User login
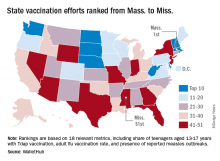
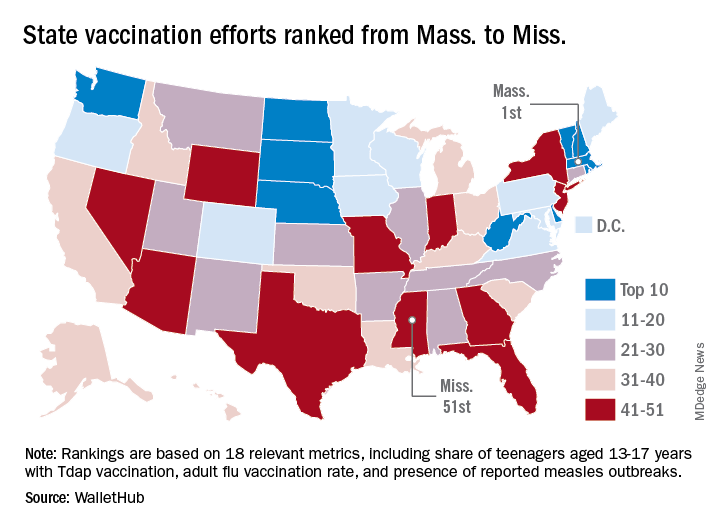
Massachusetts tops state vaccination rankings
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
according to a new analysis from personal finance website WalletHub.
The Bay State’s top finish in the “children and teenagers immunization rates” category moved it ahead of Vermont in the overall rankings, which had the highest score in each of the other two broad categories – “adult and elderly vaccination rates” and “immunization uptake disparities and influencing factors” – but only finished 15th in child/teen immunization, Wallethub reported.
The state that ranked 51st in child/teen immunization – Mississippi – also finished 51st overall, behind every other state and Washington, D.C. The rest of the bottom five consisted of Texas (50th); Florida (49th), which ranked last in the adult/elderly category; Georgia (48th); and Indiana (47th). New Mexico, however, managed to show that last is not always least by earning a mid-pack overall rank of 30 despite its last-place showing in the disparities/influencing factors category, the WalletHub analysis showed.
Scores for the three broad categories were determined using 18 relevant metrics, including influenza vaccination rate in children aged 6 months to 17 years (1st, Rhode Island; 51st, Wyoming), share of adults aged 60 years and older with zoster vaccination (1st, Vermont; 51st, Mississippi), and share of population without health insurance coverage (1st, Massachusetts; 51st, Texas), WalletHub said.
“Each state should tailor its vaccines policy to its need, with an understanding that those needs may change,” Dorit Rubinstein Reiss of the University of California Hastings College of the Law, San Francisco, told WalletHub. When parents refuse to have their children vaccinated, it’s important to remember that “the state is not denying these children schooling. It is requiring that they be protected from disease first.”
Photodermatoses: Differential diagnosis includes sunscreen allergy, connective tissue disease
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – according to Vincent DeLeo, MD, of the department of dermatology at the University of Southern California, Los Angeles, who discussed common diagnoses at the annual Coastal Dermatology Symposium.
“They’re not common bread-and-butter dermatitis, like acne rosacea. Many people feel uncomfortable trying to work out the differential diagnosis when they see someone who comes in with what either the patient or the physician thinks is a reaction to the sun,” Dr. DeLeo said in an interview at the meeting.
“Usually, the best clue is the distribution of the rash or lesion,” which often are on the backs of the hands or on the arms from the sleeves down, he added. “When you see that, you should think of a photosensitive eruption,” and consider a differential diagnosis, using “certain tests, asking questions, and looking at the morphology and distribution of the reaction.”
The most common of the photodermatoses is polymorphous light eruption, which typically occurs in people on vacation, often among those who live in temperate climates and react violently to sun overexposure. “You usually have to rule out things like connective tissue disease by getting serology,” Dr. DeLeo noted in the interview.
