User login
Food insecurity tied to migraine in young adults
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
, according to Jason M. Nagata, MD, of the University of California, San Francisco, and associates.
Data were collected from a cross-sectional, nationally representative set of 14,786 young adults in the United States aged 24-32 years who participated in the 2008 National Longitudinal Study of Adolescent to Adult Health, the investigators wrote in a research letter published in JAMA Neurology.
Food insecurity was assessed by self-report through the interview question, “In the past 12 months, was there a time when (you/your household were/was) worried whether food would run out before you would get money to buy more?” Migraine was assessed by a positive answer to the interview question, “Has a doctor, nurse, or other health care professional ever told you that you have or had migraine headaches?”
In all, 1,647 study participants (11%) reported food insecurity; the prevalence of migraine in this group was 23.9%, compared with a prevalence of 13.6% in participants who did not report food insecurity. The association between food insecurity and migraine was significant both before (odds ratio, 2.00; 95% confidence interval, 1.68-2.38; P less than .001) and after adjustment (adjusted OR, .58; 95% CI, 1.30-1.95; P less than .001).
“Health care clinicians caring for persons who experience migraine should consider screening for food insecurity as a potential contributor to migraine exacerbations and provide referrals to programs such as the Supplemental Nutrition Assistance Program [formerly the Food Stamp Program] when appropriate,” the investigators concluded (JAMA Neurol. 2019 Jun 24. doi: 10.1001/jamaneurol.2019.1663).
No conflicts of interest were reported. The study was supported by grants from the University of California Global Food Initiative Fellowship, the American Academy of Pediatrics, the American Pediatric Society, and the Norman Schlossberger Research Fund from the University of California.
FROM JAMA NEUROLOGY
Patients concerned about clinician burnout
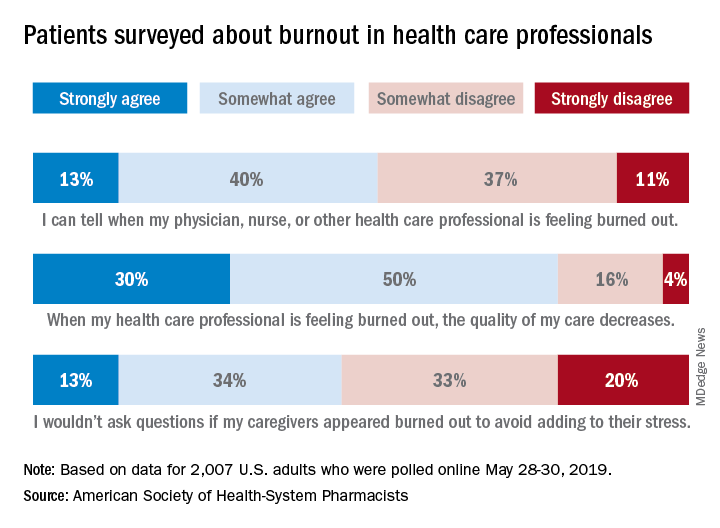
Almost three-quarters of Americans are concerned about burnout among health care professionals, according to the American Society of Health-System Pharmacists.
The public is aware “that burnout among pharmacists, physicians, nurses, and other professionals can lead to impaired attention and decreased functioning that threatens to cause medical errors and reduce safety,” the ASHP said when it released data from a survey conducted May 28-30, 2019, by the Harris Poll.
Those data show that 23% of respondents were very concerned and 51% were somewhat concerned about burnout among health care providers. Just over half (53%) of the 2,007 adults involved said that they could tell when a provider was burned out, suggesting that health care professionals “may be conveying signs of burnout to their patients without knowing it,” the society noted.
A majority of respondents (80%) felt that the quality of their care was affected when their physician, nurse, pharmacist, or other health care professional was burned out, and almost half (47%) said that they would avoid asking questions if their provider appeared burned out because they wouldn’t want to add to that person’s stress, the ASHP said.
“A healthy and thriving clinician workforce is essential to ensure optimal patient health outcomes and safety,” said Paul W. Abramowitz, PharmD, chief executive officer of the ASHP. “Within the healthcare industry, we are working to help build a culture of resilience and well-being to ensure that no patient or clinician is harmed due to burnout; but it takes a concerted effort from all entities involved – providers and healthcare organizations.”
Almost three-quarters of Americans are concerned about burnout among health care professionals, according to the American Society of Health-System Pharmacists.

