User login
More studies like VERVE needed to test live vaccines in special populations
The VERVE study highlights a crucial topic for rheumatologists treating patients in clinical practice. The traditional thinking is to inform patients never to receive live vaccines when they are using TNF (tumor necrosis factor) inhibitors to treat their autoimmune disease. The VERVE study indicates that in the case of the Zostavax vaccine, patients on this form of biologic therapy for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis can safely receive this preventive measure. This study scratches the surface on an important topic, and other studies need to follow.
Many patients on biologic therapy want to travel. Many times, international travel requires vaccination that is only in the form of a live vaccine – for example, the yellow fever vaccine. It would be useful for us to better understand whether other live vaccines can safely be administered and better inform our patients who want to travel. In addition, many times mothers with young infants are nervous if they are on biologic therapy and their children need to receive a live vaccine. They are concerned that their children will shed the live virus and they will be in jeopardy. This study highlights that this may be more of an antiquated way of thinking. We need more studies of this kind to better understand and advise our patients properly without instilling unwarranted fear.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
The VERVE study highlights a crucial topic for rheumatologists treating patients in clinical practice. The traditional thinking is to inform patients never to receive live vaccines when they are using TNF (tumor necrosis factor) inhibitors to treat their autoimmune disease. The VERVE study indicates that in the case of the Zostavax vaccine, patients on this form of biologic therapy for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis can safely receive this preventive measure. This study scratches the surface on an important topic, and other studies need to follow.
Many patients on biologic therapy want to travel. Many times, international travel requires vaccination that is only in the form of a live vaccine – for example, the yellow fever vaccine. It would be useful for us to better understand whether other live vaccines can safely be administered and better inform our patients who want to travel. In addition, many times mothers with young infants are nervous if they are on biologic therapy and their children need to receive a live vaccine. They are concerned that their children will shed the live virus and they will be in jeopardy. This study highlights that this may be more of an antiquated way of thinking. We need more studies of this kind to better understand and advise our patients properly without instilling unwarranted fear.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
The VERVE study highlights a crucial topic for rheumatologists treating patients in clinical practice. The traditional thinking is to inform patients never to receive live vaccines when they are using TNF (tumor necrosis factor) inhibitors to treat their autoimmune disease. The VERVE study indicates that in the case of the Zostavax vaccine, patients on this form of biologic therapy for rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis can safely receive this preventive measure. This study scratches the surface on an important topic, and other studies need to follow.
Many patients on biologic therapy want to travel. Many times, international travel requires vaccination that is only in the form of a live vaccine – for example, the yellow fever vaccine. It would be useful for us to better understand whether other live vaccines can safely be administered and better inform our patients who want to travel. In addition, many times mothers with young infants are nervous if they are on biologic therapy and their children need to receive a live vaccine. They are concerned that their children will shed the live virus and they will be in jeopardy. This study highlights that this may be more of an antiquated way of thinking. We need more studies of this kind to better understand and advise our patients properly without instilling unwarranted fear.
Dr. Oberstein is a practicing rheumatologist at the University of Miami Health System and is senior medical director of musculoskeletal at Modernizing Medicine in Boca Raton, Fla. She has no relevant disclosures to report.
Atezolizumab/bevacizumab may offer benefit to patients with RCC
The combination of atezolizumab plus bevacizumab may offer some benefit to patients with advanced renal cell carcinoma, especially those who are positive for programmed death-ligand 1 (PD-L1), investigators report.
The overall response rate (ORR) among such patients was 60%, compared with 19% in PD-L1–negative patients, Bradley A. McGregor, MD, clinical director for the Lank Center of Genitourinary Oncology at Dana-Farber Cancer Institute in Boston, and colleagues reported in the Journal of Clinical Oncology.
The data were presented last summer at the American Society of Clinical Oncology Annual Meeting in Chicago.
The phase 2 study comprised 60 patients, 42 of whom had variant histology RCC, and 18 of whom had clear cell RCC (ccRCC ) with at least 20% sarcomatoid differentiation. All patients had advanced renal cell carcinoma of various histologies, including papillary (12), chromophobe (10), unclassified (9), TFE3 translocation (5), collecting duct (5), and medullary (1). Most (65%) had not received prior systemic therapy.
They all received infusions of atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks. No dose modifications were allowed. Dose delays were allowed, and patients could also drop one agent and continue with the other. Treatment continued until disease progression, toxicity, or intolerable side effects.
The median number of cycles was 9.5, although the range was wide (1-42). At analysis, 15 were still on the treatment, but 45 had dropped out. Reasons were disease progression (34), death (1), toxicity (5), or unspecified (8). Six patients delayed bevacizumab doses, half because of adverse events.
After a median follow-up of 13 months, the ORR was 33%. Those with ccRCC with sarcomatoid differentiation responded best to the combination (ORR, 50%). Those with variant-histology RCC responded less robustly (ORR, 26%).
ORR varied by baseline risk category, being 33% in favorable-, 45% in intermediate-, and 11% in poor-risk patients. Median time to response was 2.7 months, median response duration was 8.9 months, and median progression-free survival was 8.3 months.
PD-L1 status was determined in 36 patients; 15 were positive. Among the positive patents, ORR was 60%, compared with 19% in PD-L1 negative patients. Response rates varied with tumor characteristics. Among patients with ccRCC with sarcomatoid differentiation, the ORR was 50% in PD-L1–positive patients and 29% in negative patients. In patients with variant histology RCC, the ORR was also better in PD-L1 positive patients (67% vs. 14%).
The most common treatment-related side effects were fatigue (35%), proteinuria (35%), musculoskeletal pain (33%), diarrhea (22%), rash (20%), hypertension (18%), pruritus (18%), thyroid dysfunction (17%), hepatitis (15%), fever (13%), and mucositis (12%). Thirty-four patients developed at least one grade 3 adverse event; there were no grade 4 or 5 toxicities. One patient died, presumably because of disease progression.
Quality of life scores were largely stable during treatment.
“The combination demonstrated responses across several subtypes of RCC, including collecting duct and medullary carcinoma, histologies that are often treated with cytotoxic chemotherapy,” the authors said. “This is notable given the generally poor prognosis and low response rate associated with variant histology RCC in trials to date.”
The study also suggests the PD-L1 status might be “intriguing as a biomarker for response to atezolizumab and bevacizumab in variant histology RCC. We plan to conduct additional correlative work, including genomic profiling and assessment of the immune microenvironment, to better elucidate markers of response and resistance,” the authors wrote.
SOURCE: McGregor BA et al. J Clin Oncol. 2019 Nov 13. doi: 10.1200/JCO.19.01882.
The combination of atezolizumab plus bevacizumab may offer some benefit to patients with advanced renal cell carcinoma, especially those who are positive for programmed death-ligand 1 (PD-L1), investigators report.