In general, these patients either present with a reaction or describe a rash that has since cleared up. When a patient is describing a problem that has disappeared, he or she may have a photo of the reaction on their phone, which can be helpful, he said at the meeting jointly presented by the University of Louisville and Global Academy for Medical Education.
Some photodermatoses are characterized by tell-tale rashes indicative of a connective tissue disease like lupus or dermatomyositis, which can be followed up with biopsy and serology to confirm a diagnosis, said Dr. DeLeo, who is also professor emeritus of dermatology at the Icahn School of Medicine at Mount Sinai, New York. Other patterns, such as blisters on the hands, should prompt tests for porphyria cutanea tarda, which requires a 24-hour urine test to confirm.
But reactions in sun-exposed areas can also be caused by drug-induced photosensitivity and drugs such as NSAIDs, antibiotics, and diuretics, among others, Dr. DeLeo said.
Another common reaction can occur with exposure to photosensitizing plant materials, resulting in blisters combined with altered pigmentation. Most frequently, these reactions are caused by exposure to limes, which contain psoralens that absorb light and produce the reaction. “That is a clinical diagnosis and we can recognize that because, like poison ivy, it is usually a streaky kind of reaction in a distinct pattern. You don’t need [additional] tests for that,” he said. This can occur, for example, after making cocktails, he said, describing an example of parents who had been mixing drinks and then picked up their child. Later, they brought the child to the ED with a hand-shaped rash, and treating physicians contacted social services out of fears of child abuse.
In some cases, patients who present with no rash can elicit the problem. Solar urticaria can produce hives on demand – ask a patient to go out in the sun and return after a few minutes, and a rash will generally appear, Dr. DeLeo advised.
Patients might want to take steps to avoid such problems and there are several options available to do so, Dr. DeLeo noted. There is some evidence of efficacy for photoprotection for an extract from a fern plant found in Central and South America, a product called Heliocare, in a study sponsored by the manufacturer, Ferndale Healthcare (J Clin Aesthet Dermatol. 2015 Feb;8[2]:19-23). Patients can also undergo two or three treatments of phototherapy for 3-4 weeks in advance of a trip in order to “harden” the skin and reduce the chances of an overreaction.
Dr. DeLeo presented a simplified guide to use when considering differential diagnoses in patients with photodermatoses.
- Solar urticaria occurs quickly upon exposure and is short lived.
- Polymorphous light eruption usually occurs during a vacation and does not affect the face.
- A sun-related eczematous rash affecting the face, which recurs, is typically photoallergy to sunscreen.
- A chronic, severe dermatitis seen in a photodistribution pattern could be chronic actinic dermatitis. The patient should undergo phototesting. A biopsy of a severe case may resemble lymphoma.
- In photosensitive individuals with an uncertain diagnosis, serology testing for connective tissue disease should be performed to rule out lupus or dermatomyositis.
- When the patient has a rash in only sun-exposed areas, as well as a history of exposure to photosensitizing drugs, the drug should be discontinued to see if the rash disappears.
- Porphyria cutanea tarda is diagnosed with a 24-hour urine test that reveals at least five times normal uroporphyrin levels, along with hemochromatosis polymorphisms. Levels of uroporphyrin that are elevated but not five times normal are suggestive of pseudoporphyria.
- Phototoxic contact dermatitis caused by plants often results in unusual distributions.
- Hispanic individuals with a photodistributed rash, especially in the presence of cheilitis, may have actinic prurigo.
Dr. DeLeo is a consultant for Estee Lauder. The annual Coastal Dermatology Symposium is jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
IHS Pediatrician Convicted on Sexual Abuse Counts
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Stanley Patrick Weber, a former Indian Health Service (IHS) pediatrician, was convicted September 27 on 8 counts of sexual abuse of 4 Native American boys under his care at the Pine Ridge Indian Reservation, Oglala Lakota County, South Dakota.
Weber was an employee of the IHS from 1986 until he resigned in 2016. During that time, although suspicions were rampant that he was abusing young patients, he was moved from one reservation to another. An investigation by The Wall Street Journal and PBS/Frontline found officials in the IHS chain of command “ignored warning signs” and tried to silence whistleblowers.
