User login
Penicillin-susceptible Streptococcus pneumoniae most common cause of bacteremic CAP
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.
The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.

The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.

The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
FROM PEDIATRICS
Measles incidence has slowed as summer begins
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.
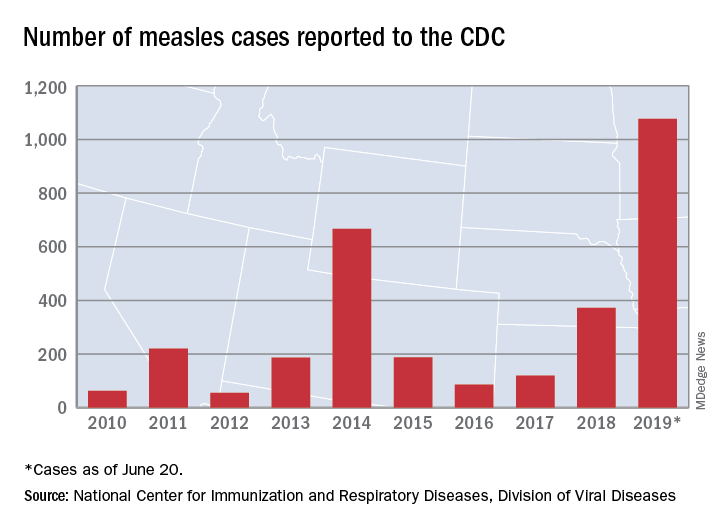
The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.

The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.

The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
Liquid biopsy assays found sensitive for NSCLC EGFR mutations
Two liquid biopsy assays show generally good concordance with the gold standard of next-generation sequencing (NGS) performed on tissue for detecting epidermal growth factor receptor (EGFR) mutations in non–small cell lung (NSCLC), finds a retrospective cohort study.
Availability of targeted therapies for EGFR-mutated NSCLC underscores the importance of detecting these molecular aberrations, note lead investigator Christi M.J. Steendam, MD, department of pulmonary diseases, Erasmus MC Rotterdam, and Amphia Hospital, Breda, the Netherlands, and coinvestigators. In addition, assessing and monitoring mutational status can provide information about resistance and better inform treatment decisions.
The investigators studied 36 patients with EGFR-mutated NSCLC who had experienced progression on their current therapy and had both tissue and plasma available. They first compared results of droplet digital polymerase chain reaction (ddPCR) and NGS for detecting primary activating EGFR mutations and the resistance p.T790M EGFR mutation (the most common resistance mechanism to first- and second-generation tyrosine kinase inhibitors in this population) in plasma-derived cell-free DNA. They then compared each assay against NGS performed on conventional tissue.
Study results showed high agreement between ddPCR and NGS, at 86% for detection of the primary activating mutation and at 94% for detection of the p.T790M mutation. Findings were similar for the quantified allele ratio (mutant alleles divided by total alleles).
Overall, 15 patients (41.7%) had some degree of discrepant results. Six had no detectable mutations in cell-free DNA, three had detectable p.T790M in plasma but not in tissue, and three others had detectable p.T790M in tissue but not in plasma.
Finally, there was generally good concordance of the cell-free DNA results and the results obtained in tissue for detection of the primary activating mutation (69% for ddPCR, 83% for NGS) and for detection of p.T790M (75% for ddPCR, 75% for NGS). Patients with discordant results tended to have intrathoracic and/or CNS progression.
“ddPCR and NGS yield comparable results, with similar sensitivity for the mutations that can be detected by both methods, and the concordance with tissue-based results is high,” Dr. Steendam and coinvestigators summarize in JCO Precision Oncology. “When searching for a resistance mechanism, NGS analysis of cell-free DNA in plasma offers a more comprehensive view than ddPCR, with comparable precision at a single mutation level. When no mutations are detected in plasma, tissue-based investigation remains desirable.”
“Our results confirm the ability to detect targetable aberrations in blood, which provides possibilities for new lines of targeted treatments in daily practice without the necessity of tissue procurement in many patients,” they conclude.
Dr. Steendam disclosed that she receives research funding from AstraZeneca (institutional) and travel, accommodations, and/or expenses from Roche, Boehringer Ingelheim, and Eli Lilly. The study did not receive any specific funding.
SOURCE: Steendam CMJ et al. JCO Precis Oncol. 2019 June 20. doi: 10.1200/PO.18.00401.
Two liquid biopsy assays show generally good concordance with the gold standard of next-generation sequencing (NGS) performed on tissue for detecting epidermal growth factor receptor (EGFR) mutations in non–small cell lung (NSCLC), finds a retrospective cohort study.
Availability of targeted therapies for EGFR-mutated NSCLC underscores the importance of detecting these molecular aberrations, note lead investigator Christi M.J. Steendam, MD, department of pulmonary diseases, Erasmus MC Rotterdam, and Amphia Hospital, Breda, the Netherlands, and coinvestigators. In addition, assessing and monitoring mutational status can provide information about resistance and better inform treatment decisions.
The investigators studied 36 patients with EGFR-mutated NSCLC who had experienced progression on their current therapy and had both tissue and plasma available. They first compared results of droplet digital polymerase chain reaction (ddPCR) and NGS for detecting primary activating EGFR mutations and the resistance p.T790M EGFR mutation (the most common resistance mechanism to first- and second-generation tyrosine kinase inhibitors in this population) in plasma-derived cell-free DNA. They then compared each assay against NGS performed on conventional tissue.