The overall response rate (ORR) among such patients was 60%, compared with 19% in PD-L1–negative patients, Bradley A. McGregor, MD, clinical director for the Lank Center of Genitourinary Oncology at Dana-Farber Cancer Institute in Boston, and colleagues reported in the Journal of Clinical Oncology.
The data were presented last summer at the American Society of Clinical Oncology Annual Meeting in Chicago.
The phase 2 study comprised 60 patients, 42 of whom had variant histology RCC, and 18 of whom had clear cell RCC (ccRCC ) with at least 20% sarcomatoid differentiation. All patients had advanced renal cell carcinoma of various histologies, including papillary (12), chromophobe (10), unclassified (9), TFE3 translocation (5), collecting duct (5), and medullary (1). Most (65%) had not received prior systemic therapy.
They all received infusions of atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks. No dose modifications were allowed. Dose delays were allowed, and patients could also drop one agent and continue with the other. Treatment continued until disease progression, toxicity, or intolerable side effects.
The median number of cycles was 9.5, although the range was wide (1-42). At analysis, 15 were still on the treatment, but 45 had dropped out. Reasons were disease progression (34), death (1), toxicity (5), or unspecified (8). Six patients delayed bevacizumab doses, half because of adverse events.
After a median follow-up of 13 months, the ORR was 33%. Those with ccRCC with sarcomatoid differentiation responded best to the combination (ORR, 50%). Those with variant-histology RCC responded less robustly (ORR, 26%).
ORR varied by baseline risk category, being 33% in favorable-, 45% in intermediate-, and 11% in poor-risk patients. Median time to response was 2.7 months, median response duration was 8.9 months, and median progression-free survival was 8.3 months.
PD-L1 status was determined in 36 patients; 15 were positive. Among the positive patents, ORR was 60%, compared with 19% in PD-L1 negative patients. Response rates varied with tumor characteristics. Among patients with ccRCC with sarcomatoid differentiation, the ORR was 50% in PD-L1–positive patients and 29% in negative patients. In patients with variant histology RCC, the ORR was also better in PD-L1 positive patients (67% vs. 14%).
The most common treatment-related side effects were fatigue (35%), proteinuria (35%), musculoskeletal pain (33%), diarrhea (22%), rash (20%), hypertension (18%), pruritus (18%), thyroid dysfunction (17%), hepatitis (15%), fever (13%), and mucositis (12%). Thirty-four patients developed at least one grade 3 adverse event; there were no grade 4 or 5 toxicities. One patient died, presumably because of disease progression.
Quality of life scores were largely stable during treatment.
“The combination demonstrated responses across several subtypes of RCC, including collecting duct and medullary carcinoma, histologies that are often treated with cytotoxic chemotherapy,” the authors said. “This is notable given the generally poor prognosis and low response rate associated with variant histology RCC in trials to date.”
The study also suggests the PD-L1 status might be “intriguing as a biomarker for response to atezolizumab and bevacizumab in variant histology RCC. We plan to conduct additional correlative work, including genomic profiling and assessment of the immune microenvironment, to better elucidate markers of response and resistance,” the authors wrote.
SOURCE: McGregor BA et al. J Clin Oncol. 2019 Nov 13. doi: 10.1200/JCO.19.01882.
The combination of atezolizumab plus bevacizumab may offer some benefit to patients with advanced renal cell carcinoma, especially those who are positive for programmed death-ligand 1 (PD-L1), investigators report.
The overall response rate (ORR) among such patients was 60%, compared with 19% in PD-L1–negative patients, Bradley A. McGregor, MD, clinical director for the Lank Center of Genitourinary Oncology at Dana-Farber Cancer Institute in Boston, and colleagues reported in the Journal of Clinical Oncology.
The data were presented last summer at the American Society of Clinical Oncology Annual Meeting in Chicago.
The phase 2 study comprised 60 patients, 42 of whom had variant histology RCC, and 18 of whom had clear cell RCC (ccRCC ) with at least 20% sarcomatoid differentiation. All patients had advanced renal cell carcinoma of various histologies, including papillary (12), chromophobe (10), unclassified (9), TFE3 translocation (5), collecting duct (5), and medullary (1). Most (65%) had not received prior systemic therapy.
They all received infusions of atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks. No dose modifications were allowed. Dose delays were allowed, and patients could also drop one agent and continue with the other. Treatment continued until disease progression, toxicity, or intolerable side effects.
The median number of cycles was 9.5, although the range was wide (1-42). At analysis, 15 were still on the treatment, but 45 had dropped out. Reasons were disease progression (34), death (1), toxicity (5), or unspecified (8). Six patients delayed bevacizumab doses, half because of adverse events.
After a median follow-up of 13 months, the ORR was 33%. Those with ccRCC with sarcomatoid differentiation responded best to the combination (ORR, 50%). Those with variant-histology RCC responded less robustly (ORR, 26%).
ORR varied by baseline risk category, being 33% in favorable-, 45% in intermediate-, and 11% in poor-risk patients. Median time to response was 2.7 months, median response duration was 8.9 months, and median progression-free survival was 8.3 months.
PD-L1 status was determined in 36 patients; 15 were positive. Among the positive patents, ORR was 60%, compared with 19% in PD-L1 negative patients. Response rates varied with tumor characteristics. Among patients with ccRCC with sarcomatoid differentiation, the ORR was 50% in PD-L1–positive patients and 29% in negative patients. In patients with variant histology RCC, the ORR was also better in PD-L1 positive patients (67% vs. 14%).
The most common treatment-related side effects were fatigue (35%), proteinuria (35%), musculoskeletal pain (33%), diarrhea (22%), rash (20%), hypertension (18%), pruritus (18%), thyroid dysfunction (17%), hepatitis (15%), fever (13%), and mucositis (12%). Thirty-four patients developed at least one grade 3 adverse event; there were no grade 4 or 5 toxicities. One patient died, presumably because of disease progression.
Quality of life scores were largely stable during treatment.
“The combination demonstrated responses across several subtypes of RCC, including collecting duct and medullary carcinoma, histologies that are often treated with cytotoxic chemotherapy,” the authors said. “This is notable given the generally poor prognosis and low response rate associated with variant histology RCC in trials to date.”
The study also suggests the PD-L1 status might be “intriguing as a biomarker for response to atezolizumab and bevacizumab in variant histology RCC. We plan to conduct additional correlative work, including genomic profiling and assessment of the immune microenvironment, to better elucidate markers of response and resistance,” the authors wrote.
SOURCE: McGregor BA et al. J Clin Oncol. 2019 Nov 13. doi: 10.1200/JCO.19.01882.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Survey: Cancer-related pain, opioid use up since 2018
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Cancer-related pain was more common among patients in 2019 than in 2018, as was the use of prescription opioids, according to the American Society of Clinical Oncology.

Patients who have/had cancer were significantly more likely to report that they were currently experiencing cancer-related pain in 2019 (19%) than in 2018 (12%), but there was only a slight increase in patients who said that they had experienced cancer-related pain in the past, the society reported in its National Cancer Opinion Survey.
