User login
‘Robust antitumor immune responses’ observed in pediatric ALL
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.
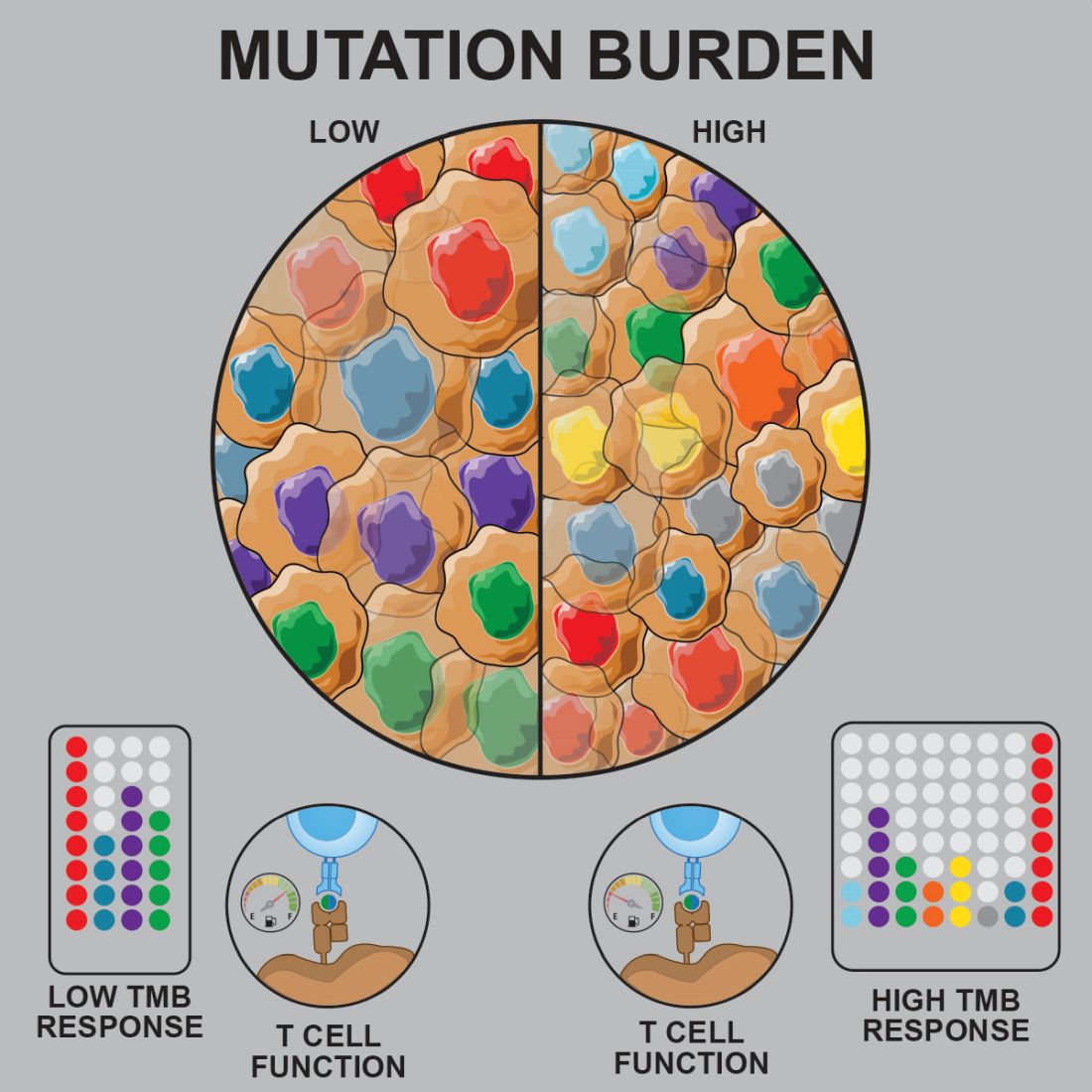
Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Pediatric acute lymphoblastic leukemia (ALL) may be more vulnerable to immunotherapies than previously thought, according to researchers.

Prior studies suggested that tumors with a low mutational burden don’t elicit strong antitumor responses and therefore aren’t very susceptible to immunotherapy.
Now, researchers have found evidence to suggest that pediatric ALL induces “robust antitumor immune responses” despite a low mutational burden. The investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Anthony E. Zamora, PhD, of St. Jude Children’s Research Hospital in Memphis, Tenn., and colleagues recounted these findings in Science Translational Medicine.
The researchers analyzed samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2) to determine how endogenous CD8+ T cells respond to patient-specific cancer neoantigens.
The investigators first assessed the ability of tumor-specific mutations and gene fusions to generate neoepitopes, or neoantigens predicted to bind patient-specific human leukocyte antigen (HLA) proteins. The team identified 5-28 neoepitopes per patient, including epitopes that spanned the fusion junction in patients with ETV6-RUNX1 fusions.
The researchers then tested whether CD8+ tumor infiltrating lymphocytes (TILs) were directly responsive to mutated neoepitopes. They observed cytokine responses across patient samples, noting that 31 of the 36 putative neoantigens tested (86%) were “immunogenic and capable of inducing robust cytokine responses.”
Next, the investigators mapped TIL responses to specific epitopes using patient-specific tetramers that corresponded to the previously identified neoepitopes. Seventeen of the 25 patient-specific tetramers (68%) bound to TILs above the background set by irrelevant HLA-matched tetramers.
“Within those responses, we observed immunodominance hierarchies among the distinct TIL populations, with a majority of tetramer-bound CD8+ T cells restricted to one or two putative neoepitopes,” the researchers noted.
The team also pointed out that seven of nine patients tested had CD8+ T cells that responded to ETV6-RUNX1.
Finally, the investigators performed transcriptional profiling of ALL-specific CD8+ TILs to assess inter- and intrapatient heterogeneity. The team identified three hierarchical clusters, which were characterized by transcriptional factors and regulators associated with:
- Functional effector CD8+ T cells (TBX21 and EOMES).
- Dysfunctional CD8+ T cells (STAT1/3/4, NR4A2/3, and BCL6).
- Exhausted CD8+ T cells (EOMES, MAF, PRDM1, and BATF).
Considering these findings together, the researchers concluded that “pediatric ALL elicits a potent neoepitope-specific CD8+ T-cell response.” Therefore, adoptive T-cell, monoclonal antibody, and targeted T-cell receptor therapies “should be explored” in pediatric ALL.
This research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
SOURCE: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Preclinical research suggests pediatric acute lymphoblastic leukemia (ALL) induces “robust antitumor immune responses” despite a low mutational burden.
Major finding: Investigators identified tumor-associated CD8+ T cells that responded to 86% of neoantigens tested and recognized 68% of neoepitopes tested.
Study details: Analysis of samples from pediatric patients with ETV-associated ALL (n = 9) or ERG-associated ALL (n = 2).
Disclosures: The research was supported by the National Institutes of Health, National Cancer Institute, National Institute of General Medical Sciences, Key for a Cure Foundation, and American Lebanese Syrian Associated Charities. The researchers disclosed patent applications and relationships with Pfizer, Amgen, and other companies.
Source: Zamora AE et al. Sci. Transl. Med. 2019 Jun 26. doi: 10.1126/scitranslmed.aat8549.
Leflunomide added to glucocorticoids reduces relapse in IgG4-related disease
MADRID – The addition of leflunomide to standard glucocorticoids (GCs) in the treatment of IgG4-related disease increases the median duration of response, reduces the proportion of patients with relapse within 12 months, and permits GCs to be tapered, according to results of a randomized trial presented at the European Congress of Rheumatology.
“The rate of adverse events with the addition of leflunomide was numerically higher, but there were no significant differences in risks of any specific adverse event,” reported Feng Huang, MD, of the department of rheumatology at Chinese People’s Liberation Army General Hospital in Beijing.
GCs are highly effective in IgG4-related disease, which is an autoimmune process driven by elevated concentrations of the antibody IgG4 in the tissue of affected organs and in the serum. It has been described in a broad array of sites, including the heart, lung, kidneys, and meninges. It has been widely recognized only in the last 10 years, according to Dr. Huang. Although most patients respond to GCs, he said the problem is that about 50% of patients relapse within 12 months and more than 90% within 3 years.
This randomized, controlled study was conducted after positive results were observed with leflunomide in a small, uncontrolled pilot study published several years ago (Intern Med J. 2017 Jun;47[6]:680-9. doi: 10.1111/imj.13430). In this randomized trial, the objectives were to confirm that leflunomide extends the relapse-free period and has acceptable safety relative to GC alone.
Patients with confirmed IgG4-related disease were enrolled. Patients randomized to GC were started on 0.5 to 0.8 mg/kg per day. A predefined taper regimen was employed in those with symptom control. Those randomized to the experimental arm received GC in the same dose and schedule plus 20 mg/day of leflunomide.
The 33 patients in each group were well matched at baseline for age, comorbidities, and disease severity.
