User login
Twitter chat recap: Take-homes from LUPUS 2019
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.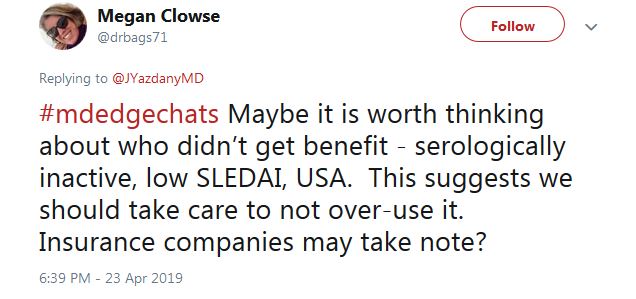
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.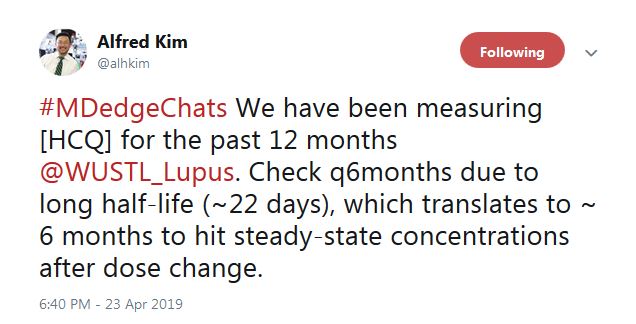
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.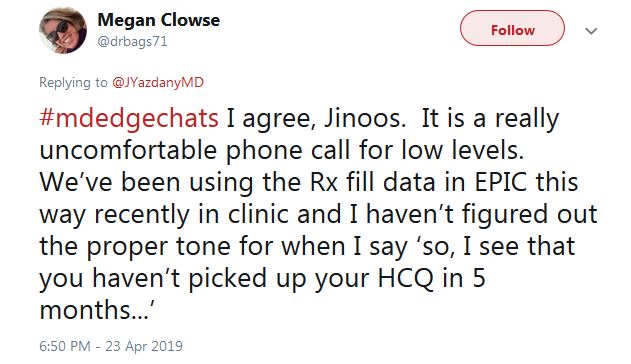
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”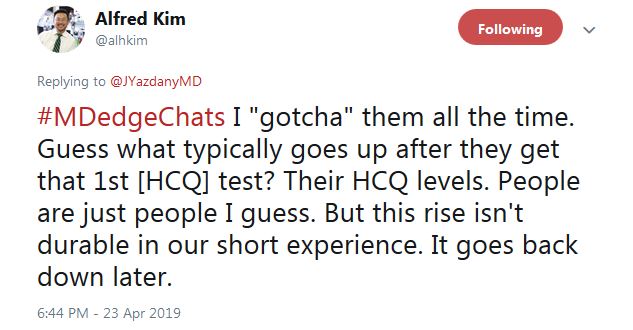
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”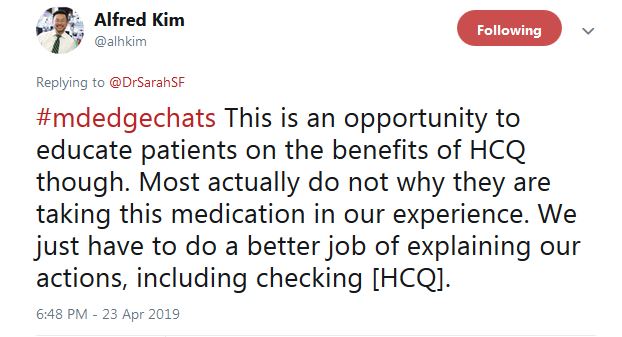
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”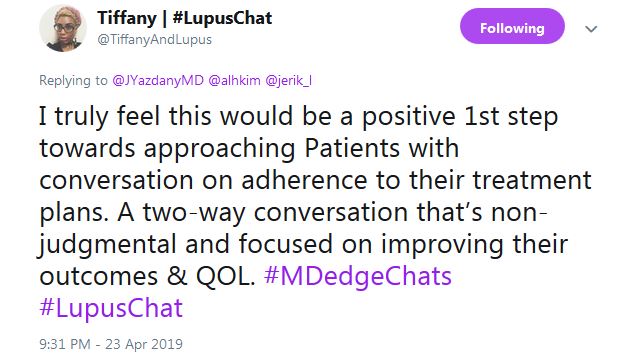
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
Despite negative trial findings, belimumab (Benlysta) remains a valid option for black lupus patients, so long as they have high disease activity and positive serology.
That was just one of the many useful messages from a robust question-and-answer session on Twitter April 23, about important findings from the recent LUPUS 2019 Congress in San Francisco. The Twitter chat was hosted by MDedge Rheumatology and led by Jinoos Yazdany, MD, and Gabriela Schmajuk, MD, both associate professors of rheumatology at the University of California, San Francisco (UCSF). The chat included scores of posts from over a dozen participants, most of them rheumatologists, and it’s worth a recap.
The EMBRACE trial
The belimumab EMBRACE trial was the first topic up to bat. The Food and Drug Administration ordered GlaxoSmithKline to conduct the trial as a condition of approval for lupus in 2011; phase 3 trials found no benefit among a small number of black subjects and even a suggestion of harm.
Although lupus is highly prevalent among black people, and outcomes are generally worse, EMBRACE was the first lupus trial to enroll an entirely black population; 345 patients were treated for a year at 10 mg/kg IV every 4 weeks. Inclusion criteria included disease activity scores of at least 8.
Overall, 49% of belimumab patients, and 42% on placebo, had a positive response, which meant a drop of 4 or more disease activity points, among other things. The difference was not statistically significant (P = .11).
However, GSK’s data showed a statistically significant benefit in favor of belimumab among people who entered with a disease activity score of at least 10 (53% vs. 41% for placebo), as well as for those with low complement levels (47% vs. 25%) and both anti–double stranded DNA antibodies and low complement (45% vs. 24%). Response rates were also significantly higher among the 57% of subjects who lived outside of the United States and Canada.
So what to make of the results?

“The EMBRACE efficacy data seems to reinforce what we already know: belimumab doesn’t work in a lot of patients, and it [has] the best chance of working in patients with higher baseline disease activity,” tweeted Dr. Yazdany.
As for prescribing, Sarah Patterson, MD, a postdoctoral fellow in the UCSF Division of Rheumatology, tweeted that the results “support the use of belimumab for black lupus patients with high disease activity” and positive serology.
For those who don’t fit the treatment profile, “we should take care to not over-use it,” said Megan Clowse, MD, an associate professor of rheumatology at Duke University, Durham, N.C., in a tweet.
The HCQ adherence fail
Poor hydroxychloroquine (HCQ) adherence came up next on Twitter. The chat participants agreed it’s a huge problem, but no one knows why. Perhaps it’s because patients don’t feel a therapeutic effect or perhaps because GI problems and other side effects are worse than doctors think. Maybe there’s simply not enough social support to encourage people to stay on the drug, even though it’s the single most important medication in lupus.
A nine-study meta-analysis presented at LUPUS 2019 suggested a solution: blood levels. The odds of nonadherence were three times higher in patients below threshold HCQ levels, and although not statistically significant, the mean lupus disease activity index score was more than 3 points higher.
A rheumatologist on the chat said that he’s already checking them.
“We have been measuring HCQ for the past 12 months” at Washington University, St. Louis, according to Alfred Kim, MD, PhD, an assistant professor of rheumatology at the school. The data are just now coming in, he said, but it seems to be catching people.
That raised another question on the chat, however: What do you do with people who aren’t down with the program? They’ll be out the door and gone with the wrong words.
“I haven’t figured out the proper tone for when I say ‘So, I see that you haven’t picked up your HCQ in 5 months,’ ” tweeted Dr. Clowse. “It is a really uncomfortable phone call.”
Dr. Kim said that “I ‘gotcha’ them all the time. Guess what typically goes up after they get that 1st HCQ test? Their HCQ levels ... But this rise isn’t durable in our short experience. It goes back down later.”
He tweeted that overall “this is an opportunity to educate patients on the benefits of HCQ ... Most actually do not [know] why they are taking this medication, in our experience.” In another tweet, Dr. Kim said “I tell them I care,” and that checking HCQ levels “is one way of demonstrating how I want to improve their outcomes.”
Tiffany from #LupusChat thought that it’s time for doctors to sit down with patient advocates and hash it out. She tweeted that “I truly feel this would be a positive 1st step ... a two-way conversation that’s non-judgmental and focused on improving” outcomes and quality of life.
Baricitinib for lupus?
The final topic was baricitinib (Olumiant).
There were modest indications of benefit at 4 mg/day oral after 6 months in a phase 2 trial with 314 people. There were also serious infections in 6% versus 2% on 2 mg/day and 1% on placebo. One patient in the 4-mg/day arm (1%) developed deep vein thrombosis (DVT), but they were positive for antiphospholipid antibodies, which raise the clot risk.
Baricitinib is FDA approved as second line at 2 mg/day for adult rheumatoid arthritis; there’s a black box warning of malignancies, thrombosis, and serious infections.
“I’m not sure” the risk-benefit is in the right direction for baricitinib in lupus. “There were a significant number of serious infections ... for not a lot of effect on” disease activity, tweeted the Twitter chat coleader, Dr. Schmajuk. In a separate tweet, she said that, even in nonlupus patients, “we are avoiding [Janus kinase inhibitors] in patients with DVT risk factors. If I were a patient, [I’m] not sure I would want to take the risk.”
“Future studies with larger sample size and longer follow up ... are needed to address some of the concerns related to DVT,” said Zahi Touma, MD, an assistant professor of rheumatology at the University of Toronto.
FROM LUPUS 2019
Three-drug regimen shows promise for refractory primary biliary cholangitis
VIENNA –
In addition to producing drops in levels of alkaline phosphatase and bilirubin, key surrogate markers for ultimate clinical benefit, the addition of bezafibrate also led to reduced pruritis among five of eight patients who had this symptom when they started on bezafibrate, Lena Smets said at the meeting, sponsored by the European Association for the Study of the Liver. Pruritis is a bothersome adverse effect from obeticholic acid (OCA) treatment that also occurs in patients with untreated primary biliary cholangitis (PBC), so the drop in pruritis in patients who started bezafibrate was notable. Overall, the triple regimen of ursodeoxycholic acid (UDCA), OCA, and bezafibrate was “well tolerated,” said Ms. Smets, a researcher at KU Leuven, Belgium.
Bezafibrate is available in Europe as a lipid-lowering treatment, especially for lowering triglycerides, so there might be a temptation to use it off label in routine practice as an add-on to UDCA and OCA in PBC patients who are not fully responsive to this dual therapy, Ms. Smets acknowledged. But she stressed that what’s needed now is a multicenter, randomized trial of bezafibrate as part of triple-therapy regimen with many more than the 10 patients included in her review.
Both UDCA and OCA have Food and Drug Administration approval for U.S. treatment of PBC. Bezafibrate is not approved for U.S. marketing, but the related agent fenofibrate has FDA approval and has shown preliminary evidence of acting like bezafibrate in PBC patients in small pilot studies or case reports, showing that “growing evidence supports the use of fibrates, but their safety has not been firmly established, and caution should be used,” according to a recent review by clinicians from the University of California, Davis (Gastroenterol Hepatol [NY]. 2018 March;14[3]:154-63).
The series of 10 PBC patients who received triple therapy at KU Leuven began as part of a cohort of 16 PBC patients treated at that center with UDCA monotherapy for an average of 6 years before entering the POISE (Phase 3 Study of Obeticholic Acid in Patients With Primary Biliary Cirrhosis) phase 3 trial that ran at KU Leuven and 57 other sites in 13 countries. POISE randomized 216 PBC patients with persistently elevated alkaline phosphatase and bilirubin levels despite UDCA treatment to added treatment with OCA. The results showed incremental benefit to these patients from a tolerable OCA acid regimen (N Engl J Med. 2016 Aug 18;375[7]631-43). The findings helped OCA (Ocaliva) get FDA marketing approval in 2016 for treatment of PBC when added to UDCA in patients not fully responsive to UDCA monotherapy.
The case for bezafibrate as an add-on to UDCA for refractory PBC patients was documented by a 2018 report from the BEZURSO (Phase 3 Study of Bezafibrate in Combination With Ursodeoxycholic Acid in Primary Biliary Cirrhosis) trial. Run at multiple centers in France, the trial randomized 100 patients on UDCA treatment to added bezafibrate or placebo, and showed that bezafibrate produced significant incremental decreases in and normalizations of alkaline phosphatase and bilirubin levels. It also had the expected effect of increasing serum creatinine level by an average of 5% (N Engl J Med. 2018 June 7;378[23]:2171-81).
Among the 16 participants in the POISE trial at KU Leuven, 13 completed that trial and then agreed to start on a triple regimen with bezafibrate added because of persistent elevations in alkaline phosphatase, and 10 patients completed 6 months on triple treatment. After 6 months, alkaline phosphatase levels reached the normal range in 5 of these 10 patients, Ms. Smets reported. Bilirubin levels also decreased in each of the 10 patients, although bilirubin had already been at a normal level in 9 of the 10 patients at the start of bezafibrate treatment, and this rate remained at 9 of 10 after 6 months. Eight of the 10 had pruritis when they started bezafibrate, and five of these eight reported decreased symptoms on treatment. Patients also showed no biochemical evidence of hepatotoxicity on the triple regimen, Ms. Smets said.
Guidelines published in 2017 from the European Association for the Study of the Liver cited evidence from small studies showing possible efficacy of fibrates as an add-on for PBC patients refractory to UDCA monotherapy, but stopped short of any endorsement of their use (J Hepatol. 2017 July;67[1]:145-72). However, guidelines from the American Association for the Study of Liver Diseases, released several months later and after publication of the BEZURSO results, said that “fibrates can be considered as off-label alternatives for patients with PBC and inadequate response to UDCA,” but also warned that “use of OCA and fibrates is discouraged in patients with decompensated liver disease (Child Pugh–Turcotte B or C)” (Hepatology. 2018 Jan;69[1]:394-419).
SOURCE: Smets L et al. J Hepatol. 2019 April;70[1]:e130.
Clinicians in Europe already use this triple therapy in appropriate patients. Bezafibrate is cheap, it has been used since the 1970s to lower triglyceride levels, and it is generally safe. Following the report of results from the BEZURSO trial in 2018, guidelines changed to accept the option of adding a fibrate to ursodeoxycholic acid and obeticholic acid.
Thomas Berg, MD, is professor and head of hepatology at University Hospital in Leipzig, Germany. He has received personal fees and research support from several companies including Intercept, the company that markets obeticholic acid (Ocaliva). He made these comments in an interview.
Clinicians in Europe already use this triple therapy in appropriate patients. Bezafibrate is cheap, it has been used since the 1970s to lower triglyceride levels, and it is generally safe. Following the report of results from the BEZURSO trial in 2018, guidelines changed to accept the option of adding a fibrate to ursodeoxycholic acid and obeticholic acid.
Thomas Berg, MD, is professor and head of hepatology at University Hospital in Leipzig, Germany. He has received personal fees and research support from several companies including Intercept, the company that markets obeticholic acid (Ocaliva). He made these comments in an interview.
Clinicians in Europe already use this triple therapy in appropriate patients. Bezafibrate is cheap, it has been used since the 1970s to lower triglyceride levels, and it is generally safe. Following the report of results from the BEZURSO trial in 2018, guidelines changed to accept the option of adding a fibrate to ursodeoxycholic acid and obeticholic acid.
Thomas Berg, MD, is professor and head of hepatology at University Hospital in Leipzig, Germany. He has received personal fees and research support from several companies including Intercept, the company that markets obeticholic acid (Ocaliva). He made these comments in an interview.
VIENNA –
In addition to producing drops in levels of alkaline phosphatase and bilirubin, key surrogate markers for ultimate clinical benefit, the addition of bezafibrate also led to reduced pruritis among five of eight patients who had this symptom when they started on bezafibrate, Lena Smets said at the meeting, sponsored by the European Association for the Study of the Liver. Pruritis is a bothersome adverse effect from obeticholic acid (OCA) treatment that also occurs in patients with untreated primary biliary cholangitis (PBC), so the drop in pruritis in patients who started bezafibrate was notable. Overall, the triple regimen of ursodeoxycholic acid (UDCA), OCA, and bezafibrate was “well tolerated,” said Ms. Smets, a researcher at KU Leuven, Belgium.
Bezafibrate is available in Europe as a lipid-lowering treatment, especially for lowering triglycerides, so there might be a temptation to use it off label in routine practice as an add-on to UDCA and OCA in PBC patients who are not fully responsive to this dual therapy, Ms. Smets acknowledged. But she stressed that what’s needed now is a multicenter, randomized trial of bezafibrate as part of triple-therapy regimen with many more than the 10 patients included in her review.
Both UDCA and OCA have Food and Drug Administration approval for U.S. treatment of PBC. Bezafibrate is not approved for U.S. marketing, but the related agent fenofibrate has FDA approval and has shown preliminary evidence of acting like bezafibrate in PBC patients in small pilot studies or case reports, showing that “growing evidence supports the use of fibrates, but their safety has not been firmly established, and caution should be used,” according to a recent review by clinicians from the University of California, Davis (Gastroenterol Hepatol [NY]. 2018 March;14[3]:154-63).
The series of 10 PBC patients who received triple therapy at KU Leuven began as part of a cohort of 16 PBC patients treated at that center with UDCA monotherapy for an average of 6 years before entering the POISE (Phase 3 Study of Obeticholic Acid in Patients With Primary Biliary Cirrhosis) phase 3 trial that ran at KU Leuven and 57 other sites in 13 countries. POISE randomized 216 PBC patients with persistently elevated alkaline phosphatase and bilirubin levels despite UDCA treatment to added treatment with OCA. The results showed incremental benefit to these patients from a tolerable OCA acid regimen (N Engl J Med. 2016 Aug 18;375[7]631-43). The findings helped OCA (Ocaliva) get FDA marketing approval in 2016 for treatment of PBC when added to UDCA in patients not fully responsive to UDCA monotherapy.
The case for bezafibrate as an add-on to UDCA for refractory PBC patients was documented by a 2018 report from the BEZURSO (Phase 3 Study of Bezafibrate in Combination With Ursodeoxycholic Acid in Primary Biliary Cirrhosis) trial. Run at multiple centers in France, the trial randomized 100 patients on UDCA treatment to added bezafibrate or placebo, and showed that bezafibrate produced significant incremental decreases in and normalizations of alkaline phosphatase and bilirubin levels. It also had the expected effect of increasing serum creatinine level by an average of 5% (N Engl J Med. 2018 June 7;378[23]:2171-81).
Among the 16 participants in the POISE trial at KU Leuven, 13 completed that trial and then agreed to start on a triple regimen with bezafibrate added because of persistent elevations in alkaline phosphatase, and 10 patients completed 6 months on triple treatment. After 6 months, alkaline phosphatase levels reached the normal range in 5 of these 10 patients, Ms. Smets reported. Bilirubin levels also decreased in each of the 10 patients, although bilirubin had already been at a normal level in 9 of the 10 patients at the start of bezafibrate treatment, and this rate remained at 9 of 10 after 6 months. Eight of the 10 had pruritis when they started bezafibrate, and five of these eight reported decreased symptoms on treatment. Patients also showed no biochemical evidence of hepatotoxicity on the triple regimen, Ms. Smets said.
Guidelines published in 2017 from the European Association for the Study of the Liver cited evidence from small studies showing possible efficacy of fibrates as an add-on for PBC patients refractory to UDCA monotherapy, but stopped short of any endorsement of their use (J Hepatol. 2017 July;67[1]:145-72). However, guidelines from the American Association for the Study of Liver Diseases, released several months later and after publication of the BEZURSO results, said that “fibrates can be considered as off-label alternatives for patients with PBC and inadequate response to UDCA,” but also warned that “use of OCA and fibrates is discouraged in patients with decompensated liver disease (Child Pugh–Turcotte B or C)” (Hepatology. 2018 Jan;69[1]:394-419).
SOURCE: Smets L et al. J Hepatol. 2019 April;70[1]:e130.
VIENNA –
In addition to producing drops in levels of alkaline phosphatase and bilirubin, key surrogate markers for ultimate clinical benefit, the addition of bezafibrate also led to reduced pruritis among five of eight patients who had this symptom when they started on bezafibrate, Lena Smets said at the meeting, sponsored by the European Association for the Study of the Liver. Pruritis is a bothersome adverse effect from obeticholic acid (OCA) treatment that also occurs in patients with untreated primary biliary cholangitis (PBC), so the drop in pruritis in patients who started bezafibrate was notable. Overall, the triple regimen of ursodeoxycholic acid (UDCA), OCA, and bezafibrate was “well tolerated,” said Ms. Smets, a researcher at KU Leuven, Belgium.
Bezafibrate is available in Europe as a lipid-lowering treatment, especially for lowering triglycerides, so there might be a temptation to use it off label in routine practice as an add-on to UDCA and OCA in PBC patients who are not fully responsive to this dual therapy, Ms. Smets acknowledged. But she stressed that what’s needed now is a multicenter, randomized trial of bezafibrate as part of triple-therapy regimen with many more than the 10 patients included in her review.
Both UDCA and OCA have Food and Drug Administration approval for U.S. treatment of PBC. Bezafibrate is not approved for U.S. marketing, but the related agent fenofibrate has FDA approval and has shown preliminary evidence of acting like bezafibrate in PBC patients in small pilot studies or case reports, showing that “growing evidence supports the use of fibrates, but their safety has not been firmly established, and caution should be used,” according to a recent review by clinicians from the University of California, Davis (Gastroenterol Hepatol [NY]. 2018 March;14[3]:154-63).
The series of 10 PBC patients who received triple therapy at KU Leuven began as part of a cohort of 16 PBC patients treated at that center with UDCA monotherapy for an average of 6 years before entering the POISE (Phase 3 Study of Obeticholic Acid in Patients With Primary Biliary Cirrhosis) phase 3 trial that ran at KU Leuven and 57 other sites in 13 countries. POISE randomized 216 PBC patients with persistently elevated alkaline phosphatase and bilirubin levels despite UDCA treatment to added treatment with OCA. The results showed incremental benefit to these patients from a tolerable OCA acid regimen (N Engl J Med. 2016 Aug 18;375[7]631-43). The findings helped OCA (Ocaliva) get FDA marketing approval in 2016 for treatment of PBC when added to UDCA in patients not fully responsive to UDCA monotherapy.
The case for bezafibrate as an add-on to UDCA for refractory PBC patients was documented by a 2018 report from the BEZURSO (Phase 3 Study of Bezafibrate in Combination With Ursodeoxycholic Acid in Primary Biliary Cirrhosis) trial. Run at multiple centers in France, the trial randomized 100 patients on UDCA treatment to added bezafibrate or placebo, and showed that bezafibrate produced significant incremental decreases in and normalizations of alkaline phosphatase and bilirubin levels. It also had the expected effect of increasing serum creatinine level by an average of 5% (N Engl J Med. 2018 June 7;378[23]:2171-81).
Among the 16 participants in the POISE trial at KU Leuven, 13 completed that trial and then agreed to start on a triple regimen with bezafibrate added because of persistent elevations in alkaline phosphatase, and 10 patients completed 6 months on triple treatment. After 6 months, alkaline phosphatase levels reached the normal range in 5 of these 10 patients, Ms. Smets reported. Bilirubin levels also decreased in each of the 10 patients, although bilirubin had already been at a normal level in 9 of the 10 patients at the start of bezafibrate treatment, and this rate remained at 9 of 10 after 6 months. Eight of the 10 had pruritis when they started bezafibrate, and five of these eight reported decreased symptoms on treatment. Patients also showed no biochemical evidence of hepatotoxicity on the triple regimen, Ms. Smets said.
Guidelines published in 2017 from the European Association for the Study of the Liver cited evidence from small studies showing possible efficacy of fibrates as an add-on for PBC patients refractory to UDCA monotherapy, but stopped short of any endorsement of their use (J Hepatol. 2017 July;67[1]:145-72). However, guidelines from the American Association for the Study of Liver Diseases, released several months later and after publication of the BEZURSO results, said that “fibrates can be considered as off-label alternatives for patients with PBC and inadequate response to UDCA,” but also warned that “use of OCA and fibrates is discouraged in patients with decompensated liver disease (Child Pugh–Turcotte B or C)” (Hepatology. 2018 Jan;69[1]:394-419).
SOURCE: Smets L et al. J Hepatol. 2019 April;70[1]:e130.
REPORTING FROM ILC 2019
Association insurance pushes on despite court ruling
When the Trump administration in June 2018 issued rules making it easier for small employers to band together to buy health insurance, “we started looking immediately,” recalled Scott Lyon, a top executive at the Small Business Association of Michigan.
Although he offered traditional small-group health insurance to his association’s employees and members, Mr. Lyon liked adding a new option for both: potentially less expensive coverage through an association health plan, which doesn’t have to meet all the rules of the Affordable Care Act (ACA).
Now, a few months in, “we’ve got 400 companies and a couple of thousand workers signed up,” said Mr. Lyon.
Most of the new enrollees joined through groups like Mr. Lyon’s or local chambers of commerce, farm bureaus, or agriculture-based cooperatives. Such groups see the plans not only as a way to offer insurance, but also as an enticement to boost membership.
In the first legal test, however, U.S. District Judge John Bates at the end of March sided with 11 states and the District of Columbia challenging the law. He invalidated a large chunk of those June rules, saying the administration issued them as an “end-run around the Affordable Care Act.”
So what now?
Unless the government seeks – which it has yet to do – and is granted a stay of the judge’s order, “plans formed under the vacated sections of the rule are illegal,” said Timothy Jost, an emeritus health law professor from Washington and Lee University, Lexington, Va.
Still, that won’t mean anything for existing plans if the states or federal regulators choose not to enforce the ruling, Mr. Jost said.
And that could cause more confusion in the marketplace.
While the states that brought the challenge are expected to enforce the ruling, some other states support broader access to association health plans, said Christopher Condeluci, an attorney who represents several such plans, including the one formed by Mr. Lyon’s group.
“These plans are not an end run around the ACA,” said Mr. Condeluci.
Association health plans already established under the administration’s rules cover “virtually” all the federal law’s essential health benefits, he said, with the exception of dental and vision care for children.
Local chamber of commerce plans are mainly continuing business as usual while watching to see if the government will appeal, said Katie Mahoney, vice president of health policy at the U.S. Chamber of Commerce.
A few, including a plan offered through the Las Vegas chamber, may limit new enrollment for sole proprietors, she said, as the judge sharply questioned whether they qualified as “employers” under federal laws.
Sole proprietors are generally individuals who own and operate their own businesses without any employees.
Judge Bates wrote that, in the regulation, the Department of Labor “stretches the definition of employer” beyond what federal law allows. The rule was designed to increase access to plans that “avoid the most stringent requirements” of the ACA.
The opinion by Judge Bates, who was appointed by President George W. Bush, is widely expected to be appealed, although the government has not yet done so.
The decision affects one pillar of a broader effort by the Trump administration to expand access to less expensive health insurance. Association plans have long been a favorite of Republicans, existing before the ACA. Supporters say they are one way to pool groups of businesses together to get better premium rates.
Still, some plans faced problems in the past, including bankruptcy or complaints that they misled consumers by not fully informing them about what is covered.
After the ACA took effect, enrollment fell, partly because many small businesses were buying new ACA plans and many existing association plans had to comply with ACA rules for small-group coverage anyway. People who ran their own businesses and had no employees qualified only for coverage through the ACA’s individual market.
But the Trump administration in June broadened the definition of those eligible to buy insurance through employer-based associations to include sole proprietors and also made it easier to form associations to offer coverage.
In addition, the changes allowed more association plans to be classified as large-employer coverage, which exempts them from some of the ACA’s requirements. For example, association plans don’t have to include all 10 of the ACA’s “essential” health benefits, such as mental health care and prescription drug coverage.
Also, unlike ACA plans, association insurers can set premium rates based on an employer’s industry, as well as taking into account the age range and gender makeup of their workforce.
In other words, association plans can charge less for companies with workforces that are generally younger and male in occupations that involve mainly desk work than for firms with mostly older workers or companies doing riskier work, such as cutting down trees or roofing.
Still, such plans must abide by other ACA provisions, including accepting people with preexisting medical conditions.
Critics, including the states that sued, say the new rules and other administration-backed changes will weaken the market for ACA plans by drawing out younger and healthier people. The states also argued that the new rules would be costly for them to administer, alleging they would have to devote more resources to preventing consumer fraud.
In Michigan, Mr. Lyon said the association his group formed, called Transcend, offers coverage to small employers and sole proprietors that is just as generous as large-group plans. It is a fully insured plan through the state’s Blue Cross Blue Shield carrier that covers a broad array of benefits, except children’s dental and vision.
“One thing we don’t want to do is sell a bag of air to our members,” said Mr. Lyon.
While some new members have reported large savings by enrolling, Mr. Lyon said association plans are not necessarily less expensive than small-group coverage. It all depends on the demographic and occupational makeup of the small business, he said.
“Our best estimate was association health plans would be the right solution for 30%-35% of the small-group world,” said Mr. Lyon. “It all has to come together. Age matters. Gender matters. It’s so specific to each company.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the Trump administration in June 2018 issued rules making it easier for small employers to band together to buy health insurance, “we started looking immediately,” recalled Scott Lyon, a top executive at the Small Business Association of Michigan.
Although he offered traditional small-group health insurance to his association’s employees and members, Mr. Lyon liked adding a new option for both: potentially less expensive coverage through an association health plan, which doesn’t have to meet all the rules of the Affordable Care Act (ACA).
Now, a few months in, “we’ve got 400 companies and a couple of thousand workers signed up,” said Mr. Lyon.
Most of the new enrollees joined through groups like Mr. Lyon’s or local chambers of commerce, farm bureaus, or agriculture-based cooperatives. Such groups see the plans not only as a way to offer insurance, but also as an enticement to boost membership.
In the first legal test, however, U.S. District Judge John Bates at the end of March sided with 11 states and the District of Columbia challenging the law. He invalidated a large chunk of those June rules, saying the administration issued them as an “end-run around the Affordable Care Act.”
So what now?
Unless the government seeks – which it has yet to do – and is granted a stay of the judge’s order, “plans formed under the vacated sections of the rule are illegal,” said Timothy Jost, an emeritus health law professor from Washington and Lee University, Lexington, Va.
Still, that won’t mean anything for existing plans if the states or federal regulators choose not to enforce the ruling, Mr. Jost said.
And that could cause more confusion in the marketplace.
While the states that brought the challenge are expected to enforce the ruling, some other states support broader access to association health plans, said Christopher Condeluci, an attorney who represents several such plans, including the one formed by Mr. Lyon’s group.
“These plans are not an end run around the ACA,” said Mr. Condeluci.
Association health plans already established under the administration’s rules cover “virtually” all the federal law’s essential health benefits, he said, with the exception of dental and vision care for children.
Local chamber of commerce plans are mainly continuing business as usual while watching to see if the government will appeal, said Katie Mahoney, vice president of health policy at the U.S. Chamber of Commerce.
A few, including a plan offered through the Las Vegas chamber, may limit new enrollment for sole proprietors, she said, as the judge sharply questioned whether they qualified as “employers” under federal laws.
Sole proprietors are generally individuals who own and operate their own businesses without any employees.
Judge Bates wrote that, in the regulation, the Department of Labor “stretches the definition of employer” beyond what federal law allows. The rule was designed to increase access to plans that “avoid the most stringent requirements” of the ACA.
The opinion by Judge Bates, who was appointed by President George W. Bush, is widely expected to be appealed, although the government has not yet done so.
The decision affects one pillar of a broader effort by the Trump administration to expand access to less expensive health insurance. Association plans have long been a favorite of Republicans, existing before the ACA. Supporters say they are one way to pool groups of businesses together to get better premium rates.
Still, some plans faced problems in the past, including bankruptcy or complaints that they misled consumers by not fully informing them about what is covered.
After the ACA took effect, enrollment fell, partly because many small businesses were buying new ACA plans and many existing association plans had to comply with ACA rules for small-group coverage anyway. People who ran their own businesses and had no employees qualified only for coverage through the ACA’s individual market.
But the Trump administration in June broadened the definition of those eligible to buy insurance through employer-based associations to include sole proprietors and also made it easier to form associations to offer coverage.
In addition, the changes allowed more association plans to be classified as large-employer coverage, which exempts them from some of the ACA’s requirements. For example, association plans don’t have to include all 10 of the ACA’s “essential” health benefits, such as mental health care and prescription drug coverage.
Also, unlike ACA plans, association insurers can set premium rates based on an employer’s industry, as well as taking into account the age range and gender makeup of their workforce.
In other words, association plans can charge less for companies with workforces that are generally younger and male in occupations that involve mainly desk work than for firms with mostly older workers or companies doing riskier work, such as cutting down trees or roofing.
Still, such plans must abide by other ACA provisions, including accepting people with preexisting medical conditions.
Critics, including the states that sued, say the new rules and other administration-backed changes will weaken the market for ACA plans by drawing out younger and healthier people. The states also argued that the new rules would be costly for them to administer, alleging they would have to devote more resources to preventing consumer fraud.
In Michigan, Mr. Lyon said the association his group formed, called Transcend, offers coverage to small employers and sole proprietors that is just as generous as large-group plans. It is a fully insured plan through the state’s Blue Cross Blue Shield carrier that covers a broad array of benefits, except children’s dental and vision.
“One thing we don’t want to do is sell a bag of air to our members,” said Mr. Lyon.
While some new members have reported large savings by enrolling, Mr. Lyon said association plans are not necessarily less expensive than small-group coverage. It all depends on the demographic and occupational makeup of the small business, he said.
“Our best estimate was association health plans would be the right solution for 30%-35% of the small-group world,” said Mr. Lyon. “It all has to come together. Age matters. Gender matters. It’s so specific to each company.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the Trump administration in June 2018 issued rules making it easier for small employers to band together to buy health insurance, “we started looking immediately,” recalled Scott Lyon, a top executive at the Small Business Association of Michigan.
Although he offered traditional small-group health insurance to his association’s employees and members, Mr. Lyon liked adding a new option for both: potentially less expensive coverage through an association health plan, which doesn’t have to meet all the rules of the Affordable Care Act (ACA).
Now, a few months in, “we’ve got 400 companies and a couple of thousand workers signed up,” said Mr. Lyon.
Most of the new enrollees joined through groups like Mr. Lyon’s or local chambers of commerce, farm bureaus, or agriculture-based cooperatives. Such groups see the plans not only as a way to offer insurance, but also as an enticement to boost membership.
In the first legal test, however, U.S. District Judge John Bates at the end of March sided with 11 states and the District of Columbia challenging the law. He invalidated a large chunk of those June rules, saying the administration issued them as an “end-run around the Affordable Care Act.”
So what now?
Unless the government seeks – which it has yet to do – and is granted a stay of the judge’s order, “plans formed under the vacated sections of the rule are illegal,” said Timothy Jost, an emeritus health law professor from Washington and Lee University, Lexington, Va.
Still, that won’t mean anything for existing plans if the states or federal regulators choose not to enforce the ruling, Mr. Jost said.
And that could cause more confusion in the marketplace.
While the states that brought the challenge are expected to enforce the ruling, some other states support broader access to association health plans, said Christopher Condeluci, an attorney who represents several such plans, including the one formed by Mr. Lyon’s group.
“These plans are not an end run around the ACA,” said Mr. Condeluci.
Association health plans already established under the administration’s rules cover “virtually” all the federal law’s essential health benefits, he said, with the exception of dental and vision care for children.
Local chamber of commerce plans are mainly continuing business as usual while watching to see if the government will appeal, said Katie Mahoney, vice president of health policy at the U.S. Chamber of Commerce.
A few, including a plan offered through the Las Vegas chamber, may limit new enrollment for sole proprietors, she said, as the judge sharply questioned whether they qualified as “employers” under federal laws.
Sole proprietors are generally individuals who own and operate their own businesses without any employees.
Judge Bates wrote that, in the regulation, the Department of Labor “stretches the definition of employer” beyond what federal law allows. The rule was designed to increase access to plans that “avoid the most stringent requirements” of the ACA.
The opinion by Judge Bates, who was appointed by President George W. Bush, is widely expected to be appealed, although the government has not yet done so.
The decision affects one pillar of a broader effort by the Trump administration to expand access to less expensive health insurance. Association plans have long been a favorite of Republicans, existing before the ACA. Supporters say they are one way to pool groups of businesses together to get better premium rates.
Still, some plans faced problems in the past, including bankruptcy or complaints that they misled consumers by not fully informing them about what is covered.
After the ACA took effect, enrollment fell, partly because many small businesses were buying new ACA plans and many existing association plans had to comply with ACA rules for small-group coverage anyway. People who ran their own businesses and had no employees qualified only for coverage through the ACA’s individual market.
But the Trump administration in June broadened the definition of those eligible to buy insurance through employer-based associations to include sole proprietors and also made it easier to form associations to offer coverage.
In addition, the changes allowed more association plans to be classified as large-employer coverage, which exempts them from some of the ACA’s requirements. For example, association plans don’t have to include all 10 of the ACA’s “essential” health benefits, such as mental health care and prescription drug coverage.
Also, unlike ACA plans, association insurers can set premium rates based on an employer’s industry, as well as taking into account the age range and gender makeup of their workforce.
In other words, association plans can charge less for companies with workforces that are generally younger and male in occupations that involve mainly desk work than for firms with mostly older workers or companies doing riskier work, such as cutting down trees or roofing.
Still, such plans must abide by other ACA provisions, including accepting people with preexisting medical conditions.
Critics, including the states that sued, say the new rules and other administration-backed changes will weaken the market for ACA plans by drawing out younger and healthier people. The states also argued that the new rules would be costly for them to administer, alleging they would have to devote more resources to preventing consumer fraud.
In Michigan, Mr. Lyon said the association his group formed, called Transcend, offers coverage to small employers and sole proprietors that is just as generous as large-group plans. It is a fully insured plan through the state’s Blue Cross Blue Shield carrier that covers a broad array of benefits, except children’s dental and vision.
“One thing we don’t want to do is sell a bag of air to our members,” said Mr. Lyon.
While some new members have reported large savings by enrolling, Mr. Lyon said association plans are not necessarily less expensive than small-group coverage. It all depends on the demographic and occupational makeup of the small business, he said.
“Our best estimate was association health plans would be the right solution for 30%-35% of the small-group world,” said Mr. Lyon. “It all has to come together. Age matters. Gender matters. It’s so specific to each company.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Predictive analytics with large data sets are being pursued to individualize IBD therapy
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
SAN FRANCISCO – Predictive analytics of large quantities of data using machine learning present a powerful tool for improving therapeutic choices, according to a summary of work performed in inflammatory bowel disease (IBD) and presented at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
This type of work is relevant to many fields of medicine, but studies conducted in IBD have provided particularly compelling evidence that predictive analytics will improve outcomes and lead to more cost effective delivery of care, according to Akbar K. Waljee, MD, MSc, an associate professor in the division of gastroenterology at University of Michigan, Ann Arbor, and a staff physician and researcher at the VA Ann Arbor Healthcare system.
“We collect large amounts of clinical data every day in the delivery of health care, but we are now only just beginning to leverage [these] data to guide treatment,” Dr. Waljee said. He has now published several papers on the role of precision analytics of big data to improve treatment choices in IBD, as well as other diseases. These analyses are relevant for determining both who to treat with a certain drug and who to not treat with it.
In one example, data from 1,080 IBD patients taking thiopurines were used to develop a machine learning algorithm that analyzed multiple readily available variables, such as a complete blood count with differential and a chemistry panel, to predict whether someone was or was not in remission. This was then used to compare the mean yearly clinical event rates (new steroids prescriptions, hospitalizations, and abdominal surgeries) between the two groups (1.08 vs. 3.95 events) to show the associated clinical benefit of using this algorithm.
“The heterogeneity of response to therapies for IBD is well established. If machine learning predicts effective choices, there will be an opportunity to accelerate the time to disease control, as well as save costs by avoiding therapies not likely to be effective,” Dr. Waljee explained.
In another example, an algorithm was developed to predict the likelihood of achieving a corticosteroid-free biologic remission at 1 year in Crohn’s disease patients when patients were evaluated 6 weeks after initiating the gut-selective biologic vedolizumab. Again, it was based on an analysis of numerous variables, including laboratory data, sex, and race. Based on the model drawn from the analysis of 472 patients, 35.8% of the patients predicted to be in corticosteroid-free biologic remission at 1 year achieved this endpoint, whereas only 6.7% of the patients predicted to fail achieved the endpoint.
“This suggests that we can use an algorithm relatively early in the course of this biologic to predict who is going to respond,” reported Dr. Waljee. Again, patients with a low likelihood of response at 6 weeks can be started on an alternative treatment, which could potentially accelerate the time to disease control and avoid the costs of an ineffective and expensive treatment.
IBD is a particularly attractive focus of precision analytics with big data. IBD has a relatively unpredictable relapsing/remitting course and a heterogeneous response to available therapies. Algorithms predictive of response circumvent the inherent delays from evaluating disease control over an extended period.
“With ever increasing concern about costs of care and access to care, these treatment algorithms promise to use resources more efficiently,” Dr. Waljee said.
EXPERT ANALYSIS FROM 2019 AGA TECH SUMMIT
Investigational laser for acne treatment targets sebaceous gland, spares dermis
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
DENVER – An investigational 1726-nm without histologic disruption to the surrounding dermis, results from a small study showed.
“It is our belief that if we can destruct the sebaceous glands, it will cure acne,” Emil A. Tanghetti, MD, said at the annual conference of the American Society for Laser Medicine and Surgery. “High-dose red light PDT [photodynamic therapy] has been used, but it causes epidermal damage and an unacceptably long recovery time. Gold and silver nanoshells with infrared light have also been used. Usually these particles do not get into the sebaceous glands and appear to result in temporary improvement by wounding the infrainfundibular region of the sebaceous gland complex. It’s been shown that the 1726-nm light can target sebum and might be able to selectively damage sebaceous glands.”
Dr. Tanghetti, of the Sacramento-based Center for Dermatology and Laser Surgery, along with R. Rox Anderson, MD, and Fernanda H. Sakamoto, MD, PhD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, are studying a 1726-nm laser being developed by Accure. This device uses robust and precise air cooling, a creative pulsing strategy to enhance the differential sebum to water absorption, and active, real-time monitoring via thermal imaging.
“The device is constructed to turn off before an unsafe temperature is reached,” Dr. Tanghetti said. “The thermal camera is looking at the treatment site as it happening. If it gets too hot, it can turn the whole device off. You can only do real-time monitoring with air cooling. You cannot do it with contract cooling or cryogen cooling.”
In a study of 10 patients with acne, the researchers developed a multiple pulse strategy to slowly and preferentially heat sebaceous glands while sparing the epidermis and the surrounding dermis. They performed 3.5-mm punch biopsies at 24, 48, and 72 hours after treatment, and evaluated them with hematoxylin and eosin staining.
From a clinical standpoint, Dr. Tanghetti and his colleagues noted small papules in the treated areas immediately, 24 hours and 72 hours after treatment. “You don’t see the epidermal damage that you would see with someone who had PDT,” he said.
Histologic evaluation of tissue specimens at 24 and 72 hours revealed destruction of sebaceous glands in the dermis characterized by loss of the definition of the sebocytes, and eosinophilic changes of the basal cell layer of these glands. The collagen surrounding the gland appeared to be preserved, with occasional small clots observed in the adjacent blood vessels. “There was no obvious damage to the surrounding dermis or other follicular structures,” Dr. Tanghetti said. “The hard thing is to differentiate complete damage [of the sebaceous gland] from partial damage. That’s something we’re working on by looking at sebum production, which is going to be the ultimate outcome.”
The study authors reported having numerous financial ties to medical device and pharmaceutical companies.
dbrunk@mdedge.com
EXPERT ANALYSIS FROM ASLMS 2019
Lessons from KEYNOTE-158 and the role of R-CHOP
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I take a look at two recent trials – one offers potential in previously-treated cervical cancer patients with poor prognosis and the other confirms the role of R-CHOP as the standard of care in diffuse large B-cell lymphoma.
Pembrolizumab in KEYNOTE-158
In an international phase 2 “basket trial,” Hyun Cheol Chung, MD, PhD, and colleagues used pembrolizumab 200 mg every 3 weeks in 98 previously treated patients with advanced cervical cancer. Almost 84% of o the patients had PD-L1 positive tumors (greater than 1%). The authors said that viral induction of malignancy leads to antigen production and upregulation of PD-1. Therefore, advanced cervical cancer patients would likely express PD-L1 on tumor cells and respond to immune checkpoint inhibitor therapy.
In this interim report, there were 12 responses (all in PD-L1 positive patients), with three complete responses. Median response duration had not been reached at median follow-up of 10.2 months. Seven of 12 responses were ongoing at 12 months. There were grade 3-4 adverse events in 12.2% of patients and no treatment-related deaths.
The study – “Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study” – was published in the Journal of Clinical Oncology (2019 April 3. doi: 10.1200/JCO.18.01265).
The encouraging results of pembrolizumab in this generally chemotherapy-refractory patient population were consistent with other small, early-phase studies investigating immune checkpoint inhibitors that led to the accelerated approval of pembrolizumab in previously treated PD-L1 advanced cervical cancer patients with progressive disease after chemotherapy.
What this means in practice
Although excitement should be tempered about an interim report of an organ-specific subset of a phase 2 international basket trial that was heavily populated by young PS 0-1 patients and generated an overall response rate of less than 15%, no conventional chemotherapy or biologic agent offers the potential of complete or prolonged response, and disease control rates of 30%.
Clinical trials should always be the first choice, but immune checkpoint inhibitors offer an attractive off-study option.
Among many single agents in National Comprehensive Cancer Network guidelines for recurrent advanced cervical cancer after first-line cisplatin-based chemotherapy, there is a reason why pembrolizumab is listed first. For patients with PD-L1 expressing tumors or MSI-H/dMMR tumors, I would use it.
Frontline therapy in DLBCL
In a large, randomized phase 3 trial, close to 500 stage III-IV patients with diffuse large B-cell lymphoma (DLBCL), including primary mediastinal B-cell lymphoma and intravascular large B-cell lymphoma, were assigned to receive either conventional R-CHOP chemotherapy or the more complex, more toxic DA-EPOCH-R regimen that appeared superior in single-institution studies and was feasible in multi-institutional phase 2 trials.
The study – “Dose-Adjusted EPOCH-R Compared With R-CHOP as Frontline Therapy for Diffuse Large B-Cell Lymphoma: Clinical Outcomes of the Phase III Intergroup Trial Alliance/CALGB 50303” – was published in the Journal of Clinical Oncology (2019 Apr 2. doi: 10.1200/JCO.18.01994).
In the study, progression-free survival and overall survival were no different for R-CHOP and DA-EPOCH-R, but – predictably – DA-EPOCH-R was more toxic and had more treatment discontinuations.
R-CHOP had better outcomes than expected. This suggests that patient-selection bias (more favorable histology, fewer high-risk subsets who required urgent therapy) may have been at work.
Further study of DA-EPOCH-R in higher IPI patients or in patients selected because of more adverse molecular features (DE phenotype, MYC+, double hit) is warranted given the poor outcomes with R-CHOP in high-risk patients, intriguing results in single institution trials of DA-EPOCH-R, and the underrepresentation of high-risk patients in the current study.
What this means in practice
Whether by virtue of the types of patients enrolled or because it is the best regimen in all DLBCL patients, R-CHOP remains the standard of care outside of a clinical trial.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers, and in expanding access to clinical trials to medically underserved populations.
Speaking at a conference? Read these tips first
Recently, I was asked to present my top public speaking tips for a group of women leaders. This is a topic near and dear to my heart, and one that I teach a number of groups, from medical students to faculty.
I also benefited from just returning from the Harvard Macy Educators Course, where Victoria Brazil, MD, an experienced emergency medicine physician from Australia, provided her top tips. Here is a mash-up of the top tips to think about for any of the speakers out there among us – with a few shout-outs for the ladies out there. Please add your own!
The Dos
- Do project power: Stand tall with a relaxed stance and shoulders back – posture is everything. This is especially important for women, who may tend to shrink their bodies, or anyone who is short. A powerful messenger is just as important as the power of the message. The same also applies to sitting down, especially if you are on a panel. Do not look like you are falling into the table.
- Do look up: Think about addressing the people in the back, not in the front row. This looks better in photos as well since you are appealing to the large audience and not the front row. Dr. Brazil’s tip came from Cate Blanchett who said that before she gives talks, she literally and physically advises “picking up your crown and put it on your head.” Not only will you feel better, you will look it too.
- Do pause strategically: The human brain needs rest to process what you are about to say. You can ask people to “think of a time” and take a pause. Or “I want you to all think about what I just said for one moment.” And TAKE a moment. But think about Emma’s pause during the March For Your Lives. Pauses are powerful and serve as a way to cement what you are saying for even the most critical crowd. Think about when anyone on their phone pauses, even if you’re on a boring conference call others will wake up and wonder what is going on and are now engaged in the talk.
- Do strategically summarize: Before you end, or in between important sections, say the following: “There are three main things you can do.” Even if someone fell asleep, they will wake up to take note. It’s a way to get folks’ attention back. There is nothing like challenging others to do something.
The Don’ts
- Don’t start with an apology for “not being an expert”: Or whatever you are thinking about apologizing for. The voice in your head does not need to be broadcast to others. Just say thank you after you are introduced, and launch in. Someone has asked you to talk, so bring your own unique expertise and don’t start with undermining yourself!
- Don’t use your slides as a crutch: Make your audience look at you and not your slides. That means at times, you may be talking and your slides will not be moving. Other times, if you are starting with a story, maybe there is no slide behind you and the screen is blacked out. Some of the most powerful moments in a talk are when slides are not being used.
- Don’t stand behind the podium if you can help it. This means ask for a wireless microphone. Most podiums will overwhelm you. If you have to use a podium, go back to the posture in the “dos.” One year, I had a leg injury and definitely used the podium, so obviously there may be times you need to use a podium; even then, try as hard as possible to make sure you are seen.
- Don’t engage grandstanders during Q&A: Invariably, you will get someone who stands up and goes into a long comment that is not a question to hear themselves speak. Insert yourself, say “thank you” and take the next question. If there is not a next question, you can add, “Before I forget, I want to share another question I am often asked which may be of help to you.” Then, answer your own question. You get the final word this way!
Happy speaking! I look forward to seeing you in warmer weather during the spring conference season.
For more posts from the Hospital Leader blog, visit hospitalleader.org.
Recently, I was asked to present my top public speaking tips for a group of women leaders. This is a topic near and dear to my heart, and one that I teach a number of groups, from medical students to faculty.
I also benefited from just returning from the Harvard Macy Educators Course, where Victoria Brazil, MD, an experienced emergency medicine physician from Australia, provided her top tips. Here is a mash-up of the top tips to think about for any of the speakers out there among us – with a few shout-outs for the ladies out there. Please add your own!
The Dos
- Do project power: Stand tall with a relaxed stance and shoulders back – posture is everything. This is especially important for women, who may tend to shrink their bodies, or anyone who is short. A powerful messenger is just as important as the power of the message. The same also applies to sitting down, especially if you are on a panel. Do not look like you are falling into the table.
- Do look up: Think about addressing the people in the back, not in the front row. This looks better in photos as well since you are appealing to the large audience and not the front row. Dr. Brazil’s tip came from Cate Blanchett who said that before she gives talks, she literally and physically advises “picking up your crown and put it on your head.” Not only will you feel better, you will look it too.
- Do pause strategically: The human brain needs rest to process what you are about to say. You can ask people to “think of a time” and take a pause. Or “I want you to all think about what I just said for one moment.” And TAKE a moment. But think about Emma’s pause during the March For Your Lives. Pauses are powerful and serve as a way to cement what you are saying for even the most critical crowd. Think about when anyone on their phone pauses, even if you’re on a boring conference call others will wake up and wonder what is going on and are now engaged in the talk.
- Do strategically summarize: Before you end, or in between important sections, say the following: “There are three main things you can do.” Even if someone fell asleep, they will wake up to take note. It’s a way to get folks’ attention back. There is nothing like challenging others to do something.
The Don’ts
- Don’t start with an apology for “not being an expert”: Or whatever you are thinking about apologizing for. The voice in your head does not need to be broadcast to others. Just say thank you after you are introduced, and launch in. Someone has asked you to talk, so bring your own unique expertise and don’t start with undermining yourself!
- Don’t use your slides as a crutch: Make your audience look at you and not your slides. That means at times, you may be talking and your slides will not be moving. Other times, if you are starting with a story, maybe there is no slide behind you and the screen is blacked out. Some of the most powerful moments in a talk are when slides are not being used.
- Don’t stand behind the podium if you can help it. This means ask for a wireless microphone. Most podiums will overwhelm you. If you have to use a podium, go back to the posture in the “dos.” One year, I had a leg injury and definitely used the podium, so obviously there may be times you need to use a podium; even then, try as hard as possible to make sure you are seen.
- Don’t engage grandstanders during Q&A: Invariably, you will get someone who stands up and goes into a long comment that is not a question to hear themselves speak. Insert yourself, say “thank you” and take the next question. If there is not a next question, you can add, “Before I forget, I want to share another question I am often asked which may be of help to you.” Then, answer your own question. You get the final word this way!
Happy speaking! I look forward to seeing you in warmer weather during the spring conference season.
For more posts from the Hospital Leader blog, visit hospitalleader.org.
Recently, I was asked to present my top public speaking tips for a group of women leaders. This is a topic near and dear to my heart, and one that I teach a number of groups, from medical students to faculty.
I also benefited from just returning from the Harvard Macy Educators Course, where Victoria Brazil, MD, an experienced emergency medicine physician from Australia, provided her top tips. Here is a mash-up of the top tips to think about for any of the speakers out there among us – with a few shout-outs for the ladies out there. Please add your own!
The Dos
- Do project power: Stand tall with a relaxed stance and shoulders back – posture is everything. This is especially important for women, who may tend to shrink their bodies, or anyone who is short. A powerful messenger is just as important as the power of the message. The same also applies to sitting down, especially if you are on a panel. Do not look like you are falling into the table.
- Do look up: Think about addressing the people in the back, not in the front row. This looks better in photos as well since you are appealing to the large audience and not the front row. Dr. Brazil’s tip came from Cate Blanchett who said that before she gives talks, she literally and physically advises “picking up your crown and put it on your head.” Not only will you feel better, you will look it too.
- Do pause strategically: The human brain needs rest to process what you are about to say. You can ask people to “think of a time” and take a pause. Or “I want you to all think about what I just said for one moment.” And TAKE a moment. But think about Emma’s pause during the March For Your Lives. Pauses are powerful and serve as a way to cement what you are saying for even the most critical crowd. Think about when anyone on their phone pauses, even if you’re on a boring conference call others will wake up and wonder what is going on and are now engaged in the talk.
- Do strategically summarize: Before you end, or in between important sections, say the following: “There are three main things you can do.” Even if someone fell asleep, they will wake up to take note. It’s a way to get folks’ attention back. There is nothing like challenging others to do something.
The Don’ts
- Don’t start with an apology for “not being an expert”: Or whatever you are thinking about apologizing for. The voice in your head does not need to be broadcast to others. Just say thank you after you are introduced, and launch in. Someone has asked you to talk, so bring your own unique expertise and don’t start with undermining yourself!
- Don’t use your slides as a crutch: Make your audience look at you and not your slides. That means at times, you may be talking and your slides will not be moving. Other times, if you are starting with a story, maybe there is no slide behind you and the screen is blacked out. Some of the most powerful moments in a talk are when slides are not being used.
- Don’t stand behind the podium if you can help it. This means ask for a wireless microphone. Most podiums will overwhelm you. If you have to use a podium, go back to the posture in the “dos.” One year, I had a leg injury and definitely used the podium, so obviously there may be times you need to use a podium; even then, try as hard as possible to make sure you are seen.
- Don’t engage grandstanders during Q&A: Invariably, you will get someone who stands up and goes into a long comment that is not a question to hear themselves speak. Insert yourself, say “thank you” and take the next question. If there is not a next question, you can add, “Before I forget, I want to share another question I am often asked which may be of help to you.” Then, answer your own question. You get the final word this way!
Happy speaking! I look forward to seeing you in warmer weather during the spring conference season.
For more posts from the Hospital Leader blog, visit hospitalleader.org.
Looking ahead: Gastroenterology devices over the next decade
SAN FRANCISCO – The gastroenterology device field has matured technically in recent years, with plenty of innovation in applications to antireflux, obesity, and colorectal polyp detection, among others, but barriers to adoption remain. The most pressing is reimbursement, which is a process that is often opaque and off-putting, especially for small companies that lack the capital to bull their way through the obstacles.

Reimbursement decisions get made on a case-by-case basis, “and a lot of times there’s a finite number of dollars in the health care system, and CMS [Centers for Medicare & Medicaid Services] and other entities are trying to limit how many things we can have. They look at data and summaries that can be somewhat biased. Plus the way the methodology works in surveying physicians is not very clear, so the overall process needs more clarity,” said Sri Komanduri, MD, AGAF, professor of medicine and surgery at Northwestern University, Chicago, and vice chair of the AGA Center for GI Innovation and Technology, in an interview at the 2019 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
The 2019 summit highlighted new technologies in its annual Shark Tank competition and brought experts from industry, academia, and regulatory agencies to San Francisco for 2 days of presentations on the challenges and opportunities in gastroenterology devices.
Lack of clarity is indeed a key challenge, agreed V. Raman Muthusamy, MD, AGAF, director of endoscopy at the University of California, Los Angeles, Health System, professor of clinical medicine at UCLA, and chair of the AGA Center for GI Innovation and Technology. “Maybe you designed a trial that you think will be adequate but the person who is ultimately making the decision on coverage doesn’t think it’s adequate, so having societies and payers really speak together with industry and innovators to get this information early rather than late could save a lot of time and money, and ultimately get these products to patients sooner,” Dr. Muthusamy said in an interview.
And he insists that the technology is ready, as evidenced in part by the Shark Tank contestants and this year’s winner. There is more to come. In the immediate future, Dr. Muthusamy anticipates use of artificial intelligence to enhance polyp detection, and perhaps assessing larger polyps. “Computers can aid us in reading things, particularly in analyzing large amounts of data which may look similar to finding a needle in a haystack, whether that’s dysplasia in a field of normal tissue, or it’s identifying a small locus of blood in an otherwise bloodless field.”
He also expects more expansion of technologies that will allow endoscopists to perform techniques that were once limited to surgeons, such as making endoscopic submucosal dissection easier to perform. “We continue to see development of endoscopic platforms that are going to allow us to become endoscopic surgeons,” said Dr. Muthusamy.
Interventional ultrasound should continue to gain traction, and Dr. Muthusamy hopes to see an endoscopic antireflux device that could provide patients a middle-ground option between medication and surgery.
But these innovations still face many obstacles to reaching patients. Getting Food and Drug Administration approval, getting a code, and reimbursement are all daunting roadblocks. “You clear one hurdle only to run into another, and if you get one of these steps wrong, and they say you have to redo a trial, you’re talking potentially millions of dollars and several years,” said Dr. Muthusamy.
That could stifle innovation, particularly among small companies. “That may be why you see a lot of smaller companies get acquired early – they don’t have the sort of capital to sustain the long road to the finish,” he said.
However, Dr. Muthusamy believes there is room for optimism, as evidenced by progress at the FDA. “If we can make the level of changes in the next decade in the reimbursement process that we’ve made in the regulatory process in the last decade, we’ll have made some real progress.”
The AGA Center for GI Innovation and Technology is working behind the scenes to guide the FDA, payers, and industry in overcoming the overcoming the obstacles inherent in the device development, approval, and adoption process. The center’s goal is to continue to advance innovation in GI, while making sure the needs of gastroenterologists and patients are met with each new technology that comes to market.
SAN FRANCISCO – The gastroenterology device field has matured technically in recent years, with plenty of innovation in applications to antireflux, obesity, and colorectal polyp detection, among others, but barriers to adoption remain. The most pressing is reimbursement, which is a process that is often opaque and off-putting, especially for small companies that lack the capital to bull their way through the obstacles.

Reimbursement decisions get made on a case-by-case basis, “and a lot of times there’s a finite number of dollars in the health care system, and CMS [Centers for Medicare & Medicaid Services] and other entities are trying to limit how many things we can have. They look at data and summaries that can be somewhat biased. Plus the way the methodology works in surveying physicians is not very clear, so the overall process needs more clarity,” said Sri Komanduri, MD, AGAF, professor of medicine and surgery at Northwestern University, Chicago, and vice chair of the AGA Center for GI Innovation and Technology, in an interview at the 2019 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
The 2019 summit highlighted new technologies in its annual Shark Tank competition and brought experts from industry, academia, and regulatory agencies to San Francisco for 2 days of presentations on the challenges and opportunities in gastroenterology devices.
Lack of clarity is indeed a key challenge, agreed V. Raman Muthusamy, MD, AGAF, director of endoscopy at the University of California, Los Angeles, Health System, professor of clinical medicine at UCLA, and chair of the AGA Center for GI Innovation and Technology. “Maybe you designed a trial that you think will be adequate but the person who is ultimately making the decision on coverage doesn’t think it’s adequate, so having societies and payers really speak together with industry and innovators to get this information early rather than late could save a lot of time and money, and ultimately get these products to patients sooner,” Dr. Muthusamy said in an interview.
And he insists that the technology is ready, as evidenced in part by the Shark Tank contestants and this year’s winner. There is more to come. In the immediate future, Dr. Muthusamy anticipates use of artificial intelligence to enhance polyp detection, and perhaps assessing larger polyps. “Computers can aid us in reading things, particularly in analyzing large amounts of data which may look similar to finding a needle in a haystack, whether that’s dysplasia in a field of normal tissue, or it’s identifying a small locus of blood in an otherwise bloodless field.”
He also expects more expansion of technologies that will allow endoscopists to perform techniques that were once limited to surgeons, such as making endoscopic submucosal dissection easier to perform. “We continue to see development of endoscopic platforms that are going to allow us to become endoscopic surgeons,” said Dr. Muthusamy.
Interventional ultrasound should continue to gain traction, and Dr. Muthusamy hopes to see an endoscopic antireflux device that could provide patients a middle-ground option between medication and surgery.
But these innovations still face many obstacles to reaching patients. Getting Food and Drug Administration approval, getting a code, and reimbursement are all daunting roadblocks. “You clear one hurdle only to run into another, and if you get one of these steps wrong, and they say you have to redo a trial, you’re talking potentially millions of dollars and several years,” said Dr. Muthusamy.
That could stifle innovation, particularly among small companies. “That may be why you see a lot of smaller companies get acquired early – they don’t have the sort of capital to sustain the long road to the finish,” he said.
However, Dr. Muthusamy believes there is room for optimism, as evidenced by progress at the FDA. “If we can make the level of changes in the next decade in the reimbursement process that we’ve made in the regulatory process in the last decade, we’ll have made some real progress.”
The AGA Center for GI Innovation and Technology is working behind the scenes to guide the FDA, payers, and industry in overcoming the overcoming the obstacles inherent in the device development, approval, and adoption process. The center’s goal is to continue to advance innovation in GI, while making sure the needs of gastroenterologists and patients are met with each new technology that comes to market.
SAN FRANCISCO – The gastroenterology device field has matured technically in recent years, with plenty of innovation in applications to antireflux, obesity, and colorectal polyp detection, among others, but barriers to adoption remain. The most pressing is reimbursement, which is a process that is often opaque and off-putting, especially for small companies that lack the capital to bull their way through the obstacles.

Reimbursement decisions get made on a case-by-case basis, “and a lot of times there’s a finite number of dollars in the health care system, and CMS [Centers for Medicare & Medicaid Services] and other entities are trying to limit how many things we can have. They look at data and summaries that can be somewhat biased. Plus the way the methodology works in surveying physicians is not very clear, so the overall process needs more clarity,” said Sri Komanduri, MD, AGAF, professor of medicine and surgery at Northwestern University, Chicago, and vice chair of the AGA Center for GI Innovation and Technology, in an interview at the 2019 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology.
The 2019 summit highlighted new technologies in its annual Shark Tank competition and brought experts from industry, academia, and regulatory agencies to San Francisco for 2 days of presentations on the challenges and opportunities in gastroenterology devices.
Lack of clarity is indeed a key challenge, agreed V. Raman Muthusamy, MD, AGAF, director of endoscopy at the University of California, Los Angeles, Health System, professor of clinical medicine at UCLA, and chair of the AGA Center for GI Innovation and Technology. “Maybe you designed a trial that you think will be adequate but the person who is ultimately making the decision on coverage doesn’t think it’s adequate, so having societies and payers really speak together with industry and innovators to get this information early rather than late could save a lot of time and money, and ultimately get these products to patients sooner,” Dr. Muthusamy said in an interview.
And he insists that the technology is ready, as evidenced in part by the Shark Tank contestants and this year’s winner. There is more to come. In the immediate future, Dr. Muthusamy anticipates use of artificial intelligence to enhance polyp detection, and perhaps assessing larger polyps. “Computers can aid us in reading things, particularly in analyzing large amounts of data which may look similar to finding a needle in a haystack, whether that’s dysplasia in a field of normal tissue, or it’s identifying a small locus of blood in an otherwise bloodless field.”
He also expects more expansion of technologies that will allow endoscopists to perform techniques that were once limited to surgeons, such as making endoscopic submucosal dissection easier to perform. “We continue to see development of endoscopic platforms that are going to allow us to become endoscopic surgeons,” said Dr. Muthusamy.
Interventional ultrasound should continue to gain traction, and Dr. Muthusamy hopes to see an endoscopic antireflux device that could provide patients a middle-ground option between medication and surgery.
But these innovations still face many obstacles to reaching patients. Getting Food and Drug Administration approval, getting a code, and reimbursement are all daunting roadblocks. “You clear one hurdle only to run into another, and if you get one of these steps wrong, and they say you have to redo a trial, you’re talking potentially millions of dollars and several years,” said Dr. Muthusamy.
That could stifle innovation, particularly among small companies. “That may be why you see a lot of smaller companies get acquired early – they don’t have the sort of capital to sustain the long road to the finish,” he said.
However, Dr. Muthusamy believes there is room for optimism, as evidenced by progress at the FDA. “If we can make the level of changes in the next decade in the reimbursement process that we’ve made in the regulatory process in the last decade, we’ll have made some real progress.”
The AGA Center for GI Innovation and Technology is working behind the scenes to guide the FDA, payers, and industry in overcoming the overcoming the obstacles inherent in the device development, approval, and adoption process. The center’s goal is to continue to advance innovation in GI, while making sure the needs of gastroenterologists and patients are met with each new technology that comes to market.
EXPERT ANALYSIS FROM THE 2019 AGA TECH SUMMIT
Circulating tumor cells predict NSCLC survival, but clinical role uncertain
GENEVA – Circulating tumor cell (CTC) count is an independent predictor of both progression-free and overall survival in patients with advanced non–small cell lung cancer (NSCLC), according to data from 550 patients.
This is the largest CTC study to date and the first to compare test results from multiple centers, reported lead author Colin Lindsay, MD, PhD, of the University of Manchester (England) and colleagues. Among the centers, investigators found minimal variability in results guiding progression-free survival and no significant differences in results predicting overall survival. These findings suggest that CTC testing could be reproducible and reliable on a large scale, Dr. Lindsay said during a presentation at the European Lung Cancer Conference; he added that this conclusion addresses a previous concern about the process.
“A slight problem with the process is still that it is semi-automated,” Dr. Lindsay said at the meeting presented by the European Society for Medical Oncology. “The machine will harvest potential cells and stain potential cells, but the end step of the process is that a trained user in each laboratory will decide which cell is a CTC and which cell isn’t a CTC, and it’s that potential for user variability that was the basis of this study.”
The retrospective study involved 550 patients with NSCLC whose samples were processed at seven centers in multiple European countries, including 209 patients whose data was previously unpublished. The investigators looked for associations between CTC count and survival using Cox regression analysis and evaluated if CTCs could add value to prognostic clinicopathologic models based on c-indices and likelihood ratio statistics. CTC count was assessed as a continuous variable and, based on previous studies, using two categorical thresholds: at least 2 cells per 7.5 mL and at least 5 cells per 7.5 mL. In addition, the investigators looked for associations between NSCLC molecular subtypes and CTC levels.
The results showed that both cutoff levels were predictive of survival, with the higher threshold carrying a poorer prognosis. For progression-free survival, CTC counts of at least 2 cells per 7.5 mL carried a hazard ratio of 1.72, whereas the 5-cell threshold had a hazard ratio of 2.21 (P less than .001 for both). Similarly, overall survival hazard ratios for the lower and higher thresholds were 2.18 and 2.75, respectively (P less than .001 for both). When baseline CTC count was added to the analysis, predictive accuracy increased further, dropping P values tenfold, down to .0001. C-index models had a more modest impact. Although minor heterogeneity was detected among centers for prediction of progression-free survival, overall survival data was broadly reliable. Dr. Lindsay noted that intercenter differences seemed to diminish with greater testing experience. No relationships were detected between molecular subtypes and CTC profiles.
“It’s always good to finish a talk with the white elephant in the room,” Dr. Lindsay said in his concluding remarks. “Is there room for CTCs in non–small cell lung cancer? I believe they have the potential to complement ctDNA work by offering a cellular context, but [CTCs] aren’t there yet for clinical roll-out.”
Invited discussant Juergen Wolf, MD, of the University Hospital Cologne (Germany) provided a similar conclusion, suggesting that CTCs have a clear place in research, but their clinical value is debatable. He noted that ctDNA, the most similar diagnostic and prognostic tool under development, has a pragmatic edge because ctDNA samples are more amenable to shipping and handling. Dr. Wolf noted that ctDNA also has been shown to have value for treatment planning, specifically for the EGFR T790M resistance mutation. This latter point tied into a larger issue described by Dr. Wolf, who suggested that in the current treatment landscape for NSCLC, predictive testing needs to be actionable.
“We cannot draw a consequence of a prognostic biomarker,” Dr. Wolf said. “In the era of personalized medicine, what we need is predictive markers, predictive of the outcome of specific therapies.”The investigators disclosed financial relationships with AstraZeneca, Novartis, Pfizer, and others.
SOURCE: Lindsay C et al. ELCC 2019, Abstract 21O.
GENEVA – Circulating tumor cell (CTC) count is an independent predictor of both progression-free and overall survival in patients with advanced non–small cell lung cancer (NSCLC), according to data from 550 patients.
This is the largest CTC study to date and the first to compare test results from multiple centers, reported lead author Colin Lindsay, MD, PhD, of the University of Manchester (England) and colleagues. Among the centers, investigators found minimal variability in results guiding progression-free survival and no significant differences in results predicting overall survival. These findings suggest that CTC testing could be reproducible and reliable on a large scale, Dr. Lindsay said during a presentation at the European Lung Cancer Conference; he added that this conclusion addresses a previous concern about the process.
“A slight problem with the process is still that it is semi-automated,” Dr. Lindsay said at the meeting presented by the European Society for Medical Oncology. “The machine will harvest potential cells and stain potential cells, but the end step of the process is that a trained user in each laboratory will decide which cell is a CTC and which cell isn’t a CTC, and it’s that potential for user variability that was the basis of this study.”
The retrospective study involved 550 patients with NSCLC whose samples were processed at seven centers in multiple European countries, including 209 patients whose data was previously unpublished. The investigators looked for associations between CTC count and survival using Cox regression analysis and evaluated if CTCs could add value to prognostic clinicopathologic models based on c-indices and likelihood ratio statistics. CTC count was assessed as a continuous variable and, based on previous studies, using two categorical thresholds: at least 2 cells per 7.5 mL and at least 5 cells per 7.5 mL. In addition, the investigators looked for associations between NSCLC molecular subtypes and CTC levels.
The results showed that both cutoff levels were predictive of survival, with the higher threshold carrying a poorer prognosis. For progression-free survival, CTC counts of at least 2 cells per 7.5 mL carried a hazard ratio of 1.72, whereas the 5-cell threshold had a hazard ratio of 2.21 (P less than .001 for both). Similarly, overall survival hazard ratios for the lower and higher thresholds were 2.18 and 2.75, respectively (P less than .001 for both). When baseline CTC count was added to the analysis, predictive accuracy increased further, dropping P values tenfold, down to .0001. C-index models had a more modest impact. Although minor heterogeneity was detected among centers for prediction of progression-free survival, overall survival data was broadly reliable. Dr. Lindsay noted that intercenter differences seemed to diminish with greater testing experience. No relationships were detected between molecular subtypes and CTC profiles.
“It’s always good to finish a talk with the white elephant in the room,” Dr. Lindsay said in his concluding remarks. “Is there room for CTCs in non–small cell lung cancer? I believe they have the potential to complement ctDNA work by offering a cellular context, but [CTCs] aren’t there yet for clinical roll-out.”
Invited discussant Juergen Wolf, MD, of the University Hospital Cologne (Germany) provided a similar conclusion, suggesting that CTCs have a clear place in research, but their clinical value is debatable. He noted that ctDNA, the most similar diagnostic and prognostic tool under development, has a pragmatic edge because ctDNA samples are more amenable to shipping and handling. Dr. Wolf noted that ctDNA also has been shown to have value for treatment planning, specifically for the EGFR T790M resistance mutation. This latter point tied into a larger issue described by Dr. Wolf, who suggested that in the current treatment landscape for NSCLC, predictive testing needs to be actionable.
“We cannot draw a consequence of a prognostic biomarker,” Dr. Wolf said. “In the era of personalized medicine, what we need is predictive markers, predictive of the outcome of specific therapies.”The investigators disclosed financial relationships with AstraZeneca, Novartis, Pfizer, and others.
SOURCE: Lindsay C et al. ELCC 2019, Abstract 21O.
GENEVA – Circulating tumor cell (CTC) count is an independent predictor of both progression-free and overall survival in patients with advanced non–small cell lung cancer (NSCLC), according to data from 550 patients.
This is the largest CTC study to date and the first to compare test results from multiple centers, reported lead author Colin Lindsay, MD, PhD, of the University of Manchester (England) and colleagues. Among the centers, investigators found minimal variability in results guiding progression-free survival and no significant differences in results predicting overall survival. These findings suggest that CTC testing could be reproducible and reliable on a large scale, Dr. Lindsay said during a presentation at the European Lung Cancer Conference; he added that this conclusion addresses a previous concern about the process.
“A slight problem with the process is still that it is semi-automated,” Dr. Lindsay said at the meeting presented by the European Society for Medical Oncology. “The machine will harvest potential cells and stain potential cells, but the end step of the process is that a trained user in each laboratory will decide which cell is a CTC and which cell isn’t a CTC, and it’s that potential for user variability that was the basis of this study.”
The retrospective study involved 550 patients with NSCLC whose samples were processed at seven centers in multiple European countries, including 209 patients whose data was previously unpublished. The investigators looked for associations between CTC count and survival using Cox regression analysis and evaluated if CTCs could add value to prognostic clinicopathologic models based on c-indices and likelihood ratio statistics. CTC count was assessed as a continuous variable and, based on previous studies, using two categorical thresholds: at least 2 cells per 7.5 mL and at least 5 cells per 7.5 mL. In addition, the investigators looked for associations between NSCLC molecular subtypes and CTC levels.
The results showed that both cutoff levels were predictive of survival, with the higher threshold carrying a poorer prognosis. For progression-free survival, CTC counts of at least 2 cells per 7.5 mL carried a hazard ratio of 1.72, whereas the 5-cell threshold had a hazard ratio of 2.21 (P less than .001 for both). Similarly, overall survival hazard ratios for the lower and higher thresholds were 2.18 and 2.75, respectively (P less than .001 for both). When baseline CTC count was added to the analysis, predictive accuracy increased further, dropping P values tenfold, down to .0001. C-index models had a more modest impact. Although minor heterogeneity was detected among centers for prediction of progression-free survival, overall survival data was broadly reliable. Dr. Lindsay noted that intercenter differences seemed to diminish with greater testing experience. No relationships were detected between molecular subtypes and CTC profiles.
“It’s always good to finish a talk with the white elephant in the room,” Dr. Lindsay said in his concluding remarks. “Is there room for CTCs in non–small cell lung cancer? I believe they have the potential to complement ctDNA work by offering a cellular context, but [CTCs] aren’t there yet for clinical roll-out.”
Invited discussant Juergen Wolf, MD, of the University Hospital Cologne (Germany) provided a similar conclusion, suggesting that CTCs have a clear place in research, but their clinical value is debatable. He noted that ctDNA, the most similar diagnostic and prognostic tool under development, has a pragmatic edge because ctDNA samples are more amenable to shipping and handling. Dr. Wolf noted that ctDNA also has been shown to have value for treatment planning, specifically for the EGFR T790M resistance mutation. This latter point tied into a larger issue described by Dr. Wolf, who suggested that in the current treatment landscape for NSCLC, predictive testing needs to be actionable.
“We cannot draw a consequence of a prognostic biomarker,” Dr. Wolf said. “In the era of personalized medicine, what we need is predictive markers, predictive of the outcome of specific therapies.”The investigators disclosed financial relationships with AstraZeneca, Novartis, Pfizer, and others.
SOURCE: Lindsay C et al. ELCC 2019, Abstract 21O.
REPORTING FROM ELCC 2019
Five enter the Shark Tank, one emerges
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
SAN FRANCISCO – All five innovative startups pitched at the Shark Tank at the 2019 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology, are in advanced stages of development, but only one is given the opportunity to be declared the winner of the competition. The ideas ranged from a smart toilet for early disease detection to a unique strategy for obesity phenotyping, but the winner by both official decision and popular vote was a smartphone app to help patients with inflammatory bowel disease (IBD) manage the condition.
“As always, this year’s Shark Tank was a highlight of the AGA Tech Summit and represents the progress our field is making when it comes to innovation. Our panel of sharks was focused on understanding the problem each innovation solved – that’s the key when determining if an idea is novel or innovation for innovation’s sake. We were impressed with all of the technologies presented, but ultimately chose the Oshi Health IBD app as our winner because of the impact it is already having on improving the health and care of IBD patients,” said V. Raman Muthusamy, MD, AGAF, chair of the AGA Center for GI Innovation and Technology.
The winner: Oshi pitches “all-in-one” IBD app
By both popular vote from those attending the AGA Tech Summit as well as the six-member Shark Tank panel, Oshi Health was selected as the 2019 Shark Tank winner for its IBD app. The app was designed to help patients track symptoms, a first step in understanding flare patterns, which differ substantially between patients and emphasize the need for a personalized plan for controlling disease.

“Since we launched last June at DDW® we have had 40,000 downloads. We are the number one IBD management app,” reported Dan Weinstein, MBA, CEO of Oshi Health.
The available app represents the first of three phases as the functionality is expanded. Currently, in addition to using the app as a tracking tool, patients can find resources to learn about their disease and to communicate with other patients about their experiences. In a second phase, information gathered by the app will be made available to physicians to provide accurate current information about disease status to better individualize therapy.
Ultimately, the app is expected to guide treatment based on information it has collected on symptom patterns and other data collected over time, although this application is further down the road and will require regulatory approval if it is designed to provide clinical advice as expected, according to Mr. Weinstein.
However, benefits have already been seen. Mr. Weinstein cited data that associated the app with a 40% improvement in medication adherence and a nearly 60-day reduction in flare duration. Calling the app “the next chapter in treat-to-target” IBD management, he believes that this is an important step forward in digital health that will improve IBD outcomes. The Shark Tank panel agreed.
Runners-up: Other potential innovations to improve GI health
With or without Shark Tank endorsement, the other four startups described in the competition are moving forward. Each is designed to address an important unmet need with the potential to improve patient outcomes, which is a criterion for their inclusion in the competition.
The smart toilet seat
One involves a technologically advanced toilet seat. The new seat is based on the fact that fecal matter provides insight into a broad array of disease states, but specimen collection is a hurdle for a variety of reasons, including patient resistance. A toilet seat developed by Toi Labs, called TrueLoo, is equipped with lighting and cameras that captures images of bowel movements and urination for subsequent analysis.
“The toilet seat sees what the eye cannot,” according to Vikram Kashyap, CEO of Toi Labs. He believes it has major potential for early detection of conditions ranging from dehydration to gastrointestinal cancer.

Others agree. According to Mr. Kashyap, executives of a chain of senior living facilities have already expressed interest in installing this seat to better monitor health among residents. The seat is bolted into position in place of any standard toilet seat. It collects images and data that are transmitted directly to a cellular network.
“Using our technology, the goal is to catch disease states early before they progress,” said Mr. Kashyap, who called the surveillance system a low-cost disease-screening tool. He believes the smart toilet seat could be of the most important disease detection devices developed in recent years.
AI to aid screening endoscopy
A third entrant in this year’s Shark Tank described a strategy to employ artificial intelligence (AI) to aid endoscopists in screening for dysplasia. The tool is called Ultivision and is being developed by a startup called Docbot. The CEO, Andrew Ninh, and a senior executive, Jason B. Samarasena, MD, outlined an idea that could be used in either screening colonoscopy or in surveillance of Barrett’s esophagus).
“Dysplasia is difficult to find. It is subtle and it is often missed. With better detection of dysplasia, artificial intelligence offers an opportunity to reduce risk of cancer,” Dr. Samarasena said.
The tool integrates seamlessly with existing endoscopic tools, according to Mr. Ninh. As tissue is visualized, the AI is programmed to highlight suspected dysplasia with a colored box to alert the endoscopist. The colonoscopy application is a more advanced stage of development and might be submitted for regulatory approval this year, he said. The same technology will be adapted for Barrett’s esophagus.
“It is like facial recognition for dysplasia,” said Dr. Samarasena.
Obesity phenotyping tool
A fourth Shark Tank entrant employs technology to phenotype obese patients to better tailor therapy. The Pheno Test, developed by Phenomix Sciences, applies “multi-omics” to a blood-based test to separate patients with obesity into four phenotypes. When therapy is tailored to the phenotype, weight loss is greater, according to Andres J. Acosta, MD, PhD, assistant professor of medicine and consultant in gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
In an initial study that compared weight loss in 55 patients treated based on phenotype with 175 patients managed with standard of care, the total body weight loss “more than doubled,” Dr. Acosta reported.
According to Dr. Acosta, obesity is driven by very different mechanisms. He described the four major phenotypes identified with his test as hungry brain (satiation signal is impaired), hungry gut (signals to eat are upregulated), emotional hunger (psychological reasons drive eating behavior), and slow metabolism (failure to burn fat at normal rates).
With the blood test, which utilizes hormones, metabolites, DNA, and other biomarkers to separate these phenotypes, treatment can be tailored appropriately, according to Dr. Acosta. His company is now seeking Food and Drug Administration clearance of the test, which he believes will have a major impact on obesity control.
Capsule diagnostic tool
The final entrant selected to participate in this year’s Shark Tank described an ingestible capsule that diagnoses diseases by detecting gases as it descends the gastrointestinal tract. The Atmo Gas Capsule from Atmo Biosciences measures gases at the source, accelerating the diagnosis of such diseases as irritable bowel syndrome (IBS) and IBD.
“By measuring gases at their source, the accuracy is far better than a breath test,” said Malcolm Hebblewhite, MBA, CEO of Atmo Biosciences. The capsule is an alternative to more invasive and expensive diagnostic tools and it is highly accurate.
Providing examples, Mr. Hebblewhite said that elevated levels of oxygen suggest a disorder of motility while an elevated level of carbon dioxide and hydrogen suggest IBS. The capsule transmits data to a small receiver and then on to a smartphone.
“The real-time data is displayed for the user with more complex information accessible by the practitioner remotely via the cloud,” Mr. Hebblewhite said. He cited several papers that have already been published documenting the potential of this technology.
“The capsule is a single-use disposable device that is not retrieved,” according to Mr. Hebblewhite. He reported that his company plans to pursue the diagnosis of motility as an initial clinical application. The diagnosis of IBS and other GI conditions will follow. Clinical studies are already planned.
REPORTING FROM 2019 AGA TECH SUMMIT
















