User login
FDA: Two repackagers issue voluntary ranitidine recall
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.
The ranitidine recall saga continues as two more companies have issued voluntary recalls of their repackaged ranitidine products because of possibly unacceptable levels of N-nitrosodimethylamine (NDMA), according to the Food and Drug Administration.
Golden State Medical Supply has recalled 150-mg and 300-mg ranitidine capsules, manufactured by Novitium, and Precision Dose has recalled a ranitidine oral solution at 150 mg/mL, manufactured by Amneal Pharmaceuticals.
“FDA has advised companies to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 ng/day or 0.32 parts per million for ranitidine),” the FDA said. The agency has “posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing.”
The FDA added that consumers taking over-the-counter ranitidine can consider alternatives such as famotidine (Pepcid), cimetidine (Tagamet), esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
Find the full press release and more information on other ranitidine recalls on the FDA website.
What’s new in hepatitis C: Four themes that dominated at the Liver Meeting
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
BOSTON – Treatment of persons who inject drugs, updates in pangenotypic direct-acting antiviral therapy, the benefits of sustained virologic response, and preemptive therapy in donor-positive organ transplantation topped the list of notable hepatitis C–related abstracts this year at the annual meeting of the American Association for the Study of Liver Diseases.
That’s according to Marc Ghany, MD, of the liver diseases branch of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, who gave a hepatitis C debrief to attendees on the final day of the meeting. Here are some of the meeting highlights as summarized by Dr. Ghany in this well-attended last-day session.
Treatment of HCV in people who inject drugs
Emerging data suggest it is feasible to treat hepatitis C virus (HCV) infection in persons who inject drugs (PWIDs); however, overcoming adherence issues remains a challenge, Dr. Ghany told attendees.
According to one study presented at AASLD by Dhiman and coauthors (Abstract 0165), decentralized care of PWIDs using direct-acting antiviral (DAA) therapy was safe and effective, even in those with cirrhosis. Authors demonstrated an “impressive” rate of sustained virologic response at 12 weeks (SVR12) of 91% by a modified intention-to-treat analysis, Dr. Ghany said; however, treatment interruptions were frequent and reduced the overall SVR rate in the study to 78%.
Other studies at the meeting looked at strategies to improve DAA efficacy in this population of patients at high risk of nonadherence, including use of a digital medicine program (Abstract 1554) and a model of care in which an internist-addiction medicine specialist evaluated opiate-dependent patients for HCV infection in a hepatology clinic (Abstract 1589).
Reinfection remains a focus of research in PWIDs. At this meeting, Janjua and coauthors reported that DAA-treated PWIDs in British Columbia had a threefold higher rate of reinfection versus non-PWIDs; however, there were no detected reinfections among PWIDs who had received uninterrupted opioid agonist therapy. “These data suggested that opioid agonist therapy should be given before and after HCV treatment in persons who inject drugs to prevent the infection,” Dr. Ghany said in his presentation.
Updates on pangenotypic DAA therapy
Jonas and coauthors (Abstract 1551) reported on the safety and efficacy of glecaprevir/pibrentasvir for 8 weeks in children with chronic HCV infection enrolled in the ongoing phase 2/3 DORA study. The SVR12 was high, according to Dr. Ghany, at 96% overall, and consistent across age cohorts from 3 to less than 12 years of age.
“In the near future, we should have a safe and effective regimen (approved) for children 3 years or older,” Dr. Ghany said. “I think this will serve us well, as we try to eliminate HCV in children, who number up to 5 million cases worldwide.”
A short course of glecaprevir/pibrentasvir is approved for patients with HCV and compensated cirrhosis, and data to support that was presented last year at The Liver Meeting; however, data were not presented on patients with genotype 3, the most difficult-to-treat genotype, Dr. Ghany said. That gap was filled at this year’s meeting with a report (Abstract LP9) showing SVR12 rates of 98.4% per protocol and 95.2% in intention-to-treat analysis.
Relationship of SVR to clinical outcomes
While the impact of sustained virologic response (SVR) on all-cause mortality is clear in patients with HCV, less is known about the effect of SVR on liver-related mortality and other outcomes, Dr. Ghany said. In one study presented here (Abstract 0039), based on analysis of a Veterans Affairs database of patients with chronic HCV infection, SVR was linked to a significant reduction in liver-related mortality, while in another report (Abstract 0037), SVR was associated with significant reductions in acute coronary syndromes, end-stage renal disease, and ischemic stroke.
Similarly, a multinational, propensity score–matched analysis (Abstract 0040) demonstrated that SVR had an impact on 5-year overall survival and liver-related survival in patients with HCV-related hepatocellular carcinoma (HCC). “For HCC patients who are candidates for HCC therapy, consideration should also be given to treating these individuals (with DAA therapy) because of the impact on overall survival,” Dr. Ghany said.
Preemptive DAA therapy in organ transplantation
Exciting new data show that preemptive therapy, given for short durations, appears to either prevent or cure HCV infection after organ transplant, said Dr. Ghany.
A retrospective analysis by Wijarnpreecha and colleagues (Abstract 0003) showed that 12 or 24 weeks of direct-acting antiviral (DAA) therapy resulted in an SVR12 for 24 out of 24 HCV-seropositive to HCV-seronegative liver transplants, while Durand and colleagues (Abstract 0042) showed that just 4 weeks of pre- and postexposure DAA prophylaxis resulted in SVR12s for 9 out of 9 HCV donor-positive, recipient-negative kidney transplants. Finally, Feld and coauthors (Abstract 0038) showed that preemptive ezetimibe with DAA therapy for 7 days prevented or rapidly cured infection in an experience that included 16 HCV-positive organ donors and 25 HCV-negative recipients.
“While these data are very encouraging, I think we do need to have long-term follow-up of these patients for graft survival, as well as the effect on wait times,” Dr. Ghany said.
Dr. Ghany reported no disclosures related to his presentation.
REPORTING FROM THE LIVER MEETING 2019
Expanding the reach of available cancer therapies
In this edition of “How I Will Treat My Next Patient,” I highlight two articles that demonstrate the safety of established treatments – nephrectomy and stereotactic ablative body radiotherapy (SABR) – in patient populations that previously may have been excluded from those treatments at many centers.
Nephrectomy in advanced RCC
Nirmish Singla, MD, and colleagues reported a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced renal cell carcinoma (RCC) who had received front- or later-line immune checkpoint inhibitor therapy (ICIs). Half had received nivolumab alone; the others received nivolumab plus ipilimumab. Surgery was performed laparoscopically in five cases (Urol Oncol. 2019 Dec;37[12]:924-31).
No patient experienced a major intraoperative complication. Four experienced postoperative complications, the majority of which were addressed with interventional radiology procedures. The median hospital stay was 4 days. One patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism and sepsis. Six of the 10 patients did not have any complications or readmissions. There were no immune-related toxicities and no wound-healing issues. ICI therapy was resumed postoperatively in six patients.
At nephrectomy (plus or minus metastatectomy), one patient achieved a response to immunotherapy in the primary tumor, and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer. All surgical margins were negative.
During a median postoperative follow-up of 180 days, nephrectomy following ICI was safe. Pathologic response in both the primary tumor and metastatic sites was encouraging.
What this means in clinical practice
In medical school, all of us are admonished not to be afraid to unlearn something and to learn something new. Historically, nephrectomy was felt to be helpful in improving overall survival in patients with advanced RCC. Effective targeted therapies and ICIs have caused us to question the role of nephrectomy and its timing, since 20%-40% of patients who have apparently localized RCC at the time of nephrectomy develop recurrences within 3 years. Preoperative therapy could mitigate potentially aggressive tumor biology, treat micrometastatic disease, and help select patients who should not be treated surgically.
In the CARMENA trial of the treatment of advanced RCC patients with the tyrosine kinase inhibitor sunitinib versus nephrectomy followed by sunitinib, most patients could avoid nephrectomy without compromising survival (N Engl J Med. 2018; 379:417-27). Results were updated at the 2019 annual meeting of the American Society of Clinical Oncology. Overall, nephrectomy was not beneficial. However, delayed nephrectomy (after sunitinib) appeared be beneficial for good responders with only one IMDC (International Metastatic RCC Database Consortium) risk factor and only one metastatic site.
The small study by Dr. Singla and colleagues illustrates that nephrectomy is feasible after ICI, plus or minus anti-CTLA4-targeted treatment, and that favorable histologic results can be achieved. With ICI plus or minus anti-CTLA4-targeted treatment, no patient had progressive disease prior to surgery. This experience is germane in view of recently updated results of the CheckMate 214 trial, showing superior overall survival, response rates, and response duration for nivolumab plus ipilimumab, in comparison with sunitinib.
There are still unresolved questions, including whether these favorable outcomes can be achieved in community practice and whether there are genomic or immunohistochemistry expression profiles to select patients who can benefit from this approach. It’s unclear whether there are practical issues that influence outcome, such as type of ICI, number of preoperative treatment cycles, and additional systemic therapies including postoperative treatment. However, the current series rings the starting bell for the study of those questions and a promising era for patients with this deadly disease.
SABR in moderately central NSCLC
SABR to peripheral, small non–small cell lung cancers (NSCLCs) produces high local control rates, with low grade 3-4 toxicity, and is an alternative to resection in patients who are unfit for surgery. In a pragmatic, community-based, prospective cohort experience in Scotland, Robert Rulach, MBChB, and colleagues, treated 50 T1-2N0M0 NSCLC patients with SABR 50-Gy in five fractions (Clin Oncol. 2019 Oct 10. doi: 10.1016/j.clon.2019.09.055). The dose and fractionation schedule was safe and effective in the phase 1/2 RTOG 0813 trial and is concordant with guidelines from the National Comprehensive Cancer Network (NCCN).
All of the tumors were moderately central, as in the RTOG trial. One patient had an additional tumor that was ultracentral. Notably, 84% of patients were deemed medically unfit for surgery.
All patients completed radiotherapy without treatment delays. Two patients died within 90 days of treatment. There were no grade 4 or grade 5 toxicities and the overall rate of grade 3 toxicity was 4%. With a median follow-up of 25.2 months, 34 patients died: 18 from causes unrelated to cancer and 16 from cancer recurrence. The median overall survival was 27 months. The 2-year overall survival rate was 67.6%, commensurate with the rate seen in RTOG 0813.
The researchers concluded that, for frail patients with centrally-located NSCLC treated uniformly in a community practice, SABR with the RTOG 0813 treatment protocol produced acceptable toxicity and overall survival comparable with the published literature.
What this means in clinical practice
The results and conclusions of the study by Dr. Rulach and colleagues are straightforward: SABR can be used for centrally-located NSCLC without producing massive hemoptysis, bronchial stricture, and fistula formation. Since the majority of patients had no histologic diagnosis, T1N0 lesions, and no routine follow-up CT scans beyond 3 months post treatment, conclusions beyond that are unjustified.
In a community-based practice, NCCN guideline–concordant SABR treatment in moderately centrally-located NSCLC was safely delivered. For the burgeoning population of medically inoperable and/or elderly NSCLC patients, this alone is reassuring for clinicians and is helpful information for patients who require and/or desire nonsurgical treatment.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two articles that demonstrate the safety of established treatments – nephrectomy and stereotactic ablative body radiotherapy (SABR) – in patient populations that previously may have been excluded from those treatments at many centers.
Nephrectomy in advanced RCC
Nirmish Singla, MD, and colleagues reported a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced renal cell carcinoma (RCC) who had received front- or later-line immune checkpoint inhibitor therapy (ICIs). Half had received nivolumab alone; the others received nivolumab plus ipilimumab. Surgery was performed laparoscopically in five cases (Urol Oncol. 2019 Dec;37[12]:924-31).
No patient experienced a major intraoperative complication. Four experienced postoperative complications, the majority of which were addressed with interventional radiology procedures. The median hospital stay was 4 days. One patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism and sepsis. Six of the 10 patients did not have any complications or readmissions. There were no immune-related toxicities and no wound-healing issues. ICI therapy was resumed postoperatively in six patients.
At nephrectomy (plus or minus metastatectomy), one patient achieved a response to immunotherapy in the primary tumor, and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer. All surgical margins were negative.
During a median postoperative follow-up of 180 days, nephrectomy following ICI was safe. Pathologic response in both the primary tumor and metastatic sites was encouraging.
What this means in clinical practice
In medical school, all of us are admonished not to be afraid to unlearn something and to learn something new. Historically, nephrectomy was felt to be helpful in improving overall survival in patients with advanced RCC. Effective targeted therapies and ICIs have caused us to question the role of nephrectomy and its timing, since 20%-40% of patients who have apparently localized RCC at the time of nephrectomy develop recurrences within 3 years. Preoperative therapy could mitigate potentially aggressive tumor biology, treat micrometastatic disease, and help select patients who should not be treated surgically.