At the end of 12 months, 50% of those treated with GC alone versus 21.2% of those treated with GC plus leflunomide had relapse. That translated into a significantly higher hazard ratio (HR) for relapse in the GC monotherapy group (HR, 1.75; P = .034).
The mean duration of remission was 7 months on the combination versus 3 months on GC alone. Dr. Huang also reported a significantly higher proportion of complete responses in the group receiving the combination.
In addition, “more patients on the combination therapy were able to adhere to the steroid-tapering schedule without relapse,” Dr. Huang reported. The rate of 54.5% of patients on combination therapy who were able to reach a daily GC dose of 5 mg/day or less proved significantly higher than the 18.2% rate seen with GC alone (P = .002).
Adverse events were reported by 54% of those on the combination versus 42% of those on monotherapy, but this difference did not reach statistical significance. The biggest differences in adverse events were the proportions of patients with infections (18.2% vs. 12.1%) and elevated liver enzymes (12.1% vs. 3.0%), both of which were more common in the combination therapy group. Neither of these differences was statistically significant.
Of patients with relapses, the most common organs involved were the salivary gland, the pancreas, and the bile ducts, each accounting for relapse in five patients. Other organs in which relapse occurred included the lacrimal gland and the skin. There were three cases of relapse characterized by retroperitoneal fibrosis.
Over the course of follow-up, new-onset diabetes mellitus occurred in 21.2% and 27.3% of the combination and GC-only groups, respectively. This difference also did not reach statistical significance.
Although this study was small with an open-label design, Dr. Huang said the data strongly suggest that a combination of leflunomide and GC is superior to GC alone. Based on these results, he said a starting dose of 20 mg/day of leflunomide is a reasonable standard in this setting.
Dr. Huang and colleagues reported no potential conflicts of interest.
SOURCE: Wang Y et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):157. Abstract OPO164, doi: 10.1136/annrheumdis-2019-eular.5717
MADRID – The addition of leflunomide to standard glucocorticoids (GCs) in the treatment of IgG4-related disease increases the median duration of response, reduces the proportion of patients with relapse within 12 months, and permits GCs to be tapered, according to results of a randomized trial presented at the European Congress of Rheumatology.
“The rate of adverse events with the addition of leflunomide was numerically higher, but there were no significant differences in risks of any specific adverse event,” reported Feng Huang, MD, of the department of rheumatology at Chinese People’s Liberation Army General Hospital in Beijing.
GCs are highly effective in IgG4-related disease, which is an autoimmune process driven by elevated concentrations of the antibody IgG4 in the tissue of affected organs and in the serum. It has been described in a broad array of sites, including the heart, lung, kidneys, and meninges. It has been widely recognized only in the last 10 years, according to Dr. Huang. Although most patients respond to GCs, he said the problem is that about 50% of patients relapse within 12 months and more than 90% within 3 years.
This randomized, controlled study was conducted after positive results were observed with leflunomide in a small, uncontrolled pilot study published several years ago (Intern Med J. 2017 Jun;47[6]:680-9. doi: 10.1111/imj.13430). In this randomized trial, the objectives were to confirm that leflunomide extends the relapse-free period and has acceptable safety relative to GC alone.
Patients with confirmed IgG4-related disease were enrolled. Patients randomized to GC were started on 0.5 to 0.8 mg/kg per day. A predefined taper regimen was employed in those with symptom control. Those randomized to the experimental arm received GC in the same dose and schedule plus 20 mg/day of leflunomide.
The 33 patients in each group were well matched at baseline for age, comorbidities, and disease severity.
At the end of 12 months, 50% of those treated with GC alone versus 21.2% of those treated with GC plus leflunomide had relapse. That translated into a significantly higher hazard ratio (HR) for relapse in the GC monotherapy group (HR, 1.75; P = .034).
The mean duration of remission was 7 months on the combination versus 3 months on GC alone. Dr. Huang also reported a significantly higher proportion of complete responses in the group receiving the combination.
In addition, “more patients on the combination therapy were able to adhere to the steroid-tapering schedule without relapse,” Dr. Huang reported. The rate of 54.5% of patients on combination therapy who were able to reach a daily GC dose of 5 mg/day or less proved significantly higher than the 18.2% rate seen with GC alone (P = .002).
Adverse events were reported by 54% of those on the combination versus 42% of those on monotherapy, but this difference did not reach statistical significance. The biggest differences in adverse events were the proportions of patients with infections (18.2% vs. 12.1%) and elevated liver enzymes (12.1% vs. 3.0%), both of which were more common in the combination therapy group. Neither of these differences was statistically significant.
Of patients with relapses, the most common organs involved were the salivary gland, the pancreas, and the bile ducts, each accounting for relapse in five patients. Other organs in which relapse occurred included the lacrimal gland and the skin. There were three cases of relapse characterized by retroperitoneal fibrosis.
Over the course of follow-up, new-onset diabetes mellitus occurred in 21.2% and 27.3% of the combination and GC-only groups, respectively. This difference also did not reach statistical significance.
Although this study was small with an open-label design, Dr. Huang said the data strongly suggest that a combination of leflunomide and GC is superior to GC alone. Based on these results, he said a starting dose of 20 mg/day of leflunomide is a reasonable standard in this setting.
Dr. Huang and colleagues reported no potential conflicts of interest.
SOURCE: Wang Y et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):157. Abstract OPO164, doi: 10.1136/annrheumdis-2019-eular.5717
MADRID – The addition of leflunomide to standard glucocorticoids (GCs) in the treatment of IgG4-related disease increases the median duration of response, reduces the proportion of patients with relapse within 12 months, and permits GCs to be tapered, according to results of a randomized trial presented at the European Congress of Rheumatology.
“The rate of adverse events with the addition of leflunomide was numerically higher, but there were no significant differences in risks of any specific adverse event,” reported Feng Huang, MD, of the department of rheumatology at Chinese People’s Liberation Army General Hospital in Beijing.
GCs are highly effective in IgG4-related disease, which is an autoimmune process driven by elevated concentrations of the antibody IgG4 in the tissue of affected organs and in the serum. It has been described in a broad array of sites, including the heart, lung, kidneys, and meninges. It has been widely recognized only in the last 10 years, according to Dr. Huang. Although most patients respond to GCs, he said the problem is that about 50% of patients relapse within 12 months and more than 90% within 3 years.
This randomized, controlled study was conducted after positive results were observed with leflunomide in a small, uncontrolled pilot study published several years ago (Intern Med J. 2017 Jun;47[6]:680-9. doi: 10.1111/imj.13430). In this randomized trial, the objectives were to confirm that leflunomide extends the relapse-free period and has acceptable safety relative to GC alone.
Patients with confirmed IgG4-related disease were enrolled. Patients randomized to GC were started on 0.5 to 0.8 mg/kg per day. A predefined taper regimen was employed in those with symptom control. Those randomized to the experimental arm received GC in the same dose and schedule plus 20 mg/day of leflunomide.
The 33 patients in each group were well matched at baseline for age, comorbidities, and disease severity.
At the end of 12 months, 50% of those treated with GC alone versus 21.2% of those treated with GC plus leflunomide had relapse. That translated into a significantly higher hazard ratio (HR) for relapse in the GC monotherapy group (HR, 1.75; P = .034).
The mean duration of remission was 7 months on the combination versus 3 months on GC alone. Dr. Huang also reported a significantly higher proportion of complete responses in the group receiving the combination.
In addition, “more patients on the combination therapy were able to adhere to the steroid-tapering schedule without relapse,” Dr. Huang reported. The rate of 54.5% of patients on combination therapy who were able to reach a daily GC dose of 5 mg/day or less proved significantly higher than the 18.2% rate seen with GC alone (P = .002).
Adverse events were reported by 54% of those on the combination versus 42% of those on monotherapy, but this difference did not reach statistical significance. The biggest differences in adverse events were the proportions of patients with infections (18.2% vs. 12.1%) and elevated liver enzymes (12.1% vs. 3.0%), both of which were more common in the combination therapy group. Neither of these differences was statistically significant.
Of patients with relapses, the most common organs involved were the salivary gland, the pancreas, and the bile ducts, each accounting for relapse in five patients. Other organs in which relapse occurred included the lacrimal gland and the skin. There were three cases of relapse characterized by retroperitoneal fibrosis.
Over the course of follow-up, new-onset diabetes mellitus occurred in 21.2% and 27.3% of the combination and GC-only groups, respectively. This difference also did not reach statistical significance.
Although this study was small with an open-label design, Dr. Huang said the data strongly suggest that a combination of leflunomide and GC is superior to GC alone. Based on these results, he said a starting dose of 20 mg/day of leflunomide is a reasonable standard in this setting.
Dr. Huang and colleagues reported no potential conflicts of interest.
