User login
Anakinra treatment for pediatric ‘cytokine storms’: Does one size fit all?
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
FROM ARTHRITIS & RHEUMATOLOGY
Parent survey sheds light on suboptimal compliance with eczema medications
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
of children with AD.
Perceived effectiveness was the main driver of this variation, Alan Schwartz PhD, and Korey Capozza, MPH, wrote in the study, published in Pediatric Dermatology.
“Responses suggest parents may be willing to use therapies with concerning side effects if they can see a clear benefit for their child’s eczema, but when anticipated improvements fail to materialize, they may change their usage, usually in the direction of using less medication or stopping,” observed Dr. Schwartz, of the University of Illinois, Chicago, and Ms. Capozza, of Global Parents for Eczema Research.
“Addressing expectations related to effectiveness, rather than concerns about medication use, may thus be more likely to lead to taking medication as directed.”
The researchers posted a 15-question survey on the Facebook page of Global Parents for Eczema Research, an international coalition of parents of children with AD. During the month that the survey was posted, 86 parents completed it; questions pertained to adherence to medications and reasons for changing treatments. The mean age of their children was 6 years, most (about 83%) had moderate or severe eczema, and about half lived in the United States.
More than half (55%) reported using the AD medications as directed. But 30% said they took or applied less than prescribed, 13% had stopped the prescribed medication altogether, and 2% took or applied more (or more often) than prescribed.
There were several reasons stated for this variance. Concern over side effects was the most common (46%) reason for not using medications as directed. The next most common reasons were that the child’s symptoms went away (28%); or the “medication was not helping or was not helping as much,” in 23%.
A lack of physician trust or not agreeing with the physician’s recommendations accounted for 18% of the concerns. The remainder thought it wasn’t important to take the medication as prescribed, it was inconvenient or too time consuming, that they forgot, it was too expensive, or they were confused about the directions.
To the question asking “What would have made you more likely to use the medication as prescribed?” the most common answer was a clearer indication of effectiveness (56%). The next most common was “access to research or evidence about benefit and side effect profile” (14%).
A good relationship between the physician and patient was associated with taking medication as directed
“Improvement in adherence to topical treatments among children with AD could yield large gains in quality-of-life improvements and reduce exposure to costlier and potentially more toxic systemic agents,” the authors noted. “Given the large, documented gains in disease improvement, and even remission, achieved with interventions that address adherence among patients with other chronic diseases, strategies that address the underlying causes for poor adherence among parents of children with atopic dermatitis stand to provide a significant, untapped benefit.”
No financial disclosures were noted.
SOURCE: Pediatr Dermatol. 2019 Aug 28. doi: 10.1111/pde.13991.
FROM PEDIATRIC DERMATOLOGY
Skip supplemental O2 in nonhypoxic ACS
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
PARIS – A massive randomized trial that included all New Zealanders with a suspected acute coronary syndrome during a 2-year period has provided definitive evidence that giving high-flow supplemental oxygen to those who are nonhypoxemic is of no clinical benefit, although it wasn’t harmful, either.

“Patients who have a normal blood oxygen saturation level are very unlikely to benefit from supplemental oxygen,” Ralph Stewart, MbChB, said in presenting the results of the NZOTACS (New Zealand Oxygen Therapy in Acute Coronary Syndromes) trial at the annual congress of the European Society of Cardiology.
“It’s amazing that oxygen has been used in patients with suspected heart attack for over 50 years, and during that time there’s never been definite evidence that it improves outcomes. And more recently some have even suggested giving high-level oxygen might actually cause harm,” observed Dr. Stewart, a cardiologist at Auckland City Hospital and the University of Auckland (New Zealand).
The primary outcome in NZOTACS was 30-day all-cause mortality. In the overall study population, the rate was 3.0% in the group assigned to the routine high-flow oxygen protocol and closely similar at 3.1% in those randomized to the conservative oxygen strategy. And there was reassuringly no signal that the liberal oxygen protocol caused any harm.
To conduct this cluster randomized crossover trial, Dr. Stewart and his coinvestigators divided New Zealand into quadrants and, taking advantage of the coordinated health care systems operative in the nation of 4.8 million, they arranged for all ambulances, emergency departments, and hospitals in each geographic region to utilize each supplemental oxygen strategy for a total of 12 months.
In the liberal oxygen strategy, patients with suspected ACS on the basis of ischemic chest pain or ECG changes received high-flow oxygen by face mask at 6-8 L/min regardless of their blood oxygen saturation (SaO2) level. The oxygen was stopped only upon clinical resolution of myocardial ischemia. In contrast, in the low-oxygen protocol, supplemental oxygen was reserved for patients with an initial SaO2 below 90%, with a target SaO2 of 90%-94%.
Roughly 90% of the nearly 41,000 study participants had a normal SaO2 of 90% or more. Their 30-day mortality was 2.1% with the high-oxygen protocol and similar at 1.9% with the conservative oxygen protocol.
In contrast, there was a suggestion of benefit for the routine liberal oxygen strategy in the subgroup of patients with ST-elevation MI. Their 30-day mortality was 8.8% with high-flow oxygen and 10.6% with the conservative oxygen protocol. The resultant 19% relative risk reduction barely missed statistical significance. There was also a trend for possible benefit of routine high-flow oxygen in the roughly 12% of NZOTACS participants with an SaO2 below 95%, a lower bar than the 90% SaO2 that defines hypoxemia. Their death rate at 30 days was 10.1% if they got supplemental oxygen and 11.1% if they only received oxygen in the event their SaO2 was below 90%. But these exploratory findings must be viewed as hypothesis-generating, and a large confirmatory study would be required, Dr. Stewart noted.
Discussant Robin Hofmann, MD, PhD, commented that, based on the NZOTACS results, he believes a couple of changes to the current ESC guidelines on management of ACS are in order. The guidelines now state that oxygen is indicated in patients with suspected ACS and hypoxemia as defined by an SaO2 below 90%, giving that recommendation a Class I Level of Evidence C. That should now be upgraded to the strongest-possible Class I A recommendation, according to Dr. Hofmann, a cardiologist at the Karolinska Institute in Stockholm.
The ESC guidelines also state that oxygen isn’t routinely recommended in patients with an SaO2 of 90% or more, rating that guidance Class III B. On the basis of NZOTACS coupled with earlier far smaller studies, that should be changed to a Class III A recommendation, meaning simply don’t do it. The hint provided by NZOTACS of a possible small benefit for oxygen in patients with an SaO2 below 95% isn’t strong enough evidence to carry the day, in Dr. Hofmann’s view.
Dr. Stewart and Dr. Hofmann reported having no financial conflicts of interest. The NZOTACS trial was funded by the National Heart Foundation of New Zealand.
SOURCE: Stewart R. ESC 2019, Hotline Session 2.
REPORTING FROM THE ESC CONGRESS 2019
Part 4: We Can All Be Leaders
Personality quizzes abound on the Internet these days; you can find out everything from which Disney Princess you are to what type of fruit you would be. But there is a serious case to be made for how your personality type influences your work. It affects how you manage others, develop leadership skills, approach conflict resolution, and manage change.1 Understanding your personality type assists in identifying your strengths, weaknesses, and areas in need of development.
My personality type is ENTP: someone who is “resourceful in solving new and challenging problems.”1 At many points in my career, I found myself in a leadership role. But truthfully, I never set out to be a leader—my career goals set me on that path. You might call me an “accidental leader.”
Recall my story about the founding of the American Academy of Nurse Practitioners (AANP; see Part 2): In the beginning, we were all encouraged to contribute whatever time and energy we could to getting the organization off the ground. I had plenty of time and energy to give. I saw the need for an NP-dedicated organization as a challenge. While I did not know my personality type at the time, I understood that I had a drive to meet challenges—those arising from the status quo and those of moving the vision for this new organization forward.
