User login
VIDEO: Physicians fall short on adequate sleep, consumption of fruits and vegetables
LOS ANGELES – Physicians appear to meet Centers for Disease Control and Prevention guidelines for exercise, but they fall short when it comes to getting enough sleep and consuming an adequate amount of fruits and vegetables.
Those are key findings from a survey of 20 Tennessee-based physicians from a variety of medical specialties whom Deepti G. Bulchandani, MD, presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
Inspired by her daughter, Eesha Nachnani, Dr. Bulchandani, an endocrinologist with Saint Thomas Medical Partners in Hendersonville, Tenn., created a survey in which physicians were asked about their nutritional habits, as well as how much sleep and exercise they were getting. The duo found that only half of the survey respondents were eating at least 1.5-2 cups of fruit and 2-3 cups of vegetables a day, as recommended by the CDC, and only half were consuming less than 2,300 mg of sodium per day. They also found that only one in four physicians were sleeping more than 7 hours a day on a regular basis. The good news? All respondents met the recommended CDC guidelines for exercise.
“What was most neglected was sleep,” Dr. Bulchandani said. “[Electronic medical reports are] taking a lot of time. We do have [work hours] protection for residents, but physicians don’t have rules that are set for them. I think that is taking a toll.”
Dr. Bulchandani reported having no financial disclosures.
LOS ANGELES – Physicians appear to meet Centers for Disease Control and Prevention guidelines for exercise, but they fall short when it comes to getting enough sleep and consuming an adequate amount of fruits and vegetables.
Those are key findings from a survey of 20 Tennessee-based physicians from a variety of medical specialties whom Deepti G. Bulchandani, MD, presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
Inspired by her daughter, Eesha Nachnani, Dr. Bulchandani, an endocrinologist with Saint Thomas Medical Partners in Hendersonville, Tenn., created a survey in which physicians were asked about their nutritional habits, as well as how much sleep and exercise they were getting. The duo found that only half of the survey respondents were eating at least 1.5-2 cups of fruit and 2-3 cups of vegetables a day, as recommended by the CDC, and only half were consuming less than 2,300 mg of sodium per day. They also found that only one in four physicians were sleeping more than 7 hours a day on a regular basis. The good news? All respondents met the recommended CDC guidelines for exercise.
“What was most neglected was sleep,” Dr. Bulchandani said. “[Electronic medical reports are] taking a lot of time. We do have [work hours] protection for residents, but physicians don’t have rules that are set for them. I think that is taking a toll.”
Dr. Bulchandani reported having no financial disclosures.
LOS ANGELES – Physicians appear to meet Centers for Disease Control and Prevention guidelines for exercise, but they fall short when it comes to getting enough sleep and consuming an adequate amount of fruits and vegetables.
Those are key findings from a survey of 20 Tennessee-based physicians from a variety of medical specialties whom Deepti G. Bulchandani, MD, presented at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
Inspired by her daughter, Eesha Nachnani, Dr. Bulchandani, an endocrinologist with Saint Thomas Medical Partners in Hendersonville, Tenn., created a survey in which physicians were asked about their nutritional habits, as well as how much sleep and exercise they were getting. The duo found that only half of the survey respondents were eating at least 1.5-2 cups of fruit and 2-3 cups of vegetables a day, as recommended by the CDC, and only half were consuming less than 2,300 mg of sodium per day. They also found that only one in four physicians were sleeping more than 7 hours a day on a regular basis. The good news? All respondents met the recommended CDC guidelines for exercise.
“What was most neglected was sleep,” Dr. Bulchandani said. “[Electronic medical reports are] taking a lot of time. We do have [work hours] protection for residents, but physicians don’t have rules that are set for them. I think that is taking a toll.”
Dr. Bulchandani reported having no financial disclosures.
REPORTING FROM AACE 2019
Older women with ESRD face higher mortality, compared with male counterparts
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
LOS ANGELES – In patients with end-stage renal disease, women older than 50 years have a significantly higher mortality, compared with their male counterparts, results from an analysis of national data showed.
“The racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of [hypertension] in patients with CKD [chronic kidney disease], are well recognized,” the study’s senior author, Ricardo Correa, MD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Whites have better control of blood pressure, compared with Hispanics or African Americans with CKD, for example. On the other hand, gender differences in the outcome of blood pressure control and mortality across the different CKD stages have been very poorly studied, with conflicting results.”
The importance of gender difference has been mostly the focus in cardiovascular diseases, he continued, with compelling data revealing a higher incidence in men than in women of similar age, and a menopause-associated increase in cardiovascular disease in women.
“Whether the same can be said for hypertension, remains to be elucidated,” said Dr. Correa, an endocrinologist who directs the diabetes and metabolism fellowship at the University of Arizona in Phoenix.
In what he said is the first study of its kind, Dr. Correa and his colleagues set out to determine if gender in the U.S. population and menopausal age affect the inpatient survival rate in hypertensive patients across different stages of CKD. They drew from the 2005-2012 National Inpatient Sample to identify 2,121,750 hospitalized hypertensive patients and compared a number of factors between men and women, including crude mortality and mortality per CKD stage, menopausal age, length of stay, and total hospital charges.
