User login
Mouthwash shows some efficacy for oral mucositis pain
Doxepin mouthwash and diphenhydramine/lidocaine/antacid (DLA) mouthwash can offer 4 hours of pain relief for cancer patients with oral mucositis, according to investigators.
Although these agents led to statistical improvements in pain, neither met predetermined clinical efficacy thresholds, reported lead author Terence T. Sio, MD, of the Mayo Clinic Hospital in Phoenix and his colleagues, who suggested that more safety and efficacy research is needed.
“Few pharmacological agents or interventions have been shown to effectively reduce the severity of radiotherapy-related oral mucositis and its associated pain,” the investigators wrote in JAMA.
They noted that this knowledge gap affects everyday practice since “more than 80% of patients develop oral mucositis during radiotherapy, and mouthwashes and systemic analgesic agents are frequently used to treat the condition.”
Small studies have shown that doxepin, a tricyclic antidepressant, could be an effective agent for oral mucositis, while a variety of DLA mouthwashes are commonly prescribed, despite a dearth of relevant Cochrane reviews or randomized placebo-controlled trials.
This background led to the present study, which included 275 patients who had developed oral mucositis while undergoing head and neck radiotherapy for cancer. The patients were randomized evenly into three mouthwash groups: placebo (2.5 mL Ora-Sweet SF oral solution and 2.5 mL of water), doxepin (25 mg in 5 mL solution), or diphenhydramine (12.5 mg in 5 mL alcohol-free solution), lidocaine (2% viscous solution), and antacid (20 mg of simethicone, 200 mg of magnesium hydroxide, and 200 mg of aluminum hydroxide in 355 mL solution). The study was divided into two cycles; in the first, patients used their assigned mouthwash once, whereas in the second cycle, which was optional, patients used their assigned treatment every 4 hours for up to 7 days.
The primary endpoint was oral mucositis pain. Multiple secondary endpoints were assessed, including patient preference for continued therapy and various adverse effects, such as drowsiness and taste. Responses were assessed using a combination of the Oral Mucositis Daily Questionnaire and the Oral Mucositis Weekly Questionnaire–Head and Neck Cancer. This modified questionnaire was conducted prior to treatment, then after treatment at 5, 15, 30, 60, 120, and 240 minutes. Pain improvements were compared by area under the curve after adjustment for baseline score. Clinical improvement was defined as a 3.5 point difference in pain score, compared with placebo.
Data analysis showed that pain in the first 4 hours decreased the most in the DLA group (11.7 points), slightly less in the doxepin group (11.6 points), and least in the placebo group (8.7 points). Compared with placebo, both treatments offered statistical improvements. DLA patients responded the most (3.0 points; P = .004), while, again, the average doxepin response was similar, albeit with a slightly higher P value. (2.9 points; P = .02). The investigators discouraged direct comparisons between the two agents because the study was not designed for this purpose.
Neither intervention met the predetermined 3.5-point threshold for clinical improvement, although the investigators suggested that some patients may have had meaningful responses.
“There is some suggestion in post hoc analyses that the findings may have been clinically relevant for some patients,” the investigators wrote, noting that responder analysis favored treatment with DLA versus placebo, but not doxepin versus placebo. “However,” they noted, “the overall clinical importance of the statistically significant primary findings remains uncertain.”
Compared with placebo, doxepin mouthwash was associated with stinging or burning, unpleasant taste, drowsiness, and fatigue. Of note, fatigue only occurred in the doxepin group, at a rate of 6%. Both treatment groups had a maximum grade 3 adverse event rate of 4%, while the placebo arm had an adverse event rate of 2%.
“Further research is needed to assess longer-term efficacy and safety for both mouthwashes,” the investigators concluded.
The study was funded by the National Cancer Institute and the Mayo Clinic Symptom Intervention Program. One investigator reported a nonfinancial support from CutisPharma. The other investigators declared no conflicts of interest.
SOURCE: Sio TT et al. JAMA. 2019 Apr 16. doi: 10.1001/jama.2019.3504.
Oral mucositis is a common and serious complication of cancer, but quality research and reliable treatments for the condition are lacking, according to Sharon Elad, DMD, of the University of Rochester (N.Y.) Medical Center and Noam Yarom, DMD, of Tel Aviv University.
“Despite the strengths of the randomized clinical trial (RCT) design in general, some studies evaluating therapies for oral mucositis have been underpowered, are of low quality, or have yielded conflicting results about the benefits of the interventions,” Dr. Elad and Dr. Yarom wrote in a JAMA editorial.
“These issues highlight the need for well-designed RCTs that test interventions for oral mucositis appropriately.”
In this context, the doctors reviewed the simultaneously published study by Sio et al., in which patients were given either of two topical therapies for oral mucositis: diphenhydramine/lidocaine/antacid mouthwash or doxepin mouthwash. Both interventions led to statistically significant improvements in pain, compared with placebo; however, these improvements were not clinically significant, according to the investigators’ predetermined threshold.
“The distinction between statistical significance and clinical importance is relevant in this study,” Dr. Elad and Dr. Yarom wrote, “and the findings suggest that pain relief was short-term and limited among many of the patients. Nevertheless, this limited effect may be beneficial if doxepin is used as a supplemental analgesic (eg, to reduce the dose of systemic opioids).”
“The severity of oral pain in oral mucositis may exceed the beneficial effect of local anesthesia,” they added. “In severe oral mucositis–associated pain, clinicians may elect to use a stronger pain medication as a first-line treatment. Optional pain management approaches include patient-controlled analgesics, topical morphine, and fentanyl transdermal patch or nasal spray.”
Dr. Elad and Dr. Yarom said that future oral mucositis studies should evaluate treatments head-to-head and against placebo, with a watchful eye for severe, adverse events, which can occur even with local treatments, because of damaged mucosal barriers that allow for systemic absorption. They also pointed out that emerging technologies such as proton-beam radiotherapy should minimize rates of mucositis. However, “until these advances are routinely used,” they wrote, “the search for an effective, safe therapy for oral mucositis and its associated pain needs to continue.”
Dr. Elad reported relationships with Falk Pharma and the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology. Dr. Yarom reported no conflicts.
Dr. Elad is a professor of dentistry and a professor of oncology at the University of Rochester (N.Y.) Medical Center. Dr. Yarom is a senior lecturer of oral medicine and the program director of the postgraduate oral medicine in the department of oral pathology and oral medicine at Tel Aviv University, as well as the director of the oral medicine clinic at Sheba Medical Center in Tel HaShomer, Israel.
Oral mucositis is a common and serious complication of cancer, but quality research and reliable treatments for the condition are lacking, according to Sharon Elad, DMD, of the University of Rochester (N.Y.) Medical Center and Noam Yarom, DMD, of Tel Aviv University.
“Despite the strengths of the randomized clinical trial (RCT) design in general, some studies evaluating therapies for oral mucositis have been underpowered, are of low quality, or have yielded conflicting results about the benefits of the interventions,” Dr. Elad and Dr. Yarom wrote in a JAMA editorial.
“These issues highlight the need for well-designed RCTs that test interventions for oral mucositis appropriately.”
In this context, the doctors reviewed the simultaneously published study by Sio et al., in which patients were given either of two topical therapies for oral mucositis: diphenhydramine/lidocaine/antacid mouthwash or doxepin mouthwash. Both interventions led to statistically significant improvements in pain, compared with placebo; however, these improvements were not clinically significant, according to the investigators’ predetermined threshold.
“The distinction between statistical significance and clinical importance is relevant in this study,” Dr. Elad and Dr. Yarom wrote, “and the findings suggest that pain relief was short-term and limited among many of the patients. Nevertheless, this limited effect may be beneficial if doxepin is used as a supplemental analgesic (eg, to reduce the dose of systemic opioids).”
“The severity of oral pain in oral mucositis may exceed the beneficial effect of local anesthesia,” they added. “In severe oral mucositis–associated pain, clinicians may elect to use a stronger pain medication as a first-line treatment. Optional pain management approaches include patient-controlled analgesics, topical morphine, and fentanyl transdermal patch or nasal spray.”