Our leadership skills are derived from everyday experiences—both the good and the bad. But we only grow if we study the consequences of those experiences to gain insights and to find new ways to manage ourselves and the team.2 Understanding your own personality and skills helps you better appreciate the differences in those you lead and understand how to direct or utilize their particular skills.
Each team member brings a set of skills, range of ideas, and problem-solving approaches to a unique situation. You should identify your team members’ strengths and promote a culture in which the whole team feels comfortable, confident, supported, and encouraged to contribute.3
How do you do that? By initiating and maintaining effective working relationships within the team and demonstrating skills in care coordination and delegation. Everyone benefits when each team member’s unique abilities are used to progress toward the goal.
In my experience, a team is most effective when the leader
- knows each team members’ professional and personal goals
- sets real priorities and commitments
- establishes clear direction
- builds rapport
- is fair with everyone
- shares knowledge and resources
- mentors others to become effective leaders.
Continue to: Another thing that the most effective leaders do is...
Another thing that the most effective leaders do is manage their time and conserve their energy and focus. Think in both short- and long-term goals. Leaders are ordinary people with extraordinary determination, but they know when to stop working and how to recharge their batteries.3,4
It is also important for leaders to acknowledge their accomplishments. All too often, we downplay the contributions we have made, the barriers we have overcome, and the sacrifices we have made to get to where we are today. Our accomplishments add to our body of experience and serve as the foundation for our growth.
Contrary to popular belief, leaders can be made. Anybody can be a leader. One just has to take the time to understand the commitment and the responsibilities. Leadership is a function of who you are, what you can do, and how you do it. Find a mission that ignites your passion, and go for it!
1. The Myers & Briggs Foundation. MBTI® Type at Work. www.myersbriggs.org/type-use-for-everyday-life/mbti-type-at-work/. Accessed September 10, 2019.
2. AZquotes. John Dewey quotes. www.azquotes.com/quote/497608. Accessed September 10, 2019.
3. Knowledge@Wharton. Three big leadership clichés—and how to rethink them. Wharton School of the University of Pennsylvania website. https://knowledge.wharton.upenn.edu/article/three-big-leadership-cliches-rethink/. Published November 26, 2018. Accessed September 10, 2019.
4. ForbesQuotes. Thoughts on the Business of Life. www.forbes.com/quotes/5477/. Accessed September 10, 2019.
Personality quizzes abound on the Internet these days; you can find out everything from which Disney Princess you are to what type of fruit you would be. But there is a serious case to be made for how your personality type influences your work. It affects how you manage others, develop leadership skills, approach conflict resolution, and manage change.1 Understanding your personality type assists in identifying your strengths, weaknesses, and areas in need of development.
My personality type is ENTP: someone who is “resourceful in solving new and challenging problems.”1 At many points in my career, I found myself in a leadership role. But truthfully, I never set out to be a leader—my career goals set me on that path. You might call me an “accidental leader.”
Recall my story about the founding of the American Academy of Nurse Practitioners (AANP; see Part 2): In the beginning, we were all encouraged to contribute whatever time and energy we could to getting the organization off the ground. I had plenty of time and energy to give. I saw the need for an NP-dedicated organization as a challenge. While I did not know my personality type at the time, I understood that I had a drive to meet challenges—those arising from the status quo and those of moving the vision for this new organization forward.
Our leadership skills are derived from everyday experiences—both the good and the bad. But we only grow if we study the consequences of those experiences to gain insights and to find new ways to manage ourselves and the team.2 Understanding your own personality and skills helps you better appreciate the differences in those you lead and understand how to direct or utilize their particular skills.
Each team member brings a set of skills, range of ideas, and problem-solving approaches to a unique situation. You should identify your team members’ strengths and promote a culture in which the whole team feels comfortable, confident, supported, and encouraged to contribute.3
How do you do that? By initiating and maintaining effective working relationships within the team and demonstrating skills in care coordination and delegation. Everyone benefits when each team member’s unique abilities are used to progress toward the goal.
In my experience, a team is most effective when the leader
- knows each team members’ professional and personal goals
- sets real priorities and commitments
- establishes clear direction
- builds rapport
- is fair with everyone
- shares knowledge and resources
- mentors others to become effective leaders.
Continue to: Another thing that the most effective leaders do is...
Another thing that the most effective leaders do is manage their time and conserve their energy and focus. Think in both short- and long-term goals. Leaders are ordinary people with extraordinary determination, but they know when to stop working and how to recharge their batteries.3,4
It is also important for leaders to acknowledge their accomplishments. All too often, we downplay the contributions we have made, the barriers we have overcome, and the sacrifices we have made to get to where we are today. Our accomplishments add to our body of experience and serve as the foundation for our growth.
Contrary to popular belief, leaders can be made. Anybody can be a leader. One just has to take the time to understand the commitment and the responsibilities. Leadership is a function of who you are, what you can do, and how you do it. Find a mission that ignites your passion, and go for it!
Personality quizzes abound on the Internet these days; you can find out everything from which Disney Princess you are to what type of fruit you would be. But there is a serious case to be made for how your personality type influences your work. It affects how you manage others, develop leadership skills, approach conflict resolution, and manage change.1 Understanding your personality type assists in identifying your strengths, weaknesses, and areas in need of development.
My personality type is ENTP: someone who is “resourceful in solving new and challenging problems.”1 At many points in my career, I found myself in a leadership role. But truthfully, I never set out to be a leader—my career goals set me on that path. You might call me an “accidental leader.”
Recall my story about the founding of the American Academy of Nurse Practitioners (AANP; see Part 2): In the beginning, we were all encouraged to contribute whatever time and energy we could to getting the organization off the ground. I had plenty of time and energy to give. I saw the need for an NP-dedicated organization as a challenge. While I did not know my personality type at the time, I understood that I had a drive to meet challenges—those arising from the status quo and those of moving the vision for this new organization forward.
Our leadership skills are derived from everyday experiences—both the good and the bad. But we only grow if we study the consequences of those experiences to gain insights and to find new ways to manage ourselves and the team.2 Understanding your own personality and skills helps you better appreciate the differences in those you lead and understand how to direct or utilize their particular skills.
Each team member brings a set of skills, range of ideas, and problem-solving approaches to a unique situation. You should identify your team members’ strengths and promote a culture in which the whole team feels comfortable, confident, supported, and encouraged to contribute.3
How do you do that? By initiating and maintaining effective working relationships within the team and demonstrating skills in care coordination and delegation. Everyone benefits when each team member’s unique abilities are used to progress toward the goal.
In my experience, a team is most effective when the leader
- knows each team members’ professional and personal goals
- sets real priorities and commitments
- establishes clear direction
- builds rapport
- is fair with everyone
- shares knowledge and resources
- mentors others to become effective leaders.
Continue to: Another thing that the most effective leaders do is...
Another thing that the most effective leaders do is manage their time and conserve their energy and focus. Think in both short- and long-term goals. Leaders are ordinary people with extraordinary determination, but they know when to stop working and how to recharge their batteries.3,4
It is also important for leaders to acknowledge their accomplishments. All too often, we downplay the contributions we have made, the barriers we have overcome, and the sacrifices we have made to get to where we are today. Our accomplishments add to our body of experience and serve as the foundation for our growth.
Contrary to popular belief, leaders can be made. Anybody can be a leader. One just has to take the time to understand the commitment and the responsibilities. Leadership is a function of who you are, what you can do, and how you do it. Find a mission that ignites your passion, and go for it!
1. The Myers & Briggs Foundation. MBTI® Type at Work. www.myersbriggs.org/type-use-for-everyday-life/mbti-type-at-work/. Accessed September 10, 2019.
