User login
Gut bacterium R. gnavus linked to lupus flares
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
SAN FRANCISCO –
Not only that, but those patients also had highly elevated antibodies to an endotoxin-like antigen released by one particular R. gnavus strain.
That antigen is “very proinflammatory, very immunogenic. We are wondering if this is actually [what drives] the immune activation that results in immune complexes in the glomeruli” of patients with lupus nephritis, said investigator Gregg Silverman, MD, a professor of medicine and pathology and head of the laboratory of B-cell immunobiology at New York University.
R. gnavus is an obligate anaerobe found in the guts of most people, but in lupus, it might be a problem.
“We are finding a very specific relationship with lupus patients and this bacteria – and this particular antibody,” Dr. Silverman explained in an interview at an international congress on systemic lupus erythematosus. “There’s an expansion of this particular bug, but also a contraction of others” as disease activity progresses.
“It speaks to an imbalance,” he added, and it suggests a role for probiotics or even fecal transplants to restore order.
“What if instead of killing the immune system” in lupus treatment, “we should be reducing or removing a single bacterium or a single molecule?” he asked.
Dr. Silverman is one of many researchers working to unravel the role of the human microbiome in both disease and health. His findings are preliminary, and, as he cautioned, correlation is not causation. But the implications are remarkable, Dr. Silverman noted.
REPORTING FROM LUPUS 2019
Self-Assessment in Family Practice
Stem cells enabling key insights into schizophrenia
ORLANDO – Stem cell models could boost schizophrenia treatment to a new level, helping to gauge the importance of both rare and common genetic variants that are difficult to study in traditional-style trials, an expert said at the annual congress of the Schizophrenia International Research Society.
The promise of the approach is particularly crucial at this point in the course of schizophrenia research, when the heritability of the disease is being repeatedly underscored in the literature, said Kristen Brennand, PhD, associate professor of neuroscience, genetics and genomics, and psychiatry at the Icahn School of Medicine at Mount Sinai, New York.
“It seems that each new paper that comes out raises that estimate of heritability and the numbers I’ve seen recently are as high as 85%,” she said. “Schizophrenia is as heritable as autism and bipolar, more heritable than BRCA1 breast cancer, it’s more heritable than alcoholism. But that heritability is highly complex.”
Highly penetrant variants that are more easily studied account for only a small sliver of this heritability. Rare variants that are harder to study account for more. And common variants – the count is up to 145 and will almost certainly grow – also play a big role.
“These are variants that all of us carry,” Dr. Brennand said. “We all carry dozens of these variants. Patients just either carry more of them or they’re hitting pathways in a different way than they’re hitting the rest of us.”
Using human-induced pluripotent stem cells (HiPSC) – grown out of skin biopsy samples from schizophrenia patients and then grown into neural progenitor cells and ultimately neurons – are much more practical for studying the genetics of these variants than case-control studies that require tens of thousands of subjects.
Researchers have found that cohorts using HiPSCs concord with the genetic findings from postmortem datasets of schizophrenia patients.
More recently, in work not yet published, she said her lab has focused on rare 2p16.3 deletions of the NRXN1 gene, finding that neuronal branching is reduced and that there is decreased neuronal activity in schizophrenia patients with these deletions.
Her lab is also using HiPSCs and clustered regularly interspaced short palindromic repeats editing to validate the function of common variants and genes linked with schizophrenia. A key finding has been that there could be important relationships between risk genes that are more distant from schizophrenia single-nucleotide polymorphisms (SNPs).
“We’re expanding the list of potential schizophrenia risk genes by considering not just immediately proximal but also distal interactions between schizophrenia SNPs,” Dr. Brennand said. “If there are 224 genes that are next to risk SNPs, you can add a few hundred more potential genes that might be coregulated by these risk SNPs.”
Harnessing the power of HiPSCs could be the gateway to precision medicine in schizophrenia, she said. A drug that might benefit, say, two out of a dozen patients would likely fail in a clinical trial, unless patient selection is improved.
“Perhaps genotype might predict clinical response, perhaps stem cell drug responsiveness might predict clinical response,” she said. “What I envision is this dream where we have patients and we genotype them, and we better understand how their DNA impacts their gene expression, and how their gene expression impacts their synaptic function, and that this might help us better understand their prognosis.”