Dr. Elad and Dr. Yarom said that future oral mucositis studies should evaluate treatments head-to-head and against placebo, with a watchful eye for severe, adverse events, which can occur even with local treatments, because of damaged mucosal barriers that allow for systemic absorption. They also pointed out that emerging technologies such as proton-beam radiotherapy should minimize rates of mucositis. However, “until these advances are routinely used,” they wrote, “the search for an effective, safe therapy for oral mucositis and its associated pain needs to continue.”
Dr. Elad reported relationships with Falk Pharma and the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology. Dr. Yarom reported no conflicts.
Dr. Elad is a professor of dentistry and a professor of oncology at the University of Rochester (N.Y.) Medical Center. Dr. Yarom is a senior lecturer of oral medicine and the program director of the postgraduate oral medicine in the department of oral pathology and oral medicine at Tel Aviv University, as well as the director of the oral medicine clinic at Sheba Medical Center in Tel HaShomer, Israel.
Oral mucositis is a common and serious complication of cancer, but quality research and reliable treatments for the condition are lacking, according to Sharon Elad, DMD, of the University of Rochester (N.Y.) Medical Center and Noam Yarom, DMD, of Tel Aviv University.
“Despite the strengths of the randomized clinical trial (RCT) design in general, some studies evaluating therapies for oral mucositis have been underpowered, are of low quality, or have yielded conflicting results about the benefits of the interventions,” Dr. Elad and Dr. Yarom wrote in a JAMA editorial.
“These issues highlight the need for well-designed RCTs that test interventions for oral mucositis appropriately.”
In this context, the doctors reviewed the simultaneously published study by Sio et al., in which patients were given either of two topical therapies for oral mucositis: diphenhydramine/lidocaine/antacid mouthwash or doxepin mouthwash. Both interventions led to statistically significant improvements in pain, compared with placebo; however, these improvements were not clinically significant, according to the investigators’ predetermined threshold.
“The distinction between statistical significance and clinical importance is relevant in this study,” Dr. Elad and Dr. Yarom wrote, “and the findings suggest that pain relief was short-term and limited among many of the patients. Nevertheless, this limited effect may be beneficial if doxepin is used as a supplemental analgesic (eg, to reduce the dose of systemic opioids).”
“The severity of oral pain in oral mucositis may exceed the beneficial effect of local anesthesia,” they added. “In severe oral mucositis–associated pain, clinicians may elect to use a stronger pain medication as a first-line treatment. Optional pain management approaches include patient-controlled analgesics, topical morphine, and fentanyl transdermal patch or nasal spray.”
Dr. Elad and Dr. Yarom said that future oral mucositis studies should evaluate treatments head-to-head and against placebo, with a watchful eye for severe, adverse events, which can occur even with local treatments, because of damaged mucosal barriers that allow for systemic absorption. They also pointed out that emerging technologies such as proton-beam radiotherapy should minimize rates of mucositis. However, “until these advances are routinely used,” they wrote, “the search for an effective, safe therapy for oral mucositis and its associated pain needs to continue.”
Dr. Elad reported relationships with Falk Pharma and the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology. Dr. Yarom reported no conflicts.
Dr. Elad is a professor of dentistry and a professor of oncology at the University of Rochester (N.Y.) Medical Center. Dr. Yarom is a senior lecturer of oral medicine and the program director of the postgraduate oral medicine in the department of oral pathology and oral medicine at Tel Aviv University, as well as the director of the oral medicine clinic at Sheba Medical Center in Tel HaShomer, Israel.
Doxepin mouthwash and diphenhydramine/lidocaine/antacid (DLA) mouthwash can offer 4 hours of pain relief for cancer patients with oral mucositis, according to investigators.
Although these agents led to statistical improvements in pain, neither met predetermined clinical efficacy thresholds, reported lead author Terence T. Sio, MD, of the Mayo Clinic Hospital in Phoenix and his colleagues, who suggested that more safety and efficacy research is needed.
“Few pharmacological agents or interventions have been shown to effectively reduce the severity of radiotherapy-related oral mucositis and its associated pain,” the investigators wrote in JAMA.
They noted that this knowledge gap affects everyday practice since “more than 80% of patients develop oral mucositis during radiotherapy, and mouthwashes and systemic analgesic agents are frequently used to treat the condition.”
Small studies have shown that doxepin, a tricyclic antidepressant, could be an effective agent for oral mucositis, while a variety of DLA mouthwashes are commonly prescribed, despite a dearth of relevant Cochrane reviews or randomized placebo-controlled trials.
This background led to the present study, which included 275 patients who had developed oral mucositis while undergoing head and neck radiotherapy for cancer. The patients were randomized evenly into three mouthwash groups: placebo (2.5 mL Ora-Sweet SF oral solution and 2.5 mL of water), doxepin (25 mg in 5 mL solution), or diphenhydramine (12.5 mg in 5 mL alcohol-free solution), lidocaine (2% viscous solution), and antacid (20 mg of simethicone, 200 mg of magnesium hydroxide, and 200 mg of aluminum hydroxide in 355 mL solution). The study was divided into two cycles; in the first, patients used their assigned mouthwash once, whereas in the second cycle, which was optional, patients used their assigned treatment every 4 hours for up to 7 days.
The primary endpoint was oral mucositis pain. Multiple secondary endpoints were assessed, including patient preference for continued therapy and various adverse effects, such as drowsiness and taste. Responses were assessed using a combination of the Oral Mucositis Daily Questionnaire and the Oral Mucositis Weekly Questionnaire–Head and Neck Cancer. This modified questionnaire was conducted prior to treatment, then after treatment at 5, 15, 30, 60, 120, and 240 minutes. Pain improvements were compared by area under the curve after adjustment for baseline score. Clinical improvement was defined as a 3.5 point difference in pain score, compared with placebo.
Data analysis showed that pain in the first 4 hours decreased the most in the DLA group (11.7 points), slightly less in the doxepin group (11.6 points), and least in the placebo group (8.7 points). Compared with placebo, both treatments offered statistical improvements. DLA patients responded the most (3.0 points; P = .004), while, again, the average doxepin response was similar, albeit with a slightly higher P value. (2.9 points; P = .02). The investigators discouraged direct comparisons between the two agents because the study was not designed for this purpose.
Neither intervention met the predetermined 3.5-point threshold for clinical improvement, although the investigators suggested that some patients may have had meaningful responses.
“There is some suggestion in post hoc analyses that the findings may have been clinically relevant for some patients,” the investigators wrote, noting that responder analysis favored treatment with DLA versus placebo, but not doxepin versus placebo. “However,” they noted, “the overall clinical importance of the statistically significant primary findings remains uncertain.”
Compared with placebo, doxepin mouthwash was associated with stinging or burning, unpleasant taste, drowsiness, and fatigue. Of note, fatigue only occurred in the doxepin group, at a rate of 6%. Both treatment groups had a maximum grade 3 adverse event rate of 4%, while the placebo arm had an adverse event rate of 2%.
“Further research is needed to assess longer-term efficacy and safety for both mouthwashes,” the investigators concluded.
The study was funded by the National Cancer Institute and the Mayo Clinic Symptom Intervention Program. One investigator reported a nonfinancial support from CutisPharma. The other investigators declared no conflicts of interest.
SOURCE: Sio TT et al. JAMA. 2019 Apr 16. doi: 10.1001/jama.2019.3504.
Doxepin mouthwash and diphenhydramine/lidocaine/antacid (DLA) mouthwash can offer 4 hours of pain relief for cancer patients with oral mucositis, according to investigators.
Although these agents led to statistical improvements in pain, neither met predetermined clinical efficacy thresholds, reported lead author Terence T. Sio, MD, of the Mayo Clinic Hospital in Phoenix and his colleagues, who suggested that more safety and efficacy research is needed.
“Few pharmacological agents or interventions have been shown to effectively reduce the severity of radiotherapy-related oral mucositis and its associated pain,” the investigators wrote in JAMA.
They noted that this knowledge gap affects everyday practice since “more than 80% of patients develop oral mucositis during radiotherapy, and mouthwashes and systemic analgesic agents are frequently used to treat the condition.”
Small studies have shown that doxepin, a tricyclic antidepressant, could be an effective agent for oral mucositis, while a variety of DLA mouthwashes are commonly prescribed, despite a dearth of relevant Cochrane reviews or randomized placebo-controlled trials.