2. AZquotes. John Dewey quotes. www.azquotes.com/quote/497608. Accessed September 10, 2019.
3. Knowledge@Wharton. Three big leadership clichés—and how to rethink them. Wharton School of the University of Pennsylvania website. https://knowledge.wharton.upenn.edu/article/three-big-leadership-cliches-rethink/. Published November 26, 2018. Accessed September 10, 2019.
4. ForbesQuotes. Thoughts on the Business of Life. www.forbes.com/quotes/5477/. Accessed September 10, 2019.
1. The Myers & Briggs Foundation. MBTI® Type at Work. www.myersbriggs.org/type-use-for-everyday-life/mbti-type-at-work/. Accessed September 10, 2019.
2. AZquotes. John Dewey quotes. www.azquotes.com/quote/497608. Accessed September 10, 2019.
3. Knowledge@Wharton. Three big leadership clichés—and how to rethink them. Wharton School of the University of Pennsylvania website. https://knowledge.wharton.upenn.edu/article/three-big-leadership-cliches-rethink/. Published November 26, 2018. Accessed September 10, 2019.
4. ForbesQuotes. Thoughts on the Business of Life. www.forbes.com/quotes/5477/. Accessed September 10, 2019.
USPSTF: Screening pregnant women for asymptomatic bacteriuria cuts pyelonephritis risk
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
FROM JAMA
PsA Fast Facts: Comorbidities



Virtual dark therapy tames manic episodes
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
REPORTING FROM ECNP 2019
Don’t Take the Fall With Head Injuries
In the early morning hours of June 10, 2009, a 77-year-old man who had been undergoing chemotherapy for multiple myeloma took sleep medication. He then fell down a flight of stairs in his split-level home.
The patient sustained a laceration to his scalp but returned to bed and waited until later that morning to call his internist for an appointment. Later that day, the physician placed 11 sutures for the scalp laceration and performed a neurologic examination; he did not note any abnormalities. The patient complained of back pain, so the physician ordered a back x-ray, which revealed a TI2 fracture that had occurred from the fall. No further treatment was provided for the scalp injury, except removal of the stitches about a week later.
Six days after the fall and doctor visit, the patient’s condition began to deteriorate rapidly, with noted slurred speech and loss of consciousness. He was transported to an emergency department, where CT revealed a massive subdural hematoma. An immediate craniotomy was performed. However, on June 27, 2009, the patient died as a result of the brain bleed.
His estate filed suit against the physician and his practice, alleging medical malpractice and violations in the standard of care. The estate alleged that the standard of care required the physician to obtain a CT scan and that, had one been performed, it would have revealed a small subdural hematoma in time for it to have been successfully treated (ie, before the massive second related bleed). The estate’s theory of the case did not rest on the presentation of clinical symptoms. A medical expert who testified for the estate stated that the subdural hematoma began at the time of the fall.
The defense denied any violations in the standard of care. The physician contended that the patient had presented with no symptoms other than a head laceration, and there were no criteria for ordering CT. Further, the defense asserted that the patient was symptom free for 6 days post-fall. According to the defense, the patient experienced a sudden arterial bleed that was not caused by the fall and would not have been revealed on CT ordered at the time of initial presentation, because it did not occur until 6 days later.
VERDICT
After a 10-day trial and 25 minutes’ deliberation, the jury returned a defense verdict.
COMMENTARY
The 25-minute deliberation suggests that terms such as “bridging veins” and “shearing injury” were unlikely bandied about in the jury room. The jury was likely dismissive of the plaintiff’s claim owing to his cancer diagnosis, and perhaps rightly so. But if we eliminate the multiple myeloma diagnosis, the jury might have decided differently.
Continue to: The defendant physician...
The defendant physician did a good job of documenting a negative neurologic exam, which helped him convince the jury that the patient did not have any signs or symptoms when first evaluated. But in this patient, was imaging to rule out intracranial bleeding indicated?
As an oversimplification, we tend to think of intracranial hemorrhage in 2 varieties: the insidious and the bold. Subdural hematomas are stealthy, they are sneaky, and they prey on the old. They step out of the shadows to cause symptoms. They are the ninjas of intracranial hemorrhage. Beware.
Epidural hematomas and subarachnoid hemorrhage (SAH) are the opposite. They classically present with a sudden and severe symptom complex: with epidural hematoma, the loss of consciousness, lucid interval, and final loss of consciousness; with SAH, the “worst in your life” thunder-clap headache, which may be heralded by a sentinel headache.1 When manifesting this way, they are brash, direct, and unsubtle to the point of being obnoxious—the Steven Stifler of intracranial bleeding.
This generalization is made to highlight the potentially sneaky nature of subdural hemorrhage. There are circumstances in which the clinical presentation of epidural hematoma and SAH will be more challenging. The question here is whether a negative initial neurologic exam can adequately screen for a potentially stealthy subdural hematoma.
Subdural hemorrhage is caused by rapidly changing velocity that may stretch and tear small bridging veins.2,3 Subdural hematoma is more common in the elderly, those who abuse alcohol, and those with a prior history of head trauma.4 As the brain shrinks with age or atrophy, the subdural space enlarges and traversing veins are stretched to cover a wider distance—rendering them vulnerable to rupture.5 These structures may also weaken as a result of low cerebrospinal fluid (intracranial hypotension); as pressure decreases (eg, from a leak), the brain’s buoyancy is reduced, causing traction on anchoring and supporting structures (eg, bridging veins).5 Injury to bridging veins can even occur as a result of a coup-contrecoup mechanism in the absence of direct physical impact.6,7 Bottom line: the injury itself may be subtle, requiring an index of suspicion to make the diagnosis.
Continue to: The case patient was...
The case patient was elderly. He had a chronic malignancy and sustained a fall down the stairs. He was taking sleeping pills, which may have slowed reflexive protective mechanisms after he started to fall (resulting in greater force imparted to his head). Multiple myeloma can predispose a patient to coagulopathy, and we don’t know in this case if this patient’s multiple myeloma made him more susceptible to bleeding—but it certainly didn’t help.8 The patient’s age, the mechanism of injury, and the history of malignancy made this a setup for hemorrhage.
Interestingly, we are not given details about how the patient looked during his suture removal. We are told the time between the initial fall and deterioration was 6 days. Scalp sutures were removed “about a week later,” which was after the deterioration—so this can’t be correct. Removing scalp sutures after 5 days seems premature, but that is the only possibility if 6 days elapsed between the fall and the deterioration.
