User login
Familial Hemiplegic Migraines & Neuropsychological Testing
In individuals with familial hemiplegic migraines (FHM), baseline and serial neuropsychological testing may help identify the potential progression and course of cognitive impairment associated with this condition. This according to a single-case study involving a male aged 24 years who recently endured an atypical, prolonged FHM episode. Researchers found:
- The patient’s overall neuropsychological functioning was intact with low average semantic fluency and processing speed.
- The patient also exhibited mild indication of executive dysfunction.
Trahan EM, Mercado JM. Familial hemiplegic migraines and baseline neuropsychological testing: A case report. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13505.
In individuals with familial hemiplegic migraines (FHM), baseline and serial neuropsychological testing may help identify the potential progression and course of cognitive impairment associated with this condition. This according to a single-case study involving a male aged 24 years who recently endured an atypical, prolonged FHM episode. Researchers found:
- The patient’s overall neuropsychological functioning was intact with low average semantic fluency and processing speed.
- The patient also exhibited mild indication of executive dysfunction.
Trahan EM, Mercado JM. Familial hemiplegic migraines and baseline neuropsychological testing: A case report. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13505.
In individuals with familial hemiplegic migraines (FHM), baseline and serial neuropsychological testing may help identify the potential progression and course of cognitive impairment associated with this condition. This according to a single-case study involving a male aged 24 years who recently endured an atypical, prolonged FHM episode. Researchers found:
- The patient’s overall neuropsychological functioning was intact with low average semantic fluency and processing speed.
- The patient also exhibited mild indication of executive dysfunction.
Trahan EM, Mercado JM. Familial hemiplegic migraines and baseline neuropsychological testing: A case report. [Published online ahead of print March 14, 2019]. Headache. doi:10.1111/head.13505.
Discuss compounded bioidentical hormones and cancer risk
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
The clinical scenario is as follows: A 62-year-old woman comes to see me for a new diagnosis of grade 1 endometrial cancer. She has a normal body mass index of 24 kg/m2, a history of four prior full-term pregnancies, no family history of malignancy, and no medical comorbidities. She is otherwise a specimen of good health, and has no clear identifiable risk factors for this malignancy. She then reports that she transitioned through menopause at age 52 years and developed severe hot flashes with sleep and mood disturbance. She did not wish to take conventional hormone replacement therapy (HT) because she had heard it causes cancer. She subsequently researched the Internet and found a provider who has been prescribing compounded bioidentical hormone therapy (CBHT) for her for the past 10 years. She submits saliva for testing of her estrogen levels, and the provider uses this data to compound the appropriate doses of “natural” estrogens and testosterone for her which she applies via vaginal or transdermal creams. She has been prescribed a progesterone suppository, but she doesn’t always take that because she doesn’t notice that it has any effect on how she feels.
My answer is, of course, I don’t know. Cancer is a complex disease with a complex array of causative and promoting factors. However, we do know that taking estrogen unopposed with adequate progesterone can cause the development of uterine cancer and its precursor state.1 If those bioidentical estrogens were effective at controlling her menopausal symptoms, they likely were effective at stimulating her endometrium at the same time.
What are compounded bioidentical hormones?
The term “bioidentical” refers to having the same molecular structure as that which is found in the human body. Examples of bioidentical estrogens include 17-beta-estradiol, estrone, and estriol – which are produced from yams and soy. Micronized progesterone is an example of a bioidentical progesterone. Many of these drugs are approved by the Food and Drug Administration, and prescribed and dispensed by conventional pharmacies.
An alternative, and increasingly popular, version of bioidentical hormones are CBHs. It should be recognized that this is a marketing, and not a scientific, term. These products utilize hormones, in some cases FDA-approved bioidentical hormones, that are broken down and blended by specialized pharmacies and reconstituted (compounded) into different, and sometimes “customized,” dosing and delivery methods (such as capsules, patches, gels, creams, lozenges, suppositories). Frequently used compounded products utilize multiple formulations of estrogens in doublets and triplets as well as progesterone, testosterone, and dehydroepiandrosterone.
How do they differ from synthetic hormones?
Distributors of CBHs state that they differ from conventional HT (synthetic and bioidentical) because of the customization process from which they promise greater efficacy and a sense of personalized medicine. The distributors frequently utilize assays from saliva, blood, vaginal secretions, and urine to measure a woman’s hormone levels, and titrate her compounded formulation based on those results. It should be noted that there is no data to support that titration of hormones to blood, salivary, or urine levels is efficacious or ensures greater safety than titration based on symptom management.
Critics of CBHT, which includes the North American Menopause Society2 and the American College of Obstetricians and Gynecologists,3 highlight that the main difference between CBHT and HT is lack of FDA regulation over the CBHT industry. Many of these agents are delivered transdermally and therefore are classified as “dietary supplements.” As such, they do not require FDA regulation or proof of safety or efficacy.
Lack of FDA approval allows CBHs to be distributed without package inserts and boxed warnings (such as the standard warnings about MI, venous thromboembolic events, and breast cancer). The absence of FDA approval also allows them to avoid FDA-regulated guarantees about purity, potency, and efficacy. Audits of CBHs have shown high rates of discrepancy between stated and measured potency, including observations of both much lower and much higher than stated strength.4
Why would dosing accuracy be important in hormone therapy prescription? If a woman taking estrogen therapy is not receiving adequate cotreatment with progesterone because of either omission or a subtherapeutic product, she increases her risk for endometrial cancer.
What drives patients’ decision to use compounded bioidentical hormones?
After the Womens’ Health Initiative study was published in 20025, all FDA-regulated estrogen preparations were required to carry specific warnings, particularly in relation to the increased risk for MI, venous thromboembolic events, and breast cancer. There was a clear uptake in use of CBHT after this study was reported. By avoiding FDA regulations, distributors of CBHTs may have avoided providing Womens’ Health Initiative information to patients. The absence of an insert with a written warning, in and of itself, makes these preparations seem safer to the patient.
But is it entirely a lack of information that drives demand for CBHTs? Surveys of current or former users suggest the motivations are more complex than that. A survey of 21 past or present CBHT users inquired about reasons for use of CBHT over conventional HT.6 Their responses were categorized as either push motivations away from conventional therapy versus pull motivations toward CBHT. About 95% of current and former users cited distrust of the biomedicine and pharmaceutical industry as reasons for use of CBHT. Fear about the safety of conventional HT, particularly with respect to cancer risk, also was strongly cited at 81%. Motivations pulling toward CBHT included its efficacy (76%) and perception that CBHT is “safer” than conventional HT (76%).
Women in this study also appreciated the tailored, individualized approach that often is associated with CBHT, in which providers spend long consultations discussing individual patient needs and concerns. They enjoy the idea of a customized blend that is created, as opposed to a standard dosing regimen, and intuitively trust the reliability of blood and saliva testing as a prescriptive tool.
Are bioidentical hormones safe with respect to cancer risk?
Hormones themselves are not inert substances, including those derived in vivo and those from plants. They have powerful effects in the human body and can promote malignant transformation or proliferation, alter metabolic pathways, stimulate vascular tone, influence coagulation pathways, along with many other effects. A hormone’s potential for deleterious effect can be present regardless of how that hormone is synthesized, procured, or prepared. While there are no data to suggest that CBHT is associated with increased cancer risk, compared with conventional HT, there are by no means any data to suggest it is safer. Unopposed compounded estrogens place women at increased risk for endometrial cancers, and the prolonged use of hormonal therapy, compounded or otherwise, after menopause increases the risk for breast cancer.
How should we counsel patients?
Patients who desire compounded bioidentical hormone preparations should be counseled that little is known about the safety of these preparations, compared with conventional hormone preparations. The fact that the components are often plant based rather than synthetic does not inherently alter their potential negative impact on biologic pathways. Patients should be educated regarding the difference between FDA-regulated products and nonregulated products so that they can understand that lack of a boxed warning on a non-FDA regulated product does not mean an absence of risk. Women should be informed of the potential inaccuracies in dosing and strength of the CBH preparations they receive.
