, a 2022 study by some of the current study researchers of the same Chinese cohort focused on the role of inflammation in combination with metabolic syndrome on colorectal cancer specifically, and found an increased risk for cancer when both metabolic syndrome and inflammation were present.
However, the reasons for this association between metabolic syndrome and cancer remain unclear, and the effect of the fluctuating nature of metabolic syndrome over time on long-term cancer risk has not been explored, the researchers wrote.
“There is emerging evidence that even normal weight individuals who are metabolically unhealthy may be at an elevated cancer risk, and we need better metrics to define the underlying metabolic dysfunction in obesity,” Sheetal Hardikar, MBBS, PhD, MPH, an investigator at the Huntsman Cancer Institute, University of Utah, said in an interview.
Dr. Hardikar, who serves as assistant professor in the department of population health sciences at the University of Utah, was not involved in the current study. She and her colleagues published a research paper on data from the National Health and Nutrition Examination Survey in 2023 that showed an increased risk of obesity-related cancer.
What New Study Adds to Related Research
Previous studies have consistently reported an approximately 30% increased risk of cancer with metabolic syndrome, Dr. Hardikar said. “What is unique about this study is the examination of metabolic syndrome trajectories over four years, and not just the presence of metabolic syndrome at one point in time,” she said.
In the new study, published in Cancer on March 11 (doi: 10.1002/cncr.35235), 44,115 adults in China were separated into four trajectories based on metabolic syndrome scores for the period from 2006 to 2010. The scores were based on clinical evidence of metabolic syndrome, defined using the International Diabetes Federation criteria of central obesity and the presence of at least two other factors including increased triglycerides, decreased HDL cholesterol, high blood pressure (or treatment for previously diagnosed hypertension), and increased fasting plasma glucose (or previous diagnosis of type 2 diabetes).
The average age of the participants was 49 years; the mean body mass index ranged from approximately 22 kg/m2 in the low-stable group to approximately 28 kg/m2 in the elevated-increasing group.
The four trajectories of metabolic syndrome were low-stable (10.56% of participants), moderate-low (40.84%), moderate-high (41.46%), and elevated-increasing (7.14%), based on trends from the individuals’ initial physical exams on entering the study.
Over a median follow-up period of 9.4 years (from 2010 to 2021), 2,271 cancer diagnoses were reported in the study population. Those with an elevated-increasing metabolic syndrome trajectory had 1.3 times the risk of any cancer compared with those in the low-stable group. Risk for breast cancer, endometrial cancer, kidney cancer, colorectal cancer, and liver cancer in the highest trajectory group were 2.1, 3.3, 4.5, 2.5, and 1.6 times higher, respectively, compared to the lowest group. The increased risk in the elevated-trajectory group for all cancer types persisted when the low-stable, moderate-low, and moderate-high trajectory pattern groups were combined.
The researchers also examined the impact of chronic inflammation and found that individuals with persistently high metabolic syndrome scores and concurrent chronic inflammation had the highest risks of breast, endometrial, colon, and liver cancer. However, individuals with persistently high metabolic syndrome scores and no concurrent chronic inflammation had the highest risk of kidney cancer.
What Are the Limitations of This Research?
The researchers of the current study acknowledged the lack of information on other causes of cancer, including dietary habits, hepatitis C infection, and Helicobacter pylori infection. Other limitations include the focus only on individuals from a single community of mainly middle-aged men in China that may not generalize to other populations.
Also, the metabolic syndrome trajectories did not change much over time, which may be related to the short 4-year study period.
Using the International Diabetes Federation criteria was another limitation, because it prevented the assessment of cancer risk in normal weight individuals with metabolic dysfunction, Dr. Hardikar noted.
Does Metabolic Syndrome Cause Cancer?
“This research suggests that proactive and continuous management of metabolic syndrome may serve as an essential strategy in preventing cancer,” senior author Han-Ping Shi, MD, PhD, of Capital Medical University in Beijing, noted in a statement on the study.
More research is needed to assess the impact of these interventions on cancer risk. However, the data from the current study can guide future research that may lead to more targeted treatments and more effective preventive strategies, he continued.