SOURCE: Wang Y et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):157. Abstract OPO164, doi: 10.1136/annrheumdis-2019-eular.5717
REPORTING FROM EULAR 2019 CONGRESS
Becoming a high-value care physician
‘Culture shift’ comes from collective efforts
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
‘Culture shift’ comes from collective efforts
‘Culture shift’ comes from collective efforts
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
Question Marks Lead to Dollar Signs
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
Despite advances, imaging of axSpA remains an adjunctive tool
MADRID – Evidence for always using imaging in an adjunctive role to clinical findings in the diagnosis and assessment of axial spondyloarthritis (axSpA) continues to grow, two experts agreed in a scientific session at the European Congress of Rheumatology.
“Imaging has to be understood in the context of other findings. With the patient history, the physical examination, and the laboratory results, the value of imaging improves substantially. Therefore, before an image is ordered it is important to ask how likely is it that a patient has axial spondylitis,” said Floris A. van Gaalen, MD, PhD, of Leiden (Netherlands) University Medical Center.
As one of the experts who participated in the scientific session, Dr. van Gaalen focused specifically on the value of x-ray and MRI in the diagnosis of axSpA, emphasizing their limited value if interpreted without clinical context. He explained that even highly experienced radiologists are fooled, particularly at early stages of disease.
Although the quality of imaging has been increasing steadily, “there is no cookbook approach with which you can guarantee a diagnosis of spondyloarthritis. Imaging can be valuable, but there is a risk of false positives because features on imaging, such as bone marrow edema, are shared with other sources of back pain,” Dr. van Gaalen said.
Considering the importance of context, Dr. van Gaalen advised clinicians against reading the radiology report without evaluating the images themselves. He said the features on imaging make more sense when they are considered at the same time as the patient’s history, symptoms, and laboratory reports.
Order imaging relevant to treatment decisions
Assigned to discuss the value of imaging for assessing progression, Xenofon Baraliakos, MD, a rheumatologist and clinical researcher at Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, Herne, Germany, offered the same message.
“It is important to consider all of the clinical information available, not just the features on imaging,” Dr. Baraliakos said. Often, MRI findings provide corroboration for other objective measures of disease status, but Dr. Baraliakos advised that imaging should be ordered only when it has the potential to alter therapy.
“What we can learn from imaging might be interesting, but the question to ask is whether it is useful,” Dr. Baraliakos said. Rather than incurring the costs of imaging for reassurance, Dr. Baraliakos recommended ordering these studies with specific objectives relevant to treatment decisions.
Neither Dr. van Gaalen nor Dr. Baraliakos denied the value of imaging, particularly MRI, to increase confidence in the diagnosis of axSpA or to guide therapy. Rather, their point was that imaging should not be considered a reliable stand-alone axSpA assessment strategy.
Clinical and imaging findings better then imaging alone
Data from a blinded radiology study presented during the same scientific session reinforced this conclusion. Led by Dr. Baraliakos and presented separately from his discussion about the adjunctive nature of imaging data in axSpA, the study showed that rheumatologists with access to both clinical and imaging data can detect a greater proportion of axSpA than radiologists working from imaging data alone.
In this study, 300 consecutive patients suspected of axSpA were enrolled. All had chronic back pain of more than 3 months’ duration. While highly experienced radiologists were asked to diagnose or rule out a diagnosis of axSpA on the basis of the MRI blinded to other clinical information, experienced rheumatologists evaluated the patients with access to all clinical, laboratory, and imaging data.
A diagnosis of axSpA was reached in 131 patients by the rheumatologists. The remaining 169 were determined not to have axSpA. Although the radiologists agreed on those with or without axSpA in 86.3% of cases, there were 31 cases (28.1%) in which rheumatologists diagnosed axSpA but radiologists did not.
In an analysis of which MRI features were considered critical by radiologists when there was agreement, they identified bone marrow edema in seven cases (7.2%). In 30 cases (30.9%), the radiologists considered the presence of chronic lesions to be critical to their diagnosis. In the remaining 69.9% of cases, radiologists were confident in their diagnosis only when both bone edema and chronic lesions were present.
Not surprisingly, the presence of chronic lesions and more pronounced bone marrow edema permitted both radiologists and rheumatologists to increase their confidence when discriminating between axSpA and non-axSpA patients.
“The combination of structural changes and bone marrow edema as assessed by MRI performed best in the process of diagnosing or ruling out axSpA in this real-life setting at our center,” Dr. Baraliakos said.
However, when only one or two features are considered, trade-offs of lower sensitivity for higher specificity or higher sensitivity for lower specificity occur. For example, although the specificity for a diagnosis of axSpA reached 99.4% when both bone marrow edema and ankylosis are present, the sensitivity of this finding was only 5.3%, according to data provided by Dr. Baraliakos. Conversely, the presence of sclerosis had a sensitivity of 81.7% but a specificity of only 43.2%.
One lesson from this analysis is that there is “increasing insecurity of only including bone marrow edema of the sacroiliac joint as the major criterion for diagnosing axSpA,” Dr. Baraliakos said. However, the larger point in the context of the earlier expert comments is that MRI findings should be considered important but insufficient for the evaluation of axSpA.
SOURCE: Baraliakos X et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):255-6. Abstract OPO344, doi: 10.1136/annrheumdis-2019-eular.5027
MADRID – Evidence for always using imaging in an adjunctive role to clinical findings in the diagnosis and assessment of axial spondyloarthritis (axSpA) continues to grow, two experts agreed in a scientific session at the European Congress of Rheumatology.
“Imaging has to be understood in the context of other findings. With the patient history, the physical examination, and the laboratory results, the value of imaging improves substantially. Therefore, before an image is ordered it is important to ask how likely is it that a patient has axial spondylitis,” said Floris A. van Gaalen, MD, PhD, of Leiden (Netherlands) University Medical Center.
As one of the experts who participated in the scientific session, Dr. van Gaalen focused specifically on the value of x-ray and MRI in the diagnosis of axSpA, emphasizing their limited value if interpreted without clinical context. He explained that even highly experienced radiologists are fooled, particularly at early stages of disease.
Although the quality of imaging has been increasing steadily, “there is no cookbook approach with which you can guarantee a diagnosis of spondyloarthritis. Imaging can be valuable, but there is a risk of false positives because features on imaging, such as bone marrow edema, are shared with other sources of back pain,” Dr. van Gaalen said.
Considering the importance of context, Dr. van Gaalen advised clinicians against reading the radiology report without evaluating the images themselves. He said the features on imaging make more sense when they are considered at the same time as the patient’s history, symptoms, and laboratory reports.
Order imaging relevant to treatment decisions
Assigned to discuss the value of imaging for assessing progression, Xenofon Baraliakos, MD, a rheumatologist and clinical researcher at Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, Herne, Germany, offered the same message.
“It is important to consider all of the clinical information available, not just the features on imaging,” Dr. Baraliakos said. Often, MRI findings provide corroboration for other objective measures of disease status, but Dr. Baraliakos advised that imaging should be ordered only when it has the potential to alter therapy.
“What we can learn from imaging might be interesting, but the question to ask is whether it is useful,” Dr. Baraliakos said. Rather than incurring the costs of imaging for reassurance, Dr. Baraliakos recommended ordering these studies with specific objectives relevant to treatment decisions.
Neither Dr. van Gaalen nor Dr. Baraliakos denied the value of imaging, particularly MRI, to increase confidence in the diagnosis of axSpA or to guide therapy. Rather, their point was that imaging should not be considered a reliable stand-alone axSpA assessment strategy.
Clinical and imaging findings better then imaging alone
Data from a blinded radiology study presented during the same scientific session reinforced this conclusion. Led by Dr. Baraliakos and presented separately from his discussion about the adjunctive nature of imaging data in axSpA, the study showed that rheumatologists with access to both clinical and imaging data can detect a greater proportion of axSpA than radiologists working from imaging data alone.
In this study, 300 consecutive patients suspected of axSpA were enrolled. All had chronic back pain of more than 3 months’ duration. While highly experienced radiologists were asked to diagnose or rule out a diagnosis of axSpA on the basis of the MRI blinded to other clinical information, experienced rheumatologists evaluated the patients with access to all clinical, laboratory, and imaging data.
A diagnosis of axSpA was reached in 131 patients by the rheumatologists. The remaining 169 were determined not to have axSpA. Although the radiologists agreed on those with or without axSpA in 86.3% of cases, there were 31 cases (28.1%) in which rheumatologists diagnosed axSpA but radiologists did not.
In an analysis of which MRI features were considered critical by radiologists when there was agreement, they identified bone marrow edema in seven cases (7.2%). In 30 cases (30.9%), the radiologists considered the presence of chronic lesions to be critical to their diagnosis. In the remaining 69.9% of cases, radiologists were confident in their diagnosis only when both bone edema and chronic lesions were present.
Not surprisingly, the presence of chronic lesions and more pronounced bone marrow edema permitted both radiologists and rheumatologists to increase their confidence when discriminating between axSpA and non-axSpA patients.
“The combination of structural changes and bone marrow edema as assessed by MRI performed best in the process of diagnosing or ruling out axSpA in this real-life setting at our center,” Dr. Baraliakos said.
