User login
Longer-lasting neuromodulators coming down the pike
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
SAN DIEGO – In the coming years, expect to see an increasing number of neuromodulators hit the market, Joel L. Cohen, MD, predicted at the annual Masters of Aesthetics Symposium.
One such product, DaxibotulinumtoxinA (Daxi), formerly known as RT002, contains a proprietary peptide that may contribute to extending its duration of action beyond currently available neuromodulator products. “Another difference for Daxi is that it does not contain human serum albumin,” said Dr. Cohen, who’s in private practice in Greenwood Village and Lone Tree, both in Colo.
In trials of the agent conducted by Revance, the manufacturer, for the treatment of moderate to severe glabellar lines, DaxibotulinumtoxinA achieved a 1-point change in results from baseline in a median of 24 weeks, while the return to baseline wrinkle severity occurred in a median of 28 weeks. According to the Revance web site, DaxibotulinumtoxinA is up for possible Food and Drug Administration approval in 2020.
Though current neuromodulators on the market may be most effective for 3-4 months, the reality is that patients often don’t come in for longer stretches of time – as there is still some degree of efficacy. Dr. Cohen shared interim data from an ongoing study that showed that at 6 months 69% of patients remain satisfied with the result of their last injection. “With Dysport, for example, even though we know the durability is to 3-4 months, we have patients who may still be happy with the results at 6 months,” he said.
Another trend he discussed is the increasing interest in QM1114, a novel, ready-to-use type A botulinum toxin formulation being developed by Galderma for the aesthetic treatment of glabellar lines. Unlike Botox, Dysport, Xeomin, and Jueveau, QM1114 is a liquid and thus does not require reconstitution.
“Myobloc is also a liquid but it is a type B botulinum toxin,” Dr. Cohen said. “It’s always been formulated as a liquid toxin, but it’s not something we can use commonly in our aesthetic practices [unless a patient is suspected of having extremely rare type A antibodies] for many reasons beyond simply it not being approved for aesthetic use. Though Myobloc kicks in faster, it spreads more, it hurts more, and it doesn’t last as long.”
In a phase 2 study presented at the 2019 World Congress of Dermatology, investigators, including Dr. Cohen, evaluated the safety and efficacy of QM1114 for the treatment of glabellar lines in 359 patients aged 23-79 years. Patients were randomly assigned to one of three single-treatment groups – 35 units, 45 units, or 60 units – or to placebo. Two weeks post treatment, wrinkle severity improved by at least two grades based on the assessment of investigators (a range from 83%-91%) and by that of treated subjects (a range from 73%-86%), compared with 6% and 8%, respectively, in the placebo group. In addition, 90%-98% of subjects rated themselves as “very satisfied” or “satisfied” with the treatment at month 1, compared with 72%-80% of subjects at month 6. Treatment-related adverse events occurred in little more than 1% of subjects in any QM1114 group and presented as mild to moderate injection-site pain, headache, eyelid ptosis, injection-site pruritus, injection-site swelling, and eyelid edema.
Dr. Cohen reported having research and financial ties to numerous pharmaceutical and device companies including Merz, Galderma, Allergan, Revance, Evolus, and Croma.
EXPERT ANALYSIS FROM MOA 2019
Semaglutide beats canagliflozin as second-line therapy for type 2 diabetes
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
BARCELONA – The glucagonlike peptide–1 receptor antagonist semaglutide (Ozempic) produced greater reductions in glycated hemoglobin and body weight than the sodium-glucose cotransporter 2 inhibitor canagliflozin (Invokana) in second-line treatment in patients with type 2 diabetes after metformin and lifestyle modifications, researchers reported at the annual meeting of the European Association for the Study of Diabetes.
The year-long SUSTAIN (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) 8 trial comparing semaglutide and canagliflozin is one of the few head-to-head comparisons of the glucagonlike peptide–1 receptor antagonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes of drugs.
Findings showed overall changes in HbA1c level from baseline to week 52 of –1.5 percentage points with semaglutide and –1.0 percentage point with canagliflozin, and changes in body weight during the same time of –5.3 kg and –4.2 kg, respectively. The estimated treatment differences were –0.49 percentage points for HbA1c (P less than .001) and –1.06 kg for body weight (P less than .0029).
A significantly higher percentage of patients receiving semaglutide also achieved HbA1c targets at 52 weeks, compared with those receiving canagliflozin: 66.1% versus 45.1%, respectively, achieved the American Diabetes Association’s target of less than 7%, and 52.8% versus 23.6% (P less than .0001) reached the lower target of 6.5% or lower, as set by the American Association of Clinical Endocrinologists.