“Current evidence based on this study and many other reports strongly suggests an increased risk for cancer associated with metabolic syndrome,” Dr. Hardikar said in an interview. The data serve as a reminder to clinicians to look beyond BMI as the only measure of obesity, and to consider metabolic factors together to identify individuals at increased risk for cancer, she said.
“We must continue to educate patients about obesity and all the chronic conditions it may lead to, but we cannot ignore this emerging phenotype of being of normal weight but metabolically unhealthy,” Dr. Hardikar emphasized.
What Additional Research is Needed?
Looking ahead, “we need well-designed interventions to test causality for metabolic syndrome and cancer risk, though the evidence from the observational studies is very strong,” Dr. Hardikar said.
In addition, a consensus is needed to better define metabolic dysfunction,and to explore cancer risk in normal weight but metabolically unhealthy individuals, she said.
The study was supported by the National Key Research and Development Program of China. The researchers and Dr. Hardikar had no financial conflicts to disclose.
FROM CANCER
Sublingual Immunotherapy Safe, Effective for Older Kids
Sublingual immunotherapy (SLIT) is as safe and effective for high-risk older children and adolescents as oral immunotherapy (OIT) is for infants and preschoolers, according to new research.
Preliminary data from a study of more than 180 pediatric patients with multiple food allergies showed that while most patients had mild symptoms, none experienced a severe grade 4 reaction during the buildup and maintenance phase of SLIT.
In addition, 70% of those tested at the end of the treatment protocol were able to tolerate 300 mg of their allergen, a success rate nearly as high as that for OIT.
The study was published in The Journal of Allergy and Clinical Immunology: In Practice.
SLIT has been used successfully in the treatment of environmental allergens such as grass and tree pollen and dust mites. In this study, researchers decided to test SLIT’s effectiveness and safety in the treatment of food allergies in older children.
“We knew that OIT is very effective and safe in infants and toddlers, but there was literature illustrating that for older, school-age kids and adolescents, OIT is not safe enough, as those older age groups tend to have higher risk of severe reaction during treatment,” senior author Edmond Chan, MD, clinical professor of allergy at the University of British Columbia and pediatric allergist at BC Children’s Hospital, both in Vancouver, British Columbia, Canada, told this news organization. “With that knowledge, we decided to explore SLIT as another first-phase therapy for the older kids.”
The investigators recruited 188 high-risk older children aged 4-18 years for multifood SLIT. Most (61.7%) participants had multiple food allergies. Approximately 68% were male, and the population’s median age was 11.3 years.
Nearly half (48.4%) of participants had atopic dermatitis, 45.2% had asthma, 58.0% had allergic rhinitis, and 2.66% had preexisting eosinophilic esophagitis.
Most (75.0%) of the children were classified as higher risk, and 23 had a history of a grade 3 or 4 reaction before beginning SLIT.
Of the 188 children who were initially enrolled in the study, 173 (92.0%) finished their SLIT buildup phase.
Because the study started when COVID-19 pandemic restrictions were in place, the SLIT protocol mandated that patients be seen virtually. The patients’ caregivers learned how to mix and administer the required doses at home using recipes specially developed by the research team that used products bought at the grocery store.
A wide variety of food allergens were treated, including peanut, other legumes, tree nuts, sesame, other seeds, egg, cow’s milk, fish, wheat, shrimp, and other allergens.
The children built up to 2-mg protein SLIT maintenance over the course of three to five visits under nurse supervision.
After 1-2 years of daily SLIT maintenance, patients were offered a low-dose oral food challenge (OFC; cumulative dose: 300 mg of protein) with the goal of bypassing OIT buildup.
Nearly all patients (93.1%) had symptoms during SLIT buildup, but most were mild grade 1 (52.1%) or 2 (40.4%) reactions. Only one patient had a grade 3 reaction. None of the patients experienced a severe grade 4 reaction.
The most common grade 1 reaction was oral itch, an expected symptom of SLIT, which occurred in 82.7% of the patients.
Four patients (2.10%) received epinephrine during buildup and went to the emergency department. All these patients returned to continue SLIT without further need for epinephrine.
To test the effectiveness of SLIT, the researchers performed 50 low-dose OFCs in 20 patients. Of these food challenges, 35 (70%) were successful, and patients were asked to start daily 300-mg OIT maintenance, thus bypassing OIT buildup.