Of the 2,121,750 patients, 1,092,931 (52%) were men and 1,028,819 (48%) were women; their mean age was 65 years. Among women, 32% had stage 3 CKD, 15% had stage 4 disease, 3% had stage 5 CKD, and 54% had end-stage renal disease (ESRD). Among men, 33% had stage 3 CKD, 13% had stage 4 disease, 3% had stage 5 CKD, and 51% had ESRD. The researchers observed that in-hospital crude mortality was significantly higher for men, compared with a matched group of women at CKD stages 3 and 4 (3.09% vs. 3.29% for CDK 3; P less than .0001 and 4.05% vs. 4.36% for CDK 4; P = .0004), yet was nonsignificant among those with ESRD (4.68% vs. 4.83%; P = .45).
When the researchers factored in menopausal age, they found that women with stage 3, 4, or 5 CKD who were aged 50 years or younger had a mortality rate similar to that of men with same stage disease, whereas women older than 50 years with ESRD had a significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001, compared with those of white, non-Hispanic descent).
“One could hypothesize that cardiac remodeling in hemodialysis women may be different than that in hemodialysis men to the extent that it affects mortality,” Dr. Correa said. “However, it is unclear if the survival benefit for dialysis men is owing to the possibility of a selection bias or not. Dialysis women may not be receiving equal access to cardiovascular procedures or surgical interventions (arteriovenous fistula, for example) or women may not be offered adequate hemodialysis to the same extent as men are. Further investigations regarding sex-based differences in dialysis treatment are required.”
He acknowledged certain limitations of the study, including its observational design. “We lacked detailed information regarding the cause of death, dialysis efficiency, types of dialysis accesses, and left ventricular hypertrophy measurements. We did not account for transitions between different hemodialysis modalities [and] we do not have information about distances or traveling time to dialysis units.”
The study’s first author was Kelvin Tran, MD. The researchers reported having no financial disclosures.
REPORTING FROM AACE 2019
Key clinical point: .
Major finding: Women older than 50 years with end-stage renal disease had significantly higher mortality, compared with their male counterparts, especially those of Asian, African American, and Hispanic descent (P less than .001 vs. those of white, non-Hispanic descent).
Study details: An observational study of more than 2 million hypertensive patients from the Nationwide Inpatient Sample.
Disclosures: Dr. Correa reported having no financial disclosures.
Depression treatment rates rose with expanded insurance coverage
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Multiple national policies designed to expand insurance coverage for mental health services in the United States likely contributed to modest increases in treatment for depression, according to an analysis of three national medical expenditure surveys.
for their depression,” wrote Jason M. Hockenberry, PhD, of Emory University in Atlanta and his associates. The study was published in JAMA Psychiatry.
To examine trends in depression treatment and spending, especially after the passage of the Mental Health Parity and Addiction Equity Act in 2008 and the Affordable Care Act in 2010, the authors analyzed responses to the 1998, 2007, and 2015 Medical Expenditure Panel Surveys (MEPSs). The final analysis included 86,216 individuals who were a mean (SD) age of 37.2 years.
From 1998 to 2015, rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 to 3.47 (95% CI, 3.16-3.79) per 100. The treated prevalence among white survey respondents was more than double that of black respondents in 2015, at 4.00 (95% CI, 3.58-4.43) per 100, compared with 1.91 (95% CI, 1.55-2.28) per 100. Though psychotherapy use declined from 1998 to 2007 and then increased slightly in 2015, the proportion of patients treated using pharmacotherapy stayed relatively constant at 81.9% (95% CI, 77.9%-85.9%) in 1998 and 80.8% (95% CI, 77.9%-83.7%) in 2015.
Total spending on outpatient depression treatment increased from $12,430,000 in 1998 to $15,554,000 in 2007, and $17,404,000 in 2015. The percentage of spending that came from self-pay decreased from 32% in 1998 to 20% in 2015. At the same time, the percentage of spending covered by Medicaid increased, from 19% in 1998 to 36% in 2015.
Dr. Hockenberry and his coauthors acknowledged the limitations of their study, including the pitfalls of relying on national surveys over long periods of time. Specifically, the MEPSs depended in part on inexact measures, such as memory of health care visits; the 2015 survey also had a response rate of only 47.7%. That said, they reinforced their findings by citing how additional surveys that assess major depression – including the 2016 National Survey on Drug Use and Health – “have found similar proportions of treated depression to what we find in the 2015 MEPS.”
The study was supported in part by the Commonwealth Fund, and Dr. Hockenberry also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
SOURCE: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
FROM JAMA PSYCHIATRY
Key clinical point: Treatment for – and spending on – depression both saw modest increases from 1998 to 2015.
Major finding: Rates of outpatient treatment for depression increased from 2.36 (95% confidence interval, 2.12-2.61) per 100 in 1998 to 3.47 (95% CI, 3.16-3.79) per 100 in 2015.
Study details: An analysis of 86,216 individuals from the 1998, 2007, and 2015 Medical Expenditure Panel Surveys.
Disclosures: The study was supported in part by the Commonwealth Fund, and the lead author also reported receiving grants from the Commonwealth Fund. No other conflicts of interest were reported.
