User login
COVID-19 in pregnant women and the impact on newborns
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
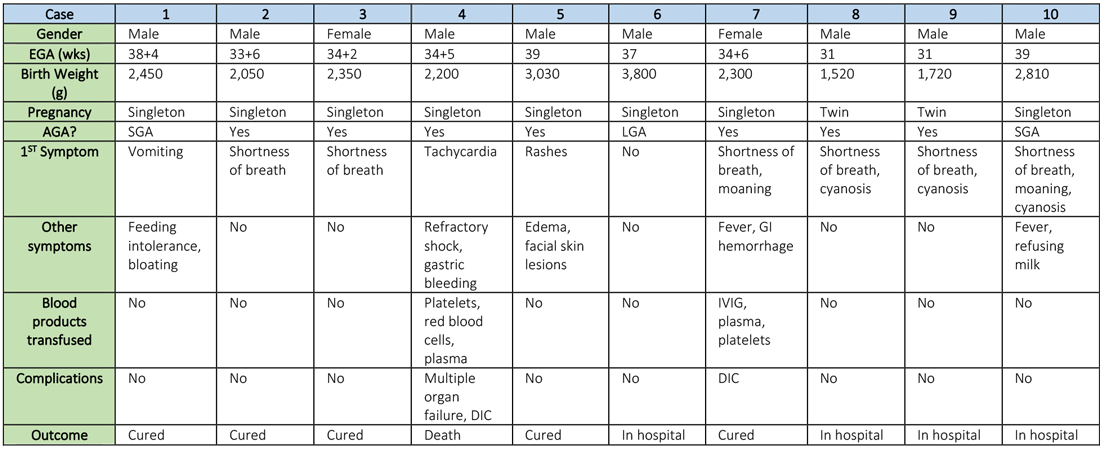
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Clinical question: How does infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pregnant mothers affect their newborns?
Background: A novel coronavirus, now named SARS-CoV-2 by the World Health Organization (previously referred to as 2019-nCoV), is currently causing a worldwide pandemic. It is believed to have originated in Hubei province, China, but is now rapidly spreading in other countries. Although its effects are most severe in the elderly, SARS-CoV-2 has been infecting younger patients, including pregnant women. The effect of COVID-19, the disease caused by SARS-CoV-2, in pregnant women on their newborn children, is unknown, as is the nature of perinatal transmission of SARS-CoV-2.
Study design: Retrospective analysis.
Setting: Five hospitals in Hubei province, China.
Synopsis: Researchers retrospectively analyzed the clinical features and outcomes of 10 neonates (including two twins) born to nine mothers with confirmed SARS-CoV-2 infection in five hospitals in Hubei province, China, during Jan. 20–Feb. 5, 2020. The mothers were, on average, 30 years of age, but their prior state of health was not described. SARS-CoV-2 infection was confirmed in eight mothers by SARS-CoV-2 nucleic acid testing (NAT). The twins’ mother was diagnosed with COVID-19 based on chest CT scan showing viral interstitial pneumonia with other causes of fever and lung infection being “excluded,” despite a negative SARS-CoV-2 NAT test.
Symptoms occurred in the following:
- Before delivery in four mothers, three of whom were treated with oseltamivir (Tamiflu) after delivery.
- On the day of delivery in two mothers, one of whom was treated with oseltamivir and nebulized inhaled interferon after delivery.
- After delivery in three mothers.
Seven mothers delivered by cesarean section and two by vaginal delivery. Prenatal complications included intrauterine distress in six mothers, premature rupture of membranes in three (5-7 hours before onset of true labor), abnormal amniotic fluid in two, “abnormal” umbilical cord in two, and placenta previa in one.
The neonates born to these mothers included two females and eight males; four were full-term and six were premature (degree of prematurity not described). Symptoms first observed in these newborns included shortness of breath (six), fevers (two), tachycardia (one), and vomiting, feeding intolerance, “bloating,” refusing milk, and “gastric bleeding.” Chest radiographs were abnormal in seven newborns, including evidence of “infection” (four), neonatal respiratory distress syndrome (two), and pneumothorax (one). Two cases were described in detail:
- A neonate delivered at 34+5/7 weeks gestational age, was admitted due to shortness of breath and “moaning.” Eight days later, the neonate developed refractory shock, multiple organ failure, disseminated intravascular coagulation requiring transfusions of platelets, red blood cells, and plasma. He died on the ninth day.