Dr. Brennand reported a financial relationship with Alkermes.
ORLANDO – Stem cell models could boost schizophrenia treatment to a new level, helping to gauge the importance of both rare and common genetic variants that are difficult to study in traditional-style trials, an expert said at the annual congress of the Schizophrenia International Research Society.
The promise of the approach is particularly crucial at this point in the course of schizophrenia research, when the heritability of the disease is being repeatedly underscored in the literature, said Kristen Brennand, PhD, associate professor of neuroscience, genetics and genomics, and psychiatry at the Icahn School of Medicine at Mount Sinai, New York.
“It seems that each new paper that comes out raises that estimate of heritability and the numbers I’ve seen recently are as high as 85%,” she said. “Schizophrenia is as heritable as autism and bipolar, more heritable than BRCA1 breast cancer, it’s more heritable than alcoholism. But that heritability is highly complex.”
Highly penetrant variants that are more easily studied account for only a small sliver of this heritability. Rare variants that are harder to study account for more. And common variants – the count is up to 145 and will almost certainly grow – also play a big role.
“These are variants that all of us carry,” Dr. Brennand said. “We all carry dozens of these variants. Patients just either carry more of them or they’re hitting pathways in a different way than they’re hitting the rest of us.”
Using human-induced pluripotent stem cells (HiPSC) – grown out of skin biopsy samples from schizophrenia patients and then grown into neural progenitor cells and ultimately neurons – are much more practical for studying the genetics of these variants than case-control studies that require tens of thousands of subjects.
Researchers have found that cohorts using HiPSCs concord with the genetic findings from postmortem datasets of schizophrenia patients.
More recently, in work not yet published, she said her lab has focused on rare 2p16.3 deletions of the NRXN1 gene, finding that neuronal branching is reduced and that there is decreased neuronal activity in schizophrenia patients with these deletions.
Her lab is also using HiPSCs and clustered regularly interspaced short palindromic repeats editing to validate the function of common variants and genes linked with schizophrenia. A key finding has been that there could be important relationships between risk genes that are more distant from schizophrenia single-nucleotide polymorphisms (SNPs).
“We’re expanding the list of potential schizophrenia risk genes by considering not just immediately proximal but also distal interactions between schizophrenia SNPs,” Dr. Brennand said. “If there are 224 genes that are next to risk SNPs, you can add a few hundred more potential genes that might be coregulated by these risk SNPs.”
Harnessing the power of HiPSCs could be the gateway to precision medicine in schizophrenia, she said. A drug that might benefit, say, two out of a dozen patients would likely fail in a clinical trial, unless patient selection is improved.
“Perhaps genotype might predict clinical response, perhaps stem cell drug responsiveness might predict clinical response,” she said. “What I envision is this dream where we have patients and we genotype them, and we better understand how their DNA impacts their gene expression, and how their gene expression impacts their synaptic function, and that this might help us better understand their prognosis.”
Dr. Brennand reported a financial relationship with Alkermes.
ORLANDO – Stem cell models could boost schizophrenia treatment to a new level, helping to gauge the importance of both rare and common genetic variants that are difficult to study in traditional-style trials, an expert said at the annual congress of the Schizophrenia International Research Society.
The promise of the approach is particularly crucial at this point in the course of schizophrenia research, when the heritability of the disease is being repeatedly underscored in the literature, said Kristen Brennand, PhD, associate professor of neuroscience, genetics and genomics, and psychiatry at the Icahn School of Medicine at Mount Sinai, New York.
“It seems that each new paper that comes out raises that estimate of heritability and the numbers I’ve seen recently are as high as 85%,” she said. “Schizophrenia is as heritable as autism and bipolar, more heritable than BRCA1 breast cancer, it’s more heritable than alcoholism. But that heritability is highly complex.”
Highly penetrant variants that are more easily studied account for only a small sliver of this heritability. Rare variants that are harder to study account for more. And common variants – the count is up to 145 and will almost certainly grow – also play a big role.
“These are variants that all of us carry,” Dr. Brennand said. “We all carry dozens of these variants. Patients just either carry more of them or they’re hitting pathways in a different way than they’re hitting the rest of us.”