In the CARMENA trial of the treatment of advanced RCC patients with the tyrosine kinase inhibitor sunitinib versus nephrectomy followed by sunitinib, most patients could avoid nephrectomy without compromising survival (N Engl J Med. 2018; 379:417-27). Results were updated at the 2019 annual meeting of the American Society of Clinical Oncology. Overall, nephrectomy was not beneficial. However, delayed nephrectomy (after sunitinib) appeared be beneficial for good responders with only one IMDC (International Metastatic RCC Database Consortium) risk factor and only one metastatic site.
The small study by Dr. Singla and colleagues illustrates that nephrectomy is feasible after ICI, plus or minus anti-CTLA4-targeted treatment, and that favorable histologic results can be achieved. With ICI plus or minus anti-CTLA4-targeted treatment, no patient had progressive disease prior to surgery. This experience is germane in view of recently updated results of the CheckMate 214 trial, showing superior overall survival, response rates, and response duration for nivolumab plus ipilimumab, in comparison with sunitinib.
There are still unresolved questions, including whether these favorable outcomes can be achieved in community practice and whether there are genomic or immunohistochemistry expression profiles to select patients who can benefit from this approach. It’s unclear whether there are practical issues that influence outcome, such as type of ICI, number of preoperative treatment cycles, and additional systemic therapies including postoperative treatment. However, the current series rings the starting bell for the study of those questions and a promising era for patients with this deadly disease.
SABR in moderately central NSCLC
SABR to peripheral, small non–small cell lung cancers (NSCLCs) produces high local control rates, with low grade 3-4 toxicity, and is an alternative to resection in patients who are unfit for surgery. In a pragmatic, community-based, prospective cohort experience in Scotland, Robert Rulach, MBChB, and colleagues, treated 50 T1-2N0M0 NSCLC patients with SABR 50-Gy in five fractions (Clin Oncol. 2019 Oct 10. doi: 10.1016/j.clon.2019.09.055). The dose and fractionation schedule was safe and effective in the phase 1/2 RTOG 0813 trial and is concordant with guidelines from the National Comprehensive Cancer Network (NCCN).
All of the tumors were moderately central, as in the RTOG trial. One patient had an additional tumor that was ultracentral. Notably, 84% of patients were deemed medically unfit for surgery.
All patients completed radiotherapy without treatment delays. Two patients died within 90 days of treatment. There were no grade 4 or grade 5 toxicities and the overall rate of grade 3 toxicity was 4%. With a median follow-up of 25.2 months, 34 patients died: 18 from causes unrelated to cancer and 16 from cancer recurrence. The median overall survival was 27 months. The 2-year overall survival rate was 67.6%, commensurate with the rate seen in RTOG 0813.
The researchers concluded that, for frail patients with centrally-located NSCLC treated uniformly in a community practice, SABR with the RTOG 0813 treatment protocol produced acceptable toxicity and overall survival comparable with the published literature.
What this means in clinical practice
The results and conclusions of the study by Dr. Rulach and colleagues are straightforward: SABR can be used for centrally-located NSCLC without producing massive hemoptysis, bronchial stricture, and fistula formation. Since the majority of patients had no histologic diagnosis, T1N0 lesions, and no routine follow-up CT scans beyond 3 months post treatment, conclusions beyond that are unjustified.
In a community-based practice, NCCN guideline–concordant SABR treatment in moderately centrally-located NSCLC was safely delivered. For the burgeoning population of medically inoperable and/or elderly NSCLC patients, this alone is reassuring for clinicians and is helpful information for patients who require and/or desire nonsurgical treatment.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I Will Treat My Next Patient,” I highlight two articles that demonstrate the safety of established treatments – nephrectomy and stereotactic ablative body radiotherapy (SABR) – in patient populations that previously may have been excluded from those treatments at many centers.
Nephrectomy in advanced RCC
Nirmish Singla, MD, and colleagues reported a single-center retrospective cohort study, assessing outcomes of 11 nephrectomies (10 radical, 1 partial) in 10 patients with advanced renal cell carcinoma (RCC) who had received front- or later-line immune checkpoint inhibitor therapy (ICIs). Half had received nivolumab alone; the others received nivolumab plus ipilimumab. Surgery was performed laparoscopically in five cases (Urol Oncol. 2019 Dec;37[12]:924-31).
No patient experienced a major intraoperative complication. Four experienced postoperative complications, the majority of which were addressed with interventional radiology procedures. The median hospital stay was 4 days. One patient died of progressive disease more than 3 months after surgery, and another died of pulmonary embolism and sepsis. Six of the 10 patients did not have any complications or readmissions. There were no immune-related toxicities and no wound-healing issues. ICI therapy was resumed postoperatively in six patients.
At nephrectomy (plus or minus metastatectomy), one patient achieved a response to immunotherapy in the primary tumor, and three of four patients who underwent resection of hepatic, pulmonary, or adrenal metastases had no detectable cancer. All surgical margins were negative.
During a median postoperative follow-up of 180 days, nephrectomy following ICI was safe. Pathologic response in both the primary tumor and metastatic sites was encouraging.
What this means in clinical practice
In medical school, all of us are admonished not to be afraid to unlearn something and to learn something new. Historically, nephrectomy was felt to be helpful in improving overall survival in patients with advanced RCC. Effective targeted therapies and ICIs have caused us to question the role of nephrectomy and its timing, since 20%-40% of patients who have apparently localized RCC at the time of nephrectomy develop recurrences within 3 years. Preoperative therapy could mitigate potentially aggressive tumor biology, treat micrometastatic disease, and help select patients who should not be treated surgically.
In the CARMENA trial of the treatment of advanced RCC patients with the tyrosine kinase inhibitor sunitinib versus nephrectomy followed by sunitinib, most patients could avoid nephrectomy without compromising survival (N Engl J Med. 2018; 379:417-27). Results were updated at the 2019 annual meeting of the American Society of Clinical Oncology. Overall, nephrectomy was not beneficial. However, delayed nephrectomy (after sunitinib) appeared be beneficial for good responders with only one IMDC (International Metastatic RCC Database Consortium) risk factor and only one metastatic site.
The small study by Dr. Singla and colleagues illustrates that nephrectomy is feasible after ICI, plus or minus anti-CTLA4-targeted treatment, and that favorable histologic results can be achieved. With ICI plus or minus anti-CTLA4-targeted treatment, no patient had progressive disease prior to surgery. This experience is germane in view of recently updated results of the CheckMate 214 trial, showing superior overall survival, response rates, and response duration for nivolumab plus ipilimumab, in comparison with sunitinib.
There are still unresolved questions, including whether these favorable outcomes can be achieved in community practice and whether there are genomic or immunohistochemistry expression profiles to select patients who can benefit from this approach. It’s unclear whether there are practical issues that influence outcome, such as type of ICI, number of preoperative treatment cycles, and additional systemic therapies including postoperative treatment. However, the current series rings the starting bell for the study of those questions and a promising era for patients with this deadly disease.
SABR in moderately central NSCLC
SABR to peripheral, small non–small cell lung cancers (NSCLCs) produces high local control rates, with low grade 3-4 toxicity, and is an alternative to resection in patients who are unfit for surgery. In a pragmatic, community-based, prospective cohort experience in Scotland, Robert Rulach, MBChB, and colleagues, treated 50 T1-2N0M0 NSCLC patients with SABR 50-Gy in five fractions (Clin Oncol. 2019 Oct 10. doi: 10.1016/j.clon.2019.09.055). The dose and fractionation schedule was safe and effective in the phase 1/2 RTOG 0813 trial and is concordant with guidelines from the National Comprehensive Cancer Network (NCCN).
All of the tumors were moderately central, as in the RTOG trial. One patient had an additional tumor that was ultracentral. Notably, 84% of patients were deemed medically unfit for surgery.
All patients completed radiotherapy without treatment delays. Two patients died within 90 days of treatment. There were no grade 4 or grade 5 toxicities and the overall rate of grade 3 toxicity was 4%. With a median follow-up of 25.2 months, 34 patients died: 18 from causes unrelated to cancer and 16 from cancer recurrence. The median overall survival was 27 months. The 2-year overall survival rate was 67.6%, commensurate with the rate seen in RTOG 0813.
The researchers concluded that, for frail patients with centrally-located NSCLC treated uniformly in a community practice, SABR with the RTOG 0813 treatment protocol produced acceptable toxicity and overall survival comparable with the published literature.
What this means in clinical practice
The results and conclusions of the study by Dr. Rulach and colleagues are straightforward: SABR can be used for centrally-located NSCLC without producing massive hemoptysis, bronchial stricture, and fistula formation. Since the majority of patients had no histologic diagnosis, T1N0 lesions, and no routine follow-up CT scans beyond 3 months post treatment, conclusions beyond that are unjustified.
In a community-based practice, NCCN guideline–concordant SABR treatment in moderately centrally-located NSCLC was safely delivered. For the burgeoning population of medically inoperable and/or elderly NSCLC patients, this alone is reassuring for clinicians and is helpful information for patients who require and/or desire nonsurgical treatment.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
Findings confirm link between methimazole and risk for acute pancreatitis
CHICAGO –
After 6 months of methimazole use, the odds ratio for acute pancreatitis was 2.02, with a nonsignificant risk elevation for propylthiouracil use after a similar duration, Laszlo Hegedüs, MD, reported at the annual meeting of the American Thyroid Association.
“Ongoing methimazole, but not propylthiouracil, use is associated with an increased risk of acute pancreatitis,” he said.
Dr. Hegedüs, professor of endocrinology and metabolism at the University of Odense (Denmark), said that the European Medicines Association has noted postmarketing reports of acute pancreatitis in patients who received the antithyroid drug methimazole, as well as its prodrug, carbimazole. The agency has accordingly contraindicated antithyroid drug use for patients who previously experienced acute pancreatitis after receiving one of these drugs, advising that methimazole should be “discontinued immediately” should a patient develop acute pancreatitis.
However, investigation of the antithyroid drug–pancreatitis association had been limited to aggregating those case reports, so Dr. Hegedüs and colleagues decided to use Danish medical record and registry data to investigate the association in a nationwide, controlled study that looked at both duration of therapy and total antithyroid drug use.
During the period from 1995-2018, a total of 118,649 patients who used antithyroid drugs were found in the 5.5 million individuals in the Statistics Denmark registry. Dr. Hegedüs and his colleagues also pulled in patient registry and national prescription registry data, as well as civil vital statistics data.
Of those who used antithyroid drugs, 103,825 patients used methimazole, and 14,824 used propylthiouracil. The researchers found 43,580 instances of hospitalization for first-time acute pancreatitis in the pooled antithyroid drug data. Of those, however, just 226 (0.5%) occurred in patients using methimazole, and 19 (0.04%) in those using propylthiouracil at the time of pancreatitis onset.
To ascertain the risk of acute pancreatitis in patients using antithyroid drugs for various durations, Dr. Hegedüs and his colleagues used a case-crossover study design. In the case-crossover technique, patients served as their own controls, because each patient was both exposed and not exposed to antithyroid drugs at some point during the study period. Antithyroid drugs are well suited to this study design, explained Dr. Hegedüs, because they are given for a limited time. A case-crossover design can be used with a small sample size and effectively controls for potentially confounding variables.
The odds ratio for acute pancreatitis in methimazole users after 3 months of exposure was 1.51, with a 95% confidence interval of 1.12-2.02. After 3 months of propylthiouracil exposure, the odds ratio for acute pancreatitis was 1.16 (95% CI 0.46-2.3). At 6 months, the odds ratio of 2.02 for methimazole was similarly statistically significant (95% CI, 1.50-2.78), whereas the odds ratio of 1.40 for propylthiouracil use was not significant (95% CI, 0.58-3.34).
The researchers also wanted to find out whether the cumulative drug dose affected the risk of acute pancreatitis, so they drew from the antithyroid drug population to conduct a case-control study. Here, the investigators matched data from four control patients to each case of acute pancreatitis. The researchers also controlled for sex, age, comorbidities, and prior use of drugs associated with pancreatitis.
Overall, 20% of the 692 methimazole users and their controls were men, as were 16% of the 108 propylthiouracil users, in the case-control study.
Just more than half of patients overall had a total dose exposure of 200 to 1,200 defined daily dose (DDD) – a measure developed by the World Health Organization to denote the assumed average adult dose per day of a medication – with about a quarter of patients receiving a total antithyroid drug dose more than 1,200 DDD and about 20% receiving a dose exposure of less than 200 DDD. The risk of acute pancreatitis did not increase with increased total exposure to antithyroid drugs.
