User login
Plant-based diet lowers risk of heart failure
according to an analysis published online in the Journal of the American College of Cardiology.
Conversely, a Southern diet, defined as favoring fried and processed foods, is associated with an increased risk of heart failure. The results support a population-based dietary strategy for decreasing the risk of incident heart failure, according to the investigators.
Campaigns to prevent heart failure often emphasize the maintenance of a healthy diet and weight; however, little research has examined the relationship between dietary patterns and incident heart failure in patients without coronary heart disease.
Kyla M. Lara, MD, postgraduate fellow of cardiology and general internal medicine at the Icahn School of Medicine at Mount Sinai, New York, and colleagues sought to analyze the associations between five dietary patterns and incident hospitalizations for heart failure among adults in the United States. They examined data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) trial, a prospective study of black and white adults who were followed from 2003-2007 to 2014. Eligible participants completed a food frequency questionnaire and had no coronary heart disease or heart failure at baseline.
The REGARDS researchers’ principal component analysis identified the following five dietary patterns: convenience (for example, Mexican and Chinese dishes and fast food), plant based (for example, vegetables, fruit, and fish), sweets (for example, desserts, breads, and candy), Southern (for example, fried food, processed meats, and sugary beverages), and alcohol/salads. Dr. Lara and colleagues chose incident heart failure hospitalization as their primary endpoint.
The investigators included 16,068 participants in their analysis. Mean age was 64 years, roughly 59% of the sample were women, and 34% were black.
After a median 8.7 years of follow-up, 363 participants had incident heart failure hospitalizations. The highest quartile of adherence to the plant-based dietary pattern was associated with a 41% lower risk of heart failure in multivariate models, compared with the lowest quartile. The highest adherence to the Southern dietary pattern was linked with a 72% higher risk of heart failure after adjustments for age, sex, race, and other potential confounders such as education, income, smoking, and physical activity.
After further adjustments for body mass index, waist circumference, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and chronic kidney disease, the association was attenuated and no longer statistically significant. Dr. Lara and colleagues found no statistically significant associations between incident heart failure with reduced or preserved ejection fraction hospitalizations and the dietary patterns. They also found no associations with the other three dietary patterns.
One researcher reported receiving research funding from Amgen and has consulted for Novartis. The other researchers reported no relevant conflicts.
SOURCE: Lara KM et al. J Am Coll Cardiol. 2019 Apr 30;73(16):2036-45.
This analysis of the REGARDS study contributes toward creating a strong evidence base for the prevention of heart failure through dietary measures, wrote Dong D. Wang, SCD, MD, a visiting scientist at Harvard School of Public Health, Boston, in an accompanying editorial. Empirically derived dietary patterns, such as those described in this study, can form the basis for recommendations easily, he added. “We usually have greater confidence when interpreting the associations with dietary patterns as causal than we have for the associations with specific nutrients or foods. Furthermore, the findings are particularly useful for making recommendations to a general population because of their use of a baseline coronary heart disease–free study population and the inclusion of black participants with greater susceptibility to heart failure. Thus, this study possesses a great potential of informing the population-level strategies for the prevention of heart failure.”
Nutritional epidemiologic studies examining subtypes of heart failure are valuable in light of the disease’s phenotypic and pathophysiological heterogeneity, Dr. Wang wrote. “These findings, if confirmed in future studies, will not only contribute to in-depth biological understanding and phenotypic refinement of heart failure, but also inform dietary prevention approaches customized for specific heart failure phenotypes. In addition, they perfectly fit into key missions of precision medicine [i.e., understanding large variability between individuals in both the development and the clinical manifestations of the specific disease, as well as variability in individual’s response to dietary, lifestyle, and pharmacological interventions].”
Dr. Wang reported no relationships relevant to the contents of this paper.
This analysis of the REGARDS study contributes toward creating a strong evidence base for the prevention of heart failure through dietary measures, wrote Dong D. Wang, SCD, MD, a visiting scientist at Harvard School of Public Health, Boston, in an accompanying editorial. Empirically derived dietary patterns, such as those described in this study, can form the basis for recommendations easily, he added. “We usually have greater confidence when interpreting the associations with dietary patterns as causal than we have for the associations with specific nutrients or foods. Furthermore, the findings are particularly useful for making recommendations to a general population because of their use of a baseline coronary heart disease–free study population and the inclusion of black participants with greater susceptibility to heart failure. Thus, this study possesses a great potential of informing the population-level strategies for the prevention of heart failure.”
Nutritional epidemiologic studies examining subtypes of heart failure are valuable in light of the disease’s phenotypic and pathophysiological heterogeneity, Dr. Wang wrote. “These findings, if confirmed in future studies, will not only contribute to in-depth biological understanding and phenotypic refinement of heart failure, but also inform dietary prevention approaches customized for specific heart failure phenotypes. In addition, they perfectly fit into key missions of precision medicine [i.e., understanding large variability between individuals in both the development and the clinical manifestations of the specific disease, as well as variability in individual’s response to dietary, lifestyle, and pharmacological interventions].”