The public is aware “that burnout among pharmacists, physicians, nurses, and other professionals can lead to impaired attention and decreased functioning that threatens to cause medical errors and reduce safety,” the ASHP said when it released data from a survey conducted May 28-30, 2019, by the Harris Poll.
Those data show that 23% of respondents were very concerned and 51% were somewhat concerned about burnout among health care providers. Just over half (53%) of the 2,007 adults involved said that they could tell when a provider was burned out, suggesting that health care professionals “may be conveying signs of burnout to their patients without knowing it,” the society noted.
A majority of respondents (80%) felt that the quality of their care was affected when their physician, nurse, pharmacist, or other health care professional was burned out, and almost half (47%) said that they would avoid asking questions if their provider appeared burned out because they wouldn’t want to add to that person’s stress, the ASHP said.
“A healthy and thriving clinician workforce is essential to ensure optimal patient health outcomes and safety,” said Paul W. Abramowitz, PharmD, chief executive officer of the ASHP. “Within the healthcare industry, we are working to help build a culture of resilience and well-being to ensure that no patient or clinician is harmed due to burnout; but it takes a concerted effort from all entities involved – providers and healthcare organizations.”
Almost three-quarters of Americans are concerned about burnout among health care professionals, according to the American Society of Health-System Pharmacists.

The public is aware “that burnout among pharmacists, physicians, nurses, and other professionals can lead to impaired attention and decreased functioning that threatens to cause medical errors and reduce safety,” the ASHP said when it released data from a survey conducted May 28-30, 2019, by the Harris Poll.
Those data show that 23% of respondents were very concerned and 51% were somewhat concerned about burnout among health care providers. Just over half (53%) of the 2,007 adults involved said that they could tell when a provider was burned out, suggesting that health care professionals “may be conveying signs of burnout to their patients without knowing it,” the society noted.
A majority of respondents (80%) felt that the quality of their care was affected when their physician, nurse, pharmacist, or other health care professional was burned out, and almost half (47%) said that they would avoid asking questions if their provider appeared burned out because they wouldn’t want to add to that person’s stress, the ASHP said.
“A healthy and thriving clinician workforce is essential to ensure optimal patient health outcomes and safety,” said Paul W. Abramowitz, PharmD, chief executive officer of the ASHP. “Within the healthcare industry, we are working to help build a culture of resilience and well-being to ensure that no patient or clinician is harmed due to burnout; but it takes a concerted effort from all entities involved – providers and healthcare organizations.”
Immunotherapy-treated NSCLC: Adverse impact of steroids driven by palliative indications
Giving steroids for indications other than cancer palliation doesn’t compromise the effectiveness of immunotherapy for advanced non–small cell lung cancer (NSCLC), suggests a single-center retrospective cohort study.
The immunosuppressant activity of corticosteroids and recent reports linking them to poorer outcomes has raised concern about their use during immunotherapy, noted Biagio Ricciuti, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. But mechanisms underpinning this association are unclear.
The investigators studied 650 patients with NSCLC treated with immunotherapy targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1), either as monotherapy or with other immunotherapy. Overall, 14.3% were receiving 10 mg or more of prednisone daily when they started the immunotherapy, a cutoff selected for study because it has been used an exclusion criterion for clinical trials.
Results reported in the Journal of Clinical Oncology showed that, compared with other patients, those who received 10 mg or more of steroids indeed had poorer median progression-free survival (2.0 vs. 3.4 months; P = .01) and overall survival (4.9 vs. 11.2 months; P less than .001).
However, when the indication for steroid therapy was considered, median progression-free survival was just 1.4 months among patients who received 10 mg or more prednisone for cancer-related palliation, compared with 4.6 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons (for example, autoimmune disease, chronic obstructive pulmonary disease flare, hypersensitivity prophylaxis, or management of noncancer pain) and 3.4 months among patients who received 0-10 mg of prednisone (P less than .001 across groups).
Similarly, median overall survival was just 2.2 months among patients who received 10 mg or more prednisone for palliative indications, but 10.7 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons and 11.2 months among patients who received less than 10 mg prednisone (P less than .001 across groups).