- A neonate delivered at 34+6 weeks gestational age and was admitted 25 minutes after delivery due to shortness of breath and “moaning.” He required 2 days of noninvasive support/oxygen therapy and was observed to later develop “oxygen fluctuations” and thrombocytopenia at 3 days of life. The neonate was treated with “respiratory support,” intravenous immunoglobulin, transfusions of platelets and plasma, hydrocortisone (5 mg/kg per day for 6 days), low-dose heparin (2 units/kg per hr for 6 days), and low molecular weight heparin (2 units/kg per hr for 6 days). He was described to be “cured” 15 days later.
All nine neonates underwent pharyngeal swabs for SARS-CoV-2 NAT, and all were negative.
Bottom line: Although data are currently very limited, neonates born to mothers with COVID-19 appear to be at risk for adverse outcomes, including fetal distress, respiratory distress, thrombocytopenia associated with abnormal liver function, and death. There was no evidence of vertical transmission in this study.
Citation: Zhu H et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020 Feb;9(1):51-60.
Dr. Chang is chief of pediatric hospital medicine at Baystate Children’s Hospital in Springfield, Mass., and associate professor of pediatrics at the University of Massachusetts, also in Springfield.
Hospitalist movers and shakers – March 2020
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
Swati Mehta, MD, recently was honored as the lone hospitalist on the National Executive Physician Council for Beryl Institute (Nashville, Tenn.). Only 24 total physicians were selected to the council. Dr. Mehta also was named the 2019 Distinguished Physician Award winner at Vituity (Emeryville, Calif.), where she is the executive director of quality and performance.
A nocturnist at Sequoia Hospital (Redwood City, Calif.), Dr. Mehta is a member of the Society of Hospital Medicine’s Patient Experience interest group.
Shannon Phillips, MD, SFHM, has been named to the National Quality Forum’s Board of Directors for 2020. The chief patient experience officer at Intermountain Healthcare (Salt Lake City, Utah), she also is a recent member of the Performance Measurement and Reporting Committee.
Dr. Phillips, whose focus at Intermountain is on catalyzing safety, quality, and experience of care, was named a 2018 Becker’s Hospital Review Hospital and Health System CXO to Know. Previously, she worked at the Cleveland Clinic, where she was its first patient safety officer and an associate chief quality officer.
Vineet Arora, MD, MHM, has been elected as a new member of the National Academy of Medicine, which honors pioneering scientific and professional achievements within the field.
An academic hospitalist at the University of Chicago, Dr. Arora specializes in improving the learning environment for her medical trainees, as well as maintaining a high level of quality, safety, and care for patients. She also is considered an expert in using social media and other new technology to enhance medical education.
The National Academy of Medicine stated that Dr. Arora’s honor was “for pioneering work to optimize resident fatigue and patient safety during long shifts.”
Edmondo Robinson, MD, SFHM, has been named senior vice president and chief digital innovation officer at Moffitt Cancer Center (Tampa, Fla.). The chief digital innovation officer position is a newly created position that the veteran physician has assumed. Dr. Robinson has 16 years’ experience in clinical and technological work.
In this new position, Dr. Robinson, a practicing academic hospitalist, will head Moffitt’s digital innovation while looking to create and test new services, programs, partnerships, and technologies.
Dr. Robinson comes to Moffitt after serving as chief transformation officer and senior vice president at ChristianaCare (Wilmington, Del.). A teacher at Sidney Kimmel Medical College, Philadelphia, Dr. Robinson was the founding medical director of ChristianaCare Hospitalist Partners.
Relias Healthcare (Tupelo, Miss.) has begun providing hospitalist and emergency medicine services for North Mississippi Health Services’ Gilmore-Amory Trauma Center. Relias, a multistate company that has partnered with more than 150 providers, now has a role at four different North Mississippi Health Services facilities.