This background led to the present study, which included 275 patients who had developed oral mucositis while undergoing head and neck radiotherapy for cancer. The patients were randomized evenly into three mouthwash groups: placebo (2.5 mL Ora-Sweet SF oral solution and 2.5 mL of water), doxepin (25 mg in 5 mL solution), or diphenhydramine (12.5 mg in 5 mL alcohol-free solution), lidocaine (2% viscous solution), and antacid (20 mg of simethicone, 200 mg of magnesium hydroxide, and 200 mg of aluminum hydroxide in 355 mL solution). The study was divided into two cycles; in the first, patients used their assigned mouthwash once, whereas in the second cycle, which was optional, patients used their assigned treatment every 4 hours for up to 7 days.
The primary endpoint was oral mucositis pain. Multiple secondary endpoints were assessed, including patient preference for continued therapy and various adverse effects, such as drowsiness and taste. Responses were assessed using a combination of the Oral Mucositis Daily Questionnaire and the Oral Mucositis Weekly Questionnaire–Head and Neck Cancer. This modified questionnaire was conducted prior to treatment, then after treatment at 5, 15, 30, 60, 120, and 240 minutes. Pain improvements were compared by area under the curve after adjustment for baseline score. Clinical improvement was defined as a 3.5 point difference in pain score, compared with placebo.
Data analysis showed that pain in the first 4 hours decreased the most in the DLA group (11.7 points), slightly less in the doxepin group (11.6 points), and least in the placebo group (8.7 points). Compared with placebo, both treatments offered statistical improvements. DLA patients responded the most (3.0 points; P = .004), while, again, the average doxepin response was similar, albeit with a slightly higher P value. (2.9 points; P = .02). The investigators discouraged direct comparisons between the two agents because the study was not designed for this purpose.
Neither intervention met the predetermined 3.5-point threshold for clinical improvement, although the investigators suggested that some patients may have had meaningful responses.
“There is some suggestion in post hoc analyses that the findings may have been clinically relevant for some patients,” the investigators wrote, noting that responder analysis favored treatment with DLA versus placebo, but not doxepin versus placebo. “However,” they noted, “the overall clinical importance of the statistically significant primary findings remains uncertain.”
Compared with placebo, doxepin mouthwash was associated with stinging or burning, unpleasant taste, drowsiness, and fatigue. Of note, fatigue only occurred in the doxepin group, at a rate of 6%. Both treatment groups had a maximum grade 3 adverse event rate of 4%, while the placebo arm had an adverse event rate of 2%.
“Further research is needed to assess longer-term efficacy and safety for both mouthwashes,” the investigators concluded.
The study was funded by the National Cancer Institute and the Mayo Clinic Symptom Intervention Program. One investigator reported a nonfinancial support from CutisPharma. The other investigators declared no conflicts of interest.
SOURCE: Sio TT et al. JAMA. 2019 Apr 16. doi: 10.1001/jama.2019.3504.
FROM JAMA
Criminalization of mental illness must stop, judge says
ORLANDO – Judge Steve Leifman, who presides over 11th judicial circuit court in Miami-Dade County, Fla., was about to take the bench several years ago when he agreed to see a couple, who then begged him to help their son, who had mental illness. Judge Leifman was about to hear his case.
The man was a Harvard-educated former psychiatrist and at first appeared healthy, but then took on a look of terror and began screaming when the judge asked him a question. Although the man was clearly psychotic, Judge Leifman had no choice but to release him to the streets – he had no authority under the law to involuntarily commit anyone to psychiatric treatment.
There was little doubt that the man would end up committing a crime and being put behind bars.
Judge Leifman, who gave the keynote address at the annual congress of the Schizophrenia International Research Society, now has made it his life’s work to reform a system in which jails are the de facto hospitals for people with mental illness.
he said. “And I don’t know why people aren’t angrier about it.”
He quoted figures that are staggering in their illustration of how mental illness has become criminalized. People with mental illnesses in the United States are 9 times more likely to be incarcerated than hospitalized, and 18 times more likely to find a bed in jail than at a state civil hospital, he said. On any given day, about 400,000 people with mental illness are in jail and 800,000 are under correctional supervision. He said that 40% of all people with mental illness in the United States will at some point come into contact with the criminal justice system.
Together, U.S. counties spend $80 billion a year on correctional costs. States spend an additional $71 billion, he said.
Judge Leifman has helped start an initiative called Stepping Up to lead reform. It’s an effort by the National Association of Counties, American Psychiatric Association Foundation, and the Council of State Government. More than 400 counties over the past few years have passed resolutions saying they’re committed to change.
Judge Leifman organized a summit, with criminal justice and health groups coming together to assess the issue, only to diagnose a system that’s “designed to fail.” Local officials have crafted a new system with links to comprehensive care for people with mental illness that make jail a last resort rather than a first stop. A key component is “crisis intervention team policing,” in which law enforcement officers are trained to identify people with mental illness, deescalate situations, and get them to proper care rather than arrest them. All 36 Miami-Dade County police departments are trained in this program, and it has eased the incarceration and recidivism rates.
“It has been a huge cultural shift,” Judge Leifman said.
“We’ve improved public safety, we’ve reduced police injuries, we’ve helped police officers get back to patrol much quicker, we’ve saved critical tax dollars, we’ve saved lives, and we’ve decriminalized mental illness,” he said. “But we still have plenty to do. Because as good as all this has been, it’s limited. ... Our state’s mental health systems are still too fragmented, they’re still antiquated, and they’re painfully underresourced. And the laws are old and they don’t reflect the science today.”
Judge Leifman reported no relevant disclosures.
ORLANDO – Judge Steve Leifman, who presides over 11th judicial circuit court in Miami-Dade County, Fla., was about to take the bench several years ago when he agreed to see a couple, who then begged him to help their son, who had mental illness. Judge Leifman was about to hear his case.
The man was a Harvard-educated former psychiatrist and at first appeared healthy, but then took on a look of terror and began screaming when the judge asked him a question. Although the man was clearly psychotic, Judge Leifman had no choice but to release him to the streets – he had no authority under the law to involuntarily commit anyone to psychiatric treatment.
There was little doubt that the man would end up committing a crime and being put behind bars.
Judge Leifman, who gave the keynote address at the annual congress of the Schizophrenia International Research Society, now has made it his life’s work to reform a system in which jails are the de facto hospitals for people with mental illness.
he said. “And I don’t know why people aren’t angrier about it.”
He quoted figures that are staggering in their illustration of how mental illness has become criminalized. People with mental illnesses in the United States are 9 times more likely to be incarcerated than hospitalized, and 18 times more likely to find a bed in jail than at a state civil hospital, he said. On any given day, about 400,000 people with mental illness are in jail and 800,000 are under correctional supervision. He said that 40% of all people with mental illness in the United States will at some point come into contact with the criminal justice system.
Together, U.S. counties spend $80 billion a year on correctional costs. States spend an additional $71 billion, he said.
Judge Leifman has helped start an initiative called Stepping Up to lead reform. It’s an effort by the National Association of Counties, American Psychiatric Association Foundation, and the Council of State Government. More than 400 counties over the past few years have passed resolutions saying they’re committed to change.
Judge Leifman organized a summit, with criminal justice and health groups coming together to assess the issue, only to diagnose a system that’s “designed to fail.” Local officials have crafted a new system with links to comprehensive care for people with mental illness that make jail a last resort rather than a first stop. A key component is “crisis intervention team policing,” in which law enforcement officers are trained to identify people with mental illness, deescalate situations, and get them to proper care rather than arrest them. All 36 Miami-Dade County police departments are trained in this program, and it has eased the incarceration and recidivism rates.
“It has been a huge cultural shift,” Judge Leifman said.
“We’ve improved public safety, we’ve reduced police injuries, we’ve helped police officers get back to patrol much quicker, we’ve saved critical tax dollars, we’ve saved lives, and we’ve decriminalized mental illness,” he said. “But we still have plenty to do. Because as good as all this has been, it’s limited. ... Our state’s mental health systems are still too fragmented, they’re still antiquated, and they’re painfully underresourced. And the laws are old and they don’t reflect the science today.”
Judge Leifman reported no relevant disclosures.
ORLANDO – Judge Steve Leifman, who presides over 11th judicial circuit court in Miami-Dade County, Fla., was about to take the bench several years ago when he agreed to see a couple, who then begged him to help their son, who had mental illness. Judge Leifman was about to hear his case.