In a multivariate analysis that adjusted for performance status and PD-L1 positivity and that used patients receiving up to 10 mg prednisone as the comparator, patients receiving 10 mg or more for cancer palliation had a trend toward high risk of progression-free survival events and a higher risk of death (hazard ratio, 1.40; P less than .06 and HR, 1.60; P = .02, respectively). In contrast, patients receiving 10 mg or more for cancer-unrelated reasons did not have elevated risks (HR, 0.62; P = .14 and HR, 0.91; P = .79, respectively).
“These data suggest that the significantly worse outcomes among patients who receive corticosteroids before immunotherapy are driven by the group of patients treated with corticosteroids for palliative oncologic symptom management, rather than by patients receiving corticosteroids for other reasons,” Dr. Ricciuti and coinvestigators wrote. “Corticosteroid use for cancer symptom management might simply correlate with patients who have adverse prognostic factors (e.g., brain metastases and poor performance status) rather than cause a clinically significant blunting of the response to [immune checkpoint inhibitors].
“Our data suggest that corticosteroids should not necessarily be decreased or discontinued before the start of immunotherapy out of a theoretical concern that corticosteroids could impair a response to immunotherapy,” the investigators concluded. “Additional mechanistic studies are needed to identify whether the use of corticosteroids affects specific aspects of the immune system necessary for immunotherapy activity.”
Dr. Ricciuti reported that he has no relevant conflicts of interest. The study did not receive any funding.
SOURCE: Ricciuti B et al. J Clin Oncol. 2019 Jun 17. doi: 10.1200/JCO.19.00189.
Giving steroids for indications other than cancer palliation doesn’t compromise the effectiveness of immunotherapy for advanced non–small cell lung cancer (NSCLC), suggests a single-center retrospective cohort study.
The immunosuppressant activity of corticosteroids and recent reports linking them to poorer outcomes has raised concern about their use during immunotherapy, noted Biagio Ricciuti, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. But mechanisms underpinning this association are unclear.
The investigators studied 650 patients with NSCLC treated with immunotherapy targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1), either as monotherapy or with other immunotherapy. Overall, 14.3% were receiving 10 mg or more of prednisone daily when they started the immunotherapy, a cutoff selected for study because it has been used an exclusion criterion for clinical trials.
Results reported in the Journal of Clinical Oncology showed that, compared with other patients, those who received 10 mg or more of steroids indeed had poorer median progression-free survival (2.0 vs. 3.4 months; P = .01) and overall survival (4.9 vs. 11.2 months; P less than .001).
However, when the indication for steroid therapy was considered, median progression-free survival was just 1.4 months among patients who received 10 mg or more prednisone for cancer-related palliation, compared with 4.6 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons (for example, autoimmune disease, chronic obstructive pulmonary disease flare, hypersensitivity prophylaxis, or management of noncancer pain) and 3.4 months among patients who received 0-10 mg of prednisone (P less than .001 across groups).
Similarly, median overall survival was just 2.2 months among patients who received 10 mg or more prednisone for palliative indications, but 10.7 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons and 11.2 months among patients who received less than 10 mg prednisone (P less than .001 across groups).
In a multivariate analysis that adjusted for performance status and PD-L1 positivity and that used patients receiving up to 10 mg prednisone as the comparator, patients receiving 10 mg or more for cancer palliation had a trend toward high risk of progression-free survival events and a higher risk of death (hazard ratio, 1.40; P less than .06 and HR, 1.60; P = .02, respectively). In contrast, patients receiving 10 mg or more for cancer-unrelated reasons did not have elevated risks (HR, 0.62; P = .14 and HR, 0.91; P = .79, respectively).
“These data suggest that the significantly worse outcomes among patients who receive corticosteroids before immunotherapy are driven by the group of patients treated with corticosteroids for palliative oncologic symptom management, rather than by patients receiving corticosteroids for other reasons,” Dr. Ricciuti and coinvestigators wrote. “Corticosteroid use for cancer symptom management might simply correlate with patients who have adverse prognostic factors (e.g., brain metastases and poor performance status) rather than cause a clinically significant blunting of the response to [immune checkpoint inhibitors].
“Our data suggest that corticosteroids should not necessarily be decreased or discontinued before the start of immunotherapy out of a theoretical concern that corticosteroids could impair a response to immunotherapy,” the investigators concluded. “Additional mechanistic studies are needed to identify whether the use of corticosteroids affects specific aspects of the immune system necessary for immunotherapy activity.”
Dr. Ricciuti reported that he has no relevant conflicts of interest. The study did not receive any funding.
