User login
Vitamin D does not improve bone density, structure in healthy patients
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
REPORTING FROM ASBMR 2019
Many institutions exceed recommended radiation doses during lung cancer screening
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A significant proportion of institutions exceed guideline-recommended dose levels for CT screening for lung cancer.
Major finding: About 21% of institutions have median volume CT dose index above American College of Radiology guidelines, and 65% have median effective dose above ACR guidelines.
Study details: A prospective study of data for 12,529 patients undergoing screening at 72 institutions.
Disclosures: The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
Source: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
Women’s residency and subspecialty choices diverging
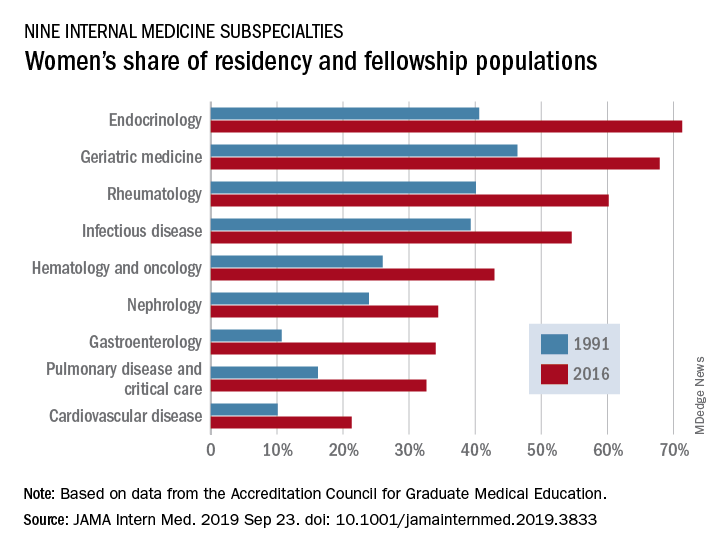
Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.

Women made up 43.2% of the internal medicine resident population in 2016, compared with 30.2% in 1991. Over that same time, however, the percentage of women in subspecialty fellowships dropped from 33.3% to 23.6%, Anna T. Stone, MD, and associates wrote in a research letter published in JAMA Internal Medicine.
“Many factors are associated with the decisions of medical students in choosing an internal medicine residency, including their sex, educational experience, views of patient care, and lifestyle perceptions. Similar considerations apply to subspecialty training,” wrote Dr. Stone of the department of cardiology at St. Vincent Hospital and Heart Center, Indianapolis, and associates.
When the investigators focused on a subset of nine internal medicine subspecialties, they saw growth: “The percentage of women entering each of the fields [residents plus fellows] increased over time, with variations between specialty and some year-to-year variations within a specialty.”
Although none of the nine subspecialties had been majority women in 1991, by 2016 women made up more than half of the residents and fellows in four: endocrinology (71.3%), geriatric medicine (67.9%), rheumatology (60.2%), and infectious disease (54.6%), according to data from the Accreditation Council for Graduate Medical Education.
And then there’s cardiology. Its low rate of participation among women – the only one of the nine subspecialties under 35% – “is an important issue that the cardiology profession should continue to address,” they wrote.
In a survey of internal medicine residents conducted by other researchers, women were more likely than men to report that they had never considered cardiology as a career choice, Dr. Stone and associates noted, and women in the survey “had different perceptions of cardiology than men.”
SOURCE: Stone AT et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3833.
FROM JAMA INTERNAL MEDICINE
Automatic reenrollment helps keep people insured
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
appearing in JAMA Internal Medicine
Researchers looked at 123,244 households in California that were enrolled in marketplace plans that exited the state in 2015. Of the 781 households that were not automatically reenrolled in other plans, the unadjusted and adjusted enrollment rates were 21.4% and 21.5%, respectively. Researchers adjusted for a variety of household characteristics, including age of the oldest household member, household size, receipt of tax credit subsidy, and other factors. Of the 122,463 with the option to reenroll, unadjusted and adjusted enrollment was 51.2%.
