User login
ISCHEMIA-CKD: No benefit for coronary revascularization in advanced CKD with stable angina
PHILADELPHIA – Coronary revascularization in patients with stage 4 or 5 chronic kidney disease (CKD) and stable ischemic heart disease accomplishes nothing constructive, according to clear-cut results of the landmark ISCHEMIA-CKD trial.
In this multinational randomized trial including 777 stable patients with advanced CKD and moderate or severe myocardial ischemia on noninvasive testing, an early invasive strategy didn’t reduce the risks of death or ischemic events, didn’t provide any significant improvement in quality of life metrics, and resulted in an increased risk of stroke, Sripal Bangalore, MD, reported at the American Heart Association scientific sessions.
“ said Dr. Bangalore, professor of medicine and director of complex coronary intervention at New York University.
This was easily the largest-ever trial of an invasive versus conservative coronary artery disease management strategy in this challenging population. For example, in the COURAGE trial of optimal medical therapy with or without percutaneous coronary intervention for stable coronary disease (N Engl J Med. 2007 Apr 12;356[15]:1503-16), only 16 of the 2,287 participants had an estimated glomerular filtration rate below 30 mL/min per 1.73 m2.
Of note, the participating study sites received special training aimed at minimizing the risk of acute kidney injury after cardiac catheterization in this at-risk population. The training included utilization of a customized left ventricular end diastolic volume–based hydration protocol, guidance on how to perform percutaneous coronary intervention with little or no contrast material, and encouragement of a heart/kidney team approach involving a cardiologist, nephrologist, and cardiovascular surgeon. This training really paid off, with roughly a 7% incidence of acute kidney injury after catheterization.
“The expected rate in such patients would be 30%-60%,” according to Dr. Bangalore.
The primary endpoint in ISCHEMIA-CKD was the rates of death or MI during 3 years of prospective follow-up. This occurred in 36.7% of patients randomized to optimal medical therapy alone and 36.4% of those randomized to an early invasive strategy. Similarly, the major secondary endpoint, comprising death, MI, and hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest, was also a virtual dead heat, occurring in about 39% of subjects. More than 27% of study participants had died by the 3-year mark regardless of how their coronary disease was managed.
The adjusted risk of stroke was 3.76-fold higher in the invasive strategy group; however, the most strokes were not procedurally related, and the explanation for the significantly increased risk in the invasively managed group remains unknown, the cardiologist said.
John A. Spertus, MD, who led the quality of life assessment in ISCHEMIA-CKD and in the 5,129-patient parent ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches), also presented at the AHA scientific sessions. He reported that, unlike in the parent study, there was no hint of a meaningful long-term quality of life benefit for revascularization plus optimal medical therapy, compared with that of optimal medical therapy alone, in ISCHEMIA-CKD.
“We have greater than 93% confidence that there is more of a quality of life effect in patients without advanced CKD than in patients with advanced CKD,” said Dr. Spertus, director of health outcomes research at the Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri, Kansas City.
Discussant Glenn L. Levine, MD, hailed ISCHEMIA-CKD as “a monumental achievement,” an ambitious, well-powered study with essentially no loss of follow-up during up to 4 years. It helps fill an utter void in the AHA/American College of Cardiology guidelines, which to date offer no recommendations at all regarding revascularization in patients with CKD because of a lack of evidence. And he considers ISCHEMIA-CKD to be unequivocally practice changing and guideline changing.
“Based on the results of ISCHEMIA-CKD, I will generally not go searching for ischemia and [coronary artery disease] in most severe and end-stage CKD patients, absent marked or unacceptable angina, and will treat them with medical therapy alone,” declared Dr. Levine, professor of medicine at Baylor College of Medicine and director of the cardiac care unit at the Michael E. DeBakey VA Medical Center, both in Houston.
He offered a few caveats. Excluded from participation in ISCHEMIA-CKD were patients with acute coronary syndrome, significant heart failure, or unacceptable angina despite optimal medical therapy at baseline, so the study results don’t apply to them. Also, the acute kidney injury rate after intervention was eye-catchingly low.
Dr. Bangalore discussed the ISCHEMIA-CKD trial and outcomes in a video interview with Medscape’s Tricia Ward.
“It seems unlikely that all centers routinely do and will exactly follow the very careful measures to limit contrast and minimize contrast nephropathy used in this study,” Dr. Levine commented.
ISCHEMIA-CKD, like its parent ISCHEMIA trial, was funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore reported having no relevant financial interests. Dr. Spertus holds the copyright for the Seattle Angina Questionnaire, which was used for quality of life measurement in the trials. Dr. Levine disclosed that he has no relations with industry or conflicts of interest.
PHILADELPHIA – Coronary revascularization in patients with stage 4 or 5 chronic kidney disease (CKD) and stable ischemic heart disease accomplishes nothing constructive, according to clear-cut results of the landmark ISCHEMIA-CKD trial.
In this multinational randomized trial including 777 stable patients with advanced CKD and moderate or severe myocardial ischemia on noninvasive testing, an early invasive strategy didn’t reduce the risks of death or ischemic events, didn’t provide any significant improvement in quality of life metrics, and resulted in an increased risk of stroke, Sripal Bangalore, MD, reported at the American Heart Association scientific sessions.
“ said Dr. Bangalore, professor of medicine and director of complex coronary intervention at New York University.
This was easily the largest-ever trial of an invasive versus conservative coronary artery disease management strategy in this challenging population. For example, in the COURAGE trial of optimal medical therapy with or without percutaneous coronary intervention for stable coronary disease (N Engl J Med. 2007 Apr 12;356[15]:1503-16), only 16 of the 2,287 participants had an estimated glomerular filtration rate below 30 mL/min per 1.73 m2.
Of note, the participating study sites received special training aimed at minimizing the risk of acute kidney injury after cardiac catheterization in this at-risk population. The training included utilization of a customized left ventricular end diastolic volume–based hydration protocol, guidance on how to perform percutaneous coronary intervention with little or no contrast material, and encouragement of a heart/kidney team approach involving a cardiologist, nephrologist, and cardiovascular surgeon. This training really paid off, with roughly a 7% incidence of acute kidney injury after catheterization.
“The expected rate in such patients would be 30%-60%,” according to Dr. Bangalore.
The primary endpoint in ISCHEMIA-CKD was the rates of death or MI during 3 years of prospective follow-up. This occurred in 36.7% of patients randomized to optimal medical therapy alone and 36.4% of those randomized to an early invasive strategy. Similarly, the major secondary endpoint, comprising death, MI, and hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest, was also a virtual dead heat, occurring in about 39% of subjects. More than 27% of study participants had died by the 3-year mark regardless of how their coronary disease was managed.
The adjusted risk of stroke was 3.76-fold higher in the invasive strategy group; however, the most strokes were not procedurally related, and the explanation for the significantly increased risk in the invasively managed group remains unknown, the cardiologist said.
John A. Spertus, MD, who led the quality of life assessment in ISCHEMIA-CKD and in the 5,129-patient parent ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches), also presented at the AHA scientific sessions. He reported that, unlike in the parent study, there was no hint of a meaningful long-term quality of life benefit for revascularization plus optimal medical therapy, compared with that of optimal medical therapy alone, in ISCHEMIA-CKD.
“We have greater than 93% confidence that there is more of a quality of life effect in patients without advanced CKD than in patients with advanced CKD,” said Dr. Spertus, director of health outcomes research at the Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri, Kansas City.
Discussant Glenn L. Levine, MD, hailed ISCHEMIA-CKD as “a monumental achievement,” an ambitious, well-powered study with essentially no loss of follow-up during up to 4 years. It helps fill an utter void in the AHA/American College of Cardiology guidelines, which to date offer no recommendations at all regarding revascularization in patients with CKD because of a lack of evidence. And he considers ISCHEMIA-CKD to be unequivocally practice changing and guideline changing.
“Based on the results of ISCHEMIA-CKD, I will generally not go searching for ischemia and [coronary artery disease] in most severe and end-stage CKD patients, absent marked or unacceptable angina, and will treat them with medical therapy alone,” declared Dr. Levine, professor of medicine at Baylor College of Medicine and director of the cardiac care unit at the Michael E. DeBakey VA Medical Center, both in Houston.
He offered a few caveats. Excluded from participation in ISCHEMIA-CKD were patients with acute coronary syndrome, significant heart failure, or unacceptable angina despite optimal medical therapy at baseline, so the study results don’t apply to them. Also, the acute kidney injury rate after intervention was eye-catchingly low.
Dr. Bangalore discussed the ISCHEMIA-CKD trial and outcomes in a video interview with Medscape’s Tricia Ward.
“It seems unlikely that all centers routinely do and will exactly follow the very careful measures to limit contrast and minimize contrast nephropathy used in this study,” Dr. Levine commented.
ISCHEMIA-CKD, like its parent ISCHEMIA trial, was funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore reported having no relevant financial interests. Dr. Spertus holds the copyright for the Seattle Angina Questionnaire, which was used for quality of life measurement in the trials. Dr. Levine disclosed that he has no relations with industry or conflicts of interest.
PHILADELPHIA – Coronary revascularization in patients with stage 4 or 5 chronic kidney disease (CKD) and stable ischemic heart disease accomplishes nothing constructive, according to clear-cut results of the landmark ISCHEMIA-CKD trial.
In this multinational randomized trial including 777 stable patients with advanced CKD and moderate or severe myocardial ischemia on noninvasive testing, an early invasive strategy didn’t reduce the risks of death or ischemic events, didn’t provide any significant improvement in quality of life metrics, and resulted in an increased risk of stroke, Sripal Bangalore, MD, reported at the American Heart Association scientific sessions.