Weber also was convicted last year and served time for abusing 2 boys at another government hospital in Montana before he arrived at Pine Ridge.
The Pine Ridge Reservation is home to some of the poorest communities in the US, with a dropout rate of > 70%, and a teen suicide rate 150% higher than America’s as a whole. Many of the youths testifying in the case described difficult childhoods, according to the Frontline program. In addition to assaulting them during health care visits, Weber lured them to his home with offers of alcohol and cash.
A White House task force was created to address the agency’s failures. In a statement, Rear Adm Michael Weahkee, IHS principal deputy director, said the IHS is “doing all we can throughout our agency to strengthen our protection for patients.” Steps taken include reinforcing with staff the prohibitions against inappropriate contact with children, reminding staff of the protections for whistleblowers, and hiring Integritas Creative Solutions to conduct a medical quality assurance review to examine whether laws, policies and procedures have been followed with regard to protecting patients from sexual abuse. The review complements work done by the Presidential Task Force on Protecting Native American Children in the Indian Health Service System and a separate review by the HHS Office of the Inspector General.
The IHS also is providing professional counseling for victims. Services do not have to be at an IHS facility or with an IHS provider. A confidential hot line has been established at 301-443-0658.
Weahkee calls the verdict “a long-awaited victory.” But, he adds, “we know that it will not completely heal the wounds inflicted on Dr. Weber’s victims.”
Vaping-associated lung injury cases exceed 1,000
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email FDAVapingSampleInquiries@fda.hhs.gov. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email LungDiseaseOutbreak@cdc.gov. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email FDAVapingSampleInquiries@fda.hhs.gov. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email LungDiseaseOutbreak@cdc.gov. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
More than 1,000 cases of vaping-associated lung injury have been reported in 48 states and the U.S. Virgin Islands, according to a telebriefing by the Centers for Disease Control and Prevention.
As of Oct. 1, there have been 1,080 confirmed and probable cases of lung injury associated with the use of e-cigarettes, or vaping, said Anne Schuchat, MD, principal deputy director of the CDC. The latest figures were also reported in a statement issued by the CDC.
Dr. Schuchat said 18 related deaths in 15 states have been confirmed, and additional deaths are under investigation.
“As we have continued to get data for additional cases, the trends we reported last week persist,” Dr. Schuchat said (MMWR. 2019 Sep 27;68[39];860-4).
“Most patients reported a history of using THC [tetrahydrocannabinol]-containing products, and most patients are male and young people.” Of the 1,080 cases identified, approximately 70% are male, roughly 80% are younger than 35 years of age, and 37% are under 21 years of age. The patients’ median age is 23 years (range, 13-75 years). Among patients who have died, the median age is 50 years (range, 27-71 years).
The CDC now has information from 578 patients on the substances used in vaping products in the 90 days before symptom onset. About 78% of these patients reported using THC-containing products, and 37% reported exclusive use of THC-containing products. Roughly 58% of patients reported using nicotine-containing products, and 17% reported exclusive use of nicotine-containing products.
“I wish we had more answers regarding the specific harmful products or components that are causing these illnesses,” Dr. Schuchat said. She noted that THC-containing products appear to be the most commonly used, but these products don’t appear to be the only culprit. Additionally, in a report released recently in the New England Journal of Medicine (2019 Sep 9. doi: 10.1056/NEJMoa1911614), THC-containing products bought “off the street” were commonly used by patients with lung injuries. However, the CDC can’t say for certain if it’s safer for consumers to buy THC-containing products from a licensed dispensary.
The CDC has deployed staff to several states to help investigate the lung injuries, reached out to the clinical community to increase awareness of the injuries, and worked with clinicians and medical examiners to review assessments of patients who have developed these injuries, including those who have died. The CDC has also convened clinical professional societies to “help strengthen the detection, reporting, and management of cases,” Dr. Schuchat said.