However, when only one or two features are considered, trade-offs of lower sensitivity for higher specificity or higher sensitivity for lower specificity occur. For example, although the specificity for a diagnosis of axSpA reached 99.4% when both bone marrow edema and ankylosis are present, the sensitivity of this finding was only 5.3%, according to data provided by Dr. Baraliakos. Conversely, the presence of sclerosis had a sensitivity of 81.7% but a specificity of only 43.2%.
One lesson from this analysis is that there is “increasing insecurity of only including bone marrow edema of the sacroiliac joint as the major criterion for diagnosing axSpA,” Dr. Baraliakos said. However, the larger point in the context of the earlier expert comments is that MRI findings should be considered important but insufficient for the evaluation of axSpA.
SOURCE: Baraliakos X et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):255-6. Abstract OPO344, doi: 10.1136/annrheumdis-2019-eular.5027
MADRID – Evidence for always using imaging in an adjunctive role to clinical findings in the diagnosis and assessment of axial spondyloarthritis (axSpA) continues to grow, two experts agreed in a scientific session at the European Congress of Rheumatology.
“Imaging has to be understood in the context of other findings. With the patient history, the physical examination, and the laboratory results, the value of imaging improves substantially. Therefore, before an image is ordered it is important to ask how likely is it that a patient has axial spondylitis,” said Floris A. van Gaalen, MD, PhD, of Leiden (Netherlands) University Medical Center.
As one of the experts who participated in the scientific session, Dr. van Gaalen focused specifically on the value of x-ray and MRI in the diagnosis of axSpA, emphasizing their limited value if interpreted without clinical context. He explained that even highly experienced radiologists are fooled, particularly at early stages of disease.
Although the quality of imaging has been increasing steadily, “there is no cookbook approach with which you can guarantee a diagnosis of spondyloarthritis. Imaging can be valuable, but there is a risk of false positives because features on imaging, such as bone marrow edema, are shared with other sources of back pain,” Dr. van Gaalen said.
Considering the importance of context, Dr. van Gaalen advised clinicians against reading the radiology report without evaluating the images themselves. He said the features on imaging make more sense when they are considered at the same time as the patient’s history, symptoms, and laboratory reports.
Order imaging relevant to treatment decisions
Assigned to discuss the value of imaging for assessing progression, Xenofon Baraliakos, MD, a rheumatologist and clinical researcher at Rheumazentrum Ruhrgebiet, Ruhr-University Bochum, Herne, Germany, offered the same message.
“It is important to consider all of the clinical information available, not just the features on imaging,” Dr. Baraliakos said. Often, MRI findings provide corroboration for other objective measures of disease status, but Dr. Baraliakos advised that imaging should be ordered only when it has the potential to alter therapy.
“What we can learn from imaging might be interesting, but the question to ask is whether it is useful,” Dr. Baraliakos said. Rather than incurring the costs of imaging for reassurance, Dr. Baraliakos recommended ordering these studies with specific objectives relevant to treatment decisions.
Neither Dr. van Gaalen nor Dr. Baraliakos denied the value of imaging, particularly MRI, to increase confidence in the diagnosis of axSpA or to guide therapy. Rather, their point was that imaging should not be considered a reliable stand-alone axSpA assessment strategy.
Clinical and imaging findings better then imaging alone
Data from a blinded radiology study presented during the same scientific session reinforced this conclusion. Led by Dr. Baraliakos and presented separately from his discussion about the adjunctive nature of imaging data in axSpA, the study showed that rheumatologists with access to both clinical and imaging data can detect a greater proportion of axSpA than radiologists working from imaging data alone.
In this study, 300 consecutive patients suspected of axSpA were enrolled. All had chronic back pain of more than 3 months’ duration. While highly experienced radiologists were asked to diagnose or rule out a diagnosis of axSpA on the basis of the MRI blinded to other clinical information, experienced rheumatologists evaluated the patients with access to all clinical, laboratory, and imaging data.
A diagnosis of axSpA was reached in 131 patients by the rheumatologists. The remaining 169 were determined not to have axSpA. Although the radiologists agreed on those with or without axSpA in 86.3% of cases, there were 31 cases (28.1%) in which rheumatologists diagnosed axSpA but radiologists did not.
In an analysis of which MRI features were considered critical by radiologists when there was agreement, they identified bone marrow edema in seven cases (7.2%). In 30 cases (30.9%), the radiologists considered the presence of chronic lesions to be critical to their diagnosis. In the remaining 69.9% of cases, radiologists were confident in their diagnosis only when both bone edema and chronic lesions were present.
Not surprisingly, the presence of chronic lesions and more pronounced bone marrow edema permitted both radiologists and rheumatologists to increase their confidence when discriminating between axSpA and non-axSpA patients.
“The combination of structural changes and bone marrow edema as assessed by MRI performed best in the process of diagnosing or ruling out axSpA in this real-life setting at our center,” Dr. Baraliakos said.
However, when only one or two features are considered, trade-offs of lower sensitivity for higher specificity or higher sensitivity for lower specificity occur. For example, although the specificity for a diagnosis of axSpA reached 99.4% when both bone marrow edema and ankylosis are present, the sensitivity of this finding was only 5.3%, according to data provided by Dr. Baraliakos. Conversely, the presence of sclerosis had a sensitivity of 81.7% but a specificity of only 43.2%.
One lesson from this analysis is that there is “increasing insecurity of only including bone marrow edema of the sacroiliac joint as the major criterion for diagnosing axSpA,” Dr. Baraliakos said. However, the larger point in the context of the earlier expert comments is that MRI findings should be considered important but insufficient for the evaluation of axSpA.
SOURCE: Baraliakos X et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):255-6. Abstract OPO344, doi: 10.1136/annrheumdis-2019-eular.5027
REPORTING FROM EULAR 2019 CONGRESS
MedPAC to Congress: End “incident-to” billing
Incident-to billing occurs when an advanced practicing registered nurse (APRN) or a physician assistant (PA) performs a service but bills Medicare under the physician’s national provider number and receives full physician fee schedule payment, as opposed to 85% of the fee under their own number.
“Medicare beneficiaries increasingly use APRNs and PAs for both primary and specialty care,” according to MedPAC’s June report. “APRNs are furnishing a larger share and a greater variety of services for Medicare beneficiaries than they did in the past. Despite this growing reliance, Medicare does not have a full accounting of the services delivered and beneficiaries treated.”
Currently, identical coding requirements obscure whether the physician or the APRN/PA is providing the service, making it difficult to track volume and quality.
MedPAC estimated that, in 2016, 17% of all nurse practitioners billed all their services as incident to, as that was the number of nurse practitioners who never appeared in the performing provider field for reimbursement but ordered services/drugs or at least one Medicare fee-for-service beneficiary.
Another 34% billed some of their services as incident to as their name appeared at least once in the performing provider they ordered services/drugs for, but ordered more services/drugs for patients where they were not listed as the performing provider.
That leaves just about half (49%) who did not billing their services as incident to.
Requiring APRNs and PAs to bill directly for all of their services provided would update Medicare’s payment policies to better reflect current clinical practice, according to the MedPAC report. “In addition to improving policy makers’ foundational knowledge of who provides care for Medicare beneficiaries, direct billing could create substantial benefits for the Medicare program, beneficiaries, clinicians, and researchers that range from improving the accuracy of the physician fee schedule, reducing expenditures, enhancing program integrity, and allowing for better comparisons between cost and quality of care provided by physicians and APRNs/PAs.”
At their October 2018 meeting, MedPAC commissioners discussed how to appropriately compensate APRNs and PAs, should incident-to billing be eliminated; they ultimately recommended maintaining the 85% rate.
The American Academy of Family Practitioners spoke out against the idea of eliminating incident-to billing.
However, lowering all APRN/PA payments to 85% of what physicians make would impact doctors in a negative way, according to AAFP President Michael Munger, MD.
Dr. Munger described primary care as a team sport, and “this is certainly going to be felt in terms of the overall mission of delivering quality care.”
Access to care also could be reduced along with the reduced payment level.
“You have to make business decisions at the end of the day,” he said in an interview. “You need to make sure that you can have adequate revenue to offset expenses, and if you are going to take a 15% cut in your revenue in, you have to look at where your expenses are, and obviously salary is your No. 1 expense. If you are not able to count on this revenue and you can’t afford to have NPs and PAs as part of the team, it is going to become an access issue for patients.”
The MedPAC commissioner saw it differently.
“Most of these clinicians are already paid at this lower rate, and yet the supply of these clinicians has increased dramatically over the last several years,” the report states, adding that the salary differential between these clinicians and physicians “is large enough that employing them likely would remain attractive even if all of their services were paid at 85% of physician fee schedule rates.”
MedPAC, as an advisory body to Congress, makes no disclosures.
Incident-to billing occurs when an advanced practicing registered nurse (APRN) or a physician assistant (PA) performs a service but bills Medicare under the physician’s national provider number and receives full physician fee schedule payment, as opposed to 85% of the fee under their own number.
