User login
CONCLUDE data inconclusive on hypoglycemia risk for degludec vs. glargine
BARCELONA – data presented at the annual meeting of the European Association for the Study of Diabetes.
During a 36-week maintenance treatment period, the rate ratio (RR) for the primary endpoint of the study – severe or confirmed symptomatic hypoglycemia, defined as a blood-glucose level of less than 3.1 mmol/L (56 mg/dL) – was 0.88 (95% confidence interval, 0.73-1.06; P = .17) in a comparison the two long-acting insulins.
There were some differences that favored insulin degludec over insulin glargine when other endpoints were considered, but because a statistical testing hierarchy was used, “no confirmatory results can be made from CONCLUDE,” one of the study investigators, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, San Diego, said during a special symposium at the meeting.
“If the primary endpoint was not achieved, the testing hierarchy stopped after the first occurrence of nonsignificance,” she explained. Although that occurred, the other endpoints could still be analyzed as they were all prespecified, she argued. These showed that during the maintenance treatment period, there were lower rates of nocturnal symptomatic hypoglycemic episodes (RR, 0.63; 95% CI, 0.48-0.84; P = .0014) and severe episodes (those requiring third-party assistance; RR, 0.20; 95% CI, 0.07-0.57; P = .0027) with insulin degludec, compared with insulin glargine.
The trial came under fire, however, from Stefano Del Prato, MD, professor of endocrinology in the department of endocrinology metabolism and chief of the section of diabetes at the University of Pisa, Italy, who provided the EASD-invited independent commentary on the study’s findings. “This is the CONCLUDE trial. You’d could expect a CONCLUDE trial to be conclusive in its conclusions!” he said, noting that there were “too many uncertainties” in the trial.
CONCLUDE was a randomized, open-label, head-to-head study of insulin degludec versus insulin glargine in 1,609 adult patients with type 2 diabetes who were inadequately treated with basal insulin with or without oral antidiabetic drugs. The aim of the trial was to evaluate the risk of hypoglycemia associated with the two long-acting insulins.
“Hypoglycemia is a very common event in both type 1 and type 2 diabetes,” Thomas Pieber, MD, Medical University Graz, Austria, reminded the audience. Severe hypoglycemia is associated with insulin use, and data show that patients with type 2 diabetes are at as much risk as are those with type 1 diabetes in the longer term (Diabetologia. 2007;50:1140-7).
The rationale for the CONCLUDE study comes from evidence from other trials, he said. Specifically, there was a pharmacodynamic/pharmacokinetic study comparing insulin degludec U200 with insulin glargine U300 that showed four times lower day-to-day variability in favor of insulin degludec (Diabetes Obes Metab. 2017;19:1032-9). Dr. Pieber noted that there was also a 30% lower potency of insulin glargine U300 versus insulin degludec U200. Those findings, together with findings from the SWITCH 2 (JAMA. 2017;318:45-56) and EDITION II (Diabetes Care. 2014;37:3235-43) trials, led to the hypothesis that there might be lower rates of hypoglycemia with insulin degludec U200 than with insulin glargine U300.
Dr. Pieber noted that one early issue with the trial was how patients’ blood glucose had been initially measured, because the blood glucose monitoring system that patients were first using seemed to display falsely higher values than what was actually expected, potentially putting patients’ safety at risk. This called for a protocol amendment (J Diabetes Sci Technol. 2019;13:498-506) and a new blood glucose monitoring system being used.
A similar proportion of patients in each group discontinued treatment during the study – 12.3% in the insulin degludec arm, and 11.4% in the insulin glargine arm – for reasons including adverse events (2.9% vs. 2.1%, respectively) and lack of efficacy (1.0% vs. 1.6%), he said.
Post hoc analyses hinted at slight benefits of insulin degludec over insulin glargine in terms of end of treatment hemoglobin A1c and the daily observed insulin dose, but less weight gain for insulin glargine.
Commenting further on the study, Dr. Del Prato said there was much debate around the pharmacokinetics and pharmacodynamic differences between insulin degludec and insulin glargine, and that the data should be interpreted “with a lot of caution.”
He said that it was not clear from the findings why there might be a lower risk of hypoglycemia with insulin degludec. He noted that the rate of diurnal hypoglycemia should be considered, and that the statistical interpretation of the data may be “a matter of uncertainty as well.”
Dr. Del Prato agreed that “prevention of hypoglycemia remained a major task in treating type 2 diabetic patients, particularly those on insulin therapy.” He proposed that differentiating between new basal insulin analogs may need “better tools for characterization of PK/PD, rigorous interpretation of the results, careful assessment of the generalizability of results from randomized controlled trials performed in selected study populations, and independent research and careful real-world studies to be performed.”
Furthermore, he said translating the potential benefit of new insulin analogs “cannot just rely on their properties, rather it requires validation over the time of the accuracy of glucose meters, and adequate patient education, and even more, education reinforcement.”
All of the speakers disclosed ties with Novo Nordisk, which funded the study, as well as other pharmaceutical companies.
SOURCES: Philis-Tsimikas A. EASD 2019, Oral Presentation 90; Pieber T. EASD 2019, Oral Presentation S38.1; Philis-Tsimikas A. EASD 2019, Oral Presentation S38.2; Del Prato S. EASD 2019. Oral Presentation S38.3.
BARCELONA – data presented at the annual meeting of the European Association for the Study of Diabetes.
During a 36-week maintenance treatment period, the rate ratio (RR) for the primary endpoint of the study – severe or confirmed symptomatic hypoglycemia, defined as a blood-glucose level of less than 3.1 mmol/L (56 mg/dL) – was 0.88 (95% confidence interval, 0.73-1.06; P = .17) in a comparison the two long-acting insulins.
There were some differences that favored insulin degludec over insulin glargine when other endpoints were considered, but because a statistical testing hierarchy was used, “no confirmatory results can be made from CONCLUDE,” one of the study investigators, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, San Diego, said during a special symposium at the meeting.
“If the primary endpoint was not achieved, the testing hierarchy stopped after the first occurrence of nonsignificance,” she explained. Although that occurred, the other endpoints could still be analyzed as they were all prespecified, she argued. These showed that during the maintenance treatment period, there were lower rates of nocturnal symptomatic hypoglycemic episodes (RR, 0.63; 95% CI, 0.48-0.84; P = .0014) and severe episodes (those requiring third-party assistance; RR, 0.20; 95% CI, 0.07-0.57; P = .0027) with insulin degludec, compared with insulin glargine.
The trial came under fire, however, from Stefano Del Prato, MD, professor of endocrinology in the department of endocrinology metabolism and chief of the section of diabetes at the University of Pisa, Italy, who provided the EASD-invited independent commentary on the study’s findings. “This is the CONCLUDE trial. You’d could expect a CONCLUDE trial to be conclusive in its conclusions!” he said, noting that there were “too many uncertainties” in the trial.
CONCLUDE was a randomized, open-label, head-to-head study of insulin degludec versus insulin glargine in 1,609 adult patients with type 2 diabetes who were inadequately treated with basal insulin with or without oral antidiabetic drugs. The aim of the trial was to evaluate the risk of hypoglycemia associated with the two long-acting insulins.
“Hypoglycemia is a very common event in both type 1 and type 2 diabetes,” Thomas Pieber, MD, Medical University Graz, Austria, reminded the audience. Severe hypoglycemia is associated with insulin use, and data show that patients with type 2 diabetes are at as much risk as are those with type 1 diabetes in the longer term (Diabetologia. 2007;50:1140-7).
The rationale for the CONCLUDE study comes from evidence from other trials, he said. Specifically, there was a pharmacodynamic/pharmacokinetic study comparing insulin degludec U200 with insulin glargine U300 that showed four times lower day-to-day variability in favor of insulin degludec (Diabetes Obes Metab. 2017;19:1032-9). Dr. Pieber noted that there was also a 30% lower potency of insulin glargine U300 versus insulin degludec U200. Those findings, together with findings from the SWITCH 2 (JAMA. 2017;318:45-56) and EDITION II (Diabetes Care. 2014;37:3235-43) trials, led to the hypothesis that there might be lower rates of hypoglycemia with insulin degludec U200 than with insulin glargine U300.
Dr. Pieber noted that one early issue with the trial was how patients’ blood glucose had been initially measured, because the blood glucose monitoring system that patients were first using seemed to display falsely higher values than what was actually expected, potentially putting patients’ safety at risk. This called for a protocol amendment (J Diabetes Sci Technol. 2019;13:498-506) and a new blood glucose monitoring system being used.
A similar proportion of patients in each group discontinued treatment during the study – 12.3% in the insulin degludec arm, and 11.4% in the insulin glargine arm – for reasons including adverse events (2.9% vs. 2.1%, respectively) and lack of efficacy (1.0% vs. 1.6%), he said.
Post hoc analyses hinted at slight benefits of insulin degludec over insulin glargine in terms of end of treatment hemoglobin A1c and the daily observed insulin dose, but less weight gain for insulin glargine.
Commenting further on the study, Dr. Del Prato said there was much debate around the pharmacokinetics and pharmacodynamic differences between insulin degludec and insulin glargine, and that the data should be interpreted “with a lot of caution.”
He said that it was not clear from the findings why there might be a lower risk of hypoglycemia with insulin degludec. He noted that the rate of diurnal hypoglycemia should be considered, and that the statistical interpretation of the data may be “a matter of uncertainty as well.”
Dr. Del Prato agreed that “prevention of hypoglycemia remained a major task in treating type 2 diabetic patients, particularly those on insulin therapy.” He proposed that differentiating between new basal insulin analogs may need “better tools for characterization of PK/PD, rigorous interpretation of the results, careful assessment of the generalizability of results from randomized controlled trials performed in selected study populations, and independent research and careful real-world studies to be performed.”
Furthermore, he said translating the potential benefit of new insulin analogs “cannot just rely on their properties, rather it requires validation over the time of the accuracy of glucose meters, and adequate patient education, and even more, education reinforcement.”
All of the speakers disclosed ties with Novo Nordisk, which funded the study, as well as other pharmaceutical companies.
SOURCES: Philis-Tsimikas A. EASD 2019, Oral Presentation 90; Pieber T. EASD 2019, Oral Presentation S38.1; Philis-Tsimikas A. EASD 2019, Oral Presentation S38.2; Del Prato S. EASD 2019. Oral Presentation S38.3.
BARCELONA – data presented at the annual meeting of the European Association for the Study of Diabetes.
