User login
‘Medicare for All’ emerges as early divide in first Democratic debate
Health care dominated early on June 26, with Sens. Elizabeth Warren (Mass.) and Cory Booker (N.J.) using questions about the economy to take aim at pharmaceutical and insurance companies. Sen. Amy Klobuchar (Minn.) emphasized the difficulties many Americans face in paying premiums.
But the candidates broke ranks on the details and not all of their claims stayed strictly within the lines.
Only two candidates — New York City Mayor Bill de Blasio and Sen. Warren — raised their hands in favor of banishing private insurance to install a government-sponsored Medicare for All approach.
Sen. Klobuchar, a single-payer skeptic, expressed concern about “kicking half of America off their health insurance in 4 years.” (That’s correct: In 2017, a majority of Americans had private coverage, with 49% getting that insurance through work, according to the Kaiser Family Foundation.)
Former Texas Rep. Beto O’Rourke, who also supports maintaining a private insurance system, outlined his own universal health care plan, based on a “Medicare for America” bill in Congress.
The single-payer talk set off other discussions about the role of health insurance and the cost of care. We fact-checked some of the biggest claims.
Sen. Warren: “The insurance companies last year alone sucked $23 billion in profits out of the health care system. $23 billion. And that doesn’t count the money that was paid to executives, the money that was spent lobbying Washington.”
We contacted Warren’s campaign, who directed us to a report from the National Association of Insurance Commissioners, a nonpartisan group of industry regulators. It supports her assessment.
The report says that in 2018, health insurers posted $23.4 billion in net earnings, or profits, compared with $16.1 billion a year prior.
This came up in the context of Warren’s support for eliminating private insurance under a Medicare for All system. However, the financing and price tag of such a system is unclear.
Sen. Booker: “The overhead for insurers that they charge is 15%, while Medicare’s overhead is only at 2%.”
This is a flawed comparison. Booker said administrative overhead eats up much more for private carriers than it does for Medicare, the government insurance program for seniors and the disabled. But Medicare piggybacks off the Social Security Administration, which covers costs of enrollment, payments, and keeping track of patients.
Also, Medicare relies on private providers for some of its programs, and overhead charges there are higher. Medicare’s overhead is less than that of private carriers, but exact figures are elusive.
The insurance companies’ trade group, America’s Health Insurance Plans (AHIP), reported in 2018 that 18.1% of private health care premiums went to non–health care services. That includes taxes of 4.7% and profits of 2.3%. The Medicare trustees reported that in 2018, total expenses were $740.6 billion, with administrative expenses of $9.9 billion. That comes to 1.3%, less than Booker said.
Sen. Warren: “I spent a big chunk of my life studying why families go broke, and one of the No. 1 reasons is the cost of health care, medical bills. And that’s not just for people who don’t have insurance. It’s for people who have insurance.”
Is the No. 1 reason people go broke the cost of health care? We’ve rated similar statements Half True — partially accurate but lacking important context.
A 2005 study Warren coauthored and a 2009 paper both found that health care expenses were a leading cause of personal bankruptcy. But these claims have come under dispute, in particular from academics who suggest that people may overstate the role medical bills play in their financial problems. Other research suggests a far narrower impact, though that, in turn, has been criticized for focusing only on adult hospitalizations.
That said, research from the Consumer Financial Protection Bureau found that medical bills are a leading cause of personal debt — in 2014, the CFPB found that nearly 20% of credit reports included a medical debt tradeline.
But Rep. Tulsi Gabbard (Hawaii) drew on examples of universal health coverage in other countries to explain why she still supported some private insurance options.
Rep. Gabbard: “If you look at other countries in the world who have universal health care, every one of them has some form of a role of private insurance.”
This is correct. Virtually every country with universal health care includes a role for private insurance. Some allow it to cover services not addressed by the national plan. Others allow it as a means to get care faster. Others heavily regulate it as a principal source of coverage.
For instance, Canada, the model for the principal Medicare for All bill, allows private insurance to address prescription drug coverage, private rooms in hospitals, and vision and dental care. (It is not allowed to compete with the government plan.) In England, about 10% of people — mostly wealthier people — elect for private coverage, which can yield faster access to care. Countries such as the Netherlands and Switzerland heavily regulate private coverage.
Beyond Medicare for All, candidates touched on strategies to bring down drug prices as well as other issues.
Sen. Klobuchar: “2,500 drug prices have gone up in double digits since [Donald Trump] took office.”
This is accurate, according to a report from Pharmacy Benefits Consultants, an industry group, which listed a number of pharmaceutical products experiencing price increases as high as 1,468%.
And the numbers are even less flattering than Sen. Klobuchar suggested.
An analysis by the Associated Press found that, between January and July 2018, more than 4,400 branded prescription drugs experienced price increases. Meanwhile, data compiled by Rx Savings Solutions found that the list price of more than 3,000 drugs went up this year.
Rep. O’Rourke: “In Texas, the single largest provider of mental health care is the county jail system.”
This is correct. Texas jails are the largest mental health care systems in the state, according to a report from the University of Texas at Austin. The Harris County jail, which includes a 108-bed unit, identifies itself as the largest mental health care facility in Texas.
This is not a Texas-specific issue. According to a 2011 NPR report, it is more common to see Americans getting mental health care in jails and prisons than in hospitals or other dedicated treatment facilities.
Election Day is 495 days away.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.
Health care dominated early on June 26, with Sens. Elizabeth Warren (Mass.) and Cory Booker (N.J.) using questions about the economy to take aim at pharmaceutical and insurance companies. Sen. Amy Klobuchar (Minn.) emphasized the difficulties many Americans face in paying premiums.
But the candidates broke ranks on the details and not all of their claims stayed strictly within the lines.
Only two candidates — New York City Mayor Bill de Blasio and Sen. Warren — raised their hands in favor of banishing private insurance to install a government-sponsored Medicare for All approach.