“Medicare beneficiaries increasingly use APRNs and PAs for both primary and specialty care,” according to MedPAC’s June report. “APRNs are furnishing a larger share and a greater variety of services for Medicare beneficiaries than they did in the past. Despite this growing reliance, Medicare does not have a full accounting of the services delivered and beneficiaries treated.”
Currently, identical coding requirements obscure whether the physician or the APRN/PA is providing the service, making it difficult to track volume and quality.
MedPAC estimated that, in 2016, 17% of all nurse practitioners billed all their services as incident to, as that was the number of nurse practitioners who never appeared in the performing provider field for reimbursement but ordered services/drugs or at least one Medicare fee-for-service beneficiary.
Another 34% billed some of their services as incident to as their name appeared at least once in the performing provider they ordered services/drugs for, but ordered more services/drugs for patients where they were not listed as the performing provider.
That leaves just about half (49%) who did not billing their services as incident to.
Requiring APRNs and PAs to bill directly for all of their services provided would update Medicare’s payment policies to better reflect current clinical practice, according to the MedPAC report. “In addition to improving policy makers’ foundational knowledge of who provides care for Medicare beneficiaries, direct billing could create substantial benefits for the Medicare program, beneficiaries, clinicians, and researchers that range from improving the accuracy of the physician fee schedule, reducing expenditures, enhancing program integrity, and allowing for better comparisons between cost and quality of care provided by physicians and APRNs/PAs.”
At their October 2018 meeting, MedPAC commissioners discussed how to appropriately compensate APRNs and PAs, should incident-to billing be eliminated; they ultimately recommended maintaining the 85% rate.
The American Academy of Family Practitioners spoke out against the idea of eliminating incident-to billing.
However, lowering all APRN/PA payments to 85% of what physicians make would impact doctors in a negative way, according to AAFP President Michael Munger, MD.
Dr. Munger described primary care as a team sport, and “this is certainly going to be felt in terms of the overall mission of delivering quality care.”
Access to care also could be reduced along with the reduced payment level.
“You have to make business decisions at the end of the day,” he said in an interview. “You need to make sure that you can have adequate revenue to offset expenses, and if you are going to take a 15% cut in your revenue in, you have to look at where your expenses are, and obviously salary is your No. 1 expense. If you are not able to count on this revenue and you can’t afford to have NPs and PAs as part of the team, it is going to become an access issue for patients.”
The MedPAC commissioner saw it differently.
“Most of these clinicians are already paid at this lower rate, and yet the supply of these clinicians has increased dramatically over the last several years,” the report states, adding that the salary differential between these clinicians and physicians “is large enough that employing them likely would remain attractive even if all of their services were paid at 85% of physician fee schedule rates.”
MedPAC, as an advisory body to Congress, makes no disclosures.
Incident-to billing occurs when an advanced practicing registered nurse (APRN) or a physician assistant (PA) performs a service but bills Medicare under the physician’s national provider number and receives full physician fee schedule payment, as opposed to 85% of the fee under their own number.
“Medicare beneficiaries increasingly use APRNs and PAs for both primary and specialty care,” according to MedPAC’s June report. “APRNs are furnishing a larger share and a greater variety of services for Medicare beneficiaries than they did in the past. Despite this growing reliance, Medicare does not have a full accounting of the services delivered and beneficiaries treated.”
Currently, identical coding requirements obscure whether the physician or the APRN/PA is providing the service, making it difficult to track volume and quality.
MedPAC estimated that, in 2016, 17% of all nurse practitioners billed all their services as incident to, as that was the number of nurse practitioners who never appeared in the performing provider field for reimbursement but ordered services/drugs or at least one Medicare fee-for-service beneficiary.
Another 34% billed some of their services as incident to as their name appeared at least once in the performing provider they ordered services/drugs for, but ordered more services/drugs for patients where they were not listed as the performing provider.
That leaves just about half (49%) who did not billing their services as incident to.
Requiring APRNs and PAs to bill directly for all of their services provided would update Medicare’s payment policies to better reflect current clinical practice, according to the MedPAC report. “In addition to improving policy makers’ foundational knowledge of who provides care for Medicare beneficiaries, direct billing could create substantial benefits for the Medicare program, beneficiaries, clinicians, and researchers that range from improving the accuracy of the physician fee schedule, reducing expenditures, enhancing program integrity, and allowing for better comparisons between cost and quality of care provided by physicians and APRNs/PAs.”
At their October 2018 meeting, MedPAC commissioners discussed how to appropriately compensate APRNs and PAs, should incident-to billing be eliminated; they ultimately recommended maintaining the 85% rate.
The American Academy of Family Practitioners spoke out against the idea of eliminating incident-to billing.
However, lowering all APRN/PA payments to 85% of what physicians make would impact doctors in a negative way, according to AAFP President Michael Munger, MD.
Dr. Munger described primary care as a team sport, and “this is certainly going to be felt in terms of the overall mission of delivering quality care.”
Access to care also could be reduced along with the reduced payment level.
“You have to make business decisions at the end of the day,” he said in an interview. “You need to make sure that you can have adequate revenue to offset expenses, and if you are going to take a 15% cut in your revenue in, you have to look at where your expenses are, and obviously salary is your No. 1 expense. If you are not able to count on this revenue and you can’t afford to have NPs and PAs as part of the team, it is going to become an access issue for patients.”
The MedPAC commissioner saw it differently.
“Most of these clinicians are already paid at this lower rate, and yet the supply of these clinicians has increased dramatically over the last several years,” the report states, adding that the salary differential between these clinicians and physicians “is large enough that employing them likely would remain attractive even if all of their services were paid at 85% of physician fee schedule rates.”
MedPAC, as an advisory body to Congress, makes no disclosures.
Risk model could help predict VTE in acute leukemia
AMSTERDAM – A new clinical prediction model can determine the risk of venous thromboembolism in patients with leukemia, according to investigators.
The scoring system, which incorporates historical, morphological, and cytologic factors, was internally validated at multiple time points over the course of a year, reported lead author, Alejandro Lazo-Langner, MD, of the University of Western Ontario, London.
“It is important that we can predict or anticipate which patients [with acute leukemia] will develop venous thrombosis so that we can develop preventions and aim for better surveillance strategies,” Dr. Lazo-Langner said at the annual congress of the European Hematology Association. Venous thromboembolism (VTE) risk modeling is available for patients with solid tumors, but a similar prognostic tool for leukemia patients has been missing.
To fill this practice gap, Dr. Lazo-Langner and colleagues conducted a retrospective cohort study involving 501 patients with acute leukemia who were diagnosed between 2006 and 2017. Of these patients, 427 (85.2%) had myeloid lineage and 74 (14.8%) had lymphoblastic disease. VTE outcomes of interest included proximal lower- and upper-extremity deep vein thrombosis; pulmonary embolism; and thrombosis of unusual sites, such as splanchnic and cerebral. Patients were followed until last follow-up, VTE, or death. Single variable and multiple variable logistic regression were used sequentially to evaluate and confirm potential predictive factors, with nonparametric bootstrapping for internal validation.
After last follow-up, 77 patients (15.3%) had developed VTE; specifically, 44 patients had upper-extremity deep vein thrombosis, 28 had lower-extremity deep vein thrombosis or pulmonary embolism, and 5 had cerebral vein thrombosis. The median time from leukemia diagnosis to VTE was approximately 2 months (64 days). Out of 20 possible predictive factors, 7 were included in the multivariable model, and 3 constitute the final model. These three factors are platelet count greater than 50 x 109/L at time of diagnosis (1 point), lymphoblastic leukemia (2 points), and previous history of venous thromboembolism (3 points).
Dr. Lazo-Langner explained that leukemia patients at high risk of VTE are those with a score of 3 or more points. Using this risk threshold, the investigators found that the overall cumulative incidence of VTE in the high-risk group was 44.0%, compared with 10.5% in the low-risk group. Temporal analysis showed a widening disparity between the two groups, from 3 months (28.8% vs. 6.3%), to 6 months (41.1% vs. 7.9%), and 12 months (42.5% vs. 9.3%).
When asked if treatment type was evaluated, Dr. Lazo-Langner said that treatment type was evaluated but proved unfruitful for the model, which is designed for universal use in leukemia.
“We did include a number of different chemotherapy regimens,” he said. “The problem is, because we included both AML [acute myeloid leukemia] and ALL [acute lymphoblastic leukemia] lineage, and the cornerstone of treatment is different for both lineages. It’s difficult to actually include what kind of chemotherapy [patients had]. For instance, it is known that anthracyclines increase risk of thrombosis, but in both lineages, you use anthracyclines, so you really cannot use that as a predictor.”
Looking to the future, the next step will be validation in other cohorts. If this is successful, then Dr. Lazo-Langner speculated that clinicians could use the scoring system to direct monitoring and treatment. For example, patients with high scores and low platelet counts could receive earlier transfusional support, while all high-risk patients could be placed under more intensive surveillance and given additional education about thrombosis.