During a 36-week maintenance treatment period, the rate ratio (RR) for the primary endpoint of the study – severe or confirmed symptomatic hypoglycemia, defined as a blood-glucose level of less than 3.1 mmol/L (56 mg/dL) – was 0.88 (95% confidence interval, 0.73-1.06; P = .17) in a comparison the two long-acting insulins.
There were some differences that favored insulin degludec over insulin glargine when other endpoints were considered, but because a statistical testing hierarchy was used, “no confirmatory results can be made from CONCLUDE,” one of the study investigators, Athena Philis-Tsimikas, MD, of Scripps Whittier Diabetes Institute, San Diego, said during a special symposium at the meeting.
“If the primary endpoint was not achieved, the testing hierarchy stopped after the first occurrence of nonsignificance,” she explained. Although that occurred, the other endpoints could still be analyzed as they were all prespecified, she argued. These showed that during the maintenance treatment period, there were lower rates of nocturnal symptomatic hypoglycemic episodes (RR, 0.63; 95% CI, 0.48-0.84; P = .0014) and severe episodes (those requiring third-party assistance; RR, 0.20; 95% CI, 0.07-0.57; P = .0027) with insulin degludec, compared with insulin glargine.
The trial came under fire, however, from Stefano Del Prato, MD, professor of endocrinology in the department of endocrinology metabolism and chief of the section of diabetes at the University of Pisa, Italy, who provided the EASD-invited independent commentary on the study’s findings. “This is the CONCLUDE trial. You’d could expect a CONCLUDE trial to be conclusive in its conclusions!” he said, noting that there were “too many uncertainties” in the trial.
CONCLUDE was a randomized, open-label, head-to-head study of insulin degludec versus insulin glargine in 1,609 adult patients with type 2 diabetes who were inadequately treated with basal insulin with or without oral antidiabetic drugs. The aim of the trial was to evaluate the risk of hypoglycemia associated with the two long-acting insulins.
“Hypoglycemia is a very common event in both type 1 and type 2 diabetes,” Thomas Pieber, MD, Medical University Graz, Austria, reminded the audience. Severe hypoglycemia is associated with insulin use, and data show that patients with type 2 diabetes are at as much risk as are those with type 1 diabetes in the longer term (Diabetologia. 2007;50:1140-7).
The rationale for the CONCLUDE study comes from evidence from other trials, he said. Specifically, there was a pharmacodynamic/pharmacokinetic study comparing insulin degludec U200 with insulin glargine U300 that showed four times lower day-to-day variability in favor of insulin degludec (Diabetes Obes Metab. 2017;19:1032-9). Dr. Pieber noted that there was also a 30% lower potency of insulin glargine U300 versus insulin degludec U200. Those findings, together with findings from the SWITCH 2 (JAMA. 2017;318:45-56) and EDITION II (Diabetes Care. 2014;37:3235-43) trials, led to the hypothesis that there might be lower rates of hypoglycemia with insulin degludec U200 than with insulin glargine U300.
Dr. Pieber noted that one early issue with the trial was how patients’ blood glucose had been initially measured, because the blood glucose monitoring system that patients were first using seemed to display falsely higher values than what was actually expected, potentially putting patients’ safety at risk. This called for a protocol amendment (J Diabetes Sci Technol. 2019;13:498-506) and a new blood glucose monitoring system being used.
A similar proportion of patients in each group discontinued treatment during the study – 12.3% in the insulin degludec arm, and 11.4% in the insulin glargine arm – for reasons including adverse events (2.9% vs. 2.1%, respectively) and lack of efficacy (1.0% vs. 1.6%), he said.
Post hoc analyses hinted at slight benefits of insulin degludec over insulin glargine in terms of end of treatment hemoglobin A1c and the daily observed insulin dose, but less weight gain for insulin glargine.
Commenting further on the study, Dr. Del Prato said there was much debate around the pharmacokinetics and pharmacodynamic differences between insulin degludec and insulin glargine, and that the data should be interpreted “with a lot of caution.”
He said that it was not clear from the findings why there might be a lower risk of hypoglycemia with insulin degludec. He noted that the rate of diurnal hypoglycemia should be considered, and that the statistical interpretation of the data may be “a matter of uncertainty as well.”
Dr. Del Prato agreed that “prevention of hypoglycemia remained a major task in treating type 2 diabetic patients, particularly those on insulin therapy.” He proposed that differentiating between new basal insulin analogs may need “better tools for characterization of PK/PD, rigorous interpretation of the results, careful assessment of the generalizability of results from randomized controlled trials performed in selected study populations, and independent research and careful real-world studies to be performed.”
Furthermore, he said translating the potential benefit of new insulin analogs “cannot just rely on their properties, rather it requires validation over the time of the accuracy of glucose meters, and adequate patient education, and even more, education reinforcement.”
All of the speakers disclosed ties with Novo Nordisk, which funded the study, as well as other pharmaceutical companies.
SOURCES: Philis-Tsimikas A. EASD 2019, Oral Presentation 90; Pieber T. EASD 2019, Oral Presentation S38.1; Philis-Tsimikas A. EASD 2019, Oral Presentation S38.2; Del Prato S. EASD 2019. Oral Presentation S38.3.
REPORTING FROM EASD 2019
Continuation of natalizumab treatment reduces risk of MS relapses during pregnancy
STOCKHOLM – according to research presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Continuation of treatment is not associated with major fetal risks.
Pregnancy influences the choice of treatment for patients with MS. Not all therapies are compatible with pregnancy; the newer, more effective treatments in particular may pose risks in this regard. “There is a need to accumulate new data on how to manage our highly active patients with MS – in particular, patients who are treated with natalizumab,” said Doriana Landi, MD, PhD, a research fellow at the University of Rome Tor Vergata.
Data suggest that suspension of natalizumab before pregnancy is associated with significant disease worsening. In 2018, Portaccio et al. found that suspending natalizumab treatment before conception is associated with a higher risk of relapses during pregnancy (Neurology. 2018 Mar 6;90[10]:e832-9). Suspending natalizumab treatment at conception, however, reduced the risk of relapse by a factor of three. Other data have suggested that continuing natalizumab treatment until the 30th week of gestation could protect mothers and guarantee fetal safety.
Comparing two cohorts of women with MS
To investigate this question further, Dr. Landi and colleagues investigated a cohort of 29 women with MS who were receiving natalizumab and became pregnant. They compared this cohort’s outcomes with those of the cohort that Portaccio et al. had studied. The investigators categorized participants in both cohorts into three groups according to the time of their last infusion of natalizumab in relation to their last menstrual period. Group 0 had their last infusion before their last menstrual period, group 1 had their last infusion during the first trimester of pregnancy, and group 2 continued treatment after the first trimester. All women restarted natalizumab post partum and had at least 12 months of follow-up after delivery.
Dr. Landi and colleagues calculated the annualized relapse rate during pregnancy and during 12 months post partum for all groups. They also compared the outcomes of newborns between groups.
Prematurity may have influenced rate of hematologic complications
In all, the researchers analyzed 90 completed pregnancies in 86 women with MS. There were no significant demographic differences between groups. The overall population’s mean age was approximately 31 years, and its median Expanded Disability Status Scale (EDSS) score was about 2. The women gave birth to 94 newborns. Mean gestational age was 38.42 weeks, mean birth weight was 2,878.08 mg, and mean length was 48.23 cm.
Group 0 included 31 patients, group 1 included 30 patients, and group 2 included 28 patients. The median interval between the last dose of natalizumab and last menstrual period was 70 days for group 0, 21 days for group 1, and 197 days for group 2. Group 2 received a median of five natalizumab infusions during pregnancy, with a median interval of 80 days between the last prepartum dose and delivery. The median interval between last prepartum dose and the first postpartum dose was 411 days in group 0, 288 in group 1, and 103 in group 2.
Most pregnancies in group 2 were exposed to natalizumab for at least 28 weeks of gestation. Women in group 2 restarted natalizumab treatment significantly earlier, compared with women in the other groups.
Group 0 had a significantly increased ARR during and after pregnancy, compared with the other groups, and group 2 had the lowest ARR during pregnancy. The mean ARR during pregnancy was 1.06 in group 0, 0.49 in group 1, and 0.09 in group 2. The ARR decreased to 0 in pregnancies exposed to natalizumab for more than 90 days. The mean ARR post partum was 0.39 in group 0, 0.23 in group 1, and 0.10 in group 2. Women in group 0 and group 1 had a higher EDSS during pregnancy than before conception.
Newborns’ mean gestational age, birth weight, and length did not differ significantly between groups. Five newborns in group 2, three of whom were premature, had anemia. This outcome is consistent with previous findings, and prematurity may be a confounding factor, said Dr. Landi. The frequency of birth defects was higher in group 2 than in the other groups, but most of them occurred in one twin, said Dr. Landi. The investigators observed malformations in one group 0 newborn (minor), four group 1 newborns (five minor and one major), and four (two minor and four major) group 2 newborns.
In pregnancy during which natalizumab treatment is prolonged until the third trimester, treatment should be restarted after delivery within 2 weeks of the last infusion to avoid potential doubled rebound, said Dr. Landi. Future studies should examine whether extended dosing is as effective as regular dosing. A larger sample size is needed to estimate risks correctly for newborns in exposed pregnancies and counsel patients appropriately.
Dr. Landi reported receiving travel funding from Biogen, Merck Serono, Sanofi-Genzyme, and Teva; honoraria for speaking from Sanofi-Genzyme and Teva; and consultation fees from Merck Serono and Teva. She is an investigator in clinical trials being conducted for Biogen, Merck Serono, Novartis, Roche, and Teva.
SOURCE: Landi D et al. ECTRIMS 2019, Abstract 338.
STOCKHOLM – according to research presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Continuation of treatment is not associated with major fetal risks.
Pregnancy influences the choice of treatment for patients with MS. Not all therapies are compatible with pregnancy; the newer, more effective treatments in particular may pose risks in this regard. “There is a need to accumulate new data on how to manage our highly active patients with MS – in particular, patients who are treated with natalizumab,” said Doriana Landi, MD, PhD, a research fellow at the University of Rome Tor Vergata.
Data suggest that suspension of natalizumab before pregnancy is associated with significant disease worsening. In 2018, Portaccio et al. found that suspending natalizumab treatment before conception is associated with a higher risk of relapses during pregnancy (Neurology. 2018 Mar 6;90[10]:e832-9). Suspending natalizumab treatment at conception, however, reduced the risk of relapse by a factor of three. Other data have suggested that continuing natalizumab treatment until the 30th week of gestation could protect mothers and guarantee fetal safety.