An additional nine OFCs that were unsuccessful were counseled to self-escalate from 80 mg or higher to 300 mg at home with medical guidance as needed.
“Our preliminary data of 20 patients and 50 low-dose oral food challenges suggest that an initial phase of 1-2 years of 2-mg daily SLIT therapy may be a safe and effective way to bypass the OIT buildup phase without the need for dozens of in-person visits with an allergist,” said Dr. Chan.
“So now we have the best of both worlds. We harness the safety of SLIT for the first 1-2 years, with the effectiveness of OIT for the remainder of the treatment period,” he said.
Adds to Evidence
Commenting on the study for this news organization, Julia Upton, MD, associate professor of pediatrics at the University of Toronto, Toronto, Ontario, Canada, said, “This study adds to the evidence that consistent, low exposure to food drives meaningful desensitization far above the daily dose.” Upton did not participate in the research.
“Prior prospective studies in SLIT demonstrated that small single-digit-milligram doses and time greatly increased the threshold of reaction. This real-world report suggests that a way to utilize that threshold increase is by switching to a commonly used maintenance dose of OIT,” said Dr. Upton.
“Although few patients have been assessed for the 300-mg challenge, this study is notable for the age group of 4-18 years, and that many of the patients had reacted to low doses in the past. It also shows that many families are capable of diluting and mixing their own immunotherapy solutions with store-bought foods under the guidance of an experienced allergy clinic,” she added.
“Overall, evidence is building that by various routes, initial small amounts with minimal updoses, plus the tincture of time, may be preferred to multiple frequent updosing from multiple perspectives, including safety, feasibility, cost, and medical resources. It will also be important to understand the preferences and goals of the patient and family as various regimens become more available,” Dr. Upton concluded.
The study was funded by BC Children’s Hospital Foundation. Dr. Chan reported receiving research support from DVB Technologies; has been a member of advisory boards for Pfizer, Miravo, Medexus, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi, Genzyme, Bausch Health, Avir Pharma, AstraZeneca, ALK, and Alladapt; and was a colead of the CSACI OIT guidelines. Dr. Upton reported research support/grants from Novartis, Regeneron, Sanofi, ALK Abello, DBV Technologies, CIHR, and SickKids Food Allergy and Anaphylaxis Program and fees from Pfizer, ALK Abello, Bausch Health, Astra Zeneca, and Pharming. She serves as an associate editor for Allergy, Asthma & Clinical Immunology and is on the Board of Directors of Canadian Society of Allergy and Clinical Immunology and the Healthcare Advisory Board of Food Allergy Canada.
A version of this article appeared on Medscape.com .
Sublingual immunotherapy (SLIT) is as safe and effective for high-risk older children and adolescents as oral immunotherapy (OIT) is for infants and preschoolers, according to new research.
Preliminary data from a study of more than 180 pediatric patients with multiple food allergies showed that while most patients had mild symptoms, none experienced a severe grade 4 reaction during the buildup and maintenance phase of SLIT.
In addition, 70% of those tested at the end of the treatment protocol were able to tolerate 300 mg of their allergen, a success rate nearly as high as that for OIT.
The study was published in The Journal of Allergy and Clinical Immunology: In Practice.
SLIT has been used successfully in the treatment of environmental allergens such as grass and tree pollen and dust mites. In this study, researchers decided to test SLIT’s effectiveness and safety in the treatment of food allergies in older children.
“We knew that OIT is very effective and safe in infants and toddlers, but there was literature illustrating that for older, school-age kids and adolescents, OIT is not safe enough, as those older age groups tend to have higher risk of severe reaction during treatment,” senior author Edmond Chan, MD, clinical professor of allergy at the University of British Columbia and pediatric allergist at BC Children’s Hospital, both in Vancouver, British Columbia, Canada, told this news organization. “With that knowledge, we decided to explore SLIT as another first-phase therapy for the older kids.”
The investigators recruited 188 high-risk older children aged 4-18 years for multifood SLIT. Most (61.7%) participants had multiple food allergies. Approximately 68% were male, and the population’s median age was 11.3 years.