“I think recognizing symptoms early is important,” Dr. Lazo-Langner said, “and that would be training not only clinicians, but also nursing personnel and the patients themselves to be aware of the symptoms, so they can actually recognize them sooner.”
The study was funded by the Canadian Institutes of Health Research. Dr. Lazo-Langner is an investigator with the Canadian Venous Thromboembolism Clinical Trials and Outcomes Research (CanVECTOR) Network.
SOURCE: Lazo-Langner A et al. EHA 2019, Abstract S1642.
AMSTERDAM – A new clinical prediction model can determine the risk of venous thromboembolism in patients with leukemia, according to investigators.
The scoring system, which incorporates historical, morphological, and cytologic factors, was internally validated at multiple time points over the course of a year, reported lead author, Alejandro Lazo-Langner, MD, of the University of Western Ontario, London.
“It is important that we can predict or anticipate which patients [with acute leukemia] will develop venous thrombosis so that we can develop preventions and aim for better surveillance strategies,” Dr. Lazo-Langner said at the annual congress of the European Hematology Association. Venous thromboembolism (VTE) risk modeling is available for patients with solid tumors, but a similar prognostic tool for leukemia patients has been missing.
To fill this practice gap, Dr. Lazo-Langner and colleagues conducted a retrospective cohort study involving 501 patients with acute leukemia who were diagnosed between 2006 and 2017. Of these patients, 427 (85.2%) had myeloid lineage and 74 (14.8%) had lymphoblastic disease. VTE outcomes of interest included proximal lower- and upper-extremity deep vein thrombosis; pulmonary embolism; and thrombosis of unusual sites, such as splanchnic and cerebral. Patients were followed until last follow-up, VTE, or death. Single variable and multiple variable logistic regression were used sequentially to evaluate and confirm potential predictive factors, with nonparametric bootstrapping for internal validation.
After last follow-up, 77 patients (15.3%) had developed VTE; specifically, 44 patients had upper-extremity deep vein thrombosis, 28 had lower-extremity deep vein thrombosis or pulmonary embolism, and 5 had cerebral vein thrombosis. The median time from leukemia diagnosis to VTE was approximately 2 months (64 days). Out of 20 possible predictive factors, 7 were included in the multivariable model, and 3 constitute the final model. These three factors are platelet count greater than 50 x 109/L at time of diagnosis (1 point), lymphoblastic leukemia (2 points), and previous history of venous thromboembolism (3 points).
Dr. Lazo-Langner explained that leukemia patients at high risk of VTE are those with a score of 3 or more points. Using this risk threshold, the investigators found that the overall cumulative incidence of VTE in the high-risk group was 44.0%, compared with 10.5% in the low-risk group. Temporal analysis showed a widening disparity between the two groups, from 3 months (28.8% vs. 6.3%), to 6 months (41.1% vs. 7.9%), and 12 months (42.5% vs. 9.3%).
When asked if treatment type was evaluated, Dr. Lazo-Langner said that treatment type was evaluated but proved unfruitful for the model, which is designed for universal use in leukemia.
“We did include a number of different chemotherapy regimens,” he said. “The problem is, because we included both AML [acute myeloid leukemia] and ALL [acute lymphoblastic leukemia] lineage, and the cornerstone of treatment is different for both lineages. It’s difficult to actually include what kind of chemotherapy [patients had]. For instance, it is known that anthracyclines increase risk of thrombosis, but in both lineages, you use anthracyclines, so you really cannot use that as a predictor.”
Looking to the future, the next step will be validation in other cohorts. If this is successful, then Dr. Lazo-Langner speculated that clinicians could use the scoring system to direct monitoring and treatment. For example, patients with high scores and low platelet counts could receive earlier transfusional support, while all high-risk patients could be placed under more intensive surveillance and given additional education about thrombosis.
“I think recognizing symptoms early is important,” Dr. Lazo-Langner said, “and that would be training not only clinicians, but also nursing personnel and the patients themselves to be aware of the symptoms, so they can actually recognize them sooner.”
The study was funded by the Canadian Institutes of Health Research. Dr. Lazo-Langner is an investigator with the Canadian Venous Thromboembolism Clinical Trials and Outcomes Research (CanVECTOR) Network.
SOURCE: Lazo-Langner A et al. EHA 2019, Abstract S1642.
AMSTERDAM – A new clinical prediction model can determine the risk of venous thromboembolism in patients with leukemia, according to investigators.
The scoring system, which incorporates historical, morphological, and cytologic factors, was internally validated at multiple time points over the course of a year, reported lead author, Alejandro Lazo-Langner, MD, of the University of Western Ontario, London.
“It is important that we can predict or anticipate which patients [with acute leukemia] will develop venous thrombosis so that we can develop preventions and aim for better surveillance strategies,” Dr. Lazo-Langner said at the annual congress of the European Hematology Association. Venous thromboembolism (VTE) risk modeling is available for patients with solid tumors, but a similar prognostic tool for leukemia patients has been missing.
To fill this practice gap, Dr. Lazo-Langner and colleagues conducted a retrospective cohort study involving 501 patients with acute leukemia who were diagnosed between 2006 and 2017. Of these patients, 427 (85.2%) had myeloid lineage and 74 (14.8%) had lymphoblastic disease. VTE outcomes of interest included proximal lower- and upper-extremity deep vein thrombosis; pulmonary embolism; and thrombosis of unusual sites, such as splanchnic and cerebral. Patients were followed until last follow-up, VTE, or death. Single variable and multiple variable logistic regression were used sequentially to evaluate and confirm potential predictive factors, with nonparametric bootstrapping for internal validation.
After last follow-up, 77 patients (15.3%) had developed VTE; specifically, 44 patients had upper-extremity deep vein thrombosis, 28 had lower-extremity deep vein thrombosis or pulmonary embolism, and 5 had cerebral vein thrombosis. The median time from leukemia diagnosis to VTE was approximately 2 months (64 days). Out of 20 possible predictive factors, 7 were included in the multivariable model, and 3 constitute the final model. These three factors are platelet count greater than 50 x 109/L at time of diagnosis (1 point), lymphoblastic leukemia (2 points), and previous history of venous thromboembolism (3 points).
Dr. Lazo-Langner explained that leukemia patients at high risk of VTE are those with a score of 3 or more points. Using this risk threshold, the investigators found that the overall cumulative incidence of VTE in the high-risk group was 44.0%, compared with 10.5% in the low-risk group. Temporal analysis showed a widening disparity between the two groups, from 3 months (28.8% vs. 6.3%), to 6 months (41.1% vs. 7.9%), and 12 months (42.5% vs. 9.3%).
When asked if treatment type was evaluated, Dr. Lazo-Langner said that treatment type was evaluated but proved unfruitful for the model, which is designed for universal use in leukemia.
“We did include a number of different chemotherapy regimens,” he said. “The problem is, because we included both AML [acute myeloid leukemia] and ALL [acute lymphoblastic leukemia] lineage, and the cornerstone of treatment is different for both lineages. It’s difficult to actually include what kind of chemotherapy [patients had]. For instance, it is known that anthracyclines increase risk of thrombosis, but in both lineages, you use anthracyclines, so you really cannot use that as a predictor.”
Looking to the future, the next step will be validation in other cohorts. If this is successful, then Dr. Lazo-Langner speculated that clinicians could use the scoring system to direct monitoring and treatment. For example, patients with high scores and low platelet counts could receive earlier transfusional support, while all high-risk patients could be placed under more intensive surveillance and given additional education about thrombosis.
“I think recognizing symptoms early is important,” Dr. Lazo-Langner said, “and that would be training not only clinicians, but also nursing personnel and the patients themselves to be aware of the symptoms, so they can actually recognize them sooner.”
The study was funded by the Canadian Institutes of Health Research. Dr. Lazo-Langner is an investigator with the Canadian Venous Thromboembolism Clinical Trials and Outcomes Research (CanVECTOR) Network.
SOURCE: Lazo-Langner A et al. EHA 2019, Abstract S1642.
REPORTING FROM EHA CONGRESS
Parent education improves quick disposal of children’s unused prescription opioids
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
SAN ANTONIO – Interventions aimed at educating parents about proper disposal methods for leftover prescription opioids and on explaining the risks of retaining opioids can increase the likelihood that parents will dispose of opioids when their children no longer need them, according to new research.
“Cost-effective disposal methods can nudge parents to dispose of their child’s leftover opioids promptly after use, but risk messaging is needed to best affect both early disposal and planned retention,” concluded Terri Voepel-Lewis, PhD, RN, of the University of Michigan, Ann Arbor, and colleagues.
“Such strategies can effectively reduce the presence of risky leftover medications in the home and decrease the risks posed to children and adolescents,” they wrote in a research poster at the annual meeting of College on Problems of Drug Dependence.
The researchers recruited 517 parents of children prescribed a short course of opioids, excluding children with chronic pain or the inability to report their pain.
The 255 parents randomly assigned to the nudge group received visual instructions on how to properly dispose of drugs while the 262 parents in the control group did not receive information on a disposal method. The groups were otherwise similar in terms of parent education, race/ethnicity, the child’s age and past opioid use, the parents’ past opioid use or misuse, whether opioids were kept in the home and whether the child’s procedure had been orthopedic/sports medicine–related.