Mednax (Sunrise, Fla.) has added Arcenio Chacon and Associated Pediatricians of Homestead, a pediatric critical care and hospital practice, as an affiliate.
Chacon and Associated Pediatricians are based out of Miami and have served Baptist Health South Florida for more than 25 years. The four-physician practice provides critical care and pediatric hospitalist services at Baptist Children’s Hospital (Miami) and hospitalist services at Miami Cancer Institute and Homestead (Fla.) Hospital.
Mednax is a health solutions company that provides subspecialty service in all 50 states. Established in 1979, Mednax partners with hospitals, health systems, and health care facilities to offer clinical services, as well as revenue cycle management, patient engagement, and perioperative improvement consulting services.
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Study identifies two distinct type 1 diabetes ‘endotypes’
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
Two histologically distinct “endotypes” of type 1 diabetes, T1DE1 and T1DE2, have been identified in children based on their age at diagnosis
The findings were published online March 15 in Diabetologia by Pia Leete, PhD, of the Institute of Biomedical and Clinical Science, University of Exeter Medical School, UK, and colleagues.
The results suggest that the immune attack is far more aggressive and the islets more inflamed in the younger-onset group (T1DE1) and less intense in the older-onset group (T1DE2), the authors explain.
“We’re extremely excited to find evidence that type 1 diabetes is two separate conditions: T1DE1 and T1DE2. The significance of this could be enormous in helping us to understand what causes the illness and in unlocking avenues to prevent future generations of children from getting type 1 diabetes,” said senior author Noel G. Morgan, PhD, also of the University of Exeter, in a statement.
Morgan added that the discovery “might also lead to new treatments if we can find ways to reactivate dormant insulin-producing cells in the older age group. This would be a significant step towards the holy grail to find a cure for some people.”
Endotypes can inform immune interventions
The study involved an immunohistological analysis of proinsulin and insulin distribution in the islets of pancreas samples recovered from 19 youth who died soon after (<2 years) onset of type 1 diabetes and from 13 with onset more than 5 years prior to harvesting. Those results were compared with C-peptide and proinsulin measurements in 171 living individuals with type 1 diabetes of longer than 5 years duration.
The Exeter team has previously reported that the immune cell profiles in the inflamed islets of children younger than 7 years of age soon after the diagnosis of type 1 diabetes seem to be distinctly different for those in children aged 13 and older at diagnosis. The younger group at diagnosis (termed “T1DE1”) retained a lower proportion of insulin-containing islets than did the older-onset group (“T1DE2”).
Those aged 7-12 at diagnosis could belong to either group, but there was no continuum. Rather, they appeared to align distinctly with one or the other “endotype,” Leete and colleagues say.
In the new analysis, proinsulin processing was aberrant to a much greater degree among children diagnosed with type 1 diabetes prior to age 7 years than among those diagnosed after age 12 years, with the profiles of proinsulin processing correlating with the previously defined immune cell profiles.
For those aged 7-12, the proinsulin distribution in islets directly correlated with their immune phenotypes, either T1DE1 or T1DE2.
And among the living patients, circulating proinsulin:C-peptide ratios were elevated in the <7-year onset group compared with the ≥13-year group, even 5 years after diagnosis.
“Together, these data imply that, when considered alongside age at diagnosis, measurement of the ratio of proinsulin to C-peptide may represent a convenient biomarker to distinguish the endotypes defined here,” Leete and colleagues say.
The two-endotype proposal isn’t meant to suggest that “a simple dichotomy will ultimately be sufficient to account for the entire heterogeneity seen in people developing type 1 diabetes,” the authors stress. Rather, additional endotypes will likely be defined as more variables are considered.
They write, “Recognition of such differences should inform the design of future immunotherapeutic interventions designed to arrest disease progression.”
The research was sponsored by Diabetes UK and JDRF.
This article first appeared on Medscape.com.
Should physicians with OUDs return to practice after treatment?