Nearly half (48.4%) of participants had atopic dermatitis, 45.2% had asthma, 58.0% had allergic rhinitis, and 2.66% had preexisting eosinophilic esophagitis.
Most (75.0%) of the children were classified as higher risk, and 23 had a history of a grade 3 or 4 reaction before beginning SLIT.
Of the 188 children who were initially enrolled in the study, 173 (92.0%) finished their SLIT buildup phase.
Because the study started when COVID-19 pandemic restrictions were in place, the SLIT protocol mandated that patients be seen virtually. The patients’ caregivers learned how to mix and administer the required doses at home using recipes specially developed by the research team that used products bought at the grocery store.
A wide variety of food allergens were treated, including peanut, other legumes, tree nuts, sesame, other seeds, egg, cow’s milk, fish, wheat, shrimp, and other allergens.
The children built up to 2-mg protein SLIT maintenance over the course of three to five visits under nurse supervision.
After 1-2 years of daily SLIT maintenance, patients were offered a low-dose oral food challenge (OFC; cumulative dose: 300 mg of protein) with the goal of bypassing OIT buildup.
Nearly all patients (93.1%) had symptoms during SLIT buildup, but most were mild grade 1 (52.1%) or 2 (40.4%) reactions. Only one patient had a grade 3 reaction. None of the patients experienced a severe grade 4 reaction.
The most common grade 1 reaction was oral itch, an expected symptom of SLIT, which occurred in 82.7% of the patients.
Four patients (2.10%) received epinephrine during buildup and went to the emergency department. All these patients returned to continue SLIT without further need for epinephrine.
To test the effectiveness of SLIT, the researchers performed 50 low-dose OFCs in 20 patients. Of these food challenges, 35 (70%) were successful, and patients were asked to start daily 300-mg OIT maintenance, thus bypassing OIT buildup.
An additional nine OFCs that were unsuccessful were counseled to self-escalate from 80 mg or higher to 300 mg at home with medical guidance as needed.
“Our preliminary data of 20 patients and 50 low-dose oral food challenges suggest that an initial phase of 1-2 years of 2-mg daily SLIT therapy may be a safe and effective way to bypass the OIT buildup phase without the need for dozens of in-person visits with an allergist,” said Dr. Chan.
“So now we have the best of both worlds. We harness the safety of SLIT for the first 1-2 years, with the effectiveness of OIT for the remainder of the treatment period,” he said.
Adds to Evidence
Commenting on the study for this news organization, Julia Upton, MD, associate professor of pediatrics at the University of Toronto, Toronto, Ontario, Canada, said, “This study adds to the evidence that consistent, low exposure to food drives meaningful desensitization far above the daily dose.” Upton did not participate in the research.
“Prior prospective studies in SLIT demonstrated that small single-digit-milligram doses and time greatly increased the threshold of reaction. This real-world report suggests that a way to utilize that threshold increase is by switching to a commonly used maintenance dose of OIT,” said Dr. Upton.
“Although few patients have been assessed for the 300-mg challenge, this study is notable for the age group of 4-18 years, and that many of the patients had reacted to low doses in the past. It also shows that many families are capable of diluting and mixing their own immunotherapy solutions with store-bought foods under the guidance of an experienced allergy clinic,” she added.
“Overall, evidence is building that by various routes, initial small amounts with minimal updoses, plus the tincture of time, may be preferred to multiple frequent updosing from multiple perspectives, including safety, feasibility, cost, and medical resources. It will also be important to understand the preferences and goals of the patient and family as various regimens become more available,” Dr. Upton concluded.
The study was funded by BC Children’s Hospital Foundation. Dr. Chan reported receiving research support from DVB Technologies; has been a member of advisory boards for Pfizer, Miravo, Medexus, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi, Genzyme, Bausch Health, Avir Pharma, AstraZeneca, ALK, and Alladapt; and was a colead of the CSACI OIT guidelines. Dr. Upton reported research support/grants from Novartis, Regeneron, Sanofi, ALK Abello, DBV Technologies, CIHR, and SickKids Food Allergy and Anaphylaxis Program and fees from Pfizer, ALK Abello, Bausch Health, Astra Zeneca, and Pharming. She serves as an associate editor for Allergy, Asthma & Clinical Immunology and is on the Board of Directors of Canadian Society of Allergy and Clinical Immunology and the Healthcare Advisory Board of Food Allergy Canada.
