User login
What are the risks of long-term PPI use for GERD symptoms in patients > 65 years?
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE-BASED ANSWER:
The use of proton pump inhibitors (PPIs) to control gastroesophageal reflux disease (GERD) is significantly associated with an increased risk of cardiovascular events such as acute myocardial infarction and myocardial ischemia, especially with treatment longer than 8 weeks (strength of recommendation [SOR]: A, systematic review of randomized, controlled trials [RCTs]). This summary is based on data extrapolated from studies on all adults because there is limited evidence that specifically addresses patients older than 65 years.
Adults taking PPIs also appear to be at increased risk of Clostridium difficile infection, community-acquired pneumonia (CAP; with use for < 30 days), and fracture (SOR: B, systematic reviews of heterogeneous prospective and retrospective observational studies).
Daily headaches • associated nausea • obesity • Dx?
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.
DISCUSSION
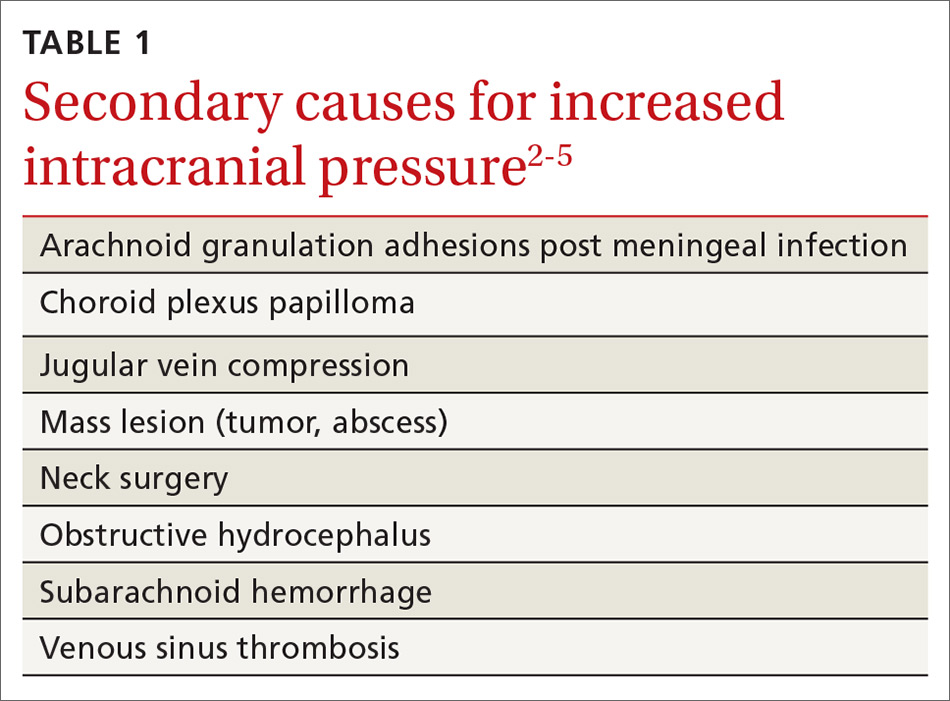
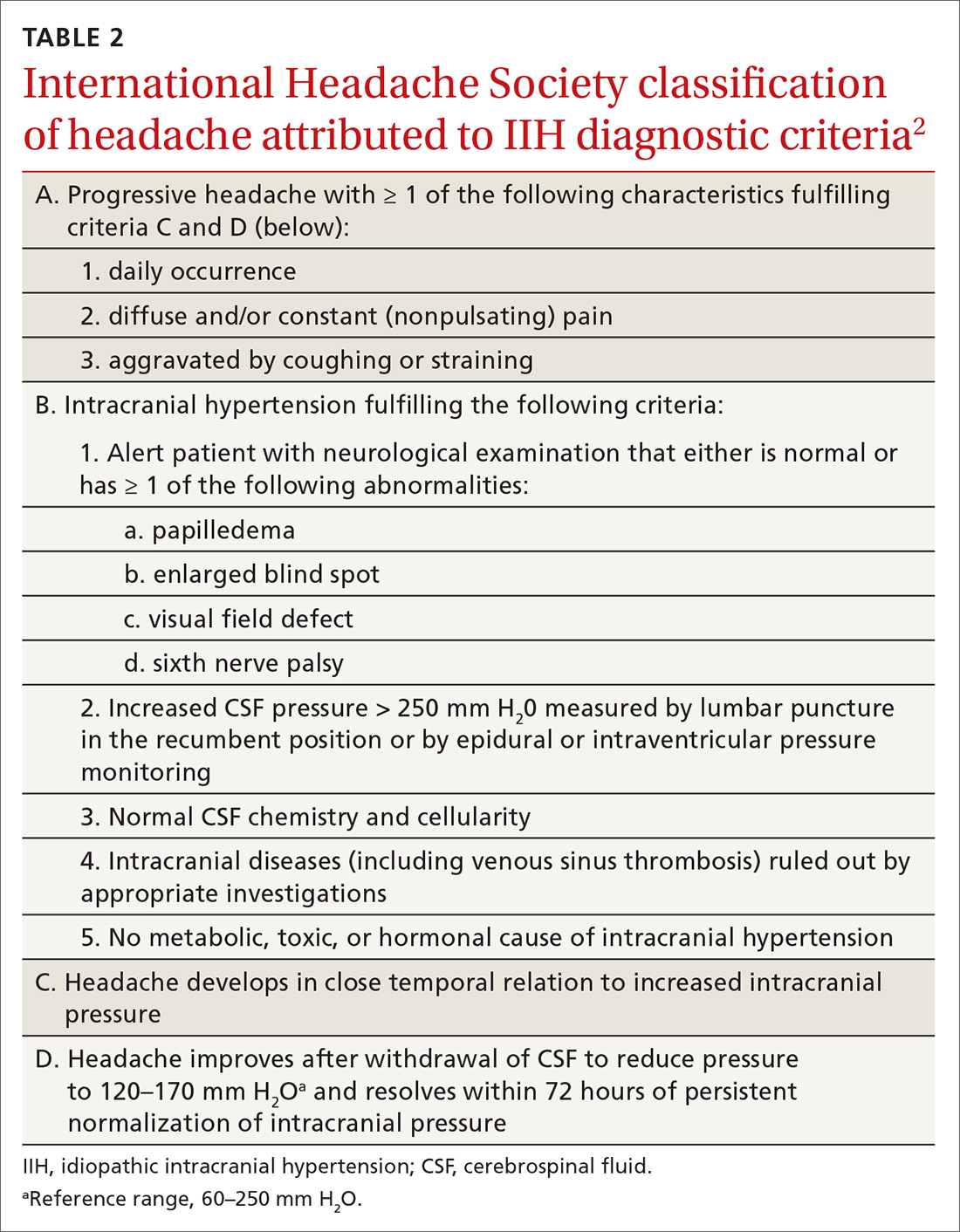
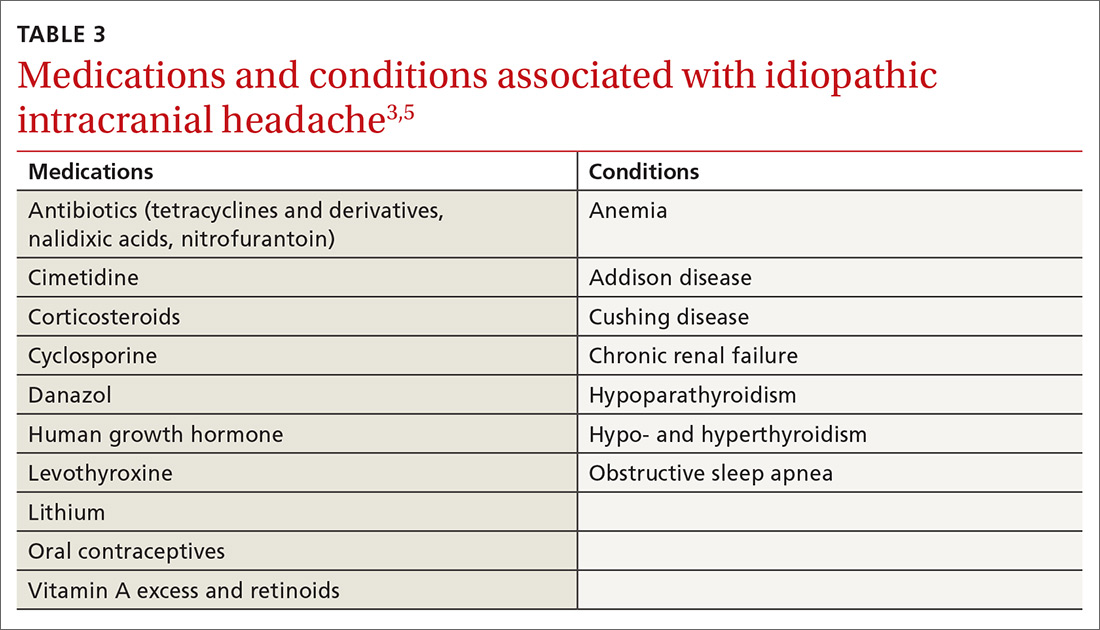
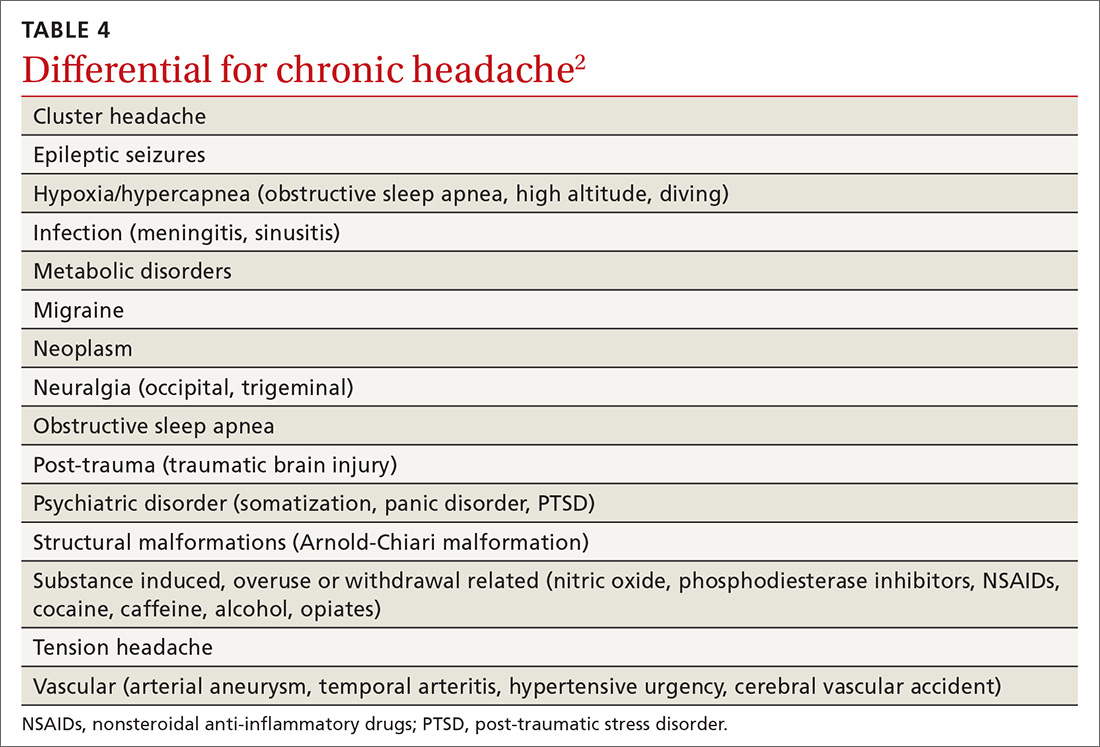
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).
Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6
Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6
Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
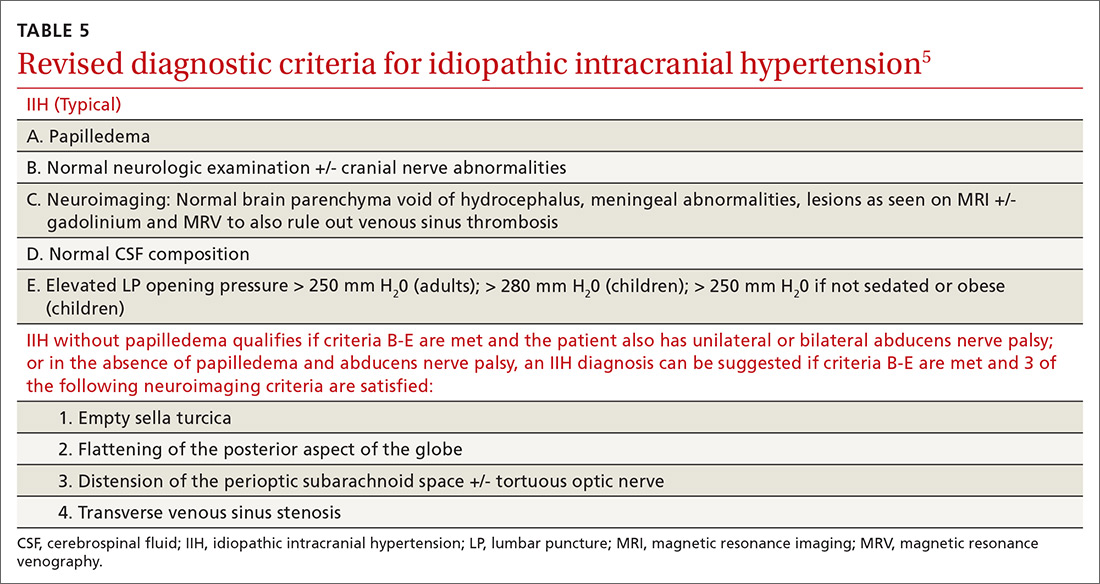
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).
TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; paltooa@mcmaster.ca
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.
DISCUSSION
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).
Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6
Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6
Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).
TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; paltooa@mcmaster.ca
THE CASE
A 22-year-old woman presented to our office complaining of headaches that started 6 weeks earlier. Initially the headache was throbbing, nonpositional, infrequent, and intermittent, lasting 15 to 45 minutes, often starting in the neck and migrating towards the right frontotemporal region. During the week prior to presentation, the headaches became daily and constant, with brief periods of relief after the patient took ibuprofen 400 mg 4 times a day as needed. The patient reported associated nausea, a sensation of pressure changes in the ears, and intermittent dimming of vision in the right eye (sometimes independent of headache). The patient denied photophobia and phonophobia. Her only medication was an oral contraceptive pill (OCP). She had no prior history of headaches.