New review points to importance of sustained recovery
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
New review points to importance of sustained recovery
New review points to importance of sustained recovery
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
HPV vaccine-chemo combo prolongs cervical cancer survival
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: There may be an overall survival benefit of combining human papillomavirus vaccination with standard-of-care chemotherapy for cervical cancer.
Major finding: The median overall survival was 16.8 months for patients with immune responses to the vaccine that were higher than the median and 11.2 months for patients with immune responses lower than the median (hazard ratio, 0.491; P = .012).
Study details: A phase 1/2 study of 77 women with HPV16-positive advanced, metastatic, or recurrent cervical cancer.
Disclosures: The study was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
Source: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
COVID-19 guidance for children’s health care providers
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com
We are in uncharted waters with national and local states of emergency, schools and most activities being shut down, and rapidly evolving strategies on managing the COVID-19 outbreak. Everyone’s anxiety is appropriately high. As health care providers for children, you are facing changes in your personal life at home and in practice, likely including setting up televisits, trying to assess which patients to see, managing staffing challenges, and facing potential cash flow issues as expenses continue but revenue may fall short. And, of course, you will address a host of novel questions and concerns from the families you care for.
Your top priorities are to stay calm while offering clear recommendations on testing, quarantine, and treatment with guidance from our federal and local public health agencies. By providing clear guidance on the medical issues, you will offer substantial reassurance to families. But even with a medical plan in place, this remains a confusing and anxiety-provoking moment, one without much precedent in most people’s lives or in our national experience. Our aim is to complement that guidance by offering you some principles to help families manage the stress and anxiety that the disruptions and uncertainties that this public health emergency has created.
Offer clear, open, regular, and child-centered communication
If you have an email mailing list of your parents, you may want to summarize information you are gathering with a note they can expect at a specified time each day. You could request them to email you questions that then can be included as an FAQ (frequently asked questions).
Most children will have noticed people wearing face masks, or dramatic scenes on the news with hospital workers in full protective gear, breathlessly reporting growing numbers of the infected and the deceased. At a minimum, they are being commanded to wash hands and to not touch their faces (which is challenging enough for adults!), and are probably overhearing conversations about quarantines and contagion as well as family concerns about jobs and family finances. Many children are managing extended school closures and some are even managing the quarantine or serious illness of a loved one. When children overhear frightening news from distressed adults, they are going to become anxious and afraid themselves. Parents should remember to find out what their children have seen, heard, or understood about what is going on, and they should correct misinformation or misunderstandings with clear explanations. They also should find out what their children are curious about. “What has you wondering about that?” is a great response when children have questions, in order to make sure you get at any underlying worry.
It is fine to not have an answer to every question. It is difficult to offer clear explanations about something that we don’t yet fully understand, and it is fine to acknowledge what we don’t know. “That’s a great question. Let’s look together at the CDC [Centers for Disease Control and Prevention] website.” Offering to look for answers or information together can be a powerful way to model how to handle uncertainty. And always couch answers with appropriate (not false) reassurance: “Children and young adults appear to be very safe from this illness, but we want to take care to protect those that are older or already sick.”
Remember most children set their anxiety level based on their parent’s anxiety, and part of being child centered in your communication includes offering information in an age-appropriate manner. Preschool-aged children (up to 5 years) still have magical thinking. They are prone to finding masks and gowns scary and to assume that school stopping may be because they did something wrong. Tell them about the new illness, and about the doctors and officials working hard to keep people safe. Reassure them about all of the adults working hard together to understand the illness and take care of people who are sick. Their sense of time is less logical, so you may have to tell them more than once. Reassure them that children do not get very sick from this illness, but they can carry and spread it, like having paint on their hands, so they need to wash their hands often to take good care of other people.
School-age children (aged roughly 5-12 years) are better equipped cognitively to understand the seriousness of this outbreak. They are built to master new situations, but are prone to anxiety as they don’t yet have the emotional maturity to tolerate uncertainty or unfairness. Explain what is known without euphemisms, be truly curious about what their questions are, and look for answers together. Often what they need is to see you being calm in the face of uncertainty, bearing the strong feelings that may come, and preserving curiosity and compassion for others.