SOURCE: Ricciuti B et al. J Clin Oncol. 2019 Jun 17. doi: 10.1200/JCO.19.00189.
Giving steroids for indications other than cancer palliation doesn’t compromise the effectiveness of immunotherapy for advanced non–small cell lung cancer (NSCLC), suggests a single-center retrospective cohort study.
The immunosuppressant activity of corticosteroids and recent reports linking them to poorer outcomes has raised concern about their use during immunotherapy, noted Biagio Ricciuti, MD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston, and coinvestigators. But mechanisms underpinning this association are unclear.
The investigators studied 650 patients with NSCLC treated with immunotherapy targeting programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1), either as monotherapy or with other immunotherapy. Overall, 14.3% were receiving 10 mg or more of prednisone daily when they started the immunotherapy, a cutoff selected for study because it has been used an exclusion criterion for clinical trials.
Results reported in the Journal of Clinical Oncology showed that, compared with other patients, those who received 10 mg or more of steroids indeed had poorer median progression-free survival (2.0 vs. 3.4 months; P = .01) and overall survival (4.9 vs. 11.2 months; P less than .001).
However, when the indication for steroid therapy was considered, median progression-free survival was just 1.4 months among patients who received 10 mg or more prednisone for cancer-related palliation, compared with 4.6 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons (for example, autoimmune disease, chronic obstructive pulmonary disease flare, hypersensitivity prophylaxis, or management of noncancer pain) and 3.4 months among patients who received 0-10 mg of prednisone (P less than .001 across groups).
Similarly, median overall survival was just 2.2 months among patients who received 10 mg or more prednisone for palliative indications, but 10.7 months among patients who received 10 mg or more prednisone for cancer-unrelated reasons and 11.2 months among patients who received less than 10 mg prednisone (P less than .001 across groups).
In a multivariate analysis that adjusted for performance status and PD-L1 positivity and that used patients receiving up to 10 mg prednisone as the comparator, patients receiving 10 mg or more for cancer palliation had a trend toward high risk of progression-free survival events and a higher risk of death (hazard ratio, 1.40; P less than .06 and HR, 1.60; P = .02, respectively). In contrast, patients receiving 10 mg or more for cancer-unrelated reasons did not have elevated risks (HR, 0.62; P = .14 and HR, 0.91; P = .79, respectively).
“These data suggest that the significantly worse outcomes among patients who receive corticosteroids before immunotherapy are driven by the group of patients treated with corticosteroids for palliative oncologic symptom management, rather than by patients receiving corticosteroids for other reasons,” Dr. Ricciuti and coinvestigators wrote. “Corticosteroid use for cancer symptom management might simply correlate with patients who have adverse prognostic factors (e.g., brain metastases and poor performance status) rather than cause a clinically significant blunting of the response to [immune checkpoint inhibitors].
“Our data suggest that corticosteroids should not necessarily be decreased or discontinued before the start of immunotherapy out of a theoretical concern that corticosteroids could impair a response to immunotherapy,” the investigators concluded. “Additional mechanistic studies are needed to identify whether the use of corticosteroids affects specific aspects of the immune system necessary for immunotherapy activity.”
Dr. Ricciuti reported that he has no relevant conflicts of interest. The study did not receive any funding.
SOURCE: Ricciuti B et al. J Clin Oncol. 2019 Jun 17. doi: 10.1200/JCO.19.00189.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Continue your VAM conversations on SVSConnect
Conversations surrounding the 2019 Vascular Annual Meeting have begun on SVSConnect. Share what you learned at your favorite session, start a discussion about the Branding Initiative or reminisce about the ‘Vascular Spectacular’ gala. Keep the conversations going and connect with other attendees you met – or didn’t meet – at the conference. Simply log in with your SVS credentials to get started. Reach out to communications@vascularsociety.org or call 312-334-2300 with questions.
Conversations surrounding the 2019 Vascular Annual Meeting have begun on SVSConnect. Share what you learned at your favorite session, start a discussion about the Branding Initiative or reminisce about the ‘Vascular Spectacular’ gala. Keep the conversations going and connect with other attendees you met – or didn’t meet – at the conference. Simply log in with your SVS credentials to get started. Reach out to communications@vascularsociety.org or call 312-334-2300 with questions.