“There is no evidence of a cumulative dose effect of either methimazole or propylthiouracil on the risk of acute pancreatitis,” said Dr. Hegedüs. However, “the warning of the European Medicines Agency seems justified,” he added. “The frequency of acute pancreatitis in acute methimazole users is of a similar magnitude [to that] reported for agranulocytosis,” a known, dire complication of antithyroid drug use. Patients should be advised of the potential complication and informed of signs and symptoms of acute pancreatitis, he said.
Dr. Hegedüs noted that the study had the advantage of using validated epidemiologic methods to look at drug exposure and outcomes at a nationwide scale. However, the registries from which the data were drawn also have limitations. The investigators could not determine the severity of hyperthyroidism, he said, and the relatively rare occurrence of acute pancreatitis meant that there was not sufficient statistical power to look at the subgroup of individuals who had Grave’s disease and to compare them with those with nodular toxic goiter.
He advised conducting a confirmatory study in an independent cohort, as well as further investigating the yet unknown mechanism of action for the link between the antithyroid drug and acute pancreatitis.
Dr. Hegedüs reported that he had no relevant conflicts of interest and reported no outside sources of funding.
Help your patients understand the symptoms, treatments and complications of pancreatitis by sharing AGA patient education at https://www.gastro.org/
SOURCE: Hegedüs, L. et al. ATA 2019, Short Call Oral Abstract 6 .
CHICAGO –
After 6 months of methimazole use, the odds ratio for acute pancreatitis was 2.02, with a nonsignificant risk elevation for propylthiouracil use after a similar duration, Laszlo Hegedüs, MD, reported at the annual meeting of the American Thyroid Association.
“Ongoing methimazole, but not propylthiouracil, use is associated with an increased risk of acute pancreatitis,” he said.
Dr. Hegedüs, professor of endocrinology and metabolism at the University of Odense (Denmark), said that the European Medicines Association has noted postmarketing reports of acute pancreatitis in patients who received the antithyroid drug methimazole, as well as its prodrug, carbimazole. The agency has accordingly contraindicated antithyroid drug use for patients who previously experienced acute pancreatitis after receiving one of these drugs, advising that methimazole should be “discontinued immediately” should a patient develop acute pancreatitis.
However, investigation of the antithyroid drug–pancreatitis association had been limited to aggregating those case reports, so Dr. Hegedüs and colleagues decided to use Danish medical record and registry data to investigate the association in a nationwide, controlled study that looked at both duration of therapy and total antithyroid drug use.
During the period from 1995-2018, a total of 118,649 patients who used antithyroid drugs were found in the 5.5 million individuals in the Statistics Denmark registry. Dr. Hegedüs and his colleagues also pulled in patient registry and national prescription registry data, as well as civil vital statistics data.
Of those who used antithyroid drugs, 103,825 patients used methimazole, and 14,824 used propylthiouracil. The researchers found 43,580 instances of hospitalization for first-time acute pancreatitis in the pooled antithyroid drug data. Of those, however, just 226 (0.5%) occurred in patients using methimazole, and 19 (0.04%) in those using propylthiouracil at the time of pancreatitis onset.
To ascertain the risk of acute pancreatitis in patients using antithyroid drugs for various durations, Dr. Hegedüs and his colleagues used a case-crossover study design. In the case-crossover technique, patients served as their own controls, because each patient was both exposed and not exposed to antithyroid drugs at some point during the study period. Antithyroid drugs are well suited to this study design, explained Dr. Hegedüs, because they are given for a limited time. A case-crossover design can be used with a small sample size and effectively controls for potentially confounding variables.
The odds ratio for acute pancreatitis in methimazole users after 3 months of exposure was 1.51, with a 95% confidence interval of 1.12-2.02. After 3 months of propylthiouracil exposure, the odds ratio for acute pancreatitis was 1.16 (95% CI 0.46-2.3). At 6 months, the odds ratio of 2.02 for methimazole was similarly statistically significant (95% CI, 1.50-2.78), whereas the odds ratio of 1.40 for propylthiouracil use was not significant (95% CI, 0.58-3.34).
The researchers also wanted to find out whether the cumulative drug dose affected the risk of acute pancreatitis, so they drew from the antithyroid drug population to conduct a case-control study. Here, the investigators matched data from four control patients to each case of acute pancreatitis. The researchers also controlled for sex, age, comorbidities, and prior use of drugs associated with pancreatitis.
Overall, 20% of the 692 methimazole users and their controls were men, as were 16% of the 108 propylthiouracil users, in the case-control study.
Just more than half of patients overall had a total dose exposure of 200 to 1,200 defined daily dose (DDD) – a measure developed by the World Health Organization to denote the assumed average adult dose per day of a medication – with about a quarter of patients receiving a total antithyroid drug dose more than 1,200 DDD and about 20% receiving a dose exposure of less than 200 DDD. The risk of acute pancreatitis did not increase with increased total exposure to antithyroid drugs.
“There is no evidence of a cumulative dose effect of either methimazole or propylthiouracil on the risk of acute pancreatitis,” said Dr. Hegedüs. However, “the warning of the European Medicines Agency seems justified,” he added. “The frequency of acute pancreatitis in acute methimazole users is of a similar magnitude [to that] reported for agranulocytosis,” a known, dire complication of antithyroid drug use. Patients should be advised of the potential complication and informed of signs and symptoms of acute pancreatitis, he said.
Dr. Hegedüs noted that the study had the advantage of using validated epidemiologic methods to look at drug exposure and outcomes at a nationwide scale. However, the registries from which the data were drawn also have limitations. The investigators could not determine the severity of hyperthyroidism, he said, and the relatively rare occurrence of acute pancreatitis meant that there was not sufficient statistical power to look at the subgroup of individuals who had Grave’s disease and to compare them with those with nodular toxic goiter.
He advised conducting a confirmatory study in an independent cohort, as well as further investigating the yet unknown mechanism of action for the link between the antithyroid drug and acute pancreatitis.
Dr. Hegedüs reported that he had no relevant conflicts of interest and reported no outside sources of funding.
Help your patients understand the symptoms, treatments and complications of pancreatitis by sharing AGA patient education at https://www.gastro.org/
SOURCE: Hegedüs, L. et al. ATA 2019, Short Call Oral Abstract 6 .
CHICAGO –
After 6 months of methimazole use, the odds ratio for acute pancreatitis was 2.02, with a nonsignificant risk elevation for propylthiouracil use after a similar duration, Laszlo Hegedüs, MD, reported at the annual meeting of the American Thyroid Association.
“Ongoing methimazole, but not propylthiouracil, use is associated with an increased risk of acute pancreatitis,” he said.
Dr. Hegedüs, professor of endocrinology and metabolism at the University of Odense (Denmark), said that the European Medicines Association has noted postmarketing reports of acute pancreatitis in patients who received the antithyroid drug methimazole, as well as its prodrug, carbimazole. The agency has accordingly contraindicated antithyroid drug use for patients who previously experienced acute pancreatitis after receiving one of these drugs, advising that methimazole should be “discontinued immediately” should a patient develop acute pancreatitis.
However, investigation of the antithyroid drug–pancreatitis association had been limited to aggregating those case reports, so Dr. Hegedüs and colleagues decided to use Danish medical record and registry data to investigate the association in a nationwide, controlled study that looked at both duration of therapy and total antithyroid drug use.
During the period from 1995-2018, a total of 118,649 patients who used antithyroid drugs were found in the 5.5 million individuals in the Statistics Denmark registry. Dr. Hegedüs and his colleagues also pulled in patient registry and national prescription registry data, as well as civil vital statistics data.
Of those who used antithyroid drugs, 103,825 patients used methimazole, and 14,824 used propylthiouracil. The researchers found 43,580 instances of hospitalization for first-time acute pancreatitis in the pooled antithyroid drug data. Of those, however, just 226 (0.5%) occurred in patients using methimazole, and 19 (0.04%) in those using propylthiouracil at the time of pancreatitis onset.
To ascertain the risk of acute pancreatitis in patients using antithyroid drugs for various durations, Dr. Hegedüs and his colleagues used a case-crossover study design. In the case-crossover technique, patients served as their own controls, because each patient was both exposed and not exposed to antithyroid drugs at some point during the study period. Antithyroid drugs are well suited to this study design, explained Dr. Hegedüs, because they are given for a limited time. A case-crossover design can be used with a small sample size and effectively controls for potentially confounding variables.
The odds ratio for acute pancreatitis in methimazole users after 3 months of exposure was 1.51, with a 95% confidence interval of 1.12-2.02. After 3 months of propylthiouracil exposure, the odds ratio for acute pancreatitis was 1.16 (95% CI 0.46-2.3). At 6 months, the odds ratio of 2.02 for methimazole was similarly statistically significant (95% CI, 1.50-2.78), whereas the odds ratio of 1.40 for propylthiouracil use was not significant (95% CI, 0.58-3.34).
The researchers also wanted to find out whether the cumulative drug dose affected the risk of acute pancreatitis, so they drew from the antithyroid drug population to conduct a case-control study. Here, the investigators matched data from four control patients to each case of acute pancreatitis. The researchers also controlled for sex, age, comorbidities, and prior use of drugs associated with pancreatitis.
Overall, 20% of the 692 methimazole users and their controls were men, as were 16% of the 108 propylthiouracil users, in the case-control study.
Just more than half of patients overall had a total dose exposure of 200 to 1,200 defined daily dose (DDD) – a measure developed by the World Health Organization to denote the assumed average adult dose per day of a medication – with about a quarter of patients receiving a total antithyroid drug dose more than 1,200 DDD and about 20% receiving a dose exposure of less than 200 DDD. The risk of acute pancreatitis did not increase with increased total exposure to antithyroid drugs.
“There is no evidence of a cumulative dose effect of either methimazole or propylthiouracil on the risk of acute pancreatitis,” said Dr. Hegedüs. However, “the warning of the European Medicines Agency seems justified,” he added. “The frequency of acute pancreatitis in acute methimazole users is of a similar magnitude [to that] reported for agranulocytosis,” a known, dire complication of antithyroid drug use. Patients should be advised of the potential complication and informed of signs and symptoms of acute pancreatitis, he said.
Dr. Hegedüs noted that the study had the advantage of using validated epidemiologic methods to look at drug exposure and outcomes at a nationwide scale. However, the registries from which the data were drawn also have limitations. The investigators could not determine the severity of hyperthyroidism, he said, and the relatively rare occurrence of acute pancreatitis meant that there was not sufficient statistical power to look at the subgroup of individuals who had Grave’s disease and to compare them with those with nodular toxic goiter.
He advised conducting a confirmatory study in an independent cohort, as well as further investigating the yet unknown mechanism of action for the link between the antithyroid drug and acute pancreatitis.
Dr. Hegedüs reported that he had no relevant conflicts of interest and reported no outside sources of funding.
Help your patients understand the symptoms, treatments and complications of pancreatitis by sharing AGA patient education at https://www.gastro.org/
SOURCE: Hegedüs, L. et al. ATA 2019, Short Call Oral Abstract 6 .
REPORTING FROM ATA 2019
Key clinical point: Patients taking methimazole are at increased risk for acute pancreatitis.
Major finding: After 6 months of methimazole use, the odds ratio for acute pancreatitis was 2.02, with a nonsignificant risk elevation for propylthiouracil use of similar duration.
Study details: Danish registry-based, case-control and case-crossover study of 118,649 patients on antithyroid drugs.
Disclosures: Dr. Hegedüs reported no outside sources of funding and no conflicts of interest.
Source: Hegedüs L et al. ATA 2019, Short Call Oral Abstract 6.
A unified app platform helps gastroenterologists achieve a digital transformation
CHICAGO – A gastroenterologist-founded tech firm is making big waves in digital health care as Rx.Health, a spinoff from Mount Sinai Hospitals, New York, partners with the American Gastroenterological Association and other professional societies to deliver health solutions to the palms of patients’ hands.
“I would make the argument that disruption doesn’t have to come from the West Coast. It can come from savvy East Coasters, as well as Midwesterners, as well as Southerners,” said Ashish Atreja, MD, MPH, chief innovation officer of medicine at Mount Sinai Hospitals, New York.
At his home institution, where Dr. Atreja also serves on the gastroenterology faculty at the Icahn School of Medicine, the discussion about digital health began as Mount Sinai experienced rapid expansion. “So that has been a learning ground for us – to say, ‘What is happening across different hospitals? How are we going to standardize care?’ ” he said, speaking at the AGA Partners in Value Meeting.
“We’re looking at digital health to do it,” and the digital initiative dovetails perfectly with the strong value-based health mission of Mount Sinai, he added. “We say that, if our hospital beds are filled, then we are failing,” although the institution’s biggest revenue stream is from inpatient care. “We really have to look beyond the four walls of the hospital to provide care.”
The digital innovation laboratory at Mount Sinai was set up about 6 years ago, making it one of the first such centers in the country. It took about a year to build a team that had the technical skills to build apps in house, but once the ball got rolling, “it has been a fascinating journey,” said Dr. Atreja.