Adolescents also will need all of this support, and can be curious about more abstract implications (political, ethical, financial). Do not be surprised when they ask sophisticated questions, but still are focused on the personal disruptions or sacrifices (a canceled dance or sports meet, concerns about academic performance). Adolescence is a time of intense preoccupation with their emerging identity and relationships; it is normal for them to experience events in a way that may seem selfish, especially if it disrupts their time with friends. Remind parents to offer compassion and validation, while acknowledging that shared sacrifice and discomfort are a part of every individual’s experience when a society must respond to such a large challenge.
Be mindful of children’s vulnerabilities
Being child centered goes beyond thinking about their age and developmental stage. Parents are the experts on their children and will know about any particular vulnerabilities to the stresses of this serious outbreak. Children who are prone to anxiety or suffer from anxiety disorders may be more prone to silent worry. It is especially important to check in with them often, find out what they know and what they are worried about, and remind them to “never worry alone.” It also is important to continue with any recommended treatment, avoiding accommodation of their anxieties, except when it is required by public health protocols (i.e., staying home from school). Children with developmental disabilities may require additional support to change behaviors (hand washing) and may be more sensitive to changes in routine. And children with learning disabilities or special services in school may require additional support or structure during a prolonged period at home.
Preserve routines and structure
Routines and predictability are important to the sense of stability and well-being of most children (and adults). While disruptions are unavoidable, preserve what routines you can, and establish some new ones. For children who are out of school for several weeks, set up a consistent home routine, with a similar wake-up and bedtime, and a “school schedule.” There may be academic activities like reading or work sheets. If the parents’ work is disrupted, they can homeschool, shoring up weak academic areas or enhancing areas of interest. Be sure to preserve time for physical activity and social connections within this new framework. Social time does not require physical proximity, and can happen by screen or phone. Physical activity should be outside if at all possible. Predictability, preserved expectations (academic and otherwise), physical exercise, social connection, and consistent sleep will go a long way in protecting everyone’s ability to manage the disruptions of this epidemic.
Find opportunity in the disruption
Many families have been on a treadmill of work, school, and activities that have left little unscheduled time or spontaneity. Recommend looking at this disruption as a rare opportunity to slow down, spend time together, listen, learn more about one another, and even to have fun. Families could play board games, card games, watch movies together, or even read aloud. They might discover it is the time to try new hobbies (knitting, learning a new language or instrument), or to teach each other new skills. You might learn something new, or something new about your children. You also will offer a model of finding the opportunity in adversity, and even offer them some wonderful memories from a difficult time.
Take care of the vulnerable and ease others’ hardships
Without a doubt, this will be a difficult time for many people, medically, financially, and emotionally. One powerful strategy to build resilience in our children and strengthen our communities is to think with children about ways to help those who are most at risk or burdened by this challenge. Perhaps they want to make cards or FaceTime calls to older relatives who may be otherwise isolated. They may want to consider ways to support the work of first responders, even just with appreciation. They may want to reach out to elderly neighbors and offer to get groceries or other needed supplies for them. Balancing appropriate self-care with a focus on the needs of those who are more vulnerable or burdened than ourselves is a powerful way to show our children how communities pull together in a challenging time; enhance their feeling of connectedness; and build resilience in them, in our families, and in our communities.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@mdedge.com
Prospective algorithm favors vaginal hysterectomy
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
based on data from a prospective study of 365 patients.
“Total vaginal hysterectomy is the most cost-effective route, with a low complication rate, and, therefore, should be performed when feasible,” wrote Jennifer J. Schmitt, DO, of the Mayo Clinic, Rochester, Minn., and colleagues.
However, algorithms to support the decision to choose vaginal hysterectomy are not widely used, they said.
To assess the optimal surgical route for hysterectomy, the researchers devised a prospective algorithm and decision tree based on history of laparotomy, uterine size, and vaginal access. The results of their study were published in Obstetrics & Gynecology.