Parents also were randomly assigned to routine care or to a Scenario-Tailored Opioid Messaging Program (STOMP). The STOMP group received tailored opioid risk information.
After a baseline survey on the child’s past pain, opioid use, misuse of opioids and risk perceptions, parents completed follow-up surveys at 7 and 14 days on opioid use, child pain, and behaviors related to retaining or disposing of opioids.
Just over a third of parents in the nudge group (34.7%) disposed of leftover opioids immediately after use, compared with 24% in the control group (odds ratio, 1.68; P = .01). Parents with the highest rate of disposal were those in the nudge group who participated in STOMP; they were more than twice as likely to dispose of opioids immediately after they were no longer needed (OR, 2.55; compared with control/non-STOMP).
A higher likelihood of disposal for parents in the nudge group alone, however, barely missed significance (OR, 1.77; P = .06) before adjustment. Parents’ intention to dispose of opioids was significantly different only among those who received STOMP education.
After the researchers controlled for child and parent factors, actual early disposal was significantly more likely in both the nudge and STOMP groups.
“Parental past opioid behaviors (kept an opioid in the home and past misuse) as well as orthopedic/sports medicine procedure were strongly associated with parents’ intention to retain [opioids],” the authors reported.
The study results revealed a divergence in parents’ intentions versus their behavior for one of the intervention groups.
“The nudge intervention improved parents’ prompt disposal of leftover prescription opioids but had no effect on planned retention rates,” the researchers reported. “In contrast, STOMP education had significant effects on early disposal behavior and planned retention. ”
Additional findings regarding parents’ past behaviors suggested that those who have kept leftover opioids or previously misused them may see the risks of doing so as low, the authors noted.
“Importantly, parents’ past prescription opioid retention behavior doubled the risk for planned retention, and their past opioid misuse more than tripled the risk,” the researchers wrote. “Assessing parents’ past behaviors and enhancing their perceptions of the real risks posed to children are important targets for risk reduction.”
The National Institute on Drug Addiction funded the research. The authors reported having no conflicts of interest.
REPORTING FROM CPDD 2019
Generalized Granuloma Annulare Responsive to Narrowband UVB
To the Editor:
Granuloma annulare (GA) is a common dermatosis that usually presents with dermal papules and annular plaques in a symmetric distribution.1 The etiology is unknown, but a delayed-type hypersensitivity reaction is the favored pathogenesis. Several systemic associations have been reported with generalized GA including diabetes mellitus, hyperlipidemia, autoimmune thyroiditis, rheumatoid arthritis, and lymphoproliferative malignancies, as well as other malignancies and viral infections such as human immunodeficiency virus and hepatitis C. Localized GA often is self-limiting, but generalized disease can be chronic and progressive. Although asymptomatic in most cases, the lesions can be cosmetically bothersome, and many patients desire treatment. There are few well-controlled studies of treatment, and most are limited to case reports and series. A review of GA treatment noted only 3 randomized studies: 2 relating to photodynamic therapy and 1 to cryosurgery. Well-accepted therapies, such as topical and intralesional corticosteroids, antimalarials, immunosuppressants, antibiotics, and phototherapy, are substantiated by lesser-quality evidence.1 Phototherapy has been studied for the treatment of GA and other disorders with altered dermal matrix deposition for which there are limited effective treatment options. UV irradiation promotes degradation of structural components of the dermis and inhibition of collagen production.2 Granuloma annulare generally is resistant to therapy. We report a case of generalized GA of long duration that responded well to phototherapy with narrowband UVB (NB-UVB).
A 60-year-old woman presented with generalized GA of 4 years’ duration that was confirmed on biopsy on 2 occasions (Figure 1). The lesions were asymptomatic but disfiguring and consisted of extensive pink, thin, annular plaques and papules on the torso, arms, and legs (Figure 2A). Apart from mild depression for which she was being treated with paroxetine and trazodone, she was otherwise healthy without evidence of thyroid disease, hyperlipidemia, or diabetes mellitus. Prior treatments for GA had included tapering courses of prednisone (up to 30 mg/d, tapered by 5 mg every 4 days) and betamethasone dipropionate cream 0.05%. She was started on NB-UVB therapy 5 times weekly in incremental doses with no adjuvant therapy. After 100 treatments, there was notable improvement with lesions becoming paler and flatter, with some involuting completely (Figure 2B). The frequency of treatment was reduced to 3 times weekly with continued improvement. An NB-UVB device was used containing 48 TL 100W/01-FS72 lamps with a mean irradiance of 2.9 mW/cm2. Her starting dose was 90 mJ/cm2. The cumulative dose after 100 treatments was 35,600 mJ/cm2. Apart from occasional mild erythema, there were no adverse effects.
Inui et al3 described the successful treatment of generalized GA with NB-UVB. A retrospective review of NB-UVB for vitiligo, pruritus, and inflammatory dermatoses included 2 cases of generalized GA that were noted to have only a minimal to mild improvement.4 Most reports relating to phototherapy of GA have focused on psoralen plus UVA (PUVA). A retrospective study of 33 patients treated with systemic PUVA showed improvement in two-thirds of patients.5 Older studies showed systemic PUVA was effective in 1 patient after 53 treatments6 and in 4 patients using a high-dose protocol7; topical PUVA was effective in 4 patients after an average of 26 treatments.8 Psoralen plus UVA bath was reported as an effective treatment of generalized GA in a child.9 UVA1 phototherapy provided good or excellent results in half of patients (10/20) studied with generalized GA; however, discontinuation of treatment resulted in early recurrence of disease.10 In general, NB-UVB has been preferred over PUVA and UVA1 due to long-term safety, tolerability, and access. Although further clinical trials are needed, our report suggests that NB-UVB could be a useful modality in generalized GA.
- Thornsberry LA, English JC III. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Inui S, Nishida Y, Itami S, et al. Disseminated granuloma annulare responsive to narrowband ultraviolet B therapy. J Am Acad Dermatol. 2005;53:532-533.
- Samson Yashar S, Gielczyk R, Scherschun L, et al. Narrow-band ultraviolet B treatment for vitiligo, pruritus, and inflammatory dermatoses. Photodermatol Photoimmunol Photomed. 2003;19:164-168.
- Browne F, Turner D, Goulden V. Psoralen and ultraviolet A in the treatment of granuloma annulare. Photodermatol Photoimmunol Photomed. 2011;27:81-84.
- Setterfield J, Huilgol SC, Black MM. Generalised granuloma annulare successfully treated with PUVA. Clin Exp Dermatol. 1999;24:458-460.
- Munchenberger S, Schopf E, Simon JC. Phototherapy with UVA-1 for generalized granuloma annulare. Arch Dermatol. 1997;133:1605.
- Grundmann-Kollmann M, Ochsendorf FR, Zollner TM, et al. Cream psoralen plus ultraviolet A therapy for granuloma annulare. Br J Dermatol. 2001;144:996-999.
- Batchelor R, Clark S. Clearance of generalized popular umbilicated granuloma annulare in a child with bath PUVA therapy. Pediatr Dermatol. 2006;23:72-74.
- Schnopp C, Tzaneva S, Mempel M, et al. UVA1 phototherapy for disseminated granuloma annulare. Photodermatol Photoimmunol Photomed. 2005;21:68-71.
To the Editor:
Granuloma annulare (GA) is a common dermatosis that usually presents with dermal papules and annular plaques in a symmetric distribution.1 The etiology is unknown, but a delayed-type hypersensitivity reaction is the favored pathogenesis. Several systemic associations have been reported with generalized GA including diabetes mellitus, hyperlipidemia, autoimmune thyroiditis, rheumatoid arthritis, and lymphoproliferative malignancies, as well as other malignancies and viral infections such as human immunodeficiency virus and hepatitis C. Localized GA often is self-limiting, but generalized disease can be chronic and progressive. Although asymptomatic in most cases, the lesions can be cosmetically bothersome, and many patients desire treatment. There are few well-controlled studies of treatment, and most are limited to case reports and series. A review of GA treatment noted only 3 randomized studies: 2 relating to photodynamic therapy and 1 to cryosurgery. Well-accepted therapies, such as topical and intralesional corticosteroids, antimalarials, immunosuppressants, antibiotics, and phototherapy, are substantiated by lesser-quality evidence.1 Phototherapy has been studied for the treatment of GA and other disorders with altered dermal matrix deposition for which there are limited effective treatment options. UV irradiation promotes degradation of structural components of the dermis and inhibition of collagen production.2 Granuloma annulare generally is resistant to therapy. We report a case of generalized GA of long duration that responded well to phototherapy with narrowband UVB (NB-UVB).