The research comes as the Centers for Medicare & Medicaid Services is contemplating the elimination of automatic reenrollment in marketplace plans.
“Elimination of automatic reenrollment would likely be associated with decreases in the number of enrollees who remain insured through the marketplaces,” research authors Coleman Drake, PhD, University of Pittsburgh, and David Anderson, Duke Univeristy, Durham, N.C., wrote in a letter (JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717).
“As an opt-out policy similar to that used in other health insurance markets such as Medicaid, automatic reenrollment may be associated with increases in continuity of coverage in the marketplaces by reducing administrative barriers to reenrollment,” the authors continued.
Dr. Drake and Mr. Anderson noted that losing automatic reenrollment was associated with a decrease in enrollment, but more study is needed particularly because the group that lost reenrollment was small.
“Households with different demographics or different experiences may have behaved differently if they had lost the option to automatically reenroll,” they state. “Losing automatic reenrollment because of a policy change rather than an insurer exit also may be associated with households behaving differently. Given the magnitude of our findings, it is critical that future studies continue investigating the association between automatic reenrollment and continuity of coverage.”
SOURCE: Coleman D, Anderson A. JAMA Inter Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3717.
FROM JAMA INTERNAL MEDICINE
Psoriasis Journal Scan: September 2019
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners.
Alariny AF, Farid CI, Elweshahi HM, Abbood SS. J Sex Med. 2019 Sep 18.
In a comparative cross-sectional study that aimed to evaluate the psychopathological and sexual aspects of psoriasis vulgaris in patients and their partners, the sample included 220 psoriasis vulgaris patients (110 males and 110 females), their consenting partners, and 220 age- and sex-matched healthy controls. The main outcome measures were frequency of depression, anxiety, low self-esteem, and sexual dysfunction in psoriasis vulgaris patients, partners, and controls; the domains of sexual function affected in the studied groups; and the etiology of erectile dysfunction in affected psoriatic males.
Ceramide- and Keratolytic-containing Body Cleanser and Cream Application in Patients with Psoriasis: Outcomes from a Consumer Usage Study.
Del Rosso JQ. J Clin Aesthet Dermatol. 2019 Jul;12(7):18-21
Ceramides are epidermal lipids that play an essential role in stratum corneum function, including maintaining physiologic permeability barrier properties. The role of ceramides in the maintenance and repair of epidermal barrier function is believed to be valuable in the treatment of psoriasis. Normalization of corneocyte desquamation and the incorporation of agents that promote desquamation to reduce hyperkeratosis are also regarded as key factors in psoriasis management. Based on results reported by the study patients, the evaluated ceramide/keratolytic-containing cream and cleanser both yielded a high level of patient acceptance regarding improvement in skin characteristics in patients with psoriasis, including when used as a combination adjunctive regimen.
Split thickness skin graft in active psoriasis in patient with clear cell variant squamous cell carcinoma.
Scupham L, Ingle A. BMJ Case Rep. 2019 Sep 16;12(9).
The case report discusses split thickness skin grafting in a patient with active psoriasis. This also reports a case of a rare variant of squamous cell carcinoma.
Epicardial Adipose Tissue Inflammation Can Cause the Distinctive Pattern of Cardiovascular Disorders Seen in Psoriasis.
Packer M. Am J Med. 2019 Sep 11.
Psoriasis is a systemic inflammatory disorder that can target adipose tissue; the resulting adipocyte dysfunction is manifest clinically as the metabolic syndrome, which is present in ≈20-40% of patients. Epicardial adipose tissue inflammation is likely responsible for a distinctive pattern of cardiovascular disorders, consisting of: accelerated coronary atherosclerosis leading to myocardial infarction, atrial myopathy leading to atrial fibrillation and thromboembolic stroke, and ventricular myopathy leading to heart failure with a preserved ejection fraction. If cardiovascular inflammation drives these risks, then treatments that focus on blood pressure, lipids and glucose will not ameliorate the burden of cardiovascular disease in patients with psoriasis, especially in those who are young and have severe inflammation. Instead, interventions that alleviate systemic and adipose tissue inflammation may not only minimize the risks of atrial fibrillation and heart failure, but may also have favorable effects on the severity of psoriasis. Viewed from this perspective, the known link between psoriasis and cardiovascular disease is not related to the influence of the individual diagnostic components of the metabolic syndrome.