Using human-induced pluripotent stem cells (HiPSC) – grown out of skin biopsy samples from schizophrenia patients and then grown into neural progenitor cells and ultimately neurons – are much more practical for studying the genetics of these variants than case-control studies that require tens of thousands of subjects.
Researchers have found that cohorts using HiPSCs concord with the genetic findings from postmortem datasets of schizophrenia patients.
More recently, in work not yet published, she said her lab has focused on rare 2p16.3 deletions of the NRXN1 gene, finding that neuronal branching is reduced and that there is decreased neuronal activity in schizophrenia patients with these deletions.
Her lab is also using HiPSCs and clustered regularly interspaced short palindromic repeats editing to validate the function of common variants and genes linked with schizophrenia. A key finding has been that there could be important relationships between risk genes that are more distant from schizophrenia single-nucleotide polymorphisms (SNPs).
“We’re expanding the list of potential schizophrenia risk genes by considering not just immediately proximal but also distal interactions between schizophrenia SNPs,” Dr. Brennand said. “If there are 224 genes that are next to risk SNPs, you can add a few hundred more potential genes that might be coregulated by these risk SNPs.”
Harnessing the power of HiPSCs could be the gateway to precision medicine in schizophrenia, she said. A drug that might benefit, say, two out of a dozen patients would likely fail in a clinical trial, unless patient selection is improved.
“Perhaps genotype might predict clinical response, perhaps stem cell drug responsiveness might predict clinical response,” she said. “What I envision is this dream where we have patients and we genotype them, and we better understand how their DNA impacts their gene expression, and how their gene expression impacts their synaptic function, and that this might help us better understand their prognosis.”
Dr. Brennand reported a financial relationship with Alkermes.
EXPERT ANALYSIS FROM SIRS 2019
Combo B-cell depletion advances in SLE
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
SAN FRANCISCO – The sequential combination of rituximab followed directly by maintenance belimumab shows considerable promise as a strategy to address the aberrant B-cell immunology present in systemic lupus erythematosus (SLE) – and thereby improve clinical outcomes, Y.K. Onno Teng, MD, PhD, reported at an international congress on systemic lupus erythematosus.
Dr. Teng, a nephrologist and clinical trialist at Leiden (the Netherlands) University, and his coworkers were pioneers of this one-two punch, in which a two-dose course of rituximab (Rituxan) is given to deplete CD20-positive B-cells, followed by long-term maintenance belimumab (Benlysta) to inhibit repopulation of specific problematic types of B-cells. The rationale for the use of belimumab here lies in the observation that the initial B-cell depletion induced by rituximab triggers a surge in B lymphocyte stimulator (BLyS), which signals the bone marrow to start making more B-cells. And belimumab famously inhibits BLyS, also known as B-cell activating factor, or BAFF.
Dr. Teng presented the 2-year extended results of Synergistic B-cell Immunomodulation in SLE (SYNBIoSe-1), a phase 2a, open-label, single-arm, proof-of-concept study whose 24-week immunologic results have previously been reported (J Autoimmun. 2018 Jul;91:45-54).
Based in part upon the encouraging SYNBIoSe-1 findings as well as the sound mechanistic rationale for this treatment strategy, the combination of rituximab and belimumab is picking up steam in the research world as a potentially important treatment advance in SLE. Currently underway in patients with nonrenal SLE is the phase 3, GlaxoSmithKline-sponsored, global BLISS-BELIEVE trial, as well as the phase 2 BEAT-LUPUS study, a University College London–based randomized trial of rituximab plus either placebo or belimumab. Also, Dr. Teng and his coworkers are now conducting SYNBIoSe-2, in which patients with lupus nephritis are being randomized to standard therapy with glucocorticoids and mycophenolate or to rituximab, belimumab, and mycophenolate.
SYNBIoSe-2 is a further exploration of the encouraging signal of efficacy for lupus nephritis noted in SYNBIoSe-1. Of the 12 participants in SYNBIoSe-1 who had baseline active lupus nephritis, 8 had a positive renal response to the rituximab/belimumab combo, including 6 patients who achieved a prolonged complete renal response through 104 weeks of follow-up.