Dr. Wang reported no relationships relevant to the contents of this paper.
This analysis of the REGARDS study contributes toward creating a strong evidence base for the prevention of heart failure through dietary measures, wrote Dong D. Wang, SCD, MD, a visiting scientist at Harvard School of Public Health, Boston, in an accompanying editorial. Empirically derived dietary patterns, such as those described in this study, can form the basis for recommendations easily, he added. “We usually have greater confidence when interpreting the associations with dietary patterns as causal than we have for the associations with specific nutrients or foods. Furthermore, the findings are particularly useful for making recommendations to a general population because of their use of a baseline coronary heart disease–free study population and the inclusion of black participants with greater susceptibility to heart failure. Thus, this study possesses a great potential of informing the population-level strategies for the prevention of heart failure.”
Nutritional epidemiologic studies examining subtypes of heart failure are valuable in light of the disease’s phenotypic and pathophysiological heterogeneity, Dr. Wang wrote. “These findings, if confirmed in future studies, will not only contribute to in-depth biological understanding and phenotypic refinement of heart failure, but also inform dietary prevention approaches customized for specific heart failure phenotypes. In addition, they perfectly fit into key missions of precision medicine [i.e., understanding large variability between individuals in both the development and the clinical manifestations of the specific disease, as well as variability in individual’s response to dietary, lifestyle, and pharmacological interventions].”
Dr. Wang reported no relationships relevant to the contents of this paper.
according to an analysis published online in the Journal of the American College of Cardiology.
Conversely, a Southern diet, defined as favoring fried and processed foods, is associated with an increased risk of heart failure. The results support a population-based dietary strategy for decreasing the risk of incident heart failure, according to the investigators.
Campaigns to prevent heart failure often emphasize the maintenance of a healthy diet and weight; however, little research has examined the relationship between dietary patterns and incident heart failure in patients without coronary heart disease.
Kyla M. Lara, MD, postgraduate fellow of cardiology and general internal medicine at the Icahn School of Medicine at Mount Sinai, New York, and colleagues sought to analyze the associations between five dietary patterns and incident hospitalizations for heart failure among adults in the United States. They examined data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) trial, a prospective study of black and white adults who were followed from 2003-2007 to 2014. Eligible participants completed a food frequency questionnaire and had no coronary heart disease or heart failure at baseline.
The REGARDS researchers’ principal component analysis identified the following five dietary patterns: convenience (for example, Mexican and Chinese dishes and fast food), plant based (for example, vegetables, fruit, and fish), sweets (for example, desserts, breads, and candy), Southern (for example, fried food, processed meats, and sugary beverages), and alcohol/salads. Dr. Lara and colleagues chose incident heart failure hospitalization as their primary endpoint.
The investigators included 16,068 participants in their analysis. Mean age was 64 years, roughly 59% of the sample were women, and 34% were black.
After a median 8.7 years of follow-up, 363 participants had incident heart failure hospitalizations. The highest quartile of adherence to the plant-based dietary pattern was associated with a 41% lower risk of heart failure in multivariate models, compared with the lowest quartile. The highest adherence to the Southern dietary pattern was linked with a 72% higher risk of heart failure after adjustments for age, sex, race, and other potential confounders such as education, income, smoking, and physical activity.
After further adjustments for body mass index, waist circumference, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and chronic kidney disease, the association was attenuated and no longer statistically significant. Dr. Lara and colleagues found no statistically significant associations between incident heart failure with reduced or preserved ejection fraction hospitalizations and the dietary patterns. They also found no associations with the other three dietary patterns.
One researcher reported receiving research funding from Amgen and has consulted for Novartis. The other researchers reported no relevant conflicts.
SOURCE: Lara KM et al. J Am Coll Cardiol. 2019 Apr 30;73(16):2036-45.
according to an analysis published online in the Journal of the American College of Cardiology.
Conversely, a Southern diet, defined as favoring fried and processed foods, is associated with an increased risk of heart failure. The results support a population-based dietary strategy for decreasing the risk of incident heart failure, according to the investigators.
Campaigns to prevent heart failure often emphasize the maintenance of a healthy diet and weight; however, little research has examined the relationship between dietary patterns and incident heart failure in patients without coronary heart disease.
Kyla M. Lara, MD, postgraduate fellow of cardiology and general internal medicine at the Icahn School of Medicine at Mount Sinai, New York, and colleagues sought to analyze the associations between five dietary patterns and incident hospitalizations for heart failure among adults in the United States. They examined data from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) trial, a prospective study of black and white adults who were followed from 2003-2007 to 2014. Eligible participants completed a food frequency questionnaire and had no coronary heart disease or heart failure at baseline.