“ said Dr. Bangalore, professor of medicine and director of complex coronary intervention at New York University.
This was easily the largest-ever trial of an invasive versus conservative coronary artery disease management strategy in this challenging population. For example, in the COURAGE trial of optimal medical therapy with or without percutaneous coronary intervention for stable coronary disease (N Engl J Med. 2007 Apr 12;356[15]:1503-16), only 16 of the 2,287 participants had an estimated glomerular filtration rate below 30 mL/min per 1.73 m2.
Of note, the participating study sites received special training aimed at minimizing the risk of acute kidney injury after cardiac catheterization in this at-risk population. The training included utilization of a customized left ventricular end diastolic volume–based hydration protocol, guidance on how to perform percutaneous coronary intervention with little or no contrast material, and encouragement of a heart/kidney team approach involving a cardiologist, nephrologist, and cardiovascular surgeon. This training really paid off, with roughly a 7% incidence of acute kidney injury after catheterization.
“The expected rate in such patients would be 30%-60%,” according to Dr. Bangalore.
The primary endpoint in ISCHEMIA-CKD was the rates of death or MI during 3 years of prospective follow-up. This occurred in 36.7% of patients randomized to optimal medical therapy alone and 36.4% of those randomized to an early invasive strategy. Similarly, the major secondary endpoint, comprising death, MI, and hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest, was also a virtual dead heat, occurring in about 39% of subjects. More than 27% of study participants had died by the 3-year mark regardless of how their coronary disease was managed.
The adjusted risk of stroke was 3.76-fold higher in the invasive strategy group; however, the most strokes were not procedurally related, and the explanation for the significantly increased risk in the invasively managed group remains unknown, the cardiologist said.
John A. Spertus, MD, who led the quality of life assessment in ISCHEMIA-CKD and in the 5,129-patient parent ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches), also presented at the AHA scientific sessions. He reported that, unlike in the parent study, there was no hint of a meaningful long-term quality of life benefit for revascularization plus optimal medical therapy, compared with that of optimal medical therapy alone, in ISCHEMIA-CKD.
“We have greater than 93% confidence that there is more of a quality of life effect in patients without advanced CKD than in patients with advanced CKD,” said Dr. Spertus, director of health outcomes research at the Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri, Kansas City.
Discussant Glenn L. Levine, MD, hailed ISCHEMIA-CKD as “a monumental achievement,” an ambitious, well-powered study with essentially no loss of follow-up during up to 4 years. It helps fill an utter void in the AHA/American College of Cardiology guidelines, which to date offer no recommendations at all regarding revascularization in patients with CKD because of a lack of evidence. And he considers ISCHEMIA-CKD to be unequivocally practice changing and guideline changing.
“Based on the results of ISCHEMIA-CKD, I will generally not go searching for ischemia and [coronary artery disease] in most severe and end-stage CKD patients, absent marked or unacceptable angina, and will treat them with medical therapy alone,” declared Dr. Levine, professor of medicine at Baylor College of Medicine and director of the cardiac care unit at the Michael E. DeBakey VA Medical Center, both in Houston.
He offered a few caveats. Excluded from participation in ISCHEMIA-CKD were patients with acute coronary syndrome, significant heart failure, or unacceptable angina despite optimal medical therapy at baseline, so the study results don’t apply to them. Also, the acute kidney injury rate after intervention was eye-catchingly low.
Dr. Bangalore discussed the ISCHEMIA-CKD trial and outcomes in a video interview with Medscape’s Tricia Ward.
“It seems unlikely that all centers routinely do and will exactly follow the very careful measures to limit contrast and minimize contrast nephropathy used in this study,” Dr. Levine commented.
ISCHEMIA-CKD, like its parent ISCHEMIA trial, was funded by the National Heart, Lung, and Blood Institute. Dr. Bangalore reported having no relevant financial interests. Dr. Spertus holds the copyright for the Seattle Angina Questionnaire, which was used for quality of life measurement in the trials. Dr. Levine disclosed that he has no relations with industry or conflicts of interest.
REPORTING FROM AHA 2019
IL-1 alpha is a new target in atopic dermatitis
MADRID – on the basis of an encouraging phase 2, proof-of-concept study involving bermekimab, an investigational monoclonal antibody directed at that cytokine.
The mechanism of benefit is unclear, although there are a number of plausible possibilities, all orbiting around the notion that AD is not only a Th2 immunity–mediated disease, but that Th1 immunity plays a role, too.
“The ultimate proof, as you will see, is that it works,” Alice B. Gottlieb, MD, PhD, observed in presenting the phase 2 study findings at the annual congress of the European Academy of Dermatology and Venereology.
Bermekimab is a monoclonal antibody cloned from human peripheral B lymphocytes. Sources of its target – IL-1 alpha – that are of therapeutic relevance include neutrophils, keratinocytes, platelets, and monocytes.
The study included 10 adults with moderate to severe AD who received a single subcutaneous injection of 200 mg of bermekimab and 28 who received 400 mg weekly for 8 weeks. Lumping together the various outcome measures employed in the study, the 400-mg dose was three times more effective than the 200-mg dose, a finding explainable by the 240% higher serum levels at 400 mg, according to Dr. Gottlieb, medical director of Mount Sinai Beth Israel Dermatology in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
This was a small, uncontrolled, proof-of-concept study; at this early stage, results painted a favorable picture of efficacy and safety. For example, scores on the Eczema Area and Severity Index dropped by a mean of 65% from baseline at week 4 of bermekimab at 400 mg/week and by 80% at week 8. Scoring Atopic Dermatitis scores dropped by about 55% at week 4 and by nearly 70% at 8 weeks. Scores on the 0-4 Investigator Global Assessment improved by 1.2 points at week 4 and 1.4 points by week 8. Dermatology Life Quality Index scores improved by a mean of 70% by week 8, at which point 61% of patients had a DLQI of 0-1, indicating that AD had no or only a slight impact on quality of life. Scores on the Hospital Anxiety and Depression Scale improved by about 45% at 4 weeks and 65% at 8 weeks.
At baseline, most patients rated their pain as 7 out of 10. By week 8, pain scores had dropped by an average of 80%, and 80% of patients experienced a 4-point drop or greater. Similarly, 80% of patients had at least a 4-point drop in their self-rated worst itch scores on a 0-10 scale. The greatest improvement in both pain and itch scores occurred in the first 4 weeks, after which further improvement continued, albeit at a slower rate.
Adverse events consisted of grade 2 wheezing in two patients, grade 1 nausea in two patients, and a 3% rate of mild injection-site reactions.
An audience member rose to comment: “This is really interesting data, and it goes against everything that we think about atopic dermatitis. But we know from allergic contact dermatitis that IL-1 is a very early signal coming out of keratinocytes. It almost makes you wonder whether that’s not a primary problem in atopic dermatitis that we haven’t realized – that the keratinocytes are under stress because of damage to the skin barrier or other functions, and by alleviating that stress by blocking IL-1, you block the progression into what we previously thought was an Th2-mediated disease, atopic dermatitis.”
“I was thinking that, too – that the keratinocytes could be playing a role in atopic dermatitis through IL-1,” Dr. Gottlieb replied.
She noted that her Mount Sinai colleague Emma Guttman-Yassky, MD, PhD, and coinvestigators have demonstrated that a monoclonal antibody that blocks IL-17C is effective in treating AD, and that IL-17C causes keratinocytes to release proinflammatory IL-1 alpha. High levels of IL-1 alpha have been shown to drive leukocyte recruitment into the skin, promote breakdown of the skin barrier through production of matrix metalloproteinase, stimulate itch by a direct effect on nerves, and cause leaky vascular endothelium.
A phase 2 study of bermekimab for treatment of AD enrolled the first patient in mid-November. In addition, Dr. Gottlieb has led a positive phase 2 study of the monoclonal antibody in patients with hidradenitis suppurativa, with a phase 3 trial in the works.
She reported receiving research funding from and serving as a consultant without personal compensation to XBiotech, which sponsored the AD and hidradenitis suppurativa studies. She has similar financial relationships with numerous pharmaceutical companies developing psoriasis medications.
MADRID – on the basis of an encouraging phase 2, proof-of-concept study involving bermekimab, an investigational monoclonal antibody directed at that cytokine.
The mechanism of benefit is unclear, although there are a number of plausible possibilities, all orbiting around the notion that AD is not only a Th2 immunity–mediated disease, but that Th1 immunity plays a role, too.
“The ultimate proof, as you will see, is that it works,” Alice B. Gottlieb, MD, PhD, observed in presenting the phase 2 study findings at the annual congress of the European Academy of Dermatology and Venereology.
Bermekimab is a monoclonal antibody cloned from human peripheral B lymphocytes. Sources of its target – IL-1 alpha – that are of therapeutic relevance include neutrophils, keratinocytes, platelets, and monocytes.
The study included 10 adults with moderate to severe AD who received a single subcutaneous injection of 200 mg of bermekimab and 28 who received 400 mg weekly for 8 weeks. Lumping together the various outcome measures employed in the study, the 400-mg dose was three times more effective than the 200-mg dose, a finding explainable by the 240% higher serum levels at 400 mg, according to Dr. Gottlieb, medical director of Mount Sinai Beth Israel Dermatology in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
This was a small, uncontrolled, proof-of-concept study; at this early stage, results painted a favorable picture of efficacy and safety. For example, scores on the Eczema Area and Severity Index dropped by a mean of 65% from baseline at week 4 of bermekimab at 400 mg/week and by 80% at week 8. Scoring Atopic Dermatitis scores dropped by about 55% at week 4 and by nearly 70% at 8 weeks. Scores on the 0-4 Investigator Global Assessment improved by 1.2 points at week 4 and 1.4 points by week 8. Dermatology Life Quality Index scores improved by a mean of 70% by week 8, at which point 61% of patients had a DLQI of 0-1, indicating that AD had no or only a slight impact on quality of life. Scores on the Hospital Anxiety and Depression Scale improved by about 45% at 4 weeks and 65% at 8 weeks.
