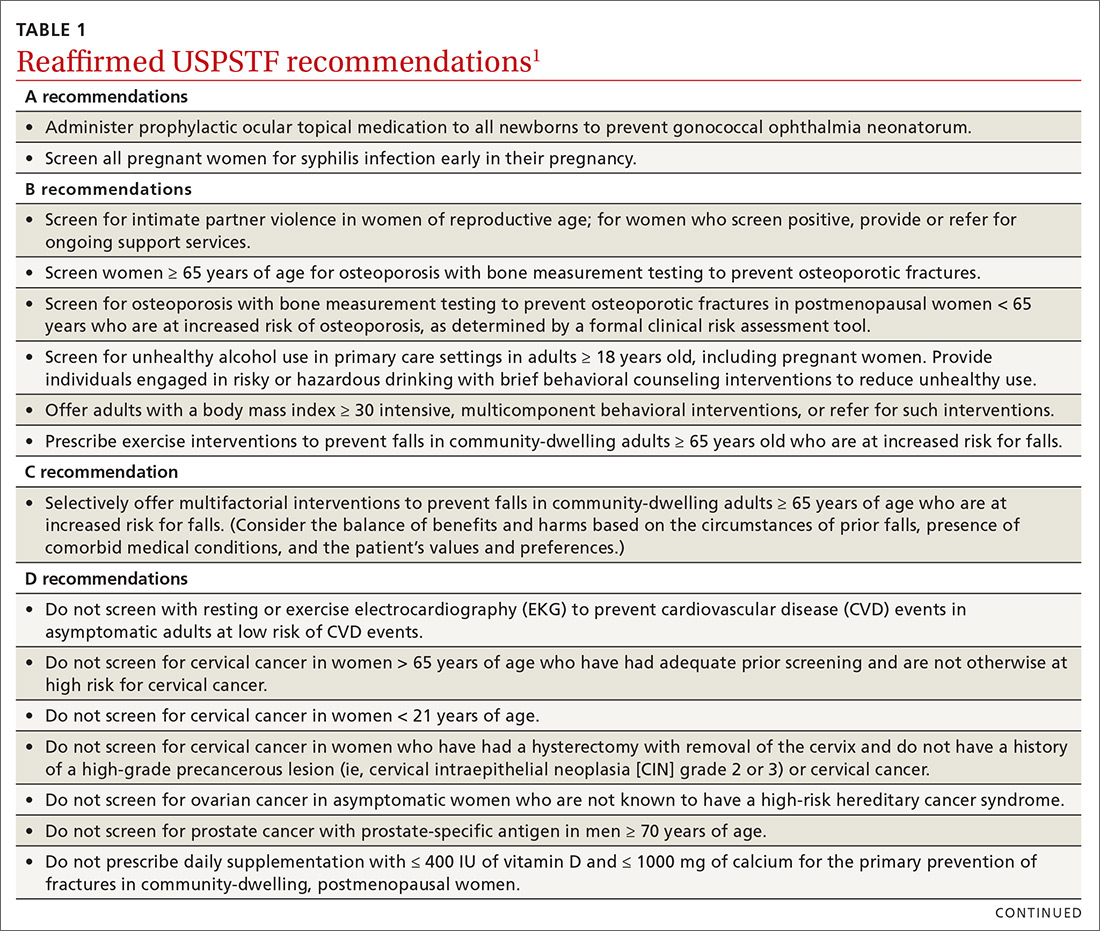
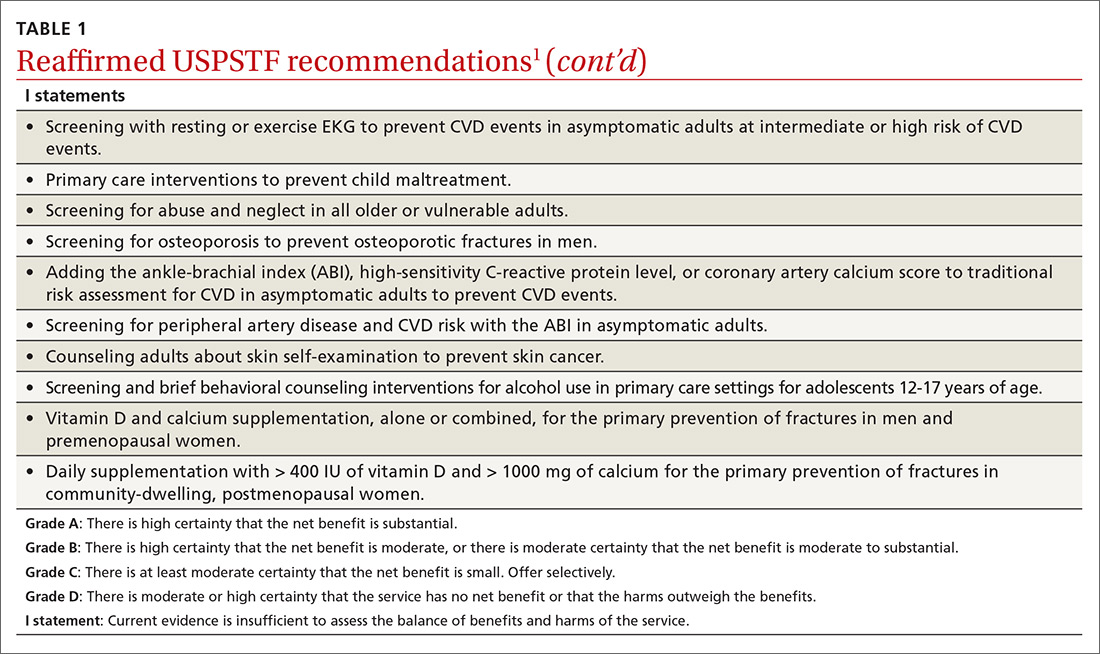
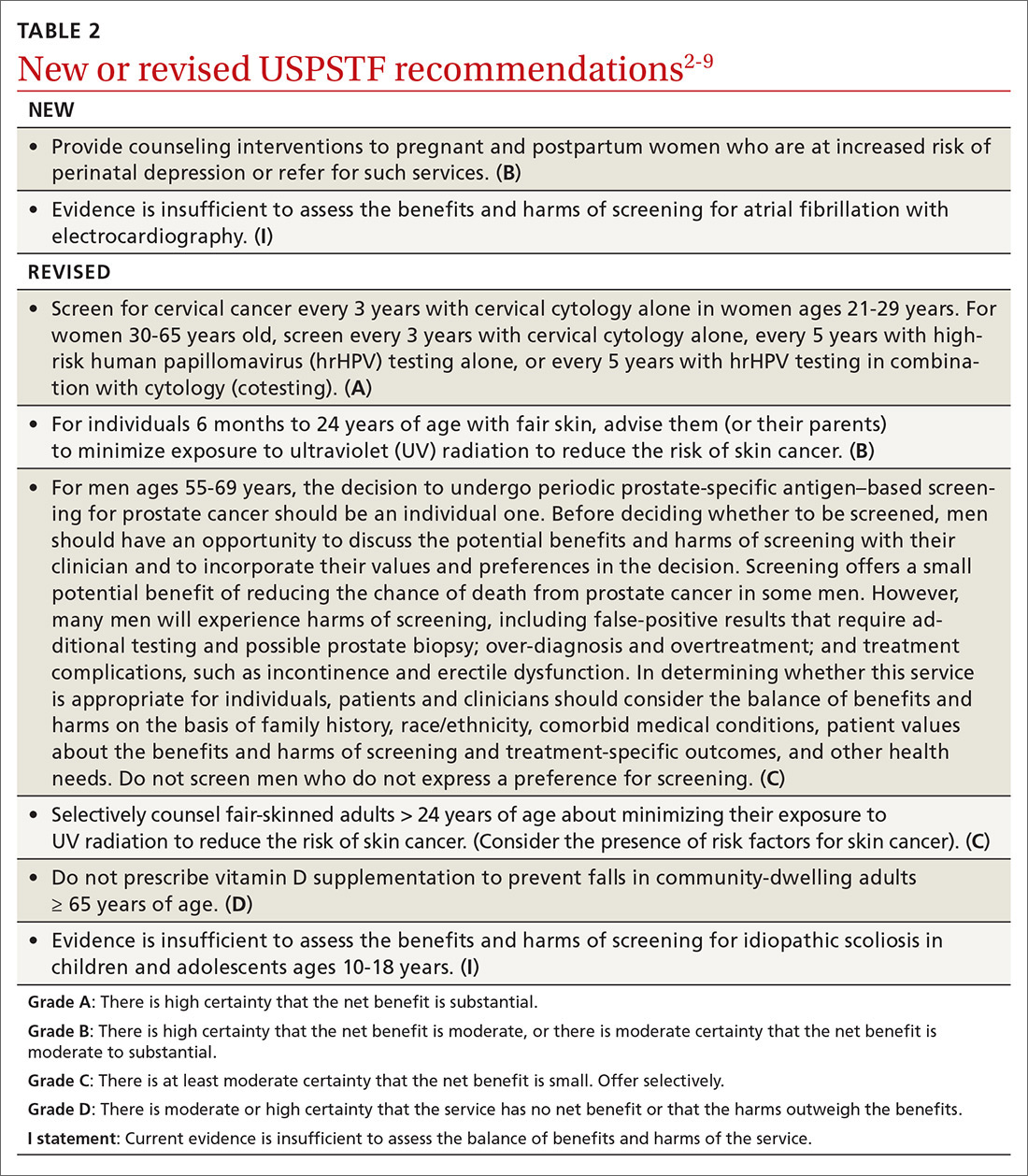
User login
Overdoses are driving down life expectancy
The average life expectancy in the United States declined from 78.9 years in 2014 to 78.6 years in 2017.1 The 2017 figure—78.6 years—means life expectancy is shorter in the United States than in other countries.1 The decline is due, in part, to the drug overdose epidemic in the United States.2 In 2017, 70,237 people died by drug overdose2—with prescription drugs, heroin, and opioids (especially fentanyl) being the major threats.3 From 2016 to 2017, overdoses from synthetic opioids, such as fentanyl, fentanyl analogs, and tramadol, increased from 6.2 to 9 per 100,000 people.2
These statistics should motivate all health care professionals to improve the general public’s health metrics, especially when treating patients with substance use disorders. But to best do so, we need a collaborative effort across many professions—not just health care providers, but also public health officials, elected government leaders, and law enforcement. To better define what this would entail, we suggest ways in which these groups could expand their roles to help reduce overdose deaths.
Health care professionals:
- implement safer opioid prescribing for patients who have chronic pain;
- educate patients about the risks of opioid use;
- consider alternative therapies for pain management; and
- utilize electronic databases to monitor controlled substance prescribing.
Public health officials:
- expand naloxone distribution; and
- enhance harm reduction (eg, syringe exchange programs, substance abuse treatment options).
Government leaders:
- draft legislation that allows the use of better interventions for treating individuals with drug dependence or those who overdose; and
- improve criminal justice approaches so that laws are less punitive and more therapeutic for individuals who suffer from drug dependence.
Law enforcement:
- supply naltrexone kits to first responders and provide appropriate training.
Kuldeep Ghosh, MD, MS
Rajashekhar Yeruva, MD
Steven Lippmann, MD
Louisville, Ky
1. National Center for Health Statistics. Table 15. Life expectancy at birth, at age 65, and at age 75, by sex, race, and Hispanic origin: United States, selected years 1900-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/hus/2016/015.pdf. Published 2016. Accessed April 24, 2019.
2. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No 329. National Center for Health Statistics. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published November 2019. Accessed April 24, 2019.
3. United States Drug Enforcement Administration. DEA releases 2018 National Drug Threat Assessment. https://www.dea.gov/press-releases/2018/11/02/dea-releases-2018-national-drug-threat-assessment-0. Published November 2, 2018. Accessed April 24, 2019.
The average life expectancy in the United States declined from 78.9 years in 2014 to 78.6 years in 2017.1 The 2017 figure—78.6 years—means life expectancy is shorter in the United States than in other countries.1 The decline is due, in part, to the drug overdose epidemic in the United States.2 In 2017, 70,237 people died by drug overdose2—with prescription drugs, heroin, and opioids (especially fentanyl) being the major threats.3 From 2016 to 2017, overdoses from synthetic opioids, such as fentanyl, fentanyl analogs, and tramadol, increased from 6.2 to 9 per 100,000 people.2
These statistics should motivate all health care professionals to improve the general public’s health metrics, especially when treating patients with substance use disorders. But to best do so, we need a collaborative effort across many professions—not just health care providers, but also public health officials, elected government leaders, and law enforcement. To better define what this would entail, we suggest ways in which these groups could expand their roles to help reduce overdose deaths.