Study results showed high agreement between ddPCR and NGS, at 86% for detection of the primary activating mutation and at 94% for detection of the p.T790M mutation. Findings were similar for the quantified allele ratio (mutant alleles divided by total alleles).
Overall, 15 patients (41.7%) had some degree of discrepant results. Six had no detectable mutations in cell-free DNA, three had detectable p.T790M in plasma but not in tissue, and three others had detectable p.T790M in tissue but not in plasma.
Finally, there was generally good concordance of the cell-free DNA results and the results obtained in tissue for detection of the primary activating mutation (69% for ddPCR, 83% for NGS) and for detection of p.T790M (75% for ddPCR, 75% for NGS). Patients with discordant results tended to have intrathoracic and/or CNS progression.
“ddPCR and NGS yield comparable results, with similar sensitivity for the mutations that can be detected by both methods, and the concordance with tissue-based results is high,” Dr. Steendam and coinvestigators summarize in JCO Precision Oncology. “When searching for a resistance mechanism, NGS analysis of cell-free DNA in plasma offers a more comprehensive view than ddPCR, with comparable precision at a single mutation level. When no mutations are detected in plasma, tissue-based investigation remains desirable.”
“Our results confirm the ability to detect targetable aberrations in blood, which provides possibilities for new lines of targeted treatments in daily practice without the necessity of tissue procurement in many patients,” they conclude.
Dr. Steendam disclosed that she receives research funding from AstraZeneca (institutional) and travel, accommodations, and/or expenses from Roche, Boehringer Ingelheim, and Eli Lilly. The study did not receive any specific funding.
SOURCE: Steendam CMJ et al. JCO Precis Oncol. 2019 June 20. doi: 10.1200/PO.18.00401.
Two liquid biopsy assays show generally good concordance with the gold standard of next-generation sequencing (NGS) performed on tissue for detecting epidermal growth factor receptor (EGFR) mutations in non–small cell lung (NSCLC), finds a retrospective cohort study.
Availability of targeted therapies for EGFR-mutated NSCLC underscores the importance of detecting these molecular aberrations, note lead investigator Christi M.J. Steendam, MD, department of pulmonary diseases, Erasmus MC Rotterdam, and Amphia Hospital, Breda, the Netherlands, and coinvestigators. In addition, assessing and monitoring mutational status can provide information about resistance and better inform treatment decisions.
The investigators studied 36 patients with EGFR-mutated NSCLC who had experienced progression on their current therapy and had both tissue and plasma available. They first compared results of droplet digital polymerase chain reaction (ddPCR) and NGS for detecting primary activating EGFR mutations and the resistance p.T790M EGFR mutation (the most common resistance mechanism to first- and second-generation tyrosine kinase inhibitors in this population) in plasma-derived cell-free DNA. They then compared each assay against NGS performed on conventional tissue.
Study results showed high agreement between ddPCR and NGS, at 86% for detection of the primary activating mutation and at 94% for detection of the p.T790M mutation. Findings were similar for the quantified allele ratio (mutant alleles divided by total alleles).
Overall, 15 patients (41.7%) had some degree of discrepant results. Six had no detectable mutations in cell-free DNA, three had detectable p.T790M in plasma but not in tissue, and three others had detectable p.T790M in tissue but not in plasma.
Finally, there was generally good concordance of the cell-free DNA results and the results obtained in tissue for detection of the primary activating mutation (69% for ddPCR, 83% for NGS) and for detection of p.T790M (75% for ddPCR, 75% for NGS). Patients with discordant results tended to have intrathoracic and/or CNS progression.
“ddPCR and NGS yield comparable results, with similar sensitivity for the mutations that can be detected by both methods, and the concordance with tissue-based results is high,” Dr. Steendam and coinvestigators summarize in JCO Precision Oncology. “When searching for a resistance mechanism, NGS analysis of cell-free DNA in plasma offers a more comprehensive view than ddPCR, with comparable precision at a single mutation level. When no mutations are detected in plasma, tissue-based investigation remains desirable.”
“Our results confirm the ability to detect targetable aberrations in blood, which provides possibilities for new lines of targeted treatments in daily practice without the necessity of tissue procurement in many patients,” they conclude.
Dr. Steendam disclosed that she receives research funding from AstraZeneca (institutional) and travel, accommodations, and/or expenses from Roche, Boehringer Ingelheim, and Eli Lilly. The study did not receive any specific funding.
SOURCE: Steendam CMJ et al. JCO Precis Oncol. 2019 June 20. doi: 10.1200/PO.18.00401.
FROM JCO PRECISION ONCOLOGY
Barrett’s esophagus endoscopies are undersampled
As many as one in five endoscopies in patients with Barrett’s esophagus are not being done according to accepted protocol, say the authors of a study published online in Gastrointestinal Endoscopy.
The Seattle biopsy protocol, which is recommended in nondysplastic Barrett’s esophagus to pick up esophageal adenocarcinoma, calls for four-quadrant biopsies at 2-cm intervals in patients without dysplasia and 1-cm intervals in patients with prior dysplasia, as well as targeted biopsies of any mucosal abnormalities.
Sachin Wani, MD, from the University of Colorado at Denver, Aurora, and coauthors used registry data to examine procedures and outcomes in 58,709 esophagogastroduodenoscopies in 53,541 patients with an endoscopic finding of, or screening indication for, Barrett’s esophagus.
They assessed protocol adherence by dividing the Barrett’s esophagus length by the number of pathology jars. A ratio of two or less was considered adherent, and the authors also allowed for a lenient rounding down, or stringent rounding up.