In short, these are difficult cases. If intracranial bleeding can be subtle and delayed, how can we be sure a patient is not experiencing a bleed? We can only apply the relevant standard of care using all the clinical information we have. The Canadian CT Head Rule and New Orleans Criteria are clinical tools designed to help providers determine when to image (see Table for details).9
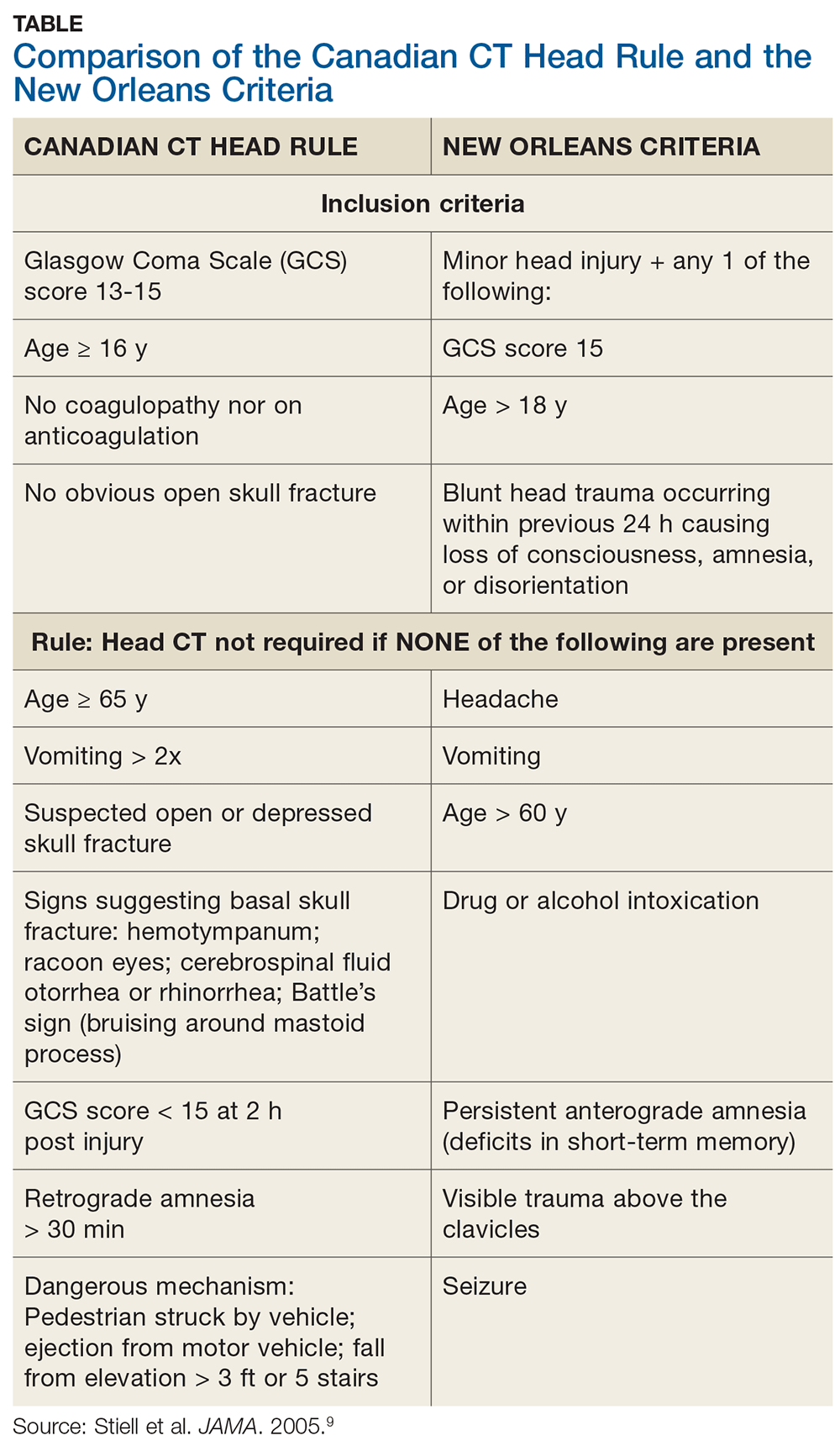
Applying the Canadian CT Head Rule to the facts of this case, we would image the patient because he fell down a “flight” of stairs (which is > 5 stairs) and he is 77 years old (older than 65). The New Orleans Criteria require head CT for minor injury with any positive findings.9 Because the patient is older than 60, he would be scanned according this rule.
In this case, the tools indicate scanning would have been appropriate. The patient’s multiple myeloma might have further impelled a decision to image. However, the jury was persuaded that the defendant’s negative neurologic exam was reasonable under the circumstances. This was likely made possible by the physician’s good recordkeeping and demonstrated genuine concern for the patient’s well-being—as well as a differing viewpoint of the patient’s age and health status.
Continue to: Finally, a word about...
Finally, a word about falls and the elderly: We’ve all heard the 80s advertising catchphrase (which lives on as a present-day meme) “I’ve fallen, and I can’t get up!” The problem is, many don’t. It would be more clinically accurate to say, “I’ve fallen, and I’ll be hospitalized for an extended period of time, then transferred to a skilled nursing facility, but I won’t survive to discharge.” The reality is that falls kill, and the severity is underestimated.10 If it were a “brain-eating amoeba,” the media would be all over it. With falls, not so much. We tend to pay less attention.
Risk factors for a fall include postural hypotension; use of benzodiazepines or other sedative-hypnotic drugs; use of ≥ 4 medications; environmental hazards for tripping; impairment in balance and transfer skills; and gait impairment.11 Home setup also contributes—loose throw rugs, uneven carpet edges, cracked sidewalks, clutter and furniture, cables and wires and cords, oh my.
Do your older patients a favor by reinforcing fall risk. Instruct them to rise slowly from seated or recumbent positions; always consider central nervous system sedation and/or the coordination-hampering properties of medications, particularly in combination. Raise the issue of home safety. A brief 10-second comment from you may plant a seed in a family member’s head to do what you cannot: scan and make safe the patient’s living environment.
1. de Falco FA. Sentinel headache. Neurol Sci. 2004;25(suppl 3):S215-S217.
2. Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120(6):1378-1384.
3. Victor M, Ropper A. Craniocerebral trauma. In: Victor M, Ropper A, eds. Adams and Victor’s Principles of Neurology. 7th ed. New York, NY: McGraw-Hill; 2001:925.
4. McBridde W. Subdural hematoma in adults: etiology, clinical features, and diagnosis. UpToDate website. www.uptodate.com/contents/subdural-hematoma-in-adults-etiology-clinical-features-and-diagnosis? search=subdural%20hematoma. Published December 10, 2018. Accessed September 23, 2019.
5. US National Library of Medicine. Subdural hematoma. Medline Plus website. https://medlineplus.gov/ency/article/000713.htm. Accessed September 23, 2019.
6. Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol. 2002;12(6):1237-1252.
7. Mayer S, Rowland L. Head injury. In: Rowland L, ed. Merritt’s Neurology. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:401.
8. Saif MW, Allegra CJ, Greenberg B. Bleeding diathesis in multiple myeloma. J Hematother Stem Cell Res. 2001;10(5):657-660.
9. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518.
10. Abdelrahman H, Almadani A, El-Menyar A, et al. Home-related falls: an underestimated mechanism of injury. J Family Community Med. 2018; 25(1):48-51.
11. Fuller GF. Falls in the elderly. Am Fam Physician. 2000;61(7):2159-2168.
In the early morning hours of June 10, 2009, a 77-year-old man who had been undergoing chemotherapy for multiple myeloma took sleep medication. He then fell down a flight of stairs in his split-level home.
The patient sustained a laceration to his scalp but returned to bed and waited until later that morning to call his internist for an appointment. Later that day, the physician placed 11 sutures for the scalp laceration and performed a neurologic examination; he did not note any abnormalities. The patient complained of back pain, so the physician ordered a back x-ray, which revealed a TI2 fracture that had occurred from the fall. No further treatment was provided for the scalp injury, except removal of the stitches about a week later.
Six days after the fall and doctor visit, the patient’s condition began to deteriorate rapidly, with noted slurred speech and loss of consciousness. He was transported to an emergency department, where CT revealed a massive subdural hematoma. An immediate craniotomy was performed. However, on June 27, 2009, the patient died as a result of the brain bleed.
His estate filed suit against the physician and his practice, alleging medical malpractice and violations in the standard of care. The estate alleged that the standard of care required the physician to obtain a CT scan and that, had one been performed, it would have revealed a small subdural hematoma in time for it to have been successfully treated (ie, before the massive second related bleed). The estate’s theory of the case did not rest on the presentation of clinical symptoms. A medical expert who testified for the estate stated that the subdural hematoma began at the time of the fall.
The defense denied any violations in the standard of care. The physician contended that the patient had presented with no symptoms other than a head laceration, and there were no criteria for ordering CT. Further, the defense asserted that the patient was symptom free for 6 days post-fall. According to the defense, the patient experienced a sudden arterial bleed that was not caused by the fall and would not have been revealed on CT ordered at the time of initial presentation, because it did not occur until 6 days later.
VERDICT
After a 10-day trial and 25 minutes’ deliberation, the jury returned a defense verdict.
