User login
In Informed Consent, Capacity Is Crucial
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
Prevalence of developmental disabilities up significantly since 2009
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.
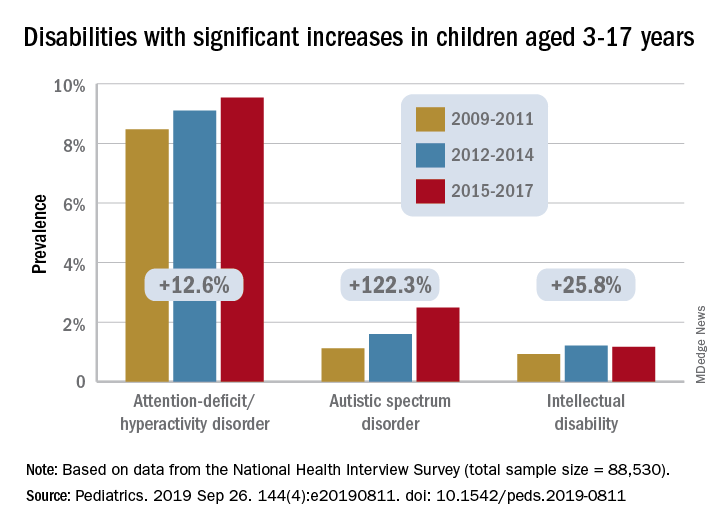
The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.

The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.

The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
FROM PEDIATRICS
Key clinical point:
Major finding: Among U.S. children aged 3-17 years, prevalence of developmental disabilities increased by 9.5% from 2009 to 2017.
Study details: The sample from the National Health Interview Survey included 88,530 children.
Disclosures: The investigators said that there was no external funding for the study and that they had no relevant financial relationships to disclose.
Source: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811.
Blips on Lips
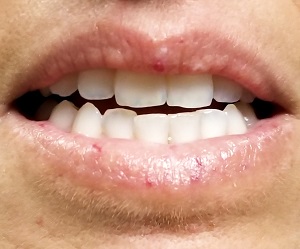
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.
EXAMINATION
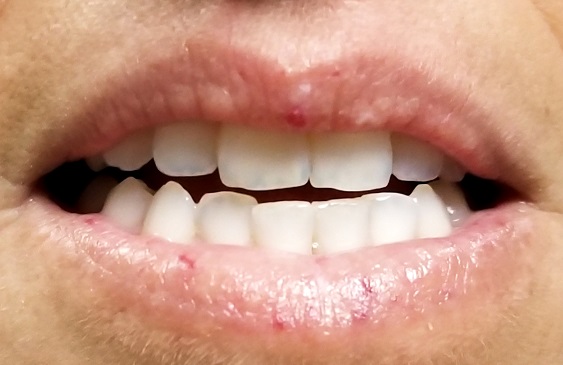
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.
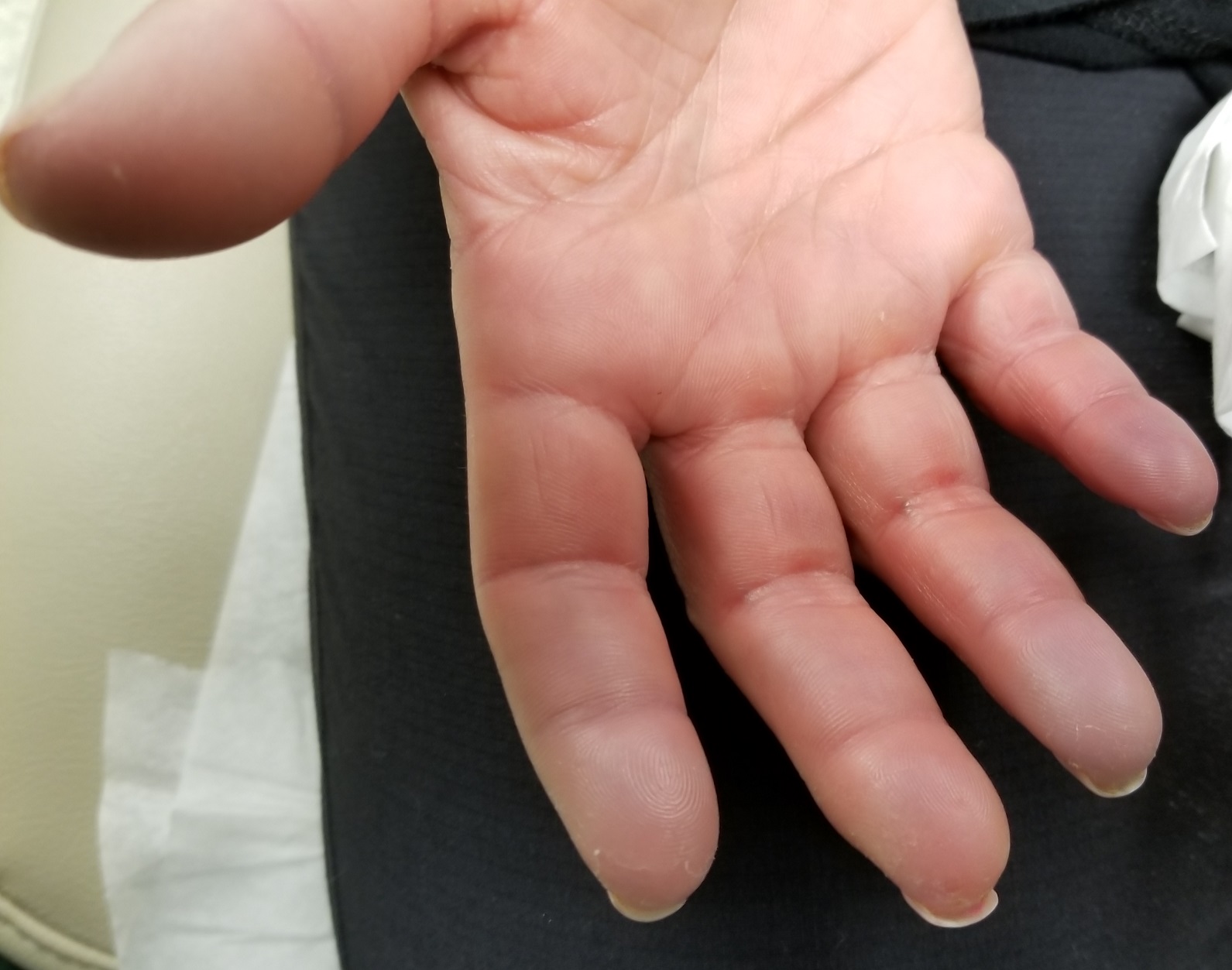
The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.

EXAMINATION
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.

The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.

EXAMINATION
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.

The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
Chronic Subjective Tinnitus
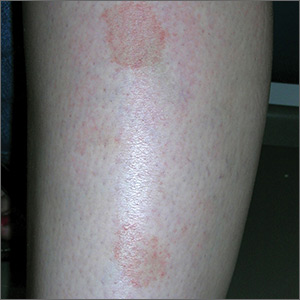
Marks on lower leg
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Auto-brewery syndrome and hangovers as ‘illnesses’
Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Biologics beyond anti-TNF therapies show promise for ulcerative colitis
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: New biologic therapies and strategies for ulcerative colitis patients continue to evolve.