Just over half the procedures in the study (51.1%) resulted in a recorded, pathology-confirmed diagnosis of Barrett’s esophagus, and the mean length of Barrett’s esophagus was 2.3 cm.
Overall, 87.8% of endoscopies were adherent according to the lenient criteria, and 82.7% were adherent by the stringent definition.
Patients with longer lengths of Barrett’s esophagus were significantly less likely to be biopsied according to the Seattle biopsy protocol guidelines, with a 31% decrease in odds of adherence with every 1-cm increase in Barrett’s esophagus length. Patients with lengths from 0-4 cm were 76.3%-80.6% adherent to biopsy protocols, but those with lengths greater than 8 cm were only 37.9%-40.6% adherent.
“These results are most concerning because we found that dysplasia detection rates also increase with increasing [Barrett’s esophagus] length,” the authors wrote. “Therefore, per unit length, patients who need it most are being biopsied least.
Older and male patients also were less likely to be biopsied according to the protocol, and an American Society of Anesthesiologists classification of three or above was a predictor of nonadherence.
“These findings may be a reflection of a higher number of comorbidities in these patients with [Barrett’s esophagus] and perceived lack of benefit of surveillance endoscopy,” the authors wrote.
They also noted a geographic effect, such that patients living in the Northeast regions were more likely to be biopsied according to protocol than were those living in Western regions.
The study was funded by the University of Colorado department of medicine. Four authors declared consultancies and research grants from the medical device sector and others.
SOURCE: Wani S et al. Gastrointest Endosc. 2019, June 11.
As many as one in five endoscopies in patients with Barrett’s esophagus are not being done according to accepted protocol, say the authors of a study published online in Gastrointestinal Endoscopy.
The Seattle biopsy protocol, which is recommended in nondysplastic Barrett’s esophagus to pick up esophageal adenocarcinoma, calls for four-quadrant biopsies at 2-cm intervals in patients without dysplasia and 1-cm intervals in patients with prior dysplasia, as well as targeted biopsies of any mucosal abnormalities.
Sachin Wani, MD, from the University of Colorado at Denver, Aurora, and coauthors used registry data to examine procedures and outcomes in 58,709 esophagogastroduodenoscopies in 53,541 patients with an endoscopic finding of, or screening indication for, Barrett’s esophagus.
They assessed protocol adherence by dividing the Barrett’s esophagus length by the number of pathology jars. A ratio of two or less was considered adherent, and the authors also allowed for a lenient rounding down, or stringent rounding up.
Just over half the procedures in the study (51.1%) resulted in a recorded, pathology-confirmed diagnosis of Barrett’s esophagus, and the mean length of Barrett’s esophagus was 2.3 cm.
Overall, 87.8% of endoscopies were adherent according to the lenient criteria, and 82.7% were adherent by the stringent definition.
Patients with longer lengths of Barrett’s esophagus were significantly less likely to be biopsied according to the Seattle biopsy protocol guidelines, with a 31% decrease in odds of adherence with every 1-cm increase in Barrett’s esophagus length. Patients with lengths from 0-4 cm were 76.3%-80.6% adherent to biopsy protocols, but those with lengths greater than 8 cm were only 37.9%-40.6% adherent.
“These results are most concerning because we found that dysplasia detection rates also increase with increasing [Barrett’s esophagus] length,” the authors wrote. “Therefore, per unit length, patients who need it most are being biopsied least.
Older and male patients also were less likely to be biopsied according to the protocol, and an American Society of Anesthesiologists classification of three or above was a predictor of nonadherence.
“These findings may be a reflection of a higher number of comorbidities in these patients with [Barrett’s esophagus] and perceived lack of benefit of surveillance endoscopy,” the authors wrote.
They also noted a geographic effect, such that patients living in the Northeast regions were more likely to be biopsied according to protocol than were those living in Western regions.
The study was funded by the University of Colorado department of medicine. Four authors declared consultancies and research grants from the medical device sector and others.
SOURCE: Wani S et al. Gastrointest Endosc. 2019, June 11.
As many as one in five endoscopies in patients with Barrett’s esophagus are not being done according to accepted protocol, say the authors of a study published online in Gastrointestinal Endoscopy.
The Seattle biopsy protocol, which is recommended in nondysplastic Barrett’s esophagus to pick up esophageal adenocarcinoma, calls for four-quadrant biopsies at 2-cm intervals in patients without dysplasia and 1-cm intervals in patients with prior dysplasia, as well as targeted biopsies of any mucosal abnormalities.
Sachin Wani, MD, from the University of Colorado at Denver, Aurora, and coauthors used registry data to examine procedures and outcomes in 58,709 esophagogastroduodenoscopies in 53,541 patients with an endoscopic finding of, or screening indication for, Barrett’s esophagus.
They assessed protocol adherence by dividing the Barrett’s esophagus length by the number of pathology jars. A ratio of two or less was considered adherent, and the authors also allowed for a lenient rounding down, or stringent rounding up.
Just over half the procedures in the study (51.1%) resulted in a recorded, pathology-confirmed diagnosis of Barrett’s esophagus, and the mean length of Barrett’s esophagus was 2.3 cm.
Overall, 87.8% of endoscopies were adherent according to the lenient criteria, and 82.7% were adherent by the stringent definition.
Patients with longer lengths of Barrett’s esophagus were significantly less likely to be biopsied according to the Seattle biopsy protocol guidelines, with a 31% decrease in odds of adherence with every 1-cm increase in Barrett’s esophagus length. Patients with lengths from 0-4 cm were 76.3%-80.6% adherent to biopsy protocols, but those with lengths greater than 8 cm were only 37.9%-40.6% adherent.