In addition, the CDC has joined with the Food and Drug Administration and other public health partners to develop a laboratory plan for “continued testing of products, aerosol testing of substances produced by the products, and clinical pathology lung specimens from patients,” Dr. Schuchat said.
The FDA is also working to gather more information about vaping-associated lung injuries. The FDA is trying to obtain “critical details” about the specific products or substances that may be involved, said Judy McMeekin, PharmD, deputy associate commissioner for regulatory affairs at the FDA.
“There does not currently appear to be one product or substance involved in all of the cases,” Dr. McMeekin said. “We are leaving no stone unturned and following all potential leads regarding any particular product, constituent, or compound that may be at issue.”
The FDA has collected more than 440 samples of vaping devices and products from 18 states. The agency is still analyzing these samples, but a preliminary analysis has shown that some products contain THC concentrations ranging from 14% to 76%, and some products contain a combination of THC and vitamin E acetate ranging from 31% to 88%.
For information about the collection of vaping products for possible testing by the FDA, email FDAVapingSampleInquiries@fda.hhs.gov. For information about collection and submission of clinical specimens for possible testing by the CDC, see the Healthcare Provider webpage.
Clinicians and health officials who have questions about this outbreak can email LungDiseaseOutbreak@cdc.gov. All others with questions about this outbreak can contact CDC-INFO at 800-232-4636 or submit information at the Contact CDC-INFO page.
AGA promotes workforce diversity in academic gastroenterology: The FORWARD program
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD
|
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
One-third of patients with severe asthma are overusing corticosteroids
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
REPORTING FROM ERS 2019
FDA approves Descovy as HIV PrEP for men and transgender women who have sex with men
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
The decision, backing the earlier recommendation of the FDA’s Antimicrobial Drugs Advisory Committee, was based upon results from DISCOVER, a pivotal, multiyear, global phase 3 clinical trial that evaluated the safety and efficacy of Descovy (emtricitabine 200 mg and tenofovir alafenamide 25-mg tablets for PrEP, compared with Truvada (emtricitabine 200 mg and tenofovir disoproxil fumarate 300-mg tablets).
DISCOVER included more than 5,300 adult cisgender men who have sex with men or transgender women who have sex with men.
In the trial, Descovy achieved noninferiority to Truvada.
Descovy has a Boxed Warning in its U.S. product label regarding the risk of posttreatment acute exacerbation of hepatitis B, according to the company.
The Descovy label also includes a Boxed Warning regarding the risk of drug resistance with PrEP use in undiagnosed early HIV-1 infection. The effectiveness of Descovy for PrEP in individuals at risk of HIV-1 from receptive vaginal sex was not tested, and thus cisgender women at risk for infection from vaginal sex were not included in the population for which the drug was approved.
The Descovy label and safety information is available here.
The FDA version of the announcement is available here.
Functional medicine offers another approach to treating psychiatric illness
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
Tocilizumab beat azathioprine for NMOSD
STOCKHOLM – according to data reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorders (TANGO) trial, explained senior investigator Fu-Dong Shi, MD, PhD, tracked the time to first NMOSD relapse for patients randomized either to tocilizumab or azathioprine. After 60 weeks of participation in the head-to-head trial, 86% of patients receiving tocilizumab remained relapse-free, compared with 56.9% of those on azathioprine. By 90 weeks of therapy, the relapse-free rate held at 86% for those receiving tocilizumab, compared with 48.1% for those on azathioprine, for a 76.4% relative reduction in risk of relapse.
Patients with concomitant autoimmune disease saw particular benefit from tocilizumab compared with azathioprine. On tocilizumab, 78.8% of those with concomitant autoimmune disease remained relapse-free at 90 weeks, a rate that did not differ significantly from that seen in those without other autoimmune disease. However, 22.3% of those with concomitant autoimmune disease who received azathioprine remained relapse-free at 90 weeks, compared with 63.5% of those without autoimmune disease.
Dr. Shi, of the Tianjin (China) Medical University General Hospital, explained that there was limited evidence for the advantage of newer disease-modifying therapies (DMTs) over such older medications as azathioprine to treat NMOSD. Therefore, he said, he and other investigators in the Chinese Medical Network for Neuroinflammation (CMNN), including first author Chao Zhang, MD, initiated a randomized, multicenter, open-label trial that compared tocilizumab to azathioprine for highly relapsing NMOSD.
A total of 118 patients were enrolled. To participate, patients had to meet the 2015 international consensus criteria for NMOSD, and have had at least two relapses in the past year or three relapses within the preceding 2 years.
After a washout period, participants were randomized 1:1 to receive daily oral azathioprine dosed at 2-3 mg/kg or intravenous tocilizumab dosed at 8 mg/kg every 4 weeks. Participants continued on the assigned regimen for at least 60 weeks.
Secondary endpoints included 12-week confirmed disability progression. Here, 35.8% of those on azathioprine saw disability progression, compared with 9.9% on tocilizumab, for a 72.5% relative risk reduction. Serum aquaporin 4-IgG titers fell further for those taking tocilizumab.
In terms of safety, 83% of those on azathioprine and 61% of those receiving tocilizumab had a treatment-related adverse event, as determined by investigator assessment. About half of patients in each group experienced moderate adverse events, and about 90% had mild adverse events in each group. However, there were twice as many severe adverse events among those taking azathioprine. There was one NMOSD-related death in each group.
Subgroup analyses looked at baseline disability scores, number of previous relapses, disease duration, and age at randomization. None of these variables made a difference in the statistically better performance of tocilizumab compared with azathioprine, with hazard ratios ranging from 0.076 to 0.343 in favor of tocilizumab for all subgroups.
Patients were almost all female (92% overall) and about 47 years old at baseline, with a 6-year history of NMOSD.
Overall, 83% of the cohort had experienced optic neuritis over the preceding 2 years, and 94% had experienced acute myelitis. Other manifestations of NMOSD, such as acute brainstem syndrome and symptomatic cerebral syndrome, were much less frequent, occurring in 4%-30% of patients.
Most patients (71%) had been on oral corticosteroids before the washout period, and 34% had been taking mycophenolate mofetil. Azathioprine was taken by 44% of patients at baseline.
Additional data captured during the TANGO trial are being analyzed and will be reported at a later date, said Dr. Shi. These include optical coherence tomography and visual evoked potentials; magnetic resonance imaging of the brain, spinal cord, and optic nerve; and tracking serum B-cell subpopulations. He added that the investigators are continuing long-term, real-world follow-up of the TANGO patient cohort.
Dr. Shi acknowledged that TANGO was limited by the lack of an independent data monitoring committee and the open-label nature of the study.
The study was funded by Tianjin Medical University; Capital Medical University, Beijing; and the National Science Foundation of China. Dr. Shi and coauthors reported no conflicts of interest.
SOURCE: Zhang C et al. ECTRIMS 2019, Abstract 140.
STOCKHOLM – according to data reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorders (TANGO) trial, explained senior investigator Fu-Dong Shi, MD, PhD, tracked the time to first NMOSD relapse for patients randomized either to tocilizumab or azathioprine. After 60 weeks of participation in the head-to-head trial, 86% of patients receiving tocilizumab remained relapse-free, compared with 56.9% of those on azathioprine. By 90 weeks of therapy, the relapse-free rate held at 86% for those receiving tocilizumab, compared with 48.1% for those on azathioprine, for a 76.4% relative reduction in risk of relapse.
Patients with concomitant autoimmune disease saw particular benefit from tocilizumab compared with azathioprine. On tocilizumab, 78.8% of those with concomitant autoimmune disease remained relapse-free at 90 weeks, a rate that did not differ significantly from that seen in those without other autoimmune disease. However, 22.3% of those with concomitant autoimmune disease who received azathioprine remained relapse-free at 90 weeks, compared with 63.5% of those without autoimmune disease.