Comparing two cohorts of women with MS
To investigate this question further, Dr. Landi and colleagues investigated a cohort of 29 women with MS who were receiving natalizumab and became pregnant. They compared this cohort’s outcomes with those of the cohort that Portaccio et al. had studied. The investigators categorized participants in both cohorts into three groups according to the time of their last infusion of natalizumab in relation to their last menstrual period. Group 0 had their last infusion before their last menstrual period, group 1 had their last infusion during the first trimester of pregnancy, and group 2 continued treatment after the first trimester. All women restarted natalizumab post partum and had at least 12 months of follow-up after delivery.
Dr. Landi and colleagues calculated the annualized relapse rate during pregnancy and during 12 months post partum for all groups. They also compared the outcomes of newborns between groups.
Prematurity may have influenced rate of hematologic complications
In all, the researchers analyzed 90 completed pregnancies in 86 women with MS. There were no significant demographic differences between groups. The overall population’s mean age was approximately 31 years, and its median Expanded Disability Status Scale (EDSS) score was about 2. The women gave birth to 94 newborns. Mean gestational age was 38.42 weeks, mean birth weight was 2,878.08 mg, and mean length was 48.23 cm.
Group 0 included 31 patients, group 1 included 30 patients, and group 2 included 28 patients. The median interval between the last dose of natalizumab and last menstrual period was 70 days for group 0, 21 days for group 1, and 197 days for group 2. Group 2 received a median of five natalizumab infusions during pregnancy, with a median interval of 80 days between the last prepartum dose and delivery. The median interval between last prepartum dose and the first postpartum dose was 411 days in group 0, 288 in group 1, and 103 in group 2.
Most pregnancies in group 2 were exposed to natalizumab for at least 28 weeks of gestation. Women in group 2 restarted natalizumab treatment significantly earlier, compared with women in the other groups.
Group 0 had a significantly increased ARR during and after pregnancy, compared with the other groups, and group 2 had the lowest ARR during pregnancy. The mean ARR during pregnancy was 1.06 in group 0, 0.49 in group 1, and 0.09 in group 2. The ARR decreased to 0 in pregnancies exposed to natalizumab for more than 90 days. The mean ARR post partum was 0.39 in group 0, 0.23 in group 1, and 0.10 in group 2. Women in group 0 and group 1 had a higher EDSS during pregnancy than before conception.
Newborns’ mean gestational age, birth weight, and length did not differ significantly between groups. Five newborns in group 2, three of whom were premature, had anemia. This outcome is consistent with previous findings, and prematurity may be a confounding factor, said Dr. Landi. The frequency of birth defects was higher in group 2 than in the other groups, but most of them occurred in one twin, said Dr. Landi. The investigators observed malformations in one group 0 newborn (minor), four group 1 newborns (five minor and one major), and four (two minor and four major) group 2 newborns.
In pregnancy during which natalizumab treatment is prolonged until the third trimester, treatment should be restarted after delivery within 2 weeks of the last infusion to avoid potential doubled rebound, said Dr. Landi. Future studies should examine whether extended dosing is as effective as regular dosing. A larger sample size is needed to estimate risks correctly for newborns in exposed pregnancies and counsel patients appropriately.
Dr. Landi reported receiving travel funding from Biogen, Merck Serono, Sanofi-Genzyme, and Teva; honoraria for speaking from Sanofi-Genzyme and Teva; and consultation fees from Merck Serono and Teva. She is an investigator in clinical trials being conducted for Biogen, Merck Serono, Novartis, Roche, and Teva.
SOURCE: Landi D et al. ECTRIMS 2019, Abstract 338.
STOCKHOLM – according to research presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Continuation of treatment is not associated with major fetal risks.
Pregnancy influences the choice of treatment for patients with MS. Not all therapies are compatible with pregnancy; the newer, more effective treatments in particular may pose risks in this regard. “There is a need to accumulate new data on how to manage our highly active patients with MS – in particular, patients who are treated with natalizumab,” said Doriana Landi, MD, PhD, a research fellow at the University of Rome Tor Vergata.
Data suggest that suspension of natalizumab before pregnancy is associated with significant disease worsening. In 2018, Portaccio et al. found that suspending natalizumab treatment before conception is associated with a higher risk of relapses during pregnancy (Neurology. 2018 Mar 6;90[10]:e832-9). Suspending natalizumab treatment at conception, however, reduced the risk of relapse by a factor of three. Other data have suggested that continuing natalizumab treatment until the 30th week of gestation could protect mothers and guarantee fetal safety.
Comparing two cohorts of women with MS
To investigate this question further, Dr. Landi and colleagues investigated a cohort of 29 women with MS who were receiving natalizumab and became pregnant. They compared this cohort’s outcomes with those of the cohort that Portaccio et al. had studied. The investigators categorized participants in both cohorts into three groups according to the time of their last infusion of natalizumab in relation to their last menstrual period. Group 0 had their last infusion before their last menstrual period, group 1 had their last infusion during the first trimester of pregnancy, and group 2 continued treatment after the first trimester. All women restarted natalizumab post partum and had at least 12 months of follow-up after delivery.
Dr. Landi and colleagues calculated the annualized relapse rate during pregnancy and during 12 months post partum for all groups. They also compared the outcomes of newborns between groups.
Prematurity may have influenced rate of hematologic complications
In all, the researchers analyzed 90 completed pregnancies in 86 women with MS. There were no significant demographic differences between groups. The overall population’s mean age was approximately 31 years, and its median Expanded Disability Status Scale (EDSS) score was about 2. The women gave birth to 94 newborns. Mean gestational age was 38.42 weeks, mean birth weight was 2,878.08 mg, and mean length was 48.23 cm.
Group 0 included 31 patients, group 1 included 30 patients, and group 2 included 28 patients. The median interval between the last dose of natalizumab and last menstrual period was 70 days for group 0, 21 days for group 1, and 197 days for group 2. Group 2 received a median of five natalizumab infusions during pregnancy, with a median interval of 80 days between the last prepartum dose and delivery. The median interval between last prepartum dose and the first postpartum dose was 411 days in group 0, 288 in group 1, and 103 in group 2.
Most pregnancies in group 2 were exposed to natalizumab for at least 28 weeks of gestation. Women in group 2 restarted natalizumab treatment significantly earlier, compared with women in the other groups.
Group 0 had a significantly increased ARR during and after pregnancy, compared with the other groups, and group 2 had the lowest ARR during pregnancy. The mean ARR during pregnancy was 1.06 in group 0, 0.49 in group 1, and 0.09 in group 2. The ARR decreased to 0 in pregnancies exposed to natalizumab for more than 90 days. The mean ARR post partum was 0.39 in group 0, 0.23 in group 1, and 0.10 in group 2. Women in group 0 and group 1 had a higher EDSS during pregnancy than before conception.
Newborns’ mean gestational age, birth weight, and length did not differ significantly between groups. Five newborns in group 2, three of whom were premature, had anemia. This outcome is consistent with previous findings, and prematurity may be a confounding factor, said Dr. Landi. The frequency of birth defects was higher in group 2 than in the other groups, but most of them occurred in one twin, said Dr. Landi. The investigators observed malformations in one group 0 newborn (minor), four group 1 newborns (five minor and one major), and four (two minor and four major) group 2 newborns.
In pregnancy during which natalizumab treatment is prolonged until the third trimester, treatment should be restarted after delivery within 2 weeks of the last infusion to avoid potential doubled rebound, said Dr. Landi. Future studies should examine whether extended dosing is as effective as regular dosing. A larger sample size is needed to estimate risks correctly for newborns in exposed pregnancies and counsel patients appropriately.
Dr. Landi reported receiving travel funding from Biogen, Merck Serono, Sanofi-Genzyme, and Teva; honoraria for speaking from Sanofi-Genzyme and Teva; and consultation fees from Merck Serono and Teva. She is an investigator in clinical trials being conducted for Biogen, Merck Serono, Novartis, Roche, and Teva.
SOURCE: Landi D et al. ECTRIMS 2019, Abstract 338.
REPORTING FROM ECTRIMS 2019
ASCT may cure follicular lymphoma for some rituximab-naive patients
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
Prompt autologous stem cell transplantation (ASCT) is often curative in rituximab-naive patients with follicular lymphoma who have experienced early failure of first-line therapy and achieved a response to second-line therapy, suggest results from a registry-based study conducted by GELTAMO (the Spanish Lymphoma and Bone Marrow Transplant Group).
“Overall, our results suggest that, whereas some patients might benefit from more aggressive therapies, such as allogenic stem cell transplantations, or novel drugs, such as immunomodulatory agents, monoclonal antibodies, phosphoinositide 3-kinase inhibitors, or even the application of bispecific T-cell engagers and chimeric antigen receptor T cells, there are a considerable number of patients in this high-risk [early therapy failure] subgroup that can be cured with ASCT, even in the absence of rituximab,” Ana Jiménez-Ubieto, MD, PhD, of the Hospital Universitario, 12 de Octubre, Madrid, Spain, and colleagues wrote.
The results are more favorable when ASCT is performed in patients experiencing early therapy failure, with less than 1 year from first relapse after primary treatment to ASCT.
“Early ASCT could be a hopeful option in patients with difficult access to rituximab,” the researchers wrote in Hematology/Oncology and Stem Cell Therapy.
Patients with follicular lymphoma who experience relapse or progression during or soon after first-line therapy have poor overall survival, and there is no standard therapy for this population, according to the researchers. Previous research has shown that ASCT prolongs survival in those who have received rituximab before transplantation, but benefit in the absence of this agent is unknown.
Dr. Jiménez-Ubieto and colleagues conducted a multicenter registry-based retrospective cohort study of 134 patients with nontransformed follicular lymphoma who underwent ASCT during 1989-2007 while in second complete or partial response to rescue chemotherapy and had not received rituximab.
Overall, 65% of the patients had experienced early therapy failure (relapse or progression within 2 years of starting first-line chemotherapy). Within this group, 78% underwent ASCT within 1 year, and 67% underwent ASCT while in second complete response. Median posttransplantation follow-up for the entire study cohort was 13.4 years.
Study results showed that patients who had experienced early therapy failure versus who had not had poorer 5-year progression-free survival (43% vs. 57%; P = .048) but similar 5-year overall survival (69% vs. 77%; P = .4). However, those patients with early therapy failure who underwent ASCT within 1 year had a statistically indistinguishable 5-year progression-free survival relative to counterparts without early therapy failure (48% vs. 66%; P = .44).
Additionally, the 48% progression-free survival seen in this subset was almost identical to the 49% seen in a historical cohort of patients with early therapy failure who similarly underwent ASCT within 1 year of first relapse but received rituximab before transplantation (Hematol Oncol. 2018;36[5]:765-72). This suggests “that the possible synergistic effect of rituximab plus ASCT is not as relevant if ASCT is offered soon in the course of the disease,” the researchers wrote.