Sen. Klobuchar, a single-payer skeptic, expressed concern about “kicking half of America off their health insurance in 4 years.” (That’s correct: In 2017, a majority of Americans had private coverage, with 49% getting that insurance through work, according to the Kaiser Family Foundation.)
Former Texas Rep. Beto O’Rourke, who also supports maintaining a private insurance system, outlined his own universal health care plan, based on a “Medicare for America” bill in Congress.
The single-payer talk set off other discussions about the role of health insurance and the cost of care. We fact-checked some of the biggest claims.
Sen. Warren: “The insurance companies last year alone sucked $23 billion in profits out of the health care system. $23 billion. And that doesn’t count the money that was paid to executives, the money that was spent lobbying Washington.”
We contacted Warren’s campaign, who directed us to a report from the National Association of Insurance Commissioners, a nonpartisan group of industry regulators. It supports her assessment.
The report says that in 2018, health insurers posted $23.4 billion in net earnings, or profits, compared with $16.1 billion a year prior.
This came up in the context of Warren’s support for eliminating private insurance under a Medicare for All system. However, the financing and price tag of such a system is unclear.
Sen. Booker: “The overhead for insurers that they charge is 15%, while Medicare’s overhead is only at 2%.”
This is a flawed comparison. Booker said administrative overhead eats up much more for private carriers than it does for Medicare, the government insurance program for seniors and the disabled. But Medicare piggybacks off the Social Security Administration, which covers costs of enrollment, payments, and keeping track of patients.
Also, Medicare relies on private providers for some of its programs, and overhead charges there are higher. Medicare’s overhead is less than that of private carriers, but exact figures are elusive.
The insurance companies’ trade group, America’s Health Insurance Plans (AHIP), reported in 2018 that 18.1% of private health care premiums went to non–health care services. That includes taxes of 4.7% and profits of 2.3%. The Medicare trustees reported that in 2018, total expenses were $740.6 billion, with administrative expenses of $9.9 billion. That comes to 1.3%, less than Booker said.
Sen. Warren: “I spent a big chunk of my life studying why families go broke, and one of the No. 1 reasons is the cost of health care, medical bills. And that’s not just for people who don’t have insurance. It’s for people who have insurance.”
Is the No. 1 reason people go broke the cost of health care? We’ve rated similar statements Half True — partially accurate but lacking important context.
A 2005 study Warren coauthored and a 2009 paper both found that health care expenses were a leading cause of personal bankruptcy. But these claims have come under dispute, in particular from academics who suggest that people may overstate the role medical bills play in their financial problems. Other research suggests a far narrower impact, though that, in turn, has been criticized for focusing only on adult hospitalizations.
That said, research from the Consumer Financial Protection Bureau found that medical bills are a leading cause of personal debt — in 2014, the CFPB found that nearly 20% of credit reports included a medical debt tradeline.
But Rep. Tulsi Gabbard (Hawaii) drew on examples of universal health coverage in other countries to explain why she still supported some private insurance options.
Rep. Gabbard: “If you look at other countries in the world who have universal health care, every one of them has some form of a role of private insurance.”
This is correct. Virtually every country with universal health care includes a role for private insurance. Some allow it to cover services not addressed by the national plan. Others allow it as a means to get care faster. Others heavily regulate it as a principal source of coverage.
For instance, Canada, the model for the principal Medicare for All bill, allows private insurance to address prescription drug coverage, private rooms in hospitals, and vision and dental care. (It is not allowed to compete with the government plan.) In England, about 10% of people — mostly wealthier people — elect for private coverage, which can yield faster access to care. Countries such as the Netherlands and Switzerland heavily regulate private coverage.
Beyond Medicare for All, candidates touched on strategies to bring down drug prices as well as other issues.
Sen. Klobuchar: “2,500 drug prices have gone up in double digits since [Donald Trump] took office.”
This is accurate, according to a report from Pharmacy Benefits Consultants, an industry group, which listed a number of pharmaceutical products experiencing price increases as high as 1,468%.
And the numbers are even less flattering than Sen. Klobuchar suggested.
An analysis by the Associated Press found that, between January and July 2018, more than 4,400 branded prescription drugs experienced price increases. Meanwhile, data compiled by Rx Savings Solutions found that the list price of more than 3,000 drugs went up this year.
Rep. O’Rourke: “In Texas, the single largest provider of mental health care is the county jail system.”
This is correct. Texas jails are the largest mental health care systems in the state, according to a report from the University of Texas at Austin. The Harris County jail, which includes a 108-bed unit, identifies itself as the largest mental health care facility in Texas.
This is not a Texas-specific issue. According to a 2011 NPR report, it is more common to see Americans getting mental health care in jails and prisons than in hospitals or other dedicated treatment facilities.
Election Day is 495 days away.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.
Health care dominated early on June 26, with Sens. Elizabeth Warren (Mass.) and Cory Booker (N.J.) using questions about the economy to take aim at pharmaceutical and insurance companies. Sen. Amy Klobuchar (Minn.) emphasized the difficulties many Americans face in paying premiums.
But the candidates broke ranks on the details and not all of their claims stayed strictly within the lines.
Only two candidates — New York City Mayor Bill de Blasio and Sen. Warren — raised their hands in favor of banishing private insurance to install a government-sponsored Medicare for All approach.
Sen. Klobuchar, a single-payer skeptic, expressed concern about “kicking half of America off their health insurance in 4 years.” (That’s correct: In 2017, a majority of Americans had private coverage, with 49% getting that insurance through work, according to the Kaiser Family Foundation.)
Former Texas Rep. Beto O’Rourke, who also supports maintaining a private insurance system, outlined his own universal health care plan, based on a “Medicare for America” bill in Congress.
The single-payer talk set off other discussions about the role of health insurance and the cost of care. We fact-checked some of the biggest claims.
Sen. Warren: “The insurance companies last year alone sucked $23 billion in profits out of the health care system. $23 billion. And that doesn’t count the money that was paid to executives, the money that was spent lobbying Washington.”
We contacted Warren’s campaign, who directed us to a report from the National Association of Insurance Commissioners, a nonpartisan group of industry regulators. It supports her assessment.