The REGARDS researchers’ principal component analysis identified the following five dietary patterns: convenience (for example, Mexican and Chinese dishes and fast food), plant based (for example, vegetables, fruit, and fish), sweets (for example, desserts, breads, and candy), Southern (for example, fried food, processed meats, and sugary beverages), and alcohol/salads. Dr. Lara and colleagues chose incident heart failure hospitalization as their primary endpoint.
The investigators included 16,068 participants in their analysis. Mean age was 64 years, roughly 59% of the sample were women, and 34% were black.
After a median 8.7 years of follow-up, 363 participants had incident heart failure hospitalizations. The highest quartile of adherence to the plant-based dietary pattern was associated with a 41% lower risk of heart failure in multivariate models, compared with the lowest quartile. The highest adherence to the Southern dietary pattern was linked with a 72% higher risk of heart failure after adjustments for age, sex, race, and other potential confounders such as education, income, smoking, and physical activity.
After further adjustments for body mass index, waist circumference, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and chronic kidney disease, the association was attenuated and no longer statistically significant. Dr. Lara and colleagues found no statistically significant associations between incident heart failure with reduced or preserved ejection fraction hospitalizations and the dietary patterns. They also found no associations with the other three dietary patterns.
One researcher reported receiving research funding from Amgen and has consulted for Novartis. The other researchers reported no relevant conflicts.
SOURCE: Lara KM et al. J Am Coll Cardiol. 2019 Apr 30;73(16):2036-45.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Plant-based and Southern diets influence the risk of heart failure.
Major finding: Adherence to a plant-based diet reduces the risk of heart failure by 41%.
Study details: An analysis of data for 16,068 participants in the REGARDS study.
Disclosures: One coauthor reported receiving research funding from Amgen and has consulted for Novartis.
Source: Lara KM et al. J Am Coll Cardiol. 2019 Apr 30;73(16):2036-45.
Factors emerge for mitigating CD19 CAR T toxicity
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
REPORTING FROM TCT 2019
Pulmonologist: In COPD, try dual therapy before adding corticosteroid
PHILADELPHIA – While triple therapy is effective for patients with chronic obstructive pulmonary disease (COPD), not all patients actually need the inhaled corticosteroid component to reduce exacerbations, a Mayo Clinic pulmonologist said at the annual meeting of the American College of Physicians.
“When you’re increasing therapy, we can go to a dual-bronchodilator combination before adding corticosteroids,” Megan Dulohery Scrodin, MD, of Mayo Clinic, Rochester, Minn., noted in a well-attended session.
That approach came as news to many internists, at least going by results of an audience poll in which 76% of attendees picked triple therapy for a 65-year-old male with COPD and frequent exacerbations despite having used a long-acting muscarinic antagonist (LAMA). Only 10% picked what Dr. Dulohery Scrodin said was optimal: to keep the patient on the LAMA, and add a long-acting beta-agonist (LABA).
“I would encourage you to do this as a stepwise process,” Dr. Dulohery Scrodin told attendees after seeing those poll results.
For a patient with minimal symptoms and few exacerbations, the best approach is a short-acting bronchodilator plus smoking cessation, avoidance of environmental triggers, and keeping up to date with vaccinations, she said.
For patients with more severe symptoms or frequent exacerbations, adding a LAMA or LABA would be warranted, along with considering pulmonary rehabilitation.
“There’s been studies comparing long-acting muscarinic antagonists to long-acting beta agonists, and the long-acting muscarinic antagonists like tiotropium seem to be superior,” she said. “So I always do a LAMA inhaler first.”
For patients still having exacerbations despite one long-acting bronchodilator, the best approach would be to add the second bronchodilator, and if that still doesn’t work, she said, add an inhaled corticosteroid and consider a pulmonary consultation for advanced therapy.
“If the patient doesn’t need an inhaled corticosteroid, we try to avoid it and use dual bronchodilator therapy,” said Dr. Dulohery Scrodin, noting that inhaled corticosteroids are associated with increased risk of pneumonia, along with other complications such as dysphonia and oral candidiasis.
In studies, single-inhaler triple therapy with fluticasone furoate, umeclidinium, and vilanterol does seem to reduce exacerbations more than LABA/LAMA combination therapy or LABA/inhaled corticosteroid treatment, but that doesn’t necessarily mean it should be automatically chosen over dual therapy, the presenter noted.
“Similar to asthma, I would do the least amount of therapy that your patient gets under control,” she told the audience.
Dr. Dulohery Scrodin reported that she had no relevant disclosures.
PHILADELPHIA – While triple therapy is effective for patients with chronic obstructive pulmonary disease (COPD), not all patients actually need the inhaled corticosteroid component to reduce exacerbations, a Mayo Clinic pulmonologist said at the annual meeting of the American College of Physicians.