SYNBIoSe-1 included 15 patients, all with severe refractory SLE as shown by a median 11-year disease duration and a baseline SLE Disease Activity Index score of 18. Two-thirds of patients achieved sustained low-level disease activity, interrupted in one case by a single major disease flare. Two patients stopped treatment because of a lack of response. Several others left the study because they were doing so well on treatment that they decided the time was right to become pregnant.
Immunologically, patients showed an 84% reduction in B-cell repopulation over the course of 2 years. Particularly striking was the long-term inhibition of double-negative B-cells and IgD-positive naive B-cells, which Dr. Teng described as “very trigger happy” in that they readily become transformed into activated antibody-producing cells.
Sustained specific reductions in anti-double-stranded DNA autoantibodies and other pathogenic antinuclear antibodies were also documented through 104 weeks.
SYNBIoSe-1 results at odds with CALIBRATE trial results
The favorable impact of the rituximab/belimumab combo on lupus nephritis seen in SYNBIoSe-1 is at odds with the results of CALIBRATE, a U.S. study in which 43 patients with active lupus nephritis despite conventional treatment were randomized open label to induction therapy with two doses of rituximab on top of standard background therapy, followed by either belimumab and prednisone or prednisone alone. In CALIBRATE, the anti-BLyS biologic didn’t improve clinical outcomes. Dr. Teng said he believes he knows why.
“There was an important difference in background immunosuppression in the two studies. We used mycophenolate in SYNBIoSe-1, while they used cyclophosphamide in CALIBRATE,” he noted. “Other investigators have shown that mycophenolate mostly depletes plasma cells, whereas cyclophosphamide is very much depleting proliferating cells, predominantly the B-cell population and to a lesser extent the plasma cell population. I think this phenomenon might explain why adding BLyS inhibition to patients treated with CellCept [mycophenolate] might be of more added value than adding it to cyclophosphamide therapy.”
Dr. Teng reported having no financial conflicts regarding the SYNBIoSe-1 study, which was funded by research grants from the Dutch Kidney Foundation and the Netherlands Organization for Health Research and Development.
REPORTING FROM LUPUS 2019
The Enuretic Child
Continuity of Care in Family Practice: Part 2: Implications of Continuity
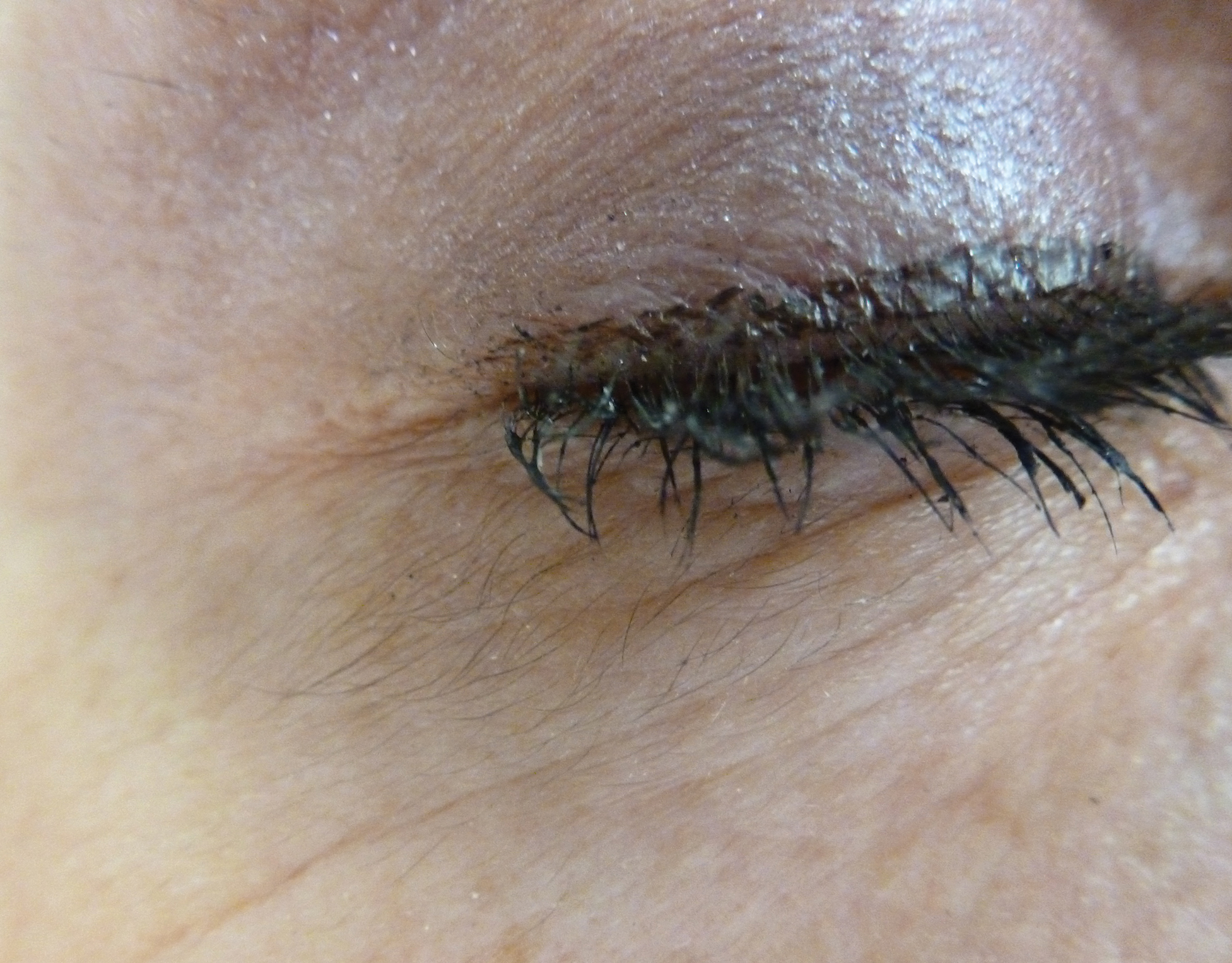
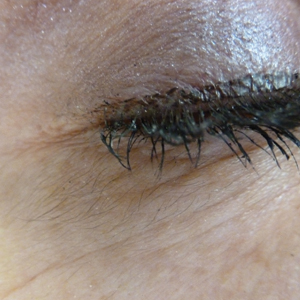