Innovation doesn’t always mean adoption
When Dr. Atreja and his colleagues took apps that were powerful data collection tools and put them out for general patient use, “We only saw 6% adoption ... because the patients forgot the names. They mistyped the names. They got lost in 60,000 apps. They forgot the activation code.
“And even if they got all of this, 20% of patients didn’t have space in their smartphones anyway.”
That’s when Dr. Atreja and his collaborators realized they didn’t really have an innovation problem, but rather a transformation problem – they needed to change the existing digital patchwork into a clinically meaningful intervention.
At this turning point, the Mount Sinai digital innovation team realized physicians could use evidence-based apps “and actually prescribe them – much the same way that you prescribe medication. ... So this was our ‘aha’ moment 3 years ago,” said Dr. Atreja.
Now, at Mount Sinai, apps are integrated with the electronic health record and can be prescribed with a few clicks. With the integrated digital prescription platform, patient activation of the apps has increased to 92%, said Dr. Atreja.
Currently, about 25 projects using this integrated system are being initiated within the Mount Sinai health system, and 35-50 external projects are underway in association with Rx.Health, a spin-off of the Mount Sinai efforts. Dr. Atreja serves as chief strategy officer for Rx.Health.
In all, 22 health systems are using the app platform at present, which bundles many facets of digital health – health education, remote monitoring, telehealth, secure messaging, to name a few.
The unified platform, said Dr. Atreja, “allows all of us – clinicians, business drivers, tech, researchers – to become creators and digital practitioners.”
Case study: Colonoscopy
After a particularly discouraging day in the endoscopy suite in which six of seven patients had inadequate bowel preparation for colonoscopy, Dr. Atreja dug a little further into Mount Sinai’s endoscopy data. “I realized we were losing one million dollars a year because of no-shows and inadequate bowel preparation,” he said.
A higher success rate could be achieved with bowel preparation if enough staff time is dedicated to repeat phone calls, he conceded. “But you are spending $300,000 just on a brute force solution” of massive staff resources, he said.
In the Mount Sinai example, when all missed opportunities are considered, “you’re looking at 4 to 5 million dollars that we’re leaking because we are not able to engage patients at the right time.”
Gastrointestinal procedures are a major source for revenue leaks, he said. Patients may miss procedures or be late; up to 1 in 4 patients may have poor bowel preparation, and sometimes patients arrive without a plan for a ride home after a procedure that requires sedation.
Other care gaps include the 30%-70% of patients who don’t return at recommended screening intervals, and patients who have positive fecal immunohistochemical testing but don’t receive a colonoscopy. Some patients have colonoscopies ordered, but never scheduled, and still others are never offered any colorectal cancer surveillance testing at all.
It’s no wonder patients are confused, said Dr. Atreja, providing an example of one center’s colonoscopy preparation instructions for split-dose polyethylene glycol bowel preparation. Patients must closely follow a full page of bullet points to be completed at precise time intervals. “One in four patients actually loses the paper by the time they need it before the procedure,” he said. Another 40% don’t look at instructions until it’s too late to prepare adequately or to line up an escort to bring them home post procedure.
This scenario, he said, shows that “it’s not in the science of medicine, but in the practice of medicine, that we are failing. ... So how about we completely change the game and create a real-time digital navigation for the patient?”
The digital alternative to the slip of paper is a real-time patient navigation tool that guides patients through the entire colonoscopy preparation process. “Based on where the patient is at that point in time, and the procedure, and the bowel prep,” the app gives the patient timely and relevant information: what the procedure is like, why bowel preparation is important, and how preparation is correctly performed, explained Dr. Atreja.
A reminder to arrange an escort arrives on the patient’s phone a full 10 days before the procedure, with subsequent nudges. Patients even receive driving and parking directions. The day before the procedure, a last-minute query checks on transportation. “So we’re working with Uber to actually make an ... integration with Uber so they can pick up the patient if they have transportation issues.”
Post procedure, patients are asked about their experiences, and a plan for appropriate patient recall is integrated into the app as well. “The best part is, this has not been designed by anyone [other] than those in the health system. Because we already know the recommended guidelines, we know the best practices.” This, he said, is where the value of digital apps is truly created.
Early evidence gleaned from a dashboard that’s part of the digital health solution from one site using the app shows a 24% improvement in bowel preparation. Importantly, the rate of aborted procedures has been cut in half, and patient satisfaction rates are at 93%.
The endoscopy suite as digital transformation center
Now, in partnership with AGA, Dr. Atreja and his collaborators are planning a roll-out to multiple sites to see whether the savings and return on investment are replicated at other endoscopy sites. The vision expands beyond reducing revenue leaks to creating “digital transformation centers,” he said.
Digital health solutions such as this afford powerful opportunity for data collection, not only for practice optimization but also for research, said Dr. Atreja. He cited the example of endoscopic retrograde cholangiopancreatography, where procedural details could be linked to postprocedural admission rates in the service of fine-tuning one of the endoscopist’s greatest procedural challenges.
“You can create all of those clinical trial networks for devices right on the fly,” he said. In devising a clinical trial for an app-based intervention for anxiety – prevalent in those with irritable bowel disease – Dr. Atreja and his colleagues opened trial enrollment at 8 a.m., hoping to enroll 20 patients. By the end of the day, over 200 had enrolled. “We over-subscribed our trial by 10 times” in 1 day using the digital platform, he said.
Dr. Atreja is currently working with the American College of Cardiology on digital solutions for home monitoring of heart failure patients. “Partnerships with other health systems and societies are key for learning and rapid transformation – a rising tide lifts all boats,” said Dr. Atreja. “Digital medicine is not digital medicine. It is medicine. Because the practice of medicine is medicine.”
Dr. Atreja reported receiving funding from AbbVie, Janssen, Pfizer, Takeda, Astrazeneca, UCB, and Roche; the RxUniverse app has been licensed from the Icahn School of Medicine at Mount Sinai to Rx.Health.
AGA has partnered with RxHealth to support gastroenterologists’ ability to provide patient care and improve patient adherence by creating up-to-date, evidenced-based digital tools that can be prescribed at point of care. Dr. John I. Allen, the Editor in Chief of GI & Hepatology News, is on the advisory board of RxHealth and recused himself from review and approval of this story. Learn more about the program and how to become a pioneer site at https://rx.health/GI or Engage@Rx.Health .
CHICAGO – A gastroenterologist-founded tech firm is making big waves in digital health care as Rx.Health, a spinoff from Mount Sinai Hospitals, New York, partners with the American Gastroenterological Association and other professional societies to deliver health solutions to the palms of patients’ hands.
“I would make the argument that disruption doesn’t have to come from the West Coast. It can come from savvy East Coasters, as well as Midwesterners, as well as Southerners,” said Ashish Atreja, MD, MPH, chief innovation officer of medicine at Mount Sinai Hospitals, New York.
At his home institution, where Dr. Atreja also serves on the gastroenterology faculty at the Icahn School of Medicine, the discussion about digital health began as Mount Sinai experienced rapid expansion. “So that has been a learning ground for us – to say, ‘What is happening across different hospitals? How are we going to standardize care?’ ” he said, speaking at the AGA Partners in Value Meeting.
“We’re looking at digital health to do it,” and the digital initiative dovetails perfectly with the strong value-based health mission of Mount Sinai, he added. “We say that, if our hospital beds are filled, then we are failing,” although the institution’s biggest revenue stream is from inpatient care. “We really have to look beyond the four walls of the hospital to provide care.”
The digital innovation laboratory at Mount Sinai was set up about 6 years ago, making it one of the first such centers in the country. It took about a year to build a team that had the technical skills to build apps in house, but once the ball got rolling, “it has been a fascinating journey,” said Dr. Atreja.
Innovation doesn’t always mean adoption
When Dr. Atreja and his colleagues took apps that were powerful data collection tools and put them out for general patient use, “We only saw 6% adoption ... because the patients forgot the names. They mistyped the names. They got lost in 60,000 apps. They forgot the activation code.
“And even if they got all of this, 20% of patients didn’t have space in their smartphones anyway.”
That’s when Dr. Atreja and his collaborators realized they didn’t really have an innovation problem, but rather a transformation problem – they needed to change the existing digital patchwork into a clinically meaningful intervention.
At this turning point, the Mount Sinai digital innovation team realized physicians could use evidence-based apps “and actually prescribe them – much the same way that you prescribe medication. ... So this was our ‘aha’ moment 3 years ago,” said Dr. Atreja.
Now, at Mount Sinai, apps are integrated with the electronic health record and can be prescribed with a few clicks. With the integrated digital prescription platform, patient activation of the apps has increased to 92%, said Dr. Atreja.
Currently, about 25 projects using this integrated system are being initiated within the Mount Sinai health system, and 35-50 external projects are underway in association with Rx.Health, a spin-off of the Mount Sinai efforts. Dr. Atreja serves as chief strategy officer for Rx.Health.
In all, 22 health systems are using the app platform at present, which bundles many facets of digital health – health education, remote monitoring, telehealth, secure messaging, to name a few.
The unified platform, said Dr. Atreja, “allows all of us – clinicians, business drivers, tech, researchers – to become creators and digital practitioners.”
Case study: Colonoscopy
After a particularly discouraging day in the endoscopy suite in which six of seven patients had inadequate bowel preparation for colonoscopy, Dr. Atreja dug a little further into Mount Sinai’s endoscopy data. “I realized we were losing one million dollars a year because of no-shows and inadequate bowel preparation,” he said.
A higher success rate could be achieved with bowel preparation if enough staff time is dedicated to repeat phone calls, he conceded. “But you are spending $300,000 just on a brute force solution” of massive staff resources, he said.
In the Mount Sinai example, when all missed opportunities are considered, “you’re looking at 4 to 5 million dollars that we’re leaking because we are not able to engage patients at the right time.”
Gastrointestinal procedures are a major source for revenue leaks, he said. Patients may miss procedures or be late; up to 1 in 4 patients may have poor bowel preparation, and sometimes patients arrive without a plan for a ride home after a procedure that requires sedation.
Other care gaps include the 30%-70% of patients who don’t return at recommended screening intervals, and patients who have positive fecal immunohistochemical testing but don’t receive a colonoscopy. Some patients have colonoscopies ordered, but never scheduled, and still others are never offered any colorectal cancer surveillance testing at all.
It’s no wonder patients are confused, said Dr. Atreja, providing an example of one center’s colonoscopy preparation instructions for split-dose polyethylene glycol bowel preparation. Patients must closely follow a full page of bullet points to be completed at precise time intervals. “One in four patients actually loses the paper by the time they need it before the procedure,” he said. Another 40% don’t look at instructions until it’s too late to prepare adequately or to line up an escort to bring them home post procedure.
This scenario, he said, shows that “it’s not in the science of medicine, but in the practice of medicine, that we are failing. ... So how about we completely change the game and create a real-time digital navigation for the patient?”
The digital alternative to the slip of paper is a real-time patient navigation tool that guides patients through the entire colonoscopy preparation process. “Based on where the patient is at that point in time, and the procedure, and the bowel prep,” the app gives the patient timely and relevant information: what the procedure is like, why bowel preparation is important, and how preparation is correctly performed, explained Dr. Atreja.
A reminder to arrange an escort arrives on the patient’s phone a full 10 days before the procedure, with subsequent nudges. Patients even receive driving and parking directions. The day before the procedure, a last-minute query checks on transportation. “So we’re working with Uber to actually make an ... integration with Uber so they can pick up the patient if they have transportation issues.”
Post procedure, patients are asked about their experiences, and a plan for appropriate patient recall is integrated into the app as well. “The best part is, this has not been designed by anyone [other] than those in the health system. Because we already know the recommended guidelines, we know the best practices.” This, he said, is where the value of digital apps is truly created.
Early evidence gleaned from a dashboard that’s part of the digital health solution from one site using the app shows a 24% improvement in bowel preparation. Importantly, the rate of aborted procedures has been cut in half, and patient satisfaction rates are at 93%.
The endoscopy suite as digital transformation center
Now, in partnership with AGA, Dr. Atreja and his collaborators are planning a roll-out to multiple sites to see whether the savings and return on investment are replicated at other endoscopy sites. The vision expands beyond reducing revenue leaks to creating “digital transformation centers,” he said.
Digital health solutions such as this afford powerful opportunity for data collection, not only for practice optimization but also for research, said Dr. Atreja. He cited the example of endoscopic retrograde cholangiopancreatography, where procedural details could be linked to postprocedural admission rates in the service of fine-tuning one of the endoscopist’s greatest procedural challenges.