The study population included 365 women aged 18 years and older who underwent hysterectomies between Nov. 24, 2015, and Dec. 31, 2017, at a single center. A total of 202 patients (55%) met criteria for a total vaginal hysterectomy using the algorithm, and 57 (15.6%) were assigned to have an examination under anesthesia followed by total vaginal hysterectomy, for a total of 259 expected vaginal hysterectomies. Ultimately, 211 (81.5%) of the patients identified as being the best candidates for having a vaginal hysterectomy underwent the procedure. Almost all of the procedures – 99.1% – were completed successfully.
The algorithm predicted that 52 patients were expected to have an examination under anesthesia followed by a robot-assisted total laparoscopic hysterectomy and 54 were expected to have an abdominal, robotic, or laparoscopic hysterectomy. A total of 46 procedures (44 robotic, when vaginal was expected and 2 abdominal, when vaginal was expected) deviated to a more invasive route than prescribed by the algorithm, and 7 procedures deviated from the algorithm-predicted robotic or abdominal procedure to total vaginal hysterectomy.
Approximately 95% of the patients were discharged within 24 hours of surgery. These patients included 7 who had vaginal surgery when a more invasive method was predicted and did not experience intraoperative complications or Accordion grade 3 complications.
“Prospective algorithm use predicts that 55.3% of all hysterectomies were expected to have an a priori total vaginal hysterectomy, which is higher than the actual total vaginal hysterectomy rate of 11.5% reported previously,” the researchers noted, and they added that vaginal hysterectomy would be associated with cost savings of $657,524 if the total hysterectomy rate was 55% instead of 11%.
The study findings were limited by several factors including an expertise bias at the center where the study was conducted, as well as the small number of patients with algorithm deviations or poor outcomes, and the lack of a control group, the researchers noted. However, the results support the use of the algorithm “in combination with educating gynecologic surgeons about the feasibility of vaginal surgery,” they said.
“Prospective use of this algorithm nationally may increase the rate of total vaginal hysterectomy and decrease health care delivery costs,” they concluded.
“The American College of Obstetricians and Gynecologists continues to recommend vaginal hysterectomy as the approach of choice whenever feasible, and although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, the rate of vaginal hysterectomy in the United States has continued to decline,” Arnold P. Advincula, MD, of Columbia University Medical Center, New York, wrote in an accompanying editorial.
Many variables beyond clinical will determine the optimal hysterectomy route, Dr. Advincula said.
“Although historical evidence demonstrates that vaginal hysterectomy is associated with better outcomes when compared with other approaches, a multitude of studies now exist that challenge this notion. Given the financial implications and overall costs of care with surgical complications and 30-day readmissions, experienced high-volume surgeons using all available routes have shown robotics to be the best surgical approach in terms of fewer postoperative complications and lowest 30-day readmission rates,” he noted. However, “one should not split hairs and subtly pit one minimally invasive option against another, but instead should work toward the goal of minimizing laparotomy, which is still performed at a high rate,” Dr. Advincula emphasized.
The study was supported in part by the National Center for Advancing Translational Science. Dr. Schmitt had no financial conflicts to disclose. Dr. Advincula disclosed serving as a consultant for AbbVie, Baxter, ConMed, Eximis Surgical, Intuitive Surgical, and Titan Medical, and performing consultancy work and receiving royalties from Cooper Surgical.
SOURCES: Schmitt JJ et al. Obstet Gynecol. 2020;135:761-9. doi: 10.1097/AOG.0000000000003725; Advincula A. Obstet Gynecol. 2020;135:759-60. doi: doi: 10.1097/AOG.0000000000003814.
FROM OBSTETRICS & GYNECOLOGY
Physicians and health systems can reduce fear around COVID-19
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.
A message from a Chief Wellness Officer
We are at a time, unfortunately, of significant public uncertainty and fear of “the coronavirus.” Mixed and inaccurate messages from national leaders in the setting of delayed testing availability have heightened fears and impeded a uniformity in responses, medical and preventive.
Despite this, physicians, nurses, and other health professionals across the country, and in many other countries, have been addressing the medical realities of this pandemic in a way that should make every one of us health professionals proud – from the Chinese doctors and nurses to the Italian intensivists and primary care physicians throughout many countries who have treated patients suffering from, or fearful of, a novel disease with uncertain transmission characteristics and unpredictable clinical outcomes.