COMMENTARY
The 25-minute deliberation suggests that terms such as “bridging veins” and “shearing injury” were unlikely bandied about in the jury room. The jury was likely dismissive of the plaintiff’s claim owing to his cancer diagnosis, and perhaps rightly so. But if we eliminate the multiple myeloma diagnosis, the jury might have decided differently.
Continue to: The defendant physician...
The defendant physician did a good job of documenting a negative neurologic exam, which helped him convince the jury that the patient did not have any signs or symptoms when first evaluated. But in this patient, was imaging to rule out intracranial bleeding indicated?
As an oversimplification, we tend to think of intracranial hemorrhage in 2 varieties: the insidious and the bold. Subdural hematomas are stealthy, they are sneaky, and they prey on the old. They step out of the shadows to cause symptoms. They are the ninjas of intracranial hemorrhage. Beware.
Epidural hematomas and subarachnoid hemorrhage (SAH) are the opposite. They classically present with a sudden and severe symptom complex: with epidural hematoma, the loss of consciousness, lucid interval, and final loss of consciousness; with SAH, the “worst in your life” thunder-clap headache, which may be heralded by a sentinel headache.1 When manifesting this way, they are brash, direct, and unsubtle to the point of being obnoxious—the Steven Stifler of intracranial bleeding.
This generalization is made to highlight the potentially sneaky nature of subdural hemorrhage. There are circumstances in which the clinical presentation of epidural hematoma and SAH will be more challenging. The question here is whether a negative initial neurologic exam can adequately screen for a potentially stealthy subdural hematoma.
Subdural hemorrhage is caused by rapidly changing velocity that may stretch and tear small bridging veins.2,3 Subdural hematoma is more common in the elderly, those who abuse alcohol, and those with a prior history of head trauma.4 As the brain shrinks with age or atrophy, the subdural space enlarges and traversing veins are stretched to cover a wider distance—rendering them vulnerable to rupture.5 These structures may also weaken as a result of low cerebrospinal fluid (intracranial hypotension); as pressure decreases (eg, from a leak), the brain’s buoyancy is reduced, causing traction on anchoring and supporting structures (eg, bridging veins).5 Injury to bridging veins can even occur as a result of a coup-contrecoup mechanism in the absence of direct physical impact.6,7 Bottom line: the injury itself may be subtle, requiring an index of suspicion to make the diagnosis.
Continue to: The case patient was...
The case patient was elderly. He had a chronic malignancy and sustained a fall down the stairs. He was taking sleeping pills, which may have slowed reflexive protective mechanisms after he started to fall (resulting in greater force imparted to his head). Multiple myeloma can predispose a patient to coagulopathy, and we don’t know in this case if this patient’s multiple myeloma made him more susceptible to bleeding—but it certainly didn’t help.8 The patient’s age, the mechanism of injury, and the history of malignancy made this a setup for hemorrhage.
Interestingly, we are not given details about how the patient looked during his suture removal. We are told the time between the initial fall and deterioration was 6 days. Scalp sutures were removed “about a week later,” which was after the deterioration—so this can’t be correct. Removing scalp sutures after 5 days seems premature, but that is the only possibility if 6 days elapsed between the fall and the deterioration.
In short, these are difficult cases. If intracranial bleeding can be subtle and delayed, how can we be sure a patient is not experiencing a bleed? We can only apply the relevant standard of care using all the clinical information we have. The Canadian CT Head Rule and New Orleans Criteria are clinical tools designed to help providers determine when to image (see Table for details).9

Applying the Canadian CT Head Rule to the facts of this case, we would image the patient because he fell down a “flight” of stairs (which is > 5 stairs) and he is 77 years old (older than 65). The New Orleans Criteria require head CT for minor injury with any positive findings.9 Because the patient is older than 60, he would be scanned according this rule.
In this case, the tools indicate scanning would have been appropriate. The patient’s multiple myeloma might have further impelled a decision to image. However, the jury was persuaded that the defendant’s negative neurologic exam was reasonable under the circumstances. This was likely made possible by the physician’s good recordkeeping and demonstrated genuine concern for the patient’s well-being—as well as a differing viewpoint of the patient’s age and health status.
Continue to: Finally, a word about...
Finally, a word about falls and the elderly: We’ve all heard the 80s advertising catchphrase (which lives on as a present-day meme) “I’ve fallen, and I can’t get up!” The problem is, many don’t. It would be more clinically accurate to say, “I’ve fallen, and I’ll be hospitalized for an extended period of time, then transferred to a skilled nursing facility, but I won’t survive to discharge.” The reality is that falls kill, and the severity is underestimated.10 If it were a “brain-eating amoeba,” the media would be all over it. With falls, not so much. We tend to pay less attention.
Risk factors for a fall include postural hypotension; use of benzodiazepines or other sedative-hypnotic drugs; use of ≥ 4 medications; environmental hazards for tripping; impairment in balance and transfer skills; and gait impairment.11 Home setup also contributes—loose throw rugs, uneven carpet edges, cracked sidewalks, clutter and furniture, cables and wires and cords, oh my.
Do your older patients a favor by reinforcing fall risk. Instruct them to rise slowly from seated or recumbent positions; always consider central nervous system sedation and/or the coordination-hampering properties of medications, particularly in combination. Raise the issue of home safety. A brief 10-second comment from you may plant a seed in a family member’s head to do what you cannot: scan and make safe the patient’s living environment.
In the early morning hours of June 10, 2009, a 77-year-old man who had been undergoing chemotherapy for multiple myeloma took sleep medication. He then fell down a flight of stairs in his split-level home.
The patient sustained a laceration to his scalp but returned to bed and waited until later that morning to call his internist for an appointment. Later that day, the physician placed 11 sutures for the scalp laceration and performed a neurologic examination; he did not note any abnormalities. The patient complained of back pain, so the physician ordered a back x-ray, which revealed a TI2 fracture that had occurred from the fall. No further treatment was provided for the scalp injury, except removal of the stitches about a week later.
Six days after the fall and doctor visit, the patient’s condition began to deteriorate rapidly, with noted slurred speech and loss of consciousness. He was transported to an emergency department, where CT revealed a massive subdural hematoma. An immediate craniotomy was performed. However, on June 27, 2009, the patient died as a result of the brain bleed.
His estate filed suit against the physician and his practice, alleging medical malpractice and violations in the standard of care. The estate alleged that the standard of care required the physician to obtain a CT scan and that, had one been performed, it would have revealed a small subdural hematoma in time for it to have been successfully treated (ie, before the massive second related bleed). The estate’s theory of the case did not rest on the presentation of clinical symptoms. A medical expert who testified for the estate stated that the subdural hematoma began at the time of the fall.
The defense denied any violations in the standard of care. The physician contended that the patient had presented with no symptoms other than a head laceration, and there were no criteria for ordering CT. Further, the defense asserted that the patient was symptom free for 6 days post-fall. According to the defense, the patient experienced a sudden arterial bleed that was not caused by the fall and would not have been revealed on CT ordered at the time of initial presentation, because it did not occur until 6 days later.
VERDICT
After a 10-day trial and 25 minutes’ deliberation, the jury returned a defense verdict.
COMMENTARY
The 25-minute deliberation suggests that terms such as “bridging veins” and “shearing injury” were unlikely bandied about in the jury room. The jury was likely dismissive of the plaintiff’s claim owing to his cancer diagnosis, and perhaps rightly so. But if we eliminate the multiple myeloma diagnosis, the jury might have decided differently.
Continue to: The defendant physician...
The defendant physician did a good job of documenting a negative neurologic exam, which helped him convince the jury that the patient did not have any signs or symptoms when first evaluated. But in this patient, was imaging to rule out intracranial bleeding indicated?