A version of this article appeared on Medscape.com .
Sublingual immunotherapy (SLIT) is as safe and effective for high-risk older children and adolescents as oral immunotherapy (OIT) is for infants and preschoolers, according to new research.
Preliminary data from a study of more than 180 pediatric patients with multiple food allergies showed that while most patients had mild symptoms, none experienced a severe grade 4 reaction during the buildup and maintenance phase of SLIT.
In addition, 70% of those tested at the end of the treatment protocol were able to tolerate 300 mg of their allergen, a success rate nearly as high as that for OIT.
The study was published in The Journal of Allergy and Clinical Immunology: In Practice.
SLIT has been used successfully in the treatment of environmental allergens such as grass and tree pollen and dust mites. In this study, researchers decided to test SLIT’s effectiveness and safety in the treatment of food allergies in older children.
“We knew that OIT is very effective and safe in infants and toddlers, but there was literature illustrating that for older, school-age kids and adolescents, OIT is not safe enough, as those older age groups tend to have higher risk of severe reaction during treatment,” senior author Edmond Chan, MD, clinical professor of allergy at the University of British Columbia and pediatric allergist at BC Children’s Hospital, both in Vancouver, British Columbia, Canada, told this news organization. “With that knowledge, we decided to explore SLIT as another first-phase therapy for the older kids.”
The investigators recruited 188 high-risk older children aged 4-18 years for multifood SLIT. Most (61.7%) participants had multiple food allergies. Approximately 68% were male, and the population’s median age was 11.3 years.
Nearly half (48.4%) of participants had atopic dermatitis, 45.2% had asthma, 58.0% had allergic rhinitis, and 2.66% had preexisting eosinophilic esophagitis.
Most (75.0%) of the children were classified as higher risk, and 23 had a history of a grade 3 or 4 reaction before beginning SLIT.
Of the 188 children who were initially enrolled in the study, 173 (92.0%) finished their SLIT buildup phase.
Because the study started when COVID-19 pandemic restrictions were in place, the SLIT protocol mandated that patients be seen virtually. The patients’ caregivers learned how to mix and administer the required doses at home using recipes specially developed by the research team that used products bought at the grocery store.
A wide variety of food allergens were treated, including peanut, other legumes, tree nuts, sesame, other seeds, egg, cow’s milk, fish, wheat, shrimp, and other allergens.
The children built up to 2-mg protein SLIT maintenance over the course of three to five visits under nurse supervision.
After 1-2 years of daily SLIT maintenance, patients were offered a low-dose oral food challenge (OFC; cumulative dose: 300 mg of protein) with the goal of bypassing OIT buildup.
Nearly all patients (93.1%) had symptoms during SLIT buildup, but most were mild grade 1 (52.1%) or 2 (40.4%) reactions. Only one patient had a grade 3 reaction. None of the patients experienced a severe grade 4 reaction.
The most common grade 1 reaction was oral itch, an expected symptom of SLIT, which occurred in 82.7% of the patients.
Four patients (2.10%) received epinephrine during buildup and went to the emergency department. All these patients returned to continue SLIT without further need for epinephrine.
To test the effectiveness of SLIT, the researchers performed 50 low-dose OFCs in 20 patients. Of these food challenges, 35 (70%) were successful, and patients were asked to start daily 300-mg OIT maintenance, thus bypassing OIT buildup.
An additional nine OFCs that were unsuccessful were counseled to self-escalate from 80 mg or higher to 300 mg at home with medical guidance as needed.
“Our preliminary data of 20 patients and 50 low-dose oral food challenges suggest that an initial phase of 1-2 years of 2-mg daily SLIT therapy may be a safe and effective way to bypass the OIT buildup phase without the need for dozens of in-person visits with an allergist,” said Dr. Chan.
“So now we have the best of both worlds. We harness the safety of SLIT for the first 1-2 years, with the effectiveness of OIT for the remainder of the treatment period,” he said.