Physical examination showed a blood pressure of 148/66 mm Hg, body mass index of 44.38, muscle tenderness in the neck and upper back, and no focal neurological findings. Funduscopic examination was unsuccessful. A working diagnosis of atypical migraine was made, but because of unilateral visual disturbance the patient was referred to Ophthalmology for further evaluation. The following day, ophthalmological consultation found bilateral papilledema and the patient was admitted to our hospitalist service via the Emergency Department. She subsequently was referred to inpatient Neurology.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of the brain and orbits with and without contrast was unremarkable. Magnetic resonance venography (MRV) with contrast of the brain showed possible stenosis at the junction of the transverse and sigmoid sinuses but no mass lesion nor venous sinus thrombosis. Lumbar puncture (LP) revealed an opening pressure of 650 mm H20 (reference range, 60–250 mm H2O).1 A diagnosis of idiopathic intracranial hypertension (IIH) was made.
DISCUSSION
IIH, previously known as pseudotumor cerebri and benign intracranial hypertension, is defined by signs and symptoms of elevated intracranial pressure (ICP) without obvious cause on neuroimaging (TABLE 12-5). It is well documented that IIH is consequential and can result in vision loss and intractable chronic headaches.5,6 Older terms such as pseudotumor cerebri and benign intracranial hypertension are therefore no longer recommended because they are considered misleading and not reflective of the severity of potential injury caused by the condition3,4,6 IIH is considered a diagnosis of exclusion requiring certain criteria to be met (TABLE 22). Although the etiology of IIH is unclear, associations have been made between IIH and various medications and conditions2-5,7 (TABLE 33,5).
Classically, IIH affects women who are obese and of childbearing age, but studies have shown that this condition also can affect men and children—albeit less frequently.3,5-7 The incidence of IIH in the general population is between 0.03 to 2.36/100,000 people per year, but in women, the incidence is 0.65 to 4.65/100,000 per year.6 Furthermore, females who are obese have an incidence of 2.7 to 19.3/100,000 per year.6
Headache is the most common symptom of IIH. Unfortunately, the differential diagnosis of headache is vast; thus, a careful history is needed to narrow the field3,5-7 (TABLE 42). Associated symptoms of transient visual changes, pulsatile tinnitus, neck and back pain, nausea, vomiting, photo/phonophobia, and findings of abducens nerve palsy or papilledema—while nonspecific— should raise suspicion for elevated ICP and IIH, especially in women who are obese.2-8 Once IIH is suspected, an urgent diagnosis and treatment is necessary to prevent permanent vision loss.3,4,6
Headache with findings of papilledema warrants neuroimaging, preferably with MRI, to rule out intracranial mass and hydrocephalus.1,2,5 MRV also is recommended to assess for intracranial venous thrombosis, an alternate cause for papilledema and increased ICP.1,2,4,5
Continue to: Recently, a classification of IIH...
Recently, a classification of IIH without papilledema has been acknowledged by the International Headache Society.2,8 Specific MRI findings have been suggested to help make this diagnosis5,9 (TABLE 55).
TREATMENT FOR IIH CAN BE MEDICAL OR SURGICAL
Medications associated with IIH should be discontinued.7 The first-line medication for IIH is acetazolamide, a carbonic anhydrase inhibitor that works in the choroid plexus to decrease cerebrospinal fluid (CSF) production and thus, lower ICP.3,6 An adult dose of 1 to 2 g/day3,4,6 is tolerated well, but can be increased to 4 g/day,10 if necessary. Weight loss via diet and exercise or bariatric surgery has been shown to be effective in patients who are obese and have been given a diagnosis of IIH.3,4
Topiramate also has been suggested as a treatment option, based on its usefulness in weight loss and because of its action as a weak carbonic anhydrase inhibitor.3,6 Also, LP has therapeutic merit—although relief is only short-term.3,6 Patients who fail medical therapy and have intractable headache or progressive visual loss appear to benefit from optic nerve sheath fenestration.3,7,8
Our patient experienced notable improvement in her headache after LP. Her OCP was discontinued, a diuretic regimen started, and weight loss counseling was provided. Prior to discharge, the patient was seen by a neuro-ophthalmologist for perimetry, a visual field test that assesses for acute vision loss and establishes a baseline for follow-up monitoring of vision.7
THE TAKEAWAY
Headache is a common condition that may be challenging to correctly diagnose. A thorough history and neurological examination, including fundoscopy, are essential in the evaluation of headache and suspected IIH. In the primary care setting, limited time, lack of mydriatic agents, suboptimal lighting, and practitioner inexperience may pose challenges for funduscopic examination. Ophthalmoscopes incorporating new technology to expand and magnify the examiner’s field of view may facilitate this exam.11 A global rise in the prevalence of obesity underscores a need for primary care providers to be compulsive about their clinical evaluation when symptoms suspicious of IIH are present. Lastly, if IIH cannot be ruled out confidently, recommend a prompt evaluation by an ophthalmologist.
CORRESPONDENCE
Aarti Paltoo, MD, MSc, CCFP, Peel Village Medical Center, 28 Rambler Drive, Brampton, Ontario L6W 1E2 Canada; paltooa@mcmaster.ca