The report says that in 2018, health insurers posted $23.4 billion in net earnings, or profits, compared with $16.1 billion a year prior.
This came up in the context of Warren’s support for eliminating private insurance under a Medicare for All system. However, the financing and price tag of such a system is unclear.
Sen. Booker: “The overhead for insurers that they charge is 15%, while Medicare’s overhead is only at 2%.”
This is a flawed comparison. Booker said administrative overhead eats up much more for private carriers than it does for Medicare, the government insurance program for seniors and the disabled. But Medicare piggybacks off the Social Security Administration, which covers costs of enrollment, payments, and keeping track of patients.
Also, Medicare relies on private providers for some of its programs, and overhead charges there are higher. Medicare’s overhead is less than that of private carriers, but exact figures are elusive.
The insurance companies’ trade group, America’s Health Insurance Plans (AHIP), reported in 2018 that 18.1% of private health care premiums went to non–health care services. That includes taxes of 4.7% and profits of 2.3%. The Medicare trustees reported that in 2018, total expenses were $740.6 billion, with administrative expenses of $9.9 billion. That comes to 1.3%, less than Booker said.
Sen. Warren: “I spent a big chunk of my life studying why families go broke, and one of the No. 1 reasons is the cost of health care, medical bills. And that’s not just for people who don’t have insurance. It’s for people who have insurance.”
Is the No. 1 reason people go broke the cost of health care? We’ve rated similar statements Half True — partially accurate but lacking important context.
A 2005 study Warren coauthored and a 2009 paper both found that health care expenses were a leading cause of personal bankruptcy. But these claims have come under dispute, in particular from academics who suggest that people may overstate the role medical bills play in their financial problems. Other research suggests a far narrower impact, though that, in turn, has been criticized for focusing only on adult hospitalizations.
That said, research from the Consumer Financial Protection Bureau found that medical bills are a leading cause of personal debt — in 2014, the CFPB found that nearly 20% of credit reports included a medical debt tradeline.
But Rep. Tulsi Gabbard (Hawaii) drew on examples of universal health coverage in other countries to explain why she still supported some private insurance options.
Rep. Gabbard: “If you look at other countries in the world who have universal health care, every one of them has some form of a role of private insurance.”
This is correct. Virtually every country with universal health care includes a role for private insurance. Some allow it to cover services not addressed by the national plan. Others allow it as a means to get care faster. Others heavily regulate it as a principal source of coverage.
For instance, Canada, the model for the principal Medicare for All bill, allows private insurance to address prescription drug coverage, private rooms in hospitals, and vision and dental care. (It is not allowed to compete with the government plan.) In England, about 10% of people — mostly wealthier people — elect for private coverage, which can yield faster access to care. Countries such as the Netherlands and Switzerland heavily regulate private coverage.
Beyond Medicare for All, candidates touched on strategies to bring down drug prices as well as other issues.
Sen. Klobuchar: “2,500 drug prices have gone up in double digits since [Donald Trump] took office.”
This is accurate, according to a report from Pharmacy Benefits Consultants, an industry group, which listed a number of pharmaceutical products experiencing price increases as high as 1,468%.
And the numbers are even less flattering than Sen. Klobuchar suggested.
An analysis by the Associated Press found that, between January and July 2018, more than 4,400 branded prescription drugs experienced price increases. Meanwhile, data compiled by Rx Savings Solutions found that the list price of more than 3,000 drugs went up this year.
Rep. O’Rourke: “In Texas, the single largest provider of mental health care is the county jail system.”
This is correct. Texas jails are the largest mental health care systems in the state, according to a report from the University of Texas at Austin. The Harris County jail, which includes a 108-bed unit, identifies itself as the largest mental health care facility in Texas.
This is not a Texas-specific issue. According to a 2011 NPR report, it is more common to see Americans getting mental health care in jails and prisons than in hospitals or other dedicated treatment facilities.
Election Day is 495 days away.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente. Politifact is owned by the nonprofit Poynter Institute for Media Studies.
Orthopedic complications in sickle cell require prompt action
FORT LAUDERDALE, FLA. – Orthopedic crises are common in patients with sickle cell disease, ranging from osteonecrosis to bone infarction, and physicians who manage these patients should know how to recognize these crises and not hesitate to consult an orthopedic surgeon early on, according to one expert at the annual meeting of the Foundation for Sickle Cell Disease Research.
“Sickle cell is a common entity in orthopedic surgery, so you shouldn’t hesitate in the hospital or outpatient settings to call for an orthopedic surgeon when you’re dealing with acute pain crises, medullary infarcts, and osteonecrosis,” said Mark W. Bridges, MD, an orthopedic surgeon with Orthopaedic Associates in Southern Florida.
Dr. Bridges noted that the femoral head is the most common location for osteonecrosis, one of the four major orthopedic manifestations of sickle cell disease that he reviewed. The others are septic arthritis, osteomyelitis, and bone infarction.
“Bone infarction is more common than osteomyelitis, and gadolinium-enhanced MRI can help to differentiate the two,” he said.
Osteonecrosis occurs when ischemic cells die, weakening the subchondral bone. Besides the femoral head, osteonecrosis commonly affects the humeral head of the shoulder and the femoral condyles of the knee. Dr. Bridges reviewed the five stages of the Ficat and Arlet classification of osteonecrosis:
- 0 – no pain, normal x-rays.
- I – pain, normal x-rays but abnormal MRI.
- II – pain, abnormal x-ray (sclerosis without collapse).
- III – pain (subchondral collapse without joint degeneration).
- IV – pain (arthritic changes with subchondral collapse).
For osteonecrosis of the shoulder, Dr. Bridges said four surgical options exist: core decompression for stages I and II; humeral head resurfacing for stages II and III; and hemiarthroplasty or total shoulder replacement for stages III and IV.
“No medical therapies are known to slow the progression,” he said.