“When you’re increasing therapy, we can go to a dual-bronchodilator combination before adding corticosteroids,” Megan Dulohery Scrodin, MD, of Mayo Clinic, Rochester, Minn., noted in a well-attended session.
That approach came as news to many internists, at least going by results of an audience poll in which 76% of attendees picked triple therapy for a 65-year-old male with COPD and frequent exacerbations despite having used a long-acting muscarinic antagonist (LAMA). Only 10% picked what Dr. Dulohery Scrodin said was optimal: to keep the patient on the LAMA, and add a long-acting beta-agonist (LABA).
“I would encourage you to do this as a stepwise process,” Dr. Dulohery Scrodin told attendees after seeing those poll results.
For a patient with minimal symptoms and few exacerbations, the best approach is a short-acting bronchodilator plus smoking cessation, avoidance of environmental triggers, and keeping up to date with vaccinations, she said.
For patients with more severe symptoms or frequent exacerbations, adding a LAMA or LABA would be warranted, along with considering pulmonary rehabilitation.
“There’s been studies comparing long-acting muscarinic antagonists to long-acting beta agonists, and the long-acting muscarinic antagonists like tiotropium seem to be superior,” she said. “So I always do a LAMA inhaler first.”
For patients still having exacerbations despite one long-acting bronchodilator, the best approach would be to add the second bronchodilator, and if that still doesn’t work, she said, add an inhaled corticosteroid and consider a pulmonary consultation for advanced therapy.
“If the patient doesn’t need an inhaled corticosteroid, we try to avoid it and use dual bronchodilator therapy,” said Dr. Dulohery Scrodin, noting that inhaled corticosteroids are associated with increased risk of pneumonia, along with other complications such as dysphonia and oral candidiasis.
In studies, single-inhaler triple therapy with fluticasone furoate, umeclidinium, and vilanterol does seem to reduce exacerbations more than LABA/LAMA combination therapy or LABA/inhaled corticosteroid treatment, but that doesn’t necessarily mean it should be automatically chosen over dual therapy, the presenter noted.
“Similar to asthma, I would do the least amount of therapy that your patient gets under control,” she told the audience.
Dr. Dulohery Scrodin reported that she had no relevant disclosures.
PHILADELPHIA – While triple therapy is effective for patients with chronic obstructive pulmonary disease (COPD), not all patients actually need the inhaled corticosteroid component to reduce exacerbations, a Mayo Clinic pulmonologist said at the annual meeting of the American College of Physicians.
“When you’re increasing therapy, we can go to a dual-bronchodilator combination before adding corticosteroids,” Megan Dulohery Scrodin, MD, of Mayo Clinic, Rochester, Minn., noted in a well-attended session.
That approach came as news to many internists, at least going by results of an audience poll in which 76% of attendees picked triple therapy for a 65-year-old male with COPD and frequent exacerbations despite having used a long-acting muscarinic antagonist (LAMA). Only 10% picked what Dr. Dulohery Scrodin said was optimal: to keep the patient on the LAMA, and add a long-acting beta-agonist (LABA).
“I would encourage you to do this as a stepwise process,” Dr. Dulohery Scrodin told attendees after seeing those poll results.
For a patient with minimal symptoms and few exacerbations, the best approach is a short-acting bronchodilator plus smoking cessation, avoidance of environmental triggers, and keeping up to date with vaccinations, she said.
For patients with more severe symptoms or frequent exacerbations, adding a LAMA or LABA would be warranted, along with considering pulmonary rehabilitation.
“There’s been studies comparing long-acting muscarinic antagonists to long-acting beta agonists, and the long-acting muscarinic antagonists like tiotropium seem to be superior,” she said. “So I always do a LAMA inhaler first.”
For patients still having exacerbations despite one long-acting bronchodilator, the best approach would be to add the second bronchodilator, and if that still doesn’t work, she said, add an inhaled corticosteroid and consider a pulmonary consultation for advanced therapy.
“If the patient doesn’t need an inhaled corticosteroid, we try to avoid it and use dual bronchodilator therapy,” said Dr. Dulohery Scrodin, noting that inhaled corticosteroids are associated with increased risk of pneumonia, along with other complications such as dysphonia and oral candidiasis.
In studies, single-inhaler triple therapy with fluticasone furoate, umeclidinium, and vilanterol does seem to reduce exacerbations more than LABA/LAMA combination therapy or LABA/inhaled corticosteroid treatment, but that doesn’t necessarily mean it should be automatically chosen over dual therapy, the presenter noted.
“Similar to asthma, I would do the least amount of therapy that your patient gets under control,” she told the audience.
Dr. Dulohery Scrodin reported that she had no relevant disclosures.