Dr. Shi, of the Tianjin (China) Medical University General Hospital, explained that there was limited evidence for the advantage of newer disease-modifying therapies (DMTs) over such older medications as azathioprine to treat NMOSD. Therefore, he said, he and other investigators in the Chinese Medical Network for Neuroinflammation (CMNN), including first author Chao Zhang, MD, initiated a randomized, multicenter, open-label trial that compared tocilizumab to azathioprine for highly relapsing NMOSD.
A total of 118 patients were enrolled. To participate, patients had to meet the 2015 international consensus criteria for NMOSD, and have had at least two relapses in the past year or three relapses within the preceding 2 years.
After a washout period, participants were randomized 1:1 to receive daily oral azathioprine dosed at 2-3 mg/kg or intravenous tocilizumab dosed at 8 mg/kg every 4 weeks. Participants continued on the assigned regimen for at least 60 weeks.
Secondary endpoints included 12-week confirmed disability progression. Here, 35.8% of those on azathioprine saw disability progression, compared with 9.9% on tocilizumab, for a 72.5% relative risk reduction. Serum aquaporin 4-IgG titers fell further for those taking tocilizumab.
In terms of safety, 83% of those on azathioprine and 61% of those receiving tocilizumab had a treatment-related adverse event, as determined by investigator assessment. About half of patients in each group experienced moderate adverse events, and about 90% had mild adverse events in each group. However, there were twice as many severe adverse events among those taking azathioprine. There was one NMOSD-related death in each group.
Subgroup analyses looked at baseline disability scores, number of previous relapses, disease duration, and age at randomization. None of these variables made a difference in the statistically better performance of tocilizumab compared with azathioprine, with hazard ratios ranging from 0.076 to 0.343 in favor of tocilizumab for all subgroups.
Patients were almost all female (92% overall) and about 47 years old at baseline, with a 6-year history of NMOSD.
Overall, 83% of the cohort had experienced optic neuritis over the preceding 2 years, and 94% had experienced acute myelitis. Other manifestations of NMOSD, such as acute brainstem syndrome and symptomatic cerebral syndrome, were much less frequent, occurring in 4%-30% of patients.
Most patients (71%) had been on oral corticosteroids before the washout period, and 34% had been taking mycophenolate mofetil. Azathioprine was taken by 44% of patients at baseline.
Additional data captured during the TANGO trial are being analyzed and will be reported at a later date, said Dr. Shi. These include optical coherence tomography and visual evoked potentials; magnetic resonance imaging of the brain, spinal cord, and optic nerve; and tracking serum B-cell subpopulations. He added that the investigators are continuing long-term, real-world follow-up of the TANGO patient cohort.
Dr. Shi acknowledged that TANGO was limited by the lack of an independent data monitoring committee and the open-label nature of the study.
The study was funded by Tianjin Medical University; Capital Medical University, Beijing; and the National Science Foundation of China. Dr. Shi and coauthors reported no conflicts of interest.
SOURCE: Zhang C et al. ECTRIMS 2019, Abstract 140.
STOCKHOLM – according to data reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
The tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorders (TANGO) trial, explained senior investigator Fu-Dong Shi, MD, PhD, tracked the time to first NMOSD relapse for patients randomized either to tocilizumab or azathioprine. After 60 weeks of participation in the head-to-head trial, 86% of patients receiving tocilizumab remained relapse-free, compared with 56.9% of those on azathioprine. By 90 weeks of therapy, the relapse-free rate held at 86% for those receiving tocilizumab, compared with 48.1% for those on azathioprine, for a 76.4% relative reduction in risk of relapse.
Patients with concomitant autoimmune disease saw particular benefit from tocilizumab compared with azathioprine. On tocilizumab, 78.8% of those with concomitant autoimmune disease remained relapse-free at 90 weeks, a rate that did not differ significantly from that seen in those without other autoimmune disease. However, 22.3% of those with concomitant autoimmune disease who received azathioprine remained relapse-free at 90 weeks, compared with 63.5% of those without autoimmune disease.
Dr. Shi, of the Tianjin (China) Medical University General Hospital, explained that there was limited evidence for the advantage of newer disease-modifying therapies (DMTs) over such older medications as azathioprine to treat NMOSD. Therefore, he said, he and other investigators in the Chinese Medical Network for Neuroinflammation (CMNN), including first author Chao Zhang, MD, initiated a randomized, multicenter, open-label trial that compared tocilizumab to azathioprine for highly relapsing NMOSD.
A total of 118 patients were enrolled. To participate, patients had to meet the 2015 international consensus criteria for NMOSD, and have had at least two relapses in the past year or three relapses within the preceding 2 years.
After a washout period, participants were randomized 1:1 to receive daily oral azathioprine dosed at 2-3 mg/kg or intravenous tocilizumab dosed at 8 mg/kg every 4 weeks. Participants continued on the assigned regimen for at least 60 weeks.
Secondary endpoints included 12-week confirmed disability progression. Here, 35.8% of those on azathioprine saw disability progression, compared with 9.9% on tocilizumab, for a 72.5% relative risk reduction. Serum aquaporin 4-IgG titers fell further for those taking tocilizumab.
In terms of safety, 83% of those on azathioprine and 61% of those receiving tocilizumab had a treatment-related adverse event, as determined by investigator assessment. About half of patients in each group experienced moderate adverse events, and about 90% had mild adverse events in each group. However, there were twice as many severe adverse events among those taking azathioprine. There was one NMOSD-related death in each group.
Subgroup analyses looked at baseline disability scores, number of previous relapses, disease duration, and age at randomization. None of these variables made a difference in the statistically better performance of tocilizumab compared with azathioprine, with hazard ratios ranging from 0.076 to 0.343 in favor of tocilizumab for all subgroups.
Patients were almost all female (92% overall) and about 47 years old at baseline, with a 6-year history of NMOSD.
Overall, 83% of the cohort had experienced optic neuritis over the preceding 2 years, and 94% had experienced acute myelitis. Other manifestations of NMOSD, such as acute brainstem syndrome and symptomatic cerebral syndrome, were much less frequent, occurring in 4%-30% of patients.
Most patients (71%) had been on oral corticosteroids before the washout period, and 34% had been taking mycophenolate mofetil. Azathioprine was taken by 44% of patients at baseline.
Additional data captured during the TANGO trial are being analyzed and will be reported at a later date, said Dr. Shi. These include optical coherence tomography and visual evoked potentials; magnetic resonance imaging of the brain, spinal cord, and optic nerve; and tracking serum B-cell subpopulations. He added that the investigators are continuing long-term, real-world follow-up of the TANGO patient cohort.
Dr. Shi acknowledged that TANGO was limited by the lack of an independent data monitoring committee and the open-label nature of the study.
The study was funded by Tianjin Medical University; Capital Medical University, Beijing; and the National Science Foundation of China. Dr. Shi and coauthors reported no conflicts of interest.
SOURCE: Zhang C et al. ECTRIMS 2019, Abstract 140.
REPORTING FROM ECTRIMS 2019
Treatment of Unresectable Stage III Non-small Cell Lung Cancer
With a recent renaissance in cancer diagnostics and treatment, there is hope for many fighting the disease. In this article, Dr. M. Patricia Rivera discusses screening and diagnostic procedures as well as the treatment options that are available for patients with unresectable stage III non-small cell lung cancer.
This article is sponsored by AstraZeneca.
US-33194 Last Updated 9/19
With a recent renaissance in cancer diagnostics and treatment, there is hope for many fighting the disease. In this article, Dr. M. Patricia Rivera discusses screening and diagnostic procedures as well as the treatment options that are available for patients with unresectable stage III non-small cell lung cancer.
This article is sponsored by AstraZeneca.
US-33194 Last Updated 9/19
With a recent renaissance in cancer diagnostics and treatment, there is hope for many fighting the disease. In this article, Dr. M. Patricia Rivera discusses screening and diagnostic procedures as well as the treatment options that are available for patients with unresectable stage III non-small cell lung cancer.
This article is sponsored by AstraZeneca.
US-33194 Last Updated 9/19