Furthermore, a significantly higher proportion of patients in the semaglutide arm achieved 10% or more weight loss by the end of the study (22.3% vs. 8.9% in the canagliflozin arm; P less than .0001), with a trend for 5% or greater weight loss favoring semaglutide (51.1% vs. 46.6%, P = .21). A post hoc analysis also showed that patients treated with semaglutide could achieve a weight loss of 15% or more (6.8% vs. 0.9% for canagliflozin, P = .0001).
“SUSTAIN 8 provides clinically relevant information regarding the head-to-head comparison of these two very commonly used glucose-lowering classes [of drugs] as second-line therapy in patients with type 2 diabetes,” lead study author Ildiko Lingvay, MD, said. The findings support the use of semaglutide as an alternative to canagliflozin when treatment intensification after metformin is needed, Dr. Lingvay and coauthors concluded in an article published simultaneously in Lancet Diabetes & Endocrinology (2019 Sep 17. doi: 10.1016/S2213-8587[19]30311-0).
Dr. Lingvay of the University of Texas in Dallas observed that both GLP-1 RAs and SGLT2 inhibitors are recommended as second-line treatment after metformin and lifestyle modifications, particularly when there is a need to minimize the risk for hypoglycemia and weight gain, and there is established cardiovascular disease. Despite their wide endorsement, however, there has really been only one other head-to-head trial that evaluated the two drug classes – the PIONEER 2 study, which compared oral semaglutide and the SGLT2 inhibitor empagliflozin (Jardiance). Another trial, DURATION-8, compared the GLP-1 RA exenatide (Byetta) or the SGLT2 inhibitor dapagliflozin (Farxiga) with an exenatide-dapagliflozin combination, but it did not directly compare the two drug classes.
SUSTAIN 8 was a phase 3b, randomized, double-blind, parallel-group, controlled trial that compared once-weekly subcutaneous semaglutide 1.0 mg and daily oral canagliflozin 300 mg as add-on treatments to metformin in 788 individuals with type 2 diabetes. Participants had to have a starting HbA1c of between 7.0% and 10.0%, to be on a stable dose of metformin, and to have an estimated glomerular filtration rate of 60 mL/min per 1.73 m3 or higher.
Of the 394 patients randomized to semaglutide, 83.3% completed the study treatment and 15.7% discontinued prematurely, most often because of adverse events (9.7%). Of the remaining 394 patients randomized to canagliflozin therapy, 87.1% completed treatment and 12.9% discontinued prematurely, again mostly for adverse events (5.1%).
Overall the rate of any adverse events (76.0% vs. 71.8%) or serious adverse events (4.6% vs. 5.3%) were similar between the semaglutide and canagliflozin groups. As expected, more gastrointestinal side effects were seen in patients treated with semaglutide than in those treated with canagliflozin (46.9% vs. 27.9%), and there were more infections in the canagliflozin group (29.1% vs. 34.5%). Hypoglycemic episodes were “very rare in this population,” Dr. Lingvay reported. Rates of severe or confirmed hypoglycemia were 1.5% and 1.3% for the respective arms.
Other findings of note were improved fasting blood lipids – with greater changes in total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides seen with semaglutide than canagliflozin. Systolic blood pressure dropped in both groups, with a greater change in the canagliflozin than semaglutide group (–5.5 mm Hg vs. –3.5 mm Hg; P = .0452).
In a substudy of SUSTAIN 8 (n = 178), which was reported separately at the meeting, both semaglutide and canagliflozin reduced total fat mass as assessed with whole-body, dual-energy x-ray absorptiometry scanning. The changes in total fat mass from baseline to week 52 were a respective –3.4 kg and –2.6, or 1.4% and 1.2%. Total lean mass changed by a respective –2.3 kg and –1.5 kg (1.2% and 1.1%), and visceral fat mass by –0.2 kg and –0.1 kg (–0.9% and 0.4%). There was no statistical significance between the groups. A post hoc analysis did show, however, that a greater drop in waist circumference might be achieved with semaglutide than with canagliflozin (–4.0 vs. –2.9 cm [–3.9% vs. –2.5%], P = .02).
“Importantly, neither treatment was associated with deleterious body composition changes, such as gains in fat mass or reductions in the total lean mass,” said Rory McCrimmon, MBChB, professor of experimental diabetes and metabolism at the University of Dundee, Scotland, when presenting the substudy findings.
“These findings are consistent with results from other body composition studies with GLP-1 RAs and SGLT2 [inhibitors],”Dr. McCrimmon said, adding that “the positive effects on total fat loss and visceral fat reduction highlight the role of semaglutide and canagliflozin as relevant treatment options for patients with type 2 diabetes.”
Novo Nordisk funded the study. Dr. Lingvay has received consulting fees from Novo Nordisk; research grants from her institution; and grants, personal fees, or both, from other companies not related to the study. Dr. McCrimmon has received personal fees from Novo Nordisk and two other companies.