Musculoskeletal ultrasound can improve referrals from dermatology to rheumatology for patients with psoriasis.
Solmaz D, Bakirci S, Al Onazi A, Al Osaimi N, Fahim S, Aydin SZ. Br J Dermatol. 2019 Sep 10.
Psoriasis affects 1-3% of the population and up to 1/3 of psoriasis patients have underlying psoriatic arthritis (PsA). Non-specific musculoskeletal complaints are even higher, being around 50%. Detecting early signs of PsA and early treatments are crucial to improve the outcomes to prevent progressive, damaging arthritis. Due to the high frequency of non-specific pain in psoriasis, it is not possible for every psoriasis patient with joint pain to be assessed by a rheumato
Check on Your Fiscal Health
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at mark@nationalaffinity.net or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at mark@nationalaffinity.net or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
The Affinity Program of expanded benefits is available to SVS members and can connect them with individual disability plans. These plans – available through Principal Life Insurance Company, Securian and Lloyds of London – provide tax-free benefits and can protect hundreds of thousands of dollars.
If interested in learning more about your disability insurance options, contact Mark Blocker at mark@nationalaffinity.net or at 949-554- 9936; he is available after-hours and on weekends. Learn more here.
Submit a MIPS Targeted Review Request by 9/30
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.
If you participated in the Merit-based Incentive Payment System (MIPS) in 2018, your performance feedback is now available for review on the Quality Payment Program website. Through a process called targeted review, MIPS eligible clinicians or groups can request for CMS to review their performance feedback and final score calculation. The MIPS payment adjustment you receive in 2020 will be based on your final score. Please refer to the QPP Access User Guide for additional details. The deadline to submit your request is 7 PM (CT), September 30, 2019.
In Bladder Cancer, New Systemic Treatments Arise
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
MINNEAPOLIS -- A new era of systemic treatment for bladder cancer has arrived, a US Department of Veterans Affairs (VA) hematologist/oncologist told colleagues, and more changes await on the horizon.
“After a historically long dry spell, you're seeing novel drugs and combinations under investigation,” said Elizabeth Henry, MD, of Edward Hines, Jr. VA Hospital and Loyola University Medical Center in Chicago, Illinois, in a presentation at the annual meeting of the Association of VA Hematology/Oncology. “Our treatment paradigm will almost certainly continue to change.”
There’s a major need for new approaches in bladder cancer, Dr. Henry said. While the median survival for patients with metastatic disease treated has risen, it remains low at about 15 months. And, she said, the 5-year survival rate is about 15% with modern treatments.
Platinum-based chemotherapy is still the first-line treatment, she said, and cisplatin-based combos remain the standard. However, many patients are not eligible to take cisplatin because of factors such as reduced performance status, impaired renal function, peripheral neuropathy, hearing loss and heart failure. “Many patients have renal insufficiency and are platinum ineligible from the get-go,” Dr. Henry said.
In these patients, immune checkpoint inhibitors are an option, but research suggests they may lead to poorer survival in those with PDL1-low tumors. In 2018, the US Food and Drug Administration (FDA) advised their use as first-line treatment only in PDL1-high, cisplatin-ineligible patients or those who can’t undergo chemotherapy, she said.
As second-line therapy after platinum treatment, Dr. Henry said, several drugs targeting the PD1/PDL1 pathway are now FDA-approved with response rates at 15% to 25%; only 1 (pembrolizumab) has level 1 evidence from a phase 3 randomized clinical trial.
Single-agent chemotherapy is an option for patients who have worsened or cannot undergo treatment with immune checkpoint inhibitors. However, according to Dr. Henry, response rates are low (about 10%-15%) and there's no prospective or randomized control trial data showing a survival benefit.