The man was a Harvard-educated former psychiatrist and at first appeared healthy, but then took on a look of terror and began screaming when the judge asked him a question. Although the man was clearly psychotic, Judge Leifman had no choice but to release him to the streets – he had no authority under the law to involuntarily commit anyone to psychiatric treatment.
There was little doubt that the man would end up committing a crime and being put behind bars.
Judge Leifman, who gave the keynote address at the annual congress of the Schizophrenia International Research Society, now has made it his life’s work to reform a system in which jails are the de facto hospitals for people with mental illness.
he said. “And I don’t know why people aren’t angrier about it.”
He quoted figures that are staggering in their illustration of how mental illness has become criminalized. People with mental illnesses in the United States are 9 times more likely to be incarcerated than hospitalized, and 18 times more likely to find a bed in jail than at a state civil hospital, he said. On any given day, about 400,000 people with mental illness are in jail and 800,000 are under correctional supervision. He said that 40% of all people with mental illness in the United States will at some point come into contact with the criminal justice system.
Together, U.S. counties spend $80 billion a year on correctional costs. States spend an additional $71 billion, he said.
Judge Leifman has helped start an initiative called Stepping Up to lead reform. It’s an effort by the National Association of Counties, American Psychiatric Association Foundation, and the Council of State Government. More than 400 counties over the past few years have passed resolutions saying they’re committed to change.
Judge Leifman organized a summit, with criminal justice and health groups coming together to assess the issue, only to diagnose a system that’s “designed to fail.” Local officials have crafted a new system with links to comprehensive care for people with mental illness that make jail a last resort rather than a first stop. A key component is “crisis intervention team policing,” in which law enforcement officers are trained to identify people with mental illness, deescalate situations, and get them to proper care rather than arrest them. All 36 Miami-Dade County police departments are trained in this program, and it has eased the incarceration and recidivism rates.
“It has been a huge cultural shift,” Judge Leifman said.
“We’ve improved public safety, we’ve reduced police injuries, we’ve helped police officers get back to patrol much quicker, we’ve saved critical tax dollars, we’ve saved lives, and we’ve decriminalized mental illness,” he said. “But we still have plenty to do. Because as good as all this has been, it’s limited. ... Our state’s mental health systems are still too fragmented, they’re still antiquated, and they’re painfully underresourced. And the laws are old and they don’t reflect the science today.”
Judge Leifman reported no relevant disclosures.
REPORTING FROM SIRS 2019
Patient complications affect surgeons adversely
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
Psychological consequences of patient complications seem to be an important occupational health issue for surgeons, according to the results of an extensive literature review published in JAMA Surgery.
Sanket Srinivasa, PhD, of North Shore Hospital, Auckland, New Zealand, and colleagues assessed studies from MEDLINE, Embase, PubMed, Web of Science, and Google Scholar that examined the consequences of complications, adverse events, or error for surgeons published up to the search date of May 1, 2018. Studies pertaining to burnout alone, studies not conducted on surgeons or surgical trainees, and review articles with no original data were excluded. This final review of consisted of nine studies (10,702 unique participants) that explored the occurrence of patient complications and their affect on surgeons’ psychological well-being and their professional and personal lives.
All of the studies indicated that surgeons were affected emotionally after patient complications, which led to adverse consequences in their professional and personal lives. The study authors identified four themes from the literature.
- The adverse emotional influence of complications (including anxiety, guilt, sadness, shame, and interference with professional and leisure activities) after intraoperative adverse events; one study diagnosed acute traumatic stress (using valid diagnostic criteria) in one-third of their participants 1 month after a major surgical complication.
- Coping mechanisms used by surgeons and trainees (including limited discussion with colleagues, exercise, artistic or creative outlets, alcohol and substance abuse); emotion-focused coping strategies reported included rationalization, seeking reassurance, blaming oneself or others, and dissociation with self-distraction. Other adaptive strategies used included engaging in artistic endeavors and exercise, although maladaptive strategies were also adopted by some, including alcohol and substance use disorder.
- Institutional support mechanisms and barriers to support (including clinical conferences, discussion with mentors, and a perception that emotional distress would be perceived as a constitutional weakness). For example, surgical trainees in one study did not believe that morbidity and mortality meetings addressed the emotional needs of trainees, and respondents in another study pointed to poor institutional support with a competitive, unsympathetic surgical culture, with the morbidity and mortality meeting being regarded as accusatory and hostile without providing support.
- The consequences of complications in future clinical practice (including changes in practice, introduction of protocols, education of staff members, and participating in root-cause analysis). Participants in several studies believed that dealing with errors and complications improved their subsequent performance, For example, 92 of 123 respondents (74.8%) in one study believed that their professional ability was not impaired after a complication, and in another study half of the surgeons did not believe they should stop operating for a brief period after an intraoperative death. “However, respondents in other studies described a combination of anxiety and shock affecting their ability to rectify the operative problem in a practical sense immediately after an intraoperative complication. Some respondents reported impairment for weeks after the incident, describing ongoing rumination, difficulties in concentration, adversely affected clinical judgment, and loss of confidence,” according to the researchers. Surgeons in another study described an initial denial and minimization of the severity of the consequence potentially delaying the necessary treatment, while some surgeons reported avoiding or stopping certain operations as well as contemplating early retirement.
“Surgeons across the studies indicated that they deal with these problems in isolation with significant personal and clinical consequences. With primum non nocere remaining a cornerstone of medical practice as applied to patients, a similar philosophy needs to be embraced by the surgical community for the betterment of health of the profession,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Srinivasa S et al. JAMA Surg. 2019 Mar 27. doi: 10.1001/jamasurg.2018.5640.
FROM JAMA SURGERY
Belimumab a bust for black SLE patients
SAN FRANCISCO – ordered by the Food and Drug Administration.
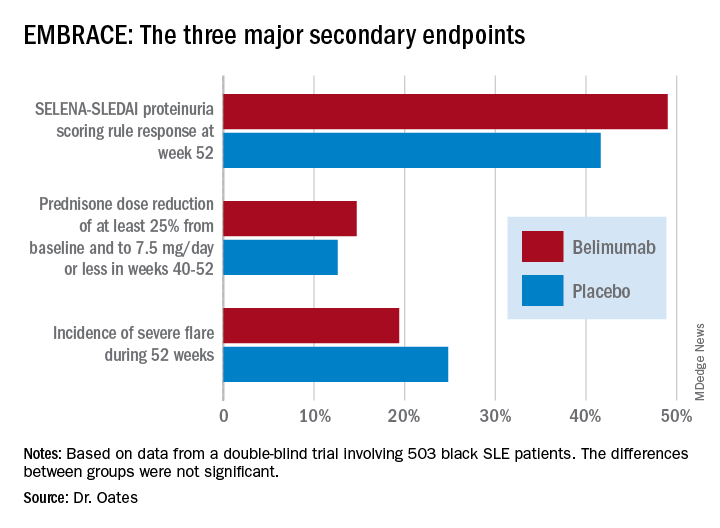
Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
SAN FRANCISCO – ordered by the Food and Drug Administration.

Numerically, the outcome trends in the EMBRACE study consistently favored belimumab (Benlysta) over placebo. And not just for all three components of the primary endpoint, but for each of the three major prespecified secondary endpoints as well. Yet not a single one of those six favorable trends attained statistical significance, Jim C. Oates, MD, reported at an international congress on systemic lupus erythematosus.
“The study did not achieve its primary endpoint, although there was a numeric advantage for patients on belimumab, with a 40% increase in the chance of response,” observed Dr. Oates, professor of medicine, director of the division of rheumatology and immunology, and vice chair for research at the Medical University of South Carolina, Charleston.
EMBRACE was a 52-week, double-blind trial in which 503 black systemic lupus erythematosus (SLE) patients were randomized 2:1 to 48 weeks of IV belimumab at the approved dose of 10 mg/kg or to placebo infusions on top of standard care background therapies. The postmarketing study was required by the FDA as a condition for the agency’s 2011 marketing approval of belimumab, a human monoclonal antibody that inhibits B-cell activating factor, also known as a B-lymphocyte stimulator. The agency request came because the three premarketing phase 3 intravenous trials, as well as the phase 3 subcutaneous belimumab trial, included only small numbers of black patients, and the results in that population were conflicting.