Health care professionals:
- implement safer opioid prescribing for patients who have chronic pain;
- educate patients about the risks of opioid use;
- consider alternative therapies for pain management; and
- utilize electronic databases to monitor controlled substance prescribing.
Public health officials:
- expand naloxone distribution; and
- enhance harm reduction (eg, syringe exchange programs, substance abuse treatment options).
Government leaders:
- draft legislation that allows the use of better interventions for treating individuals with drug dependence or those who overdose; and
- improve criminal justice approaches so that laws are less punitive and more therapeutic for individuals who suffer from drug dependence.
Law enforcement:
- supply naltrexone kits to first responders and provide appropriate training.
Kuldeep Ghosh, MD, MS
Rajashekhar Yeruva, MD
Steven Lippmann, MD
Louisville, Ky
The average life expectancy in the United States declined from 78.9 years in 2014 to 78.6 years in 2017.1 The 2017 figure—78.6 years—means life expectancy is shorter in the United States than in other countries.1 The decline is due, in part, to the drug overdose epidemic in the United States.2 In 2017, 70,237 people died by drug overdose2—with prescription drugs, heroin, and opioids (especially fentanyl) being the major threats.3 From 2016 to 2017, overdoses from synthetic opioids, such as fentanyl, fentanyl analogs, and tramadol, increased from 6.2 to 9 per 100,000 people.2
These statistics should motivate all health care professionals to improve the general public’s health metrics, especially when treating patients with substance use disorders. But to best do so, we need a collaborative effort across many professions—not just health care providers, but also public health officials, elected government leaders, and law enforcement. To better define what this would entail, we suggest ways in which these groups could expand their roles to help reduce overdose deaths.
Health care professionals:
- implement safer opioid prescribing for patients who have chronic pain;
- educate patients about the risks of opioid use;
- consider alternative therapies for pain management; and
- utilize electronic databases to monitor controlled substance prescribing.
Public health officials:
- expand naloxone distribution; and
- enhance harm reduction (eg, syringe exchange programs, substance abuse treatment options).
Government leaders:
- draft legislation that allows the use of better interventions for treating individuals with drug dependence or those who overdose; and
- improve criminal justice approaches so that laws are less punitive and more therapeutic for individuals who suffer from drug dependence.
Law enforcement:
- supply naltrexone kits to first responders and provide appropriate training.
Kuldeep Ghosh, MD, MS
Rajashekhar Yeruva, MD
Steven Lippmann, MD
Louisville, Ky
1. National Center for Health Statistics. Table 15. Life expectancy at birth, at age 65, and at age 75, by sex, race, and Hispanic origin: United States, selected years 1900-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/hus/2016/015.pdf. Published 2016. Accessed April 24, 2019.
2. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No 329. National Center for Health Statistics. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published November 2019. Accessed April 24, 2019.
3. United States Drug Enforcement Administration. DEA releases 2018 National Drug Threat Assessment. https://www.dea.gov/press-releases/2018/11/02/dea-releases-2018-national-drug-threat-assessment-0. Published November 2, 2018. Accessed April 24, 2019.