A 60-year-old woman presented with generalized GA of 4 years’ duration that was confirmed on biopsy on 2 occasions (Figure 1). The lesions were asymptomatic but disfiguring and consisted of extensive pink, thin, annular plaques and papules on the torso, arms, and legs (Figure 2A). Apart from mild depression for which she was being treated with paroxetine and trazodone, she was otherwise healthy without evidence of thyroid disease, hyperlipidemia, or diabetes mellitus. Prior treatments for GA had included tapering courses of prednisone (up to 30 mg/d, tapered by 5 mg every 4 days) and betamethasone dipropionate cream 0.05%. She was started on NB-UVB therapy 5 times weekly in incremental doses with no adjuvant therapy. After 100 treatments, there was notable improvement with lesions becoming paler and flatter, with some involuting completely (Figure 2B). The frequency of treatment was reduced to 3 times weekly with continued improvement. An NB-UVB device was used containing 48 TL 100W/01-FS72 lamps with a mean irradiance of 2.9 mW/cm2. Her starting dose was 90 mJ/cm2. The cumulative dose after 100 treatments was 35,600 mJ/cm2. Apart from occasional mild erythema, there were no adverse effects.
Inui et al3 described the successful treatment of generalized GA with NB-UVB. A retrospective review of NB-UVB for vitiligo, pruritus, and inflammatory dermatoses included 2 cases of generalized GA that were noted to have only a minimal to mild improvement.4 Most reports relating to phototherapy of GA have focused on psoralen plus UVA (PUVA). A retrospective study of 33 patients treated with systemic PUVA showed improvement in two-thirds of patients.5 Older studies showed systemic PUVA was effective in 1 patient after 53 treatments6 and in 4 patients using a high-dose protocol7; topical PUVA was effective in 4 patients after an average of 26 treatments.8 Psoralen plus UVA bath was reported as an effective treatment of generalized GA in a child.9 UVA1 phototherapy provided good or excellent results in half of patients (10/20) studied with generalized GA; however, discontinuation of treatment resulted in early recurrence of disease.10 In general, NB-UVB has been preferred over PUVA and UVA1 due to long-term safety, tolerability, and access. Although further clinical trials are needed, our report suggests that NB-UVB could be a useful modality in generalized GA.
To the Editor:
Granuloma annulare (GA) is a common dermatosis that usually presents with dermal papules and annular plaques in a symmetric distribution.1 The etiology is unknown, but a delayed-type hypersensitivity reaction is the favored pathogenesis. Several systemic associations have been reported with generalized GA including diabetes mellitus, hyperlipidemia, autoimmune thyroiditis, rheumatoid arthritis, and lymphoproliferative malignancies, as well as other malignancies and viral infections such as human immunodeficiency virus and hepatitis C. Localized GA often is self-limiting, but generalized disease can be chronic and progressive. Although asymptomatic in most cases, the lesions can be cosmetically bothersome, and many patients desire treatment. There are few well-controlled studies of treatment, and most are limited to case reports and series. A review of GA treatment noted only 3 randomized studies: 2 relating to photodynamic therapy and 1 to cryosurgery. Well-accepted therapies, such as topical and intralesional corticosteroids, antimalarials, immunosuppressants, antibiotics, and phototherapy, are substantiated by lesser-quality evidence.1 Phototherapy has been studied for the treatment of GA and other disorders with altered dermal matrix deposition for which there are limited effective treatment options. UV irradiation promotes degradation of structural components of the dermis and inhibition of collagen production.2 Granuloma annulare generally is resistant to therapy. We report a case of generalized GA of long duration that responded well to phototherapy with narrowband UVB (NB-UVB).
A 60-year-old woman presented with generalized GA of 4 years’ duration that was confirmed on biopsy on 2 occasions (Figure 1). The lesions were asymptomatic but disfiguring and consisted of extensive pink, thin, annular plaques and papules on the torso, arms, and legs (Figure 2A). Apart from mild depression for which she was being treated with paroxetine and trazodone, she was otherwise healthy without evidence of thyroid disease, hyperlipidemia, or diabetes mellitus. Prior treatments for GA had included tapering courses of prednisone (up to 30 mg/d, tapered by 5 mg every 4 days) and betamethasone dipropionate cream 0.05%. She was started on NB-UVB therapy 5 times weekly in incremental doses with no adjuvant therapy. After 100 treatments, there was notable improvement with lesions becoming paler and flatter, with some involuting completely (Figure 2B). The frequency of treatment was reduced to 3 times weekly with continued improvement. An NB-UVB device was used containing 48 TL 100W/01-FS72 lamps with a mean irradiance of 2.9 mW/cm2. Her starting dose was 90 mJ/cm2. The cumulative dose after 100 treatments was 35,600 mJ/cm2. Apart from occasional mild erythema, there were no adverse effects.
Inui et al3 described the successful treatment of generalized GA with NB-UVB. A retrospective review of NB-UVB for vitiligo, pruritus, and inflammatory dermatoses included 2 cases of generalized GA that were noted to have only a minimal to mild improvement.4 Most reports relating to phototherapy of GA have focused on psoralen plus UVA (PUVA). A retrospective study of 33 patients treated with systemic PUVA showed improvement in two-thirds of patients.5 Older studies showed systemic PUVA was effective in 1 patient after 53 treatments6 and in 4 patients using a high-dose protocol7; topical PUVA was effective in 4 patients after an average of 26 treatments.8 Psoralen plus UVA bath was reported as an effective treatment of generalized GA in a child.9 UVA1 phototherapy provided good or excellent results in half of patients (10/20) studied with generalized GA; however, discontinuation of treatment resulted in early recurrence of disease.10 In general, NB-UVB has been preferred over PUVA and UVA1 due to long-term safety, tolerability, and access. Although further clinical trials are needed, our report suggests that NB-UVB could be a useful modality in generalized GA.
- Thornsberry LA, English JC III. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Inui S, Nishida Y, Itami S, et al. Disseminated granuloma annulare responsive to narrowband ultraviolet B therapy. J Am Acad Dermatol. 2005;53:532-533.
- Samson Yashar S, Gielczyk R, Scherschun L, et al. Narrow-band ultraviolet B treatment for vitiligo, pruritus, and inflammatory dermatoses. Photodermatol Photoimmunol Photomed. 2003;19:164-168.
- Browne F, Turner D, Goulden V. Psoralen and ultraviolet A in the treatment of granuloma annulare. Photodermatol Photoimmunol Photomed. 2011;27:81-84.
- Setterfield J, Huilgol SC, Black MM. Generalised granuloma annulare successfully treated with PUVA. Clin Exp Dermatol. 1999;24:458-460.
- Munchenberger S, Schopf E, Simon JC. Phototherapy with UVA-1 for generalized granuloma annulare. Arch Dermatol. 1997;133:1605.
- Grundmann-Kollmann M, Ochsendorf FR, Zollner TM, et al. Cream psoralen plus ultraviolet A therapy for granuloma annulare. Br J Dermatol. 2001;144:996-999.
- Batchelor R, Clark S. Clearance of generalized popular umbilicated granuloma annulare in a child with bath PUVA therapy. Pediatr Dermatol. 2006;23:72-74.
- Schnopp C, Tzaneva S, Mempel M, et al. UVA1 phototherapy for disseminated granuloma annulare. Photodermatol Photoimmunol Photomed. 2005;21:68-71.
- Thornsberry LA, English JC III. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419-1428.
- Inui S, Nishida Y, Itami S, et al. Disseminated granuloma annulare responsive to narrowband ultraviolet B therapy. J Am Acad Dermatol. 2005;53:532-533.
- Samson Yashar S, Gielczyk R, Scherschun L, et al. Narrow-band ultraviolet B treatment for vitiligo, pruritus, and inflammatory dermatoses. Photodermatol Photoimmunol Photomed. 2003;19:164-168.
- Browne F, Turner D, Goulden V. Psoralen and ultraviolet A in the treatment of granuloma annulare. Photodermatol Photoimmunol Photomed. 2011;27:81-84.
- Setterfield J, Huilgol SC, Black MM. Generalised granuloma annulare successfully treated with PUVA. Clin Exp Dermatol. 1999;24:458-460.
- Munchenberger S, Schopf E, Simon JC. Phototherapy with UVA-1 for generalized granuloma annulare. Arch Dermatol. 1997;133:1605.
- Grundmann-Kollmann M, Ochsendorf FR, Zollner TM, et al. Cream psoralen plus ultraviolet A therapy for granuloma annulare. Br J Dermatol. 2001;144:996-999.
- Batchelor R, Clark S. Clearance of generalized popular umbilicated granuloma annulare in a child with bath PUVA therapy. Pediatr Dermatol. 2006;23:72-74.
- Schnopp C, Tzaneva S, Mempel M, et al. UVA1 phototherapy for disseminated granuloma annulare. Photodermatol Photoimmunol Photomed. 2005;21:68-71.
Practice Points
- The generalized variant of granuloma annulare (GA) can be persistent, sometimes lasting years to decades; treatment is not always effective.
- The safety profile and tolerability of narrowband UVB phototherapy make it a suitable treatment option for generalized GA.
What’s new in pediatric sepsis
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ESPID 2019