It is now time for physicians and other health providers in the United States to step up to the plate and model appropriate transmission-reducing behavior for the general public. This will help reduce the overall morbidity and mortality associated with this pandemic and let us return to a more normal lifestyle as soon as possible. Physicians need to be reassuring but realistic, and there are concrete steps that we can take to demonstrate to the general public that there is a way forward.
First the basic facts. The United States does not have enough intensive care beds or ventilators to handle a major pandemic. We will also have insufficient physicians and nurses if many are quarantined. The tragic experience in Italy, where patients are dying from lack of ventilators, intensive care facilities, and staff, must not be repeated here.
Many health systems are canceling or reducing outpatient appointments and increasingly using video and other telehealth technologies, especially for assessing and triaging people who believe that they may have become infected and are relatively asymptomatic. While all of the disruptions may seem unsettling, they are actually good news for those of us in healthcare. Efforts to “flatten the curve” will slow the infection spread and help us better manage patients who become critical.
So, what can physicians do?
- Make sure you are getting good information about the situation. Access reliable information and data that are widely available through the Centers for Disease Control and Prevention, the National Institutes of Health, and the World Health Organization. Listen to professional news organizations, local and national. Pass this information to your patients and community.
- Obviously, when practicing clinically, follow all infection control protocols, which will inevitably change over time. Make it clear to your patients why you are following these protocols and procedures.
- Support and actively promote the public health responses to this pandemic. Systematic reviews of the evidence base have found that isolating ill persons, testing and tracing contacts, quarantining exposed persons, closing schools and workplaces, and avoiding crowding are more effective if implemented immediately, simultaneously (ie, school closures combined with teleworking for parents), and with high community compliance.
- Practice social distancing so that you remain as much in control as you can. This will make you feel psychologically better and safer, as well as reduce the risk for transmission. Take the essential precautionary measures that we are all being asked to take. Wash your hands. Do not shake hands. Clean shared items. Do not go to large public gatherings. Minimize large group travel as much as you can. Use video to see your patients or your own doctor.
- Connect and reconnect with people you trust and love. See your family, your partner, your children, your friends. Speak to them on the phone and nourish those relationships. See how they feel and care for each other. They will be worried about you. Reassure them. Be in the moment with them and use the importance of these relationships to give yourself a chance not to overthink any fears you might have.
- Look after yourself physically. Physical fitness is good for your mental health. While White House guidelines suggest avoiding gyms, you can still enjoy long walks and outdoor activities. Take the weekend off and don’t work excessively. Sleep well – at least 7-8 hours. which has a series of really excellent meditation and relaxation tools.
- Do not panic. Uncertainty surrounding the pandemic makes all of us anxious and afraid. It is normal to become hypervigilant, especially with our nonstop media. It is normal to be concerned when we feel out of control and when we are hearing about a possible future catastrophe, especially when fed with differing sets of information from multiple sources and countries.
- Be careful with any large decisions you are making that may affect the lives of yourself and your loved ones. Think about your decisions and try to take the long view; and run them by your spouse, partner, or friends. This is not a time to be making sudden big decisions that may be driven unconsciously, in part at least, by fear and anxiety.
- Realize that all of these societal disruptions are actually good for us in health care, and they help your family and friends understand the importance of slowing the disease’s spread. That’s good for health care and good for everyone.
Finally, remember that “this is what we do,” to quote Doug Kirk, MD, chief medical officer of UC Davis Health. We must look after our patients. But we also have to look after ourselves so that we can look after our patients. We should all be proud of our work and our caring. And we should model our personal behavior to our patients and to our families and friends so that they will model it to their community networks. That way, more people will keep well, and we will have more chance of “flattening the curve” and reducing the morbidity and mortality associated with COVID-19.
Peter M. Yellowlees, MBBS, MD, is a professor in the Department of Psychiatry at the University of California, Davis. He is a longtime Medscape contributor.
This article first appeared on Medscape.com.