We should recognize that our patients strongly desire a relationship with their provider in which they are listened to, understood, and treated as individuals. If conversations regarding hormone use are approached with these principles, we will optimize the likelihood our patients are receptive to the highest quality information and not pulled in the direction of unregulated products.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported that she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References:
1. Maturitas. 2014 Jan;77(1):4-6.
2. Menopause. 2014 Dec;21(12):1298-300.
3. Fertil Steril. 2012 Aug;98(2):308-12.
4. Report: Limited FDA survey of compounded drug products (Silver Spring, Md.: U.S. Food and Drug Administration, 2009).
5. JAMA. 2002;288(3):321-33.
6. BMC Womens Health. 2017 Oct 2;17(1):97.
Surprise! MTX proves effective in psoriatic arthritis
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
MAUI, HAWAII – The first-ever, double-blind, randomized, controlled clinical trial evidence demonstrating that methotrexate indeed has therapeutic efficacy in psoriatic arthritis has come at an awkward time – on the heels of a basically negative Cochrane Collaboration systematic review as well as the latest American College of Rheumatology/National Psoriasis Foundation guidelines for treatment of psoriatic arthritis, which recommend anti–tumor necrosis factor therapy as first line, ahead of methotrexate.
The timing of the release of the SEAM-PsA randomized trial results was such that neither the Cochrane group nor the ACR/NPF guideline committee was able to consider the new, potentially game-changing study findings.
“I look at SEAM-PsA and have to say, methotrexate does seem to be an effective therapy. I think it calls into question the new guidelines, which were developed before the data were out. Now you look at this and have to ask, can you really say you should use a TNF inhibitor before methotrexate based on these results? I don’t know,” Eric M. Ruderman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He also shared other problems he has with the new guidelines, which he considers seriously flawed.
The Cochrane Collaboration Systematic Review
The Cochrane group cast a net for all randomized, controlled clinical trials of methotrexate versus placebo or another disease-modifying antirheumatic drug (DMARD). They found eight, which they judged to be of poor quality. Their conclusion: “Low-quality evidence suggests that low-dose (15 mg or less) oral methotrexate might be slightly more effective than placebo when taken for 6 months; however, we are uncertain if it is more harmful” (Cochrane Database Syst Rev. 2019 Jan 18;1:CD012722. doi: 10.1002/14651858.CD012722.pub2).
“The new Cochrane Review concludes methotrexate doesn’t seem to work that well,” observed symposium director Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego.
“That’s because it’s based on published data, and there’s been very little of that,” said Dr. Ruderman, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago.
“I think most people assume, based on clinical experience, that it does work well. It’s all we had for years and years and years,” he added.
That is, until SEAM-PsA.
SEAM-PsA
SEAM-PsA randomized 851 DMARD- and biologic-naive patients with a median 0.6-year duration of psoriatic arthritis to one of three treatment arms for 48 weeks: once-weekly etanercept at 50 mg plus oral methotrexate at 20 mg, etanercept plus oral placebo, or methotrexate plus injectable placebo.
This is a study that will reshape clinical practice for many rheumatologists, according to Dr. Kavanaugh. The hypothesis was that in psoriatic arthritis, just as has been shown to be the case in rheumatoid arthritis, the combination of a TNF inhibitor plus methotrexate would have greater efficacy than either agent alone. But the study brought a couple of major surprises.
“Methotrexate didn’t do so badly,” Dr. Kavanaugh observed. “And the combination did nothing. I would have bet that the combination would have shown methotrexate had a synergistic effect with the TNF inhibitor, especially for x-ray changes. But the combination didn’t do any better than etanercept alone.”
Make no mistake: Etanercept monotherapy significantly outperformed methotrexate monotherapy for the primary endpoint, the ACR 20 response at week 24, by a margin of 60.9% versus 50.7%. Dr. Ruderman deemed that methotrexate response rate to be quite respectable, although he bemoaned the absence of a double-placebo comparator arm. And the key secondary endpoint, the minimal disease activity response rate at week 24, was also significantly better with etanercept, at 35.9% compared with 22.9%. Moreover, both etanercept arms showed significantly less radiographic progression than with methotrexate alone.
However, that was it. There were no significant differences between etanercept and methotrexate in other secondary endpoints, including the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC), the Disease Activity in PSoriatic Arthritis (DAPSA) score, the Leeds Dactylitis Instrument (LDI), and quality of life as assessed by the 36-item Short Form Health Survey total score.
“Methotrexate showed generally good efficacy across multiple domains,” the investigators concluded (Arthritis Rheumatol. 2019 Feb 12. doi: 10.1002/art.40851).
“Another intriguing thing to come out of this study for me were the enthesitis and dactylitis results. My clinical experience suggested methotrexate wasn’t so great for that, but this study suggests that’s not true,” Dr. Ruderman said.
“There are a couple of key take-home points from this study,” according to Dr. Kavanaugh. “One is that the combination is not synergistic. When you start a rheumatoid arthritis patient on methotrexate, you try to keep him on methotrexate when you add a TNF inhibitor. This study would say there doesn’t seem like there’s a reason to do that in your psoriatic arthritis patient. And the second message is that methotrexate seems to work.”
New ACR/NPF psoriatic arthritis guidelines under fire
“The new guidelines are fuzzy, aren’t they?” Dr. Kavanaugh said in lobbing the topic over to Dr. Ruderman.
“Where do we start?” he replied, shaking his head. “These are evidence-based guidelines in an area in which there was virtually no evidence.”
Indeed, the guidelines committee proudly employed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology, which forces committee members to issue “conditional” recommendations when there’s not enough evidence to make a “strong” recommendation.
“You’re not allowed to say, ‘We don’t know, there’s not enough evidence to make a choice,’ ” Dr. Ruderman said. “The problem with these guidelines is virtually everything in it is a conditional recommendation except ‘stop smoking,’ which was a strong recommendation.
“A conditional recommendation is pretty much a fancy term for expert opinion. It’s basically everybody in the room saying, ‘This is what we think.’ And that makes guidelines challenging because as a rheumatologist, you’re an expert. The people in the room have perhaps looked at the data more carefully than you’ve drilled down into the studies, but ultimately they’ve taken care of these patients and you’ve taken care of these patients, so why is their opinion better than your opinion, if it’s an informed opinion?”
His other critique of the 28-page guidelines is they don’t include the reasoning behind the conditional recommendations.
“If the conditional recommendation is, ‘In this situation, a TNF inhibitor is preferred over an IL-17 inhibitor,’ that would be great if they had also said, ‘This is why we thought that.’ But that’s not in the paper,” Dr. Ruderman said.
He and Dr. Kavanaugh reported serving as consultants to numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
MYSTIC trial: bTMB correlates with tTMB, predicts survival in mNSCLC
ATLANTA – Blood tumor mutational burden (bTMB) predicts survival benefit in metastatic non–small cell lung cancer (mNSCLC) patients treated with first-line durvalumab plus tremelimumab versus platinum-based chemotherapy, according to findings from the open-label, phase 3 MYSTIC trial.
Specifically, in patients with bTMB based on circulating tumor DNA at levels of 20 mutations (mut)/megabase (Mb) or greater, overall survival (OS) was significantly improved with durvalumab alone and with durvalumab plus tremelimumab versus chemotherapy (hazard ratios, 0.74 and 0.49, respectively). Progression-free survival (PFS) was also improved (HRs, 0.76 and 0.53, respectively), Solange Peters, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
Among patients with bTMB less than 20 mut/Mb, the corresponding OS hazard ratios were 1.55 and 1.26, and the corresponding PFS hazard ratios were 1.22 and 1.16, said Dr. Peters, director of teaching and patient care in the area of medical oncology and thoracic malignancies at Centre Hospitalier Universitaire Vaudois, Lausanne (Switzerland) University, and president-elect of the European Society for Medical Oncology.
Study subjects were 1,118 patients with immunotherapy- and chemotherapy-naive EGFR and ALK wild-type mNSCLC who were randomized 1:1 to receive either the programmed death–ligand 1 (PD-L1) agent durvalumab, durvalumab plus the anti-CTLA4 agent tremelimumab, or chemotherapy. At a tissue TMB (tTMB) level of 10 mut/Mb or greater, those who received either durvalumab or durvalumab plus tremelimumab had better OS than did those who received chemotherapy (HRs, 0.70 and 0.72, respectively).
Further, bTMB levels of at least 16 mut/Mb correlated positively with tTMB (Spearman correlation coefficient, 0.6; Pearson correlation coefficient, 0.7), she said, adding that survival probability at 24 months was 41.7% with durvalumab plus tremelimumab versus 35.0% with durvalumab alone and 22.7% with chemotherapy in this patient subgroup.
“When you look at the subgroup of patients with lower number of mutations than 10, this phenomenon is not observed. If anything should be potentially stressed about these hazard ratios – all above 1 – is potentially that chemotherapy represents a better option in this patient population,” she said.