“These results are most concerning because we found that dysplasia detection rates also increase with increasing [Barrett’s esophagus] length,” the authors wrote. “Therefore, per unit length, patients who need it most are being biopsied least.
Older and male patients also were less likely to be biopsied according to the protocol, and an American Society of Anesthesiologists classification of three or above was a predictor of nonadherence.
“These findings may be a reflection of a higher number of comorbidities in these patients with [Barrett’s esophagus] and perceived lack of benefit of surveillance endoscopy,” the authors wrote.
They also noted a geographic effect, such that patients living in the Northeast regions were more likely to be biopsied according to protocol than were those living in Western regions.
The study was funded by the University of Colorado department of medicine. Four authors declared consultancies and research grants from the medical device sector and others.
SOURCE: Wani S et al. Gastrointest Endosc. 2019, June 11.
FROM GASTROINTESTINAL ENDOSCOPY
Imaging remission decried as ticket to RA overtreatment
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
MADRID – Defining remission in patients with rheumatoid arthritis depends on their clinical status, not on the presence or absence of inflammatory signals on ultrasound or MRI, many rheumatologists now agree.
The strong consensus that’s formed against using imaging as a criterion for RA remission was apparent at the European Congress of Rheumatology during presentation of a pending update to the EULAR recommendations for managing RA, as well as in at least two separate, invited lectures.
“Imaging is out,” proclaimed Josef S. Smolen, MD, as he spoke at the congress about the pending RA management revisions. This condemnation of imaging by ultrasound or MRI as an unsafe and misleading target for RA treatment by Dr. Smolen, professor of medicine at the Medical University of Vienna, was perhaps the most forceful statement he made while presenting the draft revision of EULAR’s RA recommendations.
The case for using ultrasound or MR to find inflammatory signatures in joints that can function as treatment targets collapsed earlier in 2019 with publication of results from IMAGINE-RA (An MRI-guided Treatment Strategy to Prevent Disease Progression in Patients With Rheumatoid Arthritis), a multicenter Danish study that randomized 200 RA patients in remission to either a conventional, disease activity–guided treatment target (in this case the DAS28-CRP [Disease Activity Score in 28 joints plus C-reactive protein]), or a treatment target that included the conventional clinical target plus treating to eliminate any bone marrow edema visualized by MRI. After 24 months of treatment, the prevalence of clinical remission and MRI remission was about the same in both arms, with no statistically significant differences. But serious adverse events in 6 patients managed by their clinical assessment compared favorably against 17 among those managed to an imaging remission endpoint, a difference that strongly hinted at dangerous overtreatment of the imaging-guided patients (JAMA. 2019 Feb 5;321[5]:461-72).
The failure of MRI assessment of inflammation to improve RA treatment in IMAGINE-RA came against the backdrop of two 2016 reports that documented the same limitation when using ultrasound to detect joint inflammation and guide treatment in RA patients. The TaSER (Targeting Synovitis in Early Rheumatoid Arthritis) study randomized 111 patients with newly diagnosed RA or undifferentiated arthritis to conventional disease activity assessment, DAS28–erythrocyte sedimentation rate, or to that plus assessment by musculoskeletal ultrasound, and found no difference in clinical or imaging outcomes (Ann Rheum Dis. 2016 Jun;75[6]:1043-50). The second report, ARCTIC (Aiming for Remission in Rheumatoid Arthritis), randomized 238 RA patients to either a tight RA control strategy based on DAS alone or based on DAS plus serial examination of joints with ultrasound. The results showed that, after 16-24 months on treatment, the two strategies produced no significant difference in the rates of sustained RA remission with no radiographic damage or swollen joints detected (BMJ. 2016 Aug 16;354:i4205).
The results from these three studies have shown that “not all inflammation seen by ultrasound or MR is pathological,” and that “no imaging technique or biomarker has shown superiority to clinical assessment as a treat-to-target” goal, Sofia Ramiro, MD, said in a talk at the congress during which she reviewed this evidence.
“Treat-to-target that takes imaging into account is high risk because it exposes patients to overtreatment, which has costs in the broad sense, safety included,” said Dr. Ramiro, a rheumatologist at Leiden (the Netherlands) University Medical Center. “I think that systematically evaluating a patient’s joint with imaging won’t have additional value, and is the wrong approach.”
A similar assessment came from Stefan Siebert, MD, during a separate lecture during the congress. He highlighted that use of ultrasound or MRI to guide treatment in these three studies consistently led to substantially higher rates of treatment escalation, treatment with biologics, and in two of the three studies a notable increase in serious adverse events. Treatment with a biologic drug was roughly twice as frequent in the imaging-guided arms of TaSER and ARCTIC, compared with the control arms in those studies, and in IMAGINE-RA, the use of a biologic drug occurred more than 20 times more often in the imaging arms, he noted. And in both TaSER and IMAGINE-RA the rate of serious adverse events was more than doubled in the imaging arms, compared with the controls.
“Just identifying inflammation [in a joint] is not enough to make a diagnosis. Inflammation is normal process, and finding it does not identify a pathological state,” noted Dr. Siebert, a rheumatologist at the University of Glasgow. “Imaging leads to overdiagnosis and overtreatment when physicians use imaging inappropriately,” he concluded.
Dr. Smolen has been a consultant to several drug companies. Dr. Ramiro has been a consultant to or speaker on behalf of AbbVie, Eli Lilly, Merck, Novartis, and Sanofi, and she has received research funding from Merck. Dr. Siebert has been a consultant to or speaker on behalf of AbbVie, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB, and he has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB.
