User login
Part 4: Misguided Research or Missed Opportunities?
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at NPEditor@mdege.com.
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at NPEditor@mdege.com.
I have been ruminating about the Bai et al article on independent billing in the emergency department (ED) for weeks.1 I keep wondering why the data analysis seems so off base. Don’t get me wrong: The data gathered from Medicare is what it is—but a key piece of information is not present in the pure numbers input to the Medicare database.
So, I continued to probe this study with my colleagues. To a person, their comments supported that the intent of the study is unclear. The authors posit their objective to be an examination of the “involvement of NPs and PAs” in emergency services, using billing data. But to use billing data as a measure of “involvement” does not tell the whole story.
Independence in billing does not mean that the care NPs and PAs are providing is “beyond their scope of practice.” Moreover, the billing does not capture whether, or to what extent, physician consultation or assistance was involved. If the NP or PA dictated the chart, then they are by default the “only” (independent) provider. However, billing independently does not mean a physician (or other provider) was not consulted about the plan of care.
Case in point: Years ago, I had a young woman present to the ED with a sore throat. Her presenting complaint was a symptom of a peritonsillar abscess. So I phoned an ENT colleague (a physician) and asked him about the best treatment and follow-up in this case. Did he make a note in or sign the chart? No. Was I the only provider of record? Yes. Was that care “independent,” if you only look at the billing (done by a coder, for the record)? Yes.
Admittedly, Bai and colleagues do add in their conclusion that “independence in billing … does not necessarily indicate [NPs’/PAs’] independence in care delivery.”1 And they do note that the true challenge in the ED is determining how best to “blend” the expertise of the three professions (MD, NP, and PA) to provide efficient and cost-effective care.
However, throughout the article, there is an underpinning of inference that NPs and PAs are potentially practicing beyond their scope. Their comment that the increase in billing for NP and PA services results in a “reduction of the proportion of emergency physicians” speaks volumes.1 Perhaps there is more concern here about ED physician job security than about independent billing!
Regardless of the intention by Bai et al—and acknowledging that the analysis they presented is somewhat interesting—I see two missed opportunities to “actionalize” the data.2 One is to use the information to identify whether a problem with billing exists (ie, is there upcharging as a result of more details contained within the electronic health record?). The second is to use the data to investigate innovative ways to improve access to care across the continuum. Essentially, how do we use the results of any data analysis in a way that can be useful? That is the real challenge.
Continue to: The biggest conclusion I've drawn...
The biggest conclusion I’ve drawn from my exploration of these study findings? The opportunity to investigate the competencies of all ED providers, with the goal of improving access and controlling costs, is there. And as the NPs and PAs providing the care, we should undertake the next research study or data analysis and not leave the research on us to other professions!
I’d love to hear your thoughts on the Bai et al study or any aspect of this 4-part discussion! Drop me a line at NPEditor@mdege.com.
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
1. Bai G, Kelen GD, Frick KD, Anderson GF. Nurse practitioners and physician assistants in emergency medical services who billed independently, 2012-2016. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.01.052. Accessed April 1, 2019.
2. The Wharton School at the University of Pennsylvania. Big data’s biggest challenge: how to avoid getting lost in the weeds. Knowledge@Wharton podcast. March 14, 2019. http://knowledge.wharton.upenn.edu/article/data-analytics-challenges. Accessed April 1, 2019.
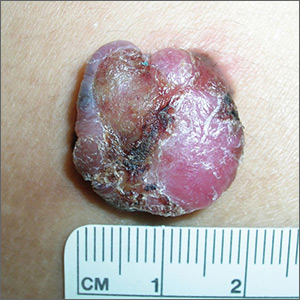
Painful lump on back
The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
CDC warns against misuse of opioid-prescribing guideline
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Officials at the Centers for Disease Control and Prevention are warning against the misapplication of the agency’s 2016 guidelines on opioid prescribing, as well as clarifying dosage recommendations for patients starting or stopping pain medications.
In a perspective published in the New England Journal of Medicine on April 24, lead author Deborah Dowell, MD, chief medical officer for the CDC’s National Center for Injury Prevention and Control, conveyed concern that some policies and practices derived from the 2016 CDC Guideline for Prescribing Opioids for Chronic Pain are inconsistent with the recommendations and often go beyond their scope.
Misapplication examples include inappropriately applying the guideline to patients in active cancer treatment, patients experiencing acute sickle cell crises, or patients experiencing postsurgical pain, Dr. Dowell wrote.
The guideline offers guidance to clinicians treating chronic pain in adults who are already receiving opioids long-term at high dosages, she noted. It includes advice on maximizing nonopioid treatment, reviewing risks associated with continuing high-dose opioids, and collaborating with patients who agree to taper dosage, among other guidance.
Any application of the guideline’s dosage recommendation that results in hard limits or “cutting off” opioids is also an incorrect use of the recommendations, according to Dr. Dowell.
While the guideline advises clinicians to start opioids at the lowest effective dosage and avoid increasing dosage to 90 morphine milligram equivalents per day or more, that statement does not suggest discontinuation of opioids already prescribed at high dosages, according to the CDC’s clarification.
The guidance also does not apply to patients receiving or starting medication-assisted treatment for opioid use disorder.
The commentary comes after a trio of organizations raised concerns that insurers are inappropriately applying the recommendations to active cancer patients when making coverage determinations.
The American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the American Society of Hematology, raised the issue in a letter to the CDC in February. In response, Dr. Dowell clarified that the recommendations are not intended to deny clinically appropriate opioid therapy to any patients who suffer chronic pain, but rather to ensure that physicians and patients consider all safe and effective treatment options.
In the perspective, Dr. Dowell wrote that the CDC is evaluating the intended and unintended impact of the 2016 opioid-prescribing guideline on clinician and patient outcomes and that the agency is committed to updating the recommendations when new evidence is available.
Tagraxofusp produces high response rate in BPDCN
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tagraxofusp produced responses in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Major finding: The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
Study details: A phase 2 trial of 47 patients, 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN.
Disclosures: The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Source: Pemmaraju N et al. N Engl J Med. 2019;380:1628-37.
Texans’ rattler diet, recycled humans, and, ahem, Puber
Endgame for arachnophobia
We think Tony Stark would like this creative solution for spider and ant phobias. Comic book movies have now infiltrated every aspect of culture, including serious scientific research. And let’s be honest – more than one scientist has been inspired to go into their fields by Bruce Banner, Stark, or maybe even Doctor Octopus (no judgment).
A group of (possibly mad) scientists has tested exposure therapy for spider and ant phobias in people by showing participants the Spider-Man and Ant-Man movies. While the viewing material may not be totally scientifically accurate, researchers found that watching seven seconds of Spider-Man 2 or Ant-Man reduced spider and ant phobia by 20%.
The participants were specifically exposed to ants and spiders in the context of the movies, so surprisingly the phobia reduction had nothing to do with Tobey Maguire or Paul Rudd.
Old poop, new discovery
Here at LOTME, we like us some good bathroom humor. And don’t worry, we won’t ever change. In this week’s edition of the Wonderful World of Poop, we take you to Texas, 1,500 years ago. The sky was bigger, the air was fresher, and the humans of the Lower Pecos region were as hardcore as you can get. A recent re-examination of coprolite samples taken from the region found one interesting chunk of poop-rock that contained an entire rattlesnake.
Now, the presence of snake bits in early human poo is not that crazy; people ate (and still eat) snakes. The appearance of a centimeter-long fang, scales, and bones, however, did take the researchers by surprise. Why would someone eat a snake? Was it an ancient way to inoculate against snake venom? Or perhaps crunchy snake fangs were the world’s earliest version of a Cheeto?
In fact, researchers hypothesized that this dietary behavior was not normal for the people of the Lower Pecos, and most likely was more ceremonial. You know, the casual eating-a-full-snake ceremony.
Will Texans embrace this ceremony of their past and start chomping on rattlers? Who’s to say? All we know is that poop is the gift that keeps on giving.
A new way to soil yourself
If you’re reading this, we can say with some certainty that you managed to survive another Tax Day. Congratulations! But there’s still Benjamin Franklin’s other ultimate certainty of life. You know … the big sleep, the last roundup, assume room temperature, buy the farm, shuffle off this mortal coil, give up the ghost, and so on.
What are you going to do about that?
A big question, for sure, so let’s just focus on the earthly remains. A company called Recompose has a new alternative to burial and cremation, something they’ve dubbed “natural organic reduction” and others have described as “human composting” or “accelerated decomposition.” In a pilot project last year at Washington State University in Pullman, the Recompose process transformed the bodies of six donors to soil in 4-7 weeks, AP reported.
The company says that natural organic reduction is much more environmentally friendly than current practices, creating a cubic yard of soil per person, and that “friends and family are welcome to take some (or all) home to grow a tree or a garden.”
A garden sounds nice, or maybe something indoors. Just think of the potted plant possibilities: daisies (to push up), a Venus flytrap (the organic reduction continues), some poison ivy (a gift for people you don’t like), or maybe roses. Who wouldn’t want to come out of death smelling like a rose?
San Francisco vs. illegal dumping
Maybe you’re not quite ready to commit to using human remains as compost to fertilize your garden. Perhaps you want to start off only using human poop as fertilizer, see how that goes before sprinkling Grandma all over your tulips.
Well, if you’re looking for a sweet deal, we’re certain San Francisco can work something out with you because, in the past 7 years, incidence of human feces in public places within the city has quintupled, rising from 5,500 reported cases in 2011 to 28,100 cases in 2018.
The problem, likely related to an increasing homeless population who can’t afford San Francisco’s exorbitant rental prices and have limited access to public restrooms, is so bad that the city commissioned a “Poop Patrol” in the summer of 2018 to wipe down some of the poorer, more suspect neighborhoods.
While the upstanding members of the Poop Patrol are almost certainly doing a fine job, it’s probably safe to say that human fecal clean-up is an industry ripe for disruption.
We look forward to the inevitable Silicon Valley start-up and for the media to hail it as “Uber, but for poop.”
Endgame for arachnophobia
We think Tony Stark would like this creative solution for spider and ant phobias. Comic book movies have now infiltrated every aspect of culture, including serious scientific research. And let’s be honest – more than one scientist has been inspired to go into their fields by Bruce Banner, Stark, or maybe even Doctor Octopus (no judgment).
A group of (possibly mad) scientists has tested exposure therapy for spider and ant phobias in people by showing participants the Spider-Man and Ant-Man movies. While the viewing material may not be totally scientifically accurate, researchers found that watching seven seconds of Spider-Man 2 or Ant-Man reduced spider and ant phobia by 20%.
The participants were specifically exposed to ants and spiders in the context of the movies, so surprisingly the phobia reduction had nothing to do with Tobey Maguire or Paul Rudd.
Old poop, new discovery
Here at LOTME, we like us some good bathroom humor. And don’t worry, we won’t ever change. In this week’s edition of the Wonderful World of Poop, we take you to Texas, 1,500 years ago. The sky was bigger, the air was fresher, and the humans of the Lower Pecos region were as hardcore as you can get. A recent re-examination of coprolite samples taken from the region found one interesting chunk of poop-rock that contained an entire rattlesnake.
Now, the presence of snake bits in early human poo is not that crazy; people ate (and still eat) snakes. The appearance of a centimeter-long fang, scales, and bones, however, did take the researchers by surprise. Why would someone eat a snake? Was it an ancient way to inoculate against snake venom? Or perhaps crunchy snake fangs were the world’s earliest version of a Cheeto?
In fact, researchers hypothesized that this dietary behavior was not normal for the people of the Lower Pecos, and most likely was more ceremonial. You know, the casual eating-a-full-snake ceremony.
Will Texans embrace this ceremony of their past and start chomping on rattlers? Who’s to say? All we know is that poop is the gift that keeps on giving.
A new way to soil yourself
If you’re reading this, we can say with some certainty that you managed to survive another Tax Day. Congratulations! But there’s still Benjamin Franklin’s other ultimate certainty of life. You know … the big sleep, the last roundup, assume room temperature, buy the farm, shuffle off this mortal coil, give up the ghost, and so on.
What are you going to do about that?
A big question, for sure, so let’s just focus on the earthly remains. A company called Recompose has a new alternative to burial and cremation, something they’ve dubbed “natural organic reduction” and others have described as “human composting” or “accelerated decomposition.” In a pilot project last year at Washington State University in Pullman, the Recompose process transformed the bodies of six donors to soil in 4-7 weeks, AP reported.
The company says that natural organic reduction is much more environmentally friendly than current practices, creating a cubic yard of soil per person, and that “friends and family are welcome to take some (or all) home to grow a tree or a garden.”
A garden sounds nice, or maybe something indoors. Just think of the potted plant possibilities: daisies (to push up), a Venus flytrap (the organic reduction continues), some poison ivy (a gift for people you don’t like), or maybe roses. Who wouldn’t want to come out of death smelling like a rose?
San Francisco vs. illegal dumping
Maybe you’re not quite ready to commit to using human remains as compost to fertilize your garden. Perhaps you want to start off only using human poop as fertilizer, see how that goes before sprinkling Grandma all over your tulips.
Well, if you’re looking for a sweet deal, we’re certain San Francisco can work something out with you because, in the past 7 years, incidence of human feces in public places within the city has quintupled, rising from 5,500 reported cases in 2011 to 28,100 cases in 2018.
The problem, likely related to an increasing homeless population who can’t afford San Francisco’s exorbitant rental prices and have limited access to public restrooms, is so bad that the city commissioned a “Poop Patrol” in the summer of 2018 to wipe down some of the poorer, more suspect neighborhoods.
While the upstanding members of the Poop Patrol are almost certainly doing a fine job, it’s probably safe to say that human fecal clean-up is an industry ripe for disruption.
We look forward to the inevitable Silicon Valley start-up and for the media to hail it as “Uber, but for poop.”
Endgame for arachnophobia
We think Tony Stark would like this creative solution for spider and ant phobias. Comic book movies have now infiltrated every aspect of culture, including serious scientific research. And let’s be honest – more than one scientist has been inspired to go into their fields by Bruce Banner, Stark, or maybe even Doctor Octopus (no judgment).
A group of (possibly mad) scientists has tested exposure therapy for spider and ant phobias in people by showing participants the Spider-Man and Ant-Man movies. While the viewing material may not be totally scientifically accurate, researchers found that watching seven seconds of Spider-Man 2 or Ant-Man reduced spider and ant phobia by 20%.
The participants were specifically exposed to ants and spiders in the context of the movies, so surprisingly the phobia reduction had nothing to do with Tobey Maguire or Paul Rudd.
Old poop, new discovery
Here at LOTME, we like us some good bathroom humor. And don’t worry, we won’t ever change. In this week’s edition of the Wonderful World of Poop, we take you to Texas, 1,500 years ago. The sky was bigger, the air was fresher, and the humans of the Lower Pecos region were as hardcore as you can get. A recent re-examination of coprolite samples taken from the region found one interesting chunk of poop-rock that contained an entire rattlesnake.
Now, the presence of snake bits in early human poo is not that crazy; people ate (and still eat) snakes. The appearance of a centimeter-long fang, scales, and bones, however, did take the researchers by surprise. Why would someone eat a snake? Was it an ancient way to inoculate against snake venom? Or perhaps crunchy snake fangs were the world’s earliest version of a Cheeto?
In fact, researchers hypothesized that this dietary behavior was not normal for the people of the Lower Pecos, and most likely was more ceremonial. You know, the casual eating-a-full-snake ceremony.
Will Texans embrace this ceremony of their past and start chomping on rattlers? Who’s to say? All we know is that poop is the gift that keeps on giving.
A new way to soil yourself
If you’re reading this, we can say with some certainty that you managed to survive another Tax Day. Congratulations! But there’s still Benjamin Franklin’s other ultimate certainty of life. You know … the big sleep, the last roundup, assume room temperature, buy the farm, shuffle off this mortal coil, give up the ghost, and so on.
What are you going to do about that?
A big question, for sure, so let’s just focus on the earthly remains. A company called Recompose has a new alternative to burial and cremation, something they’ve dubbed “natural organic reduction” and others have described as “human composting” or “accelerated decomposition.” In a pilot project last year at Washington State University in Pullman, the Recompose process transformed the bodies of six donors to soil in 4-7 weeks, AP reported.
The company says that natural organic reduction is much more environmentally friendly than current practices, creating a cubic yard of soil per person, and that “friends and family are welcome to take some (or all) home to grow a tree or a garden.”
A garden sounds nice, or maybe something indoors. Just think of the potted plant possibilities: daisies (to push up), a Venus flytrap (the organic reduction continues), some poison ivy (a gift for people you don’t like), or maybe roses. Who wouldn’t want to come out of death smelling like a rose?
San Francisco vs. illegal dumping
Maybe you’re not quite ready to commit to using human remains as compost to fertilize your garden. Perhaps you want to start off only using human poop as fertilizer, see how that goes before sprinkling Grandma all over your tulips.
Well, if you’re looking for a sweet deal, we’re certain San Francisco can work something out with you because, in the past 7 years, incidence of human feces in public places within the city has quintupled, rising from 5,500 reported cases in 2011 to 28,100 cases in 2018.
The problem, likely related to an increasing homeless population who can’t afford San Francisco’s exorbitant rental prices and have limited access to public restrooms, is so bad that the city commissioned a “Poop Patrol” in the summer of 2018 to wipe down some of the poorer, more suspect neighborhoods.
While the upstanding members of the Poop Patrol are almost certainly doing a fine job, it’s probably safe to say that human fecal clean-up is an industry ripe for disruption.
We look forward to the inevitable Silicon Valley start-up and for the media to hail it as “Uber, but for poop.”
Measuring hydroxychloroquine blood levels could inform safe, optimal dosing
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
SAN FRANCISCO – , according to an investigation of the Hopkins Lupus Cohort, an ongoing longitudinal study of lupus patients in the Baltimore area.
As innocuous as the assertions seem, they are anything but. They directly contradict a 2014 investigation from Kaiser Permanente that put the retinopathy risk after 20 years at almost 40%; that finding led directly to an American Academy of Ophthalmology recommendation to reduce the maximum hydroxychloroquine dose from 6.5 mg/kg per day ideal weight to 5 mg/kg real weight, where it remains to this day.
Meanwhile, very few rheumatologists have access to hydroxychloroquine (HCQ) blood levels because most commercial labs don’t offer them. Plasma testing is widely available, but it’s nowhere near as good, according to Michelle Petri, MD, a rheumatology professor at Johns Hopkins University, Baltimore; director of the Hopkins Lupus Cohort; and a respected authority on lupus management.
“The Kaiser Permanente study was very worrisome,” she said. “I remember that I thought it didn’t fit my practice at all; I don’t see 40%. It made me even more concerned when the ophthalmologists” reduced the dose, “because hydroxychloroquine is the most important medicine I have for my lupus patients; it is the only one that improves survival. We don’t want to scare our patients into thinking that 40% of them are going to have retinopathy.”
Dueling studies
Dr. Petri’s concerns led her and her team to launch their own investigation; they followed 537 Baltimore cohort members on HCQ as they went through eye exams by Hopkins retinopathy specialists, often with optical coherence tomography (OCT). With a sensitivity of 93% and specificity of 84%, OCT is the best screening method available.
“We found that the risk of retinopathy is not nearly as high as Kaiser Permanente found,” just 11.46% (11/96) with 16-20 years of use, and 8% (6/75) with 21 or more years. On average, “the risk is probably about 10% after 16 or more years, not 40%,” Dr. Petri said at an international congress on systemic lupus erythematosus.
Patients with “possible” retinopathy were not included in the analysis.
When asked for comment, Ronald Melles, MD, a Kaiser ophthalmologist in Redwood City, Calif.; one of the two authors on the Kaiser study; and an author on the subsequent AAO recommendations, stood by his work.
“A rate of 12% retinopathy after 16 years of use ... seems right in line with what we found.” However, “the fact that the rate went down to 8%” after 20 years does not make sense; “the longer you are on the medicine, the more likely you would be to develop the toxicity,” he said.
Maybe the fluctuation had to do with the fact that there were only 75 patients in the Hopkins study on HCQ past 20 years, whereas “we looked at 2,361 patients, and 238 were on the medication for” 20 years or more. Patients over 5.0 mg/kg per day had a 5.67-fold higher risk”of retinopathy, he said (univariate analysis, P less than .001).
Dr. Petri wasn’t buying it. The across the board recommendation was made “without any recognition that if you reduce the dose, you reduce the benefit,” she said.
A new referee: blood levels
Dr. Petri and her team also found that HCQ blood levels correlated with retinopathy, and it was a direct relationship. Patients in the highest maximum tertile (1,753-6,281 ng/mL) had a retinopathy rate of 6.7%, a good deal higher than patients in lower tertiles. It was the same story with the highest mean tertile (1,117-3,513 ng/mL). Retinopathy in that group occurred in 7.9% versus 3.7% or less in lower tertiles. The findings were statistically significant.
Patients in the third tertile “are at the greatest risk, so I reduce their dose,” but “I do not want to reduce the dose across the board” to 5 mg/kg per day; that’s overreach. The tertile approach, if it pans out, might be a better way, she said.
The problem with plasma levels is that HCQ binds to red blood cells, so plasma levels are artificially low and do not indicate the true HCQ load. For now, just one company in the United States offers HCQ blood levels: Exagen.
“We have to get the big companies to start offering” this, and “I want rheumatologists to adopt it. I am lucky at Hopkins [because] we have our own homegrown blood level assay,” she said.
Dr. Melles agreed that tracking blood level makes sense, “but the literature I am aware of has not been able to closely correlate either lupus disease activity or retinal toxicity with blood levels. Also, we have seen some patients at lower doses develop toxicity and other patients on higher doses without any detectable changes.”
Still, “we would like to see [this] studied more, perhaps with newer analytic methods,” said his coauthor on the Kaiser study, and also the lead author on the AAO guidelines, Michael Marmor, MD, professor emeritus of ophthalmology at Stanford (Calif.) University.
In the end, on the same team
Dr. Petri said there is interest among some of her fellow members of the American College of Rheumatology to work with AAO to revise the guidelines. “Until then,” she said, “I want the ophthalmologists to withdraw” them.
She’s worried about undertreatment and believes that the previous AAO guideline, up to 6.5 mg/kg per day ideal weight, was fine, “with some understanding that there are high-risk groups, such as the elderly and people with renal impairment, where the dose should be reduced.”
“No matter how obese a patient is, I cap it at 400 mg/day,” she said, and, with the luxury of HCQ blood level testing, “no matter the weight, if the person is in the upper tertile, I reduce the dose.”
Dr. Marmor agreed that “if rheumatologists prescribe 5 mg/kg real weight and do not stress compliance, some patients may indeed be underdosed.”
“However, that is a fault of the doctor and patient relationship,” he said, “not the guideline; we do not feel it ethical to prescribe higher doses which could increase toxicity in reliable patients ... just because some patients might be unreliable.”
Overall, “I have not heard complaints from rheumatologists in our area, who try hard to follow the current recommendations. ... any doctor can use the dose he or she feels is necessary for a patient. Several recent reports [also] suggest the incidence of toxicity is falling now with usage of AAO guidelines, [and] I am not aware of any data” showing that management has become less effective, he said.
In the meantime, “I assure you that AAO wants ... to serve both specialties, and will change the guidelines when there is new, defensible data,” he added.
The Hopkins team found that the risk of HCQ retinopathy was highest in men and white patients, as well as older people. Body mass index and hypertension also predicted retina issues.
“As screening tests are frequently abnormal due to causes other than HCQ ... stopping [it] based on an abnormal test without confirmation from a retinopathy expert could needlessly deprive an SLE patient of an important medication,” they said.
The Hopkins Lupus Cohort is funded by the National Institutes of Health. The physicians didn’t have any relevant disclosures.
SOURCES: Petri M et al. Lupus Sci Med. 2019;6(suppl 1). Abstracts 15 and 16.
REPORTING FROM LUPUS 2019
In chronic pain, catastrophizing contributes to disrupted brain circuitry
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
MILWAUKEE – When a patient with acute pain tumbles into a chronic pain state, many factors are at play, according to the widely accepted biopsychosocial theory of pain. Emotional, cognitive, and environmental components all contribute to the persistent and recalcitrant symptoms chronic pain patients experience.
Now, modern neuroimaging techniques show how for some, pain signals hijack the brain’s regulatory networks, allowing rumination and catastrophizing to intrude on the exteroception that’s critical to how humans interact with one another and the world. Interrupting catastrophizing with nonpharmacologic techniques yields measurable improvements – and there’s promise that a single treatment session can make a lasting difference.
“Psychosocial phenotypes, such as catastrophizing, are part of a complex biopsychosocial web of contributors to chronic pain. ,” said Robert R. Edwards, PhD, a psychologist at Brigham and Women’s Hospital/Harvard Medical School (Boston) Pain Management Center. Dr. Edwards moderated a session focused on catastrophizing at the scientific meeting of the American Pain Society.
Through magnetic resonance imaging techniques that measure functional connectivity, researchers can now see how nodes in the brain form connected networks that are differentially activated.
For example, the brain’s salience network (SLN) responds to stimuli that merit attention, such as evoked or clinical pain, Vitaly Napadow, PhD, said during his presentation. Key nodes in the SLN include the anterior cingulate cortex, the anterior insula, and the anterior temporoparietal junction. One function of the salience network, he said, is to regulate switching between the default mode network (DMN) – an interoceptive network – and the central executive network, usually active in exteroceptive tasks.
“The default mode network has been found to play an important role in pain processing,” Dr. Napadow said. These brain regions are more active in self-referential cognition – thinking about oneself – than when performing external tasks, he said. Consistently, studies have found decreased DMN deactivation in patients with chronic pain; essentially, the constant low hum of pain-focused DMN activity never turns off in a chronic pain state.
For patients with chronic pain, high levels of catastrophizing mean greater impact on functional brain connectivity, said Dr. Napadow, director of the Center for Integrative Pain NeuroImaging at the Martino Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School, Boston.
Looking at patients with chronic low back pain, he and his research team looked for connections between the DMN and the insula, which has a central role in pain processing. This connectivity was increased only in patients with high catastrophizing scores, said Dr. Napadow, with increased DMN-insula connectivity associated with increased pain scores only for this subgroup (Pain. 2019 Mar 4. doi: 10.1097/j.pain.0000000000001541).
“The model that we’re moving toward is that chronic pain leads to a blurring in the canonical network” of brain connectivity, Dr. Napadow said. “The speculation here is that the DMN-SLN linkage could be a sort of neural substrate for a common perception that chronic pain patients have – that their pain becomes part of who they are. Their interoceptive state becomes linked to the pain they are feeling: They are their pain.”
Where to turn with this information, which has large clinical implications? “Catastrophizing is a consistent risk factor for poor pain treatment outcomes, especially when we’re talking about pharmacologic treatments,” Dr. Edwards said. Also, chronic pain patients with the highest catastrophizing scores have the most opioid-related side effects, he said.
“Cognitive-behavioral therapy is potentially the most effective at reducing this risk factor,” said Dr. Edwards, noting that long-term effects were seen at 6 and 12 months post treatment. “These are significant, moderate-sized effects; there is some evidence that effects are largest in those with the highest baseline pain catastrophizing scores.”
“CBT is considered the gold standard, mainly because it’s the best studied” among treatment modalities, psychologist Beth Darnall, PhD, pointed out in her presentation. There’s evidence that other nonpharmacologic interventions can reduce catastrophizing: Psychology-informed yoga practices, physical therapy, and certain medical devices, such as high-frequency transcutaneous electric nerve stimulation units, may all have efficacy against catastrophizing and the downward spiral of chronic pain.
Still, a randomized controlled trial of CBT for pain in patients with fibromyalgia showed that the benefit, measured as reduction in pain interference with daily functioning, was almost twice as high in the high-catastrophizing group, “suggesting the potential utility of this method for patients at greatest risk,” said Dr. Edwards.
“We see a specific pattern of alterations in chronic pain similar to that seen in anxiety disorder; this suggests that some individuals are primed for the experience of pain,” said Dr. Darnall, clinical professor of anesthesiology, perioperative medicine, and pain medicine at Stanford (Calif.) University. “We are not born with the understanding of how to modulate pain and the distress it causes us.”
When she talks to patients, Dr. Darnall said: “I describe pain as being our ‘harm alarm.’ ... I like to describe it to people that ‘you have a very protective nervous system.’ ”
Dr. Darnall and her colleagues reported success with a pilot study of a single 2.5-hour-long session that addressed pain catastrophizing. From a baseline score of 26.1 on the Pain Catastrophizing Scale to a score of 13.8 at week 4, the 57 participants saw a significant decrease in mean scores on the scale (d [effect size] = 1.15).
On the strength of these early findings, Dr. Darnall and her collaborators are embarking on a randomized controlled trial ; the 3-arm comparative effectiveness study will compare a single-session intervention against 8 weeks of CBT or education-only classes for individuals with catastrophizing and chronic pain. The trial is structured to test the hypothesis that the single-session intervention will be noninferior to the full 8 weeks of CBT, Dr. Darnall said.
Building on the importance of avoiding stigmatizing and pejorative terms when talking about pain and catastrophizing, Dr. Darnall said she’s moved away from using the term “catastrophizing” in patient interactions. The one-session intervention is called “Empowered Relief – Train Your Brain Away from Pain.”
There’s a practical promise to a single-session class: Dr. Darnall has taught up to 85 patients at once, she said, adding, “This is a low-cost and scalable intervention.”
Dr. Edwards and Dr. Napadow reported funding from the National Institutes of Health, and they reported no conflicts of interest. Dr. Darnall reported funding from the NIH and the Patient-Centered Outcomes Research Institute. She serves on the scientific advisory board of Axial Healthcare and has several commercial publications about pain.
REPORTING FROM APS 2019
High-dose MTX-based chemo is well tolerated in older PCNSL patients
GLASGOW – Most older patients with primary central nervous system lymphoma (PCNSL) can tolerate high-dose methotrexate-based chemotherapy and achieve similar outcomes as younger and fitter patients, according to a retrospective analysis of 244 patients in the United Kingdom.
For older patients – at least 65 years old – who received methotrexate-based regimens, treatment-related mortality was 6.8%, which is comparable with rates seen in trials involving younger patients, reported lead author Edward Poynton, MD, of University College Hospital in London.
Specifically, Dr. Poynton cited the phase 2 IELSG32 trial, which had a treatment-related mortality rate of 6% among patients up to age 70 years. These patients were treated with the established protocol for younger patients: chemotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix) followed by autologous stem cell transplant or whole-brain radiotherapy.
Introducing Dr. Poynton’s presentation at the annual meeting of the British Society for Haematology, Simon Rule, MD, of the University of Plymouth (England), added historical context to the new findings.
“When I started in hematology ... [PCNSL] was a universally fatal disease, pretty much,” Dr. Rule said. “And then we had methotrexate, and it worked occasionally. And then we had a randomized trial, which was randomization of methotrexate plus or minus high-dose cytarabine, showing benefit.”
This combination became the benchmark against which subsequent randomized trials were measured; however, such high-intensity regimens have raised concerns about safety and efficacy in older patients, Dr. Rule said, noting that the present study serves to inform clinicians about real-world outcomes in this population.
The retrospective analysis reviewed 244 patients who were aged at least 65 years when histologically diagnosed with PCNSL at 14 U.K. tertiary centers between 2012 and 2017. All patients received first-line care of any kind, ranging from best supportive care to clinical trial therapy. Patients were grouped into three treatment cohorts divided by level of frailty. Analysis showed that these divisions correlated with age, renal function, Eastern Cooperative Oncology Group performance status, and treatment intensity.
The frail group received palliative treatment consisting of whole-brain radiotherapy, an oral alkylator, or best supportive care. The less-fit group received methotrexate in combination with rituximab, an oral alkylator, or both. The fit group was most intensively treated, receiving high-dose methotrexate and cytarabine – with or without rituximab – or MATRix.
The primary objective was overall response rate, while the secondary objectives were median overall survival and progression-free survival.
The analysis showed that 79% of patients (n = 193) received methotrexate-based therapy of some kind, with 61% receiving three or more cycles of therapy and 30% requiring dose reductions. The overall response rate was 63%.
Dr. Poynton noted that about two-thirds of patients who achieved a partial response in early assessment went on to achieve a complete response. Patients in the fit group more often responded than those who were less fit (87% vs. 65%; P = .01) and more often received consolidation therapy (42% vs. 23%; P = .01).
Fitness level was also associated with median overall survival, which was longest in the fit group at 42 months. The other two groups had dramatically shorter survival times: 8 months in the less-fit group and just 2 months in the frail group.
A closer look at the data revealed some patterns, Dr. Poynton said.
“What we see is that age at diagnosis is significantly correlated with progression-free survival but not with overall survival,” he said, noting that, in contrast, performance status was associated with both survival measures.
Methotrexate dose also impacted both survival measures. Patients who received 75% or more of their induction dose over the course of treatment had better median overall survival and progression-free survival than those who received less than 75%. Similarly, consolidation therapy improved both survival measures.
Patients aged older than 70 years who received intensive chemotherapy had a treatment-related mortality rate of 4.8%, which is lower than the overall treatment-related mortality, Dr. Poynton reported.
Considering the correlation between methotrexate dose and survival, Dr. Poynton suggested that “dose reductions should be carefully considered.”
He also noted that patients in the fit cohort who received intensive chemotherapy had comparable outcomes with younger patients in prospective trials, and yet 44% of patients older than 65 years in the real world who received high-dose methotrexate with cytarabine would have been ineligible for the IELSG32 trial.
“We’ve been able to identify this cohort of patients retrospectively,” Dr. Poynton said. “They definitely exist, and I think we need to work harder at how are going to identify these patients prospectively in the future, so we know which of our patients who are older can benefit from intensive chemotherapy and which patients won’t.”
Dr. Poynton reported having no relevant financial disclosures. His coinvestigators reported relationships with AbbVie, Merck, Takeda, Jazz Pharmaceuticals, and others.
GLASGOW – Most older patients with primary central nervous system lymphoma (PCNSL) can tolerate high-dose methotrexate-based chemotherapy and achieve similar outcomes as younger and fitter patients, according to a retrospective analysis of 244 patients in the United Kingdom.
For older patients – at least 65 years old – who received methotrexate-based regimens, treatment-related mortality was 6.8%, which is comparable with rates seen in trials involving younger patients, reported lead author Edward Poynton, MD, of University College Hospital in London.
Specifically, Dr. Poynton cited the phase 2 IELSG32 trial, which had a treatment-related mortality rate of 6% among patients up to age 70 years. These patients were treated with the established protocol for younger patients: chemotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix) followed by autologous stem cell transplant or whole-brain radiotherapy.
Introducing Dr. Poynton’s presentation at the annual meeting of the British Society for Haematology, Simon Rule, MD, of the University of Plymouth (England), added historical context to the new findings.
“When I started in hematology ... [PCNSL] was a universally fatal disease, pretty much,” Dr. Rule said. “And then we had methotrexate, and it worked occasionally. And then we had a randomized trial, which was randomization of methotrexate plus or minus high-dose cytarabine, showing benefit.”
This combination became the benchmark against which subsequent randomized trials were measured; however, such high-intensity regimens have raised concerns about safety and efficacy in older patients, Dr. Rule said, noting that the present study serves to inform clinicians about real-world outcomes in this population.
The retrospective analysis reviewed 244 patients who were aged at least 65 years when histologically diagnosed with PCNSL at 14 U.K. tertiary centers between 2012 and 2017. All patients received first-line care of any kind, ranging from best supportive care to clinical trial therapy. Patients were grouped into three treatment cohorts divided by level of frailty. Analysis showed that these divisions correlated with age, renal function, Eastern Cooperative Oncology Group performance status, and treatment intensity.
The frail group received palliative treatment consisting of whole-brain radiotherapy, an oral alkylator, or best supportive care. The less-fit group received methotrexate in combination with rituximab, an oral alkylator, or both. The fit group was most intensively treated, receiving high-dose methotrexate and cytarabine – with or without rituximab – or MATRix.
The primary objective was overall response rate, while the secondary objectives were median overall survival and progression-free survival.
The analysis showed that 79% of patients (n = 193) received methotrexate-based therapy of some kind, with 61% receiving three or more cycles of therapy and 30% requiring dose reductions. The overall response rate was 63%.
Dr. Poynton noted that about two-thirds of patients who achieved a partial response in early assessment went on to achieve a complete response. Patients in the fit group more often responded than those who were less fit (87% vs. 65%; P = .01) and more often received consolidation therapy (42% vs. 23%; P = .01).
Fitness level was also associated with median overall survival, which was longest in the fit group at 42 months. The other two groups had dramatically shorter survival times: 8 months in the less-fit group and just 2 months in the frail group.
A closer look at the data revealed some patterns, Dr. Poynton said.
“What we see is that age at diagnosis is significantly correlated with progression-free survival but not with overall survival,” he said, noting that, in contrast, performance status was associated with both survival measures.
Methotrexate dose also impacted both survival measures. Patients who received 75% or more of their induction dose over the course of treatment had better median overall survival and progression-free survival than those who received less than 75%. Similarly, consolidation therapy improved both survival measures.
Patients aged older than 70 years who received intensive chemotherapy had a treatment-related mortality rate of 4.8%, which is lower than the overall treatment-related mortality, Dr. Poynton reported.
Considering the correlation between methotrexate dose and survival, Dr. Poynton suggested that “dose reductions should be carefully considered.”
He also noted that patients in the fit cohort who received intensive chemotherapy had comparable outcomes with younger patients in prospective trials, and yet 44% of patients older than 65 years in the real world who received high-dose methotrexate with cytarabine would have been ineligible for the IELSG32 trial.
“We’ve been able to identify this cohort of patients retrospectively,” Dr. Poynton said. “They definitely exist, and I think we need to work harder at how are going to identify these patients prospectively in the future, so we know which of our patients who are older can benefit from intensive chemotherapy and which patients won’t.”
Dr. Poynton reported having no relevant financial disclosures. His coinvestigators reported relationships with AbbVie, Merck, Takeda, Jazz Pharmaceuticals, and others.
GLASGOW – Most older patients with primary central nervous system lymphoma (PCNSL) can tolerate high-dose methotrexate-based chemotherapy and achieve similar outcomes as younger and fitter patients, according to a retrospective analysis of 244 patients in the United Kingdom.
For older patients – at least 65 years old – who received methotrexate-based regimens, treatment-related mortality was 6.8%, which is comparable with rates seen in trials involving younger patients, reported lead author Edward Poynton, MD, of University College Hospital in London.
Specifically, Dr. Poynton cited the phase 2 IELSG32 trial, which had a treatment-related mortality rate of 6% among patients up to age 70 years. These patients were treated with the established protocol for younger patients: chemotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix) followed by autologous stem cell transplant or whole-brain radiotherapy.
Introducing Dr. Poynton’s presentation at the annual meeting of the British Society for Haematology, Simon Rule, MD, of the University of Plymouth (England), added historical context to the new findings.
“When I started in hematology ... [PCNSL] was a universally fatal disease, pretty much,” Dr. Rule said. “And then we had methotrexate, and it worked occasionally. And then we had a randomized trial, which was randomization of methotrexate plus or minus high-dose cytarabine, showing benefit.”
This combination became the benchmark against which subsequent randomized trials were measured; however, such high-intensity regimens have raised concerns about safety and efficacy in older patients, Dr. Rule said, noting that the present study serves to inform clinicians about real-world outcomes in this population.
The retrospective analysis reviewed 244 patients who were aged at least 65 years when histologically diagnosed with PCNSL at 14 U.K. tertiary centers between 2012 and 2017. All patients received first-line care of any kind, ranging from best supportive care to clinical trial therapy. Patients were grouped into three treatment cohorts divided by level of frailty. Analysis showed that these divisions correlated with age, renal function, Eastern Cooperative Oncology Group performance status, and treatment intensity.
The frail group received palliative treatment consisting of whole-brain radiotherapy, an oral alkylator, or best supportive care. The less-fit group received methotrexate in combination with rituximab, an oral alkylator, or both. The fit group was most intensively treated, receiving high-dose methotrexate and cytarabine – with or without rituximab – or MATRix.
The primary objective was overall response rate, while the secondary objectives were median overall survival and progression-free survival.
The analysis showed that 79% of patients (n = 193) received methotrexate-based therapy of some kind, with 61% receiving three or more cycles of therapy and 30% requiring dose reductions. The overall response rate was 63%.
Dr. Poynton noted that about two-thirds of patients who achieved a partial response in early assessment went on to achieve a complete response. Patients in the fit group more often responded than those who were less fit (87% vs. 65%; P = .01) and more often received consolidation therapy (42% vs. 23%; P = .01).
Fitness level was also associated with median overall survival, which was longest in the fit group at 42 months. The other two groups had dramatically shorter survival times: 8 months in the less-fit group and just 2 months in the frail group.
A closer look at the data revealed some patterns, Dr. Poynton said.
“What we see is that age at diagnosis is significantly correlated with progression-free survival but not with overall survival,” he said, noting that, in contrast, performance status was associated with both survival measures.
Methotrexate dose also impacted both survival measures. Patients who received 75% or more of their induction dose over the course of treatment had better median overall survival and progression-free survival than those who received less than 75%. Similarly, consolidation therapy improved both survival measures.
Patients aged older than 70 years who received intensive chemotherapy had a treatment-related mortality rate of 4.8%, which is lower than the overall treatment-related mortality, Dr. Poynton reported.
Considering the correlation between methotrexate dose and survival, Dr. Poynton suggested that “dose reductions should be carefully considered.”
He also noted that patients in the fit cohort who received intensive chemotherapy had comparable outcomes with younger patients in prospective trials, and yet 44% of patients older than 65 years in the real world who received high-dose methotrexate with cytarabine would have been ineligible for the IELSG32 trial.
“We’ve been able to identify this cohort of patients retrospectively,” Dr. Poynton said. “They definitely exist, and I think we need to work harder at how are going to identify these patients prospectively in the future, so we know which of our patients who are older can benefit from intensive chemotherapy and which patients won’t.”
Dr. Poynton reported having no relevant financial disclosures. His coinvestigators reported relationships with AbbVie, Merck, Takeda, Jazz Pharmaceuticals, and others.
REPORTING FROM BSH 2019
CD40 ligand–binding protein safely lowered RA disease activity
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
A nonantibody scaffold protein that targets the CD40 ligand appears to dampen down autoimmune responses without the thromboembolic complications seen in trials of monoclonal antibodies against the CD40 ligand.
In a paper published in Science Translational Medicine, researchers presented the results of a phase 1a study in 59 healthy volunteers and phase 1b proof-of-concept study in 57 individuals with rheumatoid arthritis. Participants received either varying dosages of CD40 ligand (CD40L)–binding protein VIB4920 – a single dose in the healthy volunteers and seven doses in the phase 1b study – or placebo.
Jodi L. Karnell, PhD, of Viela Bio in Gaithersburg, Md., and coauthors, wrote that the CD40/CD40L pathway is known to play a key role in humoral immune responses and in the pathogenesis of several autoimmune diseases.
However, previous clinical trials of compounds targeting CD40L were stopped early because of an increased risk of adverse thromboembolic events related to platelet aggregation, despite showing potential benefits in lupus and immune thrombocytopenic purpura.
Preclinical studies of VIB4920 found that it blocked the expansion of CD40L-dependent human B cells without showing any signs of platelet aggregation. The authors said the platelet aggregation had been linked to a particular region of anti-CD40L monoclonal antibodies, but VIB4920 was engineered using a protein scaffold that did not contain that region.
In healthy volunteers, researchers saw a dose-dependent suppression of antibody production and reductions in B-cell proliferation, in recall response to a T cell–dependent antigen.
In individuals with rheumatoid arthritis, more than half of those treated with the two highest dosages of VIB4920 achieved low disease activity state or clinical remission by 12 weeks. Overall, there was also a significant decrease in disease activity, and dose-dependent decreases in rheumatoid factor autoantibodies and Vectra DA biomarker score, which is a composite of twelve rheumatoid arthritis–related biomarkers.
“The consistency of improvement across a variety of clinical and laboratory outcome measures further supports the potential clinical efficacy of VIB4920,” the authors wrote.
Researchers saw a similar rate of adverse events in the placebo and treatment arms of the study.
“VIB4920 represents an alternative to monoclonal antibody–based targeting of CD40L, which does not induce platelet aggregation in vitro and demonstrates a favorable safety profile in early clinical evaluation.”
The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or of Viela Bio.
SOURCE: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: Treatment with higher doses of VIB4920 is associated with low disease activity or remission in 50% of rheumatoid arthritis patients.
Study details: Phase 1a and 1b study in 59 healthy individuals and 57 people with rheumatoid arthritis.
Disclosures: The study was funded by MedImmune. All but one author were employees of MedImmune/AstraZeneca or Viela Bio.
Source: Karnell J et al. Sci Transl Med. 2019 April 24. doi: 10.1126/scitranslmed.aar6584.
Report: Part B funds stable, hospital trust running out
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”
Medicare’s Part B trust fund is well funded and stable enough to pay physicians through the foreseeable future, according to an annual report by the Medicare Board of Trustees.
The Supplemental Medical Insurance (SMI) trust fund, which covers Medicare Part B and D, contained $104 billion in assets at the end of 2018 and is expected to be adequately financed in all years because of continued premium and general revenue income, according to the report, which was released April 22.
However, the Hospital Insurance (HI) trust fund, which funds Medicare Part A, is expected to run out by 2026, the same projection as last year, the trustees reported.
In addition, trustees said that total Medicare costs – including both HI and SMI expenditures – will grow from about 4% of gross domestic product (GDP) in 2018 to about 6% of GDP by 2038 and then increase gradually thereafter to about 6.5% of GDP by 2093.
The faster rate of growth in Medicare spending, compared with GDP growth, is attributable to a growing number of Medicare patients and increased volume and intensity of health care services, according to the report. Alone, SMI costs are projected to grow steadily from 2% of GDP in 2018 to about 4% of GDP in 2038 because of the aging population and rising health care costs.
The report delivers a dose of reality, reminding the country that the program’s main trust for hospital services can pay full benefits for only 7 more years, Seema Verma, administrator of the Centers for Medicare & Medicaid Services said.
“The Trump administration is working hard to protect and strengthen Medicare and lower costs while improving quality in order to protect the program for future generations of seniors who have paid into the program their whole lives,” Ms. Verma said in a statement. “If we do not take the fiscal crisis in Medicare seriously, we will jeopardize access to health care for millions of seniors.”
Department of Health & Human Services Secretary Alex M. Azar II said the annual report provides a sobering reminder that more work is necessary to support current and future generations of seniors.
“Instead of trying to expand Medicare into a universal entitlement that even covers wealthy Americans of working age, as some have proposed, we need to fulfill Medicare’s promise to our seniors,” Mr. Azar said in a statement, referring to proposals to expand government health care by some Democrats.
The trustees report notes that Medicare has introduced a number of initiatives to strengthen and protect the program and finalized a number of rules that advance a patient-driven health care system through competition.
“In particular, CMS is strengthening Medicare through increasing choice in Medicare Advantage and adding supplemental benefits to the program, offering more care options for people with diabetes, providing new telehealth services, and lowering prescription drug costs for seniors,” the agency stated in a press release. “CMS is also continuing work to advance policies to increase price transparency and help beneficiaries compare costs across different providers.”