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.
1. Lee SC, Lueck CJ. Cerebrospinal fluid pressure in adults. J Neuroophthalmol. 2014;34:278-283.
2. International Headache Society. Idiopathic intracranial hypertension. The International Classification of Headache Disorders. 2nd ed. Oxford, UK: Blackwell Publishing; 2003:1-232.
3. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488-494.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14:380-390.
5. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159-1165.
6. Julayanont P, Karukote A, Ruthirago D, et al. Idiopathic intracranial hypertension: ongoing clinical challenges and future prospects. J Pain Res. 2016;9:87-99.
7. Friedman DI, Digre KB. Headache medicine meets neuro-ophthalmology: exam techniques and challenging cases. Headache. 2013;53:703-716.
8. Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranaial hypertension with and without papilledema. Headache. 2009;49:185-193.
9. Digre KB. Imaging characteristics of IIH: are they reliable? Cephalagia. 2013;33:1067-1069.
10. Horton J. Acetazolamide for pseudotumor cerebri--evidence from the NORDIC trial. JAMA. 2014;311:1618-1619.
11. Petrushkin H, Barsam A, Mavrakakis M, et al. Optic disc assessment in the emergency department: a comparative study between the PanOptic and direct ophthalmoscopes. Emerg Med J. 2012;29:1007-1008.
High ankle sprains: Easy to miss, so follow these tips
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
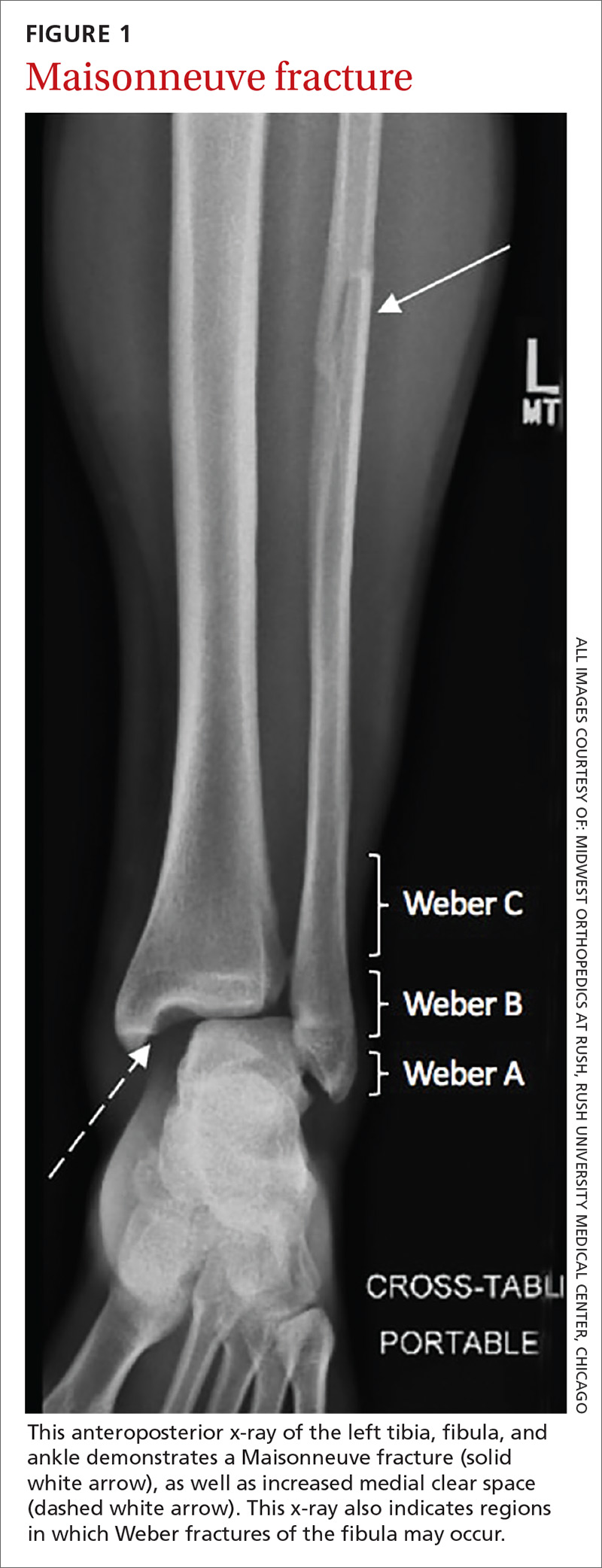
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
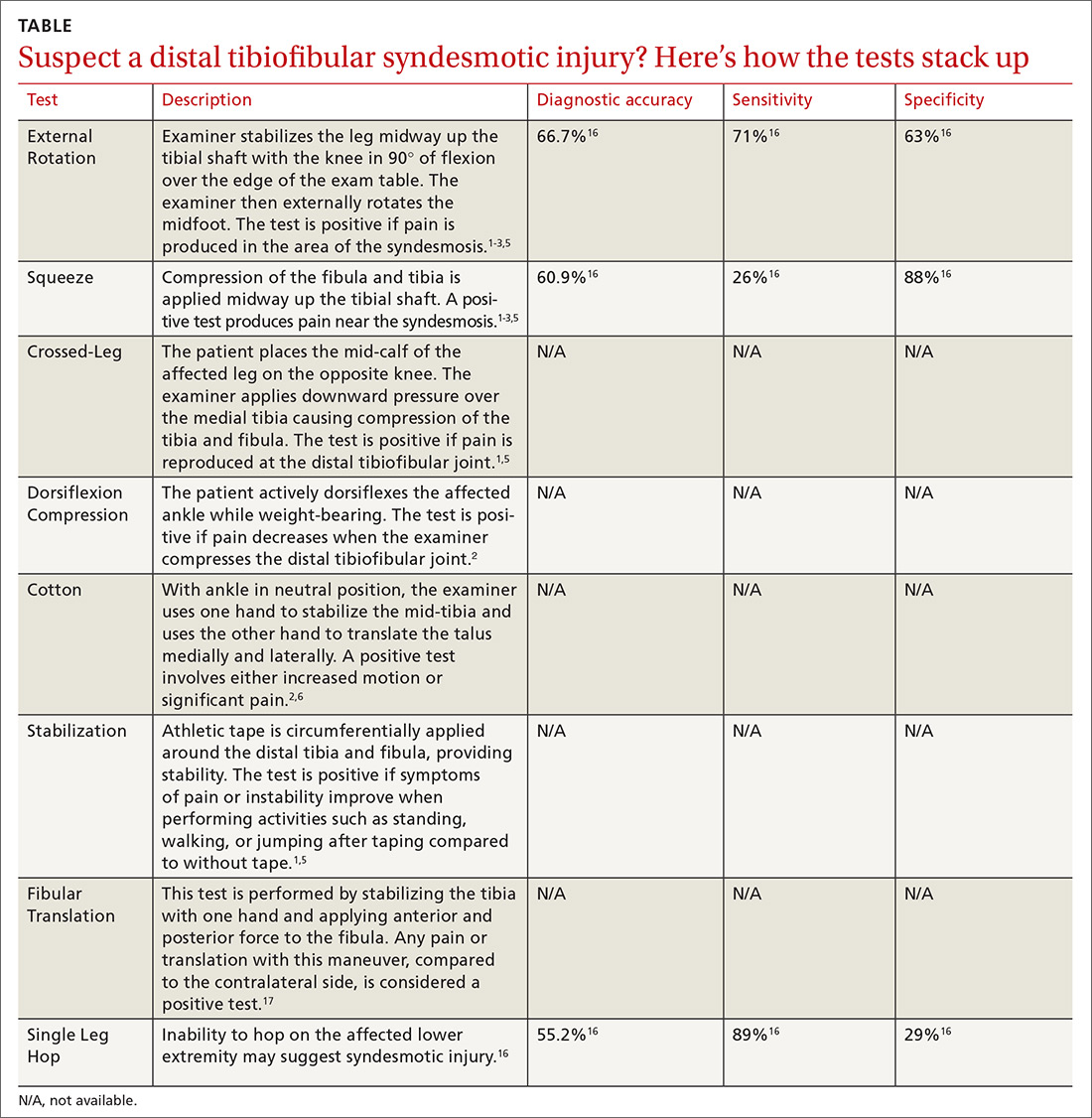
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
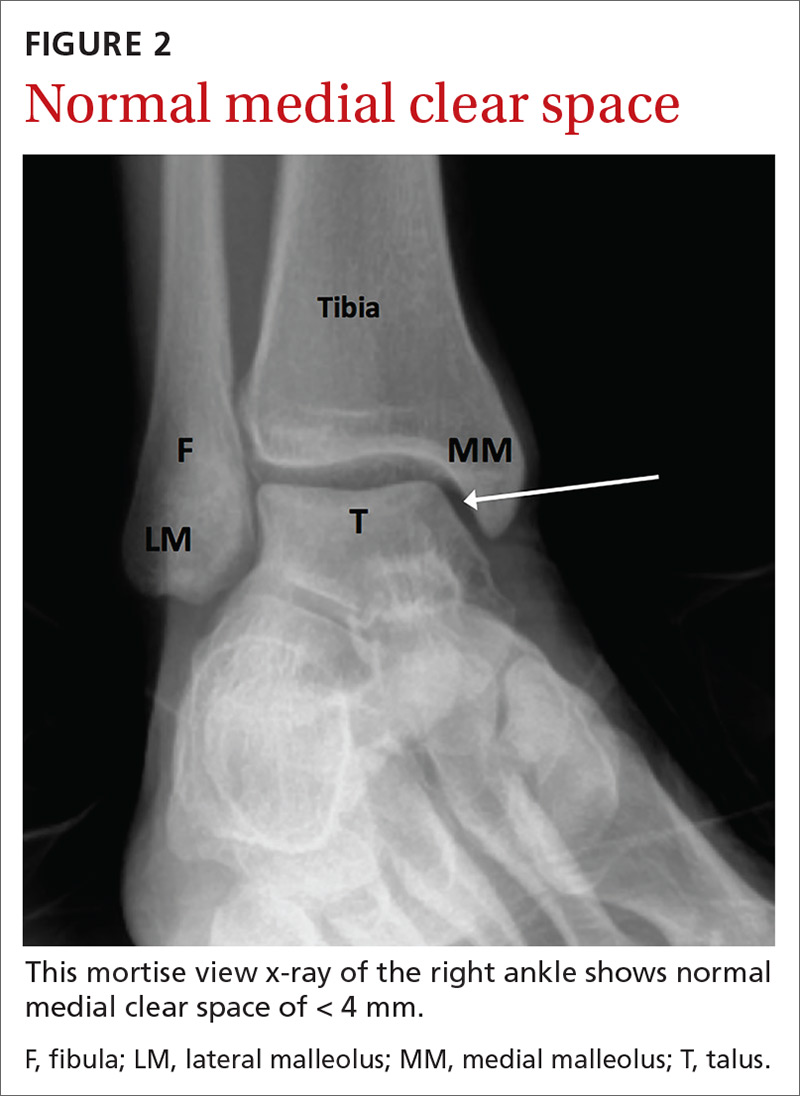
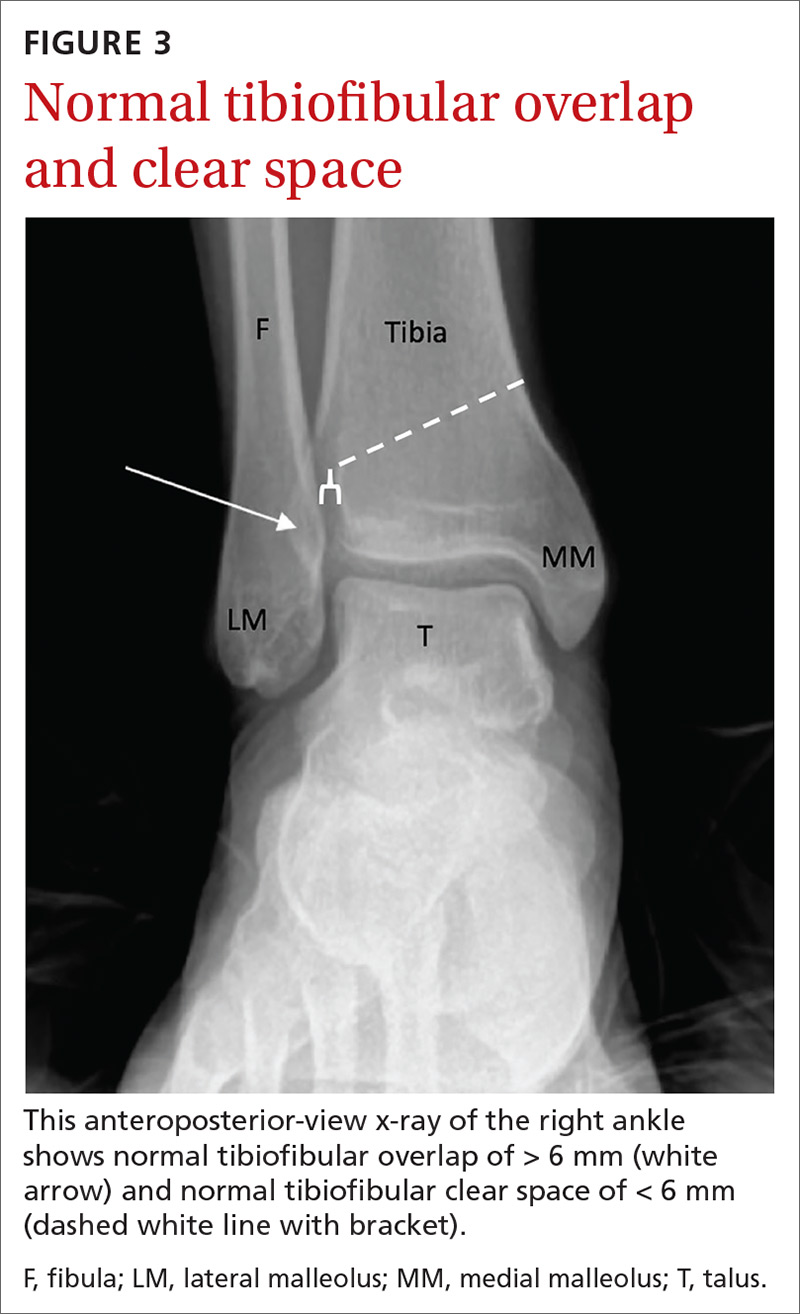
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
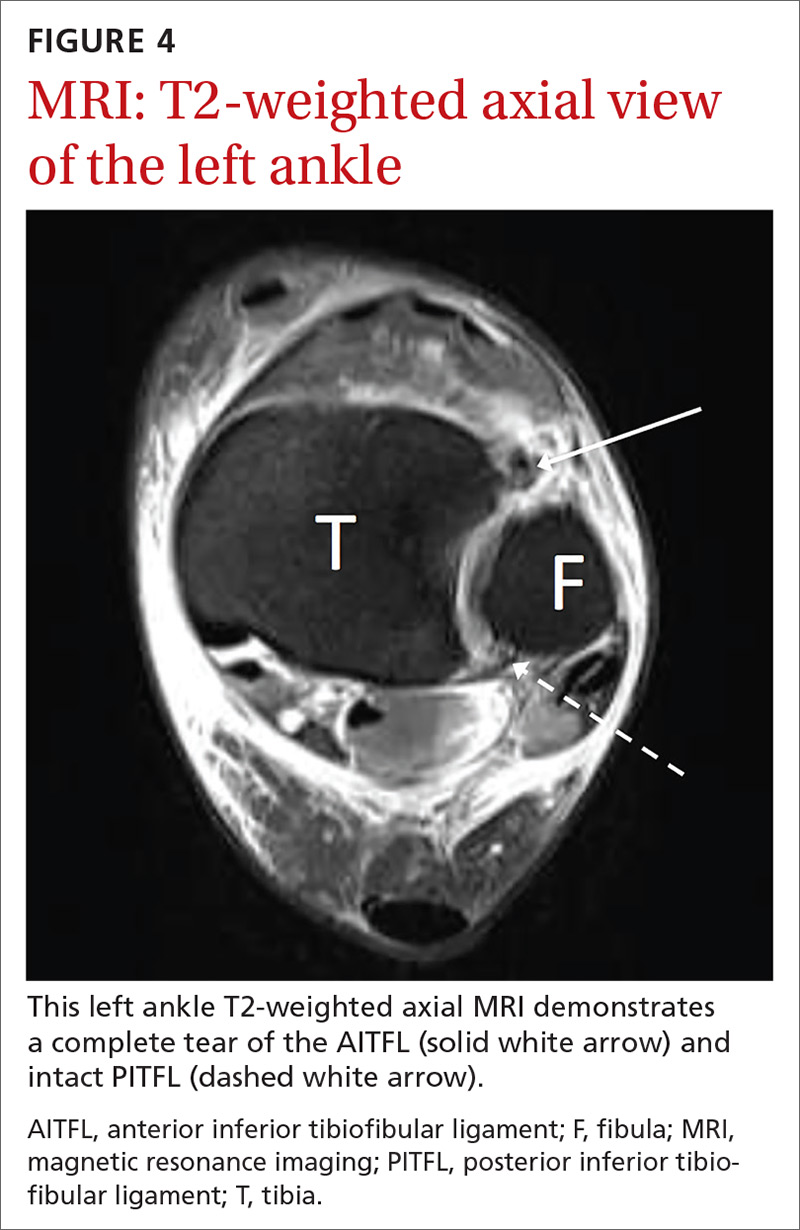
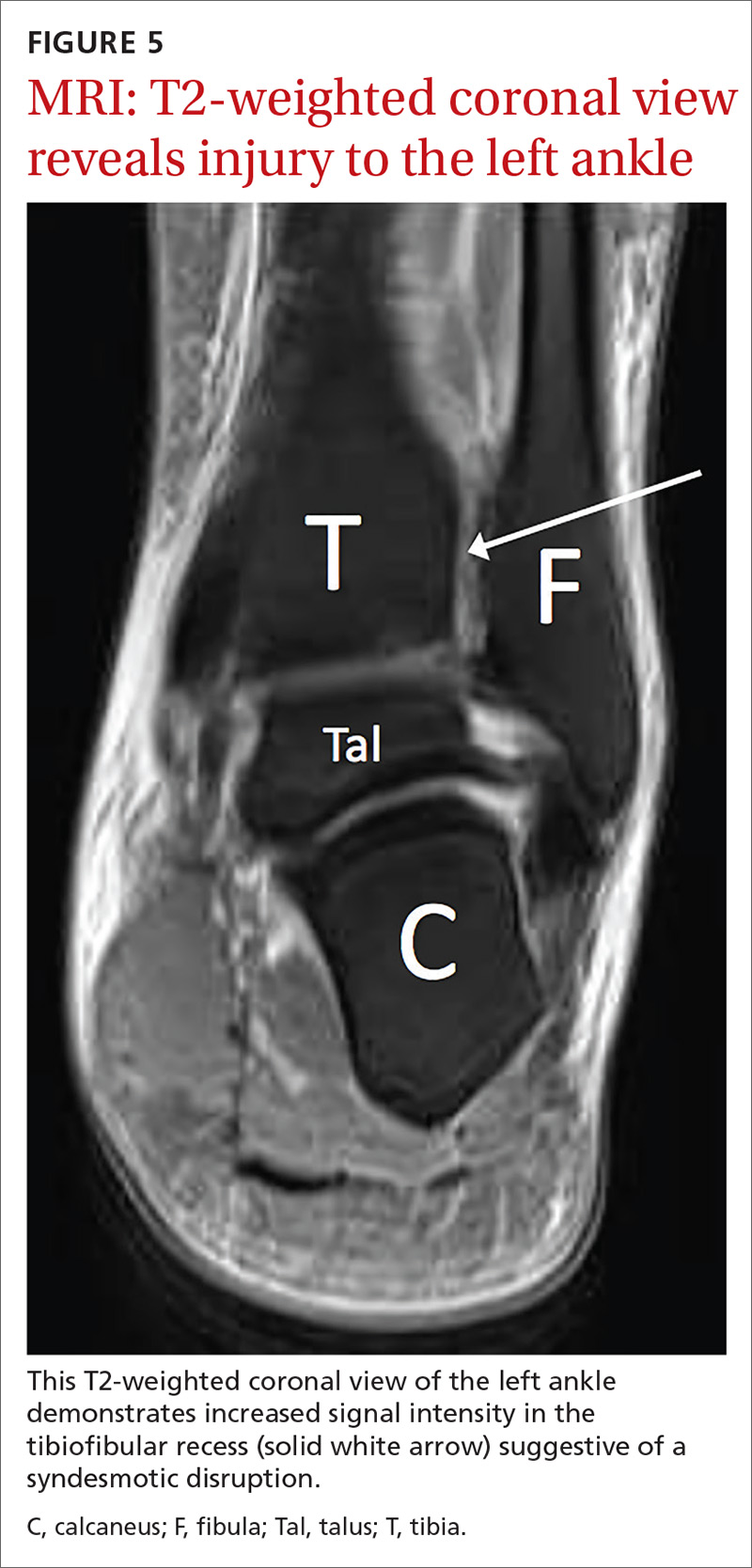
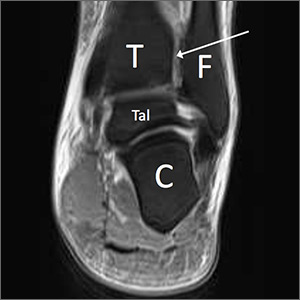
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; jack.nickless@rushortho.com.
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; jack.nickless@rushortho.com.
CASE
A 19-year-old college football player presents to your outpatient family practice clinic after suffering a right ankle injury during a football game over the weekend. He reports having his right ankle planted on the turf with his foot externally rotated when an opponent fell onto his posterior right lower extremity. He reports having felt immediate pain in the area of the right ankle and requiring assistance off of the field, as he had difficulty walking. The patient was taken to the emergency department where x-rays of the right foot and ankle did not show any signs of acute fracture or dislocation. The patient was diagnosed with a lateral ankle sprain, placed in a pneumatic ankle walking brace, and given crutches.
A high ankle sprain, or distal tibiofibular syndesmotic injury, can be an elusive diagnosis and is often mistaken for the more common lateral ankle sprain. Syndesmotic injuries have been documented to occur in approximately 1% to 10% of all ankle sprains.1-3 The highest number of these injuries occurs between the ages of 18 and 34 years, and they are more frequently seen in athletes than in nonathletes, particularly those who play collision sports, such as football, ice hockey, rugby, wrestling, and lacrosse.1-9 In one study by Hunt et al,10 syndesmotic injuries accounted for 24.6% of all ankle injuries in National Collegiate Athletic Association (NCAA) football players. Incidence continues to grow as recognition of high ankle sprains increases among medical professionals.1,5 Identification of syndesmotic injury is critical, as lack of detection can lead to extensive time missed from athletic participation and chronic ankle dysfunction, including pain and instability.2,4,6,11
Back to basics: A brief anatomy review
Stability in the distal tibiofibular joint is maintained by the syndesmotic ligaments, which include the anterior inferior tibiofibular ligament (AITFL), the posterior inferior tibiofibular ligament (PITFL), the transverse ligament, and the interosseous ligament.3-6,8 This complex of ligaments stabilizes the fibula within the incisura of the tibia and maintains a stable ankle mortise.1,4,5,11 The deep portion of the deltoid ligament also adds stability to the syndesmosis and may be disrupted by a syndesmotic injury.2,5-7,11
Mechanisms of injury: From most common to less likely
The distal tibiofibular syndesmosis is disrupted when an injury forces apart the distal tibiofibular joint. The most commonly reported means of injury is external rotation with hyper-dorsiflexion of the ankle.1-3,5,6,11 With excessive external rotation of the forefoot, the talus is forced against the medial aspect of the fibula, resulting in separation of the distal tibia and fibula and injury to the syndesmotic ligaments.2,3,5,6 Injuries associated with external rotation are commonly seen in sports that immobilize the ankle within a rigid boot, such as skiing and ice hockey.1,2,5 Some authors have suggested that a planovalgus foot alignment may place athletes at inherent risk for an external rotation ankle injury.5,6
Syndesmotic injury may also occur with hyper-dorsiflexion, as the anterior, widest portion of the talus rotates into the ankle mortise, wedging the tibia and fibula apart.2,3,5 There have also been reports of syndesmotic injuries associated with internal rotation, plantar flexion, inversion, and eversion.3,5,11 Therefore, physicians should maintain a high index of suspicion for injury to the distal tibiofibular joint, regardless of the mechanism of injury.