SOURCES: Lingvay I et al. Lancet Diabetes Endocrinol. 2019 Sep 17. doi: 10.1016/S2213-8587(19)30311-0; McCrimmon RJ et al. EASD 2019, Abstract 54.
REPORTING FROM EASD 2019
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
Role of the Nervous System in Psoriasis
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
1. Amanat M, Salehi M, Rezaei N. Neurological and psychiatric disorders in psoriasis. Rev Neurosci. 2018;29:805-813.
2. Eberle FC, Brück J, Holstein J, et al. Recent advances in understanding psoriasis [published April 28, 2016]. F1000Res. doi:10.12688/f1000research.7927.1.
3. Lee EB, Reynolds KA, Pithadia DJ, et al. Clearance of psoriasis after ischemic stroke. Cutis. 2019;103:74-76.
4. Zhu TH, Nakamura M, Farahnik B, et al. The role of the nervous system in the pathophysiology of psoriasis: a review of cases of psoriasis remission or improvement following denervation injury. Am J Clin Dermatol. 2016;17:257-263.
5. Raychaudhuri SP, Farber EM. Neuroimmunologic aspects of psoriasis. Cutis. 2000;66:357-362.
6. Kwon CW, Fried RG, Nousari Y, et al. Psoriasis: psychosomatic, somatopsychic, or both? Clin Dermatol. 2018;36:698-703.
7. Lotti T, D’Erme AM, Hercogová J. The role of neuropeptides in the control of regional immunity. Clin Dermatol. 2014;32:633-645.
8. Hall JM, Cruser D, Podawiltz A, et al. Psychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasis [published online August 30, 2012]. Dermatol Res Pract. 2012;2012:403908.
9. Raychaudhuri SK, Raychaudhuri SP. NGF and its receptor system: a new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann N Y Acad Sci. 2009;1173:470-477.
10. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243-251.
11. Levi-Montalcini R, Skaper SD, Dal Toso R, et al. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514-520.
12. Harvima IT, Viinamäki H, Naukkarinen A, et al. Association of cutaneous mast cells and sensory nerves with psychic stress in psoriasis. Psychother Psychosom. 1993;60:168-176.
13. He Y, Ding G, Wang X, et al. Calcitonin gene‐related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl). 2000;113:747-751.
14. Chu DQ, Choy M, Foster P, et al. A comparative study of the ability of calcitonin gene‐related peptide and adrenomedullin13–52 to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol. 2000;130:1589-1596.
15. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptides and general neuronal marker in psoriasis—an immunohistochemical study. Clin Exp Dermatol. 1995;20:384-389.
16. Farber EM, Nickoloff BJ, Recht B, et al. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986;14(2, pt 1):305-311.
17. Pincelli C, Fantini F, Romualdi P, et al. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98:421-427.
18. Raychaudhuri SP, Jiang WY, Farber EM. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Derm Venereol. 1998;78:84-86.
19. Pincelli C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur J Dermatol. 2000;10:85-90.
20. Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
21. Nakamura M, Toyoda M, Morohashi M. Pruritogenic mediators in psoriasis vulgaris: comparative evaluation of itch-associated cutaneous factors. Br J Dermatol. 2003;149:718-730.
22. Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38(5, pt 1):768-770.
23. Wang TS, Tsai TF. Psoriasis sparing the lower limb with postpoliomyelitis residual paralysis. Br J Dermatol. 2014;171:429-431.
24. Weiner SR, Bassett LW, Reichman RP. Protective effect of poliomyelitis on psoriatic arthritis. Arthritis Rheum. 1985;28:703-706.
25. Ostrowski SM, Belkai A, Loyd CM, et al. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530-1538.
26. Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418-420.
27. Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220-221.
28. Perlman HH. Remission of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128-129.
FDA approves oral semaglutide for HbA1c management in type 2 diabetes
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
Deep transcranial magnetic stimulation alleviates OCD symptoms
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
COPENHAGEN – High-frequency deep transcranial magnetic stimulation (dTMS) directed at the anterior cingulate cortex and medial prefrontal cortex proved to be a safe and effective nonpharmacologic treatment for symptoms of obsessive-compulsive disorder in an international, randomized, sham-controlled, double-blind clinical trial that earned the device clearance for that indication from the Food and Drug Administration.
“Deep TMS is a relatively new form of TMS that allows direct stimulation of deeper neuronal pathways than standard TMS. It induces a direct effective field at a depth of 3-5 cm below the skull, compared to less than 1.5 cm for the standard TMS figure-eight coil,” said Dr. Carmi, of Chaim Sheba Medical Center in Ramat Gan, Israel.
The brain circuitry involved in obsessive-compulsive disorder (OCD) is very well known. Multiple potential targets for intervention are available. Dr. Carmi and coinvestigators focused on the anterior cingulate cortex and medial prefrontal cortex, because this is an area that’s very much involved in OCD – it’s the generator of increased error-related negativity on the Stroop task – and it can be stimulated by dTMS, whereas standard TMS can’t reach it.