1. National Center for Health Statistics. Table 15. Life expectancy at birth, at age 65, and at age 75, by sex, race, and Hispanic origin: United States, selected years 1900-2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/hus/2016/015.pdf. Published 2016. Accessed April 24, 2019.
2. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No 329. National Center for Health Statistics. https://www.cdc.gov/nchs/products/databriefs/db294.htm. Published November 2019. Accessed April 24, 2019.
3. United States Drug Enforcement Administration. DEA releases 2018 National Drug Threat Assessment. https://www.dea.gov/press-releases/2018/11/02/dea-releases-2018-national-drug-threat-assessment-0. Published November 2, 2018. Accessed April 24, 2019.
ERRATUM
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
Does withholding an ACE inhibitor or ARB before surgery improve outcomes?
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
EVIDENCE SUMMARY
An international prospective cohort study analyzed data from 14,687 patients, 4802 of whom were on an ACEI or ARB, to study the effect on 30-day morbidity and mortality of withholding the medications 24 hours before a noncardiac surgery.1 Of the ACEI or ARB users, 26% (1245) withheld their medication and 3557 continued it 24 hours before surgery.
Large study shows benefit in withholding meds
Patients who withheld the ACEI or ARB were less likely to experience the primary composite outcome of all-cause death, stroke, or myocardial injury (150/1245 [12%] vs 459/3557 [12.9%]; adjusted relative risk [RR] = 0.82; 95% confidence interval [CI], 0.70-0.96; P = .01; number needed to treat [NNT] = 116) and intraoperative hypotension (adjusted RR = 0.80; 95% CI, 0.72-0.93; P < .001; NNT = 18). For the NNT calculation, which the investigators didn’t perform, the treatment is the number needed to withhold an ACEI or ARB to show benefit.
Smaller, weaker studies yield different results
A retrospective cohort analysis of propensity-matched ACEI users with ACEI nonusers (9028 in each group) undergoing noncardiac surgery compared intra- and postoperative respiratory complications or mortality.2 The study found no association with either 30-day mortality (odds ratio [OR] = 0.93; 95% CI, 0.73-1.19) or the composite of in-hospital morbidity and mortality (OR = 1.06; 95% CI, 0.97-1.15). Limitations included comparison of users with nonusers as opposed to an intention-to-withhold study, the retrospective nature of the study, and the fact that outcomes were gathered from ICD-9 billing codes rather than obtained prospectively.
A Cochrane review assessed the benefits and harms of perioperative ACEIs or ARBs on mortality and morbidity in adults undergoing any type of surgery.3 Seven RCTs with a total of 571 participants were included in the review. Overall, the review didn’t find evidence to support prevention of mortality, morbidity, and complications by perioperative ACEIs or ARBs because the included studies were of low and very low methodological quality, had a high risk for bias, and lacked power. Moreover, the review didn’t assess the effect of withholding ACEIs or ARBs before surgery.
A random-effects meta-analysis of 5 studies (3 randomized trials and 2 observational studies) totaling 434 patients suggested that patients receiving ACEIs or ARBs immediately before surgery were more likely to develop hypotension requiring vasopressors (RR = 1.50; 95% CI, 1.15-1.96).4 Sufficient data weren’t available to assess other outcomes, and the included studies were relatively small and generally not powered to observe clinically significant consequences nor designed to measure the incidence of patient-important outcomes.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2014 American College of Cardiology/American Heart Association Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery states that continuing ACEIs or ARBs perioperatively is reasonable (class IIa recommendation [moderate benefit of treatment relative to risk]; level of evidence [LOE], B [data from limited populations and single randomized or nonrandomized trials]). 5
The guideline also recommends that if ACEIs or ARBs are held before surgery, it is reasonable to restart them as soon as clinically feasible postoperatively (class IIa recommendation; LOE, C [data from very limited populations and consensus opinion or case studies]).
Editor’s Takeaway
The results of the large prospective cohort contradict those of previous smaller, methodologically weaker studies, and the new findings should be taken seriously.1 Nevertheless, selection bias (why did investigators stop the ACEI?) remains. Until we have a large RCT, the preop question to ask may be why not stop the ACEI?
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the Vascular Events in Noncardiac Surgery Patients Cohort Evaluation prospective cohort. Anesthesiology. 2017;126:16-27.
2. Turan A, You J, Shiba A, et al. Angiotensin converting enzyme inhibitors are not associated with respiratory complications or mortality after noncardiac surgery. Anesth Analg. 2012;114:552-560.
3. Zou Z, Yuan HB, Yang B, et al. Perioperative angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database Syst Rev. 2016;(1):CD009210.
4. Rosenman DJ, McDonald FS, Ebbert JO, et al. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319-325.
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130:e278-e333.
EVIDENCE-BASED ANSWER:
A guarded yes, because the evidence of benefit is from observational studies and applies to noncardiac surgery. Withholding angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) 24 hours before noncardiac surgery has been associated with a 30-day lower risk for all-cause death, stroke, myocardial injury, and intraoperative hypotension (18% adjusted relative risk reduction).
The finding is based on 1 international prospective cohort study and, of note, is an association and a likelihood of benefit. Confirmation would require a large randomized trial (RCT; strength of recommendation [SOR]: B, good-quality international prospective cohort study).
Guidelines are not mandates
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
Just like the 2018 hypertension treatment guidelines, the 2018 Guidelines on the Management of Blood Cholesterol developed by the American College of Cardiology and the American Heart Association (ACC/AHA) have made treatment decisions much more complicated. In this issue of JFP, Wójcik and Shapiro summarize the 70-page document to help family physicians and other primary health care professionals use these complex guidelines in everyday practice.
The good news is that not much has changed from the 2013 ACC/AHA cholesterol guidelines regarding the treatment of patients with established cardiovascular disease and diabetes mellitus, and those with familial hyperlipidemia—the groups at highest risk for major cardiovascular events. Most of these patients should be treated aggressively, and a target low-density lipoprotein of 70 mg/dL is recommended.
The new guidelines recommend using ezetimibe or a PCSK9 inhibitor if the goal of 70 mg/dL cannot be achieved with a statin alone. There is randomized trial evidence to support the benefit of this aggressive approach. Generic ezetimibe costs about $20 per month,1 but the PCSK9 inhibitors are about $500 per month,2,3 so cost may be a treatment barrier for the 2 monoclonal antibodies approved for cardiovascular prevention: evolocumab and alirocumab.
For primary prevention, the new guidelines are much more complicated. They divide cardiovascular risk into 4 tiers depending on the 10-year risk for atherosclerotic cardiovascular disease calculated using the “pooled cohort equation.” Treatment recommendations are more aggressive for those at higher risk. Although it intuitively makes sense to treat those at higher risk more aggressively, there is no clinical trial evidence to support this approach’s superiority over the simpler approach recommended in the 2013 guidelines.
I find the recommendations for screening and primary prevention in adults ages 75 and older and for children and teens to be problematic. A meta-analysis of 28 studies found no statin treatment benefit for primary prevention in those older than 70.4 And there are no randomized trials showing benefit of screening and treating children and teens for hyperlipidemia.
On a positive note, most patients do not need to fast prior to having their lipids measured.
Read the 2018 cholesterol treatment guideline summary in this issue of JFP. But as you do so, remember that guidelines are guidelines; they are not mandates for treatment. You may need to customize these guidelines for your practice and your patients. In my opinion, the simpler 2013 cholesterol guidelines remain good guidelines.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
1. Ezetimibe prices. GoodRx. www.goodrx.com/ezetimibe. Accessed April 24, 2019.
2. Dangi-Garimella S. Amgen announces 60% reduction in list price of PCSK9 inhibitor evolocumab. AJMC. October 24, 2018. https://www.ajmc.com/newsroom/amgen-announces-60-reduction-in-list-price-of-pcsk9-inhibitor-evolocumab. Accessed May 1, 2019.
3. Kuchler H. Sanofi and Regeneron cut price of Praluent by 60%. Financial Times. February 11, 2019. https://www.ft.com/content/d1b34cca-2e18-11e9-8744-e7016697f225. Accessed May 1, 2019.
4. Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials. Lancet. 2019;393:407-415.
Hyperextension of the bilateral knees in a 1-day-old neonate • no knee fractures or dislocation on x-ray • Dx?
THE CASE
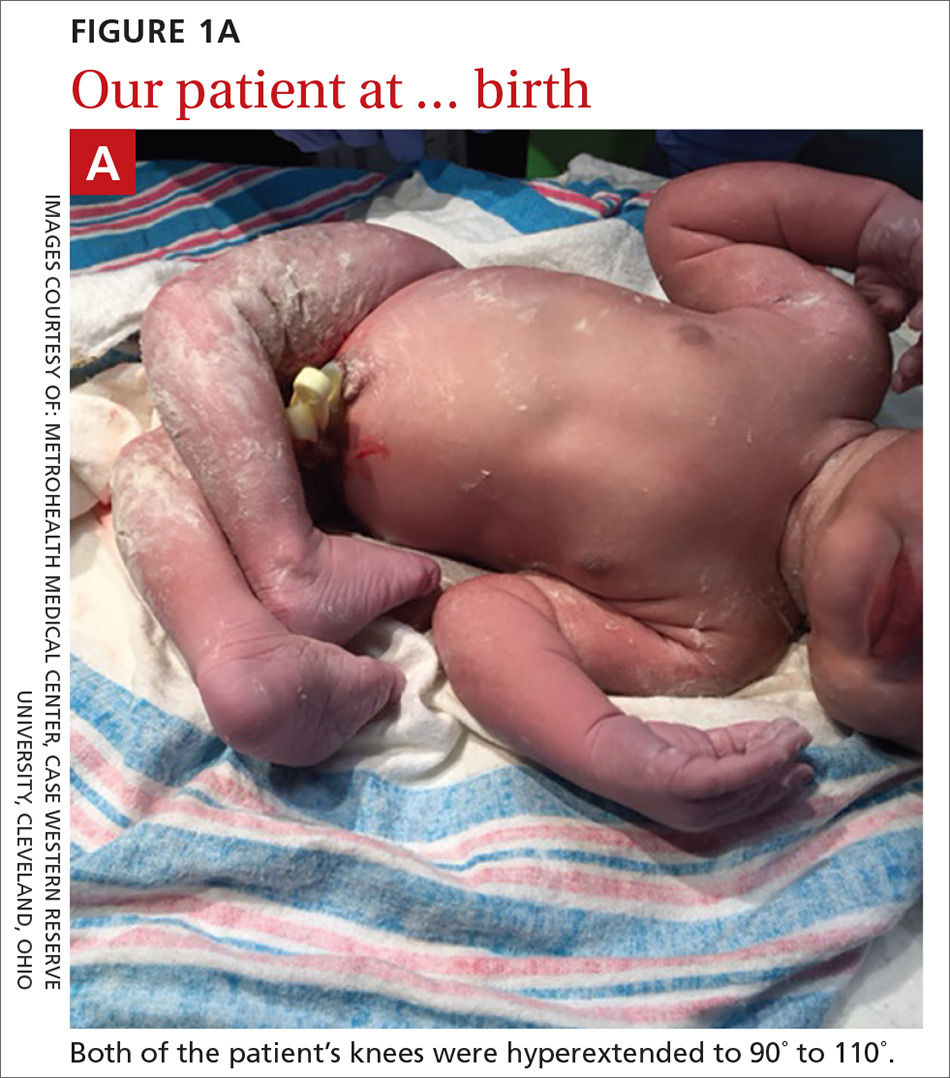
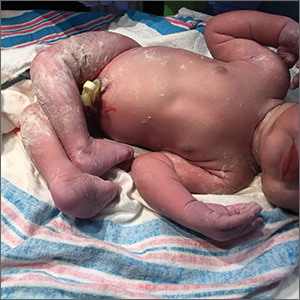
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
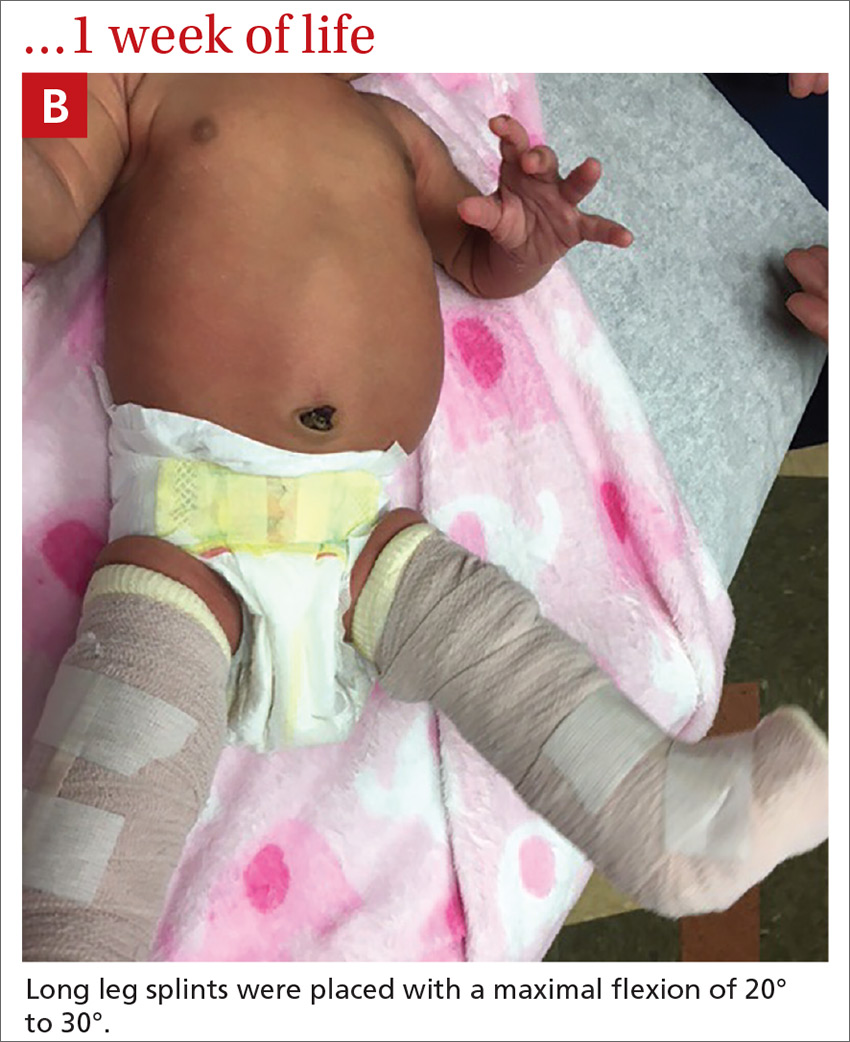
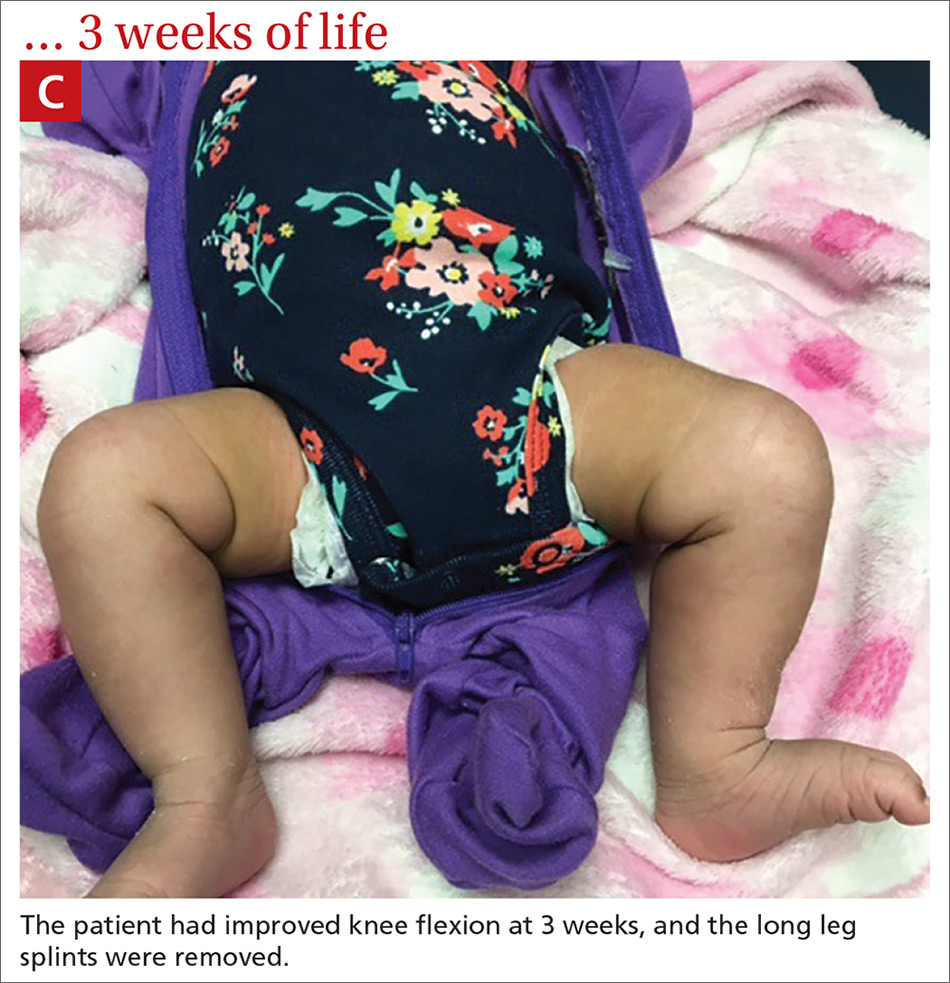
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
THE CASE
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
THE CASE
A 29-year-old G7P2315 woman gave birth to a girl at 37 weeks via spontaneous vaginal delivery. APGAR scores were 9 and 9. Birth weight was 2760 g. Cardiovascular and pulmonary examinations were normal (heart rate, 154 beats/min; respiratory rate, 52 breaths/min). Following delivery, the neonate appeared healthy, had a lusty cry, and had no visible craniofacial or cutaneous abnormalities; however, the bilateral knees were hyperextended to 90° to 110° (FIGURE 1A).
The mother had started prenatal care at 7 weeks with 10 total visits to her family physician (JD) throughout the pregnancy. Routine laboratory screening and prenatal ultrasounds (including an anatomy scan) were normal. She had a history of 3 preterm deliveries at 35 weeks, 36 weeks, and 36 weeks, respectively, and had been on progesterone shots once weekly starting at 18 weeks during the current pregnancy. She had no history of infections or recent travel. Her family history was remarkable for a sister who gave birth to a child with
THE DIAGNOSIS
The neonate tolerated passive flexion of the knees to a neutral position. Hip examination demonstrated appropriate range of movement with negative Ortolani and Barlow tests. The infant’s feet aligned correctly, with toes in the front and heels in the back, and an x-ray of the bilateral knees showed no fractures or dislocation.
Based on the clinical examination and x-ray findings, we made a diagnosis of congenital genu recurvatum. A pediatric orthopedics consultation was obtained, and the knees were placed in short leg splints in comfortable flexion to neutral on Day 1 of life. She was discharged the next day.
DISCUSSION
Congenital genu recurvatum, also known as congenital dislocation of the knee, is a rare condition involving abnormal hyperextension of the unilateral or bilateral knees with limited flexion.1 Reports in the literature are limited, but there seems to be a female predominance among known cases of congenital genu recurvatum.2 The clinical presentation varies. Finding may be isolated to the knee(s) but also can present in association with other congenital abnormalities, such as developmental dysplasia of the hip, clubfoot, and hindfoot and forefoot deformities.3,4
Diagnosis is made clinically with radiographic imaging
Diagnosis of congenital genu recurvatum is made clinically and can be confirmed via radiographic imaging of the knees.5 Clinical diagnosis requires assessment of the degree of hyperextension and palpation of the femoral condyles, which become more prominent as the severity of the hyperextension increases.6 X-rays help assess if a true dislocation or subluxation of the tibia on the femur has occurred. Based on the clinical and radiographic findings, congenital genu recurvatum typically is classified according to 3 levels of severity: grade 1 classification only involves hyperextension of the knees without dislocation or subluxation, grade 2 involves the same characteristic hyperextension along with anterior subluxation of the tibia on the femur, and grade 3 includes hyperextension with true dislocation of the tibia on the femur.1 Grades 1 and 2 on this spectrum technically are diagnosed as congenital genu recurvatum while grade 3 is diagnosed as a congenital dislocation of the knee,7 although the 2 terms are used interchangeably in the literature. We classified our case as a grade 1 congenital genu recurvatum based on the clinical and radiographic findings.
Congenital knee hyperextension has intrinsic and extrinsic causes
Hyperextension of the knees at birth may be caused by various intrinsic or extrinsic factors. Intrinsic causes may include breech position, lack of intrauterine space, trauma to the mother, quadriceps contracture or fibrosis, absence of the suprapatellar pouch, deficient or hypoplastic anterior cruciate ligament, pathological tissues, arthrogryposis, or genetic disorders such as Larsen syndrome or achondroplasia.6
Continue to: Extrinsic causes...
Extrinsic causes may include traumatic dislocation during the birthing process3 or intrauterine pressure leading to malposition of the joints. When intrauterine pressure is combined with reduced intrauterine space, this phenomenon is known as packaging disorder.6 Entanglement of the umbilical cord around the legs of the fetus during development may be another potential factor.1
The exact etiology in our patient was unknown, but we determined the cause was extrinsic based on the lack of other genetic abnormalities. We initially considered a possible connection between our patient’s diagnosis and her family history of thrombocytopenia absent radius syndrome, but it was later determined that both were isolated cases and the limb abnormalities were coincidental.
Treatment options and outcomes for extrinsic and intrinsic etiologies depend on the severity of the hyperextension and any associated abnormalities, as well as the time in which therapy is initiated.1 Reduction of the hyperextension within 24 hours of birth has been associated with excellent outcomes.8 Regardless of the cause, all cases of congenital genu recurvatum should first be treated conservatively. Evidence has suggested that conservative therapy involving early gentle manipulation of the knee combined with serial splinting and casting should be the first line of treatment.6 If initial treatment attempts fail or in cases occurring later in life, surgical interventions (eg, quadriceps release procedures such as percutaneous quadriceps recession or V-Y quadricepsplasty, proximal tibial closing-wedge, anterior displacement osteotomy) likely is warranted.6,9
Our patient. At 1 week of life, our patient’s short leg splints were replaced with long leg splints with a maximal flexion of 20° to 30° (FIGURE 1B). Weekly follow-ups with serial casting were initiated in the pediatric orthopedics clinic. At 3 weeks of life, the patient’s knee flexion had improved and the splints were removed (FIGURE 1C). Upon clinical examination, the bilateral knees were extended to a neutral position, and both could be actively and passively flexed to 90°. The patient was referred to Physical Therapy to perform range of movement exercises on the knees.
At 8 weeks of life, the bilateral legs were in full extension, and knee flexion was up to 130°. Physical therapy for knee range of movement exercise was continued on a weekly basis until 6 months of life, then twice monthly until the patient was 1 year old. Ultimately, the hyperextension was corrected, and the patient started walking at around 16 months of age. Her prognosis is good, and she will be able to participate in low-impact sports, after consulting with her orthopedist.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Congenital genu recurvatum is a rare condition that presents with abnormal hyperextension of the knee(s) with limited flexion. Early diagnosis and assessment of the severity of the hyperextension is crucial in determining the type of intervention to pursue. Conservative management entails serial casting and splinting to increase knee flexion. If conservative management fails or if the diagnosis is made later in life, surgical options often are pursued.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 MetroHealth Medical Drive, Cleveland, OH 44109; jxd114@case.edu
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
1. Donaire AR, Sethuram S, Kitsos E, et al. Congenital bilateral knee hyperextension in a well-newborn infant. Res J Clin Pediatr. 2017;1. https://www.scitechnol.com/peer-review/congenital-bilateral-knee-hyperextension-in-a-wellnewborn-infant-V63Y.php?article_id=5940. Accessed April 2, 2019.
2. Osakwe GO, Asuquo EJ, Abang EI, et al. Congenital knee dislocation: challenges in management in a low resource center. Journal of dental and medical sciences. 2016;15:78-82.
3. Katz MP, Grogono BJ, Soper KC. The etiology and treatment of congenital dislocation of the knee. J Bone Joint Surg Br. 1967;49:112-20.
4. Elmada M, Ceylan H, Erdil M, et al. Congenital dislocation of knee. Eur J Med. 2013;10:164-166.
5. Abdelaziz TH, Samir S. Congenital dislocation of the knee: a protocol for management based on degree of knee flexion. J Child Orthop. 2011;5:143-149.
6. Tiwari M, Sharma N. Unilateral congenital knee and hip dislocation with bilateral clubfoot—a rare packaging disorder. J Orthop Case Rep. 2013;3:21-24.
7. Ahmadi B, Shahriaree H, Silver CM. Severe congenital genu recurvatum. case report. J Bone Joint Surg Am. 1979;61:622-623.
8. Cheng CC, Ko JY. Early reduction for congenital dislocation of the knee within twenty-four hours of birth. Chang Gung Med J. 2010;33:266-273.
9. Youssef AO. Limited open quadriceps release for treatment of congenital dislocation of the knee. J Pediatric Orthop. 2017;37:192-198.
Erythematous swollen ear
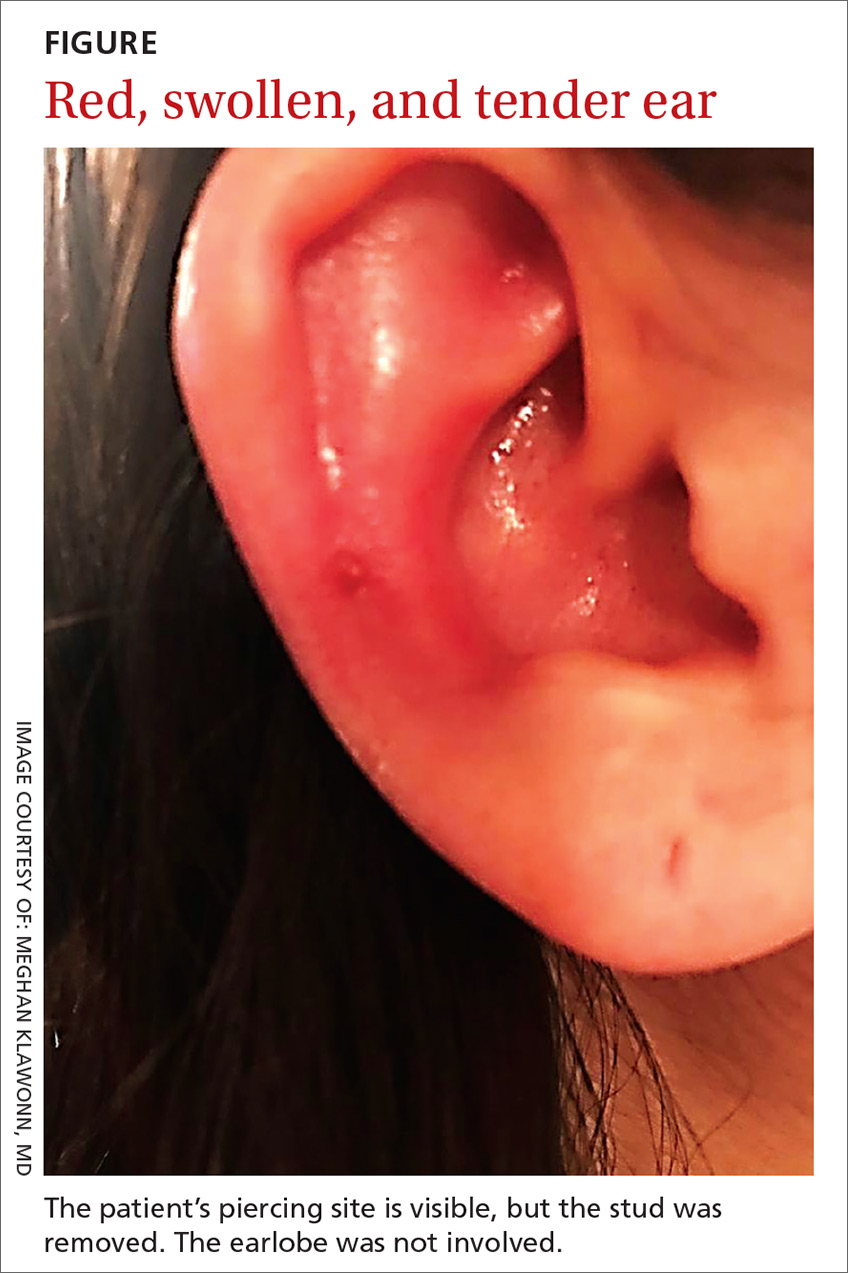
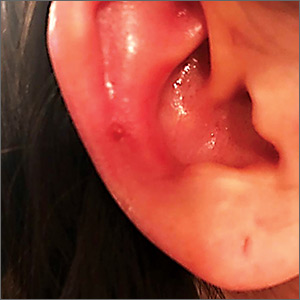
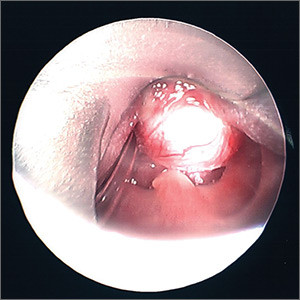
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; mhelm2@pennstatehealth.psu.edu
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; mhelm2@pennstatehealth.psu.edu
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; mhelm2@pennstatehealth.psu.edu
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
Failure to thrive in a 6-day-old neonate • intermittent retractions with inspiratory stridor • Dx?
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).
Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; Bhansen7000@gmail.com
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).
Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; Bhansen7000@gmail.com
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).
Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; Bhansen7000@gmail.com
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
Can vitamin D prevent acute respiratory infections?
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
PRACTICE CHANGER
Reduce acute respiratory tract infections in those with significant vitamin D deficiency (circulating 25-hydroxyvitamin D levels < 10 ng/mL) with daily or weekly vitamin D supplementation—not bolus vitamin D treatment.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 25 trials.
Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
Could that back pain be caused by ankylosing spondylitis?
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; carlton.j.covey.mil@mail.mil
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; carlton.j.covey.mil@mail.mil
CASE
A 38-year-old man presents to your primary care clinic with chronic low back stiffness and pain. You have evaluated and treated this patient for this complaint for more than a year. His symptoms are worse in the morning upon wakening and improve with activity and anti-inflammatory medications. He denies any trauma or change in his activity level. His medical history includes chronic insertional Achilles pain and plantar fasciopathy, both for approximately 2 years. The patient reports no systemic or constitutional symptoms, and no pertinent family history.
How would you proceed with his work-up?
Ankylosing spondylitis (AS) is a form of arthritis that primarily affects the spine and sacroiliac joints. It is the most common spondyloarthropathy (SpA)—a family of disorders that also includes psoriatic arthritis; arthritis associated with inflammatory bowel disease; reactive arthritis; and juvenile SpA.1 AS is most prevalent in Caucasians and may affect 0.1% to 1.4% of the population.2
Historically, a diagnosis of AS required radiographic evidence of inflammation of the axial spine or sacrum that manifested as chronic stiffness and back pain. However, the disease can also be mild or take time for radiographic evidence to appear. So an umbrella term emerged—axial spondyloarthritis (axSpA)—that includes both AS and the less severe form, called nonradiographic axSpA (nr-axSpA). While patients with AS exhibit radiographic abnormalities consistent with sacroiliitis, patients with early, or nr-axSpA, do not have radiographic abnormalities of the sacroiliac (SI) joint or axial spine.
In clinical practice, the distinction between AS and nr-axSpA has limited impact on the management of individual patients. However, early recognition, intervention, and treatment in patients who do not meet radiographic criteria for AS can improve patient-oriented outcomes.
The family physician (FP)’s role. It is not necessary that FPs be able to make a definitive diagnosis, but FPs should:
- be able to recognize the symptoms of inflammatory back pain (IBP);
- know which radiographic and laboratory studies to obtain and when;
- know the Assessment of SpondyloArthritis international Society (ASAS) criteria3 that assist in identifying patients at risk for axSpA; and
- know when to refer moderate- to high-risk patients to rheumatologists for assistance with the diagnosis.
FPs should have a high index of suspicion in any patient who has chronic back pain (> 3 months) with other features of SpA, and should pay special attention to young adult patients (< 45 years) who have IBP features.
Continue to: Definitive data to show...
Definitive data to show what percentage of patients with nr-axSpA progress to AS are lacking. However, early identification of AS is important, as those who go undiagnosed have increased back pain, stiffness, progressive loss of mobility, and decreased quality of life. In addition, patients diagnosed after significant sacroiliitis is visible are less responsive to treatment.4
What follows is a review of what you’ll see and the tools that will help with diagnosis and referral.
The diagnosis dilemma
In the past, the modified New York criteria have been used to define AS, but they require the presence of both clinical symptoms and radiographic findings indicative of sacroiliitis for an AS designation.5,6 Because radiographic sacroiliitis can be a late finding in axSpA and nonexistent in nr-asSpA, these criteria are of limited clinical utility.
To assist in early identification, the ASAS published criteria to classify patients with early axSpA prior to radiographic manifestations.3 While not strictly diagnostic, these criteria combine patient history that includes evidence of IBP, human leukocyte antigen (HLA)-B27 positivity, and radiography to assist health care providers in identifying patients who may have axSpA and need prompt referral to a rheumatologist.
Easy to miss, even with evidence. It takes an average of 5 to 7 years for patients with radiographic evidence of AS to receive the proper diagnosis.7 There are several reasons for this. First, the axSpA spectrum encompasses a small percentage of patients who present to health care providers with back pain. In addition, many providers overlook the signs and symptoms of IBP, which are a hallmark of the condition. And finally, as stated earlier, true criteria for the diagnosis of axSpA do not exist.
Continue to: In addition...
In addition, AS predominantly affects people in the third and fourth decades of life, but as many as 5% of patients of all ages with chronic back pain (> 3 months) can be classified as having AS.8 In patients who have IBP features, 14% can be classified as having axSpA.9 Therefore, it is important to recognize the features of IBP (TABLE 110). The presence of 4 of the 5 of IBP features has a sensitivity of 77% and a specificity of 91.7% for IBP.10
A different kind of back pain. The vast majority of patients presenting with low back pain will have features of mechanical back pain, which include improvement with rest, mild and short-lived morning stiffness and/or pain upon waking, and the absence of inflammatory markers. Those with axSpA, on the other hand, are more likely to report improvement of pain with exercise, no improvement with rest, and pain at night with improvement upon rising. While the presence of IBP features alone isn’t diagnostic for nr-axSpA or AS, such features should increase your suspicion, especially when such features are present in younger patients.
Physical exam findings
Physical exam findings are neither sensitive nor specific for the diagnosis of an axSpA disorder, but can help build a case for one. The physical exam can also assist in identifying comorbid conditions including uveitis, psoriasis, dactylitis, and enthesitis. Experts do not recommend using serial measurements of axial range of motion because they are time-consuming, and normative values are highly variable.
On examination of the peripheral joints and feet, note any swollen, tender, or deformed joints, as well as any dactylitis. Although any enthesis can be affected in axSpA, the insertional points of the Achilles and the plantar fascia are the most typical,1 so pay particular attention to these areas. On skin exam, note any evidence of psoriatic manifestations. Refer all patients with suspected uveitis to an ophthalmologist for confirmation of the diagnosis.
Lab studies: Not definitive, but helpful
No laboratory studies confirm a diagnosis of nr-axSpA or AS; however, 2 studies—C-reactive protein (CRP) and HLA-B27—are important, as levels are listed as part of ASAS’s axSpA features (TABLE 23) and are factors that should be considered when deciding whether a referral is needed (TABLE 311). As such, HLA-B27 and CRP testing should be performed in all patients suspected of having an axSpA spectrum disorder.
Continue to: HLA-B27 is...
HLA-B27 is positive in 70% to 95% of patients with axSpA and can help build a case for the disorder.6,12 CRP is useful too, as an elevated CRP has important treatment implications (more on that in a bit).6
Other diagnoses in the differential include: degenerative disc disease, lumbar spondylosis, congenital vertebral anomalies, and osteoarthritis of the SI joint, bone metastasis, or primary bone tumors.1
Start with plain x-rays. The American College of Radiology (ACR) published appropriateness criteria for obtaining x-rays in patients suspected of having axSpA.13 Plain x-rays of the spine and SI joint are recommended for the initial evaluation. Magnetic resonance imaging (MRI) of the SI joint and/or spine should be obtained if the initial x-rays are negative or equivocal. Patient symptomology and/or exam findings determine whether to include the SI joint and/or spine. If the patient has subjective and objective findings concerning for pathology of both, then an MRI of the spine and SI joint is warranted.
Alternatively, computed tomography (CT) can be substituted if MRI is unavailable. In patients with known axSpA, surveillance radiography should not occur more often than every 2 years.6
Timely referral is essential
Timely referral to a rheumatologist is an essential part of early diagnosis and treatment. Advances in treatment options for axSpA have become available in recent years and offer new hope for patients.
Continue to: As the presence of IBP...
As the presence of IBP features portends a 3-fold increase in the risk for axSpA,8 we propose an approach to the referral of patients with IBP features that deviates slightly from the ASAS algorithm. We believe it is within the scope of FPs to recognize IBP features, order appropriate ancillary studies, start a trial of nonsteroidal anti-inflammatory drugs (NSAIDs), and follow-up with patients in 2 to 4 weeks to review results and evaluate treatment response. As such, all patients < 45 years old with IBP symptoms (TABLE 110) for 3 months or longer should be sent for laboratory workup (HLA-B27, CRP) and plain radiographs of the sacroiliac joints and lumbar spine.
Older patients, patients with IBP features for < 3 months, or patients < 45 years with IBP that have negative lab testing and negative radiographs should start an exercise program, be treated with an NSAID, and be assessed for ASAS spondyloarthritis features (TABLE 23).
Any patient with positive lab testing, positive radiographs, or ≥ 1 ASAS axSpA features should be referred to Rheumatology (TABLE 311). Patients with a negative radiograph should be evaluated with an MRI of the SI joints or spine (driven by pain location) and referred to Rheumatology if positive.
Keep in mind that not all patients fit neatly into an algorithm or a classification system. Therefore, we recommend that any patient with IBP features who fails to improve after 3 months of an exercise program, for whom you have a high index of suspicion for possible axSpA spectrum disease, receive appropriate ancillary studies and referral for expert consultation.
Exercise and NSAIDs form the basis of treatment
The purpose of treating patients with a suspected axSpA spectrum disorder is to decrease pain and stiffness, improve function and quality of life, and, ideally, halt or slow progression of disease. The only modifiable predictor of progression to axSpA is smoking; as such, encourage tobacco cessation if appropriate.14
Continue to: Nonpharmacologic treatment...
Nonpharmacologic treatment, such as regular aerobic exercise and strength training, should be prescribed for all patients with axSpA.6 Regular exercise is helpful in improving lower back pain, function, and spinal mobility. Combination endurance and strength-training programs are associated with the greatest benefits, and aquatic therapy is better than land-based therapy for pain.15 That said, recommend land-based exercises over no exercise when pool-based therapy is unavailable.
NSAIDs (eg, ibuprofen 200-800 mg at variable frequency, up to a maximum dose of 2400 mg/d; naproxen 250-500 mg bid) are the core treatment for patients with axSpA, as they improve pain, function, and quality of life.6 Both traditional NSAIDs and cyclooxygenase II (COX-II) inhibitors are effective; no differences in efficacy exist between the classes.6,15,16
NSAIDs have been shown to be as safe as placebo for up to 12 weeks of continuous use in patients without gastritis or renal disease.16 In patients with a gastrointestinal comorbidity, use NSAIDs cautiously.17
If adequate pain relief is not obtained after 2 to 4 weeks of NSAID use, try a different NSAID prior to escalating treatment.6 More research is needed to evaluate the effect of NSAIDs on spinal radiographic progression of disease because of conflicting results of existing studies.16
Unlike with other rheumatologic disorders, oral glucocorticoids and traditional disease-modifying anti-rheumatic drugs (DMARDs) are not effective in axSpA and should not be prescribed.18
Continue to: Other agents
Other agents. In patients who continue to have symptoms, or cannot tolerate 12 weeks of NSAIDs, newer biologic DMARDs may be considered. Tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) have shown the best efficacy.18,19 In patients with AS, these medications improve pain and function, increase the chance of achieving partial remission of symptoms, and reduce CRP levels and MRI-detectable inflammation of the SI joint and/or spine.1,19 At this time, these medications are reserved for use in patients with clinical symptoms consistent with, and radiographic evidence of, axSpA, or in patients with nr-axSpA who have elevated CRP levels.18
For patients diagnosed with axSpA, an elevated CRP, short symptom duration (or young age), and inflammation noted on MRI seem to be the best predictors of a good response to TNFi.20 All patients in whom biologic DMARDS are considered should be referred to a rheumatologist because of cost, potential adverse effects, and stringent indications for use.
Surveil disease progression to prevent complications
We don’t yet know if progression of axSpA is linear or if the process can be slowed or halted with timely treatment. We do know that the natural history of structural progression is low in patients with early nr-axSpA.
Examples of validated online tools that can assist in measuring patient response to treatment and/or progression of disease follow.21 They can be used alone or in combination to help monitor treatment and progression of disease.
- The Ankylosing Spondylitis Disease Activity Score (ASDAS) (https://www.asas-group.org/clinical-instruments/asdas-calculator/). This measure of disease activity uses a 5-item patient assessment and CRP level measurement.
- The Bath Ankylosing Spondylitis Functional Index (BASFI) (http://basdai.com/BASFI.php). The BASFI consists of 8 items pertaining to everyday function and 2 items assessing the ability of patients to cope with everyday life.
- The Ankylosing Spondylitis Quality of Life Scale (ASQoL; http://oml.eular.org/sysModules/obxOml/docs/ID_32/ASQoL%20Questionnaire%20English.pdf).The ASQoL is an 18-item questionnaire related to the impact of disease on sleep, mood, motivation, and activities of daily living, among others.
Comorbidities. Patients with axSpA have an increased lifetime risk for cardiovascular disease, osteoporosis, fracture, inflammatory bowel disease, and iritis.6 Acute back pain in a patient with axSpA should be evaluated for a fracture and not automatically deemed an axSpA flare.13 Obtain a CT scan of the spine for all patients with known spine ankyloses who are suspected of having a fracture (because of the low sensitivity of plain radiography).13
Continue to: Prognosis
Prognosis. AS is a progressive long-term medical condition. Patients may experience progressive spinal deformity, hip joint or sacroiliac arthroses, or neurologic compromise after trauma. Reserve surgical referral for patients with spinal deformity that significantly affects quality of life and is severe or progressing despite nonpharmacologic and pharmacologic measures. Refer patients with an unstable spinal fracture for surgical intervention.6
Advise patients of available local, national, and international support groups. The National Ankylosis Spondylitis Society (NASS) based in the United Kingdom and the Spondylitis Association of America (SAA) are patient-friendly, nonprofit organizations that provide resources and information to people to help them learn about and cope with their condition.
CASE
You diagnose IBP in this patient and proceed with a work-up. You order x-rays of the back and SI joint, a CRP level, and an HLA-B27 test. X-rays and laboratory studies are negative. The patient is encouraged by your recommendation to start an aerobic and strength training home exercise program. In addition, you prescribe naproxen 500 mg bid and ask the patient to return in 1 month.
On follow-up he states that the naproxen is working well to control his pain. Upon further chart review and questioning, the patient confirms a history of chronic plantar fasciosis and psoriasis that he has controlled with intermittent topical steroids. He denies visual disturbances or gastrointestinal complaints. You refer him to a rheumatologist, where biologic agents are discussed but not prescribed at this time.
CORRESPONDENCE
Carlton J Covey, MD, FAAFP, Nellis Family Medicine Residency Program, 4700 Las Vegas Blvd. North, Nellis AFB, NV 89191; carlton.j.covey.mil@mail.mil
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84.
2. Lawrence R, Helmick C, Arnett F, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
3. Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part II); validation and final selection. Ann Rheum Dis. 2009;68:777-783.
4. Seo MR, Baek HL, Yoon HH, et al. Delayed diagnosis is linked to worse outcomes and unfavorable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397-1405.
5. van der Linden SM, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361-68.
6. National Institute for Health and Care Excellence. NICE Guideline, No. 65. Spondyloarthritis in over 16s: diagnosis and management. February 2017. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0091652/. Accessed April 24, 2019.
7. Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008:27:457-462.
8. Underwood MR, Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074-1077.
9. Strand V, Singh J. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. 2017;92:555-564.
10. Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis. 2009;68:784-788.
11. Poddubnyy D, van Tubergen A, Landewe R, et al. Development of ASAS-endorsed recommendation for the early referral of patients with a suspicion of axial spondyloarthritis. Ann Rheum Dis. 2015;74:1483-1487.
12. Rostom S, Dougados M, Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77:108-114.
13. Bernard SA, Kransdorf MJ, Beaman FD, et al. ACR appropriateness criteria chronic back pain suspected sacroiliitis-spondyloarthropathy. J Am Coll Radiol. 2017;14:S62-S70.
14. Dougados M, Demattei C, van den Berg R, et al. Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol. 2016;68:1904-1913.
15. Regel A, Sepriano A, Baraliakos X, et al. Efficacy and safety of non-pharmacological treatment: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017;3:e000397.
16. Kroon FPB, van der Burg LRA, Ramiro S, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst Rev. 2015;7:CD010952.
17. Radner H, Ramiro S, Buchbinder R, et al. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondyloarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951.
18. van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991.
19. Maxwell LJ, Zochling J, Boonen A, et al. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;4:CN005468.
20. Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282-295.
21. Zochling J. Measures of symptoms and disease status in ankylosing spondylitis. Arthritis Care Res. 2011;63:S47-S58.
PRACTICE RECOMMENDATIONS
› Evaluate all patients with back pain lasting > 3 months for inflammatory back pain features. C
› Treat all patients with confirmed or suspected axial spondyloarthritis with a trial of nonsteroidal anti-inflammatory drugs. A
› Recommend that all patients with back pain—including those with suspected axial spondyloarthritis—start an exercise program that includes both strength and aerobic activities. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
2019 USPSTF update
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.