Total joint replacement can be inevitable in these patients when total collapse of the joint occurs, but Dr. Bridges added a word of caution. “Overall when it comes down to replacing joints, there are more complications in patients that have [sickle cell disease],” he said. “Normally the complication rate is about 1%; that typically goes up to about 10% in SCD patients, but when you have a patient with end-stage disease – shoulder collapse or hip collapse – you have to do something.”
Septic arthritis is an infection within the joint space, most commonly the hip, and it affects 5% of children and 0.3% of adults with sickle cell disease (Clin Orthop Relat Res. 2010;468:1676-81).
“This is very similar to a vaso-occlusive crisis,” Dr. Bridges said.
MRI with gadolinium can help guide treatment, and blood cultures and joint aspiration can identify the infectious microbe. Staphylococcus aureus is the most common, Dr. Bridges said. Treatment consists of IV antibiotics, irrigation, and debridement.
Osteomyelitis is an infection within the bone with symptoms similar to those of septic arthritis, although osteomyelitis patients are typically sicker, he said. MRI with gadolinium is indicated in patients who don’t respond to IV fluid, oxygenation, and nonsteroidal anti-inflammatory drugs. “Try to treat them like they have vaso-occlusive crisis,” he said. Blood cultures usually suffice in these patients; bone aspiration is rarely needed, Dr. Bridges said.
The most common organisms are Staphylococcus aureus and Salmonella, and sickle cell disease patients can have infections in more than one location, Dr. Bridges noted.
“If IV antibiotics don’t work, then these patients need surgical debridement,” he added.
Adults are prone to a higher rate of complications than are children, including joint stiffness, osteonecrosis, pathologic fracture, and chronic osteomyelitis.
Ischemic marrow from vaso-occlusion can result in bone infarction. With its severe pain, swelling, erythema, and loss of motion, bone infarction can appear similar to osteomyelitis and septic arthritis, although a high-grade fever is uncommon in bone infarction. Unlike osteomyelitis, gadolinium on MRI does not enhance in bone infarction.
Treatment “consists of supportive management with pain medications, hydration, and antibiotics until osteomyelitis is ruled out,” Dr. Bridges said.
When a patient with one of these orthopedic conditions needs surgery, there are three considerations: preoperative transfusions to achieve hemoglobin level of 10 mg/dL for major procedures; no transfusions for arthroscopy and small closed reductions; and postoperative oxygenation and hydration to prevent a vaso-occlusive event and acute chest syndrome, he said.
Dr. Bridges reported having no conflicts of interest.
FORT LAUDERDALE, FLA. – Orthopedic crises are common in patients with sickle cell disease, ranging from osteonecrosis to bone infarction, and physicians who manage these patients should know how to recognize these crises and not hesitate to consult an orthopedic surgeon early on, according to one expert at the annual meeting of the Foundation for Sickle Cell Disease Research.
“Sickle cell is a common entity in orthopedic surgery, so you shouldn’t hesitate in the hospital or outpatient settings to call for an orthopedic surgeon when you’re dealing with acute pain crises, medullary infarcts, and osteonecrosis,” said Mark W. Bridges, MD, an orthopedic surgeon with Orthopaedic Associates in Southern Florida.
Dr. Bridges noted that the femoral head is the most common location for osteonecrosis, one of the four major orthopedic manifestations of sickle cell disease that he reviewed. The others are septic arthritis, osteomyelitis, and bone infarction.
“Bone infarction is more common than osteomyelitis, and gadolinium-enhanced MRI can help to differentiate the two,” he said.
Osteonecrosis occurs when ischemic cells die, weakening the subchondral bone. Besides the femoral head, osteonecrosis commonly affects the humeral head of the shoulder and the femoral condyles of the knee. Dr. Bridges reviewed the five stages of the Ficat and Arlet classification of osteonecrosis:
- 0 – no pain, normal x-rays.
- I – pain, normal x-rays but abnormal MRI.
- II – pain, abnormal x-ray (sclerosis without collapse).
- III – pain (subchondral collapse without joint degeneration).
- IV – pain (arthritic changes with subchondral collapse).
For osteonecrosis of the shoulder, Dr. Bridges said four surgical options exist: core decompression for stages I and II; humeral head resurfacing for stages II and III; and hemiarthroplasty or total shoulder replacement for stages III and IV.
“No medical therapies are known to slow the progression,” he said.
Total joint replacement can be inevitable in these patients when total collapse of the joint occurs, but Dr. Bridges added a word of caution. “Overall when it comes down to replacing joints, there are more complications in patients that have [sickle cell disease],” he said. “Normally the complication rate is about 1%; that typically goes up to about 10% in SCD patients, but when you have a patient with end-stage disease – shoulder collapse or hip collapse – you have to do something.”
Septic arthritis is an infection within the joint space, most commonly the hip, and it affects 5% of children and 0.3% of adults with sickle cell disease (Clin Orthop Relat Res. 2010;468:1676-81).
“This is very similar to a vaso-occlusive crisis,” Dr. Bridges said.
MRI with gadolinium can help guide treatment, and blood cultures and joint aspiration can identify the infectious microbe. Staphylococcus aureus is the most common, Dr. Bridges said. Treatment consists of IV antibiotics, irrigation, and debridement.
Osteomyelitis is an infection within the bone with symptoms similar to those of septic arthritis, although osteomyelitis patients are typically sicker, he said. MRI with gadolinium is indicated in patients who don’t respond to IV fluid, oxygenation, and nonsteroidal anti-inflammatory drugs. “Try to treat them like they have vaso-occlusive crisis,” he said. Blood cultures usually suffice in these patients; bone aspiration is rarely needed, Dr. Bridges said.
The most common organisms are Staphylococcus aureus and Salmonella, and sickle cell disease patients can have infections in more than one location, Dr. Bridges noted.
“If IV antibiotics don’t work, then these patients need surgical debridement,” he added.
Adults are prone to a higher rate of complications than are children, including joint stiffness, osteonecrosis, pathologic fracture, and chronic osteomyelitis.