“You can create all of those clinical trial networks for devices right on the fly,” he said. In devising a clinical trial for an app-based intervention for anxiety – prevalent in those with irritable bowel disease – Dr. Atreja and his colleagues opened trial enrollment at 8 a.m., hoping to enroll 20 patients. By the end of the day, over 200 had enrolled. “We over-subscribed our trial by 10 times” in 1 day using the digital platform, he said.
Dr. Atreja is currently working with the American College of Cardiology on digital solutions for home monitoring of heart failure patients. “Partnerships with other health systems and societies are key for learning and rapid transformation – a rising tide lifts all boats,” said Dr. Atreja. “Digital medicine is not digital medicine. It is medicine. Because the practice of medicine is medicine.”
Dr. Atreja reported receiving funding from AbbVie, Janssen, Pfizer, Takeda, Astrazeneca, UCB, and Roche; the RxUniverse app has been licensed from the Icahn School of Medicine at Mount Sinai to Rx.Health.
AGA has partnered with RxHealth to support gastroenterologists’ ability to provide patient care and improve patient adherence by creating up-to-date, evidenced-based digital tools that can be prescribed at point of care. Dr. John I. Allen, the Editor in Chief of GI & Hepatology News, is on the advisory board of RxHealth and recused himself from review and approval of this story. Learn more about the program and how to become a pioneer site at https://rx.health/GI or Engage@Rx.Health .
CHICAGO – A gastroenterologist-founded tech firm is making big waves in digital health care as Rx.Health, a spinoff from Mount Sinai Hospitals, New York, partners with the American Gastroenterological Association and other professional societies to deliver health solutions to the palms of patients’ hands.
“I would make the argument that disruption doesn’t have to come from the West Coast. It can come from savvy East Coasters, as well as Midwesterners, as well as Southerners,” said Ashish Atreja, MD, MPH, chief innovation officer of medicine at Mount Sinai Hospitals, New York.
At his home institution, where Dr. Atreja also serves on the gastroenterology faculty at the Icahn School of Medicine, the discussion about digital health began as Mount Sinai experienced rapid expansion. “So that has been a learning ground for us – to say, ‘What is happening across different hospitals? How are we going to standardize care?’ ” he said, speaking at the AGA Partners in Value Meeting.
“We’re looking at digital health to do it,” and the digital initiative dovetails perfectly with the strong value-based health mission of Mount Sinai, he added. “We say that, if our hospital beds are filled, then we are failing,” although the institution’s biggest revenue stream is from inpatient care. “We really have to look beyond the four walls of the hospital to provide care.”
The digital innovation laboratory at Mount Sinai was set up about 6 years ago, making it one of the first such centers in the country. It took about a year to build a team that had the technical skills to build apps in house, but once the ball got rolling, “it has been a fascinating journey,” said Dr. Atreja.
Innovation doesn’t always mean adoption
When Dr. Atreja and his colleagues took apps that were powerful data collection tools and put them out for general patient use, “We only saw 6% adoption ... because the patients forgot the names. They mistyped the names. They got lost in 60,000 apps. They forgot the activation code.
“And even if they got all of this, 20% of patients didn’t have space in their smartphones anyway.”
That’s when Dr. Atreja and his collaborators realized they didn’t really have an innovation problem, but rather a transformation problem – they needed to change the existing digital patchwork into a clinically meaningful intervention.
At this turning point, the Mount Sinai digital innovation team realized physicians could use evidence-based apps “and actually prescribe them – much the same way that you prescribe medication. ... So this was our ‘aha’ moment 3 years ago,” said Dr. Atreja.
Now, at Mount Sinai, apps are integrated with the electronic health record and can be prescribed with a few clicks. With the integrated digital prescription platform, patient activation of the apps has increased to 92%, said Dr. Atreja.
Currently, about 25 projects using this integrated system are being initiated within the Mount Sinai health system, and 35-50 external projects are underway in association with Rx.Health, a spin-off of the Mount Sinai efforts. Dr. Atreja serves as chief strategy officer for Rx.Health.
In all, 22 health systems are using the app platform at present, which bundles many facets of digital health – health education, remote monitoring, telehealth, secure messaging, to name a few.
The unified platform, said Dr. Atreja, “allows all of us – clinicians, business drivers, tech, researchers – to become creators and digital practitioners.”
Case study: Colonoscopy
After a particularly discouraging day in the endoscopy suite in which six of seven patients had inadequate bowel preparation for colonoscopy, Dr. Atreja dug a little further into Mount Sinai’s endoscopy data. “I realized we were losing one million dollars a year because of no-shows and inadequate bowel preparation,” he said.
A higher success rate could be achieved with bowel preparation if enough staff time is dedicated to repeat phone calls, he conceded. “But you are spending $300,000 just on a brute force solution” of massive staff resources, he said.
In the Mount Sinai example, when all missed opportunities are considered, “you’re looking at 4 to 5 million dollars that we’re leaking because we are not able to engage patients at the right time.”
Gastrointestinal procedures are a major source for revenue leaks, he said. Patients may miss procedures or be late; up to 1 in 4 patients may have poor bowel preparation, and sometimes patients arrive without a plan for a ride home after a procedure that requires sedation.
Other care gaps include the 30%-70% of patients who don’t return at recommended screening intervals, and patients who have positive fecal immunohistochemical testing but don’t receive a colonoscopy. Some patients have colonoscopies ordered, but never scheduled, and still others are never offered any colorectal cancer surveillance testing at all.
It’s no wonder patients are confused, said Dr. Atreja, providing an example of one center’s colonoscopy preparation instructions for split-dose polyethylene glycol bowel preparation. Patients must closely follow a full page of bullet points to be completed at precise time intervals. “One in four patients actually loses the paper by the time they need it before the procedure,” he said. Another 40% don’t look at instructions until it’s too late to prepare adequately or to line up an escort to bring them home post procedure.
This scenario, he said, shows that “it’s not in the science of medicine, but in the practice of medicine, that we are failing. ... So how about we completely change the game and create a real-time digital navigation for the patient?”
The digital alternative to the slip of paper is a real-time patient navigation tool that guides patients through the entire colonoscopy preparation process. “Based on where the patient is at that point in time, and the procedure, and the bowel prep,” the app gives the patient timely and relevant information: what the procedure is like, why bowel preparation is important, and how preparation is correctly performed, explained Dr. Atreja.
A reminder to arrange an escort arrives on the patient’s phone a full 10 days before the procedure, with subsequent nudges. Patients even receive driving and parking directions. The day before the procedure, a last-minute query checks on transportation. “So we’re working with Uber to actually make an ... integration with Uber so they can pick up the patient if they have transportation issues.”
Post procedure, patients are asked about their experiences, and a plan for appropriate patient recall is integrated into the app as well. “The best part is, this has not been designed by anyone [other] than those in the health system. Because we already know the recommended guidelines, we know the best practices.” This, he said, is where the value of digital apps is truly created.
Early evidence gleaned from a dashboard that’s part of the digital health solution from one site using the app shows a 24% improvement in bowel preparation. Importantly, the rate of aborted procedures has been cut in half, and patient satisfaction rates are at 93%.
The endoscopy suite as digital transformation center
Now, in partnership with AGA, Dr. Atreja and his collaborators are planning a roll-out to multiple sites to see whether the savings and return on investment are replicated at other endoscopy sites. The vision expands beyond reducing revenue leaks to creating “digital transformation centers,” he said.
Digital health solutions such as this afford powerful opportunity for data collection, not only for practice optimization but also for research, said Dr. Atreja. He cited the example of endoscopic retrograde cholangiopancreatography, where procedural details could be linked to postprocedural admission rates in the service of fine-tuning one of the endoscopist’s greatest procedural challenges.
“You can create all of those clinical trial networks for devices right on the fly,” he said. In devising a clinical trial for an app-based intervention for anxiety – prevalent in those with irritable bowel disease – Dr. Atreja and his colleagues opened trial enrollment at 8 a.m., hoping to enroll 20 patients. By the end of the day, over 200 had enrolled. “We over-subscribed our trial by 10 times” in 1 day using the digital platform, he said.
Dr. Atreja is currently working with the American College of Cardiology on digital solutions for home monitoring of heart failure patients. “Partnerships with other health systems and societies are key for learning and rapid transformation – a rising tide lifts all boats,” said Dr. Atreja. “Digital medicine is not digital medicine. It is medicine. Because the practice of medicine is medicine.”
Dr. Atreja reported receiving funding from AbbVie, Janssen, Pfizer, Takeda, Astrazeneca, UCB, and Roche; the RxUniverse app has been licensed from the Icahn School of Medicine at Mount Sinai to Rx.Health.
AGA has partnered with RxHealth to support gastroenterologists’ ability to provide patient care and improve patient adherence by creating up-to-date, evidenced-based digital tools that can be prescribed at point of care. Dr. John I. Allen, the Editor in Chief of GI & Hepatology News, is on the advisory board of RxHealth and recused himself from review and approval of this story. Learn more about the program and how to become a pioneer site at https://rx.health/GI or Engage@Rx.Health .
EXPERT ANALYSIS FROM AGA PARTNERS IN VALUE 2019
Learning about and prescribing emergency contraception
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.
FDA okays cenobamate (Xcopri) for focal epilepsy
The Food and Drug Administration has approved cenobamate (Xcopri) for the treatment of partial-onset seizures in adult patients with epilepsy.
The approval on Nov. 21 was based on results from two randomized controlled trials that included more than 600 patients, the agency said in a press release.
Together, the trials showed that the study drug at doses of 100, 200, and 400 mg significantly reduced the percentage of seizures, compared with placebo.
The FDA notes that, although the recommended maintenance dose of the drug is 200 mg/day after titration, some patients may need to be titrated up to 400 mg/day.
“Xcopri is a new option to treat adults with partial-onset seizures, which is an often difficult-to-control condition that can have a significant impact on patient quality of life,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a statement.
Adverse events
As reported by Medscape Medical News, results from one of the studies upon which this approval was based were published online last week in Lancet Neurology. The findings showed that both primary endpoints were met.
Although most treatment-emergent adverse events (AEs) were reported to be mild to moderate in severity, one of these participants receiving the 200 mg dose had a drug reaction with eosinophilia and systemic symptoms (DRESS).
The FDA noted that one patient in the other trial also died when the active drug was titrated rapidly.
In an open-label safety trial of 1,339 participants that was also reviewed by the FDA, there were no cases of DRESS when patients started cenobamate at 12.5 mg/day and the dose was adjusted every 2 weeks.
Because more patients who took the drug than those taking placebo had a shortening of the QT interval greater than 20 milliseconds, cenobamate shouldn’t be used in those with hypersensitivity to the drug “or any of the inactive ingredients in Xcopri or Familial Short QT syndrome,” the agency wrote, adding that QT shortening can be associated with ventricular fibrillation, a serious heart rhythm problem.
The FDA also noted that any patient taking an antiepileptic drug should be monitored for the emergence or worsening of depressive symptoms, suicidal thoughts or behaviors, or any other changes in mood.
The most common AEs reported in the trials were somnolence, dizziness, fatigue, and diplopia (double vision).
‘Welcome option’
“The approval of Xcopri will provide clinicians with an effective medication for our patients who are continuing to have focal [partial-onset] seizures,” Michael Sperling, MD, professor of neurology and director of the Jefferson Comprehensive Epilepsy Center, Philadelphia, and an investigator in the drug’s clinical development program, said in a press release from SK Life Science.
“It is very encouraging to see that patients receiving Xcopri saw significant reductions in frequency of seizures, with some even achieving zero seizures,” Dr. Sperling added.
“There is an urgent need to advance research and introduce new treatment options. The FDA approval of Xcopri for the treatment of partial-onset seizures is a welcome option for the epilepsy community,” Beth Lewin Dean, chief executive officer of Citizens United for Research in Epilepsy, said in the same release.
SK Life Science noted in a statement that the drug is expected to be available in the United States in the second quarter of 2020 “following scheduling review” by the Drug Enforcement Administration.
This story first appeared on Medscape.com.
The Food and Drug Administration has approved cenobamate (Xcopri) for the treatment of partial-onset seizures in adult patients with epilepsy.
The approval on Nov. 21 was based on results from two randomized controlled trials that included more than 600 patients, the agency said in a press release.
Together, the trials showed that the study drug at doses of 100, 200, and 400 mg significantly reduced the percentage of seizures, compared with placebo.
The FDA notes that, although the recommended maintenance dose of the drug is 200 mg/day after titration, some patients may need to be titrated up to 400 mg/day.
“Xcopri is a new option to treat adults with partial-onset seizures, which is an often difficult-to-control condition that can have a significant impact on patient quality of life,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a statement.
Adverse events
As reported by Medscape Medical News, results from one of the studies upon which this approval was based were published online last week in Lancet Neurology. The findings showed that both primary endpoints were met.