REPORTING FROM INTERNAL MEDICINE 2019
U.S. measles cases nearing postelimination-era high
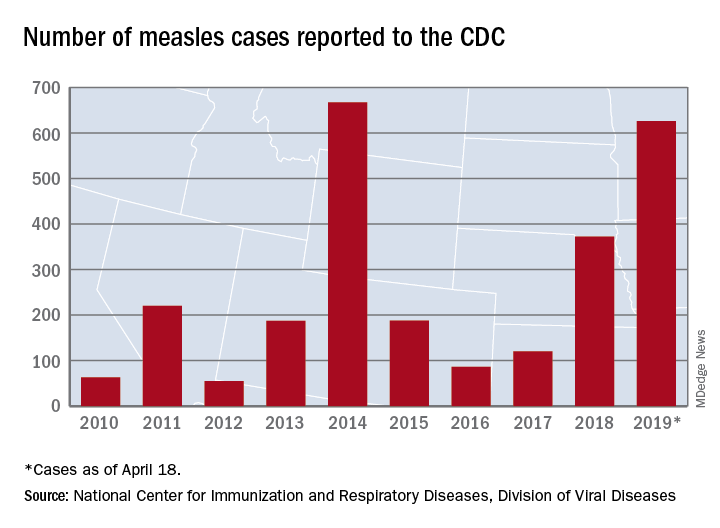
The United States has topped 600 cases of measles for 2019 and is likely to pass the postelimination high set in 2014 “in the coming weeks,” according to the Centers for Disease Control and Prevention.
The 71 new measles cases reported during the week ending April 18 bring the total for the year to 626 in 22 states, the CDC reported April 22. Two states, Iowa and Tennessee, reported their first cases last week.
Outbreaks continue in five states: one in California (Butte County), one in Michigan (Oakland County/Wayne County/Detroit), one in New Jersey (Ocean County/Monmouth County), two in New York (New York City and Rockland County), and one in Washington (Clark County/King County), the CDC said.
The most active outbreak since mid-February has been the one occurring in New York City, mainly in Brooklyn, and last week was no exception as 50 of the 71 new U.S. cases were reported in the borough.
On April 18, a judge in Brooklyn “ruled against a group of parents who challenged New York City’s recently imposed mandatory measles vaccination order,” Reuters reported. That same day, the city issued a summons, subject to a fine of $1,000 each, to three people in Brooklyn who were still unvaccinated, according to NYC Health, which also said that four additional schools would be closed for not complying with an order to exclude unvaccinated students.
On April 15, the Iowa Department of Public Health confirmed the state’s first case of measles since 2011. The individual from Northeastern Iowa had not been vaccinated and had recently returned from Israel. The state’s second case of the year, a household contact of the first individual, was confirmed on April 18.
Also on April 18, the Tennessee Department of Health confirmed its first case of the year in a resident of the eastern part of the state. Meanwhile, media are reporting that state health officials in Mississippi are investigating possible exposures on April 9 and 10 in the Hattiesburg area by the infected Tennessee man.
Outside the United States, “many countries are in the midst of sizeable measles outbreaks, with all regions of the world experiencing sustained rises in cases,” the World Health Organization said. Current outbreaks include the Democratic Republic of the Congo, Ethiopia, Georgia, Kazakhstan, Kyrgyzstan, Madagascar, Myanmar, Philippines, Sudan, Thailand, and Ukraine.
Preliminary data for the first 3 months of 2019 show that cases worldwide were up by 300% over the first 3 months of 2018: 112,163 cases vs. 28,124. The actual numbers for 2019 are expected to be considerably higher than those reported so far, and WHO estimates that, globally, less than 1 in 10 cases are actually reported.
The United States has topped 600 cases of measles for 2019 and is likely to pass the postelimination high set in 2014 “in the coming weeks,” according to the Centers for Disease Control and Prevention.
The 71 new measles cases reported during the week ending April 18 bring the total for the year to 626 in 22 states, the CDC reported April 22. Two states, Iowa and Tennessee, reported their first cases last week.
Outbreaks continue in five states: one in California (Butte County), one in Michigan (Oakland County/Wayne County/Detroit), one in New Jersey (Ocean County/Monmouth County), two in New York (New York City and Rockland County), and one in Washington (Clark County/King County), the CDC said.
The most active outbreak since mid-February has been the one occurring in New York City, mainly in Brooklyn, and last week was no exception as 50 of the 71 new U.S. cases were reported in the borough.
On April 18, a judge in Brooklyn “ruled against a group of parents who challenged New York City’s recently imposed mandatory measles vaccination order,” Reuters reported. That same day, the city issued a summons, subject to a fine of $1,000 each, to three people in Brooklyn who were still unvaccinated, according to NYC Health, which also said that four additional schools would be closed for not complying with an order to exclude unvaccinated students.
On April 15, the Iowa Department of Public Health confirmed the state’s first case of measles since 2011. The individual from Northeastern Iowa had not been vaccinated and had recently returned from Israel. The state’s second case of the year, a household contact of the first individual, was confirmed on April 18.