REPORTING FROM EULAR 2019 CONGRESS
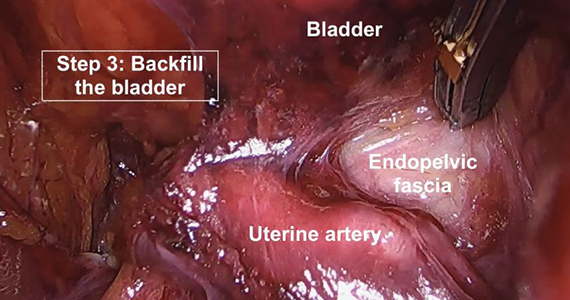
A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
CVD risk upped in postmenopausal breast cancer survivors
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
according to a new study of nearly 300 women.
Previous studies have shown that cardiovascular risk is greater among postmenopausal women treated for breast cancer compared with those without cancer, but specific risk factors have not been well studied, wrote Daniel de Araujo Brito Buttros, MD, of Paulista State University, Sao Paulo, Brazil, and colleagues.
In a study published in Menopause, the researchers evaluated several CVD risk factors in 96 postmenopausal women with breast cancer and 192 women without breast cancer, including metabolic syndrome, subclinical atherosclerosis, and heat shock proteins (HSP) 60 and 70.
Overall, breast cancer patients had significantly higher HSP60 levels and lower HSP70 levels than those of their cancer-free peers. These two proteins have an antagonistic relationship in cardiovascular disease, with HSP60 considered a risk factor for CVD, and HSP70 considered a protective factor. Average HSP60 levels for the breast cancer and control groups were 35 ng/mL and 10.8 ng/mL, respectively; average HSP70 levels were 0.5 ng/mL and 1.3 ng/mL, respectively.
Both diabetes and metabolic syndrome were significantly more common among breast cancer patients vs. controls (19.8% vs. 6.8% and 54.2% vs. 30.7%, respectively). Carotid artery plaque also was more common in breast cancer patients vs. controls (19.8% vs. 9.4%, respectively, P = 0.013).
In addition, systolic and diastolic blood pressure levels were significantly higher among the breast cancer patients, as were triglycerides and glucose.
The findings were limited by several factors including the cross-sectional design that could not prove a causal relationship between CVD risk and breast cancer, the researchers noted.
However, the results demonstrate the increased CVD risk for breast cancer patients, and “[therefore], women diagnosed with breast cancer might receive multidisciplinary care, including cardiology consultation at the time of breast cancer diagnosis and also during oncologic follow-up visits,” they said.
“Heart disease appears more commonly in women treated for breast cancer because of the toxicities of chemotherapy, radiation therapy, and use of aromatase inhibitors, which lower estrogen. Heart-healthy lifestyle modifications will decrease both the risk of recurrent breast cancer and the risk of developing heart disease,” JoAnn Pinkerton, MD, executive director of the North American Menopause Society, said in a statement. “Women should schedule a cardiology consultation when breast cancer is diagnosed and continue with ongoing follow-up after cancer treatments are completed,” she emphasized.
The researchers had no financial conflicts to disclose.
SOURCE: Buttros DAB et al. Menopause. 2019. doi: 10.1097/GME.0000000000001348.
FROM MENOPAUSE
Neurogastroenterology and motility fellowships
“So you want to be a gastroenterologist? What do you really want to do?” This is not an uncommon question that a trainee is faced with when progressing through residency and gastroenterology fellowship.
The list of possibilities includes general gastroenterology, advanced endoscopy, transplant hepatology, and neurogastroenterology and motility. From there, each subspecialty can be broken down further into organ system or a specific procedure of interest. Another necessary question is whether to pursue a career in academics or private practice. The first is for the resident who is interested in gaining experience in gastroenterology prior to starting a general gastroenterology fellowship (there are two programs that currently allow for this pathway). The other track is for those who have completed a general gastroenterology fellowship and are looking to enhance their academic careers by pursuing additional training in neurogastroenterology and motility.
There is currently a need for gastroenterologists interested in neurogastroenterology and motility. Among the most common diagnoses in an ambulatory setting, based on International Classification of Disease (ICD) coding, are abdominal pain, gastroesophageal reflux disease, constipation, nausea and vomiting, irritable bowel syndrome, functional dyspepsia, and dysphagia.1,2 While many fellows are exposed to a wide range of motility patients during general gastroenterology fellowship, there is typically not a sufficient amount of training to attain “level 2” proficiency.2,3 In an effort to help standardize training there are recommended thresholds established and advanced training in neurogastroenterology and motility can help fellows to attain that proficiency.2,3 The extra year can also help you prepare to run a motility lab, train nurses, establish lab protocols and quality standards, and manage referrals, which are important skills as a neurogastroenterology and motility specialist.
Types of programs
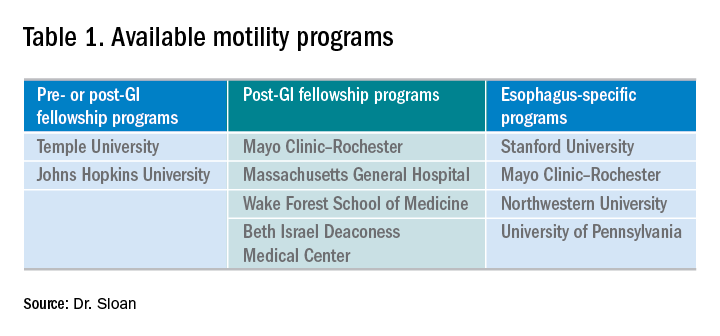
There are several different types of motility programs available. As mentioned previously, some programs afford individuals an opportunity to gain additional experience in gastroenterology before progressing to general gastroenterology fellowship. There are two programs that offer a 1-year fellowship in neurogastroenterology and motility, both prior to or after a general gastroenterology fellowship. Four programs offer 1-year neurogastroenterology and motility fellowships only after a general gastroenterology fellowship. While the neurogastroenterology and motility fellowships cover esophageal motility, there are four programs that specifically focus solely on the esophagus (Table 1).