Major finding: In UNIFI, a higher percentage of patients who received a single infusion of ustekinumab at 130 mg or 6 mg per kg of body weight achieved clinical remission at 8 weeks, compared with placebo (15.6% and 15.5%, vs. 5.3%, respectively. In VARSITY, a higher percentage of patients who received vedolizumab achieved clinical remission at 52 weeks, compared with those who received adalimumab (31.3% vs. 22.5%, respectively).
Study details: UNIFI, a phase 3 trial, included an 8-week randomized induction trial of 961 patients and a 44-week randomized-withdrawal maintenance trial. VARSITY was a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis.
Disclosures: UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
Sources: Sands B et al. N Engl J Med. 2019 Sep 25. doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25. doi: 10/1056/NEJMoa1905725.
Step-up therapy with glucocorticoids benefits black children with asthma
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Black children with poorly controlled asthma had a superior response to stepped increases in inhaled glucocorticoids, compared with stepped increases in long-acting beta2-agonists.
Major finding: A total of 46% of black children with poorly controlled asthma improved when inhaled glucocorticoids were increased.
Study details: The data come from the BARD trials, a pair of prospective, randomized, double-blind trials including 280 children aged 5-11 years with at least one grandparent identified as black and 294 adolescents and adults who had family backgrounds that were similar to the children.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
Source: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
Q&A with DDW 2019 Advancing Clinical Practice: GI Fellow-Directed Quality Improvement Projects session abstract reviewers
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
Neurostimulation device may treat vertigo in patients with vestibular migraine
according to a small, open-label study published online Sept. 25 in Neurology.
