User login
Using Social Media to Talk About Public Health Issues
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
Chronic abdominal pain, career options in industry, coding basics, and more
As many of us see patients with abdominal pain on an almost daily basis, it becomes easy to overlook the substantial long-term effects this chronic pain can have on patients. In this quarter’s In Focus article, Emily Weaver and Eva Szigethy (UPMC) explore how to utilize a multidisciplinary approach to effectively treat chronic abdominal pain, and they also highlight how chronic abdominal pain can truly be a traumatic experience for patients. This article is definitely a must-read for all practitioners.
Also in this issue of The New Gastroenterologist, Matthew Whitson (Hofstra-Northwell) provides some advice on becoming an effective educator, which is critically important, especially when making the transition from being a trainee to now having to teach trainees. Additionally, Erica Cohen and Gil Melmed (Cedars-Sinai) provide some important lessons about attempting to start an investigator-led clinical trial, which is a difficult task regardless of what career stage you’re in.
In this quarter’s DHPA-cosponsored private practice perspective, Marc Sonenshine (Atlanta Gastroenterology Associates) provides some tips for how to develop a specialized niche in private practice. And in our postfellowship pathway section, Mark Sostek (Orphomed) provides an enlightening overview of some career options in industry.
Finally, Kathleen Mueller (AskMueller Consulting, LLC) gives an overview of some coding basics, which is important knowledge, especially for trainees, and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale/Quinnipiac) provides an overview of some advanced degree programs you may consider when contemplating a career change.
Interested in contributing to The New Gastroenterologist? Have ideas for future issues? If so, please contact me at bryson.katona@pennmedicine.upenn.edu or the managing editor, Ryan Farrell, at rfarrell@gastro.org.
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
As many of us see patients with abdominal pain on an almost daily basis, it becomes easy to overlook the substantial long-term effects this chronic pain can have on patients. In this quarter’s In Focus article, Emily Weaver and Eva Szigethy (UPMC) explore how to utilize a multidisciplinary approach to effectively treat chronic abdominal pain, and they also highlight how chronic abdominal pain can truly be a traumatic experience for patients. This article is definitely a must-read for all practitioners.
Also in this issue of The New Gastroenterologist, Matthew Whitson (Hofstra-Northwell) provides some advice on becoming an effective educator, which is critically important, especially when making the transition from being a trainee to now having to teach trainees. Additionally, Erica Cohen and Gil Melmed (Cedars-Sinai) provide some important lessons about attempting to start an investigator-led clinical trial, which is a difficult task regardless of what career stage you’re in.
In this quarter’s DHPA-cosponsored private practice perspective, Marc Sonenshine (Atlanta Gastroenterology Associates) provides some tips for how to develop a specialized niche in private practice. And in our postfellowship pathway section, Mark Sostek (Orphomed) provides an enlightening overview of some career options in industry.
Finally, Kathleen Mueller (AskMueller Consulting, LLC) gives an overview of some coding basics, which is important knowledge, especially for trainees, and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale/Quinnipiac) provides an overview of some advanced degree programs you may consider when contemplating a career change.
Interested in contributing to The New Gastroenterologist? Have ideas for future issues? If so, please contact me at bryson.katona@pennmedicine.upenn.edu or the managing editor, Ryan Farrell, at rfarrell@gastro.org.
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
As many of us see patients with abdominal pain on an almost daily basis, it becomes easy to overlook the substantial long-term effects this chronic pain can have on patients. In this quarter’s In Focus article, Emily Weaver and Eva Szigethy (UPMC) explore how to utilize a multidisciplinary approach to effectively treat chronic abdominal pain, and they also highlight how chronic abdominal pain can truly be a traumatic experience for patients. This article is definitely a must-read for all practitioners.
Also in this issue of The New Gastroenterologist, Matthew Whitson (Hofstra-Northwell) provides some advice on becoming an effective educator, which is critically important, especially when making the transition from being a trainee to now having to teach trainees. Additionally, Erica Cohen and Gil Melmed (Cedars-Sinai) provide some important lessons about attempting to start an investigator-led clinical trial, which is a difficult task regardless of what career stage you’re in.
In this quarter’s DHPA-cosponsored private practice perspective, Marc Sonenshine (Atlanta Gastroenterology Associates) provides some tips for how to develop a specialized niche in private practice. And in our postfellowship pathway section, Mark Sostek (Orphomed) provides an enlightening overview of some career options in industry.
Finally, Kathleen Mueller (AskMueller Consulting, LLC) gives an overview of some coding basics, which is important knowledge, especially for trainees, and Latha Alaparthi (Gastroenterology Center of Connecticut/Yale/Quinnipiac) provides an overview of some advanced degree programs you may consider when contemplating a career change.
Interested in contributing to The New Gastroenterologist? Have ideas for future issues? If so, please contact me at bryson.katona@pennmedicine.upenn.edu or the managing editor, Ryan Farrell, at rfarrell@gastro.org.
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Exploring multidisciplinary treatments in the traumatizing aspects of chronic abdominal pain
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).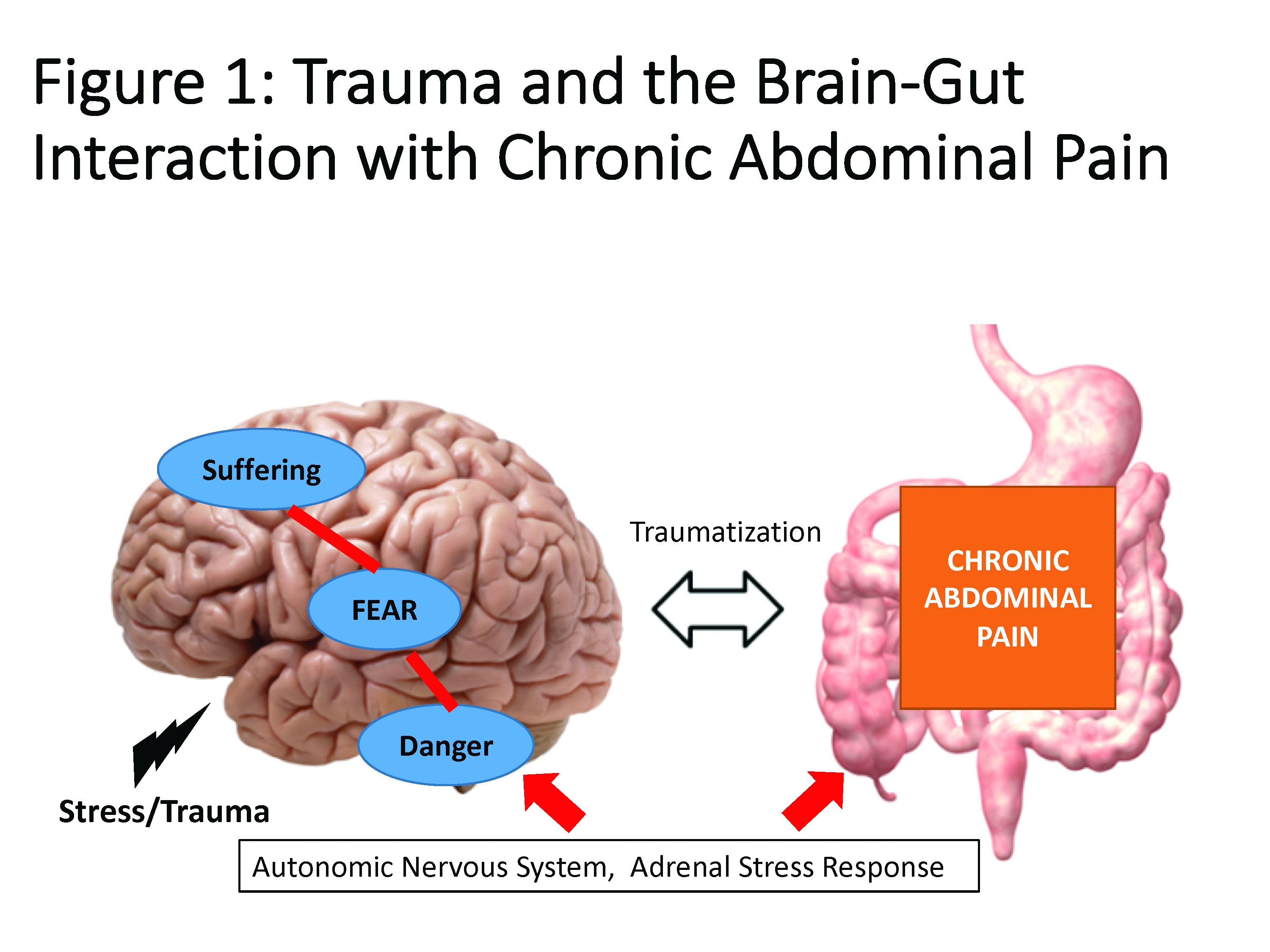
The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21
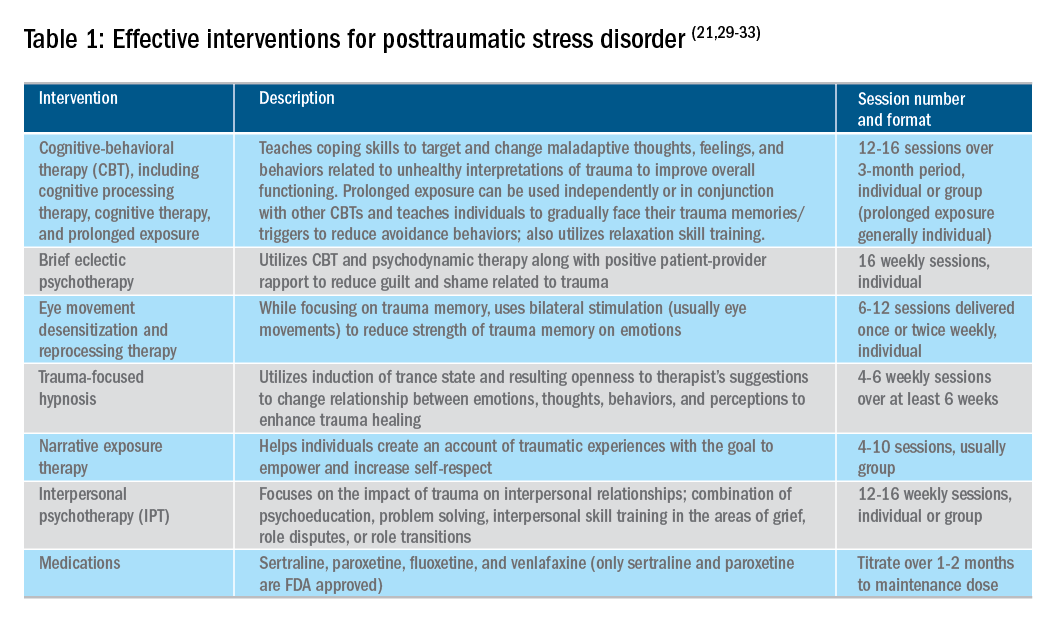
Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).
The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21

Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
Introduction
Abdominal pain is a complex phenomenon that involves unpleasant sensory and emotional experiences caused by actual or potential visceral tissue damage. As pain becomes chronic, there is a functional reorganization of the brain involved in emotional and cognitive processing leading to amplification of pain perception and associated pain suffering.1,2 With the rising recognition of the complexity of pain management in the 1960s, the treatment of pain became a recognized field of study, leading to the formation of interdisciplinary teams to treat pain. However, although efficacious, this model lacked adequate reimbursement structures and eventually subsided as opioids (which at the time were widely believed to be nonaddictive) become more prevalent.3 Not only is there a lack of empirical evidence for opioids in the management of chronic abdominal pain, there is a growing list of adverse consequences of prolonged opioid use for both the brain and gastrointestinal tract.4
Recently, there has been more clinical focus on behavioral interventions that can modulate gut pain signals and associated behaviors by reversing maladaptive emotional and cognitive brain processes.5 One such psychological process that has received little attention is the traumatizing nature of chronic abdominal pain. Chronic pain, particularly when it feels uncontrollable to patients, activates the brain’s fear circuitry and drives hyperarousal, emotional numbing, and consolidation of painful somatic memories, which become habitual and further amplify negative visceral signals.6,7 These processes are identical to the symptom manifestations of posttraumatic stress disorder (PTSD) such as intrusiveness, avoidance, negative mood and cognitions, and hyperarousal from life events. In fact, individuals with a history of other traumatizing exposures have an even higher risk of developing chronic pain disorders.8 This review has two objectives: to provide a theoretical framework for understanding chronic pain as a traumatizing experience with posttraumatic manifestations and to discuss behavioral interventions and adjunctive nonopioid pharmacotherapy embedded in multidisciplinary care models essential to reversing this negative brain-gut cycle and reducing pain-related suffering.
Trauma and chronic abdominal pain
Trauma is defined as an individual’s response to a threat to safety. Traumatized patients or those with PTSD are at higher risk for chronic abdominal pain.9 Given the strong neurobiological connection between the brain and gut that has been phylogenetically preserved, emotional (e.g., fear, terror) or physical (e.g., pain) signals represent danger, and with chronicity, there can be a kindling-related consolidation of these maladaptive neurobiological pathways leading to suffering (e.g., hopelessness, sense of failure) and disability (Figure 1).
The interrelationship between chronic pain and trauma is multifaceted and is further complicated by the traumatizing nature of chronic pain itself, when pain is interpreted as a signal that the body is sick or even dangerously ill. Patients with chronic abdominal pain may seek multiple medical opinions and often undergo extensive, unnecessary, and sometimes harmful interventions to find the cause of their pain, with fear of disability and even death driving this search for answers.
The degree to which an individual with long-lasting pain interprets their discomfort as a risk to their well-being is related to the degree of trauma they experience because of their pain.10 Indeed, many of the negative symptoms associated with posttraumatic stress are also found in those with chronic abdominal pain. Trauma impacts the fear circuitry centers of the brain, leading to altered activation of the hypothalamic-pituitary-adrenal axis and the amygdala, as well as chronic activation of the sympathetic nervous system and stress-released hormones, all of which are potential pathways that dysregulate the brain-gut relationship.11-13 Worries for safety, which are reactivated by physiological cues (e.g., GI symptoms, pain), as well as avoidance of potential triggers of GI symptoms (e.g., food, exercise, medications, and situations such as travel or scheduled events, and fear of being trapped without bathroom access), are common. Traumatized individuals can experience a foreshortened sense of the future, which may lead to decreased investment in long-term determinants of health (e.g., balanced diet, exercise, social support) and have higher rates of functional impairment and higher health care utilization.14 Negative mood, including irritability, anxiety, depression, insomnia, and impaired concentration are common in those with trauma and chronic pain and can be accompanied by internalized blame (e.g., depression, substance abuse, suicidality) or externalized blame (e.g., negative relationships with health care providers, rejection from their support or faith system). These can be worsened by an impaired sense of trust, which impacts the patient-provider relationship and other sources of social support leading to lack of behavioral activation, anhedonia, and isolation.
Another commonality is hypervigilance, as those with chronic abdominal pain are often hyperaware of physical symptoms and always “on alert” for a signal indicative of a pain flare. Anxiety and depression frequently co-occur in populations with trauma and chronic pain; these diagnoses are associated with higher rates of catastrophizing and learned helplessness, which may be exacerbated by lack of a “cure” for functional gastrointestinal disorders (FGIDs) and chronic pain.15 These factors could potentially lead to lack of engagement with treatment or, alternatively, risky or destructive attempts to cure pain including dangerous complementary alternative treatments or substance abuse to numb sensations. Another feature of trauma in chronic pain is the sense of dissociation from and lack of control over the body, sometimes induced by negative medical experiences (e.g., unwanted physical examinations, medication side effects, traumatic procedures, or hospitalizations).16,17
The importance of treating trauma in the management of chronic pain
Behavioral treatment is increasingly being recognized as an essential component in the management of any chronic pain syndrome.18 The most studied psychosocial interventions for chronic abdominal pain are cognitive-behavioral therapy (CBT) and gut-focused hypnosis. CBT is usually a problem-focused, short-term intervention that can be delivered individually in the office, via group therapy, or through virtual platforms. CBT is most effective when cognitive distortions and ineffective behaviors create emotional distress, and the therapy targets patient’s stress reactivity, visceral anxiety, catastrophizing, and inflexible coping styles.5 Gut-focused hypnosis is the second most–studied behavioral treatment for chronic abdominal pain and utilizes the trance state to make positive suggestions leading to broad and lasting physiological and psychological improvement.19 In addition to pain management, both CBT and hypnosis are efficacious treatments for PTSD.20,21

Utilizing a multidisciplinary medical team including integrated behavioral experts, such as in a patient-centered medical home, is considered the standard of care for treatment of chronic pain. The patient-provider relationship is essential, as is consistent follow-up to ensure effective symptom management and improvements in quality of life. Additionally, patient education, including a positive (i.e., clear) diagnosis and information on the brain-gut relationship, is associated with symptom improvement. In our subspecialty medical home for inflammatory bowel disease (IBD), we found that, in our patients who were on opioids for their chronic pain, engagement with our embedded behavioral and pain specialists resulted in significant reduction in opioid use and depression as well as improved self-reported quality of life.22 Gastroenterologists and advanced-practice providers operating without embedded behavioral therapists can consider referring patients to behavioral treatment (e.g., licensed clinical social workers, licensed professional counselors, marriage and family therapists, psychologists, and psychiatrists; the latter often specialize in medication management and may not offer behavioral therapy) for trauma if patients have undergone a traumatic event (e.g., exposure to any potentially life-threatening event, serious injury, or violence) at any point in their lifetime and are experiencing intrusive symptoms (e.g., memories, dreams, or flashbacks to trauma), avoidance of trauma reminders, and negative mood or hyperarousal related to traumatic events (Table 1).23
With the traumatization component of chronic abdominal pain, which can further drive maladaptive coping cycles, incorporation of trauma-informed treatment into gastroenterology clinics is an avenue toward more effective treatment. The core principles of trauma-informed care include safety, choice, collaboration, trustworthiness, and empowerment,24 and are easily aligned with patient-centered models of care such as the interdisciplinary medical home model. Incorporation of screening techniques, interdisciplinary training of clinicians, and use of behavioral providers with experience in evidenced-based treatments of trauma enhance a clinic’s ability to effectively identify and treat individuals who have trauma because of their abdominal pain.25 Additionally, the most common behavioral interventions for functional gastrointestinal disorders (FGIDs) are also efficacious in the treatment of trauma. CBT is a well-validated treatment for PTSD that utilizes exposure therapy to help individuals restructure negative beliefs shaped by their negative experience and develop relaxation skills. Hypnosis is also validated in the treatment of trauma, with the possible mechanism of action being the replacement of the negative or dissociated traumatic trance with a healthy, adaptive trance facilitated by the hypnotherapist.21
Adjunctive nonopioid medications for chronic abdominal pain
While there are few randomized controlled trials establishing efficacy of pharmacotherapy for sustained improvement of abdominal pain or related suffering, several small trials and consensus clinical expert opinion suggest partial improvement in these domains.26,27 Central neuromodulators that can attenuate chronic visceral pain include antidepressants, antipsychotics, and other central nervous system–targeted medications.26 Tricyclic antidepressants (e.g., amitriptyline, nortriptyline, imipramine, desipramine) are often first-line treatment for FGIDs.28 Serotonin noradrenergic reuptake inhibitors (e.g., duloxetine, venlafaxine, desvenlafaxine, milnacipran) are also effective in pain management. Selective serotonin reuptake inhibitors (e.g., paroxetine, fluoxetine, sertraline, citalopram, escitalopram) can be used, especially when comorbid depression, anxiety, and phobic disorders are present. Tetracyclic antidepressants (e.g., mirtazapine, mianserin, trazodone) are effective treatments for early satiety, nausea/vomiting, insomnia, and low weight. Augmenting agents are utilized when single agents do not provide maximum benefit, including quetiapine (disturbed sleep), bupropion (fatigue), aripiprazole, buspirone, and tandospirone (dyspeptic features and anxiety). Delta ligands including gabapentin and pregabalin are helpful for abdominal wall pain or fibromyalgia. Ketamine is a newer but promising pathway for treatment of pain and depression and is increasingly being utilized in outpatient settings. Additionally, partial opioid-receptor agonists including methadone and suboxone have been reported to decrease pain in addition to their efficacy in addiction recovery. Medical marijuana is another area of growing interest, and while research has yet to show a clear effect in pain management, it does appear helpful in nausea and appetite stimulation. Obtaining a therapeutic response is the first treatment goal, after which a patient should be monitored in at least 6-month intervals to ensure sustained benefits and tolerability, and if these are not met, enhancement of treatment or a slow taper is indicated. As in all treatments, a positive patient-provider relationship predicts improved treatment adherence and outcomes.26 However, while these pharmacological interventions can reduce symptom severity, there is little evidence that they reduce traumatization without adjunctive psychotherapy.29
Summary
Both behavioral and pharmacological treatment options are available for chronic abdominal pain and most useful if traumatic manifestations are assessed and included as treatment targets. A multidisciplinary approach to the treatment of chronic abdominal pain with increased screening and treatment of trauma is a promising pathway to improved care and management for patients with chronic pain. If trauma is left untreated, the benefits of otherwise effective treatments are likely to be significantly limited.
References
1. Apkarian AV et al. Prog Neurobiol. 2009 Feb;87(2):81-97.
2. Gallagher RM et al. Pain Med. 2011 Jan;12(1):1-2.
3. Collier R et al. CMAJ. 2018 Jan 8;190(1):E26-7. doi: 10.1503/cmaj.109-5523.
4. Szigethy E et al. Nature Reviews Gastroenterology & Hepatology, 2018;15:168-80.
5. Ballou S et al. Clin Transl Gastroenterol. 2017 Jan;8(1):e214.
6. Egloff N et al. J Pain Res. 2013 Nov 5;6:765-70.
7. Fashler S et al. J Pain Res. 2016 Aug 10;9:551-61.
8. McKernan LC et al. Clin J Pain. 2019 May;35(5):385-93.
9. Ju T et al. J Clin Gastroenterol. 2018 Dec 19. doi: 10.1097/MCG.0000000000001153.
10. Fishbain DA et al. Pain Med. 2017 Apr 1;18(4):711-35.
11. Martin CR et al. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-48.
12. Osadchiy V et al. Clin Gastroenterol Hepatol. 2019 Jan;17(2):322-32.
13. Brzozowski B et al. Curr Neuropharmacol. 2016 Nov;14(8):892-900.
14. Outclat SD et al. Pain Med. 2014;15(11):1872-9.
15. Asmundson GJ et al. Can J Psychiatry. 2002;Dec;47(10):930-7.
16. Taft TH et al. Inflamm Bowel Dis. 2019 Mar 7. doi: 10.1093/ibd/izz032.
17. Duckworth MP et al. International Journal of Rehabilitation and Health, 2000 Apr;5(2):129-39.
18. Scascighini L et al. Rheumatology (Oxford). 2008 May;47(5):670-8.
19. Palsson O et al. European Gastroenterology & Hepatology Review. 2010;6(1):42-6.
20. Watkins LE et al. Frontiers in Behavioral Neuroscience. 2018;12:1-9.
21. O’Toole SK et al. J Trauma Stress. 2016 Feb;29(1):97-100.
22. Goldblum Y et al. Digestive Disease Week. San Diego. 2019. Abstract in press.
23. American Psychiatric Association. Diagnostic and Statistical Manual (of Mental Disorders), Fifth Edition. Arlington, Va: American Psychiatric Publishing, 2013.
24. United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. 2018. Trauma-informed approach and trauma-specific interventions. Retrieved from samhsa.gov/nctic/trauma-interventions.
25. Click BH et al. Inflamm Bowel Dis. 2017;23(5):681-94.
26. Drossman DA et al. Gastroenterology. 2018 Mar;154(4):1140-71.
27. Thorkelson G et al. Inflamm Bowel Dis. 2016 Jun 1;22(6):1509-22.
28. Törnblom H et al. Current Gastroenterology Reports. 2018;20(12):58.
29. Watkins LE et al. Front Behav Neurosci. 2018;12:258.
30. American Psychiatric Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. 2017.
31. Bisson JI et al. Cochrane Database Syst Rev. 2013 Dec 13;(12):CD003388.
32. Department of Veterans Affairs and Department of Defense. VA/DOD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. 2017.
33. Karatzias T et al. Psychol Med. 2019 Mar 12:1-15. doi: 10.1017/S0033291719000436. Advance online publication.
Emily Weaver, LCSW, is a UPMC Total Care–IBD program senior social worker, Eva Szigethy, MD, PhD, is professor of psychiatry and medicine, codirector, IBD Total Care Medical Home, University of Pittsburgh Medical Center, departments of medicine and psychiatry.
Patients say primary care video visits are convenient, high quality
Most patients thought video visits integrated into their existing primary care practice were convenient, provided high-quality care, and strengthened their relationship with their provider, results of a recent survey show.
The video calls may reduce in-person primary care visits, particularly for the two-thirds of patients who otherwise would need to take off from work or make other arrangements to visit the office, according to researcher Mary E. Reed, DrPH, of the Kaiser Permanente Division of Research, Oakland, Calif., and coauthors.*
“Integrated video telemedicine may be a transformative tool in increasing patient-centered access to health care,” the researcher noted in a report on the survey in the Annals of Internal Medicine.
Millions of patients have used direct-to-consumer telemedicine services that are not necessarily integrated with the patient’s electronic medical records and may not provide access to the providers they are accustomed to seeing, investigators said in their report.
By contrast, “house call” video visits that were integrated into existing primary care practices may provide convenience plus access to patients’ usual providers. However, there’s only limited evidence on the patient experience with such integrations, they said.
To fill that information gap, Dr. Reed and colleagues surveyed adults with a scheduled video telemedicine visit with a primary care provider in Kaiser Permanente Northern California. A total of 1,274 patients provided responses.
The Kaiser Permanente telemedicine system allows all primary care providers to have video visits with patients. “Through Internet-connected and video-enabled computers or mobile devices, patients can join video visits from anywhere, and available clinicians include their own providers,” the researchers said in their report.
Of the patients surveyed, 87% said they scheduled a video visit because it was more convenient than other ways of receiving care. Furthermore, 93% said their video visit adequately addressed their needs, 90% said they were confident in the quality of care, and 84% agreed that the ability to have a video visit strengthened their relationship with the provider.
Of the patients who participated in the study, 67% said that, if the visit had been in person, they would have needed to have made at least one arrangement; time off from work was the issue for more than half of participants, though many cited childcare, coverage for another activity or responsibility, or arrangements with someone else to accompany them to the visit as issues.
Concerns with video visits were reported by some patients, researchers said. Twenty-one percent said they were worried that treatment would not be adequate, and 11% said they were concerned about the privacy of medical information.
Moreover, 41% of patients who had scheduled video visits said they preferred to get care in person, the survey results show.
Even so, 89% of patients said they were interested in a future video visit, according to the researchers.
Dr. Reed and coauthors declared no conflicts of interest related to this research, which was supported by a grant from Kaiser Permanente Division of Research.
SOURCE: Reed ME et al. Ann Intern Med. 2019 Apr 29. doi: 10.7326/M18-3081.
*Correction, 4/30/19: An earlier version of this article misstated the location of the Kaiser Permanente Division of Research.
Most patients thought video visits integrated into their existing primary care practice were convenient, provided high-quality care, and strengthened their relationship with their provider, results of a recent survey show.
The video calls may reduce in-person primary care visits, particularly for the two-thirds of patients who otherwise would need to take off from work or make other arrangements to visit the office, according to researcher Mary E. Reed, DrPH, of the Kaiser Permanente Division of Research, Oakland, Calif., and coauthors.*
“Integrated video telemedicine may be a transformative tool in increasing patient-centered access to health care,” the researcher noted in a report on the survey in the Annals of Internal Medicine.
Millions of patients have used direct-to-consumer telemedicine services that are not necessarily integrated with the patient’s electronic medical records and may not provide access to the providers they are accustomed to seeing, investigators said in their report.
By contrast, “house call” video visits that were integrated into existing primary care practices may provide convenience plus access to patients’ usual providers. However, there’s only limited evidence on the patient experience with such integrations, they said.
To fill that information gap, Dr. Reed and colleagues surveyed adults with a scheduled video telemedicine visit with a primary care provider in Kaiser Permanente Northern California. A total of 1,274 patients provided responses.
The Kaiser Permanente telemedicine system allows all primary care providers to have video visits with patients. “Through Internet-connected and video-enabled computers or mobile devices, patients can join video visits from anywhere, and available clinicians include their own providers,” the researchers said in their report.
Of the patients surveyed, 87% said they scheduled a video visit because it was more convenient than other ways of receiving care. Furthermore, 93% said their video visit adequately addressed their needs, 90% said they were confident in the quality of care, and 84% agreed that the ability to have a video visit strengthened their relationship with the provider.
Of the patients who participated in the study, 67% said that, if the visit had been in person, they would have needed to have made at least one arrangement; time off from work was the issue for more than half of participants, though many cited childcare, coverage for another activity or responsibility, or arrangements with someone else to accompany them to the visit as issues.
Concerns with video visits were reported by some patients, researchers said. Twenty-one percent said they were worried that treatment would not be adequate, and 11% said they were concerned about the privacy of medical information.
Moreover, 41% of patients who had scheduled video visits said they preferred to get care in person, the survey results show.
Even so, 89% of patients said they were interested in a future video visit, according to the researchers.
Dr. Reed and coauthors declared no conflicts of interest related to this research, which was supported by a grant from Kaiser Permanente Division of Research.
SOURCE: Reed ME et al. Ann Intern Med. 2019 Apr 29. doi: 10.7326/M18-3081.
*Correction, 4/30/19: An earlier version of this article misstated the location of the Kaiser Permanente Division of Research.
Most patients thought video visits integrated into their existing primary care practice were convenient, provided high-quality care, and strengthened their relationship with their provider, results of a recent survey show.
The video calls may reduce in-person primary care visits, particularly for the two-thirds of patients who otherwise would need to take off from work or make other arrangements to visit the office, according to researcher Mary E. Reed, DrPH, of the Kaiser Permanente Division of Research, Oakland, Calif., and coauthors.*
“Integrated video telemedicine may be a transformative tool in increasing patient-centered access to health care,” the researcher noted in a report on the survey in the Annals of Internal Medicine.
Millions of patients have used direct-to-consumer telemedicine services that are not necessarily integrated with the patient’s electronic medical records and may not provide access to the providers they are accustomed to seeing, investigators said in their report.
By contrast, “house call” video visits that were integrated into existing primary care practices may provide convenience plus access to patients’ usual providers. However, there’s only limited evidence on the patient experience with such integrations, they said.
To fill that information gap, Dr. Reed and colleagues surveyed adults with a scheduled video telemedicine visit with a primary care provider in Kaiser Permanente Northern California. A total of 1,274 patients provided responses.
The Kaiser Permanente telemedicine system allows all primary care providers to have video visits with patients. “Through Internet-connected and video-enabled computers or mobile devices, patients can join video visits from anywhere, and available clinicians include their own providers,” the researchers said in their report.
Of the patients surveyed, 87% said they scheduled a video visit because it was more convenient than other ways of receiving care. Furthermore, 93% said their video visit adequately addressed their needs, 90% said they were confident in the quality of care, and 84% agreed that the ability to have a video visit strengthened their relationship with the provider.
Of the patients who participated in the study, 67% said that, if the visit had been in person, they would have needed to have made at least one arrangement; time off from work was the issue for more than half of participants, though many cited childcare, coverage for another activity or responsibility, or arrangements with someone else to accompany them to the visit as issues.
Concerns with video visits were reported by some patients, researchers said. Twenty-one percent said they were worried that treatment would not be adequate, and 11% said they were concerned about the privacy of medical information.
Moreover, 41% of patients who had scheduled video visits said they preferred to get care in person, the survey results show.
Even so, 89% of patients said they were interested in a future video visit, according to the researchers.
Dr. Reed and coauthors declared no conflicts of interest related to this research, which was supported by a grant from Kaiser Permanente Division of Research.
SOURCE: Reed ME et al. Ann Intern Med. 2019 Apr 29. doi: 10.7326/M18-3081.
*Correction, 4/30/19: An earlier version of this article misstated the location of the Kaiser Permanente Division of Research.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: Of patients surveyed, 93% said their video visit adequately addressed their needs, 90% were confident in the quality of care, and 84% said it strengthened their relationship with the provider.
Study details: Survey responses from 1,274 adults with a scheduled video telemedicine visit with a primary care provider in Kaiser Permanente Northern California.
Disclosures: The authors declared no conflicts of interest related to this work, which was supported by a grant from the Kaiser Permanente Division of Research.
Source: Reed ME et al. Ann Intern Med. 2019 Apr 29. doi: 10.7326/M18-3081.
Benlysta approved for children with SLE
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
Statute of limitations in malpractice actions
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Psoriatic Arthritis Journal Scan: April 2019
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Head and neck cancer cost analysis yields simple bundled payment model
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Good Notes Can Deter Litigation
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
Pretrial screening panels: Do they reduce frivolous claims?
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.
So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.