Presentation and evaluation
Observation of the patient and visualization of the affected ankle can provide many clues. Many patients will have difficulty walking after suffering a syndesmotic injury and may require the use of an assistive device.5 The inability to bear weight after an ankle injury points to a more severe diagnosis, such as an ankle fracture or syndesmotic injury, as opposed to a simple lateral ankle sprain. Patients may report anterior ankle pain, a sensation of instability with weight bearing on the affected ankle, or have persistent symptoms despite a course of conservative treatment. Also, they can have a variable amount of edema and ecchymosis associated with their injury; a minimal extent of swelling or ecchymosis does not exclude syndesmotic injury.3
A large percentage of patients will present with a concomitant sprain of the lateral ligaments associated with lateral swelling and bruising. One study found that 91% of syndesmotic injuries involved at least 1 of the lateral collateral ligaments (anterior talofibular ligament [ATFL], calcaneofibular ligament [CFL], or posterior talofibular ligament [PTFL]).12 Patients may have pain or a sensation of instability when pushing off with the toes,5 and patients with syndesmotic injuries often have tenderness to palpation over the distal anterolateral ankle or syndesmotic ligaments.7
Continue to: A thorough examination...
A thorough examination of the ankle, including palpation of common fracture sites, is important. Employ the Ottawa Ankle Rules (see http://www.theottawarules.ca/ankle_rules) to investigate for: tenderness to palpation over the posterior 6 cm of the posterior aspects of the distal medial and lateral malleoli; tenderness over the navicular; tenderness over the base of the fifth metatarsal; and/or the inability to bear weight on the affected lower extremity immediately after injury or upon evaluation in the physician’s office. Any of these findings should raise concern for a possible fracture (see “Adult foot fractures: A guide”) and require an x-ray(s) for further evaluation.13
Perform range-of-motion and strength testing with regard to ankle dorsiflexion, plantar flexion, abduction, adduction, inversion, and eversion. Palpate the ATFL, CFL, and PTFL for tenderness, as these structures may be involved to varying degrees in lateral ankle sprains. An anterior drawer test (see https://www.youtube.com/watch?v=vAcBEYZKcto) may be positive with injury to the ATFL. This test is performed by stabilizing the distal tibia with one hand and using the other hand to grasp the posterior aspect of the calcaneus and apply an anterior force. The test is positive if the talus translates forward, which correlates with laxity or rupture of the ATFL.13 The examiner should also palpate the Achilles tendon, peroneal tendons just posterior to the lateral malleolus, and the tibialis posterior tendon just posterior to the medial malleolus to inspect for tenderness or defects that may be signs of injury to these tendons.
An associated Weber B or C fracture? Trauma causing ankle syndesmosis injuries may be associated with Weber B or Weber C distal fibula fractures.7 Weber B fractures occur in the distal fibula at the level of the ankle joint (see FIGURE 1). These types of fractures are typically associated with external rotation injuries and are usually not associated with disruption of the interosseous membrane.
Weber C fractures are distal fibular fractures occurring above the level of the ankle joint. These fractures are also typically associated with external ankle rotation injuries and include disruption of the syndesmosis and deltoid ligament.14
Also pay special attention to the proximal fibula, as syndesmotic injuries are commonly associated with a Maisonneuve fracture, which is a proximal fibula fracture associated with external rotation forces of the ankle (see FIGURE 1).1,2,4,11,14,15 Further workup should occur in any patient with the possibility of a Weber- or Maisonneuve-type fracture.
Continue to: Multiple tests...
Multiple tests are available to investigate the possibility of a syndesmotic injury and to assess return-to-sport readiness, including the External Rotation Test, the Squeeze Test, the Crossed-Leg Test, the Dorsiflexion Compression Test, the Cotton Test, the Stabilization Test, the Fibular Translation Test, and the Single Leg Hop Test (see TABLE1-3,5,6,16,17). The External Rotation Test is noted by some authors to have the highest interobserver reliability, and is our preferred test.2 The Squeeze Test also has moderate interobserver reliability.2 There is a significant degree of variation among the sensitivity and specificity of these diagnostic tests, and no single test is sufficiently reliable or accurate to diagnose a syndesmotic ankle injury. Therefore, it is recommended to use multiple physical exam maneuvers, the history and mechanism of injury, and findings on imaging studies in conjunction to make the diagnosis of a syndesmotic injury.1,16
Imaging: Which modes and when?
The initial workup should include ankle x-rays when evaluating for the possibility of a distal tibiofibular syndesmosis injury. While the Ottawa Ankle Rules are helpful in providing guidance with regard to x-rays, suspicion of a syndesmotic injury mandates x-rays to determine the stability of the joint and rule out fracture. The European Society of Sports Traumatology, Knee Surgery and Arthroscopy–European Foot and Ankle Associates (ESSKA-AFAS) recommend, at a minimum, obtaining anteroposterior (AP)- and mortise-view ankle x-rays to investigate the tibiofibular clear space, medial clear space, and tibiofibular overlap.7 Most physicians also include a lateral ankle x-ray.
If possible, images should be performed while the patient is bearing weight to further evaluate stability. Radiographic findings that support the diagnosis of syndesmotic injury include a tibiofibular clear space > 6 mm on AP view, medial clear space > 4 mm on mortise view, or tibiofibular overlap < 6 mm on AP view or < 1 mm on mortise view (see FIGURES 2 and 3).1,3,5,8 Additionally, if you suspect a proximal fibular fracture, obtain an x-ray series of the proximal tibia and fibula to investigate the possibility of a Maisonneuve injury.1,2,4,11
If you continue to suspect a syndesmotic injury despite normal x-rays, obtain stress x-rays, in addition to the AP and mortise views, to ensure stability. These x-rays include AP and mortise ankle views with manual external rotation of the ankle joint, which may demonstrate abnormalities not seen on standard x-rays. Bilateral imaging can also be useful to further assess when mild abnormalities vs symmetric anatomic variants are in question.1,7
If there is concern for an unstable injury, refer the patient to a foot and ankle surgeon, who may pursue magnetic resonance imaging (MRI) or standing computed tomography (CT).1,2,5,7 MRI is the recommended choice for further evaluation of a syndesmotic injury, as it is proven to be accurate in evaluating the integrity of the syndesmotic ligaments (see FIGURES 4 and 5).18 MRI has demonstrated 100% sensitivity for detecting AITFL and PITFL injuries, as well as 93% and 100% specificity for AITFL and PITFL tears, respectively.8 A weight-bearing CT scan, particularly axial views, can also be a useful adjunct, as it is more sensitive than standard x-rays for assessing for mild diastasis. Although CT can provide an assessment of bony structures, it is not able to evaluate soft tissue structures, limiting its utility in evaluation of syndesmotic injuries.1,7
Continue to: Although not the standard of care...
Although not the standard of care, ultrasonography (US) is gaining traction as a means of investigating the integrity of the syndesmotic ligaments. US is inexpensive, readily available in many clinics, allows for dynamic testing, and avoids radiation exposure.7 However, US requires a skilled sonographer with experience in the ankle joint for an accurate diagnosis. If the workup with advanced imaging is inconclusive, but a high degree of suspicion remains for an unstable syndesmotic injury, consider arthroscopy to directly visualize and assess the syndesmotic structures.1,2,5,7,8
Grading the severity of the injury and pursuing appropriate Tx
Typically, the severity of a syndesmotic injury is classified as fitting into 1 of 3 categories: Grade I and II injuries are the most common, each accounting for 40% of syndesmotic injuries, while 20% of high ankle sprains are classified as Grade III.12
A Grade I injury consists of a stable syndesmotic joint without abnormal radiographic findings. There may be associated tenderness to palpation over the distal tibiofibular joint, and provocative testing may be subtle or normal. These injuries are often minor and able to be treated conservatively.
A Grade II injury is associated with a partial syndesmotic disruption, typically with partial tearing of the AITFL and interosseous ligament. These injuries may be stable or accompanied by mild instability, and provocative testing is usually positive. X-rays are typically normal with Grade II injuries, but may display subtle radiographic findings suggestive of a syndesmotic injury. Treatment of Grade II injuries is somewhat controversial and should be an individualized decision based upon the patient’s age, activity level, clinical exam, and imaging findings. Therefore, treatment of Grade II syndesmotic injuries may include a trial of conservative management or surgical intervention.
A Grade III injury represents inherent instability of the distal tibiofibular joint with complete disruption of all syndesmotic ligaments, with or without involvement of the deltoid ligament. X-rays will be positive in Grade III syndesmotic injuries because of the complete disruption of syndesmotic ligaments. All Grade III injuries require surgical intervention with a syndesmotic screw or other stabilization procedure.1,6-8,15
Continue to: A 3-stage rehabilitation protocol
A 3-stage rehabilitation protocol
When conservative management is deemed appropriate for a stable syndesmotic sprain, a 3-stage rehabilitation protocol is typically utilized.
The acute phase focuses on protection, pain control, and decreasing inflammation. The patient’s ankle is often immobilized in a cast or controlled ankle movement (CAM) boot. The patient is typically allowed to bear weight in the immobilizer during this phase as long as he/she is pain-free. If pain is present with weight bearing despite immobilization, non-weight bearing is recommended. The patient is instructed to elevate the lower extremity, take anti-inflammatory medication, and ice the affected ankle. Additionally, physical therapy modalities may be utilized to help with edema and pain. Joint immobilization is typically employed for 1 to 3 weeks post-injury. In the acute phase, the patient may also work with a physical therapist or athletic trainer on passive range of motion (ROM), progressing to active ROM as tolerated.1,5,7,8,19
The patient can transition from the CAM boot to a lace-up ankle brace when he/she is able to bear full weight and can navigate stairs without pain, which typically occurs around 3 to 6 weeks post-injury.1,5,7 A pneumatic walking brace may also be used as a transition device to provide added stabilization.
In the sub-acute phase, rehabilitation may progress to increase ankle mobility, strengthening, neuromuscular control, and to allow the patient to perform activities of daily living.5-7
The advanced training phase includes continued neuromuscular control, increased strengthening, plyometrics, agility, and sports-specific drills.5 Athletes are allowed to return to full participation when they have regained full ROM, are able to perform sport-specific agility drills without pain or instability, and have near-normal strength.5-7 Some authors also advocate that a Single Leg Hop Test should be included in the physical exam, and that it should be pain free prior to allowing an athlete to return to competition.20 Both progression in physical rehabilitation and return to sport should be individualized based upon injury severity, patient functionality, and physical exam findings.
Continue to: Outcomes forecast
Outcomes forecast: Variable
The resolution of symptoms and return to competition after a syndesmotic injury is variable. In one cohort study of cadets (N = 614) at the United States Military Academy, the average time lost from a syndesmotic ankle sprain was 9.82 days (range 3-21 days).9 In a retrospective review of National Hockey League players, average time to return to competition after a syndesmotic ankle injury sprain (n = 14) was 45 days (range 6-137 days) vs 1.4 days (range 0-6 days) for lateral ankle sprains (n = 5).21 In another study, National Football League players with syndesmotic sprains (n = 36) had a mean time loss from play of 15.4 days (± 11.1 days) vs 6.5 days (± 6.5 days) of time loss from play in those with lateral ankle sprains (n = 53).22
Although there is a fair amount of variability among studies, most authors agree that the average athlete can expect to return to sport 4 to 8 weeks post-injury with conservative management.19 At least 1 study suggests that the average time to return to sport in patients with Grade III syndesmotic injuries who undergo surgical treatment with a syndesmotic screw is 41 days (range 32-48).23 The differences in return to sport may be related to severity of injury and/or type of activity.
Persistent symptoms are relatively common after conservative management of syndesmotic injuries. One case series found that 36% of patients treated conservatively had complaints of persistent mild-to-moderate ankle stiffness, 23% had mild-to-moderate pain, and 18% had mild-to-moderate ankle swelling.24 Despite these symptoms, 86% of the patients rated their ankle function as good after conservative treatment.24 In patients with persistent symptoms, other possible etiologies should be considered including neurologic injury, complex regional pain syndrome, osteochondral defect, loose body, or other sources that may be contributing to pain, swelling, or delayed recovery.
At least 1 randomized controlled trial (RCT) investigated the utility of platelet rich plasma (PRP) injections around the injured AITFL in the setting of an acute syndesmotic injury. The study showed promising results, including quicker return to play, restabilization of the syndesmotic joint, and less residual pain;25 however, the study population was relatively small (N = 16), and the authors believed that more research is required on the benefits of PRP therapy in syndesmotic injuries before recommendations can be made.
An ounce of preventionis worth a pound of cure
Although injury is not always avoidable, there are measures that can help prevent ankle sprains and facilitate return to play after injury. As previously mentioned, athletes should be able to demonstrate the ability to run, cut, jump, and perform sport-specific activities without limitations prior to being allowed to return to sport after injury.5-7,26 Additionally, issues with biomechanics and functional deficits should be analyzed and addressed. By targeting specific strength deficits, focusing on proprioceptive awareness, and working on neuromuscular control, injury rates and recurrent injuries can be minimized. One RCT showed a 35% reduction in the recurrence rate of lateral ankle sprains with the use of an unsupervised home-based proprioceptive training program.27
Continue to: Strength training...
Strength training, proprioceptive and neuromuscular control activities, and low-risk activities such as jogging, biking, and swimming do not necessarily require the use of prophylactic bracing. However, because syndesmotic injuries are associated with recurrent ankle injuries, prophylactic bracing should be used during high-risk activities that involve agility maneuvers and jumping. Substantial evidence demonstrates that the use of ankle taping or ankle bracing decreases the incidence of ankle injuries, particularly in those who have had previous ankle injuries.26 In one study (N = 450), only 3% of athletes with a history of prior ankle injuries suffered a recurrent ankle sprain when using an ankle orthosis compared with a 17% injury rate in the control group.28
More recently, 2 separate studies by McGuine et al demonstrated that the use of lace-up ankle braces led to a reduction in the incidence of acute ankle injuries by 61% among 2081 high-school football players, and resulted in a significant reduction in acute ankle injuries in a study of 1460 male and female high-school basketball players, compared with the control groups.29,30
CASE
Ten days after injuring himself, the patient returns for a follow-up exam. Despite using the walking brace and crutches, he is still having significant difficulty bearing weight. He reports a sensation of instability in the right ankle. On exam, you note visible edema of the right ankle and ecchymosis over the lateral ankle, as well as moderate tenderness to palpation over the area of the ATFL and deltoid ligament. Tenderness over the medial malleolus, lateral malleolus, fifth metatarsal, and navicular is absent. Pain is reproducible with external rotation, and a Squeeze Test is positive. There is no tenderness over the proximal tibia or fibula. The patient is neurovascularly intact.
You order stress x-rays, which show widening of the medial clear space. The patient is placed in a CAM boot, instructed to continue non–weight-bearing on the ankle, and referred to a local foot and ankle surgeon for consideration of surgical fixation.
CORRESPONDENCE
John T. Nickless, MD, Division of Primary Care Sports Medicine, Department of Orthopedic Surgery, Rush University Medical Center, 1611 W. Harrison Street, Suite 200, Chicago, IL, 60612; jack.nickless@rushortho.com.
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
1. Switaj PJ, Mendoza M, Kadakia AR. Acute and chronic Injuries to the syndesmosis. Clin Sports Med. 2015;34:643-677.
2. Scheyerer MJ, Helfet DL, Wirth S, et al. Diagnostics in suspicion of ankle syndesmotic injury. Am J Orthop. 2011;40:192-197.
3. Smith KM, Kovacich-Smith KJ, Witt M. Evaluation and management of high ankle sprains. Clin Podiatr Med Surg. 2001;18:443-456.
4. Reissig J, Bitterman A, Lee S. Common foot and ankle injuries: what not to miss and how best to manage. J Am Osteopath Assoc. 2017;117:98-104.
5. Williams GN, Allen EJ. Rehabilitation of syndesmotic (high) ankle sprains. Sports Health. 2010;2:460-470.
6. Williams GN, Jones MH, Amendola A. Syndesmotic ankle sprains in athletes. Am J Sports Med. 2007;35:1197-1207.
7. Vopat ML, Vopat BG, Lubberts B, et al. Current trends in the diagnosis and management of syndesmotic injury. Curr Rev Musculoskelet Med. 2017;10:94-103.
8. Mak MF, Gartner L, Pearce CJ. Management of syndesmosis injuries in the elite athlete. Foot Ankle Clin. 2013;18:195-214.
9. Waterman BR, Belmont PJ, Cameron KL, et al. Epidemiology of ankle sprain at the United States Military Academy. Am J Sports Med. 2010;38:797-803.
10. Hunt KJ, George E, Harris AHS, et al. Epidemiology of syndesmosis injuries in intercollegiate football: incidence and risk factors from National Collegiate Athletic Association injury surveillance system data from 2004-2005 to 2008-2009. Clin J Sport Med. 2013;23:278-282.
11. Schnetzke M, Vetter SY, Beisemann N, et al. Management of syndesmotic injuries: what is the evidence? World J Orthop. 2016;7:718-725.
12. de César PC, Ávila EM, de Abreu MR. Comparison of magnetic resonance imaging to physical examination for syndesmotic injury after lateral ankle sprain. Foot Ankle Int. 2011;32:1110-1114.
13. Ivins D. Acute ankle sprain: an update. Am Fam Physician. 2006;74:1714-1720.
14. Porter D, Rund A, Barnes AF, et al. Optimal management of ankle syndesmosis injuries. Open Access J Sports Med. 2014;5:173-182.
15. Press CM, Gupta A, Hutchinson MR. Management of ankle syndesmosis injuries in the athlete. Curr Sports Med Rep. 2009;8:228-233.
16. Sman AD, Hiller CE, Rae K, et al. Diagnostic accuracy of clinical tests for ankle syndesmosis injury. Br J Sports Med. 2015;49:323-329.
17. Amendola A, Williams G, Foster D. Evidence-based approach to treatment of acute traumatic syndesmosis (high ankle) sprains. Sports Med Arthrosc. 2006;14:232-236.
18. Hunt KJ. Syndesmosis injuries. Curr Rev Musculoskelet Med. 2013;6:304-312.
19. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
20. Miller BS, Downie BK, Johnson PD, et al. Time to return to play after high ankle sprains in collegiate football players: a prediction model. Sports Health. 2012;4:504-509.
21. Wright RW, Barile RJ, Surprenant DA, et al. Ankle syndesmosis sprains in National Hockey League players. Am J Sports Med. 2016;32:1941-1945.
22. Osbahr DC, Drakos MC, O’Loughlin PF, et al. Syndesmosis and lateral ankle sprains in the National Football League. Orthopedics. 2013;36:e1378-e1384.
23. Taylor DC, Tenuta JJ, Uhorchak JM, et al. Aggressive surgical treatment and early return to sports in athletes with grade III syndesmosis sprains. Am J Sports Med. 2007;35:1133-1138.
24. Taylor DC, Englehardt DL, Bassett FH. Syndesmosis sprains of the ankle: the influence of heterotopic ossification. Am J Sports Med. 1992;20:146-150.
25. Laver L, Carmont MR, McConkey MO, et al. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: a randomized control trial. Knee Surg Sports Traumatol Arthrosc. 2014;23:3383-3392.
26. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48:528-545.
27. Hupperets MDW, Verhagen EALM, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684.
28. Tropp H, Askling C, Gillquist J. Prevention of ankle sprains. Am J Sports Med. 1985;13:259-262.
29. McGuine TA, Brooks A, Hetzel S. The effect of lace-up ankle braces on injury rates in high school basketball players. Am J Sports Med. 2011;39:1840-1848.
30. McGuine TA, Hetzel S, Wilson J, et al. The effect of lace-up ankle braces on injury rates in high school football players. Am J Sports Med. 2012;40:49-57.
PRACTICE RECOMMENDATIONS
› Maintain a high level of suspicion for syndesmotic injury in any athlete describing an external rotation or hyper-dorsiflexion ankle injury. A
› Obtain weight-bearing anteroposterior- and mortise-view ankle x-rays in all cases of suspected syndesmotic injuries. A
› Consider stress x-rays of the affected ankle, contralateral ankle x-rays for comparison views, or advanced imaging with magnetic resonance imaging (MRI) or computed tomography if initial x-rays are unrevealing. A
› Treat stable syndesmotic injuries with conservative measures and rehabilitation. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keeping caries at bay in breastfeeding babies
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; peter.wong@sickkids.ca.
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; peter.wong@sickkids.ca.
Early childhood caries (ECCs) are a preventable public health challenge. Breastfeeding may provide early protection from ECCs. In addition, oral hygiene that begins in infancy, regular dental care visits, and a healthy diet can minimize ECC risk.
In this article we review the critical role of the family physician (FP) in reducing ECCs by promoting breastfeeding and infant oral health and addressing dental health concerns.
How ECCs develop
ECCs represent decayed, missing, or filled areas in the primary dentition of the tooth surface. The bacteria that cause them (most often Streptococcus mutans1) strongly adhere to teeth and produce acids as waste products of fermentable carbohydrate metabolism that demineralize tooth enamel and progress into the dentin. Weakened enamel and dentin can result in cavitation (ie, a dental cavity). Left untreated, caries can extend to the pulp and destroy the entire tooth. ECCs are a risk factor not only for dental caries in primary teeth, but in permanent dentition as well.
ECCs are the most common chronic disease affecting young children.1 Dental disease may begin soon after tooth eruption with detrimental effects on oral development. Almost half of children have dental caries by 5 years of age.2
ECCs represent a complex and multifactorial disease that is impacted by biomedical factors and unmet social needs. Children who are most at risk include those with low socioeconomic status, a high-sugar diet, exposure to household smoke, and limited dental care access.3 In addition, women with low education, poor oral health, and/or a lack of fluoride exposure are more likely to have children with ECCs.3 This is partly because of vertical transmission of cariogenic bacteria from caregiver to child. Horizontal transmission in daycare settings can also occur. Paternal and child oral health have not been linked.
Support breastfeeding; keep oral microbiome changes in mind
The American Academy of Pediatrics (AAP) recommends exclusive breastfeeding for the first 6 months of life, a combination of breastfeeding and complementary foods until 12 months of age, and continued breastfeeding for as long as mutually desired by mother and baby.4 The World Health Organization (WHO) recommends continued breastfeeding until 2 years of age or beyond.5 In fact, the WHO global nutrition targets for 2025 include increasing the rate of exclusive breastfeeding in the first 6 months of life to at least 50%.6
In addition to maternal, financial, and societal benefits, human milk offers nutritional and other health-related advantages for children that optimize growth and development into adulthood.4 Breastfed infants may benefit from reduction in infections and diseases, including asthma, diabetes mellitus, childhood cancer, and obesity.7 Improved neurocognitive development, intelligence, and education attainment in adulthood have also been described.8 And the rich microbiome of human milk helps to establish oral and intestinal floras9 and may mediate protection from ECCs.3
Continue to: However, as a child's oral microbiome changes...
However, as a child’s oral microbiome changes with the emergence of primary teeth and exposure to more and varied bacteria and dietary sugars, the natural sugars in human milk may become the substrate for cariogenic bacteria.3 ECCs can develop and progress rapidly. Importantly, both the practice of breastfeeding and ECC risk are modified by socioeconomic status, maternal oral health and education, and exposure to household smoking.3,7 Understanding these relationships may help you better target risk assessment and counseling efforts.
What the research tells us about breastfeeding and ECCs
Breastfeeding is hypothesized to be one of many factors that influence ECC development. However, studies on this association have had conflicting results and have not adequately controlled for major confounders, such as dietary composition, maternal and infant oral hygiene, and maternal oral health status.
So here is what we know.
Breastfeeding during the first year. In one meta-analysis involving children who breastfed for up to 12 months, those who breastfed longer within the 12-month period had a reduced risk of ECCs compared with those who breastfed for a shorter period of time,3 which implies that breast milk may be protective in the first year of life.3
Further, a 2014 study with about 500 participants found that children were more likely to have caries by 5 years of age if they breastfed for <6 months than if they breastfed for at least 6 months.10
Continue to: After the first year
After the first year. A Canadian study found an increased risk of ECCs associated with breastfeeding for longer periods of time. The study of healthy urban children reported that breastfeeding for >24 months was associated with a 2- to 3-fold increased odds of ECCs compared with shorter breastfeeding duration.11
No relationship? Lastly, a US study using National Health and Nutrition Examination Survey data found there was no evidence to suggest that breastfeeding duration was an independent risk factor for ECCs.12
A possible explanation for a link
An initial protective effect of breastfeeding against ECCs may be related to breast milk’s immunomodulatory factors and rich microbiome. Breast milk contains Lactobacilli and substances, including human casein and secretory IgA, that inhibit growth and attachment of bacteria,9 particularly the caries pathogen S mutans. Early defense against ECCs may be mediated through the establishment of a healthy oral and gut microbiome that results from exposure to breastfeeding and contact with skin, gut, and breast milk microbiomes. Later on, the child’s oral microbiome changes with the emergence of teeth and the introduction of complementary foods andother drinks.
A look at the role vitamin D plays
Vitamin D status may influence childhood dental health.13 Low maternal vitamin D levels have been associated with ECCs,14 and mothers with higher prenatal vitamin D intakes were more likely to report that their children were caries-free compared with women who had lower vitamin D intake.15 Additionally, children with severe ECCs were found to have lower vitamin D levels than cavity-free children.16 Unfortunately, only a minority of infants who are predominantly breastfed for > 6 months receive vitamin D supplementation.17
Other factors at work: Carbohydrate exposure, nocturnal feedings
Exposure to carbohydrates—the essential substrate for cariogenic bacteria—is a key factor in ECC development. Refined sugars contribute considerably to tooth decay. Frequency of feeding and feeding practices, such as prolonged nocturnal feeding (either breast or bottle) may increase ECC risk.3 Further, a major determinant of ECC risk is colonization of the infant’s mouth by cariogenic bacteria. Finally, ECC risk depends on socioeconomic status, oral hygiene, exposure to fluoride, and the mother’s oral health, education, and smoking status.3 Even birth order plays a role, with those born first having lower risk than subsequent children.18
Continue to: Breastfeeding and another area of oral health...
Breastfeeding and another area of oral health: Malocclusion
In addition to its relationship with ECCs, breastfeeding promotes adequate development of craniofacial structures (comprising the tongue, facial muscles, and jaw), which are important for smiling, emotion, and social contact. Breastfeeding may prevent the development of malocclusion (ie, a misalignment of the teeth) in primary dentition, which is a risk factor for malocclusion in adulthood.7 Although previous studies had conflicting results, a large prospective study found that breastfeeding significantly reduced the risk of moderate and severe malocclusion; however, this effect was nullified by nonnutritive sucking and pacifier use.19
Oral health recommendations: The FP’s role
ECCs are theoretically preventable. To optimize the benefits of breastfeeding and minimize ECC risk, parents should follow recommendations for their children regarding proper oral hygiene, appropriate fluoride exposure, regular dental visits, and a healthy diet.1
Be sure to advise parents to:
- avoid saliva-sharing behaviors (eg, sharing utensils with their children or cleaning a pacifier with their mouth), as these may increase early colonization of S mutans in infants;
- seek regular preventive dental care and attend to caries—both for their children and themselves; and
- use antimicrobial oral care products including xylitol-containing chewing gum to lower levels of cariogenic microorganisms in themselves and, in turn, reduce mother–child vertical transmission of S mutans.1
In addition, make sure your prenatal counseling includes a discussion of the importance of good maternal oral health and diet—including an adequate vitamin D intake—to prevent ECCs in their children.
It’s never too early to start
Providing guidance on children’s oral health can start with the first well-infant visit. FPs should perform an oral health risk assessment by 6 months of age (see the AAP’s Oral Health Risk Assessment Tool at https://www.aap.org/en-us/Documents/oralhealth_RiskAssessmentTool.pdf) and evaluate fluoride exposure. Advise parents to establish a dental home by the time the child is 12 months of age; to clean their children’s mouths after feedings (before teeth arrive) with a clean, wet, soft washcloth; and to brush their children’s teeth, once they erupt, twice daily using a soft toothbrush (TABLE 120).
Continue to: Talk to parents about...
Talk to parents about how to provide optimal exposure to fluoride, which is known to be safe and effective for the prevention of ECCs.1 Use of fluoridated toothpaste in small amounts provides the benefits of fluoride without increasing the risk of fluorosis, especially for children at risk for caries (see TABLE 221).
The US Preventive Services Task Force recommends that primary care practitioners apply fluoride varnish biannually for at least 2 years to the primary teeth of all children up to 5 years of age (Grade B evidence).22 This is particularly important for high-risk children, such as those with low-income or minority status. However, practitioners should also take into account that high cumulative fluoride intake can lead to dental fluorosis.1 Finally, tell parents to avoid giving their children sugar-containing snacks and drinks to reduce ECC risk.
CORRESPONDENCE
Peter D. Wong, MD, 303-89 Humber College Boulevard, Toronto, Ontario, Canada M9V 4B8; peter.wong@sickkids.ca.
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
1. American Academy of Pediatric Dentistry. Guideline on infant oral health care. Pediatr Dent. 2012;34:e148-e152.
2. Dye BA, Thornton-Evans G, Li X, et al. Dental caries and sealant prevalence in children and adolescents in the United States, 2011-2012. NCHS Data Brief. No. 191, March 2015. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. https://www.cdc.gov/nchs/data/databriefs/db191.pdf. Accessed January 25, 2019.
3. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
4. Eidelman AI, Schanler RJ, Johnston M, et al. Breastfeeding and the use of human milk (section on breastfeeding). Pediatrics. 2012;129:e827-e841.
5. World Health Organization. 55th World Health Assembly. Agenda Item 13.10: Infant and young child nutrition. https://www.who.int/nutrition/topics/WHA55.25_iycn_en.pdf?ua=1. May 18, 2002. Accessed January 25, 2019.
6. World Health Organization. Global Nutrition Targets 2025 Breastfeeding Policy Brief 5. http://apps.who.int/iris/bitstream/10665/149022/1/WHO_NMH_NHD_14.7_eng.pdf?ua=1. Accessed April 1, 2019.
7. Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-490.
8. Victora CG, Horta BL, de Mola CL, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. 2015;3:e199-e205.
9. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12:14-25.
10. Hong L, Levy SM, Warren JJ, et al. Infant breast-feeding and childhood caries: a nine-year study. Pediatr Dent. 2014;36:342-347.
11. Wong PD, Birken CS, Parkin PC, et al. Total breast-feeding duration and dental caries in healthy urban children. Acad Pediatr. 2017;17:310-315.
12. Iida H, Auinger P, Billings RJ, et al. Association between infant breastfeeding and early childhood caries in the United States. Pediatrics. 2007;120:e944-e952.
13. Schroth R, Rabbani R, Loewen G, et al. Vitamin D and dental caries in children. J Dent Res. 2016;95:173-179.
14. Schroth RJ, Lavelle C, Tate R, et al. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133:e1277-e1284.
15. Tanaka K, Hitsumoto S, Miyake Y, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Ann Epidemiol. 2015;25:620-625.
16. Schroth RJ, Halchuk S, Star L. Prevalence and risk factors of caregiver reported severe early childhood caries in Manitoba First Nations children: results from the RHS Phase 2 (2008-2010). Int J Circumpolar Health. 2013;72.
17. Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125:105-111.
18. Nicolau B, Marcenes W, Bartley M, et al. A life course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319-326.
19. Peres KG, Cascaes AM, Peres MA, et al. Exclusive breastfeeding and risk of dental malocclusion. Pediatrics. 2015;136:e60-e67.
20. Tinanoff N, Reisin S. Update on early childhood caries since the Surgeon General’s Report. Acad Pediatr. 2009;9:396-403.
21. Canadian Dental Association. CDA Position on Use of Fluorides in Caries Prevention. 2012. https://www.cda-adc.ca/_files/position_statements/fluoride.pdf. Accessed January 25, 2019.
22. US Preventive Services Task Force. USPSTF A and B Recommendations. https://www.uspreventiveservicestaskforce.org/Page/Name/uspstf-a-and-b-recommendations/. Accessed April 1, 2019.
PRACTICE RECOMMENDATIONS
› Promote breastfeeding as the preferred method of feeding infants. A
› Optimize pediatric oral health by reducing risk factors for dental disease and by providing parents with anticipatory guidance to prevent early childhood caries. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Aspirin for primary prevention: It depends
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
Acetylsalicylic acid has been around for nearly 200 years. It traces its history back to a French chemist (Charles Frederic Gerhardt) and 2 German chemists (Felix Hoffmann and Arthur Eichengrün) who worked at Bayer, the company that launched the pain reliever under the name “aspirin” in 1899. It is now one of the most commonly used medications in the world.
With aspirin's anti-inflammatory properties in mind, researchers conducted randomized trials for secondary prevention of heart attacks in the 1970s; low-dose aspirin was proven effective in reducing risk for a second myocardial infarction. These trials led to speculation that aspirin might be effective for primary prevention as well. Indeed, in the 1980s the large Physicians' Health Study found aspirin reduced the incidence of first heart attack in healthy physicians by 44%.1 Unfortunately, there was no reduction in mortality from heart disease and it was only effective for those older than 50.
The downside of aspirin was a slight increase in the incidence of hemorrhagic stroke and bleeding requiring transfusion. Nonetheless, many healthy adults started taking daily aspirin hoping to prevent a heart attack.
In this issue of JFP, Smith and colleagues summarize the 2016 recommendations of the US Preventive Services Task Force (USPSTF) regarding aspirin for primary prevention, as well as the 4 large aspirin prevention trials published in 2018 subsequent to the USPSTF recommendations. The USPSTF recommended aspirin for adults ages 50 to 59 with a 10-year cardiovascular risk of at least 10% (B recommendation). For those ages 60-69, the USPSTF recommendation for aspirin as primary prevention has a “C” rating, meaning that patient preference is important to consider in balancing benefit and harms. For those 70 and older, the USPSTF gave aspirin an “I” (insufficient evidence) rating because of increased risk for bleeding. It is important to note that the positive B recommendation for those ages 50-59 is based not only on cardiovascular risk reduction but also on a slight risk reduction for colon cancer for those taking aspirin for at least 10 years.
The 4 new, large randomized trials published in 2018, however, cast doubt on the USPSTF recommendations because the results of these trials were negative for the most part. The bottom line is that daily aspirin for prevention is definitely not for everyone and perhaps not for anyone except those who have established vascular disease or are at high risk for vascular disease and low risk for bleeding.
No wonder patients are confused!
Smith recommends that, before prescribing aspirin to healthy adults for prevention, we assess each individual’s personal cardiovascular and bleeding risk using an online decision tool called Aspirin-Guide (www.aspiringuide.com). I agree.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
1. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129-135.
Newborn with desquamating rash
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).
Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; jjwalker@hhsc.org
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).
Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; jjwalker@hhsc.org
A 9-day-old boy was brought to the emergency department by his mother. The infant had been doing well until his most recent diaper change when his mother noticed a rash around the umbilicus (FIGURE), genitalia, and anus.
The infant was born at term via spontaneous vaginal delivery. The pregnancy was uncomplicated; the infant’s mother was group B strep negative. Following a routine postpartum course, the infant underwent an elective circumcision before hospital discharge on his second day of life. There were no interval reports of irritability, poor feeding, fevers, vomiting, or changes in urine or stool output.
The mother denied any recent unusual exposures, sick contacts, or travel. However, upon further questioning, the mother noted that she herself had several small open wounds on the torso that she attributed to untreated methicillin-resistant Staphylococcus aureus (MRSA).
On physical examination, the infant was overall well-appearing and was breastfeeding vigorously without respiratory distress or cyanosis. He was afebrile with normal vital signs. The majority of the physical examination was normal; however, there was erythematous desquamation around the umbilical stump and genitalia with no vesicles noted. The umbilical stump had a small amount of purulent drainage and necrosis centrally. The infant had a 1-cm round, peeling lesion on the left temple (FIGURE) with a small amount of dried serosanguinous drainage and similar superficial peeling lesions at the left preauricular area and anterior chest. There was no underlying fluctuance and only minimal surrounding erythema.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Staphylococcal scalded skin syndrome
Based on the age of the patient, clinical presentation, and suspected maternal MRSA infection (with possible transmission to the infant), we diagnosed staphylococcal scalded skin syndrome (SSSS) in this patient. SSSS is rare, with annual incidence of 45 cases per million US infants under the age of 2.1 Newborns with a generalized form of SSSS commonly present with fever, poor feeding, irritability, and lethargy. This is followed by a generalized erythematous rash that initially may appear on the head and neck and spread to the rest of the body. Large, fragile blisters subsequently appear. These blisters rupture on gentle pressure, which is known as a positive Nikolsky sign. Ultimately, large sheets of skin easily slough off, leaving raw, denuded skin.2
S aureus is not part of normal skin flora, yet it is found on the skin and mucous membranes of 19% to 55% of healthy adults and children.3S aureus can cause a wide range of infections ranging from abscesses to cellulitis; SSSS is caused by hematogenous spread of S aureus exfoliative toxin. Newborns and immunocompromised patients are particularly susceptible.
Neonatal patients with SSSS most commonly present at 3 to 16 days of age.2 The lack of antitoxin antibody in neonates allows the toxin to reach the epidermis where it acts locally to produce the characteristic fragile skin lesions that often rupture prior to clinical presentation.2,4 During progression of the disease, flaky skin desquamation will occur as the lesions heal.
A retrospective review of 39 cases of SSSS identified pneumonia as the most frequent complication, occurring in 74.4% of the cases.5 The mortality rate of SSSS is up to 5%, and is associated with sepsis, superinfection, electrolyte imbalances, and extensive skin involvement.2,6
If SSSS is suspected, obtain cultures from the blood, urine, eyes, nose, throat, and skin lesions to identify the primary focus of infection.7 However, the retrospective review of 39 cases (noted above) found a positive rate of S aureus isolation of only 23.5%.5 Physicians will often have to make a diagnosis based on clinical presentation and empirically initiate broad-spectrum antibiotics while considering alternative diagnoses.
Continue to: A clinical diagnosis with a large differential
A clinical diagnosis with a large differential
While biopsy rarely is required, it may be helpful to distinguish SSSS from other entities in the differential diagnosis (TABLE2,3,7-13).
Toxic epidermal necrolysis (TEN) is a rare and life-threatening desquamating disease nearly always caused by a reaction to medications, including antibiotics. TEN can occur at any age. Fever, diffuse erythema, and extensive epidermal involvement (>30% of skin) differentiate TEN from Stevens-Johnson syndrome (SJS), which affects less than 10% of the epidermis. It is worth mentioning that TEN and SJS are now considered to be a spectrum of one disease, and an overlap syndrome has been described with 10% to 30% of skin affected.8 Diagnosis is made clinically, although skin biopsy routinely is performed.7,9
Congenital syphilis features a red or pink maculopapular rash followed by desquamation. Lesions are more common on the soles.10 Desquamation or ulcerative skin lesions should be examined for spirochetes.11 A quantitative, nontreponemal test such as the rapid plasma reagin (RPR) or the Venereal Disease Research Laboratory (VDRL) will be positive in most infants if exposed through the placenta, but antibodies will disappear in uninfected infants by 6 months of age.8
Congenital cutaneous candidiasis presents with a generalized eruption of erythematous macules, papules, and/or pustules with widespread desquamating and/or erosive dermatitis. Premature neonates with extremely low birth weight are at higher risk.13 Diagnosis is confirmed on microscopy by the presence of Candida albicans spores in skin scrapings.13
Neonatal herpes simplex virus (HSV) symptoms typically appear between 1 and 3 weeks of life, with 60% to 70% of cases presenting with classic clustering vesicles on an erythematous base.14 Diagnosis is made with HSV viral culture or polymerase chain reaction (PCR).
Continue to: SSSS should be considered a pediatrics emergency
SSSS should be considered a pediatric emergency
SSSS should be considered a pediatric emergency due to potential complications. Core measures of SSSS treatment include immediate administration of intravenous (IV) antibiotics. US population studies suggest clindamycin and penicillinase-resistant penicillin as empiric therapy.15 However, local strains and resistance patterns, including the prevalence of MRSA, as well as age, comorbidities, and severity of illness should influence antibiotic selection.
IV nafcillin or oxacillin may be used with pediatric dosing of 150 mg/kg daily divided every 6 hours for methicillin-sensitive Staphylococcus aureus (MSSA). For suspected MRSA, IV vancomycin should be considered, with an infant dose of 40 to 60 mg/kg daily divided every 6 hours.16 Fluid, electrolyte, and nutritional management should be addressed immediately. Ongoing fluid losses due to exfoliated skin must be replaced, and skin care to desquamated areas also should be addressed urgently.
Our patient. Phone consultation with an infectious disease specialist at a local children’s hospital resulted in a recommendation to treat for sepsis empirically with IV vancomycin, cefotaxime, and acyclovir. Acyclovir was discontinued once the HSV PCR came back negative. The antibiotic coverage was narrowed to IV ampicillin 50 mg/kg every 8 hours when cerebrospinal fluid and blood cultures returned negative at 48 hours, wound culture sensitivity grew MSSA, and the patient’s clinical condition stabilized. Our patient received 10 days of IV antibiotics and was discharged on oral amoxicillin 50 mg/kg divided twice daily for a total of 14 days of treatment per recommendations by the infectious disease specialist. Our patient fully recovered without any residual skin findings after completion of the antibiotic course.
CORRESPONDENCE
Jennifer J. Walker, MD, MPH, Hawaii Island Family Health Center at Hilo Medical Center, 1190 Waianuenue Ave, Hilo, HI 96720; jjwalker@hhsc.org
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
1. Staiman A, Hsu D, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in US children. Br J Dermatol. 2018;178:704-708.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505-520.
4. Ladhani S. Understanding the mechanism of action of the exfoliative toxins of Staphylococcus aureus. FEMS Immunol Med Microbiol. 2003;39:181-189.
5. Li MY, Hua Y, Wei GH, et al. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31:43-47.
6. Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39:627-633.
7. Ely JW, Seabury Stone M. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81:726-734.
8. Bastuji-Garin SB, Stern RS, Shear NH, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92.
9. Elias PM, Fritsch P, Epstein EH. Staphylococcal scalded skin syndrome. clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch Dermatol. 1977;113:207-219.
10. O’Connor NR, McLaughlin M, Ham P. Newborn skin: part I: common rashes. Am Fam Physician. 2008;77:47-52.
11. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995;8:1-21.
12. Arnold SR, Ford-Jones EL. Congenital syphilis: a guide to diagnosis and management. Paediatr Child Health. 2000;5:463-469.
13. Darmstadt GL, Dinulos JG, Miller Z. Congenital cutaneous candidiasis: clinical presentation, pathogenesis, and management guidelines. Pediatrics. 2000;105:438-444.
14. Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1-13.
15. Braunstein I, Wanat KA, Abuabara K, et al. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31:305-308.
16. Gilbert DN, Chambers HF, Eliopoulos GM, et al. The Sanford Guide to Antimicrobial Therapy. 48th ed. Sperryville, VA: Antimicrobial Therapy, Inc; 2014:56.
Subacute polyarticular arthralgias • swelling of the ankles and right knee • recent travel to the Dominican Republic • Dx?
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; Jeremy.Golding@umassmemorial.org
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; Jeremy.Golding@umassmemorial.org
THE CASE
A 78-year-old woman with a history of anxiety and hypertension presented to our family medicine residency practice in Massachusetts with subacute polyarticular arthralgias that had been present for 2 months. She complained of pain and swelling of both ankles and the right knee. She noted that her symptoms had started on a recent trip to the Dominican Republic, where she developed generalized joint pain and a fever that lasted 1 to 2 weeks and subsequently resolved with the lingering polyarthralgias. She denied any rash, constitutional symptoms, photosensitivity, headaches, photophobia, or history of tick bite. Physical examination revealed normal vital signs, notable warmth and swelling of the bilateral ankles that was worse on the right side, and swelling of the right knee with effusion—but no tenderness—to palpation.
THE DIAGNOSIS
The patient’s labwork revealed a white blood cell count of 5900/mcL (reference range, 4500–11,000/mcL), hemoglobin count of 12.5 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 230×103/mcL. Electrolytes and renal function were normal. She had an elevated erythrocyte sedimentation rate of 34 mm/h (reference range, 0–20 mm/h) and a positive antinuclear antibody (ANA) test, but no titer was reported. Anti-chikungunya IgG and IgM antibodies were positive on enzyme-linked immunosorbent assay (ELISA) serologic testing.
DISCUSSION
Chikungunya is an infectious disease that is relatively rare in the United States. Chikungunya was rarely identified in American travelers prior to 2006, but incidence increased over the next decade. In 2014, a total of 2811 cases were reported.1 Chikungunya is an RNA arbovirus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is endemic to West Africa. Within the last 2 decades, there has been an increasing number of outbreaks in India, Asia, Europe, and the Americas, where the highest incidence is in South America, followed by Central America. In the United States, almost all reported cases of chikungunya infection have been in travelers returning from endemic areas.2 The first 2 known cases of local transmission in the United States were reported in Florida in July 2014.3 Local transmission of chikungunya is significant in that it represents the possibility of a local reservoir for sustained transmission.
Disease presentation. Patients will initially complain of a high fever and severe distal polyarthralgias that usually are symmetric. The most common symptoms are polyarthralgias (87%–98% of patients), myalgias (46%–59%), and a maculopapular rash
The term chikungunya is derived from a Kimakonde (central Bantu) word meaning “that which bends up” because of the arthralgia caused by the disease. Fever usually lasts 3 to 7 days; polyarthralgia begins shortly after the onset of fever.4 Frank arthritis also may be present. Infection often exacerbates a previously damaged or diseased joint. Acute symptoms usually persist for 1 to 2 weeks, but arthralgias and arthritis can persist for months to years following resolution of the acute disease.6 In one study of 47 patients with acute chikungunya in Marseilles, France, the number of patients who were symptomatic declined from 88% to 86%, 48%, and 4% at 1, 3, 6, and 15 months, respectively.7
The differential diagnosis includes tropical infectious diseases (dengue, chikungunya, Zika, and leptospirosis) in patients who have recently traveled to the tropics and who complain of subacute polyarticular arthralgias or arthritis; locally acquired infections associated with arthralgia/arthritis such as Lyme disease and other tick-borne diseases and rickettsial infections; parvovirus B19 and other postinfectious arthritides; and rheumatologic conditions such as systemic lupus.
Clinical differentiation among dengue, chikungunya, and Zika may be difficult, although persistent frank arthritis is much more common in chikungunya than in dengue or Zika. Furthermore, conjunctivitis is present in Zika but is absent in chikungunya. Chikungunya also is more likely to cause high fever, severe arthralgia, arthritis, rash, and lymphopenia than Zika or dengue. Dengue is more likely to cause lymphopenia and hemorrhagic consequences than is chikungunya or Zika.8
Continue to: In our patient...
In our patient, dengue titers were not obtained because the duration of symptoms was thought to be more consistent with chikungunya, but testing for dengue also would have been appropriate.
The most common test for diagnosing acute chikungunya is ELISA serologic testing for IgM antibodies, which develop toward the end of the first week of infection; earlier in that first week, serum testing for viral RNA may be performed by polymerase chain reaction.
Treatment is largely supportive
Treatment of acute chikungunya is largely supportive and includes anti-inflammatory agents. To our knowledge, no antiviral agents have been shown to be effective. Postacute or chronic symptoms may require treatment with glucocorticoids or other immunomodulatory medications. A 2017 literature review of treatments for chikungunya-associated rheumatic disorders showed evidence that chloroquine was more effective than placebo for chronic pain relief. Also, adding a disease-modifying antirheumatic agent in combination with chloroquine was more effective for controlling pain and reducing disability than hydroxychloroquine monotherapy.10
Our patient was treated with ibuprofen only and experienced resolution of joint symptoms several months after the initial presentation. A repeat ANA test 12 months later was negative.
A 2009 review of the medical literature revealed a single case report of chikungunya associated with positive ANA.8 Although a positive ANA may be associated with acute viral infections, significantly elevated ANA levels typically are associated with autoimmunity. Resolution of the patient’s serum ANA 1 year later suggested that the positive ANA was not secondary to a pre-existing rheumatologic condition but rather a consequence of her body’s response to the chikungunya infection itself. Our case raises the hypothesis that, at least in some cases, chikungunya somehow stimulates a temporary autoimmune response, which may help explain why immunomodulatory medications can be effective treatment options.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Chikungunya is increasingly common in tropical and subtropical regions. Family physicians practicing in the United States should become familiar with the common patterns of presentation of viruses such as chikungunya, dengue, and Zika. Obtaining a travel history for patients presenting with arthritis improves the differential diagnosis and may even reveal the cause of the condition.
CORRESPONDENCE
Jeremy Golding, MD, 279 Lincoln Street, Worcester, MA 01605; Jeremy.Golding@umassmemorial.org
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
1. Chikungunya virus. Centers for Disease Control and Prevention website. https://www.cdc.gov/chikungunya/geo/united-states.html. Reviewed December 17, 2018. Accessed March 5, 2019.
2. Pan American Health Organization. Preparedness and response for chikungunya virus: introduction into the Americas. https://www.paho.org/hq/dmdocuments/2012/CHIKV-English.pdf. Published 2011. Accessed March 5, 2019.
3. First chikungunya case acquired in the United States reported in Florida [press release]. Atlanta, GA: Centers for Disease Control and Prevention; July 17, 2014. http://www.cdc.gov/media/releases/2014/p0717-chikungunya.html. Accessed March 5, 2019.
4. Taubitz W, Cramer JP, Kapaun A, et al. Chikungunya fever in travelers: clinical presentation and course [published online May 23, 2007]. Clin Infect Dis. 2007;45:e1-e4.
5. Thiberville SD, Moyen N, Dupuis-Maguiraga L, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345-370.
6. Burt FJ, Rolph MS, Rulli NE, et al. Chikungunya: a re-emerging virus. Lancet. 2012;379:662-671.
7. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore). 2007;86:123-137.
8. Chikungunya virus. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Reviewed December 17, 2018. Accessed March 5, 2019.
9. Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552-1563.
10. Martí-Carvajal A, Ramon-Pardo P, Javelle E, et al. Interventions for treating patients with chikungunya virus infection-related rheumatic and musculoskeletal disorders: a systematic review. PLoS One. 2017;12:e0179028.
Postpartum anxiety: More common than you think
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).
Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the
Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; veronica.a.jordan@gmail.com.
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).
Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the
Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; veronica.a.jordan@gmail.com.
THE CASE
Julia* is a 31-year-old woman, gravida 3 para 3, who presents to your office for evaluation after a recent emergency department (ED) visit. Her husband and children are with her. She is 4 months postpartum after an uncomplicated normal spontaneous vaginal delivery. She is breastfeeding her healthy baby boy and is using an intrauterine device for birth control. She went to the ED last week after “choking on a chip” while having lunch with her children. It felt like she “couldn’t breathe.” She called 911 herself. The ED evaluation was unremarkable. Her discharge diagnosis was “panic attack,” and she was sent home with a prescription for lorazepam.
Since the incident, she has been unable to eat any solid foods and has lost 7 pounds. She also reports a globus sensation, extreme fear of swallowing, insomnia, and pervasive thoughts that she could die at any moment and leave her children motherless. She has not taken the lorazepam.
She has a history of self-reported anxiety dating back to high school but no history of panic attacks. She has never been diagnosed with an anxiety disorder and has never before been prescribed anti-anxiety medication. She doesn’t have a history of postpartum depression in prior pregnancies, and a depression screening at her postpartum visit 2 months ago was negative.
●
*The patient’s name has been changed to protect her identity.
During the perinatal period, women are particularly vulnerable to affective disorders, and primary care physicians are encouraged to routinely screen for and treat depression in pregnant and postpartum women.1 However, anxiety disorders have a higher incidence than mood disorders in the general population,2 and perinatal anxiety may be more widely underrecognized and undertreated than depression.3 In addition, higher depression scores early in pregnancy have been shown to predict higher anxiety later in pregnancy.4
As family physicians, we are well-trained to recognize and treat anxiety disorders in the general patient population; however, we may lack the awareness and tools to identify these conditions in the perinatal period. Given our frequent encounters with both mom and baby in a child’s first year of life, we are uniquely positioned to promptly recognize, diagnose, and treat postpartum anxiety and thereby improve health outcomes for families.
DEFINING PERINATAL ANXIETY
Anxiety disorders (including generalized anxiety disorder, panic, phobia, and social anxiety) are the most common mental health disorders evaluated and treated in the primary care setting, with a lifetime prevalence of close to 30%.2
Continue to: A recent report from...
A recent report from the Centers for Disease Control and Prevention (CDC) estimates that 1 in 9 women experience symptoms of postpartum depression.5 The prevalence of anxiety disorders during pregnancy and the early postpartum period is not as well-known, but studies suggest that perinatal anxiety is much more prevalent than depression. In one study, generalized anxiety disorder (GAD) in the pre- and postnatal periods was 15.8% and 17.1%, respectively; an incidence far exceeding that of perinatal depression (3.9% and 4.8%, for the same periods).6 Additional evidence suggests that even more women in the postnatal period experience clinically significant levels of anxiety but do not meet full diagnostic criteria for an anxiety disorder.7
In another study, 9.5% of women met criteria for GAD at some point during pregnancy, with highest anxiety levels in the first trimester.8 Women with a history of GAD, lower education, lack of social support, and personal history of child abuse have the highest risk for postpartum anxiety. Women with a history of posttraumatic stress disorder (PTSD) may be twice as likely to develop postpartum anxiety as healthy women.9
It has been well-documented that sleep disruption—which is very common in new mothers in the postnatal period—contributes to mood and anxiety disorders.10,11
Clarifying a diagnosis of postpartum anxiety
The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5)12 specifies no diagnosis of postpartum anxiety disorder. And no standardized diagnostic criteria exist. It is likely that in some cases, postpartum anxiety represents an exacerbation of underlying GAD, and in other cases it is a situational disorder brought about by specific circumstances of the peripartum period.
The DSM-5 does, however, provide a helpful diagnostic approach. It defines a diagnosis of postpartum depression as being a variant of major depressive disorder (MDD) in which a woman must 1) meet criteria for a major depressive episode; and 2) occur during pregnancy or within 4 weeks of delivery. In practice, many clinicians extend the second requirement to include the first year postpartum.13 There is a “with anxious distress” specifier for major depression in the DSM-5, but the 2 disorders are otherwise unlinked.
Continue to: To apply the...
To apply the DSM-5 principles for postpartum depression to postpartum anxiety, a patient would need to 1) meet the diagnostic criteria for an anxiety disorder that 2) have their onset within a specified perinatal period. Variant presentations of anxiety in the postpartum period might include panic disorder and phobias, which could also interfere with a woman’s ability to care for her child.
The DSM-5 offers the following criteria for GAD12:
- excessive worry about a variety of topics
- worry that is experienced as hard to control
- worry associated with at least 3 physical or cognitive symptoms: edginess/restlessness, tiring easily, impaired concentration, irritability
- anxiety, worry, or associated symptoms that make it hard to carry out day-to-day activities and responsibilities
- symptoms that are unrelated to any other medical conditions and cannot be explained by the effect of substances including a prescription medication, alcohol, or recreational drugs
- symptoms that are not better explained by a different mental disorder.
Debilitating effects of postpartum anxiety
Many women experience some level of anxiety during pregnancy and early postpartum—anxiety that may range from normal and adaptive to debilitating.14 While the challenges of caring for a newborn are likely to bring some level of anxiety, these symptoms should be transient and not interfere with a woman’s capacity to care for her infant, herself, or her family.
Postpartum anxiety has been associated with a prior fear of giving birth, fear of death (of both mother and baby), lack of control, lack of self-confidence, and lack of confidence in the medical system.9 The experience of such ongoing disturbing thoughts or feelings of worry and tension that affect a woman’s ability to manage from day to day should indicate an illness state that deserves medical attention.
Mothers with postpartum anxiety disorders report significantly less bonding with their infants than do mothers without anxiety.15 A recent narrative review describes numerous studies that illustrate the negative effects of postpartum anxiety on bonding, breastfeeding, infant temperament, early childhood development, and conduct disorders.16 Anxious women may be less likely to initiate breastfeeding, have more challenges with breastfeeding, and even have a different milk composition.17 Women with prenatal anxiety are also more likely to stop breastfeeding prematurely.18 Children of anxious mothers may be more likely to have a difficult temperament and to display more distress.19 There are small studies demonstrating deficits in early infant development and increases in conduct disorder in the male offspring of anxious women.20
Continue to: SCREENING FOR POSTPARTUM ANXIETY
SCREENING FOR POSTPARTUM ANXIETY
Screening for perinatal depression has become standard of care, and the Edinburgh Postnatal Depression Scale (EPDS) is a widely used instrument.1 The EPDS, a 10-question self-report scale, was created and validated to screen for perinatal depression, with a cutoff of > 10/30 usually considered a positive result.
Researchers have investigated the utility of the EPDS as a screening tool for perinatal anxiety as well.21-23 These studies show some promise, but there are questions as to whether a total score or a subscale score of the EPDS is most accurate in detecting anxiety. Women with perinatal anxiety may score low on the total EPDS, yet score higher on 3 anxiety-specific questions (TABLE 123). For this reason, several studies propose an EPDS anxiety subscore or subscale (referred to as EPDS-3A).
Of note, there are some women who will score high on the subscale who do not ultimately meet the criteria for an anxiety disorder diagnosis. Clinicians should not over-interpret these scores and should always use sound clinical judgment when making a diagnosis.
Research has also focused on using the GAD 7-item (GAD-7) scale (TABLE 224),25 and on the
Family physicians may consider using the EPDS subscale if they are already using the EPDS, or adding the GAD-7 as a separate screening instrument during a postpartum visit. To date there is no one standard recommendation or screening tool.
Continue to: NONPHARMACOLOGIC TREATMENT
NONPHARMACOLOGIC TREATMENT
As one would with any patient who has situational anxiety, help new mothers find ways to increase their coping skills, reduce stress, and mobilize social supports and family resources. Given the association between sleep disruption and perinatal anxiety, counsel new mothers, especially those at high risk for postpartum anxiety, to prioritize sleep during this vulnerable time. To that end, consider recommending that they ask partners, family members, or friends to help them take care of the infant at night (or during the day). Such nonmedical interventions may be sufficient for women with mild anxiety.
Very few studies have addressed nonpharmacologic management of postpartum anxiety, but cognitive behavioral therapy (CBT) has been shown to help in managing and treating anxiety disorders outside of pregnancy.28 A few small studies indicate promise for CBT and for mindfulness-based interventions (MBIs) during pregnancy.29
A 2016 systematic review of pharmacologic and nonpharmacologic treatment of anxiety in the perinatal period found support for the use of CBT for panic disorder and specific phobias both in pregnancy and postpartum.30 A very small study found that teaching mothers to massage their preterm infants decreased maternal anxiety.31
If the patient is amenable, it is reasonable to start with behavioral interventions like CBT or MBI before pharmacologic treatment—particularly when physicians have mental health professionals embedded in their primary care team.
PHARMACOLOGIC TREATMENT
Selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are considered first-line treatment for moderate to severe anxiety disorders in the perinatal and postnatal period.
Continue to: SSRIs in pregnancy
SSRIs in pregnancy. Lacking support of randomized controlled trials, most recommendations regarding SSRIs in pregnancy come from expert consensus or cohort and case control studies. Studies have raised concerns for an increased rate of congenital heart defects among fetuses exposed to paroxetine32 and primary pulmonary hypertension with all SSRIs.33 But the absolute risks are quite small. There have also been concerns regarding low birth weight and preterm birth, but it is possible that these outcomes result from the depression itself rather than the medication.34
Unfortunately, there are very few studies evaluating the efficacy of SSRIs in treating postpartum depression35 and even fewer that specifically evaluate their effect on perinatal anxiety. Many experts believe that not treating anxiety/depression is actually more harmful than the fetal effects of SSRIs, and that SSRIs are largely safe in both pregnancy and while breastfeeding, with benefits outweighing the risks.
SSRIs while breastfeeding. SSRIs have been found to be present in varying levels in breastmilk but may or may not be present in the serum of nursing infants.36 A 2008 guideline from the American College of Obstetricians and Gynecologists lists paroxetine, sertraline, and fluvoxamine as slightly safer than fluoxetine, escitalopram, and citalopram.37 A 2015 systematic review similarly concluded that sertraline and paroxetine have the most safety data on lactation.38 Lowest effective dose is always recommended to minimize exposure.
Benzodiazepines. As in the general population, benzodiazepines should be reserved for short-term use in acute anxiety and panic because they are associated with such adverse effects as worsening of depression/anxiety and risk of dependence and overdose. Longer-acting benzodiazepines (eg, clonazepam) are generally not recommended in lactation because of reported effects on infants, including sedation. Shorter-acting benzodiazepines (eg, lorazepam) are considered safer in lactation.39
THE CASE
Julia saw her family physician 4 more times, was evaluated by an ear-nose-and-throat specialist for her throat complaints, saw a therapist for CBT and a psychiatrist for medication, had 3 more ED visits, and lost 23 pounds before she finally agreed to start an SSRI for postpartum anxiety. She screened high on the EPDS-3A (9/9) despite scoring low on the full EPDS for perinatal depression (total, 9/30).
Continue to: Because of her swallowing impediments...
Because of her swallowing impediments and because she was breastfeeding, sertraline solution was started at very small doses. It was titrated weekly to obtain therapeutic levels. By 4 weeks, her weight stabilized. By 8 weeks, she started gaining weight and sleeping better. She saw the therapist regularly to continue CBT techniques. Over the next several months she started eating a normal diet. She is currently maintained on her SSRI, is still breastfeeding, and has achieved insight into her perinatal anxiety disorder.
CORRESPONDENCE
Veronica Jordan, MD, 3569 Round Barn Cir #200, Santa Rosa, CA 95403; veronica.a.jordan@gmail.com.
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
1. O’Connor E, Rossom RC, Henninger M, et al. Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:388-406.
2. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
3. Giardinelli L, Innocenti A, Benni L, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Womens Ment Health. 2012;15:21-30.
4. Rallis S, Skouteris H, McCabe M, et al. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36-e42.
5. Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms — 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66:153-158.
6. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155.
7. Phillips J, Sharpe L, Matthey S, et al. Maternally focused worry. Arch Womens Ment Health. 2009;12:409-418.
8. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131:277-283.
9. Schlomi Polachek I, Huller Harari L, Baum M, et al. Postpartum anxiety in a cohort of women from the general population: risk factors and association with depression during last week of pregnancy, postpartum depression and postpartum PTSD. Isr J Psychiatry Relat Sci. 2014;51:128-134.
10. Bei B, Coo S, Trinder J. Sleep and mood during pregnancy and the postpartum period. Sleep Med Clin. 2015;10:25-33.
11. Lawson A, Murphy KE, Sloan E, et al. The relationship between sleep and postpartum mental disorders: a systematic review. J Affect Disord. 2015;176:65-77.
12. APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013.
13. Langan R, Goodbred AJ. Identification and management of peripartum depression. Am Fam Physician. 2016;93:852-858.
14. Ali E. Women’s experiences with postpartum anxiety disorders: a narrative literature review. Int J Womens Health. 2018;10:237-249.
15. Tietz A, Zietlow AL, Reck C. Maternal bonding in mothers with postpartum anxiety disorder: the crucial role of subclinical depressive symptoms and maternal avoidance behaviour. Arch Womens Ment Health. 2014;17:433-442.
16. Field T. Postnatal anxiety prevalence, predictors and effects on development: a narrative review. Infant Behav Dev. 2018;51:24-32.
17. Serim Demirgoren B, Ozbek A, Ormen M, et al. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J Int Med Res. 2017;45:843-848.
18. Ystrom E. Breastfeeding cessation and symptoms of anxiety and depression: a longitudinal cohort study. BMC Pregnancy Childbirth. 2012;12:36.
19. Britton JR. Infant temperament and maternal anxiety and depressed mood in the early postpartum period. Women Health. 2011;51:55-71.
20. Glasheen C, Richardson GA, Kim KH, et al. Exposure to maternal pre- and postnatal depression and anxiety symptoms: risk for major depression, anxiety disorders, and conduct disorder in adolescent offspring. Dev Psychopathol. 2013;26:1045-1063.
21. Petrozzi A, Gagliardi L. Anxious and depressive components of Edinburgh Postnatal Depression Scale in maternal postpartum psychological problems. J Perinat Med. 2013;41:343-348.
22. Bina R, Harrington D. The Edinburgh Postnatal Depression Scale: screening tool for postpartum anxiety as well? Findings from a confirmatory factor analysis of the Hebrew version. Matern Child Health J. 2016;20:904-914.
23. Matthey S, Fisher J, Rowe H. Using the Edinburgh postnatal depression scale to screen for anxiety disorders: conceptual and methodological considerations J Affect Disord. 2013;146:224-230.
24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
25. Simpson W, Glazer M, Michalski N, et al. Comparative efficacy of the Generalized Anxiety Disorder 7-Item Scale and the Edinburgh Postnatal Depression Scale as screening tools for generalized anxiety disorder in pregnancy and the postpartum period. Can J Psychiatry. 2014;59:434-440.
26. Moran TE, Polanin JR, Wenzel A. The Postpartum Worry Scale-Revised: an initial validation of a measure of postpartum worry. Arch Womens Ment Health. 2014;17:41-48.
27. Fallon V, Halford JCG, Bennett KM, et al. The Postpartum Specific Anxiety Scale: development and preliminary validation. Arch Womens Ment Health. 2016;19:1079-1090.
28. Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621-632.
29. Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Womens Ment Health. 2014;17:373-387.
30. Marchesi C, Ossola P, Amerio A, et al. Clinical management of perinatal anxiety disorders: a systematic review. J Affect Disord. 2016;190:543-550.
31. Feijó L, Hernandez-Reif M, Field T, et al. Mothers’ depressed mood and anxiety levels are reduced after massaging their preterm infants. Infant Behav Devel. 2006;29:476-480.
32. Bérard A, Iessa N, Chaabane S, et al. The risk of major cardiac malformations associated with paroxetine use during the first trimester of pregnancy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:589-604.
33. Huybrechts KF, Bateman BT, Palmsten K, et al. Antidepressant use late in pregnancy and risk of persistent pulmonary hypertension of the newborn. JAMA. 2015;313:2142-2151.
34. Cantarutti A, Merlino L, Monzani E, et al. Is the risk of preterm birth and low birth weight affected by the use of antidepressant agents during pregnancy? A population-based investigation. PLoS One. 2016;11:e0168115.
35. Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;11:CD002018.
36. Freeman MP. Postpartum depression treatment and breastfeeding. J Clin Psychiatry. 2009;70:e35.
37. ACOG Committee on Practice Bulletins—number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111:1001-1020.
38. Orsolini L, Bellantuono C. Serotonin reuptake inhibitors and breastfeeding: a systematic review. Hum Psychopharmacol. 2015;30:4-20.
39. NIH. Drugs and Lactation Database. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 26, 2019.
Should you switch the DAPT agent one month after ACS?
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
PRACTICE CHANGER
Switch to clopidogrel from one of the newer P2Y12 blockers 1 month after an acute coronary event, while continuing aspirin, to decrease bleeding events without increasing the risk of ischemic events.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT).
Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
PrEP adherence lowest among Medicaid patients, others
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
SEATTLE – Female sex, young age, residing in a rural location, black race, and Medicaid insurance were all associated with reduced adherence to HIV pre-exposure prophylaxis in a study from the Centers for Disease Control and Prevention.
Daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir (Truvada) prevents infection, but not everyone sticks with it. The investigators wanted to find out who struggles the most with adherence to help focus future intervention efforts.
The team identified 7,250 commercially insured and 349 Medicaid PrEP users in IBM MarketScan databases during 2011-2016. They tracked them from their initial PrEP prescription until there was a gap of 30 days or more in their PrEP refills, at which point they met the study’s definition of nonpersistence.
Overall, “commercially insured nonpersistent PrEP users were young, female, and rural. Medicaid insured nonpersistent users were young, female, and black,” said lead investigator Ya-Lin Huang, PhD, a health scientist in the CDC Division of HIV/AIDS Prevention.
“It is concerning that some populations with low persistence were among those with the highest rates of HIV diagnosis, such as young, black men. Research is needed to understand reasons for discontinuing PrEP. Interventions tailored for priority populations are needed to improve PrEP persistence,” she said at the Conference on Retroviruses and Opportunistic Infections.
Her team found that commercially insured patients stuck with PrEP longer than did those on Medicaid, a median of 14.5 months, with 56% still filling their prescriptions after a year, versus a median Medicaid persistence of 7.6 months, with only a third of Medicaid patients still on PrEP after 12 months.
Men were more persistent with PrEP than were women in both groups, as were older people versus younger. The median length of adherence among commercially insured patients aged 45-54 years, for instance, was 20.5 months, versus 8.6 months among people aged 18-24 years. Older PrEP users persisted longer among Medicaid patients as well, a median of 10 versus 4 months.
Also in the Medicaid group, white patients stuck with PrEP longer than did black patients, a median of 8.5 months versus 4.1 months. There were no racial differences with commercial insurance.
The findings held even when the team used gaps of 60 and 90 days, instead of 30 days, to define nonpersistence.
The study says nothing about why people stopped PrEP, or why there were such stark differences between the groups.
Perhaps, in some cases, people quit risky behavior or entered new relationships. Maybe PrEP was too expensive for some, or transportation to the clinic was an issue. Side effects might have been a problem, or people could have lost their insurance coverage, or maybe didn’t want to deal with the hassle. It’s impossible to know from the data, Dr. Huang said.
She said her team wants to figure it out, so they can help people overcome barriers to treatment, which likely vary across subgroups.
The study also was limited to patients who were enrolled in coverage at least 6 months before and 6 months after their first PrEP prescription; the investigators want to exam the situation for people with less consistent coverage, and no coverage at all.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no disclosures.
SOURCE: Huang YA et al. CROI 2019, Abstract 106.
REPORTING FROM CROI 2019