What now? Targeted approaches are entering the picture. For example, fibroblast growth factor receptor inhibitors, which target a pathway that often mutates in bladder cancer. One drug, erdafitinib (Balversa), received FDA approval earlier this year based on a phase 2 trial data that showed an objective response rate (ORR) of 40%. Dr. Henry cautioned that unusual adverse effects can occur, including hyperphosphatemia (a disorder that boosts phosphate levels), ocular toxicity (which can lead to retinal detachment), and toxicity of the skin and hair.
“Patients need to be closely followed if they're starting this as a targeted drug,” Dr. Henry said.
Anti-Nectin-4 antibody drug conjugate, which targets urothelial carcinomas with uniformly high expression of the Nectin-4 cell surface marker, also is showing promise. Recent research suggests a “remarkable” ORR of 42% and nearly 8 months duration of response, she said.
Adverse effects include rash, peripheral neuropathy, and hyperglycemia. “Overall, this is thought to be a relatively well-tolerated therapy,” she said.
In terms of other advances, “we are moving closer to an era of molecular subtype-specific therapeutic strategies,” Dr. Henry said, and the National Comprehensive Cancer Network recommends early molecular testing in stage IIIB/IV urothelial cancer. “It can help facilitate treatment decisions and prevent delays in later lines of therapy, although we're still limited by development of individualized biomarker assays for specific drugs.”
Moving forward, she said, “continued research is needed to learn how to incorporate predictive molecular profiles to optimize treatment selection.”
Dr. Henry reported no relevant disclosures.
Nerves, Neuropeptides, and the Nervous System in the Pathogenesis of Psoriasis
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2
Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15
Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19
Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
Background
Psoriasis is a complex, multifactorial, systemic disease that is associated with numerous neurologic comorbidities, including stroke, multiple sclerosis, epilepsy, migraine, restless leg syndrome, Parkinson disease, and less frequently Guillain-Barré syndrome and myasthenia gravis. Anxiety and depression also are frequently seen in patients with psoriasis.1 In recent years, heightened understanding of the pathogenesis and disease mechanisms involved in psoriasis has led to the development of therapies designed to help to control the chronic inflammation associated with the disease, such as immunobiologics and small molecules.2

Although tremendous effort has gone into elucidating the immunologic underpinnings of psoriasis (certainly a worthwhile endeavor), less attention has been given to the role the nervous system plays in its pathogenesis.3,4 Nonetheless, clinical evidence suggests that the nervous system plays an important role in the pathophysiology of psoriasis and is deserving of further investigation.3
Nerves and Neuropeptides
Psychological stress is known to exacerbate psoriasis, which points to the involvement of the nervous system in psoriasis.3,5,6 In addition to provoking the sympathetic response, psychological stressors have been shown to affect the peripheral nervous system in psoriasis by modulating the skin’s network of nerves and neuropeptides.6-11 A small study divided patients with psoriasis into low-stress and high-stress groups based on their clinical examinations and answers to questionnaires. Immunohistochemical analysis showed patients in the high-stress group had elevated levels of calcitonin gene-related peptide and vasoactive intestinal polypeptide as well as reduced levels of the neuropeptide-degrading enzyme chymase compared to the low-stress group.12 Two later studies showed calcitonin gene-related peptide stimulates keratinocyte proliferation3,13 and is found at increased levels in psoriatic skin.3,14 Similarly, higher quantities of vasoactive intestinal peptide-positive nerve fibers in the epidermis and dermis are found in psoriatic plaques compared to nonlesional and normal skin.3,15

Early research suggested that substance P (SP) released from cutaneous nerve fibers causes a local neurogenic response that elicits psoriasis in predisposed individuals.16 However, there have been conflicting reports of both higher and lower levels of SP in involved and noninvolved skin in patients with psoriasis compared with healthy individuals, making the role of SP in psoriasis ambiguous.3,15,17
Nerve growth factor (NGF), a principal mediator of neurogenic inflammation, also is suspected of playing a role in the pathogenesis of psoriasis.3,6 Studies have shown NGF prevents apoptosis of keratinocytes, activates T cells, and is found in higher levels in psoriatic skin compared to controls.3,18,19