As an oversimplification, we tend to think of intracranial hemorrhage in 2 varieties: the insidious and the bold. Subdural hematomas are stealthy, they are sneaky, and they prey on the old. They step out of the shadows to cause symptoms. They are the ninjas of intracranial hemorrhage. Beware.
Epidural hematomas and subarachnoid hemorrhage (SAH) are the opposite. They classically present with a sudden and severe symptom complex: with epidural hematoma, the loss of consciousness, lucid interval, and final loss of consciousness; with SAH, the “worst in your life” thunder-clap headache, which may be heralded by a sentinel headache.1 When manifesting this way, they are brash, direct, and unsubtle to the point of being obnoxious—the Steven Stifler of intracranial bleeding.
This generalization is made to highlight the potentially sneaky nature of subdural hemorrhage. There are circumstances in which the clinical presentation of epidural hematoma and SAH will be more challenging. The question here is whether a negative initial neurologic exam can adequately screen for a potentially stealthy subdural hematoma.
Subdural hemorrhage is caused by rapidly changing velocity that may stretch and tear small bridging veins.2,3 Subdural hematoma is more common in the elderly, those who abuse alcohol, and those with a prior history of head trauma.4 As the brain shrinks with age or atrophy, the subdural space enlarges and traversing veins are stretched to cover a wider distance—rendering them vulnerable to rupture.5 These structures may also weaken as a result of low cerebrospinal fluid (intracranial hypotension); as pressure decreases (eg, from a leak), the brain’s buoyancy is reduced, causing traction on anchoring and supporting structures (eg, bridging veins).5 Injury to bridging veins can even occur as a result of a coup-contrecoup mechanism in the absence of direct physical impact.6,7 Bottom line: the injury itself may be subtle, requiring an index of suspicion to make the diagnosis.
Continue to: The case patient was...
The case patient was elderly. He had a chronic malignancy and sustained a fall down the stairs. He was taking sleeping pills, which may have slowed reflexive protective mechanisms after he started to fall (resulting in greater force imparted to his head). Multiple myeloma can predispose a patient to coagulopathy, and we don’t know in this case if this patient’s multiple myeloma made him more susceptible to bleeding—but it certainly didn’t help.8 The patient’s age, the mechanism of injury, and the history of malignancy made this a setup for hemorrhage.
Interestingly, we are not given details about how the patient looked during his suture removal. We are told the time between the initial fall and deterioration was 6 days. Scalp sutures were removed “about a week later,” which was after the deterioration—so this can’t be correct. Removing scalp sutures after 5 days seems premature, but that is the only possibility if 6 days elapsed between the fall and the deterioration.
In short, these are difficult cases. If intracranial bleeding can be subtle and delayed, how can we be sure a patient is not experiencing a bleed? We can only apply the relevant standard of care using all the clinical information we have. The Canadian CT Head Rule and New Orleans Criteria are clinical tools designed to help providers determine when to image (see Table for details).9

Applying the Canadian CT Head Rule to the facts of this case, we would image the patient because he fell down a “flight” of stairs (which is > 5 stairs) and he is 77 years old (older than 65). The New Orleans Criteria require head CT for minor injury with any positive findings.9 Because the patient is older than 60, he would be scanned according this rule.
In this case, the tools indicate scanning would have been appropriate. The patient’s multiple myeloma might have further impelled a decision to image. However, the jury was persuaded that the defendant’s negative neurologic exam was reasonable under the circumstances. This was likely made possible by the physician’s good recordkeeping and demonstrated genuine concern for the patient’s well-being—as well as a differing viewpoint of the patient’s age and health status.
Continue to: Finally, a word about...
Finally, a word about falls and the elderly: We’ve all heard the 80s advertising catchphrase (which lives on as a present-day meme) “I’ve fallen, and I can’t get up!” The problem is, many don’t. It would be more clinically accurate to say, “I’ve fallen, and I’ll be hospitalized for an extended period of time, then transferred to a skilled nursing facility, but I won’t survive to discharge.” The reality is that falls kill, and the severity is underestimated.10 If it were a “brain-eating amoeba,” the media would be all over it. With falls, not so much. We tend to pay less attention.
Risk factors for a fall include postural hypotension; use of benzodiazepines or other sedative-hypnotic drugs; use of ≥ 4 medications; environmental hazards for tripping; impairment in balance and transfer skills; and gait impairment.11 Home setup also contributes—loose throw rugs, uneven carpet edges, cracked sidewalks, clutter and furniture, cables and wires and cords, oh my.
Do your older patients a favor by reinforcing fall risk. Instruct them to rise slowly from seated or recumbent positions; always consider central nervous system sedation and/or the coordination-hampering properties of medications, particularly in combination. Raise the issue of home safety. A brief 10-second comment from you may plant a seed in a family member’s head to do what you cannot: scan and make safe the patient’s living environment.
1. de Falco FA. Sentinel headache. Neurol Sci. 2004;25(suppl 3):S215-S217.
2. Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120(6):1378-1384.
3. Victor M, Ropper A. Craniocerebral trauma. In: Victor M, Ropper A, eds. Adams and Victor’s Principles of Neurology. 7th ed. New York, NY: McGraw-Hill; 2001:925.
4. McBridde W. Subdural hematoma in adults: etiology, clinical features, and diagnosis. UpToDate website. www.uptodate.com/contents/subdural-hematoma-in-adults-etiology-clinical-features-and-diagnosis? search=subdural%20hematoma. Published December 10, 2018. Accessed September 23, 2019.
5. US National Library of Medicine. Subdural hematoma. Medline Plus website. https://medlineplus.gov/ency/article/000713.htm. Accessed September 23, 2019.
6. Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol. 2002;12(6):1237-1252.
7. Mayer S, Rowland L. Head injury. In: Rowland L, ed. Merritt’s Neurology. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:401.
8. Saif MW, Allegra CJ, Greenberg B. Bleeding diathesis in multiple myeloma. J Hematother Stem Cell Res. 2001;10(5):657-660.
9. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518.
10. Abdelrahman H, Almadani A, El-Menyar A, et al. Home-related falls: an underestimated mechanism of injury. J Family Community Med. 2018; 25(1):48-51.
11. Fuller GF. Falls in the elderly. Am Fam Physician. 2000;61(7):2159-2168.
1. de Falco FA. Sentinel headache. Neurol Sci. 2004;25(suppl 3):S215-S217.
2. Miller JD, Nader R. Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg. 2014;120(6):1378-1384.
3. Victor M, Ropper A. Craniocerebral trauma. In: Victor M, Ropper A, eds. Adams and Victor’s Principles of Neurology. 7th ed. New York, NY: McGraw-Hill; 2001:925.
4. McBridde W. Subdural hematoma in adults: etiology, clinical features, and diagnosis. UpToDate website. www.uptodate.com/contents/subdural-hematoma-in-adults-etiology-clinical-features-and-diagnosis? search=subdural%20hematoma. Published December 10, 2018. Accessed September 23, 2019.
5. US National Library of Medicine. Subdural hematoma. Medline Plus website. https://medlineplus.gov/ency/article/000713.htm. Accessed September 23, 2019.
6. Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol. 2002;12(6):1237-1252.
7. Mayer S, Rowland L. Head injury. In: Rowland L, ed. Merritt’s Neurology. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:401.
8. Saif MW, Allegra CJ, Greenberg B. Bleeding diathesis in multiple myeloma. J Hematother Stem Cell Res. 2001;10(5):657-660.
9. Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294(12):1511-1518.
10. Abdelrahman H, Almadani A, El-Menyar A, et al. Home-related falls: an underestimated mechanism of injury. J Family Community Med. 2018; 25(1):48-51.
11. Fuller GF. Falls in the elderly. Am Fam Physician. 2000;61(7):2159-2168.
OS benefit with pembrolizumab endures long-term in advanced NSCLC
BARCELONA – First-line pembrolizumab provides a durable long-term overall survival (OS) benefit, compared with that of chemotherapy, in patients with advanced nonsquamous non–small cell lung cancer (NSCLC), according to 3-year data from the phase 3 Keynote-024 trial.