The EMBRACE results are particularly disappointing in light of the increased prevalence, severity, and mortality of SLE in black patients. However, the final word on EMBRACE isn’t in, as the data from the recently completed open-label extension phase beyond 52 weeks have not yet been analyzed.
Roughly three-quarters of EMBRACE enrollees completed the full 52 weeks of study. Withdrawal for adverse events or lack of efficacy occurred in 6.7% and 5.4%, respectively, of placebo-treated controls, and in a respective 5.4% and 4.7% of patients in the belimumab arm.
The primary study outcome at 52 weeks was the SLE Responder Index (SRI) response rate with the modified SLE Disease Activity Index (SLEDAI)–2000 scoring for proteinuria (SRI-S2K). This required at least a 4-point reduction from baseline in the Safety of Estrogens in Lupus Erythematosus – National Assessment (SELENA)-SLEDAI, no worsening in the Physician Global Assessment, as well as no new British Isles Lupus Assessment Group (BILAG) A or two new BILAG B organ domain scores. This outcome was achieved in 48.7% of the belimumab group and 41.6% of controls, for 40% greater likelihood in the active treatment arm in a logistic regression analysis, which didn’t achieve statistical significance. While the between-group difference was significant in favor of belimumab during the monthly assessments at weeks 32-44, the belimumab and placebo response rates converged thereafter.
The belimumab safety profile contained no surprises. Of note, rates of opportunistic infections, depression, and suicide or self-injury, which had been deemed adverse events of special interest based upon previous studies, were numerically lower than in controls.
The deflating EMBRACE results are sure to come under close scrutiny, since black patients with SLE have been identified as a population with a major unmet need for improved therapies. Of note, 44% of study participants were from the United States and Canada, and they had a longer disease duration, lower damage scores, and less serologically active disease than subjects from the rest of the world.
Because the results of prior phase 3 studies showed increased belimumab response rates in patients with serologically more active disease, prespecified subgroup analyses of the composite endpoint were conducted. These analyses parsed out several subgroups who were significantly more likely to achieve the primary endpoint with belimumab than with placebo. Black patients with a baseline SELENA-SLEDAI-S2K score of 10 or greater had a 52.5% response rate to belimumab, compared with 40.9% with placebo, for a 76% relative increase. Patients with a low baseline C3 and/or C4 were 200% more likely to respond to the biologic agent than placebo, by a margin of 47.2% versus 24.6%. And patients from outside North America were 80% more likely to respond to belimumab, with a 57.5% response rate, compared with 44.0% on placebo.
One audience member noted there is evidence that the use of mycophenolate appears to be advantageous in African American SLE patients and wondered if the EMBRACE subgroup on belimumab plus background mycophenolate fared significantly better than with placebo. Dr. Oates replied that, although it’s an important question, the subset analysis isn’t available yet.
The EMBRACE trial was sponsored by GlaxoSmithKline. Dr. Oates reported receiving research funding from that pharmaceutical company and several others, the National Institutes of Health, and the Department of Veterans Affairs.
SOURCE: Oates JC et al. Lupus Sci Med. 2019;6[suppl 1], Abstract 200.
REPORTING FROM LUPUS 2019
FDA approves generic naloxone spray for opioid overdose treatment
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
lfranki@mdedge.com
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
lfranki@mdedge.com
The Food and Drug Administration on April 19 approved the first generic naloxone hydrochloride nasal spray (Narcan) as treatment for stopping or reversing an opioid overdose.
“In the wake of the opioid crisis, a number of efforts are underway to make this emergency overdose reversal treatment more readily available and more accessible,” said Douglas Throckmorton, MD, deputy center director for regulatory programs in the FDA’s Center for Drug Evaluation and Research, in a press release. “In addition to this approval of the first generic naloxone nasal spray, moving forward, we will prioritize our review of generic drug applications for naloxone.”
The agency said the naloxone nasal spray does not need assembly and can be used by anyone, regardless of medical training. If the spray is administered quickly after the overdose begins, the effect of the opioid will be countered, often within minutes. However, patients should still seek immediate medical attention.
The FDA cautioned that, when used on a patient with an opioid dependence, naloxone can cause severe opioid withdrawal, characterized by symptoms such as body aches, diarrhea, tachycardia, fever, runny nose, sneezing, goose bumps, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure.
Find the full press release on the FDA website.
lfranki@mdedge.com
Short Takes
Short Takes
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Short Takes
Short Takes
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Both sleep quantity and quality is disturbed in hospitalized patients
A cross-sectional, observational, single-day study of over 2,000 hospitalized patients showed that, on average, these patients received 83 minutes less sleep time than at home. Quality of sleep – as measured by the Consensus Sleep Diary (CSD) and the Dutch-Flemish Patient-Reported-Outcomes Measurement Information System (PROMIS) Sleep Disturbance item bank – was also significantly disturbed. Sleep disruptions were most commonly caused by noise from other patients and by being awakened by hospital staff.
Citation: Wesselius H et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.2669.
Health care costs and mortality improve in Medicare beneficiaries who receive transitional care management (TCM) service
In a retrospective cohort analysis of Medicare Fee-for-Service beneficiaries, the adjusted total Medicare costs (average, $3,358 vs. $3,033) and mortality (1.6% vs 1.0%) were higher among those beneficiaries who did not receive TCM services, compared with those who did receive TCM services, in the 31-60 days following an eligible discharge; however, use of this service by clinicians remained very low.
Citation: Bindman AB et al. Changes in health care costs and mortality associated with transitional care management services after a discharge among Medicare beneficiaries. JAMA Intern Med. 2018 Jul 30. doi: 10.1001/jamainternmed.2018.2572.
Unsafe zolpidem use is common
In a review of the 2015 US Medical Expenditure Panel Survey, investigators found that up to 77% of patients prescribed zolpidem reported being prescribed longer durations and higher doses, as well as the drug being prescribed alongside other CNS depressants, despite known risks and recommended prescription and Food and Drug Administration guidelines.
Citation: Moore T et al. Assessment of patterns of potentially unsafe use of zolpidem. JAMA Intern Med. 2018 Jul 16. doi: 10.1001/jamainternmed.2018.3031.
Obsessive-compulsive personality disorder may involve brain abnormalities
A clue to the neuroanatomy of obsessive-compulsive personality disorder may lay in abnormal hippocampal or amygdalar structures, according to a new observational imaging study.
MRI showed that the right and left volumes of the hippocampus and amygdala are smaller in patients with obsessive-compulsive personality disorder than in healthy controls, researchers found.
These findings lend support to the likelihood that obsessive-compulsive personality disorder “seems to be a neurocognitive function disorder rather than a personality disorder,” Mehmet Gurkan Gurok, of Firat University, Elazig, Turkey, and his associates reported in the Journal of Clinical Neuroscience.
The authors previously had investigated volumes of hippocampus and amygdala in patients with obsessive-compulsive disorder and found these patients had “increased white matter volumes, greater left and right thalamus volumes and significantly reduced left and right orbitofrontal cortex volumes, compared with healthy controls.”
For this study, they similarly used MRI to assess hippocampal and amygdalar volumes in 16 patients with obsessive-compulsive personality disorder (inpatients and outpatients at Firat University) and in 18 healthy controls – matched for age, sex, education, and handedness – who were studying at the hospital.
The researchers used the DSM-IV and the Structured Clinical Interview for the Diagnostic Schedule for Mental Disorders-Fourth Edition for diagnoses but noted later in their limitations that “the validity of diagnosis should be questioned” for personality disorders.
Participants were excluded if they had a comorbid Axis I psychiatric diagnosis besides depression, any contraindications for MRI, history of alcohol or substance dependence within the previous 6 months, use of psychoactive medications within 4 weeks of the study, or any current severe medical conditions. In addition, neither controls nor their first-degree relatives had a history of psychiatric disorders.
The findings showed that those with obsessive-compulsive personality disorder had smaller volumes on both sides of the hippocampus, compared with healthy controls. Amygdalar volume on both the left and right sides were similarly smaller in patients with obsessive-compulsive personality disorder, compared with healthy controls (left, P = .001; right, P = .002). The authors also reported they “did not find any correlation between hippocampus and amygdala volumes and any clinical and demographic variables.”
The research was funded by Firat University. The authors reported having no conflicts of interest.