Also on April 18, the Tennessee Department of Health confirmed its first case of the year in a resident of the eastern part of the state. Meanwhile, media are reporting that state health officials in Mississippi are investigating possible exposures on April 9 and 10 in the Hattiesburg area by the infected Tennessee man.
Outside the United States, “many countries are in the midst of sizeable measles outbreaks, with all regions of the world experiencing sustained rises in cases,” the World Health Organization said. Current outbreaks include the Democratic Republic of the Congo, Ethiopia, Georgia, Kazakhstan, Kyrgyzstan, Madagascar, Myanmar, Philippines, Sudan, Thailand, and Ukraine.
Preliminary data for the first 3 months of 2019 show that cases worldwide were up by 300% over the first 3 months of 2018: 112,163 cases vs. 28,124. The actual numbers for 2019 are expected to be considerably higher than those reported so far, and WHO estimates that, globally, less than 1 in 10 cases are actually reported.
The United States has topped 600 cases of measles for 2019 and is likely to pass the postelimination high set in 2014 “in the coming weeks,” according to the Centers for Disease Control and Prevention.
The 71 new measles cases reported during the week ending April 18 bring the total for the year to 626 in 22 states, the CDC reported April 22. Two states, Iowa and Tennessee, reported their first cases last week.
Outbreaks continue in five states: one in California (Butte County), one in Michigan (Oakland County/Wayne County/Detroit), one in New Jersey (Ocean County/Monmouth County), two in New York (New York City and Rockland County), and one in Washington (Clark County/King County), the CDC said.
The most active outbreak since mid-February has been the one occurring in New York City, mainly in Brooklyn, and last week was no exception as 50 of the 71 new U.S. cases were reported in the borough.
On April 18, a judge in Brooklyn “ruled against a group of parents who challenged New York City’s recently imposed mandatory measles vaccination order,” Reuters reported. That same day, the city issued a summons, subject to a fine of $1,000 each, to three people in Brooklyn who were still unvaccinated, according to NYC Health, which also said that four additional schools would be closed for not complying with an order to exclude unvaccinated students.
On April 15, the Iowa Department of Public Health confirmed the state’s first case of measles since 2011. The individual from Northeastern Iowa had not been vaccinated and had recently returned from Israel. The state’s second case of the year, a household contact of the first individual, was confirmed on April 18.
Also on April 18, the Tennessee Department of Health confirmed its first case of the year in a resident of the eastern part of the state. Meanwhile, media are reporting that state health officials in Mississippi are investigating possible exposures on April 9 and 10 in the Hattiesburg area by the infected Tennessee man.
Outside the United States, “many countries are in the midst of sizeable measles outbreaks, with all regions of the world experiencing sustained rises in cases,” the World Health Organization said. Current outbreaks include the Democratic Republic of the Congo, Ethiopia, Georgia, Kazakhstan, Kyrgyzstan, Madagascar, Myanmar, Philippines, Sudan, Thailand, and Ukraine.
Preliminary data for the first 3 months of 2019 show that cases worldwide were up by 300% over the first 3 months of 2018: 112,163 cases vs. 28,124. The actual numbers for 2019 are expected to be considerably higher than those reported so far, and WHO estimates that, globally, less than 1 in 10 cases are actually reported.
Osimertinib again shows strength in NSCLC with leptomeningeal metastases
GENEVA – Treatment with osimertinib leads to clinically meaningful responses in about half of patients with epidermal growth factor receptor (EGFR) T790M–positive non–small cell lung cancer (NSCLC) who have asymptomatic leptomeningeal metastases, according to a post hoc analysis of patients from multiple AURA studies.
This conclusion aligns with previous encouraging findings delivered by the BLOOM trial, with the notable caveat that AURA patients received the Food and Drug Administration–approved dose of 80 mg osimertinib daily, instead of 160 mg, as given to BLOOM patients, reported lead author Myung Ju Ahn, MD, PhD, of Samsung Medical Center in Seoul, South Korea, and her colleagues.
At the European Lung Cancer Congress, invited discussant Pasi A. Jänne, MD, PhD of the Dana-Farber Cancer Institute in Boston, provided additional background for the study. “Leptomeningeal disease is really a devastating complication for our patients with lung cancer,” Dr. Jänne said, noting that effective treatments have been historically lacking; apart from osimertinib, other treatment strategies have included whole brain radiation therapy, high-dose pemetrexed, and pulsatile erlotinib, which were largely based on anecdotal evidence. Like other next-generation tyrosine kinase inhibitors, osimertinib stands apart from older agents because of its greater ability to penetrate the blood-brain barrier.
The present, retrospective analysis involved 22 patients with advanced, EGFR T790M–positive NSCLC with asymptomatic leptomeningeal metastases (LM) that was radiographically detected by blinded independent review. Patients received 80 mg osimertinib daily after progressing on another EGFR tyrosine kinase inhibitor. Follow-up brain scans were evaluated using Response Assessment in Neuro-Oncology LM criteria. Median overall survival was determined, as were progression-free survival, duration of response, and objective response rate, with these latter parameters analyzed specifically for LM disease.