As previously reported, first-line treatment with the programmed death-1 (PD-1) inhibitor significantly improved progression-free survival (PFS) and OS, compared with those of platinum-based chemotherapy, and had fewer adverse events after a median of 11.2 months of follow-up in the open-label trial. In 305 patients with advanced NSCLC, high PD-L1 expression, and an absence of targetable epidermal growth factor or anaplastic lymphoma kinase gene alterations, median PFS was 10.3 months vs. 6.0 months, and estimated OS was 80.2% vs. 72.4% in the groups, respectively (hazard ratios, 0.50 and 0.60, respectively).
At 3 years after treatment initiation, median OS was 26.3 months vs. 14.2 months in patients treated with pembrolizumab or chemotherapy (HR, 0.65), respectively, and the OS rates were 43.7% and 24.9%, Martin Reck, MD, reported at the World Conference on Lung Cancer.
This was despite 98 of 151 patients assigned to chemotherapy crossing over to pembrolizumab, Dr. Reck, head of the department of thoracic oncology and the clinical trial department in the department of thoracic oncology at the Lung Clinic Grosshansdorf (Germany), noted at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
Additionally, despite longer mean treatment duration in the pembrolizumab arm than in the chemotherapy arm (11.1 vs. 4.4 months), grade 3-5 treatment-related adverse events were less frequent with pembrolizumab than with chemotherapy (31.2% vs. 53.3%), he said.
Of 38 patients in the pembrolizumab arm who completed 2 years of therapy, 34 were alive at 3 years, and 31 (81.6%) had an objective response, including 2 who had a complete response. Median duration of response was not reached, and OS was 97.4%.
Grade 3-5 adverse events occurred in 5 (13.2%) of those patients; no fatal treatment-related adverse events occurred.
Of note, 7 of 10 patients who completed 2 years of treatment, but who subsequently progressed, experienced an objective response with a second course of pembrolizumab, and 8 remain alive, he said.
Patients in KEYNOTE-024 were randomized to receive 200 mg of pembrolizumab every 3 weeks for 2 years or investigator’s choice of platinum doublet for 4-6 cycles plus optional maintenance, with stratification by performance status, tumor histology, and region.
The findings confirm the long-term efficacy of pembrolizumab, compared with platinum-based chemotherapy, demonstrate “consistent benefit in overall survival ... despite a crossover of 65%,” and show the first signs of efficacy with reexposure to pembrolizumab at the time of progression.
“This is work in progress; currently we have data from 10 [reexposed] patients, but what we do see is clinical activity even after [reexposure] to pembrolizumab, so we do see a stabilization of response to the disease in 70% of the patients, and 50% are ongoing.”
The findings highlight a new reality: “There are really some patients who have this disease as a chronic disease induced by immunotherapy.” Dr. Reck said.
KEYNOTE-024 was funded by Merck Sharp & Dohme. Dr. Reck reported receiving personal fees/honoraria for consultancy and lectures from Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Merck Sharp & Dohme, MSD, Eli Lilly, Pfizer, AbbVie, Roche, and Novartis.
SOURCE: Reck M et al. WCLC 2019, Abstract OA14.01.
BARCELONA – First-line pembrolizumab provides a durable long-term overall survival (OS) benefit, compared with that of chemotherapy, in patients with advanced nonsquamous non–small cell lung cancer (NSCLC), according to 3-year data from the phase 3 Keynote-024 trial.
As previously reported, first-line treatment with the programmed death-1 (PD-1) inhibitor significantly improved progression-free survival (PFS) and OS, compared with those of platinum-based chemotherapy, and had fewer adverse events after a median of 11.2 months of follow-up in the open-label trial. In 305 patients with advanced NSCLC, high PD-L1 expression, and an absence of targetable epidermal growth factor or anaplastic lymphoma kinase gene alterations, median PFS was 10.3 months vs. 6.0 months, and estimated OS was 80.2% vs. 72.4% in the groups, respectively (hazard ratios, 0.50 and 0.60, respectively).
At 3 years after treatment initiation, median OS was 26.3 months vs. 14.2 months in patients treated with pembrolizumab or chemotherapy (HR, 0.65), respectively, and the OS rates were 43.7% and 24.9%, Martin Reck, MD, reported at the World Conference on Lung Cancer.
This was despite 98 of 151 patients assigned to chemotherapy crossing over to pembrolizumab, Dr. Reck, head of the department of thoracic oncology and the clinical trial department in the department of thoracic oncology at the Lung Clinic Grosshansdorf (Germany), noted at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
Additionally, despite longer mean treatment duration in the pembrolizumab arm than in the chemotherapy arm (11.1 vs. 4.4 months), grade 3-5 treatment-related adverse events were less frequent with pembrolizumab than with chemotherapy (31.2% vs. 53.3%), he said.
Of 38 patients in the pembrolizumab arm who completed 2 years of therapy, 34 were alive at 3 years, and 31 (81.6%) had an objective response, including 2 who had a complete response. Median duration of response was not reached, and OS was 97.4%.
Grade 3-5 adverse events occurred in 5 (13.2%) of those patients; no fatal treatment-related adverse events occurred.
Of note, 7 of 10 patients who completed 2 years of treatment, but who subsequently progressed, experienced an objective response with a second course of pembrolizumab, and 8 remain alive, he said.
Patients in KEYNOTE-024 were randomized to receive 200 mg of pembrolizumab every 3 weeks for 2 years or investigator’s choice of platinum doublet for 4-6 cycles plus optional maintenance, with stratification by performance status, tumor histology, and region.
The findings confirm the long-term efficacy of pembrolizumab, compared with platinum-based chemotherapy, demonstrate “consistent benefit in overall survival ... despite a crossover of 65%,” and show the first signs of efficacy with reexposure to pembrolizumab at the time of progression.
“This is work in progress; currently we have data from 10 [reexposed] patients, but what we do see is clinical activity even after [reexposure] to pembrolizumab, so we do see a stabilization of response to the disease in 70% of the patients, and 50% are ongoing.”
The findings highlight a new reality: “There are really some patients who have this disease as a chronic disease induced by immunotherapy.” Dr. Reck said.
KEYNOTE-024 was funded by Merck Sharp & Dohme. Dr. Reck reported receiving personal fees/honoraria for consultancy and lectures from Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Merck Sharp & Dohme, MSD, Eli Lilly, Pfizer, AbbVie, Roche, and Novartis.
SOURCE: Reck M et al. WCLC 2019, Abstract OA14.01.
BARCELONA – First-line pembrolizumab provides a durable long-term overall survival (OS) benefit, compared with that of chemotherapy, in patients with advanced nonsquamous non–small cell lung cancer (NSCLC), according to 3-year data from the phase 3 Keynote-024 trial.
As previously reported, first-line treatment with the programmed death-1 (PD-1) inhibitor significantly improved progression-free survival (PFS) and OS, compared with those of platinum-based chemotherapy, and had fewer adverse events after a median of 11.2 months of follow-up in the open-label trial. In 305 patients with advanced NSCLC, high PD-L1 expression, and an absence of targetable epidermal growth factor or anaplastic lymphoma kinase gene alterations, median PFS was 10.3 months vs. 6.0 months, and estimated OS was 80.2% vs. 72.4% in the groups, respectively (hazard ratios, 0.50 and 0.60, respectively).
At 3 years after treatment initiation, median OS was 26.3 months vs. 14.2 months in patients treated with pembrolizumab or chemotherapy (HR, 0.65), respectively, and the OS rates were 43.7% and 24.9%, Martin Reck, MD, reported at the World Conference on Lung Cancer.
This was despite 98 of 151 patients assigned to chemotherapy crossing over to pembrolizumab, Dr. Reck, head of the department of thoracic oncology and the clinical trial department in the department of thoracic oncology at the Lung Clinic Grosshansdorf (Germany), noted at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
Additionally, despite longer mean treatment duration in the pembrolizumab arm than in the chemotherapy arm (11.1 vs. 4.4 months), grade 3-5 treatment-related adverse events were less frequent with pembrolizumab than with chemotherapy (31.2% vs. 53.3%), he said.
Of 38 patients in the pembrolizumab arm who completed 2 years of therapy, 34 were alive at 3 years, and 31 (81.6%) had an objective response, including 2 who had a complete response. Median duration of response was not reached, and OS was 97.4%.
Grade 3-5 adverse events occurred in 5 (13.2%) of those patients; no fatal treatment-related adverse events occurred.
Of note, 7 of 10 patients who completed 2 years of treatment, but who subsequently progressed, experienced an objective response with a second course of pembrolizumab, and 8 remain alive, he said.
Patients in KEYNOTE-024 were randomized to receive 200 mg of pembrolizumab every 3 weeks for 2 years or investigator’s choice of platinum doublet for 4-6 cycles plus optional maintenance, with stratification by performance status, tumor histology, and region.
The findings confirm the long-term efficacy of pembrolizumab, compared with platinum-based chemotherapy, demonstrate “consistent benefit in overall survival ... despite a crossover of 65%,” and show the first signs of efficacy with reexposure to pembrolizumab at the time of progression.
“This is work in progress; currently we have data from 10 [reexposed] patients, but what we do see is clinical activity even after [reexposure] to pembrolizumab, so we do see a stabilization of response to the disease in 70% of the patients, and 50% are ongoing.”
The findings highlight a new reality: “There are really some patients who have this disease as a chronic disease induced by immunotherapy.” Dr. Reck said.
KEYNOTE-024 was funded by Merck Sharp & Dohme. Dr. Reck reported receiving personal fees/honoraria for consultancy and lectures from Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Merck Sharp & Dohme, MSD, Eli Lilly, Pfizer, AbbVie, Roche, and Novartis.
SOURCE: Reck M et al. WCLC 2019, Abstract OA14.01.
REPORTING FROM WCLC 2019
CBD in beauty products
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).
Cannabidiol (CBD) seems to be everywhere now. Since the Farm Bill of 2018 legalizing the cultivation of hemp was signed into law last December, many CBD-based products have hit the market. The advent of and changed the public conversation about cannabis. That, and with the surge in legal availability, its use is more commonplace now – even in elderly populations and regions of the country where products thought to be associated with the marijuana plant would have once been considered taboo. A recent Gallup poll found that 14% of Americans say they now use CBD. As the benefits of CBD are demonstrated and perceptions change, having background knowledge of the manufacturing and available data on CBD will be helpful when patients ask about these products for skin care, to provide an evidenced-based approach.
CBD is one of over a hundred phytocannabinoids, which are naturally occurring cannabinoids found in the oily resin of the flower or “bud” (and to a lesser extent the leaves) of the cannabis plant. This is opposed to synthetic cannabinoids, as well as endocannabinoids (cannabinoid receptors found in humans and animals). Both CBD and THC (delta9-tetrahydrocannabinol), another phytocannabinoid, can provide anti-inflammatory and pain-control benefits; the main difference is that THC has psychoactive effects and CBD does not.
Cannabis is a genus of flowering plants in the Cannabaceae family, made up of three primary species: Cannabis sativa, Cannabis indica, and Cannabis ruderalis. CBD can be harvested from either Cannabis sativa or Cannabis indica. People often confuse hemp as equal to Cannabis sativa species and marijuana as equal to Cannabis indica, but neither hemp or marijuana are specific strains or species of cannabis plants, they are broad classifications of cannabis that do not indicate a specific strain.
Hemp, a term used to classify varieties of cannabis that contain trace amounts of THC, has generally been used to describe nonintoxicating cannabis harvested for the industrial use of its derived products, such as textiles, paper, food (hemp seeds), building materials, and skin care. While both “hemp” and “marijuana” can produce high amounts of CBD, CBD products sourced from hemp contain 0.3% THC or less (the legal allowance), while CBD products derived from “marijuana” typically contain 5%-35% THC. Since the 2018 Farm Act legalized the production of hemp in all 50 states, but not marijuana, most CBD nationwide is sourced from hemp. CBD from a marijuana source or a product containing both CBD and over 0.3% THC can only be sold in states where marijuana is legal. At this time, 11 states have legalized marijuana.
Marijuana varieties, grown to maximize the amount or quality of THC, are selectively bred in controlled environments designed to optimize the breed’s characteristics and produce female plants that yield budding flowers. In contrast, because of hemp’s diverse uses, it is grown to maximize its size and yield and is typically grown outdoors and does not require the level of control and attention needed to grow marijuana.
While there is some debate about whether CBD derived from hemp or marijuana differs, medical observations to date are that CBD derived from either source has the same mechanism of action; however, whether CBD has more therapeutic benefits in products alone or in combination with THC and other cannabis components remains to be determined. Of note, CBD is also absent in the roots or the seeds of cannabis and hemp. While hemp seeds are a good source of protein and omega-3 fatty acids, companies that claim they derive CBD from hemp stalk, hemp seeds, or hemp seed oil are making false claims because these parts of the plants contain no CBD, no THC, and no known plant cannabinoids.
CBD binds to endocannabinoid receptor CB2, whereas THC binds to both CB1 and CB2. CB1 receptors are primarily found in the central nervous system, affecting neurotransmitters leading to CNS depression, euphoria, psychosis, impaired memory, and increased appetite and have antiemetic effects, whereas CB2 is mostly found in peripheral organs and primarily affects the immune system resulting in decreased pain and anti-inflammatory and antioxidant effects.
The skin has the highest amount and concentration of CB2 receptors in the body. As detailed in Dr. Leslie Baumann’s column “Primer on cannabis for cosmeceuticals” in Dermatology News, June 2019, skin-specific studies indicate that, when applied topically, CBD decreases sebum production and has anti-inflammatory effects. There is also evidence that CBD has antioxidant effects. Therefore, in the correct formulation, CBD may have potential in treating common sometimes debilitating skin conditions such as acne, as well as other inflammatory skin conditions.
For acne, beauty products containing CBD have the potential to help overall complexion and prevent acne scars. Because most degradation of collagen involves inflammation – whether the inflammation is secondary to excessive UV exposure, diet, poor health, or stress – the anti-inflammatory and antioxidant effects also have potential benefit in treating and preventing signs of aging. Of note, the CB2 receptor has also been shown to be upregulated in melanoma and squamous cell carcinoma. In a recent study of keratinocytes irradiated with UVA and UVB light, CBD demonstrated antioxidant activity through nuclear factor erythroid 2–related factor 2 (Nrf2) activation, as well as anti-inflammatory properties as an inhibitor of the nuclear factor NF-kappa-B. Whether topical CBD can effectively prevent or treat cutaneous tumorigenesis is promising, but large scale data are still needed.
So far, the benefits of CBD in beauty products and topical skin formulations for treatment of skin disease are based on preclinical information, and there is a corresponding lack of high-quality randomized, controlled trials that evaluate their effects on skin-specific issues. Now, with the 2018 Farm Act in place, large-scale, randomized, controlled trials with cannabinoids should be able to be performed more easily to demonstrate the dermatologic benefits of this promising compound.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
Resources
Gallup. “14% of Americans Say They Use CBD Products.” https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx.
Project CBD. “What is CBD?” www.projectcbd.org/cbd-101-what-is-cbd.
Palmieri B et al. Clin Ter. 2019 Mar-Apr;170(2):e93-e99.
Jastrząb A et al. Cells. 2019 Aug 3;8(8).