SOURCE: Gurok MG et al. J Clin Neurosci. 2019 Apr 5. doi: 10.1016/j.jocn.2019.03.060.
A clue to the neuroanatomy of obsessive-compulsive personality disorder may lay in abnormal hippocampal or amygdalar structures, according to a new observational imaging study.
MRI showed that the right and left volumes of the hippocampus and amygdala are smaller in patients with obsessive-compulsive personality disorder than in healthy controls, researchers found.
These findings lend support to the likelihood that obsessive-compulsive personality disorder “seems to be a neurocognitive function disorder rather than a personality disorder,” Mehmet Gurkan Gurok, of Firat University, Elazig, Turkey, and his associates reported in the Journal of Clinical Neuroscience.
The authors previously had investigated volumes of hippocampus and amygdala in patients with obsessive-compulsive disorder and found these patients had “increased white matter volumes, greater left and right thalamus volumes and significantly reduced left and right orbitofrontal cortex volumes, compared with healthy controls.”
For this study, they similarly used MRI to assess hippocampal and amygdalar volumes in 16 patients with obsessive-compulsive personality disorder (inpatients and outpatients at Firat University) and in 18 healthy controls – matched for age, sex, education, and handedness – who were studying at the hospital.
The researchers used the DSM-IV and the Structured Clinical Interview for the Diagnostic Schedule for Mental Disorders-Fourth Edition for diagnoses but noted later in their limitations that “the validity of diagnosis should be questioned” for personality disorders.
Participants were excluded if they had a comorbid Axis I psychiatric diagnosis besides depression, any contraindications for MRI, history of alcohol or substance dependence within the previous 6 months, use of psychoactive medications within 4 weeks of the study, or any current severe medical conditions. In addition, neither controls nor their first-degree relatives had a history of psychiatric disorders.
The findings showed that those with obsessive-compulsive personality disorder had smaller volumes on both sides of the hippocampus, compared with healthy controls. Amygdalar volume on both the left and right sides were similarly smaller in patients with obsessive-compulsive personality disorder, compared with healthy controls (left, P = .001; right, P = .002). The authors also reported they “did not find any correlation between hippocampus and amygdala volumes and any clinical and demographic variables.”
The research was funded by Firat University. The authors reported having no conflicts of interest.
SOURCE: Gurok MG et al. J Clin Neurosci. 2019 Apr 5. doi: 10.1016/j.jocn.2019.03.060.
A clue to the neuroanatomy of obsessive-compulsive personality disorder may lay in abnormal hippocampal or amygdalar structures, according to a new observational imaging study.
MRI showed that the right and left volumes of the hippocampus and amygdala are smaller in patients with obsessive-compulsive personality disorder than in healthy controls, researchers found.
These findings lend support to the likelihood that obsessive-compulsive personality disorder “seems to be a neurocognitive function disorder rather than a personality disorder,” Mehmet Gurkan Gurok, of Firat University, Elazig, Turkey, and his associates reported in the Journal of Clinical Neuroscience.
The authors previously had investigated volumes of hippocampus and amygdala in patients with obsessive-compulsive disorder and found these patients had “increased white matter volumes, greater left and right thalamus volumes and significantly reduced left and right orbitofrontal cortex volumes, compared with healthy controls.”
For this study, they similarly used MRI to assess hippocampal and amygdalar volumes in 16 patients with obsessive-compulsive personality disorder (inpatients and outpatients at Firat University) and in 18 healthy controls – matched for age, sex, education, and handedness – who were studying at the hospital.
The researchers used the DSM-IV and the Structured Clinical Interview for the Diagnostic Schedule for Mental Disorders-Fourth Edition for diagnoses but noted later in their limitations that “the validity of diagnosis should be questioned” for personality disorders.
Participants were excluded if they had a comorbid Axis I psychiatric diagnosis besides depression, any contraindications for MRI, history of alcohol or substance dependence within the previous 6 months, use of psychoactive medications within 4 weeks of the study, or any current severe medical conditions. In addition, neither controls nor their first-degree relatives had a history of psychiatric disorders.
The findings showed that those with obsessive-compulsive personality disorder had smaller volumes on both sides of the hippocampus, compared with healthy controls. Amygdalar volume on both the left and right sides were similarly smaller in patients with obsessive-compulsive personality disorder, compared with healthy controls (left, P = .001; right, P = .002). The authors also reported they “did not find any correlation between hippocampus and amygdala volumes and any clinical and demographic variables.”
The research was funded by Firat University. The authors reported having no conflicts of interest.
SOURCE: Gurok MG et al. J Clin Neurosci. 2019 Apr 5. doi: 10.1016/j.jocn.2019.03.060.
FROM THE JOURNAL OF CLINICAL NEUROSCIENCE
How to incorporate the gender wage gap into contract negotiations
PHILADELPHIA – that they need to account for: the gender wage gap.
“Find a lawyer ... that will support your fight for pay equity,” Michael Sinha, MD, advised in a presentation at the annual meeting of the American College of Physicians.
“Definitely interview them,” said Dr. Sinha of Harvard Medical School, Boston. “Get a sense of how committed they are to that cause. Seek recommendations from other women in medicine. Maybe they will point you to the person who really is committed to this cause and wants to help you.”
He also cautioned that physicians might have to help their lawyer fill in the knowledge gap. “Sometimes you need to provide that lawyer with data. There are a lot of reports that have been published from various organizations [including the ACP]. Don’t assume that the lawyer has the evidence.”
Armed with evidence, he said there is opportunity to address gender pay gaps in the contract. “You can put a lot of things into your contract, why not some of these things? If there is institutional evidence of a pay gap or a leaky promotional pipeline, you are telling them you have a problem with salary discrepancies between male and female physicians and I need to protect my own self-worth.”
Dr. Sinha recommended prospective employees develop strategies with their lawyers, which could mean letting the lawyer take the lead in the negotiations.
“Maybe that reduces stress or emotion in the process. That will preserve a good relationship with the employer,” he said. “A confident, knowledgeable lawyer may help mitigate gender differences in negotiation strategy.”
In order to help close the gender gap, Dr. Sinha said he advises his male colleagues to help female physicians by being open about their compensation, especially if compensation is not public information. “I have been encouraging male physician colleagues of mine to share that information when you are asked. Don’t make it impossible for someone to figure out.”
He noted that it is not likely that closing the gender gap by raising women’s compensation is going to result in male physicians losing money, but rather in the long run it will mean better compensation for everyone.
Dr. Sinha also had this advice for employers: “Before you do anything else, level your pay gap. ... For every physician that works in your organization, level your pay gap. Offer equitable salary packages up front to men and women. Understand your responsibilities under federal and state equal pay laws.”
When it comes to equitable compensation packages, all offerings, including salary and fringe benefits, should be offered to both male and female physicians or to neither.
“Don’t make something nonnegotiable for women but engage men in negotiations,” he said. “I think that is obvious but I have seen that happen.”
Dr. Sinha also said it is important to check your gender biases at the door before entering negotiations. Don’t view a woman asking for something as demanding or harsh when a male asking for the same exact thing is viewed as assertive and self-confident.
“Don’t fall into those traps. And if you have people that can call out your biases and help you see that, that’s important,” Dr. Sinha noted.
He also stated that hiring committees need to show diversity in terms of gender, race, and ethnicity, adding that it is “hugely important” that you do that.
The bottom line is to not force women to negotiate for equal pay, Dr. Sinha noted. “The gender gap is well documented in medicine, and you really have to do your part.”
PHILADELPHIA – that they need to account for: the gender wage gap.
“Find a lawyer ... that will support your fight for pay equity,” Michael Sinha, MD, advised in a presentation at the annual meeting of the American College of Physicians.
“Definitely interview them,” said Dr. Sinha of Harvard Medical School, Boston. “Get a sense of how committed they are to that cause. Seek recommendations from other women in medicine. Maybe they will point you to the person who really is committed to this cause and wants to help you.”
He also cautioned that physicians might have to help their lawyer fill in the knowledge gap. “Sometimes you need to provide that lawyer with data. There are a lot of reports that have been published from various organizations [including the ACP]. Don’t assume that the lawyer has the evidence.”