Conversations surrounding the 2019 Vascular Annual Meeting have begun on SVSConnect. Share what you learned at your favorite session, start a discussion about the Branding Initiative or reminisce about the ‘Vascular Spectacular’ gala. Keep the conversations going and connect with other attendees you met – or didn’t meet – at the conference. Simply log in with your SVS credentials to get started. Reach out to communications@vascularsociety.org or call 312-334-2300 with questions.
Provide feedback on Branding Initiative
The SVS Branding Initiative concepts were introduced at the Vascular Annual Meeting last week. We asked attendees to provide their feedback either at the SVS booth or through the event app. Input from members is vital in moving this forward, and we appreciate all who have shared their thoughts on the topic. If you did not have the opportunity to complete the survey, there is still time! All SVS members are encouraged to provide feedback until the survey closes on June 26. Take the survey here.
The SVS Branding Initiative concepts were introduced at the Vascular Annual Meeting last week. We asked attendees to provide their feedback either at the SVS booth or through the event app. Input from members is vital in moving this forward, and we appreciate all who have shared their thoughts on the topic. If you did not have the opportunity to complete the survey, there is still time! All SVS members are encouraged to provide feedback until the survey closes on June 26. Take the survey here.
The SVS Branding Initiative concepts were introduced at the Vascular Annual Meeting last week. We asked attendees to provide their feedback either at the SVS booth or through the event app. Input from members is vital in moving this forward, and we appreciate all who have shared their thoughts on the topic. If you did not have the opportunity to complete the survey, there is still time! All SVS members are encouraged to provide feedback until the survey closes on June 26. Take the survey here.
VAM on Demand Coming Soon
If you missed the Vascular Annual Meeting, would like to review some sessions or view the ones you missed, purchase VAM on Demand. The online library will hold audio and slide presentations of most sessions from the meeting. It will become available in four-six weeks, at which time a notification will be distributed. Attendees will pay $199 and non-attendees will pay $499. Contact the SVS Education Department for more information at education@vascularsociety.org.
If you missed the Vascular Annual Meeting, would like to review some sessions or view the ones you missed, purchase VAM on Demand. The online library will hold audio and slide presentations of most sessions from the meeting. It will become available in four-six weeks, at which time a notification will be distributed. Attendees will pay $199 and non-attendees will pay $499. Contact the SVS Education Department for more information at education@vascularsociety.org.
If you missed the Vascular Annual Meeting, would like to review some sessions or view the ones you missed, purchase VAM on Demand. The online library will hold audio and slide presentations of most sessions from the meeting. It will become available in four-six weeks, at which time a notification will be distributed. Attendees will pay $199 and non-attendees will pay $499. Contact the SVS Education Department for more information at education@vascularsociety.org.
New single-dose influenza therapy effective among outpatients
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
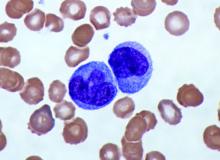
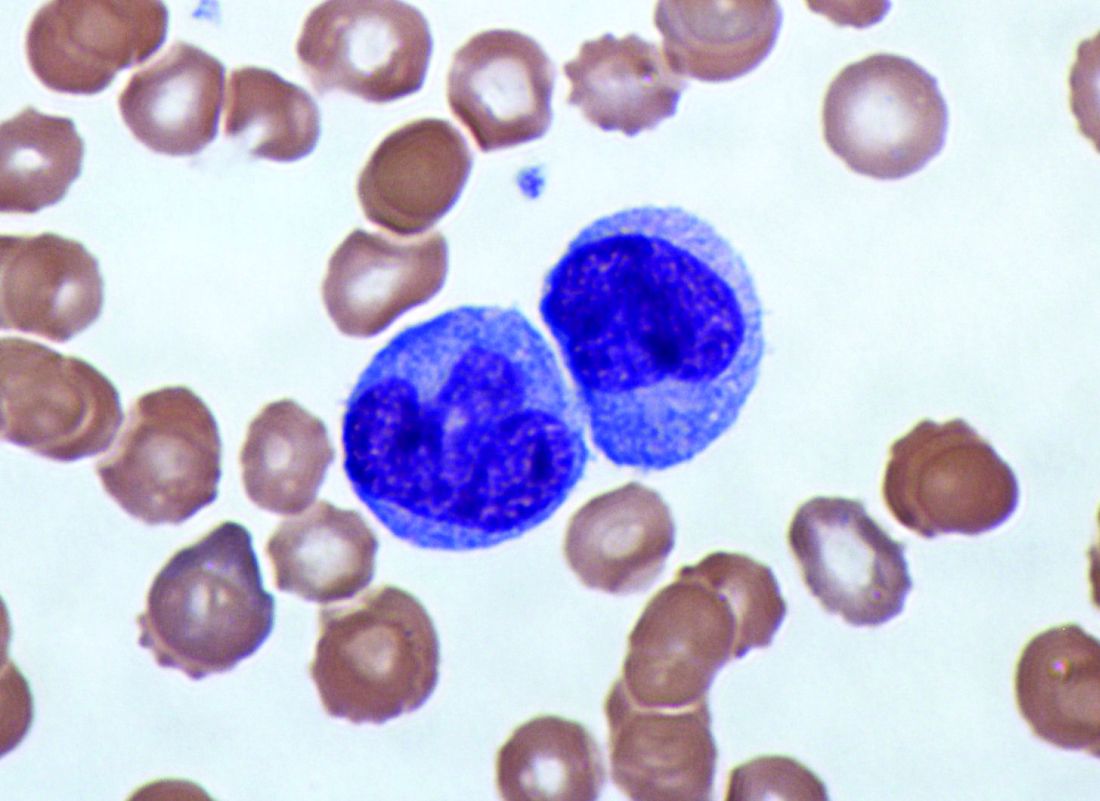
Elevated monocyte count predicts poor outcomes in idiopathic pulmonary fibrosis