Demographically, the patient population was consistent with previous AURA trials, with a predominance of Asian (82%) and female (59%) patients. Patients received treatment for a median of 7.3 months. Analysis showed that slightly more than half of patients (55%) responded to therapy, with an even split between partial (27%) and complete responders (27%). Median progression-free survival reached almost 1 year (11.1 months), while overall survival exceeded a year and a half (18.8 months), with a 1-year overall survival rate of 65%. Duration of response data are still immature. Graphical longitudinal analysis showed comparable responses between the AURA and BLOOM trials, suggesting that an 80-mg dose is likely to provide a similar efficacy to a 160-mg dose, Dr. Ahn said, although she also urged a cautionary interpretation because of study design.
Dr. Ahn described the survival statistics as “encouraging” at the meeting presented by the European Society for Medical Oncology, as the outcomes were better than those typically seen in historical controls.
Discussing these findings, Dr. Jänne suggested that the patient assessment criteria were “pretty subjective in nature.”
“You could get a score of plus one or minus one if the scans are kind of better or kind of worse,” Dr. Jänne said. “There’s really no objective criteria there.” He noted that imaging results may not reflect clinical impact of LM disease, and suggested that additional assessment criteria would have been welcome, such as assessments involving neurologic status or cerebrospinal fluid characteristics, both of which were included in the BLOOM trial.
“The real question comes, when we’re facing someone with leptomeningeal disease, is 80 milligrams as effective as 160 milligrams?” Dr. Jänne asked. “I don’t think we have the answer to that because the [AURA and BLOOM] studies are different, including different endpoints, and they’re not comparative.” He also noted that, in the United States, when met with LM disease progression, clinicians commonly increase the osimertinib dose from 80 mg to 160 mg; however, “it is without any data,” and warrants an actual clinical study.
The study was funded by AstraZeneca. The investigators reported financial relationships with Novartis, Pfizer, Roche, and others.
SOURCE: Ahn MJ et al. ELCC 2019, Abstract 105O.
GENEVA – Treatment with osimertinib leads to clinically meaningful responses in about half of patients with epidermal growth factor receptor (EGFR) T790M–positive non–small cell lung cancer (NSCLC) who have asymptomatic leptomeningeal metastases, according to a post hoc analysis of patients from multiple AURA studies.
This conclusion aligns with previous encouraging findings delivered by the BLOOM trial, with the notable caveat that AURA patients received the Food and Drug Administration–approved dose of 80 mg osimertinib daily, instead of 160 mg, as given to BLOOM patients, reported lead author Myung Ju Ahn, MD, PhD, of Samsung Medical Center in Seoul, South Korea, and her colleagues.
At the European Lung Cancer Congress, invited discussant Pasi A. Jänne, MD, PhD of the Dana-Farber Cancer Institute in Boston, provided additional background for the study. “Leptomeningeal disease is really a devastating complication for our patients with lung cancer,” Dr. Jänne said, noting that effective treatments have been historically lacking; apart from osimertinib, other treatment strategies have included whole brain radiation therapy, high-dose pemetrexed, and pulsatile erlotinib, which were largely based on anecdotal evidence. Like other next-generation tyrosine kinase inhibitors, osimertinib stands apart from older agents because of its greater ability to penetrate the blood-brain barrier.
The present, retrospective analysis involved 22 patients with advanced, EGFR T790M–positive NSCLC with asymptomatic leptomeningeal metastases (LM) that was radiographically detected by blinded independent review. Patients received 80 mg osimertinib daily after progressing on another EGFR tyrosine kinase inhibitor. Follow-up brain scans were evaluated using Response Assessment in Neuro-Oncology LM criteria. Median overall survival was determined, as were progression-free survival, duration of response, and objective response rate, with these latter parameters analyzed specifically for LM disease.
Demographically, the patient population was consistent with previous AURA trials, with a predominance of Asian (82%) and female (59%) patients. Patients received treatment for a median of 7.3 months. Analysis showed that slightly more than half of patients (55%) responded to therapy, with an even split between partial (27%) and complete responders (27%). Median progression-free survival reached almost 1 year (11.1 months), while overall survival exceeded a year and a half (18.8 months), with a 1-year overall survival rate of 65%. Duration of response data are still immature. Graphical longitudinal analysis showed comparable responses between the AURA and BLOOM trials, suggesting that an 80-mg dose is likely to provide a similar efficacy to a 160-mg dose, Dr. Ahn said, although she also urged a cautionary interpretation because of study design.
Dr. Ahn described the survival statistics as “encouraging” at the meeting presented by the European Society for Medical Oncology, as the outcomes were better than those typically seen in historical controls.
Discussing these findings, Dr. Jänne suggested that the patient assessment criteria were “pretty subjective in nature.”