Armed with evidence, he said there is opportunity to address gender pay gaps in the contract. “You can put a lot of things into your contract, why not some of these things? If there is institutional evidence of a pay gap or a leaky promotional pipeline, you are telling them you have a problem with salary discrepancies between male and female physicians and I need to protect my own self-worth.”
Dr. Sinha recommended prospective employees develop strategies with their lawyers, which could mean letting the lawyer take the lead in the negotiations.
“Maybe that reduces stress or emotion in the process. That will preserve a good relationship with the employer,” he said. “A confident, knowledgeable lawyer may help mitigate gender differences in negotiation strategy.”
In order to help close the gender gap, Dr. Sinha said he advises his male colleagues to help female physicians by being open about their compensation, especially if compensation is not public information. “I have been encouraging male physician colleagues of mine to share that information when you are asked. Don’t make it impossible for someone to figure out.”
He noted that it is not likely that closing the gender gap by raising women’s compensation is going to result in male physicians losing money, but rather in the long run it will mean better compensation for everyone.
Dr. Sinha also had this advice for employers: “Before you do anything else, level your pay gap. ... For every physician that works in your organization, level your pay gap. Offer equitable salary packages up front to men and women. Understand your responsibilities under federal and state equal pay laws.”
When it comes to equitable compensation packages, all offerings, including salary and fringe benefits, should be offered to both male and female physicians or to neither.
“Don’t make something nonnegotiable for women but engage men in negotiations,” he said. “I think that is obvious but I have seen that happen.”
Dr. Sinha also said it is important to check your gender biases at the door before entering negotiations. Don’t view a woman asking for something as demanding or harsh when a male asking for the same exact thing is viewed as assertive and self-confident.
“Don’t fall into those traps. And if you have people that can call out your biases and help you see that, that’s important,” Dr. Sinha noted.
He also stated that hiring committees need to show diversity in terms of gender, race, and ethnicity, adding that it is “hugely important” that you do that.
The bottom line is to not force women to negotiate for equal pay, Dr. Sinha noted. “The gender gap is well documented in medicine, and you really have to do your part.”
PHILADELPHIA – that they need to account for: the gender wage gap.
“Find a lawyer ... that will support your fight for pay equity,” Michael Sinha, MD, advised in a presentation at the annual meeting of the American College of Physicians.
“Definitely interview them,” said Dr. Sinha of Harvard Medical School, Boston. “Get a sense of how committed they are to that cause. Seek recommendations from other women in medicine. Maybe they will point you to the person who really is committed to this cause and wants to help you.”
He also cautioned that physicians might have to help their lawyer fill in the knowledge gap. “Sometimes you need to provide that lawyer with data. There are a lot of reports that have been published from various organizations [including the ACP]. Don’t assume that the lawyer has the evidence.”
Armed with evidence, he said there is opportunity to address gender pay gaps in the contract. “You can put a lot of things into your contract, why not some of these things? If there is institutional evidence of a pay gap or a leaky promotional pipeline, you are telling them you have a problem with salary discrepancies between male and female physicians and I need to protect my own self-worth.”
Dr. Sinha recommended prospective employees develop strategies with their lawyers, which could mean letting the lawyer take the lead in the negotiations.
“Maybe that reduces stress or emotion in the process. That will preserve a good relationship with the employer,” he said. “A confident, knowledgeable lawyer may help mitigate gender differences in negotiation strategy.”
In order to help close the gender gap, Dr. Sinha said he advises his male colleagues to help female physicians by being open about their compensation, especially if compensation is not public information. “I have been encouraging male physician colleagues of mine to share that information when you are asked. Don’t make it impossible for someone to figure out.”
He noted that it is not likely that closing the gender gap by raising women’s compensation is going to result in male physicians losing money, but rather in the long run it will mean better compensation for everyone.
Dr. Sinha also had this advice for employers: “Before you do anything else, level your pay gap. ... For every physician that works in your organization, level your pay gap. Offer equitable salary packages up front to men and women. Understand your responsibilities under federal and state equal pay laws.”
When it comes to equitable compensation packages, all offerings, including salary and fringe benefits, should be offered to both male and female physicians or to neither.
“Don’t make something nonnegotiable for women but engage men in negotiations,” he said. “I think that is obvious but I have seen that happen.”
Dr. Sinha also said it is important to check your gender biases at the door before entering negotiations. Don’t view a woman asking for something as demanding or harsh when a male asking for the same exact thing is viewed as assertive and self-confident.
“Don’t fall into those traps. And if you have people that can call out your biases and help you see that, that’s important,” Dr. Sinha noted.
He also stated that hiring committees need to show diversity in terms of gender, race, and ethnicity, adding that it is “hugely important” that you do that.
The bottom line is to not force women to negotiate for equal pay, Dr. Sinha noted. “The gender gap is well documented in medicine, and you really have to do your part.”
REPORTING FROM INTERNAL MEDICINE 2019
Update in Psoriasis: Optimizing Combination Topical Therapies to Improve Adherence and Patient Outcomes
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.
Click here to download this supplement.
A majority of patients with psoriasis are undertreated. In recent years, however, new formulations of topical medications for psoriasis have been introduced, with the goal of enhancing penetration, efficacy, and patient acceptance. Along with new systemic therapies, these treatments allow for more aggressive goals for disease clearance.
Topical medications can be used as monotherapy but combining topical agents can increase efficacy and may allow for use of lower doses with fewer adverse events. Fixed-dose combination treatments combine active ingredients in one vehicle and may improve patient adherence and acceptance by simplifying the treatment regimen.
This supplement provides physicians with education on evidence-based assessment and management of psoriasis, the combination of systemic and topical medications, and strategies for improving patient adherence to treatment regimens.
Click here to download this supplement.
Calcium supplement use linked to cancer death
PHILADELPHIA – a nutrition specialist noted at the annual meeting of the American College of Physicians.
The report, published (Ann Intern Med. 2019 Apr 9. doi: 10.7326/M18-2478) just 2 days before the start of the Internal Medicine meeting, found no mortality benefits associated with any reported dietary supplement use among nearly 31,000 adults in the National Health and Nutrition Examination Survey.
On the contrary, they found that excess calcium consumption was associated with increased risk for cancer-related deaths. Calcium supplements were specifically implicated in the excess of mortality, according to the investigators, with a rate ratio of 1.53 (95% confidence interval, 1.04-2.25) for intakes of 1,000 mg/day versus no intake.
“It’s better to get all of your vitamins from your food, over supplements,” said Marijane Hynes, MD, director of weight management at George Washington University, Washington, in a meet-the-professor session at the conference.
The amount of calcium patients are getting from food can be estimated with one rule of thumb: Multiply the number of dairy servings per day by 300 mg, Dr. Hynes said, who added that a serving is 8 ounces of milk or 1 ounce of hard cheese. Dark green vegetables, breads, cereals, and some nuts can provide 100-200 mg of calcium per day.
Calcium carbonate can be taken with food to enhance calcium absorption, according to Dr. Hynes, while calcium citrate can be taken without food, and is preferred for patients taking acid reflux medications.
Because calcium absorption is reduced at higher doses, patients who need more than 600 mg/day should be taking divided doses, she said.
Bone health goes beyond the dairy aisle, Dr. Hynes added. High vitamin K intake was linked to reduced hip fracture risk among the Framingham Heart Study participants. To get the recommended amount of vitamin K in the diet, patients can consume one or more servings of broccoli, kale, collard greens, or dark green lettuce.
Dr. Hynes reported she that had no relationships with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
These are observational data. This is not saying we put someone on calcium, and they ended up with cancer, and when you look at this whole thing it’s amazing to me that nobody is discussing the benefits that were found in patients taking magnesium, vitamin K2, and other vitamins. The other thing I would like to point out is that, for at least a decade, it has been really well established that we shouldn’t be using more than 1,000 milligrams of calcium a day, especially from a supplements source. In this study, supplemental calcium intake of 1,000 mg/d or higher was associated with increased risk of cancer death, so what’s the big deal?
The big thing with calcium is calcium comes in 7 different forms. When you eat a variety of fruits and vegetables the source of calcium you get is mixed. The problem with supplements is you are using one or maybe two forms of calcium, but if your body doesn’t recognize that form of calcium then you aren’t getting calcium and it may not be beneficial to you.
What we need to do here, in my opinion, is we need to look at the whole picture. We know that dieting alone or exercising alone does not improve outcomes. It’s the combination of diet, exercise, hormone balance, nutrients from supplements, and emotional balance that makes you healthy. Similarly, you can’t say if you just take this one nutrient you are going to improve your quality of life.
With calcium and vitamin D, you have to take vitamin K2, because vitamin K2 activates osteocalcin, a protein that rebuilds the matrix of the bone. Without vitamin K2, you can’t deposit calcium in the bones. K2 also prevents the deposition of calcium in the blood vessels.
Magnesium is another tremendously important mineral, and magnesium deficiency is the most common mineral deficiency in the United States.
Probably one of the most common causes of magnesium deficiency is the use of acid blockers. I would be very curious to know how many people were taking proton pump inhibitors or acid blockers in general. I bet you most of them were.
Derrick DeSilva Jr., MD, is an internist, practicing in Edison, N.J. He made these comments in an interview. He reported serving as a consultant for Common Sense Supplements, a company that produces dietary supplements.
These are observational data. This is not saying we put someone on calcium, and they ended up with cancer, and when you look at this whole thing it’s amazing to me that nobody is discussing the benefits that were found in patients taking magnesium, vitamin K2, and other vitamins. The other thing I would like to point out is that, for at least a decade, it has been really well established that we shouldn’t be using more than 1,000 milligrams of calcium a day, especially from a supplements source. In this study, supplemental calcium intake of 1,000 mg/d or higher was associated with increased risk of cancer death, so what’s the big deal?
The big thing with calcium is calcium comes in 7 different forms. When you eat a variety of fruits and vegetables the source of calcium you get is mixed. The problem with supplements is you are using one or maybe two forms of calcium, but if your body doesn’t recognize that form of calcium then you aren’t getting calcium and it may not be beneficial to you.
What we need to do here, in my opinion, is we need to look at the whole picture. We know that dieting alone or exercising alone does not improve outcomes. It’s the combination of diet, exercise, hormone balance, nutrients from supplements, and emotional balance that makes you healthy. Similarly, you can’t say if you just take this one nutrient you are going to improve your quality of life.
With calcium and vitamin D, you have to take vitamin K2, because vitamin K2 activates osteocalcin, a protein that rebuilds the matrix of the bone. Without vitamin K2, you can’t deposit calcium in the bones. K2 also prevents the deposition of calcium in the blood vessels.
Magnesium is another tremendously important mineral, and magnesium deficiency is the most common mineral deficiency in the United States.
Probably one of the most common causes of magnesium deficiency is the use of acid blockers. I would be very curious to know how many people were taking proton pump inhibitors or acid blockers in general. I bet you most of them were.
Derrick DeSilva Jr., MD, is an internist, practicing in Edison, N.J. He made these comments in an interview. He reported serving as a consultant for Common Sense Supplements, a company that produces dietary supplements.
These are observational data. This is not saying we put someone on calcium, and they ended up with cancer, and when you look at this whole thing it’s amazing to me that nobody is discussing the benefits that were found in patients taking magnesium, vitamin K2, and other vitamins. The other thing I would like to point out is that, for at least a decade, it has been really well established that we shouldn’t be using more than 1,000 milligrams of calcium a day, especially from a supplements source. In this study, supplemental calcium intake of 1,000 mg/d or higher was associated with increased risk of cancer death, so what’s the big deal?
The big thing with calcium is calcium comes in 7 different forms. When you eat a variety of fruits and vegetables the source of calcium you get is mixed. The problem with supplements is you are using one or maybe two forms of calcium, but if your body doesn’t recognize that form of calcium then you aren’t getting calcium and it may not be beneficial to you.
What we need to do here, in my opinion, is we need to look at the whole picture. We know that dieting alone or exercising alone does not improve outcomes. It’s the combination of diet, exercise, hormone balance, nutrients from supplements, and emotional balance that makes you healthy. Similarly, you can’t say if you just take this one nutrient you are going to improve your quality of life.
With calcium and vitamin D, you have to take vitamin K2, because vitamin K2 activates osteocalcin, a protein that rebuilds the matrix of the bone. Without vitamin K2, you can’t deposit calcium in the bones. K2 also prevents the deposition of calcium in the blood vessels.
Magnesium is another tremendously important mineral, and magnesium deficiency is the most common mineral deficiency in the United States.
Probably one of the most common causes of magnesium deficiency is the use of acid blockers. I would be very curious to know how many people were taking proton pump inhibitors or acid blockers in general. I bet you most of them were.
Derrick DeSilva Jr., MD, is an internist, practicing in Edison, N.J. He made these comments in an interview. He reported serving as a consultant for Common Sense Supplements, a company that produces dietary supplements.
PHILADELPHIA – a nutrition specialist noted at the annual meeting of the American College of Physicians.
The report, published (Ann Intern Med. 2019 Apr 9. doi: 10.7326/M18-2478) just 2 days before the start of the Internal Medicine meeting, found no mortality benefits associated with any reported dietary supplement use among nearly 31,000 adults in the National Health and Nutrition Examination Survey.
On the contrary, they found that excess calcium consumption was associated with increased risk for cancer-related deaths. Calcium supplements were specifically implicated in the excess of mortality, according to the investigators, with a rate ratio of 1.53 (95% confidence interval, 1.04-2.25) for intakes of 1,000 mg/day versus no intake.
“It’s better to get all of your vitamins from your food, over supplements,” said Marijane Hynes, MD, director of weight management at George Washington University, Washington, in a meet-the-professor session at the conference.
The amount of calcium patients are getting from food can be estimated with one rule of thumb: Multiply the number of dairy servings per day by 300 mg, Dr. Hynes said, who added that a serving is 8 ounces of milk or 1 ounce of hard cheese. Dark green vegetables, breads, cereals, and some nuts can provide 100-200 mg of calcium per day.
Calcium carbonate can be taken with food to enhance calcium absorption, according to Dr. Hynes, while calcium citrate can be taken without food, and is preferred for patients taking acid reflux medications.
Because calcium absorption is reduced at higher doses, patients who need more than 600 mg/day should be taking divided doses, she said.
Bone health goes beyond the dairy aisle, Dr. Hynes added. High vitamin K intake was linked to reduced hip fracture risk among the Framingham Heart Study participants. To get the recommended amount of vitamin K in the diet, patients can consume one or more servings of broccoli, kale, collard greens, or dark green lettuce.
Dr. Hynes reported she that had no relationships with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
PHILADELPHIA – a nutrition specialist noted at the annual meeting of the American College of Physicians.
The report, published (Ann Intern Med. 2019 Apr 9. doi: 10.7326/M18-2478) just 2 days before the start of the Internal Medicine meeting, found no mortality benefits associated with any reported dietary supplement use among nearly 31,000 adults in the National Health and Nutrition Examination Survey.
On the contrary, they found that excess calcium consumption was associated with increased risk for cancer-related deaths. Calcium supplements were specifically implicated in the excess of mortality, according to the investigators, with a rate ratio of 1.53 (95% confidence interval, 1.04-2.25) for intakes of 1,000 mg/day versus no intake.
“It’s better to get all of your vitamins from your food, over supplements,” said Marijane Hynes, MD, director of weight management at George Washington University, Washington, in a meet-the-professor session at the conference.
The amount of calcium patients are getting from food can be estimated with one rule of thumb: Multiply the number of dairy servings per day by 300 mg, Dr. Hynes said, who added that a serving is 8 ounces of milk or 1 ounce of hard cheese. Dark green vegetables, breads, cereals, and some nuts can provide 100-200 mg of calcium per day.
Calcium carbonate can be taken with food to enhance calcium absorption, according to Dr. Hynes, while calcium citrate can be taken without food, and is preferred for patients taking acid reflux medications.
Because calcium absorption is reduced at higher doses, patients who need more than 600 mg/day should be taking divided doses, she said.
Bone health goes beyond the dairy aisle, Dr. Hynes added. High vitamin K intake was linked to reduced hip fracture risk among the Framingham Heart Study participants. To get the recommended amount of vitamin K in the diet, patients can consume one or more servings of broccoli, kale, collard greens, or dark green lettuce.
Dr. Hynes reported she that had no relationships with entities producing, marketing, reselling, or distributing health care goods or services consumed by, or used on, patients.
REPORTING FROM INTERNAL MEDICINE 2019