Patients who had experienced early therapy failure achieved better overall survival if they underwent ASCT while in second complete response, as opposed to second partial response. Notably, 56% of those who underwent ASCT while in second complete response were alive at 13.7 years of follow-up and remained so long term.
The study was funded by the Foundation Research Institute at the Hospital Universitario 12 de Octubre. The researchers reported having no relevant conflicts of interest.
SOURCE: Jiménez-Ubieto A et al. Hematol Oncol Stem Cell Ther. 2019 Jul 9. doi: 10.1016/j.hemonc.2019.06.001.
FROM HEMATOLOGY/ONCOLOGY AND STEM CELL THERAPY
Using ultrasound guidance for adult abdominal paracentesis
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Background: Abdominal paracentesis is a commonly performed procedure, and with appropriate training, hospitalists can deliver similar outcomes when compared to interventional radiologists.
Study design: Position statement.
Setting: The Society of Hospital Medicine Point-of-Care Ultrasound (POCUS) Task Force developed these guidelines after reviewing available literature and voted on the appropriateness and consensus of a recommendation.
Synopsis: A total of 794 articles were screened, and 91 articles were included and incorporated into the recommendations. The 12 recommendations fall into three categories (clinical outcomes, technique, and training), and all 12 recommendations achieved consensus as strong recommendations.
To improve clinical outcomes, the authors recommended ultrasound guidance in performing paracentesis to reduce the risk of serious complications, to avoid attempting paracentesis with insufficient fluid, and to improve overall procedure success.
The authors advocated for several technique recommendations, including using the ultrasound to assess volume and location of intraperitoneal fluid, to identify the needle insertion site and confirm in multiple planes, to use color flow Doppler to identify abdominal wall vessels, to mark the insertion site immediately prior to the procedure, and to consider real-time ultrasound guidance.
When health care professionals are learning ultrasound-guided paracentesis, the authors recommended use of dedicated training sessions with simulation if available and that competency should be demonstrated before independently attempting the procedure.
Bottom line: These recommendations from SHM POCUS Task Force provides consensus guidelines on the use of ultrasound guidance when performing or learning abdominal paracentesis.
Citation: Cho J et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: A position statement of the Society of Hospital Medicine. 2019 Jan 2. doi: 10.12788/jhm.3095.
Dr. Schmit is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System, also in San Antonio.
Hormone therapy may do more harm than good for men with low PSA before early salvage radiation
For men with prostate cancer who have a low prostate-specific antigen (PSA) level prior to salvage radiation therapy (SRT), adding an antiandrogen may increase the risk of other-cause mortality by twofold or more, according to investigators.
This finding was drawn from a secondary analysis of the NRG Oncology/RTOG 9601 phase 3 trial, a practice-changing study that showed that 2 years of antiandrogen therapy with bicalutamide improved overall survival when added to SRT, compared with that of SRT alone.
Results from the present analysis paint a more complex picture, revealing that patients with low PSA levels who received hormone therapy had a higher rate of other-cause mortality, primarily because of high-grade cardiac and neurologic events, reported lead author Daniel Spratt, MD, of the University of Michigan Rogel Cancer Center, Ann Arbor, who presented findings at the annual meeting of the American Society for Radiation Oncology.
Dr. Spratt described how treatment paradigms have changed since RTOG 9601 began in 1998, which prompted a revisitation of the trial. “Almost half of the men [in the trial] had a persistently elevated PSA after they underwent their initial surgery,” Dr. Spratt said. “This is uncommonly seen today. Additionally, about 60% of patients received what we call late salvage radiation therapy, where PSA was monitored and continued to rise beyond 0.5 [ng/mL]; again, this is not what is recommended to be used today.”
In the present analysis, the investigators stratified patients by PSA level. Of the 760 men involved, 85% had a PSA of 0.2-1.5 ng/mL. Patients were further subgrouped by those with a PSA of 0.2-0.6 ng/mL and those with a very low PSA, of 0.2-0.3 ng/mL. Multiple endpoints were assessed, including overall survival, other-cause mortality, distant metastasis, and rates of grade 3-5 cardiac or neurologic events.
The analysis showed that men with a PSA greater than 1.5 ng/mL had improved survival (hazard ratio, 0.45; 95% confidence interval, 0.25-0.81) when treated with bicalutamide, but those with PSA of 1.5 ng/mL or less did not (HR, 0.87; 95% CI, 0.66-1.16). Looking more closely at patients with a PSA of 1.5 ng/mL or less, those with a PSA of 0.2-0.6 ng/mL had a twofold increased rate of other-cause mortality (subdistribution HR, 1.94; P = .009). The picture became even more concerning for patients with a PSA of 0.2-0.3 ng/mL who were treated with bicalutamide: They had a fourfold increased risk of other cause mortality (sHR, 4.14). Among the cases with elevated other-cause mortality, grade 3-4 cardiac and neurologic events were likely to be blamed.
“What is likely driving this, and of concern, is that for [patients with a] PSA of less than 1.5 [ng/mL] … there was a three- to four-and-a-half-fold increased risk of high grade cardiac events,” Dr. Spratt said.
“The current guidelines recommend that all men be offered hormone therapy when receiving salvage radiation therapy, but our data demonstrate that men with lower PSA’s are probably more harmed than helped by long-term hormone therapy,” Dr. Spratt concluded. “We now have three randomized trials with over 2,400 men in total, [none of which showed that] short- or long-term hormone therapy improves overall survival in men [with a low PSA level who receive] early salvage radiotherapy. Thus, PSA prior to salvage radiation actually is not only prognostic, it predicts who will benefit most from hormone therapy, and guidelines should now be updated to reflect this finding.”
The session moderator, Anthony Zietman, MD, of Massachusetts General Hospital, Boston, helped put the findings in perspective: “This really suggests that we’ve got to hold back a little,” Dr. Zietman said. “There are some people who really benefit [from hormone therapy], some who don’t benefit, and some who just might be harmed, so I think we can be much more thoughtful and cautious in the future. … Thirty thousand men a year are in this situation who could be receiving this treatment. You could fill Fenway Park with that many people. So there are some big downstream implications. From here on out, I’m going to be a lot more cautious with my patients.”
The study was primarily funded by AstraZeneca with additional support from the Korea Institute of Radiological and Medical Sciences. The investigators disclosed relationships with Novartis, Roche, Amgen, and others.
SOURCE: Spratt et al. ASTRO 2019, Abstract LBA1.
For men with prostate cancer who have a low prostate-specific antigen (PSA) level prior to salvage radiation therapy (SRT), adding an antiandrogen may increase the risk of other-cause mortality by twofold or more, according to investigators.
This finding was drawn from a secondary analysis of the NRG Oncology/RTOG 9601 phase 3 trial, a practice-changing study that showed that 2 years of antiandrogen therapy with bicalutamide improved overall survival when added to SRT, compared with that of SRT alone.
Results from the present analysis paint a more complex picture, revealing that patients with low PSA levels who received hormone therapy had a higher rate of other-cause mortality, primarily because of high-grade cardiac and neurologic events, reported lead author Daniel Spratt, MD, of the University of Michigan Rogel Cancer Center, Ann Arbor, who presented findings at the annual meeting of the American Society for Radiation Oncology.
Dr. Spratt described how treatment paradigms have changed since RTOG 9601 began in 1998, which prompted a revisitation of the trial. “Almost half of the men [in the trial] had a persistently elevated PSA after they underwent their initial surgery,” Dr. Spratt said. “This is uncommonly seen today. Additionally, about 60% of patients received what we call late salvage radiation therapy, where PSA was monitored and continued to rise beyond 0.5 [ng/mL]; again, this is not what is recommended to be used today.”
In the present analysis, the investigators stratified patients by PSA level. Of the 760 men involved, 85% had a PSA of 0.2-1.5 ng/mL. Patients were further subgrouped by those with a PSA of 0.2-0.6 ng/mL and those with a very low PSA, of 0.2-0.3 ng/mL. Multiple endpoints were assessed, including overall survival, other-cause mortality, distant metastasis, and rates of grade 3-5 cardiac or neurologic events.
The analysis showed that men with a PSA greater than 1.5 ng/mL had improved survival (hazard ratio, 0.45; 95% confidence interval, 0.25-0.81) when treated with bicalutamide, but those with PSA of 1.5 ng/mL or less did not (HR, 0.87; 95% CI, 0.66-1.16). Looking more closely at patients with a PSA of 1.5 ng/mL or less, those with a PSA of 0.2-0.6 ng/mL had a twofold increased rate of other-cause mortality (subdistribution HR, 1.94; P = .009). The picture became even more concerning for patients with a PSA of 0.2-0.3 ng/mL who were treated with bicalutamide: They had a fourfold increased risk of other cause mortality (sHR, 4.14). Among the cases with elevated other-cause mortality, grade 3-4 cardiac and neurologic events were likely to be blamed.
“What is likely driving this, and of concern, is that for [patients with a] PSA of less than 1.5 [ng/mL] … there was a three- to four-and-a-half-fold increased risk of high grade cardiac events,” Dr. Spratt said.
“The current guidelines recommend that all men be offered hormone therapy when receiving salvage radiation therapy, but our data demonstrate that men with lower PSA’s are probably more harmed than helped by long-term hormone therapy,” Dr. Spratt concluded. “We now have three randomized trials with over 2,400 men in total, [none of which showed that] short- or long-term hormone therapy improves overall survival in men [with a low PSA level who receive] early salvage radiotherapy. Thus, PSA prior to salvage radiation actually is not only prognostic, it predicts who will benefit most from hormone therapy, and guidelines should now be updated to reflect this finding.”
The session moderator, Anthony Zietman, MD, of Massachusetts General Hospital, Boston, helped put the findings in perspective: “This really suggests that we’ve got to hold back a little,” Dr. Zietman said. “There are some people who really benefit [from hormone therapy], some who don’t benefit, and some who just might be harmed, so I think we can be much more thoughtful and cautious in the future. … Thirty thousand men a year are in this situation who could be receiving this treatment. You could fill Fenway Park with that many people. So there are some big downstream implications. From here on out, I’m going to be a lot more cautious with my patients.”
The study was primarily funded by AstraZeneca with additional support from the Korea Institute of Radiological and Medical Sciences. The investigators disclosed relationships with Novartis, Roche, Amgen, and others.
SOURCE: Spratt et al. ASTRO 2019, Abstract LBA1.