At baseline, most patients rated their pain as 7 out of 10. By week 8, pain scores had dropped by an average of 80%, and 80% of patients experienced a 4-point drop or greater. Similarly, 80% of patients had at least a 4-point drop in their self-rated worst itch scores on a 0-10 scale. The greatest improvement in both pain and itch scores occurred in the first 4 weeks, after which further improvement continued, albeit at a slower rate.
Adverse events consisted of grade 2 wheezing in two patients, grade 1 nausea in two patients, and a 3% rate of mild injection-site reactions.
An audience member rose to comment: “This is really interesting data, and it goes against everything that we think about atopic dermatitis. But we know from allergic contact dermatitis that IL-1 is a very early signal coming out of keratinocytes. It almost makes you wonder whether that’s not a primary problem in atopic dermatitis that we haven’t realized – that the keratinocytes are under stress because of damage to the skin barrier or other functions, and by alleviating that stress by blocking IL-1, you block the progression into what we previously thought was an Th2-mediated disease, atopic dermatitis.”
“I was thinking that, too – that the keratinocytes could be playing a role in atopic dermatitis through IL-1,” Dr. Gottlieb replied.
She noted that her Mount Sinai colleague Emma Guttman-Yassky, MD, PhD, and coinvestigators have demonstrated that a monoclonal antibody that blocks IL-17C is effective in treating AD, and that IL-17C causes keratinocytes to release proinflammatory IL-1 alpha. High levels of IL-1 alpha have been shown to drive leukocyte recruitment into the skin, promote breakdown of the skin barrier through production of matrix metalloproteinase, stimulate itch by a direct effect on nerves, and cause leaky vascular endothelium.
A phase 2 study of bermekimab for treatment of AD enrolled the first patient in mid-November. In addition, Dr. Gottlieb has led a positive phase 2 study of the monoclonal antibody in patients with hidradenitis suppurativa, with a phase 3 trial in the works.
She reported receiving research funding from and serving as a consultant without personal compensation to XBiotech, which sponsored the AD and hidradenitis suppurativa studies. She has similar financial relationships with numerous pharmaceutical companies developing psoriasis medications.
MADRID – on the basis of an encouraging phase 2, proof-of-concept study involving bermekimab, an investigational monoclonal antibody directed at that cytokine.
The mechanism of benefit is unclear, although there are a number of plausible possibilities, all orbiting around the notion that AD is not only a Th2 immunity–mediated disease, but that Th1 immunity plays a role, too.
“The ultimate proof, as you will see, is that it works,” Alice B. Gottlieb, MD, PhD, observed in presenting the phase 2 study findings at the annual congress of the European Academy of Dermatology and Venereology.
Bermekimab is a monoclonal antibody cloned from human peripheral B lymphocytes. Sources of its target – IL-1 alpha – that are of therapeutic relevance include neutrophils, keratinocytes, platelets, and monocytes.
The study included 10 adults with moderate to severe AD who received a single subcutaneous injection of 200 mg of bermekimab and 28 who received 400 mg weekly for 8 weeks. Lumping together the various outcome measures employed in the study, the 400-mg dose was three times more effective than the 200-mg dose, a finding explainable by the 240% higher serum levels at 400 mg, according to Dr. Gottlieb, medical director of Mount Sinai Beth Israel Dermatology in the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
This was a small, uncontrolled, proof-of-concept study; at this early stage, results painted a favorable picture of efficacy and safety. For example, scores on the Eczema Area and Severity Index dropped by a mean of 65% from baseline at week 4 of bermekimab at 400 mg/week and by 80% at week 8. Scoring Atopic Dermatitis scores dropped by about 55% at week 4 and by nearly 70% at 8 weeks. Scores on the 0-4 Investigator Global Assessment improved by 1.2 points at week 4 and 1.4 points by week 8. Dermatology Life Quality Index scores improved by a mean of 70% by week 8, at which point 61% of patients had a DLQI of 0-1, indicating that AD had no or only a slight impact on quality of life. Scores on the Hospital Anxiety and Depression Scale improved by about 45% at 4 weeks and 65% at 8 weeks.
At baseline, most patients rated their pain as 7 out of 10. By week 8, pain scores had dropped by an average of 80%, and 80% of patients experienced a 4-point drop or greater. Similarly, 80% of patients had at least a 4-point drop in their self-rated worst itch scores on a 0-10 scale. The greatest improvement in both pain and itch scores occurred in the first 4 weeks, after which further improvement continued, albeit at a slower rate.
Adverse events consisted of grade 2 wheezing in two patients, grade 1 nausea in two patients, and a 3% rate of mild injection-site reactions.
An audience member rose to comment: “This is really interesting data, and it goes against everything that we think about atopic dermatitis. But we know from allergic contact dermatitis that IL-1 is a very early signal coming out of keratinocytes. It almost makes you wonder whether that’s not a primary problem in atopic dermatitis that we haven’t realized – that the keratinocytes are under stress because of damage to the skin barrier or other functions, and by alleviating that stress by blocking IL-1, you block the progression into what we previously thought was an Th2-mediated disease, atopic dermatitis.”
“I was thinking that, too – that the keratinocytes could be playing a role in atopic dermatitis through IL-1,” Dr. Gottlieb replied.
She noted that her Mount Sinai colleague Emma Guttman-Yassky, MD, PhD, and coinvestigators have demonstrated that a monoclonal antibody that blocks IL-17C is effective in treating AD, and that IL-17C causes keratinocytes to release proinflammatory IL-1 alpha. High levels of IL-1 alpha have been shown to drive leukocyte recruitment into the skin, promote breakdown of the skin barrier through production of matrix metalloproteinase, stimulate itch by a direct effect on nerves, and cause leaky vascular endothelium.
A phase 2 study of bermekimab for treatment of AD enrolled the first patient in mid-November. In addition, Dr. Gottlieb has led a positive phase 2 study of the monoclonal antibody in patients with hidradenitis suppurativa, with a phase 3 trial in the works.
She reported receiving research funding from and serving as a consultant without personal compensation to XBiotech, which sponsored the AD and hidradenitis suppurativa studies. She has similar financial relationships with numerous pharmaceutical companies developing psoriasis medications.
REPORTING FROM EADV 2019
Cannabis frequently is used for endometriosis pain
VANCOUVER, B.C. – with over a third reporting either current or past use, according to a new survey.
The finding comes as more and more companies are marketing CBD-containing products to women, with unsubstantiated claims about efficacy, according to Anna Reinert, MD, who presented the research at a meeting sponsored by AAGL.
Women self-reported that marijuana use was moderately effective, while the median value for CBD corresponded to “slightly effective.”
To investigate use patterns, Dr. Reinert and colleagues created a questionnaire with 55-75 questions, which followed a branching logic tree. Topics included pain history, demographics, and experience with marijuana and CBD for the purpose of controlling pelvic pain. The survey was sent to two populations: an endometriosis association mailing list, and patients at a chronic pain center in Phoenix.
About 24,500 surveys were sent out; 366 were received and analyzed. The response rate was much different between the two populations, at 1% in the endometriosis association and 16% of the clinic population. Dr. Reinert attributed the low response rate in the association sample to the continuing stigma surrounding marijuana use, citing much higher response rates to other surveys sent out by the association around the same time.
Overall, 63% of respondents said they had never used marijuana; 37% reported past or present use; 65% said they had never used CBD; and 35% reported past or present use. About 45% of marijuana users reported that its use was very effective, and 25% said it was moderately effective. About 22% of CBD users said it was very effective, and about 33% said it was moderately effective. The median values lay in the moderately effective range for marijuana, and in the slightly effective range for CBD.
The findings suggest a need for more research into the potential benefit and limitations of cannabis for pelvic pain from endometriosis, said Dr. Reinert, an obstetrician/gynecologist the University of Southern California, Los Angeles.
Until this study, evidence of efficacy of marijuana for this indication has been sparse. A report from the National Academy of Sciences showed that there is evidence that cannabis and cannabinoids have a therapeutic effect on chronic pain in adults (National Academies Press (US) 2017 Jan 12), but the report made no mention of gynecological applications. Despite this lack of evidence, surveys have shown that women of reproductive age use marijuana, and an analysis by the Ameritox Laboratory in a pain management population found that 13% of women and 19% of men tested positive for marijuana in their urine.
Still, “there is not research looking at marijuana for women with chronic health pain,” Dr. Reinert said at the meeting.
But that doesn’t stop companies from developing CBD vaginal suppositories and marketing them for menstrual pelvic discomfort, pain during sex, and other issues. Lay press articles often boost these claims, although some skeptical takes address the lack of evidence. Still, “there’s a lot on the more positive side,” she said.
That leads to a lot of interest among patients in using marijuana or CBD for symptom relief, which is part of the reason that Dr. Reinert’s team decided to examine its use and perceived efficacy. Another reason is that there is some biological basis to believe that cannabis could be helpful. There is some evidence that women with endometriosis have changes in their endocannabinoid system (Cannabis Cannabinoid Res. 2017;2:72-80), and there are clinical trials examining the impact of non-CBD, non-tetrahydrocannabinol (THC) endocannabinoid ligands.
Dr. Reinert has no financial disclosures.
VANCOUVER, B.C. – with over a third reporting either current or past use, according to a new survey.
The finding comes as more and more companies are marketing CBD-containing products to women, with unsubstantiated claims about efficacy, according to Anna Reinert, MD, who presented the research at a meeting sponsored by AAGL.