Ischemic marrow from vaso-occlusion can result in bone infarction. With its severe pain, swelling, erythema, and loss of motion, bone infarction can appear similar to osteomyelitis and septic arthritis, although a high-grade fever is uncommon in bone infarction. Unlike osteomyelitis, gadolinium on MRI does not enhance in bone infarction.
Treatment “consists of supportive management with pain medications, hydration, and antibiotics until osteomyelitis is ruled out,” Dr. Bridges said.
When a patient with one of these orthopedic conditions needs surgery, there are three considerations: preoperative transfusions to achieve hemoglobin level of 10 mg/dL for major procedures; no transfusions for arthroscopy and small closed reductions; and postoperative oxygenation and hydration to prevent a vaso-occlusive event and acute chest syndrome, he said.
Dr. Bridges reported having no conflicts of interest.
FORT LAUDERDALE, FLA. – Orthopedic crises are common in patients with sickle cell disease, ranging from osteonecrosis to bone infarction, and physicians who manage these patients should know how to recognize these crises and not hesitate to consult an orthopedic surgeon early on, according to one expert at the annual meeting of the Foundation for Sickle Cell Disease Research.
“Sickle cell is a common entity in orthopedic surgery, so you shouldn’t hesitate in the hospital or outpatient settings to call for an orthopedic surgeon when you’re dealing with acute pain crises, medullary infarcts, and osteonecrosis,” said Mark W. Bridges, MD, an orthopedic surgeon with Orthopaedic Associates in Southern Florida.
Dr. Bridges noted that the femoral head is the most common location for osteonecrosis, one of the four major orthopedic manifestations of sickle cell disease that he reviewed. The others are septic arthritis, osteomyelitis, and bone infarction.
“Bone infarction is more common than osteomyelitis, and gadolinium-enhanced MRI can help to differentiate the two,” he said.
Osteonecrosis occurs when ischemic cells die, weakening the subchondral bone. Besides the femoral head, osteonecrosis commonly affects the humeral head of the shoulder and the femoral condyles of the knee. Dr. Bridges reviewed the five stages of the Ficat and Arlet classification of osteonecrosis:
- 0 – no pain, normal x-rays.
- I – pain, normal x-rays but abnormal MRI.
- II – pain, abnormal x-ray (sclerosis without collapse).
- III – pain (subchondral collapse without joint degeneration).
- IV – pain (arthritic changes with subchondral collapse).
For osteonecrosis of the shoulder, Dr. Bridges said four surgical options exist: core decompression for stages I and II; humeral head resurfacing for stages II and III; and hemiarthroplasty or total shoulder replacement for stages III and IV.
“No medical therapies are known to slow the progression,” he said.
Total joint replacement can be inevitable in these patients when total collapse of the joint occurs, but Dr. Bridges added a word of caution. “Overall when it comes down to replacing joints, there are more complications in patients that have [sickle cell disease],” he said. “Normally the complication rate is about 1%; that typically goes up to about 10% in SCD patients, but when you have a patient with end-stage disease – shoulder collapse or hip collapse – you have to do something.”
Septic arthritis is an infection within the joint space, most commonly the hip, and it affects 5% of children and 0.3% of adults with sickle cell disease (Clin Orthop Relat Res. 2010;468:1676-81).
“This is very similar to a vaso-occlusive crisis,” Dr. Bridges said.
MRI with gadolinium can help guide treatment, and blood cultures and joint aspiration can identify the infectious microbe. Staphylococcus aureus is the most common, Dr. Bridges said. Treatment consists of IV antibiotics, irrigation, and debridement.
Osteomyelitis is an infection within the bone with symptoms similar to those of septic arthritis, although osteomyelitis patients are typically sicker, he said. MRI with gadolinium is indicated in patients who don’t respond to IV fluid, oxygenation, and nonsteroidal anti-inflammatory drugs. “Try to treat them like they have vaso-occlusive crisis,” he said. Blood cultures usually suffice in these patients; bone aspiration is rarely needed, Dr. Bridges said.
The most common organisms are Staphylococcus aureus and Salmonella, and sickle cell disease patients can have infections in more than one location, Dr. Bridges noted.
“If IV antibiotics don’t work, then these patients need surgical debridement,” he added.
Adults are prone to a higher rate of complications than are children, including joint stiffness, osteonecrosis, pathologic fracture, and chronic osteomyelitis.
Ischemic marrow from vaso-occlusion can result in bone infarction. With its severe pain, swelling, erythema, and loss of motion, bone infarction can appear similar to osteomyelitis and septic arthritis, although a high-grade fever is uncommon in bone infarction. Unlike osteomyelitis, gadolinium on MRI does not enhance in bone infarction.
Treatment “consists of supportive management with pain medications, hydration, and antibiotics until osteomyelitis is ruled out,” Dr. Bridges said.
When a patient with one of these orthopedic conditions needs surgery, there are three considerations: preoperative transfusions to achieve hemoglobin level of 10 mg/dL for major procedures; no transfusions for arthroscopy and small closed reductions; and postoperative oxygenation and hydration to prevent a vaso-occlusive event and acute chest syndrome, he said.
Dr. Bridges reported having no conflicts of interest.
EXPERT ANALYSIS FROM FSCDR 2019
ACIP adds hexavalent vaccine to VFC program
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
The pediatric hexavalent vaccine (DTaP-[inactivated poliovirus] IPV-[hepatitis B] HepB-[Haemophilis influenzae type b] Hib) should be included as an option in the Vaccines for Children (VFC) program for the infant series at ages 2, 4, and 6 months, according to unanimous votes at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The addition of the vaccine to the VFC program required no motions on the part of the committee, but involved separate votes on each component of the vaccine.
Combination vaccination has been associated with increased coverage and more likely completion of the full infant vaccine series, said Sara Oliver, MD, of the CDC’s National Center for Immunization and Respiratory Diseases.
The new vaccine is being developed jointly by Sanofi and Merck, and has been approved by the Food and Drug Administration for use in children through age 4 years.
Dr. Oliver presented evidence that the safety profile of the combination vaccine is consistent with that of the component vaccines. In addition, “use of combination vaccines can reduce the number of injections patient receive and alleviate concern associated with the number of injections,” she said. However, “considerations should include provider assessment, patient preference, and the potential for adverse events.”
although it will not be available until 2021 in order to ensure sufficient supply, Dr. Oliver noted.
The combination vaccination work group considered whether the new vaccine should be preferentially recommended for American Indian and Alaskan Native populations, but they concluded that post–dose one immunogenicity data are needed before such a preferential recommendation can be made.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
ACIP favors shared decision on pneumococcal vaccine for older adults
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
ACIP extends HPV vaccine coverage
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
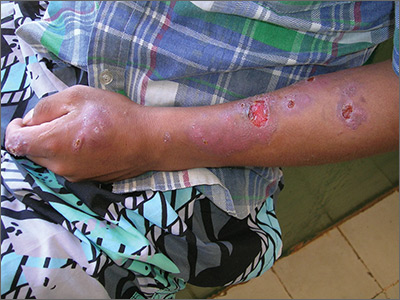
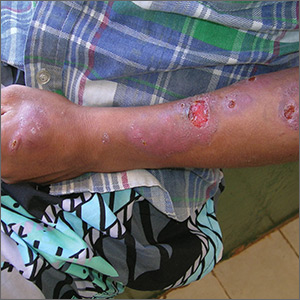
Sores on left arm
The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP noted that a pattern seemed to start on the patient’s second finger and spread up his arm. He considered that this skin disease might be secondary to sporotrichosis (a deep fungal infection, also referred to as rose gardener’s disease).
Sporotrichosis typically spreads up the arm from an inoculation of the hand from a scratch of a rose thorn. The ulcers partially resemble pyoderma gangrenosum, but the edges are neither undermined nor the color of gun metal. While sporotrichosis may be spread to humans through injuries while working with rose bushes, many other plants and animals can carry the organism Sporothrix schenckii.
The FP decided to offer a definitive diagnosis with a fungal culture since sporotrichosis treatment would require months of an oral antifungal agent. Obtaining a fungal culture would require a punch biopsy because the Sporothrix schenckii grows deeply in the tissue and is not reliably found on the skin surface. The mother and patient consented to the procedure and the FP performed a 4-mm punch biopsy on the edge of the largest ulcer on the arm. The specimen was placed in a sterile urine culture cup on sterile gauze with some saline (preservative free). (See the Watch & Learn video on “Punch biopsy.”)
It is important to note that that if the specimen had been sent in standard formalin, a culture could not be performed and histology could miss the dead organism. Clinical suspicion for sporotrichosis was so high in this case that the FP prescribed oral itraconazole 200 mg daily for the next 2 weeks while awaiting the fungal culture result.
The fungal culture grew out Sporothrix schenckii. The FP prescribed itraconazole 200 mg daily for 3 months and planned to continue the therapy until at least 2 to 4 weeks after the lesions had healed. With monthly follow-up visits, the itraconazole treatment lasted 5 months.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux, EJ, Usatine R. Pyoderma gangrenosum. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd Ed. New York, NY: McGraw-Hill; 2019:1147-1152.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Specific prednisone regimen safer than others when used with abiraterone for mCR prostate cancer
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
FROM JAMA ONCOLOGY
Obesity might be targetable driver of psoriatic arthritis progression
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
MADRID – Two sets of data presented at the European Congress of Rheumatology support the potential for weight loss to be a valuable adjunctive strategy for improving outcomes in patients with psoriatic arthritis (PsA).
One set, drawn from the ongoing PsABio observational study, correlated increasing body mass index with greater disease activity and greater disability. Another, based on patients followed for 12 months, showed that a weight loss of about 15% is associated with a significant reduction in PsA activity.
“As clinicians, we largely focus on drugs in the treatment of PsA, but these data draw attention to obesity as a potential target for improving outcomes in PsA,” said Stefan Siebert, MD, a rheumatologist at the Institute of Infection, Immunity, and Inflammation at the University of Glasgow (Scotland).
Dr. Siebert cautioned that his data show association, not causation, but he said these data add to a growing body of evidence that provide compelling support for trials to test the premise that weight loss improves outcomes.
Although not a trial, a study by Eva Klingberg, MD, PhD, of the Sahlgrenska Academy at the University of Gothenburg (Sweden) and her associates tested this premise and showed weight loss was associated with improvement in multiple PsA activity parameters 6 and 12 months after a significant weight loss program.
“This is just one study, so we need more data, but we are already using weight loss to manage PsA in obese patients in Sweden,” said Dr. Klingberg, speaking about her work in advance of the presentation. Like Dr. Siebert, she agreed that weight loss is an important potential treatment strategy in PsA.
In the observational PsABio study, which is following patients with PsA at rheumatology centers in eight European countries, the goal of its analysis was to evaluate disease activity and outcomes in relationship to baseline weight for patients starting a biologic therapy as part of standard clinical practice. Of the 917 patients evaluated, 450 started ustekinumab (Stelara) and 467 started a tumor necrosis factor inhibitor (TNFi). The researchers had weight data for 827 of these patients.
At the time of enrollment, 40% were overweight as defined by a body mass index (BMI) ranging from 25 to 29 kg/m2, and 30.4% were obese as defined by a BMI greater than 30 kg/m2. The mean baseline BMI was 28.1 kg/m2. The mean age of the study population was 49.7 years. Slightly more than half were female.
Relative to a BMI of 30 kg/m2 or less, higher BMI at baseline is shown in multiple regression analysis to be independently and significantly linked to disease activity assessed by the clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA; P = .026), to patient perception of disease impact as measured by Psoriatic Arthritis Impact of Disease (PsAID-12; P less than .0001), and to greater disability as measured with Health Assessment Questionnaire Disability Index (HAQ-DI; P less than .0001).
“There are multiple sets of data that show obesity predicts who develops PsA. Our data further show that, of patients with PsA who are candidates for a biologic, those with obesity have greater disease activity,” Dr. Siebert said. “We are using all of these expensive drugs, but I think there is now a need to also focus on lifestyle interventions, in addition to drug therapy, to reduce disease activity and improve outcomes in PsA.”
The data to be presented by Dr. Klingberg provide a step in that direction. In this study, 46 PsA patients participated in a weight-loss treatment that restricted calorie intake to 640 kcal/day, and the researchers followed 39 of these patients for 1 year. The participants averaged 56 years old, and almost two-thirds were women. All enrolled patients had to have a BMI of at least 33 kg/m2, and the actual average BMI was 35 kg/m2. The median weight loss among the 39 patients followed for 1 year after the start of a 12- to 16-week weight-loss treatment was 16.1 kg, representing about 16% of their body weight at entry.
Dr. Klingberg showed that disease activity in those who achieved and maintained weight loss after the program was significant at 6 and 12 months when measured with the Psoriatic Arthritis Response Criteria (PsARC) or the American College of Rheumatology (ACR) 20, 50, and 70 criteria. In the 39 patients followed for 12 months, 36% fulfilled PsARC, and 54%, 36%, and 15% fulfilled the ACR 20, 50, and 70 responses, respectively.
“In Sweden, any obese individual can be referred for a weight loss program because of the multiple health benefits that are associated with weight reduction,” Dr. Klingberg explained. “We were able to look at patients with PsA and show that this substantially reduces the burden of their joint disease in addition to the other health advantages of losing weight.”
An improvement in symptoms is a logical expectation from reducing the mechanical strain imposed by obesity on inflamed joints, but Dr. Klingberg is more impressed by the potential for weight loss to reduce the proinflammatory signaling generated by adipose tissue. In PsA, there is evidence that weight loss reduces disease activity in the skin, as well as the joints, which supports this link.
“We need more data to document the benefits from weight loss in patients with PsA, but I think management of the comorbidities of PsA, including obesity, is something that should already be routinely discussed with patients,” Dr. Klingberg said.
Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB. Dr. Klingberg has been an advisor to Novartis, a speaker on behalf of Lilly, and has receive research funding from Roche.
Mitchel L. Zoler contributed to this report.
SOURCE: Siebert S et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69. Abstract OP0007. doi: 10.1136/annrheumdis-2019-eular.5841; Klingberg E et al. Ann Rheum Dis. Jun 2019;78(suppl 2):69-70. Abstract OP0008. doi: 10.1136/annrheumdis-2019-eular.5551.
REPORTING FROM EULAR 2019 CONGRESS
Substantial reductions in HPV infections, CIN2+ after vaccination
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
The introduction of the human papillomavirus according to a meta-analysis of data from more than 60 million individuals worldwide.
Mélanie Drolet, PhD, from the Centre de recherche du CHU de Québec–Université Laval, and coauthors of the HPV Vaccination Impact Study Group reported the results of a systematic review and meta-analysis of 65 studies showing pre- and postvaccination frequency of at least one HPV-related endpoint published in the Lancet. The studies were conducted in 14 high-income countries, 12 of which were vaccinating only women and girls, with the results at 5-8 years published in the Lancet.
At 5-8 years after a vaccination program was implemented, there was a significant 83% reduction in the prevalence of HPV 16 and 18, both of which are targeted by the vaccine, among girls aged 13-19 years; a 66% reduction among women aged 20-24 years; and a 37% reduction in women aged 25-29 years, even though most of these women were unvaccinated.
There also were significant decreases at 5-8 years in the prevalence of HPV subtypes 31, 33, and 45, which are not included in the vaccine but against which the vaccine appears to offer cross-protection. Among girls aged 13-19 years, there was a significant 54% reduction in the prevalence of these subtypes, among women aged 20-24 years there was a nonsignificant 28% decrease, but among women aged 25-29 years, there was no significant decrease.
The analysis also found significant declines in the prevalence of cervical intraepithelial neoplasias (CINs) of grade 2 or above. At 5-9 years after vaccination was introduced, CIN2+ decreased by 51% among girls aged 15-19 years who also were screened for cervical cancer, and by 31% among women aged 20-24 years.
However, over the same time period, the rates of CIN2+ increased by a significant 19% among mostly unvaccinated women aged 25-29 years and 23% among mostly unvaccinated women aged 30-39 years, despite both groups being screened for cervical abnormalities.
While most of the countries in the study vaccinated only girls and women, two studies did find nonsignificant decreases in the prevalence of HPV 16, 18, 31, 33, and 45 among boys aged 16-19 years, but not among men aged 20-24 years.
HPV vaccination also was associated with significant declines in the incidence of anogenital warts among both males and females. In the first 4 years alone, vaccination was associated with significant reductions in anogenital wart diagnoses among females aged 15-29 years, as well as nonsignificant but “substantial” reductions in unvaccinated boys aged 15-19 years.
After 5-8 years, anogenital wart diagnoses decreased by 67% among girls aged 15-19 years, significantly by 54% among women aged 20-24 years, and 31% among women aged 25-29 years – all significant changes. Among boys aged 15-19 years, anogenital wart diagnoses decreased by a significant 48%, and among men aged 20-24 years they decreased by a significant 32%.
The decreases in anogenital wart diagnoses were even greater in countries that implemented vaccination among multiple cohorts simultaneously and achieved high vaccination coverage, compared with countries that vaccinated only one cohort at a time or had low routine vaccination coverage.
“Our study is the first to show the real-world additional benefit of multicohort HPV vaccination and high routine vaccination coverage, and the fast and substantial herd effects of vaccination in countries which implement these measures,” wrote Dr. Drolet and coauthors. “The greater impact of multicohort vaccination was similar when restricting the analyses to countries with high routine vaccination coverage.”
They pointed to the World Health Organization’s recently revised position on HPV vaccination, which now recommends vaccination of multiple cohorts of girls aged 9-14 years, although they raised the question of what might be the optimal number of age cohorts. “Number needed to vaccinate and cost-effectiveness analyses in high-income countries suggest that vaccinating multiple cohorts of individuals up to 18 years of age is highly efficient and cost effective.”
This analysis by Drolet et al. “provides compelling evidence for HPV vaccine efficacy on all outcomes explored and for almost all age strata,” Dr. Silvia de Sanjose, of PATH in Seattle, and Dr. Sinead Delany-Moretlwe of the Wits Reproductive Health and HIV Institute at the University of Witwatersrand in Johannesburg, said in an accompanying editorial (Lancet. 2019 Jun 26. doi: 10.1016/ S0140-6736[19]30549-5). This study shows just how effective HPV vaccination can be across a range of outcomes and ages, and also demonstrates the herd immunity benefits, particularly when multiple cohorts are vaccinated and there is high vaccination coverage.
One key limitation of this analysis is the lack of data from low- and middle-income countries. The data by Drolet et al. “emphasise the importance of redoubling our efforts to tackle the fiscal, supply, and programmatic barriers that currently limit HPV vaccine programmes; with these efforts, HPV vaccination could become a hallmark investment of cancer prevention in the 21st century,” Dr. de Sanjose and Dr. Delany-Moretlwe concluded.
The study was funded by WHO, Canadian Institutes of Health Research, and Fonds de recherche du Québec–Santé. No conflicts of interest were declared.
Dr. de Sanjose declared previous institutional support from Merck.
SOURCE: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
FROM THE LANCET
Key clinical point: Significant declines in HPV infections, CIN2+, and anogenital warts have occurred after the introduction of HPV vaccine programs, some because of herd effects.
Major finding: The HPV vaccination program is associated with a significant 83% reduction in the prevalence of HPV 16 and 18 among girls aged 13-19 years in 14 high-income countries.
Study details: Systematic review and meta-analysis of 65 studies involving more than 60 million individuals in 14 countries.
Disclosures: The study was funded by World Health Organization, Canadian Institutes of Health Research, and Fonds de recherche du Québec – Santé. No conflicts of interest were declared.
Source: Drolet M et al. Lancet 2019 Jun 26. doi: 10.1016/ S0140-6736(19)30298-3.
Surprise billing legislation passes Senate committee
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
A bill aimed at ending the practice of surprise billing, along with a number of other health care cost-containment measures, passed by an overwhelming majority during a mark-up session of the Senate Health, Education, Labor, and Pensions Committee.
S. 1895, the Lower Health Care Costs Act of 2019, passed 20-3; Sens. Rand Paul (R-Ky.), Bernie Sanders (D-Ver.) and Elizabeth Warren (D-Mass.) voted against it.
A summary of the bill’s provisions can be found here.
With Sen. Sanders and Sen. Warren not present, presumably out to prepare for the Democratic presidential nominee debates and voting by proxy, only Sen. Paul was present to speak against the bill. He questioned whether it would have any impact on lowering health care cost.
Among other provisions, the bill would ban all gag clauses that would keep pricing data from being released; all anticompetitive clauses in facility and insurance contracts that would otherwise limit access to higher-quality, lower-cost care; would designate a nongovernment entity focused on price transparency; and would improve the accuracy of directory information.
But government-induced transparency is not the solution, Sen. Paul said.
He called it a “fallacy” that “you can mandate transparency, and you’ll create a marketplace.” Rather, you need to create a marketplace, and transparency will naturally follow, he said.
Sen. Paul noted that just having institutions publishing prices that no one pays and prices that are not freely fluctuating “doesn’t work.”
“The irony here is that, when you have no insurance involved, you actually have a marketplace,” he said. “The people without insurance are the only true marketplace,” he said, adding that those with high deductibles would also fall into that category.
The crux of S. 1895 is protections to end so-called “surprise bills” that occur when patients receive medical services from out-of-network health care professionals at in-network hospitals. These out-of-network services are not constrained by prior agreements and can add up to tens of thousands of dollars.
“There are those who have seen the history of price controls and know that you never get what you intended,” he said, and predicted that this could lead to a shortage of physicians.
The American Medical Association also criticized the surprise billing provisions of the bill.
In a June 25 letter, the AMA noted that “the approach outlined in S. 1895 fails to address some of the fundamental reasons why surprise billing occurs – inadequate provider networks, higher patient-cost sharing requirements for out-of-network services, and noncompetitive local markets that empower plans to offer take-it-or-leave-it contracts.”
AMA also criticized the use of benchmark pricing to settle out-of-network billing issues. “By setting a payment maximum at the individual plans’ median in-network amount, insurers will have even less incentive to negotiate contracts with individual providers,” according to the letter. “They can drive down the median in-network amount by simply dropping from their networks providers who are currently paid above the median. Or, they can simply stop negotiating altogether, knowing that their financial obligation is limited to their own median in-network payment amounts.”
The Physicians Advocacy Institute agreed. In a June 26 statement, the organization stated that it remains “deeply concerned that arbitrary, government-set payment benchmarks championed by the health insurance industry will further undermine provider networks and devastate patients’ access to critical medical services.”
A collective of hospital organizations, including the Federation of American Hospitals and the American Hospital Association also opposed the use of benchmark pricing.
In a June 25 letter to committee leadership, the groups stated that they are “concerned that the rate-setting provision of the legislation is a plan-determined, nontransparent process that will upend private payment negotiation. A default rate will become the payment ceiling and remove incentives for insurers to develop comprehensive networks, as there are already increasing numbers of narrow network products offered that exclude certain types of providers.”
The bill also addresses the cost of prescription drugs, including providing clearer information about patents, ensuring a more timely access to generics, altering exclusivity rules to help get generics to market quicker, reporting requirements for price increases, and a number of other provisions aimed at increasing competition in an effort to lower drug pricing.
Other areas covered by the bill include more oversight of pharmacy benefit managers, strengthening parity in mental health laws, and a number of provisions aimed at public health and health information technology.
REPORTING FROM A SENATE COMMITTEE MEETING