Source: Hockenberry JM et al. JAMA Psychiatry. 2019 Apr 24. doi: 10.1001/jamapsychiatry.2019.0633.
Alirocumab gains indication to reduce cardiovascular risks
Alirocumab has received an updated indication from the Food and Drug Administration for reducing the overall risk of major adverse cardiovascular events in patients with a recent acute coronary event.
Alirocumab is designed to inhibit the binding of PCSK9 (proprotein convertase subtilisin/kexin type 9) to LDL receptors, thereby lowering LDL cholesterol, according to manufacturer Regeneron, which is developing alirocumab in partnership with Sanofi.
The drug was previously approved in the United States as an adjunct treatment along with diet and maximally tolerated statin therapy to help lower LDL cholesterol in adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease.
The approval of the supplemental Biologics License Application was supported by data from the ODYSSEY Outcomes trial in which 18,924 patients who had an acute coronary syndrome were randomized to alirocumab or placebo plus background high-intensity statin therapy starting at a median of 2.6 months after the event. Over 3 years’ follow-up, a composite endpoint outcome including death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or unstable angina occurred in 9.5% of alirocumab patients and 11.1% of placebo patients.
In the study, patients received subcutaneous dose of 75 mg of alirocumab every 2 weeks, which was adjusted to achieve an LDL cholesterol level of 25-50 mg/dL. The most significant benefits occurred among patients with a baseline LDL cholesterol of 100 mg/dL or higher who were taking high-intensity statins, which supports the role of LDL cholesterol reduction in improving outcomes for coronary syndrome patients, according to study investigators.
Alirocumab is given as a subcutaneous injection. The most common side effects include pain and tenderness at the injection site, and redness, itching, or swelling; some patients have reported symptoms of a common cold or flu.
More details of the ODYSSEY Outcomes trial were presented at the annual meeting of the American College of Cardiology.
Alirocumab has received an updated indication from the Food and Drug Administration for reducing the overall risk of major adverse cardiovascular events in patients with a recent acute coronary event.
Alirocumab is designed to inhibit the binding of PCSK9 (proprotein convertase subtilisin/kexin type 9) to LDL receptors, thereby lowering LDL cholesterol, according to manufacturer Regeneron, which is developing alirocumab in partnership with Sanofi.
The drug was previously approved in the United States as an adjunct treatment along with diet and maximally tolerated statin therapy to help lower LDL cholesterol in adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease.
The approval of the supplemental Biologics License Application was supported by data from the ODYSSEY Outcomes trial in which 18,924 patients who had an acute coronary syndrome were randomized to alirocumab or placebo plus background high-intensity statin therapy starting at a median of 2.6 months after the event. Over 3 years’ follow-up, a composite endpoint outcome including death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or unstable angina occurred in 9.5% of alirocumab patients and 11.1% of placebo patients.
In the study, patients received subcutaneous dose of 75 mg of alirocumab every 2 weeks, which was adjusted to achieve an LDL cholesterol level of 25-50 mg/dL. The most significant benefits occurred among patients with a baseline LDL cholesterol of 100 mg/dL or higher who were taking high-intensity statins, which supports the role of LDL cholesterol reduction in improving outcomes for coronary syndrome patients, according to study investigators.
Alirocumab is given as a subcutaneous injection. The most common side effects include pain and tenderness at the injection site, and redness, itching, or swelling; some patients have reported symptoms of a common cold or flu.
More details of the ODYSSEY Outcomes trial were presented at the annual meeting of the American College of Cardiology.
Alirocumab has received an updated indication from the Food and Drug Administration for reducing the overall risk of major adverse cardiovascular events in patients with a recent acute coronary event.
Alirocumab is designed to inhibit the binding of PCSK9 (proprotein convertase subtilisin/kexin type 9) to LDL receptors, thereby lowering LDL cholesterol, according to manufacturer Regeneron, which is developing alirocumab in partnership with Sanofi.
The drug was previously approved in the United States as an adjunct treatment along with diet and maximally tolerated statin therapy to help lower LDL cholesterol in adults with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease.
The approval of the supplemental Biologics License Application was supported by data from the ODYSSEY Outcomes trial in which 18,924 patients who had an acute coronary syndrome were randomized to alirocumab or placebo plus background high-intensity statin therapy starting at a median of 2.6 months after the event. Over 3 years’ follow-up, a composite endpoint outcome including death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or unstable angina occurred in 9.5% of alirocumab patients and 11.1% of placebo patients.
In the study, patients received subcutaneous dose of 75 mg of alirocumab every 2 weeks, which was adjusted to achieve an LDL cholesterol level of 25-50 mg/dL. The most significant benefits occurred among patients with a baseline LDL cholesterol of 100 mg/dL or higher who were taking high-intensity statins, which supports the role of LDL cholesterol reduction in improving outcomes for coronary syndrome patients, according to study investigators.
Alirocumab is given as a subcutaneous injection. The most common side effects include pain and tenderness at the injection site, and redness, itching, or swelling; some patients have reported symptoms of a common cold or flu.