In addition to pursuing an extra year of training, interested gastroenterology fellows may choose to explore a 1-month Clinical Training Program sponsored by the American Neurogastroenterology and Motility Society (ANMS) at 1 of 10 centers.
Where to find programs
Currently, there is not a singular list of neurogastroenterology and motility programs available for review as you might find with an Accreditation Council for Graduate Medical Education (ACGME) residency or fellowship. At present, the best way to identify the available programs is to search online. Motivation to select a specific program may be related to individual preference and can include geography and department expertise; this ultimately helps to create a focused list. With regard to the ANMS 1-month Clinical Training Program, the list of available programs is available on the society’s website and is for fellows currently in training who wish to incorporate neurogastroenterology and motility into their general GI fellowship.
How to apply
Advanced training in neurogastroenterology and motility is currently a non-ACGME pathway and does not offer a match process for its applicants. After identifying a program of interest, one can find specific instructions on how to apply at the programs’ websites. Typically the process involves reaching out to the program director, writing a letter of interest or personal statement, providing letters of recommendation, and interviewing. Each program has some variability in what is required and attention should be paid to the criteria listed on the specific website.
My experience
I was fortunate to have substantial exposure to esophageal motility in my general gastroenterology fellowship. Gaining this experience was invaluable and laid the foundation for my interest in neurogastroenterology and motility, and, specifically, esophageal dysmotility. My interest in neurogastroenterology and motility then collided with my desire to pursue a career in academics. Knowing the general trajectory for my future career, I began exploring the possibility of undergoing an additional 1-year fellowship early in my second year of GI fellowship. I worked closely with my program director to help define my future goals and to identify available places that would help me attain those goals. While I continued to have an interest in the esophagus, additional training in neurogastroenterology and motility would broaden my understanding and enhance my ability to manage complex patients and perform research at a tertiary care center. I investigated the different neurogastroenterology and motility fellowship programs online and followed the online application instructions. Utilizing national gastroenterology society conferences as networking opportunities, I was able to meet with the program director of my current neurogastroenterology and motility fellowship. In my third year of general gastroenterology fellowship I formally interviewed with the motility group at Johns Hopkins and was later accepted into the neurogastroenterology and motility fellowship program.
Now, nearing the end of my 1-year neurogastroenterology and motility fellowship, I reflect on my extremely positive experience. Throughout the course of the year I have been able to work with multiple GI providers — each with their own area of expertise within the field. There has been a profound exposure to a wide variety of patients with a spectrum of motility conditions covering the entire GI tract. There has been ample opportunity to read motility studies with the guidance and support of the motility faculty to further enhance my skills. The additional year has broadened my exposure to, and the management of, the biopsychosocial aspect of this specific patient population. In line with that, I have had the ability to grow with regard to my use of pharmacology and recognize which symptom might benefit from a particular neuromodulator. An emphasis was also placed on learning the gut-brain axis, and, through multidisciplinary clinics, I worked closely with other disciplines such as psychiatry and GI clinical psychology. Furthermore, the additional year has allowed me to be involved in several research projects within neurogastroenterology and motility that will undoubtedly enhance my future career.
Conclusion
Deciding to pursue an additional year in neurogastroenterology and motility has been one that has helped to give a solid direction to my budding career. It has left me confident in managing this diverse and complex patient population and has helped prepare me for a career in academic gastroenterology. For those who are interested in academic neurogastroenterology and motility, an additional fellowship can help define you as a gastroenterologist and help you to pursue the career of your dreams.
Dr. Sloan is a clinical instructor in the division of gastroenterology at Johns Hopkins in Baltimore.
References
1. Peery A. et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015;149:1731-41.e3.
2. Rao S., Parkman H. Advanced training in neurogastroenterology and gastrointestinal motility. Gastroenterology 2015;148:881-5.
3. American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association (AGA) Institute, and American Society for Gastrointestinal Endoscopy. The gastroenterology core curriculum, third edition. Gastroenterology 2007;132:2012-18.
“So you want to be a gastroenterologist? What do you really want to do?” This is not an uncommon question that a trainee is faced with when progressing through residency and gastroenterology fellowship.
The list of possibilities includes general gastroenterology, advanced endoscopy, transplant hepatology, and neurogastroenterology and motility. From there, each subspecialty can be broken down further into organ system or a specific procedure of interest. Another necessary question is whether to pursue a career in academics or private practice. The first is for the resident who is interested in gaining experience in gastroenterology prior to starting a general gastroenterology fellowship (there are two programs that currently allow for this pathway). The other track is for those who have completed a general gastroenterology fellowship and are looking to enhance their academic careers by pursuing additional training in neurogastroenterology and motility.