“You could get a score of plus one or minus one if the scans are kind of better or kind of worse,” Dr. Jänne said. “There’s really no objective criteria there.” He noted that imaging results may not reflect clinical impact of LM disease, and suggested that additional assessment criteria would have been welcome, such as assessments involving neurologic status or cerebrospinal fluid characteristics, both of which were included in the BLOOM trial.
“The real question comes, when we’re facing someone with leptomeningeal disease, is 80 milligrams as effective as 160 milligrams?” Dr. Jänne asked. “I don’t think we have the answer to that because the [AURA and BLOOM] studies are different, including different endpoints, and they’re not comparative.” He also noted that, in the United States, when met with LM disease progression, clinicians commonly increase the osimertinib dose from 80 mg to 160 mg; however, “it is without any data,” and warrants an actual clinical study.
The study was funded by AstraZeneca. The investigators reported financial relationships with Novartis, Pfizer, Roche, and others.
SOURCE: Ahn MJ et al. ELCC 2019, Abstract 105O.
GENEVA – Treatment with osimertinib leads to clinically meaningful responses in about half of patients with epidermal growth factor receptor (EGFR) T790M–positive non–small cell lung cancer (NSCLC) who have asymptomatic leptomeningeal metastases, according to a post hoc analysis of patients from multiple AURA studies.
This conclusion aligns with previous encouraging findings delivered by the BLOOM trial, with the notable caveat that AURA patients received the Food and Drug Administration–approved dose of 80 mg osimertinib daily, instead of 160 mg, as given to BLOOM patients, reported lead author Myung Ju Ahn, MD, PhD, of Samsung Medical Center in Seoul, South Korea, and her colleagues.
At the European Lung Cancer Congress, invited discussant Pasi A. Jänne, MD, PhD of the Dana-Farber Cancer Institute in Boston, provided additional background for the study. “Leptomeningeal disease is really a devastating complication for our patients with lung cancer,” Dr. Jänne said, noting that effective treatments have been historically lacking; apart from osimertinib, other treatment strategies have included whole brain radiation therapy, high-dose pemetrexed, and pulsatile erlotinib, which were largely based on anecdotal evidence. Like other next-generation tyrosine kinase inhibitors, osimertinib stands apart from older agents because of its greater ability to penetrate the blood-brain barrier.
The present, retrospective analysis involved 22 patients with advanced, EGFR T790M–positive NSCLC with asymptomatic leptomeningeal metastases (LM) that was radiographically detected by blinded independent review. Patients received 80 mg osimertinib daily after progressing on another EGFR tyrosine kinase inhibitor. Follow-up brain scans were evaluated using Response Assessment in Neuro-Oncology LM criteria. Median overall survival was determined, as were progression-free survival, duration of response, and objective response rate, with these latter parameters analyzed specifically for LM disease.
Demographically, the patient population was consistent with previous AURA trials, with a predominance of Asian (82%) and female (59%) patients. Patients received treatment for a median of 7.3 months. Analysis showed that slightly more than half of patients (55%) responded to therapy, with an even split between partial (27%) and complete responders (27%). Median progression-free survival reached almost 1 year (11.1 months), while overall survival exceeded a year and a half (18.8 months), with a 1-year overall survival rate of 65%. Duration of response data are still immature. Graphical longitudinal analysis showed comparable responses between the AURA and BLOOM trials, suggesting that an 80-mg dose is likely to provide a similar efficacy to a 160-mg dose, Dr. Ahn said, although she also urged a cautionary interpretation because of study design.
Dr. Ahn described the survival statistics as “encouraging” at the meeting presented by the European Society for Medical Oncology, as the outcomes were better than those typically seen in historical controls.
Discussing these findings, Dr. Jänne suggested that the patient assessment criteria were “pretty subjective in nature.”
“You could get a score of plus one or minus one if the scans are kind of better or kind of worse,” Dr. Jänne said. “There’s really no objective criteria there.” He noted that imaging results may not reflect clinical impact of LM disease, and suggested that additional assessment criteria would have been welcome, such as assessments involving neurologic status or cerebrospinal fluid characteristics, both of which were included in the BLOOM trial.
“The real question comes, when we’re facing someone with leptomeningeal disease, is 80 milligrams as effective as 160 milligrams?” Dr. Jänne asked. “I don’t think we have the answer to that because the [AURA and BLOOM] studies are different, including different endpoints, and they’re not comparative.” He also noted that, in the United States, when met with LM disease progression, clinicians commonly increase the osimertinib dose from 80 mg to 160 mg; however, “it is without any data,” and warrants an actual clinical study.
The study was funded by AstraZeneca. The investigators reported financial relationships with Novartis, Pfizer, Roche, and others.
SOURCE: Ahn MJ et al. ELCC 2019, Abstract 105O.
REPORTING FROM ELCC 2019