Treatment included intravenous durvalumab at a dose of 20 mg/kg every 4 weeks with or without IV tremelimumab at a dose of 1 mg/kg every 4 weeks for up to four doses (372 and 374 patients, respectively), or platinum-based chemotherapy (372 patients).
“Durvalumab ... is already approved for unresectable stage 3 NSCLC and has shown clinical activity in heavily pretreated patients with mNSCLC in the context of phase 2 and 3 previously presented trials,” Dr. Peters said, noting that the multicenter MYSTIC trial was designed to assess its potential role in the treatment of all-comers with stage IV disease.
Overall survival data from the study were presented at the 2018 ESMO Immuno-Oncology Congress, and showed that the primary endpoint of superiority of durvalumab or durvalumab plus tremelimumab versus chemotherapy for OS in patients with high PD-L1 expression (at least 25% of tumor cells expressing PD-L1) was not met. There did, however, appear to be a clinically meaningful 3-month survival benefit (HR, 0.76) with durvalumab, she said.
The idea of combining durvalumab with tremelimumab was based on the potential for “antitumor activity via nonredundant pathways,” she noted, further explaining that the interest in determining whether bTMB correlates with tTMB relates to the rapid availability and less invasive nature of the latter and the fact that bTMB measured from circulating tumor DNA, “biologically speaking, may be more representative of the heterogeneity of metastatic lesions in the context of an advanced disease.”
The current findings are from exploratory analyses looking at OS based on bTMB and tTMB at varying levels to “better understand optimal outcomes and potential contributions of tremelimumab,” she said, noting that survival benefit was observed in all subgroups defined by a high TMB (equal to or greater than vs. less than 4, 8, 12, 16, and 20 mut/Mb).
Outcomes using the bTMB cutoff of 16 or greater mut/Mb were also presented at the ESMO meeting, and showed an OS benefit with durvalumab and durvalumab plus tremelimumab versus chemotherapy in those patients; the finding was more pronounced with durvalumab plus tremelimumab (HR, 0.82 and 0.62, respectively), and the effect increased with increasing bTMB levels.
“Based on that, we decided to [use the 20 mut/Mb or greater] cutoff to conduct a subsequent analysis,” Dr. Peters said, noting again the “very significantly improved” OS and PFS for combined treatment versus chemotherapy with 20 or greater versus less than 20 mut/Mb, and the value of chemotherapy in those with lower bTMB.
Tumor response was also better with durvalumab plus tremelimumab versus chemotherapy at this cutoff; objective response rates were 29.9% and 48.4% with durvalumab and durvalumab plus tremelimumab, respectively, versus 21.4% with chemotherapy.
“Again, under this threshold [less than 20 mut/Mb], it looked like chemotherapy was offering a better response rate with 31.4% as compared to 20% and less for the immunotherapy strategy,” she said.
Also of note, the percentage of patients remaining in response at 6 and 12 months was much better in those who received immunotherapy with durvalumab plus tremelimumab versus chemotherapy; this was more pronounced in those with high TMB (85.6% vs. 14.4% at 6 months; 81.7% vs. 7.2% at 12 months).
No differences were seen in toxicity patterns in the subgroup of patients with bTMB greater than 20 mut/Mb when compared with the overall safety population from MYSTIC, she added.
“Based on this exploratory analysis, further investigation and prospective validation of bTMB as a predictive biomarker for immunotherapy is warranted and should potentially be evaluated in as many as possible future clinical trials looking at immunotherapy across various solid tumors,” Dr. Peters said.
MYSTIC is sponsored by AstraZeneca. Dr. Peters reported relationships – including receipt of honoraria or consulting fees, receipt of grant/research support, and/or speaking engagements – with numerous pharmaceutical companies.
ATLANTA – Blood tumor mutational burden (bTMB) predicts survival benefit in metastatic non–small cell lung cancer (mNSCLC) patients treated with first-line durvalumab plus tremelimumab versus platinum-based chemotherapy, according to findings from the open-label, phase 3 MYSTIC trial.
Specifically, in patients with bTMB based on circulating tumor DNA at levels of 20 mutations (mut)/megabase (Mb) or greater, overall survival (OS) was significantly improved with durvalumab alone and with durvalumab plus tremelimumab versus chemotherapy (hazard ratios, 0.74 and 0.49, respectively). Progression-free survival (PFS) was also improved (HRs, 0.76 and 0.53, respectively), Solange Peters, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
Among patients with bTMB less than 20 mut/Mb, the corresponding OS hazard ratios were 1.55 and 1.26, and the corresponding PFS hazard ratios were 1.22 and 1.16, said Dr. Peters, director of teaching and patient care in the area of medical oncology and thoracic malignancies at Centre Hospitalier Universitaire Vaudois, Lausanne (Switzerland) University, and president-elect of the European Society for Medical Oncology.
Study subjects were 1,118 patients with immunotherapy- and chemotherapy-naive EGFR and ALK wild-type mNSCLC who were randomized 1:1 to receive either the programmed death–ligand 1 (PD-L1) agent durvalumab, durvalumab plus the anti-CTLA4 agent tremelimumab, or chemotherapy. At a tissue TMB (tTMB) level of 10 mut/Mb or greater, those who received either durvalumab or durvalumab plus tremelimumab had better OS than did those who received chemotherapy (HRs, 0.70 and 0.72, respectively).
Further, bTMB levels of at least 16 mut/Mb correlated positively with tTMB (Spearman correlation coefficient, 0.6; Pearson correlation coefficient, 0.7), she said, adding that survival probability at 24 months was 41.7% with durvalumab plus tremelimumab versus 35.0% with durvalumab alone and 22.7% with chemotherapy in this patient subgroup.
“When you look at the subgroup of patients with lower number of mutations than 10, this phenomenon is not observed. If anything should be potentially stressed about these hazard ratios – all above 1 – is potentially that chemotherapy represents a better option in this patient population,” she said.
Treatment included intravenous durvalumab at a dose of 20 mg/kg every 4 weeks with or without IV tremelimumab at a dose of 1 mg/kg every 4 weeks for up to four doses (372 and 374 patients, respectively), or platinum-based chemotherapy (372 patients).
“Durvalumab ... is already approved for unresectable stage 3 NSCLC and has shown clinical activity in heavily pretreated patients with mNSCLC in the context of phase 2 and 3 previously presented trials,” Dr. Peters said, noting that the multicenter MYSTIC trial was designed to assess its potential role in the treatment of all-comers with stage IV disease.
Overall survival data from the study were presented at the 2018 ESMO Immuno-Oncology Congress, and showed that the primary endpoint of superiority of durvalumab or durvalumab plus tremelimumab versus chemotherapy for OS in patients with high PD-L1 expression (at least 25% of tumor cells expressing PD-L1) was not met. There did, however, appear to be a clinically meaningful 3-month survival benefit (HR, 0.76) with durvalumab, she said.
The idea of combining durvalumab with tremelimumab was based on the potential for “antitumor activity via nonredundant pathways,” she noted, further explaining that the interest in determining whether bTMB correlates with tTMB relates to the rapid availability and less invasive nature of the latter and the fact that bTMB measured from circulating tumor DNA, “biologically speaking, may be more representative of the heterogeneity of metastatic lesions in the context of an advanced disease.”
The current findings are from exploratory analyses looking at OS based on bTMB and tTMB at varying levels to “better understand optimal outcomes and potential contributions of tremelimumab,” she said, noting that survival benefit was observed in all subgroups defined by a high TMB (equal to or greater than vs. less than 4, 8, 12, 16, and 20 mut/Mb).
Outcomes using the bTMB cutoff of 16 or greater mut/Mb were also presented at the ESMO meeting, and showed an OS benefit with durvalumab and durvalumab plus tremelimumab versus chemotherapy in those patients; the finding was more pronounced with durvalumab plus tremelimumab (HR, 0.82 and 0.62, respectively), and the effect increased with increasing bTMB levels.
“Based on that, we decided to [use the 20 mut/Mb or greater] cutoff to conduct a subsequent analysis,” Dr. Peters said, noting again the “very significantly improved” OS and PFS for combined treatment versus chemotherapy with 20 or greater versus less than 20 mut/Mb, and the value of chemotherapy in those with lower bTMB.
Tumor response was also better with durvalumab plus tremelimumab versus chemotherapy at this cutoff; objective response rates were 29.9% and 48.4% with durvalumab and durvalumab plus tremelimumab, respectively, versus 21.4% with chemotherapy.
“Again, under this threshold [less than 20 mut/Mb], it looked like chemotherapy was offering a better response rate with 31.4% as compared to 20% and less for the immunotherapy strategy,” she said.