The retrospective, single-center study included data from 18 patients who had vestibular migraine and received acute treatment with a handheld stimulation device. The device used in the study, GammaCore, is approved for the treatment of migraine. There are no approved treatments for vestibular migraine.
“There’s a huge need for effective treatments for vestibular migraine attacks,” first author Shin C. Beh, MD, said in a news release. “People with vestibular migraine do not always have headaches, and when they do, they are often less severe than in typical migraine, so the pain-relieving drugs used for typical migraine often are not effective. People can take drugs that suppress the vertigo or the nausea, but those drugs cause drowsiness and make it hard for people to go about their usual activities.” Dr. Beh is an assistant professor of neurology and neurotherapeutics at University of Texas Southwestern Medical Center in Dallas.
The patients had an average age of about 46 years, and 16 were women. A total of 14 patients received treatment during an acute vestibular migraine attack, and 4 received treatment while they had bothersome interictal dizziness consistent with persistent perceptual postural dizziness.
After stimulation, vertigo improved in 13 of the 14 people with vestibular migraine attacks. In two of these patients, vertigo resolved completely, and five had at least a 50% improvement in vertigo symptoms. On a 0-10 scale, patients’ mean vertigo rating decreased from 5.2 before stimulation to 3.1 after stimulation.
Of five patients with vestibular migraine attacks who had headache, all experienced a reduction in headache pain. Mean headache severity decreased from 6 before stimulation to 2.4 after stimulation.
None of the four patients with interictal dizziness experienced a reduction in dizziness after stimulation.
Patients place the device against the neck to receive electrical stimulation, and patients received stimulation for 2 minutes on each side of the neck.
Participants reported a mild pulling sensation of the neck muscles during the stimulation but did not report pain or other side effects.
“Vestibular migraine is the most common neurologic cause of vertigo and can greatly interfere with a person’s daily life,” Dr. Beh said. “If these results can be confirmed with larger studies, not only could there finally be a treatment for vestibular migraine, such a treatment would also be easy to use.”
A randomized, double-blind, sham-controlled study is needed to further assess the use of noninvasive vagus nerve stimulation in treating vestibular migraine, the authors said.
The study had no targeted funding, and Dr. Beh had no relevant disclosures. Coauthor Deborah I. Friedman, MD, professor of neurology and neurotherapeutics and ophthalmology at UT Southwestern Medical Center, disclosed serving on advisory boards or speaking for pharmaceutical and medical device companies. Dr. Friedman is on the editorial advisory board of Neurology Reviews.
SOURCE: Beh SC et al. Neurology. 2019 Sep 25. doi: 10.1212/WNL.0000000000008388.
according to a small, open-label study published online Sept. 25 in Neurology.
The retrospective, single-center study included data from 18 patients who had vestibular migraine and received acute treatment with a handheld stimulation device. The device used in the study, GammaCore, is approved for the treatment of migraine. There are no approved treatments for vestibular migraine.
“There’s a huge need for effective treatments for vestibular migraine attacks,” first author Shin C. Beh, MD, said in a news release. “People with vestibular migraine do not always have headaches, and when they do, they are often less severe than in typical migraine, so the pain-relieving drugs used for typical migraine often are not effective. People can take drugs that suppress the vertigo or the nausea, but those drugs cause drowsiness and make it hard for people to go about their usual activities.” Dr. Beh is an assistant professor of neurology and neurotherapeutics at University of Texas Southwestern Medical Center in Dallas.
The patients had an average age of about 46 years, and 16 were women. A total of 14 patients received treatment during an acute vestibular migraine attack, and 4 received treatment while they had bothersome interictal dizziness consistent with persistent perceptual postural dizziness.
After stimulation, vertigo improved in 13 of the 14 people with vestibular migraine attacks. In two of these patients, vertigo resolved completely, and five had at least a 50% improvement in vertigo symptoms. On a 0-10 scale, patients’ mean vertigo rating decreased from 5.2 before stimulation to 3.1 after stimulation.
Of five patients with vestibular migraine attacks who had headache, all experienced a reduction in headache pain. Mean headache severity decreased from 6 before stimulation to 2.4 after stimulation.
None of the four patients with interictal dizziness experienced a reduction in dizziness after stimulation.
Patients place the device against the neck to receive electrical stimulation, and patients received stimulation for 2 minutes on each side of the neck.
Participants reported a mild pulling sensation of the neck muscles during the stimulation but did not report pain or other side effects.