Women self-reported that marijuana use was moderately effective, while the median value for CBD corresponded to “slightly effective.”
To investigate use patterns, Dr. Reinert and colleagues created a questionnaire with 55-75 questions, which followed a branching logic tree. Topics included pain history, demographics, and experience with marijuana and CBD for the purpose of controlling pelvic pain. The survey was sent to two populations: an endometriosis association mailing list, and patients at a chronic pain center in Phoenix.
About 24,500 surveys were sent out; 366 were received and analyzed. The response rate was much different between the two populations, at 1% in the endometriosis association and 16% of the clinic population. Dr. Reinert attributed the low response rate in the association sample to the continuing stigma surrounding marijuana use, citing much higher response rates to other surveys sent out by the association around the same time.
Overall, 63% of respondents said they had never used marijuana; 37% reported past or present use; 65% said they had never used CBD; and 35% reported past or present use. About 45% of marijuana users reported that its use was very effective, and 25% said it was moderately effective. About 22% of CBD users said it was very effective, and about 33% said it was moderately effective. The median values lay in the moderately effective range for marijuana, and in the slightly effective range for CBD.
The findings suggest a need for more research into the potential benefit and limitations of cannabis for pelvic pain from endometriosis, said Dr. Reinert, an obstetrician/gynecologist the University of Southern California, Los Angeles.
Until this study, evidence of efficacy of marijuana for this indication has been sparse. A report from the National Academy of Sciences showed that there is evidence that cannabis and cannabinoids have a therapeutic effect on chronic pain in adults (National Academies Press (US) 2017 Jan 12), but the report made no mention of gynecological applications. Despite this lack of evidence, surveys have shown that women of reproductive age use marijuana, and an analysis by the Ameritox Laboratory in a pain management population found that 13% of women and 19% of men tested positive for marijuana in their urine.
Still, “there is not research looking at marijuana for women with chronic health pain,” Dr. Reinert said at the meeting.
But that doesn’t stop companies from developing CBD vaginal suppositories and marketing them for menstrual pelvic discomfort, pain during sex, and other issues. Lay press articles often boost these claims, although some skeptical takes address the lack of evidence. Still, “there’s a lot on the more positive side,” she said.
That leads to a lot of interest among patients in using marijuana or CBD for symptom relief, which is part of the reason that Dr. Reinert’s team decided to examine its use and perceived efficacy. Another reason is that there is some biological basis to believe that cannabis could be helpful. There is some evidence that women with endometriosis have changes in their endocannabinoid system (Cannabis Cannabinoid Res. 2017;2:72-80), and there are clinical trials examining the impact of non-CBD, non-tetrahydrocannabinol (THC) endocannabinoid ligands.
Dr. Reinert has no financial disclosures.
VANCOUVER, B.C. – with over a third reporting either current or past use, according to a new survey.
The finding comes as more and more companies are marketing CBD-containing products to women, with unsubstantiated claims about efficacy, according to Anna Reinert, MD, who presented the research at a meeting sponsored by AAGL.
Women self-reported that marijuana use was moderately effective, while the median value for CBD corresponded to “slightly effective.”
To investigate use patterns, Dr. Reinert and colleagues created a questionnaire with 55-75 questions, which followed a branching logic tree. Topics included pain history, demographics, and experience with marijuana and CBD for the purpose of controlling pelvic pain. The survey was sent to two populations: an endometriosis association mailing list, and patients at a chronic pain center in Phoenix.
About 24,500 surveys were sent out; 366 were received and analyzed. The response rate was much different between the two populations, at 1% in the endometriosis association and 16% of the clinic population. Dr. Reinert attributed the low response rate in the association sample to the continuing stigma surrounding marijuana use, citing much higher response rates to other surveys sent out by the association around the same time.
Overall, 63% of respondents said they had never used marijuana; 37% reported past or present use; 65% said they had never used CBD; and 35% reported past or present use. About 45% of marijuana users reported that its use was very effective, and 25% said it was moderately effective. About 22% of CBD users said it was very effective, and about 33% said it was moderately effective. The median values lay in the moderately effective range for marijuana, and in the slightly effective range for CBD.
The findings suggest a need for more research into the potential benefit and limitations of cannabis for pelvic pain from endometriosis, said Dr. Reinert, an obstetrician/gynecologist the University of Southern California, Los Angeles.
Until this study, evidence of efficacy of marijuana for this indication has been sparse. A report from the National Academy of Sciences showed that there is evidence that cannabis and cannabinoids have a therapeutic effect on chronic pain in adults (National Academies Press (US) 2017 Jan 12), but the report made no mention of gynecological applications. Despite this lack of evidence, surveys have shown that women of reproductive age use marijuana, and an analysis by the Ameritox Laboratory in a pain management population found that 13% of women and 19% of men tested positive for marijuana in their urine.
Still, “there is not research looking at marijuana for women with chronic health pain,” Dr. Reinert said at the meeting.
But that doesn’t stop companies from developing CBD vaginal suppositories and marketing them for menstrual pelvic discomfort, pain during sex, and other issues. Lay press articles often boost these claims, although some skeptical takes address the lack of evidence. Still, “there’s a lot on the more positive side,” she said.
That leads to a lot of interest among patients in using marijuana or CBD for symptom relief, which is part of the reason that Dr. Reinert’s team decided to examine its use and perceived efficacy. Another reason is that there is some biological basis to believe that cannabis could be helpful. There is some evidence that women with endometriosis have changes in their endocannabinoid system (Cannabis Cannabinoid Res. 2017;2:72-80), and there are clinical trials examining the impact of non-CBD, non-tetrahydrocannabinol (THC) endocannabinoid ligands.
Dr. Reinert has no financial disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
OTC hormonal contraception: An important goal in the fight for reproductive justice
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
A new American College of Obstetricians and Gynecologists (ACOG) committee opinion addresses how contraception access can be improved through over-the-counter (OTC) hormonal contraception for people of all ages—including oral contraceptive pills (OCPs), progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate (DMPA). Although ACOG endorses OTC contraception, some health care providers may be hesitant to support the increase in accessibility for a variety of reasons. We are hopeful that we address these concerns and that all clinicians can move to support ACOG’s position.
Easing access to hormonal contraception is a first step
OCPs are the most widely used contraception among teens and women of reproductive age in the United States.1 Although the Affordable Care Act (ACA) mandated health insurance coverage for contraception, many barriers continue to exist, including obtaining a prescription. Only 13 states have made it legal to obtain hormonal contraception through a pharmacist.2 There also has been an increase in the number of telemedicine and online services that deliver contraceptives to individuals’ homes. While these efforts have helped to decrease barriers to hormonal contraception access for some patients, they only reach a small segment of the population. As clinicians, we should strive to make contraception universally accessible and affordable to everyone who desires to use it. OTC provision can bring us closer to this goal.
Addressing the misconceptions about contraception
Adverse events with hormonal contraception are rarer than one may think. There are few risks associated with hormonal contraception. Venous thromboembolus (VTE) is a serious, although rare, adverse effect (AE) of hormonal contraception. The rate of VTE with combined oral contraception is estimated at 3 to 8 events per 10,000 patient-years, and VTE is even less common with progestin-only contraception (1 to 5 per 10,000 patient-years). For both types of hormonal contraception, the risk of VTE is smaller than with pregnancy, which is 5 to 20 per 10,000 patient-years.3 There are comorbidities that increase the risk of VTE and other AEs of hormonal contraception. In the setting of OTC hormonal contraception, individuals would self-screen for contraindications in order to reduce these complications.
Patients have the aptitude to self-screen for contraindications. Studies looking at the ability of patients over the age of 18 to self-screen for contraindications to hormonal contraception have found that patients do appropriately screen themselves. In fact, they are often more conservative than a physician in avoiding hormonal contraceptive methods.4 Patients younger than age 18 rarely have contraindications to hormonal contraception, but limited studies have shown that they too are able to successfully self-screen.5 ACOG recommends self-screening tools be provided with all OTC combined hormonal contraceptive methods to aid an individual’s contraceptive choice.
Most patients continue their well person care. Some opponents to ACOG’s position also have expressed concern that people who access their contraception OTC will forego their annual exam with their provider. However, studies have shown that the majority of people will continue to make their preventative health care visits.6,7
We need to invest in preventing unplanned pregnancy
Currently, hormonal contraception is covered by health insurance under the ACA, with some caveats. Without a prescription, patients may have to pay full price for their contraception. However, one can find generic OCPs for less than $10 per pack out of pocket. Any cost can be prohibitive to many patients; thus, transition to OTC access to contraception also should ensure limiting the cost to the patient. One possible solution to mitigate costs is to require insurance companies to cover the cost of OTC hormonal contraceptives. (See action item below.)
Reduction in unplanned pregnancies improves public health and public expense, and broadening access to effective forms of contraception is imperative in reducing unplanned pregnancies. Every $1 invested in contraception access realizes $7.09 in savings.8 By making hormonal contraception widely available OTC, access could be improved dramatically—although pharmacist provision of hormonal contraception may be a necessary intermediate step. ACOG’s most recent committee opinion encourages all reproductive health care providers to be strong advocates for this improvement in access. As women’s health providers, we should work to decrease access barriers for our patients; working toward OTC contraception is a critical step in equal access to birth control methods for all of our patients.
Action items
Remember, before a pill can move to OTC access, the manufacturing (pharmaceutical) company must submit an application to the US Food and Drug Administration to obtain this status. Once submitted, the process may take 3 to 4 years to be completed. Currently, no company has submitted an OTC application and no hormonal birth control is available OTC. Find resources for OTC birth control access here: http://ocsotc.org/ and www.freethepill.org.