Acquired Hypertrichosis of the Periorbital Area and Malar Cheek
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
An otherwise healthy woman in her late 50s with Fitzpatrick skin type II presented to the dermatology department for a scheduled cosmetic botulinum toxin injection. Her medical history was notable only for periodic nonsurgical cosmetic procedures including botulinum toxin and dermal fillers, and she was not taking any daily systemic medications. During the preoperative assessment, subtle bilateral and symmetric hypertrichosis with darker terminal hair formation was noted on the periorbital skin and zygomatic cheek. Upon inquiry, the patient admitted to purchasing a “special eye drop” from Mexico and using it regularly. After instillation of 2 to 3 drops per eye, she would laterally wipe the resulting excess drops away from the eyes with her hands and then wash her hands. She denied a change in eye color from their natural brown but did report using blue color contact lenses. She denied an increase in hair growth elsewhere including the upper lip, chin, upper chest, forearms, and hands. She denied deepening of her voice, acne, or hair thinning.
Poster ads don’t belong in the clinic
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the last few months, I’ve received several posters. They’re always delivered by UPS, and come in a solid cardboard box to keep them from being crushed.
The boxes get opened, and once I know what they are, the whole thing gets tossed in the office recycling.
I know they’re presented as helpful patient information, with some bullet lists and glossy graphics showing brains, nerve transmitters, or patients. But the basic reality is that they’re just advertisements. Like infomercials on TV, they come across as professional and interesting, but at their heart and soul are just selling something.
No thanks.
Years ago, a company sent me a poster listing the warning signs of stroke. Although it was still an advertisement, I decided to hang it up in my exam room as a sort of public service announcement. Unfortunately, I soon discovered that any patient left staring at it for more than 1-2 minutes would start to complain of at least two of the symptoms listed. It got taken down after a few days.
I have nothing against advertising. It pays for websites, television shows, sporting events, newspapers, and magazines.
But my exam room isn’t the place for it. Patients are bombarded with direct-to-consumer advertising for many drugs in every media outlet. The doctor’s discussion room shouldn’t be one of the them.
The meeting between me and a patient should be frank, honest assessments about what should be done and what, specifically, is best for their individual case. I don’t need marketing for a drug that may or may not be appropriate, or easily covered by insurance, staring back at them.
It’s a thin line. Obviously, magazines out in my lobby are full of pharmaceutical ads, and that doesn’t bother me. But once a patient crosses the line into my consultation area it should just be between me and them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
John Fry—Researcher in Family Practice
Hydroxychloroquine adherence in SLE: worse than you thought
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
SAN FRANCISCO – Routine office measurement of hydroxychloroquine blood levels in systemic lupus erythematosus (SLE) patients accomplishes two major objectives, Nathalie Costedoat-Chalumeau, MD, asserted at an international congress on systemic lupus erythematosus.
First, measuring hydroxychloroquine levels identifies the surprisingly large number of individuals who are severely nonadherent to this cornerstone of lupus therapy despite its excellent benefit/risk ratio. Also, serial measurements coupled with brief counseling have been shown to boost poor adherence rates, noted Dr. Costedoat-Chalumeau, professor of rheumatology at Paris Descartes University.
Numerous studies have documented startlingly low adherence to hydroxychloroquine among SLE patients. Some of the same studies show prescribing physicians are often clueless as to their patients’ adherence or lack thereof.
Just how bad is the adherence problem? A recent study of 10,406 Medicaid patients with SLE who started on hydroxychloroquine showed that 85% of them were nonadherent as defined by pharmacy refill data, indicating insufficient medication on hand to cover a minimum of 80% of days during at least 1 year of follow-up.
In a novel finding, the investigators also broke down the Medicaid data month by month and identified four broad patterns of adherence/nonadherence. A total of 17% of patients were persistently adherent throughout the first year after the drug was dispensed. Another 36% were persistent nonadherers right from the get-go. A further 24% remained partially adherent, dropping down to a plateau of 30%-40% monthly adherence after the first couple of months and staying there. And 23% dropped steadily from roughly 50% adherence at month 3 to nearly total nonadherence from month 9 onward. Overall, adherence in the Medicaid cohort declined over the course of the first year (Semin Arthritis Rheum. 2018 Oct;48[2]:205-13).
Dr. Costedoat-Chalumeau was the lead investigator in a large French multicenter clinical trial known as the PLUS Study, which established that increasing the hydroxychloroquine daily dose to raise blood levels to a target of at least 1,000 ng/mL didn’t reduce the risk of flares (Ann Rheum Dis. 2013;72[11]:1786-92).
“So there is no reason to use blood drug measurements to adjust hydroxychloroquine daily dose or blood levels to prevent SLE flares. But drug levels teach us something regarding adherence,” she said.
Why routinely measuring hydroxychloroquine levels matters
Dr. Costedoat-Chalumeau and other investigators have shown that whole blood drug levels below 200 ng/mL indicate a patient is severely nonadherent. In various studies, that’s 7%-29% of SLE patients who are supposedly on hydroxychloroquine.
Also, investigators at Johns Hopkins University, Baltimore, have analyzed prospective data from the Hopkins Lupus Cohort and determined that at the first clinic visit after going on a maximum of 400 mg/day of hydroxychloroquine, only 44% of participants had a blood drug level above the 500-ng/mL threshold indicative of adherence.
Importantly, however, the Hopkins researchers also demonstrated that with repeated brief counseling of nonadherent patients as to why hydroxychloroquine is the most important medication they take for their SLE, adherence climbed in stepwise fashion with each visit in which the drug blood level was assessed: With no prior measurement, adherence was 56%; with one prior measurement, it jumped to 69%; with two, 77% of patients were adherent to hydroxychloroquine; and with three or more prior blood level checks, adherence rose to 80% (J Rheumatol. 2015 Nov;42[11]:2092-7).
It is well established that hydroxychloroquine prevents SLE flares, protects against thrombotic events, diabetes, dyslipidemia, and lupus-induced organ damage, and improves survival. Dr. Costedoat-Chalumeau’s final words on hydroxychloroquine adherence: ”Drugs don’t work in people who don’t take them.”
She reported having no financial conflicts regarding her presentation.
REPORTING FROM LUPUS 2019