“Vestibular migraine is the most common neurologic cause of vertigo and can greatly interfere with a person’s daily life,” Dr. Beh said. “If these results can be confirmed with larger studies, not only could there finally be a treatment for vestibular migraine, such a treatment would also be easy to use.”
A randomized, double-blind, sham-controlled study is needed to further assess the use of noninvasive vagus nerve stimulation in treating vestibular migraine, the authors said.
The study had no targeted funding, and Dr. Beh had no relevant disclosures. Coauthor Deborah I. Friedman, MD, professor of neurology and neurotherapeutics and ophthalmology at UT Southwestern Medical Center, disclosed serving on advisory boards or speaking for pharmaceutical and medical device companies. Dr. Friedman is on the editorial advisory board of Neurology Reviews.
SOURCE: Beh SC et al. Neurology. 2019 Sep 25. doi: 10.1212/WNL.0000000000008388.
according to a small, open-label study published online Sept. 25 in Neurology.
The retrospective, single-center study included data from 18 patients who had vestibular migraine and received acute treatment with a handheld stimulation device. The device used in the study, GammaCore, is approved for the treatment of migraine. There are no approved treatments for vestibular migraine.
“There’s a huge need for effective treatments for vestibular migraine attacks,” first author Shin C. Beh, MD, said in a news release. “People with vestibular migraine do not always have headaches, and when they do, they are often less severe than in typical migraine, so the pain-relieving drugs used for typical migraine often are not effective. People can take drugs that suppress the vertigo or the nausea, but those drugs cause drowsiness and make it hard for people to go about their usual activities.” Dr. Beh is an assistant professor of neurology and neurotherapeutics at University of Texas Southwestern Medical Center in Dallas.
The patients had an average age of about 46 years, and 16 were women. A total of 14 patients received treatment during an acute vestibular migraine attack, and 4 received treatment while they had bothersome interictal dizziness consistent with persistent perceptual postural dizziness.
After stimulation, vertigo improved in 13 of the 14 people with vestibular migraine attacks. In two of these patients, vertigo resolved completely, and five had at least a 50% improvement in vertigo symptoms. On a 0-10 scale, patients’ mean vertigo rating decreased from 5.2 before stimulation to 3.1 after stimulation.
Of five patients with vestibular migraine attacks who had headache, all experienced a reduction in headache pain. Mean headache severity decreased from 6 before stimulation to 2.4 after stimulation.
None of the four patients with interictal dizziness experienced a reduction in dizziness after stimulation.
Patients place the device against the neck to receive electrical stimulation, and patients received stimulation for 2 minutes on each side of the neck.
Participants reported a mild pulling sensation of the neck muscles during the stimulation but did not report pain or other side effects.
“Vestibular migraine is the most common neurologic cause of vertigo and can greatly interfere with a person’s daily life,” Dr. Beh said. “If these results can be confirmed with larger studies, not only could there finally be a treatment for vestibular migraine, such a treatment would also be easy to use.”
A randomized, double-blind, sham-controlled study is needed to further assess the use of noninvasive vagus nerve stimulation in treating vestibular migraine, the authors said.
The study had no targeted funding, and Dr. Beh had no relevant disclosures. Coauthor Deborah I. Friedman, MD, professor of neurology and neurotherapeutics and ophthalmology at UT Southwestern Medical Center, disclosed serving on advisory boards or speaking for pharmaceutical and medical device companies. Dr. Friedman is on the editorial advisory board of Neurology Reviews.
SOURCE: Beh SC et al. Neurology. 2019 Sep 25. doi: 10.1212/WNL.0000000000008388.
FROM NEUROLOGY
Key clinical point: Noninvasive vagus nerve stimulation may reduce the intensity of vertigo and headache in patients with acute vestibular migraine attacks.
Major finding: After stimulation, vertigo improved in 13 out of 14 people with vestibular migraine attacks. In 2 of these patients, vertigo resolved completely, and 5 had at least a 50% improvement in vertigo symptoms. On a 0-10 scale, patients’ mean vertigo rating decreased from 5.2 before stimulation to 3.1 after stimulation.
Study details: An open-label study of 18 patients with vestibular migraine.
Disclosures: The study had no targeted funding, and the first author had no relevant disclosures. A coauthor disclosed serving on advisory boards or speaking for pharmaceutical and medical device companies and is on the editorial advisory board of Neurology Reviews.
Source: Beh SC et al. Neurology. 2019 Sep 25. doi: 10.1212/WNL.0000000000008388.