Neuropeptides also may play a contributory role in the itching and Köbner phenomenon that are seen with psoriasis.3 The Köbner phenomenon refers to the formation of psoriatic lesions in uninvolved skin of patients with psoriasis following cutaneous trauma.20 Increased levels of NGF in nonlesional skin of patients with psoriasis are believed to contribute to the development of psoriatic plaques following trauma by triggering an inflammatory response that upregulates other neuropeptides, such as SP and calcitonin gene-related peptide.3 These neuropeptides generate keratinocyte proliferation, which in turn further increase NGF expression; as such, a cycle of inflammation and formation of psoriatic lesions is engendered.3,5 A noteworthy correlation also has been shown between the severity of pruritus and density of NGF-immunoreactive keratinocytes, high-affinity NGF receptors, protein gene product 9.5–immunoreactive intraepidermal fibers, and immunoreactive vessels for E-selectin.3,21
Spontaneous Clearing of Psoriasis
Spontaneous remission of psoriasis after cerebrovascular accident was first described in a case report published in 1998.22 Other cases have reported protective effects from psoriasis and psoriatic arthritis in limbs affected by poliomyelitis.23,24 Conversely, recurrence of skin lesions in areas corresponding to nervous system injury also have been reported in cases in which patients regained neurologic function; when permanent nerve damage was sustained, psoriasis did not recur,4 which confirms that peripheral nerves play a role in the pathogenesis of psoriasis.3 It is believed that peripheral nerve damage leads to reduced secretion of neuropeptides, and central nervous system injury can propagate similar downstream effects.3,25

Reports of psoriasis remission in the wake of peripheral and central nervous system injury from surgical nerve resection as well as cerebrovascular accident, as documented in the case presented here, provide clinical evidence in support of the neurocutaneous pathway’s role in psoriasis.3,4 Several reports have described clinical improvement of psoriasis following sensory cutaneous nerve damage, suggesting inflammation of the cutaneous nerves may be involved in the pathogenesis of psoriasis.3,6 Clearance of psoriatic plaques at the site of injury occurred following nerve resection; after reinnervation of the affected areas, disease recurrence occurred.6,26-28 More recently, cutaneous denervation was shown to improve acanthosis and IL-23 expression in mice with psoriasiform skin.3,25 Intradermal injections of calcitonin gene-related peptide and/or a SP agonist into the denervated areas reversed this denervation-mediated improvement.3,25
Bottom Line
This case report describes spontaneous clearing of psoriasis following a cerebrovascular accident. Improvement in psoriasis in the absence of neural inputs suggest the nervous system plays a crucial role in the development of psoriatic disease.4 A better understanding of the neuropeptides involved in the neurologic-mediated clearance of psoriasis may contribute to the development of improved targeted therapies, specifically designed to target the neurologic aspects of psoriasis.3 Neuropeptides such as nerve growth factor, calcitonin gene-related peptide, and vasoactive intestinal peptide, and possibly SP may play an important role in the pathogenesis of psoriasis and may one day be ideal targets for novel therapies.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
- Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
- Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
- Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
- Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
- Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
- Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
- Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
- Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
- Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
- Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
- He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
- Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
- Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
- Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
- Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
- Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
- Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
- Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
- Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
- Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
- Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
- Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
The Case
A 52-year-old man with psoriasis presented to the dermatology clinic for follow-up. The patient had been using topical clobetasol and apremilast with limited success; however, he had not yet tried biologic therapy. Physical examination revealed erythematous, scaly, indurated papules and plaques on the chest, abdomen, back, arms, and legs, consistent with psoriasis. Affected body surface area was approximately 10%.
Treatment
Ustekinumab was prescribed, but the patient did not pick it up from the pharmacy.
Approximately 1 month later, the patient presented to the emergency department with left-sided weakness and numbness. He was subsequently hospitalized for treatment of stroke. During hospitalization, the patient was started on lisinopril, aspirin, and atorvastatin. He also was given subcu-taneous enoxaparin with plans to initiate warfarin as an outpatient. No therapies for the treatment of psoriasis were given during his admission. Three days after being admitted, he was discharged to a skilled nursing facility.
Patient Outcome
Three months following discharge, the patient returned to the dermatology clinic for follow-up. After his stroke, he reported that his psoriasis had cleared and had not returned. Physical examination revealed his skin was clear of psoriatic lesions.
This case was adapted from Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
Clinical Trials Should Be Standard of Care
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”
MINNEAPOLIS -- Clinical trials should be considered standard of care in oncology, the president of the Association of VA Hematology/Oncology (AVAHO) declared, and he urged colleagues to bypass obstacles and embrace them in patient care.
Clinical trials help many people, not just those in the studies, as they are indicators of robust oncology programs. “There’s a significant correlation between clinical trial activity [at sites] and improvement in survival in cancer,” said oncologist Mark Klein, MD, of the Minneapolis VA Health Care System and University of Minnesota, in a presentation at the 2019 annual meeting of AVAHO.
But the numbers suggest that the VA has lagged behind on the clinical trial front, Dr. Klein said. He pointed to internal statistics from 2001 to 2003, reported in 2010, which found clinical trial participation rates among men in the VA were lower (0.37%) than those of the national rates (0.74%).
Specifically, the VA and national participation rates were 1.16% and 0.30%, respectively, for colorectal cancer, 0.67% and 0.30% for lung cancer, 0.70% and 0.47% for prostate cancer, 0.52% and 0.74% for myeloma. According to Dr. Klein, lung and prostate cancer account for about half of all cancer diagnoses in the VA.
But the participation rates were much higher among the VA hospitals that took part in clinical trials, he said, at 2% for colorectal cancer, 1.4% for lung cancer, 2.5% for prostate cancer, 18.2% for myeloma and 2.07% overall. These numbers make it clear, he said, that it’s possible to improve: “We can do better.”
Dr. Klein pointed to other numbers that suggest the VA facilities that do participate in clinical trials typically do not take part in more than 1 or 2. In 2016, he said, an analysis found that open interventional trials were in progress at 82 VA facilities. About 35 facilities had little activity with only 1 trial in progress. “It has changed since then, but not dramatically.”
Meanwhile, he said, 2016 numbers also showed that about two-thirds of open interventional trials in the VA only included a single VA site. “We weren’t playing together, having consortia and working as a team.”
There are obstacles to improvement, he said, and fixes will take time. “It probably takes 5, 10-plus years,” he said.
These barriers include:
- Lack of adequate trial offering. Changing regulations is key here;
- Strict eligibility criteria. Easing the criteria and more effective screening are helpful;
- Long distances to travel to cancer clinics. Telemedicine could make a difference on this front;
- Patient concerns about being a “guinea pig.” Patient education can help;
- Provider concerns such as worry about extra work. The VA can work to ease burdens and provide incentives for trial enrollment; and
- High costs. Study sponsors may pay for trial drugs, helping facilities to lower expenses or even reach cost neutrality.
There are more solutions to boost participation, Dr. Klein said, including education, engagement and partnerships.
On the partnership front, he highlighted NAVIGATE, an interagency consortium of the VA and the National Cancer Institute. This collaboration aims to boost enrollment of VA patients in clinical trials, he said, and there are designated NAVIGATE sites across the country from coast to coast.
A partnership between AVAHO and the National Association of Veterans’ Research and Education Foundations is also boosting clinical trials in VA oncology. Thanks to this partnership, 1 trial has completed accrual, 6 multisite trials are active, and 5 are starting up, according to Dr. Klein.
In the big picture, Dr. Klein said, it’s clear that “we’ve got a lot of barriers in the VA. Anybody who works in the VA knows that. But I’ve seen this meeting grow and grow, and I’ve seen teamwork and partnerships form.”