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
The study by Scott et al. provides evidence that monocyte count may be a “novel, simple, and inexpensive prognostic biomarker in idiopathic pulmonary fibrosis,” according to an accompanying editorial.
Progress has been made in the treatment of idiopathic pulmonary fibrosis, but patient prognosis remains “challenging to predict,” wrote Michael Kreuter, MD, of University of Heidelberg, Germany, and Toby M. Maher, MB, MSc, PhD, of Royal Brompton Hospital in London and Imperial College London. “One lesson that can be learned from other respiratory disorders is that routinely measured cellular biomarkers, such as blood eosinophil counts in chronic obstructive pulmonary disease (COPD), can predict treatment responses” (Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600[19]30050-5).
Increased blood monocyte counts in idiopathic pulmonary fibrosis may reflect disease activity, which “could explain the outcome differences,” said Dr. Kreuter and Dr. Maher. “As highlighted by the investigators themselves, before introducing assessment of monocyte counts as part of routine clinical care for individuals with idiopathic pulmonary fibrosis, the limitations of this research should be taken into account. These include uncertainty around diagnosis and disease severity in a substantial subset of the patients, and the unknown effect of medical therapies (including corticosteroids and immunosuppressant and antifibrotic drugs) on monocyte counts and prognosis.” Researchers should validate the clinical value of blood monocyte counts in existing and future cohorts and evaluate the biomarker in clinical trials.
The editorialists have received compensation and funding from various pharmaceutical companies.
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
, including hypertrophic cardiomyopathy, systemic sclerosis, and myelofibrosis, according to research published in The Lancet Respiratory Medicine.
The data indicate that “a single threshold value of absolute monocyte counts of 0.95 K/mcL could be used to identify high-risk patients with a fibrotic disease,” said Madeleine K. D. Scott, a researcher at Stanford (Calif.) University, and coauthors. The results “suggest that monocyte count should be incorporated into the clinical assessment” and may “enable more conscientious allocation of scarce resources, including lung transplantations,” they said.
While other published biomarkers – including gene panels and multicytokine signatures – may be expensive and not readily available, “absolute monocyte count is routinely measured as part of a complete blood count, an inexpensive test used in clinical practice worldwide,” the authors said.
Further study of monocytes’ mechanistic role in fibrosis ultimately could point to new treatment approaches.
A retrospective multicenter cohort study
To assess whether immune cells may identify patients with idiopathic pulmonary fibrosis at greater risk of poor outcomes, Ms. Scott and her collaborators conducted a retrospective multicenter cohort study.
They first analyzed transcriptome data from 120 peripheral blood mononuclear cell samples of patients with idiopathic pulmonary fibrosis, which they obtained from the Gene Expression Omnibus at the National Center for Biotechnology Information. They used statistical deconvolution to estimate percentages of 13 immune cell types and examined their associations with transplant-free survival. Their discovery analysis found that estimated CD14+ classical monocyte percentages above the mean correlated with shorter transplant-free survival times (hazard ratio, 1.82), but percentages of T cells and B cells did not.
The researchers then validated these results using samples from patients with idiopathic pulmonary fibrosis in two independent cohorts. In the COMET validation cohort, which included 45 patients with idiopathic pulmonary fibrosis whose monocyte counts were measured using flow cytometry, higher monocyte counts were significantly associated with greater risk of disease progression. In the Yale cohort, which included 15 patients with idiopathic pulmonary fibrosis, the 6 patients who were classified as high risk on the basis of a 52-gene signature had more CD14+ monocytes than the 9 low-risk patients did.
In addition, Ms. Scott and her collaborators looked at complete blood count values in the electronic health records of 45,068 patients with idiopathic pulmonary fibrosis, systemic sclerosis, hypertrophic cardiomyopathy, or myelofibrosis in Stanford, Northwestern, Vanderbilt, and Optum Clinformatics Data Mart cohorts.
Among patients in the COMET, Stanford, and Northwestern datasets, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (HR, 2.47) and the gender, age, and physiology index (HR, 2.06). Data from 7,459 patients with idiopathic pulmonary fibrosis “showed that patients with monocyte counts of 0.95 K/mcL or greater were at increased risk of mortality with lung transplantation as a censoring event, after adjusting for age at diagnosis and sex” in the Stanford (HR, 2.30), Vanderbilt (HR, 1.52), and Optum (HR, 1.74) cohorts. “Likewise, higher absolute monocyte count was associated with shortened survival in patients with hypertrophic cardiomyopathy across all three cohorts, and in patients with systemic sclerosis or myelofibrosis in two of the three cohorts,” the researchers said.
The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
SOURCE: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: An increased monocyte count predicts poor outcomes among patients with idiopathic pulmonary fibrosis and other fibrotic diseases.
Major finding: Among patients in three cohorts, monocyte counts of 0.95 K/mcL or greater were associated with mortality after adjustment for forced vital capacity (hazard ratio, 2.47) and the gender, age, and physiology index (HR, 2.06).
Study details: A retrospective analysis of data from 7,000 patients with idiopathic pulmonary fibrosis from five independent cohorts.
Disclosures: The study was funded by grants from the Bill & Melinda Gates Foundation, U.S. National Institute of Allergy and Infectious Diseases, and U.S. National Library of Medicine. Ms. Scott had no competing interests. Coauthors disclosed grants, compensation, and support from foundations, agencies, and companies.
Source: Scott MKD et al. Lancet Respir Med. 2019 Jun. doi: 10.1016/S2213-2600(18)30508-3.
Briefest flash of light can alter the human circadian system
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
REPORTING FROM SLEEP 2019
Key clinical point: When distributed as flashes, the human circadian system can be phase shifted by extraordinarily brief and dim light.
Major finding: The researchers observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds.
Study details: Two parallel 16-day studies involving 56 healthy men and women in their 20s and 30s.
Disclosures: The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
CF drug picks up indication for children as young as 6
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.
to include children as young as 6 years old who have cystic fibrosis.
The drug was approved in 2018 for patients aged 12 years and older who have the most common cause of the disease, two alleles for the F508del mutation in the gene that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, or at least one other CFTR mutation responsive to the combination, as listed in labeling.
The original approval was based on three phase 3, double blind, placebo-controlled trials, which demonstrated improvements in lung function and other key measures of the disease. One trial that found a 6.8% mean improvement in lung function testing over placebo at 8 weeks, and another that found a 4% improvement at 24 weeks, with fewer respiratory exacerbations and improved respiratory-related quality of life. A third trial in patients without the indicated genetic mutations was ended early for futility.
The efficacy in children under 12 years was extrapolated from those trials, plus an open-label study that found similar effects.
Labeling warns of elevated liver enzymes and cataracts in children, and notes that the drug should be taken with food that contains fat. Labeling also recommends against use with strong cytochrome P450 3A4 (CYP3A) inducers – rifampin, phenobarbital, St. John’s wort, among others – because they might reduce efficacy, and against use with CYP3A inhibitors – ketoconazole, clarithromycin, Seville oranges, grapefruit juice, etc. – because of the risk of increased exposure.
The most common side effects are headache, nausea, sinus congestion, and dizziness. The FDA has cleared a CF gene test to check for the required mutations. Symdeko is marketed by Vertex Pharmaceuticals.