Also of note, the percentage of patients remaining in response at 6 and 12 months was much better in those who received immunotherapy with durvalumab plus tremelimumab versus chemotherapy; this was more pronounced in those with high TMB (85.6% vs. 14.4% at 6 months; 81.7% vs. 7.2% at 12 months).
No differences were seen in toxicity patterns in the subgroup of patients with bTMB greater than 20 mut/Mb when compared with the overall safety population from MYSTIC, she added.
“Based on this exploratory analysis, further investigation and prospective validation of bTMB as a predictive biomarker for immunotherapy is warranted and should potentially be evaluated in as many as possible future clinical trials looking at immunotherapy across various solid tumors,” Dr. Peters said.
MYSTIC is sponsored by AstraZeneca. Dr. Peters reported relationships – including receipt of honoraria or consulting fees, receipt of grant/research support, and/or speaking engagements – with numerous pharmaceutical companies.
ATLANTA – Blood tumor mutational burden (bTMB) predicts survival benefit in metastatic non–small cell lung cancer (mNSCLC) patients treated with first-line durvalumab plus tremelimumab versus platinum-based chemotherapy, according to findings from the open-label, phase 3 MYSTIC trial.
Specifically, in patients with bTMB based on circulating tumor DNA at levels of 20 mutations (mut)/megabase (Mb) or greater, overall survival (OS) was significantly improved with durvalumab alone and with durvalumab plus tremelimumab versus chemotherapy (hazard ratios, 0.74 and 0.49, respectively). Progression-free survival (PFS) was also improved (HRs, 0.76 and 0.53, respectively), Solange Peters, MD, PhD, reported at the annual meeting of the American Association for Cancer Research.
Among patients with bTMB less than 20 mut/Mb, the corresponding OS hazard ratios were 1.55 and 1.26, and the corresponding PFS hazard ratios were 1.22 and 1.16, said Dr. Peters, director of teaching and patient care in the area of medical oncology and thoracic malignancies at Centre Hospitalier Universitaire Vaudois, Lausanne (Switzerland) University, and president-elect of the European Society for Medical Oncology.
Study subjects were 1,118 patients with immunotherapy- and chemotherapy-naive EGFR and ALK wild-type mNSCLC who were randomized 1:1 to receive either the programmed death–ligand 1 (PD-L1) agent durvalumab, durvalumab plus the anti-CTLA4 agent tremelimumab, or chemotherapy. At a tissue TMB (tTMB) level of 10 mut/Mb or greater, those who received either durvalumab or durvalumab plus tremelimumab had better OS than did those who received chemotherapy (HRs, 0.70 and 0.72, respectively).
Further, bTMB levels of at least 16 mut/Mb correlated positively with tTMB (Spearman correlation coefficient, 0.6; Pearson correlation coefficient, 0.7), she said, adding that survival probability at 24 months was 41.7% with durvalumab plus tremelimumab versus 35.0% with durvalumab alone and 22.7% with chemotherapy in this patient subgroup.
“When you look at the subgroup of patients with lower number of mutations than 10, this phenomenon is not observed. If anything should be potentially stressed about these hazard ratios – all above 1 – is potentially that chemotherapy represents a better option in this patient population,” she said.
Treatment included intravenous durvalumab at a dose of 20 mg/kg every 4 weeks with or without IV tremelimumab at a dose of 1 mg/kg every 4 weeks for up to four doses (372 and 374 patients, respectively), or platinum-based chemotherapy (372 patients).
“Durvalumab ... is already approved for unresectable stage 3 NSCLC and has shown clinical activity in heavily pretreated patients with mNSCLC in the context of phase 2 and 3 previously presented trials,” Dr. Peters said, noting that the multicenter MYSTIC trial was designed to assess its potential role in the treatment of all-comers with stage IV disease.
Overall survival data from the study were presented at the 2018 ESMO Immuno-Oncology Congress, and showed that the primary endpoint of superiority of durvalumab or durvalumab plus tremelimumab versus chemotherapy for OS in patients with high PD-L1 expression (at least 25% of tumor cells expressing PD-L1) was not met. There did, however, appear to be a clinically meaningful 3-month survival benefit (HR, 0.76) with durvalumab, she said.
The idea of combining durvalumab with tremelimumab was based on the potential for “antitumor activity via nonredundant pathways,” she noted, further explaining that the interest in determining whether bTMB correlates with tTMB relates to the rapid availability and less invasive nature of the latter and the fact that bTMB measured from circulating tumor DNA, “biologically speaking, may be more representative of the heterogeneity of metastatic lesions in the context of an advanced disease.”
The current findings are from exploratory analyses looking at OS based on bTMB and tTMB at varying levels to “better understand optimal outcomes and potential contributions of tremelimumab,” she said, noting that survival benefit was observed in all subgroups defined by a high TMB (equal to or greater than vs. less than 4, 8, 12, 16, and 20 mut/Mb).
Outcomes using the bTMB cutoff of 16 or greater mut/Mb were also presented at the ESMO meeting, and showed an OS benefit with durvalumab and durvalumab plus tremelimumab versus chemotherapy in those patients; the finding was more pronounced with durvalumab plus tremelimumab (HR, 0.82 and 0.62, respectively), and the effect increased with increasing bTMB levels.
“Based on that, we decided to [use the 20 mut/Mb or greater] cutoff to conduct a subsequent analysis,” Dr. Peters said, noting again the “very significantly improved” OS and PFS for combined treatment versus chemotherapy with 20 or greater versus less than 20 mut/Mb, and the value of chemotherapy in those with lower bTMB.
Tumor response was also better with durvalumab plus tremelimumab versus chemotherapy at this cutoff; objective response rates were 29.9% and 48.4% with durvalumab and durvalumab plus tremelimumab, respectively, versus 21.4% with chemotherapy.
“Again, under this threshold [less than 20 mut/Mb], it looked like chemotherapy was offering a better response rate with 31.4% as compared to 20% and less for the immunotherapy strategy,” she said.
Also of note, the percentage of patients remaining in response at 6 and 12 months was much better in those who received immunotherapy with durvalumab plus tremelimumab versus chemotherapy; this was more pronounced in those with high TMB (85.6% vs. 14.4% at 6 months; 81.7% vs. 7.2% at 12 months).
No differences were seen in toxicity patterns in the subgroup of patients with bTMB greater than 20 mut/Mb when compared with the overall safety population from MYSTIC, she added.
“Based on this exploratory analysis, further investigation and prospective validation of bTMB as a predictive biomarker for immunotherapy is warranted and should potentially be evaluated in as many as possible future clinical trials looking at immunotherapy across various solid tumors,” Dr. Peters said.
MYSTIC is sponsored by AstraZeneca. Dr. Peters reported relationships – including receipt of honoraria or consulting fees, receipt of grant/research support, and/or speaking engagements – with numerous pharmaceutical companies.
REPORTING FROM AACR 2019
What is your diagnosis? - May 2019
Nonsteroidal anti-inflammatory drug–induced diaphragm disease
Nonsteroidal anti-inflammatory drug (NSAID)–induced diaphragm disease is a rare cause of colonic stricture. To date, only about 50 cases have been reported. Diaphragm-like strictures occur predominantly in the right colon. The most common clinical presentations are obstructive symptoms and gastrointestinal bleeding after taking traditional NSAIDs or cyclo-oxygenase-2 inhibitors for more than 1 year.1 The thin diaphragm strictures are difficult to detect on imaging studies. They are seen during endoscopy or surgery. Concentric strictures in the right colon in the setting of chronic NSAID use are almost diagnostic of colonic diaphragm disease.2 Histopathology demonstrates submucosal fibrosis on resection specimens. Endoscopic biopsies may show lamina propria fibrosis, increased eosinophils with relative paucity of neutrophils, and even crypt distortion. The mechanism is thought to be due to contraction of scar tissue from healing concentric ulceration resulting in diaphragm-like strictures. Discontinuation of NSAIDs is recommended. Surgery is required to relieve obstruction in 75% of reported cases. Some have reported success with endoscopic balloon dilation.3
Using a 15-mm balloon, we performed endoscopic through-the-scope balloon dilation under fluoroscopy (Figures D, E). After dilation, the colonoscopy was completed to the distal ileum. There were two additional proximal concentric colonic strictures that allowed passage of the colonoscope and did not require dilation (Figure F). The patient was advised to stop diclofenac. He had no further gastrointestinal symptoms at the 3-month follow-up visit.
References
1. Wang Y-Z, Sun G, Cai F-C et al. Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract. 2016;2016:3679741
2. Püspök A, Kiener HP, Oberhuber G. Clinical, endoscopic, and histologic spectrum of nonsteroidal anti-inflammatory drug-induced lesions in the colon. Dis Colon Rectum. 2000;43:685-91.
3. Smith JA, Pineau BC. Endoscopic therapy of NSAID-induced colonic diaphragm disease: two cases and a review of published reports. Gastrointest Endosc. 2000;52:120-5.
Nonsteroidal anti-inflammatory drug–induced diaphragm disease
Nonsteroidal anti-inflammatory drug (NSAID)–induced diaphragm disease is a rare cause of colonic stricture. To date, only about 50 cases have been reported. Diaphragm-like strictures occur predominantly in the right colon. The most common clinical presentations are obstructive symptoms and gastrointestinal bleeding after taking traditional NSAIDs or cyclo-oxygenase-2 inhibitors for more than 1 year.1 The thin diaphragm strictures are difficult to detect on imaging studies. They are seen during endoscopy or surgery. Concentric strictures in the right colon in the setting of chronic NSAID use are almost diagnostic of colonic diaphragm disease.2 Histopathology demonstrates submucosal fibrosis on resection specimens. Endoscopic biopsies may show lamina propria fibrosis, increased eosinophils with relative paucity of neutrophils, and even crypt distortion. The mechanism is thought to be due to contraction of scar tissue from healing concentric ulceration resulting in diaphragm-like strictures. Discontinuation of NSAIDs is recommended. Surgery is required to relieve obstruction in 75% of reported cases. Some have reported success with endoscopic balloon dilation.3
Using a 15-mm balloon, we performed endoscopic through-the-scope balloon dilation under fluoroscopy (Figures D, E). After dilation, the colonoscopy was completed to the distal ileum. There were two additional proximal concentric colonic strictures that allowed passage of the colonoscope and did not require dilation (Figure F). The patient was advised to stop diclofenac. He had no further gastrointestinal symptoms at the 3-month follow-up visit.
References
1. Wang Y-Z, Sun G, Cai F-C et al. Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract. 2016;2016:3679741
2. Püspök A, Kiener HP, Oberhuber G. Clinical, endoscopic, and histologic spectrum of nonsteroidal anti-inflammatory drug-induced lesions in the colon. Dis Colon Rectum. 2000;43:685-91.
3. Smith JA, Pineau BC. Endoscopic therapy of NSAID-induced colonic diaphragm disease: two cases and a review of published reports. Gastrointest Endosc. 2000;52:120-5.
Nonsteroidal anti-inflammatory drug–induced diaphragm disease
Nonsteroidal anti-inflammatory drug (NSAID)–induced diaphragm disease is a rare cause of colonic stricture. To date, only about 50 cases have been reported. Diaphragm-like strictures occur predominantly in the right colon. The most common clinical presentations are obstructive symptoms and gastrointestinal bleeding after taking traditional NSAIDs or cyclo-oxygenase-2 inhibitors for more than 1 year.1 The thin diaphragm strictures are difficult to detect on imaging studies. They are seen during endoscopy or surgery. Concentric strictures in the right colon in the setting of chronic NSAID use are almost diagnostic of colonic diaphragm disease.2 Histopathology demonstrates submucosal fibrosis on resection specimens. Endoscopic biopsies may show lamina propria fibrosis, increased eosinophils with relative paucity of neutrophils, and even crypt distortion. The mechanism is thought to be due to contraction of scar tissue from healing concentric ulceration resulting in diaphragm-like strictures. Discontinuation of NSAIDs is recommended. Surgery is required to relieve obstruction in 75% of reported cases. Some have reported success with endoscopic balloon dilation.3
Using a 15-mm balloon, we performed endoscopic through-the-scope balloon dilation under fluoroscopy (Figures D, E). After dilation, the colonoscopy was completed to the distal ileum. There were two additional proximal concentric colonic strictures that allowed passage of the colonoscope and did not require dilation (Figure F). The patient was advised to stop diclofenac. He had no further gastrointestinal symptoms at the 3-month follow-up visit.
References
1. Wang Y-Z, Sun G, Cai F-C et al. Clinical features, diagnosis, and treatment strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract. 2016;2016:3679741
2. Püspök A, Kiener HP, Oberhuber G. Clinical, endoscopic, and histologic spectrum of nonsteroidal anti-inflammatory drug-induced lesions in the colon. Dis Colon Rectum. 2000;43:685-91.
3. Smith JA, Pineau BC. Endoscopic therapy of NSAID-induced colonic diaphragm disease: two cases and a review of published reports. Gastrointest Endosc. 2000;52:120-5.
A 57-year-old man presented to our hospital with a week of generalized weakness and abdominal pain.
Relevant medications included diclofenac 75 mg twice daily, aspirin 81 mg, and clopidogrel 75 mg/d. Vital signs were normal. Physical examination showed mild diffuse abdominal tenderness.
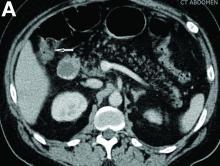
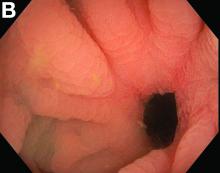
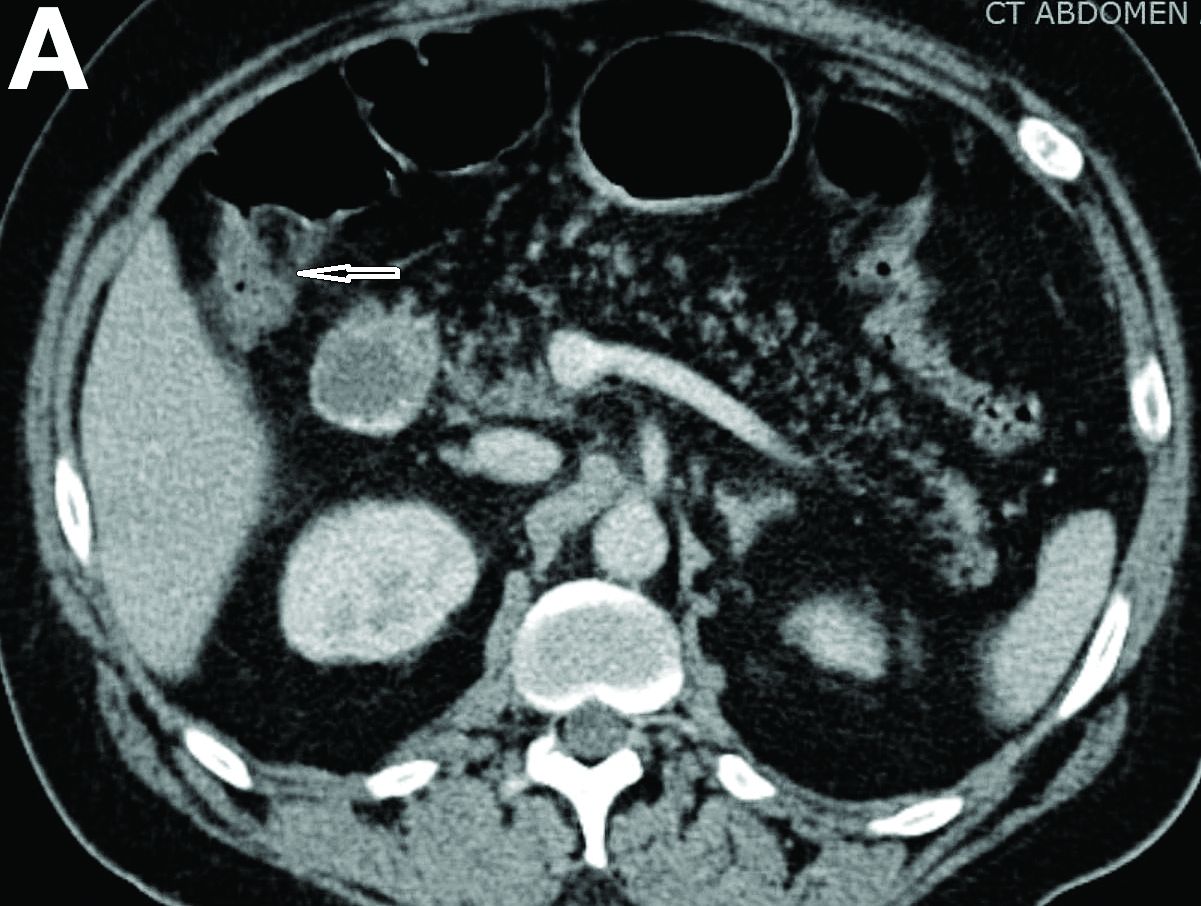
Admission blood work revealed a hemoglobin of 8.8 g/dL, decreased from a baseline hemoglobin of 12 g/dL. The patient did not have overt gastrointestinal bleeding, but tested positive for fecal occult blood. A computed tomography scan demonstrated luminal narrowing at the hepatic flexure without bowel wall thickening or obstruction (Figure A). Esophagogastroduodenoscopy was normal. Colonoscopy revealed a circumferential stricture in the right colon with an estimated diameter of 8 mm (Figure B).
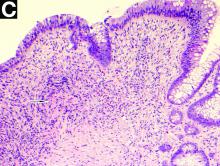
Biopsies of the stricture showed significant lamina propria fibrosis, eosinophilic infiltration, and mild crypt distortion (Figure C).
Positive psoriatic arthritis screens occur often in psoriasis patients
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Retail neurosis
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
When I stopped by last August to pick up new eyeglass lenses, Harold the optician sat alone in his shop.
“Business slow in the summer?” I asked.
Harold looked morose. “I knew it would be like this when I bought the business,” he said. “We’re open Saturdays, but summers I close at 2. Everybody’s at the Cape.”
Working in retail makes people more neurotic than necessary. I should know. I’ve been in retail for 40 years.
My patient Myrtle once explained to me how retail induces neurosis by deforming incentives. Myrtle used to work in management at a big department store. (Older readers may recall going to stores in buildings to buy things. The same readers may recall newspapers.)
“The month between Thanksgiving and Christmas makes or breaks the whole year,” Myrtle said. “If you do worse than last year, you feel bad. But if you do better than last year you also feel bad, because you worry you won’t be able to top it next year.”
She paused. “I guess that’s not a very healthy way to live, is it?”
I was too polite to agree.
Early in my career I had few patients on my schedule, maybe five on a good day. Then three of them would cancel. That was the start of my retail neurosis. Of course, I was a solo practitioner who started my own practice. The likes of me will someday be found in a museum, stuffed and mounted, along with other extinct species, under the label Medicus Cutaneous Solipsisticus (North America c. 20th century).
Over time, I got busier and dropped each of my eleven part-time jobs. By now I’ve been busy for decades, even though I’ve never had much of a waiting list. Don’t know why that is, but it no longer matters.
Except it does, psychologically. You won’t find this code in the DSM, but my working definition for the malady I describe is as follows:
Retail Neurosis (billable ICD-10 code F48.8. Other unspecified nonpsychotic mental disorders, along with writer’s block and psychasthenia):
Definition: The unquenchable fear that even the tiniest break in an endless churn of patients means that all patients will disappear later this afternoon, reverting the practice to the empty, formless void from whence it came. Other than retirement, there is no treatment for this disorder. And maybe not then either.
You might think to classify Retail Neurosis under Financial Insecurity, but that disorder has a different code. (F40.248, Fear of Failing, Life-Circumstance Problem). After all, a single well-remunerated patient (53 actinic keratoses!) can outreimburse half a dozen others with only E/M codes and big deductibles. Treat one of the former, take the rest of the hour off, and you’re financially just as well off, or even better. Yes?
No. Taking the rest of the hour off leaves you with too much time to ponder what every retailer knows: Each idle minute is another lost chance to make another sale and generate revenue. That minute (and revenue) can never be retrieved. Never!
As Myrtle would say, “Not a very healthy way to live, is it?”
Maybe not, but here as elsewhere, knowing something and fixing it are different things. Besides, brisk retail business brings a buzz, along with a sense of mastery and accomplishment, which is pleasantly addictive. Until it isn’t.
New generations of physicians and other medical providers will work in different settings than mine; they will be wage-earners in large organizations. These conglomerations bring their own neurosis-inducing incentives. Their managers measure providers’ productivity in various deforming and crazy-making ways. (See RVU-penia, ICD-10 M26.56: “Nonworking side interference.” This is actually a dental code that refers to jaw position, but billing demands creativity.) Practitioner anxieties will center on being docked for not generating enough relative value units or for failure to bundle enough comorbidities for maximizing capitation payments (e.g., Plaque Psoriasis plus Morbid Obesity plus Writer’s Block). But the youngsters will learn to get along. They’ll have to.
“Taking any time off this summer?” I asked my optician Harold.
“My wife and daughter are going out to Michigan in mid-August,” he said.
“Aren’t you going with them?”
“I can’t swing it that week,” he said. “By then, people are coming back to town, getting their kids ready for school. If I go away, I would miss some customers.”
Harold, you are my kind of guy!
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
8-week yoga wellness program feasible, beneficial in ob.gyn trainees
A yoga-based wellness program was associated with reductions in blood pressure and measures of depersonalization in a pilot study of ob.gyn. trainees, according to Shilpa Babbar, MD, of Saint Louis University, and associates.
The wellness program consisted of weekly 1-hour yoga classes over an 8-week period as well as weekly physical and nutritional challenges. The 29 people recruited to participate had their blood pressures, heart rates, and weights measured at baseline; they also took the abbreviated Maslach Burnout Inventory, the Depression Anxiety Stress Scale, and the Five Facet Mindfulness Questionnaire. These tests were repeated after the 8-week study period.
Of the 29 people who were recruited, 25 completed the study and 26 attended at least one class. Those who completed the program attended a mean of 3.8 classes, and 68% of participants attended at least half of the classes; no participant attended all classes. Participation in the weekly challenges was slightly less common, with 80% of participants engaging in at least one nutrition challenge and 60% of participants engaging in at least one physical challenge.
After the program had ended, participants had a significant decrease in the depersonalization component of burnout (P = .04), anxiety (P = .02), and systolic (P = .01) and diastolic (P = .01) blood pressures. In addition, those who attended more than 50% of classes had significantly lower systolic and diastolic blood pressures, compared with those who attended less frequently (P = .02 and P = .04, respectively). Participants also expressed increased camaraderie, appreciation, motivation, and overall training experience in a postprogram survey.
“ and overall health care system performance,” the investigators concluded.
One coauthor reported consulting with Health Insights Collaborative; no other conflicts of interest were reported.
SOURCE: Babbar S et al. Obstet Gynecol. 2019 May;133(5):994-1001.
A yoga-based wellness program was associated with reductions in blood pressure and measures of depersonalization in a pilot study of ob.gyn. trainees, according to Shilpa Babbar, MD, of Saint Louis University, and associates.
The wellness program consisted of weekly 1-hour yoga classes over an 8-week period as well as weekly physical and nutritional challenges. The 29 people recruited to participate had their blood pressures, heart rates, and weights measured at baseline; they also took the abbreviated Maslach Burnout Inventory, the Depression Anxiety Stress Scale, and the Five Facet Mindfulness Questionnaire. These tests were repeated after the 8-week study period.
Of the 29 people who were recruited, 25 completed the study and 26 attended at least one class. Those who completed the program attended a mean of 3.8 classes, and 68% of participants attended at least half of the classes; no participant attended all classes. Participation in the weekly challenges was slightly less common, with 80% of participants engaging in at least one nutrition challenge and 60% of participants engaging in at least one physical challenge.
After the program had ended, participants had a significant decrease in the depersonalization component of burnout (P = .04), anxiety (P = .02), and systolic (P = .01) and diastolic (P = .01) blood pressures. In addition, those who attended more than 50% of classes had significantly lower systolic and diastolic blood pressures, compared with those who attended less frequently (P = .02 and P = .04, respectively). Participants also expressed increased camaraderie, appreciation, motivation, and overall training experience in a postprogram survey.
“ and overall health care system performance,” the investigators concluded.
One coauthor reported consulting with Health Insights Collaborative; no other conflicts of interest were reported.
SOURCE: Babbar S et al. Obstet Gynecol. 2019 May;133(5):994-1001.
A yoga-based wellness program was associated with reductions in blood pressure and measures of depersonalization in a pilot study of ob.gyn. trainees, according to Shilpa Babbar, MD, of Saint Louis University, and associates.
The wellness program consisted of weekly 1-hour yoga classes over an 8-week period as well as weekly physical and nutritional challenges. The 29 people recruited to participate had their blood pressures, heart rates, and weights measured at baseline; they also took the abbreviated Maslach Burnout Inventory, the Depression Anxiety Stress Scale, and the Five Facet Mindfulness Questionnaire. These tests were repeated after the 8-week study period.
Of the 29 people who were recruited, 25 completed the study and 26 attended at least one class. Those who completed the program attended a mean of 3.8 classes, and 68% of participants attended at least half of the classes; no participant attended all classes. Participation in the weekly challenges was slightly less common, with 80% of participants engaging in at least one nutrition challenge and 60% of participants engaging in at least one physical challenge.
After the program had ended, participants had a significant decrease in the depersonalization component of burnout (P = .04), anxiety (P = .02), and systolic (P = .01) and diastolic (P = .01) blood pressures. In addition, those who attended more than 50% of classes had significantly lower systolic and diastolic blood pressures, compared with those who attended less frequently (P = .02 and P = .04, respectively). Participants also expressed increased camaraderie, appreciation, motivation, and overall training experience in a postprogram survey.
“ and overall health care system performance,” the investigators concluded.
One coauthor reported consulting with Health Insights Collaborative; no other conflicts of interest were reported.
SOURCE: Babbar S et al. Obstet Gynecol. 2019 May;133(5):994-1001.
FROM OBSTETRICS & GYNECOLOGY
Sleep apnea is linked with tau accumulation in the brain
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
PHILADELPHIA – according to data that will be presented at the annual meeting of the American Academy of Neurology. Tau accumulation is a biomarker of Alzheimer’s disease, and the finding suggests a possible explanation for the apparent association between sleep disruption and dementia.
“Our research results raise the possibility that sleep apnea affects tau accumulation,” said Diego Z. Carvalho, MD, of the Mayo Clinic in Rochester, Minn., in a press release. “But it is also possible that higher levels of tau in other regions may predispose a person to sleep apnea, so longer studies are now needed to solve this chicken-and-egg problem.”
Previous research had suggested an association between sleep disruption and increased risk of dementia. Obstructive sleep apnea in particular has been associated with this increased risk. The pathological processes that account for this association are unknown, however.
Dr. Carvalho and colleagues decided to evaluate whether apneas during sleep, reported by the patient or an informant, were associated with high levels of tau in cognitively normal elderly individuals. The investigators identified 288 participants in the Mayo Clinic Study of Aging for their analysis. Eligible participants were aged 65 years or older, had no cognitive impairment, had undergone tau PET and amyloid PET scans, and had completed a questionnaire that solicited information about witnessed apneas during sleep (either from patients or bed partners). Dr. Carvalho’s group took the entorhinal cortex as its region of interest because it is highly susceptible to tau accumulation. The entorhinal cortex is involved in memory, navigation, and the perception of time. They chose the cerebellum crus as their reference region.
The investigators created a linear model to evaluate the association between tau in the entorhinal cortex and witnessed apneas. They controlled the data for age, sex, years of education, body mass index, hypertension, hyperlipidemia, diabetes, reduced sleep, excessive daytime sleepiness, and global amyloid.
In all, 43 participants (15%) had witnessed apneas during sleep. Witnessed apneas were significantly associated with tau in the entorhinal cortex. After controlling for potential confounders, Dr. Carvalho and colleagues estimated a 0.049 elevation in the entorhinal cortex tau standardized uptake value ratio (95% confidence interval, 0.011–0.087; P = 0.012).
The study had a relatively small sample size, and its results require validation. Other important limitations include the absence of sleep studies to confirm the presence and severity of sleep apnea and a lack of information about whether participants already were receiving treatment for sleep apnea.
The National Institutes of Health supported the study.
SOURCE: Carvalho D et al. AAN 2019, Abstract P3.6-021.
FROM AAN 2019
Technology and the evolution of medical knowledge: What’s happening in the background
“Knowledge comes, but wisdom lingers. It may not be difficult to store up in the mind a vast quantity of facts within a comparatively short time, but the ability to form judgments requires the severe discipline of hard work and the tempering heat of experience and maturity.” – Calvin Coolidge
The information we use every day in patient care comes from one of two sources: personal experience (our own or that of another clinician) or a research study. Up until a hundred years ago, medicine was primarily a trade in which more experienced clinicians passed along their wisdom to younger clinicians, teaching them the things that they had learned during their long and difficult careers. Knowledge accrued slowly, influenced by the biased observations of each practicing doctor. People tended to remember their successes or unusual outcomes more than their failures or ordinary outcomes. Eventually, doctors realized that their knowledge base would be more accurate and accumulate more efficiently if they looked at the outcomes of many patients given the same treatment. Thus, the observational trial emerged.
As promising and important as the dawn of observational research was, it quickly became apparent that these trials had important limitations. The most notable limitations were the potential for bias and confounding variables to influence the results. Bias occurs when the opinion of the researcher influences how the result is interpreted. Confounders occur when an outcome is generated by some unexpected aspect of the patient, environment, or medication, rather than the thing that is being studied. An example of this might be a study that finds a higher mortality rate in a city by the sea than a city located inland. From these results, one might initially conclude that the sea is unhealthy. After realizing more retired people lived in the city by the sea; however, an individual would probably change his or her mind. In this example, the older age of this city’s population would be a confounding variable that drove the increased mortality in the city by the sea.
To solve the inherent problems with observational trials, the randomized, controlled trial was developed. Our reliance on information from RCTs runs so deep that it is hard to believe that the first modern clinical trial was not reported until 1948, in a paper on streptomycin in the treatment of pulmonary tuberculosis. It followed that faith in the randomized, controlled trial reached almost religious proportions, and the belief that information that does not come from an RCT should not be relied on was held by many, until recently. Why have things changed and what does this have to do with technology?
The first is an increasing recognition that, for all of their advantages, randomized trials have one nagging but critical limitation – generalizability. Randomized trials have strict inclusion and exclusion criteria. We do not have such inclusion and exclusion criteria when we take care of patients in our offices. For example, a recent trial published in the New England Journal of Medicine (2018 Dec 4. doi: 10.1056/NEJMoa1814468.), entitled “Apixaban to prevent venous thromboembolism in patients with cancer,” concluded that apixaban therapy resulted in a lower rate of venous thromboembolism than did placebo in patients starting chemotherapy for cancer. This was a large trial with more than 500 patients enrolled, and it reached an important conclusion with significant clinical implications. When you look at the details of the article, more than 1,800 patients were assessed to find the 500 patients who were eventually included in the trial. This is fairly typical of clinical trials and raises an important point: We need to be careful about how well the results of these clinical trials can be generalized to apply to the patient in front of us. This leads us to the second development that is something happening behind the scenes that each of us has contributed to.
Real-world research
As we see each patient and type information into the EHR, we add to an enormous database of medical information. That database is increasingly being used to advance our knowledge of how medicines actually work in the real world with real patients, and has already started providing answers that supplement, clarify, and even change our perspectives. The information will provide observations derived from real populations that have not been selected or influenced by the way in which a study is conducted. This new field of research is called “real-world research.”
An example of the difference between randomized controlled trial results and real-world research was published in Diabetes Care. This article examined the effectiveness of dipeptidyl peptidase 4 (DPP-4) inhibitors vs. glucagonlike peptide–1 receptor agonists (GLP-1 RAs) in the treatment of patients with diabetes. The goal of the study was to assess the difference in change in hemoglobin A1c between real-world evidence and randomized-trial evidence after initiation of a GLP-1 RA or a DPP-4 inhibitor. In RCTs, GLP-1 RAs decreased HbA1c by about 1.3% while DPP-4 inhibitors decreased HbA1c by about 0.68% (i.e., DPP-4 inhibitors were about half as effective). However, when the effects of these two diabetes drugs were examined using clinical databases in the real world, the two classes of medications had almost the same effect, each decreasing HbA1c by about 0.5%. This difference between RCT and real-world evidence might have been caused by the differential adherence to the two classes of medications, one being an injectable with significant GI side effects, and the other being a pill with few side effects.
The important take-home point is that we are now all contributing to a massive database that can be queried to give quicker, more accurate, more relevant information. Along with personal experience and randomized trials, this third source of clinical information, when used with wisdom, will provide us with the information we need to take ever better care of patients.
References
Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469-78.
Blonde L et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018 Nov;35:1763-74.
“Knowledge comes, but wisdom lingers. It may not be difficult to store up in the mind a vast quantity of facts within a comparatively short time, but the ability to form judgments requires the severe discipline of hard work and the tempering heat of experience and maturity.” – Calvin Coolidge
The information we use every day in patient care comes from one of two sources: personal experience (our own or that of another clinician) or a research study. Up until a hundred years ago, medicine was primarily a trade in which more experienced clinicians passed along their wisdom to younger clinicians, teaching them the things that they had learned during their long and difficult careers. Knowledge accrued slowly, influenced by the biased observations of each practicing doctor. People tended to remember their successes or unusual outcomes more than their failures or ordinary outcomes. Eventually, doctors realized that their knowledge base would be more accurate and accumulate more efficiently if they looked at the outcomes of many patients given the same treatment. Thus, the observational trial emerged.
As promising and important as the dawn of observational research was, it quickly became apparent that these trials had important limitations. The most notable limitations were the potential for bias and confounding variables to influence the results. Bias occurs when the opinion of the researcher influences how the result is interpreted. Confounders occur when an outcome is generated by some unexpected aspect of the patient, environment, or medication, rather than the thing that is being studied. An example of this might be a study that finds a higher mortality rate in a city by the sea than a city located inland. From these results, one might initially conclude that the sea is unhealthy. After realizing more retired people lived in the city by the sea; however, an individual would probably change his or her mind. In this example, the older age of this city’s population would be a confounding variable that drove the increased mortality in the city by the sea.
To solve the inherent problems with observational trials, the randomized, controlled trial was developed. Our reliance on information from RCTs runs so deep that it is hard to believe that the first modern clinical trial was not reported until 1948, in a paper on streptomycin in the treatment of pulmonary tuberculosis. It followed that faith in the randomized, controlled trial reached almost religious proportions, and the belief that information that does not come from an RCT should not be relied on was held by many, until recently. Why have things changed and what does this have to do with technology?
The first is an increasing recognition that, for all of their advantages, randomized trials have one nagging but critical limitation – generalizability. Randomized trials have strict inclusion and exclusion criteria. We do not have such inclusion and exclusion criteria when we take care of patients in our offices. For example, a recent trial published in the New England Journal of Medicine (2018 Dec 4. doi: 10.1056/NEJMoa1814468.), entitled “Apixaban to prevent venous thromboembolism in patients with cancer,” concluded that apixaban therapy resulted in a lower rate of venous thromboembolism than did placebo in patients starting chemotherapy for cancer. This was a large trial with more than 500 patients enrolled, and it reached an important conclusion with significant clinical implications. When you look at the details of the article, more than 1,800 patients were assessed to find the 500 patients who were eventually included in the trial. This is fairly typical of clinical trials and raises an important point: We need to be careful about how well the results of these clinical trials can be generalized to apply to the patient in front of us. This leads us to the second development that is something happening behind the scenes that each of us has contributed to.
Real-world research
As we see each patient and type information into the EHR, we add to an enormous database of medical information. That database is increasingly being used to advance our knowledge of how medicines actually work in the real world with real patients, and has already started providing answers that supplement, clarify, and even change our perspectives. The information will provide observations derived from real populations that have not been selected or influenced by the way in which a study is conducted. This new field of research is called “real-world research.”
An example of the difference between randomized controlled trial results and real-world research was published in Diabetes Care. This article examined the effectiveness of dipeptidyl peptidase 4 (DPP-4) inhibitors vs. glucagonlike peptide–1 receptor agonists (GLP-1 RAs) in the treatment of patients with diabetes. The goal of the study was to assess the difference in change in hemoglobin A1c between real-world evidence and randomized-trial evidence after initiation of a GLP-1 RA or a DPP-4 inhibitor. In RCTs, GLP-1 RAs decreased HbA1c by about 1.3% while DPP-4 inhibitors decreased HbA1c by about 0.68% (i.e., DPP-4 inhibitors were about half as effective). However, when the effects of these two diabetes drugs were examined using clinical databases in the real world, the two classes of medications had almost the same effect, each decreasing HbA1c by about 0.5%. This difference between RCT and real-world evidence might have been caused by the differential adherence to the two classes of medications, one being an injectable with significant GI side effects, and the other being a pill with few side effects.
The important take-home point is that we are now all contributing to a massive database that can be queried to give quicker, more accurate, more relevant information. Along with personal experience and randomized trials, this third source of clinical information, when used with wisdom, will provide us with the information we need to take ever better care of patients.
References
Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469-78.
Blonde L et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018 Nov;35:1763-74.
“Knowledge comes, but wisdom lingers. It may not be difficult to store up in the mind a vast quantity of facts within a comparatively short time, but the ability to form judgments requires the severe discipline of hard work and the tempering heat of experience and maturity.” – Calvin Coolidge
The information we use every day in patient care comes from one of two sources: personal experience (our own or that of another clinician) or a research study. Up until a hundred years ago, medicine was primarily a trade in which more experienced clinicians passed along their wisdom to younger clinicians, teaching them the things that they had learned during their long and difficult careers. Knowledge accrued slowly, influenced by the biased observations of each practicing doctor. People tended to remember their successes or unusual outcomes more than their failures or ordinary outcomes. Eventually, doctors realized that their knowledge base would be more accurate and accumulate more efficiently if they looked at the outcomes of many patients given the same treatment. Thus, the observational trial emerged.
As promising and important as the dawn of observational research was, it quickly became apparent that these trials had important limitations. The most notable limitations were the potential for bias and confounding variables to influence the results. Bias occurs when the opinion of the researcher influences how the result is interpreted. Confounders occur when an outcome is generated by some unexpected aspect of the patient, environment, or medication, rather than the thing that is being studied. An example of this might be a study that finds a higher mortality rate in a city by the sea than a city located inland. From these results, one might initially conclude that the sea is unhealthy. After realizing more retired people lived in the city by the sea; however, an individual would probably change his or her mind. In this example, the older age of this city’s population would be a confounding variable that drove the increased mortality in the city by the sea.
To solve the inherent problems with observational trials, the randomized, controlled trial was developed. Our reliance on information from RCTs runs so deep that it is hard to believe that the first modern clinical trial was not reported until 1948, in a paper on streptomycin in the treatment of pulmonary tuberculosis. It followed that faith in the randomized, controlled trial reached almost religious proportions, and the belief that information that does not come from an RCT should not be relied on was held by many, until recently. Why have things changed and what does this have to do with technology?
The first is an increasing recognition that, for all of their advantages, randomized trials have one nagging but critical limitation – generalizability. Randomized trials have strict inclusion and exclusion criteria. We do not have such inclusion and exclusion criteria when we take care of patients in our offices. For example, a recent trial published in the New England Journal of Medicine (2018 Dec 4. doi: 10.1056/NEJMoa1814468.), entitled “Apixaban to prevent venous thromboembolism in patients with cancer,” concluded that apixaban therapy resulted in a lower rate of venous thromboembolism than did placebo in patients starting chemotherapy for cancer. This was a large trial with more than 500 patients enrolled, and it reached an important conclusion with significant clinical implications. When you look at the details of the article, more than 1,800 patients were assessed to find the 500 patients who were eventually included in the trial. This is fairly typical of clinical trials and raises an important point: We need to be careful about how well the results of these clinical trials can be generalized to apply to the patient in front of us. This leads us to the second development that is something happening behind the scenes that each of us has contributed to.
Real-world research
As we see each patient and type information into the EHR, we add to an enormous database of medical information. That database is increasingly being used to advance our knowledge of how medicines actually work in the real world with real patients, and has already started providing answers that supplement, clarify, and even change our perspectives. The information will provide observations derived from real populations that have not been selected or influenced by the way in which a study is conducted. This new field of research is called “real-world research.”
An example of the difference between randomized controlled trial results and real-world research was published in Diabetes Care. This article examined the effectiveness of dipeptidyl peptidase 4 (DPP-4) inhibitors vs. glucagonlike peptide–1 receptor agonists (GLP-1 RAs) in the treatment of patients with diabetes. The goal of the study was to assess the difference in change in hemoglobin A1c between real-world evidence and randomized-trial evidence after initiation of a GLP-1 RA or a DPP-4 inhibitor. In RCTs, GLP-1 RAs decreased HbA1c by about 1.3% while DPP-4 inhibitors decreased HbA1c by about 0.68% (i.e., DPP-4 inhibitors were about half as effective). However, when the effects of these two diabetes drugs were examined using clinical databases in the real world, the two classes of medications had almost the same effect, each decreasing HbA1c by about 0.5%. This difference between RCT and real-world evidence might have been caused by the differential adherence to the two classes of medications, one being an injectable with significant GI side effects, and the other being a pill with few side effects.
The important take-home point is that we are now all contributing to a massive database that can be queried to give quicker, more accurate, more relevant information. Along with personal experience and randomized trials, this third source of clinical information, when used with wisdom, will provide us with the information we need to take ever better care of patients.
References
Carls GS et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40:1469-78.
Blonde L et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018 Nov;35:1763-74.