This was not only the first major clinical trial to successfully target the anterior cingulate cortex and medial prefrontal cortex using any form of TMS, it also was the first study to employ individually tailored symptom provocation using photos or a written script immediately before each treatment session. At the first patient encounter, the investigators created a list of what distressed that particular individual – for example, touching a public bathroom door handle or experiencing doubt about whether the stove had been left on – and then prior to each treatment session they deliberately provoked each study participant using representations of those triggers. The treatment, real or sham, didn’t begin until a patient’s distress level measured 4-7 on a visual analog scale.
“The idea is to deliver the treatment when the brain circuitry is aroused and not while the patient is thinking about the shopping he needs to get done after the session is over,” Dr. Carmi explained.
He was first author of the recently published pivotal study (Am J Psychiatry. 2019 May 21. doi: 10.1176/appi.ajp.2019.18101180) in which 99 adults aged up to age 65 years with OCD refractory to at least one selective serotonin reuptake inhibitor underwent real or sham dTMS every weekday for 5 consecutive weeks, plus four sessions during week 6. That’s a total of 29 sessions, featuring 2,000 magnetic stimulations per session. The study was conducted at 11 centers in the United States, Canada, and Israel. Participants had to remain on an approved drug therapy for OCD or engaged in psychotherapy throughout the study.
The primary efficacy outcome was the change in scores on the Yale-Brown Obsessive Compulsive Scale (YBOCS) from baseline to 6 weeks. Patients who received dTMS averaged a 6.0-point reduction, significantly better than the 3.3-point reduction in the sham-treatment group. The treatment response rate, as defined by at least a 30% reduction from baseline in YBOCS score, was 38% with dTMS, compared with 11% in controls. One month after the final treatment session, the response rate was 45% in the active-treatment arm, compared with less than 18% in the sham-treatment group.
In addition, 55% of patients in the active-treatment group achieved a partial response of more than a 20% reduction in YBOCS score, a rate slightly more than twice that in the sham group.
To put those findings in perspective, Dr. Carmi highlighted treatment effect–size results from OCD drug trials involving fluoxetine, fluvoxamine, sertraline, and paroxetine, all FDA-approved for treatment of OCD. The placebo-subtracted mean change in YBOCS scores in the pharmacotherapy trials were similar to the sham treatment–subtracted result in the dTMS study, with one important distinction: “In terms of change in YBOCS, it took 10-12 weeks to get those results in the drug trials, while we have shown this in a 6-week period of time,” he noted.
The only adverse effect associated with dTMS was headaches. They occurred in about one-third of the dTMS group and in a similar proportion of controls early on in the study, but they became a nonissue later.
“I have to say, we recruited 99 patients for the multicenter study, but only 2 of them dropped out because of side effects,” Dr. Carmi noted.
He reported having no financial conflicts of interest regarding the study, sponsored by Brainsway, which markets the dTMS device for the FDA-cleared indications of treatment-resistant depression and OCD.
REPORTING FROM ECNP 2019
New guideline conditionally recommends long-term home NIV for COPD patients
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
Adult insomnia associated with childhood behavioral problems
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
FROM JAMA NETWORK OPEN
Reversal agents for direct-acting oral anticoagulants
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3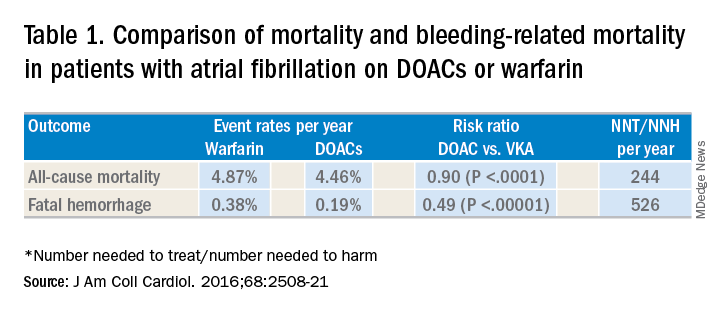
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4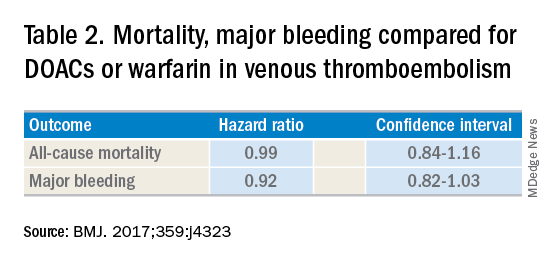
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Summary of guidelines published in the Journal of Hospital Medicine
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Early infusion of mononuclear cells may benefit stroke patients
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
FROM STEM CELLS