Although most treatment-emergent adverse events (AEs) were reported to be mild to moderate in severity, one of these participants receiving the 200 mg dose had a drug reaction with eosinophilia and systemic symptoms (DRESS).
The FDA noted that one patient in the other trial also died when the active drug was titrated rapidly.
In an open-label safety trial of 1,339 participants that was also reviewed by the FDA, there were no cases of DRESS when patients started cenobamate at 12.5 mg/day and the dose was adjusted every 2 weeks.
Because more patients who took the drug than those taking placebo had a shortening of the QT interval greater than 20 milliseconds, cenobamate shouldn’t be used in those with hypersensitivity to the drug “or any of the inactive ingredients in Xcopri or Familial Short QT syndrome,” the agency wrote, adding that QT shortening can be associated with ventricular fibrillation, a serious heart rhythm problem.
The FDA also noted that any patient taking an antiepileptic drug should be monitored for the emergence or worsening of depressive symptoms, suicidal thoughts or behaviors, or any other changes in mood.
The most common AEs reported in the trials were somnolence, dizziness, fatigue, and diplopia (double vision).
‘Welcome option’
“The approval of Xcopri will provide clinicians with an effective medication for our patients who are continuing to have focal [partial-onset] seizures,” Michael Sperling, MD, professor of neurology and director of the Jefferson Comprehensive Epilepsy Center, Philadelphia, and an investigator in the drug’s clinical development program, said in a press release from SK Life Science.
“It is very encouraging to see that patients receiving Xcopri saw significant reductions in frequency of seizures, with some even achieving zero seizures,” Dr. Sperling added.
“There is an urgent need to advance research and introduce new treatment options. The FDA approval of Xcopri for the treatment of partial-onset seizures is a welcome option for the epilepsy community,” Beth Lewin Dean, chief executive officer of Citizens United for Research in Epilepsy, said in the same release.
SK Life Science noted in a statement that the drug is expected to be available in the United States in the second quarter of 2020 “following scheduling review” by the Drug Enforcement Administration.
This story first appeared on Medscape.com.
The Food and Drug Administration has approved cenobamate (Xcopri) for the treatment of partial-onset seizures in adult patients with epilepsy.
The approval on Nov. 21 was based on results from two randomized controlled trials that included more than 600 patients, the agency said in a press release.
Together, the trials showed that the study drug at doses of 100, 200, and 400 mg significantly reduced the percentage of seizures, compared with placebo.
The FDA notes that, although the recommended maintenance dose of the drug is 200 mg/day after titration, some patients may need to be titrated up to 400 mg/day.
“Xcopri is a new option to treat adults with partial-onset seizures, which is an often difficult-to-control condition that can have a significant impact on patient quality of life,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a statement.
Adverse events
As reported by Medscape Medical News, results from one of the studies upon which this approval was based were published online last week in Lancet Neurology. The findings showed that both primary endpoints were met.
Although most treatment-emergent adverse events (AEs) were reported to be mild to moderate in severity, one of these participants receiving the 200 mg dose had a drug reaction with eosinophilia and systemic symptoms (DRESS).
The FDA noted that one patient in the other trial also died when the active drug was titrated rapidly.
In an open-label safety trial of 1,339 participants that was also reviewed by the FDA, there were no cases of DRESS when patients started cenobamate at 12.5 mg/day and the dose was adjusted every 2 weeks.
Because more patients who took the drug than those taking placebo had a shortening of the QT interval greater than 20 milliseconds, cenobamate shouldn’t be used in those with hypersensitivity to the drug “or any of the inactive ingredients in Xcopri or Familial Short QT syndrome,” the agency wrote, adding that QT shortening can be associated with ventricular fibrillation, a serious heart rhythm problem.
The FDA also noted that any patient taking an antiepileptic drug should be monitored for the emergence or worsening of depressive symptoms, suicidal thoughts or behaviors, or any other changes in mood.
The most common AEs reported in the trials were somnolence, dizziness, fatigue, and diplopia (double vision).
‘Welcome option’
“The approval of Xcopri will provide clinicians with an effective medication for our patients who are continuing to have focal [partial-onset] seizures,” Michael Sperling, MD, professor of neurology and director of the Jefferson Comprehensive Epilepsy Center, Philadelphia, and an investigator in the drug’s clinical development program, said in a press release from SK Life Science.
“It is very encouraging to see that patients receiving Xcopri saw significant reductions in frequency of seizures, with some even achieving zero seizures,” Dr. Sperling added.
“There is an urgent need to advance research and introduce new treatment options. The FDA approval of Xcopri for the treatment of partial-onset seizures is a welcome option for the epilepsy community,” Beth Lewin Dean, chief executive officer of Citizens United for Research in Epilepsy, said in the same release.
SK Life Science noted in a statement that the drug is expected to be available in the United States in the second quarter of 2020 “following scheduling review” by the Drug Enforcement Administration.
This story first appeared on Medscape.com.
Good pregnancy outcomes after laparoscopic radiofrequency ablation for fibroids
VANCOUVER – A retrospective analysis of women who became pregnant after undergoing laparoscopic radiofrequency ablation (Lap-RFA) of symptomatic myomas found no evidence that the procedure negatively impacted pregnancy or birth outcomes, according to Jay Berman, MD.
The procedure is a minimally invasive alternative to myomectomy, hysterectomy, and other surgical techniques, with minimal scarring and quick recovery. But the pivotal trials excluded women who were planning to become pregnant, and the Food and Drug Administration recommends against its use in women planning a future pregnancy because of the lack of safety and efficacy data in that population.
The procedure, which gained FDA approval in 2016, combines laparoscopic ultrasound with targeted radiofrequency to heat fibroids, which then shrink over the next few months. “There have been a lot of questions from infertility specialists regarding whether Lap-RFA can be applied to their patients because there’s very little scarring and a quick return to work. It’s really a very nice outpatient procedure for dealing with fibroids,” Dr. Berman said in an interview.
There is natural concern, however, because clinicians are uncomfortable exposing women to a pregnancy risk. “I think there’s concern from many gynecologists and reproductive endocrinologists on the pregnancy outcomes following fibroid therapy, whatever that happens to be – traditional open laparoscopic myomectomy, robotic myomectomy, all of those kinds of therapies. We were interested in looking at pregnancy outcomes following [RFA for fibroids] and whether or not C-sections would need to be recommended, similar to what you see following myomectomy, where if you enter the cavity or go through more than half of the myometrium, you recommend a C-section for that patient in subsequent pregnancies,” said Dr. Berman, who is a professor of obstetrics and gynecology at Wayne State University, Detroit.
Early case studies, mostly done in Mexico and Guatemala, found the uteri of women to be normal following Lap-RFA, he said.
The results of this study are encouraging, but are far from the final word, as the data are retrospective and small. Acessa, which provided statistical analysis for the current work, is planning a prospective study. “I don’t think there’s enough to say that the labeling should be changed, but we’re moving in that direction. There needs to be a lot more information,” Dr. Berman said at the meeting sponsored by AAGL.
The study combined data from two randomized, controlled trials in the United States and Germany; six cohort studies in the United States, Germany, and Latin America; and commercial procedures performed in the United States. The researchers relied on standardized case reports that focused primarily on maternal and infant safety, and the mode of delivery. They collected data from 38 women (mean age, 36 years) who had fibroids types 1-6 that were of a maximum 0.2-13 cm in diameter. In 19 cases, it was 5.5 cm or smaller. The number of fibroids treated ranged from 1 to 31; 19 women had one or two fibroids treated.
There were a total of 43 pregnancies, 32 of which resulted in full-term live births (74%) and there was 1 preterm birth (2.3%). All infants were healthy, and there 19 vaginal births and 13 C-sections. The reasons for the C-sections were previous C-sections or obstetric indications, such as unusual bleeding, nonprogression of labor, or abnormal fetal heartbeat. There were eight spontaneous abortions (19%) and one therapeutic abortion (2.3%), and one pregnancy was ongoing (2.3%).
Dr. Berman has been a consultant for Acessa Health, Bayer, Boston Scientific, Medtronic, and Abbvie. He has been on the speakers bureau for Acessa, Merck, Boston Scientific, Medtronic, Abbvie, and Lupin. He has performed contracted research for Acessa, Bayer, Allergan, and Obseva.
VANCOUVER – A retrospective analysis of women who became pregnant after undergoing laparoscopic radiofrequency ablation (Lap-RFA) of symptomatic myomas found no evidence that the procedure negatively impacted pregnancy or birth outcomes, according to Jay Berman, MD.
The procedure is a minimally invasive alternative to myomectomy, hysterectomy, and other surgical techniques, with minimal scarring and quick recovery. But the pivotal trials excluded women who were planning to become pregnant, and the Food and Drug Administration recommends against its use in women planning a future pregnancy because of the lack of safety and efficacy data in that population.
The procedure, which gained FDA approval in 2016, combines laparoscopic ultrasound with targeted radiofrequency to heat fibroids, which then shrink over the next few months. “There have been a lot of questions from infertility specialists regarding whether Lap-RFA can be applied to their patients because there’s very little scarring and a quick return to work. It’s really a very nice outpatient procedure for dealing with fibroids,” Dr. Berman said in an interview.
There is natural concern, however, because clinicians are uncomfortable exposing women to a pregnancy risk. “I think there’s concern from many gynecologists and reproductive endocrinologists on the pregnancy outcomes following fibroid therapy, whatever that happens to be – traditional open laparoscopic myomectomy, robotic myomectomy, all of those kinds of therapies. We were interested in looking at pregnancy outcomes following [RFA for fibroids] and whether or not C-sections would need to be recommended, similar to what you see following myomectomy, where if you enter the cavity or go through more than half of the myometrium, you recommend a C-section for that patient in subsequent pregnancies,” said Dr. Berman, who is a professor of obstetrics and gynecology at Wayne State University, Detroit.
Early case studies, mostly done in Mexico and Guatemala, found the uteri of women to be normal following Lap-RFA, he said.
The results of this study are encouraging, but are far from the final word, as the data are retrospective and small. Acessa, which provided statistical analysis for the current work, is planning a prospective study. “I don’t think there’s enough to say that the labeling should be changed, but we’re moving in that direction. There needs to be a lot more information,” Dr. Berman said at the meeting sponsored by AAGL.
The study combined data from two randomized, controlled trials in the United States and Germany; six cohort studies in the United States, Germany, and Latin America; and commercial procedures performed in the United States. The researchers relied on standardized case reports that focused primarily on maternal and infant safety, and the mode of delivery. They collected data from 38 women (mean age, 36 years) who had fibroids types 1-6 that were of a maximum 0.2-13 cm in diameter. In 19 cases, it was 5.5 cm or smaller. The number of fibroids treated ranged from 1 to 31; 19 women had one or two fibroids treated.
There were a total of 43 pregnancies, 32 of which resulted in full-term live births (74%) and there was 1 preterm birth (2.3%). All infants were healthy, and there 19 vaginal births and 13 C-sections. The reasons for the C-sections were previous C-sections or obstetric indications, such as unusual bleeding, nonprogression of labor, or abnormal fetal heartbeat. There were eight spontaneous abortions (19%) and one therapeutic abortion (2.3%), and one pregnancy was ongoing (2.3%).
Dr. Berman has been a consultant for Acessa Health, Bayer, Boston Scientific, Medtronic, and Abbvie. He has been on the speakers bureau for Acessa, Merck, Boston Scientific, Medtronic, Abbvie, and Lupin. He has performed contracted research for Acessa, Bayer, Allergan, and Obseva.
VANCOUVER – A retrospective analysis of women who became pregnant after undergoing laparoscopic radiofrequency ablation (Lap-RFA) of symptomatic myomas found no evidence that the procedure negatively impacted pregnancy or birth outcomes, according to Jay Berman, MD.
The procedure is a minimally invasive alternative to myomectomy, hysterectomy, and other surgical techniques, with minimal scarring and quick recovery. But the pivotal trials excluded women who were planning to become pregnant, and the Food and Drug Administration recommends against its use in women planning a future pregnancy because of the lack of safety and efficacy data in that population.
The procedure, which gained FDA approval in 2016, combines laparoscopic ultrasound with targeted radiofrequency to heat fibroids, which then shrink over the next few months. “There have been a lot of questions from infertility specialists regarding whether Lap-RFA can be applied to their patients because there’s very little scarring and a quick return to work. It’s really a very nice outpatient procedure for dealing with fibroids,” Dr. Berman said in an interview.
There is natural concern, however, because clinicians are uncomfortable exposing women to a pregnancy risk. “I think there’s concern from many gynecologists and reproductive endocrinologists on the pregnancy outcomes following fibroid therapy, whatever that happens to be – traditional open laparoscopic myomectomy, robotic myomectomy, all of those kinds of therapies. We were interested in looking at pregnancy outcomes following [RFA for fibroids] and whether or not C-sections would need to be recommended, similar to what you see following myomectomy, where if you enter the cavity or go through more than half of the myometrium, you recommend a C-section for that patient in subsequent pregnancies,” said Dr. Berman, who is a professor of obstetrics and gynecology at Wayne State University, Detroit.
Early case studies, mostly done in Mexico and Guatemala, found the uteri of women to be normal following Lap-RFA, he said.
The results of this study are encouraging, but are far from the final word, as the data are retrospective and small. Acessa, which provided statistical analysis for the current work, is planning a prospective study. “I don’t think there’s enough to say that the labeling should be changed, but we’re moving in that direction. There needs to be a lot more information,” Dr. Berman said at the meeting sponsored by AAGL.
The study combined data from two randomized, controlled trials in the United States and Germany; six cohort studies in the United States, Germany, and Latin America; and commercial procedures performed in the United States. The researchers relied on standardized case reports that focused primarily on maternal and infant safety, and the mode of delivery. They collected data from 38 women (mean age, 36 years) who had fibroids types 1-6 that were of a maximum 0.2-13 cm in diameter. In 19 cases, it was 5.5 cm or smaller. The number of fibroids treated ranged from 1 to 31; 19 women had one or two fibroids treated.
There were a total of 43 pregnancies, 32 of which resulted in full-term live births (74%) and there was 1 preterm birth (2.3%). All infants were healthy, and there 19 vaginal births and 13 C-sections. The reasons for the C-sections were previous C-sections or obstetric indications, such as unusual bleeding, nonprogression of labor, or abnormal fetal heartbeat. There were eight spontaneous abortions (19%) and one therapeutic abortion (2.3%), and one pregnancy was ongoing (2.3%).
Dr. Berman has been a consultant for Acessa Health, Bayer, Boston Scientific, Medtronic, and Abbvie. He has been on the speakers bureau for Acessa, Merck, Boston Scientific, Medtronic, Abbvie, and Lupin. He has performed contracted research for Acessa, Bayer, Allergan, and Obseva.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Considerations on the mode of delivery for pregnant women with hepatitis C infection
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11
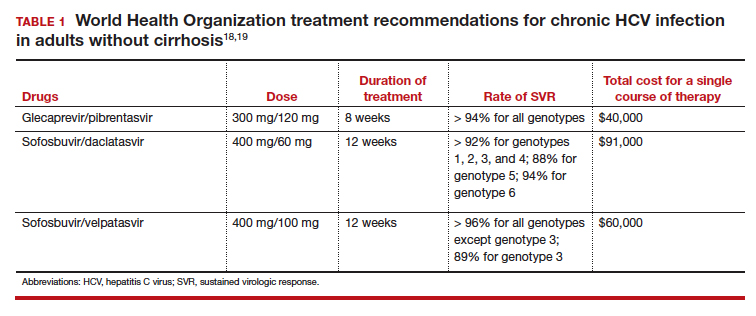
Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21
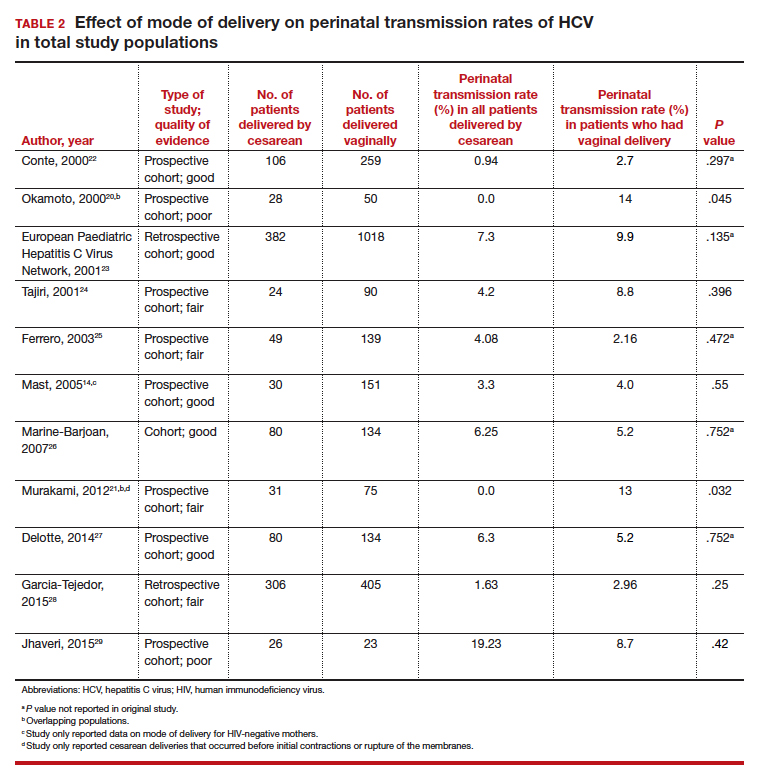
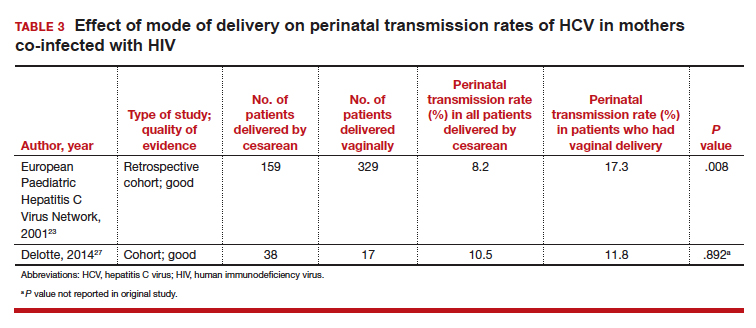
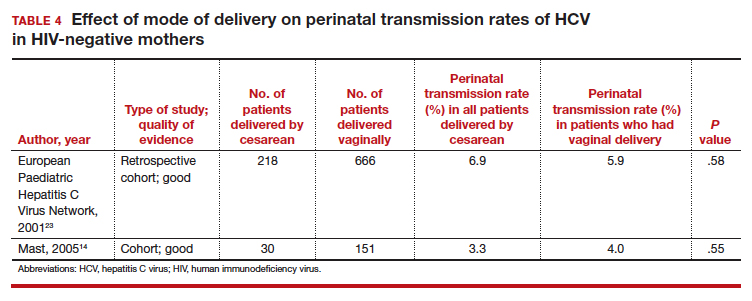
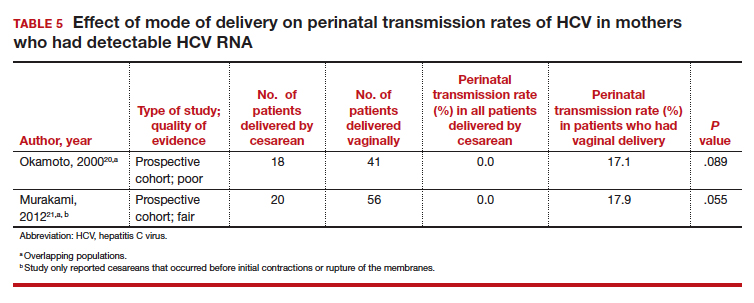
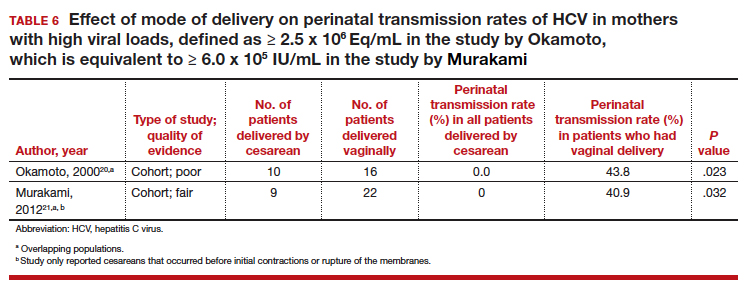
Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
CASE Pregnant woman with chronic opioid use and HIV, recently diagnosed with HCV
A 34-year-old primigravid woman at 35 weeks' gestation has a history of chronic opioid use. She previously was diagnosed with human immunodeficiency virus (HIV) infection and has been treated with a 3-drug combination antiretroviral regimen. Her most recent HIV viral load was 750 copies/mL. Three weeks ago, she tested positive for hepatitis C virus (HCV) infection. Liver function tests showed mild elevations in transaminase levels. The viral genotype is 1, and the viral load is 2.6 million copies/mL.
How should this patient be delivered? Should she be encouraged to breastfeed her neonate?
The scope of HCV infection
Hepatitis C virus is a positive-sense, enveloped, single-stranded RNA virus that belongs to the Flaviviridae family.1 There are 7 confirmed major genotypes of HCV and 67 confirmed subtypes.2 HCV possesses several important virulence factors. First, the virus's replication is prone to frequent mutations because its RNA polymerase lacks proofreading activity, resulting in significant genetic diversity. The great degree of heterogeneity among HCV leads to high antigenic variability, which is one of the main reasons there is not yet a vaccine for HCV.3 Additionally, HCV's genomic plasticity plays a role in the emergence of drug-resistant variants.4
Virus transmission. Worldwide, approximately 130 to 170 million people are infected with HCV.5 HCV infections are caused primarily by exposure to infected blood, through sharing needles for intravenous drug injection and through receiving a blood transfusion.6 Other routes of transmission include exposure through sexual contact, occupational injury, and perinatal acquisition.
The risk of acquiring HCV varies for each of these transmission mechanisms. Blood transfusion is no longer a common mechanism of transmission in places where blood donations are screened for HCV antibodies and viral RNA. Additionally, unintentional needle-stick injury is the only occupational risk factor associated with HCV infection, and health care workers do not have a greater prevalence of HCV than the general population. Moreover, sexual transmission is not a particularly efficient mechanism for spread of HCV.7 Therefore, unsafe intravenous injections are now the leading cause of HCV infection.6
Consequences of HCV infection. Once infected with HCV, about 25% of people spontaneously clear the virus and approximately 75% progress to chronic HCV infection.5 The consequences of long-term infection with HCV include end-stage liver disease, cirrhosis, and hepatocellular carcinoma.
Approximately 30% of people infected with HCV will develop cirrhosis and another 2% will develop hepatocellular carcinoma.8 Liver transplant is the only treatment option for patients with decompensated cirrhosis or hepatocellular carcinoma as a result of HCV infection. Currently, HCV infection is the leading indication for liver transplant in the United States.9
Continue to: Risk of perinatal HCV transmission...
Risk of perinatal HCV transmission
Approximately 1% to 8% of pregnant women worldwide are infected with HCV.10 In the United States, 1% to 2.5% of pregnant women are infected.11 Of these, about 6% transmit the infection to their offspring. The risk of HCV vertical transmission increases to about 11% if the mother is co-infected with HIV.12 Vertical transmission is the primary method by which children become infected with HCV.13
Several risk factors increase the likelihood of HCV transmission from mother to child, including HIV co-infection, internal fetal monitoring, and longer duration of membrane rupture.14 The effect that mode of delivery has on vertical transmission rates, however, is still debated, and a Cochrane Review found that there were no randomized controlled trials assessing the effect of mode of delivery on mother-to-infant HCV transmission.15
Serology and genotyping used in diagnosis
The serological enzyme immunoassay is the first test used in screening for HCV infection. Currently, third- and fourth-generation enzyme immunoassays are used in the United States.16 However, even these newer serological assays cannot consistently and precisely distinguish between acute and chronic HCV infections.17 After the initial diagnosis is made with serology, it usually is confirmed by assays that detect the virus's genomic RNA in the patient's serum or plasma.
The patient's HCV genotype should be identified so that the best treatment options can be determined. HCV genotyping can be accomplished using reverse transcription quantitative polymerase chain reaction (RT-qPCR) amplification. Three different RT-qPCR assessments usually are performed using different primers and probes specific to different genotypes of HCV. While direct sequencing of the HCV genome also can be performed, this method is usually not used clinically due to its technical complexity.16
Modern treatments are effective
Introduced in 2011, direct-acting antiviral therapies are now the recommended treatment for HCV infection. These drugs inhibit the virus's replication by targeting different proteins involved in the HCV replication cycle. They are remarkably successful and have achieved sustained virologic response (SVR) rates greater than 90%.11 The World Health Organization recommends several pangenotypic (that is, agents that work against all genotypes) direct-acting antiviral regimens for the treatment of chronic HCV infection in adults without cirrhosis (TABLE 1).18,19
Unfortunately, experience with these drugs in pregnant women is lacking. Many direct-acting antiviral agents have not been tested systematically in pregnant women, and, accordingly, most information about their effects in pregnant women comes from animal models.11

Continue to: Perinatal transmission rates and effect of mode of delivery...
Perinatal transmission rates and effect of mode of delivery
We compiled data from 11 studies that reported the perinatal transmission rate of HCV associated with various modes of delivery. These studies were selected from a MEDLINE literature review from 1999 to 2019. The studies were screened first by title and, subsequently, by abstract. Inclusion was restricted to randomized controlled trials, cohort studies, and case-control studies written in English. Study quality was assessed as good, fair, or poor based on the study design, sample size, and statistical analyses performed. The results from the total population of each study are reported in TABLE 2.14,20-29
Three studies separated data based on the mother's HIV status. The perinatal transmission rates of HCV for mothers co-infected with HIV are reported in TABLE 3.23,27 The results for HIV-negative mothers are reported in TABLE 4.14,23
Finally, 2 studies grouped mothers according to their HCV viral load. All of the mothers in these studies were anti-HCV antibody positive, and the perinatal transmission rates for the total study populations were reported previously in TABLE 2. The results for mothers who had detectable HCV RNA are reported in TABLE 5.20,21 High viral load was defined as
≥ 2.5 x 106 Eq/mL in the study by Okamoto and colleagues, which is equivalent to ≥ 6.0 x 105 IU/mL in the study by Murakami and colleagues due to the different assays that were used.20,21 The perinatal transmission rates for mothers with a high viral load are presented in TABLE 6.20,21





Continue to: For most, CD does not reduce HCV transmission...
For most, CD does not reduce HCV transmission
Nine of the 11 studies found that the mode of delivery did not have a statistically significant impact on the vertical transmission rate of HCV in the total study populations.14,22-29 The remaining 2 studies found that the perinatal transmission rate of HCV was lower with cesarean delivery (CD) than with vaginal delivery.20,21 When considered together, the results of these 11 studies indicate that CD does not provide a significant reduction in the HCV transmission rate in the general population.
Our review confirms the findings of others, including a systematic review by the US Preventive Services Task Force.30 That investigation also failed to demonstrate any measurable increase in risk of HCV transmission as a result of breastfeeding.
Cesarean delivery may benefit 2 groups. Careful assessment of these studies, however, suggests that 2 select groups of patients with HCV may benefit from CD:
- mothers co-infected with HIV, and
- mothers with high viral loads of HCV.
In both of these populations, the vertical transmission rate of HCV was significantly reduced with CD compared with vaginal delivery. Therefore, CD should be strongly considered in mothers with HCV who are co-infected with HIV and/or in mothers who have a high viral load of HCV.
CASE Our recommendation for mode of delivery
The patient in our case scenario has both HIV infection and a very high HCV viral load. We would therefore recommend a planned CD at 38 to 39 weeks' gestation, prior to the onset of labor or membrane rupture. Although HCV infection is not a contraindication to breastfeeding, the mother's HIV infection is a distinct contraindication.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
- Dubuisson J, Cosset FL. Virology and cell biology of the hepatitis C virus life cycle: an update. J Hepatol. 2014;61(1 suppl):S3-S13.
- Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318-327.
- Rossi LM, Escobar-Gutierrez A, Rahal P. Advanced molecular surveillance of hepatitis C virus. Viruses. 2015;7:1153-1188.
- Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826-832.
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562.
- Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041-2050.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1-39.
- Gonzalez-Grande R, Jimenez-Perez M, Gonzalez Arjona C, et al. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421-1432.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl): S58-S68.
- Spera AM, Eldin TK, Tosone G, et al. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol. 2016;8:557-565.
- Society for Maternal-Fetal Medicine; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol. 2017;217:B2-B12.
- Benova L, Mohamoud YA, Calvert C, et al. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773.
- Ghamar Chehreh ME, Tabatabaei SV, Khazanehdari S, et al. Effect of cesarean section on the risk of perinatal transmission of hepatitis C virus from HCV-RNA+/HIV- mothers: a meta-analysis. Arch Gynecol Obstet. 2011;283:255-260.
- Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880-1889.
- McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane Database Syst Rev. 2006;(4):CD005546.
- Mukherjee R, Burns A, Rodden D, et al. Diagnosis and management of hepatitis C virus infection. J Lab Autom. 2015;20:519-538.
- Araujo AC, Astrakhantseva IV, Fields HA, et al. Distinguishing acute from chronic hepatitis C virus (HCV) infection based on antibody reactivities to specific HCV structural and nonstructural proteins. J Clin Microbiol. 2011;49:54-57.
- World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. Geneva, Switzerland: World Health Organization; 2018.
- CADTH Common Drug Review. Pharmacoeconomic Review Report: Sofosbuvir/Velpatasvir/Voxilaprevir (Vosevi) (Gilead Sciences Canada, Inc): Indication: Hepatitis C infection genotype 1 to 6. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2018.
- Okamoto M, Nagata I, Murakami J, et al. Prospective reevaluation of risk factors in mother-to-child transmission of hepatitis C virus: high virus load, vaginal delivery, and negative anti-NS4 antibody. J Infect Dis. 2000;182:1511-1514.
- Murakami J, Nagata I, Iitsuka T, et al. Risk factors for mother-to-child transmission of hepatitis C virus: maternal high viral load and fetal exposure in the birth canal. Hepatol Res. 2012;42:648-657.
- Conte D, Fraquelli M, Prati D, et al. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
- European Paediatric Hepatitis C Virus Network. Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. BJOG. 2001;108:371-377.
- Tajiri H, Miyoshi Y, Funada S, et al. Prospective study of mother-to-infant transmission of hepatitis C virus. Pediatr Infect Dis J. 2001;20:10-14.
- Ferrero S, Lungaro P, Bruzzone BM, et al. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990-2000). Acta Obstet Gynecol Scand. 2003;82:229-234.
- Marine-Barjoan E, Berrebi A, Giordanengo V, et al. HCV/HIV co-infection, HCV viral load and mode of delivery: risk factors for mother-to-child transmission of hepatitis C virus? AIDS. 2007;21:1811-1815.
- Delotte J, Barjoan EM, Berrebi A, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med. 2014;27:664-670.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173-177.
- Jhaveri R, Hashem M, El-Kamary SS, et al. Hepatitis C virus (HCV) vertical transmission in 12-month-old infants born to HCV-infected women and assessment of maternal risk factors. Open Forum Infect Dis. 2015;2:ofv089.
- Cottrell EB, Chou R, Wasson N, et al. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2013;158:109-113.
Early onset of atopic dermatitis linked to poorer control
according to a study published in the Journal of the American Academy of Dermatology.
Atopic dermatitis most commonly arises in infancy but also can emerge in later childhood and even adolescence, leading to a distinction between early- and late-onset disease, wrote Joy Wan, MD, of the University of Pennsylvania, Philadelphia, and coauthors.
“Early-onset, mid-onset, and late-onset AD appear to differ in the presence of active disease over time; however, whether these groups also differ in terms of the severity of AD is unknown,” they wrote.
In this observational cohort study, 8,015 individuals with childhood-onset atopic dermatitis – 53% of whom were female – were assessed twice-yearly for up to 10 years. Nearly three-quarters (72%) of the group had early-onset atopic dermatitis – defined as onset before 2 years of age – while 19% had mid-onset disease (3-7 years) and 9% had late-onset disease (8-17 years).
The study found that older age of onset was associated with better control, such that for each additional year of age at the onset of disease, there was a 7% reduction in the odds of poorer control of disease. Those who had mid-onset disease had a 29% lower odds of poorer control compared with those with early-onset, while those with late-onset disease had a 49% lower odds of poorer control.
The likelihood of atopic dermatitis persisting beyond childhood also appeared to be linked to the age of onset. Those with mid-onset disease had a 55% lower odds of persistent atopic dermatitis, compared with those with early-onset disease, while those with late-onset disease had an 81% lower odds.
“In all 3 groups, the proportion of subjects reporting persistent AD generally declined with older age, and the differences among the 3 onset age groups were most pronounced from early adolescence onward,” the authors wrote.
They noted that there was considerable research currently focused on identifying distinct atopic dermatitis phenotypes and endotypes, and their evidence on the different disease course for early-, mid-, and late-onset disease supported this idea of disease subtypes.
“However, additional research is needed to understand whether and how early-, mid-, and late-onset AD differ molecularly or immunologically, and whether they respond differentially to treatment,” they wrote. They also suggested that the timing of onset could help identify patients who were at greater risk of persistent or poorly controlled disease, and who benefits from more intensive monitoring or treatment.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Dermatology Foundation. Three authors declared funding, consultancies, or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wan J et al. J Am Acad Dermatol. 2019 Dec;81(6):1292-9.
according to a study published in the Journal of the American Academy of Dermatology.
Atopic dermatitis most commonly arises in infancy but also can emerge in later childhood and even adolescence, leading to a distinction between early- and late-onset disease, wrote Joy Wan, MD, of the University of Pennsylvania, Philadelphia, and coauthors.
“Early-onset, mid-onset, and late-onset AD appear to differ in the presence of active disease over time; however, whether these groups also differ in terms of the severity of AD is unknown,” they wrote.
In this observational cohort study, 8,015 individuals with childhood-onset atopic dermatitis – 53% of whom were female – were assessed twice-yearly for up to 10 years. Nearly three-quarters (72%) of the group had early-onset atopic dermatitis – defined as onset before 2 years of age – while 19% had mid-onset disease (3-7 years) and 9% had late-onset disease (8-17 years).
The study found that older age of onset was associated with better control, such that for each additional year of age at the onset of disease, there was a 7% reduction in the odds of poorer control of disease. Those who had mid-onset disease had a 29% lower odds of poorer control compared with those with early-onset, while those with late-onset disease had a 49% lower odds of poorer control.
The likelihood of atopic dermatitis persisting beyond childhood also appeared to be linked to the age of onset. Those with mid-onset disease had a 55% lower odds of persistent atopic dermatitis, compared with those with early-onset disease, while those with late-onset disease had an 81% lower odds.
“In all 3 groups, the proportion of subjects reporting persistent AD generally declined with older age, and the differences among the 3 onset age groups were most pronounced from early adolescence onward,” the authors wrote.
They noted that there was considerable research currently focused on identifying distinct atopic dermatitis phenotypes and endotypes, and their evidence on the different disease course for early-, mid-, and late-onset disease supported this idea of disease subtypes.
“However, additional research is needed to understand whether and how early-, mid-, and late-onset AD differ molecularly or immunologically, and whether they respond differentially to treatment,” they wrote. They also suggested that the timing of onset could help identify patients who were at greater risk of persistent or poorly controlled disease, and who benefits from more intensive monitoring or treatment.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Dermatology Foundation. Three authors declared funding, consultancies, or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wan J et al. J Am Acad Dermatol. 2019 Dec;81(6):1292-9.
according to a study published in the Journal of the American Academy of Dermatology.
Atopic dermatitis most commonly arises in infancy but also can emerge in later childhood and even adolescence, leading to a distinction between early- and late-onset disease, wrote Joy Wan, MD, of the University of Pennsylvania, Philadelphia, and coauthors.
“Early-onset, mid-onset, and late-onset AD appear to differ in the presence of active disease over time; however, whether these groups also differ in terms of the severity of AD is unknown,” they wrote.
In this observational cohort study, 8,015 individuals with childhood-onset atopic dermatitis – 53% of whom were female – were assessed twice-yearly for up to 10 years. Nearly three-quarters (72%) of the group had early-onset atopic dermatitis – defined as onset before 2 years of age – while 19% had mid-onset disease (3-7 years) and 9% had late-onset disease (8-17 years).
The study found that older age of onset was associated with better control, such that for each additional year of age at the onset of disease, there was a 7% reduction in the odds of poorer control of disease. Those who had mid-onset disease had a 29% lower odds of poorer control compared with those with early-onset, while those with late-onset disease had a 49% lower odds of poorer control.
The likelihood of atopic dermatitis persisting beyond childhood also appeared to be linked to the age of onset. Those with mid-onset disease had a 55% lower odds of persistent atopic dermatitis, compared with those with early-onset disease, while those with late-onset disease had an 81% lower odds.
“In all 3 groups, the proportion of subjects reporting persistent AD generally declined with older age, and the differences among the 3 onset age groups were most pronounced from early adolescence onward,” the authors wrote.
They noted that there was considerable research currently focused on identifying distinct atopic dermatitis phenotypes and endotypes, and their evidence on the different disease course for early-, mid-, and late-onset disease supported this idea of disease subtypes.
“However, additional research is needed to understand whether and how early-, mid-, and late-onset AD differ molecularly or immunologically, and whether they respond differentially to treatment,” they wrote. They also suggested that the timing of onset could help identify patients who were at greater risk of persistent or poorly controlled disease, and who benefits from more intensive monitoring or treatment.
The study was partly supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Dermatology Foundation. Three authors declared funding, consultancies, or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wan J et al. J Am Acad Dermatol. 2019 Dec;81(6):1292-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY