User login
PACIFIC: Patient-reported outcomes unaffected by PD-L1 expression
GENEVA – Level of programmed death-ligand 1 (PD-L1) expression does not impact patient-reported outcomes (PROs) among those receiving durvalumab for stage III non–small cell lung cancer (NSCLC), according to a retrospective analysis of the phase III PACIFIC trial.
The findings support durvalumab for all comers regardless of PD-L1 expression, reported lead author Marina Garassino, MD, of Fondazione IRCCS – Instituto Nazionale dei Tumori in Milan, who presented findings at the European Lung Cancer Congress.
The PACIFIC trial involved 713 patients with stage III NSCLC who did not progress after platinum-based concurrent chemoradiotherapy, demonstrating both improved progression-free and overall survival. Patients were randomized 2:1 to receive either durvalumab (10 mg/kg) or placebo IV every 2 weeks for up to 1 year. Out of 713 patients involved in the trial, 63% had PD-L1 tumor expression level data available for the present analysis, allowing for subgrouping into five categories: expression level of at least 25%, less than 25%, at least 1%, less than 1%, or unknown. The investigators compared PROs from these cohorts using the European Organisation for Research and Treatment of Cancer core quality of life questionnaire and lung cancer module (EORTC QLQ-C30 and -LC13). With scores ranging from 1 to 100 points, clinically meaningful differences were defined by score changes exceeding 10 points. Changes during treatment, from baseline to week 48, were analyzed by a mixed model for repeated measures, overall responses for improvement rates by logistic regression, and hazard ratios for time to deterioration (TTD) by a stratified Cox proportional-hazards model.
The investigators found that most PROs remained consistent over time, without clinically meaningful variations between PD-L1 expression levels. However, as with the entire PACIFIC treatment population, patients in the present analysis showed changes in some PROs. At the meeting presented by the European Society for Medical Oncology, Dr. Garassino noted that it would be unrealistic to describe all PRO comparisons; instead, she presented several examples. For one, in patients receiving durvalumab, dysphagia and alopecia improved in four out of five PD-L1 subgroups and all subgroups, respectively, while patients in the placebo arm reported improvements in both measures regardless of PD-L1 expression. Other improvements tended to favor the durvalumab group; for instance, compared with other subgroups, patients with PD-L1 expression of at least 25% were more likely to report improved chest pain, physical functioning, pain, emotional functioning, and hemoptysis. In contrast, patients receiving placebo with PD-L1 expression less than 25% were more likely to report improved cough. Still, the investigators concluded that the overall picture did not suggest major differences in PROs by PD-L1 expression level, noting that global quality of life did not differ, and symptom improvement rates and time-to-deterioration measures generally aligned with the intent-to-treat population, judging by overlapping 95% confidence intervals and hazard ratios.
“These data support the PACIFIC regimen for the standard of care for stage III unresectable non–small cell lung cancer patients,” Dr. Garassino concluded.
Invited discussant Fabrice Barlesi, MD, PhD, of Aix-Marseille University, said that studies such as this one are important to ensure that investigator-implemented measures of response are calibrated to patient experiences. As an example, Dr. Barlesi noted that many clinicians would say that grade 2 diarrhea is a completely manageable adverse event, but not all patients would agree.
In this light, Dr. Barlesi said that the present findings are valuable, but they are not without flaws. He noted that 11 out of 13 symptoms from the quality of life lung cancer module were not reported, and that one-third of patients in the PACIFIC trial lacked PD-L1 expression level data.
Considering these shortcomings, and more broadly, difficulties comparing patient-reported outcome studies because of various measurement techniques, Dr. Barlesi called for standardization.
“We need to standardize the analysis of quality of life data,” Dr. Barlesi said. “We should correct for the multiplicity of tests. … we should identify some specific quality of life outcomes that we want to look at in the protocol.” He continued to suggest a variety of ideal characteristics for studies evaluating patient-reported outcomes, including defined statistical measures and protocols for missing data.
Without a standardized approach, “cross trial comparison will be a nightmare for all of us,” Dr. Barlesi said.
The study was funded by AstraZeneca. The investigators reported financial relationships with Roche, BMS, Lilly, and others.
SOURCE: Garassino et al. ELCC 2019. Abstract LBA2.
GENEVA – Level of programmed death-ligand 1 (PD-L1) expression does not impact patient-reported outcomes (PROs) among those receiving durvalumab for stage III non–small cell lung cancer (NSCLC), according to a retrospective analysis of the phase III PACIFIC trial.
The findings support durvalumab for all comers regardless of PD-L1 expression, reported lead author Marina Garassino, MD, of Fondazione IRCCS – Instituto Nazionale dei Tumori in Milan, who presented findings at the European Lung Cancer Congress.
The PACIFIC trial involved 713 patients with stage III NSCLC who did not progress after platinum-based concurrent chemoradiotherapy, demonstrating both improved progression-free and overall survival. Patients were randomized 2:1 to receive either durvalumab (10 mg/kg) or placebo IV every 2 weeks for up to 1 year. Out of 713 patients involved in the trial, 63% had PD-L1 tumor expression level data available for the present analysis, allowing for subgrouping into five categories: expression level of at least 25%, less than 25%, at least 1%, less than 1%, or unknown. The investigators compared PROs from these cohorts using the European Organisation for Research and Treatment of Cancer core quality of life questionnaire and lung cancer module (EORTC QLQ-C30 and -LC13). With scores ranging from 1 to 100 points, clinically meaningful differences were defined by score changes exceeding 10 points. Changes during treatment, from baseline to week 48, were analyzed by a mixed model for repeated measures, overall responses for improvement rates by logistic regression, and hazard ratios for time to deterioration (TTD) by a stratified Cox proportional-hazards model.
The investigators found that most PROs remained consistent over time, without clinically meaningful variations between PD-L1 expression levels. However, as with the entire PACIFIC treatment population, patients in the present analysis showed changes in some PROs. At the meeting presented by the European Society for Medical Oncology, Dr. Garassino noted that it would be unrealistic to describe all PRO comparisons; instead, she presented several examples. For one, in patients receiving durvalumab, dysphagia and alopecia improved in four out of five PD-L1 subgroups and all subgroups, respectively, while patients in the placebo arm reported improvements in both measures regardless of PD-L1 expression. Other improvements tended to favor the durvalumab group; for instance, compared with other subgroups, patients with PD-L1 expression of at least 25% were more likely to report improved chest pain, physical functioning, pain, emotional functioning, and hemoptysis. In contrast, patients receiving placebo with PD-L1 expression less than 25% were more likely to report improved cough. Still, the investigators concluded that the overall picture did not suggest major differences in PROs by PD-L1 expression level, noting that global quality of life did not differ, and symptom improvement rates and time-to-deterioration measures generally aligned with the intent-to-treat population, judging by overlapping 95% confidence intervals and hazard ratios.
“These data support the PACIFIC regimen for the standard of care for stage III unresectable non–small cell lung cancer patients,” Dr. Garassino concluded.
Invited discussant Fabrice Barlesi, MD, PhD, of Aix-Marseille University, said that studies such as this one are important to ensure that investigator-implemented measures of response are calibrated to patient experiences. As an example, Dr. Barlesi noted that many clinicians would say that grade 2 diarrhea is a completely manageable adverse event, but not all patients would agree.
In this light, Dr. Barlesi said that the present findings are valuable, but they are not without flaws. He noted that 11 out of 13 symptoms from the quality of life lung cancer module were not reported, and that one-third of patients in the PACIFIC trial lacked PD-L1 expression level data.
Considering these shortcomings, and more broadly, difficulties comparing patient-reported outcome studies because of various measurement techniques, Dr. Barlesi called for standardization.
“We need to standardize the analysis of quality of life data,” Dr. Barlesi said. “We should correct for the multiplicity of tests. … we should identify some specific quality of life outcomes that we want to look at in the protocol.” He continued to suggest a variety of ideal characteristics for studies evaluating patient-reported outcomes, including defined statistical measures and protocols for missing data.
Without a standardized approach, “cross trial comparison will be a nightmare for all of us,” Dr. Barlesi said.
The study was funded by AstraZeneca. The investigators reported financial relationships with Roche, BMS, Lilly, and others.
SOURCE: Garassino et al. ELCC 2019. Abstract LBA2.
GENEVA – Level of programmed death-ligand 1 (PD-L1) expression does not impact patient-reported outcomes (PROs) among those receiving durvalumab for stage III non–small cell lung cancer (NSCLC), according to a retrospective analysis of the phase III PACIFIC trial.
The findings support durvalumab for all comers regardless of PD-L1 expression, reported lead author Marina Garassino, MD, of Fondazione IRCCS – Instituto Nazionale dei Tumori in Milan, who presented findings at the European Lung Cancer Congress.
The PACIFIC trial involved 713 patients with stage III NSCLC who did not progress after platinum-based concurrent chemoradiotherapy, demonstrating both improved progression-free and overall survival. Patients were randomized 2:1 to receive either durvalumab (10 mg/kg) or placebo IV every 2 weeks for up to 1 year. Out of 713 patients involved in the trial, 63% had PD-L1 tumor expression level data available for the present analysis, allowing for subgrouping into five categories: expression level of at least 25%, less than 25%, at least 1%, less than 1%, or unknown. The investigators compared PROs from these cohorts using the European Organisation for Research and Treatment of Cancer core quality of life questionnaire and lung cancer module (EORTC QLQ-C30 and -LC13). With scores ranging from 1 to 100 points, clinically meaningful differences were defined by score changes exceeding 10 points. Changes during treatment, from baseline to week 48, were analyzed by a mixed model for repeated measures, overall responses for improvement rates by logistic regression, and hazard ratios for time to deterioration (TTD) by a stratified Cox proportional-hazards model.
The investigators found that most PROs remained consistent over time, without clinically meaningful variations between PD-L1 expression levels. However, as with the entire PACIFIC treatment population, patients in the present analysis showed changes in some PROs. At the meeting presented by the European Society for Medical Oncology, Dr. Garassino noted that it would be unrealistic to describe all PRO comparisons; instead, she presented several examples. For one, in patients receiving durvalumab, dysphagia and alopecia improved in four out of five PD-L1 subgroups and all subgroups, respectively, while patients in the placebo arm reported improvements in both measures regardless of PD-L1 expression. Other improvements tended to favor the durvalumab group; for instance, compared with other subgroups, patients with PD-L1 expression of at least 25% were more likely to report improved chest pain, physical functioning, pain, emotional functioning, and hemoptysis. In contrast, patients receiving placebo with PD-L1 expression less than 25% were more likely to report improved cough. Still, the investigators concluded that the overall picture did not suggest major differences in PROs by PD-L1 expression level, noting that global quality of life did not differ, and symptom improvement rates and time-to-deterioration measures generally aligned with the intent-to-treat population, judging by overlapping 95% confidence intervals and hazard ratios.
“These data support the PACIFIC regimen for the standard of care for stage III unresectable non–small cell lung cancer patients,” Dr. Garassino concluded.
Invited discussant Fabrice Barlesi, MD, PhD, of Aix-Marseille University, said that studies such as this one are important to ensure that investigator-implemented measures of response are calibrated to patient experiences. As an example, Dr. Barlesi noted that many clinicians would say that grade 2 diarrhea is a completely manageable adverse event, but not all patients would agree.
In this light, Dr. Barlesi said that the present findings are valuable, but they are not without flaws. He noted that 11 out of 13 symptoms from the quality of life lung cancer module were not reported, and that one-third of patients in the PACIFIC trial lacked PD-L1 expression level data.
Considering these shortcomings, and more broadly, difficulties comparing patient-reported outcome studies because of various measurement techniques, Dr. Barlesi called for standardization.
“We need to standardize the analysis of quality of life data,” Dr. Barlesi said. “We should correct for the multiplicity of tests. … we should identify some specific quality of life outcomes that we want to look at in the protocol.” He continued to suggest a variety of ideal characteristics for studies evaluating patient-reported outcomes, including defined statistical measures and protocols for missing data.
Without a standardized approach, “cross trial comparison will be a nightmare for all of us,” Dr. Barlesi said.
The study was funded by AstraZeneca. The investigators reported financial relationships with Roche, BMS, Lilly, and others.
SOURCE: Garassino et al. ELCC 2019. Abstract LBA2.
REPORTING FROM ELCC 2019
Treatment of Gout
Extended half-life clotting factors cut infusions, hike prices
More than a fifth of patients with hemophilia may now be using extended half-life (EHL) clotting factors, although the economic impact of these new treatments remains unclear.
Use of EHL factor VIII (FVIII) and IX (FIX) products surged from 10% of patients to 22% over an 18-month period ending in late 2017, Dr. Stacy E. Croteau and her colleagues reported in Haemophilia.
The increase appears to be mostly driven by prescribed prophylaxis rather than on-demand use of the products, wrote Dr. Croteau of Boston Children’s Hospital, and her coauthors. EHL dosages were similar to standard half-life (SHL) dosages and extended the time between infusions. But in the end, the higher cost of the EHL products actually drove up the price of prophylaxis, with a year of EHL FIX topping $1 million.
“Careful assessment of factor consumption and patient outcomes is needed to ensure general cost neutrality of this expensive therapy,” the researchers wrote. “Unless demonstrably offset by reduction in bleed doses, the net effect could be further increases in annual cost of care for this patient population.”
The study examined the use of SHL and EHL clotting factors in 7,893 adults and children with hemophilia A or B, who were being followed in the American Thrombosis and Hemostasis Network (ATHN) database. The authors sought to characterize changes in usage patterns for SHL and EHL factors, and to identify demographic and economic influences on them.
During the study, the number of patients using EHL products for both on-demand and prophylactic factor replacement increased. EHL FVIII use rose from 9% to 21%, and EHL FIX from 14% to 21%, especially among those with hemophilia B.
There were 6,437 patients with full data at both initial and final sampling. Among these, there was a 9.6% increase in the use of an EHL clotting factor by the end of the study (P less than .001). Patients with hemophilia A were less likely than hemophilia B patients to use an EHL product for prophylaxis.
While the EHL products did reduce the number of prophylactic infusions, they also cost much more, the investigators found.
The standard dose of SHL FVIII is 40 IU/g infused three times a week. The projected cost of 156 annual infusions is $690,144. EHL FVII, dosed at 50 IU/kg, cuts infusions to twice a week. The annual projected cost of the 104 infusions is $753,480.
The standard dose of SHL FIX is 67 IU/kg, infused twice a week. The annual projected cost of 104 infusions is $697,497. EHL FIX, dosed at 75 IU/Kg, halves the number of infusions. But the price for those 52 treatments exceeds $1 million ($1,015,560). Despite the cost, however, just 43 patients switched from an EHL product to a SHL factor product during the study period.
Insurance type appeared to have little influence on the choice of SHL or EHL clotting factors. Across payer types, a similar proportion of patients started using them, and 71% were covered by private insurance or Medicaid.
The study was funded HTRS/ATHN Dataset Research Engagement and a DREAM Award from the Hemostasis and Thrombosis Research Society. Dr. Croteau reported consulting for Bayer, Bioverativ, Biomarin, CSL-Behring, and other companies.
SOURCE: Croteau SE et al. Haemophilia. 2019 Apr 17. doi: 10.1111/hae.13758.
More than a fifth of patients with hemophilia may now be using extended half-life (EHL) clotting factors, although the economic impact of these new treatments remains unclear.
Use of EHL factor VIII (FVIII) and IX (FIX) products surged from 10% of patients to 22% over an 18-month period ending in late 2017, Dr. Stacy E. Croteau and her colleagues reported in Haemophilia.
The increase appears to be mostly driven by prescribed prophylaxis rather than on-demand use of the products, wrote Dr. Croteau of Boston Children’s Hospital, and her coauthors. EHL dosages were similar to standard half-life (SHL) dosages and extended the time between infusions. But in the end, the higher cost of the EHL products actually drove up the price of prophylaxis, with a year of EHL FIX topping $1 million.
“Careful assessment of factor consumption and patient outcomes is needed to ensure general cost neutrality of this expensive therapy,” the researchers wrote. “Unless demonstrably offset by reduction in bleed doses, the net effect could be further increases in annual cost of care for this patient population.”
The study examined the use of SHL and EHL clotting factors in 7,893 adults and children with hemophilia A or B, who were being followed in the American Thrombosis and Hemostasis Network (ATHN) database. The authors sought to characterize changes in usage patterns for SHL and EHL factors, and to identify demographic and economic influences on them.
During the study, the number of patients using EHL products for both on-demand and prophylactic factor replacement increased. EHL FVIII use rose from 9% to 21%, and EHL FIX from 14% to 21%, especially among those with hemophilia B.
There were 6,437 patients with full data at both initial and final sampling. Among these, there was a 9.6% increase in the use of an EHL clotting factor by the end of the study (P less than .001). Patients with hemophilia A were less likely than hemophilia B patients to use an EHL product for prophylaxis.
While the EHL products did reduce the number of prophylactic infusions, they also cost much more, the investigators found.
The standard dose of SHL FVIII is 40 IU/g infused three times a week. The projected cost of 156 annual infusions is $690,144. EHL FVII, dosed at 50 IU/kg, cuts infusions to twice a week. The annual projected cost of the 104 infusions is $753,480.
The standard dose of SHL FIX is 67 IU/kg, infused twice a week. The annual projected cost of 104 infusions is $697,497. EHL FIX, dosed at 75 IU/Kg, halves the number of infusions. But the price for those 52 treatments exceeds $1 million ($1,015,560). Despite the cost, however, just 43 patients switched from an EHL product to a SHL factor product during the study period.
Insurance type appeared to have little influence on the choice of SHL or EHL clotting factors. Across payer types, a similar proportion of patients started using them, and 71% were covered by private insurance or Medicaid.
The study was funded HTRS/ATHN Dataset Research Engagement and a DREAM Award from the Hemostasis and Thrombosis Research Society. Dr. Croteau reported consulting for Bayer, Bioverativ, Biomarin, CSL-Behring, and other companies.
SOURCE: Croteau SE et al. Haemophilia. 2019 Apr 17. doi: 10.1111/hae.13758.
More than a fifth of patients with hemophilia may now be using extended half-life (EHL) clotting factors, although the economic impact of these new treatments remains unclear.
Use of EHL factor VIII (FVIII) and IX (FIX) products surged from 10% of patients to 22% over an 18-month period ending in late 2017, Dr. Stacy E. Croteau and her colleagues reported in Haemophilia.
The increase appears to be mostly driven by prescribed prophylaxis rather than on-demand use of the products, wrote Dr. Croteau of Boston Children’s Hospital, and her coauthors. EHL dosages were similar to standard half-life (SHL) dosages and extended the time between infusions. But in the end, the higher cost of the EHL products actually drove up the price of prophylaxis, with a year of EHL FIX topping $1 million.
“Careful assessment of factor consumption and patient outcomes is needed to ensure general cost neutrality of this expensive therapy,” the researchers wrote. “Unless demonstrably offset by reduction in bleed doses, the net effect could be further increases in annual cost of care for this patient population.”
The study examined the use of SHL and EHL clotting factors in 7,893 adults and children with hemophilia A or B, who were being followed in the American Thrombosis and Hemostasis Network (ATHN) database. The authors sought to characterize changes in usage patterns for SHL and EHL factors, and to identify demographic and economic influences on them.
During the study, the number of patients using EHL products for both on-demand and prophylactic factor replacement increased. EHL FVIII use rose from 9% to 21%, and EHL FIX from 14% to 21%, especially among those with hemophilia B.
There were 6,437 patients with full data at both initial and final sampling. Among these, there was a 9.6% increase in the use of an EHL clotting factor by the end of the study (P less than .001). Patients with hemophilia A were less likely than hemophilia B patients to use an EHL product for prophylaxis.
While the EHL products did reduce the number of prophylactic infusions, they also cost much more, the investigators found.
The standard dose of SHL FVIII is 40 IU/g infused three times a week. The projected cost of 156 annual infusions is $690,144. EHL FVII, dosed at 50 IU/kg, cuts infusions to twice a week. The annual projected cost of the 104 infusions is $753,480.
The standard dose of SHL FIX is 67 IU/kg, infused twice a week. The annual projected cost of 104 infusions is $697,497. EHL FIX, dosed at 75 IU/Kg, halves the number of infusions. But the price for those 52 treatments exceeds $1 million ($1,015,560). Despite the cost, however, just 43 patients switched from an EHL product to a SHL factor product during the study period.
Insurance type appeared to have little influence on the choice of SHL or EHL clotting factors. Across payer types, a similar proportion of patients started using them, and 71% were covered by private insurance or Medicaid.
The study was funded HTRS/ATHN Dataset Research Engagement and a DREAM Award from the Hemostasis and Thrombosis Research Society. Dr. Croteau reported consulting for Bayer, Bioverativ, Biomarin, CSL-Behring, and other companies.
SOURCE: Croteau SE et al. Haemophilia. 2019 Apr 17. doi: 10.1111/hae.13758.
FROM HAEMOPHILIA
Intermittent fasting tied to positive physiological effects
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
EXPERT ANALYSIS FROM INTERNAL MEDICINE 2019
Teen e-cigarette use: A public health crisis
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at pdnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at pdnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
After 2 decades of steady decline in adolescent and young adult use of tobacco products, e-cigarettes have dramatically altered the landscape of substance use in youth. E-cigarette use among teens has been on the rise for years but the recent exponential increase is unprecedented. From 2017 to 2018, adolescent e-cigarette use had the largest year-to-year increase (78%, from 12% to 21%) of any individual substance or class of substances at any time during the past 2 decades of nationwide monitoring.1 This has appropriately caught the nation’s attention. In 2016, Surgeon General Vivek H. Murthy, MD, commissioned an extensive report about electronic cigarettes, and in 2018 Surgeon General Jerome Adams, MD, MPH, issued an advisory declaring e-cigarettes a public health crisis for adolescents.2
E-cigarettes have received attention as a possible boon to adult cigarette smokers seeking a less hazardous product. We can consider the use of tobacco products along a continuum from smoked tobacco, dual use (both smoked tobacco and electronic nicotine delivery), electronic nicotine delivery only, and finally, nonuse. For some adults, transitioning from smoked tobacco products to electronic delivery systems has been a step toward less overall harm from substance use, with a small minority of that population going on to achieve abstinence from all nicotine products.3 For youth and teens, the story has been the opposite. With the rapid rise of e-cigarettes, adolescents overwhelmingly have been moving in the wrong direction at each potential step along this continuum.4 Less than 8% of teens who use e-cigarettes indicated that smoking cessation is a factor in their use.5 An estimated 1.3 million U.S. teens now are dependent or at high risk for dependence upon nicotine because of e-cigarette use. Furthermore, these teens are at a fourfold higher risk of progression to cigarette use, compared with their peers.6
One product in particular gives us information as to why this trend has accelerated so rapidly. Juul, now the sales leader among electronic nicotine delivery systems, rose from approximately 25% to a dominant 75% of market share in just over 1 fiscal year after a social media campaign targeted toward youth and young adults. The device is shaped like an elongated flash drive, is marketed as “sleek,” “looking cool,” and being “super easy” to use. This product touts its use of nicotine salts that can deliver higher concentrations of nicotine more rapidly to mimic the experience of smoking a cigarette as closely as possible. The fruity flavors in Juul “pods” and many other devices also appeal to teens. Many youth are left misinformed, thinking they are using a relatively harmless alternative to cigarettes.
E-cigarette use in youth carries many risks. Among the physical risks is exposure to harmful chemicals (even if less numerous than smoked tobacco products) such as diacetyl (a known cause of bronchiolitis obliterans, or “popcorn lung”), formaldehyde, acrolein, benzene, and metals such as nickel, tin and lead.7 “Safer than cigarettes” is a low bar indeed. Cognitive and emotional risks of early nicotine exposure include poor focus and attention, permanent lowering of impulse control, and a higher risk of mood and anxiety disorders.
Furthermore, nicotine is a gateway drug, with a clearly understood molecular basis for how it can potentiate the effects of later used substances, especially stimulants such as cocaine.8 The gateway and priming effect is compounded for youth because of ongoing brain development and plasticity during teen years. E-cigarette use also is associated with other risk behaviors including a manyfold higher likelihood of binge drinking, having multiple sexual partners in a short period of time, and using other substances such as cannabis, cocaine, methamphetamine, and heroin or nonprescribed opioids.9 An electronic system for vaporization also presents a risk for use of other substances. In just 1 year from 2017 to 2018, marijuana “vaping” increased by more than 50% among all ages surveyed.10
Pediatric health care providers are essential educators for both teens and parents regarding the risks of e-cigarette use. Many youth don’t know what they’re using; 66% of youth reported that the vapors they were inhaling contained only flavoring. Only 13% reported they were inhaling nicotine.10 In stark contrast to these self-reports, all Juul “pods” contain nicotine. As has been a pattern with nationwide surveys of substance use for decades, adolescent use is inversely correlated with perception of risk; 70% of 8th-12th graders do not foresee great harm in regular e-cigarette use. In addition, adolescents use substances less often when they know their parents disapprove. Parents also must be taught about the risks of e-cigarette use and can be provided with resources and taught effective strategies if they have difficulty communicating their disapproval to their children.
Age-appropriate screening in primary care settings must include specific language regarding the use of electronic cigarettes, with questions about “vaping” and “juuling.” Discussions with teens may be more effective with emphasis on issues that resonate with youth such as the financial cost, loss of freedom when dependence develops, and the fact that their generation is once again being targeted by the tobacco industry. Referral for further treatment, including individual and group therapy as well as family-focused interventions, should be considered for teens who use daily, use other substances regularly, or could benefit from treatment for co-occurring mental health disorders.
Electronic cigarette use should not be recommended as a smoking cessation strategy for teens.11 Pediatric health care providers must advocate for regulation of these products, including increasing the legal age of purchase and banning flavoring in e-cigarettes products, Internet sales, and advertisements targeted to youth.
The rapid rise in e-cigarette use among teens is of great concern. As with all classes of substances, early initiation of nicotine drastically increases the risk of developing a substance use disorder and portends a prolonged course and greater accumulation of adverse consequences. There is an urgent need for education, prevention, and early identification of e-cigarette use to protect the current and future well-being of children and adolescents.
Dr. Jackson is assistant professor of psychiatry at the University of Vermont, Burlington. He said he had no relevant financial disclosures. Email Dr. Jackson at pdnews@mdedge.com.
References
1. MMWR Morb Mortal Wkly Rep. 2018;67:1276-7.
2. e-cigarettes.surgeongeneral.gov
3. N Engl J Med 2019;380:629-37.
4. Pediatrics. 2018 Dec; 142(6):e20180486.
5. MMWR Morb Mortal Wkly Rep 2018;67:196-200.
6. JAMA Pediatr. 2017 Aug 1;171(8):788-97.
7. “Public health consequences of e-cigarettes” (Washington, DC: National Academies Press, January 2018).
8. N Engl J Med 2014;371:932-43.
9. N Engl J Med 2019;380:689-90.
10. MMWR Morb Mortal Wkly Rep. 2016 Jan 8;64(52):1403-8.
11. Pediatrics. 2019 Feb;143(2). pii: e20183652.
Hip T scores can guide duration of osteoporosis therapy
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
according to Serge Ferrari, MD, and his colleagues.
Using 10 years of follow-up data from 1,343 women who took denosumab in the FREEDOM trial, Dr. Ferrari and his colleagues determined that a T score of at least –2.5 would be an appropriate target for this decision.
“A T-score unit increase of 1.0 was associated with a significant reduction in fracture risk for T scores up to, but no greater than, –2.0, suggesting that a T-score threshold of at least –2.0 would be an appropriate target for therapy to maximize treatment,” said Dr. Ferrari of the University of Geneva and his colleagues. “Further improvements in bone mineral density were not associated with major additional changes in 1-year nonvertebral fracture incidence.”
The findings “highlight the importance of the relationship between hip T score and fracture risk, which is maintained during long-term therapy with denosumab. Regular monitoring of bone mineral density during therapy may be useful to determine when fracture risk has reached a minimal threshold; treatment could therefore be suspended and/or consolidated, as in the case of a reversible therapy such as denosumab.”
SOURCE: Ferrari S et al. J Bone Miner Res. 2019 Mar 28. doi: 10.1002/jbmr.3722.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
The genesis of vaginal anomalies
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
According to our guest author Marc R. Laufer, MD, the “development of the female genital tract is a complex process that is dependent upon a series of events involving cellular differentiation, migration, fusion, and canalization. Failure of any one of these processes results in a congenital anomaly.”1
In 1933, A.K. Koff coined the terms sinovaginal bulb and vaginal plate. He proposed that the upper 80% of the vagina is derived from Müllerian epithelium and the lower 20% derived from urogenital sinus epithelium.2 In 1957, D. Bulmer proposed that vaginal epithelium derives solely from urogenital sinus epithelium.3 And in 2017, Robboy et al. supported Bulmer’s proposal that human vaginal epithelium derives solely from urogenital sinus epithelium and differs from mouse vaginal development.4
Beginning at 3 weeks of embryogenesis and continuing into the second trimester of pregnancy, development of the female genital tract takes place. The sinovaginal bulbs originate in the urogenital sinus at the distal aspect of the Müllerian tubercle. At approximately 13 weeks, these two solid evaginations grow out of the pelvic part of the urogenital sinus and proliferate into the caudal end of the uterovaginal canal to become a solid vaginal plate. Degeneration of the central cells of this vaginal plate, which occur in a cephalad direction, enables creation of the lower vagina. Canalization is generally completed by 20 weeks’ gestation.
Agenesis or absence of the lower vagina is usually associated with normal development of the upper vagina, cervix, uterus, and ovaries. It is the result of abnormal development of the sinovaginal bulbs and vaginal plate.
The hymenal membrane separates the vaginal lumen from the urogenital sinus. Secondary to degeneration of the central epithelial cells, the hymen typically ruptures, leaving a thin fold of mucous membrane around the vaginal introitus. Hymenal anatomic variants include microperforate, septate, or cribriform. They occur secondary to incomplete degeneration of the central portion of the hymen.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston. Dr. Laufer is an acclaimed physician, surgeon, clinical researcher, author, and teacher, and it is truly my pleasure to welcome him to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois at Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He reported no disclosures relevant to this Master Class. Email him at pdnews@mdedge.com.
References
1. Laufer M. Congenital anomalies of the hymen and vagina. Uptodate (accessed April 2019).
2. Contrib Embryol. 1933 Sep;24(140):59-91.
3. J Anat. 1957 Oct;91(4):490-509.
4. Differentiation. 2017 Sep-Oct;97:9-22.
Vaginal anomalies and their surgical correction
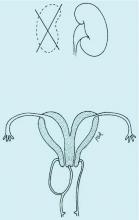
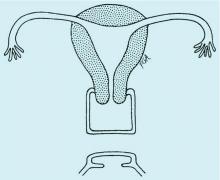
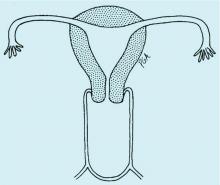
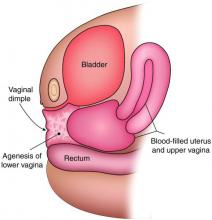
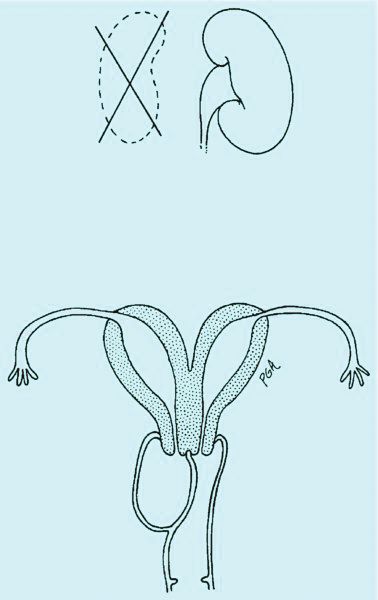
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
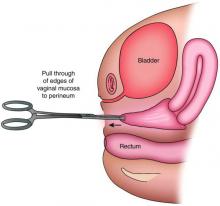
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Congenital obstructive anomalies of the vagina are unusual and can be challenging to diagnose and manage. Two of the most challenging are obstructive hemivagina with ipsilateral renal agenesis (Figure 1a) and agenesis of the lower vagina (Figure 1b), the latter of which must be differentiated most commonly from imperforate hymen (Figure 1c). Evaluation and treatment of these anomalies is dependent upon the age of the patient, as well as the symptoms, and the timing of treatment should be individualized.
Agenesis of the lower vagina
Agenesis of the lower vagina and imperforate hymen may present either in the newborn period as a bulging introitus caused by mucocolpos from vaginal secretions stimulated by maternal estradiol or during adolescence at the time of menarche. In neonates, it often is best not to intervene when obstructive anomalies are suspected as long as there is no fever; pain; or compromise of respiration, urinary and bowel function, and other functionality. It will be easier to differentiate agenesis of the lower vagina and imperforate hymen – the latter of which is one of the most common obstructive lesions of the female genital tract – later on. And if the hymen remains imperforate, the mucus will be reabsorbed and the patient usually will remain asymptomatic until menarche.
In the adolescent time period, both anomalies often are identified when the patient presents with pelvic pain – usually cyclic pelvic pain with primary amenorrhea. Because the onset of menses typically occurs 2-3 years after the onset of estrogenization and breast development, evaluating breast development can help us to determine the timing of expected menarche. An obstructive anomaly should be suspected in an adolescent who presents with pain during this time period, after evaluation for an acute abdomen (Figure 2a).
When a vaginal orifice is visualized upon evaluation of the external genitalia and separation of the labia, a higher anomaly such as a transverse vaginal septum should be suspected. When an introitus cannot be visualized, evaluation for an imperforate hymen or agenesis of the lower vagina is necessary (Figure 1b and 1c).
The simplest way to differentiate imperforate hymen from agenesis of the lower vagina is with visualization of the obstructing tissue on exam and usage of transperitoneal ultrasound. With the transducer placed on the vulva, we can evaluate the distance from the normal location of an introitus to the level of the obstruction. If the distance is in millimeters, then typically there is an imperforate hymen. If the distance is larger – more than several millimeters – then the differential diagnosis typically is agenesis of the lower vagina, an anomaly that results from abnormal development of the sinovaginal bulbs and vaginal plate.
The distance as measured by transperitoneal ultrasound also will indicate whether or not pull-through vaginoplasty (Figure 2b) – our standard treatment for lower vaginal agenesis – is possible using native vaginal mucosa from the upper vagina. Most commonly, the distance is less than 5 cm and we are able to make a transverse incision where the hymenal ring should be located, carry the dissection to the upper vagina, drain the obstruction, and mobilize the upper vaginal mucosa, suturing it to the newly created introitus to formulate a patent vaginal tract.
A rectoabdominal examination similarly can be helpful in making the diagnosis of lower vaginal agenesis and in determining whether there is enough tissue available for a pull-through procedure (Figures 2a and 2b). Because patients with this anomaly generally have normal cyclic pituitary-ovarian-endometrial function at menarche, the upper vagina will distend with blood products and secretions that can be palpated on the rectoabdominal exam. If the obstructed vaginal tissue is not felt with the rectal finger at midline, the obstructed agenesis of the vagina probably is too high for a straightforward pull-through procedure. Alternatively, the patient may have a unicornuate system with agenesis of the lower vagina; in this case, the obstructed upper vaginal tissue will not be in the midline but off to one side. MRI also may be helpful for defining the pelvic anatomy.
The optimal timing for a pull-through vaginoplasty (Figure 2b) is when a large hematocolpos (Figure 2a) is present, as the blood acts as a natural expander of the native vaginal tissue, increasing the amount of tissue available for a primary reanastomosis. This emphasizes the importance of an accurate initial diagnosis. Too often, obstructions that are actually lower vaginal agenesis are presumed to be imperforate hymen, and the hematocolpos is subsequently evacuated after a transverse incision and dissection of excess tissue, causing the upper vagina to retract and shrink. This mistake can result in the formation of a fistulous tract from the previously obstructed upper vagina to the level of the introitus.
The vaginoplasty is carried out with the patient in the dorsal lithotomy position. A Foley catheter is placed into the bladder to avoid an inadvertent anterior entry into the posterior wall of the bladder, and the labia are grasped and pulled down and out.
The hymenal tissue should be visible. A transverse incision is made, with electrocautery, where the introitus should be located, and a dissection is carried out to reach the obstructed upper vaginal tissue. Care is needed to keep the dissection in the midline and avoid the bladder above and the rectum below. In cases in which it is difficult to identify the area of obstruction, intraoperative ultrasound can be helpful. A spinal needle with a 10-cc syringe also can be used to identify a track through which to access the fluid.
The linear incision then is made with electrocautery and the obstructed hemivagina is entered. Allis clamps are used to grasp the vaginal mucosa from the previously obstructed upper vagina to help identify the tissue that needs to be mobilized. The tissue then is further dissected to free the upper vagina, and the edges are pulled down to the level of the introitus with Allis clamps. “Relaxing” incisions are made at 1, 5,7, and 11 o’clock to avoid a circumferential scar. The upper vaginal mucosa is sewn to the newly created introitus with a 2-0 vicryl suture on a UR6 (a smaller curved urology needle).
When the distance from normal introitus location to obstruction is greater than 5 cm, we sometimes use vaginal dilators to lessen the distance and reach the obstruction for a pull-through procedure. Alternatively, the upper vagina may be mobilized from above either robotically or laparoscopically so that the upper vaginal mucosa may be pulled down without entering the bladder. Occasionally, with greater distances over 5 cm, the vaginoplasty may require utilization of a buccal mucosal graft or a bowel segment.
Intraoperative ultrasound can be especially helpful for locating the obstructed vagina in women with a unicornuate system because the upper vagina will not be in the midline and ultrasound can help determine the appropriate angle for dissection.
Prophylactic antibiotics initiated postoperatively are important with pull-through vaginoplasty, because the uterus and fallopian tubes may contain blood (an excellent growth media) and because there is a risk of bacteria ascending into what becomes an open system.
Postoperatively, we guide patients on the use of flexible Milex dilators (CooperSurgical) to ensure that the vagina heals without restenosis. The length of postoperative dilation therapy can vary from 2-12 months, depending on healing. The dilator is worn 24 hours a day, 7 days a week, and is removed only for urination, defecation, and cleaning. With adequate postoperative dilation, patients will have normal sexual and reproductive function, and vaginal delivery should be possible.
Obstructed hemivagina
An obstructed hemivagina, an uncommon Müllerian duct anomaly, occurs most often with ipsilateral renal agenesis and is commonly referred to as OHVIRA. Because the formation of the reproductive system is closely associated with the development of the urinary system, it is not unusual for renal anomalies to occur alongside Müllerian anomalies and vaginal anomalies. There should be a high index of suspicion for a reproductive tract anomaly in any patient known to have a horseshoe kidney, duplex collecting system, unilateral renal agenesis, or other renal anomaly.
Patients with obstructed hemivagina typically present in adolescence with pelvic pain or dysmenorrhea, and commonly are misdiagnosed as having endometriomas or vaginal cysts. On vaginal examination, the obstructed hemivagina may be visualized as a bulge coming from the lateral vaginal sidewall. While only one cervix is appreciated on a vaginal exam, an ultrasound examination often will show two uteri and two cervices. MRI also is helpful for diagnosis.
Obstructed hemivagina requires surgical correction to open the obstruction, excise the excess vaginal tissue, and create one vagina with access to the second cervix. Great care must be taken to avoid not only the bladder and rectum but the cervices. It is not unusual for the two cervices to be at different levels, with one cervix sharing medial aspects of the vaginal wall of the second vagina (Figure 1a). The tissue between the two cervices should be left in place to avoid compromising their blood supply.
We manage this anomaly primarily through a single-stage vaginoplasty. For the nonobstructed side to be visualized, a longitudinal incision into the obstructed hemivagina should be made at the point at which it is most easily palpated. As with agenesis of the lower vagina, the fluid to be drained tends to be old menstrual blood that is thick and dark brown. It is useful to set up two suction units at the time of surgery because tubing can become clogged.
The use of vaginal side wall retractors helps with visualization. Alternatively, I tend to use malleable abdominal wall retractors vaginally, as they can be bent to conform to the angle needed and come in different widths. When it is difficult to identify the area of obstruction, a spinal needle with a 10-cc syringe again can be used to identify a track for accessing the fluid. The linear incision then is made with electrocautery, and the obstructed hemivagina is entered.
Allis clamps are used to grasp the vaginal mucosa from the previously obstructed hemivagina to help identify the tissue that needs to be excised. Once the fluid is evacuated, a finger also can be placed into the obstructed vagina is help identify excess tissue. This three-dimensional elliptical area is similar to a septum but becomes the obstructed hemivagina as it attaches to the vaginal wall (Figure 1a).
Retrograde menses and endometriosis occur commonly with obstructive anomalies like obstructed hemivagina and agenesis of the lower vagina, but laparoscopy with the goal of treating endometriosis is not indicated. We discourage its use at the time of repair because there is evidence that almost all endometriosis will completely resorb on its own once the anomalies are corrected.1,2
As with repair of lower vagina agenesis, antibiotics to prevent an ascending infection should be taken after surgical correction of obstructed hemivagina. Patients with obstructed hemivagina can have a vaginal delivery if there are no other contraindications. Women with obstructed hemivagina and ipsilateral renal anomaly have essentially two unicornuate systems and thus are at risk of breech presentation and preterm delivery.
Dr. Laufer is chief of the division of gynecology, codirector of the Center for Young Women’s Health, and director of the Boston Center for Endometriosis, all at Boston Children’s Hospital. He also is professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
References
1. Am J Obstet Gynecol. 1986;154:39.
2. J Pediatr Adolesc Gynecol. 2010;23(2):e89.
Survival after squamous recurrence may be unaffected by immune status
Patients who have recurrence of cutaneous squamous cell cancer of the head and neck (cSCC-HN) tend to have poor outcomes regardless of immune status, according to investigators.
Patients with surgically unsalvageable recurrent disease had the worst outcomes, reported lead author Lillian Sun, of the Cleveland Clinic, and her colleagues.
These findings support more intensive upfront therapy for patients with cSCC-HN, the investigators wrote in JAMA Dermatology. They noted that most patients with cSCC have good outcomes, with less than 5% experiencing recurrence or distant metastasis; however, “there is a subset of patients with adverse pathologic features and a more aggressive clinical course,” the investigators pointed out, “with substantially higher rates of locoregional recurrence (13%-41%) and distant metastasis (7%-16%) after surgical resection.”
According to the investigators, previous research has identified several patient factors that predict poor outcomes, such as invasiveness and differentiation, as well as chronic immunosuppression. To build on these data, and, in particular, to determine if immune suppression was linked with poor outcomes, the investigators conducted a retrospective analysis of 205 patients with cSCC-HN.
From this cohort, 72 patients had disease recurrence after surgery and radiotherapy. The average age of the patients was 71 years. About half were immunosuppressed (55.6%). On average, disease recurrence occurred slightly earlier in immunosuppressed patients (9.1 months) than in immunocompetent patients (10.1 months). Most patients had locoregional recurrence first, at rates of 65.6% and 77.5% among immunocompetent and immunosuppressed patients, respectively. Irrespective of immune status, median overall survival was 8.4 months and the 1-year overall survival rate was 43.2%. In contrast with previous findings, immune status was not statistically associated with median overall survival; immunocompetent patients did tend to live longer (12.9 months) than immunosuppressed patients (8.0 months), but this difference carried a P value of .90.
The investigators found that surgical candidacy after recurrence had the strongest impact on survival. Patients with surgically salvageable disease had a median overall survival of 26.1 months, compared with just 4.7 months for those who were not amenable to surgical salvage (P = .01). Among patients with unsalvageable disease, again, immune status did not have a significant impact on outcome.
“This study demonstrates that survival in this population is poor,” the investigators concluded. “Although we hypothesized that immunosuppressed status would be a significant contributor to outcomes in these patients, similar to findings in the upfront treatment setting, the current study suggests that this is not the case.”
The investigators reported clinical trial support from Genentech, Merck, and Bristol-Myers Squibb.
SOURCE: Sun et al. JAMA Derm. 27 Feb 2019. doi: 10.1001/jamadermatol.2018.5453.
Patients who have recurrence of cutaneous squamous cell cancer of the head and neck (cSCC-HN) tend to have poor outcomes regardless of immune status, according to investigators.
Patients with surgically unsalvageable recurrent disease had the worst outcomes, reported lead author Lillian Sun, of the Cleveland Clinic, and her colleagues.
These findings support more intensive upfront therapy for patients with cSCC-HN, the investigators wrote in JAMA Dermatology. They noted that most patients with cSCC have good outcomes, with less than 5% experiencing recurrence or distant metastasis; however, “there is a subset of patients with adverse pathologic features and a more aggressive clinical course,” the investigators pointed out, “with substantially higher rates of locoregional recurrence (13%-41%) and distant metastasis (7%-16%) after surgical resection.”
According to the investigators, previous research has identified several patient factors that predict poor outcomes, such as invasiveness and differentiation, as well as chronic immunosuppression. To build on these data, and, in particular, to determine if immune suppression was linked with poor outcomes, the investigators conducted a retrospective analysis of 205 patients with cSCC-HN.
From this cohort, 72 patients had disease recurrence after surgery and radiotherapy. The average age of the patients was 71 years. About half were immunosuppressed (55.6%). On average, disease recurrence occurred slightly earlier in immunosuppressed patients (9.1 months) than in immunocompetent patients (10.1 months). Most patients had locoregional recurrence first, at rates of 65.6% and 77.5% among immunocompetent and immunosuppressed patients, respectively. Irrespective of immune status, median overall survival was 8.4 months and the 1-year overall survival rate was 43.2%. In contrast with previous findings, immune status was not statistically associated with median overall survival; immunocompetent patients did tend to live longer (12.9 months) than immunosuppressed patients (8.0 months), but this difference carried a P value of .90.
The investigators found that surgical candidacy after recurrence had the strongest impact on survival. Patients with surgically salvageable disease had a median overall survival of 26.1 months, compared with just 4.7 months for those who were not amenable to surgical salvage (P = .01). Among patients with unsalvageable disease, again, immune status did not have a significant impact on outcome.
“This study demonstrates that survival in this population is poor,” the investigators concluded. “Although we hypothesized that immunosuppressed status would be a significant contributor to outcomes in these patients, similar to findings in the upfront treatment setting, the current study suggests that this is not the case.”
The investigators reported clinical trial support from Genentech, Merck, and Bristol-Myers Squibb.
SOURCE: Sun et al. JAMA Derm. 27 Feb 2019. doi: 10.1001/jamadermatol.2018.5453.
Patients who have recurrence of cutaneous squamous cell cancer of the head and neck (cSCC-HN) tend to have poor outcomes regardless of immune status, according to investigators.
Patients with surgically unsalvageable recurrent disease had the worst outcomes, reported lead author Lillian Sun, of the Cleveland Clinic, and her colleagues.
These findings support more intensive upfront therapy for patients with cSCC-HN, the investigators wrote in JAMA Dermatology. They noted that most patients with cSCC have good outcomes, with less than 5% experiencing recurrence or distant metastasis; however, “there is a subset of patients with adverse pathologic features and a more aggressive clinical course,” the investigators pointed out, “with substantially higher rates of locoregional recurrence (13%-41%) and distant metastasis (7%-16%) after surgical resection.”
According to the investigators, previous research has identified several patient factors that predict poor outcomes, such as invasiveness and differentiation, as well as chronic immunosuppression. To build on these data, and, in particular, to determine if immune suppression was linked with poor outcomes, the investigators conducted a retrospective analysis of 205 patients with cSCC-HN.
From this cohort, 72 patients had disease recurrence after surgery and radiotherapy. The average age of the patients was 71 years. About half were immunosuppressed (55.6%). On average, disease recurrence occurred slightly earlier in immunosuppressed patients (9.1 months) than in immunocompetent patients (10.1 months). Most patients had locoregional recurrence first, at rates of 65.6% and 77.5% among immunocompetent and immunosuppressed patients, respectively. Irrespective of immune status, median overall survival was 8.4 months and the 1-year overall survival rate was 43.2%. In contrast with previous findings, immune status was not statistically associated with median overall survival; immunocompetent patients did tend to live longer (12.9 months) than immunosuppressed patients (8.0 months), but this difference carried a P value of .90.
The investigators found that surgical candidacy after recurrence had the strongest impact on survival. Patients with surgically salvageable disease had a median overall survival of 26.1 months, compared with just 4.7 months for those who were not amenable to surgical salvage (P = .01). Among patients with unsalvageable disease, again, immune status did not have a significant impact on outcome.
“This study demonstrates that survival in this population is poor,” the investigators concluded. “Although we hypothesized that immunosuppressed status would be a significant contributor to outcomes in these patients, similar to findings in the upfront treatment setting, the current study suggests that this is not the case.”
The investigators reported clinical trial support from Genentech, Merck, and Bristol-Myers Squibb.
SOURCE: Sun et al. JAMA Derm. 27 Feb 2019. doi: 10.1001/jamadermatol.2018.5453.
FROM JAMA DERMATOLOGY
HAVEN 4: Monthly emicizumab shows value
For many patients with hemophilia A, with or without inhibitors, a monthly emicizumab injection is enough to ensure a high level of bleed control, based on results from the ongoing HAVEN 4 trial.
Most patients reported three or fewer treated bleeds, while slightly more than half had no treated bleeds at all, according to lead author Steven W. Pipe, MD, of the University of Michigan, Ann Arbor, and his colleagues. The investigators noted that results from this trial have already led to approval of a monthly dosing schedule in the United States and several other countries.
“This convenient regimen has the potential to improve the care of patients by decreasing their treatment burden, and increasing uptake and adherence to effective prophylaxis, which is known to decrease the development of debilitating secondary complications,” the investigators wrote. The report is in The Lancet Haematology.
The data were collected at 20 centers in 8 countries. Eligibility required that patients have severe congenital hemophilia A (less than 1% normal FVIII activity), or hemophilia A with FVIII inhibitors and concurrent treatment with bypassing agents or FVIII concentrates.
An initial run-in cohort that included seven patients assessed pharmacokinetics and safety. These patients received 6 mg/kg of emicizumab subcutaneously every 4 weeks for at least 24 weeks. After this group showed good responses, 41 additional patients were enrolled in an expansion cohort, which involved an initial loading phase of weekly doses at 3 mg/kg for the first month, followed by monthly dosing at 6 mg/kg for at least 6 months (24 weeks).
The efficacy endpoint of the study was bleed prevention, as measured by treated target joint bleeds, treated joint bleeds, treated spontaneous bleeds, all bleeds (untreated and treated), and annualized bleed rates for treated bleeds.
In the expansion cohort, the median number of bleeds in the 24-week period preceding enrollment was five. In the same group, five patients (12%) had FVIII inhibitors and 61% of patients exhibited at least one target joint.
After a median treatment of 25.6 weeks, the model-based annualized bleed rate for treated bleeds was 2.4, while the median annualized bleed rate was zero.
Slightly more than half of the patients (56.1%) reported no treated bleeds, 90% of patients reported 0-3 treated bleeds, and 85% of patients did not require treatment for targeted joint bleeds.
When untreated bleeds were included, the model-based annualized bleed rate was 4.5, while the median annualized bleed rate was 2.1. Almost one-third of patients (29%) had no bleeding events of any kind and most (80%) had 0-3 treated or untreated bleeds.
Overall, treatment was well tolerated, with no patients withdrawing from the study, discontinuing treatment, or requiring dose modifications. Laboratory parameters remained stable throughout. The most common treatment-related adverse event was injection-site reaction (22%), followed distantly by pre-syncope, chills, rash, and erythema, each of which occurred in 2% of patients.
“Overall, the results of HAVEN 4 are consistent with the findings of other HAVEN studies,” the investigators wrote. “The option of treatment with emicizumab every 4 weeks broadens the range of administration frequencies and allows clinicians to tailor treatment to each patient’s needs and preferences.”
F. Hoffman-La Roche and Chugai funded the study. The investigators reported financial relationships with the study sponsors and other companies.
SOURCE: Pipe SW et al. Lancet Haem. 2019 Apr 16. doi: 10.1016/S2352-3026(19)30054-7.
For many patients with hemophilia A, with or without inhibitors, a monthly emicizumab injection is enough to ensure a high level of bleed control, based on results from the ongoing HAVEN 4 trial.
Most patients reported three or fewer treated bleeds, while slightly more than half had no treated bleeds at all, according to lead author Steven W. Pipe, MD, of the University of Michigan, Ann Arbor, and his colleagues. The investigators noted that results from this trial have already led to approval of a monthly dosing schedule in the United States and several other countries.
“This convenient regimen has the potential to improve the care of patients by decreasing their treatment burden, and increasing uptake and adherence to effective prophylaxis, which is known to decrease the development of debilitating secondary complications,” the investigators wrote. The report is in The Lancet Haematology.
The data were collected at 20 centers in 8 countries. Eligibility required that patients have severe congenital hemophilia A (less than 1% normal FVIII activity), or hemophilia A with FVIII inhibitors and concurrent treatment with bypassing agents or FVIII concentrates.
An initial run-in cohort that included seven patients assessed pharmacokinetics and safety. These patients received 6 mg/kg of emicizumab subcutaneously every 4 weeks for at least 24 weeks. After this group showed good responses, 41 additional patients were enrolled in an expansion cohort, which involved an initial loading phase of weekly doses at 3 mg/kg for the first month, followed by monthly dosing at 6 mg/kg for at least 6 months (24 weeks).
The efficacy endpoint of the study was bleed prevention, as measured by treated target joint bleeds, treated joint bleeds, treated spontaneous bleeds, all bleeds (untreated and treated), and annualized bleed rates for treated bleeds.
In the expansion cohort, the median number of bleeds in the 24-week period preceding enrollment was five. In the same group, five patients (12%) had FVIII inhibitors and 61% of patients exhibited at least one target joint.
After a median treatment of 25.6 weeks, the model-based annualized bleed rate for treated bleeds was 2.4, while the median annualized bleed rate was zero.
Slightly more than half of the patients (56.1%) reported no treated bleeds, 90% of patients reported 0-3 treated bleeds, and 85% of patients did not require treatment for targeted joint bleeds.
When untreated bleeds were included, the model-based annualized bleed rate was 4.5, while the median annualized bleed rate was 2.1. Almost one-third of patients (29%) had no bleeding events of any kind and most (80%) had 0-3 treated or untreated bleeds.
Overall, treatment was well tolerated, with no patients withdrawing from the study, discontinuing treatment, or requiring dose modifications. Laboratory parameters remained stable throughout. The most common treatment-related adverse event was injection-site reaction (22%), followed distantly by pre-syncope, chills, rash, and erythema, each of which occurred in 2% of patients.
“Overall, the results of HAVEN 4 are consistent with the findings of other HAVEN studies,” the investigators wrote. “The option of treatment with emicizumab every 4 weeks broadens the range of administration frequencies and allows clinicians to tailor treatment to each patient’s needs and preferences.”
F. Hoffman-La Roche and Chugai funded the study. The investigators reported financial relationships with the study sponsors and other companies.
SOURCE: Pipe SW et al. Lancet Haem. 2019 Apr 16. doi: 10.1016/S2352-3026(19)30054-7.
For many patients with hemophilia A, with or without inhibitors, a monthly emicizumab injection is enough to ensure a high level of bleed control, based on results from the ongoing HAVEN 4 trial.
Most patients reported three or fewer treated bleeds, while slightly more than half had no treated bleeds at all, according to lead author Steven W. Pipe, MD, of the University of Michigan, Ann Arbor, and his colleagues. The investigators noted that results from this trial have already led to approval of a monthly dosing schedule in the United States and several other countries.
“This convenient regimen has the potential to improve the care of patients by decreasing their treatment burden, and increasing uptake and adherence to effective prophylaxis, which is known to decrease the development of debilitating secondary complications,” the investigators wrote. The report is in The Lancet Haematology.
The data were collected at 20 centers in 8 countries. Eligibility required that patients have severe congenital hemophilia A (less than 1% normal FVIII activity), or hemophilia A with FVIII inhibitors and concurrent treatment with bypassing agents or FVIII concentrates.
An initial run-in cohort that included seven patients assessed pharmacokinetics and safety. These patients received 6 mg/kg of emicizumab subcutaneously every 4 weeks for at least 24 weeks. After this group showed good responses, 41 additional patients were enrolled in an expansion cohort, which involved an initial loading phase of weekly doses at 3 mg/kg for the first month, followed by monthly dosing at 6 mg/kg for at least 6 months (24 weeks).
The efficacy endpoint of the study was bleed prevention, as measured by treated target joint bleeds, treated joint bleeds, treated spontaneous bleeds, all bleeds (untreated and treated), and annualized bleed rates for treated bleeds.
In the expansion cohort, the median number of bleeds in the 24-week period preceding enrollment was five. In the same group, five patients (12%) had FVIII inhibitors and 61% of patients exhibited at least one target joint.
After a median treatment of 25.6 weeks, the model-based annualized bleed rate for treated bleeds was 2.4, while the median annualized bleed rate was zero.
Slightly more than half of the patients (56.1%) reported no treated bleeds, 90% of patients reported 0-3 treated bleeds, and 85% of patients did not require treatment for targeted joint bleeds.
When untreated bleeds were included, the model-based annualized bleed rate was 4.5, while the median annualized bleed rate was 2.1. Almost one-third of patients (29%) had no bleeding events of any kind and most (80%) had 0-3 treated or untreated bleeds.
Overall, treatment was well tolerated, with no patients withdrawing from the study, discontinuing treatment, or requiring dose modifications. Laboratory parameters remained stable throughout. The most common treatment-related adverse event was injection-site reaction (22%), followed distantly by pre-syncope, chills, rash, and erythema, each of which occurred in 2% of patients.
“Overall, the results of HAVEN 4 are consistent with the findings of other HAVEN studies,” the investigators wrote. “The option of treatment with emicizumab every 4 weeks broadens the range of administration frequencies and allows clinicians to tailor treatment to each patient’s needs and preferences.”
F. Hoffman-La Roche and Chugai funded the study. The investigators reported financial relationships with the study sponsors and other companies.
SOURCE: Pipe SW et al. Lancet Haem. 2019 Apr 16. doi: 10.1016/S2352-3026(19)30054-7.
FROM THE LANCET HAEMATOLOGY