Adds to Evidence
Commenting on the study for this news organization, Julia Upton, MD, associate professor of pediatrics at the University of Toronto, Toronto, Ontario, Canada, said, “This study adds to the evidence that consistent, low exposure to food drives meaningful desensitization far above the daily dose.” Upton did not participate in the research.
“Prior prospective studies in SLIT demonstrated that small single-digit-milligram doses and time greatly increased the threshold of reaction. This real-world report suggests that a way to utilize that threshold increase is by switching to a commonly used maintenance dose of OIT,” said Dr. Upton.
“Although few patients have been assessed for the 300-mg challenge, this study is notable for the age group of 4-18 years, and that many of the patients had reacted to low doses in the past. It also shows that many families are capable of diluting and mixing their own immunotherapy solutions with store-bought foods under the guidance of an experienced allergy clinic,” she added.
“Overall, evidence is building that by various routes, initial small amounts with minimal updoses, plus the tincture of time, may be preferred to multiple frequent updosing from multiple perspectives, including safety, feasibility, cost, and medical resources. It will also be important to understand the preferences and goals of the patient and family as various regimens become more available,” Dr. Upton concluded.
The study was funded by BC Children’s Hospital Foundation. Dr. Chan reported receiving research support from DVB Technologies; has been a member of advisory boards for Pfizer, Miravo, Medexus, Leo Pharma, Kaleo, DBV, AllerGenis, Sanofi, Genzyme, Bausch Health, Avir Pharma, AstraZeneca, ALK, and Alladapt; and was a colead of the CSACI OIT guidelines. Dr. Upton reported research support/grants from Novartis, Regeneron, Sanofi, ALK Abello, DBV Technologies, CIHR, and SickKids Food Allergy and Anaphylaxis Program and fees from Pfizer, ALK Abello, Bausch Health, Astra Zeneca, and Pharming. She serves as an associate editor for Allergy, Asthma & Clinical Immunology and is on the Board of Directors of Canadian Society of Allergy and Clinical Immunology and the Healthcare Advisory Board of Food Allergy Canada.
A version of this article appeared on Medscape.com .
THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Inside the 2024 AAD Acne Guidelines: New Therapies Join Old Standbys
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
FROM AAD 2024
Semaglutide Curbs MASLD Severity in People Living With HIV
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
Semaglutide improved metabolic dysfunction–associated steatotic liver disease (MASLD) among people living with HIV, and in some cases resolved it completely, according to results from the SLIM LIVER study presented by the AIDS Clinical Trials Group (ACTG) at this year’s Conference on Retroviruses and Opportunistic Infections (CROI) 2024 Annual Meeting in Denver.
Furthermore, although muscle volume decreased with weight loss, participants did not experience significant changes in muscle quality or physical function.
‘A First’
SLIM LIVER is the first study evaluating semaglutide as a treatment of MASLD among people living with HIV.
The phase 2b, single-arm pilot study enrolled adults living with HIV who were virally suppressed and had central adiposity, insulin resistance or prediabetes, and steatotic liver disease.
Participants self-injected semaglutide weekly at increasing doses until they reached a 1-mg dose at week 4. At 24 weeks, the study team assessed changes in participants’ intra-hepatic triglyceride content using magnetic resonance imaging-proton density fat fraction.
The primary analysis results from SLIM LIVER were reported in an oral presentation, “Semaglutide Reduces Metabolic-Associated Steatotic Liver Disease in People With HIV: The SLIM LIVER Study,” on March 5 by Jordan E. Lake, MD, MSc, of UTHealth Houston.
A subgroup analysis of the study was provided in a poster, “Effects of Semaglutide on Muscle Structure and Function in the SLIM LIVER Study,” presented on March 4 by Grace L. Ditzenberger, PT, DPT, of the University of Colorado Anschutz Medical Campus in Aurora.
In the primary analysis, the median age of the 49 participants was 52 years, 43% were women (cisgender and transgender), the mean body mass index was 35, 39% were Hispanic and 33% were Black/African American, and 82% were taking antiretroviral therapy that included an integrase inhibitor.
Liver fat was reduced by an average of 31%, with 29% of participants experiencing a complete resolution (5% or less liver fat) of MASLD. They also experienced weight loss, reduced fasting blood glucose, and reduced fasting triglycerides, consistent with effects observed in studies of semaglutide in people without HIV.
The sub-analysis of the 46 participants for whom muscle measurements were available showed that muscle volume (measured in the psoas) decreased but with no significant change in physical function.
Semaglutide was generally well tolerated, with an adverse event profile similar to that seen in individuals without HIV.
The most common adverse events were gastrointestinal (ie, nausea, diarrhea, vomiting, and abdominal pain). Two participants experienced more significant adverse events possibly related to semaglutide but were able to continue in the study.
All participants completed the full 24 weeks of therapy at the originally prescribed dose.
Potential Impact
“Even at the low dose of 1 mg every week, most participants lost significant weight, and weight loss was closely associated with improvements in MASLD,” Dr. Lake said. “Additional research will assess the secondary effects of semaglutide on systemic inflammation and metabolism and determine whether semaglutide may have unique risks or benefits for people living with HIV.”
“These findings have the potential to have a significant impact on the health and quality of life of people living with HIV,” added ACTG Chair Judith Currier, MD, MSc, University of California Los Angeles.
The SLIM LIVER study was sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), with additional funding from UTHealth Houston McGovern School of Medicine. ACTG is a clinical trials network focused on HIV and other infectious diseases, funded by NIAID and collaborating institutes of the US National Institutes of Health.
No conflicts of interest were reported.
A version of this article appeared on Medscape.com.
FROM CROI 2024
Nurse-Led Strategy Reduces Cholesterol, BP in HIV
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A multicomponent strategy of nurse-led communication, home blood pressure monitoring, evidence-based treatment algorithms, and electronic health record tools improved systolic blood pressure (SBP) and non–high-density lipoprotein (non-HDL) cholesterol levels in people living with HIV.
METHODOLOGY:
- Investigators assessed if EXTRA-CVD, a nurse-led multicomponent intervention for preventing cardiovascular diseases (CVD), could effectively improve SBP and non-HDL cholesterol levels in people living with HIV whose viral replication has been controlled effectively using antiretroviral therapy.
- They recruited 297 individuals (median age, 59 years; 20.9% women) from three academic HIV clinics in the United States with an HIV-1 viral load < 200 copies/mL who were diagnosed with both hypertension and hypercholesterolemia.
- Participants were randomly assigned to either the EXTRA-CVD intervention group or a control group comprising individuals who received general prevention education.
- SBP (the primary outcome) was calculated as the mean of two SBP measurements obtained 1 minute apart, and non-HDL cholesterol (the secondary outcome) was calculated as total cholesterol minus HDL cholesterol.
TAKEAWAY:
- Participants in the intervention vs control group reported having significantly lower SBP as early as 4 months after the nurse-led strategy (mean difference, −6.4 mm Hg; P = .002), with the improvements sustaining until 12 months (mean difference, −4.2 mm Hg; P = .04).
- At 12 months, participants in the intervention group showed a 16.9-mg/dL (P < .001) reduction in non-HDL cholesterol levels compared with those in the control group.
- The nurse-led strategy led to a greater reduction in SBP in women with HIV vs men living with HIV (5.9 mm Hg greater SBP difference at 12 months), with the difference being clinically meaningful but not statistically significant.
- This nurse-led strategy did not increase the risk for adverse events in people living with HIV.
IN PRACTICE:
“Although the EXTRA-CVD intervention was limited to BP and cholesterol, nurse-led case management might be beneficial for a range of other primary care conditions in HIV clinics. If HIV clinics choose to implement EXTRA-CVD, they might consider adding staff trained in other chronic comorbidities and/or health promotion activities,” the authors noted.
SOURCE:
This study was led by Christopher T. Longenecker, MD, University of Washington School of Medicine, Seattle, and published online on March 5, 2024, in JAMA Network Open.
LIMITATIONS:
Because this trial was conducted at well-resourced, major academic HIV clinics, the results may not be applicable to other populations, such as smaller community-based clinics or HIV care outside the United States. The sensitivity analyses performed in this study may not have fully accounted for the bias introduced by the differential attrition in the intervention group.
DISCLOSURES:
This study was supported by grants from the National Institutes of Health (NIH). The authors declared receiving grants and personal fees from or having other ties with the NIH and other sources.
A version of this article appeared on Medscape.com.