There is currently a need for gastroenterologists interested in neurogastroenterology and motility. Among the most common diagnoses in an ambulatory setting, based on International Classification of Disease (ICD) coding, are abdominal pain, gastroesophageal reflux disease, constipation, nausea and vomiting, irritable bowel syndrome, functional dyspepsia, and dysphagia.1,2 While many fellows are exposed to a wide range of motility patients during general gastroenterology fellowship, there is typically not a sufficient amount of training to attain “level 2” proficiency.2,3 In an effort to help standardize training there are recommended thresholds established and advanced training in neurogastroenterology and motility can help fellows to attain that proficiency.2,3 The extra year can also help you prepare to run a motility lab, train nurses, establish lab protocols and quality standards, and manage referrals, which are important skills as a neurogastroenterology and motility specialist.
Types of programs
There are several different types of motility programs available. As mentioned previously, some programs afford individuals an opportunity to gain additional experience in gastroenterology before progressing to general gastroenterology fellowship. There are two programs that offer a 1-year fellowship in neurogastroenterology and motility, both prior to or after a general gastroenterology fellowship. Four programs offer 1-year neurogastroenterology and motility fellowships only after a general gastroenterology fellowship. While the neurogastroenterology and motility fellowships cover esophageal motility, there are four programs that specifically focus solely on the esophagus (Table 1).

In addition to pursuing an extra year of training, interested gastroenterology fellows may choose to explore a 1-month Clinical Training Program sponsored by the American Neurogastroenterology and Motility Society (ANMS) at 1 of 10 centers.
Where to find programs
Currently, there is not a singular list of neurogastroenterology and motility programs available for review as you might find with an Accreditation Council for Graduate Medical Education (ACGME) residency or fellowship. At present, the best way to identify the available programs is to search online. Motivation to select a specific program may be related to individual preference and can include geography and department expertise; this ultimately helps to create a focused list. With regard to the ANMS 1-month Clinical Training Program, the list of available programs is available on the society’s website and is for fellows currently in training who wish to incorporate neurogastroenterology and motility into their general GI fellowship.
How to apply
Advanced training in neurogastroenterology and motility is currently a non-ACGME pathway and does not offer a match process for its applicants. After identifying a program of interest, one can find specific instructions on how to apply at the programs’ websites. Typically the process involves reaching out to the program director, writing a letter of interest or personal statement, providing letters of recommendation, and interviewing. Each program has some variability in what is required and attention should be paid to the criteria listed on the specific website.
My experience
I was fortunate to have substantial exposure to esophageal motility in my general gastroenterology fellowship. Gaining this experience was invaluable and laid the foundation for my interest in neurogastroenterology and motility, and, specifically, esophageal dysmotility. My interest in neurogastroenterology and motility then collided with my desire to pursue a career in academics. Knowing the general trajectory for my future career, I began exploring the possibility of undergoing an additional 1-year fellowship early in my second year of GI fellowship. I worked closely with my program director to help define my future goals and to identify available places that would help me attain those goals. While I continued to have an interest in the esophagus, additional training in neurogastroenterology and motility would broaden my understanding and enhance my ability to manage complex patients and perform research at a tertiary care center. I investigated the different neurogastroenterology and motility fellowship programs online and followed the online application instructions. Utilizing national gastroenterology society conferences as networking opportunities, I was able to meet with the program director of my current neurogastroenterology and motility fellowship. In my third year of general gastroenterology fellowship I formally interviewed with the motility group at Johns Hopkins and was later accepted into the neurogastroenterology and motility fellowship program.
Now, nearing the end of my 1-year neurogastroenterology and motility fellowship, I reflect on my extremely positive experience. Throughout the course of the year I have been able to work with multiple GI providers — each with their own area of expertise within the field. There has been a profound exposure to a wide variety of patients with a spectrum of motility conditions covering the entire GI tract. There has been ample opportunity to read motility studies with the guidance and support of the motility faculty to further enhance my skills. The additional year has broadened my exposure to, and the management of, the biopsychosocial aspect of this specific patient population. In line with that, I have had the ability to grow with regard to my use of pharmacology and recognize which symptom might benefit from a particular neuromodulator. An emphasis was also placed on learning the gut-brain axis, and, through multidisciplinary clinics, I worked closely with other disciplines such as psychiatry and GI clinical psychology. Furthermore, the additional year has allowed me to be involved in several research projects within neurogastroenterology and motility that will undoubtedly enhance my future career.
Conclusion
Deciding to pursue an additional year in neurogastroenterology and motility has been one that has helped to give a solid direction to my budding career. It has left me confident in managing this diverse and complex patient population and has helped prepare me for a career in academic gastroenterology. For those who are interested in academic neurogastroenterology and motility, an additional fellowship can help define you as a gastroenterologist and help you to pursue the career of your dreams.
Dr. Sloan is a clinical instructor in the division of gastroenterology at Johns Hopkins in Baltimore.
References
1. Peery A. et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015;149:1731-41.e3.
2. Rao S., Parkman H. Advanced training in neurogastroenterology and gastrointestinal motility. Gastroenterology 2015;148:881-5.
3. American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association (AGA) Institute, and American Society for Gastrointestinal Endoscopy. The gastroenterology core curriculum, third edition. Gastroenterology 2007;132:2012-18.
“So you want to be a gastroenterologist? What do you really want to do?” This is not an uncommon question that a trainee is faced with when progressing through residency and gastroenterology fellowship.
The list of possibilities includes general gastroenterology, advanced endoscopy, transplant hepatology, and neurogastroenterology and motility. From there, each subspecialty can be broken down further into organ system or a specific procedure of interest. Another necessary question is whether to pursue a career in academics or private practice. The first is for the resident who is interested in gaining experience in gastroenterology prior to starting a general gastroenterology fellowship (there are two programs that currently allow for this pathway). The other track is for those who have completed a general gastroenterology fellowship and are looking to enhance their academic careers by pursuing additional training in neurogastroenterology and motility.