When asked about methods used to manage pain, nausea, and other symptoms, patients diagnosed with cancer most often said that they had not used anything in the last 12 months, although this response was significantly less common in 2019 (48%) than in 2018 (55%). Over-the-counter pain relievers were the most common method used (24% in 2019 and 22% in 2018), followed by vitamins/minerals/herbs (18% in 2019 and 17% in 2018), ASCO said.
Prescription opioids were the third most popular choice for symptom management both years, but use was significantly higher in 2019 (17%) than in 2018 (12%). Also showing a significant increase from 2018 to 2019 was use of medical marijuana, which went from 5% to 10%, ASCO said.
The survey was conducted online for ASCO by the Harris Poll from July 9 to Aug. 10, 2019. The total sample consisted of 4,815 U.S. adults, of whom 1,009 had been diagnosed with cancer.
Regular use of disinfectants at work associated with increased risk of COPD
“Clinicians should be aware of this new risk factor and systematically look for sources of exposure to cleaning products and disinfectants in addition to other occupational exposures in patients with COPD,” wrote Orianne Dumas, PhD, of the Université de Versailles St-Quentin-en-Yvelines (France) and coauthors. The study was published in JAMA Network Open.
To determine if regular use of disinfectants had a negative impact on respiratory health, the researchers analyzed data from 73,262 active female nurses who had no history of COPD and completed questionnaires every 2 years for the Nurses’ Health Study II. Their mean age at baseline was 54.7. Exposure to commonly used disinfectants was evaluated by a job-task-exposure matrix (JTEM) specific to nurses.
Between 2009 and 2015, 582 nurses reported incident physician-diagnosed COPD. Weekly use of disinfectants was associated with COPD incidence (adjusted hazard ratio 1.35; 95% confidence interval, 1.14-1.59). Additional associations were found in nurses who used disinfectants to clean surfaces (AHR, 1.38; 95% CI, 1.13-1.68) and to clean medical instruments (AHR, 1.31; 95% CI, 1.07-1.61). High-level exposure to certain disinfectants – including glutaraldehyde, bleach, hydrogen peroxide, alcohol, and quaternary ammonium compounds – were significantly associated with increased risk of COPD incidence.
The authors acknowledged their study’s limitations, including the JTEM only assessing exposure to seven of the major cleaning products commonly used in health care. In addition, detailed data on exposure to disinfectants was not available before 2009. However, they added that, because the study has been ongoing since 1989, it could be expected that women who had been nurses for decades had “already accumulated a long history of exposure.”
The study was supported in part by grants from the Centers for Disease Control and Prevention and the National Institutes of Health. Five of the authors reported receiving grants from the CDC’s National Institute for Occupational Safety and Health (NIOSH); one additional author reported being a consultant on a NIOSH grant and receiving personal fees from a health care system. No other conflicts of interest were reported.
SOURCE: Dumas O et al. JAMA Netw Open. 2019 Oct 18. doi: 10.1001/jamanetworkopen.2019.13563.
“Clinicians should be aware of this new risk factor and systematically look for sources of exposure to cleaning products and disinfectants in addition to other occupational exposures in patients with COPD,” wrote Orianne Dumas, PhD, of the Université de Versailles St-Quentin-en-Yvelines (France) and coauthors. The study was published in JAMA Network Open.
To determine if regular use of disinfectants had a negative impact on respiratory health, the researchers analyzed data from 73,262 active female nurses who had no history of COPD and completed questionnaires every 2 years for the Nurses’ Health Study II. Their mean age at baseline was 54.7. Exposure to commonly used disinfectants was evaluated by a job-task-exposure matrix (JTEM) specific to nurses.
Between 2009 and 2015, 582 nurses reported incident physician-diagnosed COPD. Weekly use of disinfectants was associated with COPD incidence (adjusted hazard ratio 1.35; 95% confidence interval, 1.14-1.59). Additional associations were found in nurses who used disinfectants to clean surfaces (AHR, 1.38; 95% CI, 1.13-1.68) and to clean medical instruments (AHR, 1.31; 95% CI, 1.07-1.61). High-level exposure to certain disinfectants – including glutaraldehyde, bleach, hydrogen peroxide, alcohol, and quaternary ammonium compounds – were significantly associated with increased risk of COPD incidence.
The authors acknowledged their study’s limitations, including the JTEM only assessing exposure to seven of the major cleaning products commonly used in health care. In addition, detailed data on exposure to disinfectants was not available before 2009. However, they added that, because the study has been ongoing since 1989, it could be expected that women who had been nurses for decades had “already accumulated a long history of exposure.”
The study was supported in part by grants from the Centers for Disease Control and Prevention and the National Institutes of Health. Five of the authors reported receiving grants from the CDC’s National Institute for Occupational Safety and Health (NIOSH); one additional author reported being a consultant on a NIOSH grant and receiving personal fees from a health care system. No other conflicts of interest were reported.
SOURCE: Dumas O et al. JAMA Netw Open. 2019 Oct 18. doi: 10.1001/jamanetworkopen.2019.13563.
“Clinicians should be aware of this new risk factor and systematically look for sources of exposure to cleaning products and disinfectants in addition to other occupational exposures in patients with COPD,” wrote Orianne Dumas, PhD, of the Université de Versailles St-Quentin-en-Yvelines (France) and coauthors. The study was published in JAMA Network Open.
To determine if regular use of disinfectants had a negative impact on respiratory health, the researchers analyzed data from 73,262 active female nurses who had no history of COPD and completed questionnaires every 2 years for the Nurses’ Health Study II. Their mean age at baseline was 54.7. Exposure to commonly used disinfectants was evaluated by a job-task-exposure matrix (JTEM) specific to nurses.
Between 2009 and 2015, 582 nurses reported incident physician-diagnosed COPD. Weekly use of disinfectants was associated with COPD incidence (adjusted hazard ratio 1.35; 95% confidence interval, 1.14-1.59). Additional associations were found in nurses who used disinfectants to clean surfaces (AHR, 1.38; 95% CI, 1.13-1.68) and to clean medical instruments (AHR, 1.31; 95% CI, 1.07-1.61). High-level exposure to certain disinfectants – including glutaraldehyde, bleach, hydrogen peroxide, alcohol, and quaternary ammonium compounds – were significantly associated with increased risk of COPD incidence.
The authors acknowledged their study’s limitations, including the JTEM only assessing exposure to seven of the major cleaning products commonly used in health care. In addition, detailed data on exposure to disinfectants was not available before 2009. However, they added that, because the study has been ongoing since 1989, it could be expected that women who had been nurses for decades had “already accumulated a long history of exposure.”
The study was supported in part by grants from the Centers for Disease Control and Prevention and the National Institutes of Health. Five of the authors reported receiving grants from the CDC’s National Institute for Occupational Safety and Health (NIOSH); one additional author reported being a consultant on a NIOSH grant and receiving personal fees from a health care system. No other conflicts of interest were reported.
SOURCE: Dumas O et al. JAMA Netw Open. 2019 Oct 18. doi: 10.1001/jamanetworkopen.2019.13563.
FROM JAMA NETWORK OPEN
Kaposi Sarcoma in a Patient With Postpolio Syndrome
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
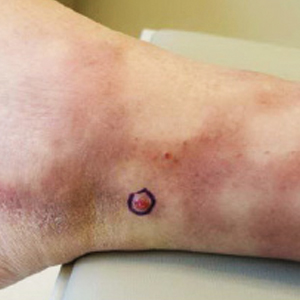
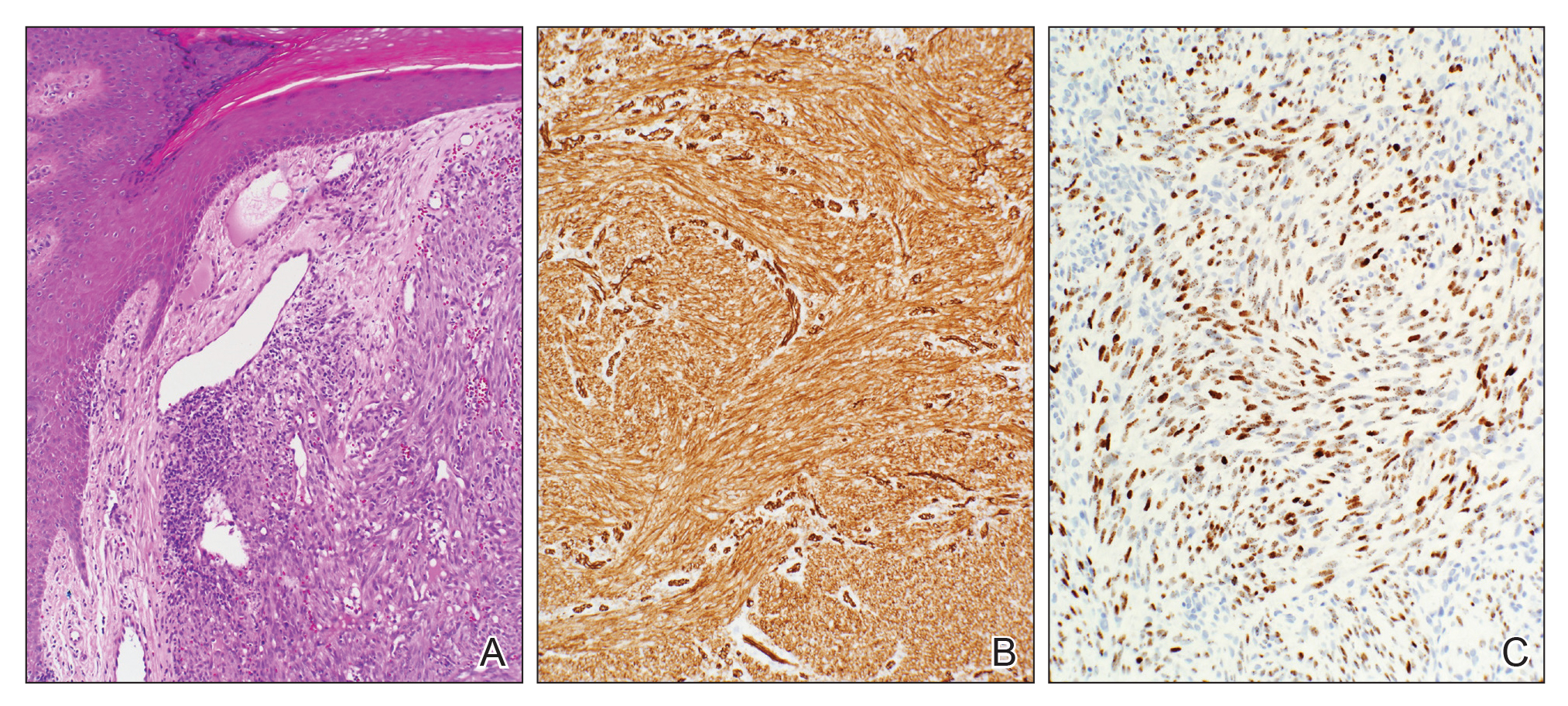
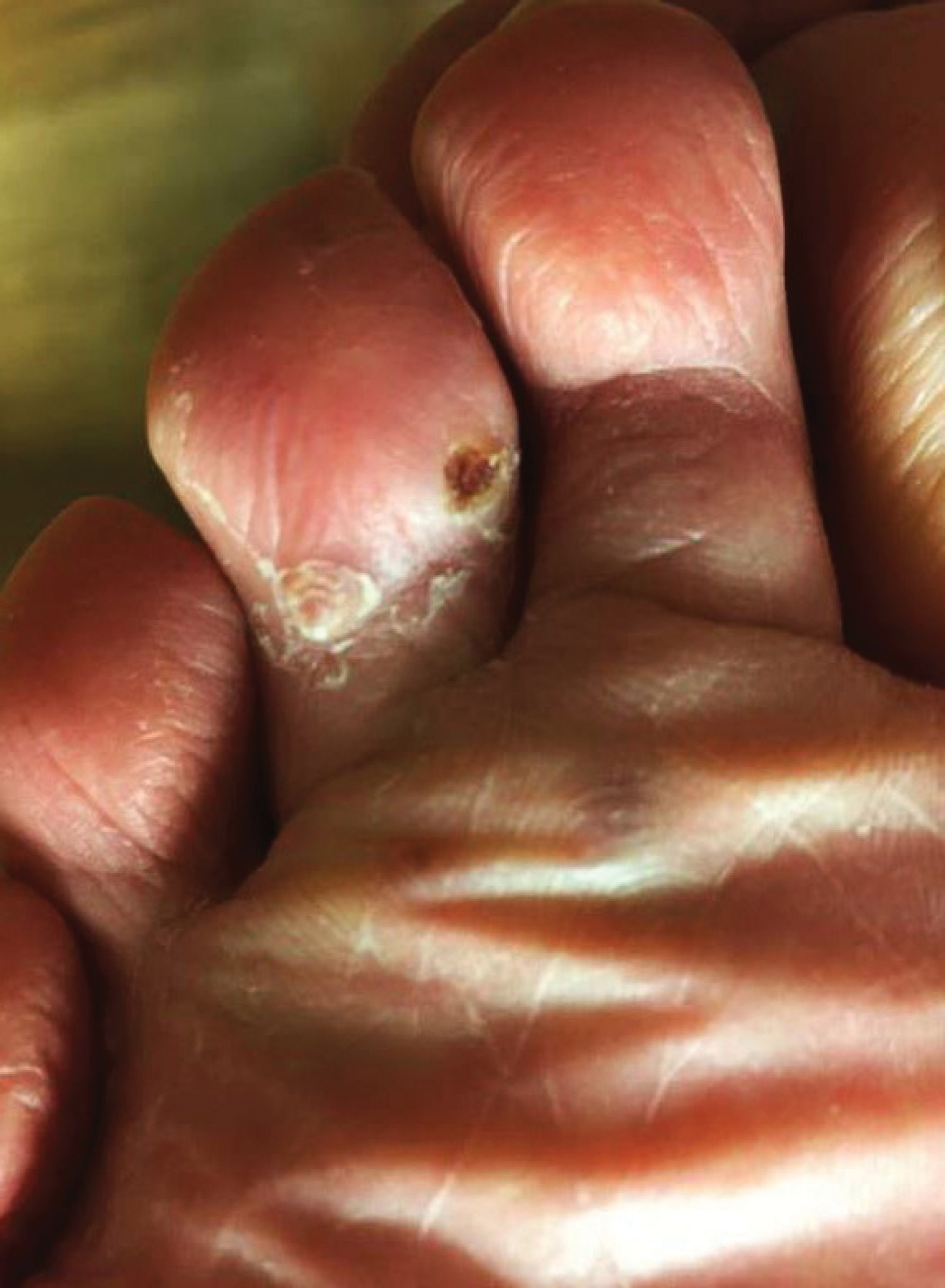
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.
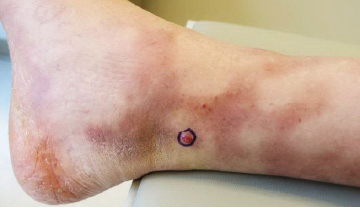
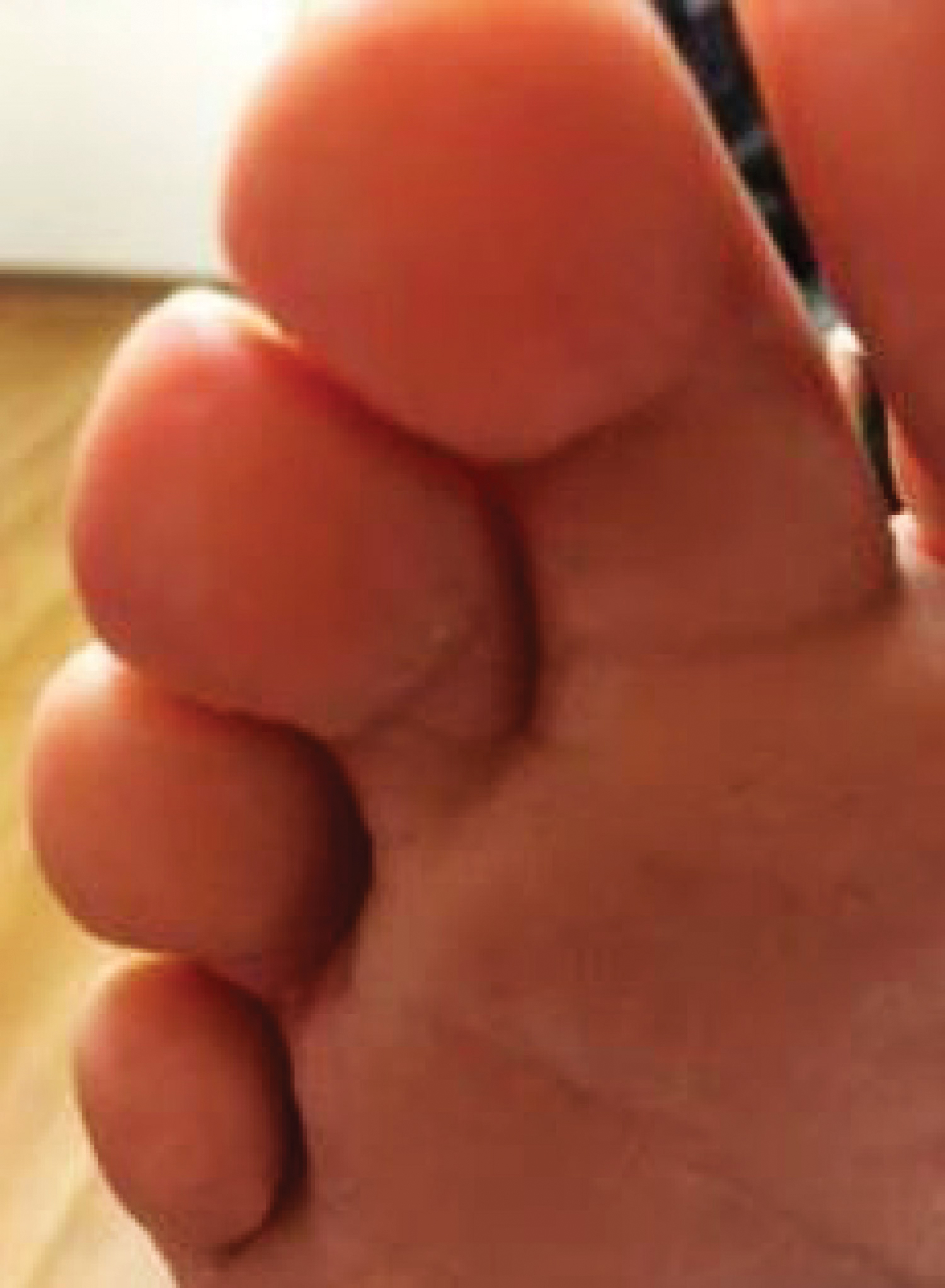
Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).
Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
Kaposi sarcoma (KS) is a low-grade vascular tumor that is rare among the general US population, with an incidence rate of less than 1 per 100,000.1 The tumor is more common among certain groups of individuals due to geographic differences in the prevalence of KS-associated herpesvirus (also referred to as human herpesvirus 8) as well as host immune factors.2 Kaposi sarcoma often is defined by the patient's predisposing characteristics yielding the following distinct epidemiologic subtypes: (1) classic KS is a rare disease affecting older men of Mediterranean descent; (2) African KS is an endemic cancer with male predominance in sub-Saharan Africa; (3) AIDS-associated KS is an often aggressive AIDS-defining illness; and (4) iatrogenic KS occurs in patients on immunosuppressive therapy.3 When evaluating a patient without any of these risk factors, the clinical suspicion for KS may be low. We report a patient with postpolio syndrome (PPS) who presented with KS of the right leg, ankle, and foot.
A 77-year-old man with a distant history of paralytic poliomyelitis presented for an annual skin examination with concern for a new lesion on the right ankle. The patient had a history of PPS primarily affecting the right leg. Physical examination revealed residual weakness in an atrophic right lower extremity with a mottled appearance and mild pitting edema to the knee. Two red, dome-shaped, vascular papules were appreciated on the medial aspect of the right ankle (Figure 1), and a shave biopsy of the larger papule was performed. Microscopic examination of the biopsy specimen was consistent with KS (Figure 2). This patient had no history of human immunodeficiency virus or immunosuppressive therapy and was not of Mediterranean descent.


Because KS is a radiosensitive vascular neoplasm and radiation therapy (RT) alone can achieve local control,4 the patient was treated with 6 megaelectron-volt electron-beam RT. He received 30 Gy in 10 fractions to the affected area of the medial ankle. The patient tolerated RT well. Three weeks after completing treatment, he was found to have mild lichenification on the right medial ankle with no clinical evidence of disease. Four months later, he presented with multiple additional vascular papules on the right third toe and in the interdigital web space (Figure 3). Shave biopsy of one of these lesions was consistent with KS. Contrast computed tomography of the chest, abdomen, and pelvis was performed, revealing no evidence of metastatic disease. The patient was treated with 30 Gy in 15 fractions using opposed lateral 6 megaelectron-volt photon fields to the entire right lower extremity below the knee to treat all of the skin affected by the PPS. His posttreatment course was complicated by edema in the affected leg that resolved after daily pneumatic compression. He had no evidence of residual or recurrent disease 6 months after completing RT (Figure 4).


Cutaneous KS is a human herpesvirus 8-positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.4 Our patient did not have any classic risk factors for KS, but his disease did arise in the setting of a right lower extremity that was notably affected by PPS. Postpolio syndrome is characterized by muscle atrophy due to denervation of the motor unit.5 Bruno et al6 found that such deficits in motor innervation could lead to impairments in venous outflow causing cutaneous venous congestion. Acroangiodermatitis clinically resembles KS but is a benign reactive vasoproliferative disorder and is well known to occur in the lower extremities as a sequela of chronic venous insufficiency.7 A case of bilateral lower extremity pseudo-KS was reported in a patient with notable PPS.8 A report of 2 patients describes KS arising in the setting of chronic venous insufficiency without any classic risk factors.9 Therefore, patients with PPS characterized by venous insufficiency may represent a population at increased risk for KS.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
- Surveillance, Epidemiology, and End Results (SEER) Program. US Population Data--1969-2017. https://seer.cancer.gov/popdata/. Published January 2019. Accessed November 25, 2019.
- Uldrick TS, Whitby D. Update on KSHV epidemiology, kaposi sarcoma pathogenesis, and treatment of saposi sarcoma. Cancer Lett. 2011;305:150-162.
- Schwartz RA, Micali G, Nasca MR, et al. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179-206.
- Arnold HL, Odom RB, James WD, et al. Andrews' Diseases of the Skin: Clinical Dermatology. Philadelphia, PA: Saunders; 1990.
- Boyer FV, Tiffreau V, Rapin A, et al. Post-polio syndrome: pathophysiological hypotheses, diagnosis criteria, drug therapy. Ann Phys Rehabil Med. 2010;53:34-41.
- Bruno RL, Johnson JC, Berman WS. Vasomotor abnormalities as post-polio sequelae: functional and clinical implications. Orthopedics. 1985;8:865-869.
- Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86:239-240.
- Rotbart G. Kaposi's disease and venous insufficiency. Phlebologie. 1978;31:439-443.
- Que SK, DeFelice T, Abdulla FR, et al. Non-HIV-related kaposi sarcoma in 2 Hispanic patients arising in the setting of chronic venous insufficiency. Cutis. 2015;95:E30-E33.
Practice Points
- Cutaneous Kaposi sarcoma (KS) is a human herpesvirus 8–positive tumor of endothelial origin typically seen in older men of Mediterranean or African descent and among immunosuppressed patients.
- In addition, patients with postpolio syndrome characterized by venous insufficiency may represent a population at increased risk for KS.
- Kaposi sarcoma is a radiosensitive vascular neoplasm, and radiation therapy can achieve local control.
Alkermes submits NDA for new schizophrenia, bipolar I treatment
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Could your patient benefit from a breast CA risk-reducing med?
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
Researchers describe first cases of episodic visual snow associated with migraine
Episodic visual snow was tied to migraine attacks in a case series of three adults who denied any visual snow outside of the migraines, based on data collected at an outpatient headache center.
Visual snow, a condition in which patients experience visual distortion of tiny, flickering dots resembling analog television static, is often a comorbid condition in migraine patients with and without aura. However, “to our knowledge, this is the first report of patients with an episodic form of visual snow strictly occurring with migraine attacks,” wrote Julius Hodak, MD, of the University of Bern (Switzerland) and colleagues.
In a research letter published in JAMA Neurology, the investigators described 3 adults with histories of migraine but no aura who presented to a tertiary headache center between January 2016 and December 2017, in addition to 1,934 adults with migraine but no visual snow. The three patients initially presented with headaches, and neurologic and MRI results were normal.
Two patients experienced black and white episodic visual snow and one experienced black and yellow visual snow. In one patient, visual snow occurred for less than 2 minutes before and during a migraine attack. The other two patients experienced visual snow during the entire migraine attack.
Based on these patients, the researchers proposed distinguishing episodic visual snow from the distinct disorder of visual snow syndrome, in which patients experience continuous visual snow and other visual symptoms.
In addition, the cases were notable because of the lack of aura in the patients, the researchers wrote.
“In clinical practice, a detailed history in patients reporting visual flickering is therefore necessary to differentiate aura from [episodic visual snow],” they added, because an aura diagnosis would affect patient guidance on contraception use or the timing of triptans.
Dr. Hodak had no financial conflicts to disclose. The study was supported by Deutsche Migräne-und Kopfschmerzgesellschaft, Eye on Vision Foundation, and Baasch-Medicus Foundation.
SOURCE: Hodak J et al. JAMA Neurol. 2019 Nov 25. doi: 10.1001/jamaneurol.2019.4050.
Episodic visual snow was tied to migraine attacks in a case series of three adults who denied any visual snow outside of the migraines, based on data collected at an outpatient headache center.
Visual snow, a condition in which patients experience visual distortion of tiny, flickering dots resembling analog television static, is often a comorbid condition in migraine patients with and without aura. However, “to our knowledge, this is the first report of patients with an episodic form of visual snow strictly occurring with migraine attacks,” wrote Julius Hodak, MD, of the University of Bern (Switzerland) and colleagues.
In a research letter published in JAMA Neurology, the investigators described 3 adults with histories of migraine but no aura who presented to a tertiary headache center between January 2016 and December 2017, in addition to 1,934 adults with migraine but no visual snow. The three patients initially presented with headaches, and neurologic and MRI results were normal.
Two patients experienced black and white episodic visual snow and one experienced black and yellow visual snow. In one patient, visual snow occurred for less than 2 minutes before and during a migraine attack. The other two patients experienced visual snow during the entire migraine attack.
Based on these patients, the researchers proposed distinguishing episodic visual snow from the distinct disorder of visual snow syndrome, in which patients experience continuous visual snow and other visual symptoms.
In addition, the cases were notable because of the lack of aura in the patients, the researchers wrote.
“In clinical practice, a detailed history in patients reporting visual flickering is therefore necessary to differentiate aura from [episodic visual snow],” they added, because an aura diagnosis would affect patient guidance on contraception use or the timing of triptans.
Dr. Hodak had no financial conflicts to disclose. The study was supported by Deutsche Migräne-und Kopfschmerzgesellschaft, Eye on Vision Foundation, and Baasch-Medicus Foundation.
SOURCE: Hodak J et al. JAMA Neurol. 2019 Nov 25. doi: 10.1001/jamaneurol.2019.4050.
Episodic visual snow was tied to migraine attacks in a case series of three adults who denied any visual snow outside of the migraines, based on data collected at an outpatient headache center.
Visual snow, a condition in which patients experience visual distortion of tiny, flickering dots resembling analog television static, is often a comorbid condition in migraine patients with and without aura. However, “to our knowledge, this is the first report of patients with an episodic form of visual snow strictly occurring with migraine attacks,” wrote Julius Hodak, MD, of the University of Bern (Switzerland) and colleagues.
In a research letter published in JAMA Neurology, the investigators described 3 adults with histories of migraine but no aura who presented to a tertiary headache center between January 2016 and December 2017, in addition to 1,934 adults with migraine but no visual snow. The three patients initially presented with headaches, and neurologic and MRI results were normal.
Two patients experienced black and white episodic visual snow and one experienced black and yellow visual snow. In one patient, visual snow occurred for less than 2 minutes before and during a migraine attack. The other two patients experienced visual snow during the entire migraine attack.
Based on these patients, the researchers proposed distinguishing episodic visual snow from the distinct disorder of visual snow syndrome, in which patients experience continuous visual snow and other visual symptoms.
In addition, the cases were notable because of the lack of aura in the patients, the researchers wrote.
“In clinical practice, a detailed history in patients reporting visual flickering is therefore necessary to differentiate aura from [episodic visual snow],” they added, because an aura diagnosis would affect patient guidance on contraception use or the timing of triptans.
Dr. Hodak had no financial conflicts to disclose. The study was supported by Deutsche Migräne-und Kopfschmerzgesellschaft, Eye on Vision Foundation, and Baasch-Medicus Foundation.
SOURCE: Hodak J et al. JAMA Neurol. 2019 Nov 25. doi: 10.1001/jamaneurol.2019.4050.
FROM JAMA NEUROLOGY
Key clinical point: Episodic visual snow in patients with migraines appears to be distinct from an aura.
Major finding: Three patients with histories of migraine without aura reported episodic visual snow that occurred only at the onset or during a migraine attack.
Study details: The data come from a case series of 3 adults with episodic visual snow and migraine and 1,934 patients with migraine only seen at an outpatient headache center.
Disclosures: Dr. Hodak had no financial conflicts to disclose. The study was supported by Deutsche Migräne-und Kopfschmerzgesellschaft, Eye on Vision Foundation, and Baasch-Medicus Foundation.
Source: Hodak J et al. JAMA Neurol. 2019 Nov 25. doi: 10.1001/jamaneurol.2019.4050.
‘Brain enhancement’ supplements sold online may illegally contain piracetam
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
, according to an analysis of products sold online.
Sales of so-called ‘brain enhancement’ supplements exceeded $640 million in 2015 in the United States alone, but little is known about the risks of these dietary supplements, Pieter A. Cohen, MD, of the Cambridge Health Alliance in Somerville, Mass., and his coauthors wrote in a research letter published online Nov. 25 in JAMA Internal Medicine.
Piracetam is prescribed in many European countries for cognitive impairment and other disorders, the authors said. There is limited evidence for its efficacy, and the United States does not permit its sale as a dietary supplement.
Using the search terms “piracetam” and “dietary supplement,” researchers identified five brands of supplements sold online and analyzed 10 samples from these. Their chemical analysis revealed that eight samples from four brands contained piracetam, ranging from 831 mg to 1,452 mg per recommended serving size, and 85%-118% of the amount on the product’s label.
“Our findings demonstrate that, even after the FDA rejected an application to market piracetam as a new supplement ingredient, the drug was nevertheless introduced into the marketplace,” the authors wrote.
The authors calculated that, if consumers followed the recommended dosage on the labels of these products, they could be exposed to up to 11,283 mg of piracetam per day.
For comparison, prescription piracetam in Europe is commonly found in 800-mg and 1,200-mg tablets, and the recommended daily dose for cognitive disorders ranges from 2,400 to 4,800 mg per day, adjusted for renal function.
The authors commented that piracetam is associated with side effects at pharmaceutical dosages, including anxiety, insomnia, agitation, depression, drowsiness, and weight gain. However, the risk associated with higher doses, particularly in the elderly and those with renal insufficiency, are unknown.
“Until the law governing supplements is reformed such that products adulterated with drugs can be effectively removed from the market, clinicians should advise patients that supplements marketed as cognitive enhancers may contain prohibited drugs at supratherapeutic doses,” the authors wrote.
One author declared research support from two organizations unrelated to the study. No conflicts of interest were declared.
SOURCE: Cohen P et al. JAMA Int Med. 2019 Nov 25. doi: 10.1001/jamainternmed.2019.5507.
FROM JAMA INTERNAL MEDICINE
Omalizumab proves effective for severe pediatric atopic dermatitis
A new study has found that omalizumab (Xolair) reduced severity and improved quality of life in pediatric patients with severe atopic dermatitis.
“Future work with an even larger sample size, a longer duration, and higher-affinity versions of omalizumab would clarify the precise role of anti-IgE therapy and its ideal target population,” wrote Susan Chan, MD, of Guy’s and St. Thomas’ NHS Foundation Trust in London and her coauthors. The study was published in JAMA Pediatrics.
To determine the benefits of omalizumab in reducing immunoglobulin E levels and thereby treating severe childhood eczema, the researchers launched the Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT). This randomized clinical trial recruited 62 patients between the ages of 4 and 19 years with severe eczema, which was defined as a score over 40 on the objective Scoring Atopic Dermatitis (SCORAD) index. They received 24 weeks of treatment with either omalizumab (n = 30) or placebo (n = 32) followed by 24 weeks of follow-up. Participants had a mean age of 10.3 years.
After 24 weeks, the adjusted mean difference in objective SCORAD index between the two groups was –6.9 (95% confidence interval, –12.2 to –1.5; P = .01) and significantly favored omalizumab therapy. The adjusted mean difference for the Eczema Area and Severity Index (–6.7; 95% CI, –13.2 to –0.1) also favored omalizumab. In regard to quality of life, after 24 weeks the Children’s Dermatology Life Quality Index/Dermatology Life Quality Index favored the omalizumab group with an adjusted mean difference of –3.5 (95% CI, –6.4 to –0.5).
In an accompanying editorial, Ann Chen Wu, MD, of Harvard Medical School in Boston noted that the results of the study from Chan et al. were promising but “more questions need to be answered before the drug can be used to treat atopic dermatitis in clinical practice” (JAMA Pediatr. 2019 Nov. 25. doi: 10.1001/jamapediatrics.2019.4509).
Her initial concern was price; she acknowledged that “omalizumab is a costly intervention” but said atopic dermatitis is also costly, raising the question as to whether the high costs of both justify treatment. In addition, omalizumab as treatment can come with both benefits and harms. Severe atopic dermatitis can decrease quality of life and, though omalizumab appears to be safe, there are adverse effects and logistical burdens to overcome.
More than anything, she recognized the need to prioritize, wondering what level of atopic dermatitis patients would truly benefit from this level of treatment. “Is using a $100,000-per-year medication for an itchy condition an overtreatment,” she asked, “or a lifesaver?”
The study was funded by the National Institute for Health Research Efficacy and Mechanism Evaluation Programme and Guy’s and St. Thomas’ Charity. The authors had numerous financial disclosures, including receiving grants from the NIHR EME Programme and Guy’s and St. Thomas’ Charity along with active and placebo drugs from Novartis for use in the study. Dr. Wu reported receiving a grant from GlaxoSmithKline.
SOURCE: Chan S et al. JAMA Pediatr. 2019 Nov 25. doi: 10.1001/jamapediatrics.2019.4476.
A new study has found that omalizumab (Xolair) reduced severity and improved quality of life in pediatric patients with severe atopic dermatitis.
“Future work with an even larger sample size, a longer duration, and higher-affinity versions of omalizumab would clarify the precise role of anti-IgE therapy and its ideal target population,” wrote Susan Chan, MD, of Guy’s and St. Thomas’ NHS Foundation Trust in London and her coauthors. The study was published in JAMA Pediatrics.
To determine the benefits of omalizumab in reducing immunoglobulin E levels and thereby treating severe childhood eczema, the researchers launched the Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT). This randomized clinical trial recruited 62 patients between the ages of 4 and 19 years with severe eczema, which was defined as a score over 40 on the objective Scoring Atopic Dermatitis (SCORAD) index. They received 24 weeks of treatment with either omalizumab (n = 30) or placebo (n = 32) followed by 24 weeks of follow-up. Participants had a mean age of 10.3 years.
After 24 weeks, the adjusted mean difference in objective SCORAD index between the two groups was –6.9 (95% confidence interval, –12.2 to –1.5; P = .01) and significantly favored omalizumab therapy. The adjusted mean difference for the Eczema Area and Severity Index (–6.7; 95% CI, –13.2 to –0.1) also favored omalizumab. In regard to quality of life, after 24 weeks the Children’s Dermatology Life Quality Index/Dermatology Life Quality Index favored the omalizumab group with an adjusted mean difference of –3.5 (95% CI, –6.4 to –0.5).
In an accompanying editorial, Ann Chen Wu, MD, of Harvard Medical School in Boston noted that the results of the study from Chan et al. were promising but “more questions need to be answered before the drug can be used to treat atopic dermatitis in clinical practice” (JAMA Pediatr. 2019 Nov. 25. doi: 10.1001/jamapediatrics.2019.4509).
Her initial concern was price; she acknowledged that “omalizumab is a costly intervention” but said atopic dermatitis is also costly, raising the question as to whether the high costs of both justify treatment. In addition, omalizumab as treatment can come with both benefits and harms. Severe atopic dermatitis can decrease quality of life and, though omalizumab appears to be safe, there are adverse effects and logistical burdens to overcome.
More than anything, she recognized the need to prioritize, wondering what level of atopic dermatitis patients would truly benefit from this level of treatment. “Is using a $100,000-per-year medication for an itchy condition an overtreatment,” she asked, “or a lifesaver?”
The study was funded by the National Institute for Health Research Efficacy and Mechanism Evaluation Programme and Guy’s and St. Thomas’ Charity. The authors had numerous financial disclosures, including receiving grants from the NIHR EME Programme and Guy’s and St. Thomas’ Charity along with active and placebo drugs from Novartis for use in the study. Dr. Wu reported receiving a grant from GlaxoSmithKline.
SOURCE: Chan S et al. JAMA Pediatr. 2019 Nov 25. doi: 10.1001/jamapediatrics.2019.4476.
A new study has found that omalizumab (Xolair) reduced severity and improved quality of life in pediatric patients with severe atopic dermatitis.
“Future work with an even larger sample size, a longer duration, and higher-affinity versions of omalizumab would clarify the precise role of anti-IgE therapy and its ideal target population,” wrote Susan Chan, MD, of Guy’s and St. Thomas’ NHS Foundation Trust in London and her coauthors. The study was published in JAMA Pediatrics.
To determine the benefits of omalizumab in reducing immunoglobulin E levels and thereby treating severe childhood eczema, the researchers launched the Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT). This randomized clinical trial recruited 62 patients between the ages of 4 and 19 years with severe eczema, which was defined as a score over 40 on the objective Scoring Atopic Dermatitis (SCORAD) index. They received 24 weeks of treatment with either omalizumab (n = 30) or placebo (n = 32) followed by 24 weeks of follow-up. Participants had a mean age of 10.3 years.
After 24 weeks, the adjusted mean difference in objective SCORAD index between the two groups was –6.9 (95% confidence interval, –12.2 to –1.5; P = .01) and significantly favored omalizumab therapy. The adjusted mean difference for the Eczema Area and Severity Index (–6.7; 95% CI, –13.2 to –0.1) also favored omalizumab. In regard to quality of life, after 24 weeks the Children’s Dermatology Life Quality Index/Dermatology Life Quality Index favored the omalizumab group with an adjusted mean difference of –3.5 (95% CI, –6.4 to –0.5).
In an accompanying editorial, Ann Chen Wu, MD, of Harvard Medical School in Boston noted that the results of the study from Chan et al. were promising but “more questions need to be answered before the drug can be used to treat atopic dermatitis in clinical practice” (JAMA Pediatr. 2019 Nov. 25. doi: 10.1001/jamapediatrics.2019.4509).
Her initial concern was price; she acknowledged that “omalizumab is a costly intervention” but said atopic dermatitis is also costly, raising the question as to whether the high costs of both justify treatment. In addition, omalizumab as treatment can come with both benefits and harms. Severe atopic dermatitis can decrease quality of life and, though omalizumab appears to be safe, there are adverse effects and logistical burdens to overcome.
More than anything, she recognized the need to prioritize, wondering what level of atopic dermatitis patients would truly benefit from this level of treatment. “Is using a $100,000-per-year medication for an itchy condition an overtreatment,” she asked, “or a lifesaver?”
The study was funded by the National Institute for Health Research Efficacy and Mechanism Evaluation Programme and Guy’s and St. Thomas’ Charity. The authors had numerous financial disclosures, including receiving grants from the NIHR EME Programme and Guy’s and St. Thomas’ Charity along with active and placebo drugs from Novartis for use in the study. Dr. Wu reported receiving a grant from GlaxoSmithKline.
SOURCE: Chan S et al. JAMA Pediatr. 2019 Nov 25. doi: 10.1001/jamapediatrics.2019.4476.
FROM JAMA PEDIATRICS