- Talk to your state representatives about why both OTC birth control access and direct pharmacy availability are important to increasing access and decreasing disparities in reproductive health care. Find your local and federal representatives here and check the status of OCP access in your state here.
- Representative Ayanna Pressley (D-MA) and Senator Patty Murray (D-WA) both have introduced legislation—the Affordability is Access Act (HR 3296/S1847)—to ensure insurance coverage for OTC contraception. Call your representative and ask them to cosponsor this legislation.
- Be mindful of legislation that promotes OTC OCPs but limits access to some populations (minors) and increases cost sharing to the patient. This type of legislation can create harmful barriers to access for some of our patients
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
- Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Rep. 2012;(60):1-25.
- Free the pill. What’s the law in your state? Ibis Reproductive Health website. http://freethepill.org/statepolicies. Accessed November 15, 2019.
- U.S. Food and Drug Administration. FDA Drug Safety Communication: updated information about the risk of blood clots in women taking birth control pills containing drospirenone. https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed November 15, 2019.
- Grossman D, Fernandez L, Hopkins K, et al. Accuracy of self-screening for contraindications to combined oral contraceptive use. Obstet Gynecol. 2008;112:572e8.
- Williams R, Hensel D, Lehmann A, et al. Adolescent self-screening for contraindications to combined oral contraceptive pills [abstract]. Contraception. 2015;92:380.
- Hopkins K, Grossman D, White K, et al. Reproductive health preventive screening among clinic vs. over-the-counter oral contraceptive users. Contraception. 2012;86:376-382.
- Grindlay K, Grossman D. Interest in over-the-counter access to a progestin-only pill among women in the United States. Womens Health Issues. 2018;28:144-151.
- Frost JJ, Sonfield A, Zolna MR, et al. Return on investment: a fuller assessment of the benefits and cost savings of the US publicly funded family planning program. Milbank Q. 2014;92:696-749.
Open enrollment 2020: Activity down on Healthcare.gov
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.
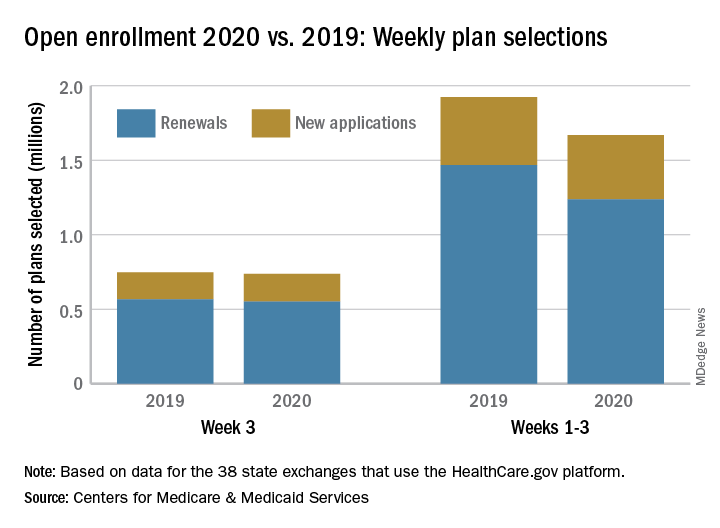
CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.

CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
The number of health insurance plans selected on Healthcare.gov during week 3 of the 2020 open enrollment was down slightly, compared with week 2 of this year and week 3 of last year, according to the Centers for Medicare & Medicaid Services.

CMS said Nov. 20, 2019, in its weekly enrollment snapshot. This number represents a slight dip in the number of plans selected during week 2, which was 754,967, according to a statement from the CMS.
The breakdown for week 3 looks like this: 550,706 consumers renewed existing coverage and 186,646 people who were not covered in 2019 selected new plans for 2020. For week 2, the first full week of this year’s open enrollment, the corresponding numbers were 558,962 and 196,005. Adding in the 2 days of week 1 brings the cumulative count to 1,669,401 plans selected for the year, the CMS reported. Last year, the total after week 3 was 1,924,476.
There are 38 states using the Healthcare.gov platform this year, and CMS reported their plan selection totals for the first time. Florida had the most plans selected with 463,066 this season, followed by Texas with 229,167 and Georgia with 105,653. California and New York do not use the federal market exchange.
The weekly reports “provide point-in-time estimates of weekly plan selections, call center activity, and visits to HealthCare.gov or CuidadoDeSalud.gov,” the CMS noted, so “the final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations.”
Newer IL-17 inhibitors make their case in phase 3 nonradiographic axial spondyloarthritis trials
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
A major gap in interleukin-17 inhibitor (IL-17i) therapy for axial spondyloarthritis (axSpA) was evidence of efficacy in nonradiographic axSpA. At ACR 2019, we saw two different IL-17i studies showing efficacy in nr-axSpA patients. Now we know that both secukinumab and ixekizumab are effective in the full spectrum of axSpA patients (ankylosing spondylitis [AS] and nr-axSpA).
The majority of clinicians would consider both AS and nr-axSpA to be driven by common processes and so drugs that are effective on one should have the same effect in the other as well. Hence the results are not a big surprise. In certain places, an approved indication for use may be important especially for reimbursement purposes. These results are likely to have maximal impact there.
The COAST-X study on ixekizumab was designed in a way similar to that of the C-axSpAnd study with certolizumab pegol. There was an extended 52-week placebo arm to study the natural history of nr-axSpA patients who are not actively treated with biologics. This design was necessary to respond to the Food and Drug Administration’s concern that, in the absence of this prolonged observation on placebo, we cannot be sure that nr-axSpA patients are not spontaneously remitting (not due to biologics).
However, the results here did surprise me. Unlike in the C-axSpAnd trial where only 13% of actively treated patients (on certolizumab pegol) switched to open-label treatment, in the COAST-X study 40% of patients on both doses of ixekizumab opted for open-label treatment. The number of patients moving out of the placebo arm was around 60% (similar in both studies). There are no straightforward factors evidently explaining this discrepancy. Between 15% and 25% of patients who switched had achieved the primary endpoint of ASAS40. Does this reflect that ASAS40 is not acceptable to patients? As the results show responses plateaued after week 16, it could be that the patients who switched might have done so well into the 52-week observation period.
Patients in the COAST-X study had slightly longer disease duration and marginally lower HLA-B27 prevalence (both factors may indicate lower chance of treatment response).
The primary endpoint of ASAS40 was met at weeks 16 and 52 with significantly higher rates seen with ixekizumab than with placebo. Again the response seems to plateau around 16 weeks with minimal gain up to week 52.
The results from the secukinumab PREVENT study are very similar to those of the COAST-X study showing the superiority of secukinumab over placebo in treating nr-axSpA patients. Interestingly, if we do not use the loading dose for secukinumab, there does not seem to be any difference from standard treatment with loading. This may have economic and administrative implications on the decision to use loading doses of secukinumab. We should carefully consider the MEASURE 4 trial results before making decisions on the utility of loading doses. In the MEASURE 4 study on AS patients, although there was no difference between load and no load arms of secukinumab (around 60% ASAS20 response in both arms), there was no significant gain above placebo with both doses. (The primary endpoint was not met.) This is likely due to the high placebo response (47% ASAS20 response). Similarly, we see a high placebo response in the COAST-X study as well, with an ASAS40 response rate of about 40% in active secukinumab arms vs. 30% in the placebo arm.
The number of patients dropping out over the 52-week follow-up period was not discussed in the PREVENT trial presentation.
There is not much here to favor one IL-17i over the other.
Dr. Haroon is codirector of the Spondylitis Program at University Health Network and associate professor of medicine and rheumatology at the University of Toronto. He is chair of the scientific committee of the Spondyloarthritis Research and Treatment Network. He disclosed serving as a consultant for Amgen, AbbVie, Janssen, Lilly, Novartis, and UCB.
FDA approves Givlaari for treatment of acute hepatic porphyria
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The Food and Drug Administration has approved givosiran (Givlaari) for the treatment of adult patients with acute hepatic porphyria, a genetic disorder that causes buildup of porphyrin molecules.
“This buildup can cause acute attacks, known as porphyria attacks, which can lead to severe pain and paralysis, respiratory failure, seizures, and mental status changes. These attacks occur suddenly and can produce permanent neurological damage and death. Prior to today’s approval, treatment options have only provided partial relief from the intense unremitting pain that characterizes these attacks,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
Approval for givosiran is based on results from a clinical trial of 94 patients with acute hepatic porphyria. Patients who received givosiran experienced 70% fewer porphyria attacks that required hospitalization, urgent health care visits, or home intravenous hemin injections compared with patients who received a placebo.
The most common adverse events associated with givosiran were nausea and injection site reactions. Patients receiving the medication should be monitored for anaphylactic reaction and renal function, and liver function should be tested before and periodically during treatment.
“The drug approved today can treat this disease by helping to reduce the number of attacks that disrupt the lives of patients,” said Dr. Pazdur, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
COAST-X top-line results: Ixekizumab improves nonradiographic axSpA vs. placebo
ATLANTA – Adding ixekizumab (Taltz) to conventional background medications significantly improved the signs and symptoms of nonradiographic axial spondyloarthritis (axSpA) in the randomized, double-blind, placebo-controlled phase 3 COAST-X trial.
The high-affinity interleukin-17A monoclonal antibody ixekizumab “has shown efficacy in ankylosing spondylitis – also called radiographic axial spondyloarthritis – [and] it recently was approved by the [Food and Drug Administration] for the treatment of active ankylosing spondylitis,” said Atul Deodhar, MD, explaining that COAST-X sought to assess its efficacy in patients with active nonradiographic axSpA and objective evidence of inflammation. He presented the results of the trial at the annual meeting of the American College of Rheumatology.
Of 303 adults with an established diagnosis of axSpA who met Assessment of Spondyloarthritis International Society (ASAS) classification criteria and who were enrolled in the 52-week trial, 105 were randomized to receive background medications plus placebo, and 102 and 96 received background medications plus ixekizumab every 2 or 4 weeks, respectively. The primary endpoint of a 40% improvement in ASAS response criteria (ASAS 40) was reached at week 16 by 19% of the placebo-group patients and by 35% and 40% of the 2- and 4-week ixekizumab-group patients, and at week 52 by 13%, 30%, and 31% of the patients in the groups, respectively, Dr. Deodhar reported.
Additionally, “all major secondary endpoints were met for each ixekizumab regimen, both at week 16 and week 52,” said Dr. Deodhar, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland.
For example, Ankylosing Spondylitis Disease Activity Score (ASDAS) at week 16 declined by 0.6 with placebo, 1.3 with 2-week ixekizumab dosing, and 1.1 points with 4-week ixekizumab dosing; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Functional Index changes were –1.5, –2.5, and –2.2, and –1.3, –2.3 and –2.0; Short Form–36 physical component score changes were 5.3, 8.0, and 8.1 points; and MRI sacroiliac joint Spondyloarthritis Research Consortium of Canada score changes were –0.3, –4.5 and –3.4, in the groups, respectively.
“ASDAS less than 2.1 – low disease activity – was achieved by 32% and 27% [in the 2- and 4-week ixekizumab groups] versus 12% in the placebo [group],” he said, noting that similarly significant results were seen at week 52.
Notably, the differences in ASAS 40 response rates between the treatment and placebo groups were observed beginning at week 1, and “a notable proportion” of patients who escaped to the open-label 2-week ixekizumab group, as allowed per study protocol starting at week 16, had an ASAS 40 response at the time of escape; the ASAS 40 response rates at that time were 6.5%, 16.7%, 25% in the groups, respectively, and the rates increased further on open-label ixekizumab, he said.
Study participants were adults diagnosed with axSpA by a physician and treated for at least 3 months. Inclusion criteria also included BASDAI score of at least 4, back pain score of at least 4, inflammation as evidenced by sacroiliitis on MRI or elevated C-reactive protein levels of greater than 5 mg/L, and inadequate response or intolerance to at least two NSAIDs.
Ixekizumab in both treatment groups was given at a dose of 80 mg, and changes to conventional background medications, including NSAIDs, conventional synthetic disease-modifying antirheumatic drugs, analgesics, and low-dose corticosteroids, were allowed, as was escape to open-label ixekizumab given every 2 weeks at investigators’ discretion after week 16.
Ixekizumab treatment was well tolerated; the frequency of serious adverse events and AEs leading to treatment discontinuation was low and similar across all arms, Dr. Deodhar said.
For example, treatment-emergent AEs occurred in 55.7%, 77.5%, and 65.6% of patients, serious AEs occurred in 1.0%, 1.0%, and 2.1%, and AE-related discontinuations occurred in 1.9%, 1.0%, and 1.0% or patients in the groups, respectively.
No deaths occurred and no new safety signals were identified.
“The results demonstrate, for the first time, that blocking IL-17A is a potential treatment option for patients with nonradiographic axSpA,” he concluded.
COAST-X was sponsored by Eli Lilly. Dr. Deodhar and most coauthors reported receiving research grants and/or honoraria for consulting or speaking from Eli Lilly and other pharmaceutical companies. Four authors are current employees and shareholders of Eli Lilly.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2729.
ATLANTA – Adding ixekizumab (Taltz) to conventional background medications significantly improved the signs and symptoms of nonradiographic axial spondyloarthritis (axSpA) in the randomized, double-blind, placebo-controlled phase 3 COAST-X trial.
The high-affinity interleukin-17A monoclonal antibody ixekizumab “has shown efficacy in ankylosing spondylitis – also called radiographic axial spondyloarthritis – [and] it recently was approved by the [Food and Drug Administration] for the treatment of active ankylosing spondylitis,” said Atul Deodhar, MD, explaining that COAST-X sought to assess its efficacy in patients with active nonradiographic axSpA and objective evidence of inflammation. He presented the results of the trial at the annual meeting of the American College of Rheumatology.
Of 303 adults with an established diagnosis of axSpA who met Assessment of Spondyloarthritis International Society (ASAS) classification criteria and who were enrolled in the 52-week trial, 105 were randomized to receive background medications plus placebo, and 102 and 96 received background medications plus ixekizumab every 2 or 4 weeks, respectively. The primary endpoint of a 40% improvement in ASAS response criteria (ASAS 40) was reached at week 16 by 19% of the placebo-group patients and by 35% and 40% of the 2- and 4-week ixekizumab-group patients, and at week 52 by 13%, 30%, and 31% of the patients in the groups, respectively, Dr. Deodhar reported.
Additionally, “all major secondary endpoints were met for each ixekizumab regimen, both at week 16 and week 52,” said Dr. Deodhar, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland.
For example, Ankylosing Spondylitis Disease Activity Score (ASDAS) at week 16 declined by 0.6 with placebo, 1.3 with 2-week ixekizumab dosing, and 1.1 points with 4-week ixekizumab dosing; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Functional Index changes were –1.5, –2.5, and –2.2, and –1.3, –2.3 and –2.0; Short Form–36 physical component score changes were 5.3, 8.0, and 8.1 points; and MRI sacroiliac joint Spondyloarthritis Research Consortium of Canada score changes were –0.3, –4.5 and –3.4, in the groups, respectively.
“ASDAS less than 2.1 – low disease activity – was achieved by 32% and 27% [in the 2- and 4-week ixekizumab groups] versus 12% in the placebo [group],” he said, noting that similarly significant results were seen at week 52.
Notably, the differences in ASAS 40 response rates between the treatment and placebo groups were observed beginning at week 1, and “a notable proportion” of patients who escaped to the open-label 2-week ixekizumab group, as allowed per study protocol starting at week 16, had an ASAS 40 response at the time of escape; the ASAS 40 response rates at that time were 6.5%, 16.7%, 25% in the groups, respectively, and the rates increased further on open-label ixekizumab, he said.
Study participants were adults diagnosed with axSpA by a physician and treated for at least 3 months. Inclusion criteria also included BASDAI score of at least 4, back pain score of at least 4, inflammation as evidenced by sacroiliitis on MRI or elevated C-reactive protein levels of greater than 5 mg/L, and inadequate response or intolerance to at least two NSAIDs.
Ixekizumab in both treatment groups was given at a dose of 80 mg, and changes to conventional background medications, including NSAIDs, conventional synthetic disease-modifying antirheumatic drugs, analgesics, and low-dose corticosteroids, were allowed, as was escape to open-label ixekizumab given every 2 weeks at investigators’ discretion after week 16.
Ixekizumab treatment was well tolerated; the frequency of serious adverse events and AEs leading to treatment discontinuation was low and similar across all arms, Dr. Deodhar said.
For example, treatment-emergent AEs occurred in 55.7%, 77.5%, and 65.6% of patients, serious AEs occurred in 1.0%, 1.0%, and 2.1%, and AE-related discontinuations occurred in 1.9%, 1.0%, and 1.0% or patients in the groups, respectively.
No deaths occurred and no new safety signals were identified.
“The results demonstrate, for the first time, that blocking IL-17A is a potential treatment option for patients with nonradiographic axSpA,” he concluded.
COAST-X was sponsored by Eli Lilly. Dr. Deodhar and most coauthors reported receiving research grants and/or honoraria for consulting or speaking from Eli Lilly and other pharmaceutical companies. Four authors are current employees and shareholders of Eli Lilly.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2729.
ATLANTA – Adding ixekizumab (Taltz) to conventional background medications significantly improved the signs and symptoms of nonradiographic axial spondyloarthritis (axSpA) in the randomized, double-blind, placebo-controlled phase 3 COAST-X trial.
The high-affinity interleukin-17A monoclonal antibody ixekizumab “has shown efficacy in ankylosing spondylitis – also called radiographic axial spondyloarthritis – [and] it recently was approved by the [Food and Drug Administration] for the treatment of active ankylosing spondylitis,” said Atul Deodhar, MD, explaining that COAST-X sought to assess its efficacy in patients with active nonradiographic axSpA and objective evidence of inflammation. He presented the results of the trial at the annual meeting of the American College of Rheumatology.
Of 303 adults with an established diagnosis of axSpA who met Assessment of Spondyloarthritis International Society (ASAS) classification criteria and who were enrolled in the 52-week trial, 105 were randomized to receive background medications plus placebo, and 102 and 96 received background medications plus ixekizumab every 2 or 4 weeks, respectively. The primary endpoint of a 40% improvement in ASAS response criteria (ASAS 40) was reached at week 16 by 19% of the placebo-group patients and by 35% and 40% of the 2- and 4-week ixekizumab-group patients, and at week 52 by 13%, 30%, and 31% of the patients in the groups, respectively, Dr. Deodhar reported.
Additionally, “all major secondary endpoints were met for each ixekizumab regimen, both at week 16 and week 52,” said Dr. Deodhar, professor of medicine in the division of arthritis and rheumatic diseases at Oregon Health & Science University, Portland.
For example, Ankylosing Spondylitis Disease Activity Score (ASDAS) at week 16 declined by 0.6 with placebo, 1.3 with 2-week ixekizumab dosing, and 1.1 points with 4-week ixekizumab dosing; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Functional Index changes were –1.5, –2.5, and –2.2, and –1.3, –2.3 and –2.0; Short Form–36 physical component score changes were 5.3, 8.0, and 8.1 points; and MRI sacroiliac joint Spondyloarthritis Research Consortium of Canada score changes were –0.3, –4.5 and –3.4, in the groups, respectively.
“ASDAS less than 2.1 – low disease activity – was achieved by 32% and 27% [in the 2- and 4-week ixekizumab groups] versus 12% in the placebo [group],” he said, noting that similarly significant results were seen at week 52.
Notably, the differences in ASAS 40 response rates between the treatment and placebo groups were observed beginning at week 1, and “a notable proportion” of patients who escaped to the open-label 2-week ixekizumab group, as allowed per study protocol starting at week 16, had an ASAS 40 response at the time of escape; the ASAS 40 response rates at that time were 6.5%, 16.7%, 25% in the groups, respectively, and the rates increased further on open-label ixekizumab, he said.
Study participants were adults diagnosed with axSpA by a physician and treated for at least 3 months. Inclusion criteria also included BASDAI score of at least 4, back pain score of at least 4, inflammation as evidenced by sacroiliitis on MRI or elevated C-reactive protein levels of greater than 5 mg/L, and inadequate response or intolerance to at least two NSAIDs.
Ixekizumab in both treatment groups was given at a dose of 80 mg, and changes to conventional background medications, including NSAIDs, conventional synthetic disease-modifying antirheumatic drugs, analgesics, and low-dose corticosteroids, were allowed, as was escape to open-label ixekizumab given every 2 weeks at investigators’ discretion after week 16.
Ixekizumab treatment was well tolerated; the frequency of serious adverse events and AEs leading to treatment discontinuation was low and similar across all arms, Dr. Deodhar said.
For example, treatment-emergent AEs occurred in 55.7%, 77.5%, and 65.6% of patients, serious AEs occurred in 1.0%, 1.0%, and 2.1%, and AE-related discontinuations occurred in 1.9%, 1.0%, and 1.0% or patients in the groups, respectively.
No deaths occurred and no new safety signals were identified.
“The results demonstrate, for the first time, that blocking IL-17A is a potential treatment option for patients with nonradiographic axSpA,” he concluded.
COAST-X was sponsored by Eli Lilly. Dr. Deodhar and most coauthors reported receiving research grants and/or honoraria for consulting or speaking from Eli Lilly and other pharmaceutical companies. Four authors are current employees and shareholders of Eli Lilly.
SOURCE: Deodhar A et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2729.
REPORTING FROM ACR 2019
Dealing with off-label medical device use in vascular surgery
Off-label device use is common in vascular surgery, but few studies address off-label uses through both surgical and legal perspectives, according to Wei Li, MD, of the University of Maryland School of Medicine, Baltimore. Dr. Li will discuss the medical-legal landscape of off-label device use in her presentation on Friday morning.
She and her colleagues assessed the publicly accessible LexisNexis legal database and Manufacturer and User Facility Device Experience (MAUDE) from January 2012 to December 2017. Jury verdict and case law searches within the LexisNexis were performed in order to identify representative cases and related legal doctrines for entries related to three (off-label) stents deployed in the superficial femoral artery.
They categorized and compared the reported adverse events for all three stents.
Although off-label device use is both legal and unregulated, it can carry potential legal implications on billing practices and subsequent medical malpractice liability, according to the researchers.
They found that off-label device use was more widespread in the pediatric patient population because of an unmet demand that can require Humanitarian Device Exemption. Among 497 total entries of reportable adverse events in MAUDE, for the three stents, they found significant differences, and they also found that the highest malfunction was associated with stent delivery. No deaths were reported with off-label use.
Dr. Li will discuss how vascular specialists need to have more in-depth knowledge about the off-label devices they use to minimize the chance of complications. Their investigation found no evidence reportable adverse events bear a direct relationship with Food and Drug Administration–approved indications related to the three superficial femoral artery stents in question.
Off-label device use is common in vascular surgery, but few studies address off-label uses through both surgical and legal perspectives, according to Wei Li, MD, of the University of Maryland School of Medicine, Baltimore. Dr. Li will discuss the medical-legal landscape of off-label device use in her presentation on Friday morning.
She and her colleagues assessed the publicly accessible LexisNexis legal database and Manufacturer and User Facility Device Experience (MAUDE) from January 2012 to December 2017. Jury verdict and case law searches within the LexisNexis were performed in order to identify representative cases and related legal doctrines for entries related to three (off-label) stents deployed in the superficial femoral artery.
They categorized and compared the reported adverse events for all three stents.
Although off-label device use is both legal and unregulated, it can carry potential legal implications on billing practices and subsequent medical malpractice liability, according to the researchers.
They found that off-label device use was more widespread in the pediatric patient population because of an unmet demand that can require Humanitarian Device Exemption. Among 497 total entries of reportable adverse events in MAUDE, for the three stents, they found significant differences, and they also found that the highest malfunction was associated with stent delivery. No deaths were reported with off-label use.
Dr. Li will discuss how vascular specialists need to have more in-depth knowledge about the off-label devices they use to minimize the chance of complications. Their investigation found no evidence reportable adverse events bear a direct relationship with Food and Drug Administration–approved indications related to the three superficial femoral artery stents in question.
Off-label device use is common in vascular surgery, but few studies address off-label uses through both surgical and legal perspectives, according to Wei Li, MD, of the University of Maryland School of Medicine, Baltimore. Dr. Li will discuss the medical-legal landscape of off-label device use in her presentation on Friday morning.
She and her colleagues assessed the publicly accessible LexisNexis legal database and Manufacturer and User Facility Device Experience (MAUDE) from January 2012 to December 2017. Jury verdict and case law searches within the LexisNexis were performed in order to identify representative cases and related legal doctrines for entries related to three (off-label) stents deployed in the superficial femoral artery.
They categorized and compared the reported adverse events for all three stents.
Although off-label device use is both legal and unregulated, it can carry potential legal implications on billing practices and subsequent medical malpractice liability, according to the researchers.
They found that off-label device use was more widespread in the pediatric patient population because of an unmet demand that can require Humanitarian Device Exemption. Among 497 total entries of reportable adverse events in MAUDE, for the three stents, they found significant differences, and they also found that the highest malfunction was associated with stent delivery. No deaths were reported with off-label use.
Dr. Li will discuss how vascular specialists need to have more in-depth knowledge about the off-label devices they use to minimize the chance of complications. Their investigation found no evidence reportable adverse events bear a direct relationship with Food and Drug Administration–approved indications related to the three superficial femoral artery stents in question.
Fewer people are dying from systemic sclerosis at younger ages
ATLANTA – Patients with systemic sclerosis aged 44 years and younger in the United States now have mortality comparable to that of the general population in that age group, according to recent results presented at the annual meeting of the American College of Rheumatology.
“Mortality for scleroderma has steadily decreased in younger ages for the last 5 decades,” Ram R. Singh, MD, professor of medicine, pathology, and laboratory medicine at the University of California, Los Angeles, said in his presentation.
Using the Centers for Disease Control and Prevention’s National Vital Statistics System database, Dr. Singh and colleagues analyzed data of adults with systemic sclerosis (SSc) and identified 46,798 adults who died between 1968 and 2015. They divided the adults with and without SSc into three different age groups: 44 and younger, 45-64, and 65 and older. The researchers performed a joinpoint trend analysis, calculating the annual percent change (APC) and average APC as well as the age-standardized mortality rate (ASMR) in each age group.
In 1968, 466 deaths were attributed to SSc, compared with 1,195 deaths in 2015. Between 1968 and 2015, there was a 19% cumulative percentage increase in SSc-related deaths, compared with a 44% decrease in mortality not attributed to SSc; when the researchers analyzed the ratio of SSc-related ASMR to non-SSc-related ASMR, there was an increase of 112%, Dr. Singh said.
When analyzing the mortality of adults with SSc by age group during 1968-2015 using the CDC’s database, Dr. Singh and colleagues found 5,457 deaths in adults 44 and younger (11.7%), 18,395 deaths in adults aged 45-64 (39.3%), and 22,946 deaths in adults aged 65 and older (49.0%), compared with totals for the general population of 10.3 million deaths in adults aged 44 and younger (9.7%), 20.8 million deaths in adults 45-64 years (19.6%), and 74.8 million deaths in adults aged 65 and older (70.6%).
Over the 48-year period, there were three major trends in SSc-related ASMR, Dr. Singh noted. In the first trend period between 1968 and 1988, there was a 1.0% increase per year (95% confidence interval, 0.6%-1.4%). The second trend period, lasting until 2000, saw a 2.2% increase per year (95% CI, 1.6%-2.7%), while the SSc-related ASMR declined by 2.6% per year in the third trend period from 2001 to 2015 (95% CI, –3.1% to –2.2%).
The percentage of annual deaths for adults with SSc decreased between 1968 and 2015, from 23.4% to 5.7%, and the average APC was greater among adults aged 44 and younger with SSc (–2.2%; 95% CI, –2.4% to –2.0%) than for adults without SSc in the same age group (–1.5%; 95% CI, –1.9% to –1.1%).
There was a cumulative 60% decrease in the ASMR of adults with SSc aged 44 and younger between 1968 and 2015 from an ASMR of 1.0 per million (95% CI, 0.8%-1.2%) to an ASMR of 0.4 per million (0.3-0.5). Adults aged 45-64 years with SSc had a cumulative 20.3% decrease in ASMR over the same time period, with an ASMR of 5.9 per million in 1968 (95% CI, 5.2-6.7) and an ASMR of 4.7 per million in 2015 (95% CI, 4.2-5.2). However, adults aged 65 and older with SSc had a 187% cumulative increase in ASMR, with an ASMR of 5.4 per million in 1968 (95% CI, 4.4-6.5) and an ASMR of 15.5 per million in 2015 (95% CI, 14.3-16.6). Adults with non-SSc-related deaths between 1968 and 2015 had a 50.0% cumulative decrease in ASMR in the group aged 44 and under, a 48.0% cumulative decrease in the 45-year to 64-year-old group, and a 42.1% decrease in the 65-year-old or older group.
The ratio of SSc to non-SSc ASMRs between 1968 and 2015 in the group aged 44 and younger declined 20.0%, whereas there was a 53.1% cumulative increase in the 45- to 64-year-old group and a 395.4% cumulative increase in the 65-year-old and older group. In the oldest group, the APC increased by 3.9% each year for 33 years (95% CI, 3.7%-4.1%) before declining by 1.6% until 2015 (95% CI, –2.0 to –1.3). In contrast, the APC for adults 44 and younger never significantly increased over the 48 years, Dr. Singh noted.
“Increasing scleroderma mortality in older age could be due to improving survival and/or increasing age of onset of scleroderma,” he said.
Dr. Singh reported no conflicts of interest.
SOURCE: Yen E et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 825.
ATLANTA – Patients with systemic sclerosis aged 44 years and younger in the United States now have mortality comparable to that of the general population in that age group, according to recent results presented at the annual meeting of the American College of Rheumatology.
“Mortality for scleroderma has steadily decreased in younger ages for the last 5 decades,” Ram R. Singh, MD, professor of medicine, pathology, and laboratory medicine at the University of California, Los Angeles, said in his presentation.
Using the Centers for Disease Control and Prevention’s National Vital Statistics System database, Dr. Singh and colleagues analyzed data of adults with systemic sclerosis (SSc) and identified 46,798 adults who died between 1968 and 2015. They divided the adults with and without SSc into three different age groups: 44 and younger, 45-64, and 65 and older. The researchers performed a joinpoint trend analysis, calculating the annual percent change (APC) and average APC as well as the age-standardized mortality rate (ASMR) in each age group.
In 1968, 466 deaths were attributed to SSc, compared with 1,195 deaths in 2015. Between 1968 and 2015, there was a 19% cumulative percentage increase in SSc-related deaths, compared with a 44% decrease in mortality not attributed to SSc; when the researchers analyzed the ratio of SSc-related ASMR to non-SSc-related ASMR, there was an increase of 112%, Dr. Singh said.
When analyzing the mortality of adults with SSc by age group during 1968-2015 using the CDC’s database, Dr. Singh and colleagues found 5,457 deaths in adults 44 and younger (11.7%), 18,395 deaths in adults aged 45-64 (39.3%), and 22,946 deaths in adults aged 65 and older (49.0%), compared with totals for the general population of 10.3 million deaths in adults aged 44 and younger (9.7%), 20.8 million deaths in adults 45-64 years (19.6%), and 74.8 million deaths in adults aged 65 and older (70.6%).
Over the 48-year period, there were three major trends in SSc-related ASMR, Dr. Singh noted. In the first trend period between 1968 and 1988, there was a 1.0% increase per year (95% confidence interval, 0.6%-1.4%). The second trend period, lasting until 2000, saw a 2.2% increase per year (95% CI, 1.6%-2.7%), while the SSc-related ASMR declined by 2.6% per year in the third trend period from 2001 to 2015 (95% CI, –3.1% to –2.2%).
The percentage of annual deaths for adults with SSc decreased between 1968 and 2015, from 23.4% to 5.7%, and the average APC was greater among adults aged 44 and younger with SSc (–2.2%; 95% CI, –2.4% to –2.0%) than for adults without SSc in the same age group (–1.5%; 95% CI, –1.9% to –1.1%).
There was a cumulative 60% decrease in the ASMR of adults with SSc aged 44 and younger between 1968 and 2015 from an ASMR of 1.0 per million (95% CI, 0.8%-1.2%) to an ASMR of 0.4 per million (0.3-0.5). Adults aged 45-64 years with SSc had a cumulative 20.3% decrease in ASMR over the same time period, with an ASMR of 5.9 per million in 1968 (95% CI, 5.2-6.7) and an ASMR of 4.7 per million in 2015 (95% CI, 4.2-5.2). However, adults aged 65 and older with SSc had a 187% cumulative increase in ASMR, with an ASMR of 5.4 per million in 1968 (95% CI, 4.4-6.5) and an ASMR of 15.5 per million in 2015 (95% CI, 14.3-16.6). Adults with non-SSc-related deaths between 1968 and 2015 had a 50.0% cumulative decrease in ASMR in the group aged 44 and under, a 48.0% cumulative decrease in the 45-year to 64-year-old group, and a 42.1% decrease in the 65-year-old or older group.
The ratio of SSc to non-SSc ASMRs between 1968 and 2015 in the group aged 44 and younger declined 20.0%, whereas there was a 53.1% cumulative increase in the 45- to 64-year-old group and a 395.4% cumulative increase in the 65-year-old and older group. In the oldest group, the APC increased by 3.9% each year for 33 years (95% CI, 3.7%-4.1%) before declining by 1.6% until 2015 (95% CI, –2.0 to –1.3). In contrast, the APC for adults 44 and younger never significantly increased over the 48 years, Dr. Singh noted.
“Increasing scleroderma mortality in older age could be due to improving survival and/or increasing age of onset of scleroderma,” he said.
Dr. Singh reported no conflicts of interest.
SOURCE: Yen E et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 825.
ATLANTA – Patients with systemic sclerosis aged 44 years and younger in the United States now have mortality comparable to that of the general population in that age group, according to recent results presented at the annual meeting of the American College of Rheumatology.
“Mortality for scleroderma has steadily decreased in younger ages for the last 5 decades,” Ram R. Singh, MD, professor of medicine, pathology, and laboratory medicine at the University of California, Los Angeles, said in his presentation.
Using the Centers for Disease Control and Prevention’s National Vital Statistics System database, Dr. Singh and colleagues analyzed data of adults with systemic sclerosis (SSc) and identified 46,798 adults who died between 1968 and 2015. They divided the adults with and without SSc into three different age groups: 44 and younger, 45-64, and 65 and older. The researchers performed a joinpoint trend analysis, calculating the annual percent change (APC) and average APC as well as the age-standardized mortality rate (ASMR) in each age group.
In 1968, 466 deaths were attributed to SSc, compared with 1,195 deaths in 2015. Between 1968 and 2015, there was a 19% cumulative percentage increase in SSc-related deaths, compared with a 44% decrease in mortality not attributed to SSc; when the researchers analyzed the ratio of SSc-related ASMR to non-SSc-related ASMR, there was an increase of 112%, Dr. Singh said.
When analyzing the mortality of adults with SSc by age group during 1968-2015 using the CDC’s database, Dr. Singh and colleagues found 5,457 deaths in adults 44 and younger (11.7%), 18,395 deaths in adults aged 45-64 (39.3%), and 22,946 deaths in adults aged 65 and older (49.0%), compared with totals for the general population of 10.3 million deaths in adults aged 44 and younger (9.7%), 20.8 million deaths in adults 45-64 years (19.6%), and 74.8 million deaths in adults aged 65 and older (70.6%).
Over the 48-year period, there were three major trends in SSc-related ASMR, Dr. Singh noted. In the first trend period between 1968 and 1988, there was a 1.0% increase per year (95% confidence interval, 0.6%-1.4%). The second trend period, lasting until 2000, saw a 2.2% increase per year (95% CI, 1.6%-2.7%), while the SSc-related ASMR declined by 2.6% per year in the third trend period from 2001 to 2015 (95% CI, –3.1% to –2.2%).
The percentage of annual deaths for adults with SSc decreased between 1968 and 2015, from 23.4% to 5.7%, and the average APC was greater among adults aged 44 and younger with SSc (–2.2%; 95% CI, –2.4% to –2.0%) than for adults without SSc in the same age group (–1.5%; 95% CI, –1.9% to –1.1%).
There was a cumulative 60% decrease in the ASMR of adults with SSc aged 44 and younger between 1968 and 2015 from an ASMR of 1.0 per million (95% CI, 0.8%-1.2%) to an ASMR of 0.4 per million (0.3-0.5). Adults aged 45-64 years with SSc had a cumulative 20.3% decrease in ASMR over the same time period, with an ASMR of 5.9 per million in 1968 (95% CI, 5.2-6.7) and an ASMR of 4.7 per million in 2015 (95% CI, 4.2-5.2). However, adults aged 65 and older with SSc had a 187% cumulative increase in ASMR, with an ASMR of 5.4 per million in 1968 (95% CI, 4.4-6.5) and an ASMR of 15.5 per million in 2015 (95% CI, 14.3-16.6). Adults with non-SSc-related deaths between 1968 and 2015 had a 50.0% cumulative decrease in ASMR in the group aged 44 and under, a 48.0% cumulative decrease in the 45-year to 64-year-old group, and a 42.1% decrease in the 65-year-old or older group.
The ratio of SSc to non-SSc ASMRs between 1968 and 2015 in the group aged 44 and younger declined 20.0%, whereas there was a 53.1% cumulative increase in the 45- to 64-year-old group and a 395.4% cumulative increase in the 65-year-old and older group. In the oldest group, the APC increased by 3.9% each year for 33 years (95% CI, 3.7%-4.1%) before declining by 1.6% until 2015 (95% CI, –2.0 to –1.3). In contrast, the APC for adults 44 and younger never significantly increased over the 48 years, Dr. Singh noted.
“Increasing scleroderma mortality in older age could be due to improving survival and/or increasing age of onset of scleroderma,” he said.
Dr. Singh reported no conflicts of interest.
SOURCE: Yen E et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 825.
REPORTING FROM ACR 2019