For men with prostate cancer who have a low prostate-specific antigen (PSA) level prior to salvage radiation therapy (SRT), adding an antiandrogen may increase the risk of other-cause mortality by twofold or more, according to investigators.
This finding was drawn from a secondary analysis of the NRG Oncology/RTOG 9601 phase 3 trial, a practice-changing study that showed that 2 years of antiandrogen therapy with bicalutamide improved overall survival when added to SRT, compared with that of SRT alone.
Results from the present analysis paint a more complex picture, revealing that patients with low PSA levels who received hormone therapy had a higher rate of other-cause mortality, primarily because of high-grade cardiac and neurologic events, reported lead author Daniel Spratt, MD, of the University of Michigan Rogel Cancer Center, Ann Arbor, who presented findings at the annual meeting of the American Society for Radiation Oncology.
Dr. Spratt described how treatment paradigms have changed since RTOG 9601 began in 1998, which prompted a revisitation of the trial. “Almost half of the men [in the trial] had a persistently elevated PSA after they underwent their initial surgery,” Dr. Spratt said. “This is uncommonly seen today. Additionally, about 60% of patients received what we call late salvage radiation therapy, where PSA was monitored and continued to rise beyond 0.5 [ng/mL]; again, this is not what is recommended to be used today.”
In the present analysis, the investigators stratified patients by PSA level. Of the 760 men involved, 85% had a PSA of 0.2-1.5 ng/mL. Patients were further subgrouped by those with a PSA of 0.2-0.6 ng/mL and those with a very low PSA, of 0.2-0.3 ng/mL. Multiple endpoints were assessed, including overall survival, other-cause mortality, distant metastasis, and rates of grade 3-5 cardiac or neurologic events.
The analysis showed that men with a PSA greater than 1.5 ng/mL had improved survival (hazard ratio, 0.45; 95% confidence interval, 0.25-0.81) when treated with bicalutamide, but those with PSA of 1.5 ng/mL or less did not (HR, 0.87; 95% CI, 0.66-1.16). Looking more closely at patients with a PSA of 1.5 ng/mL or less, those with a PSA of 0.2-0.6 ng/mL had a twofold increased rate of other-cause mortality (subdistribution HR, 1.94; P = .009). The picture became even more concerning for patients with a PSA of 0.2-0.3 ng/mL who were treated with bicalutamide: They had a fourfold increased risk of other cause mortality (sHR, 4.14). Among the cases with elevated other-cause mortality, grade 3-4 cardiac and neurologic events were likely to be blamed.
“What is likely driving this, and of concern, is that for [patients with a] PSA of less than 1.5 [ng/mL] … there was a three- to four-and-a-half-fold increased risk of high grade cardiac events,” Dr. Spratt said.
“The current guidelines recommend that all men be offered hormone therapy when receiving salvage radiation therapy, but our data demonstrate that men with lower PSA’s are probably more harmed than helped by long-term hormone therapy,” Dr. Spratt concluded. “We now have three randomized trials with over 2,400 men in total, [none of which showed that] short- or long-term hormone therapy improves overall survival in men [with a low PSA level who receive] early salvage radiotherapy. Thus, PSA prior to salvage radiation actually is not only prognostic, it predicts who will benefit most from hormone therapy, and guidelines should now be updated to reflect this finding.”
The session moderator, Anthony Zietman, MD, of Massachusetts General Hospital, Boston, helped put the findings in perspective: “This really suggests that we’ve got to hold back a little,” Dr. Zietman said. “There are some people who really benefit [from hormone therapy], some who don’t benefit, and some who just might be harmed, so I think we can be much more thoughtful and cautious in the future. … Thirty thousand men a year are in this situation who could be receiving this treatment. You could fill Fenway Park with that many people. So there are some big downstream implications. From here on out, I’m going to be a lot more cautious with my patients.”
The study was primarily funded by AstraZeneca with additional support from the Korea Institute of Radiological and Medical Sciences. The investigators disclosed relationships with Novartis, Roche, Amgen, and others.
SOURCE: Spratt et al. ASTRO 2019, Abstract LBA1.
FROM ASTRO 2019
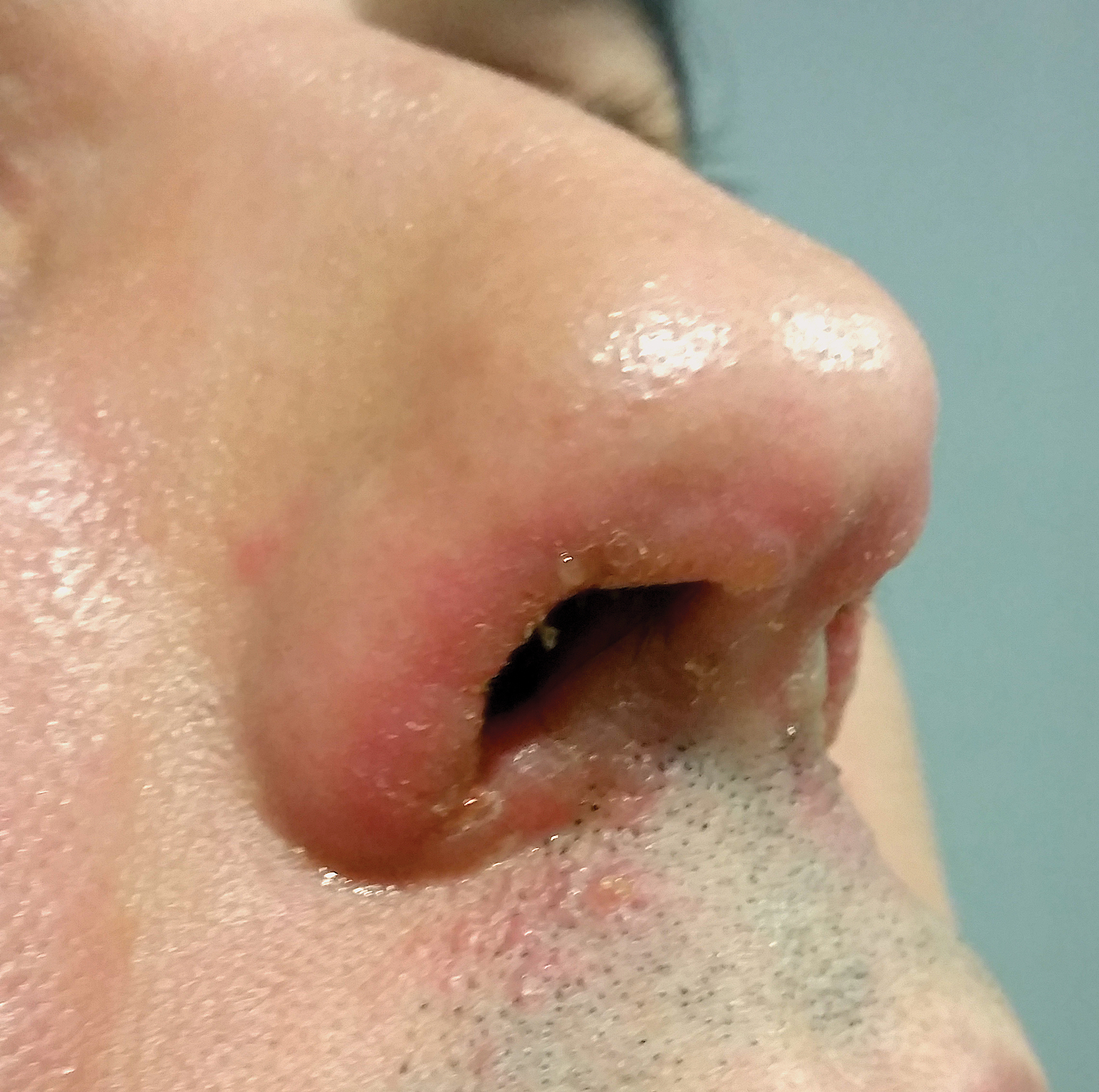
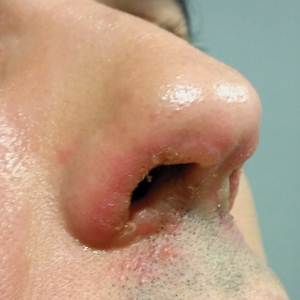
Symmetrical Pruriginous Nasal Rash
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
The Diagnosis: Irritant Contact Dermatitis
A slang term for volatile alkyl nitrites, poppers are inhaled for recreational purposes. They produce rapid-onset euphoria and sexual arousal, as well as relax anal and vaginal sphincters, facilitating sexual intercourse. Alkyl nitrites initially were developed to treat coronary disease and angina but were replaced by more potent drugs.1 Because of their psychoactive effects and smooth muscle relaxation properties, they are widely used by homosexual and bisexual men.1-3 The term poppers was originated by the sound generated when the glass vials are crushed; currently, they also may be found in other formats.1
Nausea, hypotension, and headache are mild common adverse effects of volatile alkyl nitrites1; cardiac arrhythmia, oxidative hemolysis,4 and poppers maculopathy5,6 with permanent eye damage also have been reported.7 On the skin, volatile alkyl nitrites induce irritant contact dermatitis that heals without scarring, characteristically involving the face and upper thoracic region, as they are volatile vapors.2 However, the reaction can occur elsewhere. There have been reports of contact dermatitis on other locations, such as the thigh or the ankle, due to vials broken while stored in pockets or on the cuff of the socks.1 There also is a report of irritant contact dermatitis manifesting as a penile ulcer.3 Albeit rare, allergic contact dermatitis to volatile alkyl nitrites and other nitrites also can occur.8
The abuse of alkyl nitrites may increase the risk for sexually transmitted infections (STIs), as they may decrease safer sexual practices and increase the propensity to engage in risky sexual behavior. It has been suggested to screen for STIs in patients with history of volatile alkyl nitrite use. In the past, volatile alkyl nitrites were believed to be a potential vector of human immunodeficiency virus.9 Other popular drugs used in social context or "club drugs," such as 3,4-methylenedioxymethamphetamine, gamma hydroxybutyrate, methamphetamine, and ketamine, do not produce irritant dermatitis as an adverse cutaneous reaction.10 The differential diagnosis in our patient included herpes simplex virus and contagious impetigo1 as well as bullous lupus erythematosus and periorificial dermatitis; however, the clinical picture, acute onset of the reaction, and the patient's medical history were critical in making the correct diagnosis.
The patient was treated with topical hydrocortisone and fusidic acid cream twice daily for 7 days with complete response. Sexually transmitted infection screening was unremarkable. We suggest performing an STI workup on patients with history of volatile alkyl nitrite use.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
- Schauber J, Herzinger T. 'Poppers' dermatitis. Clin Exp Dermatol. 2012;37:587-588.
- Foroozan M, Studer M, Splingard B, et al. Facial dermatitis due to inhalation of poppers [in French]. Ann Dermatol Venereol. 2009;136:298-299.
- Latini A, Lora V, Zaccarelli M, et al. Unusual presentation of poppers dermatitis. JAMA Dermatol. 2017;153:233-234.
- Shortt J, Polizzotto MN, Opat SS, et al. Oxidative haemolysis due to 'poppers'. Br J Haematol. 2008;142:328.
- Davies AJ, Kelly SP, Naylor SG, et al. Adverse ophthalmic reaction in poppers users: case series of 'poppers maculopathy'. Eye (Lond). 2012;26:1479-1486.
- Davies AJ, Kelly SP, Bhatt PR. 'Poppers maculopathy'--an emerging ophthalmic reaction to recreational substance abuse. Eye (Lond). 2012;26:888.
- Vignal-Clermont C, Audo I, Sahel JA, et al. Poppers-associated retinal toxicity. N Engl J Med. 2010;363:1583-1585.
- Bos JD, Jansen FC, Timmer JG. Allergic contact dermatitis to amyl nitrite ('poppers'). Contact Dermatitis. 1985;12:109.
- Stratford M, Wilson PD. Agitation effects on microbial cell-cell interactions. Lett Appl Microbiol. 1990;11:1-6.
- Romanelli F, Smith KM, Thornton AC, et al. Poppers: epidemiology and clinical management of inhaled nitrite abuse. Pharmacotherapy. 2004;24:69-78.
A 44-year-old man was referred to the department of dermatology for a pruriginous nasal rash. Physical examination revealed vesicles with clear content and crusts symmetrically in both nostrils and philtra. The remainder of the examination was otherwise unremarkable. The patient reported inhalation of poppers the prior night during a party. No history of connective tissue diseases was present. The patient was in overall good health with no fever or chills.
U.S. adults eating fewer carbs, more protein and fat
American diets have improved in the last several decades, with declines in consumption of low-quality carbohydrates and increases in plant protein and healthy fats, based on data from a nationally representative sample of 43,996 adults.
Changes in the economy, food policies, and food processing can affect diet over time, but trends in consumption of macronutrients in the U.S. have not been well studied, wrote Zhilei Shan, MD, of Harvard T.H. Chan School of Public Health, Boston, and colleagues.
In a study published in JAMA, the researchers reviewed data from nine consecutive cycles of the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016 to determine trends in macronutrient intake. The study population included adults aged 20 years and older who could provide at least 1 valid dietary recall questionnaire. The average age was 47 years, and 52% were women.
Overall, total carbohydrate consumption decreased from 52.5% to 50.5%, the estimated energy from high-quality carbohydrates increased from 7.42% to 8.65%, and the estimated energy from low-quality carbohydrates decreased from 45.1% to 41.8%. Total protein consumption increased from 15.5% to 16.4%, and plant protein consumption increased from 5.38% to 5.76%. Total fat consumption increased from 32.0% to 33.2%, including a 0.36% increase in saturated fatty acids from 11.5% to 11.9% and a 0.65% increase in polyunsaturated fatty acids from 7.58% to 8.23%.
Although the changes in total carbohydrates, total protein, and total fat were significant, 42% of energy intake came from low-quality carbohydrates and 10% came from saturated fat, the researchers noted. Also, the overall diet quality as measured by the Healthy Eating Index 2015 (HEI-2015) improved from 55.7 in 1999 to 57.7 in 2016. The HEI-2015 measures how diet data adheres to recommendations in the 2015-2020 Dietary Guidelines for Americans on a scale of 0-100.
“Improvements in intakes of whole grains and plant protein are encouraging, but how much do popular foods such as pizza, fast food sandwiches and burgers, and foods categorized as ‘snacks’ and ‘desserts’ contribute to the U.S. eating pattern?” wrote Linda Van Horn, PhD, RDN, and Marilyn C. Cornelis, PhD, of Northwestern University, Chicago, in an editorial accompanying the study. They cited a closer look at the NHANES data in the USDA’s “What We Eat in America” report and noted the differences in ethnicity with regard to macronutrient consumption.
“Non-Hispanic whites consume more alcohol, pizza, and fast food sandwiches (24% of total energy), while non-Hispanic blacks consume more salty snacks and sweet desserts (17% of total energy), and Hispanics consume more sugar-sweetened beverages (8% of total energy) and the least alcohol compared with the other racial/ethnic groups,” they said. “Public health efforts to educate, inform, and incent better adherence to the recommended nutrient-dense food groups are clearly needed,” they emphasized.
The study findings were limited by several factors, including the use of self-reports and changes in dietary assessments over time, the researchers noted. However, the results are strengthened by the large sample size and suggest improvements in the overall American diet, but also highlight the need for continuing public education and intervention, they said.
The study was supported by the National Institutes of Health and the Young Scientists Fund of the National Natural Science Foundation of China. Dr. Shan had no financial conflicts to disclose. Dr. Van Horn disclosed being a member of the 2020 US Dietary Guidelines Advisory Committee.
SOURCE: Shan Z et al. JAMA. 2019. doi: 10.1001/jama.2019.13771.
American diets have improved in the last several decades, with declines in consumption of low-quality carbohydrates and increases in plant protein and healthy fats, based on data from a nationally representative sample of 43,996 adults.
Changes in the economy, food policies, and food processing can affect diet over time, but trends in consumption of macronutrients in the U.S. have not been well studied, wrote Zhilei Shan, MD, of Harvard T.H. Chan School of Public Health, Boston, and colleagues.
In a study published in JAMA, the researchers reviewed data from nine consecutive cycles of the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016 to determine trends in macronutrient intake. The study population included adults aged 20 years and older who could provide at least 1 valid dietary recall questionnaire. The average age was 47 years, and 52% were women.
Overall, total carbohydrate consumption decreased from 52.5% to 50.5%, the estimated energy from high-quality carbohydrates increased from 7.42% to 8.65%, and the estimated energy from low-quality carbohydrates decreased from 45.1% to 41.8%. Total protein consumption increased from 15.5% to 16.4%, and plant protein consumption increased from 5.38% to 5.76%. Total fat consumption increased from 32.0% to 33.2%, including a 0.36% increase in saturated fatty acids from 11.5% to 11.9% and a 0.65% increase in polyunsaturated fatty acids from 7.58% to 8.23%.
Although the changes in total carbohydrates, total protein, and total fat were significant, 42% of energy intake came from low-quality carbohydrates and 10% came from saturated fat, the researchers noted. Also, the overall diet quality as measured by the Healthy Eating Index 2015 (HEI-2015) improved from 55.7 in 1999 to 57.7 in 2016. The HEI-2015 measures how diet data adheres to recommendations in the 2015-2020 Dietary Guidelines for Americans on a scale of 0-100.
“Improvements in intakes of whole grains and plant protein are encouraging, but how much do popular foods such as pizza, fast food sandwiches and burgers, and foods categorized as ‘snacks’ and ‘desserts’ contribute to the U.S. eating pattern?” wrote Linda Van Horn, PhD, RDN, and Marilyn C. Cornelis, PhD, of Northwestern University, Chicago, in an editorial accompanying the study. They cited a closer look at the NHANES data in the USDA’s “What We Eat in America” report and noted the differences in ethnicity with regard to macronutrient consumption.
“Non-Hispanic whites consume more alcohol, pizza, and fast food sandwiches (24% of total energy), while non-Hispanic blacks consume more salty snacks and sweet desserts (17% of total energy), and Hispanics consume more sugar-sweetened beverages (8% of total energy) and the least alcohol compared with the other racial/ethnic groups,” they said. “Public health efforts to educate, inform, and incent better adherence to the recommended nutrient-dense food groups are clearly needed,” they emphasized.
The study findings were limited by several factors, including the use of self-reports and changes in dietary assessments over time, the researchers noted. However, the results are strengthened by the large sample size and suggest improvements in the overall American diet, but also highlight the need for continuing public education and intervention, they said.
The study was supported by the National Institutes of Health and the Young Scientists Fund of the National Natural Science Foundation of China. Dr. Shan had no financial conflicts to disclose. Dr. Van Horn disclosed being a member of the 2020 US Dietary Guidelines Advisory Committee.
SOURCE: Shan Z et al. JAMA. 2019. doi: 10.1001/jama.2019.13771.
American diets have improved in the last several decades, with declines in consumption of low-quality carbohydrates and increases in plant protein and healthy fats, based on data from a nationally representative sample of 43,996 adults.
Changes in the economy, food policies, and food processing can affect diet over time, but trends in consumption of macronutrients in the U.S. have not been well studied, wrote Zhilei Shan, MD, of Harvard T.H. Chan School of Public Health, Boston, and colleagues.
In a study published in JAMA, the researchers reviewed data from nine consecutive cycles of the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016 to determine trends in macronutrient intake. The study population included adults aged 20 years and older who could provide at least 1 valid dietary recall questionnaire. The average age was 47 years, and 52% were women.
Overall, total carbohydrate consumption decreased from 52.5% to 50.5%, the estimated energy from high-quality carbohydrates increased from 7.42% to 8.65%, and the estimated energy from low-quality carbohydrates decreased from 45.1% to 41.8%. Total protein consumption increased from 15.5% to 16.4%, and plant protein consumption increased from 5.38% to 5.76%. Total fat consumption increased from 32.0% to 33.2%, including a 0.36% increase in saturated fatty acids from 11.5% to 11.9% and a 0.65% increase in polyunsaturated fatty acids from 7.58% to 8.23%.
Although the changes in total carbohydrates, total protein, and total fat were significant, 42% of energy intake came from low-quality carbohydrates and 10% came from saturated fat, the researchers noted. Also, the overall diet quality as measured by the Healthy Eating Index 2015 (HEI-2015) improved from 55.7 in 1999 to 57.7 in 2016. The HEI-2015 measures how diet data adheres to recommendations in the 2015-2020 Dietary Guidelines for Americans on a scale of 0-100.
“Improvements in intakes of whole grains and plant protein are encouraging, but how much do popular foods such as pizza, fast food sandwiches and burgers, and foods categorized as ‘snacks’ and ‘desserts’ contribute to the U.S. eating pattern?” wrote Linda Van Horn, PhD, RDN, and Marilyn C. Cornelis, PhD, of Northwestern University, Chicago, in an editorial accompanying the study. They cited a closer look at the NHANES data in the USDA’s “What We Eat in America” report and noted the differences in ethnicity with regard to macronutrient consumption.
“Non-Hispanic whites consume more alcohol, pizza, and fast food sandwiches (24% of total energy), while non-Hispanic blacks consume more salty snacks and sweet desserts (17% of total energy), and Hispanics consume more sugar-sweetened beverages (8% of total energy) and the least alcohol compared with the other racial/ethnic groups,” they said. “Public health efforts to educate, inform, and incent better adherence to the recommended nutrient-dense food groups are clearly needed,” they emphasized.
The study findings were limited by several factors, including the use of self-reports and changes in dietary assessments over time, the researchers noted. However, the results are strengthened by the large sample size and suggest improvements in the overall American diet, but also highlight the need for continuing public education and intervention, they said.
The study was supported by the National Institutes of Health and the Young Scientists Fund of the National Natural Science Foundation of China. Dr. Shan had no financial conflicts to disclose. Dr. Van Horn disclosed being a member of the 2020 US Dietary Guidelines Advisory Committee.
SOURCE: Shan Z et al. JAMA. 2019. doi: 10.1001/jama.2019.13771.
FROM JAMA
A quarter of ICU admissions caused by substance abuse
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
according to results from a retrospective chart review.
The abuse of illicit drugs topped substance abuse–related ICU stays, accounting for 13% of total admissions, which represented 11% of total charges.
“We conducted a study to provide updated data on ICU utilization and costs related to licit and illicit abuse at a large county hospital,” wrote Donald Westerhausen, MD, of Indiana University, Indianapolis, and colleagues. The findings were reported in Chest .
The single-center study comprised 594 patients who were admitted for prescription, alcohol, or illicit drug use between May 2017 and October 2017. The team used laboratory data, in addition to medical history, to define substance abuse–related admissions.
A total of 611 admissions occurred during the 6-month study period. The researchers collected information on patient demographics, hospital charges, insurance coverage, and other clinical parameters.
After analysis, they found that patients admitted for substance abuse were generally younger than were those admitted for other reasons (44 years vs. 59 years; P less than .001). In addition, patients were more often male (64% vs. 48%), had greater mortality (14%), and experienced longer hospital stays (median, 6 days).
In total, 25.7% of ICU admissions were related to substance abuse, which comprised 23.1% of charges. In particular, 9.5% and 2.9% of admissions were related to alcohol use and prescription drugs, which represented 7.6% and 4.2% of total charges, respectively.
“Polysubstance abuse was the most frequent subcategory of illicit and prescription drug admissions,” the researchers wrote.
They acknowledged two limitations of the study: the short duration and single-center design. Future studies should account for seasonal differences in ICU admissions, they noted.
“Identifying and accurately describing the landscape of this current health crisis will help us take appropriate action in the future,” they concluded.
No funding sources were reported. The authors did not disclose any conflicts of interest.
SOURCE: Westerhausen D et al. Chest. 2019 Sep 5. doi: 10.1016/j.chest.2019.08.2180.
FROM CHEST
Meta-analysis supports hormone therapy-targeted therapy combo for advanced breast cancer
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
“This meta-analysis solidifies an increasingly accepted role for CDK4/6 inhibitors in the upfront treatment of postmenopausal women with hormone receptor–positive, HER2-negative metastatic breast cancer,” Azadeh Nasrazadani, PhD, MD, and Adam M. Brufsky, MD, PhD, wrote in an editorial published in Lancet Oncology. “These agents are starting to displace more toxic chemotherapy agents as frontline treatments for estrogen receptor–positive metastatic breast cancer in general practice because of, to some extent, their similar efficacy with substantially more favorable side-effect profiles.”
Nonetheless, chemotherapy still has a role in this population, typically in the later-line setting and in patients experiencing a visceral crisis, they noted. However, the better overall response rate seen with a chemotherapy regimen (paclitaxel plus bevacizumab), compared with a combination targeted and hormonal therapy regimen (palbociclib plus letrozole), may hinge on the specific CDK4/6 inhibitor. “Future studies are needed to identify the appropriate setting and sequence for chemotherapy in these patients,” they maintained.
Outcomes were similar for comparable regimens containing the three currently approved CDK4/6 inhibitors, but individual resistance mechanisms have yet to be elucidated, according to Dr. Nasrazadani and Dr. Brufsky. In addition, it is unclear how these agents may affect duration of response to subsequent therapies, as evidence of a broad overall survival benefit is lacking.
“With the ever-increasing range of targeted therapies gaining approval for metastatic breast cancer, the clinical and research community continues to move further away from a monotherapy approach,” they concluded. “Trials are currently in progress evaluating the role of inhibitors of phosphoinositide 3-kinase, mammalian target of rapamycin, and MEK, among others. How these agents will compare to previously frontline chemotherapy, and whether we will gain more success with the combinations of these targeted therapies in addition to CDK4/6 inhibitors remains to be seen.”
Dr. Nasrazadani is a fellow in the division of hematology/oncology at the University of Pittsburgh Medical Center–Magee-Women’s Hospital. Dr. Brufsky is associate chief of the division of hematology/oncology and codirector of the Comprehensive Breast Cancer Center at Magee-Women’s Hospital. Dr. Brufsky reported receiving personal fees from numerous pharmaceutical companies; Dr. Nasrazadani reported no conflicts of interest.
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
Combined hormone therapy and targeted therapy should be the first choice for most postmenopausal women with recently diagnosed hormone receptor–positive, HER2-negative metastatic breast cancer, new data suggest.
Although guidelines support use of hormone therapies with or without targeted therapies in this population, up-front chemotherapy is still commonly used even in the absence of a visceral crisis, noted lead investigator Mario Giuliano, MD, PhD, of the department of clinical medicine and surgery at the University of Naples (Italy) Federico II, and colleagues.
The investigators undertook a systematic review and network meta-analysis of 140 randomized, controlled trials among 50,029 postmenopausal patients with hormone receptor–positive, HER2-negative metastatic breast cancer treated in the first- and/or second-line setting.
Study results, reported in Lancet Oncology, showed that relative to standard hormone therapy alone, the combination of hormone therapy with a targeted therapy – a CDK4/6 inhibitor, an mammalian target of rapamycin inhibitor, or an indicated phosphoinositide 3-kinase inhibitor – had significantly better efficacy, reducing the risk of progression-free survival events by more than half. In addition, no chemotherapy regimen, with or without targeted therapy, significantly outperformed the combination of hormone therapy with a CDK4/6 inhibitor.
Meanwhile, the hormone therapy–targeted therapy combinations had manageable toxicity, with the severity of adverse effects intermediate between that of hormone therapy alone and that of chemotherapy with or without targeted therapies.
“This study is, to our knowledge, the first to compare the efficacy and activity of all currently available chemotherapy and hormone therapy regimens, in combination with or without targeted therapies,” Dr. Giuliano and coinvestigators wrote. “[O]ur results corroborate the treatment algorithms recommended by the official oncology guidelines, supporting the use of new combinations of hormone therapies plus targeted therapies in the first-line or second-line setting in patients with hormone receptor-positive, HER2-negative metastatic breast cancer without visceral crisis.”
For the study, the investigators identified relevant phase 2 and phase 3 randomized, controlled trials published between 2000 and 2017, with the addition of several recently reported trials such as BOLERO-6 (JAMA Oncol. 2018;4:1367-74). Of the 140 trials ultimately included, 114 were used in the analysis of progression-free survival and time to progression, and 135 were used in the analysis of overall response.
Study results showed that when anastrozole (Arimidex) alone was the comparator, progression-free survival was significantly better with palbociclib (Ibrance) plus letrozole (Femara) (hazard ratio for events, 0.42); ribociclib (Kisqali) plus letrozole (HR, 0.43); abemaciclib (Verzenio) plus anastrozole or letrozole (HR, 0.42); palbociclib plus fulvestrant (Faslodex) (HR, 0.37); ribociclib plus fulvestrant (HR, 0.48); abemaciclib plus fulvestrant (HR, 0.44); everolimus (Afinitor) plus exemestane (Aromasin) (HR, 0.42); and, in patients with a PIK3CA mutation, the PI3K inhibitor alpelisib (Piqray) plus fulvestrant (HR, 0.39).
Several chemotherapy-based regimens, including anthracycline- and taxane-containing regimens, were also superior to anastrozole alone (hazard ratios, 0.41-0.47).
When palbociclib plus letrozole was the comparator, no chemotherapy or hormone therapy regimen yielded significantly better progression-free survival.
For the outcome of overall response, paclitaxel (Taxol) plus bevacizumab (Avastin) was the only clinically relevant regimen that significantly improved the likelihood of response relative to palbociclib plus letrozole (odds ratio, 8.95).
Dr. Giuliano reported that he receives honoraria from Amgen, AstraZeneca, Celgene, Eisai, Eli Lilly, Novartis, Pfizer,and Roche. The study did not receive any funding.
SOURCE: Giuliano M et al. Lancet Oncol. 2019 Sep 4. doi: 10.1016/S1470-2045(19)30420-6.
FROM LANCET ONCOLOGY
Sensitive, responsive parenting improves child language skills
Parental sensitive responsiveness, more than warmth, was associated with better child language skills, according to a meta-analysis in Pediatrics.
Sheri Madigan, PhD, of the department of psychology at the University of Calgary (Alta.) and the Alberta Children’s Hospital Research Institute, also in Calgary, and colleagues brought together 37 studies of the association of either parental sensitive responsiveness, warmth, or both with children’s language skills. The studies ranged in sample sizes from 9 mother-child dyads to 1,026. For the purposes of the study, sensitive responsiveness was defined as “a parent’s ability to perceive and interpret the child’s signals and cues and to respond to those signals and cues promptly and appropriately,” and warmth was defined as “caregiver physical affection or their positive affective quality during contact and involvement with the child.”
Across 36 samples with a total of 7,315 dyads, an association was seen between sensitive responsiveness and child language, with a correlation coefficient of 0.26 (95% confidence interval, 0.21-0.33), whereas the analysis exploring parental warmth with 13 samples (1,961 dyads) found a somewhat weaker association, with a correlation coefficient of 0.16 (95% CI, 0.09-0.21), according to the investigators.
“A sensitive-responsive parent can build on the moment-to-moment shifts in children’s attention, providing a finely tuned enhancement to the child’s experience,” the researchers suggested in regard to the greater effect seen with sensitive responsiveness. “Neural development is thought to occur through internalization of these finely tuned, reciprocal interactions. Warmth, on the other hand, does not involve contingency or reciprocity.”
Interestingly, moderator analyses revealed variations in effect sizes according to socioeconomic status – with greater effect sizes associated with lower socioeconomic status.
“A possible interpretation of this finding is that maternal sensitive responsiveness is particularly advantageous to children’s language when they are raise in socially disadvantaged families,” they wrote, noting that such an interpretation would be in line with previous research that showed “the protective effect of high-quality parent-child interactions in the context of adversity.”
Limitations of the analysis include that meta-analyses of observational studies are correlational in nature and are generally unable to demonstrate inferences about causality. Another is that the studies in this analysis were limited to children who developed in typical fashion, which limits generalizability to children with language delay, intellectual disability, autism, or hearing/vision difficulties. The analysis was also limited to only mother-child dyads, so the effects of paternal parenting are absent.
“The findings indicate a moderate association between sensitive-responsive parenting and children’s language skills,” the researchers concluded. “Sensitive responsiveness is a modifiable risk that has been successfully trained in parents in randomized trials and shown to improve language development of children.”
A grant from the Social Sciences and Humanities Research Council was awarded to the two of the study’s authors. Two authors’ individual contributions were supported by funding from the Alberta Children’s Hospital Foundation. The authors indicated they have no financial relationships relevant to this article or conflicts of interest to disclose.
SOURCE: Madigan S et al. Pediatrics. 2019 Sep 24. doi: 10.1542/peds.2018-3556.
Parental sensitive responsiveness and warmth, as explored in the meta-analysis by Madigan et al., is important, but so is the overall “language nutrition” children receive, wrote Heidi M. Feldman, MD, PhD.
“Pediatric clinicians routinely counsel families about food nutrition. We should address language nutrition with similar urgency,” Dr. Feldman suggested.
She pointed out that the two features of parenting explored in the meta-analysis are only part of the picture; other ingredients in language nutrition include the quantity of child-directed speech, the quality of the language input, and the nature of caregiver interactions with children, “beyond responsivity and warmth.”
“Meta-analyses on the topic of language development are extremely helpful,” Dr. Feldman wrote. “However, now we also need well-designed treatment studies to inform us about the nature and intensity of interventions to improve language-learning environments and child outcomes.”
Dr. Feldman , who is with the department of pediatrics at Stanford (Calif.) University, made these comments in an accompanying editorial in Pediatrics ( doi: 10.1542/peds.2019-2157 ). She had no financial relationships related to the commentary nor potential conflicts of interest to disclose.
Parental sensitive responsiveness and warmth, as explored in the meta-analysis by Madigan et al., is important, but so is the overall “language nutrition” children receive, wrote Heidi M. Feldman, MD, PhD.
“Pediatric clinicians routinely counsel families about food nutrition. We should address language nutrition with similar urgency,” Dr. Feldman suggested.
She pointed out that the two features of parenting explored in the meta-analysis are only part of the picture; other ingredients in language nutrition include the quantity of child-directed speech, the quality of the language input, and the nature of caregiver interactions with children, “beyond responsivity and warmth.”
“Meta-analyses on the topic of language development are extremely helpful,” Dr. Feldman wrote. “However, now we also need well-designed treatment studies to inform us about the nature and intensity of interventions to improve language-learning environments and child outcomes.”
Dr. Feldman , who is with the department of pediatrics at Stanford (Calif.) University, made these comments in an accompanying editorial in Pediatrics ( doi: 10.1542/peds.2019-2157 ). She had no financial relationships related to the commentary nor potential conflicts of interest to disclose.
Parental sensitive responsiveness and warmth, as explored in the meta-analysis by Madigan et al., is important, but so is the overall “language nutrition” children receive, wrote Heidi M. Feldman, MD, PhD.
“Pediatric clinicians routinely counsel families about food nutrition. We should address language nutrition with similar urgency,” Dr. Feldman suggested.
She pointed out that the two features of parenting explored in the meta-analysis are only part of the picture; other ingredients in language nutrition include the quantity of child-directed speech, the quality of the language input, and the nature of caregiver interactions with children, “beyond responsivity and warmth.”
“Meta-analyses on the topic of language development are extremely helpful,” Dr. Feldman wrote. “However, now we also need well-designed treatment studies to inform us about the nature and intensity of interventions to improve language-learning environments and child outcomes.”
Dr. Feldman , who is with the department of pediatrics at Stanford (Calif.) University, made these comments in an accompanying editorial in Pediatrics ( doi: 10.1542/peds.2019-2157 ). She had no financial relationships related to the commentary nor potential conflicts of interest to disclose.
Parental sensitive responsiveness, more than warmth, was associated with better child language skills, according to a meta-analysis in Pediatrics.
Sheri Madigan, PhD, of the department of psychology at the University of Calgary (Alta.) and the Alberta Children’s Hospital Research Institute, also in Calgary, and colleagues brought together 37 studies of the association of either parental sensitive responsiveness, warmth, or both with children’s language skills. The studies ranged in sample sizes from 9 mother-child dyads to 1,026. For the purposes of the study, sensitive responsiveness was defined as “a parent’s ability to perceive and interpret the child’s signals and cues and to respond to those signals and cues promptly and appropriately,” and warmth was defined as “caregiver physical affection or their positive affective quality during contact and involvement with the child.”
Across 36 samples with a total of 7,315 dyads, an association was seen between sensitive responsiveness and child language, with a correlation coefficient of 0.26 (95% confidence interval, 0.21-0.33), whereas the analysis exploring parental warmth with 13 samples (1,961 dyads) found a somewhat weaker association, with a correlation coefficient of 0.16 (95% CI, 0.09-0.21), according to the investigators.
“A sensitive-responsive parent can build on the moment-to-moment shifts in children’s attention, providing a finely tuned enhancement to the child’s experience,” the researchers suggested in regard to the greater effect seen with sensitive responsiveness. “Neural development is thought to occur through internalization of these finely tuned, reciprocal interactions. Warmth, on the other hand, does not involve contingency or reciprocity.”
Interestingly, moderator analyses revealed variations in effect sizes according to socioeconomic status – with greater effect sizes associated with lower socioeconomic status.
“A possible interpretation of this finding is that maternal sensitive responsiveness is particularly advantageous to children’s language when they are raise in socially disadvantaged families,” they wrote, noting that such an interpretation would be in line with previous research that showed “the protective effect of high-quality parent-child interactions in the context of adversity.”
Limitations of the analysis include that meta-analyses of observational studies are correlational in nature and are generally unable to demonstrate inferences about causality. Another is that the studies in this analysis were limited to children who developed in typical fashion, which limits generalizability to children with language delay, intellectual disability, autism, or hearing/vision difficulties. The analysis was also limited to only mother-child dyads, so the effects of paternal parenting are absent.
“The findings indicate a moderate association between sensitive-responsive parenting and children’s language skills,” the researchers concluded. “Sensitive responsiveness is a modifiable risk that has been successfully trained in parents in randomized trials and shown to improve language development of children.”
A grant from the Social Sciences and Humanities Research Council was awarded to the two of the study’s authors. Two authors’ individual contributions were supported by funding from the Alberta Children’s Hospital Foundation. The authors indicated they have no financial relationships relevant to this article or conflicts of interest to disclose.
SOURCE: Madigan S et al. Pediatrics. 2019 Sep 24. doi: 10.1542/peds.2018-3556.
Parental sensitive responsiveness, more than warmth, was associated with better child language skills, according to a meta-analysis in Pediatrics.
Sheri Madigan, PhD, of the department of psychology at the University of Calgary (Alta.) and the Alberta Children’s Hospital Research Institute, also in Calgary, and colleagues brought together 37 studies of the association of either parental sensitive responsiveness, warmth, or both with children’s language skills. The studies ranged in sample sizes from 9 mother-child dyads to 1,026. For the purposes of the study, sensitive responsiveness was defined as “a parent’s ability to perceive and interpret the child’s signals and cues and to respond to those signals and cues promptly and appropriately,” and warmth was defined as “caregiver physical affection or their positive affective quality during contact and involvement with the child.”
Across 36 samples with a total of 7,315 dyads, an association was seen between sensitive responsiveness and child language, with a correlation coefficient of 0.26 (95% confidence interval, 0.21-0.33), whereas the analysis exploring parental warmth with 13 samples (1,961 dyads) found a somewhat weaker association, with a correlation coefficient of 0.16 (95% CI, 0.09-0.21), according to the investigators.
“A sensitive-responsive parent can build on the moment-to-moment shifts in children’s attention, providing a finely tuned enhancement to the child’s experience,” the researchers suggested in regard to the greater effect seen with sensitive responsiveness. “Neural development is thought to occur through internalization of these finely tuned, reciprocal interactions. Warmth, on the other hand, does not involve contingency or reciprocity.”
Interestingly, moderator analyses revealed variations in effect sizes according to socioeconomic status – with greater effect sizes associated with lower socioeconomic status.
“A possible interpretation of this finding is that maternal sensitive responsiveness is particularly advantageous to children’s language when they are raise in socially disadvantaged families,” they wrote, noting that such an interpretation would be in line with previous research that showed “the protective effect of high-quality parent-child interactions in the context of adversity.”
Limitations of the analysis include that meta-analyses of observational studies are correlational in nature and are generally unable to demonstrate inferences about causality. Another is that the studies in this analysis were limited to children who developed in typical fashion, which limits generalizability to children with language delay, intellectual disability, autism, or hearing/vision difficulties. The analysis was also limited to only mother-child dyads, so the effects of paternal parenting are absent.
“The findings indicate a moderate association between sensitive-responsive parenting and children’s language skills,” the researchers concluded. “Sensitive responsiveness is a modifiable risk that has been successfully trained in parents in randomized trials and shown to improve language development of children.”
A grant from the Social Sciences and Humanities Research Council was awarded to the two of the study’s authors. Two authors’ individual contributions were supported by funding from the Alberta Children’s Hospital Foundation. The authors indicated they have no financial relationships relevant to this article or conflicts of interest to disclose.
SOURCE: Madigan S et al. Pediatrics. 2019 Sep 24. doi: 10.1542/peds.2018-3556.
FROM PEDIATRICS
Key clinical point:
Major finding: The correlation coefficient for parental sensitive responsiveness and child language skills was 0.27 (95% confidence interval, 0.21-0.33).
Study details: A meta-analysis of 37 studies that ranged in sample sizes from 9 to 1,026 mother-child dyads.
Disclosures: A grant from the Social Sciences and Humanities Research Council was awarded to the two of the study’s authors. Two authors’ individual contributions were supported by funding from the Alberta Children’s Hospital Foundation. The authors indicated they have no financial relationships relevant to this article or conflicts of interest to disclose.
Source: Madigan S et al. Pediatrics. 2019 Sep 24. doi: 10.1542/peds.2018-3556.