More details of the ODYSSEY Outcomes trial were presented at the annual meeting of the American College of Cardiology.
Which Comes First: The Mood Disorder or the Inflammation?
Mood disorders and cardiovascular disease (CVD) are often linked—1 mechanism may be common underlying low-grade inflammation. Specifically, studies have found a consistent association between circulating levels of pro-inflammatory cytokines with both mood disorders and CVD, say researchers from Lausanne University Hospital and Bern University Hospital, in Switzerland and National Institute of Mental Health in Maryland. They suggest that influence may be oneway: Mood disorders may lead to inflammation, but inflammation may not be a risk factor for the onset of mood disorders.
Noting that much of the research on inflammatory markers and CVD has focused on dysthymia, the researchers decided to conduct a study to investigate any association between atypical subtype of dysthymia and increased levels of inflammatory markers. They analyzed data from 3,118 participants who underwent comprehensive somatic and psychiatric evaluations at baseline and a mean of 5.5 years later. Current and remitted mood disorders included bipolar and major depressive disorders (MDD); subtypes included atypical, melancholic, and combinations of those.
After adjusting for confounders, they found current combined MDD was associated with increased high sensitivity C-reactive protein (hsCRP) levels and decreased IL-6 levels. Current atypical MDD was associated with increased hsCRP levels at follow-up. Moreover, remitted melancholic MDD was associated with decreased IL-6 levels at follow-up.
The major finding, the researchers say, was the association between the current atypical subtype of MDD at baseline with increased levels of hsCRP at follow-up. By contrast, inflammatory levels at baseline were not associated with subsequent atypical MDD at follow-up. What this suggests is that the disorder is causally related to increased inflammation, rather than inflammation increasing the mood disorder.
The finding of unidirectional association seems to be specific to the atypical subtype of MDD, the researchers add, which is characterized by somatic symptoms, including sleep, energy, and eating behavior.
Mood disorders and cardiovascular disease (CVD) are often linked—1 mechanism may be common underlying low-grade inflammation. Specifically, studies have found a consistent association between circulating levels of pro-inflammatory cytokines with both mood disorders and CVD, say researchers from Lausanne University Hospital and Bern University Hospital, in Switzerland and National Institute of Mental Health in Maryland. They suggest that influence may be oneway: Mood disorders may lead to inflammation, but inflammation may not be a risk factor for the onset of mood disorders.
Noting that much of the research on inflammatory markers and CVD has focused on dysthymia, the researchers decided to conduct a study to investigate any association between atypical subtype of dysthymia and increased levels of inflammatory markers. They analyzed data from 3,118 participants who underwent comprehensive somatic and psychiatric evaluations at baseline and a mean of 5.5 years later. Current and remitted mood disorders included bipolar and major depressive disorders (MDD); subtypes included atypical, melancholic, and combinations of those.
After adjusting for confounders, they found current combined MDD was associated with increased high sensitivity C-reactive protein (hsCRP) levels and decreased IL-6 levels. Current atypical MDD was associated with increased hsCRP levels at follow-up. Moreover, remitted melancholic MDD was associated with decreased IL-6 levels at follow-up.
The major finding, the researchers say, was the association between the current atypical subtype of MDD at baseline with increased levels of hsCRP at follow-up. By contrast, inflammatory levels at baseline were not associated with subsequent atypical MDD at follow-up. What this suggests is that the disorder is causally related to increased inflammation, rather than inflammation increasing the mood disorder.
The finding of unidirectional association seems to be specific to the atypical subtype of MDD, the researchers add, which is characterized by somatic symptoms, including sleep, energy, and eating behavior.
Mood disorders and cardiovascular disease (CVD) are often linked—1 mechanism may be common underlying low-grade inflammation. Specifically, studies have found a consistent association between circulating levels of pro-inflammatory cytokines with both mood disorders and CVD, say researchers from Lausanne University Hospital and Bern University Hospital, in Switzerland and National Institute of Mental Health in Maryland. They suggest that influence may be oneway: Mood disorders may lead to inflammation, but inflammation may not be a risk factor for the onset of mood disorders.
Noting that much of the research on inflammatory markers and CVD has focused on dysthymia, the researchers decided to conduct a study to investigate any association between atypical subtype of dysthymia and increased levels of inflammatory markers. They analyzed data from 3,118 participants who underwent comprehensive somatic and psychiatric evaluations at baseline and a mean of 5.5 years later. Current and remitted mood disorders included bipolar and major depressive disorders (MDD); subtypes included atypical, melancholic, and combinations of those.
After adjusting for confounders, they found current combined MDD was associated with increased high sensitivity C-reactive protein (hsCRP) levels and decreased IL-6 levels. Current atypical MDD was associated with increased hsCRP levels at follow-up. Moreover, remitted melancholic MDD was associated with decreased IL-6 levels at follow-up.
The major finding, the researchers say, was the association between the current atypical subtype of MDD at baseline with increased levels of hsCRP at follow-up. By contrast, inflammatory levels at baseline were not associated with subsequent atypical MDD at follow-up. What this suggests is that the disorder is causally related to increased inflammation, rather than inflammation increasing the mood disorder.
The finding of unidirectional association seems to be specific to the atypical subtype of MDD, the researchers add, which is characterized by somatic symptoms, including sleep, energy, and eating behavior.
Coding and payment changes could hit GIs in 2021
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at jmanthey@gastro.org .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).
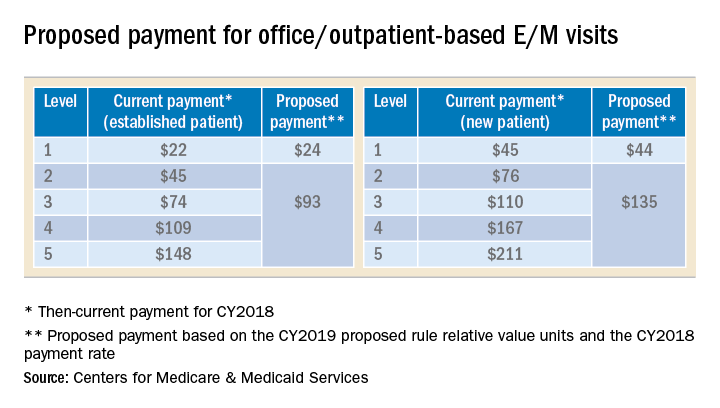
In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at jmanthey@gastro.org .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).

In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
Welcome to the new Practice Management Toolbox.
The AGA Practice Management and Economics Committee (PMEC) is pleased to host an updated Practice Management Toolbox column featuring contemporary GI practice management issues and news. As chair of the PMEC, I am excited to bring you this content on behalf of my colleagues on the committee. Each month we will highlight a timely topic relevant to gastroenterologists in practice. The AGA and PMEC strive to be at the forefront of changes to the field of gastroenterology, providing you with tools and resources to succeed. If there is an article topic you would like to suggest, please reach out to Jacob Manthey, Practice and Quality Manager at jmanthey@gastro.org .
Anton Decker, MD, AGAF
Chair, Practice Management and Economics Committee
Last year, Medicare began laying groundwork for major changes to coding and payment for common evaluation and management (E/M) services and two high-volume GI endoscopy procedures beginning January 1, 2021 with expected adoption by commercial payers. Learn about these potential changes now to help prepare your practice for the financial impact.
2021 E/M Changes: New guidelines, new payments
The Centers for Medicare and Medicaid Services (CMS), also commonly referred to as Medicare, announced in its 2019 Physician Fee Schedule proposed rule that it wanted to reduce administrative burden and improve payment accuracy for office/outpatient new and established patient codes (99201-99205 and 99211-99215) by paying level 2–5 codes at a single payment rate and simplifying documentation to support only a level 2 E/M visit, except when using time for documentation (Table).

In the original proposal, those who reported mostly level 2 and 3 E/M visits would have experienced modest payment increases while those who reported mostly level 4 and 5 E/M visits would have endured payment cuts between 20%-40%. Ultimately, the physician community, including AGA and its sister societies, opposed the proposed payment consolidation and pressured CMS not to finalize most of its proposed changes and preserve the current payment rates. The 2019 MPFS final rule made no changes to the relative values for office/outpatient new and established patient codes 99201-99205 and 99211-99215, but did outline a new plan “for paying a single rate for E/M office/outpatient visit levels 2 through 4 for established and new patients while maintaining the payment rate for E/M office/outpatient visit level 5 in order to better account for the care and needs of complex patients.” CMS agreed to continue to accept input on improvements to the proposal before CMS’ planned implementation in 2021.
A proposal to simplify E/M guidelines within Current Procedural Terminology (CPT) and preserve the individual levels of the new and established patient office/outpatient E/M codes, except 99201 which was proposed for deletion, was presented to the American Medical Association (AMA) CPT Editorial Panel, the body responsible for creating and maintaining CPT codes, and approved at its February 2019 meeting. The approved changes will not be publicly available until the CPT 2021 book is released in August 2020. In the meantime, the AMA Specialty Society Relative-value scale Update Committee (RUC) will make recommendations to CMS on potential new relative values for the E/M codes.
It is unclear whether CMS will accept the AMA CPT Editorial Panel’s changes and potential new values or move forward with the plan for three levels of E/M for office/outpatient new and established patient codes. However, any changes to the current guidelines will undoubtedly involve a learning curve for both physicians and coders and it is unclear whether approximately four months from the time the 2021 CPT book is released and the time the new rates will be implemented on January 1, 2021 is enough to master the changes and update internal systems. In addition, any changes to reimbursement will impact each practice’s bottom line.
2021 potential payment changes for CPT codes 43239 and 45385
In the same proposed rule, CMS announced that an unnamed party had nominated seven CPT codes, including esophagogastroduodenoscopy (EGD) with biopsy (CPT code 43239) and colonoscopy with snare polypectomy (CPT code 45385), as potentially overvalued and recommended reducing their reimbursement based on data from the 2017 Urban Institute report for CMS. The AGA and its sister societies pointed out to CMS major flaws in the Urban Institute study’s methods that should have prevented its use as evidence that the codes were misvalued and we provided data from the GI societies’ robust sample of physicians to support the current values.
In the 2019 MPFS final rule, CMS revealed Anthem, a major U.S. health insurance company, as the nominating party sparking concern that this unprecedented development may result in other payers using the flawed Urban Institute study to influence CMS to revalue other services.
Codes CMS identified as potentially misvalued in the 2019 MPFS final rule were referred to the RUC for resurvey of physician work and practice expense for consideration at the April 2019 RUC meeting. The AGA and its sister societies conducted a survey of a random sample of our memberships during February and March and presented our recommendations based on the data we collected. CMS’ proposed values will be published in July 2020 in the 2021 MPFS proposed rule and finalized in the final rule that November.
Next steps
CMS will announce changes to E/M coding and documentation guidelines and any new payment changes to CPT codes 43239 and 45385 in the 2021 MPFS proposed rule in July 2020. Be prepared to use this information to model the financial impact to your practice so you can determine what, if any changes, should be made. Contact your coding and billing staff, consultants and software providers to find out how they plan to implement any changes. Additional E/M training may be required for your providers and staff. The GI Societies remain vigilant and continue to advocate on the behalf of its members to advise and shape these policy evaluations and changes.
Dr. Kuo is assistant professor, director of the Center for Neurointestinal Health, GI Unit, Massachusetts General Hospital, Harvard Medical School, Boston; AGA CPT Advisor; he has no conflicts of interest. Dr. Mehta is assistant professor, Perelman School of Medicine; associate chief innovation officer, Penn Medicine, Philadelphia; AGA RUC Advisor; he has no conflicts of interest.
EU authorization recommended for buprenorphine implant
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
The European Medicines Agency announced April 26 that its human medicines committee has recommended granting a marketing authorization for Sixmo, a long-lasting implant delivering buprenorphine as treatment for opioid use disorder (OUD).
This recommendation is a step toward making the product available to patients with OUD in the European Union, according to a press release from the EMA. Safety and efficacy of the implant were studied in three trials with a total of 628 patients.
Standard treatment of OUD includes psychological and social counseling, as well as substitution opioid therapy – such as methadone or buprenorphine. The Sixmo implant involves four small rods implanted in the patient’s upper arm under local anesthetic.
The most common adverse events associated with the medicine were in keeping with the known events associated with buprenorphine – headache, constipation, and insomnia. Insertion and removal were associated with pain, severe itching, and hematoma at the implant site.
The full release can be found on the EMA website.
Medical cannabis relieved pain, decreased opioid use in elderly
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
FROM AAN 2019
FDA approves new etanercept biosimilar, Eticovo
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
Tenofovir disoproxil treated HBV with fewer future HCCs
VIENNA – Treatment of individuals chronically infected with hepatitis B virus (HBV) with the nucleotide analog tenofovir disoproxil fumarate significantly linked with a substantial cut in the incidence of hepatocellular carcinoma (HCC) compared with those who received the nucleoside analog entecavir, according to a review of more than 29,000 Hong Kong patients.
This is the second reported study to find that association. In January 2019, a study of more than 24,000 Korean residents chronically infected with HBV showed a similar, statistically significant link between treatment with tenofovir disoproxil fumarate (Viread) and a lower incidence of HCC compared with patients treated with entecavir (Baraclude) (JAMA Oncol. 2019 Jan;5[1]:30-6), Grace L.H. Wong, MD, said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
However, another report published just a few days before Dr. Wong spoke failed to find an association between tenofovir disoproxil treatment of HBV and the subsequent rate of HCC compared with patients treated with entecavir. That study comprised nearly 2,900 HBV patients treated at any of four Korean medical centers (J Hepatol. 2019 Apr. doi: 10.1016/j.jhep.2019.03.028).
Dr. Wong noted that although current guidelines from EASL cite both tenofovir disoproxil and entecavir (as well as tenofovir alafenamide [Vemlidy]) as first-line treatments for chronic HBV infection (J Hepatol. 2017 Aug;67[2]:370-98), some evidence suggests that tenofovir disoproxil might produce effects subtly different from those of entecavir.
At the meeting in Vienna, for example, a report on 176 Japanese patients with chronic HBV showed that those who were treated with a nucleotide analog such as tenofovir disoproxil produced higher serum levels of interferon-lamda3 compared with patients treated with entecavir, and increased levels of this interferon could improve clearance of HBV surface antigen (J Hepatol. 2019 April;70[1]:e477). The most recent EASL guidelines for treatment of chronic hepatitis B infection also list tenofovir disoproxil, entecavir, and tenofovir alafenamide as preferred agents (Hepatology. 2018 April;67[4]:1560-99).
The data Dr. Wong and her associates analyzed came from health records kept for about 80% of Hong Kong’s population in the Clinical Data Analysis and Recording System of the Hospital Authority of Hong Kong. From January 2010 to June 2018, this database included 28,041 consecutive patients chronically infected with HBV and treated with entecavir, and 1,309 consecutive patients treated with tenofovir disoproxil. These numbers excluded patients treated for less than 6 months, patients coinfected with hepatitis C or D virus, patients with cancer diagnosed or a liver transplanted before or during their first 6 months on treatment, and patients previously treated with an interferon or nucleos(t)ide.
During an average follow-up of 2.8 years of tenofovir disoproxil treatment, 8 patients developed HCC, and during an average follow-up of 3.7 years of entecavir treatment, 1,386 patients developed HCC, reported Dr. Wong, a hepatologist and professor of medicine at the Chinese University of Hong Kong.
In a multivariate analysis that adjusted for demographic and clinical differences, treatment with tenofovir disoproxil linked with a statistically significant 68% reduced rate of HCC development compared with the entecavir-treated patients, she said. In a propensity score–weighted analysis, tenofovir disoproxil linked with a statistically significant 64% reduced rate of incident HCC, and in a propensity score–matched analysis tenofovir disoproxil linked with a 58% reduced rate of HCC, although in this analysis, which excluded many of the entecavir-treated patients and hence had less statistical power, the difference just missed statistical significance.
As an additional step to try to rule out the possible effect of unadjusted confounders, Dr. Wong and associates analyzed the links between tenofovir disoproxil and entecavir treatment and two negative-control outcomes, the incidence of lung cancer and the incidence of acute myocardial infarction. Neither of these outcomes showed a statistically significant link with one of the HBV treatments, suggesting that the link between treatment and HCC incidence did not appear because of an unadjusted confounding bias, Dr. Wong said. The Hong Kong database did not include enough patients treated with tenofovir alafenamide to allow assessment of this drug, she added.
Dr. Wong has been an adviser to Gilead and a speaker for Abbott, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Roche. Tenofovir disoproxil fumarate is marketed by Gilead, and entecavir is marketed by Bristol-Myers Squibb.
SOURCE: Wong GL et al. J Hepatol. 2019 April;70[1]:e128.
VIENNA – Treatment of individuals chronically infected with hepatitis B virus (HBV) with the nucleotide analog tenofovir disoproxil fumarate significantly linked with a substantial cut in the incidence of hepatocellular carcinoma (HCC) compared with those who received the nucleoside analog entecavir, according to a review of more than 29,000 Hong Kong patients.
This is the second reported study to find that association. In January 2019, a study of more than 24,000 Korean residents chronically infected with HBV showed a similar, statistically significant link between treatment with tenofovir disoproxil fumarate (Viread) and a lower incidence of HCC compared with patients treated with entecavir (Baraclude) (JAMA Oncol. 2019 Jan;5[1]:30-6), Grace L.H. Wong, MD, said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
However, another report published just a few days before Dr. Wong spoke failed to find an association between tenofovir disoproxil treatment of HBV and the subsequent rate of HCC compared with patients treated with entecavir. That study comprised nearly 2,900 HBV patients treated at any of four Korean medical centers (J Hepatol. 2019 Apr. doi: 10.1016/j.jhep.2019.03.028).
Dr. Wong noted that although current guidelines from EASL cite both tenofovir disoproxil and entecavir (as well as tenofovir alafenamide [Vemlidy]) as first-line treatments for chronic HBV infection (J Hepatol. 2017 Aug;67[2]:370-98), some evidence suggests that tenofovir disoproxil might produce effects subtly different from those of entecavir.
At the meeting in Vienna, for example, a report on 176 Japanese patients with chronic HBV showed that those who were treated with a nucleotide analog such as tenofovir disoproxil produced higher serum levels of interferon-lamda3 compared with patients treated with entecavir, and increased levels of this interferon could improve clearance of HBV surface antigen (J Hepatol. 2019 April;70[1]:e477). The most recent EASL guidelines for treatment of chronic hepatitis B infection also list tenofovir disoproxil, entecavir, and tenofovir alafenamide as preferred agents (Hepatology. 2018 April;67[4]:1560-99).
The data Dr. Wong and her associates analyzed came from health records kept for about 80% of Hong Kong’s population in the Clinical Data Analysis and Recording System of the Hospital Authority of Hong Kong. From January 2010 to June 2018, this database included 28,041 consecutive patients chronically infected with HBV and treated with entecavir, and 1,309 consecutive patients treated with tenofovir disoproxil. These numbers excluded patients treated for less than 6 months, patients coinfected with hepatitis C or D virus, patients with cancer diagnosed or a liver transplanted before or during their first 6 months on treatment, and patients previously treated with an interferon or nucleos(t)ide.
During an average follow-up of 2.8 years of tenofovir disoproxil treatment, 8 patients developed HCC, and during an average follow-up of 3.7 years of entecavir treatment, 1,386 patients developed HCC, reported Dr. Wong, a hepatologist and professor of medicine at the Chinese University of Hong Kong.
In a multivariate analysis that adjusted for demographic and clinical differences, treatment with tenofovir disoproxil linked with a statistically significant 68% reduced rate of HCC development compared with the entecavir-treated patients, she said. In a propensity score–weighted analysis, tenofovir disoproxil linked with a statistically significant 64% reduced rate of incident HCC, and in a propensity score–matched analysis tenofovir disoproxil linked with a 58% reduced rate of HCC, although in this analysis, which excluded many of the entecavir-treated patients and hence had less statistical power, the difference just missed statistical significance.
As an additional step to try to rule out the possible effect of unadjusted confounders, Dr. Wong and associates analyzed the links between tenofovir disoproxil and entecavir treatment and two negative-control outcomes, the incidence of lung cancer and the incidence of acute myocardial infarction. Neither of these outcomes showed a statistically significant link with one of the HBV treatments, suggesting that the link between treatment and HCC incidence did not appear because of an unadjusted confounding bias, Dr. Wong said. The Hong Kong database did not include enough patients treated with tenofovir alafenamide to allow assessment of this drug, she added.
Dr. Wong has been an adviser to Gilead and a speaker for Abbott, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Roche. Tenofovir disoproxil fumarate is marketed by Gilead, and entecavir is marketed by Bristol-Myers Squibb.
SOURCE: Wong GL et al. J Hepatol. 2019 April;70[1]:e128.
VIENNA – Treatment of individuals chronically infected with hepatitis B virus (HBV) with the nucleotide analog tenofovir disoproxil fumarate significantly linked with a substantial cut in the incidence of hepatocellular carcinoma (HCC) compared with those who received the nucleoside analog entecavir, according to a review of more than 29,000 Hong Kong patients.
This is the second reported study to find that association. In January 2019, a study of more than 24,000 Korean residents chronically infected with HBV showed a similar, statistically significant link between treatment with tenofovir disoproxil fumarate (Viread) and a lower incidence of HCC compared with patients treated with entecavir (Baraclude) (JAMA Oncol. 2019 Jan;5[1]:30-6), Grace L.H. Wong, MD, said at the meeting, sponsored by the European Association for the Study of the Liver (EASL).
However, another report published just a few days before Dr. Wong spoke failed to find an association between tenofovir disoproxil treatment of HBV and the subsequent rate of HCC compared with patients treated with entecavir. That study comprised nearly 2,900 HBV patients treated at any of four Korean medical centers (J Hepatol. 2019 Apr. doi: 10.1016/j.jhep.2019.03.028).
Dr. Wong noted that although current guidelines from EASL cite both tenofovir disoproxil and entecavir (as well as tenofovir alafenamide [Vemlidy]) as first-line treatments for chronic HBV infection (J Hepatol. 2017 Aug;67[2]:370-98), some evidence suggests that tenofovir disoproxil might produce effects subtly different from those of entecavir.
At the meeting in Vienna, for example, a report on 176 Japanese patients with chronic HBV showed that those who were treated with a nucleotide analog such as tenofovir disoproxil produced higher serum levels of interferon-lamda3 compared with patients treated with entecavir, and increased levels of this interferon could improve clearance of HBV surface antigen (J Hepatol. 2019 April;70[1]:e477). The most recent EASL guidelines for treatment of chronic hepatitis B infection also list tenofovir disoproxil, entecavir, and tenofovir alafenamide as preferred agents (Hepatology. 2018 April;67[4]:1560-99).
The data Dr. Wong and her associates analyzed came from health records kept for about 80% of Hong Kong’s population in the Clinical Data Analysis and Recording System of the Hospital Authority of Hong Kong. From January 2010 to June 2018, this database included 28,041 consecutive patients chronically infected with HBV and treated with entecavir, and 1,309 consecutive patients treated with tenofovir disoproxil. These numbers excluded patients treated for less than 6 months, patients coinfected with hepatitis C or D virus, patients with cancer diagnosed or a liver transplanted before or during their first 6 months on treatment, and patients previously treated with an interferon or nucleos(t)ide.
During an average follow-up of 2.8 years of tenofovir disoproxil treatment, 8 patients developed HCC, and during an average follow-up of 3.7 years of entecavir treatment, 1,386 patients developed HCC, reported Dr. Wong, a hepatologist and professor of medicine at the Chinese University of Hong Kong.
In a multivariate analysis that adjusted for demographic and clinical differences, treatment with tenofovir disoproxil linked with a statistically significant 68% reduced rate of HCC development compared with the entecavir-treated patients, she said. In a propensity score–weighted analysis, tenofovir disoproxil linked with a statistically significant 64% reduced rate of incident HCC, and in a propensity score–matched analysis tenofovir disoproxil linked with a 58% reduced rate of HCC, although in this analysis, which excluded many of the entecavir-treated patients and hence had less statistical power, the difference just missed statistical significance.
As an additional step to try to rule out the possible effect of unadjusted confounders, Dr. Wong and associates analyzed the links between tenofovir disoproxil and entecavir treatment and two negative-control outcomes, the incidence of lung cancer and the incidence of acute myocardial infarction. Neither of these outcomes showed a statistically significant link with one of the HBV treatments, suggesting that the link between treatment and HCC incidence did not appear because of an unadjusted confounding bias, Dr. Wong said. The Hong Kong database did not include enough patients treated with tenofovir alafenamide to allow assessment of this drug, she added.
Dr. Wong has been an adviser to Gilead and a speaker for Abbott, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Roche. Tenofovir disoproxil fumarate is marketed by Gilead, and entecavir is marketed by Bristol-Myers Squibb.
SOURCE: Wong GL et al. J Hepatol. 2019 April;70[1]:e128.
REPORTING FROM ILC 2019