There is currently a need for gastroenterologists interested in neurogastroenterology and motility. Among the most common diagnoses in an ambulatory setting, based on International Classification of Disease (ICD) coding, are abdominal pain, gastroesophageal reflux disease, constipation, nausea and vomiting, irritable bowel syndrome, functional dyspepsia, and dysphagia.1,2 While many fellows are exposed to a wide range of motility patients during general gastroenterology fellowship, there is typically not a sufficient amount of training to attain “level 2” proficiency.2,3 In an effort to help standardize training there are recommended thresholds established and advanced training in neurogastroenterology and motility can help fellows to attain that proficiency.2,3 The extra year can also help you prepare to run a motility lab, train nurses, establish lab protocols and quality standards, and manage referrals, which are important skills as a neurogastroenterology and motility specialist.
Types of programs
There are several different types of motility programs available. As mentioned previously, some programs afford individuals an opportunity to gain additional experience in gastroenterology before progressing to general gastroenterology fellowship. There are two programs that offer a 1-year fellowship in neurogastroenterology and motility, both prior to or after a general gastroenterology fellowship. Four programs offer 1-year neurogastroenterology and motility fellowships only after a general gastroenterology fellowship. While the neurogastroenterology and motility fellowships cover esophageal motility, there are four programs that specifically focus solely on the esophagus (Table 1).

In addition to pursuing an extra year of training, interested gastroenterology fellows may choose to explore a 1-month Clinical Training Program sponsored by the American Neurogastroenterology and Motility Society (ANMS) at 1 of 10 centers.
Where to find programs
Currently, there is not a singular list of neurogastroenterology and motility programs available for review as you might find with an Accreditation Council for Graduate Medical Education (ACGME) residency or fellowship. At present, the best way to identify the available programs is to search online. Motivation to select a specific program may be related to individual preference and can include geography and department expertise; this ultimately helps to create a focused list. With regard to the ANMS 1-month Clinical Training Program, the list of available programs is available on the society’s website and is for fellows currently in training who wish to incorporate neurogastroenterology and motility into their general GI fellowship.
How to apply
Advanced training in neurogastroenterology and motility is currently a non-ACGME pathway and does not offer a match process for its applicants. After identifying a program of interest, one can find specific instructions on how to apply at the programs’ websites. Typically the process involves reaching out to the program director, writing a letter of interest or personal statement, providing letters of recommendation, and interviewing. Each program has some variability in what is required and attention should be paid to the criteria listed on the specific website.
My experience
I was fortunate to have substantial exposure to esophageal motility in my general gastroenterology fellowship. Gaining this experience was invaluable and laid the foundation for my interest in neurogastroenterology and motility, and, specifically, esophageal dysmotility. My interest in neurogastroenterology and motility then collided with my desire to pursue a career in academics. Knowing the general trajectory for my future career, I began exploring the possibility of undergoing an additional 1-year fellowship early in my second year of GI fellowship. I worked closely with my program director to help define my future goals and to identify available places that would help me attain those goals. While I continued to have an interest in the esophagus, additional training in neurogastroenterology and motility would broaden my understanding and enhance my ability to manage complex patients and perform research at a tertiary care center. I investigated the different neurogastroenterology and motility fellowship programs online and followed the online application instructions. Utilizing national gastroenterology society conferences as networking opportunities, I was able to meet with the program director of my current neurogastroenterology and motility fellowship. In my third year of general gastroenterology fellowship I formally interviewed with the motility group at Johns Hopkins and was later accepted into the neurogastroenterology and motility fellowship program.
Now, nearing the end of my 1-year neurogastroenterology and motility fellowship, I reflect on my extremely positive experience. Throughout the course of the year I have been able to work with multiple GI providers — each with their own area of expertise within the field. There has been a profound exposure to a wide variety of patients with a spectrum of motility conditions covering the entire GI tract. There has been ample opportunity to read motility studies with the guidance and support of the motility faculty to further enhance my skills. The additional year has broadened my exposure to, and the management of, the biopsychosocial aspect of this specific patient population. In line with that, I have had the ability to grow with regard to my use of pharmacology and recognize which symptom might benefit from a particular neuromodulator. An emphasis was also placed on learning the gut-brain axis, and, through multidisciplinary clinics, I worked closely with other disciplines such as psychiatry and GI clinical psychology. Furthermore, the additional year has allowed me to be involved in several research projects within neurogastroenterology and motility that will undoubtedly enhance my future career.
Conclusion
Deciding to pursue an additional year in neurogastroenterology and motility has been one that has helped to give a solid direction to my budding career. It has left me confident in managing this diverse and complex patient population and has helped prepare me for a career in academic gastroenterology. For those who are interested in academic neurogastroenterology and motility, an additional fellowship can help define you as a gastroenterologist and help you to pursue the career of your dreams.
Dr. Sloan is a clinical instructor in the division of gastroenterology at Johns Hopkins in Baltimore.
References
1. Peery A. et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015;149:1731-41.e3.
2. Rao S., Parkman H. Advanced training in neurogastroenterology and gastrointestinal motility. Gastroenterology 2015;148:881-5.
3. American Association for the Study of Liver Diseases, American College of Gastroenterology, American Gastroenterological Association (AGA) Institute, and American Society for Gastrointestinal Endoscopy. The gastroenterology core curriculum, third edition. Gastroenterology 2007;132:2012-18.
Sexual assault in military linked to sexual pain
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
FROM OBSTETRICS & GYNECOLOGY
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE